Abstract
Human monkeypox virus is spreading globally, and more information is required about its epidemiological and clinical disease characteristics in endemic countries. We report the investigation of an outbreak in November 2021 in Central African Republic (CAR). The primary case, a hunter, fell ill after contact with a non-human primate at the frontier between forest and savannah. The ensuing investigation in a small nearby town concerned two families and four waves of inter-human transmission, with 14 confirmed cases, 11 suspected cases and 17 non-infected contacts, and a secondary attack rate of 59.5% (25/42). Complications were observed in 12 of the 19 (63.2%) confirmed and suspected cases with available clinical follow-up data: eight cases of bronchopneumonia, two of severe dehydration, one corneal ulcer, one abscess, two cutaneous superinfections, and six cutaneous sequelae (cheloid scars, or depigmentation). There was one death, giving a case fatality ratio of 1/25 (4.0%) for confirmed and suspected cases. This outbreak, with the largest number of confirmed cases ever described in CAR, confirms the potential severity of the disease associated with clade I monkeypox viruses, and highlights the need for rapid control over virus circulation to prevent the further national and international spread of infection.
Keywords: mpox, Monkeypox virus, Central African Republic, Surveillance, Outbreak investigation, Zoonosis, Emerging infectious diseases
Highlights
-
•
Importance of epidemiological description of mpox outbreak in endemic settings
-
•
Extension of mpox outbreak size in Central Africa with 4 waves of secondary interhuman transmission
-
•
Extension of mpox epidemic towards urban and connected areas highlighting the risk of dissemination of the clade I
-
•
Persistence of a high morbidity and mortality with the clade I in Central Africa
1. Introduction
Before the ongoing worldwide human monkeypox virus (hMPXV) outbreak leading to the WHO declaration of a Public Health Emergency of International Concern mpox (previously monkeypox) was considered a rare emerging disease caused by a zoonotic virus from the Poxviridae family endemic to forested regions of Central and West Africa. Since the first recognized case of mpox in a human in 1970 in Basankusu territory [1], endemic hMPXV circulation has occurred in the Democratic Republic of Congo (DRC), Republic of Congo (RoC), Central African Republic (CAR), and Nigeria (since the major outbreak in 2017). Sporadic independent mpox cases have also been reported in Cameroon, Côte d'Ivoire, Gabon, Sierra Leone, and Liberia [2]. The number of outbreaks has been increasing globally over the last few decades [2], and in CAR since 2018 [3]. An expansion of the affected geographic area towards sub-Sahelian savannah zones in Sudan has been observed since 2005 [4] and to urban areas in Nigeria since 2017 [5]. Moreover, expansion outside the African continent with exported cases in animals [6,7] or humans [8] was sporadically reported until the 2022 mpox outbreak, in which >68,000 confirmed cases have occurred in humans in >99 non-endemic countries [9,10]. These exportations involve solely the West African clade/clade II of the hMPXV, the Congo Basin clade/clade I remaining confined to African settings.
Typically, in African countries in which mpox is endemic, the disease has an incubation period of 5 to 21 days, followed by the appearance of a disseminated maculopapular rash and disabling general clinical signs, such as asthenia or fever. Clinical complications, such as corneal ulceration and encephalitis, may occur, and the case fatality ratio (CFR) ranges from 3.6% (West African clade) to 10.6% (Congo Basin clade) [2]. In endemic countries, the introduction of the disease into human communities is thought to occur via zoonotic transmission from an unidentified animal reservoir, with small rodents and tree squirrels the principal suspects [11]. The virus has also been identified in chimpanzee feces and flies in Côte d'Ivoire, suggesting environmental contamination [12]. Secondary human-to-human transmission explains a large proportion of human cases, and household transmission chains of as many as seven successive events have been reported in Africa [[13], [14], [15]]. Secondary interhuman transmission occurs either directly, through contact with skin, bodily fluids, or droplets, or indirectly, through fomites or contaminated material, such as clothes or bed linen [16].
The recent worldwide emergence of mpox should alert us to the global health risk posed by this virus, the most significant Orthopoxvirus since smallpox eradication. Interestingly, the ongoing multinational outbreak, which began in May 2022, differs from previously reported outbreaks in its spread principally among men who have sex with men (MSM) [17], and a clinical presentation dominated by ano-genital lesions [9], with few hospitalizations and fewer deaths [18]. Transmission seems to occur primarily during sex, probably through skin-to-skin contact or possibly through contact with semen [19], a mode of transmission already suspected during the 2017 Nigerian outbreak [20].
The description of African epidemics remains relevant for the purposes of comparison, so that appropriate responses can be implemented in each region affected. Furthermore, if the country multinational outbreak spreads into other population groups, the experience of African countries in dealing with community transmission will be of global relevance. Despite CAR being one of the most affected countries, together with DRC, Nigeria and RoC [2], before the current outbreak, little information has been published on mpox outbreaks in this country, and recent descriptions of investigations of mpox outbreaks in Africa are relatively rare [5,[20], [21], [22], [23], [24], [25], [26], [27]]. We describe here the largest ever outbreak of confirmed human mpox in the CAR, which occurred in November 2021 in Mambéré Kadéi prefecture.
2. Methods
2.1. Outbreak investigation/data collection
Mpox is a notifiable disease in the CAR, monitored, since 2001, through a national surveillance system coordinated by the Institut Pasteur de Bangui (IPB) under the aegis of the Ministry of Public Health and Population. Field healthcare workers are regularly informed about the disease and the importance of rapidly identifying cases. For all suspected cases, a blood, or pus sample is sent to the IPB, which serves as the national reference center for mpox. Following virological confirmation, an outbreak investigation team is deployed in the field for a more complete investigation of cases and their contacts through specific case report questionnaires and the collection of additional specimens. The questionnaires are paper surveys administered in a local language (Sango) by representatives of the IPB. Information is collected on participant demographics, socioeconomic status, education, and contact with wildlife or other human cases. Swab samples from pus or lesions and 3 to 5 mL whole-blood samples are collected from each suspected case by a trained practitioner using sterile techniques. Case definitions for the CAR were adapted from international recommendations from the CDC and WHO and were very similar to those used by the Nigerian CDC: individuals with a history of fever and maculopapular rash involving the palms and soles and virological confirmation (monkeypox virus PCR-positive) were considered to be confirmed cases, those with a history of fever and maculopapular rash on the palms and soles but without virological confirmation were considered to be suspected cases and individuals without skin lesions exposed to a case during the last three weeks were considered to be contacts [28]. The index case was defined as the first human case identified in a village; this case may or may not have been the primary human case, defined as the initial cases, presumed to have been contaminated by contact with an animal source [29,30]. In the absence of easily identifiable vaccination scars for the determination of smallpox vaccination status, individuals born before 1980 were considered to have been vaccinated. Clinical severity was estimated by assessing the severity of the skin involvement, by summing the total number of lesions and scars. The disease was classified as mild (≤25 lesions), moderate (26–100 lesions), severe (101–250 lesions), or serious (>250 lesions) [29]. All cases receive symptomatic and supportive care in accordance with international guidelines for the management of mpox disease [31]. In these specific outbreak investigations, three successive field missions were performed (Fig. 2), with the medical investigator remaining on-site for several weeks.
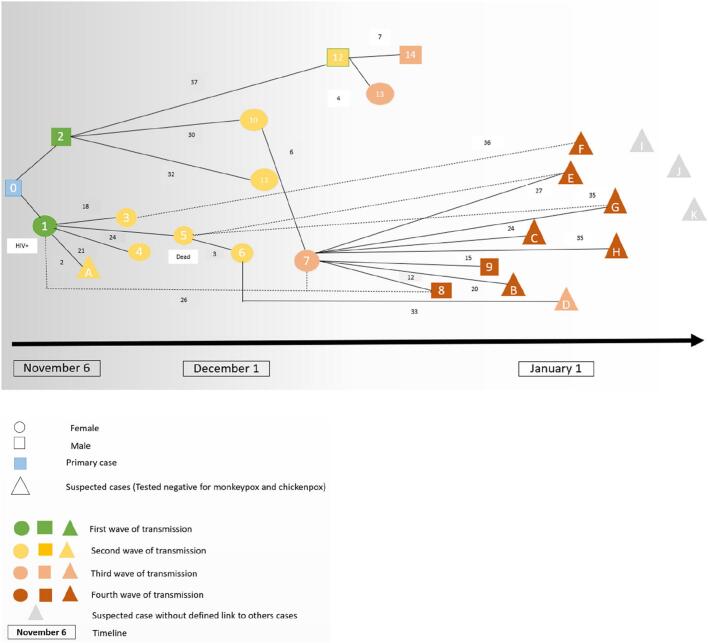
Fig. 2.
Hypothetic pattern of monkeypox virus transmission during the Bania outbreak, CAR 2021–2022.
Circles correspond to female individuals, squares to male individuals, and triangles to suspected cases. Case 0 is the primary case. Cases are organized according to the date of illness onset and the four waves of transmission (first wave: green, second wave: yellow, third wave: coral, fourth wave: brown). The numbers indicate the number of days between disease onset in two consecutive patients in the line of transmission (rash-to-rash interval). The dotted lines correspond to less reliable presumed chains of transmission. The solid lines correspond to more reliable chains of transmission. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Laboratory procedures
The recommended method of mpox diagnosis is based on virological confirmation by PCR detection of the virus in the pus or crusts of lesions [32,33]. Samples are also tested for the presence of virus, by the intracranial inoculation of suckling mice [34]. The CAR national surveillance program uses quantitative and conventional PCR targeting the hemagglutinin gene and part of the A-type inclusion body gene with generic and Congo Basin primers [35], as in previous outbreak investigations.
2.3. Statistical analysis
We assumed that transmission occurs mostly from a symptomatic person, although presymptomatic transmission can also occur, based on the recent documentation of high viral loads in patients before the onset of a rash [36]. Transmission chains were determined from patient and family interviews and the notes of the medical investigator. A transmission chain is established when there is a direct link between two cases with a reliable incubation period (5 to 21 days) [2,15]. Waves of transmission correspond to successive human-to-human transmissions. The serial (rash-to-rash) interval is the time from the appearance of a rash in a case to the onset of rash in a secondary case in a direct chain of transmission. In cases of multiple exposure, the rash-to-rash interval was calculated by considering the most plausible transmission chain. Rash onset was chosen for the determination of the serial interval, because this symptom is more specific than fever for the clinical diagnosis of mpox and is easier for individuals and their families to recollect [15]. The secondary attack rate (SAR) is defined as the probability of an infection occurring among close contacts of a case and is calculated as the number of cases among contacts over the total number of contacts at risk. Continuous data are presented as medians and interquartile ranges (IQR), and nominal data are presented as percentages. All statistical analyses were performed with Stata 15.0 (StataCorp, College Station, TX, USA).
2.3.1. Ethical considerations
These outbreak investigations took place within the framework of the national surveillance program for mpox in the CAR, and authorization to use the data for research purposes was obtained from the Institutional review board (IRB) of Institut Pasteur Paris (Autorization number IRB00006966) on January 10, 2020, and from the CES of the Université de Bangui (Comité Ethique et Scientifique) on February 21, 2021.
3. Results
3.1. Outbreak investigation
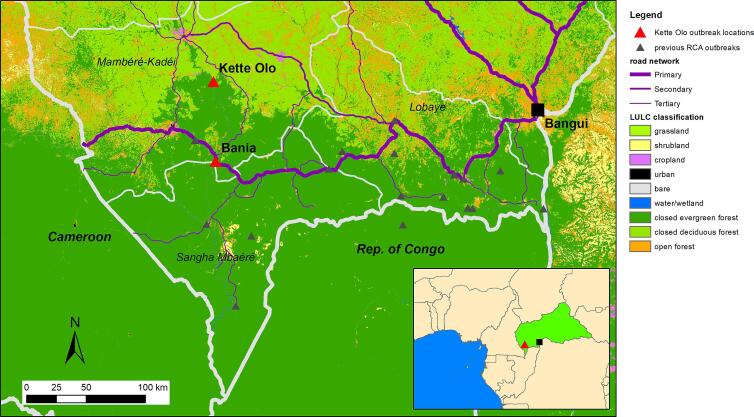
Between November 2021 and January 2022, an outbreak of human mpox occurred in Bania, Mambéré Kadéi prefecture. Bania is a town of 5000 inhabitants, surrounded by primary forest, on a busy road leading to Berbérati and then to Cameroon (Fig. 1). This area is known to have important mining (diamonds and golds) and logging industry activities. This is the first time that this area of CAR has been affected by a mpox epidemic, suggesting a potential geographic shift of epidemic areas towards peri-savannah areas (Fig. 1).
Fig. 1.
Geolocalization of the site of contamination (primary case), and of current and previous mpox outbreaks, CAR, 2021–2022.
In total, 42 individuals were investigated in this outbreak: 14 confirmed mpox cases, 11 suspected cases, and 17 contacts among family and neighbors from the same concession (Table 1).
Table 1.
Characteristics of cases and contacts, Central African Republic, Bania (Mambéré Kadéi prefecture), 2021–2022.
| Individual | Age/Sex | Relation to primary case | Date of symptom onset | Occupation | Reported contact with animals | Reported contact with human cases | Days from rash to sample | Blood sample | Pus sample | Monkeypox PCR results | Varicella-Zoster Virus PCR results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Confirmed cases | |||||||||||
| 0 | 40/M | Index case | X/11/2021 | hunter | NHP | No | ND | ND | ND | ND | ND |
| 1 | 27/F | Spouse of 0 | 06/11/2021 | farmer | NK | Yes | 32 | Yes | ND | Positive | Negative |
| 2 | 22/M | Brother of 0 | 07/11/2021 | farmer/ merchant |
No | Yes | 31 | Yes | ND | Positive | Negative |
| 3 | 38/F | Sister-in-law of 0 | 24/11/2021 | farmer | NHP | Yes | 11 | Yes | ND | Positive | Negative |
| 4 | 5/F | Daughter of 3 | 27/11/2021 | child | No | Yes | 11 | Yes | ND | Positive | Negative |
| 5 | 23/F | Daughter of 3 | 30/11/2021 | NK | No | Yes | 8 | Yes | Pus | Positive | Negative |
| 6 | 27/F | Daughter-in-law of 3 | 02/12/2021 | farmer | No | Yes | 22 | Yes | ND | Positive | Negative |
| 7 | 22/F | Daughter of 3 | 08/12/2021 | farmer | No | Yes | 5 | Yes | ND | Positive | Negative |
| 8 | 5/M | Nephew of 2 | 20/12/2021 | child | No | Yes | 4 | Yes | ND | Positive | Negative |
| 9 | 40/M | Husband of 3 | 23/12/2021 | farmer | No | Yes | 11 | Yes | ND | Positive | Negative |
| 10 | 17/F | Spouse of 2 | 02/12/2021 | pupil | No | Yes | 6 | Yes | ND | Positive | Negative |
| 11 | 4/F | Niece of 2 | 06/12/2021 | child | No | Yes | 9 | Yes | ND | Positive | Negative |
| 12 | 23/M | Brother of 2 | 13/12/2021 | farmer | No | Yes | 0 | Yes | ND | Positive | Negative |
| 13 | 4/F | Sister-in-law of 2 | 16/12/2021 | child | No | Yes | 1 | Yes | ND | Positive | Negative |
| 14 | 13/M | Nephew of 2 | 19/12/2021 | pupil | No | Yes | 3 | Yes | ND | Positive | Negative |
| Individual | Age/Sex | Relation to primary case | Date of symptom onset | Occupation | Reported contact with animals | Reported contact with human cases | Days from rash to sample | Blood sample | Pus sample | Monkeypox PCR results | Varicella-Zoster Virus PCR results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Suspected cases | |||||||||||
| A | 5/F | Niece of 1 | 08/11/2021 | child | No | Yes | 44 | Yes | ND | Negative | Negative |
| B | 2/F | Niece of 7 | 28/12/2021 | child | No | Yes | 2 | Yes | ND | Negative | Negative |
| C | 5/F | Sister of 7 | 01/01/2022 | child | No | Yes | 37 | ND | Pus | Negative | Negative |
| D | 4/F | Daughter of 6 | 04/01/2022 | child | No | Yes | 3 | Yes | ND | Negative | Negative |
| E | 6 m/M | Brother of 7 | 04/01/2022 | child | Dead NHP/duiker | Yes | 33 | Yes | Pus | Negative | Negative |
| F | 10/F | Niece of 3 | 05/01/2022 | pupil | No | Yes | 41 | Yes | Pus | Negative | Negative |
| G | 3/M | Neighbors of 7 | 12/01/2022 | child | Dead duiker | Yes | 25 | ND | ND | Negative | Negative |
| H | 38/F | Sister of 7 | 12/01/2022 | farmer | Dead NHP/dead squirrel | Yes | 25 | Yes | ND | Negative | Negative |
| I | 34/F | NK | 15/01/2021 | NK | No | No | 22 | Yes | ND | Negative | Negative |
| J | 8 m/F | NK | 18/01/2022 | child | No | Yes | 15 | ND | ND | Negative | Negative |
| K | 20/F | NK | 20/01/2022 | farmer | No | No | 17 | Yes | ND | Negative | Negative |
| Individual | Age/Sex | Relation to primary case | Date of symptom onset | Occupation | Reported contact with animals | Reported contact with human cases | Date of samples | Blood sample | Pus sample | Monkeypox PCR results | Varicella-Zoster Virus PCR results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Contacts | |||||||||||
| C1 | 40/M | NK | NA | hunter/fisherman | No | Yes | 14/12/2021 | Yes | ND | Negative | Negative |
| C2 | 1/F | NK | NA | child | No | Yes | 22/12/2021 | Yes | ND | Negative | Negative |
| C3 | 25/M | NK | NA | farmer | No | Yes | 13/12/2021 | Yes | ND | Negative | Negative |
| C4 | 27/M | NK | NA | mine worker | No | Yes | 05/01/2022 | Yes | ND | Negative | Negative |
| C5 | 18/M | NK | NA | mine worker | No | Yes | 03/01/2022 | Yes | ND | Negative | Negative |
| C6 | 30/F | NK | NA | farmer | No | Yes | 13/12/2021 | Yes | ND | Negative | Negative |
| C7 | 20/M | NK | NA | mine worker | No | Yes | 03/01/2022 | Yes | ND | Negative | Negative |
| C8 | X/M | NK | NA | child | No | Yes | 05/01/2022 | Yes | ND | Negative | Negative |
| C9 | 26/M | NK | NA | mine worker | No | Yes | 03/10/2022 | Yes | ND | Negative | Negative |
| C10 | 23/M | NK | NA | mine worker | No | Yes | 22/12/2021 | Yes | ND | Negative | Negative |
| C11 | X/M | NK | NA | NK | NK | NK | 15/12/2021 | Yes | ND | Negative | Negative |
| C12 | X/M | NK | NA | NK | NK | NK | 15/12/2021 | Yes | ND | Negative | Negative |
| C13 | X/M | NK | NA | NK | NK | NK | 13/12/2021 | Yes | ND | Negative | Negative |
| C14 | X/F | NK | NA | NK | NK | NK | 13/12/2021 | Yes | ND | Negative | Negative |
| C15 | 41/F | NK | NA | farmer | NHP | Yes | 06/02/2022 | Yes | ND | Negative | Negative |
| C16 | 18/F | NK | NA | Housewife | No | Yes | 22/12/2021 | Yes | ND | Negative | Negative |
| C17 | 35/M | NK | NA | mine worker | No | Yes | 08/02/2022 | Yes | ND | Negative | Negative |
F: Female; M: Male; NK: Not known; ND: Not done; NA: Not applicable; NHP: Non-human primate.
The primary case was a 40-year-old hunter (case 0), who fell ill during a trip to the forest 100 km north of Bania, in Kette Olo (Fig. 1). He reported contact with a non-human primate (NHP) in the forest, which was followed by the appearance of typical cutaneous lesions, for which he received traditional care. His wife (case 1) and his brother (case 2) joined him in the forest to care for him. His wife fell ill on November 6 (case 1) and returned home, whereas his brother fell sick on November 7 (case 2) (Fig. 2, Fig. S1, Table 1). These three cases were housed in the homes of three different relatives on their return. Due to the severity of the disease, all three cases were transported to the healthcare center by their relatives. Two different lines of transmission and four waves of transmission were identified. One line of transmission began with the wife of the index case (case 1), who contaminated her sister (case 3) and her three daughters (cases 4, 5 and 7) who visited her in the hospital. The other began with the brother of the index case (case 2), who contaminated his wife (case 10), another brother (case 12), his sister-in-law (case 13) and two children of his family (cases 11 and 14) (Fig. 2, Fig. S1). The outbreak lasted 75 days, with a median (IQR) serial interval of 24 (12−32) days. The secondary attack rate throughout the family concession was 59.5% (25/42). The confirmed and suspected cases were exposed to diseased individuals through meal sharing (83.3%), hospital visits (70.8%), living in the same household (68.4%), and sexual contact (16.4%).
In addition to the initial exposure of the index case to a NHP, four of the other individuals investigated reported exposure to animals, including NHPs, duikers, and squirrels (dead or alive) (Table 1). These contacts may have been facilitated by participation in caterpillar collecting camps (47.6%) and the butchering of bushmeat (33.3%). The consumption of bushmeat (90.5%) or edible caterpillars (47.6%) during the preceding three weeks was reported by confirmed and suspected cases. The family considered the occurrence of disease to be linked to prior contact with freshwater fish with eruptive lesions during this specific seasonal period in which fish were the main source of protein, which led to a ban on fish consumption. The remoteness of the site at which the initial spillover is suspected to have occurred made it impossible to investigate the animal reservoir (Fig. 1).
3.2. Confirmed and suspected mpox cases characteristics
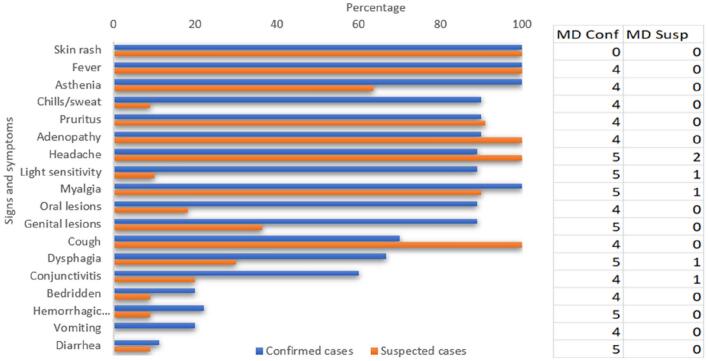
In total, 18 of the 25 confirmed and suspected cases (72%) were female. The median (IQR) age of the cases was 18.5 (5–27) years (range: 2–40 years). Most cases were children (52.2%) or farmers (37.8%) (Table 1, Table 2). Diagnosis was established from a blood sample for 21 ((84%) cases, a pus sample for four (16%). Two (10.0%) cases presented with a serious rash, six (30.0%) had a severe form, 11 (55.0%) had a moderate form, and 1 (5.0%) had a mild form (Table 2, Table S2). In total, 20 (95.2%) cases presented typical lymphadenopathy (Fig. 3, Fig. 4, Table S2) and three (15%) presented hemorrhagic lesions (Table S2). Twelve of the 19 cases for which close clinical follow-up data were available (63.2%) presented one or several clinical complications: eight cases of bronchopneumonia, two of severe dehydration, one corneal ulcer, two cutaneous superinfections, one abscess (Fig. 4) and six cutaneous sequelae (cheloid scars, or depigmentation) (Table S2). Only one death was reported, a young woman with no known underlying condition, resulting in a CFR of 4.0% (1/25). One HIV coinfection was reported, with dehydration as the only complication. Twelve coinfections with Plasmodium were also detected.
Table 2.
Epidemiological and clinical characteristics of confirmed cases, suspected cases and contacts from the outbreak investigation, Bania, CAR 2021–2022 (n = 42).
| Characteristics | Confirmed cases (n = 14) | Suspected cases (n = 11) | Contacts (n = 17) | P value |
|---|---|---|---|---|
| Sex | P= 0.02 | |||
| Female | 9 (64.3 %) | 9 (81.8 %) | 5 (29.4 %) | |
| Male | 5 (35.7 %) | 2 (18.2 %) | 12 (70.6 %) | |
| Missing data | 0 | 0 | 0 | |
| P=0.12 | ||||
| Age (years), median (IQR) | 22 (5-27) | 5 (2-20) | 25.5 (19-32.5) | |
| Age groups (years) | P=0.14 | |||
| 0-9 | 4 (28.6 %) | 7 (63.6 %) | 1 (8.3 %) | |
| Oct-19 | 2 (14.3 %) | 1 (9.1 %) | 2 (16.7 %) | |
| 20-29 | 6 (42.9 %) | 1 (9.1 %) | 5 (41.7 %) | |
| > 30 | 2 (14.3 %) | 2 (18.2 %) | 4 (33.3 %) | |
| Missing data | 0 | 0 | 5 | |
| Occupation | P=0.01 | |||
| Farmer | 6 (46.2 %) | 2 (20 %) | 3 (23.1 %) | |
| Child | 5 (38.5 %) | 7 (70 %) | 2 (15.4 %) | |
| Hunter | 0 (0 %) | 0 (0 %) | 1 (7.7 %) | |
| Mine worker | 0 (0 %) | 0 (0 %) | 6 (46.2 %) | |
| Merchant | 1 (7.7 %) | 0 (0 %) | 0 (0 %) | |
| Other | 1 (7.7 %) | 1 (10 %) | 1 (7.7 %) | |
| Missing data | 1 | 1 | 4 | |
| Sample types | P=0.19 | |||
| Pus sample | 1 (7.1%) | 3 (27.3 %) | 1 (5.9 %) | |
| Blood sample | 13 (92.9 %) | 8 (72.7 %) | 16 (94.1 %) | |
| Missing data | 0 | 0 | 0 | |
| Rash severity | ||||
| Mild (5-25 lesions) | 0 (0 %) | 1 (9.1 %) | P=0.09 | |
| Moderate (26–100 lesions) | 3 (33.3 %) | 8 (72.7 %) | ||
| Severe (101–250 lesions) | 4 (44.4 %) | 2 (18.2 %) | ||
| Serious (>250 lesions) | 2 (22.2 %) | 0 (0 %) | ||
| Missing data | 5 | 0 | ||
| Genital lesions | P=0.02 | |||
| Yes | 8 (88.9 %) | 4 (36.4 %) | ||
| No | 1 (11.1 %) | 7 (63.6 %) | ||
| Missing data | 5 | 0 | ||
| Oral lesions | P=0.01 | |||
| Yes | 8 (88.9 %) | 2 (18.2 %) | ||
| No | 1 (11.1 %) | 9 (81.8 %) | ||
| Missing data | 5 | 0 | ||
| Palm involvement | P=0.99 | |||
| Yes | 9 (90 %) | 10 (90.9 %) | ||
| No | 1 (10 %) | 1 (9.1%) | ||
| Missing data | 4 | 0 | ||
| P=0.99 | ||||
| Sole involvement | ||||
| Yes | 8 (88.9%) | 10 (90.9 %) | ||
| No | 1 (11.1%) | 1 (9.1 %) | ||
| Missing data | 5 | 0 | ||
Fig. 3.
Signs and symptoms in the confirmed and suspected cases of mpox, Bania, CAR 2021–2022 (MD missing data).
Fig. 4.
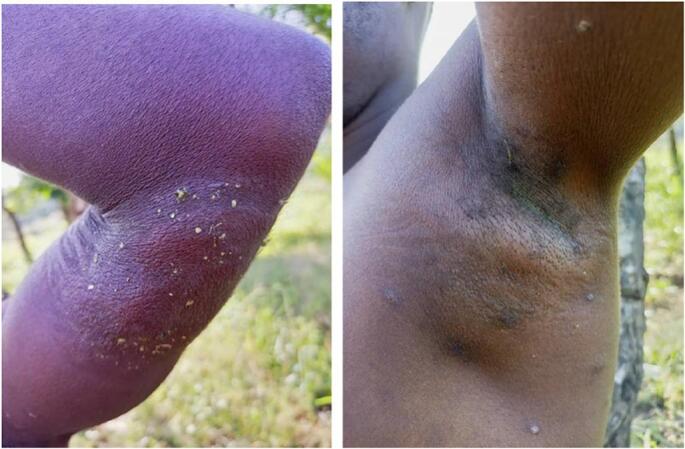
A. Cutaneous abscess in a confirmed case of mpox, Bania, CAR, 2021–2022.
B. Axillary adenopathy in a different confirmed case of mpox, Bania, CAR, 2021–2022.
Photo credits: Dr. Festus Mbrenga.
4. Discussion
This outbreak is the largest ever described in CAR in terms of the number of confirmed cases. In a recent report of 40 outbreaks since 2001 in this country [3], the median outbreak size was 3 (IQR: 1–5) cases. The size of this outbreak may be explained by the multiple (four) waves of inter-human transmission, exceeding the number of waves observed in previous outbreaks in CAR [21] and approaching the largest ever number of waves reported in a country in which mpox is endemic: seven, in DRC [14,15]. In this outbreak, individuals who subsequently became infected reported sharing meals with mpox cases, as previously highlighted in the DRC [37,38]. Visits to hospitalized mpox cases were also frequently reported, as elsewhere [14,27,39], leading to an unusually high SAR [2], at a value approached by only one study in DRC [15]. Sexual intercourse with cases was also reported in this study and may represent an underestimated mode of transmission in endemic countries, in light of the presence of genital lesions in the patients studied here and those studied in Nigeria [20,40], and the acknowledged role of sexual intercourse in the current worldwide epidemic [19,41]. This finding suggests that efforts should be made to increase the awareness of populations about the risks associated with direct contact with patients presenting skin rashes, and that isolation and control measures, particularly in hospitals, should be reinforced in CAR.
No pus samples were collected from eight of the 11 suspected cases, which may have led to an underestimation of the total number of confirmed mpox cases. This lack of appropriate samples may be due to a lack of sampling materials (swabs) and a lack of staff training in remote areas of CAR. Improvements of these aspects are required for future outbreak investigations, because monkeypox virus detection in pus and crust samples is more sensitive than detection in blood samples [36,42].
This outbreak began with the infection of a hunter in an area in which the edge of the forest meets the savannah. No cases had ever been reported in this area in the past. It is, therefore, difficult to determine whether this case reflects an extension of the area in which monkeypox virus circulates, better coverage by the national mpox surveillance system, or simply an anecdotal report. The further extension of the outbreak to a nearby small town (5000 inhabitants) is unusual for CAR and may reflect the potential for virus circulation in urbanized areas, as previously reported for mpox in Nigeria [5,43,44] and for Ebola in DRC [45,46], with the additional risk of local spread and international export. The only zoonotic contact mentioned by the primary case before the onset of symptoms was with a non-human primate. Certain tree squirrel species are considered to be the reservoir of mpox [47] and are suspected of involvement in transmissions to humans, but monkeys can accidentally become infected [12,48] and then infect humans. Interestingly, the local population attributed the source of infection to local fishes with a skin rash, possibly due to water pollution by the artisanal gold mining activities occurring during this season in this area. The persistence of uncertainty about the animal reservoir [44] highlights the need for zoological and anthropological studies to improve our understanding of the local epidemiology of disease.
This outbreak serves as a reminder of the severity of mpox infections in Central Africa, with complications in 65% of cases, and a case fatality rate of 4%. These findings are consistent with previous reports [2,3] and may partly reflect the greater severity of clade I infections than of infections with viruses from clades IIa [2,39,49] and 2b [36,41], the greater vulnerability of locally infected populations with a high proportion of children and immunosuppressed individuals [5], and the limited healthcare resources in these remote areas. The trend towards mpox disease causing larger epidemics in more connected parts of Central Africa is a matter of public health concern for this subregion and beyond. The use of a One Health approach to improve our understanding of the zoonosis will make it possible to provide tailored prevention messages.
Authors statement
CB, AF and EN designed the original research question. FM and EM lead the outbreak investigation. EM, CM, XK, BS and EG were responsible for laboratory analyses. JND and CGL were responsible for data entry. CB, LS and AF analysed and interpretated the epidemiological and clinical data. CB and AF wrote the first draft of article. JL realize the map. CVP, AG, JCM took part to the Afripox project. All authors contributed to data interpretation, revised and completed the manuscript and agreed to its final version.
Declaration of Competing Interest
No conflicts of interest to declare.
Acknowledgment
We would like to thank all the confirmed and suspected cases and contacts, and the healthcare workers who participated in the outbreak investigations.
We thank the Central African Ministry of Public Health and Population and the national World Health Organization team for their collaboration and constant support for the investigation of mpox outbreaks.
Financial support for this study was provided by the French Agence Nationale de Recherche (ANR 2019CE-35), the PTR (Projets Transversaux de Recherche PTR 218-19) fund from Institut Pasteur Paris, the INCEPTION project (PIA/ANR-16-CONV-005) and the SCOR Foundation for Science.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100523.
Appendix A. Supplementary data
Supplementary material
Data availability
The data that has been used is confidential.
References
- 1.Ladnyj I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 2.Bunge E.M., et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besombes C., Mbrenga F., Schaeffer L., Malaka C., Gonofio E., Landier J., et al. National monkeypox surveillance, Central African Republic, 2001–2021. Emerg. Infect. Dis. 2022 Dec doi: 10.3201/eid2812.220897. [date cited]. (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Formenty P., et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg. Infect. Dis. 2010;16:1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yinka-Ogunleye A., et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect. Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds M.G., et al. Spectrum of infection and risk factors for human Monkeypox, United States, 2003. Emerg. Infect. Dis. 2007;13:1332–1339. doi: 10.3201/eid1309.070175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ligon B.L. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin. Pediatr. Infect. Dis. 2004;15:280–287. doi: 10.1053/j.spid.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauldin M.R., et al. Exportation of Monkeypox virus from the African continent. J. Infect. Dis. 2022;225:1367–1376. doi: 10.1093/infdis/jiaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bragazzi N.L., et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J. Med. Virol. 2022;jmv.27931 doi: 10.1002/jmv.27931. [DOI] [PubMed] [Google Scholar]
- 10.https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- 11.Haddad N. The presumed receptivity and susceptibility to monkeypox of European animal species. Infect. Dis. Now. 2022;S2666991922001221 doi: 10.1016/j.idnow.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrono L.V., et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat. Microbiol. 2020;5:955–965. doi: 10.1038/s41564-020-0706-0. [DOI] [PubMed] [Google Scholar]
- 13.Kantele A., Chickering K., Vapalahti O., Rimoin A.W. Emerging diseases-the monkeypox epidemic in the Democratic Republic of the Congo. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016;22:658–659. doi: 10.1016/j.cmi.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Learned L.A., et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 2005;73:428–434. [PubMed] [Google Scholar]
- 15.Nolen L.D., et al. Extended human-to-human transmission during a Monkeypox outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 2016;22:1014–1021. doi: 10.3201/eid2206.150579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkinson B., et al. Infection-competent monkeypox virus contamination identified in domestic settings following an imported case of monkeypox into the UK. medRxiv. 2022;2022.06.27.22276202 doi: 10.1101/2022.06.27.22276202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vivancos R., et al. Community transmission of monkeypox in the United Kingdom, April to may 2022. Eurosurveillance. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sah R., et al. First Monkeypox deaths outside Africa: no room for complacency. Ther. Adv. Infect. Dis. 2022;9 doi: 10.1177/20499361221124027. 204993612211240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antinori A., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, may 2022. Eurosurveillance. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogoina D., et al. The 2017 human monkeypox outbreak in Nigeria—report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besombes C., et al. Intrafamily transmission of Monkeypox virus, Central African Republic, 2018. Emerg. Infect. Dis. 2019;25:1602–1604. doi: 10.3201/eid2508.190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthet N., et al. Maculopapular lesions in the Central African Republic. Lancet. 2011;378:1354. doi: 10.1016/S0140-6736(11)61142-2. [DOI] [PubMed] [Google Scholar]
- 23.Kalthan E., et al. Epidémie de 12 cas de maladie à virus monkeypox dans le district de Bangassou en République Centrafricaine en décembre 2015. Bull. Soc. Pathol. Exot. 2016;109:358–363. doi: 10.1007/s13149-016-0516-z. [DOI] [PubMed] [Google Scholar]
- 24.Kalthan E., et al. Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Méd. Mal. Infect. 2018;48:263–268. doi: 10.1016/j.medmal.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laudisoit A. 2016. Monkeypox Outbreak Investigation Aketi. [Google Scholar]
- 26.Froeschl G., Kayembe P.K. Pox-like lesions and haemorrhagic fever in two concurrent cases in the Central African Republic: case investigation and management in difficult circumstances. Pan Afr. Med. J. 2015;22 doi: 10.11604/pamj.2015.22.23.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakoune E., et al. A nosocomial outbreak of human Monkeypox in the Central African Republic. Open Forum Infect. Dis. 2017;4:ofx168. doi: 10.1093/ofid/ofx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.https://ncdc.gov.ng/diseases/info/M
- 29.Doshi R.H., et al. Monkeypox rash severity and animal exposures in the Democratic Republic of the Congo. EcoHealth. 2020;17:64–73. doi: 10.1007/s10393-019-01459-7. [DOI] [PubMed] [Google Scholar]
- 30.Jezek Z., Grab B., Szczeniowski M.V., Paluku K.M., Mutombo M. Human monkeypox: secondary attack rates. Bull. World Health Organ. 1988;66:465–470. [PMC free article] [PubMed] [Google Scholar]
- 31.Nigerian Centre for Disease Control Interim National Guidelines for Monkeypox Outbreak Response. 2017. https://ncdc.gov.ng/diseases/info/Mhttps://www.ncdc.gov.ng/themes/common/docs/protocols/50_1508912430.pdf (2017)
- 32.Durski K.N., et al. Emergence of Monkeypox — west and Central Africa, 1970–2017. MMWR Morb. Mortal. Wkly Rep. 2018;67:306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berthet N., et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci. Rep. 2021;11:13085. doi: 10.1038/s41598-021-92315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of monkeypox virus west African and Congo Basin strain DNA. J. Virol. Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adler H., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 2022;S1473309922002286 doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quiner C.A., et al. Presumptive risk factors for monkeypox in rural communities in the Democratic Republic of the Congo. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamfum J.-J.M., et al. Introduction of Monkeypox into a community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015;93:410–415. doi: 10.4269/ajtmh.15-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogoina D., Yinka-Ogunleye A. Sexual history of human monkeypox patients seen at a tertiary hospital in Bayelsa, Nigeria. Int. J. STD AIDS. 2022;33:928–932. doi: 10.1177/09564624221119335. [DOI] [PubMed] [Google Scholar]
- 41.Girometti N., et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health Centre in London, UK: an observational analysis. Lancet Infect. Dis. 2022;S147330992200411X doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen P.-Y., Ajisegiri W.S., Costantino V., Chughtai A.A., MacIntyre C.R. Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017–2020. Emerg. Infect. Dis. 2021;27 doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haider N., et al. Increased outbreaks of monkeypox highlight gaps in actual disease burden in sub-Saharan Africa and in animal reservoirs. Int. J. Infect. Dis. 2022;122:107–111. doi: 10.1016/j.ijid.2022.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pigott D.M., et al. Mapping the zoonotic niche of Ebola virus disease in Africa. eLife. 2014;3 doi: 10.7554/eLife.04395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malvy D., McElroy A.K., de Clerck H., Günther S., van Griensven J. Ebola virus disease. Lancet. 2019;393:936–948. doi: 10.1016/S0140-6736(18)33132-5. [DOI] [PubMed] [Google Scholar]
- 47.Khodakevich L., et al. The role of squirrels in sustaining monkeypox virus transmission. Trop. Geogr. Med. 1987;39:115–122. [PubMed] [Google Scholar]
- 48.Radonić A., et al. Fatal Monkeypox in wild-living sooty Mangabey, Côte d'Ivoire, 2012. Emerg. Infect. Dis. 2014;20:1009–1011. doi: 10.3201/eid2006.131329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Likos A.M. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data that has been used is confidential.