Abstract
Individuals with autism spectrum disorder (ASD) frequently present with impairments in motor skills (e.g., limb coordination, handwriting and balance), which are observed across the lifespan but remain largely untreated. Many adults with ASD may thus experience adverse motor outcomes in aging, when physical decline naturally occurs. The ‘hand knob’ of the sensorimotor cortex is an area that is critical for motor control of the fingers and hands. However, this region has received little attention in ASD research, especially in adults after midlife. The hand knob area of the precentral (PrChand) and postcentral (PoChand) gyri was semi-manually delineated in 49 right-handed adults (25 ASD, 24 typical comparison [TC] participants, aged 41–70 years). Using multimodal (T1-weighted, diffusion-weighted, and resting-state functional) MRI, we examined the morphology, ipsilateral connectivity and laterality of these regions. We also explored correlations between hand knob measures with motor skills and autism symptoms, and between structural and functional connectivity measures. Bayesian analyses indicated moderate evidence of group effects with greater right PrChand volume and reduced leftward laterality of PrChand and PoChand volume in the ASD relative to TC group. Furthermore, the right PoC-PrChand u-fibers showed increased mean diffusivity in the ASD group. In the ASD group, right u-fiber volume positively correlated with corresponding functional connectivity but did not survive multiple comparisons correction. Correlations of hand knob measures and behavior were observed in the ASD group but did not survive multiple comparisons correction. Our findings suggest that morphological laterality and u-fiber connectivity of the sensorimotor network, putatively involved in hand motor/premotor function, may be diminished in middle-aged adults with ASD, perhaps rendering them more vulnerable to motor decline in old age. The altered morphology may relate to atypical functional motor asymmetries found in ASD earlier in life, possibly reflecting altered functional asymmetries over time.
Keywords: autism, middle-age, sensorimotor, hemispheric asymmetry, u-fibers
1. Introduction
Autism spectrum disorder (ASD) is characterized by deficits in social communication, atypical patterns of restricted and repetitive behaviors and sensory abnormalities (American Psychiatric Association, 2013). Impairments in the motor domain are not included in the diagnostic criteria (American Psychiatric Association, 2013). The proportion of individuals with ASD at risk of a motor impairment, however, is estimated to be as high as 86.9% (Bhat, 2020; Licari et al., 2020). Motor difficulties in ASD are also seldomly reported by diagnosing clinicians (Licari et al., 2020) and remain largely untreated (Bhat, 2020). Motor impairments in individuals with ASD are observed across a wide range of domains (e.g., gross and fine motor coordination, postural stability, balance and grip strength), and are often related to difficulties in motor planning and increased neural noise in the sensorimotor system (Gowen & Hamilton, 2013). Fine motor impairment in ASD, in particular, is well-documented and has been reported in infancy as early as 6 months (Iverson et al., 2019; Libertus et al., 2014; Sacrey et al., 2018), at the toddler stage (Landa & Garrett-Mayer, 2006; Lloyd et al., 2013), in childhood (Fuentes et al., 2009; Green et al., 2009; Jasmin et al., 2009; Provost et al., 2007), adolescence (Fuentes et al., 2010; Pan, 2014) and into middle-age (Linke et al., 2019; Thompson et al., 2017; Travers et al., 2015, 2017).
Motor skills are essential for daily living activities required to independently care for oneself, such as eating, dressing, and walking. In children and adults with ASD, positive correlations of manual motor skills with daily living skills, and of motor coordination with daily living skills and adaptive behavior, have been reported (Bremer & Cairney, 2018; Travers et al., 2017). In aging, the ability to perform these activities is widely used as one indicator of health and disability outcomes (Katz, 1983). Manual motor skills are also found to be predictors of functional disability (Rantanen et al., 1999), dependency (Ostwald et al., 1989) and even cognitive impairment (Curreri et al., 2018) in the elderly. In individuals with ASD, there is some evidence that manual motor skills may lag increasingly behind with age (Travers et al., 2017). In an accelerated longitudinal study involving individuals with ASD aged 5–39 years at their first visit, Travers and colleagues (2017) noted that after around age 15 years, grip strength and finger tapping speed in the ASD group diverged further from a typically developing group (with a larger gap in performance than at a younger age). Thus, early motor impairments may be compounded by increased motor problems in adolescence and adulthood in ASD. Reports of an increased prevalence of movement disorders (e.g., parkinsonism) in middle-aged and older adults with ASD have emerged from direct assessment (Starkstein et al., 2015), insurance records (Croen et al., 2015), and brief self-screening (Geurts et al., 2021); however, the literature on aging in ASD remains extremely limited (Piven & Rabins, 2011).
The anatomical ‘hand knob’ landmark is a characteristic knob-like shape formed by the central sulcus that defines subareas of the pre and postcentral gyri (Yousry et al., 1997). The hand knob areas elicit hand movements when electrically stimulated (Penfield & Boldrey, 1937) and consistently show activation during hand movements (Sun et al., 2016; Yousry et al., 1997) in the contralateral hemisphere. Although they are considered to be primarily specialized for hand motor execution, there is evidence of involvement in premotor functions beyond basic motor execution, such as motor planning and coordination. For example, cytoarchitecture of the dorsal premotor cortex (corresponding to Brodmann area 6) extends to portions of the rostral precentral gyrus including the hand knob area (Geyer et al., 1996; Geyer, Matelli, Luppino, & Zilles, 2000; Rademacher et al., 2001; Rademacher, Rademacher, Caviness, Steinmetz, & Galaburda, 1993; L. E. White et al., 1997). Stimulation studies of the pre and postcentral hand knob areas show substantial overlap between representations of the hand and other body parts (e.g., mouth, arm, face) in typical individuals (Catani, 2017; Penfield & Boldrey, 1937), and a recent study proposed that the hand knob area may be involved in compositional coding of movement across limbs (Willett et al., 2020).
Neuroimaging studies on the hand knob area have mainly been limited to the topics of manual preference (Amunts et al., 1996; Dassonville et al., 1997; S Rose et al., 2012; Volkmann et al., 1998), hand paresis (Gass, Szabo, Behrens, Rossmanith, & Hennerici, 2001; Peters et al., 2009; Stephen Rose, Guzzetta, Pannek, & Boyd, 2011) and neurophysiology (Hallett, 2007). To our knowledge, only one MRI study has specifically investigated the hand knob area in ASD, focusing exclusively on the short-range association white matter tracts (i.e., u-fibers) (Thompson et al., 2017). Using deterministic tractography, Thompson and colleagues found evidence of compromised microstructure, specifically reduced fractional anisotropy and increased mean and radial diffusivity, bilaterally in the u-fibers connecting ipsilateral postcentral and precentral hand knob area in right-handed adults (aged 18–45 years) with ASD relative to a control group. Of the limited literature on middle-aged adults with ASD, reduced resting-state functional connectivity of intra and interhemispheric primary sensorimotor and premotor cortical areas has been observed by our group (Linke et al., 2019). This appears to be consistent with the reduced postcentral to precentral u-fiber structural integrity reported in the slightly younger adults with ASD. However, whether these differences in the u-fiber microstructural indices in ASD continue to be seen in middle-age, and how structural and functional connectivity measures of these regions are related, is unknown.
Although not specifically examining the hand knob area, previous MRI studies have found grey matter abnormalities in the pre and postcentral gyri in ASD. Meta-analyses of voxel-based morphometry studies involving a wide age range from children to adults (including middle-age) have reported decreased grey matter density of the left precentral gyrus (Cauda et al., 2011; Nickl-Jockschat et al., 2012) and left postcentral gyrus (DeRamus & Kana, 2015) in ASD compared with typical development, and increased grey matter density of the right precentral gyrus in adults (18–52 years) with ASD (DeRamus & Kana, 2015). Moreover, an altered trajectory with age in grey matter density of right precentral gyrus has been observed in ASD. While a typically developing group showed a u-shaped curve from age 8 to 50 years, a gradual linear decline was seen in the ASD group (Greimel et al., 2013). This pattern of results raises the possibility of reduced grey matter asymmetry in the pre- and postcentral gyri in adults with ASD. A previous large sample study on morphological asymmetry, however, did not reveal any group differences in asymmetry in these regions in ASD (Postema et al., 2019). It is possible, however, that laterality varies in different portions of the relatively large regions of the pre and postcentral gyri, and the effect of reduced asymmetry is more localized (e.g., within the hand knob).
The present multimodal MRI study is the first to examine the morphology, structural and functional connectivity, and asymmetry of the hand knob area and links with both motor skills and autism symptoms in middle-aged adults with ASD. Based on the previous literature, we hypothesized reduced asymmetry in volume (accompanied by decreased left and/or increased right volume) in the pre- (PrChand) and postcentral (PoChand) hand knob area, and decreased connectivity between the pre- and post-central hand knob (PoC-PrChand) in ASD. We also hypothesized that greater leftward laterality of structure (i.e., hand knob volume and u-fiber tract integrity) and functional connectivity would be correlated with improved motor skills and less severe autism symptoms. Additionally, we examined the superior and inferior segments of the pre- and postcentral gyri above and below the hand knob area, to determine the specificity of any diagnostic group differences within these gyri. Links between PoC-PrChand structural and functional connectivity were also investigated.
2. Methods
2.1. Participants
Seventy-three participants (35 ASD, 37 typical comparison [TC]), aged 40–70 years, were recruited from San Diego County communities through referrals from autism clinics, service providers (e.g., day programs, group homes) and local advertisement as part of an ongoing longitudinal study. Only individuals with idiopathic ASD and without epilepsy were eligible for participation. TC participants had no personal or family history of autism. All participants had no personal history of other genetic or neurological conditions or serious mental illness. Seventeen datasets were excluded after quality assessment of the imaging data (12 were affected by a hardware issue, 2 scans were incomplete, 3 had low quality anatomical images due to noise, motion or artifacts – see below) and 3 participants were excluded for incidental brain findings. As our study included laterality measures for motor regions, left-handed participants (1 ASD, 2 TC) were excluded. The final sample consisted of 25 ASD and 24 TC participants. For the fMRI analyses, 7 participants were further excluded (4 ASD and 2 TC participants for image artifacts or excessive head motion; 1 ASD participant had no fMRI data). Behavioral measures, including Bruininks Motor Ability Test (BMAT; Bruininks & Bruininks, 2012) scores, for the majority of these participants were previously presented in (Linke et al., 2019).
All ASD diagnoses were based on best clinical judgement by a clinical psychologist according to DSM-5 criteria (American Psychiatric Association, 2013), and supported by module 4 of the Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2) (Lord et al., 2002) (current ADOS scores were not available for one participant due to administrator error, however this individual met ASD criteria on a research-related ADOS within the preceding 5 years). Intelligence quotient (IQ) was assessed with the Wechsler Abbreviated Scale of Intelligence, 2nd edition (WASI-II) (Wechsler, 2011). Dichotomous hand preference (left/ambidextrous or right) was determined for all participants based on a personal history questionnaire completed by the participant or caregiver in response to the question “With which hand does s/he eat, brush teeth, write, etc.?”, in line with (Perelle & Ehrman, 2005). The Edinburgh Handedness Inventory (EHI) (Oldfield, 1971) was used to measure degree of handedness and was available for 23 ASD and 23 TC participants. The EHI consists of a self-report checklist in which participants indicate their hand preference (left, right, or if really indifferent, both left and right) across ten different actions (e.g., “write a letter with a pencil”, “throw a baseball”, “strike a match”), and a final score is calculated as follows: (# right - # left) / (# right + # left) x 100. The self-reported EHI scores corresponded to dichotomous hand preference for all participants who completed the EHI. Motor function was assessed using the short form version of the BMAT (Bruininks & Bruininks, 2012). Five subcategories of motor behavior were evaluated: fine motor (e.g., drawing a line through a curved path), manual dexterity (e.g., transferring pennies), coordination (e.g., bouncing and catching a ball), balance and mobility (e.g., standing on one leg), and strength and flexibility (e.g., grip strength).
The study was approved by the institutional review boards of the San Diego State University and University of California, San Diego. All participants were screened for MRI contraindications (e.g., claustrophobia, ferrous material in body). All participants or their conservators provided written informed consent before participation, and participants were compensated for their time.
2.2. Image acquisition
MRI data were collected at the Center for Functional Magnetic Resonance Imaging (CFMRI, University of California, San Diego) on a 3T GE Discovery MR750 scanner using a 32-channel head coil. A high resolution anatomical T1-weighted image (repetition time [TR]=8.776 ms, echo time [TE]=3.656 ms, 8° flip angle, 320 x 320 matrix, 0.8mm3 resolution) was acquired using an MPRAGE sequence with real-time prospective motion correction (PROMO; White et al., 2010). Diffusion-weighted images were acquired with 46 non-colinear directions at b=1500 (13 ASD and 9 TC participants) or 1000 s/mm2 (12 ASD and 15 TC participants) and 6 interspersed volumes at b=0 s/mm2 (TR=4000ms, TE=89ms, multi-band factor=3, 27 slices/band, 24cm field of view [FOV], 1.7 mm isotropic resolution) with reversed (anterior-posterior) phase encoding to correct for susceptibility distortions and improve signal-to-noise ratio. Resting-state functional MRI images were acquired using a multi-band EPI sequence (2 runs x 6 minute duration each, TR=800ms, TE=35ms, multi-band factor=8, 52° flip angle, 20.8cm FOV, 2mm3 resolution) with a 20s reverse phase-encoded calibration scan pair using identical imaging parameters to correct for susceptibility-induced distortions. Before each functional MRI scan, participants were given the following instructions: “Keep your eyes on the cross. Let your mind wander, relax, but please stay as still as you can. Try not to fall asleep.” Eye status was monitored throughout the duration of the scan using an in-bore camera to ensure participant compliance.
2.3. Data preprocessing
FreeSurfer 5.3.0 was used to preprocess the anatomical images using the standard pipeline (i.e., skull stripping, intensity normalization, gray-white matter segmentation) and to perform cortical surface reconstruction (Dale et al., 1999; Fischl et al., 1999). The anatomical images and surfaces were visually inspected, and any images with inaccurate surfaces or excessive image artifacts (e.g., ghosting, ringing) were excluded. For both diffusion-weighted and functional MRI data, susceptibility-induced off-resonance fields were estimated and corrected from the reversed phase-encoded image pairs using TOPUP (Andersson et al., 2003). For the diffusion-weighted images, we first applied denoising by eliminating the noise component in the patch-level PCA-domain (Veraart et al., 2016). Eddy current and susceptibility-induced distortions, inter- and intra-volume head motion and signal dropout incorporating information from both individual slices and multi-band slice groupings (Andersson et al., 2017) were then corrected in a single interpolation step using EDDY from the FMRIB Software Library (FSL) 5.0.11 (Andersson et al., 2016; Andersson & Sotiropoulos, 2016). The b-vectors were rotated according to the motion realignment. Functional MRI preprocessing included rigid-body realignment for motion correction and non-linear registration to Montreal Neurological Institute (MNI) space (SPM12) and scrubbing of motion outliers, band-pass filtering using a temporal filter of 0.008–0.08 Hz, and nuisance regression (including motion parameters and their derivatives as well as time-series derived from cerebrospinal fluid and white matter) using the Functional Connectivity (Conn) Toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012).
For purposes of group matching, in-scanner head motion was quantified in two ways for the diffusion-weighted images: average root mean squared displacement excluding in the phase-encoding direction across all brain voxels with respect to the first volume (absolute RMSD) and with respect to the preceding volume (relative RMSD). RMSD was also calculated from the fMRI realignment parameters and averaged across runs to quantify head motion during the functional MRI scans.
2.4. Delineation of the hand knob regions of interest
Given the interindividual variability in the location (Sun et al., 2012) and morphology (Caulo et al., 2007) of the hand knob along the central sulcus, the regions of interest (ROIs) for the pre (PrChand) and postcentral (PoChand) hand knob areas were semi-manually delineated in each participant. Automatic parcellations of the pre and postcentral gyri (Desikan et al., 2006) were performed for each participant and used as starting regions, from which PrChand and PoChand were subsequently delineated, to ensure that the final ROIs correspond with consistent pre and post-central cortical boundaries and with grey matter tissue. From the starting parcels, manual segmentations of the hand knob ROIs were performed by one trained operator (AB). Fsleyes (McCarthy, 2018) was used to visualize and edit the ROIs with the participants’ T1-weighted image underlay. First, the hand knob landmark, commonly identified as an omega (but also epsilon or null) shape on the precentral gyrus (Caulo et al., 2007), was located in the axial plane in each hemisphere (Figure 1A). Then the starting regions were manually edited by removing areas surrounding the hand knob (i.e., supero-posterior and infero-anterior to the hand knob [e.g., omega] landmark) until only the hand knob remained (Figure 1B). As the hand knob was most identifiable on the precentral gyrus, delineation of the precentral hand knob (PrChand) was performed first and the boundaries of the postcentral hand knob (PoChand) were extrapolated following a perpendicular line from the superior and inferior limits of PrChand to the adjacent postcentral gyrus. The T1-weighted images were rigid-body registered to diffusion space using a boundary-based approach (Greve & Fischl, 2009) and the parcellated regions were registered using the same registration matrix with nearest neighbor interpolation. All operators were trained by an experienced anatomist (JH) until agreement was reached on the location and spatial extent of the ROIs in 10 practice participants. All operators were blind to the participants’ diagnostic group.
Figure 1.
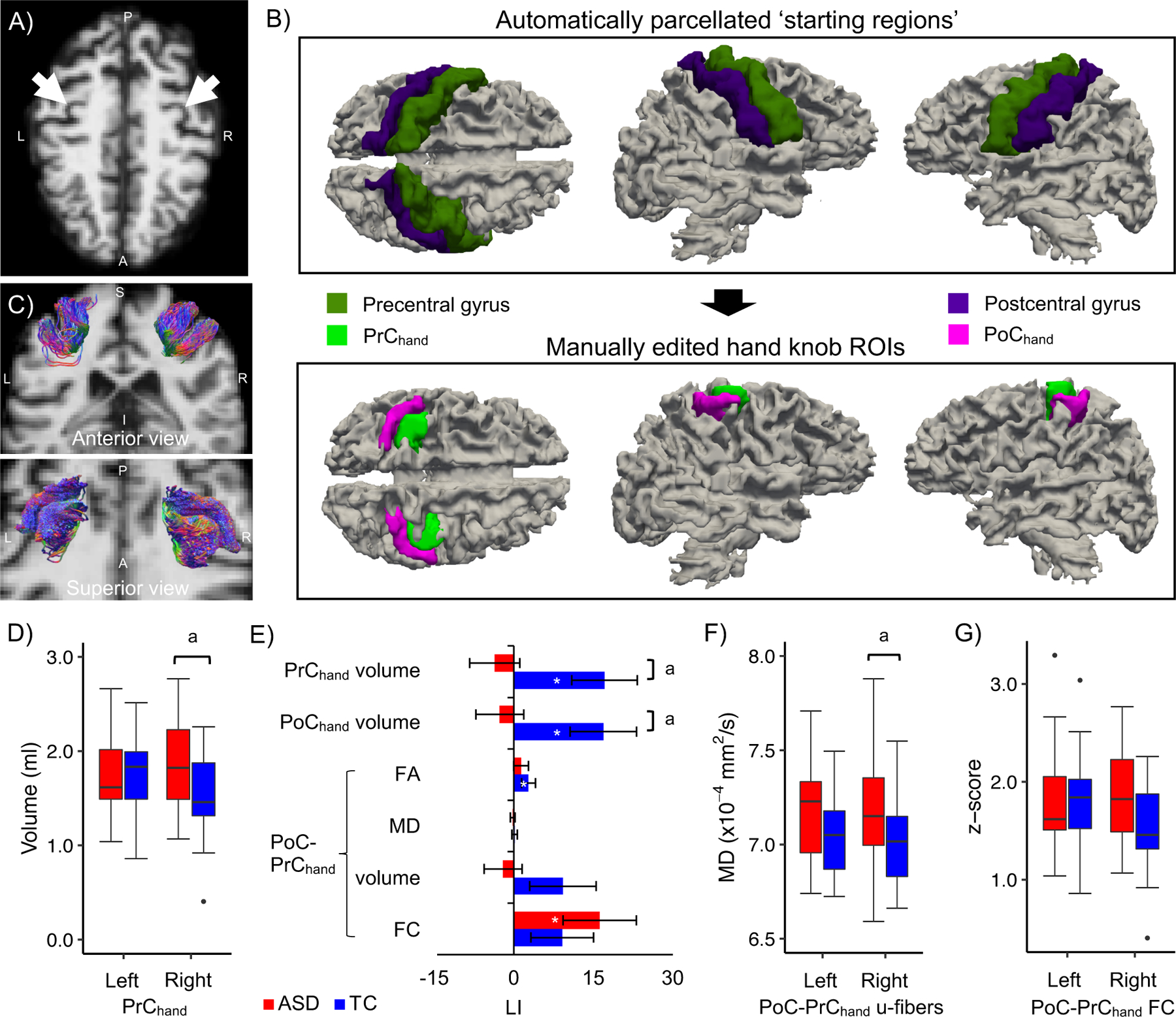
A-C) Identification and delineation of the hand knob regions and u-fibers. A) The typical omega-shaped landmark (white arrows) used to identify the hand knob in the axial plane of a representative participant with ASD (aged 49 years). B) The automatically parcellated pre and postcentral regions (upper row) were manually edited to define the pre (PrChand) and postcentral (PoChand) hand knob regions of interest (lower row). C) Streamline tractography of the u-fiber tracts connecting ipsilateral PoChand and PrChand from the same participant. D-G) Alterations in hand knob morphometry, laterality and PoC-PrC connectivity in middle-aged adults with ASD. The plots show D) increased right PrChand volume, E) reduced laterality of PrChand and PoChand knob volume (LI of other hand knob measures are also shown), and F) increased mean diffusivity (MD) in the right PoC-PrChand u-fiber tract in the ASD group. G) Medium size group effects were also observed for left PoC-PrChand u-fiber MD (increased in ASD) and right PoC-PrChand FC (decreased in ASD). a q<.10 significance level; * LI measures that differed from zero (p<.05, uncorrected). Standard errors of the means are shown in the bar plot.
To assess intra-rater reliability, the hand knob regions were delineated a second time by author AB in a subset of 15 participants. To assess inter-rater reliability, a second trained operator (CC) delineated hand knob regions in the same subset of participants. Intra-class correlation coefficients (ICC) with two-way random effects showed good overall intra-rater reliability between hand knob volume measurements (PrChand: average measure ICC=.647, p=.003; PoChand: average measure ICC=.823, p<.001) and their laterality indices [LI] (LI PrChand: average measure ICC=.755, p=.006; LI PoChand: average measure ICC=.828, p=.001). Between-rater reliability was also good overall (PrChand: average measure ICC=.667, p=.002; PoChand: average measure ICC=.855, p<.001; LI PrChand: average measure ICC=.770, p=.005.; LI PoChand: average measure ICC=.785, p=.003). Additionally, we calculated overlap coefficients (OC) for PrChand and PoChand which showed good spatial correspondence within (PrChand: mean OC [min, max]=.85 [.53, .99]; PoChand: .91 [.70, .99]) and between (PrChand: .87 [.74, .99]; PoChand: .88 [.55, .96]) raters.
Since the hand knob ROIs were delineated in the native diffusion space and therefore could be subject to variations in brain alignment with respect to the cardinal axes, we extracted the root mean squared deviation in roll, pitch and yaw rotations (RMSDrot) from the affine transformations between diffusion and MNI space for each participant. Independent t-tests showed that the groups marginally differed in RMSDrot (p=.082; TC mean [SD]: 9.66 [3.60]; ASD: 7.97 [3.05]). This measure was included as an additional covariate for the anatomical measures in our statistical model to control for brain image rotation.
2.5. Fiber tractography and diffusion scalar maps
Fiber tractography was performed using the MRtrix3 software package (Tournier et al., 2019). Response functions were estimated for each tissue type (grey matter, white matter and cerebrospinal fluid) using an unsupervised algorithm (Dhollander et al., 2019). Fiber orientation distributions were estimated using multi-tissue constrained spherical deconvolution (Jeurissen et al., 2014), and intensity normalization was applied across tissue-specific distributions to minimize spurious peaks driven by single-tissue intensities. Sensorimotor (PoC-PrC) u-fibers were obtained by performing anatomically constrained probabilistic tractography (Smith et al., 2012) on the fiber orientation distributions using ipsilateral PrChand and PoChand as both seed and target regions, that were reversed to track in both directions to control for biases in tracking direction, with 5000 randomly placed seeds per voxel (minimum length=10mm, maximum length=100mm, 45° angle threshold, step size=1mm). The reverse-track pairs were combined to create the final tracts (for an example, see Figure 1C) and were consistent with descriptions of these u-fibers in previous studies (Catani et al., 2012; Guevara et al., 2017; Pron et al., 2021; Rojkova et al., 2015; Viganò et al., 2019).
The diffusion data were fitted to the tensor model at each voxel using weighted least squares to create diffusion tensor imaging scalar maps (fractional anisotropy [FA] and mean diffusivity [MD]). The scalar maps were harmonized across acquisition protocols (b-value) using an empirical Bayes-based method (Johnson et al., 2007) shown to be robust for small sample sizes and biased samples (ComBat; Fortin et al., 2017), and effective in b-value harmonization (Karayumak et al., 2019).
2.6. Morphological, connectivity and laterality measures
The PrChand and PoChand ROI volumes of each participant were calculated as the total volume of voxels occupied by each ROI in the subjects’ diffusion space using FSLstats. In subsequent tests of surface area (SA) and cortical thickness (CT), the regions were re-registered to anatomical space, projected to the cortical mid-surface, smoothed with a Gaussian kernel (full width at half maximum=6mm) and threshold at 20% probability in FreeSurfer. The threshold was chosen to maximize areal coverage while minimizing overlap between PrChand and PoChand. SA and CT were averaged across the ROI.
The u-fiber tract measures were extracted by averaging the mean value along streamlines from the diffusion scalar maps. The volume of each u-fiber tract was calculated as the total volume of voxels occupied by the tract.
Blood oxygenation level-dependent time-series from the same individual-subject ROIs (warped to MNI standard space) were averaged across voxels, and FC was estimated between ipsilateral PrChand and PoChand using Fisher z-transformed bivariate Pearson correlations.
To analyze laterality of the anatomical and connectivity features, a laterality index (LI) was calculated for all measures as follows: , where positive values indicate lateralization in the dominant (left) hemisphere for motor function. Note that this differs from the EHI scores where positive values indicate dominant (right) hand motor function.
Additionally, for each participant, total brain volume was calculated from the tissue maps segmented from the b0 image using FSL’s FAST (Zhang et al., 2001) as the total volume of grey and white matter voxels.
In order to determine the anatomical specificity of our findings, analyses were performed on the pre- and postcentral gyri parcels above (PrCupper and PoCupper, respectively) and below (PrClower and PoClower, respectively) the hand knob using the same methods for extracting and analyzing the hand knob measures. (See Supplementary Figures 1–2 for the upper and lower PrC and PoC parcels and upper and lower PoC-PrC u-fibers in relation to the hand knob parcels in a representative subject).
2.7. Statistical analysis
Independent samples t-tests and Chi-square tests were used to compare diagnostic groups on demographic, confounding variables (e.g., in-scanner head motion) and motor performance. One-way analyses of covariance (ANCOVAs) were used to test for effects of diagnosis controlling for age (morphological measures); age, relative RMSD and a dummy variable coding for b-value protocol (u-fiber measures); and age and average RMSD (FC measures). Total brain volume (TBV) (morphological measures) and tract volume (diffusion measures), known to bias diffusion measures due to partial volume effects (Vos et al., 2011), were additionally included in the model to test potential confounding factors. ANCOVA Bayes Factors (BFs) were calculated for the diagnosis effects to quantify the strength of evidence for the presence of the effects. Since this cohort (41–70 year-olds) is relatively unique in the literature, we also tested a model including a group by age interaction term, although with the caveat that results are based on cross-sectional data and thus remain exploratory. One-sample t-tests (two-tailed) against a null-hypothesis of 0 were used to test for laterality of each hand knob measure in the diagnostic groups separately, in addition to analyzing LI for group differences. One TC participant was deemed an outlier (>3 standard deviations from the group mean) and was removed from subsequent analyses of the left PoC-PrChand u-fiber tract and the tract’s LI. We controlled for multiple comparisons using the false discovery rate (FDR) (Benjamini & Hochberg, 1995) at q<.05. BFs are presented to supplement the p-values, considering fundamental issues with null hypothesis significance testing (Wasserstein & Lazar, 2016), and were interpreted using the rules of thumb from (Kass & Raftery, 1995), where 1–3 is anecdotal, 3–20 is moderate and 20–150 strong evidence.
Exploratory Pearson partial correlations were performed between the hand knob measures and BMAT subscales (Manual Dexterity, Coordination, and Strength and Flexibility) in each group separately, partialing out the same variables as described for the ANCOVAs above. Due to the limited range of scores in the Fine Motor and Balance and Mobility BMAT subscales in both groups, these were not included in our analyses. Given the ordinal nature of the ADOS-2 measures, Spearman rank-order partial correlations were performed between hand knob measures and the Social Affect, Restricted and Repetitive Behavior [RRB], and Comparison [CS] scores of the ADOS-2 in the ASD group, partialling out the same variables as described for the ANCOVAs above. We applied FDR correction for all hand knob measures and subscales of each behavioral domain (18 x 3 = 54 comparisons) per group.
To explore relationships between structural and functional connectivity, partial Pearson correlations were performed between sensorimotor hand knob u-fiber and functional connectivity measures ipsilaterally, controlling for age, relative RMSD (dMRI motion), average RMSD (fMRI motion) and b-value protocol in each diagnostic group, separately. For each group, we tested for correlations between FC and each of FA, MD and volume. This was done separately for the left and right hemispheres and for the LI measures, resulting in 9 correlations per group. These 9 were included in FDR correction (separately by group).
Statistical analyses were conducted in R (Core R Team, 2019) and plots were created using the package ggplot2 (Wickham, 2016). Bayesian analyses were performed using the statistical software JASP (JASP Team, 2021).
3. Results
The diagnostic groups did not differ on age, sex, degree of handedness, IQ, motion dropout or absolute RMSD (Table 1; all ps>.05). The ASD group had lower performance in Manual Dexterity, Coordination and Strength and Flexibility compared with the TC group (Table 1). As the groups marginally differed in relative RMSD, this motion measure was included as a covariate in analyses examining diffusion-weighted imaging measures. While the percent difference between groups in motion dropout was 43.1%, it was well below 1% of all slices in all participants, and affected slices were replaced with predicted values during preprocessing with the EDDY tool (Andersson et al., 2016). The groups also did not differ with regard to age, sex or in-scanner motion when b-value protocols were examined separately (see Supplementary Table 1). The groups did not differ in TBV. The sample included in the FC analyses did not differ between groups on age (p=.641), sex (p=.691), degree of handedness (p=.464), IQ (non-verbal: p=.752, verbal: p=.328) or motion (p=.170).
Table 1.
Participant characteristics and group matching.
| ASD (n = 25) | TC (n = 24) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean ± SD | [Range] | Mean ± SD | [Range] | p * | % diff | |
| Sex (M/F) | 20/5 | 21/3 | 0.70 | -- | ||
| Age (years) | 51.7 ± 6.9 | [41–67] | 51.6 ± 7.0 | [41–70] | 0.95 | 0.1 |
| EHI† | 81 ± 30 | [11–100] | 86 ± 18 | [40–100] | 0.47 | −1.6 |
| WASI-II Verbal IQ | 103 ± 24 | [52–160] | 114 ± 14 | [85–144] | 0.07 | −2.4 |
| Non-verbal IQ | 108 ± 22 | [56–138] | 112 ± 12 | [90–135] | 0.36 | −1.1 |
| Full-scale IQ | 106 ± 23 | [51–143] | 115 ± 12 | [92–138] | 0.09 | −2.0 |
| ADOS-2‡ Social Affect | 10.2 ± 3.6 | [5–19] | -- | -- | -- | |
| RRB | 3.8 ± 1.8 | [1–8] | -- | -- | -- | |
| Comparison Score | 7.4 ± 1.8 | [3–10] | -- | -- | -- | |
| BMAT§ Fine Motor | 13 ± 1 | [8–15] | 14 ± 1 | [12–14] | 0.11 | −0.9 |
| Manual Dexterity | 12 ± 3 | [2–16] | 14 ± 1 | [12–17] | <.01 | −4.3 |
| Coordination | 9 ± 2 | [4–10] | 10 ± 1 | [7–10] | <.01 | −3.2 |
| Strength & Flexibility | 14 ± 4 | [6–18] | 17 ± 2 | [10–18] | <.01 | −4.9 |
| Balance & Mobility | 9 ± 1 | [7–9] | 9 ± 1 | [6–9] | 0.89 | −0.1 |
| Total brain volume (cm3) | 1516 ± 185 | [1085–1783] | 1493 ± 138 | [1096–1686] | 0.61 | 0.4 |
| Motion dropout (% slices) | 0.05 ± 0.13 | [0–0.64] | 0.01 ± 0.12 | [0–0.06] | 0.13 | 43.1 |
| dMRI absolute RMSD | 1.34 ± 1.17 | [0.34–5.34] | 1.13 ± 0.59 | [0.43–2.78] | 0.44 | 4.2 |
| dMRI relative RMSD | 0.43 ± 0.26 | [0.16–1.03] | 0.31 ± 0.11 | [0.17–0.59] | 0.05 | 7.8 |
| fMRI RMSD (average across runs)¶ | 0.10 ± 0.05 | [0.04–0.19] | 0.09 ± 0.03 | [0.04–0.19] | 0.17 | 4.7 |
T-tests; Chi-Square test for sex.
EHI unavailable for 3 participants (2 ASD, 1 TC).
1 ASD participant did not score above the ADOS-2 Total cutoff but met clinical judgment for ASD under DSM-5 criteria; ADOS-2 scores were unavailable for 1 ASD participant due to administrator error (see text).
BMAT was not completed in 2 participants (1 ASD, 1 TC).
2 participants (1 ASD, 1 TC) were excluded for excessive image artifacts, 4 participants were excluded to match for head motion (3 ASD, 1 TC) and 1 ASD participant did not have functional MRI data.
Abbreviations: EHI=Edinburgh Handedness Inventory; WASI-II=Wechsler Abbreviated Scale of Intelligence, 2nd edition; ADOS-2=Autism Diagnostic Observation Scale, 2nd edition; RRB=Restricted and Repetitive Behaviors; BMAT=Bruininks Motor Assessment Scale – Short form; RMSD=average voxel-wise root mean square displacement (head motion); diff=difference between groups.
3.1. Hand knob morphology and laterality
The hand knob ROIs were successfully delineated in all participants and the proportion of each morphological variant (80.6% omega, 17.3% epsilon and 2.0% null; Supplementary Table 2) was in line with a previous study in young adults (Caulo et al., 2007).
ANCOVAs revealed moderate evidence of group effects as indicated by Bayesian analyses (BF>3) but none survived FDR correction. The right PrChand volume was increased in the ASD compared with the TC group with a medium effect size (Figure 1D; Table 2), while no group difference was found for left PrChand volume. Inclusion of a group by age interaction term suggested a medium interaction effect in right PrChand volume (trending positively and negatively with age in the ASD and TC groups, respectively; Supplementary Figure 3A and Supplementary Table 3) in addition to the group effect.
Table 2.
Results of the ANCOVAs performed on the hand knob measures and their group means and standard deviations.
| Region | Measure | df | BF | p | q | Partial η2 | ASD mean ± SD | TC mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Left PrChand | Volume | 1, 46 | 0.3 | 0.96 | 0.97 | <0.01 | 1.80 ± 0.52 | 1.79 ± 0.49 |
| Right PrChand | Volume | 1, 46 | 4.3a | 0.01 | 0.08 | 0.13 | 1.85 ± 0.46 | 1.52 ± 0.43 |
| Left PoChand | Volume | 1, 46 | 0.5 | 0.26 | 0.39 | 0.03 | 2.32 ± 0.48 | 2.52 ± 0.70 |
| Right PoChand | Volume | 1, 46 | 0.6 | 0.21 | 0.38 | 0.03 | 2.39 ± 0.55 | 2.17 ± 0.67 |
| PrChand | LI Volume | 1, 46 | 4.8a | 0.01 | 0.08 | 0.13 | −3.6 ± 24.7 | 17.1 ± 30.2 |
| PoChand | LI Volume | 1, 46 | 3.7a | 0.02 | 0.08 | 0.12 | −2.6 ± 22.5 | 16.9 ± 30.7 |
| Left PoC-PrChand u-fibers | FA | 1, 43 | 0.9 | 0.14 | 0.29 | 0.04 | 0.36 ± 0.02 | 0.37 ± 0.02 |
| MD | 1, 43 | 1.5 | 0.04 | 0.13 | 0.09 | 7.16 ± 0.24 | 7.03 ± 0.22 | |
| Volume | 1, 43 | 0.3 | 0.97 | 0.97 | <0.01 | 9.94 ± 2.37 | 9.65 ± 1.97 | |
| Right PoC-PrChand u-fibers | FA | 1, 44 | 0.3 | 0.90 | 0.97 | <0.01 | 0.35 ± 0.03 | 0.36 ± 0.03 |
| MD | 1, 44 | 3.4a | 0.02 | 0.08 | 0.10 | 7.18 ± 0.28 | 7.01 ± 0.23 | |
| Volume | 1, 44 | 0.7 | 0.24 | 0.39 | 0.03 | 10.03 ± 1.97 | 9.03 ± 2.28 | |
| PoC-PrChand u-fibers | LI FA | 1, 43 | 0.4 | 0.39 | 0.5 | 0.02 | 1.4 ± 6.9 | 2.8 ± 6.3 |
| LI MD | 1, 43 | 0.3 | 0.75 | 0.9 | 0.00 | −0.2 ± 2.2 | 0.2 ± 2.5 | |
| LI Volume | 1, 43 | 0.8 | 0.13 | 0.29 | 0.05 | −2.0 ± 2.3 | 9.3 ± 30.1 | |
| Left PoC-PrChand | FC | 1, 38 | 0.6 | 0.13 | 0.29 | 0.06 | 1.30 ± 0.42 | 1.51 ± 0.50 |
| Right PoC-PrChand | FC | 1, 38 | 0.6 | 0.05 | 0.13 | 0.10 | 1.12 ± 0.43 | 1.39 ± 0.46 |
| PoC-PrChand | LI FC | 1, 38 | 0.3 | 0.34 | 0.47 | 0.02 | 16.2 ± 30.9 | 9.1 ± 27.7 |
BF>3, indicating moderate evidence of an effect.
Volume in ml, MD in (x 10−4 mm2/s).
Using one-sample t-tests we observed significant leftward volume asymmetry of PrChand and PoChand in the TC (p=.011; Figure 1E) but not in the ASD group. For the laterality indices, Bayesian analyses indicated moderate evidence of diagnosis effects for both PrChand and PoChand volume but did not survive FDR correction (ASD<TC; Figure 1E; Table 2). These group effects remained when additionally controlling for TBV, RMSDrot, and when we ran the analysis on only strongly right-handed individuals (EHI scores ≥80; remaining sample=17 ASD, 19 TC), to ensure the effect was not driven by weakly right-handed individuals (Supplementary Table 4). There were no group by age interaction effects for the laterality indices (Supplementary Table 3).
Post hoc ANCOVAs on CT and SA were run to further investigate the regions that showed group differences in hand knob volume. There was a significant group effect in right PrChand SA (p=.012, partial η2=.129, ASD>TC), but not CT (p=.237, partial η2=.030). For the laterality indices, small to medium group effects were observed in SA (PrChand: p=.014, partial η2=.125; PoChand: p=.063, partial η2=.073; both ASD<TC), in contrast to CT (PrChand: p=.856, partial η2=.001; PoChand: p=.238, partial η2=.030).
3.2. Hand knob PoC-PrC structural and functional connectivity
Bayesian analyses revealed moderate evidence of a diagnosis effect in MD of right PoC-PrChand tract, which was increased in the ASD compared to TC group, but it did not survive FDR correction (Figure 1F; Table 2). The effect remained when additionally controlling for tract volume (p=.017). A similar trend was observed in MD for the left PoC-PrChand tract with a small effect size but showed weak evidence as suggested by the BF (Figure 1F; Table 2). However, this is noteworthy given that group differences in these u-fiber tracts bilaterally have been previously reported in ASD. We also observed a medium effect size of group in right PoC-PrChand FC, which was decreased in the ASD relative to TC group, but similarly showed weak evidence as indicated by the BF (Figure 1G; Table 2). A medium group by age interaction effect was observed for the right u-fiber volume (trending positively and negatively with age in the ASD and TC groups, respectively; Supplementary Figure 3B and Supplementary Table 3). This interaction effect largely went away when right PrChand volume, which showed a similar group by age pattern, was included as a covariate (p=.114).
One-sample t-tests revealed leftward laterality of FA in the TC (p=.046; Figure 1E), but not in the ASD group, and leftward laterality of PoC-PrChand FC in the ASD group (p=.030; Figure 1E) that was not found in the TC group. No group differences were found in LI of PoC-PrChand u-fiber or FC measures. A group by age interaction effect was observed in LI PoC-PrChand FC (trending negatively and positively with age in the ASD and TC groups, respectively; Supplementary Figure 3C and Supplementary Table 3). A small to medium group by age interaction effect was observed for left PoC-PrChand FC with age trends in the same direction as LI (negative and positive in the ASD and TC groups, respectively), but did not survive FDR correction.
3.3. Upper and lower pre and post-central volume and connectivity
The results of the upper and lower pre and postcentral measures are shown in Supplementary Table 5. While there were no significant group differences after FDR correction, a few group effects of medium size were observed in u-fiber and FC measures (see Supplementary Table 5).
3.4. Correlations between hand knob measures and motor skills
The results of the partial correlation analyses with the BMAT subscales are reported in Supplementary Table 6. As BMAT Coordination scores were at ceiling for almost all TC participants, this measure was excluded from further analysis in the TC group. We observed three associations in the ASD group at the uncorrected p<.05 level, however, they did not survive FDR correction. These were medium effect sizes that have not been previously reported in the literature. These exploratory findings include positive correlations between LI PrChand volume and Manual Dexterity scores, and between right PoChand volume and Coordination scores, and a negative correlation between LI PoC-PrChand u-fiber FA and Coordination scores, in the ASD group (Supplementary Table 6). No associations were found between PoC-PrChand FC and motor skills measures or with the Strength and Flexibility subscale in either group.
3.5. Correlations between hand knob measures and autism symptoms
The results of the partial correlation analyses with the ADOS-2 sub-scores are reported in Supplementary Table 7. While several associations at the uncorrected p<.05 level were observed: between right PoC-PrChand u-fiber volume and the Comparison score (negative relationship), between right PoC-PrChand u-fiber volume and LI PoC-PrChand MD with the RRB sub-score (both showed a negative relationship), and between LI PoC-PrChand u-fiber FA and the Comparison score (positive relationship), none survived FDR correction (see Supplementary Table 7).
3.6. Correlations between PoC-PrChand structural and functional connectivity
The results of the partial correlation analyses between structural and functional connectivity measures are shown in Supplementary Table 8. A positive association between right PoC-PrChand u-fiber volume and right PoC-PrChand FC in the ASD group only (partial r(16)=.647, p=.007, q=.06; Supplementary Figure 5), but did not survive FDR correction. No associations between structural and functional connectivity measures were observed in the TC group.
4. Discussion
To our knowledge, this is the first study to combine morphological, connectivity and asymmetry measures of the hand knob area in adults with ASD. Consistent with our hypotheses, we found moderate evidence that: 1) typical leftward asymmetry in PrChand and PoChand volume was reduced, 2) right PrChand volume was increased, and 3) right PoC-PrChand u-fibers showed evidence of compromised microstructure (increased MD), in the ASD group. Together, our findings suggest that the neural circuitry of the hand knob area is altered in middle-aged adults with ASD, with reduced lateralization in morphology and decreased support for efficient communication between its sensory and motor regions. We also observed, in the ASD group, associations between the hand knob measures and behavior (motor skills and autism symptoms), and a positive relationship between corresponding right PoC-PrChand u-fiber volume and FC before FDR correction. Group by age interactions were also found, however, due to the small sample size these are interpreted with caution.
4.1. Reduced asymmetry of the hand knob area in ASD
In typical individuals, handedness is associated with a deeper central sulcus, particularly in its dorsal portion, in the hemisphere contralateral to the dominant hand (Amunts et al., 1996, 2000; Cykowski et al., 2008; Klöppel et al., 2010; Sun et al., 2012). A deeper sulcus would presumably indicate an increased dorsal pre and postcentral cortical volume. Indeed, in this right-handed sample, we found that PrChand and PoChand volume were significantly left-lateralized in the TC group and this effect was primarily driven by SA rather than CT, consistent with previous findings. Higher rates of mixed or left-handedness have been reported in individuals with ASD, together estimated to be ~42% when adjusting for publication bias (Markou et al., 2017), compared with ~13% in the general population (Partida et al., 2020). This would be expected to lead to reduced leftward or increased rightward volume laterality in the ASD population (i.e., consistent with our observed group effects). In our study, however, left-handed individuals were excluded and the diagnostic groups were matched on degree of handedness (i.e., EHI scores), to avoid skewing of group findings. As an added safeguard, we performed additional analyses restricted to the strongly right-handed individuals (excluding individuals with EHI<80) and found medium to large effect sizes in this more restricted sample, confirming that these results were not driven by the weakly right-handed individuals.
Our finding of increased right PrChand volume in middle-aged and older adults with ASD is consistent with previous reports of increased grey matter density in right pericentral cortex found at the level of the hand knob in younger adults with ASD (DeRamus & Kana, 2015; Ecker et al., 2012). Our findings of reduced asymmetry in PrChand and PoChand volumes are also consistent with previous unilateral findings of increased right and/or decreased left grey matter density and volume in the pre- and postcentral gyri in ASD (Cauda et al., 2011; DeRamus & Kana, 2015; Ecker et al., 2012; Mahajan et al., 2016; Nickl-Jockschat et al., 2012; Säisänen et al., 2019), which may be interpreted in the context of reduced asymmetry. To our knowledge, morphological asymmetry has only been examined in a few studies on ASD relative to typical development (Herbert et al., 2005; Postema et al., 2019; Säisänen et al., 2019). A mega-analysis (n=1774) from the ENIGMA consortium by Postema et al. (2019) did not detect any group differences in asymmetry of cortical thickness or surface area for the larger (extending beyond the hand knob) precentral or postcentral gyral regions. However, it was done in a mostly younger sample (median age = 13 years, age range = 2–64 years), thus their results may be biased towards the younger ages, which may account for the null findings. In a small-sample (n=17) study of boys with Asperger syndrome relative to typical controls, Säisänen and colleagues (2019) found no difference in asymmetry of grey matter density in a manually placed ROI centered on the hand motor area, which was determined using navigated transcranial magnetic stimulation. These studies may suggest that differences in morphological asymmetry of the hand knob area are not present at a young age in ASD. But note that Herbert et al. (2005), albeit in a small sample (n=31), observed reduced asymmetry in volume of the postcentral (but not the precentral) gyrus in children with ASD. Although not directly examining asymmetry, Mahajan and colleagues (2016) found increased left precentral grey matter volume and surface area in children with ASD and comorbid ADHD and increased right precentral grey matter volume and surface area in the group with ASD without ADHD, relative to the control group. Therefore, morphological asymmetry findings in children with ASD may present differently with co-occurring conditions. Altogether, these findings also raise the possibility that reduced morphological asymmetry in the hand knob may emerge in adulthood.
The group by age interaction we observed for right PrChand volume may also suggest that the group difference (ASD>TC) tends to become more pronounced with age as volume decreases in the TC group. A more gradual age-related decrease in right precentral volume from age 8 to 50 years has been observed in ASD relative to typical development (Greimel et al., 2013). Oddly, in the ASD group in our data, a positive correlation in right PrChand volume with age was observed. In healthy aging, cortical volume, thickness, and surface area are known to decrease with advancing age (Storsve et al., 2014). However, as this is a cross-sectional sample, and an increase in volume is unlikely at this age, it may be that the correlation is driven by a few older participants in the ASD group with particularly large volumes. The right u-fiber tract volume displayed a similar group by age interaction, but it was largely due to the increased right PrChand volume resulting in larger tract volume. We also observed a group by age interaction in LI PoC-PrChand FC, such that laterality was trending negatively with age in the ASD but not the TC group and may indicate an earlier than expected reduction in left premotor FC with age (Wu et al., 2007).
While no group differences were observed in LI PoC-PrChand structural or functional connectivity, we did detect leftward asymmetry of FA in u-fibers in the TC (but not ASD) group and leftward asymmetry of PoC-PrChand FC in the ASD (but not TC) group. These contrasting findings for FA and FC may suggest that different MRI modalities are sensitive to different aspects of these anomalies, such as efferent versus afferent connections, or with FC sensitive to more effortful processing (e.g., in left-lateralized function for predictive motor control (Mutha et al., 2012)). While overall supportive of altered asymmetry in cortical sensorimotor systems in ASD, these latter findings must be treated with caution given the lack of significant group differences.
As with language lateralization, functional lateralization of the motor system is widely viewed to be advantageous for neural communication by optimizing conduction time and wiring capacity (Karolis et al., 2019). Reduced asymmetry in morphology of the hand knob area may relate to atypical rightward asymmetry in FC of motor networks observed in children and adolescents with ASD (Cardinale et al., 2013; Floris et al., 2016). Increased recruitment of right pericentral and premotor areas during motor learning has also been reported in young adults with ASD (Müller et al., 2004). We hypothesize that these atypical motor functional patterns observed in ASD earlier in life could, over time, lead to morphological changes in the hand knob area in adults with ASD.
By delineating the hand knob regions, we were able to perform analyses on different sub-parcels of the pre and postcentral gyri. We observed significant leftward volume asymmetry only in PrChand and PoChand, and not in the upper and lower sub-parcels in the TC group indicating that this asymmetry is largely specific to the hand knob region. This is consistent with previous morphometric studies in right-handed individuals (Amunts et al., 1996, 2000; Cykowski et al., 2008; S Rose et al., 2012). As we did not detect group differences in LI in the upper and lower parcels of the pre and postcentral gyri, this may suggest that the effects of reduced asymmetry in morphology in middle-aged adults with ASD are specific to the hand knob area.
4.2. Decreased PoC-PrChand u-fiber connectivity in ASD
Our findings also suggest altered microstructure of right PoC-PrChand u-fibers and a similar trend in left PoC-PrChand u-fibers. Increased MD in the ASD group, which may reflect axonal degeneration or possibly inflammation (Alexander et al., 2007), is in line with a previous report of increased MD (and reduced FA) of PoC-PrChand u-fibers bilaterally in younger adults with ASD (Thompson et al., 2017).
This potential reduction in structural connectivity between somatosensory (PoChand) and motor (PrChand) hand knob areas in ASD could have important functional implications. Diffusion tractography does not permit the distinction between afferent and efferent connections, but bidirectional connections between primary somatosensory and primary motor cortex have been demonstrated using axonal tracing in mouse (Matyas et al., 2010), and these could have distinct functional relevance. Based on evidence in monkeys (Pavlides et al., 1993) and humans (Kumar et al., 2019; Veldman et al., 2018), feedforward PoChand-PrChand connections are likely to play an important role in learning new motor skills. These u-fibers are also likely critical for fine motor control as impairment in these connections can affect grasp stability (Shinoura et al., 2005). Indeed, atypical grasp kinematics have been reported in adults with ASD (Cook et al., 2013). Future studies should directly test whether these u-fibers are linked to aspects of motor learning and fine motor control (e.g., handwriting difficulties (Grace et al., 2017)) in ASD, particularly in early childhood when development of these skills is most critical. Reciprocal PrChand-PoChand connections may also exert top-down influences on somatosensory cortex (e.g., M2 to S1 (Manita et al., 2015)) and could potentially contribute to atypical sensory perception in ASD (Manita et al., 2015; Umeda et al., 2019).
These u-fibers may be particularly important for maintaining fine motor skills during aging. One study found that FA of superficial white matter underlying the right pre and postcentral gyri positively correlated with finger tapping performance, and declining FA mediated age-related decline in fine motor performance in healthy adults aged 18–86 years (Nazeri et al., 2015). Within-network resting-state FC in sensorimotor networks is also found to be decreased in healthy older adults compared to healthy young adults (Zonneveld et al., 2019), and may impact in particular the left premotor cortex (Wu et al., 2007). Although it did not survive FDR correction, a group by age interaction effect was observed in left PoC-PrChand FC with a negative age trend in the ASD (but not the TC) group and could suggest an earlier than normal decline in sensorimotor FC. This finding is consistent with previous work from our group that showed reduced FC between the larger precentral and postcentral gyri bilaterally in the same cohort of middle-aged adults with ASD (Linke et al., 2019).
4.3. Correlations with motor skills
No significant correlations were found between hand knob measures and motor skills in either diagnostic group after correction for multiple comparisons. There were, however, correlations of medium effect size in the ASD group including an expected association between left-lateralized PrChand volume and better manual dexterity skills, but also associations between right hemisphere hand knob measures (increased right PoChand volume and right-lateralized FA of PoC-PrChand u-fibers) and motor coordination skills in adults with ASD. Interestingly, a similar pattern of associations between manual dexterity skills (on a pegboard task) and right hemisphere u-fiber measures has been reported in adults with ASD, which contrasted with left hemisphere u-fiber associations observed in the TC group (Thompson et al., 2017). We hypothesize that these atypical right PoChand and u-fiber correlations with motor performance in adults with ASD could reflect an unusual reliance on on-line corrective sensorimotor processes in the right hemisphere (Mutha et al., 2012).
4.4. Correlations with autism symptom severity
No correlations between hand knob measures with symptom severity survived FDR correction. However, associations between greater right PoC-PrChand u-fiber volume, reflecting more substantial structural connections, and lower severity of current autism symptoms (as measured by ADOS-2 Comparison Score and RRB; partial r=−.584 and −.505, respectively), in the ASD group, offer preliminary evidence that these u-fibers may play a compensatory role in regulating motor behaviors associated with ASD (e.g., hand flapping, finger flicking).
4.5. Correlations between structural and functional connectivity
We found a positive association between corresponding right u-fiber tract volume and FC in the ASD group before FDR correction. This may reflect greater coupling between structural and functional connectivity possibly due to a prolonged increased participation of right hemisphere motor networks in the ASD group; a relationship that was not present in the TC group. Previous electrophysiological evidence has shown that activity-dependent mechanisms can produce structural changes in the white matter into adulthood (Sampaio-Baptista & Johansen-Berg, 2017).
4.6. Limitations
The small sample size of our study is one limiting factor. While our hand knob u-fiber findings replicate (and extend) a previous ASD study in a younger group of adults (Thompson et al., 2017), our novel laterality and morphology findings of the hand knob area and assessment of their regional specificity in adults with ASD will require confirmation in larger samples. In particular, it is not known whether these findings are generalizable to left-handed adults with ASD. Given that these data are cross-sectional, the group by age interaction analyses also need to be interpreted with caution, as longitudinal data is required to reliably determine age-related changes. The lack of significant associations between the hand knob u-fiber measures and motor skills may be due to a limited range of scores on the BMAT Short Form. Therefore, assessments which better capture the full range of motor function should be used in future studies. As intra-subject variability of motor task performance increases with aging (Hunter et al., 2016), repeat measurements may increase the reliability of motor measures in middle-aged and older adults.
5. Conclusions
Our multi-modal MRI findings suggest that there is reduced morphological asymmetry and compromised microstructure of the PoC-PrC u-fibers of the hand knob area in middle-aged adults with ASD. In the ASD group, we observed a positive link between the right PoC-PrChand u-fibers and their corresponding FC but it did not survive multiple comparisons correction. Our morphological findings may relate to atypical functional asymmetries found in children and young adults with ASD (Cardinale et al., 2013; Floris et al., 2016), and could additionally reflect these atypically lateralized functional motor circuits over time. Given that manual motor function typically begins to decline in middle-age (Hoogendam et al., 2014; Ranganathan et al., 2001) and can significantly impact quality of life, longitudinal follow-up is needed.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health R01 MH103494. The authors would like to thank all of the study participants.
Footnotes
Declarations of interest: none.
Data and research statement
Data used in the preparation of this manuscript are available from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health (NIH) to provide a national resource to support the sharing of federally-funded data for accelerating research. Data can be accessed via https://nda.nih.gov/. Dataset identifier: #2291. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH.
Study procedures and analyses were not pre-registered prior to the research being conducted. We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
6 References
- Alexander AL, Lee JE, Lazar M, & Field AS (2007). Diffusion tensor imaging of the brain. Neurotherapeutics : The Journal of the American Society for Experimental NeuroTherapeutics, 4(3), 316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (Ed.). (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (5th ed.). American Psychiatric Association Publishing. [Google Scholar]
- Amunts K, Jäncke L, Mohlberg H, Steinmetz H, & Zilles K (2000). Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia, 38(3), 304–312. 10.1016/S0028-3932(99)00075-5 [DOI] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, & Zilles K (1996). Asymmetry in the human motor cortex and handedness. NeuroImage, 4(3), 216–222. 10.1006/nimg.1996.0073 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, & Bastiani M (2017). Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. NeuroImage, 152(February), 450–466. 10.1016/j.neuroimage.2017.02.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Zsoldos E, & Sotiropoulos SN (2016). Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage, 141, 556–572. 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, & Ashburner J (2003). How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, & Sotiropoulos SN (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B, 57(1), 289–300. 10.2307/2346101 [DOI] [Google Scholar]
- Bhat AN (2020). Is motor impairment in autism spectrum disorder distinct from developmental coordination disorder? A report from the SPARK study. Physical Therapy, 100(4), 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E, & Cairney J (2018). The Interrelationship Between Motor Coordination and Adaptive Behavior in Children With Autism Spectrum Disorder. Frontiers in Psychology, 9(November), 1–7. 10.3389/fpsyg.2018.02350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruininks R, & Bruininks B (2012). Bruininks Motor Abilities Test Pearson. [Google Scholar]
- Cardinale RC, Shih P, Fishman I, Ford LM, & Müller RA (2013). Pervasive rightward asymmetry shifts of functional networks in autism spectrum disorder. JAMA Psychiatry, 70(9), 975–982. 10.1001/jamapsychiatry.2013.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M (2017). A little man of some importance. Brain, 140(11), 3055–3061. 10.1093/brain/awx270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell’acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, & Thiebaut de Schotten M (2012). Short frontal lobe connections of the human brain. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 48(2), 273–291. 10.1016/j.cortex.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Cauda F, Geda E, Sacco K, D’Agata F, Duca S, Geminiani G, & Keller R (2011). Grey matter abnormality in autism spectrum disorder: An activation likelihood estimation meta-analysis study. Journal of Neurology, Neurosurgery and Psychiatry, 82(12), 1304–1313. 10.1136/jnnp.2010.239111 [DOI] [PubMed] [Google Scholar]
- Caulo M, Briganti C, Mattei PA, Perfetti B, Ferretti A, Romani GL, Tartaro A, & Colosimo C (2007). New morphologic variants of the hand motor cortex as seen with MR imaging in a large study population. American Journal of Neuroradiology, 28(8), 1480–1485. 10.3174/ajnr.A0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JL, Blakemore SJ, & Press C (2013). Atypical basic movement kinematics in autism spectrum conditions. Brain, 136(9), 2816–2824. 10.1093/brain/awt208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, & Kripke C (2015). The health status of adults on the autism spectrum. Autism, 19(7), 814–823. 10.1177/1362361315577517 [DOI] [PubMed] [Google Scholar]
- Curreri C, Trevisan C, Carrer P, Facchini S, Giantin V, Maggi S, Noale M, De Rui M, Perissinotto E, Zambon S, Crepaldi G, Manzato E, & Sergi G (2018). Difficulties with Fine Motor Skills and Cognitive Impairment in an Elderly Population: The Progetto Veneto Anziani. Journal of the American Geriatrics Society, 66(2), 350–356. 10.1111/jgs.15209 [DOI] [PubMed] [Google Scholar]
- Cykowski MD, Coulon O, Kochunov PV, Amunts K, Lancaster JL, Laird AR, Glahn C, & Fox PT (2008). The Central Sulcus : an Observer-Independent Characterization of Sulcal Landmarks and Depth Asymmetry. Cerebral Cortex, 18(September 2008), 1999–2009. 10.1093/cercor/bhm224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical Surface-Based Analysis I: Segmentation and Surface Reconstruction. NeuroImage, 9, 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu X-H, Ugurbil K, Kim S-G, & Ashe J (1997). Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci, 94, 14015–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRamus TP, & Kana RK (2015). Anatomical likelihood estimation meta-analysis of grey and white matter anomalies in autism spectrum disorders. NeuroImage: Clinical, 7, 525–536. 10.1016/j.nicl.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, & Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31, 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Dhollander T, Mito R, Raffelt D, & Connelly A (2019). Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. Proc. Intl. Soc. Mag. Reson. Med, May 11–16, 555. [Google Scholar]
- Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, Catani M, Jezzard P, Barnes A, Bailey AJ, Williams SC, & Murphy DGM (2012). Brain Anatomy and Its Relationship to Behavior in Adults With Autism Spectrum Disorder. Archives of General Psychiatry, 69(2), 195–209. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999). Cortical Surface-Based Analysis II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage, 9, 195–207. [DOI] [PubMed] [Google Scholar]
- Floris DL, Barber AD, Nebel MB, Martinelli M, Lai MC, Crocetti D, Baron-Cohen S, Suckling J, Pekar JJ, & Mostofsky SH (2016). Atypical lateralization of motor circuit functional connectivity in children with autism is associated with motor deficits. Molecular Autism, 7(35), 1–14. 10.1186/s13229-016-0096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, Roalf DR, Satterthwaite TD, Gur RC, Gur RE, Schultz RT, Verma R, & Shinohara RT (2017). Harmonization of multi-site diffusion tensor imaging data. NeuroImage, 161(August), 149–170. 10.1016/j.neuroimage.2017.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, & Bastian AJ (2009). Children with autism show specific handwriting impairments. Neurology, 73(19), 1532–1537. 10.1212/WNL.0b013e3181c0d48c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, & Krieger K (2010). Perceptual reasoning predicts handwriting impairments in adolescents with autism. Neurology, 75, 1825–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass A, Szabo K, Behrens S, Rossmanith C, & Hennerici M (2001). A diffusion-weighted MRI study of acute ischemic distal arm paresis. Neurology, 57, 1589–1594. 10.1212/WNL.59.4.650 [DOI] [PubMed] [Google Scholar]
- Geurts HM, McQuaid GA, Begeer S, & Wallace GL (2021). Self-reported parkinsonism features in older autistic adults: A descriptive study. Autism, 1–13. 10.1177/13623613211020183 [DOI] [PMC free article] [PubMed]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Bürgel U, Klingberg T, Larsson J, Zilles K, & Roland PE (1996). Two different areas within the primary motor cortex of man. Letters To Nature, 382(29), 805–807. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, & Zilles K (2000). Functional neuroanatomy of the primate isocortical motor system. Anatomy and Embryology, 202, 443–474. papers://11220527-b037–458d-8d7a-1b9030c00b53/Paper/p4746%5Cnpapers://11220527-b037–458d-8d7a-1b9030c00b53/Paper/p4009 [DOI] [PubMed] [Google Scholar]
- Gowen E, & Hamilton A (2013). Motor abilities in autism: A review using a computational context. Journal of Autism and Developmental Disorders, 43(2), 323–344. 10.1007/s10803-012-1574-0 [DOI] [PubMed] [Google Scholar]
- Grace N, Enticott PG, Johnson BP, & Rinehart NJ (2017). Do Handwriting Difficulties Correlate with Core Symptomology, Motor Proficiency and Attentional Behaviours? Journal of Autism and Developmental Disorders, 47(4), 1006–1017. 10.1007/s10803-016-3019-7 [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, & Baird G (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine and Child Neurology, 51, 311–316. 10.1111/j.1469-8749.2008.03242.x [DOI] [PubMed] [Google Scholar]
- Greimel E, Nehrkorn B, Schulte-Rüther M, Fink GR, Nickl-Jockschat T, Herpertz-Dahlmann B, Konrad K, & Eickhoff SB (2013). Changes in grey matter development in autism spectrum disorder. Brain Structure and Function, 218(4), 929–942. 10.1007/s00429-012-0439-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, & Fischl B (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48(1), 63–72. 10.1016/j.neuroimage.2009.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara M, Román C, Houenou J, Duclap D, Poupon C, Mangin JF, & Guevara P (2017). Reproducibility of superficial white matter tracts using diffusion-weighted imaging tractography. NeuroImage, 147(November 2016), 703–725. 10.1016/j.neuroimage.2016.11.066 [DOI] [PubMed] [Google Scholar]
- Hallett M (2007). Transcranial Magnetic Stimulation: A Primer. Neuron, 55(2), 187–199. 10.1016/j.neuron.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, Bakardjiev AI, Hodgson J, Takeoka M, Makris N, & Caviness VS (2005). Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain, 128(1), 213–226. 10.1093/brain/awh330 [DOI] [PubMed] [Google Scholar]
- Hoogendam YY, van der Lijn F, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Ikram MA, & van der Geest JN (2014). Older age relates to worsening of fine motor skills: A population based study of middle-aged and elderly persons. Frontiers in Aging Neuroscience, 6(SEP), 1–7. 10.3389/fnagi.2014.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Pereira HM, & Keenan KG (2016). The aging neuromuscular system and motor performance. Journal of Applied Physiology, 121, 982–995. 10.1152/japplphysiol.00475.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, Shic F, Wall CA, Chawarska K, Curtin S, Estes A, Gardner JM, Hutman T, Landa RJ, Levin AR, Libertus K, Messinger DS, Nelson CA, Ozonoff S, Sacrey LAR, Sheperd K, Stone WL, Tager-Flusberg HB, Wolff JJ, … Young GS (2019). Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. Journal of Abnormal Psychology, 128(1), 69–80. 10.1037/abn0000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin E, Couture M, McKinley P, Reid G, Fombonne E, & Gisel E (2009). Sensori-motor and Daily Living Skills of Preschool Children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 39, 231–241. 10.1007/s10803-008-0617-z [DOI] [PubMed] [Google Scholar]
- JASP Team. (2021). JASP (0.16)
- Jeurissen B, Tournier JD, Dhollander T, Connelly A, & Sijbers J (2014). Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage, 103, 411–426. 10.1016/j.neuroimage.2014.07.061 [DOI] [PubMed] [Google Scholar]
- Johnson WE, Li C, & Rabinovic A (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8(1), 118–127. 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- Karayumak SC, Bouix S, Ning L, James A, Crow T, Shenton M, Kubicki M, & Rathi Y (2019). Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. NeuroImage, 184(August 2018), 180–200. 10.1016/j.neuroimage.2018.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolis VR, Corbetta M, & Thiebaut de Schotten M (2019). The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nature Communications, 10(1), 1–9. 10.1038/s41467-019-09344-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RE, & Raftery AE (1995). Bayes factors. Journal of the American Statistical Association, 90(430), 773–795. 10.1080/01621459.1995.10476572 [DOI] [Google Scholar]
- Katz S (1983). Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. Journal of the American Geriatrics Society, 31(12), 721–727. 10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed] [Google Scholar]
- Klöppel S, Mangin J-F, Vongerichten A, Frackowiak RSJ, & Siebner HR (2010). Nurture versus Nature : Long-Term Impact of Forced Right-Handedness on Structure of Pericentral Cortex. Journal of Neuroscience, 30(9), 3271–3275. 10.1523/JNEUROSCI.4394-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Manning TF, & Ostry DJ (2019). Somatosensory cortex participates in the consolidation of human motor memory. PLoS Biology, 17(10), 1–21. 10.1371/journal.pbio.3000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, & Garrett-Mayer E (2006). Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry and Allied Disciplines, 47(6), 629–638. 10.1111/j.1469-7610.2006.01531.x [DOI] [PubMed] [Google Scholar]
- Libertus K, Sheperd KA, Ross SW, & Landa RJ (2014). Limited Fine Motor and Grasping Skills in 6-Month-Old Infants at High Risk for Autism 85(6), 2218–2231. 10.1111/cdev.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licari MK, Alvares GA, Varcin K, Evans KL, Cleary D, Reid SL, Glasson EJ, Bebbington K, Reynolds JE, Wray J, & Whitehouse AJO (2020). Prevalence of Motor Difficulties in Autism Spectrum Disorder: Analysis of a Population-Based Cohort. Autism Research, 13, 298–306. 10.1002/aur.2230 [DOI] [PubMed] [Google Scholar]
- Linke A, Kinnear M, Kohli J, Fong C, Lincoln A, Carper R, & Müller R-A (2019). Impaired motor skills and atypical functional connectivity of the sensorimotor system in 40–65 year old adults with Autism Spectrum Disorders. Neurobiology of Aging, 85, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M, MacDonald M, & Lord C (2013). Motor skills of toddlers with autism spectrum disorders. Autism, 17(2), 133–146. 10.1177/1362361311402230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, & Risi S (2002). The autism diagnostic observation scale (ADOS) Los Angeles, CA: Western Psychological. [Google Scholar]
- Mahajan R, Dirlikov B, Crocetti D, & Mostofsky SH (2016). Motor Circuit Anatomy in Children with Autism Spectrum Disorder With or Without Attention Deficit Hyperactivity Disorder. Autism Research, 9(1), 67–81. 10.1186/s40945-017-0033-9.Using [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manita S, Suzuki T, Homma C, Matsumoto T, Odagawa M, Yamada K, Ota K, Matsubara C, Inutsuka A, Sato M, Ohkura M, Yamanaka A, Yanagawa Y, Nakai J, Hayashi Y, Larkum ME, & Murayama M (2015). A Top-Down Cortical Circuit for Accurate Sensory Perception. Neuron, 86(5), 1304–1316. 10.1016/j.neuron.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Markou P, Ahtam B, & Papadatou-Pastou M (2017). Elevated Levels of Atypical Handedness in Autism: Meta-Analyses. Neuropsychology Review, 27(3), 258–283. 10.1007/s11065-017-9354-4 [DOI] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, & Petersen CCH (2010). Motor control by sensory cortex. Science, 330, 1240–1244. [DOI] [PubMed] [Google Scholar]
- McCarthy P (2018). FSLeyes
- Müller RA, Cauich C, Rubio MA, Mizuno A, & Courchesne E (2004). Abnormal activity patterns in premotor cortex during sequence learning in autistic patients. Biological Psychiatry, 56(5), 323–332. 10.1016/j.biopsych.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Mutha PK, Haaland KY, & Sainsburg RL (2012). The effects of brain lateralization on motor control and adaptation. J Mot Behav, 44(6), 455–469. 10.1080/00222895.2012.747482.THE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazeri A, Chakravarty MM, Rajji TK, Felsky D, Rotenberg DJ, Mason M, Xu LN, Lobaugh NJ, Mulsant BH, & Voineskos AN (2015). Superficial white matter as a novel substrate of age-related cognitive decline. Neurobiology of Aging, 36(6), 2094–2106. 10.1016/j.neurobiolaging.2015.02.022 [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Habel U, Michel TM, Manning J, Laird AR, Fox PT, & Schneider F (2012). Brain Structure Anomalies in Autism Spectrum Disorder — A Meta-Analysis of VBM Studies Using Anatomic Likelihood Estimation. Human Brain Mapping, 33, 1470–1489. 10.1002/hbm.21299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Ostwald SK, Snowdon DA, Rysavy SDM, Keenan NL, & Kane RL (1989). Manual Dexterity as a Correlate of Dependency in the Elderly. Journal of the American Geriatrics Society, 37(10), 963–969. 10.1111/j.1532-5415.1989.tb07282.x [DOI] [PubMed] [Google Scholar]
- Pan C (2014). Motor proficiency and physical fitness in adolescent males with and without autism spectrum disorders. Autism, 18(2), 156–165. 10.1177/1362361312458597 [DOI] [PubMed] [Google Scholar]
- Partida GC, Tung JY, Eriksson N, Albrecht E, Aliev F, Ole A, Barroso I, Beckmann JS, Boks MP, Dorret I, Boyd HA, Breteler MMB, Campbell H, Daniel I, Geller F, Gieger C, Giegling I, Gordon SD, Loos RJF, … Medland SE (2020). Genome-wide association study identifies 49 common genetic variants associated with handedness . Nature Human Behaviour, 1–10. [DOI] [PMC free article] [PubMed]
- Pavlides C, Miyashita E, & Asanuma H (1993). Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. Journal of Neurophysiology, 70(2), 733–741. 10.1152/jn.1993.70.2.733 [DOI] [PubMed] [Google Scholar]
- Penfield W, & Boldrey E (1937). Somatic Motor and Sensory Representation in the cerebral cortex of man as studied by electrical stimulation. Brain: A Journal of Neurology, 60, 389–443. 10.1093/brain/60.4.389 [DOI] [Google Scholar]
- Perelle IB, & Ehrman L (2005). On the Other Hand. Behavior Genetics, 35(3), 343–350. 10.1007/s10519-005-3226-z [DOI] [PubMed] [Google Scholar]
- Peters N, Müller-Schunk S, Freilinger T, Düring M, Pfefferkorn T, & Dichgans M (2009). Ischemic stroke of the cortical “hand knob” area: Stroke mechanisms and prognosis. Journal of Neurology, 256(7), 1146–1151. 10.1007/s00415-009-5104-8 [DOI] [PubMed] [Google Scholar]
- Piven J, & Rabins P (2011). Autism spectrum disorders in older adults: Toward defining a research agenda. Journal of the American Geriatrics Society, 59(11), 2151–2155. 10.1111/j.1532-5415.2011.03632.x [DOI] [PubMed] [Google Scholar]
- Postema MC, van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Filho GB, Calderoni S, Calvo R, Daly E, Deruelle C, Di Martino A, Dinstein I, Duran FLS, Durston S, Ecker C, Ehrlich S, Fair D, Fedor J, … Francks C (2019). Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nature Communications, 10(1), 1–12. 10.1038/s41467-019-13005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pron A, Deruelle C, & Coulon O (2021). U‑shape short‑range extrinsic connectivity organisation around the human central sulcus. Brain Structure and Function, 226(1), 179–193. 10.1007/s00429-020-02177-5 [DOI] [PubMed] [Google Scholar]
- Provost B, Lopez BR, & Heimerl S (2007). A Comparison of Motor Delays in Young Children: Autism Spectrum Disorder, Developmental Delay, and Developmental Concerns. Journal of Autism and Developmental Disorders, 37, 321–328. 10.1007/s10803-006-0170-6 [DOI] [PubMed] [Google Scholar]
- Rademacher J, Bürgel U, Geyer S, Schormann T, Schleicher A, Freund HJ, & Zilles K (2001). Variability and asymmetry in the human precentral motor system: A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain, 124(11), 2232–2258. 10.1093/brain/124.11.2232 [DOI] [PubMed] [Google Scholar]
- Rademacher J, Rademacher J, Caviness VS, Steinmetz H, & Galaburda AM (1993). Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cerebral Cortex, 3(4), 313–329. 10.1093/cercor/3.4.313 [DOI] [PubMed] [Google Scholar]
- Ranganathan VK, Siemionow V, Sahgal V, & Yue GH (2001). Effects of aging on hand function. Journal of the American Geriatrics Society, 49(11), 1478–1484. 10.1046/j.1532-5415.2001.4911240.x [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, & White L (1999). Midlife hand grip strength as a predictor of old age disability. Journal of the American Medical Association, 281(6), 558–560. 10.1001/jama.281.6.558 [DOI] [PubMed] [Google Scholar]
- Rojkova K, Volle E, Urbanski M, Humbert F, Dell???Acqua F, & Thiebaut De Schotten M (2015). Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Structure and Function, 221(3), 1751–1766. 10.1007/s00429-015-1001-3 [DOI] [PubMed] [Google Scholar]
- Rose S, Rowland T, Pannek K, Baumann F, Coulthard A, McCombe P, & Henderson R (2012). Structural hemispheric asymmetries in the human precentral gyrus hand representation. Neuroscience, 210, 211–221. 10.1016/j.neuroscience.2012.02.044 [DOI] [PubMed] [Google Scholar]
- Rose Stephen, Guzzetta A, Pannek K, & Boyd R (2011). MRI Structural Connectivity , Disruption of Primary Sensorimotor Pathways , and Hand Function in Cerebral Palsy 1(4). 10.1089/brain.2011.0034 [DOI] [PubMed] [Google Scholar]
- Sacrey LR, Zwaigenbaum L, Bryson S, Brian J, & Smith IM (2018). The reach-to-grasp movement in infants later diagnosed with autism spectrum disorder: a high-risk sibling cohort study. Journal of Neurodevelopmental Disorders, 10(41), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säisänen L, Määttä S, Julkunen P, Niskanen E, Kallioniemi E, Gröhn H, Kemppainen S, Lakka TA, Lintu N, Eloranta AM, Vanninen R, Makkonen I, & Könönen M (2019). Functional and structural asymmetry in primary motor cortex in Asperger syndrome: a navigated TMS and imaging study. Brain Topography, 32(3), 504–518. 10.1007/s10548-019-00704-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C, & Johansen-Berg H (2017). White Matter Plasticity in the Adult Brain. Neuron, 96(6), 1239–1251. 10.1016/j.neuron.2017.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Yamada R, Kodama T, Takahashi M, & Yagi K (2005). Fibers connecting the primary motor and sensory areas play a role in grasp stability of the hand. NeuroImage, 25(3), 936–941. 10.1016/j.neuroimage.2004.12.060 [DOI] [PubMed] [Google Scholar]
- Smith RE, Tournier J-D, Calamante F, & Connelly A (2012). Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage, 62(3), 1924–1938. 10.1016/j.neuroimage.2012.06.005 [DOI] [PubMed] [Google Scholar]
- Starkstein S, Gellar S, Parlier M, Payne L, & Piven J (2015). High rates of parkinsonism in adults with autism. Journal of Neurodevelopmental Disorders, 7(1), 1–11. 10.1186/s11689-015-9125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, & Walhovd KB (2014). Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: Regions of accelerating and decelerating change. Journal of Neuroscience, 34(25), 8488–8498. 10.1523/JNEUROSCI.0391-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZY, Klöppel S, Rivière D, Perrot M, Frackowiak R, Siebner H, & Mangin JF (2012). The effect of handedness on the shape of the central sulcus. NeuroImage, 60(1), 332–339. 10.1016/j.neuroimage.2011.12.050 [DOI] [PubMed] [Google Scholar]
- Sun ZY, Pinel P, Riviere D, Moreno A, Dehaene S, & Mangin J-F (2016). Linking morphological and functional variability in hand movement and silent reading. Brain Structure and Function, 221, 3361–3371. 10.1007/s00429-015-1106-8 [DOI] [PubMed] [Google Scholar]
- Team, R. C. (2019). R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing. http://www.r-project.org/ [Google Scholar]
- Thompson A, Murphy D, Dell’Acqua F, Ecker C, McAlonan G, Howells H, Baron-Cohen S, Lai MC, & Lombardo MV (2017). Impaired Communication Between the Motor and Somatosensory Homunculus Is Associated With Poor Manual Dexterity in Autism Spectrum Disorder. Biological Psychiatry, 81, 211–219. 10.1016/j.biopsych.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh CH, & Connelly A (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202(August), 116137. 10.1016/j.neuroimage.2019.116137 [DOI] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Duffield TC, Prigge MDB, Froehlich AL, Lange N, Alexander AL, & Lainhart JE (2017). Longitudinal development of manual motor ability in autism spectrum disorder from childhood to mid-adulthood relates to adaptive daily living skills. Developmental Science, 20(4), 1–15. 10.1111/desc.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Tromp DPM, Adluru N, Destiche D, Samsin D, Froehlich A, Prigge MDB, Duffield TC, Lange N, Alexander AL, & Lainhart JE (2015). Brainstem White Matter Predicts Individual Differences in Manual Motor Difficulties and Symptom Severity in Autism. Journal of Autism and Developmental Disorders, 45(9), 3030–3040. 10.1007/s10803-015-2467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda T, Isa T, & Nishimura Y (2019). The somatosensory cortex receives information about motor output. Science Advances, 5(7). 10.1126/sciadv.aaw5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman MP, Maurits NM, Zijdewind I, Maffiuletti NA, van Middelkoop S, Mizelle JC, & Hortobágyi T (2018). Somatosensory electrical stimulation improves skill acquisition, consolidation, and transfer by increasing sensorimotor activity and connectivity. Journal of Neurophysiology, 120(1), 281–290. 10.1152/jn.00860.2017 [DOI] [PubMed] [Google Scholar]
- Veraart J, Novikov D, Christiaens D, Ades-aron B, Sijbers J, & Fieremans E (2016). Denoising of diffusion MRI using random matrix theory Jelle. Neuroimage, 142, 394–406. 10.1177/0164027515620239.Perceived [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò L, Fornia L, Rossi M, Howells H, Leonetti A, Puglisi G, Conti Nibali M, Bellacicca A, Grimaldi M, Bello L, & Cerri G (2019). Anatomo-functional characterisation of the human “hand-knob”: A direct electrophysiological study. Cortex, 113, 239–254. 10.1016/j.cortex.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Volkmann J, Schnitzler A, Witte OW, & Freund HJ (1998). Handedness and asymmetry of hand representation in human motor cortex. Journal of Neurophysiology, 79(4), 2149–2154. 10.1152/jn.1998.79.4.2149 [DOI] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, & Leemans A (2011). Partial volume effect as a hidden covariate in DTI analyses. NeuroImage, 55(4), 1566–1576. 10.1016/j.neuroimage.2011.01.048 [DOI] [PubMed] [Google Scholar]
- Wasserstein RL, & Lazar NA (2016). The ASA’s Statement on p-Values: Context, Process, and Purpose. American Statistician, 70(2), 129–133. 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence Pearson. [Google Scholar]
- White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, & Purves D (1997). Structure of the human sensorimotor system. I: Morphology and cytoarchitecture of the central sulcus. Cerebral Cortex, 7(1), 18–30. 10.1093/cercor/7.1.18 [DOI] [PubMed] [Google Scholar]
- White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, Kuperman J, & Dale A (2010). PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magnetic Resonance in Medicine, 63(1), 91–105. 10.1002/mrm.22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis Springer-Verlag New York. [Google Scholar]
- Willett FR, Deo DR, Avansino DT, Rezaii P, Hochberg LR, Henderson JM, & Shenoy KV (2020). Hand Knob Area of Premotor Cortex Represents the Whole Body in a Compositional Way. Cell, 181, 396–409. 10.1016/j.cell.2020.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Hallett M, Chen Y, Li K, & Chan P (2007). Aging influence on functional connectivity of the motor network in the resting state. Neuroscience Letters, 422(3), 164–168. 10.1016/j.neulet.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, & Winkler P (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain, 120, 141–157. 10.1093/brain/120.1.141 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, & Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- Zonneveld HI, Pruim RH, Bos D, Vrooman HA, Muetzel RL, Hofman A, Rombouts SA, van der Lugt A, Niessen WJ, Ikram MA, & Vernooij MW (2019). Patterns of functional connectivity in an aging population: The Rotterdam Study. NeuroImage, 189(January), 432–444. 10.1016/j.neuroimage.2019.01.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


