Abstract:
Persistent pulmonary hypertension of the newborn (PPHN) is characterized by pulmonary arterial remodeling mainly because of apoptosis resistance and excessive proliferation of pulmonary artery smooth muscle cells (PASMCs). Sildenafil is a phosphodiesterase-5 inhibitor. Some reports have shown that sildenafil exerts protective effects against PPHN. However, the function of sildenafil in PPHN and the underlying molecular mechanisms is not clear. Here, we revealed that sildenafil effectively suppressed hypoxia-induced PASMC proliferation and apoptosis inhibition (P < 0.05). Also, sildenafil obviously reduced ventricular hypertrophy, and inhibited pulmonary vascular remodeling in the PPHN model (P < 0.05). Moreover, sildenafil treatment significantly attenuated the induction of Notch3 and Hes1 induced by hypoxia treatment (P < 0.05). Furthermore, overexpression of Notch3 abolished the reduction of PASMC proliferation and promotion of PASMC apoptosis induced by sildenafil under hypoxia (P < 0.05), whereas knockdown of Notch3 had an opposite effect (P < 0.05). Together, our study demonstrates that sildenafil shows a potential benefit against the development of PPHN by inhibiting Notch3 signaling, providing a strategy for treating PPHN in the future.
Key Words: persistent pulmonary hypertension of the newborn, sildenafil, proliferation, apoptosis, Notch3 signaling
INTRODUCTION
Persistent pulmonary hypertension of the newborn (PPHN) is a life-threatening clinical disorder of newborn infants, seen in about 2 per 1000 liveborn infants.1,2 It is characterized by sustained elevation of pulmonary vascular resistance, extrapulmonary shunting from right to left across persistent fetal channels, and severe hypoxemia.2 Newborns with PPHN are at risk of severe complications, including chronic lung disease, asphyxia, neurodevelopmental sequelae, and death.3 The major histopathological feature of PPHN is pulmonary vascular remodeling.4 The abnormal proliferation and apoptosis resistance of pulmonary artery smooth muscle cells (PASMCs) has been tightly associated with vascular remodeling in hypoxia-induced PPHN. Therefore, reduction of PASMCs proliferation or elevation of PASMCs apoptosis using therapeutic agents may represent a promising strategy for PPHN treatment.
Sildenafil, an inhibitor of phosphodiesterase type 5 (PDE5) with high selectivity, enhances intracellular cyclic guanosine monophosphate (cGMP) levels by suppressing PDE5 activity and cGMP breakdown.5 cGMP accumulation results in a series of cellular changes that conclude with a reduction in the levels of intracellular calcium and the relaxation of smooth muscles.6 PDE5 inhibition is approved for erectile dysfunction, and although it is expressed in smooth muscles of the vascular system, it has no effect on systemic blood pressure. In addition, several studies show that the PDE5 inhibitor sildenafil facilitates vasodilatation in the pulmonary vascular bed, decreases the proliferation of PASMCs, and induces apoptosis.7–9 These properties have been used for the treatment of adult patients with pulmonary hypertension. Recently, sildenafil has been reported to treat the newborns with PPHN.6,10 However, the molecular mechanisms of sildenafil in PPHN remains unknown.
The aim of this study was to investigate the role of sildenafil in the hypoxia-stimulated the proliferation and apoptosis of rat PASMCs.
METHODS
Animal Experiment
All procedures and protocols were approved by the Committee on the Ethics of Animal Experiments at Qilu Children's Hospital of ShanDong University (approval no: ETYY-2019208). Eight-week-old healthy female Sprague-Dawley rats (weight, 250–300g) were mated overnight (day 0 of gestation). A combination of hypoxia and indomethacin was used to establish the rat model of PPHN, as previously described.11 In brief, on day 11 of gestation, pregnant rats were randomly assigned to 7 groups (n = 5/group).
The normoxia group: pregnant rats were subcutaneously injected with normal saline once a day for 10 days. During days 19–21 of gestation, pregnant rats were housed under standard normoxic condition (oxygen concentration, 21%), and intraperitoneally injected with isotonic saline twice a day.
The PPHN model group: pregnant rats were subcutaneously injected with normal saline once a day for 10 days. During days 19–21 of gestation, pregnant rats were subjected to hypoxic condition (oxygen concentration, 10 ± 0.5%), and intraperitoneally injected with 0.5 mg/kg indomethacin twice a day.
The sildenafil group (50 mg/kg; SDNF50): pregnant rats were subcutaneously injected with 50 mg/kg sildenafil once a day for 10 days. During days 19–21 of gestation, pregnant rats were subjected to hypoxic condition (oxygen concentration, 10 ± 0.5%), and intraperitoneally injected with 0.5 mg/kg indomethacin twice a day.
The sildenafil group (100 mg/kg; SDNF100): pregnant rats were subcutaneously injected with 100 mg/kg sildenafil once a day for 10 days. During days 19–21 of gestation, pregnant rats were subjected to hypoxic condition (oxygen concentration, 10 ± 0.5%), and intraperitoneally injected with 0.5 mg/kg indomethacin twice a day.
The sildenafil group (200 mg/kg; SDNF200): pregnant rats were subcutaneously injected with 200 mg/kg sildenafil once a day for 10 days. During days 19–21 of gestation, pregnant rats were subjected to hypoxic condition (oxygen concentration, 10 ± 0.5%), and intraperitoneally injected with 0.5 mg/kg indomethacin twice a day.
The sildenafil + Ad-Notch3 group (200 mg/kg; SDNF200): pregnant rats were subcutaneously injected with 200 mg/kg sildenafil once a day for 10 days, and received a tail vein injection of Ad-Notch3 adenovirus (1.0 × 109 plaque forming unit). During days 19–21 of gestation, pregnant rats were subjected to hypoxic condition (oxygen concentration, 10 ± 0.5%), and intraperitoneally injected with 0.5 mg/kg indomethacin twice a day.
The Ad-Notch3 shRNA group: pregnant rats were subcutaneously injected with normal saline once a day for 10 days and received a tail vein injection of Ad-Notch3 shRNA adenovirus (1.0 × 109 plaque forming unit). During days 19–21 of gestation, pregnant rats were subjected to hypoxic condition (oxygen concentration, 10 ± 0.5%), and intraperitoneally injected with 0.5 mg/kg indomethacin twice a day.
On day 22 of gestation, the fetuses were delivered by cesarean section. All fetuses (n = 30/group) were anesthetized with 3% pentobarbital sodium (60 mg/kg intraperitoneally). After fetuses were sacrificed by cervical dislocation, hearts and lungs were removed and prepared for further examination. The right ventricle (RV), left ventricle (LV), and ventricular septum (S) were separated and weighed. The RV hypertrophy index (RVHI) was calculated by measuring the weight ratio of the RV to the LV and the S (RV/[LV + S]).
Hematoxylin and Eosin (H&E) Staining
Lung tissues were paraffin-embedded and cut into 4-μm-thick sections. Sections were deparaffinized in graded alcohol solutions and xylene, and stained with H&E. Sections were photographed using a light microscope (Olympus, Tokyo, Japan).
Plasmid, siRNAs, and Transfection
Plasmids expressing Notch3 were designed and constructed by Jennio Biotech (Guangzhou, China). Notch3 siRNA was obtained from Ribobio (Guangzhou, China). Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) was used to transfect plasmids and siRNAs into PASMCs.
Cell Isolation, Culture, and Hypoxia-Induced Cell Proliferation Assay (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2-H-tetrazolium bromide Assay)
Rat lungs were cut into small pieces. PASMCs affixed tightly to the culture plates, and were cultured in Dulbecco's modified eagle's medium (DMEM, GIBCO, Grand Island, NY) with 10% fetal bovine serum (Gibco). The purity and identity of PASMCs were confirmed by a-alpha-smooth muscle (α-SMA) antibody. PASMCs (3 × 104 cells/mL) were seeded into the 96-well plates with 100 µL in each well. Cells were then grouped into normoxia, hypoxia, and hypoxia+sildenafil (concentrations of 10, 50, and 100 nM of sildenafil were adopted), totaling 5 groups. The normoxic group was grown at 37°C in 95% O2, 5% CO2 condition, and the hypoxic groups were maintained in 3% oxygen condition for 24, 48, 72, and 96 hours, respectively. For further investigating whether the Notch3 signaling pathway participated in the antiproliferative and proapoptotic effects of sildenafil, Notch3 siRNA or plasmid was transfected into the PASMCs. PASMCs were divided into normoxia, hypoxia, hypoxia + sildenafil (100 nM), hypoxia + Notch3 siRNA (50 nmol/L), and hypoxia + sildenafil (100 nM) + Notch3 plasmid (2ug/mL), totaling 5 groups. After cells were exposed to normoxia or hypoxia conditions for 24, 48, 72, and 96 hours, 20 µL of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide solution (5 mg/mL) was added each well. After cultivation for 4 hours at 37°C, 200 µL of dimethyl sulfoxide was added to each well. The absorbance at 450 nm was determined using Epoch Microplate Spectrophotometer (Bio Tek, Winooski, VT).
Quantitative Real-Time PCR
TRIzol reagent (Invitrogen, Carlsbad, CA) was used to extract the total RNA from tissues and cells. RNA was reverse-transcribed into complementary DNA by MMLV reverse transcriptase (Promega, Madison, WI). qRT-PCR was performed using SYBR qPCR Mix (Promega) and an ABI 7900HT System (Applied Biosystems, Foster City, CA). β-actin was served as an endogenous control gene for normalizing Notch3 and Hes1 expression. Fold change of gene expression was expressed as 2−ΔΔCT. Primer sequences in our study are listed as follows: Notch3 primers: forward 5′-ATAAACCTTCAACTGCTCAGTA GTT-3′, reverse 5′-ATTATATCAGCTGGCATTTTCGT-3′; Hes1 primers: forward 5′-GCCAATTTGCCTTTCTCATC-3′, reverse 5′-GAGGTGGGCTAGGGACTTTA-3′; β-actin primers: forward 5′-TCTACGAGGGTTATGCCCTT-3′, reverse 5′-GTCACGGACGATTTCACG-3′.
Cell Apoptosis Analysis
Cell apoptosis was assessed using an fluorescein 5-isothiocyanate (FITC)-labeled Annexin V (Annexin V-FITC) apoptosis detection kit (Beyotime, Jiangsu, China). Cells were harvested and resuspended in 100 µL of serum-free medium. After suspension, cells were double-stained with 5-µL Annexin V-FITC and 5-µL propidium iodide, and incubated for 15 minutes at room temperature in the dark. Subsequently, cell apoptosis was evaluated by flow cytometry (Becton Dickinson, Franklin Lakes, NJ), and analyzed with Flowjo 7.6 software (TreeStar Inc, Ashland, OR). Living, dead, early apoptotic, and apoptotic cells were distinguished, with the focus laid on the relative proportion of early and late apoptotic cells.
Cell Cycle Analysis
The harvested cells were resuspended in 1000 µL of serum-free medium. Then, cells were fixed with 75% ethanol for 4 hours at 4°C, and then the supernatant was discarded, followed by incubation with 2 μg/mL of RNase I (Sigma-Aldrich) for 30 minutes at 37°C. After incubation with 10 μg/mL of PI (Sigma-Aldrich) for 30 minutes at 4°C, cells were washed with cold phosphate buffered solution 3 times, and then cell cycle was analyzed by using flow cytometry (Becton Dickinson). The percentage of cells in the phase of G0/G1, S, or G2/M was calculated using the Flowjo 7.6 software (TreeStar Inc).
Western Blotting
RIPA buffer (Thermo Fisher Scientific, Waltham, MA) was used to extract total protein from tissues and cells. The concentration of protein was measured using the bicinchoninic acid kit (Beyotime). 20 μg of protein sample was fractionated by 10% SDS-PAGE, and then transferred to PVDF membranes (Millipore, Bedford, MA). Protein was blocked using 5% skim milk for 2 hours. The primary antibody Notch3 (1:2000; Invitrogen), Hes1 (1:3000; Invitrogen), or GAPDH (1:5000; Invitrogen) was used for incubation of membranes overnight at 4°C. The membranes were washed 3 times, and incubated with secondary antibody (1:5000; Invitrogen) for 1 hour at room temperature. Immunoreactivity was visualized using an enhanced chemiluminescence kit (Millipore). GAPDH was used as the internal reference.
Statistical Analysis
All quantitative data are reported as the mean ± SD. Statistical significance was analyzed using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. Statistical analysis of data was performed using GraphPad Prism 7.0. Differences were considered statistically significant at P < 0.05.
RESULTS
Sildenafil Ameliorates Pulmonary Artery Structural Remodeling in a Rat Model of PPHN
We investigated whether sildenafil attenuates pulmonary arterial remodeling in a rat model of PPHN. As shown in Figure 1A, PPHN group caused a significant enhancement in RVHI. Sildenafil administered at both 100 mg/kg and 200 mg/kg alleviated the elevation of RVHI induced by cotreatment with hypoxia and indomethacin. Next, the PPHN group exhibited a significant increase in the wall thickness of pulmonary arteries, changed that were reversed by sildenafil administration (Fig. 1B).
FIGURE 1.

Effects of sildenafil on the PPHN model. A, RVHI in the indicated rats (n = 30). B, Representative histopathological changes in the indicated rat lung tissues (n = 30). Data are expressed as the mean ± SD. *P < 0.05 compared with the normoxia group; #P < 0.05, compared with the model group.
Sildenafil Inhibited Cell Proliferation and Induced Apoptosis in Rat PASMCs Under Hypoxia
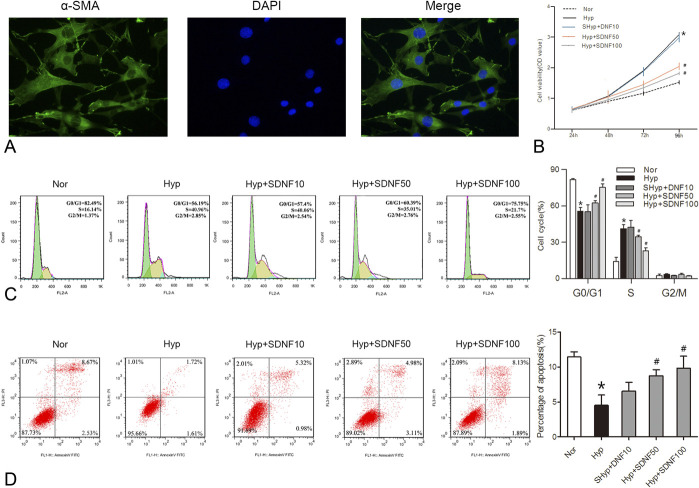
Hypoxia-induced PASMC proliferation and apoptosis inhibition is the significant feature of hypoxia-induced PPHN.12 Therefore, we examined whether sildenafil protected PPHN by repressing PASMC proliferation and inducing apoptosis. We first isolated and identified PASMCs from the rat pulmonary artery. As shown in Figure 2A, immunofluorescence staining of a-SMA displayed that the cytoplasm of cells was rich in green myofilament, which confirmed that the separated cells were PASMCs. Furthermore, hypoxia markedly accelerated the cell viability of PASMCs, as shown in Figure 2B, and sildenafil suppressed the hypoxia-induced PASMCs proliferation. Consistent with the results in Figure 2C, hypoxia exhibited a significant decrease in the percentage of G0/G1 phase cells and an increase in the percentage of S phase cells, while sildenafil markedly enhanced the proportion of cells in the G0/G1 phase and reduced the S phase cell population. Simultaneously, hypoxia obviously inhibited the apoptosis index of PASMCs, and the inhibitory effect was abolished by sildenafil treatment (Fig. 2D).
FIGURE 2.
Effects of sildenafil on the proliferation and apoptosis of rat PASMCs under hypoxia. A, Immunofluorescence identification of PASMCs with α-SMA. B, The effect of sildenafil on hypoxia-induced PASMC proliferation. C, Representative images and quantification of PASMC cell cycle distribution. D, Representative images and quantification of PASMC apoptosis. Data are expressed as the mean ± SD, n = 4. *P < 0.05 compared with the normoxia group; #P < 0.05, compared with the hypoxic group.
Sildenafil Suppresses the Activation of Notch3 Signaling Pathway In Vitro and In Vivo
It was reported that Notch3 was implicated in the pathophysiology of pulmonary vascular remodeling in PAH.13 Moreover, sildenafil has been reported to regulate Notch3 signaling pathway.14 To explore whether sildenafil can reduce the expression of Notch3 in the hypoxia-induced PASMCs and a rat model of PPHN, the expression of Notch3 was detected. Notch3 mRNA and protein expression levels were increased in the hypoxia-induced PASMCs and lung from the PPHN rat, as predicted, and sildenafil reversed the increased the mRNA and protein expression of Notch3 in the hypoxia-exposed PASMCs and lung from the PPHN rat (Fig. 3A-D). Hes1 has reported to be a downstream gene of Notch3 signaling pathway. We also evaluated the effect of sildenafil on the expression of Hes1 in vitro and in vivo, and found that Hes1 expression was upregulated at both mRNA and protein levels in the hypoxia-exposed PASMCs and lung from the PPHN rat, whereas these effects were significantly reversed by sildenafil administration (Fig. 3A-D).
FIGURE 3.

Effects of sildenafil on the Notch3 signaling pathway in vitro and in vivo. A-D, The mRNA and protein expression levels of Notch3 and Hes1 in the hypoxia-exposed PASMCs and lung from the PPHN rat. Data are expressed as the mean ± SD, n = 4. *P < 0.05 compared with the normoxia group; #P < 0.05, compared with the hypoxic group. $P < 0.05, compared with the hypoxic+sildenafil (SDNF) group. Data are expressed as the mean ± SD, n= 4. *P < 0.05 compared with the normoxia group; #P < 0.05, compared with the model group. $P < 0.05, compared with the model + SDNF group.
Overexpression of Notch3 Reverses the Sildenafil-Mediated Decrease in PASMC Proliferation, and Increase in PASMC Apoptosis Under Hypoxia
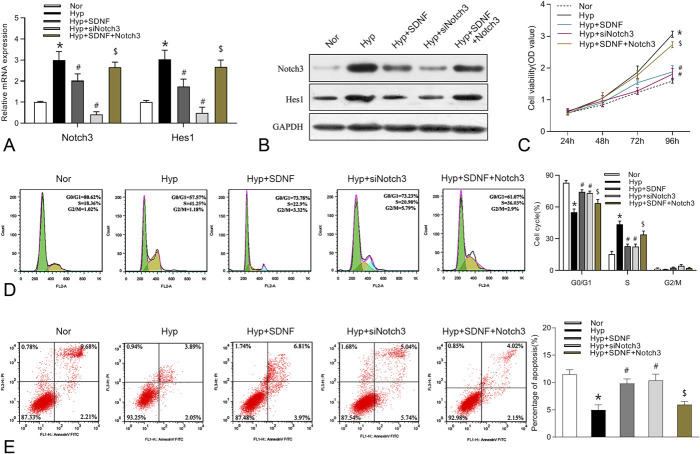
After the confirmation that sildenafil could modulate the Notch3 signaling pathway in PASMCs, we investigated whether sildenafil could regulate the proliferation and apoptosis of PASMCs by modulating the Notch3 signaling pathway. PASMCs were treated with Notch3 siRNA or plasmid and sildenafil under hypoxia, and then Notch3 mRNA and protein levels were examined. Figure 4A, B showed that overexpression of Notch3 significantly abolished the decreased the mRNA and protein levels of Notch3 and Hes1 induced by sildenafil under hypoxia, while knockdown of Notch3 further aggravated hypoxia-induced Notch3 and Hes1 mRNA and protein levels. Furthermore, enforced expression of Notch3 remarkedly restored the inhibition of PASMC growth and promotion of PASMC apoptosis induced by sildenafil under hypoxia, while silencing of Notch3 further facilitated hypoxia-induced cell growth and apoptosis inhibition (Fig. 4C-E).
FIGURE 4.
Effects of Notch3 on the proliferation and apoptosis of rat PASMCs under hypoxia. A-B, The mRNA and protein expression levels of Notch3 and Hes1 in PASMCs treated with Notch3 siRNA (50 nmol/L) or plasmid (2ug/mL) and sildenafil (100 nM) under hypoxia. C-E, The effects of Notch3 on cell viability, cell cycle progression, and apoptosis. Data are expressed as the mean ± SD, n = 4. *P < 0.05 compared with the normoxia group; #P < 0.05, compared with the hypoxic group. $P < 0.05, compared with the hypoxic+ SDNF group.
Enforced Expression of Notch3 Abolished the Protective Effect of Sildenafil on PPHN In Vivo
To further verify whether Notch3 could attenuate the protective effect of sildenafil on the PPHN rat model, the rescue experiment was performed. As expected, Notch3 overexpression overturned the repressive effects on the expression of Notch3 and Hes1 mediated by sildenafil (Fig. 5A, B). Silencing of Notch3 resulted in a decrease in the expression of Notch3 and Hes1 compared with the PPHN group (Fig. 5A, B). Moreover, the downregulation of RVHI and wall thickness of pulmonary arteries induced by sildenafil was restored by Notch3 elevation (Fig. 5C, D). However, Notch3 knockdown had higher RVHI and wall thickness of pulmonary arteries than those in the PPHN group (Fig. 5C, D).
FIGURE 5.
Effects of Notch3 on the PPHN model. A-B, Notch3 and Hes1 mRNA and protein expression levels in the lung from the indicated rats. C, RVHI in the indicated rats (n = 30). D, Representative histopathological changes in the lung from the indicated rats (n = 30). Data are expressed as the mean ± SD. *P < 0.05 compared with the normoxia group; #P < 0.05, compared with the model group. $P < 0.05, compared with the model + SDNF group.
DISCUSSION
PPHN is a progressive and complex disease with high mortality and morbidity.15 Abnormal pulmonary arterial remodeling plays a crucial role in the development of this disease. It is well known that hypoxia-induced hyperproliferation of PASMCs is a cause of pulmonary vascular remodeling, but the underlying mechanisms are still unclear. In this study, we demonstrated that sildenafil repressed hypoxia-induced rat PASMC proliferation and apoptosis inhibition. Meanwhile, sildenafil also ameliorated experimental PPHN. Moreover, sildenafil significantly attenuated the upregulation of Notch3 and Hes1 induced by hypoxia treatment. In addition, the effects of sildenafil could be markedly abolished by overexpression of Notch3. The present findings suggest that sildenafil may be a potentially effective drug target for the treatment of PPHN, helping us to further understand the pathogenesis of PPHN.
Sildenafil is a potent and selective PDE5 inhibitor, and has been proposed as a new strategy for PPHN treatment.16 Animal studies have demonstrated that sildenafil significantly repressed the pulmonary arterial remodeling, RVSP, and RVHI, as well as limited the increase in pulmonary artery medial thickness in the chronic hypoxia-induced pulmonary hypertension (PAH) rat model.17,18 Several studies have reported that sildenafil is safe during pregnancy in different species, including rat.7,19,20 Antenatal sildenafil has been reported to improve pathological features of PPHN in experimental congenital diaphragmatic hernia, such as decreased pulmonary vascular density, pulmonary artery remodeling, and RVH.7,19,20 Consistent with the aforementioned studies, this study showed that sildenafil protected rat from pulmonary artery remodeling in a model of PPHN. However, the secondary effects of sildenafil through the mother to the offsprings need further investigation. In arterial disease, vascular smooth muscle cells (VSMCs) are typically static. The proliferation of VSMCs serves a vital role in chronic hypoxia-induced PAH or PPHN.21–23 Thus, our study aimed to examine whether sildenafil could exert an ameliorative effect on the remodeling of pulmonary vascular by inhibition of PASMC proliferation. Previous studies have reported that sildenafil treatment ameliorated platelet-derived growth factor-mediated proliferation of PASMCs by an interaction between the cGMP-protein kinase G and the cAMP-protein kinase A activated pathways, causing inhibition of platelet-derived growth factor-mediated ERK activation in human PASMCs.24 Moreover, sildenafil also exerted an antiproliferative effect induced via hypoxia or ET-1 in the PASMCs.16,25 Consistently, our data revealed that hypoxia treatment accelerated the viability and cell cycle progression and impeded the apoptosis of PASMCs, whereas sildenafil attenuated these effects. Collectively, the results demonstrated that sildenafil inhibited cell viability and cell cycle progression, and induced apoptosis, thereby reversing vascular remodeling in response to hypoxic conditions.
Increasing evidence suggests that sildenafil plays a crucial role in cell growth, migration, angiogenesis, invasion, differentiation, and apoptosis by various signaling pathways, including PI3-K/Akt/GSK-3,26 MAPK,27,28 and nitric oxide and guanosine 3′,5′-cyclic monophosphate29 signaling. Recent evidence indicates that intraamniotic sildenafil treatment caused downregulation of Notch3 in the nitrofen model of congenital diaphragmatic hernia.14 However, whether sildenafil could regulate the Notch3 signaling pathway in hypoxia-induced PPHN is not clear. In our study, sildenafil reduced the expression of Notch3 in vitro and in vivo induced by hypoxia. Notch3 is a member of the Notch family receptors.30 Notch3 is predominantly expressed in VSMCs, and regulates multiple behaviors of VSMCs such as proliferation, migration, invasion, and apoptosis.31 A recent study has indicated that Notch3 is upregulated in the lung tissues from clinic PAH patients and animal models of PAH.32 Another study demonstrates that blockade of Notch3 activity using gamma secretase inhibitor blocks the development of PAH in the animal model by inhibiting the proliferation of PASMCs.33 However, whether sildenafil could affect PASMC proliferation and apoptosis by regulating the Notch3 signaling pathway remains unclear. In this study, overexpression of Notch3 reversed sildenafil-mediated decrease in the proliferation of PASMCs and increase in apoptosis under hypoxia, while loss of Notch3 further accelerated hypoxia-induced cell proliferation and apoptosis suppression. Meanwhile, upregulation of Notch3 reversed the inhibitory effects of sildenafil on the RVHI and wall thickness of pulmonary arteries. However, downregulation of Notch3 increased the RVHI and wall thickness of pulmonary arteries compared with the PPHN group.
Upon Notch3 activation, the released Notch3 intracellular domain (NICD3) by activation of Notch3 translocates to the nucleus to form an active transcriptional complex with CBF-1/RBP-Jk to modulate the transcription of effector genes such as the Hes/Hey families.31,33 Hes family members play an important role in the pulmonary vasculature. Upregulation of Notch3 intracellular domain3 in PASMCs from monocrotaline-treated rats was associated with enhancement of Hes1 expression and a concurrent reduction in the expression of p27 Kip1, thereby regulating cell cycle progression and cell proliferation.13 This study demonstrated that overexpression of Notch3 in rat PASMCs and the PPHN model upregulated Hes1 to reverse sildenafil-mediated the reduction of PASMC proliferation and promotion of apoptosis under hypoxia, as well as the RVHI and wall thickness of pulmonary arteries.
CONCLUSIONS
Our data suggested that sildenafil seems to exert its effects via suppressing excessive the proliferation of rat PASMCs and pulmonary vascular remodeling by inactivation of Notch3 signaling pathway. Thus, sildenafil may be a promising new therapeutic drug for the treatment of PPHN.
Footnotes
The author reports no conflicts of interest.
L. Kang made plans for the experiment. X. Liu conducted the experiments. Z. Li supported their method. X. Li and Y. Han joined the discussion. L. Kang wrote the manuscript. X. Li reviewed and revised the manuscript. C. Liu and C. Zhao collected and analyzed the data.
L. Kang, Z. Li, X. Li, and C. Zhao hold doctorates in medicine and philosophy.
X. Liu, Y. Han, C. Liu, and X. Li are masters in medicine.
Availability of data and material: The data used to support the findings of this study are available from the corresponding author upon request.
Ethics approval: All procedures and protocols were approved by the Committee on the Ethics of Animal Experiments at Zhejiang University (approval no: ETYY-2019208).
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics approval: All procedures and protocols were approved by the Committee on the Ethics of Animal Experiments at Zhejiang University (approval no: ETYY-2019208).
REFERENCES
- 1.Wedgwood S, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells. Am J Physiology Lung Cell Mol Physiol. 2015;309:L196–L203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair J, Lakshminrusimha S. Update on PPHN: mechanisms and treatment. Semin Perinatology. 2014;38:78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen J, Hedegaard ER, Simonsen U, et al. Current and future treatments for persistent pulmonary hypertension in the newborn. Basic Clin Pharmacol Toxicol. 2018;123:392–406. [DOI] [PubMed] [Google Scholar]
- 4.Farrow KN, Wedgwood S, Lee KJ, et al. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol. 2010;174:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhariwal AK, Bavdekar SB. Sildenafil in pediatric pulmonary arterial hypertension. J Postgrad Med. 2015;61:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonca L, Tulloh R. Sildenafil in infants and children. Children (Basel). 2017;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luong C, Rey-Perra J, Vadivel A, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation. 2011;123:2120–2131. [DOI] [PubMed] [Google Scholar]
- 8.Broughton BRS, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiology-Lung Cell Mol Physiol. 2008;294:L797–L806. [DOI] [PubMed] [Google Scholar]
- 9.Yamamura A, Yagi S, Ohara N, Tsukamoto K. Calcilytics enhance sildenafil-induced antiproliferation in idiopathic pulmonary arterial hypertension. Eur J Pharmacol. 2016;784:15–21. [DOI] [PubMed] [Google Scholar]
- 10.Evers PD, Critser PJ, Cash M, et al. Cost-utility of sildenafil for persistent pulmonary hypertension of the newborn. Am J Perinatol. 2020;38:1505–1512. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, Fu J, Yao L, et al. Altered expression of PPAR-γ and TRPC in neonatal rats with persistent pulmonary hypertension. Mol Med Rep. 2017;16:1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Nie X, Zhu J, et al. NDUFA4L2 in smooth muscle promotes vascular remodeling in hypoxic pulmonary arterial hypertension. J Cel Mol Med. 2021;25:1221–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris HE, Neves KB, Montezano AC, et al. Notch3 signalling and vascular remodelling in pulmonary arterial hypertension. Clin Sci. 2019;133:2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okolo FC, Zhang G, Rhodes J, Potoka DA. Intra-amniotic sildenafil treatment modulates vascular smooth muscle cell phenotype in the nitrofen model of congenital diaphragmatic hernia. Sci Rep. 2018;8:17668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J, Zhang L, Lian L, et al. CircATP2B4 promotes hypoxia-induced proliferation and migration of pulmonary arterial smooth muscle cells via the miR-223/ATR axis. Life Sci. 2020;262:118420. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Wang J, Zhao L, et al. Sildenafil inhibits human pulmonary artery smooth muscle cell proliferation by decreasing capacitative Ca2+ entry. J Pharmacol Sci. 2008;108:71–78. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Yin N, Hu L, et al. Sildenefil increases connexin 40 in smooth muscle cells through activation of BMP pathways in pulmonary arterial hypertension. Int J Clin Exp Pathol. 2014;7:4674–4684. [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, He W, Ye L, et al. Targeted delivery of sildenafil for inhibiting pulmonary vascular remodeling. Hypertension. 2019;73:703–711. [DOI] [PubMed] [Google Scholar]
- 19.Lemus-Varela MdL, Soliz A, Gómez-Meda BC, et al. Antenatal use of bosentan and/or sildenafil attenuates pulmonary features in rats with congenital diaphragmatic hernia. World J Pediatr. 2014;10:354–359. [DOI] [PubMed] [Google Scholar]
- 20.Russo FM, De Bie F, Hodges R, et al. Sildenafil for antenatal treatment of congenital diaphragmatic hernia: from bench to bedside. Curr Pharm Des. 2019;25:601–608. [DOI] [PubMed] [Google Scholar]
- 21.Yao J, Fang X, Zhang C, et al. Astragaloside IV attenuates hypoxia‑induced pulmonary vascular remodeling via the Notch signaling pathway [Corrigendum]. Mol Med Rep. 2021;25:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz A, Olorundami OA, Teng RJ, et al. Decreased OLA1 (Obg-Like ATPase-1) expression drives ubiquitin-proteasome pathways to downregulate mitochondrial SOD2 (superoxide dismutase) in persistent pulmonary hypertension of the newborn. Hypertension. 2019;74:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johar D, Magdeldin S. Application of laser scanning cytometry in vascular smooth muscle remodeling. Hypertens Res. 2018;41:869–885. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Yang L, Shen J, et al. The antiproliferative effect of sildenafil on pulmonary artery smooth muscle cells is mediated via upregulation of mitogen-activated protein kinase phosphatase-1 and degradation of extracellular signal-regulated kinase 1/2 phosphorylation. Anesth Analgesia. 2007;105:1034–1041. table of contents. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Li JF, Zhao L, et al. Inhibition of SOC/Ca2+/NFAT pathway is involved in the anti-proliferative effect of sildenafil on pulmonary artery smooth muscle cells. Respir Res. 2009;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Zhang ZG, Zhang RL, Chopp M. Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab. 2005;25:1150–1158. [DOI] [PubMed] [Google Scholar]
- 27.Pyriochou A, Zhou Z, Koika V, et al. The phosphodiesterase 5 inhibitor sildenafil stimulates angiogenesis through a protein kinase G/MAPK pathway. J Cel Physiol. 2007;211:197–204. [DOI] [PubMed] [Google Scholar]
- 28.Dhayade S, Kaesler S, Sinnberg T, et al. Sildenafil potentiates a cGMP-dependent pathway to promote melanoma growth. Cel Rep. 2016;14:2599–2610. [DOI] [PubMed] [Google Scholar]
- 29.Bolnick JM, Kilburn BA, Bolnick AD, et al. Sildenafil stimulates human trophoblast invasion through nitric oxide and guanosine 3', 5'-cyclic monophosphate signaling. Fertil Sterility. 2015;103:1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Zhang X, Leathers R, et al. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009;15:1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Zhang Y, Jiang H, et al. Activation of Notch3 promotes pulmonary arterial smooth muscle cells proliferation via Hes1/p27Kip1 signaling pathway. FEBS Open Bio. 2015;5:656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Q, Xu H, Yang X, et al. Notch activation of Ca(2+)-sensing receptor mediates hypoxia-induced pulmonary hypertension. Hypertens Res. 2017;40:117–129. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Dai S, Cheng X, et al. Notch3 signaling activation in smooth muscle cells promotes extrauterine growth restriction-induced pulmonary hypertension. Nutr Metab Cardiovasc Dis. 2019;29:639–651. [DOI] [PubMed] [Google Scholar]