Abstract
Skin areas exposed to ultraviolet radiation (UV) from sunlight are more prone to photoaging than unexposed areas evidenced by several signs which include skin dryness, irregular pigmentation, lentigines, hyperpigmentation, wrinkling, and decreased elasticity. Plant-based natural product ingredients with therapeutic potential against skin photoaging are gaining more attention. This article aims the reviewing the research work done in exploring the cellular and molecular mechanisms involved in UV-induced skin photoaging, followed by summarizing the mechanistic insights involved in its therapeutics by natural product-based ingredients. In the mechanistic section of the convoluted procedure of photoaging, we described the effect of UV radiation (UVR) on different cellular macromolecules (direct damage) and subsequently, the deleterious consequences of UVR-generated reactive oxygen species (indirect damage) and signaling pathways activated or inhibited by UV induced ROS generation in various cellular pathologies of skin photoaging like inflammation, extracellular matrix degradation, apoptosis, mitochondrial dysfunction, and immune suppression. We also discussed the effect of UV radiation on the adipose tissue, and transient receptor potential cation channel V of photoaging skin. In the past few decades, mechanistic studies performed in this area have deciphered various therapeutic targets, opening avenues for different available therapeutic options against this pathological condition. So the remaining portion of the review deals with various natural product-based therapeutic agents available against skin photodamage.
Keywords: Ultraviolet radiations, Skin, Photoaging, Molecular mechanism, Natural products
1. Introduction
-Irradiation of Ultraviolet (UV) radiations to human skin over some time leads to a pathological condition characterized by various changes at morphological, histological, biochemical, and molecular levels and is termed photoageing [1]. The term photoaging was coined in 1986 by Kligman and Kligman and was interchangeably used with the term dermatoheliosis [2]. For the pathogenesis of photoaging, UV radiations (photons) need to be absorbed by chromophores (First law of photochemistry) in the skin followed by a series of photochemical events to bring about the clinical signs of photoaging and photo carcinogenesis [3]. UV radiations mainly consist of three components UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (100–280 nm) [4]. UVC radiations are also called shortwave or ionizing radiations and are highly energetic [5]. These radiations do not reach the earth's surface, as atmospheric gases like Ozone absorb these, thereby protecting the life forms from the ionizing effect of this component of UV radiations [6]. UV-A and UV-B radiations pass through the ozone layer and reach the earth's surface [7]. However, these radiations lack ionization potential due to their less energy. Still, these can alter the chemical bonding of various macro and micro molecules hence disturbing their structure and normal functioning [8]. Niels Ryberg Finsen, a Danish physician and scientist, for the first time in his study “Om Lysates Indvirkninger paa Huden” (“On the effects of light on the skin”), and “Om Anvendelse i Medicine af koncentrerede kemiske Lysstraaler (“The use of concentrated chemical light rays in medicine”) showed that light could be beneficial and harmful to the skin. This study served as a model for the research of photoaging and photodamage for the next fifty years [9]. The photoaging process occurs due to repetitive exposure to chronic ultraviolet radiation to the skin and involves mainly three types of skin cells, i.e., fibroblasts, keratinocytes, and infiltrating neutrophils [10]. The main symptoms of photoaged skin involve laxity, deep wrinkles, increased fragility, and dryness [11]. Microscopic observations have revealed that photoaged skin has increased epidermal thickness and shows the accumulation of dystrophic elastin and disorganized collagen in the deeper epidermal layer [12]. At cellular levels, photoaged skin has changed the morphology of keratinocytes with irregular morphology and loss of polarity [10]. The changes -induced by UV -irradiation are similar to that of the chronological changes [13]. The degree of photoaging may vary; depending on skin type, ethnicity, and geographic location [14]. Susceptibility of skin to the UV induced damage depends upon the content of melanin a particular skin type, of Fitzpatrick scale, contains. According to which, skin types I, II, and III are more susceptible to photoaging than type IV, V and VI [15,16] (see Table 1).
Table 1.
Summarizes the therapeutic agents and their mechanism of action against skin photoaging.
| S.No | Ingredients | Name of Plant | Mechanism of action | Experimental Model | References |
|---|---|---|---|---|---|
| 1. | Ginsenoside | Ginseng | Suppression of UV -induced apoptosis by inducing DNA repair | HaCaT cells,Xpc− knockout mouse keratinocytess | [17] |
| 2. | Aucubin | Eucommia ulmoides | Protection against UV-B -induced oxidative stress | Human skin fibroblaststs | [18] |
| 3. | Vanillin and Vanillic acid | Origanum vulgare | Antioxidative characteristics and inhibition of α-MSH stimulated melanogenesis | B16F0 melanoma | [19] |
| 4. | Xanthorrhizol | Curcuma xanthorrhiza Roxb. | Protection against photoaging | Human skin fibroblasts | [20] |
| 5. | Parthenolide | Feverfew (Tanacetum parthenium) | Prevention of UV-B mediated skin photoaging via NFkB inhibition | HaCaT cell line, Normal epidermal melanocytes, Human normal fibroblast |
[21] |
| 6. | Acteoside | Clerodendron trichotomum Thunberg | Inhibition of α-MSH -induced melanin production via inactivation of adenyl cyclase | B16 melanoma | [22] |
| 7. | Proanthocyanidins | Grape Seed | Inhibition of UV -induced oxidative stress via MAPK and NF-kB | Epidermal keratinocytes | [23] |
| 8. | Flavanone glycosides | Citrus hassaku | Inhibition of melanogenesis | B16 melanoma | [24] |
| 9. | Xanthorrhizol | Curcuma xanthorrhiza Roxb. | Protection against photoaging | Human skin fibroblasts | [20] |
| 10. | Amla extract | Emblica officinalis | Effect against UV-B -induced photoaging | Human skin fibroblasts | [25] |
| 11. | Naringenin | Grape fruit | Protection against UV-B -induced apoptosis by removal of cyclobutane pyrimidine dimers | HaCaT cells | [26] |
| 12. | Chloroform extract | Moricandia arvensis | Inhibition of growth of melanoma cells and promoting differentiation | B16 F0 melanoma cells | [27] |
| 13. | Xanthohumol | Humulus lupulus | Inhibition of melanogenesis | B16 F10 melanoma cells | [28] |
| 14. | Epigallocatechin gallate | Green tea | Protection against photoaging via interfering with MAPK responsive pathways | Human dermal fibroblasts | [29] |
| 15. | Polypodium leucotomos extract | Polypodium leucotomos | Photoprotection by inhibition of ROS production and prevention of DNA damage |
Hairless rat model | [30] |
| 16. | Labisia pumila extract | Labisia pumila | Anti-photoaging effects | Normal Human dermal fibroblasts | [31] |
| 17. | Delphinidin, an Anthocyanidin | Pigmented Fruits and Vegetables |
Protection against UV-B- mediated oxidative stress and apoptosis | Human HaCaT Keratinocytes and SKH-1 hairless mouse |
[32] |
| 18. | Artocarpanone | Artocarpus heterophyllus | Inhibition of melanin biosynthesis | B16 melanoma | [33] |
| 19. | Dichloromethane fraction | Cimicifuga heracleifolia | Inhibition of melanin synthesis by activating the ERK or AKT signaling pathway | B16 F10 melanoma | [34] |
| 20. | S. lycopersicum fruit extract | Solanum lycopersicum | Anticarcinogenic Effects | Swiss Albino and C57 Bl Mice | [35] |
| 21. | Silymarin, a flavonoid | Silybum marianum | Inhibitio of UV -induced apoptosis | Human malignant melanoma A375-S2 cells | [36] |
| 22. | Galloylated Tannins | Witch Hazel (Hamamelis virginiana) | Antioxidant, cytotoxic, & antiproliferative | HaCat, SK-Mel 28 melanoma | [37] |
| 23. | PCF, a Polypeptide |
Chlamys farreri | Inhibitory effect on UV-B--induced apoptosis and DNA damage | Normal human dermal fibroblasts | [38] |
| 24. | Root Extract | Pothomorphe umbellata | Inhibition of Skin Matrix Metalloproteinases | Albino hairless mice, | [39] |
| 25. | Punica granatum extract | Punica granatum | Protection against photoaging | Human skin fibroblasts | [40] |
| 26. | Galloylated Tannins, | (Hamamelis virginiana) | antioxidant, cytotoxic, & anti proliferative properties | HaCaT and SK-Mel 28 melanoma cell lines | [34] |
| 27. | catechins and proanthacyanidin flavinols | Coca | ROS scavenging ability | Fibroblasts and Keratinocytes | [39] |
| 28. | Sulphoaphane (SFN) | Broccoli | Skin erythema | HaCaT Keratinocytes | [41] |
| 29. | Glycyrrhizic acid | mitogen-activated protein kinases, nuclear factor kappa B and mitochondrial apoptotic pathway oxidative stress mediated DNA damage |
HaCaT Keratinocytes Primary dermal fibroblasts (HDF) |
[42] [43] |
|
| 30. | Trigonalline | Funigreek seeds | relieving the UV-B -induced endoplasmic reticulum (ER) oxidative stress Protecting the skin cells and BALB/c mice against UV-B induced odidative DNA damage via modulation of PI3K-AKT-Nrf2 Pathway. |
HaCaT and HDF HDF cells and BALB/c mice |
[44,45] [46] |
Here, we describe the advanced knowledge about the mechanistic insights involved in the various pathologies associated with skin photoaging. The summarizing of the mechanistic signaling pathways in this review can help the researchers to find the various therapeutic targets for the treatment of the disease and disease management. Finally, we also highlighted various plant based natural product ingredients as therapeutics for ultraviolet radiation induced premature skin ageing. The reason for incorporating the natural product based therapeutic agents for skin photoageing is that, in topical drug delivery system the demand for the incorporation of natural product based ingredients in sunscreen formulations is increasing nowadays because of their photoprotective, antioxidant and risk free properties to prophylate UV induced skin photodamage and skin cancers.
2. Materials and methods
Various search engines such as Google Scholar, PubMed, Science Direct and Scopus were used to search the research articles related to the mechanisms of UV-induced skin photoaging and phytochemicals involved in its therapeutics, with the help of some key words like skin, photoaging, ultraviolet radiation, extracellular matrix, fibroblast, phytochemicals etc. The reviewed research articles were cited properly by using endnote 20 software.
3. The pathological mechanisms of skin photoaging
3.1. Interaction of UV radiations with skin
Dermal extracellular matrix of skin comprises of a complicated network of several macromolecules, which include collagen and elastic fibers, glycoproteins, and glycosaminoglycans and provide strength and flexibility to the skin [47]. The primary effect of chronic UV radiations in skin is the reduction of dermal extracellular matrix by the degradation of most abundant type I and type III collagen, and is considered to be primarily responsible for the wrinkled appearance of photoaged skin [48].
After absorption of UV radiations by the skin, various pathologies associated with skin photoaging appear due to their direct and indirect interactions with the biomolecules of the epidermis and dermis [49]. UV radiation is not absorbed by terrestrial molecular oxygen; hence it is safe for inhalation [50]. UV-B radiations do not penetrate deeply to the skin. Various cellular biomolecules like DNA, amino acids of proteins with aromatic rings, NADH, NADPH, flavins, quinones, porphyrins, carotenoids, urocanic acid, eu-or pheomelanin, and lipids of the epidermis of the skin [51] and is responsible mainly for producing the sun [52]. Studies have shown that UV-A radiations are 1000 times less effective than UV-B radiations and do not produce sun burns [53]. However, the UV-A portion of the UV radiation spectrum is also involved in the pathogenesis of skin photodamage and photoaging, possibly because it penetrates both epidermis and dermis [49] and degrades the extracellular matrix material, which is the most important factor for maintaining the proper skin health. Melanin, one of the important chromophores of skin, can convert the energy of UV radiations into other forms like heat, thereby protecting the skin from UV -induced skin damage [54]. The extent of protection by melanin depends upon the distribution and density of melanin secreted by melanocytes between keratinocytes [55]. For this reason, studies have shown that people with darker skin are 500 times more protected than people with fair skin phenotype against the risk of UV -induced non-melanoma type of skin cancer [56].
3.2. Effect of UV radiations on DNA, RNA, and proteins
UVR can damage the various cellular components by direct and indirect mechanisms [57]. Alteration of DNA, RNA, and protein structure and their function accelerates the process of photoageing [58]. UV-B is that component of the ultraviolet spectrum that can damage the skin by direct mechanisms. Although the stratum corneum of the epidermis absorbs a significant portion of UV-B radiation, the attenuated UV-B radiation intensity reaches the viable cells of the epidermis, causing their biological damage (mainly DNA damage). UV-B absorption leads to the formation of cyclobutane-pyrimidine dimers (CPDs) and pyrimidine-pyrimidone (6-4) photoproducts by the appearance of bonds between pyrimidine bases and their adjacent counterparts at sites rich in methylated cytosines (mC) of DNA [59]. Methylation of cytosines mainly occurs at 5′-CG-3′(CpG) sequences, and mutations at methylated CpG sites by solar UV radiations are referred to as “solar-UV signature” [60,61]. Already unstable 6–4 pyrimidine–pyrimidone or 6–4 pyrimidine–pyrimidinone (6-4 PP), when -irradiated with UV radiations of wavelength around 320 nm are converted to a more unstable form called Dewar valence isomers [62]. These three types of pre-mutagenic lesions; CPD, (6-4)-PP and Dewar valence isomers, give rise to UV-specific mutations if not appropriately repaired by nucleotide excision repair (NER) mechanism [63]. UV -irradiation give rise to most oncogenic CPDs (Thymine-cytosine (T = C) and cytosine-cytosine (C C) dimmers); since the same type of CPDs are found in tumor suppressor P53 gene of UV -irradiated cancer cells [64]. CPD, (6-4)-PP, and Dewar isomers lead to the apoptosis of healthy keratinocytes by inhibiting polymerase transcription, causing replication arrest. However, other types of mutations can lead to the tumor formation of keratinocytes by inducing their uncontrolled growth. Besides DNA, absorption of UVR by mRNA and other types of RNA at transient stages of transcription and translation can lead to the synthesis of dysfunctional proteins Epidermal proteins rich in aromatic amino acids such as tryptophan (Trp), tyrosine (Tyr) cystine, and disulfide-bonded cysteine; predominantly absorb UV-B radiations and undergo photoionization to give rise excited state species or direct radical formation (type I photo-oxidation) [65]. Studies have shown that long-lived triplet states of Trp and Tyr reduce the disulfide bonds, and the resultant disulfide radical anions (RSSR-.) formed can react with oxygen to form superoxide anions (O2-.) [66]. Excited Trp and Tyr can also respond in various other ways leading to the degradation and inducing oxidative stress of various cellular components of skin [57]. In Type II, photo-oxidation modification in protein structures is caused by their interaction with singlet oxygen, which is generated through natural and artificial UV excited photosensitizers. Both types of photo-oxidation can affect proteins rich in Trp and cysteine. Modified proteins by UV show altered function, whether it is an enzymatic protein or any structural component. Modified proteins are very harmful to the cell when they form aggregates and are responsible for many diseases, including skin aging. Cells have their internal defense mechanism to counteract the UV damaged proteins, which comprise either an anti-oxidative defense system or degrade damaged proteins by ubiquitin [64].
3.3. UV radiation generated reactive oxygen species (ROS) and skin photoaging
Besides direct skin damage, UV -irradiation also indirectly damages the skin by generating Reactive Oxygen Species (ROS) [51]. ROS produced by various endogenous photo-sensitizers is regarded as one of the primary responses of UV -induced damage to the skin [67]. ROS is generated as a byproduct during various cellular metabolic reactions, especially in mitochondria, and is categorized as - superoxide anion (O2−), hydroxyl radical (OH−), peroxyl radical (ROO.), hydrogen peroxide (H2O2). Accumulation of excessive ROS in cells is very harmful and can lead to the pathogenesis of various diseases and conditions like cancer, aging, atherosclerosis, neurodegenerative diseases etc [68]. Cells have an internal defense system in the form of enzymatic and non-enzymatic antioxidants that maintain ROS equilibrium. Any change favoring the increase in cellular ROS due to disturbed balance between available cellular antioxidants and generated cellular ROS creates a pathological condition in the cells termed “Oxidative stress,” damaging cellular macromolecules like DNA, proteins lipids, etc. [69]. One of the endogenous antioxidant enzymes, Superoxide dismutase, can convert superoxide anion (O2−) into hydrogen peroxide (H2O2). H2O2 readily crosses the cell membrane and causes local damage [70] by the generation of highly reactive hydrogen radical (OH−) when it comes in combination with transitional Fe (II) [71]. Hydrogen peroxide generated in the cells can also be decomposed into the water by the next available endogenous antioxidant enzyme Catalase [72].
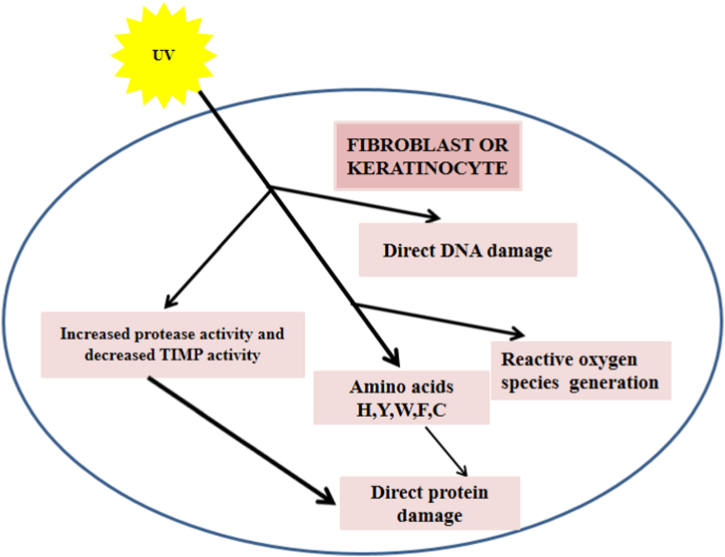
UV -induced Oxidative stress of the skin by impairing internal antioxidant defense mechanism followed by the consequent increase of reactive oxygen species in both epidermis and dermis result in initiating and prolonging the pathologies associated with photoaging. The skin's epidermis contains various photosensitizers like DNA, aromatic amino acids, NADH, NADPH, flavins, quinones, porphyrins, carotenoids, urocanic acid, eu- or pheomelanin, and lipids, which absorb most of the portion of UV-B radiations [73]. Melanin and Uroconic acid may act as photoprotective shields by absorbing most of the UV-B radiations but may also prove harmful to skin cells when urocanic acid is photo-isomerized to its cis isomer upon excessive UV-B absorption and initiate the ROS production. Further, human skin keratinocytes contain nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which catalyzes the conversion of molecular oxygen to superoxide anion upon UV–B -irradiation. UV -irradiation exposure to the skin results in skin tanning due to enhanced melanin synthesis, and this synthetic process of melanin is followed by ROS generation [74]. UV -irradiation of skin cells also results in photoionization of aromatic amino acids like tryptophan, tyrosine, and cysteine, causing further accumulation of ROS. UV -induced ROS in skin cells contributes to the etiology of photoaging by causing local inflammation, initiating signaling pathways of cell growth and proliferation, and deteriorating the structure of connective tissue of extracellular matrix (ECM) [75] (Fig. 1).
Fig. 1.
Potential mechanism of skin photoageing. UV -irradiation affect the Skin fibroblasts and keratinocytes by various different mechanisms. (1) Direct DNA damage (2) Reactive Oxygen Species (ROS) generation (3) Direct protein damage.
Fig. 1 summarizes the potential mechanism of skin photoageing.
3.3.1. Various signaling pathways activated by UV-B generated ROS
Studies have revealed that UV-B -induced photoaging progression is due to the ROS -induced activation of various signaling pathways of skin cells [75]. Activated signaling pathways may cause the initiation and appearance of various histological, cytological, and molecular pathologies like connective tissue degradation, persistent inflammation, apoptosis, DNA damage (keratinocytes, fibroblasts, and infiltrating neutrophils) by induction of their respective transcription factors. Various ROS -induced signaling pathways and their initiated events of photoaging etiology are described below.
3.3.1.1. UV-B generated ROS activates MAPK pathway in skin cells
Signaling proteins of MAPK pathway mainly belong to the family of serine/threonine protein kinases. These are sub-classified into various types like extracellular signal-regulated kinases (ERK), p38 MAPK (p38 kinase), and c-Jun NH2-terminal kinases (JNK) and are activated by different stimuli [76,77]. ERK pathway is stimulated by mitogenic stimuli, whereas p38 and JNK MAPKs are activated by stress stimuli, including UV-B radiations [78]. Studies have shown that oxidative stress plays a key role in triggering the MAPK pathway responses; this has been confirmed by using various antioxidant agents, which showed that their application results in the inactivation of MAPK sub-pathways [79]. ROS results in the phosphorylation of cytokine receptors, EGFR and Ras, resulting in the phosphorylation of various upstream proteins of MAPK sub-pathways. Different upstream proteins of p38 MAPKs like MEK3 and MEK6) are phosphorylated by already phosphorylated cytokine receptors, ultimately resulting in the activation of the p38 MAPK sub pathway. Upstream proteins of JNK and ERK sub pathways are MEK4 and MEK7 and MEK1 and MEK2, respectively. Activated MAPK proteins enter the nucleus where they activate various transcription factors like AP-1, NF-κB, COX-2, c-Myc and finally induce MAPK pathway-induced Photodamage [[80], [81], [82]].
3.3.1.2. MAPK pathway, as a mechanistic link for UV-B -induced inflammation
Inflammation is among the primary responses of UV -induced skin photodamage, where the UV-B generated ROS plays a critical role by activating the MAPK pathway, causing increased infiltration of inflammatory mediators and epidermal hyperplasia [83]. Keratinocytes are considered as the main skin cell types responsible for the secretion of cytokines by activating the p38/JNK pathway after UV-B -irradiation [84]. UV-B -irradiated keratinocytes mainly secrete pro-inflammatory cytokines like IL-1, TNF-α, IL-6, and mediators like ICAM-1and COX-2. UV-B -induced inflammation is initiated by the interaction of different components of IL1 complex like IL-1α and IL-1β with their receptors [85]. Severe sun burning also results in the secretion of one more pro-inflammatory cytokine (IL6), and its level in serum reaches its peak by about 12 h after UV-B –irradiation [86]. Cytokines, besides their role in UV-B -induced inflammation, are also involved in the production of different types of matrix metalloproteinases (MMPs) by stimulating fibroblasts [87]. Increased production of IL-6 after UV-B irradiation leads to the increased expression of MMP1 and MMP9, thus contributing in the degradation of extracellular matrix [88].
Besides the proinflammatory cytokines, prostaglandins and thromboxanes also play an essential role in UV-B -induced inflammation. In epidermal cells irradiated with UV-B radiations, activation of p38/JNK MAPKs results in the upregulation of COX-2 isoform of cyclooxygenase (COX) gene, which is responsible for the production of prostaglandin 2 (PGE-2) [89,90].
3.3.1.3. UV-B -irradiation of skin and extracellular matrix
Skin Extracellular Matrix, comprising collagen, elastin, and fibrillin, help clinicians and researchers to get comparative knowledge about the degree of photoageing, as the amount of extracellular matrix is inversely proportional to the rate of photoageing when exposed to UV-B radiations [91]. UV-B radiations decrease the extracellular matrix content of skin either due to altered collagen synthesis or degradation of collagen [88].
Activation of the TGF-β gene is responsible for dermal collagen synthesis [92]. The mechanism behind dermal collagen synthesis involves the interaction of TGF-β with TGF-β cell-surface receptor complexes (TβR I-III). Interaction of TGF-β with TβR II results in activation of serine/threonine kinase activity of TβRI, resulting in phosphorylation and activation of Smad2 and Smad3 (transcription factors). Smad4, another transcription factor, combined with the phosphrorylated Smad2 and Smad3, forms a complex that translocates to the nucleus. It activates the TGF-β gene by binding its promoter region and inducing collagen synthesis. UV-B -irradiation results in the activation of another transcription factor, Smad 7, which synergizes the action of TGF-β, thus inhibiting TGF-β/Smad 2-3 signaling pathway and blocking collagen synthesis [[93], [94], [95]], thereby increasing the resultant skin collagen and loss of skin integrity.
It is well established that UV-B -irradiation leads to the degradation of collagen by activating different types of MMPs [96]. Mechanistic studies have shown that the UV-B activated MAPK pathway causes the stimulation of some downstream transcription factors like c-Jun and activating transcription factor-2 (ATF-2). Homodimerization or heterodimerization of stimulated c-Jun with another transcription factor c-fos, results in functional activator protein (AP-1) complex formation. Functional activator protein (AP-1) binds to its response element, resulting in activation of various MMPs, including collagenase, stromelysin, and gelatinase [96,97]. These MMPs collectively affect the skin's structural integrity by degrading collagen content. Functional AP-1 transcription factor plays a vital role in the degradation of skin collagen and is very sensitive to UV-B radiations, even at sub erythemogenic dose of UV-B (∼0.1 minimal erythema dose, MED) can stimulate it [98].
3.3.1.4. UV-B -irradiation and nuclear factor NF-κB pathway
NF-κB is a family of transcription factors and its members are classified into five “Rel” proteins including RelA (p65), c-Bel, RelB, NF-κB1 (p50/p105) and NF-κB2 (p52/p100) [99]. The most important function of these transcription factors is to regulate UV-B -induced inflammation, immune response, cell proliferation, and differentiation [100]. Members of the NF-κB family members remain inactive by binding to an inhibitory protein, IκB, in the cellular cytoplasm. This inhibitory effect of IκB gets eliminated by the phosphorylation of a kinase protein, IκB kinase (IKK), causing ubiquitination and proteasomal degradation of IκB [101]. Various factors cause the activation of NF-κB in cellular cytoplasm; ROS generated by UV-B -irradiation is the most prominent, suggesting ROS plays a critical role in activating IκB kinase (IKK). Activated members of NF-κB enter the nucleus, where they initiate their transcription processes by binding to their respective DNA regulatory sequences [102,103].
3.3.1.5. UV-B -irradiation and apoptosis
UV-B -irradiation results in the appearance of Sunburn Cells (SC) in the epidermis of the skin. SCs are skin keratinocytes undergoing apoptosis showing distinct morphological features like pyknotic nuclei with eosinophilic cytoplasm, mitochondrial swelling, and rupture [104]. Molecular mechanisms underlying the SCs formation involve the initiation of various downstream pathways from UV-B -induced DNA damage, UV-B activated TNF-α, Fas and UV-B generated ROS [105]. It has been reported that UV-B-generated ROS results in the apoptosis of keratinocytes by the activation of the intrinsic or mitochondrial apoptotic pathway [106]. In the intrinsic apoptotic pathway, disruption of the outer mitochondrial membrane results in the release of cytochrome C to the cytosol, where it activates the apoptosome complex. The Activated apoptosome complex results in recruiting and activating procaspase 9 to Caspase 9, which activates the effector protein Caspase 3. Caspase 3 acts as a cutting knife leading to the cleavage of various cellular components, including DNA [107]. This pathway is controlled by multiple pro-apoptotic and anti-apoptotic members of the B-cell lymphoma (Bcl2) family, including Bax, Bim, Bak and Bcl2, Bcl-xl Mcl-1, respectively [108]. Megastore formation is stimulated by the p38 MAPK pathway, which helps redistribute Bax protein from cytosol to mitochondria. Cytochrome c, in turn, is released from mitochondria into the cytosol and triggers the execution phase of apoptosis [109].
3.3.2. UV-A generated ROS and skin photoaging
The longest UV-A wavelengths have relatively deeper penetration properties and effect the all cutaneous layers. The damaging effects of UV-A irradiation is primarily initiated by the generation of ROS, mediated by numerous endogenous chromophores and the subsequent oxidative stress is majorly responsible for the numerous cellular and molecular alterations like lipid peroxidation, DNA lesions, protein carbonylation and activation subsequent intracellular signaling pathways. These cellular and molecular alterations induce mutations, apoptosis, dermis remodeling, inflammatory reactions and abnormal immune responses [110,111]. Further evidences have shown that various external stimulators, such as pollutants and visible light (Vis), were shown to synergistic effects with UVA rays. Studies at gene expression level have shown that UV-A radiation induce the alteration of several genes approving the fact that dermal fibroblasts are highly sensitive to UVA radiation [[112], [113], [114]]. UVA radiation also appears to induce the pigmentary changes of skin which are the major signs of skin photoaging in Asians [115].
3.3.3. UV -irradiation and mitochondrial dysfunction
Evidences support that normal functioning of the mitochondria is closely connected to the skin health. UVR -induced mitochondrial dysfunction plays a vital role in skin photoaging, which can be explained by the “Defective Powerhouse Model of Premature Skin Ageing'. According to this model, accumulation of UV -induced mitochondrial DNA (mDNA) mutations leads to disturbed flow of electrons across the mitochondrial electron transport chain, which causes decreased oxygen consumption and, consequently, fewer ATPs are produced in dermal fibroblasts [116]. Because of reduced energy production, there are interrelated steps of Caspase activation, membrane depolarization, and cytochrome C release from mitochondria. These processes affect various cellular processes such as cell migration and cell division, also lead to increased mitochondrial oxidative stress levels because of a many-fold increase in ROS production at mitochondrial complexes [117,118]. Enzyme Endogenous antioxidant system of mitochondria, including glucose-6-phosphate dehydrogenase, thioredoxin reductase, glutathione–S-transferases, and peroxidase, is their best defense system against increased levels of ROS. This defense system is disturbed by UV radiations as it targets nrf2, which is the master regulator of this endogenous defense system [119]. It is for this reason, clinicians and estheticians are focusing on mitochondria as therapeutic target to treat skin photoaging disorder.
3.4. Immunosuppression in skin photoaging
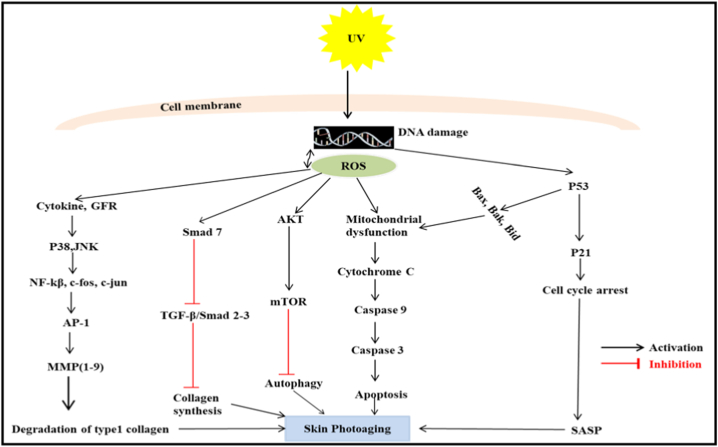
Studies have shown that UV radiations induce immune-suppression in skin cells and patients with immune-suppressed cells are more susceptible to developing tumors because of poor local immune surveillance [120]. Mechanistic study of UVR -induced immune suppression shows that UV -irradiation affects both cellular and humoral immune responses. UV radiations decrease the number of epidermal Langerhans cells (LC) and affect their functions like cellular migration and antigen presentation in lymph nodes, thereby affecting their crucial role in local immune surveillance [121]. UV radiations induce the isomerization of trans urocanic acid to cis urocanic acid in LC cells which is probably responsible for their impaired migration and antigen presentation [122]. Besides, studies have also shown that UV radiation leads to the loss of co-stimulatory molecule (B7), which is also responsible for its impaired activity [123]. It has also been established that UV -irradiation triggers the release of IL-10, an immunosuppressive cytokine from keratinocytes in response to cis-UCA [124]. UV -induced depletion of LC and release of pro-inflammatory cytokines also cause the activation of regulatory T cells (TREG), which cause the polarization of Th1/Th2 balance toward Th2. Predominant Th2 level further induces another immunosuppressive cytokine, IL-12, from epidermal LC (Fig. 2) [125].
Fig. 2.
Describes the signaling pathways responsible for UV-A/B response in skin cells. This figure describes the various signaling pathways and their important protein markers responsible for UV-A/B -induced pathologies in skin cells like inflammation, Extracellular matrix degradation and apoptosis.
Fig. 2: Describes the signaling pathways responsible for UV-A/B induc n ed skin photoageing.
3.5. UV irradiation and skin adipose tissue
Skin adipose tissue consists of dermal white adipose tissue (DWAT) and subcutaneous white adipose tissue (SWAT), both playing an influential role in skin photoaging. DWAT, lying in the reticular dermis of the skin, connect the skin surface and the subcutaneous fat, by forming a “fat bridge” between them, thus connecting the skin surface which i directly exposed to the UV with the deeper fat layer [126,127].
Frequent and excessive irradiation of skin to UV radiation leads to reduction in DWAT layer thickness and simultaneous formation of skin fibrosis, probably by a mechanism of adipocyte–myofibroblast transition [126, 128]. Replacement of DWAT layer by skin fibrosis result in coarse skin structure which ultimately leads to wrinkling of skin [126, 128]. Besides DWAT, UV radiation also modifies the SWAT metabolism as well, where there is significantly reduced accumulation of free fatty acid and triglycerides than the unexposed skin of the same individual [126, 128]. The possible reason might be that UV irradiation trigger the release of some soluble factors IL-6, IL-8, and monocyte chemotactic protein-3 which diffuse into SWAT and alter its metabolism [126,129]. Reduction in the accumulation of the free fatty acid and triglycerides of SWAT result in the thinning and sagging of connective tissue, which ultimately lead to the skin atrophy and wrinkle formation [126,130].
3.6. Role of MicroRNA (miRNA) and advanced glycation end products (AGEs) in skin photoaging
Evidences revealed that after DNA replication, the primary role of DNA methyltransferase 1 (DNMT1), is to conserve the methylation state of the daughter strands to make certain that the methylated strand was passed on to the offspring cells [131]. However, highly efficient analysis has shown that methylation levels and patterns of global genome are changed during skin aging [131]. Further studies confirmed that miRNA 217 and MiR–23a–3p regulate the senescence of human skin fibroblasts by directly targeting the DNA methyltransferase1 and enzymes related to Hyaluronic acid (HA), a major component of the dermal matrix, synthesis in human fibroblasts, respectively suggesting that miRNA play a vital role in skin photoageing [132,133]. This was further supported by the evidence that UV-B irradiation modify the expression of miR-34 family in the dorsal skin of mouse simultaneous with the imperfect regulation of collagen structure, which results in loss of skin strength and elasticity [134]. Thus miRNA can act as potential target for therapeutics of skin photoageing.
Glycation of proteins, which is done by reaction of reducing sugars with free amino groups on proteins and other molecules resulting in the reversible generation of reactive intermediates which ultimately convert to irreversible AGEs, is one of distinguished marker of aged skin. Dermal matrix and cytoskeleton proteins are more vulnerable to glycation, resulting in tissue stiffening and loss of skin elasticity [134]. Studies have revealed that glycated elastin fibres among the extracellular proteins aggregate abnormally and interact with lysozymes in the solar elastosis skin, indicating that glycation is involved in skin photoaging [135].
3.7. Role of transient receptor potential cation channel V (TRPV) in skin photoaging
TRP ion channel, responsible for various sensory responses like heat, cold, pain, stress, vision, and taste are widely distributed in the peripheral and central nervous system. They function by allowing the cations to pass through the cell membrane non-selectively. TRP ion channel consists of more than 30 subfamilies, among which TRPV subfamily plays crucial roles in the process of skin photoaging [136] Mechanistic links involved in the TRPV ion channel subfamily and skin photoageing varies according to the type of cell line used.
Irradiation of human dermal fibroblasts with UV-B results in the increase of ca2+ ion concentration via TRPV1, which leads to the degradation of nuclear factor-E2-related factor 2 (Nrf2) disturbing the intracellular redox homeostasis which produces the oxidative stress of the cell and ultimately results in skin photoaging [137,138]. When HaCaT cells are irradiated with UV radiation, it activates the Src kinase which in turn induces the trafficking of TRPV1 from intracellular vesicles to the cell membrane [126,139]. It has also been observed that TRPV1 activation results in the upregulation of MMP-1 thus contributing in natural-aging and photo aging of skin [129]. Role of TGF-β pathway in regulating the TRPV3 is a matter of concern, however, it has been observed that TRPV3 channels contribute in maintaining the normal skin health by reducing ECM production by the activation of the TRPV3/Thymic stromal lymphopoietin/Smad2/3 pathways in dermal fibroblasts [126]. Role of TRPVs in the regulation of skin photoaging demands more concern especially by carrying the animal tests to check their true effectiveness.
4. Therapeutic Approaches for the amelioration of UV -induced photoaging by plant-based natural products; An emerging therapy
Considering the harmful effects of UV radiation on skin health, especially Photodamage (a spectrum of skin disorders ranging from sunburns, premature aging to carcinogenesis), it becomes imperative to develop suitable therapeutic options [140]. Photodamage has assumed tremendous significance in the beauty care industry and the clinic in recent times. The past few decades have witnessed significant progress in establishing various therapeutic targets of Photodamage, which can develop proper therapeutic regimens with satisfactory outcomes. Currently, different therapeutic options are available; however, patients discontinue their use because of their undesired effects. For example; drugs of the first line therapy which include mainly topical retinoids (tretinoin, tazarotene, adapalene, etc), besides their antiphotoageing effects are associated with several side effects like skin irritation, redness, scaling, dryness, burning, stinging and peeling [141]. Use of chemical peels as antiphotoageing drugs harm the patient by inducing the hypo- or hyperpigmentation, infection, and scarring of the skin [142]. Although Photodynamic photorejuvenation therapy (PDT) has the reversal effects on skin photoaging but the patient experiences pain during illumination [2,143,144]. So, the researchers and industry mainly focus on treatments with more benefits and no side effects. This has diverted their attention towards ‘Plant based Natural Products [145,146]. Natural product-based ingredients are added on a large scale to cosmetic items to increase their beatification potential [147]. These natural products are rich in polyphenols, flavonoids, and antioxidants which have UV-generated free radical scavenging ability and relieve the skin from various harmful effects of UVR [148].
Studies have shown that various natural products can lower the risk of UV-B -induced skin photodamage by acting upon the various therapeutic targets. Fucoidan is a sulfated polysaccharide obtained from Brown algae and can inhibit the UV-B activated ERK pathways, thus preventing the cells from expressing MMP1 [149]. Studies show that the various cellular and molecular pathologies of UV-B -irradiated skin cells like generation of ROS, inflammation, and DNA damage can be prevented by luteolin, a flavonoid, by targeting the MAPK pathway [150]. UV-B -irradiation also leads to the generation of singlet molecular oxygen, which has severe cellular metabolic consequences and can be prevented by using a flavinoid, β-carotene [151]. Pathologies associated with skin photoaging are prevented by Parthenolide treatment, a sesquiterpene, by inhibiting the NF-κB pathway [152]. Experiments on immortalized HaCaT keratinocytes have shown that pomegranate polyphenol extract has shown satisfactory results in preventing UV-B -induced oxidative stress and has inhibitory effects on the markers of skin photodamage [153]. Manteeena and Katyar have established that proanthocyanidins obtained from grape seeds have an inhibitory effect on UV -induced oxidative stress via MAPK and NF-kB in epidermal keratinocytes [154]. Anthocyanins largely extracted from Bog blueberry (Vaccinium ulginosum L.) have also been shown to have anti photoaging effects in the human dermal fibroblast cell line. In vitro studies on various skin cell lines like HaCaT cell line, Normal epidermal melanocytes, and Human normal fibroblasts have shown that parthenolide extracted from Feverfew (Tanacetum parthenium) prevents the UV-B mediated skin photoaging by blocking the NF-κB pathway [21]. Liang et al., in 2013 carried out both In vitro and In vivo studies on HaCaT cells and Xpc− knockout mouse respectively and showed that Ginsenoside obtained from Ginseng has anti-photoaging properties by suppressing UV -induced apoptosis [17]. Studies on Human dermal fibroblasts have shown that Aucubin obtained from Eucommia ulmoides has protective effects against UV-B -induced oxidative stress [18]. Xanthorrhizol, another natural product obtained from Curcuma xanthorrhiza Roxb, also showed photoprotective effects in Human skin fibroblasts [155]. In vitro studies on Human dermal fibroblasts shows that UV-B activated MAPK activation pathways can also be blocked by using a green tea obtained flavinoid, Epigallocatechin gallate. Studies on a hairless rat model have shown that Polypodium leucotomos extract prevents UV-generated ROS and DNA damage. Delphinidin, an anthocyanidin, obtained from pigmented fruits and vegetables, provides protection against UV-B- mediated oxidative stress and apoptosis as shown in both in vitro and in vivo studies by using Human HaCaT Keratinocytes and SKH-1 hairless mouse as experimental models, respectively [32]. Silybum marianum, a source of a very potent flavonoid, Silymarin, inhibits UV-induced apoptosis in Human malignant melanoma [36]. Experiments on UV -irradiated HaCaT and SK-Mel 28 melanoma cell lines have revealed that Galloylated Tannins, obtained from Witch Hazel (Hamamelis virginiana), have antioxidant, cytotoxic & anti-proliferative properties [37]. PCF, a polypeptide obtained from Chlamys farreri, has been reported to show an inhibitory effect on UV-B -induced apoptosis and DNA damage in normal human dermal fibroblasts [38]. Extract from roots of Pothomorphe umbellate inhibits the expression of skin matrix metalloproteinases in UV-B -irradiated Albino hairless mice [39]. Punica granatum extract also protects photoaging as explored in Human skin fibroblast cells [40]. Genistein, a well-known isoflavone, derived from soybean, exerts a photoprotective effect and is also a very potent agent against photocarcinogenesis [83]. Another isoflavone, Silymarin, and its most active principle, silibinin derived from milk thistle (Silybum marianum), show photoprotective properties by preventing ROS generation and decreasing the infiltration of CD11b + lymphocytes to UV -irradiated areas [156]. An extract of grape seeds presents a group of substances called proanthocyanidins, also known as condensed tannins; it has antitumor activity in response to UV -irradiation due to its antioxidant and anti-inflammatory properties in mice [23]. The ingredients of Coca extract mainly include various polyphenols like catechins and proanthocyanidin flavanols and have ROS scavenging ability in UV -irradiated skin [157]. Broccoli is mainly enriched with Sulphoaphane (SFN), which decreases the UV -induced skin erythema [41]. Glycyrrhizic acid (GA), a marker compound of licorice and triterpene, prevents ultraviolet-B--induced Photodamage by interacting with various signaling pathways like mitogen-activated protein kinases, NF-kB, and mitochondrial apoptotic pathway [42]. Umar SA et al., in 2019 further showed that oxidative stress-mediated the application of GA can prevent DNA damage through modulating autophagy in UV-B -irradiated Human primary dermal fibroblasts (HDF) [158]. Trigonelline, a marker compound of Fenugreek seeds, also acts as an anti photoaging agent by relieving the UV-B -induced endoplasmic reticulum (ER) oxidative stress as studied in Immortalized Human Keratinocyte cell line (HaCaT) and BALB/C mice [159]. Recently, Tanveer et al. (2022), showed the genoprotective role of trigonelline in skin cells and BALB/c mice against UV-B irradiation via modulation of PI3K-AKT-Nrf2 Pathway [46].
5. Conclusions and future perspectives
Skin is cosmetically and aesthetically a vital organ, so the injury of skin photoaging is a great concern which cannot be ignored [160]. It is for this reason that researchers are more focused on understanding the mechanisms involved in skin photoaging for its applicability in aesthetics and clinic. So far, we have discussed the available research related to the mechanisms of skin photoaging, which involve alteration of cellular macromolecules by UV irradiation, oxidative stress and signaling pathways activated by oxidative stress in photoaged skin, mechanisms involved in UV induced inflammation, apoptosis, immune suppression, mitochondrial dysfunction, reduction of adipose tissue, accumulation of AGEs and activation of TRPV ion channels. In future, it is the need of the hour to explore more about the mechanisms involved in skin photoaging and find the interaction of the target molecules with the existing signaling pathways because all the signaling mechanisms triggered by UV irradiation do not work independently, instead they are cross talked. This will add more opportunities to find additional therapeutics targets of skin photoaging.
By utilizing the available therapeutic targets of skin photoaging, all attempts are being made to prevent skin photoaging either by ingestion or topical application of plant based natural product ingredients because of their very less or no side effects, their easy availability, instead of the synthetic ones available [[161], [162], [163]]. They are encouraged for the application in pharmaceutical and cosmetic industries as natural sunscreens, anti-cancer agents, activators of cell proliferation and anti-photoaging molecules. It is pertinent to mention that the aforementioned anti photoaging natural products are based on experimental studies only (In vitro and In vivo), so there is a need for future research in clinical trials to have knowledge about their dosage, safety, and efficacy and select the most efficient ingredient lead for humans by utilizing the high throughput analysis.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Council of Scientific and Industrial Research (CSIR), New Delhi, India vide Project No. HCP-007 and Department of Biotechnology (DBT), Ministry of Science and Technology, New Delhi, Government of India, vide Project No. BT/PR22668/TRM/120/154/2017 (GAP-2166).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
References
- 1.Sayama A., et al. Morphological and biochemical changes during aging and photoaging of the skin of C57BL/6J mice. J. Toxicol. Pathol. 2010;23(3):133–139. doi: 10.1293/tox.23.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han A., Chien A.L., Kang S. Photoaging. Dermatologic clinics. 2014;32(3):291–299. doi: 10.1016/j.det.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Trautinger F. Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin. Exp. Dermatol. 2001;26(7):573–577. doi: 10.1046/j.1365-2230.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- 4.Sklar L.R., et al. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem. Photobiol. Sci. 2013;12(1):54–64. doi: 10.1039/c2pp25152c. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton A.E. Ultraviolet radiation and plants: burning questions. Plant Cell. 1992;4(11):1353. doi: 10.1105/tpc.4.11.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter A., Wuttke S., Zacher K. Two years of in situ UV measurements at dallmann laboratory/jubany station. Synopsis of research performed at the dallmann laboratory and jubany station (Antarctica) Rep. Polar Res. 2008;571:12–19. [Google Scholar]
- 7.Narayanan D.L., Saladi R.N., Fox J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010;49(9):978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 8.Grune T., et al. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem. Biophys. Res. Commun. 2003;305(3):709–718. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- 9.Kulka M. In: Mechanisms And Treatment of Photoaging and Photodamage. Using Old Solutions to New Problems–Natural Drug Discovery in the 21st Century. Kulka M., editor. InTech; Croatia: 2013. pp. 255–276. [Google Scholar]
- 10.Yaar M., Gilchrest B. Ageing and photoageing of keratinocytes and melanocytes. Clin. Exp. Dermatol. 2001;26(7):583–591. doi: 10.1046/j.1365-2230.2001.00895.x. [DOI] [PubMed] [Google Scholar]
- 11.Fisher G.J., et al. Molecular mechanisms of photoaging in human skin in vivo and their prevention by all‐trans retinoic acid. Photochem. Photobiol. 1999;69(2):154–157. doi: 10.1562/0031-8655(1999)069<0154:mmopih>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Im A.-R., et al. Skin moisturizing and antiphotodamage effects of tyndallized Lactobacillus acidophilus IDCC 3302. J. Med. Food. 2018;21(10):1016–1023. doi: 10.1089/jmf.2017.4100. [DOI] [PubMed] [Google Scholar]
- 13.Laga A.C., Murphy G.F. The translational basis of human cutaneous photoaging: on models, methods, and meaning. Am. J. Pathol. 2009;174(2):357–360. doi: 10.2353/ajpath.2009.081029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vierkötter A., Krutmann J. Environmental influences on skin aging and ethnic-specific manifestations. Derm. Endocrinol. 2012;4(3):227–231. doi: 10.4161/derm.19858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandel R., et al. Skin photoaging and the role of antioxidants in its prevention. Int. Sch. Res. Notices. 2013;2013 doi: 10.1155/2013/930164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green A., et al. Factors associated with premature skin aging (photoaging) before the age of 55: a population-based study. Dermatology. 2011;222(1):74–80. doi: 10.1159/000322623. [DOI] [PubMed] [Google Scholar]
- 17.Liang J., et al. Ginsenoside Rb1 attenuates oxygen-glucose deprivation-induced apoptosis in SH-SY5Y cells via protection of mitochondria and inhibition of AIF and cytochrome c release. Molecules. 2013;18(10):12777–12792. doi: 10.3390/molecules181012777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho J.N., et al. Protective effects of aucubin isolated from Eucommia ulmoides against UVB-induced oxidative stress in human skin fibroblasts. Biol. Pharm. Bull. 2005;28(7):1244–1248. doi: 10.1248/bpb.28.1244. [DOI] [PubMed] [Google Scholar]
- 19.Chou T.H., et al. Antioxidative characteristics and inhibition of α‐melanocyte‐stimulating hormone‐stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp. Dermatol. 2010;19(8):742–750. doi: 10.1111/j.1600-0625.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 20.Oh H.I., et al. The effect of xanthorrhizol on the expression of matrix metalloproteinase‐1 and type‐I procollagen in ultraviolet‐irradiated human skin fibroblasts. Phytother Res. 2009;23(9):1299–1302. doi: 10.1002/ptr.2768. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K., et al. Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor κB inhibitor, parthenolide. J. Pharmacol. Exp. Therapeut. 2005;315(2):624–630. doi: 10.1124/jpet.105.088674. [DOI] [PubMed] [Google Scholar]
- 22.Song H.S., Sim S.S. Acetoside inhibits α‐MSH‐induced melanin production in B16 melanoma cells by inactivation of adenyl cyclase. J. Pharm. Pharmacol. 2009;61(10):1347–1351. doi: 10.1211/jpp/61.10.0011. [DOI] [PubMed] [Google Scholar]
- 23.Mantena S.K., Katiyar S.K. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Radic. Biol. Med. 2006;40(9):1603–1614. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Itoh K., et al. Inhibitory effects of Citrus hassaku extract and its flavanone glycosides on melanogenesis. Biol. Pharm. Bull. 2009;32(3):410–415. doi: 10.1248/bpb.32.410. [DOI] [PubMed] [Google Scholar]
- 25.Fujii T., et al. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J. Ethnopharmacol. 2008;119(1):53–57. doi: 10.1016/j.jep.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 26.El‐Mahdy M.A., et al. Naringenin protects HaCaT human keratinocytes against UVB‐induced apoptosis and enhances the removal of cyclobutane pyrimidine dimers from the genome†. Photochem. Photobiol. 2008;84(2):307–316. doi: 10.1111/j.1751-1097.2007.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skandrani I., et al. Chloroform extract from Moricandia arvensis inhibits growth of B16‐F0 melanoma cells and promotes differentiation in vitro. Cell Prolif. 2010;43(5):471–479. doi: 10.1111/j.1365-2184.2010.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo J.-H., et al. Effect of xanthohumol on melanogenesis in B16 melanoma cells. Exp. Mol. Med. 2008;40(3):313–319. doi: 10.3858/emm.2008.40.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae J.-Y., et al. (−) Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food Chem. Toxicol. 2008;46(4):1298–1307. doi: 10.1016/j.fct.2007.09.112. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez S., Gilaberte Y., Philips N. Mechanistic insights in the use of a Polypodium leucotomos extract as an oral and topical photoprotective agent. Photochem. Photobiol. Sci. 2010;9(4):559–563. doi: 10.1039/b9pp00156e. [DOI] [PubMed] [Google Scholar]
- 31.Choi H.-k., et al. Labisia pumila extract protects skin cells from photoaging caused by UVB irradiation. J. Biosci. Bioeng. 2010;109(3):291–296. doi: 10.1016/j.jbiosc.2009.08.478. [DOI] [PubMed] [Google Scholar]
- 32.Afaq F., et al. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Invest. Dermatol. 2007;127(1):222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 33.Arung E.T., Shimizu K., Kondo R. Inhibitory effect of artocarpanone from Artocarpus heterophyllus on melanin biosynthesis. Biol. Pharm. Bull. 2006;29(9):1966–1969. doi: 10.1248/bpb.29.1966. [DOI] [PubMed] [Google Scholar]
- 34.Jang J.Y., et al. Dichloromethane fraction of Cimicifuga heracleifolia decreases the level of melanin synthesis by activating the ERK or AKT signaling pathway in B16F10 cells. Exp. Dermatol. 2009;18(3):232–237. doi: 10.1111/j.1600-0625.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal R., et al. Anticarcinogenic effects of Solanum lycopersicum fruit extract on Swiss albino and C57 Bl mice. Asian Pac. J. Cancer Prev. APJCP. 2009;10(3):379–381. [PubMed] [Google Scholar]
- 36.Li L.-H., et al. Silymarin prevents UV irradiation-induced A375-S2 cell apoptosis. Biol. Pharm. Bull. 2004;27(7):1031–1036. doi: 10.1248/bpb.27.1031. [DOI] [PubMed] [Google Scholar]
- 37.Touriño S., et al. Highly galloylated tannin fractions from witch hazel (Hamamelis virginiana) bark: electron transfer capacity, in vitro antioxidant activity, and effects on skin-related cells. Chem. Res. Toxicol. 2008;21(3):696–704. doi: 10.1021/tx700425n. [DOI] [PubMed] [Google Scholar]
- 38.Ding B.-X., Wang C.-B. Inhibitory effect of polypeptides from Chlamys farreri on UVB-induced apoptosis and DNA damage in normal human dermal fibroblasts in vitro. Acta Pharmacol. Sin. 2003;24(10):1006–1010. [PubMed] [Google Scholar]
- 39.Ropke C.D., et al. In vitro and in vivo inhibition of skin matrix metalloproteinases by Pothomorphe umbellata root extract. Photochem. Photobiol. 2006;82(2):439–442. doi: 10.1562/2005-06-29-RA-596. [DOI] [PubMed] [Google Scholar]
- 40.Park H.M., et al. Extract of Punica granatum inhibits skin photoaging induced by UVB irradiation. Int. J. Dermatol. 2010;49(3):276–282. doi: 10.1111/j.1365-4632.2009.04269.x. [DOI] [PubMed] [Google Scholar]
- 41.Saw C.L., et al. Impact of Nrf2 on UVB‐induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol. Carcinog. 2011;50(6):479–486. doi: 10.1002/mc.20725. [DOI] [PubMed] [Google Scholar]
- 42.Afnan Q., et al. Glycyrrhizic acid prevents ultraviolet‐B‐induced photodamage: a role for mitogen‐activated protein kinases, nuclear factor kappa B and mitochondrial apoptotic pathway. Exp. Dermatol. 2016;25(6):440–446. doi: 10.1111/exd.12964. [DOI] [PubMed] [Google Scholar]
- 43.Umar S.A., et al. Glycyrrhizic acid prevents oxidative stress mediated DNA damage response through modulation of autophagy in ultraviolet-B-irradiated human primary dermal fibroblasts. Cell. Physiol. Biochem. 2019;53(1):242–257. doi: 10.33594/000000133. [DOI] [PubMed] [Google Scholar]
- 44.Lone A., et al. Trigonelline, a naturally occurring alkaloidal agent protects ultraviolet-B (UV-B) irradiation induced apoptotic cell death in human skin fibroblasts via attenuation of oxidative stress, restoration of cellular calcium homeostasis and prevention of endoplasmic reticulum (ER) stress. J. Photochem. Photobiol., B. 2019;202:111720. doi: 10.1016/j.jphotobiol.2019.111720. 111720. [DOI] [PubMed] [Google Scholar]
- 45.Lone N.A., et al. Inhibition of ultraviolet‐B radiation induced photodamage by trigonelline through modulation of mitogen activating protein kinases and nuclear factor‐κb signaling axis in skin. Photochem. Photobiol. 2021;97(4):785–794. doi: 10.1111/php.13369. [DOI] [PubMed] [Google Scholar]
- 46.Tanveer M.A., et al. Experimental Gerontology; 2022. Trigonelline, a Plant Derived Alkaloid Prevents Ultraviolet-B-Induced Oxidative DNA Damage in Primary Human Dermal Fibroblasts and BALB/c Mice via Modulation of Phosphoinositide 3-Kinase-Akt-Nrf2 Signalling axis. [DOI] [PubMed] [Google Scholar]
- 47.Silver F.H., Siperko L.M., Seehra G.P. Mechanobiology of force transduction in dermal tissue. Skin Res. Technol. 2003;9(1):3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- 48.Sárdy M. Role of matrix metalloproteinases in skin ageing. Connect. Tissue Res. 2009;50(2):132–138. doi: 10.1080/03008200802585622. [DOI] [PubMed] [Google Scholar]
- 49.D'Orazio J., et al. UV radiation and the skin. Int. J. Mol. Sci. 2013;14(6):12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Gruijl F., Leun J. Environment and health: 3. Ozone depletion and ultraviolet radiation. CMAJ (Can. Med. Assoc. J.) 2000;163(7):851–855. [PMC free article] [PubMed] [Google Scholar]
- 51.Svobodova A., Walterova D., Vostalova J. Ultraviolet light induced alteration to the skin. Biomed. Papers-Palacky Univer. Olomouc. 2006;150(1):25. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 52.Forestier S. Rationale for sunscreen development. J. Am. Acad. Dermatol. 2008;58(5):S133–S138. doi: 10.1016/j.jaad.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 53.Diffey B.L. What is light? Photodermatol. Photoimmunol. Photomed. 2002;18(2):68–74. doi: 10.1034/j.1600-0781.2002.180203.x. [DOI] [PubMed] [Google Scholar]
- 54.Brenner M., Hearing V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008;84(3):539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cichorek M., et al. Skin melanocytes: biology and development. Adv. Dermatol. Allerg. Postȩpy Dermatologii I Alergologii. 2013;30(1):30. doi: 10.5114/pdia.2013.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortonne J.P. Photoprotective properties of skin melanin. Br. J. Dermatol. 2002;146:7–10. doi: 10.1046/j.1365-2133.146.s61.3.x. [DOI] [PubMed] [Google Scholar]
- 57.Pattison D.I., Davies M.J. Cancer: Cell Structures, Carcinogens and Genomic Instability. Springer; 2006. Actions of ultraviolet light on cellular structures; pp. 131–157. [DOI] [PubMed] [Google Scholar]
- 58.Lee L.-Y., Liu S.-X. Pathogenesis of photoaging in human dermal fibroblasts. Int. J. Dermatol. Venereol. 2020;3(1):37–42. [Google Scholar]
- 59.Kim S.-i., Jin S.-G., Pfeifer G.P. Formation of cyclobutane pyrimidine dimers at dipyrimidines containing 5-hydroxymethylcytosine. Photochem. Photobiol. Sci. 2013;12(8):1409–1415. doi: 10.1039/c3pp50037c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikehata H., Ono T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011;52(2):115–125. doi: 10.1269/jrr.10175. [DOI] [PubMed] [Google Scholar]
- 61.Wurtmann E.J., Wolin S.L. RNA under attack: cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol. 2009;44(1):34–49. doi: 10.1080/10409230802594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clingen P.H., et al. Induction of cyclobutane pyrimidine dimers, pyrimidine (6-4) pyrimidone photoproducts, and Dewar valence isomers by natural sunlight in normal human mononuclear cells. Cancer Res. 1995;55(11):2245–2248. [PubMed] [Google Scholar]
- 63.Brash D.E., et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA. 1991;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kriegenburg F., et al. Redox control of the ubiquitin-proteasome system: from molecular mechanisms to functional significance. Antioxidants Redox Signal. 2011;15(8):2265–2299. doi: 10.1089/ars.2010.3590. [DOI] [PubMed] [Google Scholar]
- 65.Watson R.E., et al. Damage to skin extracellular matrix induced by UV exposure. Antioxidants Redox Signal. 2014;21(7):1063–1077. doi: 10.1089/ars.2013.5653. [DOI] [PubMed] [Google Scholar]
- 66.Bent D., Hayon E. Excited state chemistry of aromatic amino acids and related peptides. I. Tyrosine. J. Am. Chem. Soc. 1975;97(10):2599–2606. doi: 10.1021/ja00843a002. [DOI] [PubMed] [Google Scholar]
- 67.Karran P., Brem R. Protein oxidation, UVA and human DNA repair. DNA Repair. 2016;44:178–185. doi: 10.1016/j.dnarep.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uttara B., et al. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Birben E., et al. Oxidative stress and antioxidant defense. World Allergy Organiz. J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lephart E.D. Skin aging and oxidative stress: equol's anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016;31:36–54. doi: 10.1016/j.arr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Fukai T., Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants Redox Signal. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2015;15(1):1–22. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kammeyer A., Luiten R. Oxidation events and skin aging. Ageing Res. Rev. 2015;21:16–29. doi: 10.1016/j.arr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Chen H., Weng Q.Y., Fisher D.E. UV signaling pathways within the skin. J. Invest. Dermatol. 2014;134(8):2080–2085. doi: 10.1038/jid.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y., Fisher G.J. Ultraviolet (UV) light irradiation induced signal transduction in skin photoaging. J. Dermatol. Sci. Suppl. 2005;1(2):S1–S8. [Google Scholar]
- 76.Afaq F., Ahmad N., Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22(58):9254–9264. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- 77.Sharma M.R., Werth B., Werth V.P. Animal models of acute photodamage: comparisons of anatomic, cellular and molecular responses in C57BL/6J, SKH1 and Balb/c mice. Photochem. Photobiol. 2011;87(3):690–698. doi: 10.1111/j.1751-1097.2011.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim A.L., et al. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J. Invest. Dermatol. 2005;124(6):1318–1325. doi: 10.1111/j.0022-202X.2005.23747.x. [DOI] [PubMed] [Google Scholar]
- 79.Rodríguez-Rojas F., et al. MAPK pathway under chronic copper excess in green macroalgae (Chlorophyta): involvement in the regulation of detoxification mechanisms. Int. J. Mol. Sci. 2019;20(18):4546. doi: 10.3390/ijms20184546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bode A.M., Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. Signal. 2003;2003(167):re2. doi: 10.1126/stke.2003.167.re2. re2. [DOI] [PubMed] [Google Scholar]
- 81.Muthusamy V., Piva T.J. The UV response of the skin: a review of the MAPK, NFκB and TNFα signal transduction pathways. Arch. Dermatol. Res. 2010;302(1):5. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- 82.Pfundt R., et al. In situ demonstration of phosphorylated c‐jun and p38 MAP kinase in epidermal keratinocytes following ultraviolet B irradiation of human skin. J. Pathol.: J. Patholog. Soci. Great Britain Ireland. 2001;193(2):248–255. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH780>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 83.Afaq F., K Katiyar S. Polyphenols: skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011;11(14):1200–1215. doi: 10.2174/13895575111091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ansel J., et al. Cytokine modulation of keratinocyte cytokines. J. Invest. Dermatol. 1990;94(6):s101–s107. doi: 10.1111/1523-1747.ep12876053. [DOI] [PubMed] [Google Scholar]
- 85.Ansel J.C., Luger T.A., Green I. The effect of in vitro and in vivo UV irradiation on the production of ETAF activity by human and murine keratinocytes. J. Invest. Dermatol. 1983;81(6) doi: 10.1111/1523-1747.ep12522862. [DOI] [PubMed] [Google Scholar]
- 86.Clydesdale G.J., Dandie G.W., Muller H.K. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol. Cell Biol. 2001;79(6):547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 87.Hwang E., et al. A comparative study of baby immature and adult shoots of Aloe vera on UVB‐induced skin photoaging in vitro. Phytother Res. 2013;27(12):1874–1882. doi: 10.1002/ptr.4943. [DOI] [PubMed] [Google Scholar]
- 88.Pittayapruek P., et al. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016;17(6):868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simmler C., Antheaume C., Lobstein A. Antioxidant biomarkers from Vanda coerulea stems reduce irradiated HaCaT PGE-2 production as a result of COX-2 inhibition. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tripp C.S., et al. Epidermal COX-2 induction following ultraviolet irradiation: suggested mechanism for the role of COX-2 inhibition in photoprotection. J. Invest. Dermatol. 2003;121(4):853–861. doi: 10.1046/j.1523-1747.2003.12495.x. [DOI] [PubMed] [Google Scholar]
- 91.Rittié L., Fisher G.J. Natural and sun-induced aging of human skin. Cold Spring Harbor Perspect. Med, 2015;5(1):a015370. doi: 10.1101/cshperspect.a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verrecchia F., Mauviel A. Transforming growth factor-β and fibrosis. World J. Gastroenterol.: WJG. 2007;13(22):3056. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piek E., Heldin C.H., Dijke P.T. Specificity, diversity, and regulation in TGF‐β superfamily signaling. Faseb. J. 1999;13(15):2105–2124. [PubMed] [Google Scholar]
- 94.Quan T., et al. Ultraviolet irradiation alters transforming growth factor β/Smad pathway in human skin in vivo. J. Invest. Dermatol. 2002;119(2):499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- 95.Yaar M., Gilchrest B.A. Photoageing: mechanism, prevention and therapy. Br. J. Dermatol. 2007;157(5):874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 96.Scharffetter–Kochanek K., et al. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000;35(3):307–316. doi: 10.1016/s0531-5565(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 97.Kajanne R., et al. EGF‐R regulates MMP function in fibroblasts through MAPK and AP‐1 pathways. J. Cell. Physiol. 2007;212(2):489–497. doi: 10.1002/jcp.21041. [DOI] [PubMed] [Google Scholar]
- 98.Tong T., et al. α-Ionone protects against UVB-induced photoaging in human dermal fibroblasts. Molecules. 2019;24(9):1804. doi: 10.3390/molecules24091804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.López-Camarillo C., et al. Protein kinases and transcription factors activation in response to UV-radiation of skin: implications for carcinogenesis. Int. J. Mol. Sci. 2012;13(1):142–172. doi: 10.3390/ijms13010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oeckinghaus A., Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harbor Perspect. Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.May M.J., Ghosh S. Seminars in Cancer Biology. Elsevier; 1997. Rel/NF-κB and IκB proteins: an overview. [Google Scholar]
- 102.Solt L.A., May M.J. The IκB kinase complex: master regulator of NF-κB signaling. Immunol. Res. 2008;42(1–3):3. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blackwell T.S., Christman J.W. The role of nuclear factor-κ B in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol. 1997;17(1):3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 104.McStay C. What is the pathophysiology of sunburn. Medscape, Nov. 2018;8 [Google Scholar]
- 105.Assefa Z., et al. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim. Biophys. Acta, Rev. Cancer. 2005;1755(2):90–106. doi: 10.1016/j.bbcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 106.Assefa Z., et al. Ultraviolet B radiation-induced apoptosis in human keratinocytes: cytosolic activation of procaspase-8 and the role of Bcl-2. FEBS Lett. 2003;540(1–3):125–132. doi: 10.1016/s0014-5793(03)00238-2. [DOI] [PubMed] [Google Scholar]
- 107.Nys K., et al. A p38MAPK/HIF-1 pathway initiated by UVB irradiation is required to induce noxa and apoptosis of human keratinocytes. J. Invest. Dermatol. 2010;130(9):2269–2276. doi: 10.1038/jid.2010.93. [DOI] [PubMed] [Google Scholar]
- 108.Van Laethem A., et al. Apoptosis signal regulating kinase-1 connects reactive oxygen species to p38 MAPK-induced mitochondrial apoptosis in UVB-irradiated human keratinocytes. Free Radic. Biol. Med. 2006;41(9):1361–1371. doi: 10.1016/j.freeradbiomed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 109.Van Laethem A., et al. Activation of p38 MAPK is required for Bax translocation to mitochondria, cytochrome c release and apoptosis induced by UVB irradiation in human keratinocytes. Faseb. J. 2004;18(15):1946–1948. doi: 10.1096/fj.04-2285fje. [DOI] [PubMed] [Google Scholar]
- 110.Battie C., et al. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014;23:7–12. doi: 10.1111/exd.12388. [DOI] [PubMed] [Google Scholar]
- 111.Ou-Yang H., et al. A chemiluminescence study of UVA-induced oxidative stress in human skin in vivo. J. Invest. Dermatol. 2004;122(4):1020–1029. doi: 10.1111/j.0022-202X.2004.22405.x. [DOI] [PubMed] [Google Scholar]
- 112.Puri P., et al. Effects of air pollution on the skin: a review. Indian J. Dermatol., Venereol. Leprol. 2017;83:415. doi: 10.4103/0378-6323.199579. [DOI] [PubMed] [Google Scholar]
- 113.Manisalidis I., et al. Environmental and health impacts of air pollution: a review. Front. Public Health. 2020:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McDaniel D., Farris P., Valacchi G. Atmospheric skin aging—contributors and inhibitors. J. Cosmet. Dermatol. 2018;17(2):124–137. doi: 10.1111/jocd.12518. [DOI] [PubMed] [Google Scholar]
- 115.Chung J.H. Photoaging in asians. Photodermatol. Photoimmunol. Photomed. 2003;19(3):109–121. doi: 10.1034/j.1600-0781.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 116.Krutmann J., Schroeder P. Journal of Investigative Dermatology Symposium Proceedings. Elsevier; 2009. Role of mitochondria in photoaging of human skin: the defective powerhouse model. [DOI] [PubMed] [Google Scholar]
- 117.Balcázar M., et al. Bases for treating skin aging with artificial mitochondrial transfer/transplant (AMT/T) Front. Bioeng. Biotechnol. 2020;8:919. doi: 10.3389/fbioe.2020.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sreedhar A., Aguilera-Aguirre L., Singh K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020;11(6):1–14. doi: 10.1038/s41419-020-2649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Birch‐Machin M., Bowman A. Oxidative stress and ageing. Br. J. Dermatol. 2016;175:26–29. doi: 10.1111/bjd.14906. [DOI] [PubMed] [Google Scholar]
- 120.Seite S., et al. Alterations in human epidermal Langerhans cells by ultraviolet radiation: quantitative and morphological study. Br. J. Dermatol. 2003;148(2):291–299. doi: 10.1046/j.1365-2133.2003.05112.x. [DOI] [PubMed] [Google Scholar]
- 121.Katiyar S.K. UV-induced immune suppression and photocarcinogenesis: chemoprevention by dietary botanical agents. Cancer Lett. 2007;255(1):1–11. doi: 10.1016/j.canlet.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stremnitzer C. uniwien; 2010. The Roles of Urocanic Acid in the Response of Epidermal Cells to UVB. [Google Scholar]
- 123.Chomiczewska-Skóra D., et al. Effects of ultraviolet radiation on Langerhans cells. Cent. Eur. J. Immunol. 2013;38:393–398. [Google Scholar]
- 124.Zak‐Prelich M., et al. cis‐Urocanic acid does not induce the expression of immunosuppressive cytokines in murine keratinocytes¶. Photochem. Photobiol. 2001;73(3):238–244. doi: 10.1562/0031-8655(2001)073<0238:cuadni>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 125.Burkholder B., et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta, Rev. Cancer. 2014;1845(2):182–201. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 126.Geng R., et al. Boosting the photoaged skin: the potential role of dietary components. Nutrients. 2021;13(5):1691. doi: 10.3390/nu13051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kruglikov I.L., Scherer P.E. Skin aging: are adipocytes the next target? Aging (Albany NY) 2016;8(7):1457. doi: 10.18632/aging.100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee J., et al. Ultraviolet A regulates adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via up-regulation of Kruppel-like factor 2. J. Biol. Chem. 2010;285(42):32647–32656. doi: 10.1074/jbc.M110.135830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chon S.-H., Pappas A. Differentiation and characterization of human facial subcutaneous adipocytes. Adipocyte. 2015;4(1):13–21. doi: 10.4161/21623945.2014.955402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kruglikov I.L., Scherer P.E. Dermal adipocytes and hair cycling: is spatial heterogeneity a characteristic feature of the dermal adipose tissue depot? Exp. Dermatol. 2016;25(4):258–262. doi: 10.1111/exd.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie H.-f., et al. miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1. Cell Death Dis. 2017;8(3):e2663. doi: 10.1038/cddis.2017.75. e2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang B., et al. Microrna-217 modulates human skin fibroblast senescence by directly targeting DNA methyltransferase 1. Oncotarget. 2017;8(20) doi: 10.18632/oncotarget.16509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Röck K., et al. miR-23a-3p causes cellular senescence by targeting hyaluronan synthase 2: possible implication for skin aging. J. Invest. Dermatol. 2015;135(2):369–377. doi: 10.1038/jid.2014.422. [DOI] [PubMed] [Google Scholar]
- 134.Blackstone B.N., et al. Skin biomechanics and miRNA expression following chronic UVB irradiation. Adv. Wound Care. 2020;9(3):79–89. doi: 10.1089/wound.2019.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang S., Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi: 10.1177/0963689717725755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee Y.M., Kang S.M., Chung J.H. The role of TRPV1 channel in aged human skin. J. Dermatol. Sci. 2012;65(2):81–85. doi: 10.1016/j.jdermsci.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 137.Huang K.-F., et al. Ultraviolet B irradiation induced Nrf2 degradation occurs via activation of TRPV1 channels in human dermal fibroblasts. Free Radic. Biol. Med. 2019;141:220–232. doi: 10.1016/j.freeradbiomed.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 138.Han A., et al. Sulfuretin, a natural Src family kinases inhibitor for suppressing solar UV-induced skin aging. J. Funct.Foods. 2019;52:442–449. [Google Scholar]
- 139.Liu Y., et al. Molecular mechanisms of marine-derived natural compounds as photoprotective strategies. Int. Immunopharm. 2022;111 doi: 10.1016/j.intimp.2022.109174. [DOI] [PubMed] [Google Scholar]
- 140.Korać R.R., Khambholja K.M. Potential of herbs in skin protection from ultraviolet radiation. Phcog. Rev. 2011;5(10):164. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kligman A.M. Current status of topical tretinoin in the treatment of photoaged skin. Drugs Aging. 1992;2(1):7–13. doi: 10.2165/00002512-199202010-00002. [DOI] [PubMed] [Google Scholar]
- 142.Mohiuddin A.K. Skin lightning & management of hyperpigmentation. Int. J. Res. Biol. Pharm. 2019;2(2):1–37. [Google Scholar]
- 143.Berneburg M., Plettenberg H., Krutmann J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed.: Rev. Art. 2000;16(6):239–244. doi: 10.1034/j.1600-0781.2000.160601.x. [DOI] [PubMed] [Google Scholar]
- 144.Poon F., Kang S., Chien A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015;31(2):65–74. doi: 10.1111/phpp.12145. [DOI] [PubMed] [Google Scholar]
- 145.Ganceviciene R., et al. Skin anti-aging strategies. Derm. Endocrinol. 2012;4(3):308–319. doi: 10.4161/derm.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li J.W.-H., Vederas J.C. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325(5937):161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 147.Mahesh S.K., Fathima J., Veena V.G. Natural Bio-Active Compounds. Springer; 2019. Cosmetic potential of natural products: industrial applications; pp. 215–250. [Google Scholar]
- 148.Nichols J.A., Katiyar S.K. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010;302(2):71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moon H.J., et al. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008;31(2):284–289. doi: 10.1248/bpb.31.284. [DOI] [PubMed] [Google Scholar]
- 150.Wölfle U., et al. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011;50(9):1081–1093. doi: 10.1016/j.freeradbiomed.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 151.Terao J., Minami Y., Bando N. Singlet molecular oxygen-quenching activity of carotenoids: relevance to protection of the skin from photoaging. J. Clin. Biochem. Nutr. 2010;48(1):57–62. doi: 10.3164/jcbn.11-008FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tanaka K., et al. Protecting skin photoaging by NF-κB inhibitor. Curr. Drug Metabol. 2010;11(5):431–435. doi: 10.2174/138920010791526051. [DOI] [PubMed] [Google Scholar]
- 153.Zaid M.A., et al. Inhibition of UVB‐mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem. Photobiol. 2007;83(4):882–888. doi: 10.1111/j.1751-1097.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 154.Bae J.Y., et al. Bog blueberry anthocyanins alleviate photoaging in ultraviolet‐B irradiation‐induced human dermal fibroblasts. Mol. Nutr. Food Res. 2009;53(6):726–738. doi: 10.1002/mnfr.200800245. [DOI] [PubMed] [Google Scholar]
- 155.Oh H.I., et al. The effect of xanthorrhizol on the expression of matrix metalloproteinase‐1 and type‐I procollagen in ultraviolet‐irradiated human skin fibroblasts. Phytother Res.: Int. J. Dev. Pharmacolog. Toxicolog. Eva. Nat. Prod. Deriv. 2009;23(9):1299–1302. doi: 10.1002/ptr.2768. [DOI] [PubMed] [Google Scholar]
- 156.Helfrich Y.R., Sachs D.L., Voorhees J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 2008;20(3):177. [PubMed] [Google Scholar]
- 157.Heinrich U., et al. Green tea polyphenols provide photoprotection, increase microcirculation, and modulate skin properties of women. J. Nutr. 2011;141(6):1202–1208. doi: 10.3945/jn.110.136465. [DOI] [PubMed] [Google Scholar]
- 158.Umar S.A., et al. Glycyrrhizic acid prevents oxidative stress mediated DNA damage response through modulation of autophagy in ultraviolet-B-irradiated human primary dermal fibroblasts. Cell. Physiol. Biochem. 2019;53:242–257. doi: 10.33594/000000133. [DOI] [PubMed] [Google Scholar]
- 159.Naikoo S., Tasduq S.A. Trigonelline, a naturally occurring alkaloidal agent protects ultraviolet-B (UV-B) irradiation induced apoptotic cell death in human skin fibroblasts via attenuation of oxidative stress, restoration of cellular calcium homeostasis and prevention of endoplasmic reticulum (ER) stress. J. Photochem. Photobiol. B Biol. 2020;202 doi: 10.1016/j.jphotobiol.2019.111720. [DOI] [PubMed] [Google Scholar]
- 160.Farage M.A., et al. Characteristics of the aging skin. Adv. Wound Care. 2013;2(1):5–10. doi: 10.1089/wound.2011.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Działo M., et al. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016;17(2):160. doi: 10.3390/ijms17020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Iwu M.W., Duncan A.R., Okunji C.O. vol. 457. ASHS Press; Alexandria, VA: 1999. p. 462. (New Antimicrobials of Plant Origin. Perspectives on New Crops and New Uses). [Google Scholar]
- 163.Ikram M., Javed B., Raja N.I. Biomedical potential of plant-based selenium nanoparticles: a comprehensive review on therapeutic and mechanistic aspects. Int. J. Nanomed. 2021;16:249. doi: 10.2147/IJN.S295053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.