Abstract
In this study, Arthrospira fusiformis previously isolated from Lake Mariout (Alexandria, Egypt) was cultivated in the laboratory using a medium for pharmaceutical grade Arthrospira, named as Amara and Steinbüchel medium. Hot water extract of the Egyptian Spirulina was prepared by autoclaving dried biomass in distilled water at 121°C for 15 min. This algal water extract was analyzed by GC-MS to evaluate its volatile compounds and fatty acids composition. The antimicrobial activity of phycobiliprotein extract from Arthrospira fusiformis using phosphate buffer was evaluated against thirteen microbial strains (two Gram-positive bacteria, eight Gram-negative bacteria, one yeast, and two filamentous fungi). The major components of fatty acids in the hot extract of Egyptian A. fusiformis were hexadecanoic acid (palmitic acid, 55.19%) and octadecanoic acid (stearic acid, 27.14%). The main constituents of its volatile compounds were acetic acid (43.33%) and oxalic acid (47.98%). The most potent antimicrobial effect of phycobiliprotein extract was obtained against two Gram-negative bacteria Salmonella typhi and Proteus vulgaris, filamentous fungus Aspergillus niger, and the pathogenic yeast Candida albicans (all of which showed MIC values of 58.1 μg/ml). Escherichia coli and Salmonella typhimurium come second in their susceptibility to the phycobiliprotein extract from Arthrospira fusiformis and Serratia marcescens and Aspergillus flavus are the least in susceptibility, with MIC values of 116.2 and 232.5 μg/ml, respectively, while phycobiliprotein extract has no antibacterial effect on methicillin-resistant as well as susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Shigella sonnei. These findings confirmed the nutritional value of Egyptian A. fusiformis isolated from Lake Mariout and suggest the potential use of this strain as an ingredient in the cooking of some foods to increase the level of stearic acid and palmitic acid. Moreover, its effective antibacterial activities against some important and highly resistant to antibiotics bacterial pathogens in addition to its antifungal effects recommend the therapeutic use of its biomass.
1. Introduction
The filamentous blue-green microalga Arthrospira fusiformis (commercially known as Spirulina platensis) was proved to have various nutritional and medicinal properties and was considered a “miracle from the sea” or “superfood” by scientific communities. This edible, microscopic, and alkalophilic cyanobacterium belongs to the microalgae class Cyanophyta [1]. It contains higher amounts of vitamins, protein, minerals, and the like than any other single-cell protein [2, 3]. It has photoautotrophic as well as auxotrophic growth since it has the capability to utilize organic compounds in dark and grow autotrophically in light [4, 5]. Interestingly, there is no need to cook or specially treat Spirulina to increase its protein availability, which makes its production simple and preserves its valuable constituents including polyunsaturated fatty acids and vitamins [6].
Due to the nutritional value and pharmaceutical activities of Arthrospira fusiformis, it has been used for human and animal nutrition for centuries. This microalga is marketed worldwide in many stores of healthy foods [7–9]. It represents a good supply of bioactive ingredients for the diet including essential amino acids and fatty acids, high protein content (60–70%), pigments of photosynthesis, minerals, carotenoids, and vitamins [10, 11]. Several studies have reported the use of Arthrospira fusiform as a food ingredient (ingredient in pasta and enriched bread) or food additive (added to freeze-dried yogurts) [12–14].
In general, Arthrospira fusiformis colonizes unique marine environments that several other microorganisms cannot colonize, such as alkaline saline lakes, which have a pH value of up to 11.0 [1]. It also exists in various habitats such as freshwater, marches, brackish water, seawater, soils, waters of domestic and industrial uses, and thermal springs [15–19]. Arthrospira fusiformis exhibits various pharmaceutical activities including antiviral [20, 21], antibacterial [22–24], anti-inflammatory [25–27], antioxidant [24, 28, 29], and anticancer activities [30]. Moreover, it plays a significant role in the treatment of wastewater, the fixation of CO2, as well as the production of biofuels, food colors, and methane [31–33].
Five solvents have been commonly used to extract the bioactive ingredients (with antioxidant and antimicrobial activities) of Arthrospira fusiformis including ethyl acetate, dichloromethane, methanol, hexane, and petroleum ether [24, 34]. Abedin and Taha [35] tested supernatant, hexane, and methanolic extracts of Tolypothrix ceytonica, Anabaena oryza, and Spirulina platensis for antifungal activity against various phytopathogenic fungi (Aspergillus niger, Fusarium verticillioides, Aspergillus flavus, Alternaria brassicae, and Helminthosporium sp.) and fungus causing human disease (Candida albicans). They found that Spirulina platensis extracts have the highest antifungal effect on tested fungi. Kaushik and Chauhan [36] reported that methanol, ethyl acetate, hexane, and dichloromethane extracts of Spirulina platensis could inhibit the growth of Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Salmonella typhi. They showed that methanolic extract has the most potent antibacterial activity, while all extracts have no antibacterial effect on Klebsiella pneumoniae [36]. In 2010, Sharaf et al. [20] isolated and authenticated a new Egyptian Arthrospira fusiformis strain from the brackish Lake Mariout at the southwest of Alexandria city. The phosphate buffer and hot water extracts of this strain were confirmed to have antiherpetic activity (could inhibit the multiplication of herpesvirus before and during virus infection of host cells) [20, 21].
Najdenski et al. [37] confirmed that ethanol extracts and fatty acids from Arthrospira fusiformis have in vitro antibacterial activity against Staphylococcus aureus and Streptococcus pyogenes. Additionally, phycobiliproteins isolated from Arthrospira fusiformis were proved to have antimicrobial activity [37]. Ould Bellahcen et al. [38] isolated essential oil by hydrodistillation from Spirulina platensis collected from Lake Foum Elouad, Laayoune (South of Morocco) and cultivated in the laboratory using Zarrouk's medium. The essential oil was analyzed by GC/MS and GC/FID. They reported that the major components of essential oils in the Moroccan Spirulina were heptadecane (41.7%) and tetradecane (25.3%) [38]. They also reported for the first time in the Moroccan Spirulina, the presence of geosmin; a key component of odor in cyanobacteria [38].
A new medium was formulated by Amara and Steinbüchel [39] for pharmaceutical grade Arthrospira. The formulated medium was derived from a combination of George's and Zarrouk's media. Amara and Steinbüchel (A-St) medium at concentrations of 1.5–2x (high saline medium) has the ability to inhibit the growth of different forms of cyanobacteria and microalgae including Chlorella, whereas only Arthrospira could survive in this salinity.
The current work aimed to identify volatile compounds and fatty acids composition extracted by hot water from Egyptian Arthrospira fusiformis that was previously isolated from Lake Mariout and authenticated by Sharaf et al. [20]. The hot water extract was analyzed by GC-MS to predict its volatile compounds and fatty acids composition if Arthrospira is consumed as an ingredient in cooking or as a hot extract. Additionally, the in vitro antimicrobial activities of crude phycobiliprotein sodium phosphate extract of Egyptian Arthrospira fusiformis were evaluated against thirteen microbial strains.
2. Materials and Methods
2.1. Microalga Strain
The extreme alkaliphilicArthrospira fusiformis strain used in this study was isolated from the brackish Lake Mariout at the southwest of Alexandria city (Latitude 31.08011° or 31°4′48″ north, longitude 29.79562° or 29° 47′44″ east, Elevation −26 feet, Open location code 8G3F3QJW +26, GeoNames ID 352723). Arthrospira fusiformis was identified via sequencing and analysis of the phycocyanin intergenic spacer region (PC-IGS) in the phycocyanin gene (Accession CBA13040, phycocyanin alpha subunit, partial from Limnospira fusiformis LM) by Sharaf et al. [20].
2.2. Growth Medium
The composition of Amara and Steinbüchel (A-St) medium 1.5x is as follows: 13.82 g/l NaHCO3, 10.71 g/l NaCO3, and 0.75 g/l K2HPO4 (part A); 2.25 g/l NaNO3, 0.85 g/l K2SO4, 1.5 g/l NaCl, 0.22 g/l MgSO4.7H2O, 0.011 g/l CaCl2.2H2O, 0.012 g/l FeSO4.2H2O, and 0.1 g/l EDTA-Na2.2H2O (part B); 0.02 g/l ferric citrate (part C); and 0.1 g/l peptone, and 0.01 g/l yeast extract (part D) [39]. All parts of this medium except for part D were filter sterilized separately through a syringe filter of 0.22 μm pore size from TPP (St. Louis, MO), while part D was autoclaved. This medium at 1.5x concentration was used for cultivation of the investigated Arthrospira fusiformis strain to inhibit the growth of Chlorella which was contaminating the seed culture of Arthrospira.
2.3. Growth Conditions
Arthrospira fusiformis was cultivated in Amara and Steinbüchel medium in 20 l bottles. These bottles were aerated and agitated through a sterilized plastic tube using an air pump (150 bubbles/min) [40]. Bottles were incubated at 25°C and light from a florescent lamp/sunlight for 9 days at pH 9.3.
2.4. Collection of Microalga
The microalga was collected from 20 l cultivation bottles, washed ten times with double distilled water and dispersed in a mesh bottom frame, and dried at 40°C in an oven until the moisture content reached approximately 13%. The samples were then homogenized using a grinder prior to the extraction method.
2.5. Preparation of Water Extract from Microalga
Distilled water (100 ml) was added to 1 g of the dried samples, and the samples were autoclaved at 121°C for 15 min to remove microbes [41, 42]. The autoclaved samples were centrifuged at 2220 ×g for 10 min, and then the supernatant was collected as the water extract solution and lyophilized [43]. We used hot water extraction only to analyze the components consumed by people if they use Spirulina as a hot extract or food ingredient in cooking. Algal hot water extract was scanned by GC-MS (Scion GC-456, Netherlands) in Central Laboratory for Scientific Services and Environmental Assessment of SRTA-City (Alexandria, Egypt) to evaluate its volatile compounds and fatty acids composition.
2.6. Extraction of Phycobiliproteins
The dried biomass (1 g) was homogenized with 0.1 M sodium phosphate buffer (pH 7) and repeated freezing (at −20°C for 3 h) and thawing (at 4°C for 5 min) were done in dark. Subsequently, the mixture was centrifuged at 10,000g and 4°C for 20 min to separate clear supernatant that contains phycobiliproteins [44]. A supernatant sample was run on 12.5% SDS-PAGE. The absorbance of supernatant samples was evaluated at wavelengths 600, 610, 615, 620, 630, 640, 650, and 652 nm (OPTIZEN Scan UV/VIS spectrophotometer, KLab Co., Daejeon, Republic of Korea) for C-phycocyanin.
The concentration of C-phycocyanin was calculated as previously reported by Siegelman and Kycia [45] as follows:
| (1) |
where A615 is the absorbance measured at 615 nm and A652 is the absorbance measured at 652 nm.
2.7. Test Microorganisms for Antimicrobial Activity
The antimicrobial activity of phycobiliprotein extract from Arthrospira fusiformis was tested against thirteen microbial strains. Previously identified methicillin-resistantStaphylococcus aureus (MRSA) clinical isolate obtained from the blood of a patient at Almery University Hospital (Alexandria, Egypt) was used in this study [46]. Candida albicans ATCC 10231 and Staphylococcus aureus ATCC 25923 strains were obtained from Becton Dickinson (France). Salmonella typhi ATCC 19430, Escherichia coli ATCC 25922, Salmonella typhimurium LT2, and Shigella sonnei ATCC 25931 were purchased from an American-type culture collection (ATCC, USA). Aspergillus niger, Aspergillus flavus, Klebsiella pneumonia, Pseudomonas aeruginosa, Serratia marcescens, and Proteus vulgaris were collected from Mycology Center of Al-Azhar University (Cairo, Egypt), and Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Assiut Branch (Egypt). A culture aliquot (100 μl) of each strain of bacteria was added to Luria Bertani (LB) broth, incubated overnight at 37°C, and then stored in 20% glycerol at −80°C to be used as seeds stock [47]. Stock cultures of C. albicans, A. niger, and A. flavus were maintained on potato dextrose agar (PDA) overnight at 30°C for C. albicans and at 25°C for 5 days for A. niger and A. flavus [47]. To evaluate antibacterial activity, cation-adjusted Mueller–Hinton (CAMH) broth and Mueller–Hinton agar were used, while antifungal activity was evaluated using potato dextrose broth and potato dextrose agar.
2.8. Antimicrobial Activities of Phycobiliprotein Extract from Arthrospira fusiformis
Susceptibility screening of test microorganisms to phycobiliprotein extract from Arthrospira fusiformis and different antibacterial and antifungal standards was performed using the agar well diffusion technique on Mueller-Hinton agar and Potato dextrose agar for bacteria and fungi, respectively [47]. Plates were overlaid with 100 μl of an overnight culture of each bacterial pathogen at a cell density of 106 CFU/ml, and 100 μl of an overnight culture of C. albicans at a cell density of 104 cells/ml based on McFarland turbidity standard [47, 48]. Potato dextrose agar plates were overlaid with 1 ml fungal suspension (6 mm disc of the fungal growth suspended in sterile water) of 5 days old culture of A. niger and A. flavus. Then, wells were cut in the agar media by using a sterile 6 mm cork borer and filled with 100 μl of phycobiliprotein extract at a concentration of 0.93 mg/ml. Fusidic acid standard disc obtained from Mast Diagnostics (Merseyside, UK), chloramphenicol from Bioshop (Ontario, Canada), or amphotericin-B purchased from HyClone (Logan, Utah, USA) were used as a positive control, while sterile water was used as a negative control. The culture plates were kept at 4°C for 2 h to allow proper diffusion of tested antimicrobials through the inoculated media before being incubated at 30°C and 37°C for 24 h in the case of C. albicans and bacterial cultures, respectively, and for 5 days at 25°C in case of fungal cultures. The presence of the inhibition zones was examined and recorded in (mm) of three replicates.
2.9. Broth Microdilution Susceptibility Assay
The minimum inhibitory concentrations (MICs) of phycobiliprotein extract from Arthrospira fusiformis against test pathogens were determined by broth microdilution technique [49]. Two 96-well microtiter plates (Greiner, Frickenhausen, Germany) were inoculated with test microorganisms that were susceptible to the phycobiliprotein extract in the agar well diffusion assay, and then 100 μl of CAMH broth or potato dextrose broth containing phycobiliprotein extract in two-fold serial dilutions were added. The concentrations of phycobiliprotein extract ranged from 58.12 μg/ml to 0.93 mg/ml. The plate inoculated with bacteria and C. albicans was incubated at 30°C for 24 h, whereas the plate inoculated with fungi was incubated at 25°C for 5 days. The MICs were determined by measuring the absorbance at 600 nm to test bacterial pathogens and C. albicans and calculating fungal sporulation using a hemocytometer. All MIC determinations were performed in duplicate. The MIC was defined as the lowest concentration at which growth was completely inhibited. Bacteria in CAMH broth and fungi in potato dextrose broth were used as control.
2.10. Statistical Analysis
The susceptibility assay of test microorganisms to phycobiliprotein extract from Arthrospira fusiformis was carried out in triplicate and the obtained results were demonstrated as mean ± SD of triplicate. Data analysis was done by using Student's t-test and McNemar's test. A P value of less than 0.05 was regarded as statistically significant.
3. Results
3.1. GC-MS Analysis of Algal Hot Water Extract
The percentage composition of the volatile compounds and fatty acids identified in the hot water extract of Arthrospira fusiformis isolated from Lake Mariout and cultivated in Amara and Steinbüchel medium is presented in Table 1. A total of two fatty acids, one fatty acid methyl ester, and three volatile compounds were identified. The major components of A. fusiformis fatty acids were hexadecanoic acid (palmitic acid, 55.19%) and octadecanoic acid (stearic acid, 27.14%). The main constituents of its volatile compounds were acetic acid (43.33%) and oxalic acid (47.98%). GC-MS chromatograms of fatty acids and volatile compounds composition of algal hot water extract are presented in Figures S1 and S2. GC-MS chromatogram of algal hot water extract revealed the presence of a secondary metabolite or bioactive (2H-Pyran, tetrahydro-2-(12-pentadecynyloxy), and monoterpenoid (3,7-dimethyl-1,6-octadiene).
Table 1.
The percentage composition of the volatile compounds and fatty acids identified in hot water extract of Arthrospira fusiformis isolated from lake Mariout.
| No | Compounds | Type | Percentage (%) |
|---|---|---|---|
| 1 | Methyl tetradecanoate | Fatty acid methyl ester | 10.31 |
| 2 | 9-Octadecenoic acid methyl ester | Fatty acid methyl ester | 0 |
| 3 | Hexadecanoic acid, methyl ester (palmitic acid) | Fatty acid | 55.19 |
| 4 | Phthalic acid, cyclobutyl decyl ester | Natural phthalate esters | 2.77 |
| 5 | 2H-pyran, tetrahydro-2-(12-pentadecynyloxy) | Secondary metabolites (bioactive) | 3.7 |
| 6 | 9,12-octadecadienoic acid, methyl ester (linoleic acid) | Fatty acid | 0 |
| 7 | 3,7-Dimethyl-1,6-octadiene | Monoterpenoids found in the essential oils | 0.89 |
| 8 | Octadecanoic acid, methyl ester (stearic acid) | Fatty acid | 27.14 |
| 9 | Acetic acid | Volatile compound | 43.33 |
| 10 | Oxalic acid, isobutyl pentyl ester | Volatile compound | 47.98 |
| 11 | Benzo [1,3] dioxole-5-carboxylic acid | Volatile compound | 0 |
| 12 | Isoamyl nitrite | Volatile compound | 8.69 |
3.2. Extraction of Phycobiliproteins
The phycobiliproteins extraction using 0.1 M sodium phosphate buffer (pH 7) yielded 0.93 ± 0.1 mg/ml phycobiliproteins. SDS-PAGE analysis of phycobiliproteins extract is presented in Figure 1. The visible spectrum (at wavelengths 600–650 nm) of C-phycocyanin in phycobiliproteins extract is illustrated in Figure 2. The concentration of C-phycocyanin (mg/ml) calculated by the equation of Siegelman and Kycia [45] was 0.308. The purity of C-phycocyanin in phycobiliproteins extract calculated by dividing A615 (=2.094) by A280 (=2.55) was 0.82.
Figure 1.
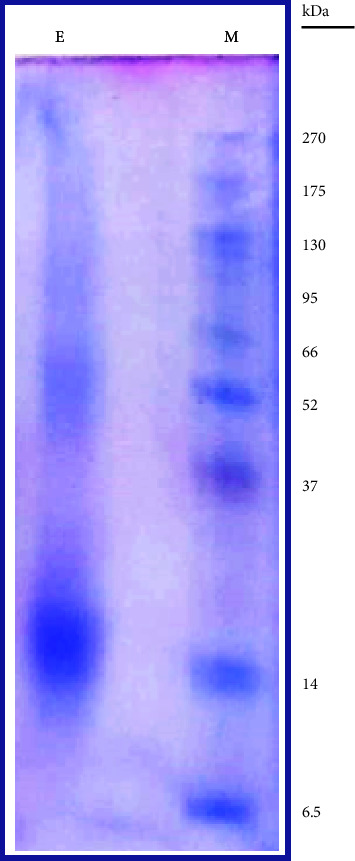
12.5% SDS-PAGE analysis of crude phycobiliproteins extract. M: protein molecular weight marker. E: phycobiliproteins extract containing a dense band of more than 14 kDa corresponding to phycocyanin subunits.
Figure 2.
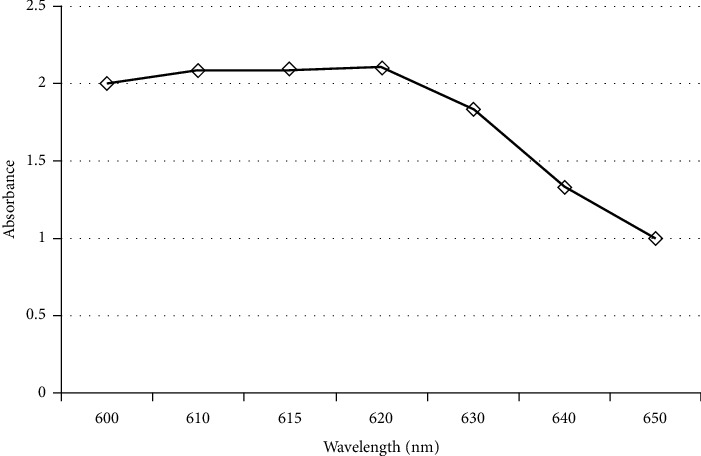
Visible spectrum (at wavelengths 600–650 nm) of C-phycocyanin in phycobiliproteins extract.
3.3. Antimicrobial Activities of Phycobiliprotein Extract from Arthrospira fusiformis
The test microorganisms differed in their susceptibility to phycobiliprotein extract from Arthrospira fusiformis. The Gram-positive bacteria MRSA and S. aureus in addition to the Gram-negative bacteria P. aeruginosa, K. pneumonia, and S. sonnei were resistant (no inhibition zones) to the phycobiliprotein extract. The most potent antimicrobial effect of phycobiliprotein extract was obtained against two Gram-negative bacteria Salmonella typhi and Proteus vulgaris, filamentous fungus Aspergillus niger, and the yeast Candida albicans (with the largest inhibition zone of 20 mm) as recorded in Table 2. Test microorganisms that showed susceptibility (inhibition zones) to phycobiliprotein extract from Arthrospira fusiformis are demonstrated in Figure S3.
Table 2.
Susceptibility of test microorganisms to phycobiliprotein extract from Arthrospira fusiformis.
| Test strains | Mean diameter of inhibition zonea (mean ± SD) | |||
|---|---|---|---|---|
| Phycobiliprotein extract | C | AMP | FC | |
| MRSA | R | R | NT | 22 ± 1.63 |
| S. aureus | R | 14.66 ± 1.24 | NT | NT |
| E. coli | 9 ± 0.81 | 14 ± 1.63 | NT | NT |
| S. typhi | 19.66 ± 0.94 | 18.33 ± 0.47 | NT | NT |
| S. typhimurium | 10.33 ± 0.47 | 22 ± 1.63 | NT | NT |
| P. aeruginosa | R | 8 ± 0.81 | NT | NT |
| K. pneumonia | R | R | NT | NT |
| S. sonnei | R | 12 ± 0.81 | NT | NT |
| P. vulgaris | 14 ± 0.81 | 24.33 ± 0.47 | NT | NT |
| S. marcescens | 5.33 ± 0.47 | 14.66 ± 0.47 | NT | NT |
| C. albicans | 20 ± 1.63 | NT | 13 ± 0.81 | NT |
| A. niger | 12.66 ± 0.47 | NT | 14.33 ± 0.47 | NT |
| A. flavus | 7.66 ± 0.47 | NT | 7.66 ± 0.47 | NT |
aMean of three assays; C-chloramphenicol and FC-fusidic acid antibacterial standards at concentrations of 50 and 10 μg/ml, respectively; AMP-amphotericin-B antifungal standard at a concentration of 100 μg/ml; R-resistant (no inhibition zone); NT-not tested.
3.4. Broth Microdilution Susceptibility Assay
Results of broth microdilution susceptibility assay revealed that S. typhi, P. vulgaris, C. albicans, and A. niger were the most susceptible test organisms with minimal inhibition concentration of 58.1 μg/ml, while the Gram-negative bacterium S. marcescens and the filamentous fungus A. flavus were the least susceptible ones (MIC of 232.5 μg/ml) (Table 3).
Table 3.
MIC values of phycobiliprotein extract from Arthrospira fusiformis against tested susceptible pathogens.
| Test strains | MIC values (μg/ml) |
|---|---|
| E. coli | 116.2 |
| S. typhi | 58.1 |
| S. typhimurium | 116.2 |
| P. vulgaris | 58.1 |
| S. marcescens | 232.5 |
| C. albicans | 58.1 |
| A. niger | 58.1 |
| A. flavus | 232.5 |
4. Discussion
The prokaryotic microalga Arthrospira fusiformis was isolated from soda lakes, seawater, as well as freshwater [50]. Commercial production of this microalga started about three decades ago and currently, Arthrospira fusiformis or Spirulina is widely consumed in vitamin supplements, nutraceuticals, and pharmaceuticals besides other applications in food dyes, aquaculture, and fish food [8, 30, 38, 51]. Arthrospira fusiformis provides its consumer with high protein content (percent composition of 60–70 by wet weight), which is considered as a complete protein (comprising eight essential amino acids such as isoleucine, leucine, in addition to valine, nevertheless with lower cysteine, methionine, and lysine content compared to standard proteins and ten nonessential amino acids) [10, 11, 52]. It also provides carbohydrates, polyunsaturated fatty acids, vitamins, essential minerals, bioactive peptides, and pigments [53, 54]. Arthrospira fusiformis contains predominantly two phycobiliproteins (C-phycocyanin and allophycocyanin) [55]. Both phycobiliproteins have various applications as potential pharmaceuticals in oxidative stress-induced diseases and promising phycocyanin-based anticancer treatments [56, 57]. Furthermore, phycobiliproteins isolated from Arthrospira fusiformis were proved to have antimicrobial activity [37]. C-phycocyanin is a blue natural pigment commonly used as a natural colorant for food additive purposes and has also anti-inflammatory activities [58, 59]. Even fatty acids from Arthrospira fusiformis were found to have in vitro antibacterial activity against Staphylococcus aureus and Streptococcus pyogenes [37]. The effective health benefits of Arthrospira fusiformis have been extensively detailed in many scientific reports [10, 20, 21, 23, 26–30, 60–62].
In 2010, Sharaf et al. [20] isolated a new Arthrospira fusiformis strain from the brackish Lake Mariout at the southwest of Alexandria city in Egypt. The strain was proved to have unique tolerance for salinity, and its phosphate buffer and hot water extracts could directly inactivate herpes simplex viral particles before infection of host cells. They recommended applying Spirulina crude extracts as a treatment for recurrent herpetic infection [20].
In this study, we identified volatile compounds and fatty acids composition extracted by hot water from Arthrospira fusiformis isolated by Sharaf et al. [20] and tested the antimicrobial activities of its phycobiliprotein extract using sodium phosphate buffer. The obtained results revealed that two fatty acids, one fatty acid methyl ester, and three volatile compounds were identified in the hot water extract of Arthrospira fusiformis. The major components of its fatty acids were palmitic acid (55.19%), and stearic acid (27.14%). Our results support and agree with a previous report by Abd El-Hameed et al. [63], which demonstrated that adding Spirulina platensis (Arthrospira fusiformis) to raw and cooked spaghetti leads to an increase of five fatty acids including palmitic acid, and stearic acid. They also observed that the fatty acid profile of spaghetti prepared with the incorporation of Spirulina platensis has a higher resistance to the thermal treatment applied during the cooking [63]. The main constituents of volatile compounds in hot extract were acetic acid (43.33%), and oxalic acid (47.98%). GC-MS chromatogram of algal hot water extract also revealed the presence of a secondary metabolite, 2H-Pyran, tetrahydro-2-(12-pentadecynyloxy, with a percentage of 3.7. This secondary metabolite has potential application as an inhibitor to non-small cell lung cancer as well as antibacterial and antioxidant activities [64, 65].
In the present study, results obtained from antimicrobial activities assays of the phycobiliprotein extract of Arthrospira fusiformis revealed that eight of the tested bacterial and fungal pathogens were susceptible to the phycobiliprotein extract but the most potent antimicrobial effects were observed against Salmonella typhi, Proteus vulgaris, Aspergillus niger, and Candida albicans. No antibacterial effect was found on methicillin-resistant as well as susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Shigella sonnei. These results agree in part with previous reports by Murugan [66], and Zamani et al. [67], which showed that C-phycocyanin of Arthrospira fusiformis has antifungal activities against Aspergillus niger, Aspergillus flavus, and Candida albicans. These results also are in agreement with previous studies by Sun et al. [22], Sadeghi et al. [23], and Abdel-Moneim et al. [24], which confirmed antibacterial activities of Spirulina platensis extracts, proteins, and peptides against selected pathogenic bacteria. However, our data disagree with the previous study by Sarada et al. [68], which demonstrated that C-phycocyanin from Spirulina platensis (Nordstedt) Geitler could inhibit the growth of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus.
The results obtained from this work suggest the potential use of biomass of A. fusiformis isolated from Lake Mariout as a food additive. Our findings combined with the results obtained by Sharaf et al. [20, 21] also recommend that A. fusiformis isolated from Lake Mariout is a potential antimicrobial against some bacterial and fungal pathogens and antiviral therapeutic.
5. Conclusion
There is a growing interest in the use of microalga Arthrospira fusiformis not only for food applications but also as safe pharmaceutical products with antimicrobial and antioxidant activities as well as corrective properties against tumor growth, anemia, and malnutrition. Therefore, we cultivated a previously isolated Egyptian Arthrospira fusiformis in the laboratory and analyzed its volatile compounds and fatty acids composition in the hot extract. We used hot water extraction only to analyze the components (volatile compounds and fatty acids) consumed by people if they use Spirulina as a hot extract or food ingredient in cooking. The antimicrobial activity of its phycobiliprotein extract was also evaluated against thirteen pathogens, eight of these pathogens were susceptible to the phycobiliprotein extract. The major fatty acids found were palmitic acid and stearic acid, which suggests the promising application of Egyptian A. fusiformis biomass as an ingredient in the cooking of some foods such as pasta or bread to increase the level of the two most commonly consumed saturated fatty acids; stearic acid and palmitic acid. Overall, our findings revealed that A. fusiformis isolated from Lake Mariout has nutritional as well as effective antimicrobial properties and suggest the potential therapeutic use of its biomass.
Acknowledgments
The authors are grateful to Neama Mahmoud Fattouh Rezk, Chemist in the Protein Research Department, Genetic Engineering and Biotechnology Research Institute, City of Scientific Research and Technological Applications, for her support and help in conducting visible spectrophotometry scanning and antimicrobial assays.
Contributor Information
Nawal Abd El-Baky, Email: nelbaky@srtacity.sci.eg.
Amro A. Amara, Email: amroamara@web.de.
Data Availability
Data are contained within the article and supplementary materials.
Ethical Approval
Not applicable.
Consent
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Gamal M. Hamad and Nawal Abd El-Baky contributed equally to this work. N. A. E. B. and A. A. A. conceived the research topic and designed the research. M. M. S. and A. A. A. isolated and identified the microalga strain. G. M. H. conducted hot water extraction and GC-MS experiments and collected their data. N. A. E. B. conducted the remaining experimental work. N. A. E. B. collected and analyzed data. G. M. H., N. A. E. B., and A. A. A. wrote the manuscript. A. A. A. proofreads and revives the manuscript. N. A. E. B. finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Supplementary Materials
Figure S1: GC-MS chromatogram of fatty acids composition of algal hot water extract. Figure S2: GC-MS chromatogram of volatile compounds composition of algal hot water extract. Figure S3: Test microorganisms that showed susceptibility to phycobiliprotein extract from Arthrospira fusiformis.
References
- 1.Venkataraman L. Spirulina platensis (Arthrospira): physiology, cell biology and biotechnology, edited by avigad vonshak. Journal of Applied Phycology . 1997;9:295–296. [Google Scholar]
- 2.Samarakoon K., Jeon Y.-J. Bio-functionalities of proteins derived from marine algae—a review. Food Research International . 2012;48(2):948–960. doi: 10.1016/j.foodres.2012.03.013. [DOI] [Google Scholar]
- 3.Batista A. P., Gouveia L., Bandarra N. M., Franco J. M., Raymundo A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Research . 2013;2:164–173. doi: 10.1016/j.algal.2013.01.004. [DOI] [Google Scholar]
- 4.Mühling M., Belay A., Whitton B. A. Screening Arthrospira (Spirulina) strains for heterotrophy. Journal of Applied Phycology . 2005;17(2):129–135. doi: 10.1007/s10811-005-7214-8. [DOI] [Google Scholar]
- 5.Busnel A., Samhat K., Gérard E., et al. Development and validation of a screening system for characterizing and modeling biomass production from cyanobacteria and microalgae: application to Arthrospira platensis and Haematococcus pluvialis. Algal Research . 2021;58 doi: 10.1016/j.algal.2021.102386.102386 [DOI] [Google Scholar]
- 6.Akhtar N., Ahmeda M. M., Sarker N., Mahbuba K. R., Sarker A. M. Growth response of Spirulina platensis in papaya skin extract and antimicrobial activities of Spirulina extracts in different culture media. Bangladesh Journal of Scientific and Industrial Research . 2012;47(2):147–152. doi: 10.3329/bjsir.v47i2.11445. [DOI] [Google Scholar]
- 7.Chirasuwan N., Chaikhalan R., Ruengjitchatchawalya M., Bunnag B., Tanticharoen M. Anti HSV-1 activity of Spirulina platensis polysaccharide. Kasetsart Journal . 2007;41:311–318. [Google Scholar]
- 8.Habib M. A. B., Parvin M., Huntington T. C., Hasan M. R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. FAO Fisheries And Aquaculture Circular. No. 1034 . Rome, Italy: FAO; 2008. [Google Scholar]
- 9.Karkos P. D., Leong S. C., Karkos C. D., Sivaji N., Assimakopoulos D. A. Spirulina in clinical practice: evidence-based human applications. Evidence-based Complementary and Alternative Medicine . 2011;2011:1–4. doi: 10.1093/ecam/nen058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capelli B., Cysewski G. R. Potential health benefits of Spirulina microalgae: a review of the existing literature. Nutrafoods . 2010;9(2):19–26. doi: 10.1007/bf03223332. [DOI] [Google Scholar]
- 11.Vo T. S., Ngo D. H., Kim S. K. Chapter 19-Nutritional and pharmaceutical properties of microalgal spirulina. In: Kim S. K., editor. Handbook of Marine Microalgae . Cambridge, MA, USA: Academic Press; 2015. pp. 299–308. [Google Scholar]
- 12.Zouari N., Abid M., Fakhfakh N., et al. Blue-green algae (Arthrospira platensis) as an ingredient in pasta: free radical scavenging activity, sensory and cooking characteristics evaluation. International Journal of Food Sciences and Nutrition . 2011;62(8):811–813. doi: 10.3109/09637486.2011.582461. [DOI] [PubMed] [Google Scholar]
- 13.Ak B., Av¸saro˘glu E., I¸sık O., Özyurt G., Kafkas E., Uslu L. Nutritional and physicochemical characteristics of bread enriched with microalgae Spirulina platensis. International Journal of Engineering Research in Africa . 2016;6:p. 9. [Google Scholar]
- 14.Yamaguchi S. K. F., Moreira J. B., Costa J. A. V., de Souza C. K., Bertoli S. L., Carvalho L. F. d. Evaluation of adding Spirulina to freeze-dried yogurts before fermentation and after freeze-drying. Industrial Biotechnology . 2019;15(2):89–94. doi: 10.1089/ind.2018.0030. [DOI] [Google Scholar]
- 15.Ould Bellahcen T., Bouchabchoub A., Massoui M., El Yachioui M. Culture et production de Spirulina platensis dans les eaux usées domestiques. Larhyss Journal . 2013;14:107–122. [Google Scholar]
- 16.Probst A. J., Castelle C. J., Singh A., et al. Genomic resolution of a cold subsurface aquifer community provides metabolic insights for novel microbes adapted to high CO2 concentrations. Environmental Microbiology . 2017;19(2):459–474. doi: 10.1111/1462-2920.13362. [DOI] [PubMed] [Google Scholar]
- 17.Bouma-Gregson K., Olm M. R., Probst A. J., Anantharaman K., Power M. E., Banfield J. F. Impacts of microbial assemblage and environmental conditions on the distribution of anatoxin-a producing cyanobacteria within a river network. The ISME Journal . 2019;13(6):1618–1634. doi: 10.1038/s41396-019-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcorta J., Alarcón-Schumacher T., Salgado O., Díez B. Taxonomic novelty and distinctive genomic features of hot spring cyanobacteria. Frontiers in Genetics . 2020;11 doi: 10.3389/fgene.2020.568223.568223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro-Severyn J., Pardo-Esté C., Mendez K. N., et al. Living to the high extreme: unraveling the composition, structure, and functional insights of bacterial communities thriving in the Arsenic-Rich Salar de Huasco Altiplanic ecosystem. Microbiology Spectrum . 2021;9(1) doi: 10.1128/spectrum.00444-21.e0044421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharaf M., Amara A., Aboul-Enein A., et al. Molecular authentication and characterization of the antiherpetic activity of the cyanobacterium Arthrospira fusiformis. Pharmazie . 2010;65(2):132–136. [PubMed] [Google Scholar]
- 21.Sharaf M., Amara A., Aboul-Enein A., Helmi S., Ballot A., Schnitzler P. Antiherpetic efficacy of aqueous extract of the cyanobacterium Arthrospira fusiformis from Chad. Pharmazie . 2013;68(5):376–380. [PubMed] [Google Scholar]
- 22.Sun Y., Chang R., Li Q., Li B. Isolation and characterization of an antibacterial peptide from protein hydrolysates of Spirulina platensis. European Food Research and Technology . 2016;242(5):685–692. doi: 10.1007/s00217-015-2576-x. [DOI] [Google Scholar]
- 23.Sadeghi S., Jalili H., RanaeiSiadat S. O., Sedighi M. Anticancer and antibacterial properties in peptide fractions from hydrolyzed Spirulina protein. Journal of Agriculture, Science and Technology . 2018;20:673–683. [Google Scholar]
- 24.Abdel-Moneim A.-M. E., El-Saadony M. T., Shehata A. M., et al. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi Journal of Biological Sciences . 2022;29(2):1197–1209. doi: 10.1016/j.sjbs.2021.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao T. K., Water J. V. d., Gershwin M. E. Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. Journal of Medicinal Food . 2005;8(1):27–30. doi: 10.1089/jmf.2005.8.27. [DOI] [PubMed] [Google Scholar]
- 26.Vaz B. D. S., Moreira J. B., Morais M. G. d., Costa J. A. V. Microalgae as a new source of bioactive compounds in food supplements. Current Opinion in Food Science . 2016;7:73–77. doi: 10.1016/j.cofs.2015.12.006. [DOI] [Google Scholar]
- 27.Al Fattah Amara A. A. The Antisickling effect of the Arthrospira platensis bilins for liver protection: a modeling, hypothesis, and food for thought. SOJ Biochemistry . 2017;3:1–12. doi: 10.15226/2376-4589/3/1/00123. [DOI] [Google Scholar]
- 28.Wu Q., Liu L., Miron A., Klimova B., Wan D., Kuca K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Archiv für Toxikologie . 2016;90(8):1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 29.Moreira J. B., Lim L.-T., Zavareze E. D. R., Dias A. R. G., Costa J. A. V., Morais M. G. d. Antioxidant ultrafine fibers developed with microalga compounds using a free surface electrospinning. Food Hydrocolloids . 2019;93:131–136. doi: 10.1016/j.foodhyd.2019.02.015. [DOI] [Google Scholar]
- 30.Shao W., Ebaid R., El-Sheekh M., Abomohra A., Eladel H. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): an overview. Grasas Y Aceites . 2019;70(1):p. e292. doi: 10.3989/gya.0690181. [DOI] [Google Scholar]
- 31.Stengel D. B., Connan S., Popper Z. A. Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnology Advances . 2011;29(5):483–501. doi: 10.1016/j.biotechadv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz R., Magro C. D., Colla L. M. Aplicações ambientais de microalgas. Revista CIATEC-UPF . 2012;4(1):48–60. doi: 10.5335/ciatec.v4i1.2393. [DOI] [Google Scholar]
- 33.Milano J., Ong H. C., Masjuki H. H., et al. Microalgae biofuels as an alternative to fossil fuel for power generation. Renewable and Sustainable Energy Reviews . 2016;58:180–197. doi: 10.1016/j.rser.2015.12.150. [DOI] [Google Scholar]
- 34.Özdemir G., Ulku Karabay N., Dalay M. C., Pazarbasi B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytotherapy Research . 2004;18(9):754–757. doi: 10.1002/ptr.1541. [DOI] [PubMed] [Google Scholar]
- 35.Abedin R. M., Taha M. H. Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of medium components by Plackett-Burman design for antimicrobial activity of Spirulina platensis. Global Journal of Biotechnology and Biochemisty . 2008;3:22–31. [Google Scholar]
- 36.Kaushik P., Chauhan A. In vitro antibacterial activity of laboratory grown culture of Spirulina platensis. Indian Journal of Microbiology . 2008;48(3):348–352. doi: 10.1007/s12088-008-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Najdenski H. M., Gigova L. G., Iliev I. I., et al. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. International Journal of Food Science and Technology . 2013;48(7):1533–1540. doi: 10.1111/ijfs.12122. [DOI] [Google Scholar]
- 38.Ould Bellahcen T., Cherki M., Sánchez J. A. C., Cherif A., El Amrani A. Chemical composition and antibacterial activity of the essential oil of Spirulina platensis from Morocco. Journal of Essential Oil Bearing Plants . 2019;22(5):1265–1276. doi: 10.1080/0972060X.2019.1669492. [DOI] [Google Scholar]
- 39.Amara A. A., Steinbüchel A. New medium for pharmaceutical grade Arthrospira. International Journal of Bacteriology . 2013;2013:9. doi: 10.1155/2013/203432.203432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anaga A., Abu G. O. A laboratory scale cultivation of Chlorella and Spirulina using waste effluent from a fertilizer company in Niger. Bioresource Technology . 1996;58(1):93–95. doi: 10.1016/s0960-8524(96)90004-8. [DOI] [Google Scholar]
- 41.Kuda T., Tsunekawa M., Goto H., Araki Y. Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. Journal of Food Composition and Analysis . 2005;18(7):625–633. doi: 10.1016/j.jfca.2004.06.015. [DOI] [Google Scholar]
- 42.Kuda T., Tsunekawa M., Hishi T., Araki Y. Antioxidant properties of dried kayamo-nori, a brown alga Scytosiphon lomentaria (Scytosiphonales, Phaeophyceae) Food Chemistry . 2005;89(4):617–622. doi: 10.1016/j.foodchem.2004.03.020. [DOI] [Google Scholar]
- 43.Rastian Z., Mehranian M., Vahabzadeh F., Sartavi K. Antioxidant activity of extract from a brown alga, Sargassum boveanum. African Journal of Biotechnology . 2007;6(24):2740–2745. doi: 10.5897/ajb2007.000-2438. [DOI] [Google Scholar]
- 44.Sharmaa G., Sarana S., Puri N., Jasuja N. D., kumar M. Optimization, purification and characterization of phycocyanin from Spirulina platensis. International Journal of Applied and Pure Science and Agriculture (IJAPSA) . 2016;2:1–20. [Google Scholar]
- 45.Siegelman H., Kycia J. H. Alga biliproteins. In: Hellebust J. A., Craigie J. S., editors. Handbook of Phycological Methods: Physiological and Biochemical Methods . Cambridge, MA, USA: Cambridge University Press; 1978. pp. 72–78. [Google Scholar]
- 46.Redwan E. M., El-Baky N. A., Al-Hejin A. M., et al. Significant antibacterial activity and synergistic effects of camel lactoferrin with antibiotics against methicillin-resistantStaphylococcus aureus (MRSA) Research in Microbiology . 2016;167(6):480–491. doi: 10.1016/j.resmic.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Abd El-Baky N. Differential antimicrobial effectiveness of camel lactoferrin-oleic acid and bovine lactoferrin-oleic acid complexes against several pathogens. SOJ Biochemistry . 2018;4:1–9. doi: 10.15226/2376-4589/4/1/00128. [DOI] [Google Scholar]
- 48.Heritage J., Evan S., Killington R. A. Introductory Microbiology . Cambridge, NY, USA: Cambridge University Press; 1996. [Google Scholar]
- 49.Cockerill F. R., Wikler M. A., Alder J., et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard; CLSI Document M07-A9 . Pennsylvania, PA, USA: Clinical and Laboratory Standard Institute); 2012. [Google Scholar]
- 50.Agustini T. W., Suzery M., Sutrisnanto D., Ma’ruf W. F. Comparative study of bioactive substances extracted from fresh and dried Spirulina sp. Procedia Environmental Sciences . 2015;23:282–289. doi: 10.1016/j.proenv.2015.01.042. [DOI] [Google Scholar]
- 51.Belay A., Ota Y., Miyakawa K., Shimamatsu H. Current knowledge on potential health benefits of Spirulina. Journal of Applied Phycology . 1993;5(2):235–241. doi: 10.1007/bf00004024. [DOI] [Google Scholar]
- 52.Benelhadj S., Gharsallaoui A., Degraeve P., Attia H., Ghorbel D. Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chemistry . 2016;194:1056–1063. doi: 10.1016/j.foodchem.2015.08.133. [DOI] [PubMed] [Google Scholar]
- 53.Yücetepe A., Özçelik B. Bioactive peptides isolated from microalgae Spirulina platensis and their biofunctional activities. Akademik Gıda . 2016;14:412–417. [Google Scholar]
- 54.Soni R. A., Sudhakar K., Rana R. S. Spirulina – from growth to nutritional product: a review. Trends in Food Science and Technology . 2017;69:157–171. doi: 10.1016/j.tifs.2017.09.010. [DOI] [Google Scholar]
- 55.Patil G., Chethana S., Madhusudhan M. C., Raghavarao K. S. M. S. Fractionation and purification of the phycobiliproteins from Spirulina platensis. Bioresource Technology . 2008;99(15):7393–7396. doi: 10.1016/j.biortech.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 56.Braune S., Krüger-Genge A., Kammerer S., Jung F., Küpper J.-H. Phycocyanin from Arthrospira platensis as potential anti-cancer drug: review of in vitro and in vivo studies. The Life . 2021;11(2):p. 91. doi: 10.3390/life11020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dagnino-Leone J., Figueroa C. P., Castañeda M. L., et al. Phycobiliproteins: s. Computational and Structural Biotechnology Journal . 2022;20:1506–1527. doi: 10.1016/j.csbj.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chentir I., Hamdi M., Li S., Doumandji A., Markou G., Nasri M. Stability, bio-functionality and bio-activity of crude phycocyanin from a two-phase cultured Saharian Arthrospira sp. Strain. Algal Research . 2018;35:395–406. doi: 10.1016/j.algal.2018.09.013. [DOI] [Google Scholar]
- 59.Pez Jaeschke D., Rocha Teixeira I., Damasceno Ferreira Marczak L., Domeneghini Mercali G. Phycocyanin from Spirulina: a review of extraction methods and stability. Food Research International . 2021;143 doi: 10.1016/j.foodres.2021.110314.110314 [DOI] [PubMed] [Google Scholar]
- 60.Gogineni V., Hamann M. T. Marine natural product peptides with therapeutic potential: chemistry, biosynthesis, and pharmacology. Biochimica et Biophysica Acta (BBA) - General Subjects . 2018;1862(1):81–196. doi: 10.1016/j.bbagen.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bilal T., Altiner A. Effect of Spirulina Platensis on some serum markers, performance metrics and organ weights, in rats fed with hydrogenated vegetable oil and/or cholesterol. VMPH . 2020;1(2):60–69. doi: 10.31559/vmph2020.1.2.6. [DOI] [Google Scholar]
- 62.Żymańczyk-Duda E., Samson S. O., Brzezińska-Rodak M., Klimek-Ochab M. Versatile applications of cyanobacteria in biotechnology. Microorganisms . 2022;10 doi: 10.3390/microorganisms10122318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abd El-Hameed M. K., Abou El-Maatti S. M., El-Saidy S. M. E., Ahmed S. M. Effect of adding Spirulina platensis in pasta products (spaghetti) Zagazig Journal of Agricultural Research . 2018;45(1):293–300. doi: 10.21608/zjar.2018.49852. [DOI] [Google Scholar]
- 64.Senthilraja P., Kayitare J., Manivel G., Manikandaprabhu S., Krishnamurthy A. Potential compound derived from Catharanthus roseus to inhibit non small cell lung cancer (NSCLC) International Journal of Research in Ayurveda and Pharmacy . 2015;6(2):265–271. doi: 10.7897/2277-4343.06254. [DOI] [Google Scholar]
- 65.El-Sayed N. N. E., Zaki M. E. A., Al-Hussain S. A., et al. Synthesis and evaluation of some new 4H-Pyran derivatives as antioxidant, antibacterial and anti-HCT-116 cells of CRC, with molecular docking, antiproliferative, apoptotic and ADME investigations. Pharmaceuticals . 2022;15(7):p. 891. doi: 10.3390/ph15070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murugan T. Screening for antifungal and antiviral activity of C-phycocyanin from Spirulina platensis. Journal of Pharmacy Research . 2011;4:4161–4163. [Google Scholar]
- 67.Zamani N., Fazilati M., Salavati H., Koohi-Dehkordi M. Evaluation of antifungal activity of purified phycocyanin from Spirulina platensis cultured in Iran against Candida albicans. Experimental Animal Biology . 2020;8:57–66. [Google Scholar]
- 68.Sarada D. V. L., Sreenath Kumar C., Rengasamy R. Purified C-phycocyanin from Spirulina platensis (Nordstedt) Geitler: a novel and potent agent against drug resistant bacteria. World Journal of Microbiology and Biotechnology . 2011;27(4):779–783. doi: 10.1007/s11274-010-0516-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: GC-MS chromatogram of fatty acids composition of algal hot water extract. Figure S2: GC-MS chromatogram of volatile compounds composition of algal hot water extract. Figure S3: Test microorganisms that showed susceptibility to phycobiliprotein extract from Arthrospira fusiformis.
Data Availability Statement
Data are contained within the article and supplementary materials.


