ABSTRACT
Neurovisual involvement has been reported in a number of patients with severe SARS-CoV-2 disease (COVID-19), mainly among adult patients. In children, such involvement has been reported in rare cases, often in those presenting with severe forms of COVID-19. The aim of this work is to explore the association between mild COVID-19 and neurovisual manifestations. We report the cases of three previously healthy children who developed neurovisual manifestations following mild acute COVID-19, analysing the clinical phenotype, the latency between the onset of acute COVID-19 and neurovisual involvement, and the kinetic of resolution. Our patients developed different clinical patterns, including visual impairment and ophthalmoplegia. In two cases, these clinical features occurred during acute COVID-19, while in the third patient their development was delayed after 10 days from disease onset. Furthermore, the dynamics of resolution were different, with one patient showing remission after 24 hours, the second after 30 days, and the third showing persistence of the strabismus after 2 months of follow-up. The spreading of COVID-19 among the paediatric population will probably lead to an increase of atypical disease forms, including those presenting with neurovisual involvement. Therefore, a better knowledge of the pathogenic and clinical features of these manifestations is warranted.
KEYWORDS: Neurovisual manifestations, COVID-19, paediatric ophthalmology, opthalmoplegia, neuro-COVID19
Introduction
Since the first stages of the coronavirus 2019 (COVID-19) pandemic, it has been demonstrated that SARS-CoV-2 has a pleiotropic effect on the central nervous system. PTSD in parents of children with severe diseases: a systematic review to face Covid-19 impact.1 Indeed, acute SARS-CoV-2 infection can be associated with a high variety of signs and symptoms deriving from neurological involvement, including headache, anosmia, ageusia, behavioural disturbances and anxiety, as well as seizures, movement disorders, cerebrovascular disease, and acute encephalitis.2,3 However, neurovisual involvement has been reported only in few cases, mostly in adult patients,4 whereas it is extremely rare in children. Herein, we describe the cases of three previously healthy children who developed neurovisual manifestations, including visual impairment and ophthalmoplegia, following acute COVID-19 and discuss the potential pathogenic and clinical implications. Each patient underwent a careful ophthalmological examination at symptom onset and during follow-up, which included ophthalmoscopy, Lang test for stereopsis and LEA symbol test or Snellen charts to measure visual acuity.
Case presentations
Case 1
The first patient is a 2-year-old boy with a previously unremarkable medical history, who was admitted to the emergency department due to the development of monocular convergent strabismus of the right eye after 2 days of fever. No other symptoms were reported. Neurological examination did not show any pathological findings, as well as the remaining physical assessment. A swab for SARS-CoV-2 polymerase chain reaction (PCR) was performed and was positive. An ophthalmological evaluation did not find any abnormalities, except for convergent strabismus of the right eye and diplopia, which was noticed by showing different images to the child on the Lang test and asking him to point at them. No refractive error was noticed. In order to exclude secondary causes for the clinical picture observed, contrast-enhanced brain magnetic resonance imaging (MRI) of the brain and orbits was performed, which was normal.
A week later, repeat examination showed that his right eye was straight, but that he had developed a left convergent strabismus which persisted, although improved, at 2-months follow-up. In addition, he had a partial deficit in left eye abduction and a loss of stereopsis.
Case 2
The second patient is a 5-year-old boy, with an unremarkable past medical history, who developed mild SARS-CoV-2 infection, characterised by fever and vomiting with early spontaneous resolution. Ten days from disease onset, he developed acute monocular convergent strabismus of the right eye and diplopia. A swab for SARS-CoV-2 PCR was performed and was positive. Neurological and physical examination were unremarkable, while ophthalmological examination revealed mild 1 dioptre myopia in the right eye but normal corrected visual acuity in each eye measured with the LEA symbol test, astigmatism, right comitant esophoria, and diplopia, which spontaneously resolved after 24 hours. During the 2 months of follow-up he did not experience any relapses.
Case 3
The third patient is an 8-year-old boy with a previously unremarkable past medical history who developed fever, headache, myalgia, and a reduction of visual acuity and perception. He reported seeing object as bigger or smaller than usual. In particular, he remembered that ‘the television looked smaller and more distant from the couch than usual’ on one or two occasions and that posters in his room ‘appeared bigger than they actually were’ as he was staring at them. The visual symptoms accompanied the onset of headaches but were not complained during his initial assessment or during hospitalisation.
On ophthalmological assessment he complained of with diplopia associated with right eye exophoria. The LEA symbol test revealed a mild loss of visual acuity (9/10 with the right eye and 9/10 with the left eye). The fundus examination and neurological assessment were normal. He tested positive for SARS-CoV-2. Brain MRI performed during the acute phase was normal.
While the acute infection resolved without complications, the diplopia and loss of visual acuity persisted for 1 month after the clinical resolution. An ophthalmological follow-up evaluation, performed 1 month after disease onset, revealed a completely restored visual acuity and normal ophthalmological examination.
Discussion
The spectrum of neuro-COVID-19 has been widely explored in the adult population, displaying a greater prevalence among patients with severe disease and potentially leading to severe complications including ischaemic and haemorrhagic stroke.5 The clinical course of neuro-COVID is usually milder in children.6 Concerning the ophthalmological involvement, there are anecdotal reports in adults of ophthalmoplegia, cranial nerve palsies, diplopia, ptosis (in the context of suspected Fisher syndrome or myasthenia gravis), and rare forms of optic neuritis triggered by the virus.7
In children, neurovisual involvement during COVID-19 has been reported only in a few cases, mostly in the form of optic neuritis,8 in patients showing elevated intracranial pressure or in those diagnosed with the multisystem inflammatory syndrome in children,9 a hyperinflammatory condition that can develop 4–6 weeks after acute COVID-19. Cases of cranial nerve palsy in children with mild COVID-19 have been previously reported, often in patients with significant neuro-metabolic comorbidities.10
We have presented two cases of strabismus and a case of acute visual impairment associated with mild acute SARS-CoV-2 infection and without known predisposing factors for the development of neurological or immune disorders. Interestingly, the symptoms appeared with different timings from the onset of acute COVID-19, being observed at disease onset in Cases 1 and 3 and after 10 days in Case 2. Additionally, the children had a different outcome regarding the resolution of the clinical picture, with symptoms being only transient in Case 2 and persisting during a 1-month follow-up in Cases 1 and 3.
Of note, Case 3 is presented with symptoms recalling the clinical picture known as Alice in Wonderland syndrome (AIWS),11 which presents with aschematia and dysmetropsia and has been mainly linked to migraine and different infectious diseases, mostly Epstein–Barr virus (EBV).12 However, differently from AIWS, serological testing performed in Case 3 did not reveal signs of previous EBV infection. To our knowledge, this is the first reported case of AIWS associated with SARS-CoV-2 infection.
Although strabismus, visual impairment, diplopia, and cranial nerve palsies are rare findings in children with COVID-19, the spreading of SARS-CoV-2 infection among the paediatric population will probably lead to the increasing recognition of atypical presentation of COVID-19 in children, including neuro-ophthalmological manifestations. Furthermore, the impact of the new viral variants on these complications has still to be defined.
The aetiology underlying neurovisual involvement in COVID-19 has not been completely elucidated, although the neurotropism of SARS-CoV-2, its ability to directly induce neuroinflammation, and the interaction between the pathogen and the host immune system are all likely to play an important role (Figure 1).13 Concerning this, it is possible that differences in the innate and adaptive immune response between adults and children14 could partly explain the different incidences of these manifestations across age groups. Immune-mediated mechanisms could result in cranial nerve palsy and impairment in muscular activity (i.e. of the lateral rectus or abducens nerve palsy in all three cases), with also even direct involvement of the optic nerve, as hypothesised for explaining the clinical picture in Case 3. The individual genetic and immunological background could be also implicated in explaining the different clinical presentations and the dynamics of the symptom resolution observed in the described patients.
Figure 1.
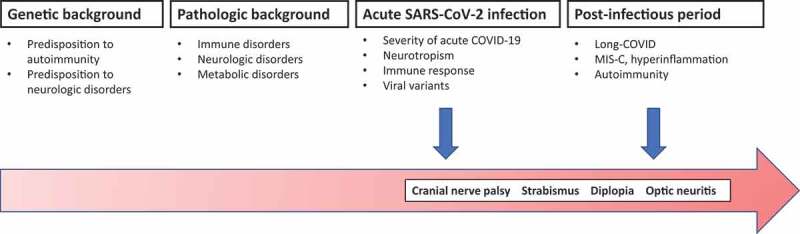
Proposed pathogenesis of neurovisual manifestations in COVID-19.
MIS-C = Multisystem inflammatory syndrome in children.
Current knowledge regarding the molecular mechanisms responsible for the SARS-CoV-2-induced immune dysregulation suggests that in patients not showing spontaneous resolution, immunomodulatory treatments (particularly, corticosteroids) could represent valid therapeutic strategies.
Hopefully, a better characterisation of the neuro-ophthalmological complications of paediatric COVID-19, the specific predisposing factors, and the clinical course will help in improving the healthcare response also against this rare disease complication.
Funding Statement
This work has been partially supported by grant from the IRCCS Stella Maris Foundation (Ricerca Corrente, and the 5 × 1000 voluntary) contributions, Italian Ministry of Health. (RB, TF).
Author contribution
AO, AB, AS, FO, and RB conceptualised this work. GC, MV, AS, MED, and TF drafted the initial paper, which was critically revised by AO, RB, MCR, RC, and DP. All the authors approved the final version of this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Corsi M, Orsini A, Pedrinelli V, et al. “PTSD in parents of children with severe diseases: a systematic review to face Covid-19 impact.” Ital J Pediatr. 2021;47(1):1–7. doi: 10.1186/s13052-021-00957-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orsini A, Corsi M, Santangelo A, et al. Challenges and management of neurological and psychiatric manifestations in SARS-CoV-2 (COVID-19) patients. Neurol Sci. September 2020;41(9):2353–2366. doi: 10.1007/s10072-020-04544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orsini A, Corsi M, Pedrinelli V, et al. Post-traumatic stress, anxiety, and depressive symptoms in caregivers of children tested for COVID-19 in the acute phase of the Italian outbreak. J Psychiatr Res. 2021;135:256–263. doi: 10.1016/j.jpsychires.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold DM, Galetta SL.. Neuro-ophthalmologic complications of coronavirus disease 2019 (COVID-19). Neurosci Lett. January 18, 2021;742:135531. doi: 10.1016/j.neulet.2020.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon DW, Schober ME. Acute central and peripheral nervous system injury associated with coronavirus disease 2019: recognition and treatment strategies. Curr Opin Pediatr. December 1, 2021;33(6):591–596. doi: 10.1097/MOP.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y, Walser SA, Asghar SJ, Jain R, Mainali G, Kumar A. A comprehensive review of neurologic manifestations of COVID-19 and management of pre-existing neurologic disorders in children. J Child Neurol. March 2021;36(4):324–330. doi: 10.1177/0883073820968995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luís ME, Hipólito-Fernandes D, Mota C, et al. A review of neuro-ophthalmological manifestations of human coronavirus infection. Eye Brain. October 30, 2020;12:129–137. doi: 10.2147/EB.S268828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baccarella A, Linder A, Spencer R, et al. Increased intracranial pressure in the setting of multisystem inflammatory syndrome in children, associated with COVID-19. Pediatr Neurol. February 2021;115:48–49. doi: 10.1016/j.pediatrneurol.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha A, Dwivedi D, Dwivedi A, Bajaj N. Optic neuritis as a presenting symptom of post-COVID-19 multisystem inflammatory syndrome in children (MIS-C). Indian J Pediatr. December 2021;88(12):1269. doi: 10.1007/s12098-021-03921-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonardi V, Meneghesso D, Debertolis G, Pin JN, Nosadini M, Sartori S. Isolated third cranial nerve palsy and COVID-19 infection in a child. Pediatr Neurol. 2021. Jul;120:11. doi: 10.1016/j.pediatrneurol.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rastogi RG, Vander Pluym J, Lewis KMA. Visual snow, and “Alice in Wonderland” syndrome in childhood. Semin Pediatr Neurol. 2016;23(1):14–17. doi: 10.1016/j.spen.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Mastria G, Mancini V, Viganò A, Di Piero V. Alice in wonderland syndrome: a clinical and pathophysiological review. Biomed Res Int. 2016;2016:8243145. doi: 10.1155/2016/8243145. Epub December 27, 2016. PMID: 28116304; PMCID: PMC5223006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finsterer J, Scorza FA. Clinical and pathophysiologic spectrum of neuro-COVID. Mol Neurobiol. August 2021;58(8):3787–3791. doi: 10.1007/s12035-021-02383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costagliola G, Spada E, Consolini R. Age-related differences in the immune response could contribute to determine the spectrum of severity of COVID-19. Immun Inflamm Dis. June 2021;9(2):331–339. doi: 10.1002/iid3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]


