ABSTRACT
In response to microbiota colonization, the intestinal epithelia of many animals exhibit increased rates of cell proliferation. We used gnotobiotic larval zebrafish to identify a secreted factor from the mutualist Aeromonas veronii that is sufficient to promote intestinal epithelial cell proliferation. This secreted A. veronii protein is a homologue of the Vibrio cholerae GlcNAc binding protein GbpA, which was identified as a chitin-binding colonization factor in mice. GbpA was subsequently shown to be a lytic polysaccharide monooxygenase (LPMO) that can degrade recalcitrant chitin. Our phenotypic characterization of gbpA deficient A. veronii found no alterations in these cells’ biogeography in the zebrafish intestine and only a modest competitive disadvantage in chitin-binding and colonization fitness when competed against the wild-type strain. These results argue against the model of GbpA being a secreted adhesin that binds simultaneously to bacterial cells and GlcNAc, and instead suggests that GbpA is part of a bacterial GlcNAc utilization program. We show that the host proliferative response to GbpA occurs in the absence of bacteria upon exposure of germ-free zebrafish to preparations of native GbpA secreted from either A. veronii or V. cholerae or recombinant A. veronii GbpA. Furthermore, domain 1 of A. veronii GbpA, containing the predicted LPMO activity, is sufficient to stimulate intestinal epithelial proliferation. We propose that intestinal epithelial tissues upregulate their rates of renewal in response to secreted bacterial GbpA proteins as an adaptive strategy for coexisting with bacteria that can degrade glycan constituents of the protective intestinal lining.
KEYWORDS: Aeromonas, Vibrio, N-acetylglucosamine, GlcNAc binding protein, GbpA, intestinal epithelial proliferation, microbiota, gnotobiotic, larval, zebrafish
Introduction
Host-associated microbes, collectively called the microbiota, are critical for the development and physiological function of their host animals1,2. This complex assemblage of microorganisms contributes to host health in ways that range from stimulating host metabolism to promoting immune system maturation3. One way in which microbial communities influence host health is by stimulating cell proliferation of the mucosal epithelia on which they reside. This impact of the microbiota is apparent when comparing epithelial cell proliferation rates of animals raised in the presence (conventionally reared, CV) or absence (germ free, GF) of microbes. For example, GF mice have reduced rates of skin epithelial cell renewal4. The animal digestive tract typically houses the most abundant microbial population in the body and correspondingly the intestinal epithelium shows marked increases in epithelial cell proliferation in CV relative to GF animals, as has been reported in young and adult mice5,6, larval zebrafish7–9, and larval and adult fruit flies10,11. However, the mechanisms underlying microbiota-induced intestinal epithelial cell proliferation are incompletely understood12.
Previously, we showed that Aeromonas veronii, a common member of the zebrafish intestinal microbiota13, secretes an unknown factor(s) that is sufficient to promote epithelial proliferation in the developing intestine of GF zebrafish8. The gnotobiotic zebrafish model offers the ability to manipulate the presence14 and genetics15 of resident microbes in the larval zebrafish, which, combined with the optical transparency and sophisticated genetic tools of the zebrafish model, make it a powerful system to identify bacterial factors that influence aspects of animal tissue development and homeostasis13,16. Here, we use gnotobiotic zebrafish to identify a secreted Aeromonas factor that stimulates intestinal epithelial proliferation, which we show is a homologue of the Vibrio cholerae N-acetylglucosamine-binding protein A (GbpA)17.
V. cholerae GbpA was discovered in a screen for bacterial mutants with impaired adhesion to cultured intestinal epithelial cells17 and gbpA-deficient V. cholerae was also shown to be defective for binding to chitin-rich zooplankton and chitin-coated beads. Chitin is a complex polymer composed of β-(1→4)-linked N-acetylglucosamine (GlcNAc) monomers. GlcNAc is also a major O-linked glycan component of intestinal mucins, providing a biochemical basis for the parallel binding of V. cholerae to chitin- and mucin-rich surfaces. In a neonatal mouse model of infection, ΔgbpA V. cholerae were recovered from the intestines at lower levels and correspondingly caused less pathology17,18. The defective colonization of ΔgbpA V. cholerae was interpreted to be a consequence of its defective adhesion to intestinal epithelia, although such an adhesion defect was not demonstrated during intestinal infection. Unexpectedly for an adhesin, the GbpA protein was found primarily in the culture supernatant rather than associated with the cell surface17.
Structural and biochemical analysis of GbpA revealed it to consist of four domains: two chitin-binding domains (domains 1 and 4) and a middle region that can bind to Vibrio cells (domains 2 and 3)19. Domain 4 resembles a chitin binding domain from Serratia marcescens chitinase B, whereas domain 1 shares homology with the AA10 family of chitin-degrading lytic polysaccharide monooxygenases (LPMOs)20 and was subsequently shown to be a functional LPMO21. The importance of this enzymatic activity of GbpA for V. cholerae colonization or pathogenesis has not been explored. Here, we demonstrate that full-length GbpA and its LPMO-containing domain 1 induce intestinal epithelial cell proliferation independently of bacterial colonization or adhesion. These findings contribute to a growing appreciation for roles of LPMOs in microbial-host interactions22.
Materials and methods
Animals
All experiments with zebrafish were performed using protocols approved by the University of Oregon Institutional Animal Care and Use Committee and following standard protocols23. Zebrafish husbandry, veterinary care, and equipment used to generate zebrafish for this study were provided by Aquatic Animal Care Services at the University of Oregon, Eugene, OR. Experiments were conducted in the University of Oregon Zebrafish Facility or in the Guillemin laboratory. Embryonic and larval zebrafish were maintained in tissue culture flasks or petri dishes in Embryo Medium, a 4 parts per thousand salt solution made by mixing 5.25 grams Instant Ocean per 1 liter of dechlorinated water. Fish at post-larval stages were maintained in tanks in the University of Oregon Zebrafish Facility on system water. Facility system water quality parameter ranges are 650 to 950 microsiemens/cm2 conductivity, 7.2 to 7.8 pH, 0 ppm ammonia, 0 ppm nitrites, 5 to 30 ppm nitrates, 30 to 100 ppm alkalinity. Water quality conductivity, pH, and temperature are continuously monitored by programmable logic controllers (PLCs) attached to aquaculture tank probes. Water quality tests for ammonia, nitrites, nitrates are performed using a colorimetric kit (Freshwater Master Test Kit, Aquarium Pharmaceuticals, Inc., Chalfont, PA). Alkalinity tests are performed using a freshwater alkalinity colorimeter (Model HI775 Freshwater Alkalinity Colorimeter, Hanna Instruments, Smithfield, RI). Water quality adjustments for conductivity and pH are made by PLC-controlled dosing pumps dispensing salt solution (Instant Ocean, Spectrum Brands, Blacksburg, VA) in the case of conductivity and basic solution (ProLine Sodium Bicarbonate, Pentair Aquatic Eco-Systems, Apopka, FL) in the case of pH. Water quality temperature is primarily provided by building HVAC room air handlers and supplemented by immersed heaters located in aquaculture tanks. Municipal water filtered through reverse osmosis membranes is pumped into aquaculture tanks to replace water lost from automatic particle filter washes, spills, and evaporation. WT (Ab/Tu) zebrafish were reared at 28°C. GF embryos were derived by surface sterilization of the chorions and maintained as previously described24. Experiments were performed on larvae ranging from 4 dpf (~3.7 mm body length) to 8 dpf (~4.7 mm body length), as specified. No exogenous food was provided to the larvae during the duration of the experiments. CV controls were clutch mates of the GF derived embryos that were not subjected to surface sterilization and were reared in parallel.
Experimental bacterial strains
Aeromonas veronii strain HM21 was originally isolated by Joerg Graf from the medical leech and has been extensively characterized, including a complete genome sequence25. To create the A. veronii ΔgbpA strain, a vector containing a kanamycin resistance cassette was transformed into SM10 E. coli. Conjugation between wild-type A. veronii HM21RS and the vector carrying SM10 E. coli strain was carried out, allowing the kanamycin resistance gene to replace the gbpA locus in A. veronii via allelic exchange. Candidate gbpA deletion strains were selected for loss of the plasmid and maintenance of kanamycin resistance. Insertion of the kanamycin cassette into the gbpA locus was verified in these candidates by PCR. Fluorescently marked derivatives of these strains were engineered with an established Tn7 transposon-based approach26. Briefly, a cassette containing the constitutively active synthetic promoter Ptac cloned upstream of genes encoding dTomato or superfolder GFP was chromosomally inserted at the attTn7 locus to generate A. veronii attTn7::Ptac-sfGFP and A. veronii ΔgbpA attTn7::Ptac-dTomato. Joerg Graf provided the A. veronii Δt2ss mutant and isogenic complementation strain A. veronii Δt2ss+T2SS 27,28. Ron Taylor provided V. cholerae classical O1 isolate CG842 and isogenic mutant V. cholerae ΔgbpA and complementation strain V. cholerae ΔgbpA+pGbpA 17.
To assay growth on minimal medium supplemented with 0.4% colloidal chitin or GlcNAc, WT and ∆gbpA Aeromonas were normalized to an OD600 of 0.005 and incubated with shaking for 24 hr at 30°C. The size and density of colloidal chitin scatters the incoming light used to monitor growth kinetics in a plate-based growth assay, leading to inconsistent readings. Thus, to determine the impact of GbpA on Aeromonas growth on colloidal chitin, each strain was grown in culture tubes and bacterial growth samples aliquoted after allowing the colloidal chitin to settle from solution. This method produced consistent and reliable measurements of bacterial growth.
GbpA expression constructs and protein purification
The UniProt ID for GbpA from A. veronii strain HM21 is: A0A7Z3TUS8 and the UniProt ID for GbpA from V. cholerae strain ATCC 39315 is: Q9KLD5. To generate a plasmid for the expression of unmodified A. veronii GbpA, PCR product of the gbpA ORF inclusive of the stop codon was generated by using the primers CBPf (gcatcatatggcagcaaaaatccatc)/CBPr1 (gcatctcgagtcacttcagctcaatccaggctt). The PCR product was cloned into the NdeI and XhoI sites of plasmid pET21b (Novagen). To generate a plasmid for the expression of a cleavable GST tagged GbpA, PCR product of the gbpA ORF lacking the secretion signal and inclusive of the stop codon were generated by using the primers GSTCBPf (gcatgaattccacggctacatcagccagccc)/GSTCBPr (gcatctcgagtcatcacttcagctcaatccagg) and cloned into the EcoR1 and Xho1 sites of pGEX6p1. The plasmids were then transformed into E. coli BL21 (DE3) RIL-CodonPlus cells (Stratagene).
Protein purification was achieved using a glutathione Sepharose 4B column (GE Healthcare) following the recommended protocol. Glutathione S-transferase (GST) cleavage was achieved after elution of GbpA from the column by adding 1 unit of PreScission protease enzyme and incubation overnight at 4°C per the instructions of the manufacturer (GE Healthcare).
CFS preparation
Cultures of A. veronii strain HM2128 in tryptic soy broth (TSB) and V. cholerae 17 in Luria broth (LB) with 0.02% arabinose, pH 6.5, were grown at 30°C for 17 h on a rotary shaker at 170 rpm. Overnight cultures of E. coli BL21 (DE3) were grown at 37°C in LB supplemented with 100 μg/ml ampicillin for plasmid maintenance, diluted 1:50 into 50 ml fresh LB/ampicillin, and grown at 37°C until OD600 reached ~0.5. IPTG was then added to a final concentration of 1 mM to induce expression of GbpA. The culture was grown with IPTG for 2–3 hours at 30°C. This resulted in a CFS dominated by GbpA, as confirmed via sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie Brilliant Blue staining, which revealed a dark band of the expected size for GbpA. This band was absent from BL21 CFS carrying an empty pET-21b vector.
Cultures prepared as above were spun at 5,600 × g for 10 min at 4°C to pellet cells, and the supernatant was passed through a 0.22-μM filter (Corning) on ice. CFS was concentrated through an Amicon Ultra-15 spin concentrator to remove small products, which were toxic to the zebrafish larvae. Protein concentration was determined by Bradford assay. All CFS exposures were performed using ~500 ng/mL total protein.
Chitin binding of Aeromonas cells and GbpA protein
To assay Aeromonas binding to chitin, magnetic chitin resin (NEB #E8036S) was prepared by washing three times in PBS. Input bacterial suspensions of WT A. veronii or A. veronii ΔgbpA dTomato (109 colony forming units (CFU)/mL) were applied to the resin and incubated for 30 min or 1 hr at 30°C with gentle rotation. The resin was washed three times with PBS to remove unbound bacteria and finally resuspended in PBS supplemented with 0.4% GlcNAc (Chem-Impex #01427) to release bacteria attached to the resin. Samples were plated on tryptic soy agar (TSA) to calculate the output CFUs and the percent of bacteria attached calculated ([CFUsoutput/CFUsinput]*100). The competitive binding assay was conducted similarly except equal amounts of WT A. veronii and A. veronii ΔgbpA dTomato were mixed prior to chitin resin exposure and the CFUs were determined for each strain by visualizing the dTomato expressing colonies using a fluorescent microscope.
To assay GbpA protein binding to chitin, cell-free supernatants (CFS) were collected from E. coli BL21 (DE3) or V. cholera following induction of gbpA expression as outlined above in CFS preparation with E. coli carrying the empty vector (pET-21b) serving as a control. Chitin binding was assayed as described previously17. Briefly, normalized CFS was applied to chitin resin and incubated for 1 hr and the flow through (FT) collected. The resin was washed 5× in PBS, resuspended in 2× protein loading buffer, and boiled for 5 minutes. Proteins in the FT and C fractions were separated via SDS-PAGE and visualized using Coomassie Brilliant Blue.
Ammonium sulfate fractionation
Ammonium sulfate fractionation was performed on un-concentrated, sterile CFS from 50 mL overnight cultures by slowly adding 100% ammonium sulfate until desired concentration was achieved. These solutions were prepared at 4°C. Precipitated proteins were collected from the 30–40%, 40–50%, 50–60% and 60–70% ammonium sulfate fractions. Precipitated proteins were collected from each fraction by centrifugation at 4°C and 14,000 g for 15 min. The proteins were resuspended in cold embryo medium (EM) and dialyzed for 2–3 hr at 4°C before adding them to 6 days post fertilization (dpf) GF larvae at a final concentration of 500 ng/mL. Pro-proliferative activity was observed in the 50–60% fraction. Hemolysis was assessed by spotting the fractions on blood agar plates.
Labeling and quantification of proliferating cells
7 dpf larvae were immersed overnight in 100 μg/mL 5-ethynyl-2′-deoxyuridine (EdU) solution (A10044; Invitrogen) for 16 h before termination of the experiment at 8 dpf. Larvae were fixed in 4% paraformaldehyde for 24 hr at 4°C, processed for paraffin embedding, and cut into 7-μm sections. For EdU detection, slides were processed according to the Click-iT EdU Cell Proliferation Assay Kit (C35002; Molecular Probes). Samples were imaged on a Nikon Eclipse TE 2000-V inverted microscope equipped with a Photometrics Coolsnap camera. EdU-labeled nuclei within the intestinal epithelium were counted over 30 serial 7-μm sections beginning at the esophageal-intestinal junction and proceeding caudally into the bulb. Analysis of this extended region was necessary because of the stochastic patterns of cell proliferation. The absolute numbers of labeled cells varied between trials. Despite these differences in the absolute numbers of labeled cells, the proportional trends of proliferating cells between treatments were consistent and reproducible between trials.
Colonization assay
Bacteria were added to GF flasks at 4 dpf at a final concentration of 106 CFUs/mL and incubated with the larvae for 48 hr at 28°C. Larvae were sacrificed at 6 dpf, immediately before the gut was removed, following our standard dissection protocol29, and homogenized in a small sample of sterile EM. Dilutions of this gut slurry were plated onto tryptic soy agar and allowed to incubate overnight at 30°C. Colonies from each gut were quantified. A minimum of 10 guts per mono-association or di-association were analyzed.
Light sheet microscopy
Gnotobiotic larval zebrafish were prepared for light sheet imaging as described by Jemielita et al30. Briefly, larvae are anesthetized in dishes filled with sterile EM and tricaine methanesulfonate (MS-222) at 120 μg/ml. They were then moved to melted agarose gel and pulled into glass capillaries where the gel was allowed to cool. The capillaries were mounted on a sample holder and a plug of gel containing each live larval zebrafish was extruded into a sample chamber filled with sterile EM and MS-222. Fluid in the sample chamber was maintained at 28°C.
Light sheet fluorescence microscopy was carried out using a home-built light sheet microscope based on the design of Keller et al31. Two coherent sapphire lasers (488 nm and 561 nm) were rapidly scanned using a galvanometer to create a thin sheet at the focus of an imaging objective. This thin sheet was used to excite fluorescence in specimens. The excitation light was captured as the sample was moved through the sheet, creating a three-dimensional image. To image the entire larval zebrafish gut, four sub-regions were imaged and subsequently registered. A complete image of the gut can be captured in two minutes in two colors with single-micron spacing between planes. All exposure times were 30 ms and laser power in both colors was set to 5 mW.
Statistical analysis
Statistical analysis was performed in Prism 9. For comparison of two treatment groups, t-tests were performed. For comparisons across multiple treatment groups, one- and two-way ANOVA analyses were performed as appropriate.
Results
A. veronii requires the Type II Secretion System for production of a secreted factor that induces intestinal epithelial proliferation
Earlier work from our group demonstrated that A. veronii strain HM21 produced an unknown secreted factor(s) that was sufficient to promote cell proliferation in 8 dpf GF larvae, as measured by the number of cells labeled during a 16 hour period of exposure to the nucleotide analog EdU within a defined 210 μm region of the anterior intestine immediately caudal to the esophageal-intestinal junction7 (Figure 1(a,b)). This factor(s) was present in cell-free supernatant (CFS) from A. veronii HM21 grown overnight in TSB and fractionated through a spin column to remove small molecular weight material, suggesting the factor(s) was unlikely to be a metabolite and could be a secreted protein7. Many Gram-negative bacteria employ the Type II Secretion System (T2SS) to secrete biologically active proteins into the extracellular environment. To test whether the pro-proliferative factor(s) were substrates of the T2SS, we added CFS from an A. veronii strain lacking a functional T2SS (∆t2ss)27 or the complement of this strain with restored T2SS function (∆t2ss + t2ss)27 to the aquatic environment of 6 dpf GF larval zebrafish and assayed cell proliferation at 8 dpf. We observed that while the CFS from A. veronii with a functional T2SS promoted cell proliferation in GF fish similarly to fish with a conventional microbiota, the CFS from the ∆t2ss strain was unable to promote cell proliferation above GF levels (Figure 1c). This observation suggests that the pro-proliferative factor(s) is a protein secreted by the T2SS.
Figure 1.
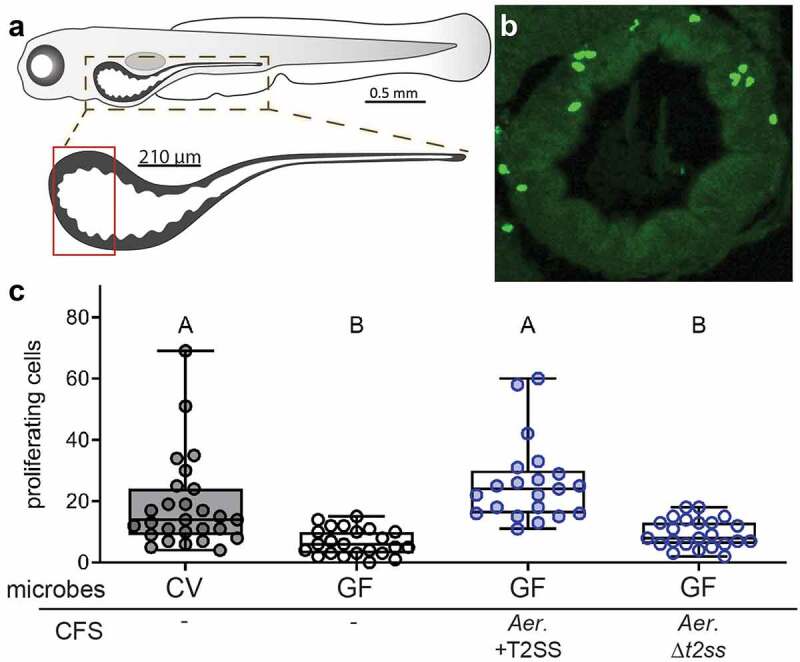
The T2SS of A. veronii is required for secretion of a pro-proliferative factor that stimulates intestinal epithelial cell proliferation. (a) Schematic of the larval zebrafish intestine, highlighting the proximal 210 μm region in which proliferative epithelial cells were quantified, as marked by incorporation of the nucleotide analog EdU. (b) Representative transverse section of the proximal zebrafish intestine stained to reveal cells that incorporated EdU.(c) Quantification of proximal intestinal epithelial cell proliferation in 8 dpf CV larvae and 8 dpf GF larvae untreated or exposed from 6 dpf to CFS from A. veronii with a functional or deleted T2SS. Boxplot whiskers represent range. Groups with different letter designations are statistically different with a p value of < 0.05 whereas groups with the same letter are not significantly different.
The A. veronii pro-proliferative factor is encoded by gbpA
To identify candidate pro-proliferative proteins secreted by A. veronii, we analyzed a mass spectrometry dataset we had generated of the abundant proteins in the CFS from the ∆t2ss and the complementation strains16, focusing on proteins present in the complementation strain and absent in the deletion strain. To further narrow the number of candidate proteins, we fractionated the CFS using ammonium sulfate precipitation, tested these fractions for pro-proliferative activity, and analyzed their composition on Coomassie stained protein gels. We found that the pro-proliferative activity was concentrated in a fraction that appeared to contain a single dominant protein species of approximately 55 kd (Figure 2a). Only two of our candidate proteins identified by mass spectrometry were close to this molecular weight: a hemolysin and a homologue of the V. cholerae GbpA secreted protein17. We determined that hemolytic activity, assayed on blood agar plates, was concentrated in a fraction lacking pro-proliferative activity (Figure 2a). We therefore turned our attention to GbpA.
Figure 2.
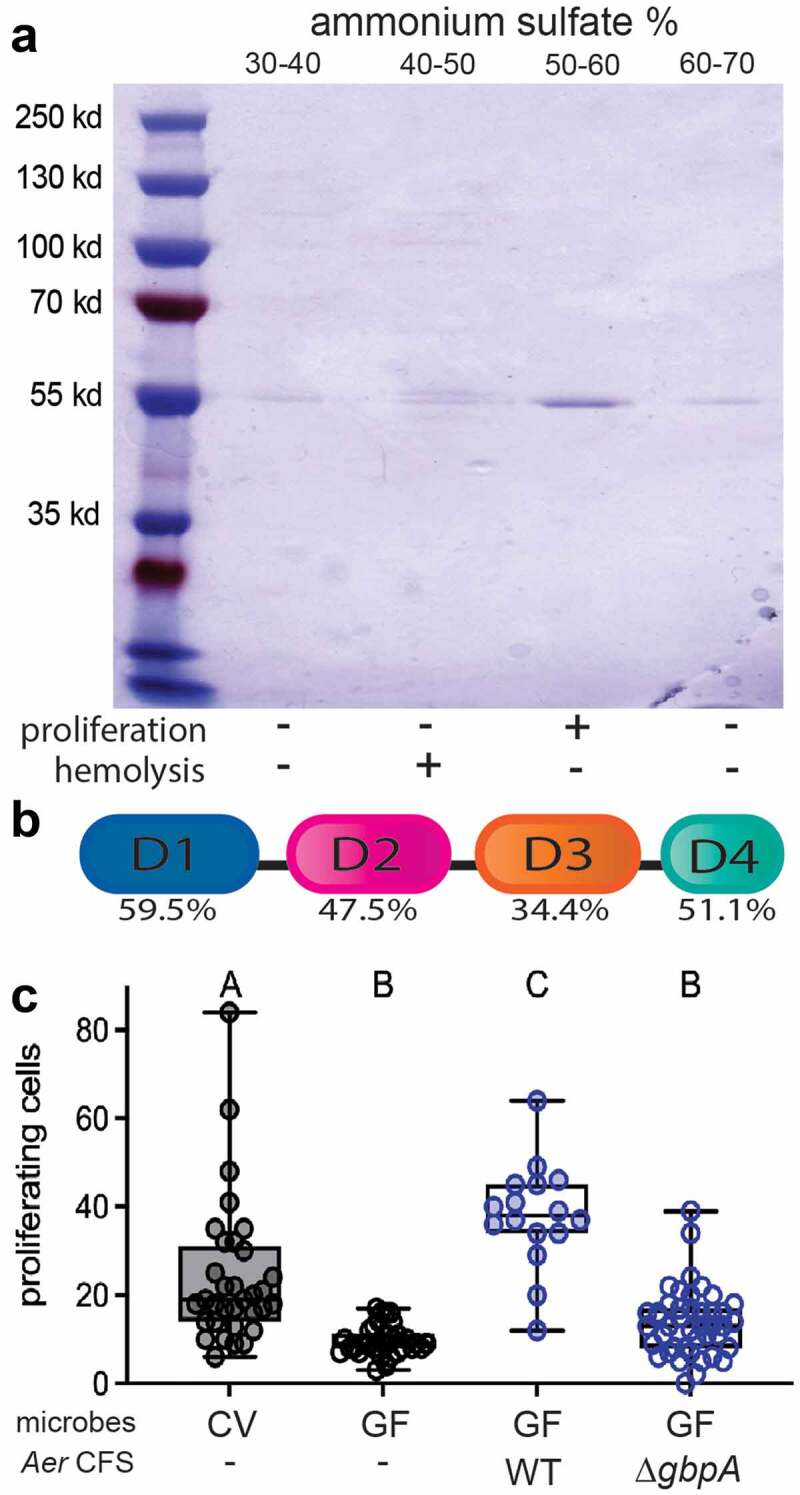
A. veronii gbpA encodes the secreted pro-proliferative factor that stimulates intestinal epithelial cell proliferation. (a0 The major protein constituents of ammonium sulfate fractions of A. veronii CFS separated by SDS-PAGE and visualized with Coomassie Brilliant Blue, with the corresponding proliferation and hemolysis activity of each fraction indicated below. (b) Schematic of the shared domain architecture of A. veronii and V. cholerae GbpA proteins, with the amino acid identity indicated for each of the four protein domains. (c) Quantification of proximal intestinal epithelial cell proliferation in 8 dpf CV larvae and 8 dpf GF larvae untreated or exposed from 6 dpf to CFS from WT or ∆gbpA A. veronii. Boxplot whiskers represent range. Groups with different letter designations are statistically different with a p value of < 0.05 whereas groups with the same letter are not significantly different.
GbpA from A. veronii has a similar predicted domain architecture to V. cholerae GbpA, with low identity putative cell surface-binding domains [domain 2 (aa 201–300) and 3 (aa 309–403)] sandwiched between higher identity N-terminal LPMO domain [domain 1 (aa 25–188)] and C-terminal carbohydrate-binding module (CBM) domain [domain 4 (aa 427–468)] (Figure 2b). The LPMO and CBM domains share 60% and 51% sequence similarities, respectively, while domains 2 and 3 are 48% and 34% similar. The Aeromonas LPMO domain contains two conserved copper-coordinating histidine residues that have been shown to be necessary for the oxidation reaction of LPMOs.
To test whether GbpA was necessary for the pro-proliferative activity in the CFS from A. veronii, we generated an isogenic strain of HM21 A. veronii in which the gbpA open reading frame was replaced by a kanamycin resistance cassette (gbpA:kan, ∆gbpA). CFS was collected from the WT and ∆gbpA strains and added to the aquatic environment of 6 dpf GF larvae. Whereas the WT CFS elicited a robust proliferative response, the CFS from the ∆gbpA strain failed to induce proliferation above the level observed in GF larvae (Figure 2c), demonstrating that gbpA is required for the proliferative response elicited by A. veronii CFS.
Colonization of the zebrafish intestine by Aeromonas or Vibrio does not require gbpA
The V. cholerae ∆gbpA strain was previously found to exhibit reduced binding to chitin beads following 30 minutes of incubation, as assayed by immunofluorescent microscopy, and reported as number of bacterial cells per bead17. We performed a similar chitin bead-binding assay with both WT and ∆gbpA A. veronii strains, assessing the fraction of bacteria recovered from chitin beads after both 30 minutes and 1 hour of incubation, using dilution plating. Only about 5% of the population of WT A. veronii bound chitin bead at 30 minutes, and this fraction reduced to about 3% by 1 hour (Figure 3a). The ∆gbpA population exhibited a lower fraction of chitin bead binding, approximately 0.8%, at both time points (Figure 3a). GbpA was suggested to confer binding of V. cholerae cells by a mechanism whereby the protein is first secreted into the extracellular environment, unassociated with the bacterial cell surface, and then subsequently binds to both the bacterial cell and GlcNAc through different protein domains19,21. A prediction of this model is that co-incubation of WT and ∆gbpA bacteria should rescue chitin-binding defects associated with the ∆gbpA mutant, since the predominant form of GbpA from the WT strain is in solution in the culture medium and equivalently accessible to WT or ∆gbpA cells. Arguing against this mechanism, we found no change in the percentage of the ∆gbpA population that is bound to chitin beads when co-incubated with WT cells (Figure 3a). This failure of the WT cells to complement the ∆gbpA cells’ chitin-binding defect was also reflected in the competitive indices, calculated as the ratio of the ∆gbpA to WT cells recovered from the chitin beads in the mixed incubations, normalized to the ratio of the two strains added initially (Figure 3b).
Figure 3.
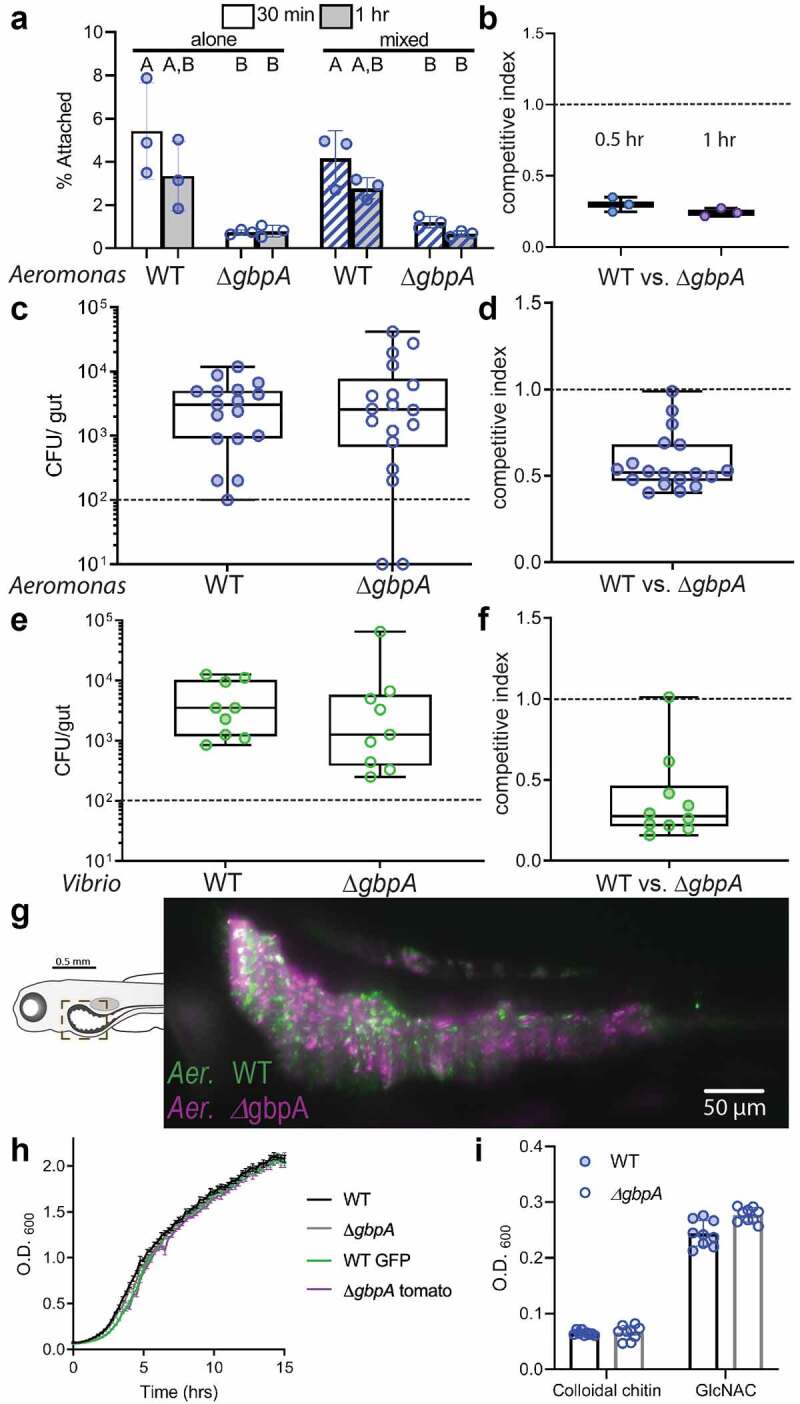
Colonization of the zebrafish intestine by A. veronii and V. cholerae does not require gbpA. (a) Binding of WT and ∆gbpa A. veronii to chitin beads, quantified as the percent of total bacteria, after either 0.5 hr (white bars) or 1 hour (gray bars) of incubation. Bacterial strains were added to chitin beads alone (solid bars) or mixed with the other strain (striped bars). Boxplot whiskers represent range. Groups with different letter designations are statistically different with a p value of < 0.05 whereas groups with the same letter are not significantly different. (b) Competitive index of ∆gbpa versus WT A. veronii recovered from chitin beads. (c) A. veronii CFUs recovered at 6 dpf following inoculation of GF zebrafish with individual strains at 4 dpf. (d) Competitive index of ∆gbpa versus WT A. veronii recovered at 6 dpf following co-inoculation of GF zebrafish with the two strains at 4 dpf. (e) V. cholerae CFUs recovered at 6 dpf following inoculation of GF zebrafish with individual strains at 4 dpf. (f) Competitive index of ∆gbpa versus WT V. cholerae strains recovered at 6 dpf fish following co-inoculation of GF zebrafish with the two strains at 4 dpf. (g) Light sheet micrograph of zebrafish intestine colonized with WT (green) and ∆gbpa (purple) A. veronii in the proximal intestinal region indicated in the schematic. (h) Growth curves measuring OD600 for each A. veronii strain grown in TSB. (i) Final OD600 measurement for WT and ∆gbpa strains grown on colloidal chitin and GlcNAc.
In human pathogenic V. cholerae strains of both the classic and El Tor biotypes, gbpA is required for colonization of the neonatal mouse intestine17,18. To test whether gbpA was required for A. veronii colonization of the zebrafish intestine, we inoculated the aquatic environment of GF zebrafish on 4dpf, and on 6dpf we assessed the bacterial colony forming units (CFU) per intestine. We observed that after 48 hours of inoculation, both WT and ∆gbpA strains colonized 6 dpf larval intestines to similar levels, demonstrating that gbpA does not play a crucial role in zebrafish gut colonization (Figure 3c).
Certain colonization factors are required only under circumstances of bacterial competition. To test whether A. veronii gbpA was required for competitive colonization of the zebrafish intestinal, we co-inoculated WT and ∆gbpA strains at equal concentrations to the aquatic environment of GF zebrafish at 4dpf and measured the number of colonizing bacteria of each strain at 6dpf. We calculated the competitive index as the ratio of ∆gbpA to WT strains recovered at 6 dpf normalized to the ratio inoculated at 4 dpf. We observed a modest competitive disadvantage of the ∆gbpA strain when competing against the WT strain (Figure 3d).
Vibrio species are normal residents of the zebrafish intestine and human-derived V. cholerae can colonize larval zebrafish32,33. We therefore tested a ∆gbpA strain of V. cholerae that exhibited a mouse colonization defect17 in our gnotobiotic zebrafish assay. As with the A. veronii strains, we found that when inoculated into GF zebrafish at 4 dpf and assayed at 6 dpf, both the V. cholerae ∆gbpA and WT strains colonized to similar levels (Figure 3e). When the two V. cholerae strains were co-inoculated into 4 dpf GF larvae, we noted a modest competitive disadvantage of the ∆gbpA strain after 48 hours when competing against the WT strain (Figure 3f).
GbpA has been shown to promote the adhesion of V. cholerae to cultured intestinal epithelial cells and intestinal tissue explants17,18. To test whether gbpA was important for A. veronii distribution in the zebrafish intestine, we generated fluorescently labeled strains of both WT (tn7:GFP) and the ∆gbpA mutant (gbpA:kan, tn7:dTomato) and imaged these strains in the intestines of live 6 dpf larval zebrafish using light sheet microscopy34. We observed no significant difference in the distribution of the two strains relative to each other or along the zebrafish intestine (Figure 3g). Neither strain exhibited an epithelial proximal distribution but instead were found in bacterial aggregates of a range of sizes within the intestinal lumen, consistent with other imaging we have performed on Aeromonas strains in the larval zebrafish intestine30,34,35.
To test for a requirement for gbpA for growth in vitro, we compared the growth curves of WT and the genetically manipulated A. veronii strains in nutrient-rich medium (TSB). Relative to the WT strain, neither the gbpA:kan insertion nor the fluorescent protein expression insertions produced any growth defects (Figure 3h). We next tested whether gbpA was required for A. veronii growth in minimal medium with colloidal chitin as the sole carbon source. Under this condition, A. veronii grew more slowly, but after 24 hours there was no difference in the population size reached by the WT and ∆gbpA strains (Figure 3i). Similarly, the ∆gbpA strain grew to WT levels in medium supplemented with GlcNAc. The lack of a ∆gbpA growth defect on chitin was not unexpected since A. veronii produces several predicted chitinases. According to the CAZy database, A. veronii HM21 genome encodes 4 chitinases belonging to the glycoside hydrolase family 18 (GH18) and 2 belonging to GH19 (Supplemental Table S1), all of which were found among the T2SS-dependent secretome, along with GbpA16. Collectively, the analysis of ∆gbpA shows the strain to have a slight defect in chitin binding and a slight competitive disadvantage in larval zebrafish intestinal colonization, which is not complemented by the presence of GbpA-producing WT cells. Our findings argue against a role for GbpA in Aeromonas epithelial adhesion in the larval zebrafish intestine.
Secreted GbpA from Aeromonas and Vibrio promotes intestinal cell proliferation
Having ruled out a role for GbpA in promoting intestinal epithelial cell proliferation through facilitating Aeromonas adhesion to the intestinal epithelium or promoting Aeromonas colonization, we next explored whether secreted GbpA protein was sufficient to promote intestinal cell proliferation in larval zebrafish. We cloned the A. veronii gbpA gene and introduced it on an inducible high copy plasmid into E. coli, which lacks any gbpA homologues in its genome. Upon induction, a 55 kd protein was the major protein species in the E. coli + pGbpA CFS, which was absent in the CFS of E. coli containing just the empty expression vector. To test whether this recombinant A. veronii GbpA had similar chitin-binding activity as the V. cholerae GbpA protein17, we performed chitin-binding assays with both proteins. We used CFS from E. coli expressing high levels of A. veronii GbpA and applied this material to chitin beads. We then collected the flow through (FT) as well as the fraction retained on the chitin beads (C). We observed that A. veronii GbpA was absent from the FT fraction and retained entirely with the chitin bead (Figure 4a). We observed a similar binding to chitin beads when we used CFS from the complementation strain of V. cholerae ΔgbpA, pGbpA that expresses V. cholerae GbpA at high levels from a plasmid pGbpA 17. Consistent with their similar domain architectures, these results indicate the A. veronii and V. cholerae GbpA proteins share the biochemical property of chitin binding.
Figure 4.
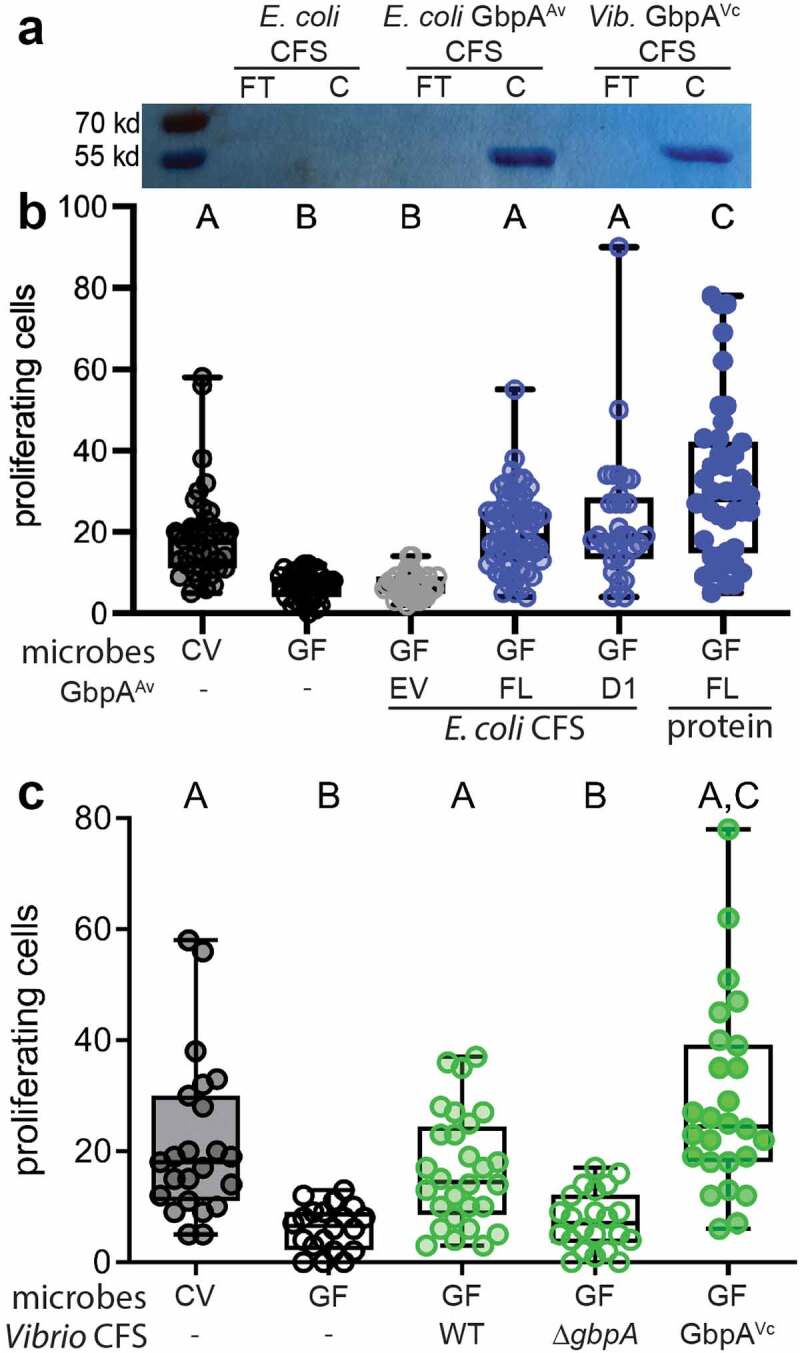
Secreted GbpA proteins are sufficient to increase intestinal epithelial proliferation in GF zebrafish. (a) CSF from engineered E. coli expressing no recombinant protein or IPTG-inducible GbpAAv and from the V. cholerae gbpA complementation strain expressing arabinose-inducible GbpAVc were incubated with chitin beads, rinsed, and the protein content of the flow through (FT) and chitin bead (C) fractions were separated by SDS-PAGE and visualized with Coomassie Brilliant Blue. (b) Quantification of proximal intestinal epithelial cell proliferation in 8 dpf CV larvae and 8 dpf GF larvae untreated or exposed from 6 dpf to CFS of E. coli expressing full length GbpAAv, domain 1 of GbpAAv, or purified full length GbpAAv protein. (c) Quantification of proximal intestinal epithelial cell proliferation in 8 dpf CV larvae and 8 dpf GF larvae untreated or exposed from 6 dpf to CFS of WT V. cholerae, the gbpA deletion strain, and the gbpA complementation strain expressing arabinose-inducible GbpAVc. Boxplot whiskers represent range. Groups with different letter designations are statistically different with a p value of < 0.05 whereas groups with the same letter are not significantly different.
We next tested the capacity of recombinant A. veronii GbpA to induce intestinal epithelial proliferation in GF zebrafish larvae. We collected the CFS from the E. coli strains expressing A. veronii GbpA or the empty vector and added each to the aquatic environment of 6 dpf GF larvae. We observed that whereas the control CFS had no effect on cell proliferation of 8 dpf GF larvae, the GbpA-enriched CFS promoted cell proliferation to levels like those observed in CV larvae (Figure 4b).
We next explored whether the LPMO-containing domain 1 of A. veronii GbpA was sufficient to induce intestinal epithelial cell proliferation. We cloned domain 1 (D1) (aa 25–188) of A. veronii GbpA into the inducible expression construct and used a similar strategy to collect CFS from E. coli enriched for this protein domain. When used to treat GF larvae from 6 to 8 dpf, this CFS containing D1-induced cell proliferation to a similar extent as the full-length GbpA from A. veronii, indicating that this domain is sufficient to induce the proliferative response in the intestinal epithelium (Figure 4b).
To verify that the pro-proliferative activity detected in the E. coli CFS was indeed GbpA, we purified a recombinant version of the protein from E. coli CFS using a GST tag, which was then removed by proteolysis. When we applied purified GbpA to 6 dpf GF larvae, we observed high levels of cell proliferation at 8 dpf (Figure 4b).
Given the similarity between A. veronii and V. cholerae GbpA, we next tested whether V. cholerae GbpA could also induce a proliferative response in the GF larval zebrafish intestine. We collected CFS from the WT, ∆gbpA mutant, and the complementation (∆gbpA, pGbpA) V. cholerae strains17 and applied these each to 6 dpf GF fish. Similar to our observations with A. veronii, we observed that the WT and complementation CFS promoted CV-like levels of cell proliferation at 8 dpf, while the mutant CFS did not promote cell proliferation (Figure 4c). This observation suggests that the pro-proliferative activity observed for secreted A. veronii GbpA is shared across other GbpA-like proteins produced by resident intestinal bacteria.
Discussion
Since early descriptions of epithelial cell renewal in the intestines of GF mice6, the microbiota has been appreciated as a source of pro-proliferative stimuli that elevates homeostatic rates of intestinal epithelial cell proliferation in many animals. The nature of the microbiota-derived molecules with this activity is incompletely understood. Several bacterial metabolites have been shown to elevate intestinal epithelial proliferation rates in mice and fruit flies, including reactive oxygen species36, indoles37, and polyamines38. Our previous characterization of intestinal epithelial proliferation in gnotobiotic larval zebrafish indicated that A. veronii, a prominent bacterial colonizer of the zebrafish intestine, stimulated a proliferative response through secreted factors of greater molecular weight than these metabolites7. Here we show that a pro-proliferative factor secreted by A. veronii is a homologue of the V. cholerae LPMO GbpA.
The best characterized bacterial proteins that elicit intestinal epithelial cell proliferation are protein toxins of bacterial pathogens. For example, the Helicobacter pylori oncogenic virulence factor CagA induces expansion of Lgr5+ stem cells during infection of the gastric epithelium39 and transgenic expression of CagA is sufficient to increase epithelial cell proliferation in both zebrafish40 and fruit fly41 intestines. These pathogen-associated cell proliferative responses, however, are distinct from responses to microbiota colonization in that they are typically associated with inflammation and hypertrophic expansion of the tissue. The proliferative response to the microbiota in the larval zebrafish intestinal epithelium occurs even when tumor necrosis factor (TNF) signaling is blocked7, in contrast to the epithelial proliferation in a zebrafish model of spontaneous intestinal inflammation and dysbiosis, which is prevented by interfering with TNF signaling42.
GbpA was previously characterized as a virulence factor of human pathogenic V. cholerae strains and implicated in disease by a proposed adhesion mechanism of the secreted protein acting to crosslink bacterial cells to GlcNAc moieties on intestinal mucins17–19. We generated gbpA deficient A. veronii to explore the function of this gene in bacterial-host interactions in the larval zebrafish intestine. We found that ∆gbpA A. veronii exhibited reduced binding to chitin beads, as reported for V. cholerae, but when we further explored this phenotype in a co-incubation assay with our WT and ∆gbpA A. veronii strains, we found that this defect was not rescued in trans by GbpA from the WT cells. This unexpected result challenges the current model that GbpA functions primarily as an adhesin, since the predominantly soluble GbpA in the extracellular environment should be readily accessed by both WT and ∆gbpA mutant cells. Additional evidence challenging the idea that GbpA’s major function is as a chitin adhesin comes from a previous study showing that a V. cholerae fliA mutant upregulates gbpA expression but is defective for chitin binding compared to the WT strain due to downregulation of the cell surface associated adhesin FrhA43,44. We also found no evidence for GbpA conferring a colonization advantage to either A. veronii or V. cholerae in the larval zebrafish intestine when introduced in mono-associations, but a slight competitive disadvantage in the presence of GbpA-secreting WT strains. Finally, we found no evidence that GpbA expression altered the intestinal biogeography of A. veronii, which normally colonizes the intestinal lumen in cellular aggregates. Our observations argue against the model that A. veronii utilizes GpbA as an adhesin to colonize GlcNAc-rich surfaces. Instead, we hypothesize that GbpA is part of a GlcNAc utilization program that A. veronii deploys when GlcNAc is an advantageous carbon source. In support of this idea, V. cholerae has been shown to upregulate gbpA in the presence of GlcNAc and chitin oligosaccharides45. We hypothesize that the modest chitin-binding defects of the ∆gbpA strain reflect physiological differences due to alterations in nutrient processing and acquisition. Although we observed no growth defect of the A. veronii ∆gbpA strain relative to WT when grown on colloidal chitin as a sole carbon source, we note that this strain secretes many chitin degrading enzymes, which likely confer redundant functions and highlight the importance of chitin-utilization for this bacterium.
The importance of GbpA in A. veronii cellular physiology is consistent with a recent characterization of Pseudomonas aeruginosa mutants lacking the gene for chitin-binding protein D (CbpD), an LPMO with similar N and C terminal chitin-binding domains as GbpA46. The authors showed that cbpD deficient P. aeruginosa have markedly altered transcriptomes and proteomes as compared with WT cells grown in various media. They further show that ∆cbpD P. aeruginosa have reduced survival in blood and pathogenicity in mouse tissues, which the authors attribute to the cells’ altered metabolic state and reduced capacity to metabolize host-produced hydrogen peroxide.
Independent of possible fitness advantages conferred by GbpA to A. veronii during host colonization, we show that the secreted protein stimulates epithelial proliferation in the GF larval intestine. This pro-proliferative activity is recapitulated by the LPMO-containing domain 1 of the protein and by the related V. cholerae GbpA. We hypothesize that a consequence of A. veronii secreting GbpA in the intestine as part of a GlcNAc utilization program is that the enzymatic activity or byproducts are sense by the host through innate immune pathways that monitor intestinal mucosal integrity or glycan composition. We have shown that the host proliferative response to the microbiota requires the innate immune adaptor Myd88, but that activation of innate immune signaling with bacterial lipopolysaccharide (LPS) is not sufficient to elicit intestinal epithelial cell proliferation7. In the zebrafish intestine, GbpA would have access to chitin as a component of the zebrafish intestinal lining47. Chitin is a feature of most invertebrate intestines and is found in many non-mammalian vertebrate intestines, where it coexists in varied proportions with a meshwork of glycan-rich mucins48. Even in mammalian intestines that lack a chitin layer, the intestinal mucus contains ample GlcNAc polysaccharides that could be cleaved by bacterial secreted LPMOs.
As a bacterial protein that can target the intestinal lining, GbpA represents an example of a Microbial Associated Competitive Activity (MACA), a term we coined to describe microbial activities important for competitive fitness in multi-species communities that are sensed by host tissues as sources of information for regulating programs of development and repair49. In this regard, the intestinal epithelial proliferation observed upon microbiota colonization is not an outcome that host-associated bacteria LPMOs evolved to evoke, but rather an adaptation of the host tissue to bolster epithelial renewal programs in the face of degradative enzymes secreted by specific constituents of its intestinal microbiota. The MACA framework explains how specific secreted proteins from microbiota members can have profound impacts on host tissue development and physiology and how different nonhomologous proteins can elicit similar effects by executing similar activities. Future studies of GbpA will test whether it elicits host epithelial proliferative response via its LPMO activity and whether specific byproducts of its enzymatic reaction or depletion of its co-substrates dioxygen or hydrogen peroxide are sensed by the host to induce epithelial renewal programs.
Supplementary Material
Acknowledgments
We thank Joerg Graf for the generous gift of A. veronii strains, Ron Taylor for the generous gift of V. cholerae strains, JT Neal for the micrograph of EdU-labelled intestine, Erika Mittge for assistance with gnotobiotic zebrafish experiments, W. Zac Stephens for assistance with A. veronii genetics, the UO Histology Facility for tissue sectioning and Rose Sockol and the UO Zebrafish Facility for maintenance of zebrafish lines.
Funding Statement
Research reported in this publication was supported by the NIH under award numbers [F32DK096755] (to AVB), [5T32GM007413] (to SVB), [F32DK124033] (to TJS), and 1R01 CA176579, [1P50GM098911], and [1P01GM125576] (to KG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure statement
AVB and KG are patent holders for the use of GbpA, patent number 9,044,434, issued 06/02/2015.
Author contributions
AVB: conceptualization, methodology, investigation, original draft preparation; SV: visualization, original draft preparation; TJS: investigation (chitin-binding assay), manuscript review and editing; SLL: investigation (light sheet microscopy), visualization; KG: conceptualization, funding acquisition, supervision, original draft preparation, review and editing.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and the supplementary materials.
Abbreviations
- GlcNAc
N-acetylglucosamine
- GbpA
GlcNAc binding protein A
- LPMO
lytic polysaccharide monooxygenase
- CFS
cell free supernatant
- CV
conventionally reared
- GF
germ free
- WT
wild type
- CFU
colony forming unity
- TSB
tryptic soy broth
- TSA
tryptic soy agar
- LB
Luria broth
- EM
embryo medium
- MS-222
tricaine methanesulfonate
- dpf
days post fertilization
- EdU
5-ethynyl-2′-deoxyuridine
- SDS-PAGE
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis
- ANOVA
analysis of variance
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2183686
References
- 1.Bosch TCG, McFall-Ngai M.. Animal development in the microbial world: re-thinking the conceptual framework. Curr Top Dev Biol. 2021;141:399–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh KT, Guillemin K. The impacts of microbiota on animal development and physiology. In: Rook GAW, Lowry CA, editors. Evolution, biodiversity, and a reassessment of the hygiene hypothesis. 2022. p. 177–196. doi: 10.1007/978-3-030-91051-8_6. [DOI] [Google Scholar]
- 3.Massaquoi MS, Kong G, Chillin D, Hamilton MK, Melancon E, Eisen JS, Guillemin K. Global host responses to the microbiota at single cell resolution in gnotobiotic zebrafish. BioRXiv. 2022. doi: 10.1101/2022.03.28.486083. [DOI] [Google Scholar]
- 4.Wang G, Sweren E, Liu H, Wier E, Alphonse MP, Chen R, Islam N, Li A, Xue Y, Chen J, et al. Bacteria induce skin regeneration via IL-1β signaling. Cell Host Microbe. 2021;29(5):777–791.e6. doi: 10.1016/j.chom.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abo H, Chassaing B, Harusato A, Quiros M, Brazil JC, Ngo VL, Viennois E, Merlin D, Gewirtz AT, Nusrat A, et al. Erythroid differentiation regulator-1 induced by microbiota in early life drives intestinal stem cell proliferation and regeneration. Nat Commun. 2020;11(1):513. doi: 10.1038/s41467-019-14258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrams GD, Bauer H, Sprinz H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963;12:355–364. [PubMed] [Google Scholar]
- 7.Cheesman SE, Neal JT, Mittge E, Seredick BM, Guillemin K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc Natl Acad Sci U S A. 2011;108(supplement_1):4570–4577. doi: 10.1073/pnas.1000072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. PNAS. 2004;101(13):4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2016;127(2):423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broderick NA, Buchon N, Lemaitre B, McFall-Ngai MJ. Microbiota-induced changes in drosophila melanogaster host gene expression and gut morphology. mBio. 2014;5(3):1–13. doi: 10.1128/mBio.01117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, et al. Symbiotic Lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. Embo J. 2013;32(23):3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones TA, Guillemin K. Racing to stay put: how resident microbiota stimulate intestinal epithelial cell proliferation. Curr Pathobiol Rep. 2018;6(1):23–28. doi: 10.1007/s40139-018-0163-0. [DOI] [Google Scholar]
- 13.Hill JH, Franzosa EA, Huttenhower C, Guillemin K. A conserved bacterial protein induces pancreatic beta cell expansion during zebrafish development. Elife. 2016;5:18. doi: 10.7554/eLife.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melancon E, Gomez De La Torre Cann S, Sichel S, Kelly M, Wiles TJ, Rawls JF, Eisen JS, Guillemin K. Best practices for germ-free derivation and gnotobiotic zebrafish husbandry. Methods Cell Biology. 2015;155:1683–1695. doi: 10.1016/bs.mcb.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiles TJ, Wall ES, Schlomann BH, Hay EA, Parthasarathy R, Guillemin K. Modernized tools for streamlined genetic manipulation and comparative study of wild and diverse proteobacterial lineages. mBio. 2018;9(5). doi: 10.1128/mBio.01877-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolig AS, Sweeney EG, Kaye LE, DeSantis MD, Perkins A, Banse AV, Hamilton MK, Guillemin K. A bacterial immunomodulatory protein with lipocalin-like domains facilitates host–bacteria mutualism in larval zebrafish. Elife. 2018;7. doi: 10.7554/eLife.37172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirn TJ, Jude BA, Taylor RK. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature. 2005;438(7069):863–866. doi: 10.1038/nature04249. [DOI] [PubMed] [Google Scholar]
- 18.Bhowmick R, Ghosal A, Das B, Koley H, Saha DR, Ganguly S, Nandy RK, Bhadra RK, Chatterjee NS. Intestinal adherence of Vibrio cholerae involves a coordinated interaction between colonization factor GbpA and mucin. Infect Immun. 2008;76(11):4968–4977. doi: 10.1128/IAI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong E, Vaaje-Kolstad G, Ghosh A, Hurtado-Guerrero R, Konarev PV, Ibrahim AFM, Svergun DI, Eijsink VGH, Chatterjee NS, van Aalten DMF, et al. The Vibrio cholerae colonization factor GbpA possesses a modular structure that governs binding to different host surfaces. PLoS Pathog. 2012;8(1):e1002373. doi: 10.1371/journal.ppat.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Book AJ, Yennamalli RM, Takasuka TE, Currie CR, Phillips GN, Fox BG. Evolution of substrate specificity in bacterial AA10 lytic polysaccharide monooxygenases. Biotechnol Biofuels. 2014;7(1):109. doi: 10.1186/1754-6834-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loose JSM, Forsberg Z, Fraaije MW, Eijsink VGH, Vaaje-Kolstad G. A rapid quantitative activity assay shows that the Vibrio cholerae colonization factor GbpA is an active lytic polysaccharide monooxygenase. FEBS Lett. 2014;588(18):3435–3440. doi: 10.1016/j.febslet.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Vandhana TM, Reyre J-L, Sushmaa D, Berrin J-G, Bissaro B, Madhuprakash J. On the expansion of biological functions of lytic polysaccharide monooxygenases. New Phytol. 2022;233(6):2380–2396. doi: 10.1111/nph.17921. [DOI] [PubMed] [Google Scholar]
- 23.The Zebrafish Book . A guide for the laboratory use of zebrafish (Danio rerio). 5th ed. Eugene: University of Oregon Press. https://zfin.org/cgi-bin/webdriver. [Google Scholar]
- 24.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2(6):371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graf J, Nelson MC, Colston SM, Dunning Hotopp JC. Closed genome sequence of Aeromonas veronii strain Hm21, an isolate from the medicinal leech Hirudo verbana. Microbiol Resour Announc. 2020;9(42):20–21. doi: 10.1128/MRA.00922-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi K-H, Gaynor JB, White KG, Lopez C, Bosio CM, Karkhoff-Schweizer RR, Schweizer HP. A Tn7-based broad-range bacterial cloning and expression system. Nat Methods. 2005;2(6):443–448. doi: 10.1038/NMETH765. [DOI] [PubMed] [Google Scholar]
- 27.Maltz M, Graf J. The Type II Secretion System is essential for erythrocyte lysis and gut colonization by the leech digestive tract symbiont Aeromonas veronii. Appl Environ Microbiol. 2011;77(2):597–603. doi: 10.1128/AEM.01621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graf J. Symbiosis of Aeromonas veronii biovar sobria and Hirudo medicinalis, the medicinal leech: a novel model for digestive tract associations. Infect Immun. 1999;67(1):1–7. doi: 10.1128/IAI.67.1.1-7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milligan-Myhre K, Charette JR, Phennicie RT, Stephens WZ, Rawls JF, Guillemin K, Kim CH. Methods Cell Biology. 2011;105:87–116. doi: 10.1016/B978-0-12-381320-6.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jemielita M, Taormina MJ, Burns AR, Hampton JS, Rolig AS, Guillemin K, Parthasarathy R. Spatial and temporal features of the growth of a bacterial species colonizing the zebrafish gut. mBio. 2014;5(6). doi: 10.1128/mBio.01751-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EHK. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science. 2008;322(5904):1065–1069. doi: 10.1126/science.1162493. [DOI] [PubMed] [Google Scholar]
- 32.Logan SL, Thomas J, Yan J, Baker RP, Shields DS, Xavier JB, Hammer BK, Parthasarathy R. The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc Natl Acad Sci U S A. 2018;115(16):E3779–3787. doi: 10.1073/pnas.1720133115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, Neely MN, Withey JH. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol. 2014;80(5):1710–1717. doi: 10.1128/AEM.03580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taormina MJ, Jemielita M, Stephens WZ, Burns AR, Troll JV, Parthasarathy R, Guillemin K. Investigating bacterial-animal symbioses with light sheet microscopy. Biol Bull. 2012;223(1):7–20. doi: 10.1086/BBLv223n1p7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiles TJ, Jemielita M, Baker RP, Schlomann BH, Logan SL, Ganz J, Melancon E, Eisen JS, Guillemin K, Parthasarathy R, et al. Host gut motility promotes competitive exclusion within a model intestinal microbiota. PLoS Biol. 2016;14(7):e1002517. doi: 10.1371/journal.pbio.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reedy AR, Luo L, Neish AS, Jones RM. Commensal microbiota-induced redox signaling activates proliferative signals in the intestinal stem cell microenvironment. Development. 2019;146:dev171520. doi: 10.1242/dev.171520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell DN, Swimm A, Sonowal R, Bretin A, Gewirtz AT, Jones RM, Kalman D. Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proc Natl Acad Sci U S A. 2020;117(35):21519–21526. doi: 10.1073/pnas.2003004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura A, Kurihara S, Takahashi D, Ohashi W, Nakamura Y, Kimura S, Onuki M, Kume A, Sasazawa Y, Furusawa Y, et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat Commun. 2021;12(1). doi: 10.1038/s41467-021-22212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigal M, Rothenberg ME, Logan CY, Lee JY, Honaker RW, Cooper RL, Passarelli B, Camorlinga M, Bouley DM, Alvarez G, et al. Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148(7):1392–404.e21. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 40.Neal JT, Peterson TS, Kent ML, Guillemin K. H. pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Dis Model Mech. 2013;6:802–810. doi: 10.1242/dmm.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones TA, Hernandez DZ, Wong ZC, Wandler AM, Guillemin K, Monack DM. The bacterial virulence factor CagA induces microbial dysbiosis that contributes to excessive epithelial cell proliferation in the Drosophila gut. PLoS Pathog. 2017;13(10):1–20. doi: 10.1371/journal.ppat.1006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolig AS, Mittge EK, Ganz J, Troll JV, Melancon E, Wiles TJ, Alligood K, Stephens WZ, Eisen JS, Guillemin K, et al. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. 2017;15(2):e2000689. doi: 10.1371/journal.pbio.2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitts G, Giglio KM, Zamorano-Sánchez D, Park JH, Townsley L, Cooley RB, Wucher BR, Klose KE, Nadell CD, Yildiz FH, et al. A conserved regulatory circuit controls large adhesins in Vibrio cholerae. mBio. 2019;10(6). doi: 10.1128/mBio.02822-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syed KA, Beyhan S, Correa N, Queen J, Liu J, Peng F, Satchell KJF, Yildiz F, Klose KE. The Vibrio cholerae flagellar regulatory hierarchy controls expression of virulence factors. J Bacteriol. 2009;191(21):6555–6570. doi: 10.1128/JB.00949-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meibom KL, Li XB, Nielsen AT, Wu C-Y, Roseman S, Schoolnik GK. The Vibrio cholerae chitin utilization program. Proceedings of the National Academy of Sciences. 2004;101(8):2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Askarian F, Uchiyama S, Masson H, Sørensen HV, Golten O, Bunæs AC, Mekasha S, Røhr ÅK, Kommedal E, Ludviksen JA, et al. The lytic polysaccharide monooxygenase CbpD promotes Pseudomonas aeruginosa virulence in systemic infection. Nat Commun. 2021;12(1). doi: 10.1038/s41467-021-21473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang JW, Fernandez J, Sohn JJ, Amemiya CT. Chitin is endogenously produced in vertebrates. Current Biology. 2015;25(7):897–900. doi: 10.1016/j.cub.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakashima K, Kimura S, Ogawa Y, Watanabe S, Soma S, Kaneko T, Yamada L, Sawada H, Tung C-H, Lu T-M, et al. Chitin-based barrier immunity and its loss predated mucus-colonization by indigenous gut microbiota. Nat Commun. 2018;24(1). doi: 10.1038/s41467-018-05884-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiles TJ, Guillemin K. Patterns of partnership: surveillance and mimicry in host-microbiota mutualisms. Curr Opin Microbiol. 2020;54:87–94. doi: 10.1016/j.mib.2020.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and the supplementary materials.


