Abstract
A subset of endometrial endometrioid carcinomas (EECs) with low-grade histology recur with poor outcomes. Published evidence suggests that poor outcomes may be associated with loss of expression of ER-alpha (ER-α) as well as with β-Catenin-1 (CTNNB1) and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations. This study reports on institutional experience with the incidence of recurrence in low-grade EEC and their association with CTNNB1 and KRAS mutations as well as estrogen/progesterone receptor (ER/PR) expression. Forty-eight (8.5%) out of 568 cases of low-grade EEC with biopsy-proven recurrence were identified; and were analyzed by immunohistochemistry for ER, PR, p53, MMR protein, and mutation analysis for exon 3 of the CTNNB1 and exon 2 of KRAS in relation to recurrence type, local or distant metastasis/recurrence. Twenty-three patients (4%) developed local, and 25 patients (4.4%) developed distant metastases/recurrence. Decreased expression or loss of ER/PR was found in 17/44 (38.6%) patients with recurrence. Eighty-four percent of patients with low-grade EEC and local recurrence had CTNNB1 mutations. Seventy-three percent of patients with distant metastasis/recurrence had KRAS mutations. The association of these mutations with the type of recurrence was statistically significant for both. Five cases with the morphology of low-grade EEC were reclassified as mesonephric-like carcinoma and were universally characterized by distant metastasis/recurrence, loss of ER/PR expression, large tumor size, absence of CTNNB1 mutations, and the presence of KRAS mutations. In low-grade EEC, CTNNB1 and KRAS mutations are associated with local recurrence and distant metastasis/recurrence, respectively, suggesting that these 2 different progression types may be conditioned by tumor genotype. ER/PR immunohistochemistry may be helpful in identifying poor performers in low-grade EEC. Furthermore, identification of the decreased expression or loss of ER/PR in tumors with low-grade histology should prompt consideration of mesonephric-like carcinoma, which is a more aggressive tumor than the low-grade EEC. KRAS mutations were associated with distant metastasis/recurrence in tumors with and without mesonephric-like phenotype.
Key Words: low-grade endometrial endometrioid carcinoma, mesonephric-like carcinoma, disease recurrence, estrogen/progesterone receptor, KRAS, CTNNB1
There are more than 800,000 cases of endometrial carcinoma (EC) annually in North America, and the incidence is increasing.1 Disease recurrence, either local recurrence (LR) or distant metastasis (DM), occurs in ~15% of the patients diagnosed with endometrial endometrioid carcinoma (EEC) and those with non-endometrioid histology.2–4 Within the EEC group, tumors with low-grade histology (low-grade EEC) are associated with better prognostic outcomes than those with non-endometrioid histology. However, ~5% to 10% of patients with low-grade EEC will have either LR or DM.5–7
Endometrial endometrioid carcinoma (EEC) usually occurs in obese women, estrogen-driven and it is mostly positive for estrogen receptors (ER) and progesterone receptors (PR). Approximately 18% to 20% of EC are ER and PR negative and are usually higher-grade tumors with a higher incidence of lymph node involvement, recurrence, relatively poor prognosis, and survival.8–12 However, most of these studies evaluated all histologic subtypes and grades. A recent study demonstrated that loss of ER/PR occurs in 4% of low-grade EEC, and it is an independent risk factor for recurrence and death.13 Mesonephric-like adenocarcinoma (MLC) is a recently described rare variant of EC that has morphologic overlap with low-grade EEC. MLC has a very high incidence of metastatic disease and recurrence. Loss of ER/PR expression and KRAS mutation is a common feature in MLC.14–17 This under-recognized variant of EC may be misclassified as low-grade EEC and recur as metastatic disease. None of the studies have examined MLC in their ER/PR negative cohort. Furthermore, recent studies have demonstrated a higher prevalence of exon 3 CTNNB1 (β-catenin) mutations in a subset of low-grade EEC with worse overall survival.18–21
The main objective of the study was to determine the incidence of local and distant metastasis/recurrence in low-grade EEC in a single institution and to assess the status of KRAS, exon 3 CTNNB1 mutations and ER/PR expression in low-grade EEC with recurrence. Another objective was to determine whether the decreased expression or loss of ER/PR is helpful in the identification of MLC that may have been misclassified as low-grade EEC in this recurrent cohort.
MATERIALS AND METHODS
Ethics Statements
This retrospective study was approved by the biomedical ethics review board for the University of Saskatchewan.
Case Selection
Pathology reports indicating a diagnosis of EEC were obtained from the pathology database of the Saskatoon Health Region from January 2007 to December 2017. In total, 656 EEC cases were identified. Eighty-eight of these cases were either International Federation of Gynecology and Obstetrics (FIGO) grade III EEC, clear cell carcinoma, or had a higher stage disease, including ovarian involvement (synchronous or metastatic), and were excluded. A total of 568 cases were reported as FIGO grades I and II and stages I and II. Forty-eight of the 568 low-grade EEC cases had biopsy-proven local or distant recurrences (including locoregional/distant lymph node metastasis) during a follow-up period of 48–156 months (median follow-up 86 mo). All 48 cases of recurrence were reviewed by 3 pathologists (RC, RK, and CHL). The pathologic assessment included tumor size, grade, depth of invasion, lymph vascular invasion (LVI), FIGO stage, and expressions of the ER, PR, p53 protein, and MMR protein by immunohistochemistry.
Immunohistochemistry
Immunohistochemistry (IHC) for ER, PR, p53, and MMR (MSH-2, MSH-6, MLH-1, and PMS-2) was performed on representative formalin-fixed/paraffin-embedded whole tissue sections from the hysterectomy specimens (Table 1). Cases with suspected mesonephric-like carcinoma features were further characterized using IHC for CD10, GATA-3, and TTF-1. ER and PR were scored as 0% to 100% (continuous variable) by estimating the percentage of positive nuclei. The intensity of the nuclear staining was also recorded as weak, moderate, or strong. Completely negative or cases showing weak focal staining with a total of less than 50% cells positive were considered as negative (high-intermediate risk).22,23 The p53, MLH-1, MSH-2, MSH-6, PMS-2, GATA-3, CD10, TTF-1, and CD10 expressions were assessed according to criteria described by Kobel, Kalloger, and Pors and colleagues, respectively.24–26 All assessments were performed by 2 pathologists independently, and discordance was reconciled by consensus.
TABLE 1.
Immunohistochemistry Protocols on Dako Autostainer Link 48 With EnVision Flex Detection System
| Primary antibody | Clone (source) | Dilution | Antigen retrieval (20 min in PT Link) | Primary Ab incubation time |
|---|---|---|---|---|
| Estrogen Receptor (ER) | EP1 (Dako/Agilent) | 1/50 | High-20 min | 30 min |
| Progesterone Receptor (PR) | 16 (Leica/Novocastra) | 1/200 | High-20 min | 30 min |
| P53 | DO-7 (Dako/Agilent) | Prediluted 50/50 | High-20 min | 30 min+15 min Linker |
| MLH-1 | ES05 (Dako) | 1/80 | High-20 min | 30 min+15 min Linker |
| MSH-2 | FE11 (Dako) | 1/40 | High-20 min | 30 min+15 min Linker |
| MSH-6 | EP49 (Dako) | 1/100 | High-20 min | 30 min |
| PMS-2 | EP51 (Dako) | 1/20 | High-20 min | 30 min |
| CD10 | 56C6 (Dako) | Prediluted | High-20 min | 30 min |
| GATA-3 | L50-823 (Cell Marque) | 1/250 | High-20 min | 30 min |
| TTF-1 | SPT 24 (Leica) | 1/25 | High-20 min | 30 min |
Gene Mutational Analysis for KRAS and CTNNB1
Before the gene mutational analysis, hematoxylin and eosin sections were examined to identify areas enriched (>70%) with tumor cells. Two 6-mm tissue cores from these tumor-rich areas were obtained from the corresponding formalin-fixed/paraffin-embedded tissue blocks. DNA was extracted using the PureLink Genomic DNA Kit (#K1820; Invitrogen) according to the manufacturer’s instructions. Adequate amounts of DNA for molecular analyses were available for 41 study cohort cases (LR, 19; DM, 22). KRAS (exon 2) and CTNNB1 (exon 3) were amplified using the PCR Master Mix (cat# K0171; Thermo Scientific, Ottawa, Ontario, Canada) and the following primers27,28 in a 50-µL polymerase chain reaction (PCR) solution according to the manufacturer’s protocol.
CTNNB1, exon 3
F: 5’-ATTTGATGGAGTTGGACATGGC-3’
R: 5’-CCAGCTACTTGTTCTTGAGTGAAG-3’
KRAS, codons 12 and exon 2
F: 5’-TAAGGCCTGCTGAAAATGACTG-3’
R: 5’-TGGTCCTGCACCAGTAATATGC-3’
PCR amplicons were separated by gel electrophoresis, followed by extraction and purification using the QIAquick PCR & Gel Cleanup Kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. Purified DNA (up to 10 µg) was subsequently used for Sanger sequencing (Applied Genomics Core and Molecular Biology Facility; University of Alberta), and data were analyzed using BioEdit Sequence Alignment Editor, version 7.2.5. For KRAS and CTNNB1 mutations, a threshold of ≥25% was used to assess the presence/absence of point mutations.
Statistical Analysis
The statistical significance of all differences was assessed using the Fisher exact test. Statistical significance was set at P<.05.
RESULTS
Clinical Pathologic Features of 48 Cases With Local Recurrence or Distant Metastasis
From the cohort of 656 EEC cases, 86.58% (N=568) were classified as low-grade (grade I/II) tumors, while the remainder (n=88) were high-grade/high-stage. Of those with low-grade EEC, 48 (8.5%) patients developed biopsy-proven recurrence during the median follow-up of 86 months, with 23 (4.0%) considered LR and 25 (4.4%) DM (including lymph node involvement). LR cases spread to the vaginal vault, mesentery, bowel, bladder, or abdominal wall. The first site of distant metastasis was also recorded. Distant sites included the lungs (n=12), liver (n=4), brain (n=2), bone (n=2), and lymph nodes (n=5) (axillary and supraclavicular lymph nodes, n=3; para-aortic lymph nodes, n=2). The mean time for local recurrence was 38.2 months (median 12 mo). The mean time for distant recurrence was 33.3 months (median 18 mo).
The clinicopathological data for these 48 patients is summarized in Table 2. The patients in the LR group were significantly younger than those in the DM group (median age, 59 (range 35 to 69 y) vs. 66 y (range 50 to 94 y), P=0.04). No significant differences were found between the 2 groups in terms of the mean BMI, tumor size, FIGO grade, depth of invasion, FIGO stage, procedure type, lymph vascular space invasion, ER/PR, and MMR protein loss status. Expression of MMR proteins, including MSH-2, MSH-6, MLH-1, and PMS-2, detected by IHC, showed evidence of abnormalities in 8 of 48 (16.7%) cases. Six of these 8 cases showed loss of MLH-1 expression due to hypermethylation of the promoter, and the other cases had germline mutations in the MSH-6 and MSH-2 genes. No sub-clonal loss of MMR protein expression was observed.
TABLE 2.
Low-grade EEC: Patients’ and Tumor Characteristics and Comparison of the Local Recurrence and Distant Metastasis Groups With Respect to Clinical, and Pathologic Characteristics
| Patient characteristics | Local recurrence (n=23), n (%) | Distant metastasis (n=25), n (%) | P |
|---|---|---|---|
| Mean age >60 y | 16 (70) | 10 (40) | 0.04 |
| Mean BMI >30 kg/m2 | 14 (61) | 12 (40) | 0.37 |
| Type of procedure | |||
| 1) abdominal hysterectomy | 14 (61) | 13 (52) | 0.55 |
| 2) total laparoscopic hysterectomy | 9 (39) | 12 (48) | 0.76 |
| Tumor size ≥4 cm | 11 (48) | 18 (72) | 0.09 |
| Depth of invasion (>50% myometrial invasion) | 14 (61) | 14 (56) | 0.73 |
| FIGO stage (mean) | 1.4 | 1.8 | 0.16 |
| Lymphovascular invasion | 7 (30) | 13 (52) | 0.13 |
| MMR deletion | 5 (22) | 3 (12) | 0.37 |
| Time to recurrence at 1 y | 11 (48) | 12 (48) | 0.99 |
BMI indicates body mass index; FIGO, International Federation of Gynecology and Obstetrics; MMR, mismatch repair; NS, not significant.
Estrogen and Progesterone Receptor Expression
In total, 27/44 study cohort cases showed diffuse, strong nuclear staining with ER and PR assays (>70%–90% of nuclei; Fig. 1), whereas 17/44 (38.64%) study cohort cases were negative for ER and PR (Table 3). Of these cases, ten were completely negative for ER/PR. Of the remaining 7 cases: 3 showed weak 50% nuclear staining for ER, and 30% for PR, 2 of these cases were MMRd (mismatch repair deficient). Three cases showed weak nuclear staining in 20% to 25% of tumor cells for ER and PR, and 1 of these was MMRd. Only 1 of the cases showed 20% nuclear staining for ER and was negative for PR.
FIGURE 1.
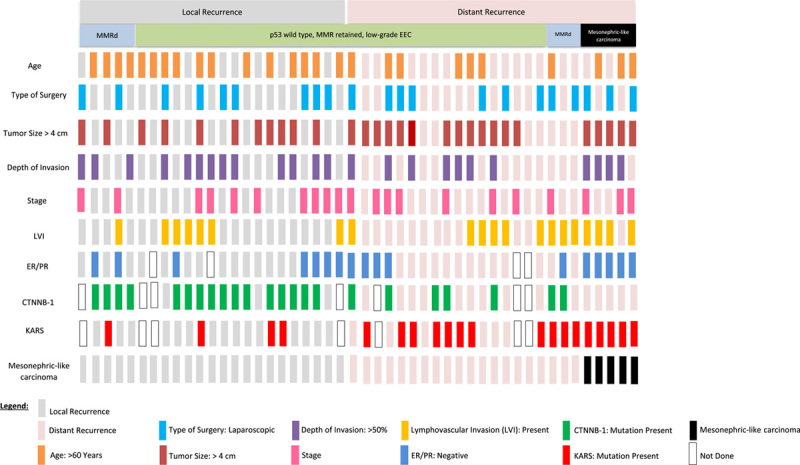
Summary of the relationship between KRAS and CTNNB1 mutations, ER/PR protein expression, and clinical pathologic characteristics of low-grade endometrial endometrioid carcinoma with local and distant recurrences. Each column represents a patient. All 5 mesonephric-like carcinomas are grouped at the far right.
TABLE 3.
Comparison of the Local Recurrence and Distant Metastasis Groups with Respect to ER/PR, KRAS, and CTNNB1 Mutations
| Patient characteristics | Local recurrence (n=23), n (%) | Distant metastasis (n=25), n (%) | P |
|---|---|---|---|
| Loss of ER and/or PR expression | 7/23 (30) | 10/25 (40) | 0.19 |
| CTNNB1 mutation | 16/19 (84) | 7/22 (32) | 0.0013 |
| KRAS mutation | 4/19 (21) | 16/22 (73) | 0.0016 |
Mesonephric-like Carcinoma (MLC)
Since loss of ER/PR expression has been reported to be a common feature of mesonephric-like carcinoma, we assessed a panel of immunohistochemical markers (TTF-1, GATA-3, and CD10) in all ER/PR negative cases. Five cases were positive for 2 or all 3 IHC markers. Three cases were diffusely and strongly positive for TTF-1, 3 showed variable positivity for GATA-3, and 3 showed focal apical and luminal CD10 expression (Fig. 2). The remaining samples were negative for CD10, GATA-3, and TTF-1. In accordance with the diagnostic criteria described in a 2016 publication,14 upon review of histology and IHC results, we reclassified 5 cases as MLC. On histology alone, we correctly identified only 2 cases that had the predominant tubular pattern (Case 2 and 3; Fig. 2). These tubules were lined by cuboidal-columnar cells that carried round-oval, medium-sized vesicular nuclei with occasional grooves and intranuclear inclusions. Other 3 cases showed a predominately glandular (endometrioid) pattern. These glands were lined by columnar cells with nuclear pseudo-stratification, which likely prompted to label these tumors as low-grade EEC. In some sections, a mixture of histologic patterns, including tubules with focal dense eosinophilic secretions, glandular patterns, papillae, and focal solid nests, were noted. Considering these findings, we reviewed the histology of all cases in our study cohort and performed additional IHC, and no additional MLC-like cases were identified. All 5 cases of mesonephric-like carcinoma were found in the group with distant metastases and accounted for 20% of cases in this group. These cases had large tumor size (5/5, >4.0 cm) and frequent LVI (4/5). These clinical features are consistent with the results of 2 previous studies that focused on the characterization of MLC.15,16
FIGURE 2.
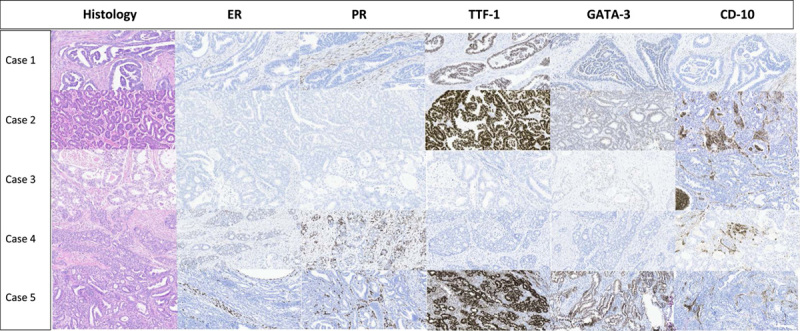
Histopathology and IHC findings in 5 cases of mesonephric-like carcinoma. Cases 1–3 and 5: Negative for ER and PR. Case 4: Focal positivity (<50%) for ER and PR. TTF-1: Diffuse and strong nuclear staining in cases 1, 2, 5, and lack of expression in cases 3 and 4. GATA-3: Weak to strong nuclear staining in cases 1, 2, 3, and 5, and lack of expression in case 4. CD10: Focal apical and luminal staining in cases 2–5, and lack of expression in case 1. ER indicates estrogen receptor; PR, progesterone receptor.
Correlation Between KRAS and CTNNB1 Mutations With the Pattern of Recurrences
Twenty-three of 41 cases (56.1%) harbored mutations in exon 3 of CTNNB1, with the frequency being significantly higher in the LR group than in the DM group (16/19, 84.2% vs. 7/22, 31.8%, P=0.0013). 20 (48.8%) had KRAS mutations in exon 2, with the frequency being significantly higher in the DM group than in the LR group (16/22, 72.7% vs. 4/19, 21.1%, P=0.0016) (Table 3). The frequency of KRAS mutation in low-grade EEC after excluding MLC cases was 65%. Furthermore, 8 cases (LR, 4; DM, 4) had both KRAS and CTNNB1 mutations, of which 3 were MMRd.
DISCUSSION
The incidence of recurrences in our cohort of low-grade EEC was 7.63% (43/563) after excluding 5 cases of MLC, and this frequency was comparable to those in other published studies (2.6%–14.2%).3–5 Similarly, the finding that the tumor size (≥4 cm) and the presence of LVI were significantly correlated with disease recurrence in low-grade EEC are also congruent with previously published studies.29–31
The lack of strong and uniform ER/PR expression in EC has been previously described, with frequency ranging from 7% to 21% of EC (endometrioid and non-endometrioid histology).8–11,32–38 Frequencies of loss of ER/PR expression vary from 3.8% to 7%, where all grades of EEC were included.11,13,33 Incidence of loss of ER/PR, specifically in low-grade recurrent EEC, is not known. Specifically, when the loss of ER/PR expression in low-grade EEC was less than 4%, the percentage of cases that recur is not reported in the literature to date. We identified this phenotype in 12/39 (30%) recurrent low-grade EEC after excluding five MLC cases. Our data suggest that decreased expression or loss of ER/PR by immunohistochemistry may serve as a useful prognostic biomarker to identify low-grade EEC that may recur locally or have distant metastasis. It has been shown that the lack of ER/PR expression in EC as well as in EEC correlates significantly with poor clinical outcomes.8,11,33,39 Recently, it was proposed that immunodetection of ER/PR can be used to select patients with EC for lymph node sampling,9,11,22,32,34 results reported in this manuscript concurred with the observations that decreased expression or loss of ER/PR was very important to identify ~30% of low-grade EEC with poor prognosis. Another significant observation of this study was that using a higher cut-off value, the sensitivity of ER/PR negative tumors can be improved.
The 1% and 10% cut-off values for ER/PR nuclear staining used in earlier studies were similar to ASCO/CAP guidelines for breast cancer.40 ER/PR status in breast cancer is a predictive marker, and it has been established at a level that provides benefit to patients treated with endocrine therapy. Hormone therapy is also used in palliative settings for recurrent EEC with a similar cut-off value for ER/PR nuclear staining of >1% as in breast cancer. The cut-off value of ER/PR positivity for its association with EEC is very important for its use as a prognostic and predictive marker. Two recent studies demonstrated a significant association between a higher cut-off value for ER/PR positivity in EEC.22,23 Weinberger et al22 suggested that optimal cut-off values to distinguish between low-risk and high-risk tumors for ER and PR were >78% and >88%, respectively. Recently, van Weelden et al41 reported a cut-off value of >50% for ER/PR positivity for a higher response rate (50%) and optimal clinical benefit rate (75%) with hormone therapy in EEC, suggesting that tumors that show strong diffuse expression of ER and PR are biologically different than those that express ER and PR at a much lower level and heterogeneously. In our study, we have also noticed a bimodal type of expression of ER and PR and have used a more conservative cut-off value of 50% to designate cases as positive or negative. Large, prospective, multicenter studies on properly fixed prebiopsy/posthysterectomy specimens are needed to determine the optimal cut-off value that best segregates patients for prognostic and predictive purposes for ER/PR in EEC. However, for diagnostic purposes, our results suggest that the cut-off of 50% is useful as 2 of the 5 cases of MLC tumors also showed weak focal expression of ER/PR. Our results demonstrated that using this simple approach, we may identify poor performer low-grade EEC as well as MLC-like cases with overlapping morphology with low-grade EEC that may have been otherwise missed.
The molecular mechanisms underlying the downregulation of ER/PR expression in EEC are unclear. ER/PR negative EECs often express markers of epithelial-mesenchymal transition, including SNAIL1, SNAIL2, TWIST1, ZEB1, ZEB2, and sonic hedgehog, as well as markers of the tumor growth factor-β pathway, all of which promote motility, invasiveness, and metastatic potential.12,42 Recently, Terakawa et al43 found that estradiol effectively repressed the clonal expansion of preneoplastic epithelial cells and their malignant progression in a mouse model. This concept has been further supported by observations that microcystic elongated and fragmented foci in EEC show reduced expression of ER/PR, suggesting an association between decreased ER/PR expression, LVI, and lymph node metastasis.44 Furthermore, KRAS mutations are more prevalent in EEC with the microcystic elongated and fragmented pattern, suggesting a possible association between KRAS mutations, loss of ER/PR expression, epithelial-mesenchymal transition, and lymph node metastasis.45,46
A review of the mutational status of CTNNB1 and KRAS in our study cohort of low-grade EEC confirmed that they do show a reciprocal trend, similar to what has been described for all EC.47 In the literature, mutations in exon 3 of the CTNNB1 gene occur in 13% to 44% of EECs18–20; and at a higher rate (60-87%) in low-grade EEC with a worse clinical outcome.18–20,48–50 Our study also demonstrated a strong association between the mutations in CTNNB1 and KRAS with the type of recurrence. CTNNB1 mutations were associated with LR and KRAS mutations with DM. The dual mutations in some cases with MMRd could potentially represent passenger events.
Mutations in exon 3 of CTNNB1 promote the stabilization of β-catenin proteins, leading to its subsequent nuclear translocation and increased transcriptional activation of a host of target genes, including MYC, MMP7, and CCND1.51 The reason why mutations in CTNNB1 promote LR as opposed to DM requires further studies. Nonetheless, the finding that CTNNB1-mutated tumors did not always show increased nuclear expression of β-Catenin-120 could suggest that the defect associated with these mutations may possibly involve the cell adhesion functions of β-catenin-1, which may facilitate the trans-tubal migration of neoplastic cells for LR. This is supported by previous studies that also found a higher prevalence of CTNNB1 mutations in ovarian endometriosis-associated endometrioid carcinoma.52–55
Previous studies have shown that KRAS mutations correlated with lymph node metastasis and short survival in patients with EC,56,57 whereas Caduff et al58 found no association with clinical pathologic parameters or survival. Although none of our cases showed mucinous differentiation, KRAS mutations are also prevalent in mucinous carcinoma of the endometrium (71.4-86%) and complex papillary mucinous lesions of the endometrium.59 A few studies suggest that pure mucinous, endometrioid carcinoma is frequently associated with poor prognostic factors, including deep myometrial invasion and lymph node metastasis.60,61 We provide additional evidence that KRAS mutations are associated with DM in patients with low-grade EEC as well as in MLC. Recently, Lac et al62 provided evidence that KRAS mutations existed in histologically normal endometrium and that the prevalence of this genetic abnormality increased with aging. These findings suggest that it is difficult to implicate KRAS mutations as a more direct pathway to DM but rather point to a likelihood of a multi-step process that possibly includes the deregulation of 1 or more downstream targets of the KRAS signaling pathway. The molecular basis for a strong association between KRAS mutations and DM in low-grade EEC requires further investigation.
Our findings also suggest that MLC, having substantial morphologic overlap with low-grade EEC, could be underdiagnosed. As such, all cases of MLC in our study were initially diagnosed as low-grade EEC. Our results highlight that no expression or very low expression of ER/PR in low-grade EEC should trigger suspicion of an investigation for MLC.
A major limitation of the study is that it is a retrospective, single-institution study of biopsy-proven, recurrent low-grade, low-stage EEC and therefore has a small sample size. Some cases that were lost to follow-up or did not have a biopsy of recurrent disease could have been missed. The molecular classification is incomplete as POLE mutation analysis was not performed on these cases. The 50% cut-off for ER/PR positivity was based on the cut-off value that clearly differentiated strongly positive and weakly/focally positive cases of recurrent EEC. The significance of focal expression of ER/PR in low-grade EEC is not known. The study demonstrated the association between decreased expression of ER/PR and recurrence. Keeping in mind that about 5% to 10% of low-grade EEC recur, the results suggest that almost all low-grade EEC that show loss of ER/PR will recur. Therefore, decreased expression and/or complete loss of ER/PR are very important to characterize in low-grade EEC to diagnose their probability to recur. Future, large multi-institutional studies are required to determine the optimal cut-off point for ER/PR for prognostic and predictive purposes.
In summary, the study showed a strong association of CTNNB1 And KRAS mutations with LR and DM, respectively, thus linking genotype to the type of recurrence. The demonstrated reciprocal relationship between KRAS and CTNNB1 mutations may suggest the existence of 2 independent pathways of tumor initiation and progression in low-grade EEC. In low-grade EEC, the bimodal ER/PR expression pattern supports it as a prognostic marker to identify low-grade EEC that are likely to recur. Identification of ER/PR low/negative tumors should also prompt consideration of the MCL subtype, which has a high risk of disease recurrence.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Rajni Chibbar, Email: tinashilpi@shaw.ca.
Sabrina Foerstner, Email: sfoerstn@ualberta.ca.
Janarathnee Suresh, Email: jak770@mail.usask.ca.
Richa Chibbar, Email: chibbar@ualberta.ca.
Alexandre Piche, Email: acp465@mail.usask.ca.
Deeksha Kundapur, Email: deekshakundapur@gmail.com.
Rani Kanthan, Email: rani.kanthan@saskhealthauthority.ca.
Cheng Han Lee, Email: chlee2@ualberta.ca.
Anita Agrawal, Email: a.agrawal@queensu.ca.
Raymond Lai, Email: rlai@ualberta.ca.
REFERENCES
- 1.SEER Cancer Stat Facts: Uterine cancer. National cancer institute, Bethesda, MD. Accessed June 2021. https://seer.cancer.gov/statfacts/html/corp.html
- 2.Sasada S, Yunokawa M, Takehara Y, et al. Baseline risk of recurrence in stage I-II endometrial carcinoma. J Gynecol Oncol. 2018;29:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis SR, Ager BJ, Do OA, et al. Recurrent early-stage endometrial cancer: Patterns of recurrence and results of salvage therapy. Gynecol Oncol. 2019;154:38–44. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi A, Matsuura M, Matoda M, et al. Clinicopathological features of early and late recurrence of endometrial carcinoma after surgical resection. Int J Gynecol Cancer. 2017;27:967–972. [DOI] [PubMed] [Google Scholar]
- 5.Lavazzo C, Gkegkes ID, Vrachnis N. Early recurrence of early stage endometrioid endometrial carcinoma: possible etiologic pathways and management options. Maturitas. 2014;78:155–159. [DOI] [PubMed] [Google Scholar]
- 6.Stasenko M, Feit N, Lee SSK, et al. Clinical patterns and genomic profiling of recurrent ‘ultra-low risk’ endometrial cancer. Int J Gynecol Cancer. 2020;30:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laban M, El-Swaify ST, Ali SH, et al. The prediction of recurrence in low-risk endometrial cancer: Is it time for a paradigm shift in adjuvant therapy? Reprod Sci. 2022;29:1068–1085. [DOI] [PubMed] [Google Scholar]
- 8.Jongen V, Briët J, de Jong R, et al. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol Oncol. 2009;112:537–542. [DOI] [PubMed] [Google Scholar]
- 9.Trovik J, Wik E, Werner HM, et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicenter trial. Eur J Cancer. 2013;49:3431–3441. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhao D, Gong C, et al. Prognostic role of hormone receptors in endometrial cancer: a systematic review and meta-analysis. World J Surg Oncol. 2015;13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backes FJ, Walker CJ, Goodfellow PJ, et al. Estrogen receptor-alpha as a predictive biomarker in endometrioid endometrial cancer. Gynecol Oncol. 2016;141:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weelden WJ, van der Putten LJM, Inda MA, et al. Oestrogen receptor pathway activity is associated with outcome in endometrial cancer. Br J Cancer. 2020;123:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan J, Xie L, Luo X, et al. The prognostic significance of estrogen and progesterone receptors in grade I and II endometrioid endometrial adenocarcinoma: hormone receptors in risk stratification. J Gynecol Oncol. 2019;30:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFarland M, Quick CM, McCluggage WG. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology. 2016;68:1013–1020. [DOI] [PubMed] [Google Scholar]
- 15.Na K, Kim HS. Clinicopathologic and Molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am J Surg Pathol. 2019;43:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Euscher ED, Bassett R, Duose DY, et al. Mesonephric-like carcinoma of the endometrium: a subset of endometrial carcinoma with an aggressive behavior. Am J Surg Pathol. 2020;44:429–443. [DOI] [PubMed] [Google Scholar]
- 17.Mirkovic J, McFarland M, Garcia E, et al. Targeted genomic profiling reveals recurrent KRAS mutations in mesonephric-like adenocarcinomas of the female genital tract. Am J Surg Pathol. 2018;42:227–233. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Patel L, Mills GB, et al. Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst. 2014;106:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurnit KC, Kim GN, Fellman BM, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early-stage endometrial cancer patients at increased risk of recurrence. Mod Pathol. 2017;30:1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costigan DC, Dong F, Nucci MR, et al. Clinicopathologic and immunohistochemical correlates of CTNNB1 mutated endometrial endometrioid carcinoma. Int J Gynecol Pathol. 2020;39:119–127. [DOI] [PubMed] [Google Scholar]
- 21.Moroney MR, Davies KD, Wilberger AC, et al. Molecular markers in recurrent stage I, grade 1 endometrioid endometrial cancers. Gynecol Oncol. 2019;153:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberger V, Bednarikova M, Hausnerova J, et al. A novel approach to preoperative Risk stratification in endometrial cancer: The added value of immunohistochemical markers. Front Oncol. 2019;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Weelden WJ, Reijnen C, Küsters-Vandevelde HVN, et al. The cutoff for estrogen and progesterone receptor expression in endometrial cancer revisited: a European Network for Individualized Treatment of Endometrial Cancer collaboration study. Hum Pathol. 2021;109:80–91. [DOI] [PubMed] [Google Scholar]
- 24.Köbel M, Ronnett BM, Singh N, et al. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol. 2019;38(suppl 1):S123–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalloger SE, Allo G, Mulligan AM, et al. Use of mismatch repair immunohistochemistry and microsatellite instability testing: exploring Canadian practices. Am J Surg Pathol. 2012;36:560–569. [DOI] [PubMed] [Google Scholar]
- 26.Pors J, Cheng A, Leo JM, et al. A comparison of GATA3, TTF1, CD10, calretinin in identifying mesonephric and mesonephric-like carcinoma of the gynecologic tract. Am J Surg Path. 2018;42:1596–1606. [DOI] [PubMed] [Google Scholar]
- 27.Machin P, Catasus L, Pons C, et al. CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol. 2002;33:206–212. [DOI] [PubMed] [Google Scholar]
- 28.Ardighieri L, Zeppernick F, Hannibal CG, et al. Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J Pathol. 2014;232:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prat J. Prognostic parameters of endometrial carcinoma. Hum Pathol. 2004;35:649–662. [DOI] [PubMed] [Google Scholar]
- 30.Del Carmen MG, Boruta DM, 2nd, Schorge JO. Recurrent endometrial cancer. Clin Obstet Gynecol. 2011;54:266–277. [DOI] [PubMed] [Google Scholar]
- 31.Esselen KM, Boruta DM, del Carmen M, et al. Defining prognostic variables in recurrent endometrioid endometrial cancer: a 15-year single-institution review. Int J Gynecol Cancer. 2011;21:1078–1083. [DOI] [PubMed] [Google Scholar]
- 32.Vrede SW, van Weelden WJ, Visser NCM, et al. Immunohistochemical biomarkers are prognostically relevant in addition to the ESMO-ESGO-ESTRO risk classification in endometrial cancer. Gynecol Oncol. 2021;161:787–794. [DOI] [PubMed] [Google Scholar]
- 33.Stelloo E, Nout RA, Osse EM, et al. Improved risk assessment by integrating molecular and clinical pathological factors in early-stage endometrial cancer-combined analysis of PORTEC cohorts. Clin Cancer Res. 2016;22:4215–4224. [DOI] [PubMed] [Google Scholar]
- 34.van der Putten LJM, Visser NCM, van de Vijver K, et al. Added value of estrogen receptor, progesterone receptor, and L1 cell adhesion molecule expression to histology-based endometrial carcinoma recurrence prediction models: an ENITEC collaboration study. Int J Gynecol Cancer. 2018;28:514–523. [DOI] [PubMed] [Google Scholar]
- 35.Geels YP, van der Putten LJM, van Tilborg AAG, et al. Immunohistochemical profiles of endometrioid endometrial carcinomas with and without metastatic disease. Appl Immunohistochem Mol Morphol. 2018;26:173–179. [DOI] [PubMed] [Google Scholar]
- 36.Salama A, Arafa M, ElZahaf E, et al. Potential role for a panel of immunohistochemical markers in the management of endometrial carcinoma. J Pathol Transl Med. 2019;53:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrone E, De Felice F, Capasso I, et al. The immunohistochemical molecular risk classification in endometrial cancer: A pragmatic and high-reproducibility method. Gynecol Oncol. 2022;163:585–593. [DOI] [PubMed] [Google Scholar]
- 38.Jiang P, Huang Y, Tu Y, et al. Combining clinicopathological parameters and molecular indicators to predict lymph node metastasis in endometrioid type endometrial adenocarcinoma. Front Oncol. 2021;11:682925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karnesis AN, Leung S, Magrill J, et al. Evaluation of endometrial carcinoma prognostic immunohistochemistry markers in the context of molecular classification. J Pathol Clin Res. 2017;3:279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and Progesterone receptor testing in breast cancer: ASCO/CAP Guideline Update. J Clin Oncol. 2020;38:1346–1366. [DOI] [PubMed] [Google Scholar]
- 41.van Weelden WJ, Lalisang RI, Bulten J, et al. Impact of hormonal biomarkers on response to hormonal therapy in advanced and recurrent endometrial cancer. Am J Obstet Gynecol. 2021;225:407.e1–407.e16. [DOI] [PubMed] [Google Scholar]
- 42.Wik E, Ræder MB, Krakstad C, et al. Lack of estrogen receptor-α is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res. 2013;19:1094–1105. [DOI] [PubMed] [Google Scholar]
- 43.Terakawa J, Serna VA, Taketo MM, et al. Ovarian insufficiency and CTNNB1 mutations drive malignant transformation of endometrial hyperplasia with altered PTEN/PI3K activities. Proc Natl Acad Sci U S A. 2019;116:4528–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart CJ, Little L. Immunophenotypic features of MELF pattern invasion in endometrial adenocarcinoma: evidence for epithelial-mesenchymal transition. Histopathology. 2009;55:91–101. [DOI] [PubMed] [Google Scholar]
- 45.Stewart CJ, Amanuel B, Grieu F, et al. KRAS mutation and microsatellite instability in endometrial adenocarcinomas showing MELF-type myometrial invasion. J Clin Pathol. 2010;63:604–608. [DOI] [PubMed] [Google Scholar]
- 46.Stewart CJ, Brennan BA, Leung YC, et al. MELF pattern invasion in endometrial carcinoma: association with low grade, myoinvasive endometrioid tumours, focal mucinous differentiation and vascular invasion. Pathology. 2009;41:454–459. [DOI] [PubMed] [Google Scholar]
- 47.Cancer Genome Atlas Research Network,, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe T, Nanamiya H, Kojima M, et al. Clinical relevance of oncogenic driver mutations identified in endometrial carcinoma. Transl Oncol. 2021;14:101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang HN, Kuo CW, Lin MC, et al. Frequent CTNNB1 or PIK3CA mutations occurred in endometrial endometrioid adenocarcinoma with high levels of microsatellite instability and loss of MSH2/MSH6 expression. Appl Immunohistochem Mol Morphol. 2020;28:284–289. [DOI] [PubMed] [Google Scholar]
- 50.Ruz-Caracuel I, López-Janeiro Á, Heredia-Soto V, et al. Clinicopathological features and prognostic significance of CTNNB1 mutation in low-grade, early-stage endometrial endometrioid carcinoma. Virchows Arch. 2021;479:1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Travaglino A, Raffone A, Gencarelli A, et al. Relationship between morular metaplasia and squamous differentiation in endometrial carcinoma. Pathol Res Pract. 2021;217:153307. [DOI] [PubMed] [Google Scholar]
- 53.Tomohiro M, Matsumoto T, Miura R, et al. Alterations in β-catenin, microsatellite instability, and HNF-1β levels are independently associated with ovarian endometriosis-associated tumorigenesis. Hum Pathol. 2019;89:10–23. [DOI] [PubMed] [Google Scholar]
- 54.Hollis RL, Thomson JP, Stanley B, et al. Molecular stratification of endometrioid ovarian carcinoma predicts clinical outcome. Nat Commn. 2020;11:4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zyla RE, Olkhov-Mitsel E, Amemiya Y, et al. CTNNB1 mutations and aberrant β-catenin expression in ovarian endometrioid carcinoma: Correlation with patient outcome. Am J Surg Pathol. 2021;45:68–76. [DOI] [PubMed] [Google Scholar]
- 56.Ito K, Watanabe K, Nasim S, et al. K-ras point mutations in endometrial carcinoma: effect on outcome is dependent on age of patient. Gynecol Oncol. 1996;63:238–246. [DOI] [PubMed] [Google Scholar]
- 57.Fujimoto I, Shimizu Y, Hirai Y, et al. Studies on ras oncogene activation in endometrial carcinoma. Gynecol Oncol. 1993;48:196–202. [DOI] [PubMed] [Google Scholar]
- 58.Caduff RF, Johnston CM, Frank TS. Mutations of the Ki-ras oncogene in carcinoma of the endometrium. Am J Pathol. 1995;146:182–188. [PMC free article] [PubMed] [Google Scholar]
- 59.Alomari A, Abi-Raad R, Buza N, Hui P, et al. Frequent KRAS mutation in complex mucinous epithelial lesions of the endometrium. Mod Pathol. 2014;5:675–680. [DOI] [PubMed] [Google Scholar]
- 60.Musa F, Huang M, Adams B, et al. Mucinous histology is a risk factor for nodal metastases in endometrial cancer. Gynecol Oncol. 2012;125:541–545. [DOI] [PubMed] [Google Scholar]
- 61.Galic V, Schiavone MB, Herzog TJ, et al. Prognostic significance of mucinous differentiation of endometrioid adenocarcinoma of the endometrium. Cancer Invest. 2013;31:500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lac V, Nazeran TM, Tessier-Cloutier B, et al. Oncogenic mutations in histologically normal endometrium: the new normal? J Pathol. 2019;249:173–181. [DOI] [PubMed] [Google Scholar]


