Abstract
Background
Evidence-based guidelines for the management of systemic therapy naïve oligometastatic renal cell carcinoma (RCC) are lacking. This prospective phase II single-arm trial evaluated the potential of stereotactic ablative radiotherapy (SAbR) to provide longitudinal disease control while preserving quality of life in patients with systemic therapy naïve oligometastatic RCC.
Patients and Methods
RCC patients with ≤3 extracranial metastases were eligible. SAbR was administered longitudinally to all upfront and, as applicable, subsequent metastases. The study was powered to achieve a primary objective of freedom from systemic therapy >1 year in >60% of patients (using Clopper and Pearson methodology). Secondary endpoints included progression-free survival (PFS), defined as time from first SAbR to progression not amenable to SAbR (local failure at SAbR-treated sites; new metastases not amenable to SAbR; >3 new metastases; or brain metastases); patient-reported quality of life (QOL) metrics; local control (LC) rates; toxicity; cancer-specific survival (CSS); and overall survival (OS).
Results
Twenty-three patients received SAbR to 33 initial and 57 total sites. Median follow-up was 21.7 months (interquartile range 16.3–30.3). Exceeding the pre-specified 60% benchmark, freedom from systemic therapy at one-year was 91.3% (95%CI: 69.5, 97.8). One-year PFS was 82.6% (95%CI: 60.1, 93.1). QOL was largely unaffected. LC was 100%. There were no grade 3/4 toxicities, but there was one death due to immune-related colitis three months after SAbR while on subsequent checkpoint inhibitor therapy where a SAbR contribution could not be excluded. One-year CSS and OS were both 95.7% (95%CI: 72.9, 99.4).
Conclusions
SAbR for oligometastatic RCC was associated with meaningful longitudinal disease control while preserving quality of life. These data support further evaluation of SAbR for systemic therapy naïve oligometastatic RCC.
Patient Summary
Sequential stereotactic radiation therapy can safely and effectively control metastatic kidney cancer with limited spread for over a year without compromising patients’ quality of life.
Take Home Message
This phase II trial provides prospective evidence that sequential SAbR to all sites of oligometastatic RCC while the disease remains oligometastatic in a selected (and predefined) systemic therapy naïve patient population offers disease control in >90% of patients while maintaining health-related quality of life.
Keywords: oligometastasis, RCC, renal cell carcinoma, SAbR, SBRT, stereotactic radiation
Introduction
There were an estimated 431,288 new cases and 179,368 deaths due to kidney cancer worldwide in 2020.(1) Over 40% of patients with renal cell carcinoma (RCC), the most common type of kidney cancer, develop metastatic disease.(2) Systemic therapy with immune checkpoint inhibitors (ICI), tyrosine kinase inhibitors (TKI), or more recently a combination of the two is the standard of care for metastatic RCC (mRCC).(3–7) Unfortunately, most patients develop progressive disease over time and systemic therapy is frequently associated with significant toxicity and deterioration of quality of life. At progression, a change in systemic therapy becomes necessary and some patients exhaust treatment options.
Metastatic RCC represents a wide spectrum of disease aggressiveness. For example, patients with International Metastatic Database Consortium (IMDC) high risk disease may succumb in less than one year, while those with favorable risk disease may have smoldering progression over many years.(8, 9) Patients may also present with widely disseminated disease or oligometastatic disease. While several definitions exist, oligometastatic disease is defined by the European Organization for Research and Treatment of Cancer (EORTC) as ≤5 metastases involving ≤3 organs. For patients with oligometastatic disease, metastasis-directed therapy is an option, but prospective data are lacking and there is currently no standard-of-care.(10)
Based on our previous preclinical and clinical experience, we hypothesized that stereotactic ablative radiation (SAbR) may control oligometastatic RCC.(11, 12) Overall, SAbR is safe and effective offering >90% local control (LC) of RCC metastases at one year.(13–15) In our experience of longitudinal SAbR administration for oligometastatic RCC that remains oligometastatic and amenable to SAbR, SAbR offered disease control while preserving quality of life and delayed systemic therapy. Specifically, we previously treated 47 systemic therapy naïve patients with SAbR to 88 metastases and observed LC rates of 91.5%, unhampered quality of life, and a median time to start of systemic therapy (TTST) of 15.2 months.(12) By postponing systemic therapy with its associated toxicities, SAbR may directly benefit patients and extend their quality of life. Furthermore, the duration of first line systemic therapy appeared to be unaffected by the earlier introduction of SAbR suggesting that SAbR did not undermine the effectiveness of subsequent drug therapy and may have additive survival benefit.(12) These results prompted a systematic prospective evaluation of SAbR in a clinical trial. In this study we sought to determine whether SAbR administered longitudinally to patients with systemic therapy naïve oligometastatic RCC was able to delay systemic therapy by >1 year in at least 60% of patients.
Methods
Study design and participants
This is a single-arm, phase II trial conducted with approval of the Institutional Review Board (IRB) at a university medical center and affiliated county hospital. The study enrolled systemic therapy naïve patients with oligometastatic RCC that provided written informed consent. Eligibility criteria included patients at least 18 years of age with pathologically proven RCC, in a favorable or intermediate IMDC risk group, who were systemic therapy naïve with three or fewer extracranial sites of metastatic disease that were all amenable to SAbR (see study protocol in Supplement for a complete list of eligibility criteria).
Treatment Technique
Patients underwent computed tomography (CT)-based simulation with customized immobilization and tumor motion assessment and management, as needed. All metastatic lesions were treated with SAbR, which was the local therapy evaluated in the study. SAbR treatment planning and delivery were performed via standard techniques with onboard cone beam CT, as previously described.(16) Treatment regimens consisted of ≥25Gy x 1 fraction, ≥12Gy x 3 fractions, or ≥8Gy x 5 fractions to the periphery of the target to cover >95% of the target volume. The dose/fractionation schedule was selected based on proximity of organs at risk of toxicity and remained at the discretion of the treating radiation oncologist. Sequential SAbR to additional sites of metastatic disease was allowed.
Outcomes
The primary objective was a clinically meaningful delay in the time to start of systemic therapy (TTST) of >1 year in at least 60% of patients. TTST was defined as the time from the first day of SAbR to the start of systemic therapy. Radiographic imaging was performed at three-month intervals. The decision to initiate systemic therapy was based on a clinical assessment and was subjective. This was complemented by a secondary endpoint involving protocol-defined progression-free survival (PFS). Defined as progressive disease not amenable to sequential SAbR, PFS was specified in the protocol as time from first day of SAbR to: local failure at SAbR-treated sites; new metastases not amenable to SAbR; >3 new metastases; or brain metastases.
Other secondary endpoints included local control (LC) rates and associated median response duration (defined as the time from the date of response until progression using Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1(17) principals); time from first day of SAbR to disease progression not amenable to further local therapy (TTP); health-related quality of life (QOL); progression-free survival with subsequent systemic therapy (PFS-ST); cancer-specific survival (CSS); overall survival (OS); and toxicity. Toxicities were assessed via the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 scale. Health-related QOL assessments were performed at three-month intervals and involved the EuroQol Group’s five-level measure (EQ-5D-5L), the Functional Assessment of Cancer Therapy – General (FACT-G), and the Functional Assessment of Cancer Therapy – Kidney Symptom Index (FKSI).
Statistical analysis
Descriptive statistics were provided as medians and interquartile ranges for continuous measures and as frequencies and percentages for categorical measures. For TTST and PFS, patients without events were censored at last follow-up. A sample size of 23 subjects achieved 80% power at a 0.10 significance level to detect a difference of 20% in 1-year TTST compared to historical estimates (40.5%).(18, 19) Kaplan-Meier curves were used to visualize survival over time and to calculate one-year survival estimates. Paired t-tests were used to test for changes in quality-of-life questionnaire responses. All tests were two-sided and performed at the 0.05 significance level via SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Twenty-three patients were enrolled between August 2018 and February 2021. Patient and treatment characteristics are summarized in Table 1. All patients were systemic therapy naïve. Prior focal therapies were allowed. Most patients had localized disease at initial presentation (87%). 22/23 (96%) patients had nephrectomy, while the remaining patient who was not a surgical candidate received SAbR to the primary RCC. Nine patients (30.1%) had prior focal therapies for metastases (radiation and/or surgery). The median time from the treatment of the primary to SAbR on the trial was 32.3 months. Twenty-three patients were treated with SAbR to 33 total lesions upfront, and ten patients received SAbR to 24 subsequent sites of oligometastatic disease, for a total of 57 treated lesions. Lung (47%), abdominal lymph nodes (11%), and non-spine bone (7.0%) were the most common sites for SAbR. The metastatic phenotype of the patients based on the STARS staging system is outlined in Table 1.(20)
Table 1.
Patient and treatment characteristics
| N (%) | |
|---|---|
| (n = 23 patients) | |
| Median age, years (IQR) | 71 (61–73) |
| Sex | |
| Male | 19 (82.6%) |
| Female | 4 (17.4%) |
| Race/Ethnicity | |
| White, Non-Hispanic | 21 (91.3%) |
| White, Hispanic | 2 (8.7%) |
| IMDC group | |
| Favorable | 17 (73.9%) |
| Intermediate | 6 (26.1%) |
| Histology | |
| Clear cell RCC | 19 (82.6%) |
| Chromophobe RCC | 2 (8.7%) |
| Papillary RCC | 2 (8.7%) |
| Tumor Grade | |
| 1 | 1 (4.3%) |
| 2 | 6 (26.1%) |
| 3 | 13 (56.5%) |
| 4 | 1 (4.3%) |
| NA (Chromophobe RCC) | 2 (8.7%) |
| Primary tumor control | |
| Nephrectomy | 22 (95.7%) |
| SAbR | 1 (4.3%) |
| AJCC T stage at initial diagnosis | |
| 1 | 4 (17.4%) |
| 2 | 3 (13.0%) |
| 3 | 16 (69.6%) |
| AJCC N stage at initial diagnosis | |
| 0/X | 22 (95.7%) |
| 1 | 1 (4.3%) |
| AJCC M stage at initial diagnosis | |
| 0 | 20 (87.0%) |
| 1 | 3 (13.0%) |
| STARS Stage | |
| A | 7 (30.4%) |
| B | 13 (56.5%) |
| C | 0 (0.0%) |
| D | 3 (13.0%) |
| E | 0 (0.0%) |
| Pattern of metastatic disease | |
| Metachronous | 16 (69.6%) |
| Synchronous | 7 (30.4%) |
| Prior therapy of metastases | |
| Prior SAbR | 4 (17.4%) |
| Prior resection | 3 (13.0%) |
| Prior resection & SAbR | 2 (8.7%) |
| Median baseline SLD, cm (IQR) | 2.4 (1.3–3.3) |
| Number of SAbR sites, initial | |
| 1 | 15 (65.2%) |
| 2 | 6 (26.1%) |
| 3 | 2 (8.7%) |
| Number of SAbR sites, total | |
| 1 | 8 (34.8%) |
| 2 | 7 (30.4%) |
| 3 | 3 (13.0%) |
| 4–7 | 5 (21.7%) |
| All sites treated with SAbR | (n = 57 lesions) |
| Lung | 27 (47.4%) |
| Abdominal LN | 6 (10.5%) |
| Bone, non-spine | 4 (7.0%) |
| Spine | 4 (7.0%) |
| Kidney | 3 (5.3%) |
| Adrenal | 3 (5.3%) |
| Liver | 3 (5.3%) |
| Thoracic LN | 3 (5.3%) |
| Pancreas | 1 (1.8%) |
| Renal bed | 1 (1.8%) |
| Chest wall | 1 (1.8%) |
| Psoas | 1 (1.8%) |
| SAbR fractionation | |
| 1 fraction | 3 (5.3%) |
| 3 fractions | 39 (68.4%) |
| 5 fractions | 15 (26.3%) |
| Median/mode dose, Gy (range) | |
| 1 fraction | 25/25 (20–25) |
| 3 fractions | 36/36 (36–39) |
| 5 fractions | 40/40 (35–40) |
Abbreviations: AJCC, American Joint Committee on Cancer staging 8th edition; IMDC, International Metastatic RCC Database Consortium; IQR, interquartile range; LN, lymph node; RCC, renal cell carcinoma; SAbR, stereotactic ablative radiotherapy; SLD, sum of the longest diameters.
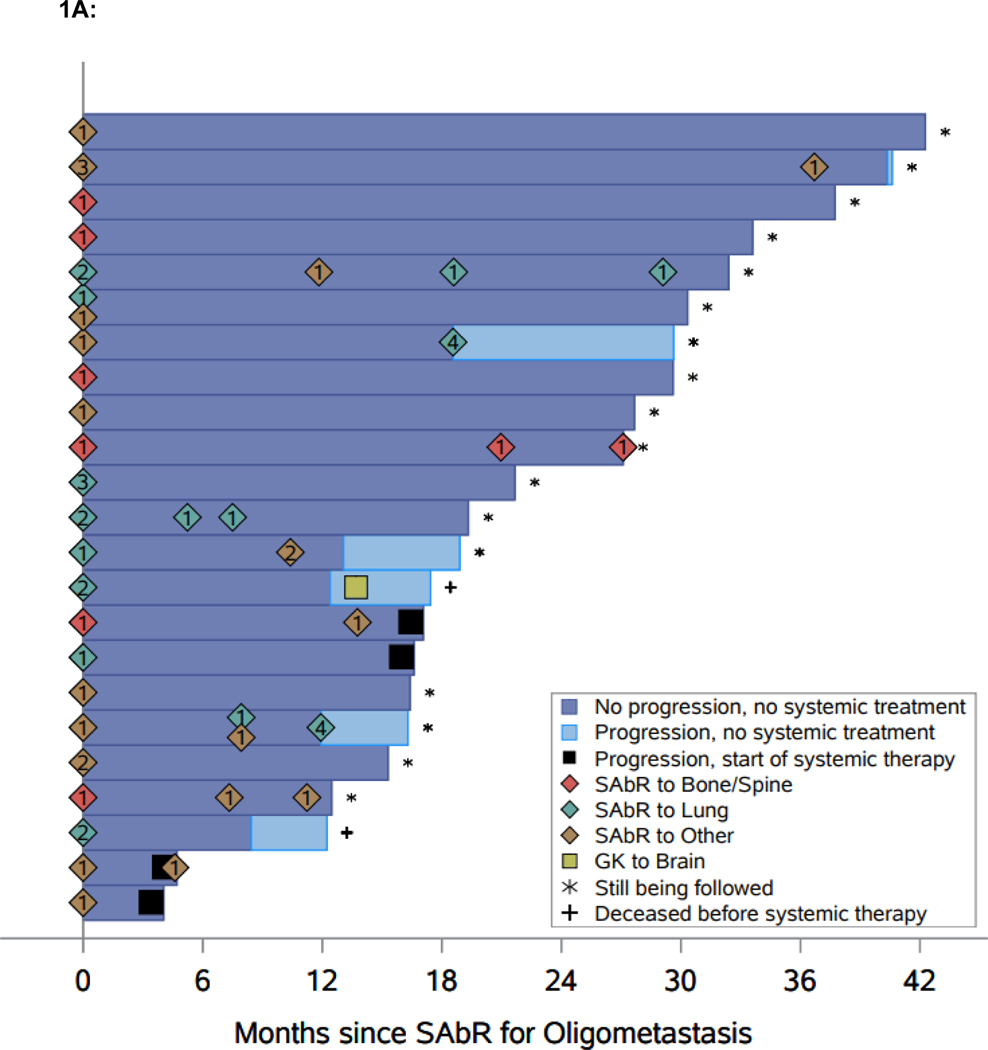
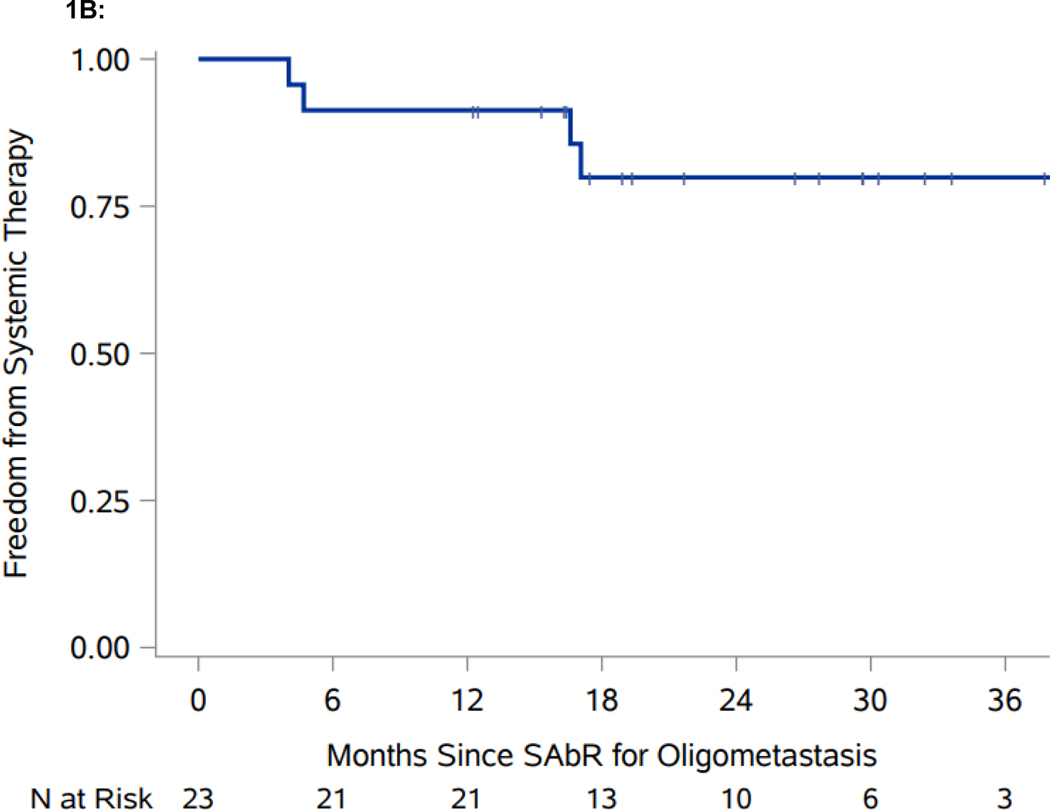
Median follow-up for the whole cohort is 21.7 months (interquartile range 16.3–30.3). Median follow-up for patients censored for the primary outcome of TTST is 26.6 months (interquartile range 16.4–32.4). Freedom from systemic therapy at one-year is 91.3% (95% CI: 69.5, 97.8), which exceeds the prespecified benchmark of 60% (Figure 1A). Median TTST has not yet been reached (Figure 1B). SAbR treated lesions had LC rates of 57/57 (100%). The one-year TTP was 87.0% (95% CI: 64.8, 95.6), which captures patients who had progressive disease but did not start systemic therapy. While limited by small numbers, ad hoc analyses failed to show an association between the interval from treatment of primary to SAbR for oligometastases and TTST (p = 0.4) (Supplemental Table 1), and overall survival (p = 0.075). Four patients progressed to systemic treatment; one patient started axitinib+pembrolizumab, one patient started lenvatinib+pembrolizumab, and two patients started ipilimumab+nivolumab (ipi/nivo). One patient on ipi/nivo had toxicity after 23 days and was switched to a different systemic therapy and the other patient discontinued ipi/nivo after one cycle due to a flare of underlying ankylosing spondylitis.
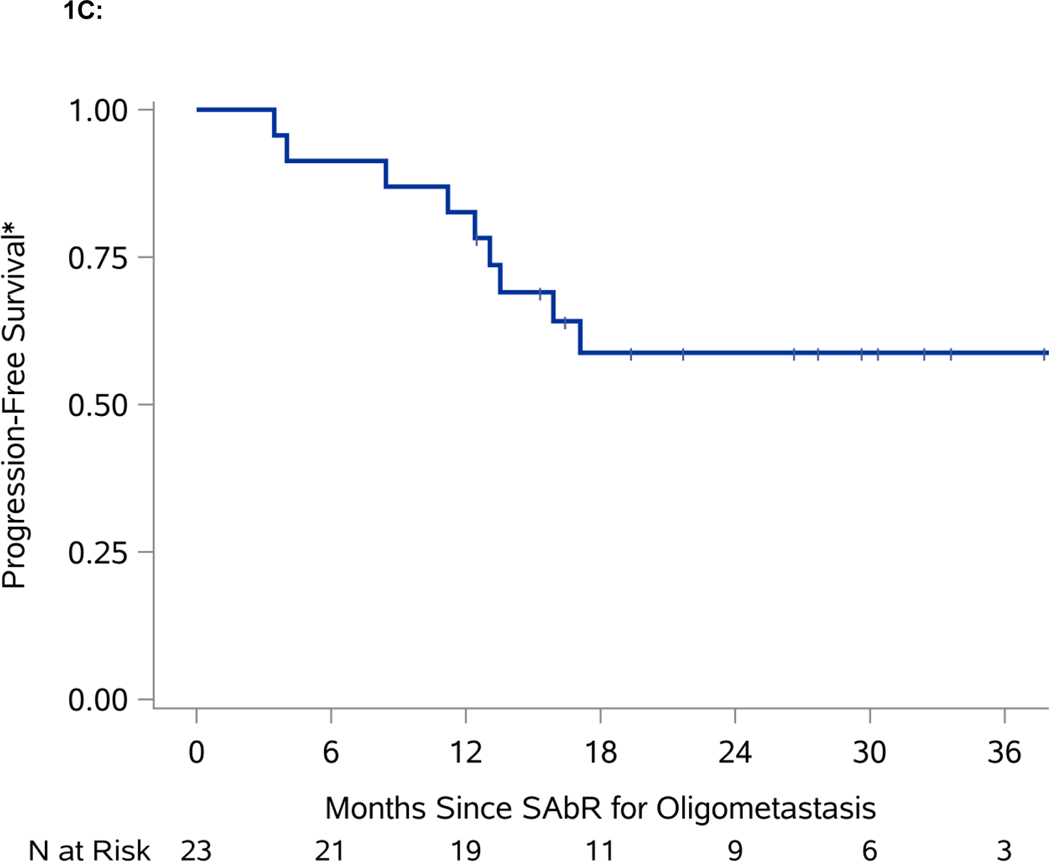
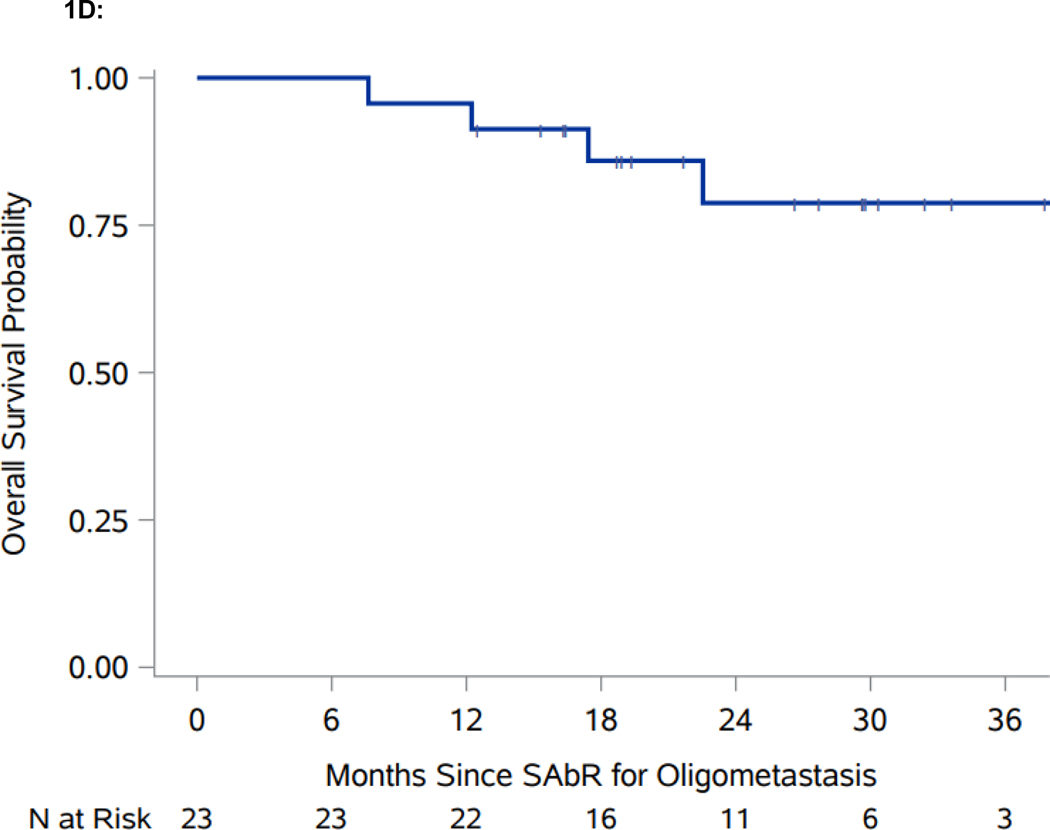
Figure 1.
Swimmer plot and Kaplan-Meier analyses of freedom from systemic therapy, progression-free survival (PFS), and overall survival (OS). A. Swimmer plot representation from onset of SAbR. Dark blue bars indicate time after SAbR without progression and systemic treatment; light blue bars show time after progression prior to start of systemic therapy with associated interventions. Diamonds indicate SAbR with numbers representing number of sites, and colors referring to location. Black squares indicate the start of systemic therapy. Asterisks indicate ongoing follow-up. Plus sign (+) indicates patients who died. GK refers to Gamma Knife radiosurgery. B. Kaplan-Meier estimate of freedom from systemic therapy. C. Kaplan-Meier estimate of PFS. D. Kaplan-Meier estimate of OS. *PFS defined as time from first SAbR to progression not amenable to SAbR, local failure at SAbR-treated sites, >3 new metastases, or brain metastases.
In addition to the four patients who progressed and started systemic treatment, six more patients progressed according to the protocol definition: two patients had >3 additional lesions (each had 4 additional metastases), which despite being amenable and indeed treated by SAbR represented a progression event; three patients developed new metastatic disease not amenable to SAbR; and one patient developed a brain metastasis treated with Gamma Knife radiosurgery. The one-year PFS for the whole cohort was 82.6% (95% CI: 60.1, 93.1). A robust estimate of median PFS is not yet available, as only two patients were still at risk at the currently observed median PFS (Figure 1C). The one-year OS for the cohort is 95.7% (95% CI: 72.9, 99.4) (Figure 1D).
Treatment-related toxicities are summarized in Table 2. One patient who received SAbR to the right kidney later experienced grade 1 ipsilateral moderate hydronephrosis and ureteral stricture without impaired renal function. One patient, who received SAbR to a right adrenal metastasis and a retroperitoneal metastatic nodule, had treatment-related grade 2 vomiting. No grade 3 or 4 toxicities were observed. One patient developed immune-related colitis while on subsequent treatment with ipilimumab/nivolumab and colonic perforation requiring partial colectomy, which showed an autoimmune infiltrate as well as the presence of cytomegalovirus. Unfortunately, the patient subsequently developed septic shock, opted for hospice care, and died soon thereafter. Although multifactorial, a contribution of SAbR to the eventual death of this patient, who had undergone radiation to an abdominal lymph node 3 months earlier, could not be excluded and therefore this death was assessed as possibly related to SAbR.
Table 2.
Summary of treatment-related toxicities
| AE-Terminology | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Abdominal pain | 1 | 1 | ||||
| Alkaline phosphatase increased | 3 | 3 | ||||
| Back pain | 1 | 1 | ||||
| Cough | 1 | 1 | ||||
| Death | 1 | 1 | ||||
| Dyspepsia | 1 | 1 | ||||
| Fatigue | 3 | 3 | ||||
| Joint range of motion decreased: Hip | 1 | 1 | ||||
| Musculoskeletal and connective tissue disorder – Other: muscle tightness | 1 | 1 | ||||
| Nausea | 5 | 5 | ||||
| Pneumonitis | 1 | 1 | ||||
| Pneumonitis (Radiation) | 1 | 1 | ||||
| Productive cough | 1 | 1 | ||||
| Renal and urinary disorders – Other: Ureteral stricture | 1 | 1 | ||||
| Renal and urinary disorders –Other: Hydronephrosis | 1 | 1 | ||||
| Vomiting | 1 | 1 | 2 | |||
| Total: | 23 | 1 | 0 | 0 | 1 | 25 |
Grade 1: Mild; Grade 2: Moderate; Grade 3: Severe or medically significant but not immediately life threatening; Grade 4: Life threatening consequences; Grade 5: Death related to the adverse event.
Two other patients died before systemic therapy. One patient died during follow-up due to congestive heart failure unrelated to RCC. After receiving SAbR to two right lung lesions, another patient subsequently developed a brain metastasis (a progression event), which was treated with Gamma Knife radiosurgery. This patient later developed multifocal disease progression in the thoracic spine and was deemed unfit for systemic therapy due to cardiomyopathy, cirrhosis, and declining performance status. A fourth patient died after progressing on the trial and Ipilimumab/Nivolumab on elected hospice care of causes unrelated to RCC or SAbR.
Health-related QOL outcomes for all patients with available follow-up data (n=19) are summarized in Table 3 and Supplemental Figure 1. Positive mean differences indicate that the QOL index measured by the questionnaire increased from baseline to follow-up. Conversely, negative mean differences indicate that the QOL index decreased from baseline to follow-up. Although long-term data is limited, most of the questionnaire responses showed no significant change in QOL from baseline, with the exceptions of the FKSI at three months (p = 0.035) and the FACT-G total score at 15 months (p = 0.042), neither of which reached the minimally important difference (MID) for the respective tools(21, 22).
Table 3.
Questionnaire responses comparing quality of life at baseline and follow-up
| Baseline vs. 3 months |
Baseline vs. 9 months |
Baseline vs. 15 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean difference (95 % CI) | P | N | Mean difference (95 % CI) | P | N | Mean difference (95 % CI) | P | |
|
| |||||||||
| FACT-G | 19 | −1.4 (−3.1, 0.3) | 0.099 | 12 | −0.3 (−3.3, 2.7) | 0.81 | 10 | −2.4 (−4.7, −0.1) | 0.042 |
| PWB | 19 | −0.1 (−3.5, 3.2) | 0.93 | 12 | −0.6 (−5.3, 4.2) | 0.79 | 10 | −1.3 (−5.0, 2.4) | 0.45 |
| SWB | 19 | −0.2 (−2.4, 2.0) | 0.84 | 12 | 1.2 (−0.9, 3.2) | 0.23 | 10 | 0.9 (−2.1, 3.9) | 0.52 |
| EWB | 19 | −1.8 (−4.8, 1.3) | 0.24 | 12 | −0.8 (−3.3, 1.8) | 0.54 | 10 | −1.1 (−4.3, 2.1) | 0.45 |
| FWB | 19 | −3.5 (−11.4, 4.3) | 0.36 | 12 | −0.5 (−10.8, 9.8) | 0.92 | 10 | −3.9 (−13.3, 5.6) | 0.38 |
| FKSI | 18 | −4.1 (−7.9, −0.3) | 0.035 | 12 | −0.1 (−4.1, 3.8) | 0.94 | 10 | −2.6 (−6.8, 1.6) | 0.20 |
| EQ-5D-5L | 19 | 2.6 (−8.0, 13.2) | 0.62 | 12 | 2.1 (−4.0, 8.2) | 0.50 | 9 | −3.9 (−13.1, 5.3) | 0.36 |
Abbreviations: EWB, emotional well-being; FACT-G, Functional Assessment of Cancer Therapy – General; FKSI, Functional Assessment of Cancer Therapy Kidney Cancer Symptom Index; FWB, functional well-being; PWB, physical well-being; SWB, social well-being.
Discussion
This single-arm phase II trial met its primary endpoint with freedom from systemic therapy at one-year of 91.3%, which exceeds the pre-specified benchmark of 60%. Overall, the study evaluated 23 patients. At 71, median age was slightly higher than median age of RCC diagnosis. Different histologies were allowed, but ~80% had clear cell histology. All patients had received definitive treatment to the primary tumor. In most patients, metastases developed metachronously (70%) and almost 40% had had prior focal therapy. None had received systemic therapy, and nearly 75% were in a favorable IMDC risk group. The median time from treatment of the primary to SAbR was 32.3 months. Over 90% of patients received SAbR to one or two metastases initially, and 10 received sequential SAbR. Radiated metastases remained controlled over the study period, SAbR was well tolerated and did not affect QOL, and systemic therapy could be effectively delayed in most patients. Thus, the current study highlights a potential new strategy for systemic therapy naïve RCC patients with oligometastatic disease where systemic therapy can be delayed with longitudinal SAbR.
This study was designed with the goal of developing a treatment strategy for patients with oligometastatic RCC for whom there is not a standard-of-care. Our premise was that focal therapies may be advantageous over systemic therapies when the disease remains localized and amenable to such approaches. Accordingly, it seemed fitting to deploy SAbR sequentially while the disease remained amenable to focal therapy. This posed, however, some challenges. The appearance of new metastases would ordinarily be regarded as a progression event. However, while in the context of systemic therapy, new metastases definitively represent a failure of the systemic therapy, that is not necessarily the case for focal therapies. Failure of focal therapy, could be argued, should involve progression at the treated site. Progression elsewhere does not necessarily signify failure of the focal therapy (at least in the same way as it represents a failure of systemic therapy). Accordingly, we modified the PFS definition as time from first day of SAbR to local failure at SAbR-treated sites or new metastases not amenable to SAbR. We also included in this definition non-oligometastatic progression. In other words, if patients developed metastases that would have excluded them from enrollment in the study in the first place because they did not meet pre-specified criteria for oligometastatic RCC, they were regarded as no longer qualifying for the intended SAbR purpose and as having progression. Thus, patients with >3 new metastases were regarded as having “progression” and that was the case also for patients developing brain metastases (despite that fact that radiation is the standard of care for brain metastases). Nevertheless, this is a higher risk patient population and this was consistent with the study qualifying criterion of ≤3 extracranial metastases.
Another challenge was establishing the primary endpoint. While the modified PFS had the advantage of being clearly defined, in reality, this was a surrogate for the ultimate goal of delaying systemic therapy. However, setting the primary endpoint on delaying systemic therapy presented some challenges. The endpoint could be accurately measured, but it could not be precisely defined. This was a clinical judgement. Of note, the same approach was previously utilized by Rini et al. in their active surveillance study(9). Because of the subjective nature of our endpoint, it is important to evaluate also how patients performed with respect to the more objective modified PFS endpoint, in particular since the trial was positive. Both endpoints aligned: freedom from systemic therapy at one-year was 91.3% (95% CI: 69.5, 97.8), freedom from progression at one year was 87.0% (95% CI: 64.8, 95.6). These data add credence to the notion that sequential SAbR for oligometastatic RCC may be a viable strategy.
Because of the subjective nature of the primary endpoint, applying these results to clinical practice can be challenging. However, inasmuch as the endpoint aligned with PFS, modified PFS criteria could be reasonably used as an alternative. There were 6 patients where the modified PFS criteria and start of systemic therapy did not match. These patients had a progression event, but did not start systemic therapy right away. While 3 of these patients did reasonably well remaining free of systemic therapy ≥4 months and were being followed at the time of study review, 2 others were deceased and they did not receive systemic therapy. Thus, it is not clear that clinical judgement is better than modified PFS criteria.
SAbR for oligometastatic cancer is an emerging concept. Chalkidou et al. recently showed a high OS rate and low toxicity with SAbR for oligometastatic cancers of multiple histologies. For the 10% of patients with mRCC in their cohort, they found a 95.2% and 82.4% OS rate at one year and two years, respectively,(23) which is quite similar to the 1-year OS survival of 95.7% in this study. A prospective study of 30 patients with either inoperable primary RCC (five patients), an isolated metastasis (six patients) or oligometastatic RCC (19 patients) treated with SAbR showed a 98% LC rate, toxicities in the 1–2 grade range in 90% of cases, and a median OS of 32 months.(13) The phase II SAbR-COMET trial randomized patients with metastatic cancer and ≤5 metastases to standard of care or standard of care plus SAbR and showed better OS in the SAbR arm.(24)
A key advantage of the SAbR approach is the ability to preserve QOL by postponing the start of systemic therapy. In our study, QOL was preserved. QOL metrics did not show a significant decline in QOL at three-, nine-, and 15-month follow-up with the exception of the FKSI at 3 months and the FACT-G at 15 months (Table 3). The FKSI assessment transiently declined at 3 months with recovery at 9 and 15 months, while the general FACT-G showed a marginal decline at 15 months. Neither the specific FACT subgroups nor the FKSI and EQ-5D-5L detected a decline at that time point. Both of the 3-month FKSI and the 15-month FACT-G declines were within the reported MID for the respective tools(21, 22).
Another potential advantage of SAbR is the lack of grade 3/4 toxicities. While current first line systemic therapies are associated with grade ≥3 toxicity in 46–83% of patients,(3–7) in our study, SAbR had one grade ≥3 toxicity (4%). There was one death due to an immune-related colitis event three months after SAbR during subsequent checkpoint inhibitor therapy where a SAbR contribution could not be excluded. Caution should be taken with SAbR administration to sites in proximity to the gastrointestinal tract in particular since patients may later receive ICI or TKIs which may exacerbate the risk of perforation. Both retrospective and prospective phase I studies suggest that concurrent ICI and SAbR is safe, but there is limited experience.(25, 26) A phase I trial by Dengina et al. evaluated patients with mRCC treated with SAbR while on TKI and ICI and found no grade ≥2 toxicities at a median follow-up of 8 months.(27) However, the safety of SAbR alongside systemic therapy is an ongoing topic of discussion,(28–31) and more prospective studies are needed. In our study, the median age was higher than the general population (71 years-old), which may also favor SAbR given the reduced risks of toxicities.
This study expands upon a recently reported phase 2 feasibility study of patients with oligometastatic mRCC similarly treated with sequential SAbR.(32) Both studies sought to identify favorable patients and excluded patients with aggressive disease. However, there are some important distinctions. The study by Tang et al. included both systemic therapy naïve as well as on-treatment patients. These patient populations are, in our opinion, different and we previously reported a study focusing on RCC patients with oligoprogressive disease - patients on systemic therapy with a limited number of progressive sites amenable to SAbR.(33) In addition, the study by Tang et al. did not meet the pre-specified co-primary endpoint of efficacy. However, local control rates, toxicity and durable control were quite similar to our study. To our knowledge, our is the first study to report the impact of sequential SAbR in systemic therapy naïve RCC patients with oligometastatic disease.
There are also significant differences with the RAPPORT trial. While both our study and the RAPPORT trial focused on patients with a limited number of metastases (≤3 or ≤5, respectively), the RAPPORT trial included patients on systemic therapy and assessed the impact of combining SAbR with an ICI, which was administered subsequently. The RAPPORT trial eavluated 30 RCC patients with ≤5 metastases treated with SAbR upfront followed by 8 cycles of pembrolizumab.(34)
Oligometastatic RCC does not have a clear standard of care. Surgery, SAbR, active surveillance and systemic therapy are all contenders. Rini et al. reported a phase 2 trial of asymptomatic, treatment naïve, patients with mRCC who underwent active surveillance to delay the start of systemic therapy where the median time on surveillance was 14.9 months.(9) In our study, which involves a different patient population, median TTST is at least 17.1 months (data not mature). A number of retrospective series show that patients with oligometastases may be managed with metastasectomy.(35) (36) The approach may particularly benefit lower-risk patients with long intervals from diagnosis of the primary tumor to development of metastases,(10) and may be applied iteratively while metastases remain limited.(37) While it is difficult to draw firm conclusions from these retrospective studies as (i) oligometastatic RCC differs from other mRCC presentations and likely impacts patient outcomes; and (ii) patients amenable to the intervention/repeated interventions may differ from those that are not, there may be benefit from aggressive local therapy for oligometastatic RCC.
Here we investigated a novel concept, sequential SAbR for systemic therapy naïve oligometastatic RCC. We hypothesized that SAbR could provide disease control in select patients whose disease remained oligometastatic and amenable to SAbR. Only patients with IMDC favorable- or intermediate-risk oligometastatic RCC were eligible and all patients had received treatment to their primary tumor. Two thirds of the patients presented with metachronous disease and the median time from treatment of the primary to SAbR was 32.3 months
There are several limitations to this study, including most significantly the lack of a control arm. Additional limitations include the small size, single institution experience and the relatively short follow-up. Nevertheless, the study is helpful by identifying a patient population who may not need to start systemic therapy right away, which can be managed safely with longitudinal SAbR while preserving their quality of life.
Conclusions
This study shows that SAbR is safe, associated with high local control rates and does not undermine quality of life in patients with oligometastatic RCC, where this intervention was associated with substantial delays in the need for systemic therapy. This prospective phase II trial justifies the evaluation of longitudinal SAbR as a potential new standard of care for oligometastatic RCC in a randomized phase III clinical trial.
Supplementary Material
Acknowledgement:
The authors acknowledge the patients that participated in the study and their families. The authors acknowledge the support and cooperation from individuals at Parkland Health and Hospital System, Dallas, TX, USA for voluntarily participating in the study intervention, surveys, data collection and in clinical decision support. Dr. Jonathan Feinberg contributed to the scientific editing of the manuscript. They have no conflicts of interest relevant to this article.
Funding:
Alana Christie, Kevin Courtney and James Brugarolas are funded by P50CA196516. Raquibul Hannan is funded by American Cancer Society RSG-16-004-01-CCE.
Footnotes
Conflicts of Interest: None
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. Epub 2021/02/05. doi: 10.3322/caac.21660. PubMed PMID: 33538338. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1994;12(1):206–12. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B, CheckMate I. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2018;378(14):1277–90. Epub 2018/03/22. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T, Investigators K-. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116–27. Epub 2019/02/20. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH Jr., Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1103–15. Epub 2019/02/20. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juarez VM, Hsieh JJ, Basso U, Shah AY, Suarez C, Hamzaj A, Goh JC, Barrios C, Richardet M, Porta C, Kowalyszyn R, Feregrino JP, Zolnierek J, Pook D, Kessler ER, Tomita Y, Mizuno R, Bedke J, Zhang J, Maurer MA, Simsek B, Ejzykowicz F, Schwab GM, Apolo AB, Motzer RJ, CheckMate ERI. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2021;384(9):829–41. Epub 2021/03/04. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grunwald V, Hutson TE, Kopyltsov E, Mendez-Vidal MJ, Kozlov V, Alyasova A, Hong SH, Kapoor A, Alonso Gordoa T, Merchan JR, Winquist E, Maroto P, Goh JC, Kim M, Gurney H, Patel V, Peer A, Procopio G, Takagi T, Melichar B, Rolland F, De Giorgi U, Wong S, Bedke J, Schmidinger M, Dutcus CE, Smith AD, Dutta L, Mody K, Perini RF, Xing D, Choueiri TK, Investigators CT. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384(14):1289–300. Epub 2021/02/23. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 8.Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, Bjarnason GA, Srinivas S, Pal SK, Yuasa T, Smoragiewicz M, Donskov F, Kanesvaran R, Wood L, Ernst DS, Agarwal N, Vaishampayan UN, Rha SY, Choueiri TK, Heng DY. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. The lancet oncology. 2015;16(3):293–300. Epub 2015/02/16. doi: 10.1016/S1470-2045(14)71222-7. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Dorff TB, Elson P, Rodriguez CS, Shepard D, Wood L, Humbert J, Pyle L, Wong YN, Finke JH, Rayman PA, Larkin JM, Garcia JA, Plimack ER. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol. 2016;17(9):1317–24. Epub 2016/08/09. doi: 10.1016/S1470-2045(16)30196-6. PubMed PMID: 27498080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabestani S, Marconi L, Hofmann F, Stewart F, Lam TB, Canfield SE, Staehler M, Powles T, Ljungberg B, Bex A. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15(12):e549–61. Epub 2014/12/03. doi: 10.1016/S1470-2045(14)70235-9. [DOI] [PubMed] [Google Scholar]
- 11.Walsh L, Stanfield JL, Cho LC, Chang CH, Forster K, Kabbani W, Cadeddu JA, Hsieh JT, Choy H, Timmerman R, Lotan Y. Efficacy of ablative high-dose-per-fraction radiation for implanted human renal cell cancer in a nude mouse model. Eur Urol. 2006;50(4):795–800; discussion Epub 2006/04/25. doi: 10.1016/j.eururo.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Schoenhals J, Christie A, Mohamad O, Wang C, Bowman I, Singla N, Hammers H, Courtney K, Bagrodia A, Margulis V, Desai N, Garant A, Choy H, Timmerman R, Brugarolas J, Hannan R. Stereotactic Ablative Radiation Therapy (SAbR) Used to Defer Systemic Therapy in Oligometastatic Renal Cell Cancer. International journal of radiation oncology, biology, physics. 2019;105(2):367–75. Epub 2019/08/05. doi: 10.1016/j.ijrobp.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svedman C, Sandstrom P, Pisa P, Blomgren H, Lax I, Kalkner KM, Nilsson S, Wersall P. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45(7):870–5. Epub 2006/09/20. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 14.Zaorsky NG, Lehrer EJ, Kothari G, Louie AV, Siva S. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): a meta-analysis of 28 studies. Eur Urol Oncol. 2019;2(5):515–23. Epub 2019/07/16. doi: 10.1016/j.euo.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Christie A, Lin MH, Jung M, Weix D, Huelsmann L, Kuhn K, Meyer J, Desai N, Kim DWN, Pedrosa I, Margulis V, Cadeddu J, Sagalowsky A, Gahan J, Laine A, Xie XJ, Choy H, Brugarolas J, Timmerman R, Hannan R. Safety and Efficacy of Stereotactic Ablative Radiation Therapy for Renal Cell Carcinoma Extracranial Metastases. Int J Radiat Oncol Biol Phys. 2017;98(1):91–100. Epub 2017/06/08. doi: 10.1016/j.ijrobp.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyengar P, Kavanagh BD, Wardak Z, Smith I, Ahn C, Gerber DE, Dowell J, Hughes R, Abdulrahman R, Camidge DR, Gaspar LE, Doebele RC, Bunn PA, Choy H, Timmerman R. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol. 2014;32(34):3824–30. Epub 2014/10/29. doi: 10.1200/JCO.2014.56.7412. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. Epub 2000/02/03. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8. Epub 2010/01/27. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31. Epub 2013/08/24. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 20.Zaorsky NG, Wang X, Garrett SM, Lehrer EJ, Lin C, DeGraff DJ, Spratt DE, Trifiletti DM, Kishan AU, Showalter TN, Park HS, Yang JT, Chinchilli VM, Wang M. Pan-cancer analysis of prognostic metastatic phenotypes. Int J Cancer. 2022;150(1):132–41. Epub 20210827. doi: 10.1002/ijc.33744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health and quality of life outcomes. 2003;1:79. Epub 2003/12/18. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella D, Yount S, Brucker PS, Du H, Bukowski R, Vogelzang N, Bro WP. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health. 2007;10(4):285–93. Epub 2007/07/25. doi: 10.1111/j.1524-4733.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- 23.Chalkidou A, Macmillan T, Grzeda MT, Peacock J, Summers J, Eddy S, Coker B, Patrick H, Powell H, Berry L, Webster G, Ostler P, Dickinson PD, Hatton MQ, Henry A, Keevil S, Hawkins MA, Slevin N, van As N. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. The lancet oncology. 2021;22(1):98–106. Epub 2021/01/03. doi: 10.1016/S1470-2045(20)30537-4. [DOI] [PubMed] [Google Scholar]
- 24.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Bauman GS, Warner A, Senan S. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–8. Epub 2019/04/16. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 25.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, Basu S, Curran MA, Cabanillas ME, Subbiah V, Fu S, Tsimberidou AM, Karp D, Gomez DR, Diab A, Komaki R, Heymach JV, Sharma P, Naing A, Hong DS. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res. 2017;23(6):1388–96. Epub 2016/11/01. doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamad O, Diaz de Leon A, Schroeder S, Leiker A, Christie A, Zhang-Velten E, Trivedi L, Khan S, Desai NB, Laine A, Albuquerque K, Iyengar P, Arriaga Y, Courtney K, Gerber DE, Hammers H, Choy H, Timmerman R, Brugarolas J, Hannan R. Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy. Oncoimmunology. 2018;7(7):e1440168. Epub 2018/06/15. doi: 10.1080/2162402X.2018.1440168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dengina N, Mitin T, Gamayunov S, Safina S, Kreinina Y, Tsimafeyeu I. Stereotactic body radiation therapy in combination with systemic therapy for metastatic renal cell carcinoma: a prospective multicentre study. ESMO Open. 2019;4(5):e000535. Epub 2019/11/02. doi: 10.1136/esmoopen-2019-000535. PubMed PMID: 31673426; PMCID: PMC6802957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sha CM, Lehrer EJ, Hwang C, Trifiletti DM, Mackley HB, Drabick JJ, Zaorsky NG. Toxicity in combination immune checkpoint inhibitor and radiation therapy: A systematic review and meta-analysis. Radiother Oncol. 2020;151:141–8. Epub 2020/07/28. doi: 10.1016/j.radonc.2020.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Verma V, Cushman TR, Tang C, Welsh JW. Toxicity of radiation and immunotherapy combinations. Adv Radiat Oncol. 2018;3(4):506–11. Epub 2018/10/30. doi: 10.1016/j.adro.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng X, Feng R, Yang L, Xing L, Yu J. The Role of Radiation Oncology in Immuno-Oncology. Oncologist. 2019;24(Suppl 1):S42–S52. Epub 2019/03/02. doi: 10.1634/theoncologist.2019-IO-S1-s04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchelebi LT, Batchelder E, Wang M, Lehrer EJ, Drabick JJ, Sharma N, Machtay M, Trifiletti DM, Zaorsky NG. Radiotherapy and Receptor Tyrosine Kinase Inhibition for Solid Cancers (ROCKIT): A Meta-Analysis of 13 Studies. JNCI Cancer Spectr. 2021;5(4):pkab050. Epub 2021/08/06. doi: 10.1093/jncics/pkab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang C, Msaouel P, Hara K, Choi H, Le V, Shah AY, Wang J, Jonasch E, Choi S, Nguyen QN, Das P, Prajapati S, Yu Z, Khan K, Powell S, Murthy R, Sircar K, Tannir NM. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-centre, feasibility, phase 2 trial. The lancet oncology. 2021. Epub 2021/11/01. doi: 10.1016/S1470-2045(21)00528-3. [DOI] [PubMed] [Google Scholar]
- 33.Hannan R, Christensen M, Hammers H, Christie A, Paulman B, Lin D, Garant A, Arafat W, Courtney K, Bowman I, Cole S, Sher D, Ahn C, Choy H, Timmerman R, Brugarolas J. Phase II Trial of Stereotactic Ablative Radiation for Oligoprogressive Metastatic Kidney Cancer. Eur Urol Oncol. 2021. Epub 20211228. doi: 10.1016/j.euo.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siva S, Bressel M, Wood ST, Shaw MG, Loi S, Sandhu SK, Tran B, A AA, Lewin JH, Cuff KE, Liu HY, Moon D, Goad J, Wong LM, LimJoon M, Mooi J, Chander S, Murphy DG, Lawrentschuk N, Pryor D. Stereotactic Radiotherapy and Short-course Pembrolizumab for Oligometastatic Renal Cell Carcinoma-The RAPPORT Trial. Eur Urol. 2022;81(4):364–72. Epub 20211223. doi: 10.1016/j.eururo.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(6):2261–6. [DOI] [PubMed] [Google Scholar]
- 36.Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117(13):2873–82. doi: 10.1002/cncr.25836. [DOI] [PubMed] [Google Scholar]
- 37.van der Poel HG, Roukema JA, Horenblas S, van Geel AN, Debruyne FM. Metastasectomy in renal cell carcinoma: A multicenter retrospective analysis. European urology. 1999;35(3):197–203. doi: 19849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.