Abstract
Purpose:
To characterize the change of ganglion cell complex (GCC) thickness and macula vessel density in eyes diagnosed as being glaucoma suspect eyes with ocular hypertension (OHT) or glaucomatous optic neuropathy (GON).
Design:
Prospective, longitudinal study.
Participants:
83 eyes (24 healthy eyes, 30 OHT eyes, and 29 GON eyes) of 65 subjects who had at least 3 visits were included from the Diagnostic Innovations in Glaucoma Study. The mean follow-up was at least 3 years.
Methods:
Optical coherence tomography angiography (OCTA)-based vessel density and OCT-based structural thickness of the 3×3 mm2 GCC scan slab were evaluated at each visit. The rates of vessel density and thickness change were compared across diagnostic groups using linear mixed-effects model.
Main Outcome Measures:
Change rates of macula GCC thickness and superficial macula vessel density.
Results:
Significant mean rates of both GCC thinning and macula vessel density loss were detectable in OHT and GON groups. 49.1% of the individual suspect eyes showed significant loss (P<0.05) either with vessel density or GCC thickness. Of the GON eyes, 51.7% showed only significant GCC loss, while 17.2% showed only significant vessel density loss. Both significant GCC loss and vessel density loss was detectable in 31.0% of the GON eyes. Vessel density loss was faster than GCC thinning in half of the suspect eyes based on percent loss analysis. The age and scan quality adjusted GCC thinning rates of the OHT group (−0.59 μm/year, P=0.025) and GON group (−0.79 μm/year, P=0.058) were faster than healthy group (−0.11 μm/year), while the rate of vessel density loss was not significantly different among the diagnostic groups (all P>0.2). Higher mean intraocular pressure during follow-up was associated with a faster rate of GCC thinning in the OHT group (P=0.065) and GON groups (P=0.015), but was not associated with the rate of vessel density decrease.
Conclusions:
While the rate of GCC thinning was faster on average in suspect eyes than in healthy eyes, some suspect eyes had significant loss of vessel density and faster vessel density loss than GCC thinning. OCT and OCTA are complementary and useful for evaluating eyes with OHT or GON.
Keywords: glaucoma, macula, OCTA, OCT, longitudinal
Precis
Progressive macula ganglion cell complex (GCC) thinning and microvasculature loss are detectable in glaucoma suspect eyes. GCC thinning is faster on average in suspect eyes than in healthy eyes. Microvasculature loss could be faster than GCC thinning in suspect eyes.
The diagnosis of glaucoma in clinical practice largely depends on identification of characteristic structural changes to the optic nerve head (ONH), and the visual field (VF) 24–2 test pattern is as close to a gold standard as there is for glaucoma.1–3 However, there is growing evidence that early glaucomatous damage involves the macula, even in the eyes with normal 24–2 VF test,3 namely glaucoma suspect. This is well supported by a study that demonstrated abnormal central VF by 10–2 VF test in 35% and 39% of ocular hypertension (OHT) and glaucomatous optic neuropathy (GON) eyes, respectively.4 Nevertheless, glaucoma specialists have largely ignored the macula as a site of early glaucomatous damage.3
The macula, defined as a radius of 8 degree central region, represents less than 2% of the retinal area but contains more than 30% of the retina ganglion cells (RGCs)5, is of primary importance for daily functioning, and loss in that region is strongly associated with self-reported quality of life.6, 7 Apoptosis of RGCs and their axons in glaucoma is responsible for VF defects. Therefore, loss of RGCs and nerve fibers in macula, represented as thinning of corresponding retina layers, is likely to be a good predictor of glaucomatous central VF deterioration.8 It has been demonstrated that optical coherence tomography (OCT) thickness of segmented macula shows good quantitative agreement with the histological thickness of corresponding retina layer, thus allows monitoring of macula neural loss.9
RGCs in the macula depend on regional capillary networks to meet their high metabolic requirements. If insufficient ocular blood flow has a central role in apoptotic RGC death, as has been suggested,10 assessment of macular microvasculature might provide another option for early detection of glaucomatous damage. By using optical coherence tomography angiography (OCTA), a technique that can non-invasively evaluate retina microvasculature integrity,11 recent studies have provided evidences of microvasculature decrease within the macula in glaucoma eyes.12–15 However, the possible role of the microvasculature impairment as an indicator of progressive macula damage at the earliest stage in the pathophysiology of glaucoma is still unclear.16
The current study aimed to characterize the progressive change of OCT-based macula ganglion cell complex (GCC) thickness and OCTA-based macula vessel density in glaucoma suspect eyes with OHT or GON, in order to identify the detectable indicator for the earliest glaucomatous damage.
Methods
Participants
Healthy subjects and glaucoma suspect patients, including OHT and GON17 were recruited from the longitudinal Diagnostic Innovations in Glaucoma Study (DIGS).18 The Institutional Review Boards of the University of California San Diego approved the protocol, and the methodology adheres to the tenets of the Declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all participants.
Inclusion criteria for DIGS were open angles with gonioscopy, a best-corrected visual acuity of 20/40 or better, a spherical refraction within ±5.0 diopters (D), and cylinder correction within ±3.0 D. Subjects were excluded if they had axial length (AL) > 26.5 mm, history of ocular trauma, or a history of intraocular surgery (except for uncomplicated glaucoma and cataract surgery). The follow-up of eyes that had uncomplicated glaucoma and cataract surgery were continued at least 3 months postoperatively. Subjects with secondary causes of elevated intraocular pressure (IOP), other intraocular eye disease, or other diseases affecting VF or who were using medications known to affect VF sensitivity also were excluded. Other information such as race, age, systemic disease history, and central corneal thickness (CCT) also was collected.
Healthy subjects had bilateral (1) IOP≤21 mmHg with no history of elevated IOP; (2) normal appearing optic disc, intact neuroretinal rim and retina nerve fiber layer (RNFL); and (3) a minimum of two reliable normal 24–2 VFs, defined as a pattern standard deviation (PSD) within 95% confidence limits and a glaucoma hemifield test (GHT) result within normal limits.19 OHT was defined as eyes had (1) a history of elevated IOP which was defined as IOP > 21 mmHg18; (2) normal appearing optic disc, intact neuroretinal rim and RNFL; and (3) without evidence of repeatable glaucomatous 24–2 VF damage. Glaucomatous VF damage was defined as a GHT outside normal limits and a PSD outside 95% normal limits, which were confirmed on at least 2 consecutive, reliable (fixation losses and false-negatives≤ 33% and false-positives≤15%) tests.12, 15 GON was defined as eyes having an optic disc appearance that is suspicious of glaucoma but without evidence of repeatable glaucomatous 24–2 VF damage. A suspicious appearing optic disc was defined as a disc with observable excavation, neuroretinal rim narrowing or notching, or a localized or diffuse RNFL defect suggestive of glaucoma with stereophotographs.18 Stereophotographs were reviewed with a stereoscopic viewer (Screen- VU stereoscope; PS Manufacturing, Portland, OR) by 2 or more experienced graders masked to the subjects’ identity and to other test results. The methodology used to grade optic disc photographs at the UCSD Optic Disc Reading Center has been provided elsewhere.20 Only photographs of adequate quality were included. Discrepancies between the 2 graders were resolved by consensus or adjudication by a third experienced grader.
Follow-up
All included eyes had at least 3 visits with OCTA and spectral-domain optical coherence tomography (SD-OCT) imaging examinations that qualified for inclusion, and at least 1.5 years of follow-up. Participants were evaluated at 6-month intervals. At the baseline visit and at each annual follow-up visit, subjects underwent a complete ophthalmologic examination including slit-lamp biomicroscopy, IOP measurement, dilated stereoscopic fundus examination, and stereophotography of the ONH.18 VF testing by standard automated perimetry (Humphrey Field Analyzer; 24–2 Swedish interactive threshold algorithm; Carl Zeiss Meditec, Jena, Germany), OCTA imaging and SD-OCT imaging were completed at baseline and every visit during follow-up.
Optical Coherence Tomography Angiography and Spectral-Domain Optical Coherence Tomography
OCTA and SD-OCT imaging used the AngioVue imaging system (Optovue, Inc., Fremont, CA, USA) which is an angiographic platform implemented on an existing commercially available SD-OCT platform. This system can provide both thickness and vascular measurements simultaneously with default eye tracking. The acquired OCT and OCTA volume of the AngioVue scan was automatically segmented by the AngioVue software (version 2017.1.0.151), and thickness and vascular analyses were derived from the same slab with exact registration of the analyzed regions.
Macula 3 × 3 mm2 scans centered on the fovea were acquired using the AngioVue OCTA system. OCTA-based vessel density and OCT-based thickness measures were calculated from the same scan slab, as follows. The split-spectrum amplitude-decorrelation angiography method was used to capture the dynamic motion of the red blood cells and provide a high-resolution 3D visualization of perfused retinal vasculature. Vessel density was calculated as the percent area occupied by flowing blood vessels in the selected region. The retinal layers of each scan were automatically segmented by the AngioVue software to visualize the superficial retinal capillary plexuses in a slab from the internal limiting membrane to the inner plexiform layer −10 μm. For this study, whole en-face image vessel density (wiVD) was derived from the entire 3×3 mm2 scan and parafoveal vessel density (pfVD) was measured in an annular region centered on the fovea with an inner diameter of 1 mm and outer diameter of 3 mm. The parafoveal region was separated into 90-degree intervals as 4 sectors (superior, inferior, temporal, and nasal). The macula cube scanning protocol measured the GCC thickness of the same scan slab as the OCTA scan. GCC thickness analysis regions of whole image (wiGCC) and parafoveal (pfGCC), as well as the sectors, were the same as that in the OCTA vessel density analysis.
OCTA and SD-OCT images underwent quality review according to the Imaging Data Evaluation and Analysis (IDEA) Reading Center standard protocol on all scans processed with standard AngioVue software (version 2017.1.0.151)14. Only good-quality images were included. Poor quality images were defined as images with (1) low scan quality as quality index (QI) less than 4, (2) poor clarity, (3) residual motion artifacts visible as irregular vessel pattern or disc boundary on the enface angiogram, (4) image cropping or local weak signal due to vitreous opacity, or (5) off-center or segmentation errors that could not be corrected. Quality control enabled assessment of identical macular layers for all visits.
Statistical Analysis
Continuous and categorical data were presented as mean (95% confidence interval, CI) and count (%). The statistical significance of differences in patient characteristics across diagnosis was determined by Fisher’s exact test for categorical variables. Mixed-effects modeling was used to compare ocular parameters among groups. Models were fit with ocular measurements as response variable and diagnostic group as fixed effects. Measurements of bilateral eyes were nested within subject to account for the fact that eyes from the same individual are more likely to have similar measurements.21, 22
Macula GCC thickness and vessel density trajectories were estimated using linear mixed effects models, with random eye-within-subject intercepts and independent random slopes-within-eye. These models include fixed effects for baseline age, QI, diagnosis group, time, and a diagnosis group and time interaction. A forward-selection procedure was introduced for the inclusion of additional fixed covariates with the P value <0.1 in univariate analysis (mean IOP, CCT and race). Percent loss of GCC thickness and macula vessel density was calculated as [1- (raw measurement / mean value of the same measurement of healthy eyes)] ×100 (unit, %)14, and rates of percent loss of GCC thickness and macula vessel density were evaluated. The unit of the normalized rates is %/year meaning annual percent change of the healthy baseline.
Linear regression models were used to evaluate the association between GCC thickness thinning rate and macula vessel density loss rate, and between the rates and putative predictive factors such as age and mean IOP.
Statistical analyses were performed using statistical software Stata 14.2 (StataCorp LLC, College Station, TX). P ≤ 0.05 was considered statistically significant.
Results
83 eyes were included, consisting of 24 healthy eyes, 30 OHT eyes, and 29 GON eyes. The mean follow-up time and number of visits were 3.0 years and 4.2 visits for healthy eyes and OHT eyes, and 3.5 years and 4.8 visits for GON eyes, respectively. Table 1 summarizes the baseline characteristics of the study subjects. At baseline, there was no significant difference among the groups in terms of gender and self-reported history of diabetes or system hypertension. The groups differed by race, age, CCT, baseline IOP, mean IOP during follow-up, IOP-lowering treatment ratio, baseline RNFL thickness, GCC thickness and macula vessel density. The healthy group was youngest and had the highest proportion of African American. The OHT group had the thickest CCT and highest baseline and mean IOP. The majority of OHT eyes and GON eyes were under IOP-lowering treatment (76.7% and 93.1%, respectively). Baseline RNFL thickness, GCC thickness and macula vessel density showed progressive worsening from healthy to OHT to GON.
Table 1.
Demographics and Ocular Characteristics of Study Population
| A. Healthy | B. OHT | C. GON | P value | |||
|---|---|---|---|---|---|---|
| A vs. B | B vs. C | A vs. C | ||||
| By subject (No.) | 17 | 24 | 24 | |||
| Gender (M/F) | 4/13 | 14/10 | 10/14 | 0.341 | ||
| Race, No. (%) | 0.016 | |||||
| Caucasian | 4 (23.5%) | 21 (87.5%) | 17 (70.8%) | |||
| African American | 9 (52.9%) | 3 (12.5%) | 4 (16.7%) | |||
| Other | 4 (23.5%) | 3 (12.5%) | ||||
| Self-reported history of Diabetes, No. (%) | 1 (5.9%) | 3 (12.5%) | 1 (4.2%) | 0.751 | ||
| Self-reported history of Hypertension, No. (%) | 8 (47.1%) | 14 (58.3%) | 14 (58.3%) | 0.501 | ||
| By Eye (No.) | 24 | 30 | 29 | |||
| Baseline age | 54.0 (48.3, 59.7) | 65.4 (62.4, 68.4) | 66.1 (60.9, 71.4) | 0.002 | 0.834 | 0.007 |
| Axial Length (mm) | 24.1 (23.6, 24.7) | 24.8 (24.1, 25.6) | 24.5 (24.1, 24.9) | 0.166 | 0.450 | 0.334 |
| CCT (μm) | 532.5 (520.5, 544.5) | 560.0 (545.1, 575.0) | 532.3 (516.1, 548.6) | 0.011 | 0.032 | 0.985 |
| DH history No.(%) | 0 | 2(6.7%) | 3(10.3%) | 0.074 | ||
| Baseline IOP (mmHg) | 14.7 (13.7, 15.8) | 17.5 (16.0, 18.9) | 16.5 (14.4, 18.6) | 0.008 | 0.521 | 0.206 |
| Mean IOP during follow-up (mmHg) | 14.4 (13.4, 15.5) | 18.4 (17.0, 19.8) | 15.8 (14.4, 17.4) | <.0001 | 0.044 | 0.228 |
| Topical IOP lowering medications No.(%) | 0 | 23(76.7%) | 27(93.1%) | 0.002 | ||
| Baseline RNFL thickness (μm) | 90.5 (80.5, 100.4) | 82.5 (77.4, 87.7) | 77.7 (72.2, 83.3) | 0.199 | 0.289 | 0.046 |
| OCTA visits | 4.2 (3.7, 4.6) | 4.2 (3.7, 4.6) | 4.8 (4.2, 5.3) | 1.000 | 0.123 | 0.101 |
| OCTA follow-up (years) | 3.0 (2.6, 3.4) | 3.0 (2.6, 3.4) | 3.5 (3.2, 3.7) | 0.996 | 0.169 | 0.127 |
| Baseline scan quality index | 7.4 (7.0, 7.8) | 7.0 (6.6, 7.4) | 6.8 (6.4, 7.3) | 0.136 | 0.571 | 0.059 |
| Baseline wiVD (%) | 47.7 (46.1, 49.2) | 46.0 (44.4, 47.7) | 45.6 (44.4, 46.8) | 0.201 | 0.709 | 0.064 |
| Baseline pfVD (%) | 50.5 (49.1, 51.9) | 48.8 (47.1, 50.4) | 48.3 (47.1, 50.4) | 0.147 | 0.670 | 0.033 |
| Baseline wiGCC (μm) | 101.1 (97.1, 105.2) | 100.1 (96.8, 103.4) | 93.7 (89.4, 98.0) | 0.749 | 0.044 | 0.034 |
| Baseline pfGCC (μm) | 106.0 (102.0, 110.1) | 105.8 (102.2, 109.3) | 98.8 (94.4, 103.3) | 0.942 | 0.039 | 0.044 |
Results are shown in mean (95% confident interval) for numerical variables and No. (%) for categorical variables. Categorical variables were compared using Fisher’s exact test, and overall P values are shown. Linear mixed model was used for comparison of numerical ocular parameters, and post-hoc P values are presented. Values with statistical significance are shown in bold.
Abbreviations: OHT, ocular hypertension; GON, glaucomatous optic neuropathy; M, male; F, female; CCT, central corneal thickness; IOP, intraocular pressure; RNFL, retinal nerve fiber layer; OCTA, optical coherence tomography angiography; wiVD, whole image vessel density.
Age and scan quality index (QI) adjusted rates of global and regional GCC thickness thinning and macula vessel density loss are presented in Table 2 and Table 3. In all three diagnostic groups, age and QI adjusted global rates of macula vessel density loss were significantly different from zero (all P<0.01), while the vessel density loss rates were not significantly different among the groups. OHT and GON groups showed significant or borderline significant GCC thinning (0.05<P<0.1 for parafoveal region of OHT group, others P≤0.05); GCC thinning rates of OHT and GON group were faster than healthy group in both age and QI adjusted model and multivariate model with extra factors (race, mean IOP, and CCT) adjusted. For sectoral evaluation, the significant faster GCC thinning rate was shown in parafoveal temporal and nasal sectors (borderline significant in parafoveal superior sector), while not in parafoveal inferior sectors. All sectoral GCC thinning rates were not significantly different between OHT group and GON group (all P> 0.1). On the other hand, sectoral vessel density loss rate was not different among the three groups. Rates of GCC thickness loss and macula vessel density loss by sector are illustrated in Figure 1 and 2.
Table 2.
Age and Scan Quality adjusted Global Rate of Macula Ganglion Cell Complex Thickness Thinning and Vessel Density Loss in Healthy, OHT and GON Eyes
| A. Healthy | B. OHT | C. GON | P Value# (a, b) | |||
|---|---|---|---|---|---|---|
| Mean (95%CI), P Value* | Mean (95%CI), P Value* | Mean (95%CI), P Value* | A vs. B | B vs. C | A vs. C | |
| Ganglion Cell Complex Thickness Thinning Rate (μm/year) | ||||||
| Whole image | −0.11 (−0.49, 0.28), 0.596 | −0.59 (−1.18, −0.00), 0.050 | −0.79 (−1.42, −0.15), 0.015 | 0.025, 0.027 | 0.593, 0.581 | 0.058, 0.061 |
| Parafoveal | −0.16 (−0.54, 0.22), 0.407 | −0.52 (−1.12, 0.09), 0.092 | −0.82 (−1.45, −0.19), 0.011 | 0.046, 0.055 | 0.672, 0.688 | 0.084, 0.090 |
| Vessel density Loss Rate (%/year) | ||||||
| Whole image | −0.82 (−1.21, −0.42), <.0001 | −0.77 (−1.19, −0.34), <.0001 | −0.93 (−1.27, −0.59), <.0001 | 0.559, 0.465 | 0.423, 0.362 | 0.840, 0.875 |
| Parafoveal | −0.79 (−1.23, −0.35), <.0001 | −0.66 (−1.07, −0.24), 0.002 | −0.77 (−1.12, −0.42), <.0001 | 0.298, 0.220 | 0.490, 0.399 | 0.666, 0.624 |
Abbreviations: OHT, ocular hypertension; GON, glaucomatous optic neuropathy; CI, confidence interval
P value represents whether the mean rate is significantly different from zero.
P value represents whether the mean rate is significantly different between groups, a, age and QI adjusted; b, age, race, mean IOP, CCT, and QI adjusted.
Values with statistical significance (P≤0.05) or borderline significance (0.05<P<0.1) are shown in bold.
Table 3.
Age and Scan Quality adjusted Regional Rate of Macula Ganglion Cell Complex Thickness Thinning and Vessel Density Loss in Healthy, OHT and GON Eyes
| A. Healthy | B. OHT | C. GON | P Value# (a, b) | |||
|---|---|---|---|---|---|---|
| Mean (95%CI), P Value* | Mean (95%CI), P Value* | Mean (95%CI), P Value* | A vs. B | B vs. C | A vs. C | |
| Ganglion Cell Complex Thickness Thinning Rate (μm/year) | ||||||
| Inferior | −0.28 (−0.67, 0.11), 0.158 | −0.09 (−1.08, 0.90), 0.858 | −0.65 (−1.42, 0.12), 0.098 | 0.677, 0.700 | 0.775, 0.770 | 0.379, 0.379 |
| Superior | −0.08 (−0.51, 0.34), 0.705 | −0.58 (−1.13, −0.03), 0.038 | −0.96 (−1.52, −0.40), 0.001 | 0.083, 0.098 | 0.995, 0.950 | 0.088, 0.091 |
| Temporal | −0.01 (−0.37, 0.36), 0.976 | −0.64 (−1.35, 0.07), 0.076 | −0.68 (−1.28, −0.09), 0.024 | 0.017, 0.022 | 0.374, 0.384 | 0.051, 0.059 |
| Nasal | −0.23 (−0.66, 0.21), 0.306 | −0.48 (−1.14, 0.18), 0.154 | −0.90 (−1.61, −0.18), 0.014 | 0.019, 0.023 | 0.288, 0.296 | 0.074, 0.083 |
| Vessel density Loss Rate (%/year) | ||||||
| Inferior | −0.75 (−1.26, −0.25), 0.003 | −0.45 (−0.87, −0.03), 0.037 | −0.65 (−1.05, −0.25), 0.001 | 0.219, 0.160 | 0.293, 0.225 | 0.763, 0.733 |
| Superior | −0.83 (−1.36, −0.30), 0.002 | −0.66 (−1.17, −0.16), 0.009 | −0.67 (−1.09, −0.25), 0.002 | 0.346, 0.228 | 0.771, 0.600 | 0.437, 0.396 |
| Temporal | −0.68 (−1.01, −0.34), <.0001 | −0.78 (−1.22, −0.33), 0.001 | −0.85 (−1.24, −0.47), <.0001 | 0.973, 0.838 | 0.673, 0.525 | 0.622, 0.638 |
| Nasal | −0.94 (−1.44, −0.43), <.0001 | −0.70 (−1.14, −0.26), 0.002 | −0.81 (−1.13, −0.49), <.0001 | 0.100, 0.100 | 0.482, 0.496 | 0.263, 0.235 |
Abbreviations: OHT, ocular hypertension; GON, glaucomatous optic neuropathy; CI, confidence interval
P value represents whether the mean rate is significantly different from zero.
P value represents whether the mean rate is significantly different between groups, a, age and QI adjusted; b, age, race, mean IOP, CCT, and QI adjusted.
Values with statistical significance (P≤0.05) or borderline significance (0.05<P<0.1) are shown in bold.
Figure 1.
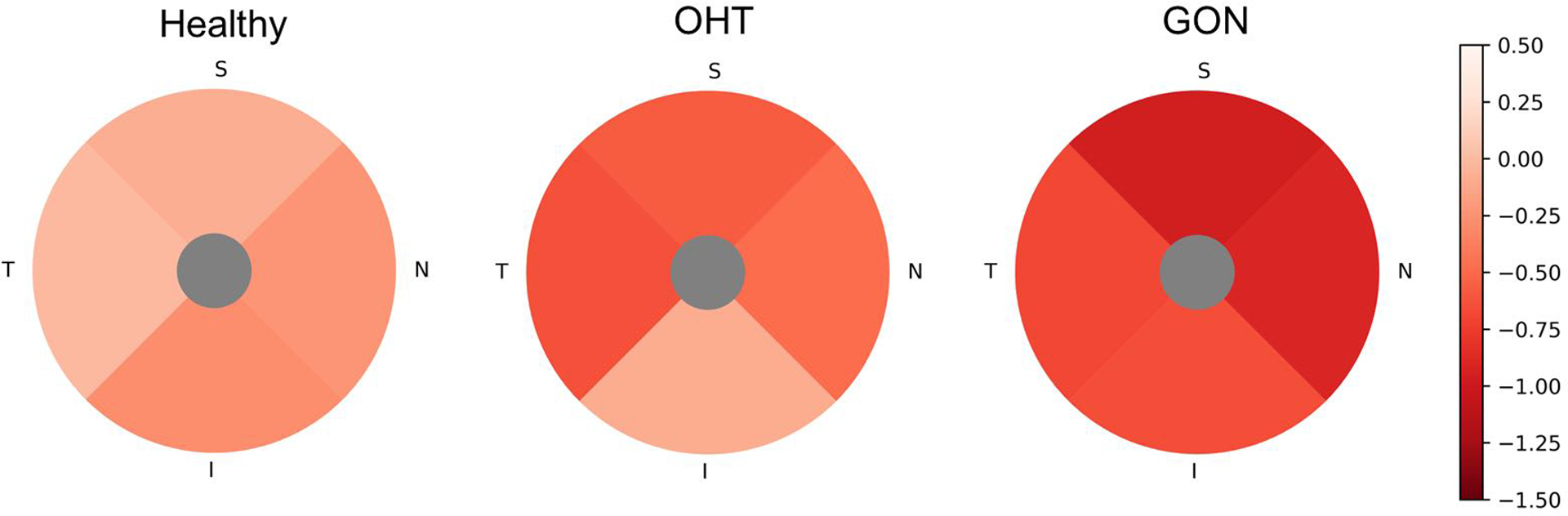
Parafoveal sectoral age and QI adjusted rates of ganglion cells complex (GCC) thinning of Healthy, ocular hypertension (OHT), and glaucomatous optic neuropathy (GON) eyes. The hotter color represents the faster thinning. The order from slowest to fastest is: Healthy, TSNI; OHT, INST; GON, ITNS. T, temporal; S, superior; N, nasal; I, inferior. Data are presented as the age and scan quality adjusted GCC thickness thinning rate (μm/year).
Figure 2.
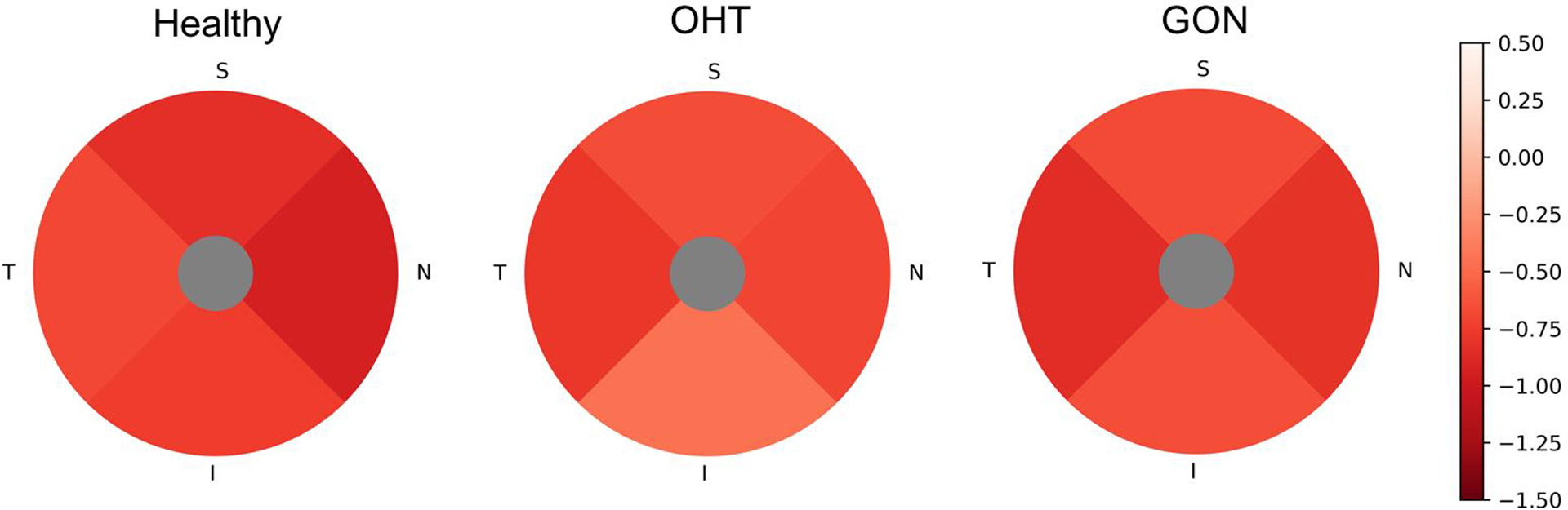
Parafoveal sectoral age and QI adjusted rates of macular vessel density loss of Healthy, ocular hypertension (OHT), and glaucomatous optic neuropathy (GON) eyes. The hotter color represents the faster loss. The order from slowest to fastest is: Healthy, TISN; OHT, ISNT; GON, ISNT. T, temporal; S, superior; N, nasal; I, inferior. Data are presented as the age and scan quality adjusted vessel density loss rate (%/year).
Association between rates of GCC thickness thinning and vessel density loss is presented in Table 4. Significant associations were found in parafoveal temporal and nasal sectors of OHT eyes- the sectors that showed significantly faster GCC thinning rates comparing with healthy eyes.
Table 4.
Association Between Rates of Ganglion Cell Complex Thinning and Vessel Density Loss
| OHT | GON | |||
|---|---|---|---|---|
| R2 | P value | R2 | P value | |
| Whole image | 7.91/14.80 | 0.128/0.105 | 2.54/3.68 | 0.601/0.561 |
| Parafoveal | 7.96/7.60 | 0.178/0.213 | 0.45/1.05 | 0.807/0.732 |
| Inferior | 3.10/3.80 | 0.133/0.065 | 0.52/1.21 | 0.770/0.686 |
| Superior | 1.36/0.37 | 0.386/0.712 | 6.99/7.58 | 0.314/0.317 |
| Temporal | 15.73/14,15 | 0.030/0.038 | 0.06/0.06 | 0.921/0.920 |
| Nasal | 17.81/20.09 | 0.014/0.011 | 0.34/0 | 0.796/0.983 |
Abbreviations: OHT, ocular hypertension; GON, glaucomatous optic neuropathy. Values with statistical significance are shown in bold. Results from analyses using original rates and percent loss rates are presented successively.
In order to directly compare GCC thinning rate with macula vessel density decrease rate, percent loss of GCC thickness and macula vessel density was calculated and the rates compared within each diagnostic group, but no significant difference was found in all groups (global and regional)(Data not shown). In addition, 29 individual eyes out of all 59 (49.1%) suspect eyes had significant loss either with vessel density or GCC. 15 (51.7 %) of the GON eyes showed only significant GCC loss, while only significant vessel density loss was found in 5 (17.2 %) GON eyes. Both significant GCC loss and vessel density loss were detectable in 9 GON eyes (31.0%). In the suspect eyes, 12 OHT eyes and 15 GON eyes (45.8%) showed faster GCC thinning than vessel density loss.
Table 5 summarizes the results of univariable models assessing the effect of each putative predictive factor on whole image GCC thinning and macula vessel density decrease over time in OHT and GON groups. GCC thinning rates could be affected by mean IOP during follow-up in both OHT and GON groups (borderline significance in OHT group), while no factor showed significant effect on macula vessel density loss rate. Each 1 mmHg higher mean IOP resulted in 0.07 μm/year and 0.10 μm/year faster rate of GCC thinning in OHT and GON eyes, respectively.
Table 5.
Effect of Putative Predictive Factor on Rates of Whole Image Ganglion Cell Complex Thickness and Vessel Density Loss
| OHT (Coefficients (95% CI), P) | GON (Coefficients (95% CI), P) | |||
|---|---|---|---|---|
| GCC Thickness | Vessel Density | GCC Thickness | Vessel Density | |
| Baseline age, per year older | 0.02 (−0.02, 0.07), 0.237 | −0.01 (−0.07, 0.05), 0.739 | 0.02 (−0.01, 0.04), 0.114 | −0.004 (−0.02, 0.01), 0.526 |
| Race, C | 0.17 (−0.53, 0.87), 0.636 | 0.21 (−0.42, 0.85), 0.509 | 0.05 (−0.35, 0.45), 0.810 | 0.14 (−0.09, 0.47), 0.241 |
| Gender, male | 0.11 (−0.64, 0.85), 0.777 | 0.50 (−0.27, 1.27), 0.206 | −0.06 (−0.69, 0.58), 0.861 | 0.001 (−0.46, 0.47), 0.995 |
| Mean IOP, per 1mmHg higher | −0.07 (−0.15, 0.004), 0.065 | 0.04 (−0.05, 0.13), 0.754 | −0.10 (−0.18, −0.02), 0.015 | 0.01 (−0.08, 0.10), 0.827 |
| CCT, per 1 μm thicker | −0.004 (−0.02, 0.01), 0.621 | 0.001 (−0.01, −0.01), 0.894 | −0.00 (−0.01, 0.01), 0.993 | −0.002 (−0.01, 0.005), 0.560 |
| AL, per 1 mm longer | 0.27 (−0.15, 0.68), 0.213 | 0.18 (−0.08, 0.44), 0.177 | −0.06 (−0.38, 0.25), 0.694 | −0.04 (−0.27, 0.19), 0.748 |
Abbreviations: OHT, ocular hypertension; GON, glaucomatous optic neuropathy; CI, confidence interval; C, Caucasian; CCT, central corneal thickness; IOP, intraocular pressure; AL, axial length. Values with statistical significance are shown in bold.
Discussion
With a mean follow-up time of more than 3 years, this study found that both GCC thinning and macula vessel density loss over time were detectable in glaucoma suspect eyes with OHT or GON. GCC thinning rates, but not vessel density loss rate, were faster on average in glaucoma suspect eyes than in healthy eyes. Vessel density loss rates were associated with GCC thinning rates in the sectors (nasal and temporal sectors in OHT eyes) that showed significant faster GCC thinning.
Although there has been an increasing interest in the evaluation of glaucomatous macula damage,23 only a few prior studies investigated on presence of early macula microvasculature damage in glaucoma.14, 24, 25 Consistent with a prior study,14 the baseline measurements in the current study showed a significant reduction of macula vessel density in GON eyes. This reduction was only found in GON eyes rather than OHT eyes. Similarly, only GON eyes showed significant GCC thickness decrease comparing with healthy eyes. This finding suggests that both macula thickness and vasculature are already impaired and detectable when glaucomatous ONH damage happens before VF shows defect.
Early detection and close monitoring of glaucomatous damage are important for advancing ocular hypotensive treatment to minimize irreversible vision loss, in particular, to avoid progression of glaucoma suspect to perimetric glaucoma. The ability to detect glaucoma progression by functional test vs. structure measurement is known to be significantly influenced by the severity of disease. Eyes with less severe disease at baseline have a higher chance of being detected as progressing by SD-OCT compared with VF.26 However, the role of OCTA for detection of early progression is yet unclear. To enhance monitoring of glaucoma suspect eyes, it is important to better characterize the glaucomatous changes that can be detected early in the course of the glaucoma continuum. It has been reported that the mean percent loss of macula vessel density was significantly less than GCC thickness in early perimetric glaucoma eyes (MD better than −6 dB),14 and more than two-thirds of the patients had less perfusion loss compared to structural thickness loss in the macula.27 This thickness/microvascular mismatch suggest that structural thinning may be more readily detectable than microvascular damage in the macula in early glaucoma. A prior longitudinal study found borderline significant faster GCC thinning rate of GON eyes compared with healthy eyes, but comparable vessel density loss rate.15 The current study, with longer follow-up time and inclusion of OHT eyes, found faster GCC thinning rate and comparable vessel density loss rate in OHT and GON eyes compared with healthy eyes, although both progressive GCC thinning and vessel density loss were detectable (significant slopes from 0). This result further suggests that macula thinning is more likely to be found than microvasculature drop-out in early stage of glaucoma. Therefore, monitoring GCC thinning should be useful to capture the earliest progression in glaucoma suspect eyes. However, it should be noted that the above inference cannot be generalized to the whole glaucoma continuum and to all glaucoma patients. Compared with the GCC thinning rate, particularly in perimetric glaucoma eyes, a prior study showed better correlation between macula vessel density loss rate and VF deterioration.15 In the current study, although there was no significant difference overall between the percent loss rates of GCC and vessel density in both the OHT and GON groups, only significant vessel density loss and faster percent loss rate of vessel density than GCC were found in some suspect eyes. This suggests progression of vessel density loss might be detected prior to GCC thinning in these suspect eyes. The biologic basis for these difference is unclear, and perhaps possible differences (e.g. vascular- vs intraocular pressure- related damage, or both) among patients account for these observations. Or perhaps, the non-neural tissue in the GCC resulted in less detectable change than that observed with vessel density.28
Macula OCT scan probability map is suggested to reliably detect glaucomatous damage.29 As described by Hood and associates in a model describing the topographic relationship between macular and ONH damage in glaucoma, macular damage was typically more extensive in the inferior region which associated with local RNFL thinning in a narrow region of the disc called macular vulnerability zone.3, 9 This pattern is easiest to see in the patients with moderate glaucoma.3 However, it is unknown if it is applicable to all stages of glaucoma. Similar to a longitudinal study that evaluated sectoral thickness loss of macular ganglion cell-inner plexiform layer in perimetric glaucoma,17 our study found that parafoveal temporal sector showed the fastest loss rate of both GCC and vessel density in OHT eyes and the fastest loss rate of vessel density in GON eyes, suggesting active structure change in this region in glaucoma suspect eyes. Another possible explanation is that the scan areas from which the thickness maps derived are different, due to different OCT instrument and software.30 The macula scan area was only 3×3 mm2 in the current study. Therefore, RGCs whose axons comprise the vulnerable region of circumpapillary RNFL are likely underrepresented in this scan. However, the adoption of the 3×3 mm2 macula region facilitated an evaluation of both OCTA and OCT measurements from the same scan slab.31 With this advantage, we found significant association of GCC thinning rate and macula vessel density loss rate in the parafoveal temporal and nasal sectors in OHT eyes, where accelerated GCC thinning was detected.
There are several limitations to the current study. According to a previous study, only about 8% of the suspect eyes developed a VF defect and eventually progressed to perimetric glaucoma with up to 4-year follow-up.32 Thus, the macula changes evaluated in this study may be underestimated, compared with “true” glaucomatous change. With lengthier longitudinal studies to identify eyes that progress to perimetric glaucoma, it may be possible to identify whether GCC or vessel density provides a better differentiation to detect the true progressors. In addition, as mentioned above, the 3×3 mm2 scan that was used in the current study was likely insufficient to portray an integrated picture of glaucomatous damage in macula. A larger 6×6 mm2 macula scan of AngioVue is now available. It has better diagnostic accuracy compared to 3×3 mm2 scans for differentiating mild glaucoma from healthy eyes.33 And, since wiVD and wiGCC were derived from the entire scan including the foveal avascular zone (FAZ) which has increased diameter in glaucoma34, measurements from the smaller scan could be affected more by increased FAZ compared to that the larger scan. It is possible that the larger field could provide more information and better show the changes in different regions.9, 35 However, longitudinal data with 6×6 mm2 scan for pre-perimetric eyes are not yet available. Third, in perimetric glaucoma, GCC thinning was found following a similar pattern as circumpapillary RNFL thinning in the classic arcuate regions. In general, the GCC thinning was more apparent in the inferior as opposed to the superior retina. In addition, the thinning was more extreme on the temporal side of the fovea.9, 29 The macula scan protocol, used in the current study for GCC thickness and vessel density measurement, separated the parafoveal region into 90-degree intervals as 4 sectors (superior, inferior, temporal, and nasal). Although this protocol, which is mainly for imaging of retina diseases, is not ideal for topographical evaluation of glaucomatous macula damage, it is generally available in a clinic setting. Last, although significant findings were obtained, the sample sizes of the current study are relatively small. The power of the current analysis has been further estimated using simulations. With the power was set at 80% and the current follow-up time, a sample size of 22 is needed to detect a significant negative slope of both GCC thinning and vessel density loss in the cohort (with significance level 0.05). While our sample size was enough to detect differences in the rates of slope among the groups, larger sample size and longer follow-up time will be needed for evaluating the significance of each slope of individual eye as well as to identify the true progressors that become perimetric glaucoma. With continuous using of OCTA in glaucoma, longitudinal studies with larger sample size could be expected to address these issues.
In conclusion, both progressive GCC thinning and macula vessel density loss were detectable in glaucoma suspect eyes with OHT or GON. GCC thinning rates in glaucoma suspect eyes were faster on average than in healthy eyes and were associated with vessel density loss rates and IOP. However, vessel density loss was faster than GCC thinning in a sizable proportion of suspect eyes. OCTA and OCT appear to be complementary and both can be useful for monitoring glaucoma suspect eyes.
Financial Support:
National Institutes of Health/National Eye Institute Grants EY029058, EY11008, EY027510, and EY026574, Core Grant P30EY022589, an unrestricted grant from Research to Prevent Blindness (New York, NY), and participant retention incentive grants in the form of glaucoma medication at no cost from Novartis/Alcon Laboratories Inc, Allergan, Akorn, and Pfizer Inc.
Commercial Disclosures:
Huiyuan Hou: none
Sasan Moghimi: none
Alireza Kamalipour: none
Eren Ekici: none
Won Hyuk Oh: none
James A. Proudfoot: none
Nevin El-Nimri: none
Rafaella C. Penteado: none
Takashi Nishida: none
Ryan Caezar David: none
Robert N. Weinreb: Research support- National Eye Institute, Carl Zeiss Meditec, Centervue, Heidelberg Engineering, Konan, Bausch & Lomb; Consultant- Allergan, Eyenovia, Nicox; Patent- Toromedes, Carl Zeiss Meditec
Reference
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311(18):1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363(9422):1711–20. [DOI] [PubMed] [Google Scholar]
- 3.Hood DC. Imaging Glaucoma. Annu Rev Vis Sci 2015;1:51–72. [DOI] [PubMed] [Google Scholar]
- 4.De Moraes CG, Hood DC, Thenappan A, et al. 24–2 Visual Fields Miss Central Defects Shown on 10–2 Tests in Glaucoma Suspects, Ocular Hypertensives, and Early Glaucoma. Ophthalmology 2017;124(10):1449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol 1990;300(1):5–25. [DOI] [PubMed] [Google Scholar]
- 6.Abe RY, Diniz-Filho A, Costa VP, et al. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology 2016;123(3):552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumberg DM, De Moraes CG, Prager AJ, et al. Association Between Undetected 10–2 Visual Field Damage and Vision-Related Quality of Life in Patients With Glaucoma. JAMA Ophthalmol 2017;135(7):742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeimer R, Asrani S, Zou S, et al. Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping. A pilot study. Ophthalmology 1998;105(2):224–31. [DOI] [PubMed] [Google Scholar]
- 9.Hood DC, Raza AS, de Moraes CG, et al. Glaucomatous damage of the macula. Prog Retin Eye Res 2013;32:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akil H, Chopra V, Al-Sheikh M, et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. British Journal of Ophthalmology 2018;102(4):515–9. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20(4):4710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarmohammadi A, Zangwill LM, Manalastas PIC, et al. Peripapillary and Macular Vessel Density in Patients with Primary Open-Angle Glaucoma and Unilateral Visual Field Loss. Ophthalmology 2017;125(4):578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou H, Moghimi S, Zangwill LM, et al. Inter-eye Asymmetry of Optical Coherence Tomography Angiography Vessel Density in Bilateral Glaucoma, Glaucoma Suspect, and Healthy Eyes. Am J Ophthalmol 2018;190:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou H, Moghimi S, Zangwill LM, et al. Macula Vessel Density and Thickness in Early Primary Open-Angle Glaucoma. Am J Ophthalmol 2019;199:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou HY, Moghimi S, Proudfoot JA, et al. Ganglion Cell Complex Thickness and Macular Vessel Density Loss in Primary Open-Angle Glaucoma. Ophthalmology 2020;127(8):1043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansouri K Optical coherence tomography angiography and glaucoma: searching for the missing link. Expert Rev Med Devices 2016;13(10):879–80. [DOI] [PubMed] [Google Scholar]
- 17.Hammel N, Belghith A, Weinreb RN, et al. Comparing the Rates of Retinal Nerve Fiber Layer and Ganglion Cell-Inner Plexiform Layer Loss in Healthy Eyes and in Glaucoma Eyes. Am J Ophthalmol 2017;178:38–50. [DOI] [PubMed] [Google Scholar]
- 18.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci 2016;57(9):OCT451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Archives of ophthalmology 2009;127(9):1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alencar LM, Zangwill LM, Weinreb RN, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophthalmol Vis Sci 2010;51(7):3531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci 2009;50(4):1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Tatham AJ, Weinreb RN, et al. Relationship between ganglion cell layer thickness and estimated retinal ganglion cell counts in the glaucomatous macula. Ophthalmology 2014;121(12):2371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triolo G, Rabiolo A, Shemonski ND, et al. Optical Coherence Tomography Angiography Macular and Peripapillary Vessel Perfusion Density in Healthy Subjects, Glaucoma Suspects, and Glaucoma Patients. Invest Ophthalmol Vis Sci 2017;58(13):5713–22. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Yu J, Kong X, et al. Macular microvasculature alterations in patients with primary open-angle glaucoma: A cross-sectional study. Medicine (Baltimore) 2016;95(33):e4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe RY, Diniz-Filho A, Zangwill LM, et al. The Relative Odds of Progressing by Structural and Functional Tests in Glaucoma. Invest Ophthalmol Vis Sci 2016;57(9):OCT421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirasawa K, Smith CA, West ME, et al. Discrepancy in Loss of Macular Perfusion Density and Ganglion Cell Layer Thickness in Early Glaucoma. American Journal of Ophthalmology 2021;221:39–47. [DOI] [PubMed] [Google Scholar]
- 28.Moghimi S, Bowd C, Zangwill LM, et al. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hood DC. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhende M, Shetty S, Parthasarathy MK, Ramya S. Optical coherence tomography: A guide to interpretation of common macular diseases. Indian J Ophthalmol 2018;66(1):20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan KH, Lam AKN, Leung CK. Optical Coherence Tomography Angiography Compared With Optical Coherence Tomography Macular Measurements for Detection of Glaucoma. JAMA Ophthalmol 2018;136(8):866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology 2014;121(7):1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penteado RC, Bowd C, Proudfoot JA, et al. Diagnostic Ability of Optical Coherence Tomography Angiography Macula Vessel Density for the Diagnosis of Glaucoma Using Difference Scan Sizes. Journal of Glaucoma 2020;29(4):245–51. [DOI] [PubMed] [Google Scholar]
- 34.Choi J, Kwon J, Shin JW, et al. Quantitative optical coherence tomography angiography of macular vascular structure and foveal avascular zone in glaucoma. PLoS One 2017;12(9):e0184948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hood DC. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res 2017;57:46–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


