Keywords: CGRP, GPCR, migraine, neuropeptide, trigeminal nerve
Abstract
Calcitonin gene-related peptide (CGRP) is a neuropeptide with diverse physiological functions. Its two isoforms (α and β) are widely expressed throughout the body in sensory neurons as well as in other cell types, such as motor neurons and neuroendocrine cells. CGRP acts via at least two G protein-coupled receptors that form unusual complexes with receptor activity-modifying proteins. These are the CGRP receptor and the AMY1 receptor; in rodents, additional receptors come into play. Although CGRP is known to produce many effects, the precise molecular identity of the receptor(s) that mediates CGRP effects is seldom clear. Despite the many enigmas still in CGRP biology, therapeutics that target the CGRP axis to treat or prevent migraine are a bench-to-bedside success story. This review provides a contextual background on the regulation and sites of CGRP expression and CGRP receptor pharmacology. The physiological actions of CGRP in the nervous system are discussed, along with updates on CGRP actions in the cardiovascular, pulmonary, gastrointestinal, immune, hematopoietic, and reproductive systems and metabolic effects of CGRP in muscle and adipose tissues. We cover how CGRP in these systems is associated with disease states, most notably migraine. In this context, we discuss how CGRP actions in both the peripheral and central nervous systems provide a basis for therapeutic targeting of CGRP in migraine. Finally, we highlight potentially fertile ground for the development of additional therapeutics and combinatorial strategies that could be designed to modulate CGRP signaling for migraine and other diseases.
CLINICAL HIGHLIGHTS.
Calcitonin gene-related peptide (CGRP) is a neuropeptide with diverse physiological functions. Approved therapeutics for migraine treatment and prevention that target CGRP signaling are a recent success story. This review provides an historical perspective on CGRP, its role as a neuromodulator, its receptors and physiological functions in the cardiovascular, gastrointestinal, pulmonary, immune, and reproductive systems, and its metabolic effects. We highlight how CGRP may cause migraine and how CGRP therapeutics may prove useful for additional diseases, including arthritis, hypertension, cardiovascular hypertrophy, rosacea, Raynaud’s phenomenon, airway diseases, gastrointestinal disorders, metabolic diseases, and possibly even COVID.
1. INTRODUCTION
1.1. Overview
The interest in calcitonin gene-related peptide (CGRP) has blossomed in the past few years as a result of the bench-to-bedside success story of CGRP-based therapeutics for preventing and treating migraine. This success has raised many questions, such as just how is CGRP working, can CGRP-based drugs be used for other disorders, and will those drugs be safe for long-term use? It is in the context of these migraine-driven questions that we have delved into the multifaceted functions of CGRP across multiple organ systems that include and go beyond migraine. We cover basic fundamentals, such as the mechanisms and sites of CGRP expression, with particular focus on the nervous system. To better understand how CGRP is working, we discuss the unusual features of the CGRP receptor family, including a second CGRP receptor and how these receptors provide future drug targets not just for migraine but for other disorders that may involve CGRP activities in diverse physiological systems.
1.2. History and Discovery
CGRP is now recognized as an important multifunctional neuropeptide, but its original discovery had nothing to do with function. Rather, it was discovered as one of the first examples of alternative RNA processing. In the early 1980s, a new perspective on gene regulation was revealed by the alternative splicing and polyadenylation of viral transcripts. The dogma that one gene generates one RNA was no longer true, but it remained to be seen whether alternative RNA processing was a viral oddity designed to pack more information into the relatively limited genome of a virus or if it was applicable to cellular genes as well. The first documentation that alternative RNA processing occurred with cellular transcripts came from immunoglobulin M (IgM) heavy chain that switched IgM from a membrane-bound to a secreted form. At about this time, Amara and Rosenfeld noticed that serial passage of medullary thyroid carcinoma tumors led to a decrease of calcitonin mRNA that was coupled to an increase of a different, but related, mRNA species (1). The initial name for this alternative transcript was pseudo-cal, which fortunately was soon discarded. Subsequent studies documented that alternative RNA splicing removed the exon encoding calcitonin to allow inclusion of new downstream exons encoding CGRP and noncoding sequences, along with a new polyadenylation site. In recognition of its origin from the calcitonin gene, this new transcript was named “calcitonin gene-related peptide” (2).
Thus, CGRP is notable as the second example of mammalian alternative RNA processing (FIGURE 1). Since those early days, alternative RNA processing is now recognized as a common theme that greatly expands the diversity of gene products from the mammalian genome. Expression of CGRP as a peptide was quickly confirmed by CGRP immunoreactivity in discrete regions of the central and peripheral nervous systems that suggested activities in cardiovascular, integrative, and gastrointestinal (GI) systems (3). Indeed, we now know that CGRP-containing nerve fibers innervate every major organ system of the body and that endogenous CGRP-expressing cells are also sometimes present in those organs.
Figure 1.
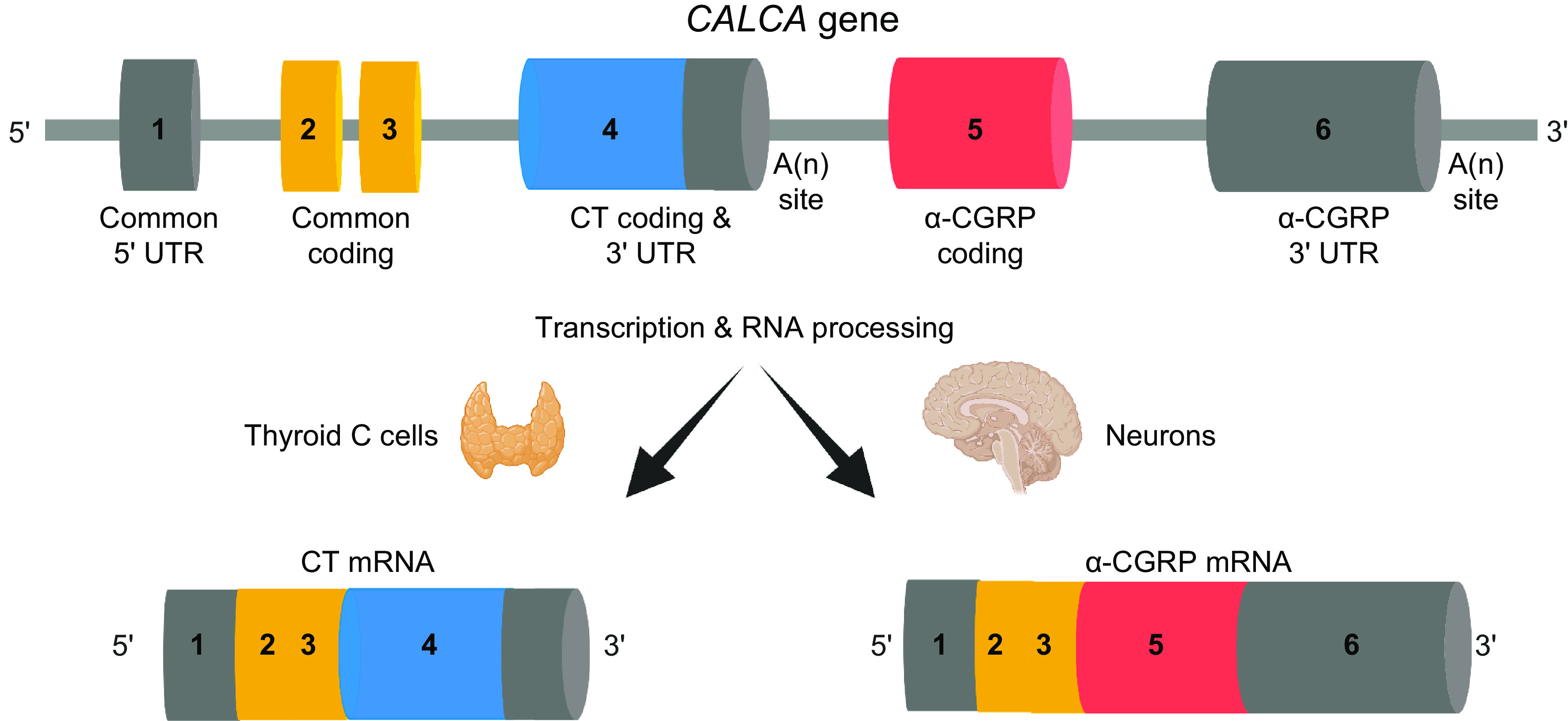
RNA processing of CALCA RNA to yield calcitonin (CT) and α-calcitonin gene-related peptide (CGRP). The CALCA gene contains 6 exons. Exon 1 is a 5′ untranslated region (UTR) common to both calcitonin and α-CGRP mRNAs. Exons 2 and 3 encode NH2-terminal sequences common to both calcitonin and α-CGRP propeptides. Exon 4 encodes calcitonin-specific sequences and 3′ UTR, followed by a cleavage and polyadenylation [A(n)] site. Exon 5 encodes α-CGRP-specific sequences that are spliced onto exon 3. Exon 6 is a 3′ UTR specific to α-CGRP mRNA and is followed by an A(n) site. Calcitonin mRNA is predominantly made in thyroid C cells, and α-CGRP mRNA is predominantly made in neurons of the central and peripheral nervous system, as well as in neuroendocrine and other cells. β-CGRP is encoded by a separate gene (CALCB), not shown. Image created with BioRender.com, with permission.
CGRP has been most studied as a neuropeptide, given its abundant expression in sensory neurons, but it is also expressed in nonneuronal cells. Nonneuronal sources of CGRP that have been reported include diffuse neuroendocrine and endocrine cells found in the thyroid (C cells) (4), lung and respiratory tract epithelia (5–7), small intestine mucosal endocrine cells (8), scattered adrenal chromaffin cells (3, 9), pancreatic islets (3), and Merkel cells in the skin (10). In addition to endocrine cells, CGRP is synthesized and secreted from other nonneuronal cells, including epithelial cells (11), adipocytes (12, 13), keratinocytes (14), endothelial cells (15, 16), white blood cells (see sect. 5.5) (17, 18), cardiac fibroblasts (19), and liver parenchymal cells (20). In some of these cell types, expression was only observed under inflammatory or other stress conditions. In general, the biological significance of nonneuronal CGRP is not clear. Likewise, although it seems unlikely that CGRP has significant endocrine activity, in part based on its fairly rapid degradation in human plasma (21), plasma CGRP levels are elevated under certain conditions, including sepsis (see sect. 5.5.1) and pregnancy (see sect. 5.8.1), possibly from perivascular spillover (22).
1.3. CGRP Family Members
CGRP is a member of a gene family of six related peptides: calcitonin, α-CGRP, β-CGRP, amylin, adrenomedullin (AM), and adrenomedullin 2 (AM2; also known as intermedin) (FIGURE 2) (23). The mature form of CGRP is a 37-amino acid peptide with an NH2-terminal disulfide bond and an amidated COOH terminus (FIGURE 2). Both regions are required for receptor activation. This structure is conserved across all members of the CGRP gene family. In addition to the six canonical members, there are also some related peptides not found in rodents or humans and other unidentified immunoreactive peptides that might be considered distant family members (24). There is also the question of whether the precursor peptide of calcitonin, pro-calcitonin, should be considered a family member since it has been reported to act as a partial agonist at the CGRP receptor (25) and has been implicated in sepsis and other inflammatory pathologies (12, 26, 27).
Figure 2.
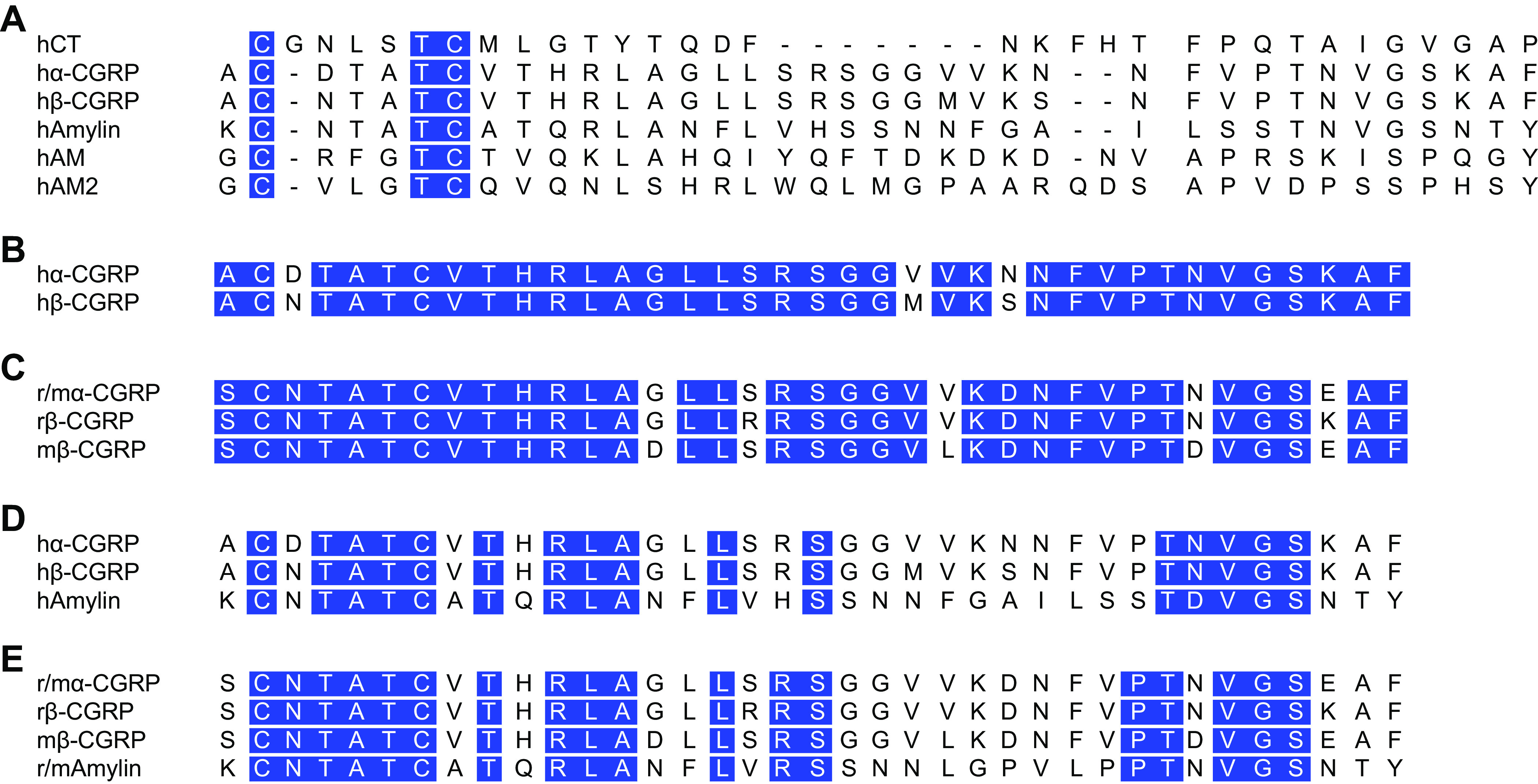
Amino acid sequence alignment of calcitonin gene-related peptide (CGRP) and related peptides from human and rat. In A, the human (h) calcitonin (CT) peptide family are shown, omitting the NH2-terminal extensions of adrenomedullin (AM) and adrenomedullin 2 (AM2). Note that the early nomenclature referred to human α- and β-CGRP as CGRP I and CGRP II, respectively. B and C align the CGRPs to illustrate their similarities and differences. D and E compare the CGRPs to amylin. Mouse and rat α-CGRP/amylin are identical and are therefore noted as “r/m” in the figure. In all peptides, a disulfide bond is formed between the 2 NH2-terminal cysteines, and they each have a COOH-terminal amide (not shown). In all panels, identical amino acids between sets of sequences are highlighted in blue.
The CGRP family peptides share overlapping, yet distinct, biological activities and expression patterns (24, 28, 29). Although this review is focused on CGRP, a broad overview of what CGRP and its family members share is warranted. In general, the CGRP family peptides are all modulators that help maintain homeostasis. What is a modulator? A modulator is a molecule, often a peptide, that either increases or decreases a signal sent by other molecules. This is most often thought of as modulation of neurotransmission, yet as seen throughout this review, for CGRP this modulation is not limited to the nervous system.
Within the CGRP family, the two most closely related peptides are α-CGRP and β-CGRP (FIGURE 2). The α- and β-isoforms are expressed from two genes. Human CALCA (rodent Calca) encodes α-CGRP, which we will often refer to simply as CGRP. Human CALCB (rodent Calcb) encodes β-CGRP and differs from α-CGRP by only a few amino acids dependent upon species. The α- and β-CGRP peptides have nearly indistinguishable activities yet are differentially regulated and expressed in an overlapping pattern (30–32). Although sometimes β-CGRP is referred to as the enteric form of CGRP, this is misleading because both α- and β-CGRP are found in the GI system and both are found in the central nervous system (CNS), as discussed in sects. 3.5 and 5.7. Indeed, given the overlap in expression and the inability of any current antisera to distinguish α- from β-CGRP, a caveat of nearly all CGRP studies is that we really do not know the relative contributions from one gene versus the other. Recent genetic studies implicating the β-CGRP gene in human diseases discussed in sect. 2.1.2 are a reminder that both α- and β-CGRP are important.
Two members of the CGRP family that are clearly distinct from CGRP in structure and receptor affinities but have overlapping activities are AM and AM2. Of note, like CGRP, both AM and AM2 are potent vasodilators and are cardioprotective (33–35). Interestingly, infusion of AM can induce migraine in susceptible patients, similar to CGRP (36). However, unlike CGRP the source of AM and AM2 is primarily nonneuronal. Within the vasculature these peptides are synthesized and released from the endothelium instead of perivascular neurons. Beyond the vasculature, AM and AM2 are expressed in the heart and other tissues that overlap but are distinct from CGRP (37, 38). As discussed in sect. 4, both AM and AM2 signal through the calcitonin receptor-like receptor (CLR)/receptor activity-modifying protein (RAMP) 2 and CLR/RAMP3 receptors, which are similar but distinct from the canonical CGRP receptor composed of CLR/RAMP1. Signaling from CLR/RAMP2 is required for angiogenesis during embryogenesis (39–41). Both AM and AM2 have been suggested as therapeutic targets for diseases such as myocardial infarction and cancer, but to date there are not yet any approved AM-based drugs (35, 42, 43).
The family member that is most similar to CGRP is amylin (FIGURE 2). Human and rodent amylin share very high sequence identity to CGRP. Most notably, the two peptides also share a common receptor, AMY1 (23). Amylin is noteworthy as a glucose-lowering hormone produced by the pancreas (44). A synthetic form of human amylin, pramlintide, was approved in 2005 for treating diabetes (45). Interestingly, headache has been reported as an adverse event in healthy subjects infused with pramlintide (46). As described in sect. 6.6, a recent study reported that infusion of pramlintide causes migraine in migraine patients that is similar to infusion of CGRP (47). Similarly, both CGRP and amylin can cause migraine-like symptoms in mice (47, 48), which provides proof of principle that these two peptides also have overlapping functional roles.
1.4. General Features of CGRP as a Neuropeptide
An inherent feature of CGRP is that it is a neuropeptide. However, the line between a neuropeptide and a neuroendocrine peptide is blurry. Most neuropeptides, including CGRP, are also released from endocrine cells and can act on both neural and nonneural targets. As our definition of endocrine cells and tissues expands, the definition of “neuroendocrine” is becoming even broader. Although strictly speaking CGRP is perhaps better classified as a neuroendocrine peptide, we will refer to it simply as a neuropeptide, since to date that best describes its functions.
Humans have >100 known neuropeptides, many of which fall into the same classification quandary with CGRP, that can be grouped in about two dozen families (49). The pleiotropic potential of neuropeptides was best stated by an early pioneer in the field, Candace Pert: “As our feelings change, this mixture of peptides travels throughout your body and your brain. And they’re literally changing the chemistry of every cell in your body” (50). With this perspective, there are two basic features of neuropeptide expression and pharmacology relevant to CGRP: posttranslational processing and activation of receptors by volume transmission over a relatively large distance.
1.4.1. Posttranslational processing.
CGRP and neuropeptides in general are processed from precursor proteins and released from vesicles. The CGRP precursor protein is proteolytically cleaved and modified by addition of a COOH-terminal amide group that is required for CGRP binding to its receptors and for biological activity (51–54). Hence detection of CGRP RNA or peptide immunoreactivity does not necessarily mean fully modified and functional CGRP. Fortunately, the enzyme required for peptide amidation (PAM) is present in many cell types. For example, 3T3 fibroblasts have low but detectable PAM that is sufficient to modify peptides even from the constitutive secretory pathway (55).
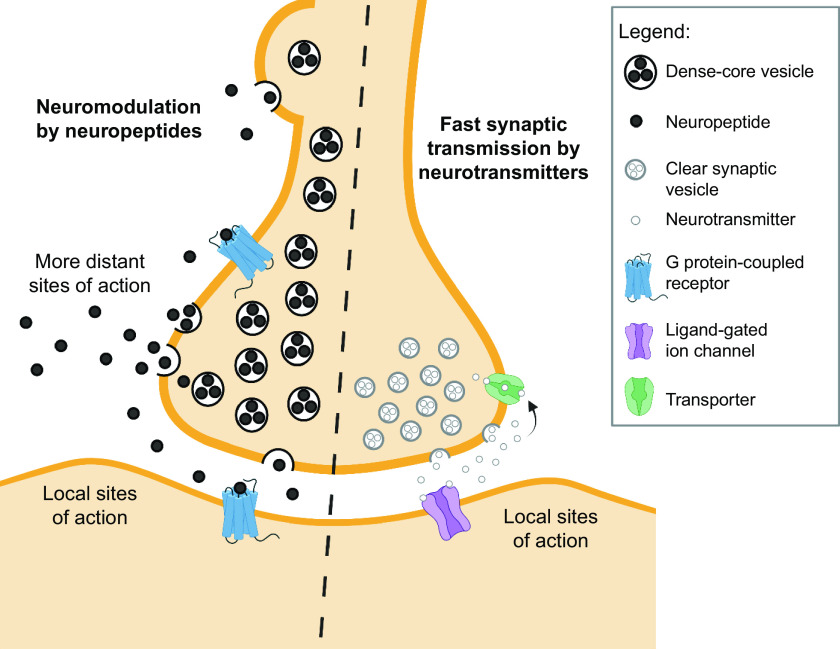
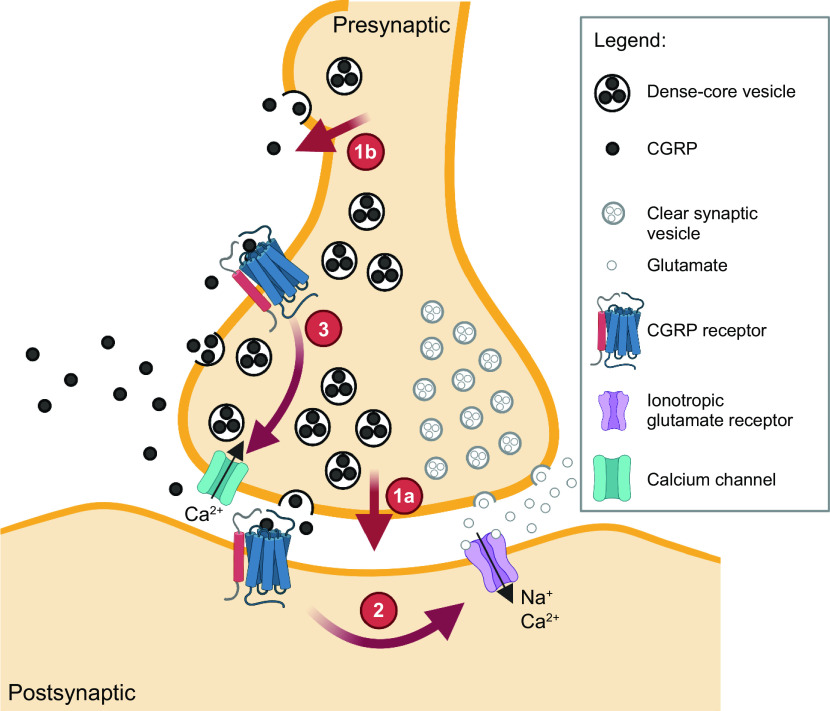
Proteolytic processing of CGRP occurs at dibasic residues that are generally recognized by several proteases, such as furin (56, 57). A feature of the processing pathway that was recognized early on was that it can generate not just one but a portfolio of peptides from a precursor peptide. In this way, along with alternative RNA processing, multiple neuropeptides can be generated from the Calca gene (56), including propeptides containing calcitonin or CGRP sequences and NH2-terminal and COOH-terminal peptides that lack calcitonin or CGRP sequences (25, 58). Processing of CGRP occurs as a progressive mechanism from the endoplasmic reticulum into dense core secretory vesicles (FIGURE 3). The secretory vesicles are transported down the axon, which can take hours or even a day for longer axons. Once released, CGRP and other peptides are only slowly removed from the extracellular space because of the lack of reuptake machinery, such as the pumps that rapidly remove neurotransmitters from the synaptic cleft.
Figure 3.
Neuropeptides versus neurotransmitters. Neuromodulation by volume transmission of neuropeptides compared with fast synaptic transmission by classical neurotransmitters. Release of neuropeptides from cell bodies, axonal varicosities, and synapses allows a broad diffusion or volume transmission of neuropeptides (released from dense core vesicles) to more distant sites of action than the more locally restricted classical neurotransmitters (released from clear synaptic vesicles). Image created with BioRender.com, with permission.
1.4.2. Activation of receptors by volume transmission.
A distinguishing feature of CGRP and all neuropeptides is volume transmission. Volume transmission is diffusion-driven distribution into extracellular fluid and cerebrospinal fluid (CSF) over a relatively large distance (59) (FIGURE 3). This feature of neuropeptides is driven by the fact that they can be released from dense core vesicles both at synapses in conjunction with release of neurotransmitters in small clear synaptic vesicles (60), as well as from nonsynaptic sites at the cell body and varicosities in axons (57, 61, 62). The capability for nonsynaptic release allows CGRP action over a larger area than classical neurotransmitters released only at the synaptic cleft (59). However, diffusion can be limited by physical barriers, such as within the meninges as shown by the relatively poor diffusion of CGRP between the dura mater and pia mater (63). The ongoing development of new technologies to measure diffusion within extracellular fluids (64) will advance our understanding of volume transmission of CGRP and other peptides in the brain.
A second feature of volume transmission of CGRP that is not unique to neuropeptides, but still important, is the large number of peptides per dense core vesicle. As a result, each vesicle releases a large bolus of CGRP. There have been surprisingly few measurements of the number of peptides within that bolus. One of us recently attempted to calculate this number (49). Using the available data (56, 65), we calculated ∼1.2 × 104 peptides per dense core vesicle, which agrees with an estimated ∼104 molecules of vasopressin per dense core vesicle in hypothalamic neurons (66). The number of vesicles released per cell and per stimulus will of course vary, but a rate of ∼103 vesicles/s was estimated from capacitance measurements of isolated hypothalamic neurons (67), which have an estimated 106 dense core vesicles and release ∼107 molecules/s (66). A conservative estimate is that many hundreds of vesicles will be released from a neuron over a timescale of seconds. Thus, millions of neuropeptides are likely released in a burst from a single neuron. The relevance of this fact is that it is likely that drugs used to sequester a peptide, such as the CGRP-binding monoclonal antibodies used as migraine therapeutics, are unlikely to be able to bind all the peptides released in this large bolus. Rather, the consequence is likely to be not a complete block of transmission but rather a reduced volume of CGRP transmission so that the released CGRP will have a reduced impact on its targets.
Finally, CGRP and nearly all neuropeptides act at G protein-coupled receptors (GPCRs) (FIGURE 3). This is an important distinction from ion channel-coupled receptors, since GPCR signaling is consistent with a slower modulatory response compared to ion channel signaling. The CGRP receptors have relatively high ligand affinities (nanomolar Kds) compared with classical neurotransmitter receptors. This allows small amounts of diffused CGRP over a relatively large distance to activate receptors. In summary, the combination of volume transmission with high-affinity receptors allows CGRP to potentially be active at relatively large distances at relatively low concentrations.
2. REGULATION OF CGRP LEVELS
2.1. Transcriptional Regulation
Given the importance of CGRP in migraine and possibly other pathologies, a key question is how are CGRP levels normally controlled? The genes encoding α-CGRP and β-CGRP are in tandem (in opposite directions) on human chromosome 11 but are differentially regulated. α-CGRP and β-CGRP are encoded by human CALCA and CALCB, respectively. Human and rodent α-CGRP genes have been much more extensively studied than β-CGRP, but recent studies described below remind us that β-CGRP is important, too.
2.1.1. Transcriptional regulatory elements and factors.
The CALCA and Calca promoters are regulated by two distinct elements: a proximal cyclic AMP response element (CRE) and a distal cell-specific enhancer. In contrast to CALCA/Calca, the regulatory elements of CALCB and Calcb have not been identified, although we do know that the genes are differentially expressed in the nervous system (30, 68) and differentially regulated by extracellular signals (32).
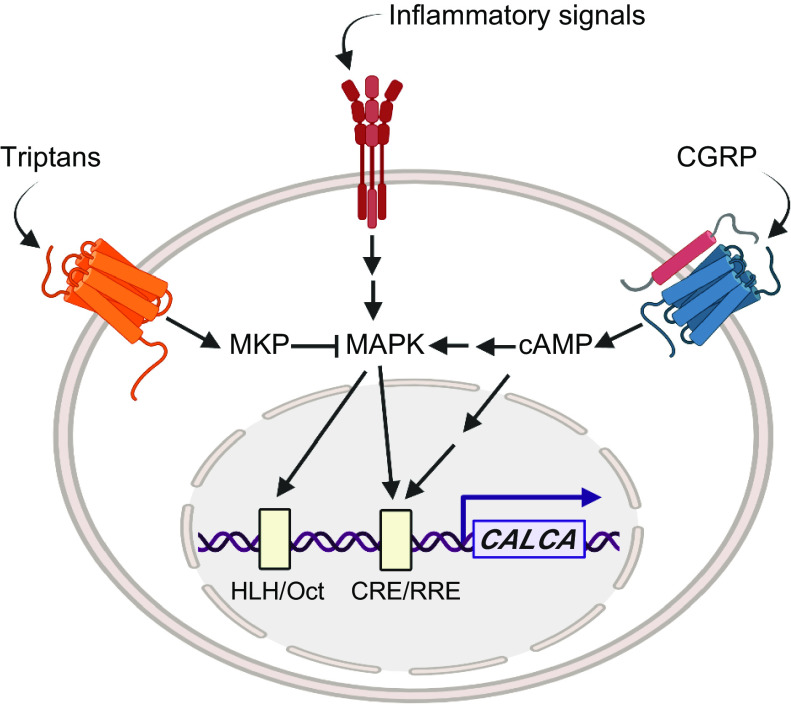
Cell-specific expression of CALCA/Calca has been studied with reporter genes in cell cultures and transgenic mice. A 1.7-kb 5′ flanking region of rat Calca was sufficient to direct expression primarily to thyroid C cells and to a lesser degree to dorsal root ganglia (DRG), spinal cord ventral horn, and the brain of transgenic mice (69). Later studies using cultured neurons indicated that a 1.25-kb 5′ flanking region was sufficient to drive a reporter gene in CGRP-positive neurons (70). Both fragments contain the proximal and distal elements. The proximal element (−255 to −72 bp in CALCA) is an inducible enhancer activated by cAMP, Ras, and nerve growth factor (NGF) signaling pathways but does not contribute to cell specificity (71–74) (FIGURE 4). The distal element is responsible for cell-specific CGRP gene expression (75, 76), and was narrowed down to an 18-bp element (at about −1 kb) containing overlapping helix-loop-helix (HLH) and octamer motifs (77) (FIGURE 4). Electrophoretic mobility shift assays revealed complexes bound to each motif, including the homeodomain protein Oct 1 at the octamer motif that was initially termed octamer-binding protein 1 (OB1, as a nod to Obi-Wan of Star Wars fame) and a cell-specific complex termed OB2 (77). The 18-bp element was capable of driving reporter gene expression specifically in neurons (78), and disruption of the HLH motif reduced the 1.25-kb promoter activity (79). These results indicate that the 18-bp element of the distal element has strong cell-specific enhancer activity. In addition, the 18-bp element can be stimulated by mitogen-activated protein kinase (MAPK) signaling pathways, including those activated by proinflammatory cytokines (80) (FIGURE 4). CGRP can directly activate these pathways to boost its own expression and potentially increase CGRP actions in a positive feedback loop (81) (FIGURE 4). These pathways may contribute to the elevated CGRP levels in diseases involving inflammatory signals, such as sepsis and possibly migraine.
Figure 4.
Regulation of CALCA gene expression. CALCA transcription is stimulated by cAMP and mitogen-activated protein kinase (MAPK) signaling pathways that are activated and repressed by extracellular signals. Calcitonin gene-related peptide (CGRP) and other ligands that act via GPCRs coupled to Gαs increase cAMP, which activates a kinase cascade leading to activation of factors at the CRE/RRE enhancer. The cAMP cascade can also activate MAPK signaling pathways that lead to activation of factors at the helix-loop-helix (HLH)/Oct enhancer. Inflammatory signals, such as tumor necrosis factor α (TNF-α) and other cytokines, activate MAPK signaling pathways leading to activation of factors at the HLH/Oct and CRE/RRE enhancers. Triptans, which act via inhibitory GPCRs, can activate MKP-1, which inhibits MAPK and hence reduces enhancer activity. Glucocorticoids, retinoic acid, and vitamin D3 also repress the gene by less defined mechanisms that inhibit the enhancers (not shown). The CGRP receptor shown represents a generic CGRP-responsive receptor, not specifically the canonical receptor or AMY1. Image created with BioRender.com, with permission.
Transcription factors that bind the 18-bp enhancer are a heterodimer of upstream stimulatory factor (USF1 and USF2) bound to the HLH motif (79) and forkhead protein FoxA2 bound to the octamer motif (OB2 complex) (82). Mutation studies confirmed the importance of these factors and showed that Oct 1 (OB1 complex) was not required. Importantly, these two DNA motifs were required to be adjacent for full enhancer activity (77), which suggests that USF and FoxA2 act synergistically for transcriptional activation. Besides their DNA binding ability, physiological roles of these transcription factors were demonstrated through small interfering (si)RNA-mediated knockdown of USF and FoxA2 (78, 82). However, a puzzle was that because the 18-bp enhancer is a cell-specific enhancer, transcription factors bound to this enhancer were expected to be neural and endocrine specific. Yet FoxA2, although present in the CGRP-positive cell lines, is predominantly expressed in the liver and is not detected in adult brain (83) or trigeminal ganglia (TG) neurons (K. Y. Park and A. F. Russo, unpublished data). Whether another FoxA2-like protein acts at the enhancer in neural cells remains to be seen. The other transcription factor, USF, is expressed in the adult brain (84) and TG neurons (78) but is not neural or endocrine specific. However, it should be noted that USF levels were higher in TG neurons than glia (78). The possibility that USF may be rate limiting for CGRP gene expression is supported by the relatively weak binding of USF to its nonconsensus site within the 18-bp enhancer and the ability of MAPK to further activate USF (79) (FIGURE 4). Thus, relatively high levels of USF in neurons along with MAPK stimulation may be necessary to activate a less-than-optimal USF-binding site, which could then contribute to CGRP neural specificity (78).
Although much is known about the cell-specific elements and factors, the story is not complete. The relative expression of the reporter gene in the transgenic mouse line described above was not determined (69). Another study did find that the 2-kb CALCA promoter was able to drive expression of a luciferase reporter gene predominantly in the brain, spinal cord, and neurons of sensory ganglia (A. Kuburas and A. F. Russo, unpublished observations). However, in the brain, expression was broadly distributed in a pattern that did not match the relative levels of endogenous CGRP RNA. This suggests that additional elements, such as a repressor element that restricts panneuronal expression, may lie outside the 2-kb promoter. This is consistent with a report that mice with Cre recombinase inserted into the Calca locus have widespread expression of a Cre-dependent reporter gene in the brain, most likely due to Calca expression during embryogenesis (85). Thus, the final story of neural-specific CGRP gene expression remains to be finished but is likely to involve epigenetic mechanisms, possibly from evolutionarily conserved elements 10 to 35 kb flanking the CALCA locus (A. F. Russo, unpublished data).
2.1.2. Epigenetic regulation of CALCA and CALCB.
The involvement of epigenetic mechanisms to restrict CALCA gene expression was suggested 30 years ago by findings that CALCA was silenced by DNA methylation in some tumors but not those that express CGRP (86, 87). These early studies reported multiple CpG islands from about −1.8 kb into exon 1 and possibly intron 2 of the human CALCA gene (86, 88). However, correlation of the methylation status with CALCA gene expression was not clear; some negative tissues and cell lines express low levels of CALCA, and the analysis was limited by the technology of the era, which only recognized a subset of CpG sites. Thus, although there was a general correlation between methylation and a more closed chromatin structure (89), the key methylation sites were not known. Subsequently, epigenetic induction of Calca was investigated in TG satellite glia (90). This study identified a hypermethylated CpG island flanking the 18-bp enhancer that was surrounded by hypoacetylated chromatin in glia and nonexpressing cell lines. Treatment with a DNA methylation inhibitor induced calcitonin mRNA in glial cultures, and with a histone deacetylase inhibitor there was a synergistic ∼80-fold increase in calcitonin and ∼3-fold increase in CGRP mRNA. Thus, epigenetic mechanisms can keep the Calca gene silent in satellite glia.
Recently, epigenetic regulation of CALCB has been examined. Sustained stimulation by the inflammatory cytokine tumor necrosis factor α (TNF-α), which stimulates Calca expression (80), led to demethylation of CALCB so that the gene was sensitized to low doses of TNF-α (91). This mechanism involved a nearby primate-specific retroviral element, which highlights that mice will not always reveal human mechanisms. Although this study was done only in HEK293F cells, it does portend a potential for epigenetic modulation of CALCB, in addition to CALCA. Furthermore, it is a good reminder that although the TG is 90% α-CGRP, β-CGRP still represents 10% of the signal (30). It is also a reminder that the two genes can be differentially regulated (32).
2.1.3. Regulation of CALCA by multiple signaling pathways.
Several stimuli that upregulate CALCA expression go through MAPK pathways (FIGURE 4). As noted above, in contrast to CALCA, very little is known about CALCB regulation. However, the recent report that CALCB is stimulated by inflammatory signals (91) (see sect. 2.1.2), suggests that CALCB may also be upregulated by MAPK pathways. NGF treatment increased CGRP mRNA levels (92) via MAPK pathways (93, 94). Cytokines such as TNF-α and interleukin (IL)-1β also activated MAPK pathways to increase CGRP levels in neurons (80, 95). Finally, nitric oxide (NO) increased CGRP promoter activity, possibly through MAPK (96). Interestingly, repeated treatments with a NO donor (nitroglycerin) also increased trigeminal CGRP levels in vivo, which was prevented by a delta opioid receptor agonist (97). The DNA element responsible for MAPK stimulation of CALCA was pinpointed to the 18-bp cell-specific enhancer since mutation of the USF binding site prevented MAPK stimulation (94) and siRNA knockdown of USF compromised MAPK stimulation (78). In other systems, USF was shown to be phosphorylated by p38 MAPK (98). In summary, MAPK pathways are a critical signal transduction pathway that increases CGRP gene expression.
Even before MAPK, one of the earliest identified regulators of CALCA was cAMP and protein kinase A (PKA) (99) (FIGURE 4). An inhibitor of PKA decreased NGF- and cAMP-stimulated CGRP promoter activity (100) and CGRP-activated promoter activity (81). Protein kinase C (PKC) was also shown to be involved in CGRP gene expression (99, 101, 102). Combination of a PKC activator (phorbol ester) and cAMP increased calcitonin and CGRP mRNA levels in an additive way, implying that PKC and PKA signaling pathways worked independently (99). On the other hand, inhibition of MAPK-stimulated CGRP promoter by a dominant-negative form of CREB (100) suggests that there is a possible cross talk between PKA and at least MAPK pathways.
The question remains as to whether CGRP autoregulates its own levels. CGRP treatment of DRG neurons, as well as cultured myotubes, activated the cAMP/PKA signaling pathway, a known pathway to increase CGRP gene expression (103–105) (FIGURE 4). Similar signaling mechanisms have been demonstrated in the trigeminal system (81, 106). Importantly, CGRP treatment increased CGRP mRNA levels and promoter activity in cultured TG neurons (81). This autostimulation was inhibited by CGRP receptor antagonists, which verifies activation via a CGRP receptor. Although very few neurons in the TG coexpress CGRP and the canonical CGRP receptor (107–109), the finding that the AMY1 CGRP receptor is in small-diameter TG neurons known to express CGRP (110) is suggestive that autoregulation may occur in the TG. This possibility is strongly supported by the recent finding that small-diameter TG neurons coexpress both CGRP and the calcitonin receptor (CTR) subunit of the AMY1 receptor (111). In addition to TG neurons, colocalization of CGRP and CGRP receptor subunits has been reported in Purkinje neurons of the cerebellum (112).
A variable for autoregulation that is often overlooked is nervous system plasticity, which can alter the receptor and peptide expression landscape. CGRP receptor subunits are dynamically regulated by stress (113), and a marked increase in the number of TG neurons activated by CGRP was recently observed after repeated nitroglycerin treatments (a migraine trigger) (114). Hence, dynamic and/or multiple receptor expressions support the possibility of an autocrine positive feedback that would sustain CGRP synthesis in the ganglia and elsewhere.
CGRP gene expression is known to be repressed by several steroidlike molecules and serotonin autoreceptors (FIGURE 4). The vitamin A metabolite retinoic acid decreased calcitonin and CGRP mRNA level in the CA77 thyroid C cell line (115), and the negative-response cis-element was identified to be the 18-bp cell-specific enhancer (116). Likewise, the synthetic glucocorticoid dexamethasone reduced CGRP mRNA and promoter activity in a cell-specific manner that was dependent on the 18-bp enhancer (117). Another agent that represses CGRP gene expression is the calcium homeostasis hormone vitamin D. Vitamin D decreased calcitonin mRNA level in parathyroid-thyroid glands of rats in a time- and dose-dependent manner (118). This repressive effect of vitamin D required both the proximal CRE and the distal enhancer in TT thyroid C cells (119), possibly by interrupting interactions between CREB and the enhancer binding proteins (120). Taken together, it is likely that retinoic acid, glucocorticoids, and vitamin D repress the CGRP gene by interfering with USF and FoxA2 at the 18-bp enhancer.
Serotonin receptor (5-HT1B/D/F) agonists that include triptan drugs used for migraine treatment also repress the CGRP gene (121) (FIGURE 4). The well-known migraine drug sumatriptan is an agonist of these receptors and decreases plasma CGRP (122). The 5-HT1 agonists decreased phosphorylation of extracellular signal-regulated protein kinase (ERK) and JNK MAP kinases and decreased CGRP mRNA in the CA77 thyroid C cell line (94, 123). Unexpectedly, sumatriptan inhibited MAPK signaling pathways via an abnormally prolonged low-level elevation of intracellular Ca2+ (124), which in turn elevated a dual-specificity phosphatase (MAPK phosphatase-1) (94). It is possible that use of triptans to inhibit dual-specificity phosphatases may be useful for treating other diseases, such as obesity and diabetes (125–128). However, the time course of sumatriptan action in migraine patients precludes transcriptional regulation as the drug mechanism for abortive treatment of migraine. Instead, it is more likely that the mechanism is via inhibiting CGRP release (129–132). The first clear evidence for triptan inhibition of CGRP release was shown in patients by Goadsby and Edvinsson (133), who showed that CGRP levels in the external jugular outflow of migraine patients were reduced concomitant with headache relief following sumatriptan administration (see sect. 6.2.1). Although speculative, it is possible that the actions of sumatriptan on CGRP gene transcription may have more long-term effects on patients, such as contributing to chronic headaches associated with medication overuse (70, 134). In this scenario, repeated triptan usage might reduce CGRP levels, which then leads to a rebound of elevated CGRP and migraine upon triptan withdrawal.
2.2. Posttranscriptional Regulation
An understanding of CGRP alternative splicing was long an elusive holy grail that appears to have been finally resolved. The splice choice is largely determined by a family of RNA-binding proteins enriched in several tissues, including the brain (135, 136). Early studies on the calcitonin/CGRP splice mechanism had implicated an intronic site in the third intron (upstream of the calcitonin-specific exon 4) (137). Later studies implicated an intronic site in the fourth intron (138, 139) and a splice enhancer within exon 4 bound by a protein called SRp55 (140). Another study pointed to intronic sequences upstream of exon 5 (141). In the following years, further studies showed that, not surprisingly, it is likely a combination of these sites that contributes to alternative splicing of the calcitonin/CGRP transcript. The best evidence points to an exon exclusion mechanism mediated by Fox-1 and Fox-2 RNA binding proteins (not to be confused with transcription factor FoxA2) (135, 136). These Fox proteins can prevent inclusion of the calcitonin-specific exon 4 by binding to silencer elements in exon 4 and in the upstream intron 3. As a result, Fox proteins prevent calcitonin exon splicing by two distinct steps in the spliceosome complex (135). Along with binding of Hu proteins to the downstream intron 4 site (142), this provides a redundant and tightly regulated alternative splicing mechanism that leads to the preferential production of CGRP in neurons.
Thus, the decision of whether to include or exclude the calcitonin exon (exon 4) is regulated by a balance of positive and negative regulation mediated by many proteins but especially by the Fox-1 and Fox-2 proteins. A caveat of these studies is that to date the splice choice can be modulated in cell culture, but no evidence of that modulation by Fox proteins occurring in vivo has been fully documented. To be clear, a splice switch between calcitonin and CGRP can occur in vivo, since it was the switch upon serial passages of medullary thyroid carcinoma tumors that originally led to the discovery of CGRP. Whether such a switch in calcitonin/CGRP splicing occurs physiologically or underlies any pathologies remains to be seen.
2.3. Control of Release
CGRP release is regulated by several mechanisms. Most relevant clinically is inhibition by 5-HT1B/D/F agonists that include the triptan migraine drugs. Notably, acute sumatriptan treatment reduces plasma CGRP levels in migraine patients (133). Inhibition by triptans has been reported in several preclinical models (24), including the recently approved 5-HT1F agonist (143). Inhibition of CGRP release has also been reported in response to other agents, most notably adenosine (144) and TRPC4 agonists (145). Conversely, stimulation of release by several agents, including cytokines, NO, and purinergic receptors, has been reported (see sect. 5.2.2). CGRP release is also indirectly stimulated by microRNA miR-34a-5p via an IL-1β inflammatory pathway in rat TG neurons (146). Interestingly, this microRNA is reportedly elevated in serum during acute migraine attacks (147) and interictally in chronic migraine patients (148).
An important point to remember for CGRP release is that it can occur from both synaptic and nonsynaptic sites, which allows volume transmission as described in sect. 1.4.2 (49). In particular, the role of cell body transmission may be very relevant within the TG, as discussed in sect. 5.2. Recent studies have suggested that intraganglionic CGRP transmission plays a role in activation of the trigeminovascular pathway by acute exposure to nasal TRPA1 agonists associated with migraine (149). Interestingly, TG cell bodies are key sites of TRPA1-mediated allodynia following nitroglycerin stimulation in mice (150). Paracrine signaling of CGRP within the ganglia has also been demonstrated between satellite glia and neurons (151). As with cell bodies, CGRP release from varicosities and terminals may act in a paracrine manner on multiple targets to influence afferent signals from sensory tissues such as the eyes, ears, nose, and mouth (152).
2.4. Degradation and Clearance
Although long lived compared with neurotransmitters, neuropeptide actions are eventually terminated by extracellular proteases. The half-life of most peptides is on the order of minutes in the plasma (59). However, an untapped area of research is the more biologically relevant half-life of peptides in extracellular or cerebrospinal fluid. For example, the half-life of CGRP in the plasma is 7 min (21), whereas in the skin CGRP actions can be on the order of hours, suggesting a longer half-life (153, 154).
CGRP degradation has largely been studied by incubating synthetic CGRP from different species with purified enzymes, cocktails of enzymes, tissue lysates such as spinal cord, or fluids such as plasma and CSF (155–164). Collectively, such studies have identified many different enzymes that are capable of cleaving CGRP into smaller fragments, and a range of different cleavage sites occur within the peptide. Enzymes implicated in CGRP degradation include neutral endopeptidase, human lung mast cell tryptase, insulin-degrading enzyme, plasmin, thrombin, matrix metalloproteinase 2, trypsin, and endothelin-converting enzyme (ECE). There is a general consensus that cleavage commonly occurs between the following pairs of amino acids: Arg11-Leu12, Gly14-Leu15, Ser17-Arg18, Arg18-Ser19, Lys24-Asn25, and Asn26-Phe27. Cleavage between Cys7 and Val8 has also been reported. Over the range of individual enzymes and studies, there are also other reported cleavage sites, such as Glu35-Ala36 and Ala36-Phe37. However, which of these serve as physiological mechanisms for controlling CGRP activity is less clear, though none of the resulting peptide fragments would be expected to be biologically active, based on currently understood structure-function relationships for CGRP (see sect. 4.4). Degradation by ECE-1 has been linked to regulation of the receptor once internalized into endosomes, whereby this promotes recycling of the receptor back to the cell surface (165). Therefore, although the peptide may be degraded, this degradation can prompt resensitization of the system to CGRP, enabling the receptor to bind a new intact CGRP molecule again at the cell surface. CGRP uptake into nerve terminals could also contribute to CGRP clearance (166). In addition to degradation, CGRP clearance occurs through the kidney and to a smaller extent the liver, based on the limited data available (167, 168). The vast majority of studies have investigated α-CGRP degradation or have not specified the form used. One study that compared human α-CGRP and β-CGRP found that both were cleaved by matrix metalloproteinase 2 (169). However, the amino acid sequence differences between human α-CGRP and β-CGRP (positions 3, 22, 25) or rat α-CGRP and β-CGRP (positions 17, 35) (FIGURE 2) could potentially result in altered recognition by different proteases, thus differentially affecting degradation and therefore function of each peptide. This possibility requires experimental confirmation.
3. SITES OF CGRP EXPRESSION
3.1. Overview and Context for Interpreting Expression Studies
Understanding exactly where CGRP is located, and how this relates to where its receptors are found, is vital for understanding its functions. CGRP is broadly distributed throughout the central and peripheral nervous systems of vertebrates. There are regions of the brain with relatively high abundance, such as the amygdala, parabrachial nucleus (PBN), and locus coeruleus (FIGURE 5) (108, 110, 112, 170–219). Other well-known sites containing CGRP are the dorsal horn of the spinal cord, the perivascular neural network of blood vessels, and sensory nerves. However, beneath these broad statements lie several layers of complexity that create challenges for defining the precise details at a cellular level. Many of these complexities also apply to receptor expression studies that are considered in sect. 4.
Figure 5.
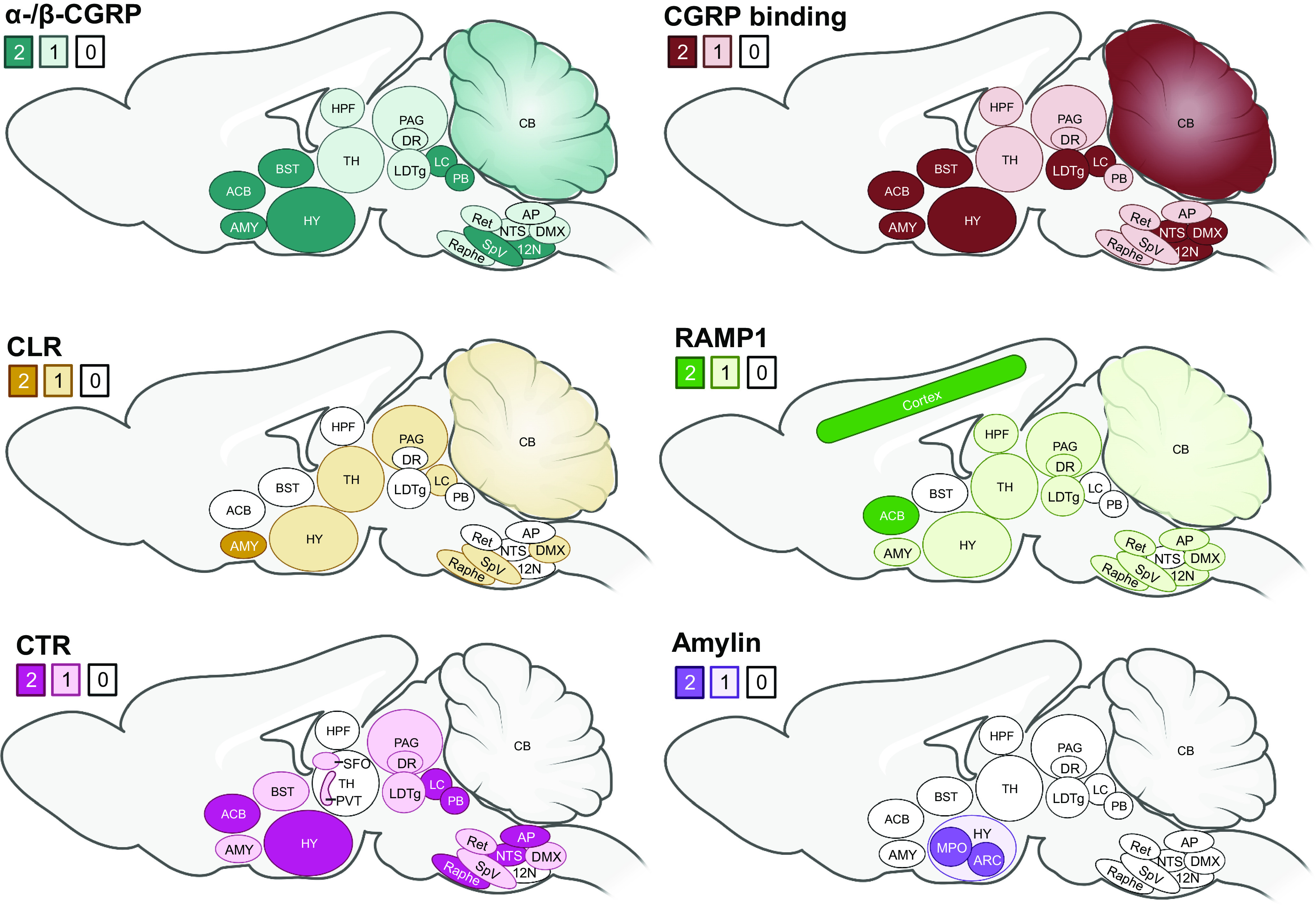
Sites of α-/β-calcitonin gene-related peptide (CGRP), amylin, CGRP binding (125I-CGRP), and receptor subunit expression in the brain. Calcitonin is not shown as it is not detected in the brain. CLR, calcitonin receptor-like receptor; CTR, calcitonin receptor; HY, hypothalamus; LDTg, laterodorsal tegmental nucleus; RAMP1, receptor activity-modifying protein 1; TH, thalamus; see TABLE 1 for other abbreviations. The shaded numbers reflect approximate differences in abundance or number of binding sites, with 2 reflecting the most and 0 the least. Much data are not clear-cut. For binding, this largely relates to radiolabeled forms of CGRP in autoradiography. For peptide and receptor subunits, this is a composite of mRNA and immunoreactivity across multiple studies. The data are mostly from rodent and primate from the following citations: rodent CGRP (see TABLE 1), primate CGRP (170–175), CGRP binding (170, 172, 176–182), CLR (108, 112, 170, 171, 176, 183–194), RAMP1 (108, 110, 112, 170, 171, 176, 183, 184, 186, 187, 192–200), CTR (110, 195, 197, 198, 201–211), and amylin (207, 212–219). Image created with BioRender.com, with permission.
In this review we use the term “expression” broadly to indicate both RNA and peptide. Since peptides are generally located in remote fibers as well as the cell body, and mRNA can sometimes be transported from the cell body (220), mRNA and protein expression data may not always be consistent. Adding to these challenges is that the interpretation of peptide or protein data can be influenced by several confounding factors. For example, definitively determining which isoforms of CGRP are expressed is complicated by the fact that antibodies are unlikely to distinguish between α-CGRP and β-CGRP because of their extremely high amino acid sequence identity. Therefore, it is challenging to obtain specific information about only one of these peptides, and about their relationships to one another. Another significant issue for the CGRP field is the potential for cross-reactivity between amylin and CGRP antibodies because of their high amino acid sequence identity, which is concentrated in certain regions of the peptide sequences (FIGURE 2). This becomes more important as the abundance of the peptide increases, with even low-level cross-reactivity for the nontarget peptide becoming amplified where it is expressed at high levels, as recently discussed (221, 222). Antibodies are nevertheless commonly employed in measuring peptide expression, but they can additionally bind nonspecifically to unrelated proteins. Ideally all antibodies would be carefully validated, but this is often not the case (223). In interpreting the data described below, it is important to recognize that not all of the studies used well-characterized probes, and that the evidence presented must be taken in the light of the potential limitations of the tools used. Hence, data obtained with antibodies are more accurately reported as CGRP-like immunoreactivity rather than CGRP expression. However, for simplicity we use the term “CGRP” or “CGRP immunoreactivity” rather than CGRP-like immunoreactivity in this review.
A further important consideration for all studies is that rodents are not always the same as humans. For example, recent studies have revealed that the spinal projections of peptidergic (CGRP) and nonpeptidergic (P2X3R) DRG nociceptors are different between mice and humans. In mice the two subsets of nociceptors are distinct, whereas in humans they largely overlap (224, 225). We briefly mention species considerations in sect. 3.5.
In the subsequent text, the sites of expression of CGRP in general are first covered, followed by a separate review of the sites of β-CGRP expression and of how this expression may differ from that of α-CGRP, where the information is available (see sect. 3.4). The reasons for not specifically discussing α-CGRP and β-CGRP by location and for separating β-CGRP to its own section are that 1) antibodies cannot separate these two peptides, and thus this distinction cannot be made, and 2) β-CGRP is rarely studied compared to α-CGRP, and so there are not sufficient data to provide a detailed comparison. The data for CGRP in major peripheral ganglia and CNS of rats and mice are detailed in TABLE 1. Some of these areas are then illustrated in FIGURE 5, in relation to CGRP binding sites and sites of receptor subunit expression.
Table 1.
CGRP mRNA and peptide expression in neural sites of rat and mouse
| Region | mRNA | Peptide |
|
|---|---|---|---|
| Immunoreactivity | Location | ||
| TG | Y (3, 30) | Y (3, 5, 108, 111, 177, 226–230) | CF |
| DRG | Y (231–234) | Y (227, 230, 232, 235) | CF |
| Spinal cord | |||
| Dorsal horn | Y (236) | Y (3, 230, 232, 236–238) | CF |
| Ventral horn | Y (231, 233, 236) | Y (3, 230, 232, 238) | CF |
| Sensory cranial nuclei | |||
| Mesencephalic trigeminal [V, VII, IX, X] | N (231) | Y (193, 239) | C |
| Principal trigeminal [V, VII, IX, X] | Y (229, 240) | F | |
| Vestibular [VIII] | Y (3, 230, 240, 241) | CF | |
| Cochlear [VIII] | Y (3, 229, 230, 240) | CF | |
| Spinal trigeminal [V, VII, IX, X] (SpV) | Y (3, 108, 171, 229, 238, 240, 241) | CF | |
| Nucleus of the solitary tract [VII, IX, X] (NTS) | ? (231) | Y (3, 193, 229, 230, 238, 240, 242) | CF |
| Motor cranial nuclei | |||
| Oculomotor [III] | Y (30, 231, 243, 244) | Y (230, 238, 240–242, 244) | CF |
| Accessory oculomotor (Edinger–Westphal) [III] | ? | Y (240, 242) | C |
| Trochlear [IV] | Y (30, 231, 244) | Y (230, 240, 242, 244) | C |
| Trigeminal [V] | Y (30, 231, 244) | Y (229, 230, 238, 240–242, 244) | C |
| Abducens [VI] | Y (30, 231, 244) | Y (230, 240, 242, 244) | C |
| Facial [VII] | Y (30, 231, 244) | Y (3, 193, 230, 238, 240–242, 244) | CF |
| Salivatory [VII, IX] | Y (245) | F | |
| Hypoglossal [XII] (12N) | Y (30, 231, 244) | Y (3, 193, 230, 238, 240–242, 244) | CF |
| Dorsal nucleus of the vagus [X] (DMX) | ? | Y (193, 242) | CF |
| Nucleus ambiguus [IX, X] | Y (30, 231, 244) | Y (3, 229–231, 238, 240–242, 244) | CF |
| Medulla oblongata | |||
| Gigantocellular reticular nucleus | Y (193, 240) | CF | |
| Cuneate/external cuneate nucleus | Y (244) | Y (229, 230, 240) | CF |
| Intercalated nucleus of the medulla oblongata | Y (244) | ? (244) | |
| Nucleus of Roller | Y (244) | ? (244) | |
| Reticular nucleus (Ret) | Y (244) | Y (229, 230, 240, 242) | CF |
| Raphe magnus nucleus | Y (193) | C | |
| Dorsal raphe nucleus | Y (240) | F | |
| Other raphe nuclei | ? (244) | N (240) | |
| Gracile nucleus | Y (193, 229, 230, 240) | CF | |
| Area postrema (AP) | Y (230, 240) | CF | |
| Pons | |||
| Lateral lemniscus | Y (244) | Y (230, 240, 244) | CF |
| Pontine nuclei | ? (244) | Y (193, 230, 242, 244) | C |
| Pontine reticular nucleus | Y (244) | Y (230, 244) | F |
| Ventral nucleus of the lateral lemniscus | Y (244) | Y (242, 244) | C |
| Dorsal nucleus of the lateral lemniscus | Y (244) | Y (230, 244) | F |
| Superior olive | Y (30, 231, 244) | Y (230, 238, 240, 242, 244) | CF |
| Central gray of the pons | ? (244) | Y (230) | C |
| Locus coeruleus (LC) | Y (193, 230, 241) | CF | |
| Cerebellum | Y (112, 193, 231, 238, 240, 242, 246) | CF | |
| Midbrain | |||
| Central periaqueductal gray (PAG) | ? (243, 244) | Y (229,230, 241, 244) | C |
| Ventral periaqueductal gray (PAG) | Y (243) | ||
| Parabigeminal nucleus | Y (244) | Y (240, 244) | CF |
| Cuneiform nucleus | Y (244) | Y (230, 240, 244) | CF |
| Ventral tegmental nucleus (Gudden) | ? (244) | Y (230, 244) | F |
| Dorsal tegmental nucleus/area | ? (244) | Y (230, 241, 244) | CF |
| Parabrachial nucleus/area (PB) | Y (30, 231, 244) | Y (3, 229, 230, 238, 240, 244, 247) | CF |
| Kölliker–Fuse nucleus | Y (244) | Y (229, 244) | C |
| Inferior colliculus | Y (230, 231, 238, 240) | CF | |
| Red nucleus | Y (193, 230) | CF | |
| Central nucleus of the inferior colliculus | ? (244) | Y (230) | C |
| Superior colliculus | Y (238, 240) | CF | |
| Forebrain | |||
| Organum vasculosum lamina terminalis | Y (230) | F | |
| Subfornical organ (SFO) | Y (230, 238) | F | |
| Medial preoptic nucleus/area (MPO) | Y (244) | Y (230, 238, 240, 244) | CF |
| Periventricular hypothalamic nucleus/zone | ? (244) | Y (230, 238, 244) | F |
| Lateral hypothalamic area | Y (244) | Y (3, 240, 244) | CF |
| Anterior hypothalamic area | ? (244) | Y (230, 238, 244) | C |
| Posterior hypothalamic nucleus | N (240) | ||
| Ventromedial hypothalamic area | Y (240) | F | |
| Paraventricular hypothalamic nuclei | ? (244) | Y (193, 230, 240, 244) | CF |
| Arcuate hypothalamic nucleus (ARC) | Y (244) | Y (230, 238, 240, 244) | CF |
| Mammillary body/supramammillary region | Y (230, 240) | F | |
| Zona incerta | Y (244) | Y (240, 244, 247–249) | F |
| Dorsomedial hypothalamic nucleus | ? (244) | Y (230, 238, 244) | C |
| Perifornical nucleus | ? (244) | Y (230, 244) | C |
| Premammillary nucleus, ventral part | ? (244) | Y (230, 244) | CF |
| Ventromedial thalamic nucleus | ? (244) | Y (238, 240, 247, 249) | CF |
| Centromedial thalamic nucleus | Y (193, 229, 247, 249) | CF | |
| Centrolateral thalamic nucleus | Y (248) | F | |
| Mediodorsal thalamic nucleus | Y (247–249) | F | |
| Laterodorsal thalamic nucleus | Y (248) | F | |
| Paracentral thalamic nucleus | Y (244) | Y (230, 244, 248, 249) | F |
| Ventroposterior thalamic nucleus, parvicellular | ? (244) | Y (230, 244, 247–250) | CF |
| Ventroposterior medial thalamic nucleus | Y (247–249) | F | |
| Ventroposterior lateral thalamic nucleus | Y (248) | F | |
| Subparafascicular thalamic nucleus | Y (244) | Y (229, 230, 244, 247, 249) | CF |
| Lateral subparafascicular thalamic nucleus | Y (244) | Y (230, 244) | C |
| Parafascicular thalamic nucleus | Y (244) | Y (230, 244, 247, 249) | CF |
| Paraventricular thalamic nucleus (PVT) | Y (193, 238, 240, 247, 249) | CF | |
| Posterior thalamic nuclear group | Y (244) | Y (230, 244, 247–249) | F |
| Lateroposterior thalamic nucleus | Y (248) | F | |
| Posterior intralaminar thalamic nucleus | Y (244) | Y (230, 244) | C |
| Peripeduncular nucleus | Y (30, 231, 244) | Y (3, 229,230, 238, 240, 247, 249) | CF |
| Geniculate body | Y (230) | F | |
| Central gray matter | Y (230, 247, 249) | CF | |
| Hippocampal formation (HPF) | |||
| CA1–CA4 | Y (244) | Y (193, 230) | C |
| Dentate gyrus (ventral) | Y (193, 230, 240) | CF | |
| Septal area (medial, lateral) | Y (3, 193, 230, 238, 240, 242) | F | |
| Bed nucleus of the stria terminalis/nucleus accumbens (BST/ACB), stria terminalis | Y (244) | Y (3, 230, 238, 240, 242, 244) | F |
| Globus pallidus | Y (3, 238, 240) | F | |
| Caudate-putamen | Y (3, 229, 230, 238, 240) | F | |
| Medial corticohypothalamic tract | Y (244) | ? (230) | |
| Olfactory bulb/tract/nuclei | Y (244) | Y (230) | F |
| Amygdala (AMY) | ? (244) | Y (3, 229, 230, 238, 240, 244, 248) | CF |
| Primary olfactory cortex | ? (244) | Y (230) | C |
| Infralimbic prefrontal area | Y (3) | ||
| Insular cortex | Y (3, 230, 238, 240, 249) | F | |
| Perirhinal cortex | Y (3, 249) | F | |
| Entorhinal cortex | Y (230) | C | |
| Cortical layers II–VI | Y (193, 246) | C | |
The identification of calcitonin gene-related peptide (CGRP) in a particular area is indicated as Y or N, indicating yes or no, respectively. “?” indicates” either none or very low expression. In these studies it is possible that expression could be localized to a very few cells, but in early publications it is not possible to discern this level of detail from the images. It is difficult to ascribe some regions as Y or N. Some studies are conflicting, either reporting presence or absence of CGRP. These have been described as “Y” because of the overall consensus, but the references that also report an absence have also been cited for completeness. For peptide expression, this is then further defined as cell body (C) or fiber (F) immunoreactivity from histological studies. The table lists ganglia, followed by cranial nerve nuclei (associated cranial nerves shown in brackets) and then brain regions from brain stem forward. The citations provide a representative sample from a large volume of literature.
3.2. Nervous System
3.2.1. Peripheral ganglia.
One of the earliest known sites of CGRP expression was the TG, a finding that has been replicated in many independent studies (3, 5, 226–228, 251–253). Numerous reports have led to the consensus that CGRP mRNA and peptide are robustly expressed in the TG and DRG; they are detected in ∼30–50% of neuronal cell bodies. Several representative studies are cited in TABLE 1. Most CGRP-positive neurons are small or of medium size; the largest neurons (>45 µm in diameter) tend not to be CGRP positive (107, 108, 111, 227). Some mRNA data suggest that CGRP is more highly expressed in the DRG than in the TG (254). In DRG, CGRP has been reported in C, Aβ, and Aδ fiber neurons (255). CGRP immunoreactivity in the TG and DRG typically manifests as dense cytoplasmic staining (in small neurons) or granular staining (in the vesicles of medium-large neurons), consistent with its role as a neuropeptide. Expression of CGRP is commonly observed in fibers as pearl-like CGRP immunoreactivity (107, 108, 111, 256). Few detailed comparisons of CGRP abundance or cellular localization across the three trigeminal nerve branches are available, but expression has been noted in all three (257). In the case of the DRG, CGRP is found at the cervical, thoracic, lumbar, and sacral levels (258). CGRP is also expressed in the nodose ganglia (227). The expression of CGRP in glial cells of the TG and DRG is inconsistent, with CGRP mRNA and immunoreactivity reported as present or absent in different studies, although in most studies glia do not appear to be CGRP positive (3, 47, 226, 254, 259, 260). Nonetheless, glial CGRP expression may be subject to regulation by inflammatory stimuli, and thus glia may be an additional source of CGRP under certain conditions (90, 261). Glial CGRP expression within the TG could contribute to paracrine cross talk and neural sensitization (151, 256).
3.2.2. Central nervous system.
As is evident from TABLE 1, CGRP expression in the CNS is very widespread. This expression extends from the brain stem through to the cortex and encompasses discrete nuclei and extensive fiber networks. CGRP is abundant in sensory and motor cranial nuclei. A large body of research has focused on the relationship between CGRP expression and brain regions that contribute to migraine. For example, CGRP mRNA and pearllike immunoreactivity are prevalent in the fibers of the spinal trigeminal nucleus in rodents and humans (171, 262, 263). In general, these studies have been highly concordant, but, unsurprisingly for such a large volume of data, there are also inconsistencies. For example, expression in the cerebellum can be ambiguous. One study reported that the dendritic arbors of Purkinje cells in the molecular layer were often CGRP positive. Also, a low to moderate density of CGRP-immunoreactive fibers was detected in the nucleus cerebellaris lateralis; such fibers were also present in other cerebellar nuclei but were less conspicuous there. This study also reported detecting a few CGRP fibers in the cerebellar white matter (240). However, mRNA was not detectable in Purkinje cells (231). A comparison of CGRP immunoreactivity in human and rhesus monkey cerebellum showed immunoreactivity in Purkinje cells, both in the cytoplasm of cell bodies and in dendrites, and within cells in the molecular layer (170). Interestingly, not all Purkinje cells contained CGRP. TABLE 1 provides additional citations for studies that have examined CGRP immunoreactivity in rodent cerebellum. It may be that overall expression levels are quite low in the cerebellum but there are discrete cells or locations that contain more substantial levels of CGRP immunoreactivity. Data for the thalamus are also difficult to interpret (TABLE 1). In part, this is due to the fact that the thalamus contains many nuclei the nomenclature for which has changed significantly over time. Most studies report that CGRP is detected in fibers in the thalamus, but cell body staining has also been reported (193). A further consideration is whether immunoreactivity is associated with neurons or other cell types. For example, one study reported CGRP in the rat cortex, but it was colocalized with alpha smooth muscle actin and Claudin-5, suggesting that it is present in cortical blood vessels rather than neurons (264).
3.2.2.1. spinal cord.
The spinal cord is innervated by a dense network of fibers that are immunoreactive for CGRP. These are located in laminae I and II of the dorsal horn, which includes fibers originating in the TG and DRG. Cell bodies immunoreactive for CGRP are also present in the spinal cord. In the dorsal horn, CGRP-expressing excitatory interneurons have been reported (265). The sensory expression of CGRP has held the greatest prominence in recent years, but it is important to recognize that CGRP is also found in motor neurons. Large cells immunoreactive for CGRP are found in the ventral horn of the spinal cord. Large CGRP-positive neurons are noted in areas associated with innervation of large muscles (258). CGRP mRNA is also found in motoneurons of the ventral horn, indicating that CGRP is actually synthesized in these cells (266). Similar patterns of expression have been observed across species, at the cervical, lumbar, and thoracic levels of the spinal cord. Spinal cord CGRP has been quantified across dorsal and ventral cervical, thoracic, and lumbar regions (172).
3.3. Other Sites of Expression
As a consequence of its presence in nerve fibers, CGRP can be found in many locations throughout the body. To avoid duplication, we describe many sites of expression in relation to a particular function elsewhere in this review. Other sites of expression could reflect functions that have not been deeply explored. Examples include the tongue (fibers), soft and hard palates, epiglottis, and esophagus (267). Similarly, the nonneuronal sites briefly covered in sect. 1.2 merit further consideration.
3.4. Specific Consideration of β-CGRP Expression
Although the distributions of α-CGRP and β-CGRP predominantly overlap, β-CGRP has been reported as the major isoform in the enteric nervous system (31, 232). However, this does not mean that this is its exclusive site of expression. The high amino acid sequence identity of the CGRPs has made it difficult to develop specific probes for each peptide, but the available data show that β-CGRP expression is widespread. The two isoforms differ in relative abundance, and occasional reports have suggested the exclusive expression of only one form at particular sites. Expression in the enteric nervous system is described in sect. 5.7 on gastrointestinal physiology (see also summary in FIGURE 6) (30, 31, 232). Other sites of expression are outlined here, and some are summarized in FIGURE 6. The cited literature covers human and rodent.
Figure 6.
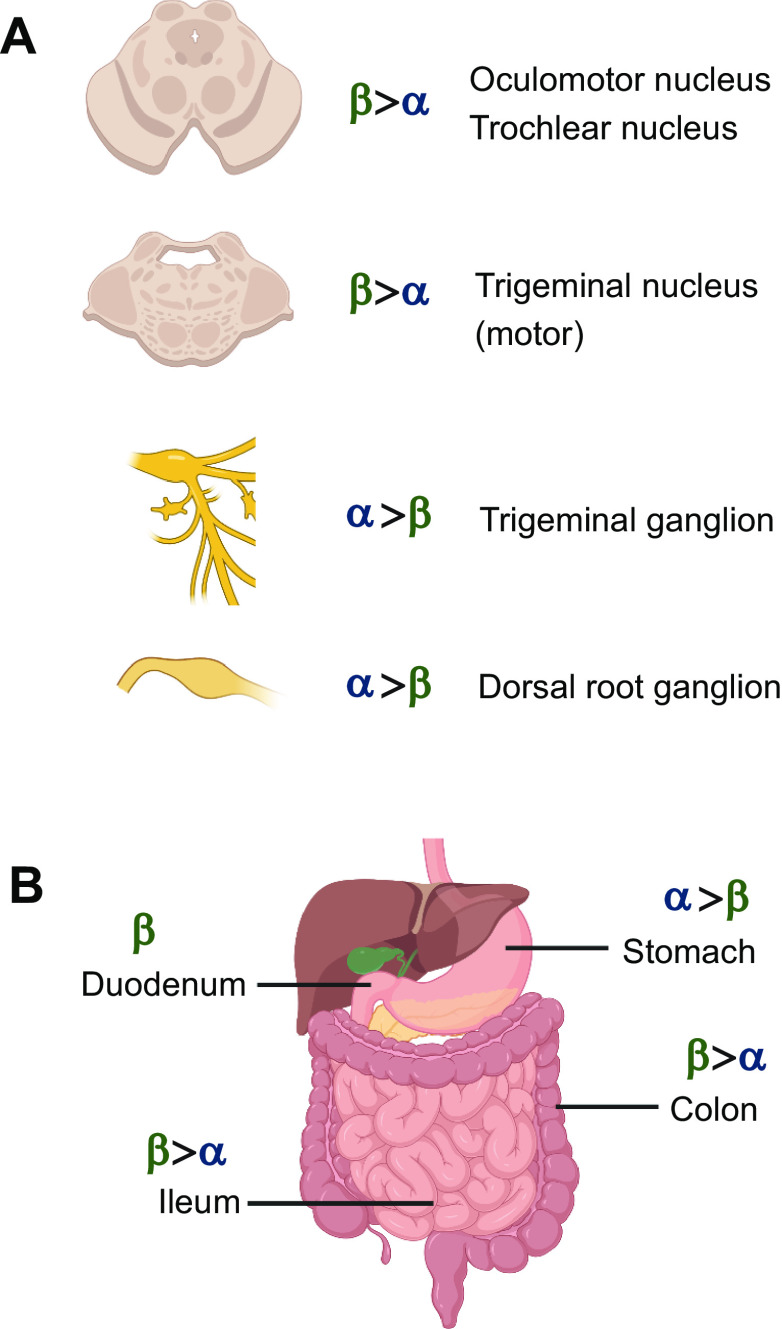
Selected sites of expression of α-calcitonin gene-related peptide (CGRP) and β-CGRP, with relative expression indicated. A: sites of expression in the nervous system. B: sites of expression in the gastrointestinal tract, including expression from extrinsic and intrinsic nerves. In many locations, such as the trigeminal ganglion and dorsal root ganglion, α-CGRP expression exceeds β-CGRP expression, whereas in other locations the reverse is true. Note that human anatomy is shown for simplicity but the majority of supporting literature is rat and mouse (30, 31, 232). Image created with BioRender.com, with permission.
Some studies have been particularly informative with respect to the expression of the two isoforms. For example, in their seminal work, Amara and colleagues showed that β-CGRP mRNA was expressed in the TG, lateral medulla, hypothalamus, and, to a lesser extent, the midbrain. Cerebellar signal was thought to be nonspecific. Hybridization to probes specific for each mRNA was observed in the TG; the motor nuclei of the third (oculomotor), fourth (trochlear), fifth (trigeminal), seventh, tenth, and twelfth cranial nerves; cells of the ventral horn (motoneurons); and other brain nuclei including the parabrachial and peripeduncular (30). Among the many sites of expression, the hybridization signal was greater in the TG for α-CGRP. This is consistent with other mRNA data suggesting that the expression of β-CGRP can be up to 10-fold lower than that of α-CGRP (232, 268,269). In contrast, in the nuclei of the third, fourth, and fifth cranial nerves the β-CGRP hybridization signal was greater than that for α-CGRP (30). In other areas, the signals were often equivalent (30). The mRNA encoding β-CGRP is also found in the DRG and spinal cord (7, 31, 233, 234, 266, 270). Recent studies using RNA-seq also identified β-CGRP in sensory ganglia (268, 271).
Peptide, rather than mRNA, data tell a similar story, supporting the evidence that β-CGRP is widely expressed. For example, important early data indicated that β-CGRP is present in the spinal cord, cerebellar cortex, and thalamus and that the concentration of β-CGRP is higher than that of α-CGRP in the cerebellum and thalamus (272). Data from α-CGRP knockout mice suggest that α-CGRP and β-CGRP are typically coexpressed, although the relative quantities of each varies (232). Interestingly, β-CGRP was expressed primarily in small neuronal cell bodies in the DRG as puncta, indicating a presence in vesicles.
Intriguing reports of differential or regulated expression of β-CGRP suggest that greater scrutiny of its functions may be worthwhile. For example, cultured normal human epidermal keratinocytes had between ∼8- and 12-fold greater β-CGRP than α-CGRP mRNA expression, depending on the culture conditions (14). Similarly, epidermal keratinocytes derived from two different transgenic mouse lines (BMP4 and noggin) had greater β-CGRP than α-CGRP mRNA expression (∼3.5-fold) (14).
3.5. Comparison Between Species and Sexes
The broad anatomical distribution of CGRP appears to be largely similar across species (221, 273). This includes expression in the spinal cord, TG, spinal trigeminal tract, PBN, and thalamus (47, 107, 172, 174, 274).
For many early studies, either the sex of animals used was not reported or only males were used. This makes it difficult to draw comparisons between male and female. There is, however, some evidence suggesting that CGRP expression is sexually dimorphic and that it is regulated by estrogen (275, 276). Peripheral inflammation due to administration of complete Freund adjuvant increased CGRP expression in female TG to a greater degree than in male mice (277). In female mice, significantly fewer neurons in the DRG were CGRP positive than in males (275). This difference appeared to be linked to activation of the estrogen receptor, which is coexpressed in DRG neurons. Consistent with this possibility, CGRP expression in the same neurons of ovariectomized females was similar to that in males. CGRP expression was lowered when ovariectomized mice were treated with estrogen, mimicking the lower-level expression observed in intact females (275). In the medulla of female rats, levels of CGRP mRNA were significantly higher than in males (191). In addition, the number of CGRP mRNA and peptide-positive cells in the medial preoptic nucleus was higher in female than in male rats (278, 279). The reverse was apparent in some hypothalamic regions, with the number of CGRP-positive cells higher in males than in females (280). Thus, there appear to be regional differences in CGRP levels between male and female rodents. Additional considerations on CGRP and sex are given in sects. 5.8 and 6.3.
4. CGRP RECEPTORS
4.1. Overview and Historical Perspective
Like the CGRP peptides themselves, CGRP binding sites are found centrally and peripherally (281). Two factors are important for weighing the relevance of binding sites: abundance and relative affinity. Sites in which the density of CGRP binding sites is high include the cerebellum, nucleus accumbens, substantia nigra, spinal cord, atria, vas deferens, and spleen (FIGURE 5). High-affinity binding sites are found in the brain (e.g., the cerebellum; FIGURE 5) and peripheral tissues including in the heart (atria and ventricles), liver, spleen, skeletal muscle, lung, and lymphocytes (281, 282). CGRP binding is sensitive to guanine nucleotides, suggesting that its receptors are members of the cell surface-localized GPCR superfamily. More specifically, receptor activation causes cAMP levels to increase, suggesting that they are of the Gαs-coupled type. Deconvoluting the molecular composition of these CGRP binding sites proved challenging, with several false starts over a number of years. Some of the literature still refers to other receptors that have been proposed as CGRP receptors, although the experimental evidence does not support this classification (28).
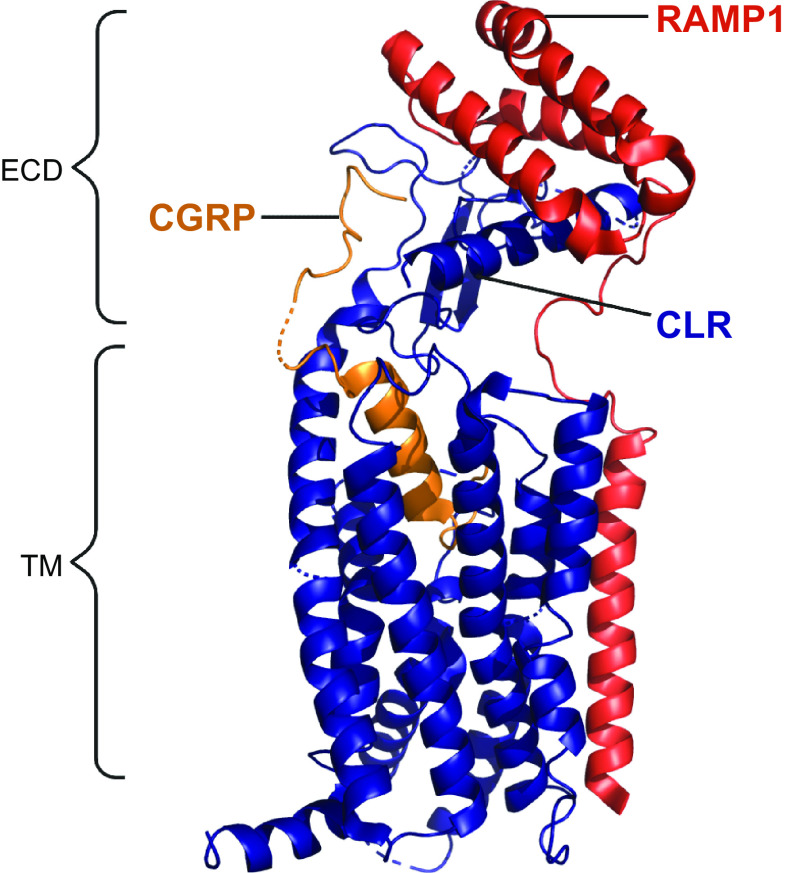
In the early-mid 1990s, the most likely GPCR candidate for a high-affinity CGRP receptor was the calcitonin receptor-like receptor (CLR). This was because of its high level of amino acid sequence identity to the CTR, whose cognate ligand, calcitonin, itself shares amino acids with CGRP (FIGURE 2) (283–285). However, it took several more years to discover that CLR required an accessory protein, receptor activity-modifying protein (RAMP)1, to reach the cell surface and to bind CGRP with high affinity (286). This combination of CLR and RAMP1 was then put forward as a likely candidate for the “CGRP1” receptor previously reported in the literature (28). This receptor was potently activated by CGRP, was effectively antagonized by the antagonist peptide fragment CGRP8-37, and could be activated by AM as well as by CGRP, though with lower potency. The tissue distribution of CLR and RAMP1 aligns with the presence of CGRP binding sites (273, 282). For example, there was a statistically significant correlation between RAMP1 mRNA and CGRP binding across eight rat tissues (heart atria and ventricles, cerebellum, spinal cord, liver, spleen, vas deferens, lung) (282). Thus the CLR/RAMP1 complex was formally ratified as “the CGRP receptor” in 2002 (FIGURE 7) (28).
Figure 7.
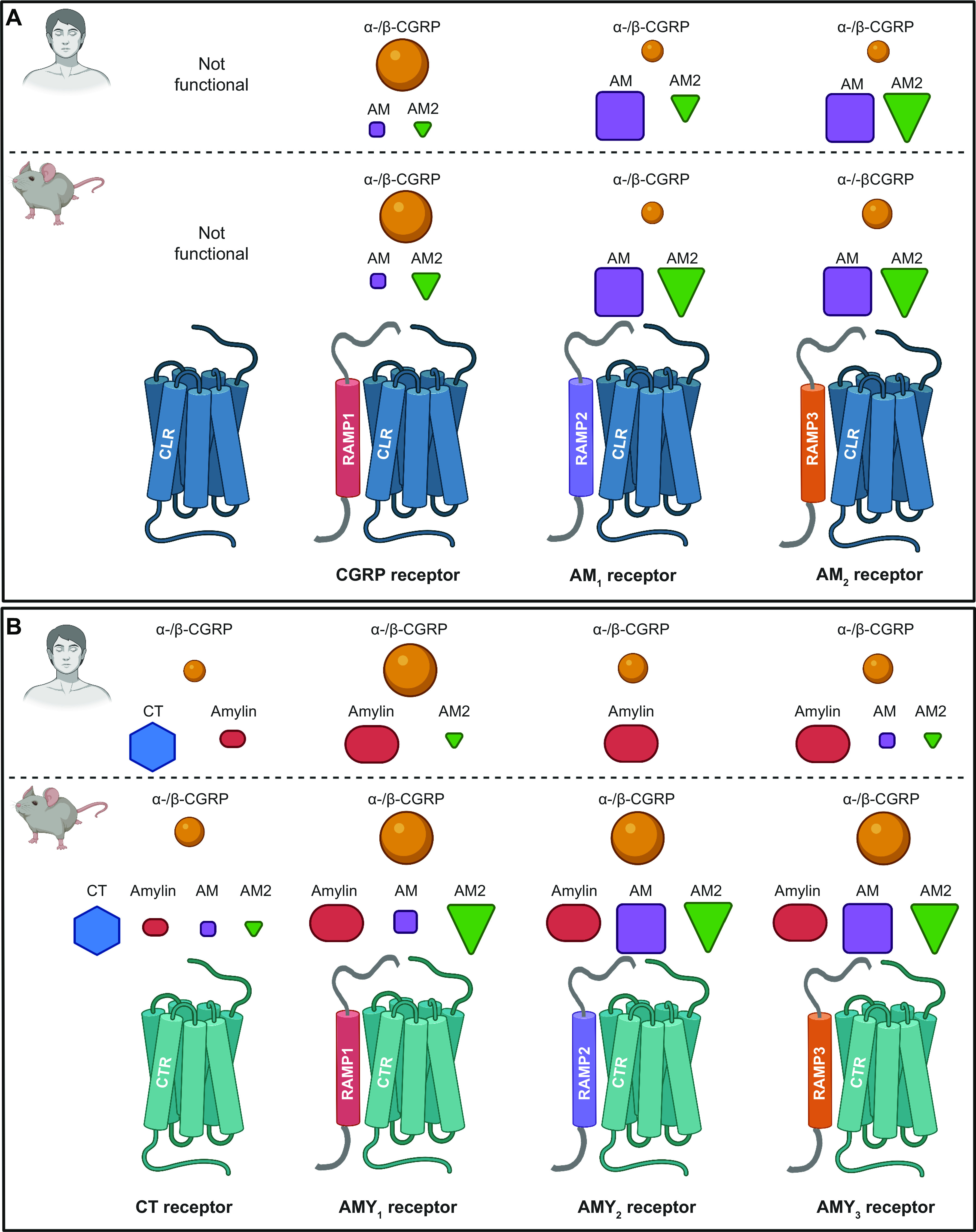
Members of the calcitonin family of receptors, showing their molecular composition and relative preferences for endogenous agonist peptides, comparing human and mouse pharmacology. A: calcitonin receptor-like receptor (CLR)-based receptors with associated receptor activity-modifying proteins (RAMPs). B: calcitonin receptor (CTR)-based receptors with associated RAMPs. For A and B, CLR or CTR combines with either RAMP1, RAMP2, or RAMP3 to form receptors for calcitonin gene-related peptide (CGRP), adrenomedullin (AM), adrenomedullin 2 (AM2), calcitonin (CT), or amylin. In A and B, the agonist pharmacology at human receptors is shown at top above the dashed line and at mouse receptors at bottom. The relative size of the peptide ligand symbol indicates the relative potency. A large symbol indicates that this is the most potent/cognate ligand or is approximately equal in potency to the cognate ligand, a medium-sized symbol indicates a ligand that is approximately ≤10-fold less potent than the cognate/most potent ligand for that receptor, and the smallest-sized symbol represents a ligand that is between 10- and 100-fold less potent than the cognate/most potent ligand for that receptor. In all cases, other ligands have been tested, but their potency is >100-fold less potent than the cognate/most potent ligand for each receptor at which they have been tested and they are therefore not shown. A broad consensus is given, using species-matched ligands where possible and using data from multiple studies, as summarized in www.guidetopharmacology.org. Thus, at the human CGRP receptor, CGRP (α-CGRP and β-CGRP) is between 10- and 100-fold more potent than AM or AM2. Mouse receptors are activated by more ligands. CLR is functional only with RAMP, as it cannot reach the cell surface without it (286, 287). CTR can act as a receptor for calcitonin without RAMP. Splice variants of CTR can also partner with RAMPs to create additional receptor subtypes; the figure represents CT(a). Image created with BioRender.com, with permission.
We now know that the CLR/RAMP1 complex is an important receptor for CGRP. However, numerous activities of CGRP cannot easily be blocked by CGRP8-37 and/or have functional behavior that does not precisely match that of the CLR/RAMP1 complex. These can be found in the literature as pharmacological phenotypes consistent with a “CGRP2” receptor (288–295). This nomenclature is not currently used because it continues to be difficult to assign a molecular identity to endogenous receptors that exhibit such profiles, but the existence of “noncanonical” CGRP receptor pharmacology is as valid now as when it was first described. Detailed pharmacological profiling of receptors that are related to CLR/RAMP1 shows that several potential candidates could explain functional “CGRP2” receptor phenotypes.
4.2. Molecular Composition
Identification of the CLR/RAMP1 complex as a high-affinity CGRP receptor was accompanied by the identification of two further RAMPs, RAMP2 and RAMP3 (286). At around the same time, RAMPs were found to associate with the CTR (296, 297). Efforts to deconvolute the agonist pharmacology of these receptors led to the current classification scheme, which is summarized in FIGURE 7 (23, 28). Accordingly, it is now well established that the calcitonin/CGRP peptide-receptor family comprises two GPCRs (CLR and CTR) with or without (for CTR) the three RAMPs. CLR with RAMP1 forms the canonical CGRP receptor, whereas CLR with RAMP2 or RAMP3 generates the AM1 and AM2 receptors, respectively. This nomenclature was developed based on evidence that the preferred ligands for these receptors were CGRP and AM peptides, respectively. Since then, however, the AM2 peptide was discovered and collated data from multiple studies indicate that, like AM, it is capable of activating all three receptors but seems to have a slight preference for the AM2 receptor when considering human receptors, upon which nomenclature is based (FIGURE 7) (23). In time, the nomenclature could be updated to reflect this, but additional work is needed to confirm that the AM2 peptide is the endogenous ligand for the AM2 receptor (23). An additional player in the function of the CGRP receptor in particular is receptor component protein (RCP), which appears to help the receptor to couple to G proteins (298). Studies on RCP are ongoing, but interpretation of RCP functions as a modulator of receptor signaling may be complicated by the possibility of nuclear functions, since RCP has been reported to be a subunit of RNA polymerase III (https://www.uniprot.org/uniprot/O75575).
CTR is a functional receptor for calcitonin in the absence of RAMPs. When CTR interacts with a RAMP, it forms the AMY1, AMY2, or AMY3 receptor, respectively (FIGURE 7). This nomenclature reflects the high affinity of amylin for each of these receptors. However, CGRP is also a high-affinity ligand for the AMY1 receptor, as further detailed in sect. 4.3. This receptor has been part of the “CGRP2” receptor conversation for many years (288, 289, 299).
Further diversity within this receptor family is apparent from the CTR (polymorphic and splice) variants, and when their combination with RAMPs is considered further subtypes of receptor are possible: AMY1(a), AMY1(b), and so on (28). This nomenclature reflects the CTR splice variant CT(a) or CT(b), respectively. The CTR splice variants differ to some extent in their functional properties and can also differ between species, and in general they are poorly studied (28, 300, 301). Notably, this can affect CGRP pharmacology (302). In humans, an additional splice variant that lacks the initial 47 amino acids of the receptor NH2 terminus is especially well activated by CGRP (302). The significance of this in physiological terms is unknown, but it indicates the potential importance of CTR splice variants in the activities of peptides beyond calcitonin and amylin.
4.3. Pharmacology: Agonists and Antagonists, and Species Differences
The agonist and antagonist pharmacology of CGRP and related receptors is regularly updated and provided as a resource online via www.guidetopharmacology.org. From time to time, consensus statements are published in collaboration with the International Union of Pharmacology Receptor Nomenclature Committee (NC-IUPHAR) to update the overall status of receptor pharmacology and nomenclature. The reviews to note are Ref. 28, which provides the original nomenclature; Ref. 288, which discusses considerations for CGRP1 and CGRP2 receptors; and Ref. 23, which provides more recent updates.
4.3.1. Agonist pharmacology.
FIGURE 7 shows the broad consensus for the relative agonist potency of endogenous ligands at human receptors. From most to least potent, the agonists of the CGRP receptor are α-CGRP and β-CGRP, AM and AM2. Although capable of activating the CGRP receptor at high concentrations, amylin is a weak agonist of the human CGRP receptor, being >100-fold weaker than CGRP at activating this receptor (23). The AM receptors are both activated by AM and AM2. CTR is potently activated by calcitonin and to a lesser degree by amylin, but the potency and/or affinity of the latter increases in the presence of each RAMP. Importantly in the context of CGRP, RAMP1 in particular increases the affinity of the CTR for CGRP. The result is the AMY1 receptor, which the collective evidence suggests is equipotently activated by CGRP and by amylin (23). The pharmacology of β-CGRP is very similar to that of α-CGRP, but an analysis for several literature studies showed that on average β-CGRP is approximately fivefold more potent than α-CGRP at the AMY1 receptor (23).
Importantly, although the pharmacology of the human receptors is the most widely discussed, it is not conserved for all receptors across species, and this must be kept in mind in interpreting the actions of CGRP in model species such as mice and rats. Whereas the RAMP1-based CGRP and AMY1 receptors are important targets for CGRP in rats and mice, receptors containing RAMP3 also stand out (287, 303–306). The rat and mouse AM2 receptors are activated more potently by CGRP than is the human AM2 receptor. Similarly, the AMY3 receptor gains CGRP activity in rats and mice relative to that in the human receptor (287, 305). It is unclear how many of these receptors contribute physiologically to the actions of CGRP in mice and rats, but it is evident that CGRP has the potential to mediate effects through receptors beyond CLR/RAMP1. It is therefore important to understand where each receptor is expressed and to use multiple tools to test them for a given effect. FIGURE 7 illustrates the pharmacological differences of endogenous agonists at human and mouse receptors.
4.3.2. Antagonist pharmacology.
4.3.2.1. selectivity considerations.
Antagonists are important pharmacological tools for understanding receptor function. In the case of the CGRP receptor family, peptide antagonists as well as small-molecule antagonists and a receptor-targeted antibody that acts as an antagonist have become available, principally as a consequence of migraine drug development (307). Antagonists are useful in primarily two ways. First, they can be used to determine whether an endogenous peptide contributes to a particular effect. Second, they have the potential to be used to identify which specific receptor mediates a particular effect (308). For example, if CGRP produces an effect that is blocked by an antagonist that is highly selective for the CLR/RAMP1 CGRP receptor, then it can be reasonably concluded that this receptor mediates the effect. However, the word “selective” is absolutely key here. The antagonist must be able to block the dose/concentration of CGRP at a concentration at which it antagonizes only the CLR/RAMP1 receptor. That is, a meaningful result depends on the antagonist being sufficiently selective for that receptor. “Selective” is somewhat subjective and context dependent. Is a threefold difference in affinity between receptors selective? What about ∼10- or 100-fold? In a precisely controlled system, a relatively small difference in the ability of an antagonist to block a response could provide definitive results. In a whole organism, many more variables are at play and a larger difference is usually needed. What is absolutely key is the dose/concentration used. If an antagonist is 100-fold better at antagonizing receptor A than receptor B yet produces full antagonism at a certain dose, then if that dose is used in an experiment the antagonist cannot be used to distinguish between receptors A and B because both will be blocked. Thus, an antagonist is only as good as its selectivity profile and the dose used.
4.3.2.2. peptide antagonists.
On this basis, most peptide antagonists of CGRP family receptors cannot be considered as tools that are useful in distinguishing between individual receptors because they are not sufficiently selective (23). The peptides tend to be truncated forms of the full-length endogenous peptide. For example, CGRP8-37 is a truncated form of CGRP that lacks the first seven amino acids, which are known to impart receptor agonism (309). Although CGRP8-37 has higher affinity at the CGRP receptor than at the AMY1 receptor, this difference is not sufficient to distinguish between them (110, 287, 299, 310–313). Some peptide antagonists can, however, distinguish between pools of receptors reasonably well. A good example is AC187. This is a peptide inspired by the amylin and calcitonin amino acid sequences. It only weakly antagonizes CLR-based receptors but potently antagonizes CTR-based receptors (110, 287, 299, 310, 311, 314). When used at an appropriate concentration, it can distinguish between these populations of receptors. However, it cannot distinguish between individual AMY receptor subtypes. This is not surprising, as most peptide antagonists were developed based on the sequences of endogenous ligands, which themselves activate multiple receptors. Thus, hope often lies in small-molecule antagonists that were specifically developed to have high affinity for a particular individual receptor. The suite of small-molecule CGRP receptor antagonists are prime examples of this and are discussed in sect. 4.3.2.3. That said, drug development of peptidergic CGRP receptor antagonists is being explored, for example, FE 205030 and variants of CGRP8-37 that have been lipidated to prolong half-life (315, 316).
4.3.2.3. nonpeptide antagonists.
Many nonpeptide/small molecules have been reported to be CGRP receptor antagonists. They have many different chemical structures and have been pharmacologically characterized to various degrees (317–321). When they were first being developed, the molecular structure of the CGRP receptor was not known and the pharmaceutical industry played a large role in identifying the molecular components of the CGRP receptor (284, 286, 319). A useful tool at the time was a cell line (SK-N-MC) that endogenously expresses the CGRP receptor and from which RAMP1 was ultimately cloned (286). These cells were, and still are, widely used as a tool for screening and studying CGRP receptor antagonists (312, 322–325). This poses some challenges because it makes it difficult to compare antagonist action in SK-N-MCs, which have endogenous CGRP receptor, with that of related receptors that are usually heterologously expressed in cell lines such as HEK sublines, CHOs, or Cos7s (317). Another complicating factor is that, given the rather complex molecular composition of the members of the CGRP receptor family, antagonists are not often profiled under comparable conditions. Thus, there are many molecules, but the picture of their receptor pharmacology and selectivity profiles is incomplete.
The “gepant” class of chemically related CGRP receptor antagonists are the best understood. These include olcegepant (BIBN4096BS), telcagepant (MK-0974), rimegepant (BMS-927711, BHV-3000), ubrogepant (MK-1602), atogepant (MK-8031), and zavegepant (formerly vazegepant, BHV-3500). These antagonists have high affinity for primate CGRP receptors and low to negligible affinity for the AM1 and AM2 or calcitonin receptors (311, 312, 319, 326–331). For those gepants for which receptor-bound structures are available, interactions occur with CLR and with amino acids on RAMP1, and this has two consequences. First, because of species differences in RAMP1, these antagonists often have lower affinity for rodent versus primate receptors (311, 319, 326, 328, 332). Thus, in rodent models they need to be used at higher concentrations to achieve antagonism, and often this is not practical or meaningful. Second, these antagonists are able to antagonize not only the CGRP receptor but also the AMY1 receptor (106, 311, 317, 333). TABLE 2 provides a summary of the receptor pharmacology of several gepants.
Table 2.
CGRP receptor antagonists and their ability to antagonize CGRP and AMY1 receptors
| A2/KB or IC50 |
Approximate Fold Selectivity |
||||
|---|---|---|---|---|---|
| CGRP receptor |
AMY1 receptor |
||||
| h-αCGRP | h-αCGRP | h-Amylin, m/r-Amylin | CGRP receptor over AMY1 receptor with CGRP or (amylin) | Reference | |
| Erenumab | 2.3 nM [SK-N-MC] | – | – | – | (334) |
| 74 nM [SK-N-MC] | – | 100 nM [HEK293S] | – | (335) | |
| 0.014 nM [oocytes] | 0.45 nM [oocytes] | – | 30-fold | (336) | |
| Ubrogepant | 0.08 nM [HEK293] | – | 8.32 nM [HEK293] | (100-fold) | (328) |
| Rimegepant | 0.28 nM [Cos7] | 8.51 nM [Cos7] | 33.11 nM [Cos7] | 30-fold (120-fold) | (333) |
| Telcagepant | 1.00 nM [Cos7] | 43.65 nM [Cos7] | – | 40-fold | (110) |
| 1.20 nM [Cos7] | 42.66 nM [Cos7] | – | 40-fold | (106) | |
| – | – | 257 nM [HEK293S] | – | (335) | |
| Olcegepant | 0.02 nM [Cos7] | – | – | – | (332) |
| 0.10 nM [Cos7] | 13.18 nM [Cos7] | 199.54 nM [Cos7] | 130-fold (2,000-fold) | (106) | |
| 0.19 nM [Cos7] | – | 36.3 nM [Cos7] | (200-fold) | (311) | |
| 0.22 nM [Cos7] | 58.88 nM [Cos7] | – | 250-fold | (110) | |
| Atogepant | 0.026 nM [HEK293] | – | 2.4 nM [HEK293] | (90-fold) | (319)* |
| Zavegepant | 0.022 nM [SK-N-MC] | – | – | – | (337) |
“–” indicates data not found. A2/KB values (bold font) or IC50 values have been converted from the original pA2/pKB or pIC50 values, followed by the cell type in which each experiment was performed in square brackets. When no A2/KB could be found, IC50 data from the antagonist inhibiting an agonist-induced functional response stimulated by a fixed concentration of agonist were used in its place. Fold selectivity was calculated where comparable data exist between receptors. Each line represents a matched study. Italics are used to indicate that a mouse/rat peptide was used, rather than human. *This reference does not describe methodology; therefore we cannot definitively describe these as A2 or IC50. CGRP, calcitonin gene-related peptide.
The antibody antagonist erenumab has a pharmacological profile similar to the gepants. It has high affinity at the CGRP receptor, and there is some evidence that it can also antagonize the AMY1 receptor, presumably by virtue of its ability to bind to RAMP1, though not all studies are universal in detecting AMY1 receptor antagonism (see sect. 4.4) (335, 336, 338, 339) (TABLE 2). One aspect that is particularly important in measuring antagonism at the AMY1 receptor in the context of CGRP is that CGRP should be used as the agonist, rather than amylin. This is because agonist and signaling pathway-dependent antagonism of this receptor has been reported (106, 333, 336). For example, amylin seems more difficult to antagonize at this receptor than CGRP, and thus where amylin is used as the agonist this is likely to overestimate selectivity between the CGRP and AMY1 receptor (339). Like-for-like comparisons should be made such that CGRP is used as the agonist at both receptors for more accurate determination of selectivity. This issue is thoroughly discussed in a recent minireview (317).
The significance of AMY1 binding by erenumab or gepants for efficacy or side effects is not yet clear but is a potentially important consideration for using any of these as either pharmacological tools or agents to study biological mechanisms (317, 335, 339–341).
4.4. Mechanisms of Ligand Binding and Receptor Activation
The mechanisms whereby CGRP binds to the CGRP receptor are now reasonably well understood. This information helps guide our study of the mechanism of action of drugs that block CGRP; the design of potentially improved drugs; and the development of new ligands for CGRP receptors.
In the case of CGRP, broadly speaking, the CGRP COOH terminus (with its important COOH-terminal amide) binds to the extracellular domain of the receptor (FIGURE 8). This positions the peptide NH2 terminus for binding to the extracellular loops and transmembrane bundle of the receptor to trigger a conformational change and produce receptor activation. Crystal structures of the isolated extracellular domain complex of CLR and RAMP1 bound to a modified CGRP COOH-terminal fragment identified specific amino acids that are required for binding (343). These data shed light on how both CLR and RAMP1 contribute to the binding of CGRP to the extracellular portion of the receptor. However, cryo-electron microscopy has revealed that the interactions between the remainder of the CGRP peptide and the receptor do not directly involve RAMP1 but only CLR (342, 344). Emerging evidence points to RAMP acting allosterically to enable peptide recognition (344, 345). Individual amino acid substitutions in both the peptide and receptor subunits have provided strong support for this mode of binding (343, 346–353). These studies also provide insights into how the receptors differentiate between endogenous ligands.
Figure 8.
Structural model of the active calcitonin gene-related peptide (CGRP) receptor, comprising calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein (RAMP)1, with CGRP bound. The extracellular domain (ECD) and transmembrane bundle (TM) are both involved in binding CGRP. Gαs was bound in the structure [PDB:6e3y (342)] but is not shown in this figure. Image created with BioRender.com, with permission.
Structures, site-directed mutagenesis, and molecular modeling have also provided insights into receptor antagonism. A crystal structure of erenumab bound to the isolated extracellular domain complex of CLR and RAMP1 shows that this antibody, like CGRP itself, is capable of binding to both components of the receptor (338). Ten of the 18 amino acids in this region of CLR that were reported as binding erenumab are identical in CTR. There are also some structures of small-molecule CGRP receptor antagonists bound to the CGRP receptor (320, 354). These also show that both CLR and RAMP1 contribute to binding of antagonists. One RAMP1 amino acid in particular, amino acid 74, is notable in that it is responsible for appreciable species selectivity, i.e., for the gepants having higher affinity for primate versus rodent receptors (311, 326, 328, 331, 334). Studies of receptor dynamics are also providing insights into CGRP binding and the mechanism of action of the gepants (344, 355).
4.5. Receptor Signaling and Regulation
4.5.1. Intracellular signaling pathways.
CGRP receptor signaling has previously been reviewed in some depth (356, 357). According to a substantial body of evidence, CGRP receptors interact with G proteins to produce intracellular signaling. This signaling can be modulated by RCP (358). The best known is activation of Gs, leading to activation of adenylate cyclase and an increase in intracellular cAMP production. Downstream, this can activate PKA and other kinases. A general overview of CGRP signaling is shown in FIGURE 9. In transfected cell models in vitro, the CLR/RAMP1 and CTR/RAMP1 CGRP-responsive receptors seem to be capable of activating a similar set of signaling molecules including ERK (348, 359–364). Although signaling patterns seem to be similar in these cellular models, how this may translate into more physiologically relevant models is largely unknown because few studies have attempted to probe AMY1 receptor signaling. In part this is because of the difficulties of determining the molecular identity of CGRP family receptors in cells and tissues with endogenous receptor. Hence, CGRP signaling is often studied, but it is not always paired with molecular analysis that could define which receptor is mediating the signaling. In trigeminal neurons, where both CGRP and AMY1 receptors are expressed, cAMP production, CREB phosphorylation, and p38 MAP kinase phosphorylation all occur in response to CGRP (106, 110, 114, 313). Calcium signaling also occurs but only in relatively few neurons, although this number can be upregulated (114, 365). An interesting observation in trigeminal neurons was that the degree of antagonism mediated by olcegepant differed according to the intracellular signaling pathway measured (106). Thus CGRP-stimulated CREB phosphorylation was more potently blocked than CGRP-stimulated cAMP production. The implications of this are unclear. ERK phosphorylation in response to CGRP has been observed in primary cells, such as primary human cardiomyocytes (366).
Figure 9.
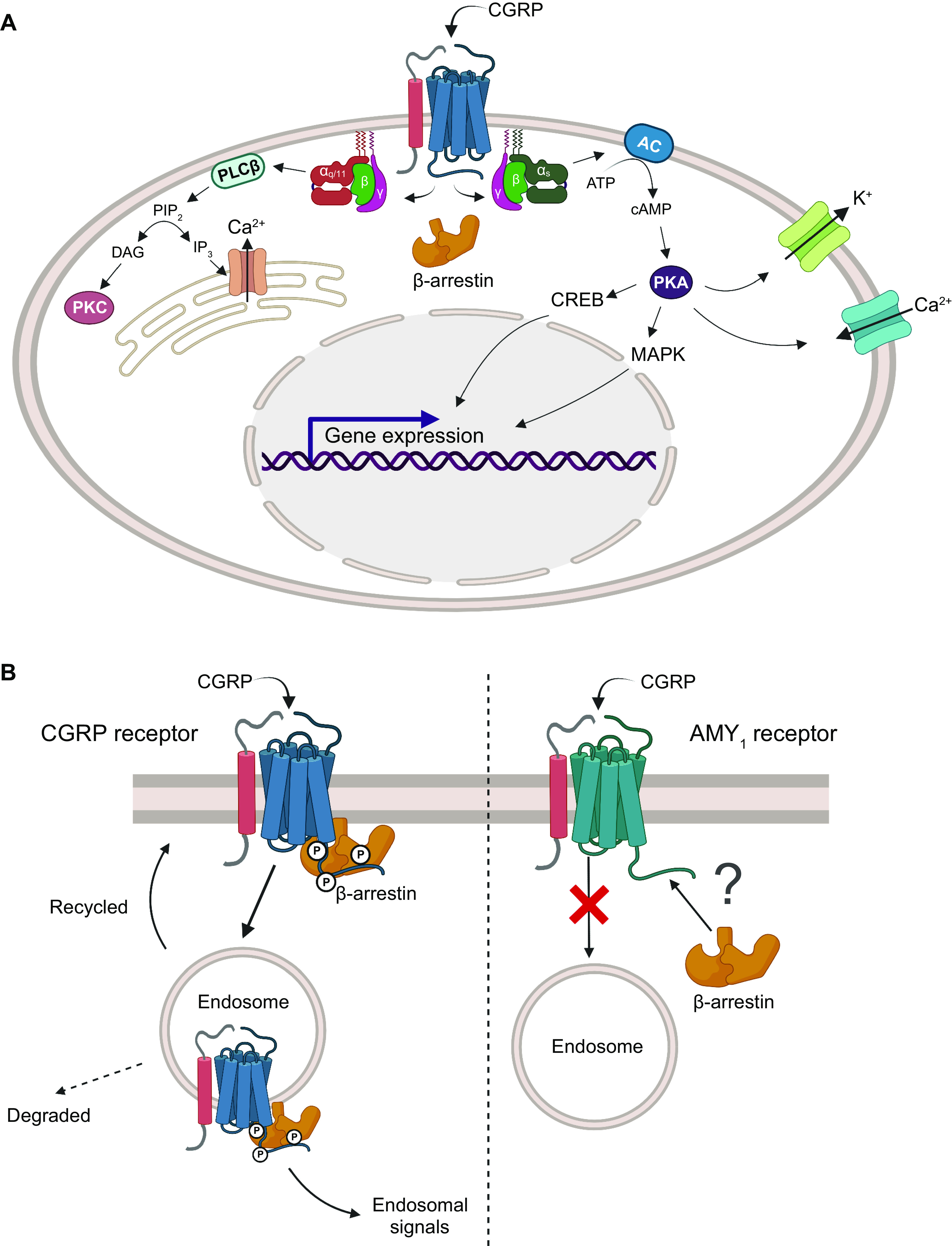
Acute calcitonin gene-related peptide (CGRP) signaling and regulation. A: an overview of pathways commonly activated by CGRP is shown. In many cases, activation of a signaling pathway by CGRP cannot be specifically assigned to the CGRP or AMY1 receptor. Signaling from the cell surface receptors, not endosomal receptors, is shown. B: an overview of receptor fate, after cell surface stimulation. The CGRP receptor (left) recruits β-arrestin and is internalized into endosomes. For the AMY1 receptor (right) internalization is not clearly evident, and it is unclear whether the receptor is phosphorylated or recruits β-arrestin. Image created with BioRender.com, with permission. AC, adenylate cyclase; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; MAPK, mitogen-activated protein kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PLC, phospholipase C.
4.5.2. Receptor regulation after stimulation.
Regulatory control of GPCRs following their activation is necessary to ensure that cells respond appropriately to endogenous ligands. Several processes and mechanisms exert longer-term effects on receptor function. Specifically, GPCR activation is usually followed by association with GPCR kinases (GRKs), to phosphorylate serine and threonine residues on intracellular receptor sites. This is followed by recruitment of β-arrestin (FIGURE 9), whose canonical role is to mediate receptor desensitization, internalization, and intracellular trafficking. In addition to GRK-mediated modifications that lead to desensitization, GPCRs can be phosphorylated by second messenger-dependent kinases (PKA and PKC) to similar effect. These mechanisms act to dampen signaling, and internalized GPCRs were originally thought to be inactive but there is now good evidence that GPCRs can promote signaling from internalized GPCRs in endosomes. In the context of the CGRP receptor (CLR/RAMP1), each of these mechanisms occurs, as has been recently reviewed (335, 367–370). Of relevance to pain, CGRP receptor signaling from endosomes was linked to persistent activation of nociceptive neurons in the spinal cord (367) and Schwann cells surrounding nociceptive fibers (371). Several useful reviews can be consulted for detailed information on CGRP receptor regulation and endosomal signaling (369, 370), but we also highlight a few key points here. Once the CGRP receptor is internalized, its fate, i.e., targeting to a recycling or degradation pathway, appears to depend on the concentration of CGRP. Because of the challenges in understanding the CGRP concentration at a particular site of receptor expression, it is difficult to know how this might translate into effects in migraine, for example, or at different physiological sites of action. An interesting aspect of the control of these processes is that ECE-1, a peptidase located in endosomes, affects CGRP receptor regulation by cleaving CGRP, and this leads to recycling of the receptor (165, 370, 372). This mechanism may occur in blood vessels (372). Although a broad set of overall mechanisms occur for the CGRP receptor, site-specific subtleties in regulatory behavior likely have an impact on our understanding of whether, when, and for how long the receptors are active and in which cell compartment, i.e., cell surface or within endosomes.
Moving beyond the canonical CGRP receptor, we also have the AMY1 receptor to consider. Two studies have now suggested that this receptor appears to be regulated differently from CLR/RAMP1 after CGRP exposure (335, 368). Specifically, the AMY1 receptor appears to be more resistant than CLR/RAMP1 to agonist-mediated internalization (FIGURE 9). On the other hand, CTR alone and when cotransfected with RAMPs can interact with cellular components that are involved in receptor trafficking (361). As it is now known that signaling pathways from the plasma membrane and endosomes can be distinct (373), differential internalization of these two receptors could have functional implications for CGRP activity. Furthermore, such differences could contribute to how anti-CGRP drugs act. The CGRP receptor antibody erenumab can prevent cell surface signaling of both receptors (335, 374) but does not produce ligand-dependent endocytosis (335, 374). It is not known how the gepants may affect receptor trafficking. More studies are needed to determine how both receptors are regulated after stimulation by CGRP. To date, most studies have been conducted only in transfected cell systems, and therefore their relevance to endogenous systems is not known. However, they highlight that if CGRP indeed acts via both receptors in vivo, differential regulation of each receptor could be a so far unappreciated mechanism for fine-tuning the effects of CGRP in different cells or under different circumstances.
A further consideration for the regulation of either receptor is how its expression might change in response to either long-term challenge with ligand or chronic ligand depletion. For example, should CGRP be upregulated in disease, what effect would this have on receptor expression levels or the availability of the receptor at the cell surface? If the CGRP receptor is internalized but the AMY1 receptor is not, does this mean that CGRP can still signal via AMY1 if its canonical receptor is not available? Alternatively, if the levels of CGRP are reduced and it is prevented from activating its receptors, could upregulating the receptor(s) serve as a compensatory mechanism? These questions require detailed investigation.
4.6. Sites of Receptor Expression (Binding, Subunits)
Receptor expression can be considered from several different perspectives. Much information about CGRP receptor expression has been gleaned from extensive studies using radiolabeled forms of CGRP. These studies are of two major types: studies of receptor binding in tissue preparations such as membranes and autoradiography-based studies of receptors in tissue sections. These provide valuable information about global expression patterns of receptors that bind CGRP with high affinity, as summarized in FIGURE 5 (172, 179, 181, 375–379). The ability of different unlabeled ligands to displace labeled peptide can then provide insights into the relative pharmacological properties of the binding sites. In some studies, labeled forms of antagonists have also been used (330). Collectively, these studies tell us that high-affinity CGRP binding sites are widely distributed through the brain and body. For example, comparison of CGRP binding in membrane preparations from a panel of rat tissues showed that high-affinity CGRP binding sites were present in heart (atria and ventricles), cerebellum, spinal cord, liver, vas deferens, lung, and spleen. Of these sites, the one with the greatest ligand binding capacity was spleen, followed by lung and liver/spinal cord, as shown by Chakravarty and colleagues (282). The presence of binding sites alone does not provide direct insight into molecular identity. This typically requires analysis of mRNA or protein from a location of interest. Again, using the study of Chakravarty and colleagues as an example, the presence of binding sites was correlated with the sites of expression of the CLR and RAMP mRNAs, to gain insight into which receptor(s) may be responsible for the binding sites (282). This can give a broad picture but does not provide detailed insights at the cellular and protein levels.
A particular challenge with the CGRP receptors relates to their dimeric nature; it is necessary to know whether both receptor components are expressed in the same sites at the same time. In neurons, there is the added consideration of whether expression is in the cell body or fiber and how this relates to where the receptor ligand is found. Generally speaking, there is a good correlation between the presence of CLR, RAMP1, CGRP, and high-affinity CGRP binding sites. As reviewed by Hendrikse et al. (273), many sites in the CNS are positive for all of these, implying that CGRP acts locally on the canonical CGRP receptor. These sites include spinal cord, the medulla of the brain stem, hypothalamus, thalamus, and amygdala (273). However, there are also some interesting mismatches, especially with regard to the relative hybridization signal of CLR mRNA and CGRP binding sites (190, 380). There are many more locations of high-affinity CGRP binding sites than there are sites where CLR mRNA is robustly detected (FIGURE 5). This could be because only a small amount of mRNA is required to be translated into functional protein or because the mRNA probes were not sensitive. However, these data could also be telling us that CLR-based receptors are responsible for CGRP binding only in certain locations or only partially responsible for CGRP binding. This would mean that CTR-based receptors could make up the difference. Consistent with this, CTR is also located at sites of high-affinity CGRP binding and/or sites of CGRP expression, such as the locus coeruleus and PBN (204, 205, 273).
The identification of sites of expression of receptor protein generally relies on the use of antibodies. Ideally, antibodies recognizing each protein would be used in the same tissue sections to colocalize RAMP and the GPCR, either CLR or CTR. Whereas many studies have been published for RAMP1 and CLR, the number for CTR is comparatively much smaller. Many of these studies have been comprehensively reviewed (273, 307, 381). Regions such as the TG have been studied in particular detail to show that RAMP1, CLR, and also CTR are sometimes detected (107, 110, 111, 194, 307, 382). Key points for interpreting these studies are the following. First, it is necessary to understand receptor expression at the level of the receptor complex. In other words, staining for either CLR or RAMP1 expression is not sufficient to conclude that a CGRP receptor is present because neither is an exclusive partner of the other (they also interact with other RAMPs or other GPCRs, respectively) (383, 384). Second, it is important that the specificity of any antibody has been validated, i.e., that off-target proteins are not being detected. A number of validation pillars outline best practice in antibody characterization (223), and ideally several of these should be used. This includes the use of knockout models to confirm that antibody immunoreactivity is lost in the knockout because antibodies used as research tools are not nearly as specific for the targets that they are designed to detect. This was recently highlighted in a study using a commercially available RAMP1 antibody for Western blotting (385). Although transfected cell controls showed that the antibody is able to detect RAMP1 at its expected molecular weight (see online supplement in Ref. 385), the immunoblots also revealed a higher-molecular weight band that was present in all cells, including cells not expressing RAMP1, and was therefore nonspecific. The additional protein that the antibody detects remains unidentified, but should it be present in a tissue sample it would not be possible to determine whether the immunoreactivity detected by this RAMP1 antibody was indeed RAMP1 or this off-target protein. Higher-molecular weight bands than those expected from RAMP1 are often explained by the authors as RAMP1 dimers, but the controls in the aforementioned study indicate that this is not the case for this antibody and therefore this is not a conclusion that should be drawn without rigorous testing (385). This study and antibody are only one example among many. Our analysis of the collective literature for protein localization by CLR and RAMP antibodies, including RAMP1, revealed that the tools used were not sufficiently validated to say with confidence that the immunoreactivity reflects the expression of these proteins. Until such validation is done and we have greater confidence in the data, reports of receptor expression should be considered preliminary. Of note, for some CTR antibodies knockout mouse controls are available, and thus data from these studies can be considered as more robust (204, 386, 387). There is a great deal still to be done in determining the molecular identity of CGRP binding sites and whether these comprise CLR with RAMP1 or other receptors. An overview of the current situation for RAMP1, CLR, and CTR expression in relation to CGRP binding sites and CGRP expression is shown in FIGURE 5. It is worth noting that calcitonin is not produced in the brain and amylin is only produced in discrete locations. Hence, are many CGRP binding sites in the brain actually explained by CTR-based receptors?
These issues aside, there are some interesting considerations that should be followed up as the tools allow. A change in binding sites has been reported with aging (388, 389). For example, in a comparison of 125I-Tyr-rat CGRP binding sites between aged (22 mo) and young (2 mo) rats, there were site-specific differences in CGRP binding, increasing with age in some locations and decreasing in others. This suggests that there may be age-related adaption in CGRP binding sites (389). There are also the open questions of where CGRP is expressed in relation to its receptors and under what circumstances it might be acting in an autocrine or paracrine manner (114, 166, 256, 390). This aspect is highlighted in sect. 2.1.3 in the context of a recent study that has shown that CTR and CGRP colocalize in mouse, rat, and human TG neurons, suggesting that a CTR-based receptor such as the AMY1 receptor could underlie some autocrine actions of CGRP (111).
Taking a holistic view of the collective data of CGRP and CGRP receptor subunit expression, although there are many studies that consider one or more aspects of expression of a functional CGRP signaling unit, there is none that includes all aspects (CGRP, RAMP1, CLR, and CTR) at a level of granularity that gives detailed spatial information at a cellular level. Additional well-validated tools are required to determine with confidence the expression of all these components in relation to one another.
5. PHYSIOLOGICAL FUNCTIONS
5.1. Overview
The main message of this section is that CGRP generally plays a compensatory and protective role in the body. Even when the levels of CGRP are elevated, such as in migraine, it is useful to remember that its role is to protect the body. In other words, CGRP should not be classified as just a “bad peptide.” Rather, we need just enough CGRP, not too much or not too little. The relative safety of the CGRP-based migraine drugs to date (sects. 5.3.1, 5.8.1, and 6.6) suggests that the optimal range of CGRP levels is fairly broad, perhaps because of physiological redundancy of family members (e.g., AM, AM2) (sect. 1.3) and receptors (e.g., canonical, AMY1) (sect. 4.2), along with the intrinsic features of neuropeptide dispersion by volume transmission (section 1.4.2). With this perspective in mind, in this section we review the normal physiological functions of CGRP, with occasional references to relevant pathologies.
5.2. Nervous System
5.2.1. Central nervous system.
5.2.1.1. central sensitization.
Despite over three decades of research on CGRP, we have likely only scratched the surface in our understanding of CGRP actions in the CNS. So far, it is clear that CGRP contributes to central sensitization as a neuromodulator of glutamatergic signaling and that it works by both presynaptic and postsynaptic mechanisms (FIGURE 10) (391–393). In general, this involves second messenger-mediated activation of presynaptic calcium channels and postsynaptic glutamate receptors in multiple regions of the brain. These actions have been studied in neurons of the spinal and medullary dorsal horns, amygdala, and anterior cingulate and insular cortices. To date, the evidence points to CGRP acting via cAMP-dependent pathways (394) that trigger immediate and long-term changes in protein function and gene expression, specifically those changes that underlie the neuroplasticity of central sensitization (395, 396).
Figure 10.
Modulation of neuronal signaling by calcitonin gene-related peptide (CGRP). CGRP is released from a neuron at the synapse (1a) and from nonsynaptic sites (e.g., a varicosity) (1b). The released CGRP binds postsynaptic receptors to produce modulatory effects at the synapse by increasing glutamate receptor signaling (2) and binds presynaptic receptors in a paracrine or autocrine manner to activate calcium channels (3). The CGRP receptor shown represents a generic CGRP-responsive receptor, not specifically the canonical receptor or AMY1. Image created with BioRender.com, with permission.
The outcomes of CGRP release in the CNS can be broadly grouped as 1) nociceptive signaling and 2) affective and aversive behaviors. However, this organization is somewhat arbitrary, and there is certainly overlap. For example, CGRP-mediated nociceptive and aversive actions likely overlap in the transmission of sensations from the posterior thalamus and PBN to the insular cortex and amygdala (391, 397).
5.2.1.2. nociceptive signaling.
Nociceptive signaling from the DRG and TG to the thalamus and cortex is perhaps the best-understood pathway involving CGRP neuromodulation. CGRP and its receptors are widely expressed along the entire pain axis, and the involvement of CGRP in pain is nicely covered in a recent comprehensive review (396). The role of CGRP in sensitization to pain is especially notable in migraine (398). This section is organized along the pain axis, from the spinal cord to the cortex.
5.2.1.2.1. Spinal cord and spinal trigeminal nucleus.
In the spinal dorsal horn, CGRP can enhance glutamate transmission by presynaptic mechanisms that lead to central sensitization (395, 399). The CGRP receptor was reported to be on presynaptic fibers and a few cell bodies (171, 237, 399, 400). Functionally, CGRP promotes trafficking of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) class of glutamate receptors to the cell surface and potentiates the effects of substance P on both AMPA receptors and the N-methyl-d-aspartate (NMDA) class of glutamate receptors (395). Thus, CGRP could potentially lead to central sensitization, such as that seen following intrathecal injection of CGRP, which causes mechanical allodynia (401). In addition to acting on glutamate receptors, CGRP has been reported to interact cooperatively with the vesicular glutamate transporter VGLUT2 in the spinal cord, thereby promoting heat hyperalgesia, pain, and itch in mice (402, 403).
As in the dorsal horn, in the superficial laminae of the spinal trigeminal nucleus caudalis (TNC) CGRP is a stimulatory neuromodulator of nociceptive transmission. Although the TNC is conceptually similar to the dorsal spinal cord, the two regions should be considered independently because of differences in the input from the DRG and TG (256). A series of in vivo experiments nicely demonstrated that CGRP released from central terminals in the TNC modulated glutamatergic second-order nociceptive neurons (404–406). Infusion of the TNC with CGRP increased the firing rate of a subset of neurons, and systemically administered CGRP receptor antagonists could inhibit both spontaneous and stimulated firing. Interestingly, the antagonists could also block activation by local application of glutamate, which suggested a postsynaptic site of action (404). These observations have been extended by in vitro studies of mouse brain stem slices, which showed that CGRP increases neuronal activity in the spinal trigeminal nucleus by activating presynaptic calcium channels and at a higher concentration by an apparently postsynaptic mechanism to facilitate glutamatergic transmission (407). A puzzle in the field has been that despite these functional data indicating postsynaptic mechanisms, expression data for CGRP receptors indicated that they were present only on presynaptic terminals and not on cell bodies or dendrites of postsynaptic second-order neurons (108, 171). This suggested a paracrine presynaptic mechanism in the trigeminal nucleus (108, 408). However, a more recent study using a different antibody that recognizes both the RAMP1 and CLR receptor subunits reported binding in the monkey TNC at both presynaptic and postsynaptic sites (194). In that study, confocal and electron microscopy showed that the receptor was located predominantly on cell bodies, dendrites, and, to a lesser degree, presynaptic axon terminals. The reason for the discrepancy between the localization studies is not clear but may relate to a difference in epitope availability, because the receptor antibody also detected CGRP receptors on primate dural mast cells and such immunoreactivity had not been seen in human cells (409). In summary, CGRP can act in the dorsal horns of the spinal cord and medulla to increase glutamatergic transmission, most likely by both presynaptic and postsynaptic mechanisms.
5.2.1.2.2. Thalamus.
The thalamus is an integrative brain center that evaluates input from many sites before relaying the information on to higher cortical regions. In particular, posterior thalamic nuclei have been linked to somatosensory pain signals. Roles for the thalamus in pain and other symptoms of migraine are supported by clinical and preclinical studies (410). Clinical studies have linked extracranial allodynia to thalamic functional magnetic resonance imaging signals from mechanical and thermal stimulation (411), and preclinical studies in rats have shown that mechanical and thermal stimulation of the dura activates neurons in the posterior nucleus (Po) and ventral posteromedial nucleus (VPM) of the thalamus (411, 412). The importance of the thalamus for headache and trigeminovascular system pathology was shown by a tracer study looking at projections of fibers from second-order neurons in the trigeminal nucleus to nuclei in the pons and midbrain, including the PBN and periaqueductal gray, and to the contralateral posterior thalamic nuclei, including the VPM (413). The functional importance of CGRP in the thalamus was nicely demonstrated by Goadsby and colleagues (192), who showed that injection of a CGRP receptor antagonist into the rat VPM reduced trigeminovascular nociceptive signals.
Importantly, the posterior thalamic region was shown to harbor neurons that receive convergent input from both non-image-forming retinal neurons and second-order, dural-sensitive trigeminal neurons (414, 415). As such, these thalamic neurons integrated visual and nociceptive signals that were then relayed to the somatosensory and visual cortices, and this presumably led to photophobia in patients. In humans, the connection between the retina and thalamic pulvinar nuclei (human posterior thalamus) was confirmed by diffusion magnetic resonance (416). Although a role for CGRP in this convergent signaling was not initially shown, subsequent studies in mice demonstrated that injection of the posterior thalamic region with CGRP was sufficient to induce light-aversive behavior even in dim light, a response that is analogous to photophobia in migraine patients (248).
5.2.1.2.3. Anterior cingulate and insular cortices.
CGRP plays a modulatory role in cortical regions. To date, studies have focused on the anterior cingulate cortex (ACC) and insular cortex, where CGRP and its receptors are found (249, 417). Early studies indicated that CGRP in the ACC may contribute to central sensitization to pain states (418). More recently, it was shown that CGRP increased glutamatergic signaling in the ACC (417). Using microelectrode arrays, the authors found that CGRP potentiated synaptic transmission by increasing NMDA receptor-mediated excitatory postsynaptic currents. The actions of CGRP were blocked by inhibitors of adenylate cyclase and PKA, indicating that a cAMP mechanism is active, as is the case in spinal neurons. These results suggest that blocking CGRP in the ACC might be helpful for treating chronic pain. However, another group found the opposite results, with CGRP apparently decreasing nociceptive signaling in the ACC (419). Future studies are needed to resolve how CGRP modulates pain in the ACC.
The insular cortex is an integrating forebrain region involved in pain perception and other higher brain functions (420, 421). Here, as in the ACC, CGRP can enhance excitatory glutamatergic signaling (422). However, in contrast to the effects in the ACC, the application of CGRP to slices of mouse insular cortical tissue potentiated evoked excitatory postsynaptic currents that were independent of the NMDA receptor. Given that CGRP decreased paired-pulse facilitation and increased the frequency, but not the amplitude, of the currents, a presynaptic mechanism was likely involved. Consistent with findings from other studies, CGRP-mediated synaptic potentiation in the insular cortex required cAMP pathways.
5.2.1.2.4. Hypothalamic and brain stem descending modulation pathways.
CGRP is involved not only in ascending but also descending modulation, affecting pathways from the raphe and posterior hypothalamus that can increase nociception (423, 424). A network of bidirectional trafficking between the hypothalamus and the limbic and brain stem nuclei provides an attractive mechanism for “perpetual feedback” that could maintain a migraine attack for a prolonged period of time (425). The role of the brain stem in migraine, including the involvement of CGRP, was recently nicely reviewed by Holland and colleagues (381). The role of the hypothalamus in migraine was also recently reviewed (426). Two nuclei are of particular interest with respect to descending modulation of trigeminovascular nociceptive signals: the hypothalamic paraventricular nucleus (PVN), which is involved in the stress response, and the hypothalamic A11 dopaminergic nucleus, which sends descending signals to the trigeminal nucleus (424, 427).
5.2.1.3. affective and aversive behaviors.
CGRP has been implicated in affective behaviors of anxiety and fear as well as aversive behaviors to food and light, which both overlap with pain responses. In these roles, CGRP is known to modulate synaptic transmission in the extended amygdala complex, including the bed nucleus of the stria terminalis (BST), which causes fear and anxiety-like responses (428, 429). In particular, transmission of CGRP from the lateral PBN to the central nucleus of the amygdala (CeA) is associated with central sensitization and pain-related behavior (430), along with aversive responses. Within the CeA, CGRP increases the amplitude of the postsynaptic current that is generated by NMDA receptors. This sensitization requires the activation of PKA and involves phosphorylation of the NR1 subunit of NMDA receptors (428, 431). Recently, Neugebauer and colleagues found sex differences in CGRP actions in the CeA in a rat chronic neuropathic pain model in which a CGRP receptor antagonist reduced mechanical hypersensitivity in both sexes but showed female-predominant effects on affective and anxiety-like behaviors (432). Behavioral evidence has implicated CGRP signaling within the BST, part of the extended amygdala, in anxiety. In addition, it points to the possibility that CGRP in the BST might modulate behavioral and neuroendocrine responses to stress (429, 433). Similar effects were seen with the stress-responsive neuropeptide corticotropin releasing factor (CRF), and CGRP can cause CRF release from the hypothalamus (434). Thus, the anxiogenic effects of CGRP in the BST appear to be mediated by CRF.
Aversive responses to certain stimuli are often linked with anxiety and pain responses. The roles of CGRP in the parabrachial and thalamic circuitry stand out in this context. The PBN is a region in the dorsal pons, and in general it contributes to many homeostatic and interoceptive sensory functions. Among the diverse sensory inputs to the PBN are second-order nociceptive neurons from the spinal cord and trigeminal nucleus. Interestingly, there are also direct monosynaptic connections from the TG, including those from the dura, which have been implicated in CGRP signaling in migraine (435). Within the PBN, CGRP-containing neurons act as a neuromodulatory alarm to higher brain regions (436). Pioneering work by Palmiter and colleagues (437–439) demonstrated that CGRP neurons in the PBN are critical for transmitting aversive signals in response to a variety of pathophysiological stimuli, including taste aversion and cancer anorexia. Optogenetic studies established that CGRP-containing PBN neurons that project to the CeA or the BST can induce the learning, maintenance, and expression of aversion to certain foods. The CGRP-containing PBN neurons receive inputs from area postrema neurons that are activated by a transforming growth factor (TGF)-β cytokine family member whose expression is elevated during infection or pathophysiological states (440). Silencing the CGRP neurons in the PBN prevented the aversive and anorexic effects of this cytokine. Thus, the CGRP-positive PBN neurons provide a link between pathophysiological signals and taste aversion.
The posterior thalamic nuclear region has long been associated with the processing of somatosensory and visceral pain signals. A major advance was a study in 2010 by Burstein and colleagues (415) demonstrating that posterior thalamic nuclei (primarily the Po and VPM) are sites of integration of light signals from intrinsically photosensitive retinal ganglion cells and signals from the dura of the meninges. This study was relevant because a hallmark of migraine is light sensitivity that is referred to as photophobia. In mice, photophobia-like behavior can be induced by injection of CGRP, either centrally (441–443) or peripherally (444). Interestingly, either injection of CGRP or optogenetic stimulation of the posterior thalamic nuclei in mice could evoke light-aversive behavior, even in dim light (248). Because the light aversion assay was historically used to investigate anxiety-like responses in animals, anxiety in a light-independent open field assay was used to rule out contributions from a general state of anxiety. The lack of an effect of CGRP injection or optogenetic stimulation in the dorsal hippocampus was used as an anatomical control. Thus, stimulation of posterior thalamic nuclei can initiate light-aversive signals in mice, and these may be modulated by CGRP to cause photophobia in migraine.
5.2.1.4. summary.
In summary, CGRP can act as a positive neuromodulator at the synapse to enhance glutamatergic signals. These actions can be by either presynaptic signals that open calcium channels or postsynaptic signals that increase NMDA and AMPA glutamate receptor signaling (FIGURE 10). However, since the current CGRP-based antagonists and antibodies are likely acting outside the blood-brain barrier, whether therapeutic targeting of these central sites may lead to adverse side effects not seen with the current drugs remains to be seen.
5.2.2. Peripheral nervous system.
5.2.2.1. peripheral sensitization.
Outside the CNS, CGRP is released from peripheral neurons onto cellular targets ranging from blood vessels to immune cells. In particular, release of inflammatory mediators in the skin and meninges can feed back onto sensory fibers and lead to peripheral sensitization of those nerves. These actions, as well as signaling within the enteric nervous system, are covered elsewhere in this review. In this section we limit the discussion to peripheral actions of CGRP, within sensory ganglia of the peripheral nervous system, and how those actions might lead to peripheral sensitization. As with central sensitization, the role of CGRP in peripheral sensitization is likely to be especially important in migraine (398).
CGRP transmits signals to neurons and satellite glia in sensory ganglia, and this can lead to peripheral sensitization. It should be noted that although we tend to group the DRG and TG together, there are functional differences between them (256). Overall, the importance of satellite glia in neural transmission is becoming more appreciated (445). This role of CGRP actions in satellite glia in the TG leading to peripheral sensitization has been studied (446). Along this line, a role for CGRP on astrocytes and microglia in the spinal cord after nerve injury has been proposed (447), although reports of CGRP receptor immunostaining in glial cells in the spinal trigeminal nucleus and Schwann cells around afferent fibers are inconsistent (108, 171, 194).
5.2.2.2. trigeminal ganglia cross talk.
A number of studies on the responses of cultured cells to various signals within the TG have led to suggestions of intense cross talk and interplay that involves CGRP, purinergic receptors, NO, and inflammatory cytokines. A comprehensive review of these signals and intracellular mechanisms was recently published (151).
CGRP released from TG neurons can act in a paracrine manner to increase purine-gated channel activity in pain signaling. Specifically, CGRP increases ATP-gated purinergic P2Y receptor signaling in satellite glia (448) and P2X3 receptors in other neurons (449, 450). CGRP control of the purinergic P2X3 receptor involves two mechanisms (450). CGRP can act directly on neurons to initiate a cAMP signaling cascade that activates the P2X3 gene, and CGRP can act indirectly, first activating the neurotrophin brain derived-neurotrophic factor (BDNF) gene in satellite glia, with release of the latter then stimulating the expression of P2X3 in neurons. Like CGRP levels, those of BDNF are elevated during migraine (451), suggesting that these two factors act together in augmenting purinergic receptors and contribute to peripheral sensitization in migraine. It is possible that feedback of signals from BDNF or P2X3 receptors leads to increased CGRP synthesis to activate pathways that increase CGRP transcription (81, 82, 124). In support of this notion, activation of P2X3 receptors causes CGRP release in cultured TG (452). The interplay between purinergic receptors and CGRP could promote depolarization of trigeminal afferents and transmission of nociceptive stimuli (453), leading to peripheral sensitization.
CGRP can stimulate the expression and release of NO and cytokines in satellite glia (151). Treatment of primary TG cultures with CGRP led to increases in the levels of multiple cytokines, including TNF-α and IL-1β (454, 455). In a multifaceted positive feedback loop, NO (96) and TNF-α (80) can increase neuronal CGRP gene transcription and peptide secretion, and satellite glia activated by NO or IL-1β released compounds that further increased CGRP release by cultured TG neurons (456). Infusion of glycerol trinitrate (GTN), which generates NO, was followed by an increase in CGRP levels and neuronal nitric oxide synthase (nNOS)-immunoreactive neurons in rats (457). The same treatment also increased the number of neurons expressing RAMP1 (458), the rate-limiting component of the CGRP receptor in the ganglion (81). A further connection between NO and CGRP was discovered with a mouse model of medication overuse headache in which NO activated the sodium channel Nav1.9 to trigger CGRP secretion leading to vasodilation and mast cell degranulation (459). In addition, not only NO, but the gaseous transmitter H2S, which together with NO produces nitrosyl (NO−), might play a role in the ganglia by stimulating the release of CGRP (460, 461). The significance of these interactions is supported by the dependence of NO-mediated facial allodynia in mice on TRPA1 receptor channels and the related association with signs of oxidative stress in TG neurons (150). Thus, cytokines and NO can create paracrine positive feedback loops with CGRP that involve glia and neurons in the TG.
Finally, there is the possibility that CGRP can directly autoregulate its own synthesis and release by an autocrine positive feedback loop. Studies with cultured TG neurons have shown that CGRP can increase the activity of reporter genes containing the CGRP promoter and release of substance P (which is colocalized with CGRP in vesicles) (81, 110). Of note, all studies to date agree that the canonical CGRP receptor is not present on trigeminal C fibers, which are the predominant source of CGRP (107, 108, 194). However, the second CGRP receptor, AMY1, appears to be present on C fibers, based on the presence of CTR and RAMP1 on TG neurons with small cell body sizes consistent with C fibers (110, 151) and coexpression of CTR with CGRP (111). Thus, a combination of paracrine and autocrine CGRP mechanisms is likely to contribute to peripheral sensitization in the ganglia.
5.2.2.3. summary.
In summary, these observations indicate that CGRP could function as an autocrine and paracrine factor to stimulate nearby glial cells and neurons, which in turn could feed back with signal molecules like NO and cytokines to further stimulate CGRP synthesis and release. This would generate a perfect storm of positive feedback loops sensitizing TG neurons, which may contribute to pain exacerbation, as in migraine.
5.3. Cardiovascular System
CGRP has many cardiovascular activities within the heart and numerous vascular beds (FIGURE 11). In this review, we focus on recent advances rather than trying to encompass all of these cardiovascular activities since they have been very well covered in other reviews. In particular, we point the reader to excellent reviews by Brain and colleagues (22, 462–464) and others (465–468).
Figure 11.
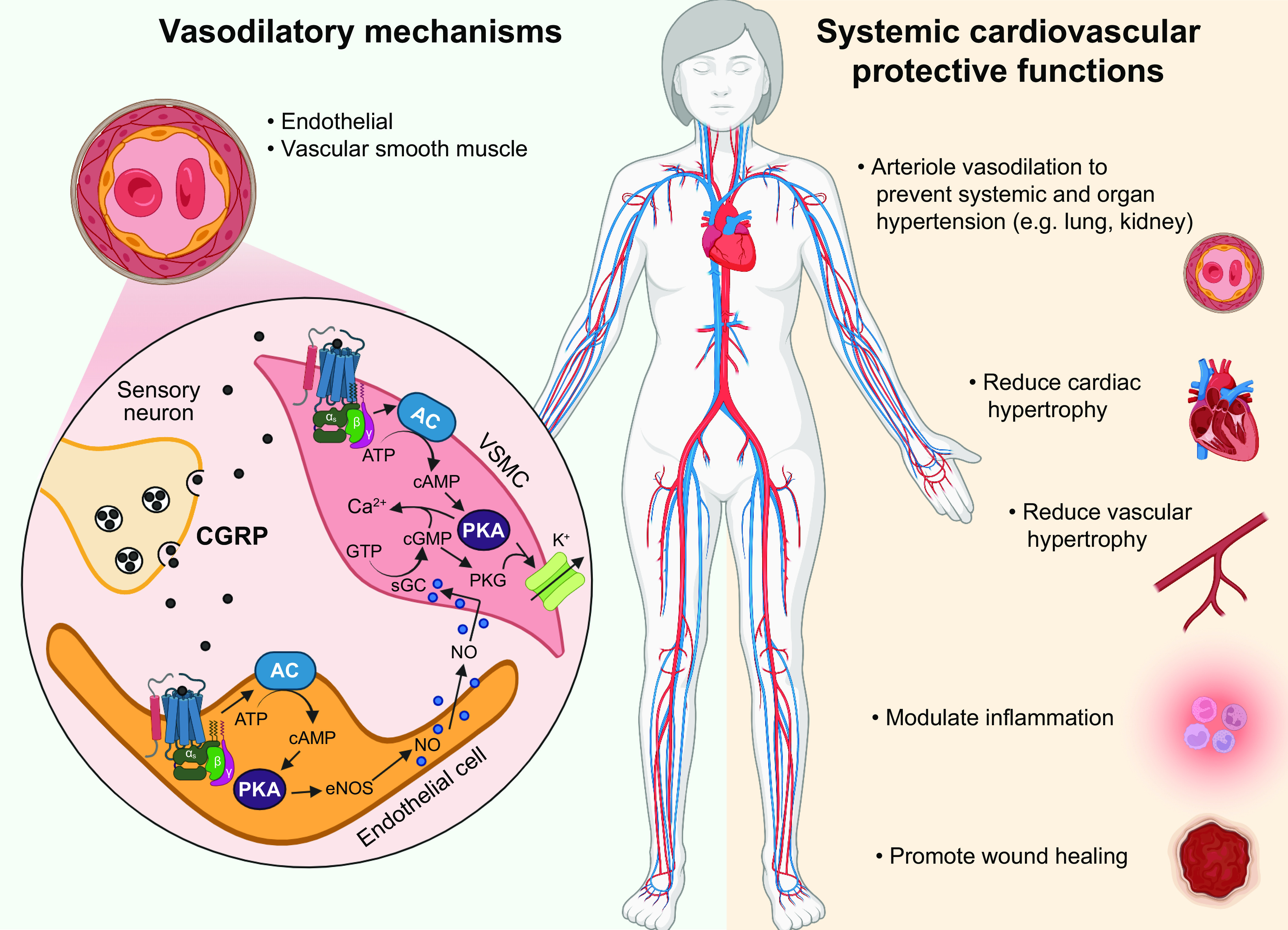
Calcitonin gene-related peptide (CGRP) in the cardiovascular system. Overview of CGRP’s actions in the cardiovascular system, with local vasodilatory mechanisms on left and systemic actions that are generally protective on right. For vasodilation, CGRP released from perivascular sensory neurons activates signaling pathways in vascular smooth muscle cells (VSMCs) that activate K+ channels to cause relaxation and endothelial cells to release nitric oxide (NO), which also causes VSMC relaxation. The systemic cardiovascular protective functions include vasodilation and other actions. AC, adenylate cyclase; eNOS, endothelial nitric oxide synthase; PKA, protein kinase A; PKG, protein kinase G; sGC, soluble guanylate cyclase. Image created with BioRender.com, with permission.
5.3.1. Compensatory vasodilation.
The best-known physiological role of CGRP is to mediate increased blood flow by causing vasodilation (FIGURE 11). CGRP was found to be the most potent vasodilatory peptide soon after its discovery (153). In the microvasculature, its potency is ∼10-fold higher than that of the most potent prostaglandins and 10–100 times higher than those of acetylcholine and substance P (153). Furthermore, the vasodilation is longer lasting (up to 6 h) than that triggered by other vasodilators (469). CGRP-induced vasodilation is seen in cerebral, coronary, and kidney vascular beds (462). Positive inotropic and chronotropic heart responses are also observed (470, 471), possibly due to hypotension caused by direct actions on cardiac muscle and reflex sympathetic nervous activity (470).
How does CGRP cause vasodilation? In the peripheral arterial vessels, it reduces blood pressure by both an NO-dependent mechanism in endothelial cells and an NO-independent mechanism in smooth muscle (462). The mechanism of CGRP action in vascular smooth muscle is cAMP-mediated phosphorylation, which leads to opening of ATP-sensitive potassium (K+) channels, resulting in relaxation (FIGURE 11). The mechanism of CGRP action on vascular endothelial cells is also cAMP dependent but involves the production of NO, which then diffuses into the smooth muscle to activate cGMP production and relaxation (463). It is clear that α-CGRP and β-CGRP have similar vasodilatory activities; there are reports of α-CGRP having more potent hemodynamic effects in Wistar rats and humans (472, 473).
Despite its potent actions on the vasculature, CGRP does not appear to play a pivotal role in the normal homeostatic control of systemic blood pressure. This is best illustrated by the fact that in mice CGRP receptor antagonism or CGRP knockout does not generally affect resting blood pressure or heart rate (463). However, there are conflicting reports of elevated mean arterial pressure and heart rate from different CGRP knockout strains (474), which still remains a puzzle. Nonetheless, by most criteria, CGRP appears to be a compensatory peptide that responds to pathophysiological challenges in an attempt to restore normal cardiovascular functions. This evidence, mainly from rodent studies, covers a range of models of cardiovascular disease, such as hypertension and heart failure (463, 464).
A compensatory role of CGRP in maintaining vascular tone is consistent with the relatively good safety profiles of CGRP- and CGRP receptor-blocking monoclonal antibodies and CGRP receptor antagonists (gepants) (468, 475). In clinical trials there was not any increased incidence of hypertension when the CGRP receptor antibody erenumab was compared with placebo, and postmarketing patient reports of adverse events, including hypertension, were generally low (476). However, because vasodilation by CGRP is likely to be a safeguard during cerebral and cardiac ischemic events, inhibition of CGRP could potentially worsen heart attack and stroke outcomes. Thus, from the beginning there has been concern about prescribing CGRP inhibitors to patients with a history of cardio- or cerebrovascular disease (477–480). This includes patients with small vessel diseases, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), who often have migraine (481). In addition, the point has been made that careful monitoring is needed even for those without a previous risk of ischemia (482). Indeed, the US Food and Drug Administration (FDA) has included hypertension in the warnings and precaution section of the label for erenumab (antibody that inhibits the CGRP receptor) based on postmarketing adverse events (483). The exposure-adjusted incidence of hypertension was fairly low at 0.144 per 100 patient-years (476), and whether this applies to the other CGRP-based drugs and antibodies it is too early to tell. Thus, despite the relatively safe adverse effects profile, continued caution is warranted (484).
5.3.2. Protective roles of CGRP.
CGRP has protective activities in the cardiovascular system. The ability to protect against hypertension is not surprising given its vasodilator activity. However, this protection also extends to vascular hypertrophy and oxidative stress (485). Using a mouse model of sustained hypertension with cardiovascular remodeling, Brain and colleagues (385) showed that α-CGRP knockout mice had a worsened phenotype, indicating that endogenous α-CGRP is protective. Furthermore, exogenous CGRP rescued α-CGRP knockout mice from both hypertension and cardiovascular remodeling. These studies were extended with a novel, long-lasting (t½ ≥7 h) acylated α-CGRP analog, which was able to reduce cardiovascular disease phenotypes in two mouse models of hypertension and heart failure in vivo. The analog mediated antihypertensive effects, attenuated cardiac remodeling, and increased angiogenesis and cell survival (486). Similarly, the long-acting CGRP analog improved recovery from myocardial perfusion in rats following chronic occlusion of the coronary artery in rats (487).
At the cellular level, CGRP has multiple effects on vascular cells that are unrelated to vascular tone. These actions can be angiogenic, proliferative, antiproliferative, proinflammatory, or anti-inflammatory and may be protective from hypertensive and ischemic heart conditions (366, 390, 488). For example, independent of effects on vascular tone, CGRP treatment of cultured cells isolated from rat aortic smooth muscle led to decreased cell proliferation and increased apoptosis via cAMP-dependent pathways, effects that could potentially decrease restenosis after aortic angioplasty (489).
Interestingly, cardiac protection might be mediated by CGRP produced by cardiac fibroblasts (19). An autocrine role for fibroblast-derived CGRP was shown by its ability to suppress cardiac fibrosis in a rat model. Ironically, a TRPA1 trigger that is associated with increased CGRP release in rodent models of migraine also led to increases in CGRP levels and reduced fibrosis. Similarly, atrial cardiomyocytes have been suggested to have the potential to release calcitonin, which could then act in a paracrine manner to limit fibrosis that would likely lead to atrial fibrillation (490). Although this study focused on the actions of calcitonin, it is reasonable to speculate that CGRP would have similar effects on atrial fibrosis. Thus, these data indicate that CGRP has protective effects on the cardiovascular system beyond neuron-mediated vasodilation, and this involves autocrine and possibly paracrine actions in the heart.
5.4. Pulmonary System
5.4.1. Expression.
CGRP is present throughout the airway, and CGRP receptors are expressed on pulmonary artery cells, epithelial goblet cells, and submucosal gland cells (463) as well as innate lymphoid immune cells (491). CGRP-containing nerve fibers that innervate the airways originate primarily from DRG and nodose-jugular ganglia (492, 493). CGRP is also expressed locally in pulmonary neuroendocrine cells (494) (FIGURE 12). These neuroendocrine cells are epithelial cells that originate from basal stem cells and share many features with sensory neurons, including responding to environmental cues.
Figure 12.
Calcitonin gene-related peptide (CGRP) in the lung. Sensory nerves containing CGRP (black circles) lie within the submucosa, where they innervate blood vessels and the airway epithelium, including submucosal glands. CGRP is also released from pulmonary neuroendocrine cells (PNECs). Among the actions of CGRP is CFTR-dependent glandular fluid secretion that likely protects the airway from secondary infection, stimulation of mucus secretion from goblet cells, and stimulation of glandular stem cell progenitors, such as basal cells, to proliferate to become transient amplifying cells that help ameliorate epithelial injuries. Image created with BioRender.com, with permission.
5.4.2. Functions.
CGRP can induce both serous secretions from submucosal glands (495) and mucus secretion from goblet cells (496) (FIGURE 12). This dual activity, along with complexities of immune modulation within the lung, may help explain some of the controversy as to whether inhibiting CGRP will have beneficial or detrimental pulmonary consequences (493, 496). Indeed, the answer is still not clear, and examples of both paradigms are given below.
There is good evidence that elevated CGRP plays a protective function in the lung. It causes vasodilation of pulmonary arteries and protects against hypoxia-induced tissue remodeling in human pulmonary hypertension (497). Similarly, gene transfer of CGRP into the lungs of mice protected against pulmonary resistance by cAMP-mediated vasodilation (498). Importantly, CGRP increases CFTR-dependent secretion of watery mucus and promotes the differentiation of lung stem cells to ameliorate the pulmonary effects of cystic fibrosis (495) (FIGURE 12). Recently, CGRP was shown to help mitigate hypoxic damage to lung epithelial cells (499). The functional role of CGRP was shown by ablation of CGRP-expressing neuroendocrine cells, which increased hypoxia-mediated epithelial injury that could be rescued by CGRP administration. Consequently, in cases of hypoxia, airway stem cells respond by proliferating and differentiating into neuroendocrine cells that secrete CGRP. This suggests that the elevated neuroendocrine cells and elevated CGRP reported in lung pathologies, such as asthma and cystic fibrosis, is not causing damage but rather is a compensatory response to ameliorate the damage (499). Thus, an elevation of CGRP after airway injury can stimulate secretions, stem cell proliferation, and protection against injuries.
However, in contrast to a protective function, the elevated CGRP levels found in the lungs of patients with respiratory diseases have been fodder for the argument that CGRP is detrimental to pulmonary functions. A recent study using transgenic mice that mimic neuroendocrine cell hyperplasia of infancy, a rare childhood disease, found that the mice had excess fluid in their lungs, increased endothelium permeability, and hypoxemia, which is similar to the more common condition of acute respiratory distress syndrome (500). In this model, CGRP receptor antagonists were beneficial for pulmonary functions, including oxygen exchange. One interpretation of these apparently contradictory findings is that CGRP is beneficial, but when in large excess, it appears that too much of a good thing may be detrimental to the lung.
5.5. Immune and Hematopoietic Systems
5.5.1. Expression and functions.
Both CGRP and receptors are widely distributed in the immune system. Sensory fibers containing CGRP are in the bone marrow, thymus, spleen, lymph nodes, and skin, as well as near immune cells in the lungs and gut (501). In addition to being synthesized by nerves, CGRP is produced by immune cells. These include lymphocytes, monocytes, and macrophages (501). The relative contribution of neuron- versus immune-generated CGRP is not known. Receptors for CGRP are found on many immune cells, including T and B lymphocytes, macrophages, mast cells, and dendritic cells (501). Indeed, within the bone marrow CGRP can act on hematopoietic progenitors (502) to increase the pool of stem cells described below. Also notable is the fact that CGRP not only acts locally at receptors on immune cells but additionally transmits information to the CNS from immune-related organs (501).
CGRP has both pro- and anti-inflammatory activities within the immune system (501, 503). Of note, immunomodulation by CGRP has been reported in preclinical models of diabetes (504), sepsis (505), experimental autoimmune encephalomyelitis (506), Crohn’s disease (507), and colitis (508–510). For a review of CGRP functions in the immune system, see Ref. 501.
The actions of CGRP in the immune system are also relevant to the skin, where it affects not only a host of immune cell types but also a variety of epidermal cell types such as keratinocytes, melanocytes, and fibroblasts (511). In vitro, CGRP promotes division of human epidermal stem cells (512), and in skin models it leads to enhanced keratinocyte proliferation and an increase in thickness of the epidermis, demonstrating a role for CGRP in modulating epidermal morphogenesis and keratinocyte growth (513). As such, CGRP is believed to help with wound repair. However, it has also been linked to several skin disorders, ranging from rosacea (see sect. 7.3) to atopic and contact dermatitis (511).
5.5.2. Mast and other immune cell activities.
Among the interactions of CGRP with immune cells, those with mast cells are the best studied. The CGRP connection to mast cells has been explored mostly for its potential relevance to migraine and was recently reviewed (514). CGRP causes degranulation of dural mast cells and the release of proinflammatory cytokines and inflammatory agents such as histamine. This leads to peripheral sensitization of nearby sensory neurons. Of note, one study reported that human mast cells lack CGRP receptors, whereas another that is more recent and used a different antibody indicates that primate mast cells do have canonical CGRP receptors (194). Furthermore, human and rodent dural mast cells have MRGPRX2/B2 receptors that can bind peptides, such as substance P (515) and pituitary adenylate cyclase activating peptide (PACAP) (516), and hence may possibly bind CGRP. These receptors and possibly the AMY1 receptor provide potential mechanisms for CGRP activation of meningeal mast cells. In this regard, the interactions of CGRP with mast cells in other tissues, especially in the GI system, are likely to be very important (501). Although we have focused on CGRP and mast cells in the meninges, the actions of CGRP on other immune cells in the meninges and elsewhere are likely to be equally important. Macrophages, dendritic cells, B cells, and various T cells are all present (517–519) and shown to be resident within the meninges (520). Given that all these cell types have CGRP receptors, it will be interesting to learn whether CGRP has physiological and/or pathological actions on these cells.
In particular, the effects of CGRP on T cells have been well documented. The connection between CGRP and T cells was first proposed in 1988 (521) and has been reviewed in all its complexities (501). Overall, CGRP seems to promote a TH2-type immunity, leading to increased production of IL-4 and decreased TH1-associated cytokines interferon-γ (IFN-γ) and IL-2. Ganor and colleagues (522–524) found that CGRP has antiviral activity that inhibits the transfer of human immunodeficiency virus type 1 (HIV-1) to T cells. This inhibition is mediated by CGRP receptor-mediated activation of a noncanonical NF-κB/STAT4 signaling pathway on mucosal antigen-presenting Langerhans cells, and this in turn leads to viral degradation and blocks conjugation of these cells with T cells. This subsequently causes degradation of the virus in proteasomes, ultimately reducing HIV-1 infection of T cells (522). In summary, CGRP in the immune system is a ripe area for future therapeutic discoveries.
5.5.3. Hematopoietic stem cells.
CGRP receptors are found on mouse bone marrow cells, which suggests a direct role for CGRP modulation of immune cell differentiation (525). Hematopoietic stem cells stay in the bone marrow until recruited to proliferate and migrate out of their stem cell niche. The role of CGRP in hematopoiesis in response to stress signals, such as those released in response to tissue injury, bleeding, and inflammation, was shown with RAMP1 knockout mice. The enhanced hematopoiesis induced by CGRP reduced the number of immature hematopoietic cells and increased the numbers of granulocytes, monocytes, and lymphocytes, all of which could contribute to resolution of the stress and/or the anti-inflammatory responses (526).
Recently it was found that CGRP from nociceptor neurons drives the mobilization of hematopoietic stem cells in response to granulocyte colony-stimulating factor (G-CSF) (527). CGRP acts directly on the stem cells, via a cAMP-mediated pathway, to promote migration from the stem cell niche. Targeting of CGRP could therefore affect the yield of hematopoietic stem cells. In addition, an unexpected action of CGRP on hematopoietic cells was observed in a mouse model of sickle cell disease in which CGRP appears to protect against vaso-occlusive episodes and chronic organ damage (528). Thus, therapeutic agents that increase CGRP may improve procedures for stem cell transplantation and sickle cell disease treatments.
In conclusion, CGRP plays important roles in immune functions, not only as a means of relaying information to the CNS but also as a local mediator of immune responses. These responses have been best characterized in dendritic, mast, and T cells, yet the presence of CGRP receptors on other cells, such macrophages, suggests that we have only seen the tip of the CGRP iceberg of immunomodulatory actions. These diverse functions extend even to amplification of bone marrow stem cells. Thus, the potential impact of CGRP in the immune system must be kept in mind when evaluating possible complications from CGRP inhibition (FDA 2020, https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/april-june-2020-potential-signals-serious-risksnew-safety-information-identified-fda-adverse-event; Ref. 529).
5.6. Metabolic Effects of CGRP in Adipose and Muscle
There is now a reasonable body of evidence to support a role for CGRP in several aspects of metabolism (530). At the whole organism level, several studies have administered exogenous CGRP and monitored food intake, body weight, and other related parameters. For example, intraperitoneal injection of C57BL/6 mice with CGRP led to reductions in food intake and total energy expenditure (531). A CGRP analog that is modified to have a prolonged half-life led to increased energy expenditure and reduced food intake in normal rats and had positive metabolic effects in diet-induced obese rats and type 2 diabetic ob/ob mice (532). Other studies similarly showed that either central or peripheral administration of CGRP reduced food intake (434, 533–535). One might expect that CGRP knockout mice would have the opposite phenotype, yet α-CGRP knockout mice have reduced weight gain and increased energy expenditure (530, 536–538). A similar phenotype was observed in adult mice in which sensory neurons had been ablated and in which CGRP activity was pharmacologically reduced with receptor antagonists or a CGRP antibody (539–541).
Several mechanisms could account for these effects of CGRP. Interrogation of neural circuitry has suggested that CGRP neurons might control meal termination (542, 543). Peripherally, CGRP affects muscle function. It is present at the neuromuscular junction, where it can modulate the actions of acetylcholine (544–549). CGRP has been proposed as an “exerkine” because of association between CGRP levels in the plasma or muscle and exercise or the degree of cardiorespiratory fitness (465, 550, 551). Furthermore, CGRP is known to affect muscle lipolysis. Rat soleus muscle isolated from rats that was incubated with rat α-CGRP caused a biphasic increase in muscle free fatty acid (FFA) concentrations, with a concomitant decrease in muscle triglyceride levels (552). CGRP also causes lipolysis in adipose tissue and adipocyte cell models (530, 537, 539, 553, 554). From a biological perspective it is puzzling why a single peptide is associated with opposing sides of the metabolism coin. Distinct central and peripheral mechanisms might be at play. A recent review highlighted the interconnectivity between migraine circuits and food intake (555) as described in sect. 7.7.
5.7. Gastrointestinal System
A link between CGRP and the GI system was suggested by the very first report on the distribution of CGRP (556). CGRP is expressed in CNS nuclei involved with ingestive behavior and in extrinsic sensory neurons innervating the GI tract and intrinsic neurons of the enteric nervous system (557) (FIGURE 13). The enteric nervous system includes a rich network of CGRP-containing nerves in both the myenteric and submucosal plexuses. CGRP is also located in immune cells of the GI tract. CGRP receptors in the GI tract are present at various locations, including the vasculature, muscle layers, mucosa, endocrine cells, immune cells, and nerves. CGRP has several GI functions described below, but most notably it increases intestinal motility (peristalsis) and secretion (ions and water).
Figure 13.
Extrinsic and intrinsic calcitonin gene-related peptide (CGRP)-containing neurons of the intestine. Extrinsic sensory neurons from the dorsal root ganglion (DRG) contain primarily α-CGRP and innervate all layers of the intestinal wall: intrinsic enteric neurons in myenteric and submucosal plexuses, blood vessels in the submucosal plexus, circular and longitudinal smooth muscle layers, and the mucosa, including microfold (M) cells in Peyer’s patches and mucus-producing goblet cells in the colon. Intrinsic enteric neurons contain primarily β-CGRP and are found in both the myenteric and submucosal plexuses. Both α- and β-CGRP neurons innervate the blood vessels. Image created with BioRender.com, with permission.
5.7.1. Expression of α-CGRP and β-CGRP.
Both α-CGRP and β-CGRP are present in the GI tract, although they are expressed by different sets of neurons (FIGURE 13). α-CGRP is predominantly in the sensory fibers from the DRG and vagal ganglia, whereas β-CGRP is predominantly in the intrinsic enteric neurons (31). β-CGRP-positive neurons have also been found in the adventitia of rat mesenteric branch arteries (558). Thus, both α- and β-CGRP neurons innervate the blood vessels as well as muscular and mucosal layers throughout the GI tract.
5.7.2. Functions.
In general, antagonist and knockout mouse studies suggest that CGRP plays a protective role in the GI system. For example, treatment with the antagonist CGRP8-37 worsened ulcerative colitis (508) and increased mucosal lesions caused by toxins (indomethacin or ethanol); in CGRP knockout mice, the healing of gastric ulcers was delayed; and agents that promote CGRP release (TRPV1 agonists) protect the GI mucosa (559, 560). Furthermore, knockout of either α-CGRP (Calca) or β-CGRP (Calcb) revealed a protective role for CGRP in experimentally induced colitis (dextran sulfate sodium salt) (510). Although another study using the same colitis model but different Calca knockout mice found no differences from wild type (561), when the same research group used another colitis model the results supported heightened susceptibility of the Calca knockout mice to injury (562), supporting an overall anti-inflammatory, and thus protective, role for CGRP in the colon. Given that CGRP is involved in multiple physiological GI processes including motility, secretion, and immune functions (563), it is not surprising that altered CGRP activity can contribute to dysmotility and disruption of the mucosal barrier (560). Yet CGRP actions in the GI system are complex, as illustrated by an RNA interference (RNAi)-mediated knockdown of the CLR subunit of the CGRP receptor that changed but did not prevent CGRP actions on motility and inflammation (564). Furthermore, as discussed in sect. 7.7, it has been suggested that CGRP receptor antagonists might be useful as spasmolytic, antidiarrheal, anti-inflammatory, and antinociceptive drugs for inflammatory bowel disease (565). To address this complexity, we have divided the physiological functions of CGRP in the GI system based on motility, secretion, and mucosal homeostasis.
5.7.2.1. motility.
The muscle layers of the GI tract contain both intrinsic and extrinsic CGRP fibers that can facilitate peristaltic movements and churning (566). However, the effects of CGRP on GI motility are complex. Whereas in the stomach CGRP inhibits gastric emptying (gastroparesis) (567), in the intestines CGRP had heterogeneous effects (both stimulatory and inhibitory) on intestinal motility (568), whereas other studies have reported primarily stimulatory effects (569). The complexity of CGRP actions is further illustrated by an in vitro study reporting that CGRP caused contraction of mouse intestinal longitudinal muscle that was preceded by a transient inhibition and that a very high concentration (10−6 M) only inhibited contraction (570).
CGRP promotes peristalsis by two mechanisms: a direct relaxant effect on longitudinal and circular muscles and indirect effects involving the release of other neurotransmitters and consequent relaxation or contraction of those muscles (571). This was shown with the use of the CGRP receptor antagonist CGRP8–37, which inhibited both ascending contraction and descending relaxation of isolated intestinal muscles during the peristaltic wave (572). CGRP from both extrinsic sensory nerves and intrinsic enteric neurons appears to modulate peristalsis (572, 573). Thus, blocking CGRP actions is predicted to slow transit time through the intestine, consistent with clinical reports of constipation in some migraine patients treated with the CGRP blockers (574, 575). A recent study reported that intestinal transit rates were decreased in transgenic mice expressing human RAMP1 by treatment with CGRP receptor antibodies or CGRP receptor small-molecule antagonists, but not CGRP antibodies, which suggested the possibility of opposing actions of CGRP at different receptors (339). On the other hand, a phase IV clinical trial that measured motility in patients before and after treatment with a CGRP antibody or CGRP receptor antibody failed to find statistically significant differences in transit times (576). Nonetheless, it is clear that elevated release of CGRP from intestinal neurons, for example during an inflammatory response, could contribute to increased intestinal motility and diarrhea, as has been observed in mice (577) and humans (578).
5.7.2.2. secretion.
The effects of CGRP on secretion have been studied in the stomach and intestines. Early studies established that CGRP acts as a potent inhibitor of gastric secretions by releasing somatostatin from D cells, which in turn inhibits the release of gastrin (579–581). It should be noted that only β-CGRP was reported to inhibit gastric acid secretion in humans, which was apparently independent of somatostatin (582). In the colon, CGRP has biphasic stimulatory and inhibitory actions (583). The antisecretory effect at low concentrations is most likely mediated by CGRP-induced somatostatin release. Thus, CGRP elicits both indirect antisecretory and direct secretory effects in the colonic epithelia.
An understudied mechanism by which CGRP is likely to stimulate fluid secretion in the intestines may involve the CFTR ion channel. Studies in the lung demonstrated that CGRP induces CFTR-dependent chloride current in airway epithelia and increased CFTR-dependent secretions in the submucosal glands (495). Whether CGRP activates CFTR in the intestines remains to be established, but it seems likely because in studies predating the discovery of either CFTR or the CGRP receptor CGRP was reported to stimulate chloride secretion from colonic epithelia (584). Such a mechanism would be consistent with CGRP increasing intestinal fluid secretion and diarrhea.
5.7.2.3. mucosal homeostasis.
An important GI role of CGRP is mucosal homeostasis by both modulation of blood flow and direct actions on mucosal cells involved in the balance of bacteria in the mucosa. In the GI tract, CGRP-containing nerve fibers surround mucosal and submucosal blood vessels that express CGRP receptors (585, 586). Functionally, CGRP has been shown to be a potent vasodilator that increases mucosal blood flow in the stomach of rats by a mechanism involving local release of NO (587). Increased mucosal blood flow stimulated by CGRP release has been shown to protect against disruption of the mucosal barrier (560, 588, 589). The protective role of CGRP against acute GI injuries has been reviewed (590).
Beyond blood flow, neuropeptides such as CGRP influence the microbiome at the mucosal interface in health and disease, including migraine (591, 592). A major advance in understanding the role of CGRP in the gut-brain axis is the recent finding that CGRP can influence the balance of commensal and pathogenic bacteria in the mucosa (593). In response to Salmonella, nociceptive DRG neurons that innervate the intestine can modulate the microbiota to resist infection by releasing CGRP into the mucosa. CGRP then strengthens the epithelia at Peyer’s patches by suppressing microfold (M) epithelial cells and supporting filamentous host bacteria to reduce Salmonella infection. In addition, CGRP plays an important role in GI mucosal homeostasis by acting on various immune cell types that are responsible for GI inflammation. CGRP reduces gastric inflammation by inhibiting dendritic cells, neutrophils, and macrophages while upregulating the release of the cytokine IL-10 (594). Most recently, the protective actions of CGRP released from nociceptive neurons were expanded to include mucus production by goblet cells that strengthens the gut mucosal barrier and helps protect against colitis (595). Conversely, CGRP may cause gastric inflammation by stimulating T cells and promoting the release of proinflammatory cytokines such as IFN-γ. Nonetheless, the weight of the evidence suggests that CGRP protects the integrity of the GI mucosa (589). These protective functions of CGRP set the stage for the implications of therapeutic modulation of CGRP.
5.8. Reproductive System
5.8.1. Females.
CGRP effects in the female reproductive tract are overall beneficial for fetal and maternal health (596). CGRP along with the related AM and AM2 peptides play multiple roles in reproductive biology (596, 597). These include fetal-maternal vascular circulation, implantation, uterine receptivity, placentation, and endometriosis. CGRP is naturally elevated during pregnancy in humans (598) and in both maternal and fetal circulation during late pregnancy in rodents, where it is proposed to regulate utero/placental blood flow and other vascular changes (599). CGRP decreases vascular tone during pregnancy in a mechanism that may be mediated in part by a sex steroid-induced increase in the vascular sensitivity to CGRP (600) and increased levels of the CGRP receptor during pregnancy (601). The mechanism of the increased sensitivity of the human female uterine artery to CGRP, AM, and AM2 has recently been shown to involve a complex interplay of NO and endothelium-derived factors (602).
Consequently, CGRP appears to help maintain the low fetoplacental vascular resistance in normal human pregnancies, and hence an inadequate CGRP vasodilatory response may contribute to the pathophysiology of hypertensive disorders during pregnancy, such as preeclampsia (603). The importance of this CGRP-dependent vascular relaxation is highlighted by the finding that vessels from human preeclamptic placentas have decreased CGRP receptors and decreased relaxation in response to CGRP, suggesting that CGRP-dependent vascular relaxation may be compromised in preeclamptic pregnancies (604). This concern is validated by a finding that near-term infusion of a CGRP receptor antagonist (CGRP8-37) in pregnant rats reduced the weight of pups and increased the mortality rate. This suggests that endogenous CGRP may play a potent vasodilator role in maintaining normal fetoplacental development, survival, and vascular adaptations during pregnancy (605). In addition to vasodilation of uterine arteries, CGRP can also relax the uterine muscle (596), indicating a role of CGRP in both vascular adaptations and uterus tension during pregnancy.
Hence, a serious concern is whether prolonged attenuation of CGRP actions by the monoclonal antibodies or receptor antagonists (gepants) may affect pregnancy (480). This is of special importance, as anti-CGRP therapies are being increasingly used by female migraine patients during their reproductive years. Although there is a paucity of clinical safety information on these drugs during pregnancy, a recent review has summarized preclinical studies and the status of patient reports (606). Preclinical studies, beginning with the monoclonal antibody erenumab (607), are encouraging since none of the CGRP agents caused observable developmental effects at clinical doses, although some effects were observed at extremely high doses. Although there has not yet been a human pregnancy clinical trial, the World Health Organization has maintained a database of suspected adverse reactions following maternal exposure to the monoclonal antibodies before or during pregnancy (606). The incidences of miscarriage and congenital anomalies were similar to rates in the general population, which is reassuring so far. In addition, there were no adverse effects in two case reports. Although it is hoped that there will be no negative long-term effects of these medications during pregnancy, there should be continued caution about long-term antagonism of CGRP until longitudinal safety data are obtained.
5.8.2. Males.
In the male reproductive system, sensory nerve fibers containing CGRP are present in rat penile tissue (608), although the presence of receptors and the functional significance is less clear. CGRP likely regulates testicular blood flow (609), and its reported regulation of electrolyte and fluid secretion in the epididymis may help with maturation and storage of spermatozoa (610). CGRP can also cause penile erection in cats (611) and possibly humans (612).
6. CGRP IN MIGRAINE
6.1. What Is Migraine?
Migraine is more than just a bad headache. It is a neurological disease characterized by episodic attacks of headache and associated symptoms (132, 613, 614). Migraine often begins with premonitory signs, such as yawning, that precede the headache. In ∼20–30% of patients, there is also an aura, a transient set of neurological symptoms that most often affect vision. These early symptoms are thought to originate from effects in the hypothalamus, brain stem, and cortex (381, 615). The headache that develops is characterized by generally moderate to severe pain, throbbing, localization to one side of the head, and intensification with movement. It is associated with nausea, vomiting, and abnormal sensitivity to light (photophobia) and to noise (phonophobia). It can also be accompanied by heightened sensitivity to odors (osmophobia) and to touch (allodynia). Vestibular symptoms of dizziness or vertigo can also be present. Whether sensitivity to internal body signals (interoception) is also increased has not been studied. Collectively, the symptoms that accompany migraine from the premonitory stage through the headache phase suggest that multiple systems in the CNS function abnormally during an episode (615–617).
6.2. Evidence for CGRP Actions in Migraine
Over the past three decades, CGRP has been fully documented to be a key player in migraine pathogenesis (24, 392, 468, 618). Briefly, three lines of clinical evidence described below support this conclusion.
6.2.1. Elevated CGRP.
The first indication that CGRP is involved in migraine was a pioneering study in 1990 by Edvinsson and Goadsby, who discovered that CGRP levels in the jugular outflow were elevated during migraine attacks (619). Importantly, this elevation in CGRP levels was reduced by sumatriptan, coincident with pain relief (133). Since these initial discoveries, elevated CGRP has been detected in several bodily fluids: saliva (620), and tears (621) during and between acute attacks and plasma of chronic migraine patients between attacks (622). The increases in CGRP levels in saliva are counteracted by the administration of a triptan (623), and those in tears and plasma are also counteracted by treatments (621, 624). A recent small but thorough month-long longitudinal study of episodic migraine patients found that CGRP levels in the saliva were elevated between attacks and were even higher during the headache, although elevated CGRP was not observed in all patients (625), and further studies are certainly needed (626). CGRP was also reported to be elevated in both the cerebrospinal fluid (CSF) (627, 628) and internal jugular vein blood (629) of chronic migraine patients. It should be noted that CSF and internal jugular blood CGRP measurements do not necessarily reflect CGRP release from the brain because rodent studies have shown that these fluids may also include CGRP released from trigeminal afferents in the pia (630) and to a small degree even from the dura (63). However, these findings of elevated CGRP are contradicted by other reports of a lack of CGRP elevation (ictal or interictal) during episodic or chronic migraine (631–634), as summarized in Refs. 635, 636. Thus, although CGRP levels are likely to be elevated in migraine, it remains unclear whether that elevation can consistently be detected and thus serve as a reliable biomarker of migraine (637, 638). A recent review provides a detailed summary of clinical studies that have measured CGRP in various body fluids and some of the associated methodological challenges (639).
There are many reasons that may account for these discrepant findings, including differences in the severity of the attack, technical challenges of measuring CGRP, and the fact that migraine is a heterogeneous disorder that is not likely to always involve elevated CGRP. In addition, there is the conceptual challenge that CGRP in the blood, saliva, or tears is not a direct measure of CGRP at its presumed sites of action (e.g., perivascular fluid, meningeal fluid, intraganglionic interstitial fluid) but rather is only representative of those levels. CGRP is not believed to have any endocrine activity, in part based on its fairly rapid biexponential degradation in human plasma (fast decay 7-min and slow decay 26-min half-life) (21). Hence, CGRP measured in clinical studies is diluted from sites of release and is unstable, which confounds accurate measurements. Furthermore, the current lack of standardized methods for data collection and sample processing is a problem (640). A recent study is a step in the right direction for describing the protocol and outlining the pitfalls of a particular assay, and more studies like this are necessary (641). In addition to migraine, CGRP has been proposed to be a biomarker for other disorders and conditions (642, 643), but, as with migraine, the data have not been persuasive.
6.2.2. CGRP induction of migraine.
The second line of clinical evidence supporting a strong causal link between CGRP and migraine came from Olesen and colleagues (644), who reported that intravenous injection of CGRP caused moderate to severe headaches that often met the criteria for experimentally induced migraine. Subsequent studies confirmed this groundbreaking finding. On average, ∼65% (range of ∼50–77% across studies) of the patients developed a migraine-like attack and ∼84% (79–100%) developed a delayed headache with or without the other migraine symptoms (644–648). In contrast to patients, control subjects developed only an immediate mild headache, characterized by a fullness-of-head feeling (646, 649). These findings suggest that migraine patients are more sensitive to CGRP, and they served as the rationale for generating CGRP-sensitized transgenic mice for preclinical studies (441, 443), although it should be noted that there was no difference in human peripheral microvasculature between migraine and control subjects (650). Furthermore, CGRP-induced migraines were reversed by administration of a triptan (645). However, CGRP infusion was not effective in familial hemiplegic migraine 1 (FHM1) patients (651, 652) and did not induce an aura (646) or prodrome (647). This indicates that, not surprisingly, other factors in addition to CGRP are involved in migraine pathogenesis.
6.2.3. Efficacy of CGRP-based therapeutics.
The third and most persuasive line of evidence in support of a role for CGRP in migraine is the clinical efficacy of at least seven drugs that target CGRP. CGRP-blocking drugs have been approved by the FDA for use in both acute and preventative treatments. Acute treatments are designed to relieve or stop the progression, or the pain and impairment, of an attack once it has begun. Preventative therapy is given, even in the absence of a headache, to reduce the frequency and severity of anticipated attacks. Until the development of CGRP-based therapeutics, acute and preventative drugs fell into two distinct categories, i.e., triptans were only effective for acute treatment and not as preventative drugs. Now, CGRP-blocking drugs can be given to either prevent an attack or stop an ongoing attack, which further emphasizes the importance of CGRP in both the initiation and maintenance of migraine pathology.
A major advance in the development of CGRP-based therapeutics for migraine was the decision by four pharmaceutical companies to generate monoclonal antibodies against either the CGRP receptor or the CGRP ligand. The rationale for using antibodies was the desire to develop a better preventative drug, especially in light of the reported liver toxicity in some patients following repeated treatments with the first generation of small-molecule CGRP receptor antagonists (telcagepant, MK-3207) (653, 654). The efficacy of telcagepant and another early drug, olcegepant (655), in clinical trials provided the proof of principle that blocking CGRP could stop a migraine, yet a strategy to avoid liver detoxification pathways was needed. Thus, monoclonal antibodies were developed since they are cleared by a different mechanism of nonspecific endocytosis in cells and proteolysis in the liver and reticuloendothelial system that avoids the liver toxicity issue. Four monoclonal antibodies (eptinezumab, erenumab, fremanezumab, galcanezumab) are approved for migraine prevention. Erenumab targets the CGRP receptor, and the others target CGRP. Although not designed as an acute treatment, the fast delivery of eptinezumab by intravenous infusion can also treat an existing migraine attack within 0.5–1 h (656).
More recently, a suite of small-molecule CGRP receptor antagonists, which are generically referred to as gepants, has been developed that complement the monoclonal antibodies. At present two gepants (rimegepant, ubrogepant) are approved for the acute treatment of migraine, and two have been approved for preventative treatment (atogepant, rimegepant). The gepants are taken orally, although a gepant that can be given as a nasal spray [zavegepant (BHV-3500)] is in clinical trials. For all these treatments, it is important to keep in mind that the antibodies and gepants most likely only reduce, rather than block, CGRP actions (657), because of the bolus release of peptides (sect. 1.4.2), and if the agents are not fully blocking both receptors (sect. 4.3.2), this could also play a role.
6.3. CGRP and Sex Bias of Migraine
An unanswered question is whether CGRP contributes to the pronounced sex bias of migraine in females. Starting during puberty, migraine occurs in women three to four times more often than in men (658). This bias decreases after menopause, which suggests that fluctuations in ovarian steroid hormones, especially estrogen and progesterone, play a role. Although a number of studies have attempted to identify a link between CGRP and sex hormones, a clear picture has yet to emerge from preclinical or clinical studies (658, 659). A recent report that dural application of CGRP causes facial allodynia only in female rodents is very exciting (660). Other studies involving systemic or intraganglionic administration of CGRP in rodents have reported that both sexes are affected (48, 441, 442, 444, 661, 662), yet in some of these the effects were more pronounced in female rodents. For example, when the sensitive automated grimace assay was used, a squint response was seen only in female mice at the lowest dose of CGRP (48). The discrepant sex results could potentially be explained by CGRP having multiple sites of action (e.g., in the dura and the TG) and the dura being more responsive to CGRP in females and the TG being responsive in both males and females. Future studies on sex-dependent CGRP actions are likely to provide new insights into migraine mechanisms.
6.4. Potential Sites and Mechanisms of CGRP Action in Migraine
Despite the clear proof that CGRP is involved in migraine, we still do not know the site(s) of action of either CGRP or the drugs (392, 393, 618, 663, 664). Coupled with the question of site of action is that of how CGRP causes migraine. In short, the answers for both questions are not fully known. However, we believe that the evidence points to CGRP acting at multiple sites, both within and outside the CNS. Ironically, this uncertainty, at least with respect to the sites of drug action in migraine, is not limited to CGRP; although triptans have been used for >20 years, their sites of action remain controversial (665). Both peripheral and central CGRP mechanisms are plausible.
6.4.1. Peripheral CGRP actions.
It is very likely that CGRP acts in the periphery in migraine (177, 405, 664, 666–668). Most notably, peripheral inhibition of CGRP by antibody therapies is sufficient to treat migraine in many patients, even though only a very small amount of antibody can cross the blood-brain barrier in rodents (669, 670). However, caution must be exercised in ascribing a solely peripheral site of action of the antibodies. First off, the circumventricular organs of the brain lack a blood-brain barrier, and at least one of these sites, the area postrema, is able to bind CGRP (FIGURE 5). Second, human imaging and sensory reflex and evoked responses have shown that erenumab affects the CNS (671, 672). Whether this is because of a direct effect of the small amount of antibody in the CNS or to an indirect effect due to peripheral modulation is an open question. In addition, peripherally administered CGRP can cause migraine in many patients, as described above. In this regard, preclinical light aversion data with transgenic mice indicate that CGRP acts by different mechanisms in the CNS than in the periphery, suggesting at least in mice that peripherally administered CGRP acts in the periphery and is not directly acting in the CNS (444).
The generally agreed-upon site of peripheral CGRP action in migraine is the trigeminovascular system, including the meninges, TG, and nodes of Ranvier along trigeminal fibers (132, 408, 673–675) (FIGURE 14). Trigeminal primary afferents innervate pial and dural meningeal vessels, and efferent projections synapse with second-order neurons in the trigeminocervical complex from the C3 dorsal horn to the caudal and interpolar parts of the spinal trigeminal nucleus (676, 677). Secondary neurons in the trigeminocervical complex project to the thalamus, where ascending input is integrated and relayed to higher cortical areas. Parasympathetic output from the sphenopalatine ganglia also contributes to the system via reflex communication with the TG (678).
Figure 14.
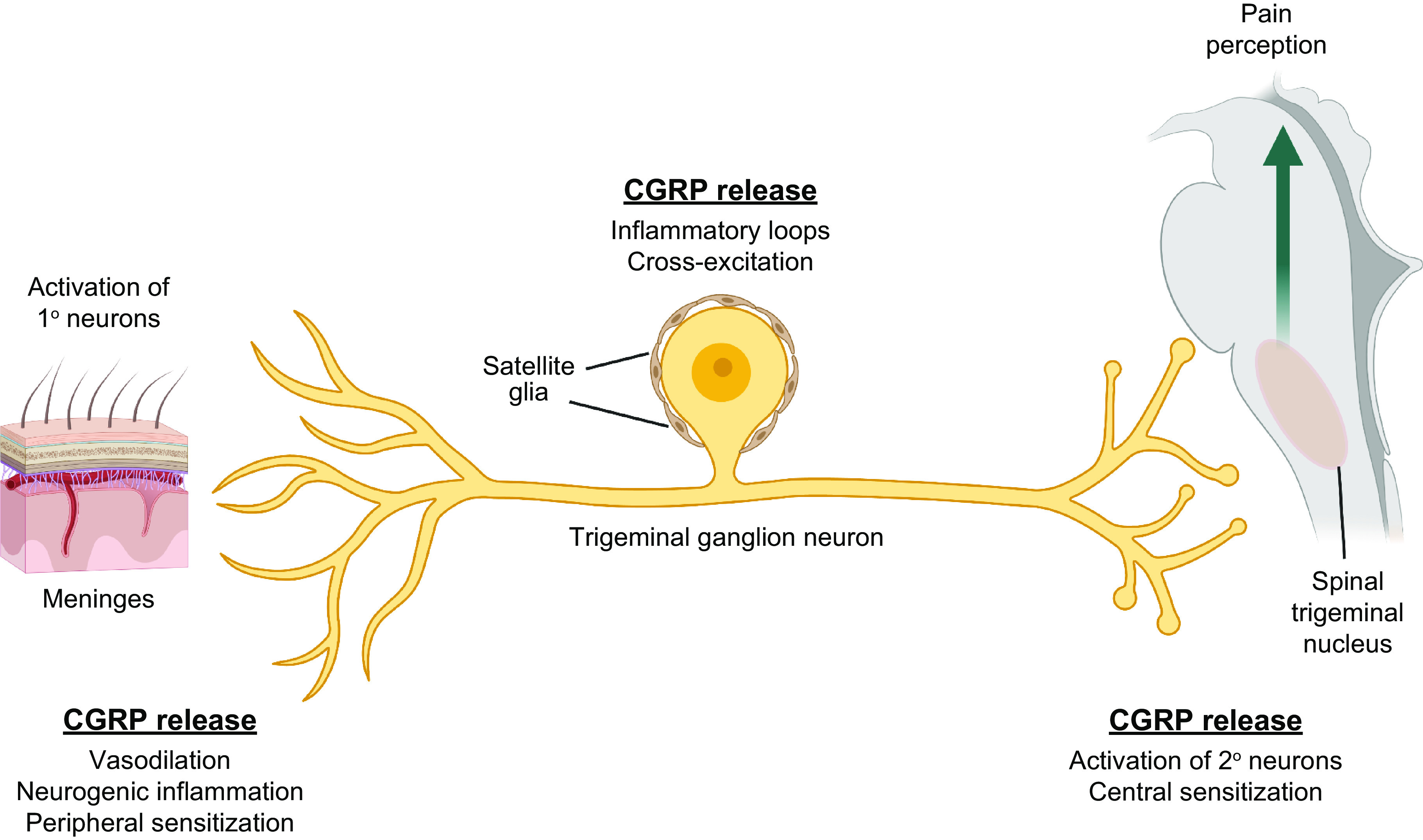
Calcitonin gene-related peptide (CGRP) in the trigeminovascular system. CGRP acts at 3 distinct regions within the trigeminovascular system. Upon activation, afferent trigeminal fibers release CGRP in the dura and pia layers of the meninges. CGRP actions on blood vessels, resident immune cells, glial Schwann cells, and trigeminal fibers can cause vasodilation and neurogenic inflammation and potentially lead to further CGRP release and peripheral sensitization. Within the ganglia, CGRP can act on satellite glia and neural cell bodies to initiate inflammatory loops and cross-signaling that could further CGRP release and excitation of nociceptive cell bodies. In the spinal trigeminal nucleus, CGRP modulates glutamatergic signals that can lead to activation of second-order neurons and central sensitization. These second-order neurons project to higher brain regions, leading to pain perception. Image created with BioRender.com, with permission.
Activation of the trigeminovascular system most likely involves peripheral mechanisms, including local release of inflammatory mediators and agents released during neurogenic inflammation (674, 679). However, during cortical spreading depression (CSD) trigeminovascular activation can also be initiated by central mechanisms, possibly by inflammatory signals (678, 680, 681). To be clear, the role of inflammatory signals is not proven in humans, and given the episodic nature of migraine the inflammatory signals must be transient, except perhaps in chronic migraine (674, 682). With respect to cellular targets in the trigeminovascular system, Schwann glial cells have recently been shown to be an important target of CGRP. With cell-selective deletion of the RAMP1 gene, it was shown that trigeminal CGRP acts on surrounding Schwann cells to cause periorbital mechanical allodynia via endosomal signaling involving NO, reactive oxygen formation, and activation of TRPA1 channels (371). Thus, this CGRP pathway involving glial cells initiates a feedforward mechanism that sustains allodynia associated with neurogenic inflammation, akin to the TG mechanisms described in sect. 5.2.2.2.
6.4.1.1. ROLE OF THE VASCULATURE?
A question that cannot be ignored is whether the vasodilatory role of CGRP on meningeal vessels is important in migraine. The prevailing view is that migraine is a neural disorder, as opposed to a vascular disorder, and that any vascular changes should be considered an epiphenomenon (683–685). Clearly, migraine is a neural disorder, yet it seems premature to rule out a vascular component (645). Notably, like CGRP, other vasodilators (e.g., NO, PACAP, VIP) can induce delayed migraine-like headaches when injected into people. Although a causal role for vasodilation from these studies was initially discarded (686), an evolving perspective is that perhaps it is not a coincidence that so many migraine-inducing agents are vasodilators (613). In this regard, the possible roles of meningeal and cerebral vasculature in migraine have been recently reviewed (514) and the evidence for and against neural and vascular models has been well debated (687–689), with recent human studies resurrecting the debate (690, 691). Here we focus on how the two models may not be mutually exclusive.
As proposed by others, we suggest that vascular actions of CGRP in migraine may extend beyond just vasodilation (390, 488, 692). In this scenario, CGRP and other vasoactive triggers of migraine are proposed to act on the vascular endothelium and smooth muscle that initiate bidirectional communication between vessels and neurons independent of changes in vascular tone. A recent preclinical study attempted to directly test the role of vasodilation in CGRP actions by measuring light-aversive behavior (analogous to photophobia) in mice after systemic blood pressure was clamped by coadministration of vasoconstrictors (693). Perhaps not surprisingly, the results were complicated, varying by constrictor. Collectively they indicate that vasodilation is not required for a migraine-like symptom, with the caveat that systemic changes may not reflect meningeal vessel tone. However, the moderating effects of some vasoconstrictors on CGRP-induced light aversion indicate that although changes in vascular tone are not required they might be a contributing factor. Thus, we propose that whereas vascular events alone are not necessary to induce migraine symptoms, they may contribute, by a combination of mechanisms, to the modulation of nociceptor signaling to the brain stem.
6.4.2. Central CGRP actions.
Although the evidence in support of peripheral sites of CGRP and drug action is strong, it does not rule out central actions. For example, small amounts of peripherally administered CGRP or drugs can enter the CNS, and/or their actions at brain circumventricular organs that lack a blood-brain barrier may be sufficient to trigger biological activity (663). Indeed, although the peripheral and central hypotheses of migraine origin remain actively debated (679, 694–697), the possibility that CGRP contributes with both peripheral and central actions seems likely.
Central actions of CGRP are supported by preclinical data demonstrating that this peptide contributes to central sensitization, as well as by data indicating that it plays roles in sensitivity to light, sound, touch, and possibly smell. In addition, CGRP may play a role in vestibular symptoms. Injection of the mouse lateral ventricles or posterior thalamic nuclei with CGRP has been shown to heighten sensitivity to light (248, 441–443). The phenotype is strikingly similar to that caused by peripheral injection of CGRP (444), but genetic evidence from overexpression of the RAMP1 CGRP receptor subunit clearly shows that it occurs by a distinct mechanism: specifically, CGRP receptors are rate limiting for intracerebroventricular (central) but not intraperitoneal (peripheral) CGRP administration (444). Likewise, injection of the guinea pig cochlea with CGRP led to an increase in auditory nerve activity (698). Together with findings from CGRP knockout mice (699, 700), these results show that CGRP released by olivocochlear neurons can enhance auditory nerve activity. Whether this leads to phonophobia-like behavior has not been directly tested. CGRP was shown to play a role in touch hypersensitivity following peripheral and central administration of CGRP (396, 401, 701). The role of CGRP in osmophobia is relatively unexplored, but it is intriguing that trigeminal nerves expressing CGRP project into the olfactory bulb (702). Likewise, the possibility that CGRP may play a role in vestibular symptoms of migraine is not known but is suggested by preclinical studies with CGRP knockout mice that show a reduction in the vestibuloocular reflex (703) and altered otolith functions (704), which together may contribute to balance problems. Based on these data, we propose that CGRP can act in the CNS to potentiate sensory signals of light, sound, touch, and smell, which are heightened in migraine (FIGURE 15) (24). This sensitization is proposed to act in concert with peripheral sensitization in a reinforcing positive feedback loop (FIGURE 15).
Figure 15.
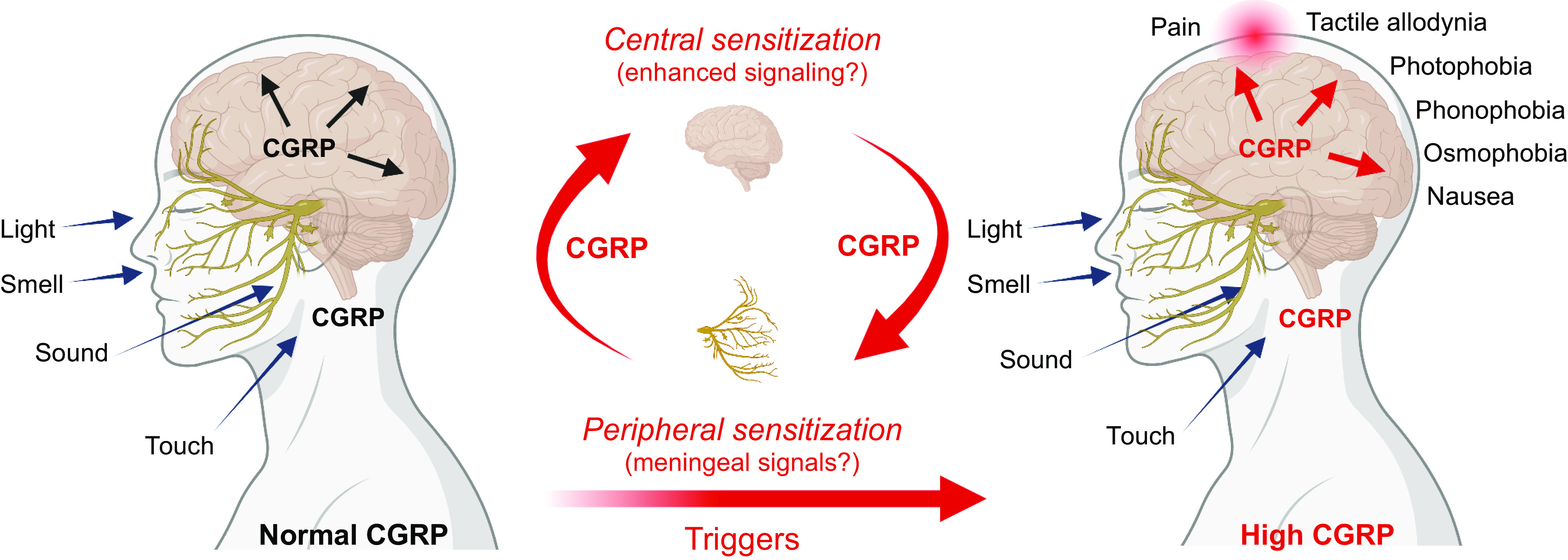
Proposed roles of central and peripheral calcitonin gene-related peptide (CGRP) in migraine. Normal levels of CGRP in the central nervous system (CNS) and peripheral nervous system (PNS) are proposed to modulate sensory inputs. When central or peripheral CGRP levels are elevated, perhaps in response to CNS signals, such as repeated cortical spreading depression (CSD) events, or peripheral signals, such as an altered trigeminovascular microenvironment, peripheral and central sensitization can occur, possibly in a reinforcing loop of positive feedback and feedforward signals between the PNS and CNS as indicated. The increased signaling leads to heightened sensitivity to sensory input that is manifested as photophobia and other migraine symptoms, with pain occurring possibly as a protective response to drive the person to reduce sensory input.
6.4.2.1. CGRP AND CSD?
A migraine phenomenon within the CNS that may possibly involve CGRP is CSD, which is associated with the aura phase of migraine (705). CSD is a self-propagating wave of neuronal and glial depolarization that slowly spreads over the cortex, followed by a prolonged suppression of neuronal activity. CSD has been shown to activate meningeal nociceptors (678, 680, 681), presumably by causing the diffusion of substances released from the cortex, such as glutamate, K+, H+, and ATP. In this mechanism, CGRP could be released from peripheral terminals in the pia and help trigger neurogenic inflammation in the dura (706). This mechanism is consistent with the loss of CSD-induced neurogenic inflammation by sensory denervation of the meninges (678) and with evidence that trigeminal fibers have collaterals that innervate both the pia and dura (707). CGRP could also be released from cortical neurons within the CNS, where it might influence neural activity and/or vascular tone (708).
Although to date there is no clear connection between CGRP and CSD, there are several intriguing observations involving the vasculature and other studies, which have recently been reviewed (708). In particular, a study with rat cortical brain slices revealed that endogenous CGRP was released during CSD and that a CGRP receptor antagonist significantly reduced CSD magnitude (709). Similar findings have been reported with intracerebral ventricular perfusion of an anti-CGRP antibody into rats reducing susceptibility to CSD (710, 711). Another preliminary study reported that systemic administration of the CGRP receptor antagonist olcegepant inhibited repetitive CSD events and altered the vascular response to CSD (712). Likewise, case reports of the CGRP receptor antibody preventing the aura phase of migraine suggest that CGRP may contribute to aura (713). However, on the other hand, CGRP infusion does not cause aura (646), and systemic application of MK-8825, a potent antagonist at rat CGRP receptors, failed to block CSD and did not alter changes in cerebral blood flow but did attenuate pain behaviors (187). In this regard, a recent preclinical study showed that CSD-induced facial hyperalgesia, photophobia, and hypomotility are ameliorated by sumatriptan and the CGRP receptor antagonist olcegepant in mice (714). Yet, while the anti-CGRP antibody fremanezumab given intravenously in rats was able to prevent activation of central trigeminovascular neurons induced by CSD, it did not prevent CSD (715) or prevent CSD-induced vascular changes (716). Given these apparently contradictory reports, one possibility is that CGRP mainly acts as a vascular modulator of CSD by actions mainly within the CNS and contributes to subsequent pain responses in the meninges. In this role, there could be a bidirectional relationship where changes in vascular tone can modulate neuronal activity, which has been termed vascular-neuro coupling (717, 718). Furthermore, pathological inverse coupling has been associated with CSD after traumatic brain injury (719). Together these data indicate that CGRP may modulate CSD propagation and modulate downstream nociceptive responses, but it is not required to trigger CSD events.
Another thread connecting CSD and CGRP is that CSD increases reactive oxygen species (ROS), which can increase CGRP synthesis and release. CSD-induced ROS were observed in the cortex and TG in rats (720, 721). The generation of ROS is relevant because ROS were shown to cause epigenetic activation of the CGRP gene in trigeminal glia (261) and trigger CGRP release from cultured DRG neurons (721). Furthermore, repeated in vivo CSD events, which are common after mild traumatic brain injury, are known to increase CGRP RNA and peptide levels in the rat cortex (722). Thus, elevation of CGRP synthesis by repeated CSD events, such as after mild traumatic brain injury, might contribute to an increased susceptibility to migraine.
6.4.3. Model: peripheral and central CGRP actions form bidirectional loops in migraine.
We propose that the actions of CGRP in migraine are not necessarily only peripheral or only central but rather both (FIGURE 15). Together peripheral and central actions have the potential to form a bidirectional circle of information flow between the trigeminovasculature and CNS. Such communication between the periphery and the brain has been indicated by imaging studies showing central effects of the predominantly peripheral-acting therapeutic CGRP antibodies (671, 672). A central tenet of this model is that CGRP acts at the intersection of peripheral inflammation and central modulation events in migraine. This hypothesis is consistent with the well-established roles of CGRP in vasodilation, neurogenic inflammation, and nociceptive modulation and with the expression of CGRP and its receptors in the right places in the trigeminovasculature and CNS. The ability of peripheral CGRP to initiate migraine and the ability of peripheral inhibition of CGRP to prevent migraine indicate that peripheral CGRP is both sufficient and necessary in at least a subset of patients. Conversely, the ability of central CGRP to initiate migraine-like symptoms in mice and the ability of central inhibition of CGRP to reduce those symptoms indicate that central CGRP is sufficient and necessary to trigger at least some preclinical symptoms. According to our model, it is possible that patients who do not respond well to peripherally acting drugs would respond better to centrally acting CGRP-targeted counterparts.
We further speculate that, as a result of CGRP-mediated heightened sensitivity, the pain of migraine is a protective response to reduce sensory input and avoid “overloading” the system to the point that a seizure would be triggered. In this scenario, the pain of migraine is a mechanism to get the person’s attention, much as pain from a cut finger protects against further damage. In migraine, pain causes the patient to reduce sensory input by going to a dark room and avoiding smells, sounds, and interactions with other people. Indeed, the best natural treatment for a migraine attack is often sleep, which is the ultimate reduction in sensory input. Future studies on the links between migraine and sleep are likely to shed light on this connection.
6.5. Migraine and CGRP Genetics
At present the genetic landscape of migraine is like a Monet painting: from a distance a picture can be seen, but up close it is just a jumble of color dabs. Indeed, from a distance we know that migraine has a strong genetic component, but when looking at specific genes their roles are not so clear. Nonetheless, with the discovery of gene variants that are predominantly associated with glutamate neurotransmission, synaptic plasticity, and the vasculature (723–726), the genetic components that cause a predisposition to migraine have begun to emerge.
The genetic component is most clear in the rare migraine disorders caused by mutations in single genes (monogenic), such as FHM1–3. In contrast, even though common migraine is known to often run in families, it is associated with allelic variations in many genes (polygenic), so Mendelian patterns are not clear (727). In addition, studies in twins and their families indicate that migraine involves equivalent combinations of genetic and environmental (epigenetic) factors (728–730). Although the existence of polygenic and epigenetic factors makes clear identification of causal migraine mutations challenging, progress has been made by genome-wide association studies (GWASs). These have identified nearly 100 candidate genes, many of which have been discovered in multiple studies (724).
Strikingly, until the past year none of the candidates identified by GWAS was either CGRP or a CGRP receptor gene (731). Now, CALCA and/or CALCB have been identified as migraine risk alleles, based on two studies (725, 726, 732). One of these was a GWAS study of >100,000 migraine cases and identified 123 risk loci, of which 86 were novel (725, 726). The new risk loci included the CALCA/CALCB genes. The resolution of this study was not sufficient to distinguish between CALCA and CALCB, which is a good reminder that a limitation of all GWAS studies is that a variant does not necessarily shed light on which nearby gene is associated with a disease (724). Nonetheless, it is interesting that, as observed in a previous large study (723), both vascular and CNS tissue/cell types were enriched for the migraine-associated variants, supporting the hypothesis that neurovascular mechanisms underlie migraine pathophysiology. The second study focused on susceptibility loci identified by a multiethnic genome-wide meta-analysis (732). This analysis revealed a strong association between the CALCB locus and common migraine. It also showed that susceptibility loci were enriched for genes that are expressed in vascular tissue and in the GI system, supporting both the neurovascular hypothesis and possibly a gut-brain axis connection in migraine.
6.6. Future Directions for Targeting the CGRP Axis in the Treatment of Headache Disorders
Could CGRP therapeutics be useful for headaches other than migraine? Some evidence suggests that CGRP could contribute to not only migraine but also other headache disorders such as cluster headache and posttraumatic headache. However, these areas are not nearly as well established as migraine. Of note, galcanezumab showed efficacy and is approved by the FDA for the treatment of episodic cluster headache but not for chronic cluster headache, where a phase 3 trial failed to meet primary and secondary end points (733, 734). Preclinical and clinical studies of posttraumatic headache following traumatic brain injury are implicating CGRP to some degree, but the case for using anti-CGRP agents in these situations, while suggestive, is not yet clear (735–743).
Given that the current CGRP-based drugs are effective in only about half of patients, the question arises of whether different anti-CGRPs can be combined effectively. Regarding the general question of combinatorial therapy for migraine, there are good arguments both pro and con (744, 745). In our opinion, given the multifactorial nature of migraine, a combinatorial approach seems logical. Indeed, there is considerable interest in the merits of either combining two CGRP therapies or combining a CGRP therapy and a non-CGRP therapy.
There is mounting evidence that addition of an acute gepant treatment on top of a preventative monoclonal antibody treatment is effective for some people. A small randomized trial supports the safety of such an approach (746, 747), although this conclusion must be tempered by these being fairly small trials and the long-term effects of dual treatments are not yet known. The concept of giving an acute “boost” to the inhibitory actions of the monoclonals makes sense, especially at the end of the antibody treatment when protection may be wearing off (748–751).
Another question is whether prior failure of one anti-CGRP precludes efficacy of another such agent or of a triptan. A recent study reported that prior treatment responses to a CGRP monoclonal antibody (type was not specified) and onabotulinumtoxinA injections are good predictors of a good response to a CGRP receptor antagonist (ubrogepant) (752). Conversely, the lack of a response to the antibody (or onabotulinumtoxinA) was a predictor that the patient would not respond well to the antagonist. Given the shared mechanisms of the CGRP antibodies and antagonists, this correlation makes sense. However, as with the family of triptan drugs, it seems likely that there will be patients who respond to one antibody or gepant but not another. Along this line, response to a triptan was also found to be a positive predictor of response to erenumab (753). This therapeutic connection between triptans and CGRP is consistent with the functional connection of triptans reducing CGRP release (133). Furthermore, preclinical studies have shown that triptans can block CGRP-induced light aversion (442, 444) and that nitroglycerin-induced symptoms can be blocked by both triptans and CGRP monoclonal antibodies (754). Thus, given the overlap between the mechanisms of CGRP antagonists and triptans, would dual therapy with these drugs be beneficial? At present, this is an open question; like the arguments for dual therapy with both a CGRP-based monoclonal antibody and a CGRP receptor antagonist, the same logic suggests that dual therapy with a CGRP-based drug and a triptan would be therapeutically beneficial. Similarly, would treatment with a CGRP-based drug and a neurokinin-1 (NK1) receptor antagonist to target both CGRP and substance P be beneficial? Although the lack of translation of rodent NK1 pain findings to humans is a serious concern, it is interesting that combination was considerably more effective than the individual treatments in mice (755).
Regarding dual treatments of a CGRP-based drug and a different therapeutic, there is growing evidence from open-label and anecdotal reports that adding onabotulinumtoxinA and a CGRP monoclonal antibody reduces the number of migraine days for some patients (756–760). These reports are consistent with evidence that the CGRP antibodies inhibit trigeminal Aδ nerve fibers whereas onabotulinumtoxinA primarily inhibits vesicular fusion and release of neuropeptides, including CGRP, from trigeminal C nerve fibers (761). Hence, combining onabotulinumtoxinA with anti-CGRPs may be beneficial for some patients with chronic migraine.
Thus, although there are interesting case reports and supportive trial results are emerging, at this stage there is no solid ground upon which to base recommendations (746, 762). Nonetheless, based on the data so far, we predict that combinatorial therapy of multiple CGRP-based drugs and combinations with other non-CGRP drugs will prove to be useful and safe for most patients. The need for such combinatorial strategies is supported by the presence of two CGRP receptors (canonical and AMY1) (763) that may have different signaling pathways from the cell surface and endosomes (367–369), the peripherally restricted sites of action of the antibodies and gepants, and the challenge of blocking the large bolus of peptide release from secretory granules, as discussed above (49). Finally, it will be important to carefully analyze the clinical data for patterns that may emerge as more patients are treated with anti-CGRPs. For example, it will be interesting to determine how long it takes for headache to return, and whether it worsens, once a patient stops taking the drug, because there are suggestions that wear-off may occur for some patients, and rebound headache is also possible if the receptor system is subject to regulation (764, 765).
To be clear, migraine is not solely due to altered CGRP signaling. This is clear from the observations that the approved drugs targeting CGRP (antibodies and gepants) are effective in only ∼40–50% of patients (468, 766, 767) and CGRP injections induce migraine-like attacks in only ∼65% of patients (644–648). Thus, other molecules need to be targeted for developing additional therapies. As a starting point, other CGRP family members and their receptors should be considered. One candidate is the other CGRP receptor, AMY1, described above (110). Indeed, a recent study confirmed that a synthetic amylin peptide, pramlintide, provoked migrainelike attacks in patients (47). Like CGRP, amylin levels are elevated in chronic migraine patients between attacks (768). Likewise, similar to CGRP, amylin injection into mice also caused migraine-like symptoms of light aversion and cutaneous hypersensitivity (47) and a grimace squint indicative of pain (48). In both humans and mice, the magnitude of responses to pramlintide or amylin was less than with CGRP. These studies provide proof of principle that activation of amylin receptors, most likely AMY1 that is shared with CGRP, is sufficient to cause migraine. This conclusion is consistent with amylin and CGRP having shared binding sites and communication between brain nuclei. For example, area postrema neurons can respond to both amylin and CGRP and project to CGRP-containing neurons in the PBN that mediate nausea-associated behaviors (769). In this regard, future studies on the relative roles of the AMY1 receptor and the canonical CGRP receptor in migraine-associated nausea and other symptoms should prove interesting. Likewise, the AM receptors CLR/RAMP2 and CLR/RAMP3 should also be considered, given the ability of AM to induce migraine-like attacks similar to CGRP (36, 47). Beyond CGRP family members, a plethora of sensory, sympathetic, and parasympathetic neuropeptides [e.g., PACAP (754, 770–775), orexin (776, 777), oxytocin (778)] are likely to play roles. In addition, altered central and/or peripheral signaling by nonpeptide mechanisms [e.g., glutamate receptors (779, 780), PAR2 receptors (781, 782)] is also likely to contribute to migraine pathogenesis.
7. CGRP IN OTHER THERAPEUTIC AREAS
The extensive description of CGRP and migraine reflects the current situation of multiple approved drugs for this indication. This is an example where dampening CGRP activity is the desired effect. However, over the years many other therapeutic indications have been proposed for CGRP manipulation. Some of these also seek to dampen CGRP activity, suggesting that the current anti-CGRP drugs could have wider applications. Others seek to increase activity of the CGRP system to achieve clinical benefit. In some therapeutic areas there is not yet a clear consensus of the direction to take.
7.1. Other Painful Conditions Including Arthritis
There is evidence that CGRP is involved in pain disorders other than headache (396). For example, nonheadache pain has commonly been associated with increased CGRP levels (783). This suggests that the anti-CGRP drugs that have been developed for migraine could have broader utility. Neuropathic pain is one such area of interest, but none of the clinical studies of anti-CGRPs to date has provided solid support for this as a therapeutic strategy (396). A recent systematic review of trigeminal neuropathic pain concluded that it is not possible to draw firm conclusions about the role of CGRP from the available data and that further research is needed (784). However, a retrospective analysis of a small number of patients treated with erenumab suggested that CGRP blockade could be beneficial in treating refractory trigeminal neuralgia (785).
Taking arthritis as a further example, there is evidence to suggest that CGRP accumulates in synovial fluid and contributes to the pathology and that in preclinical models the reduction of CGRP activity is beneficial (786–789). Interestingly, CGRP expression in patients with knee osteoarthritis was higher in women than men, and in women it correlated positively with pain severity (790). Results are available for one CGRP monoclonal antibody in patients with mild to moderate knee osteoarthritis (791). This trial measured change from baseline in the Western Ontario and McMaster Universities Osteoarthritis Index pain subscore for galcanezumab compared to placebo as the primary outcome, over 8 wk of treatment (2 doses). The study was terminated after interim analysis because of inadequate efficacy. However, it is too early to say whether or not this rules out CGRP as a target for arthritis. Many factors need to be considered, such as whether the disease stage was appropriate and whether the number of patients completing the trial was sufficient. Furthermore, a longer dosing period may have been required to achieve benefit. Neurofibromatosis and temporomandibular disorders are additional candidate conditions for which the clinical benefit of reducing CGRP activity should be investigated (792, 793).
7.2. Cardiovascular Diseases
As noted in sect. 5.3, CGRP and, more recently, calcitonin are recognized as playing a protective role in the heart and vasculature by reducing hypertrophy and hypertension. Thus, administration of stabilized CGRP analogs or therapeutic agents that stimulate CGRP actions may prove useful to reduce end-organ damage such as renal fibrosis (486). Elevated CGRP may also help reduce cardiovascular risks during pregnancy such as during preeclampsia (see sect. 5.8.1) (463, 603, 604).
7.3. Flushing/Rosacea
Hot flushes (flashes) are often reported by women during menopause and can also occur in men being treated for prostate cancer. These characteristically occur in the face, neck, and upper torso. In some individuals these can persist for many years. Several studies have reported elevated CGRP during hot flushes in women (794). Although a clear correlation between CGRP levels and flushing has been made, until recently there was little data supportive of a causative association. However, a more recent study in ovariectomized mice reported that a CGRP receptor antagonist (MK-8825) was able to inhibit flushlike temperature increases that occur in response to exercise. In mice with a genetic reduction of α-CGRP, flushlike temperature increases were also reduced (795). Therefore, it is possible that CGRP-blocking drugs could be of value in preventing hot flushes. Rosacea is associated with flushing and neurogenic inflammation, and thus potentially with elevation of CGRP levels (796, 797). Indeed, CGRP levels (α and β) have been reported to be substantially elevated in the erythematotelangiectatic subtype of rosacea (798).
7.4. Raynaud’s Phenomenon
Raynaud’s phenomenon occurs in 3–5% of the population. This condition can be either a primary or secondary condition response to stressors, particularly to cold. The digits are affected, and symptoms include vasospasm, ischemia, and pain. CGRP has been linked to this condition in several ways. Skin biopsy samples indicate that perivascular CGRP immunoreactivity is lower in patients with Raynaud’s phenomenon than in healthy control subjects (799, 800). Interestingly, patients may be more sensitive to CGRP (801), and they are able to respond to CGRP injection with increased blood flow (802). Despite this, however, their responsiveness at low temperature seems to be diminished (803). Clinical benefit has been achieved in two pilot clinical studies of CGRP infusion (804, 805). Longer-acting CGRP agonists are probably needed, but this is an area that could be worth pursuing. Use of the anti-CGRP drugs for migraine is now producing a few case reports of exacerbated or new-onset Raynaud’s phenomenon (806–808). A recent study analyzed the available data from the World Health Organization pharmacovigilance database to determine whether there is an emerging association between Raynaud’s phenomenon and the use of anti-CGRP drugs. As of January 31, 2022, there were 99 reports containing the term “Raynaud’s phenomenon” and 58 reports under the term “peripheral coldness.” The study concluded that there was disproportionate reporting of Raynaud’s phenomenon involving anti-CGRP drugs compared with triptans and beta-blockers, other migraine drugs that were used as comparators (809).
7.5. Pulmonary Diseases
CGRP has both pro- and anti-inflammatory effects in asthma and acute lung injury models (492). In response to injury, allergens, or irritants, nociceptors and pulmonary neuroendocrine cells secrete CGRP, but whether this is beneficial or harmful is often not clear (810). For example, CGRP has long been suspected to contribute to asthma, primarily because of reports of its airway-constricting capacity and its ability to increase mucus secretion (496, 811). Specifically, inhibition of CGRP released from pulmonary endocrine cells has been proposed to alleviate some of the mucus phenotypes in allergic asthma (491). Yet despite all the reports of CGRP driving proinflammatory responses, others support anti-inflammatory responses in airway allergies (492). For example, preclinical studies on models of acute lung injury suggest that blocking CGRP activity may actually be beneficial. In a sheep model of smoke injury, the symptoms of airway hyperemia, pulmonary transvascular flux, and impaired pulmonary gas exchange were ameliorated by the CGRP receptor antagonist olcegepant (812). Likewise, in CGRP knockout mice acid-induced injury, edema, and respiratory failure were attenuated (813).
Thus, it is not clear whether blocking CGRP actions in the lung will be beneficial or harmful, and this is especially relevant to current trials testing CGRP receptor antagonism as potential therapeutic for coronavirus 2019 (COVID-19), as discussed next.
7.6. COVID and CGRP
Given the importance of COVID-19 in society and with its multifactorial pathology including headache and pain in the acute phase and at later stages of “long COVID,” the question emerges of whether CGRP may be involved in COVID-19 pathology. There is emerging evidence that COVID-19 may interact with the peripheral nervous system by multiple mechanisms to cause peripheral sensitization that promotes pain (814). Whether CGRP is directly involved remains to be seen, but seems likely given CGRP’s role in peripheral sensitization and the finding that the ACE2 receptor necessary for SARS-CoV-2 infection is present on CGRP-containing sensory neurons (814). In particular, headache is a common symptom of COVID-19, and the headache often has migraine-like features (815–817). Studies have provided different hypotheses on the pathophysiology of COVID-19 headache in the acute phase, involving immune-inflammatory mechanisms (818–820) as well as the direct viral damage to the nervous system (821, 822).
Interestingly, COVID-19 patients with headache are more likely to have loss of smell (anosmia), which led to an interesting hypothesis by Messlinger and colleagues (822) suggesting that the SARS-CoV-2 virus activates trigeminal nerve fibers that project to the dura and nasal mucosa, leading to headache and anosmia in COVID-19. Indeed, earlier studies reported that trigeminal activation is increased in patients with acquired anosmia (823). In this hypothesis, the activated trigeminal fibers release CGRP, which then causes release of cytokines and other inflammatory agents that in turn can feed back to release more CGRP. This could lead to peripheral sensitization in the dura and lead to headache. However, in the olfactory system the opposite occurs, since trigeminal CGRP inhibits olfactory receptors in the epithelium (824) and inhibits neurotransmission within the olfactory bulb (825). Thus, vigorous activation of trigeminal afferents affected by SARS-CoV-2 may contribute to both headache and concomitant anosmia.
In addition to pain, headache, and anosmia, CGRP may also be involved in the pulmonary symptoms of COVID-19. As noted above, CGRP has both inflammatory and anti-inflammatory actions, so there are arguments that CGRP might either attenuate or exacerbate COVID-19 pathology in the pulmonary system. Nonetheless, a CGRP receptor antagonist is currently in clinical trials in an effort to reduce lung inflammation in COVID-19. The hypothesis is built on CGRP’s role in increasing the cytokine IL-6 and shifting T-cell phenotypes (826). However, given CGRP’s protective functions against pulmonary vascular remodeling and hypertension and subsequent heart failure in preclinical models of chronic hypoxia in the cardiovascular system, there are concerns that CGRP receptor antagonists may have cardiopulmonary adverse effects in COVID-19 patients (827).
7.7. Gastrointestinal Diseases
To a large degree, the renewed interest in GI CGRP has been driven by the role of CGRP in migraine. Two recent reviews describe how CGRP may contribute to the clinical comorbidity of GI symptoms and migraine (563, 828). The diagnostic symptoms of migraine include nausea and vomiting (614) and are experienced by 73% and 29% of patients, respectively (829). Other GI symptoms may be present with migraine as well. These include upper and lower GI comorbidities such as diarrhea, constipation, gastroesophageal reflux, and gastroparesis (555, 829, 830). As discussed in sect. 5.7, some of these symptoms may be linked to CGRP.
An interesting hypothesis was put forth by Goadsby and colleagues (555), suggesting that presumed appetite-associated triggers of migraine may actually be a consequence of premonitory symptoms preceding the headache. For example, skipping meals, which is reported by many people as a migraine trigger, might instead be a consequence of premonitory GI discomforts, such as gastroparesis, that affect appetite. Aurora and colleagues (831) recently reviewed the intriguing links between the gut and brain in migraine. Within this context, it has been suggested that the gut microbiota might play a role in migraine via the gut-brain axis (832). How CGRP might affect this axis is an open question.
A connection between migraine and GI dysfunction is further supported by the comorbidity of migraine with GI disorders (555, 830). These disorders include inflammatory bowel disease, irritable bowel syndrome (IBS), functional dyspepsia (indigestion), and gastroparesis (831). Indeed, GI disorders such as IBS are the most common comorbidities associated with migraine (591). It has long been speculated that CGRP receptor antagonists could be used to treat inflammatory bowel and other GI diseases (565). Recent preclinical studies support the possibility that inhibiting CGRP signaling may have beneficial effects for patients with IBS. A monoclonal anti-CGRP antibody was shown to reverse stress-induced changes in visceral hypersensitivity of the colon, suggesting that targeting CGRP may be an effective approach for treating IBS-related stress-induced visceral pain (833).
On the other hand, GI symptoms in migraine can also be caused by inhibiting CGRP. Most notably, constipation has been reported by a small but significant number of patients after treatment with the CGRP receptor antibody erenumab (3–4%) (834) or the CGRP receptor antagonist atogepant (7–8%) (575). These effects are a reminder that the long-term effects of reducing CGRP actions should be considered (478). Consequently, both elevated CGRP in migraine and therapeutic CGRP receptor antagonism can potentially lead to GI dysfunction.
Interestingly, common genetic polymorphisms near CALCB have been reported to contribute to diverticular disease (835), although whether this involves higher or lower expression is not known. Nonetheless, it is intriguing that a recent multiethnic genome-wide association meta-analysis identified CALCB as a susceptibility locus for migraine, leading the authors to speculate that large intestine disorders might be associated with migraine (732). This speculation was based on a tissue expression quantitative trait loci (eQTL) analysis that identified the sigmoid colon as the main tissue in which expression was affected by migraine-associated variants. This was closely followed by the esophagus muscularis and gastroesophageal junction. A second eQTL analytical tool using only the European database identified arteries as a main tissue, in agreement with a prior study that highlighted migraine variants as predominantly expressed in vascular tissue (723). These associations support a genetic origin of comorbidity of neurovascular and GI diseases and the need for further studies of the role of CGRP in the gut-brain axis in health and disease.
In summary, CGRP plays an important role in the GI system. Its importance is highlighted by the side effects from the use of anti-CGRP drugs in migraine therapy. Yet at the same time, the comorbidity of migraine and GI disorders is raising awareness that perhaps CGRP-targeted therapies could also be useful for the treatment of IBS and other common and disabling GI pathologies.
7.8. Metabolic Diseases (Diabetes, Obesity)
Consistent with the various metabolic effects (sect. 5.6), it has been proposed that the CGRP system might be an appropriate therapeutic target for diabetes and/or obesity (836). However, the somewhat contradictory findings regarding the underlying biology leave unclear whether the CGRP system might be most effectively manipulated with an agonist or an antagonist. The ultimate test may be whether any patterns emerge in clinical data with anti-CGRP therapy for migraine, but so far this has not been the case (837). Formal clinical trials that are sufficiently powered to test hypotheses around metabolism would be needed to draw firm conclusions.
8. CONCLUSIONS AND FUTURE DIRECTIONS
In conclusion, CGRP is a multifunctional neuropeptide with diverse physiological functions that span nearly every organ system. In these functions, a unique feature of CGRP and its family members is the use of a core GPCR that requires an accessory protein (RAMP). The mélange of GPCRs (CLR, CTR) and RAMPs (RAMP1, 2, 3) leads to pharmacological complexity and physiological overlap between CGRP and its family members (e.g., calcitonin, amylin, AM) that are only now beginning to unfold. In this regard, a major gap is that our understanding of CGRP pharmacology is mostly based on overexpressed receptors in heterologous cells. Future studies with endogenous receptors in native tissue should prove informative. Close attention should be paid to all receptor subunits to try and build a more complete picture of which receptor may be producing an effect. In particular, the AMY1 receptor should be considered as a contributor to CGRP actions. Similarly, the β-CGRP isoform should not be ignored and should be considered in its own right.
A major advance in the field has been the approved therapeutics for migraine treatment and prevention that target CGRP signaling. These drugs have greatly improved the quality of life for many patients. Yet despite their clinical efficacy the mechanisms by which CGRP acts in migraine remain mostly speculative. Thus, the field is ripe for preclinical and clinical studies to understand how and where CGRP acts in migraine and other diseases. In migraine, we should keep our eyes open to central actions of CGRP and peripheral actions of CGRP on immune cells and vasculature. Indeed, for all diseases, closer examination of CGRP activities in the cardiovascular system, GI tract, and pulmonary and immune systems and its metabolic effects suggest that modulating CGRP will be a useful strategy for a number of diseases in addition to migraine.
GRANTS
A.F.R. was supported by grants from the NIH (RF1 NS113839; R01 NS075599) and VA Medical Center (1 I01 RX003523). D.L.H. was also supported by a grant from the NIH (RF1 NS113839).
DISCLAIMERS
The contents of this article do not represent the views of the VA or the United States Government.
DISCLOSURES
D.L.H. has received research support from Living Cell Technologies and AbbVie and has acted as an advisor, speaker, or consultant for Lilly, Teva, Merck, and Amgen. A.F.R. has received research support from Lundbeck and has acted as an advisor or consultant for Lilly, AbbVie, Amgen, Novartis, Lundbeck, and Schedule One Therapeutics.
AUTHOR CONTRIBUTIONS
A.F.R. and D.L.H. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of our labs for many stimulating discussions over the years. We thank Erica Hendrikse for providing the precursors to the rat brain expression figures. All figures except FIGURE 2 were generated with BioRender, with permission. We thank Michael Garelja for contributions to the development of FIGURES 2 AND 7.
REFERENCES
- 1. Rosenfeld MG, Amara SG, Roos BA, Ong ES, Evans RM. Altered expression of the calcitonin gene associated with RNA polymorphism. Nature 290: 63–65, 1981. doi: 10.1038/290063a0. [DOI] [PubMed] [Google Scholar]
- 2. Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298: 240–244, 1982. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304: 129–135, 1983. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- 4. Grunditz T, Ekman R, Håkanson R, Rerup C, Sundler F, Uddman R. Calcitonin gene-related peptide in thyroid nerve fibers and C cells: effects on thyroid hormone secretion and response to hypercalcemia. Endocrinology 119: 2313–2324, 1986. doi: 10.1210/endo-119-5-2313. [DOI] [PubMed] [Google Scholar]
- 5. Uddman R, Luts A, Sundler F. Occurrence and distribution of calcitonin gene-related peptide in the mammalian respiratory tract and middle ear. Cell Tissue Res 241: 551–555, 1985. doi: 10.1007/BF00214575. [DOI] [PubMed] [Google Scholar]
- 6. Johnson DE, Wobken JD. Calcitonin gene-related peptide immunoreactivity in airway epithelial cells of the human fetus and infant. Cell Tissue Res 250: 579–583, 1987. doi: 10.1007/BF00218949. [DOI] [PubMed] [Google Scholar]
- 7. Tsukiji J, Sango K, Udaka N, Kageyama H, Ito T, Saito H, Horie H, Inoue S, Kitamura H, Hagiwara E, Ikeda H, Okubo T, Ishigatsubo Y. Long-term induction of beta-CGRP mRNA in rat lungs by allergic inflammation. Life Sci 76: 163–177, 2004. doi: 10.1016/j.lfs.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 8. Timmermans JP, Scheuermann DW, Barbiers M, Adriaensen D, Stach W, Van Hee R, De Groodt-Lasseel MH. Calcitonin gene-related peptide-like immunoreactivity in the human small intestine. Acta Anat (Basel) 143: 48–53, 1992. doi: 10.1159/000147227. [DOI] [PubMed] [Google Scholar]
- 9. Kuramoto H, Kondo H, Fujita T. Calcitonin gene-related peptide (CGRP)-like immunoreactivity in scattered chromaffin cells and nerve fibers in the adrenal gland of rats. Cell Tissue Res 247: 309–315, 1987. doi: 10.1007/bf00218312. [DOI] [PubMed] [Google Scholar]
- 10. García-Caballero T, Gallego R, Rosón E, Fraga M, Beiras A. Calcitonin gene-related peptide (CGRP) immunoreactivity in the neuroendocrine Merkel cells and nerve fibres of pig and human skin. Histochemistry 92: 127–132, 1989. doi: 10.1007/BF00490231. [DOI] [PubMed] [Google Scholar]
- 11. Wang W, Jia L, Wang T, Sun W, Wu S, Wang X. Endogenous calcitonin gene-related peptide protects human alveolar epithelial cells through protein kinase Cepsilon and heat shock protein. J Biol Chem 280: 20325–20330, 2005. doi: 10.1074/jbc.M413864200. [DOI] [PubMed] [Google Scholar]
- 12. Linscheid P, Seboek D, Zulewski H, Keller U, Müller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology 146: 2699–2708, 2005. doi: 10.1210/en.2004-1424. [DOI] [PubMed] [Google Scholar]
- 13. Timper K, Grisouard J, Radimerski T, Dembinski K, Peterli R, Häring A, Frey DM, Zulewski H, Keller U, Müller B, Christ-Crain M. Glucose-dependent insulinotropic polypeptide (GIP) induces calcitonin gene-related peptide (CGRP)-I and procalcitonin (Pro-CT) production in human adipocytes. J Clin Endocrinol Metab 96: E297–E303, 2011. doi: 10.1210/jc.2010-1324. [DOI] [PubMed] [Google Scholar]
- 14. Hou Q, Barr T, Gee L, Vickers J, Wymer J, Borsani E, Rodella L, Getsios S, Burdo T, Eisenberg E, Guha U, Lavker R, Kessler J, Chittur S, Fiorino D, Rice F, Albrecht P. Keratinocyte expression of calcitonin gene-related peptide beta: implications for neuropathic and inflammatory pain mechanisms. Pain 152: 2036–2051, 2011. doi: 10.1016/j.pain.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye F, Deng PY, Li D, Luo D, Li NS, Deng S, Deng HW, Li YJ. Involvement of endothelial cell-derived CGRP in heat stress-induced protection of endothelial function. Vascul Pharmacol 46: 238–246, 2007. doi: 10.1016/j.vph.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16. Zhou Z, Hu CP, Wang CJ, Li TT, Peng J, Li YJ. Calcitonin gene-related peptide inhibits angiotensin II-induced endothelial progenitor cells senescence through up-regulation of klotho expression. Atherosclerosis 213: 92–101, 2010. doi: 10.1016/j.atherosclerosis.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 17. Xing L, Guo J, Wang X. Induction and expression of beta-calcitonin gene-related peptide in rat T lymphocytes and its significance. J Immunol 165: 4359–4366, 2000. doi: 10.4049/jimmunol.165.8.4359. [DOI] [PubMed] [Google Scholar]
- 18. Bracci-Laudiero L, Aloe L, Caroleo MC, Buanne P, Costa N, Starace G, Lundeberg T. Endogenous NGF regulates CGRP expression in human monocytes, and affects HLA-DR and CD86 expression and IL-10 production. Blood 106: 3507–3514, 2005. doi: 10.1182/blood-2004-10-4055. [DOI] [PubMed] [Google Scholar]
- 19. Li W, Zhang Z, Li X, Cai J, Li D, Du J, Zhang B, Xiang D, Li N, Li Y. CGRP derived from cardiac fibroblasts is an endogenous suppressor of cardiac fibrosis. Cardiovasc Res 116: 1335–1348, 2020. doi: 10.1093/cvr/cvz234. [DOI] [PubMed] [Google Scholar]
- 20. Bracq S, Clement B, Pidoux E, Moukhtar MS, Jullienne A. CGRP is expressed in primary cultures of human hepatocytes and in normal liver. FEBS Lett 351: 63–66, 1994. doi: 10.1016/0014-5793(94)00823-X. [DOI] [PubMed] [Google Scholar]
- 21. Kraenzlin ME, Ch’ng JL, Mulderry PK, Ghatei MA, Bloom SR. Infusion of a novel peptide, calcitonin gene-related peptide (CGRP) in man. Pharmacokinetics and effects on gastric acid secretion and on gastrointestinal hormones. Regul Pept 10: 189–197, 1985. doi: 10.1016/0167-0115(85)90013-8. [DOI] [PubMed] [Google Scholar]
- 22. Kee Z, Kodji X, Brain SD. The role of calcitonin gene related peptide (CGRP) in neurogenic vasodilation and its cardioprotective effects. Front Physiol 9: 1249, 2018. doi: 10.3389/fphys.2018.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hay DL, Garelja ML, Poyner DR, Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br J Pharmacol 175: 3–17, 2018. doi: 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol 55: 533–552, 2015. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sexton PM, Christopoulos G, Christopoulos A, Nylen ES, Snider RH Jr, Becker KL. Procalcitonin has bioactivity at calcitonin receptor family complexes: potential mediator implications in sepsis. Crit Care Med 36: 1637–1640, 2008. doi: 10.1097/CCM.0b013e318170a554. [DOI] [PubMed] [Google Scholar]
- 26. Müller B, White JC, Nylén ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab 86: 396–404, 2001. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- 27. Arora S, Singh P, Singh PM, Trikha A. Procalcitonin levels in survivors and nonsurvivors of sepsis: systematic review and meta-analysis. Shock 43: 212–221, 2015. doi: 10.1097/SHK.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 28. Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 54: 233–246, 2002. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 29. Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem 279: 7264–7274, 2004. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- 30. Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science 229: 1094–1097, 1985. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- 31. Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience 25: 195–205, 1988. doi: 10.1016/0306-4522(88)90018-8. [DOI] [PubMed] [Google Scholar]
- 32. Russo AF, Nelson C, Roos BA, Rosenfeld MG. Differential regulation of the coexpressed calcitonin/alpha-CGRP and beta-CGRP neuroendocrine genes. J Biol Chem 263: 5–8, 1988. doi: 10.1016/S0021-9258(19)57346-X. [DOI] [PubMed] [Google Scholar]
- 33. Kato J, Kitamura K. Bench-to-bedside pharmacology of adrenomedullin. Eur J Pharmacol 764: 140–148, 2015. doi: 10.1016/j.ejphar.2015.06.061. [DOI] [PubMed] [Google Scholar]
- 34. Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett 556: 53–58, 2004. doi: 10.1016/S0014-5793(03)01368-1. [DOI] [PubMed] [Google Scholar]
- 35. Zhang SY, Xu MJ, Wang X. Adrenomedullin 2/intermedin: a putative drug candidate for treatment of cardiometabolic diseases. Br J Pharmacol 175: 1230–1240, 2018. doi: 10.1111/bph.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghanizada H, Al-Karagholi MA, Arngrim N, Mørch-Rasmussen M, Walker CS, Hay DL, Ashina M. Effect of adrenomedullin on migraine-like attacks in patients with migraine: a randomized crossover study. Neurology 96: e2488–e2499, 2021. doi: 10.1212/WNL.0000000000011930. [DOI] [PubMed] [Google Scholar]
- 37. Takahashi K, Kikuchi K, Maruyama Y, Urabe T, Nakajima K, Sasano H, Imai Y, Murakami O, Totsune K. Immunocytochemical localization of adrenomedullin 2/intermedin-like immunoreactivity in human hypothalamus, heart and kidney. Peptides 27: 1383–1389, 2006. doi: 10.1016/j.peptides.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 38. Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 21: 138–167, 2000. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 39. Dackor R, Fritz-Six K, Smithies O, Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem 282: 18094–18099, 2007. doi: 10.1074/jbc.M703544200. [DOI] [PubMed] [Google Scholar]
- 40. Dackor RT, Fritz-Six K, Dunworth WP, Gibbons CL, Smithies O, Caron KM. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol Cell Biol 26: 2511–2518, 2006. doi: 10.1128/MCB.26.7.2511-2518.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional adrenomedullin gene. Proc Natl Acad Sci USA 98: 615–619, 2001. doi: 10.1073/pnas.98.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsuruda T, Kato J, Kuwasako K, Kitamura K. Adrenomedullin: continuing to explore cardioprotection. Peptides 111: 47–54, 2019. doi: 10.1016/j.peptides.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 43. Tanaka M, Koyama T, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Kawate H, Liu T, Xian X, Imai A, Zhai L, Hirabayashi K, Owa S, Yamauchi A, Igarashi K, Taniguchi S, Shindo T. The endothelial adrenomedullin-RAMP2 system regulates vascular integrity and suppresses tumour metastasis. Cardiovasc Res 111: 398–409, 2016. doi: 10.1093/cvr/cvw166. [DOI] [PubMed] [Google Scholar]
- 44. Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev 67: 564–600, 2015. doi: 10.1124/pr.115.010629. [DOI] [PubMed] [Google Scholar]
- 45. Younk LM, Mikeladze M, Davis SN. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin Pharmacother 12: 1439–1451, 2011. doi: 10.1517/14656566.2011.581663. [DOI] [PubMed] [Google Scholar]
- 46. Heise T, Heinemann L, Heller S, Weyer C, Wang Y, Strobel S, Kolterman O, Maggs D. Effect of pramlintide on symptom, catecholamine, and glucagon responses to hypoglycemia in healthy subjects. Metabolism 53: 1227–1232, 2004. doi: 10.1016/j.metabol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 47. Ghanizada H, Al-Karagholi MA, Walker CS, Arngrim N, Rees T, Petersen J, Siow A, Mørch-Rasmussen M, Tan S, O’Carroll SJ, Harris P, Skovgaard LT, Jørgensen NR, Brimble M, Waite JS, Rea BJ, Sowers LP, Russo AF, Hay DL, Ashina M. Amylin analog pramlintide induces migraine-like attacks in patients. Ann Neurol 89: 1157–1171, 2021. doi: 10.1002/ana.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rea BJ, Davison A, Ketcha MJ, Smith KJ, Fairbanks AM, Wattiez AS, Poolman P, Kardon RH, Russo AF, Sowers LP. Automated detection of squint as a sensitive assay of sex-dependent calcitonin gene-related peptide and amylin-induced pain in mice. Pain 163: 1511–1519, 2022. doi: 10.1097/j.pain.0000000000002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Russo AF. Overview of neuropeptides: awakening the senses? Headache 57, Suppl 2: 37–46, 2017. doi: 10.1111/head.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pert C. Molecules of Emotion: the Science behind Mind-Body Medicine. New York: Scribner, 1997. [Google Scholar]
- 51. Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu Rev Neurosci 15: 57–85, 1992. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- 52. Lindberg I, Glembotski CC. Physiological signaling in the absence of amidated peptides. Proc Natl Acad Sci USA 116: 19774–19776, 2019. doi: 10.1073/pnas.1914001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Powers KG, Ma XM, Eipper BA, Mains RE. Identifying roles for peptidergic signaling in mice. Proc Natl Acad Sci USA 116: 20169–20179, 2019. doi: 10.1073/pnas.1910495116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O’Connell JP, Kelly SM, Raleigh DP, Hubbard JA, Price NC, Dobson CM, Smith BJ. On the role of the C-terminus of alpha-calcitonin-gene-related peptide (alpha CGRP). The structure of des-phenylalaninamide37-alpha CGRP and its interaction with the CGRP receptor. Biochem J 291: 205–210, 1993. doi: 10.1042/bj2910205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eipper BA, Green CB, Mains RE. Expression of prohormone processing enzymes in neuroendocrine and non-neuroendocrine cells. J Natl Cancer Inst Monogr 13: 163–168, 1992. [PubMed] [Google Scholar]
- 56. Mains RE, Eipper BA. Peptides. In: Basic Neurochemistry, Molecular, Cellular and Medical Aspects, edited by Siegel G. Philadelphia, PA: Lippincott-Raven, 1999. [Google Scholar]
- 57. Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Toxicol 48: 393–423, 2008. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burns DM, Forstrom JM, Friday KE, Howard GA, Roos BA. Procalcitonin’s amino-terminal cleavage peptide is a bone-cell mitogen. Proc Natl Acad Sci USA 86: 9519–9523, 1989. doi: 10.1073/pnas.86.23.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van den Pol AN. Neuropeptide transmission in brain circuits. Neuron 76: 98–115, 2012. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nusbaum MP, Blitz DM, Marder E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat Rev Neurosci 18: 389–403, 2017. doi: 10.1038/nrn.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eiden LE. Signaling during exocytosis. In: Handbook of Cell Signaling, edited by Bradshaw RA. New York: Elsevier Science, 2003, p. 375–392. [Google Scholar]
- 62. Bost A, Shaib AH, Schwarz Y, Niemeyer BA, Becherer U. Large dense-core vesicle exocytosis from mouse dorsal root ganglion neurons is regulated by neuropeptide Y. Neuroscience 346: 1–13, 2017. doi: 10.1016/j.neuroscience.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 63. Risch M, Vogler B, Dux M, Messlinger K. CGRP outflow into jugular blood and cerebrospinal fluid and permeance for CGRP of rat dura mater. J Headache Pain 22: 105, 2021. doi: 10.1186/s10194-021-01320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xiong H, Lacin E, Ouyang H, Naik A, Xu X, Xie C, Youn J, Wilson BA, Kumar K, Kern T, Aisenberg E, Kircher D, Li X, Zasadzinski JA, Mateo C, Kleinfeld D, Hrabetova S, Slesinger PA, Qin Z. Probing neuropeptide volume transmission in vivo by simultaneous near-infrared light-triggered release and optical sensing. Angew Chem Int Ed Engl 61: e202206122, 2022. doi: 10.1002/anie.202206122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nordmann JJ, Morris JF. Method for quantitating the molecular content of a subcellular organelle: hormone and neurophysin content of newly formed and aged neurosecretory granules. Proc Natl Acad Sci USA 81: 180–184, 1984. doi: 10.1073/pnas.81.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7: 126–136, 2006. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 67. Soldo BL, Giovannucci DR, Stuenkel EL, Moises HC. Ca2+ and frequency dependence of exocytosis in isolated somata of magnocellular supraoptic neurones of the rat hypothalamus. J Physiol 555: 699–711, 2004. doi: 10.1113/jphysiol.2003.051136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Steenbergh PH, Höppener JW, Zandberg J, Lips CJ, Jansz HS. A second human calcitonin/CGRP gene. FEBS Lett 183: 403–407, 1985. doi: 10.1016/0014-5793(85)80819-X. [DOI] [PubMed] [Google Scholar]
- 69. Baetscher M, Schmidt E, Shimizu A, Leder P, Fishman MC. SV40 T antigen transforms calcitonin cells of the thyroid but not CGRP-containing neurons in transgenic mice. Oncogene 6: 1133–1138, 1991. [PubMed] [Google Scholar]
- 70. Durham PL, Dong PX, Belasco KT, Kasperski J, Gierasch WW, Edvinsson L, Heistad DD, Faraci FM, Russo AF. Neuronal expression and regulation of CGRP promoter activity following viral gene transfer into cultured trigeminal ganglia neurons. Brain Res 997: 103–110, 2004. doi: 10.1016/j.brainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 71. de Bustros A, Ball DW, Peters R, Compton D, Nelkin BD. Regulation of human calcitonin gene transcription by cyclic AMP. Biochem Biophys Res Commun 189: 1157–1164, 1992. doi: 10.1016/0006-291X(92)92325-R. [DOI] [PubMed] [Google Scholar]
- 72. Monla YT, Peleg S, Gagel RF, Monia YT. Cell type-specific regulation of transcription by cyclic adenosine 3′,5′-monophosphate-responsive elements within the calcitonin promoter. Mol Endocrinol 9: 784–793, 1995. doi: 10.1210/mend.9.7.7476962. [DOI] [PubMed] [Google Scholar]
- 73. Watson A, Latchman D. The cyclic AMP response element in the calcitonin/calcitonin gene-related peptide gene promoter is necessary but not sufficient for its activation by nerve growth factor. J Biol Chem 270: 9655–9660, 1995. doi: 10.1074/jbc.270.16.9655. [DOI] [PubMed] [Google Scholar]
- 74. Thiagalingam A, De Bustros A, Borges M, Jasti R, Compton D, Diamond L, Mabry M, Ball DW, Baylin SB, Nelkin BD. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol Cell Biol 16: 5335–5345, 1996. doi: 10.1128/MCB.16.10.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Peleg S, Abruzzese RV, Cote GJ, Gagel RF. Transcription of the human calcitonin gene is mediated by a C cell-specific enhancer containing E-box-like elements. Mol Endocrinol 4: 1750–1757, 1990. doi: 10.1210/mend-4-11-1750. [DOI] [PubMed] [Google Scholar]
- 76. Ball DW, Compton D, Nelkin BD, Baylin SB, de Bustros A. Human calcitonin gene regulation by helix-loop-helix recognition sequences. Nucleic Acids Res 20: 117–123, 1992. doi: 10.1093/nar/20.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tverberg LA, Russo AF. Regulation of the calcitonin/calcitonin gene-related peptide gene by cell-specific synergy between helix-loop-helix and octamer-binding transcription factors. J Biol Chem 268: 15965–15973, 1993. doi: 10.1016/S0021-9258(18)82346-8. [DOI] [PubMed] [Google Scholar]
- 78. Park KY, Russo AF. Control of the calcitonin gene-related peptide enhancer by upstream stimulatory factor in trigeminal ganglion neurons. J Biol Chem 283: 5441–5451, 2008. doi: 10.1074/jbc.M708662200. [DOI] [PubMed] [Google Scholar]
- 79. Lanigan TM, Russo AF. Binding of upstream stimulatory factor and a cell-specific activator to the calcitonin/calcitonin gene-related peptide enhancer. J Biol Chem 272: 18316–18324, 1997. doi: 10.1074/jbc.272.29.18316. [DOI] [PubMed] [Google Scholar]
- 80. Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem 96: 65–77, 2006. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci 27: 2693–2703, 2007. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Viney TJ, Schmidt TW, Gierasch W, Sattar AW, Yaggie RE, Kuburas A, Quinn JP, Coulson JM, Russo AF. Regulation of the cell-specific calcitonin/calcitonin gene-related peptide enhancer by USF and the Foxa2 forkhead protein. J Biol Chem 279: 49948–49955, 2004. doi: 10.1074/jbc.M406659200. [DOI] [PubMed] [Google Scholar]
- 83. Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 5: 193–208, 2004. doi: 10.1016/j.modgep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 84. Sirito M, Lin Q, Maity T, Sawadogo M. Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res 22: 427–433, 1994. doi: 10.1093/nar/22.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 503: 111–114, 2013. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Baylin SB, Höppener JW, de Bustros A, Steenbergh PH, Lips CJ, Nelkin BD. DNA methylation patterns of the calcitonin gene in human lung cancers and lymphomas. Cancer Res 46: 2917–2922, 1986. [PubMed] [Google Scholar]
- 87. Baylin SB, Fearon ER, Vogelstein B, de Bustros A, Sharkis SJ, Burke PJ, Staal SP, Nelkin BD. Hypermethylation of the 5′ region of the calcitonin gene is a property of human lymphoid and acute myeloid malignancies. Blood 70: 412–417, 1987. [PubMed] [Google Scholar]
- 88. Broad PM, Symes AJ, Thakker RV, Craig RK. Structure and methylation of the human calcitonin/alpha-CGRP gene. Nucleic Acids Res 17: 6999–7011, 1989. doi: 10.1093/nar/17.17.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Bustros A, Nelkin BD, Silverman A, Ehrlich G, Poiesz B, Baylin SB. The short arm of chromosome 11 is a “hot spot” for hypermethylation in human neoplasia. Proc Natl Acad Sci USA 85: 5693–5697, 1988. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Park KY, Fletcher JR, Raddant AC, Russo AF. Epigenetic regulation of the calcitonin gene-related peptide gene in trigeminal glia. Cephalalgia 31: 614–624, 2011. doi: 10.1177/0333102410391487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhao Z, Zhang Z, Li J, Dong Q, Xiong J, Li Y, Lan M, Li G, Zhu B. Sustained TNF-alpha stimulation leads to transcriptional memory that greatly enhances signal sensitivity and robustness. Elife 9: e61965, 2020. doi: 10.7554/eLife.61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 337: 362–364, 1989. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- 93. Moriguchi T, Gotoh Y, Nishida E. Activation of two isoforms of mitogen-activated protein kinase kinase in response to epidermal growth factor and nerve growth factor. Eur J Biochem 234: 32–38, 1995. doi: 10.1111/j.1432-1033.1995.032_c.x. [DOI] [PubMed] [Google Scholar]
- 94. Durham PL, Russo AF. Serotonergic repression of mitogen-activated protein kinase control of the calcitonin gene-related peptide enhancer. Mol Endocrinol 12: 1002–1009, 1998. doi: 10.1210/mend.12.7.0135. [DOI] [PubMed] [Google Scholar]
- 95. Hou L, Li W, Wang X. Mechanism of interleukin-1 beta-induced calcitonin gene-related peptide production from dorsal root ganglion neurons of neonatal rats. J Neurosci Res 73: 188–197, 2003. doi: 10.1002/jnr.10651. [DOI] [PubMed] [Google Scholar]
- 96. Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci 23: 2057–2066, 2006. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Moye LS, Siegersma K, Dripps I, Witkowski W, Mangutov E, Wang D, Scherrer G, Pradhan AA. Delta opioid receptor regulation of calcitonin gene-related peptide dynamics in the trigeminal complex. Pain 162: 2297–2308, 2021. doi: 10.1097/j.pain.0000000000002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Galibert MD, Carreira S, Goding CR. The Usf-1 transcription factor is a novel target for the stress-responsive p38 kinase and mediates UV-induced tyrosinase expression. EMBO J 20: 5022–5031, 2001. doi: 10.1093/emboj/20.17.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. deBustros A, Baylin SB, Levine MA, Nelkin BD. Cyclic AMP and phorbol esters separately induce growth inhibition, calcitonin secretion, and calcitonin gene transcription in cultured human medullary thyroid carcinoma. J Biol Chem 261: 8036–8041, 1986. doi: 10.1016/S0021-9258(19)57508-1. [DOI] [PubMed] [Google Scholar]
- 100. Freeland K, Liu YZ, Latchman DS. Distinct signalling pathways mediate the cAMP response element (CRE)-dependent activation of the calcitonin gene-related peptide gene promoter by cAMP and nerve growth factor. Biochem J 345: 233–238, 2000. doi: 10.1042/bj3450233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cote GJ, Abruzzese RV, Lips CJ, Gagel RF. Transfection of calcitonin gene regulatory elements into a cell culture model of the C cell. J Bone Miner Res 5: 165–171, 1990. doi: 10.1002/jbmr.5650050210. [DOI] [PubMed] [Google Scholar]
- 102. de Bustros A, Baylin SB, Berger CL, Roos BA, Leong SS, Nelkin BD. Phorbol esters increase calcitonin gene transcription and decrease c-myc mRNA levels in cultured human medullary thyroid carcinoma. J Biol Chem 260: 98–104, 1985. doi: 10.1016/S0021-9258(18)89699-5. [DOI] [PubMed] [Google Scholar]
- 103. Seybold VS, McCarson KE, Mermelstein PG, Groth RD, Abrahams LG. Calcitonin gene-related peptide regulates expression of neurokinin1 receptors by rat spinal neurons. J Neurosci 23: 1816–1824, 2003. doi: 10.1523/JNEUROSCI.23-05-01816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Choi RC, Ting AK, Lau FT, Xie HQ, Leung KW, Chen VP, Siow NL, Tsim KW. Calcitonin gene-related peptide induces the expression of acetylcholinesterase-associated collagen ColQ in muscle: a distinction in driving two different promoters between fast- and slow-twitch muscle fibers. J Neurochem 102: 1316–1328, 2007. doi: 10.1111/j.1471-4159.2007.04630.x. [DOI] [PubMed] [Google Scholar]
- 105. Anderson LE, Seybold VS. Calcitonin gene-related peptide regulates gene transcription in primary afferent neurons. J Neurochem 91: 1417–1429, 2004. doi: 10.1111/j.1471-4159.2004.02833.x. [DOI] [PubMed] [Google Scholar]
- 106. Walker CS, Raddant AC, Woolley MJ, Russo AF, Hay DL. CGRP receptor antagonist activity of olcegepant depends on the signalling pathway measured. Cephalalgia 38: 437–451, 2018. doi: 10.1177/0333102417691762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 169: 683–696, 2010. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 108. Lennerz JK, Rühle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol 507: 1277–1299, 2008. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- 109. Tajti J, Uddman R, Möller S, Sundler F, Edvinsson L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst 76: 176–183, 1999. doi: 10.1016/S0165-1838(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 110. Walker CS, Eftekhari S, Bower RL, Wilderman A, Insel PA, Edvinsson L, Waldvogel HJ, Jamaluddin MA, Russo AF, Hay DL. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol 2: 595–608, 2015. doi: 10.1002/acn3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rees TA, Russo AF, O’Carroll SJ, Hay DL, Walker CS. CGRP and the calcitonin receptor are co-expressed in mouse, rat and human trigeminal ganglia neurons. Front Physiol 13: 860037, 2022. doi: 10.3389/fphys.2022.860037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Edvinsson L, Eftekhari S, Salvatore CA, Warfvinge K. Cerebellar distribution of calcitonin gene-related peptide (CGRP) and its receptor components calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1) in rat. Mol Cell Neurosci 46: 333–339, 2011. doi: 10.1016/j.mcn.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 113. Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther 109: 173–197, 2006. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 114. Guo Z, Czerpaniak K, Zhang J, Cao YQ. Increase in trigeminal ganglion neurons that respond to both calcitonin gene-related peptide and pituitary adenylate cyclase-activating polypeptide in mouse models of chronic migraine and posttraumatic headache. Pain 162: 1483–1499, 2021. doi: 10.1097/j.pain.0000000000002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Russo AF, Lanigan TM, Sullivan BE. Neuronal properties of a thyroid C-cell line: partial repression by dexamethasone and retinoic acid. Mol Endocrinol 6: 207–218, 1992. doi: 10.1210/mend.6.2.1569964. [DOI] [PubMed] [Google Scholar]
- 116. Lanigan TM, Tverberg LA, Russo AF. Retinoic acid repression of cell-specific helix-loop-helix-octamer activation of the calcitonin/calcitonin gene-related peptide enhancer. Mol Cell Biol 13: 6079–6088, 1993. doi: 10.1128/mcb.13.10.6079-6088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tverberg LA, Russo AF. Cell-specific glucocorticoid repression of calcitonin/calcitonin gene-related peptide transcription. Localization to an 18-base pair basal enhancer element. J Biol Chem 267: 17567–17573, 1992. doi: 10.1016/S0021-9258(19)37080-2. [DOI] [PubMed] [Google Scholar]
- 118. Naveh-Many T, Silver J. Regulation of calcitonin gene transcription by vitamin D metabolites in vivo in the rat. J Clin Invest 81: 270–273, 1988. doi: 10.1172/JCI113305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Peleg S, Abruzzese RV, Cooper CW, Gagel RF. Down-regulation of calcitonin gene transcription by vitamin D requires two widely separated enhancer sequences. Mol Endocrinol 7: 999–1008, 1993. doi: 10.1210/mend.7.8.8232320. [DOI] [PubMed] [Google Scholar]
- 120. Russo AF, Gagel R. Vitamin D control of the calcitonin gene in thyroid C cells. In: Vitamin D, edited by Feldman D. San Diego, CA: Elsevier Academic Press, 2005. [Google Scholar]
- 121. Durham PL, Russo AF. New insights into the molecular actions of serotonergic antimigraine drugs. Pharmacol Ther 94: 77–92, 2002. doi: 10.1016/S0163-7258(02)00173-0. [DOI] [PubMed] [Google Scholar]
- 122. Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 25: 179–183, 2005. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 123. Durham PL, Sharma RV, Russo AF. Repression of the calcitonin gene-related peptide promoter by 5-HT1 receptor activation. J Neurosci 17: 9545–9553, 1997. doi: 10.1523/JNEUROSCI.17-24-09545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Durham PL, Russo AF. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci 23: 807–815, 2003. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Seternes OM, Kidger AM, Keyse SM. Dual-specificity MAP kinase phosphatases in health and disease. Biochim Biophys Acta Mol Cell Res 1866: 124–143, 2019. doi: 10.1016/j.bbamcr.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Roth Flach RJ, Bennett AM. Mitogen-activated protein kinase phosphatase-1—a potential therapeutic target in metabolic disease. Expert Opin Ther Targets 14: 1323–1332, 2010. doi: 10.1517/14728222.2010.528395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nunes-Xavier C, Romá-Mateo C, Ríos P, Tárrega C, Cejudo-Marín R, Tabernero L, Pulido R. Dual-specificity MAP kinase phosphatases as targets of cancer treatment. Anticancer Agents Med Chem 11: 109–132, 2011. doi: 10.2174/187152011794941190. [DOI] [PubMed] [Google Scholar]
- 128. Hoppstädter J, Ammit AJ. Role of dual-specificity phosphatase 1 in glucocorticoid-driven anti-inflammatory responses. Front Immunol 10: 1446, 2019. doi: 10.3389/fimmu.2019.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Durham PL, Russo AF. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J Neurosci 19: 3423–3429, 1999. doi: 10.1523/JNEUROSCI.19-09-03423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Eltorp CT, Jansen-Olesen I, Hansen AJ. Release of calcitonin gene-related peptide (CGRP) from guinea pig dura mater in vitro is inhibited by sumatriptan but unaffected by nitric oxide. Cephalalgia 20: 838–844, 2000. doi: 10.1046/j.1468-2982.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- 131. Arvieu L, Mauborgne A, Bourgoin S, Oliver C, Feltz P, Hamon M, Cesselin F. Sumatriptan inhibits the release of CGRP and substance P from the rat spinal cord. Neuroreport 7: 1973–1976, 1996. doi: 10.1097/00001756-199608120-00023. [DOI] [PubMed] [Google Scholar]
- 132. Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med 346: 257–270, 2002. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 133. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33: 48–56, 1993. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 134. Diener HC, Katsarava Z. Medication overuse headache. Curr Med Res Opin 17, Suppl 1: s17–s21, 2001. doi: 10.1185/0300799039117003. [DOI] [PubMed] [Google Scholar]
- 135. Zhou HL, Lou H. Repression of prespliceosome complex formation at two distinct steps by Fox-1/Fox-2 proteins. Mol Cell Biol 28: 5507–5516, 2008. doi: 10.1128/MCB.00530-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhou HL, Baraniak AP, Lou H. Role for Fox-1/Fox-2 in mediating the neuronal pathway of calcitonin/calcitonin gene-related peptide alternative RNA processing. Mol Cell Biol 27: 830–841, 2007. doi: 10.1128/MCB.01015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Emeson RB, Hedjran F, Yeakley JM, Guise JW, Rosenfeld MG. Alternative production of calcitonin and CGRP mRNA is regulated at the calcitonin-specific splice acceptor. Nature 341: 76–80, 1989. doi: 10.1038/341076a0. [DOI] [PubMed] [Google Scholar]
- 138. Lou H, Gagel RF. Alternative RNA processing–its role in regulating expression of calcitonin/calcitonin gene-related peptide. J Endocrinol 156: 401–405, 1998. doi: 10.1677/joe.0.1560401. [DOI] [PubMed] [Google Scholar]
- 139. Lou H, Yang Y, Cote GJ, Berget SM, Gagel RF. An intron enhancer containing a 5′ splice site sequence in the human calcitonin/calcitonin gene-related peptide gene. Mol Cell Biol 15: 7135–7142, 1995. doi: 10.1128/MCB.15.12.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tran Q, Roesser JR. SRp55 is a regulator of calcitonin/CGRP alternative RNA splicing. Biochemistry 42: 951–957, 2003. doi: 10.1021/bi026753a. [DOI] [PubMed] [Google Scholar]
- 141. Roesser JR. Both U2 snRNA and U12 snRNA are required for accurate splicing of exon 5 of the rat calcitonin/CGRP gene. RNA 10: 1243–1250, 2004. doi: 10.1261/rna.5210404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhu H, Hasman RA, Barron VA, Luo G, Lou H. A nuclear function of Hu proteins as neuron-specific alternative RNA processing regulators. Mol Biol Cell 17: 5105–5114, 2006. doi: 10.1091/mbc.e06-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Labastida-Ramírez A, Rubio-Beltrán E, Haanes KA, Chan KY, Garrelds IM, Johnson KW, Danser AH, Villalón CM, MaassenVanDenBrink A. Lasmiditan inhibits calcitonin gene-related peptide release in the rodent trigeminovascular system. Pain 161: 1092–1099, 2020. doi: 10.1097/j.pain.0000000000001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Goadsby PJ, Hoskin KL, Storer RJ, Edvinsson L, Connor HE. Adenosine A1 receptor agonists inhibit trigeminovascular nociceptive transmission. Brain 125: 1392–1401, 2002. doi: 10.1093/brain/awf141. [DOI] [PubMed] [Google Scholar]
- 145. Cohen CF, Prudente AS, Berta T, Lee SH. Transient receptor potential channel 4 small-molecule inhibition alleviates migraine-like behavior in mice. Front Mol Neurosci 14: 765181, 2021. doi: 10.3389/fnmol.2021.765181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang H, Zhang XM, Zong DD, Ji XY, Jiang H, Zhang FZ, He SD. miR-34a-5p up-regulates the IL-1beta/COX2/PGE2 inflammation pathway and induces the release of CGRP via inhibition of SIRT1 in rat trigeminal ganglion neurons. FEBS Open Bio 11: 300–311, 2021. doi: 10.1002/2211-5463.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Andersen HH, Duroux M, Gazerani P. Serum microRNA signatures in migraineurs during attacks and in pain-free periods. Mol Neurobiol 53: 1494–1500, 2016. doi: 10.1007/s12035-015-9106-5. [DOI] [PubMed] [Google Scholar]
- 148. Greco R, De Icco R, Demartini C, Zanaboni AM, Tumelero E, Sances G, Allena M, Tassorelli C. Plasma levels of CGRP and expression of specific microRNAs in blood cells of episodic and chronic migraine subjects: towards the identification of a panel of peripheral biomarkers of migraine? J Headache Pain 21: 122, 2020. doi: 10.1186/s10194-020-01189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang L, Kunkler PE, Knopp KL, Oxford GS, Hurley JH. Role of intraganglionic transmission in the trigeminovascular pathway. Mol Pain 15: 1744806919836570, 2019. doi: 10.1177/1744806919836570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Marone IM, De Logu F, Nassini R, De Carvalho Goncalves M, Benemei S, Ferreira J, Jain P, Li Puma S, Bunnett NW, Geppetti P, Materazzi S. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 141: 2312–2328, 2018. doi: 10.1093/brain/awy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia 39: 1661–1674, 2019. doi: 10.1177/0333102418786261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Carr R, Frings S. Neuropeptides in sensory signal processing. Cell Tissue Res 375: 217–225, 2019. doi: 10.1007/s00441-018-2946-3. [DOI] [PubMed] [Google Scholar]
- 153. Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature 313: 54–56, 1985. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 154. Brain SD, Williams TJ. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature 335: 73–75, 1988. doi: 10.1038/335073a0. [DOI] [PubMed] [Google Scholar]
- 155. Mulderry PK, Ghatei MA, Bloom SR. In vitro production and characterisation of low molecular weight forms of calcitonin gene-related peptide immunoreactivity from rat thyroid. Biochem Biophys Res Commun 144: 883–890, 1987. doi: 10.1016/S0006-291X(87)80047-5. [DOI] [PubMed] [Google Scholar]
- 156. Walls AF, Brain SD, Desai A, Jose PJ, Hawkings E, Church MK, Williams TJ. Human mast cell tryptase attenuates the vasodilator activity of calcitonin gene-related peptide. Biochem Pharmacol 43: 1243–1248, 1992. doi: 10.1016/0006-2952(92)90498-8. [DOI] [PubMed] [Google Scholar]
- 157. Tam EK, Caughey GH. Degradation of airway neuropeptides by human lung tryptase. Am J Respir Cell Mol Biol 3: 27–32, 1990. doi: 10.1165/ajrcmb/3.1.27. [DOI] [PubMed] [Google Scholar]
- 158. Ruchon AF, Marcinkiewicz M, Ellefsen K, Basak A, Aubin J, Crine P, Boileau G. Cellular localization of neprilysin in mouse bone tissue and putative role in hydrolysis of osteogenic peptides. J Bone Miner Res 15: 1266–1274, 2000. doi: 10.1359/jbmr.2000.15.7.1266. [DOI] [PubMed] [Google Scholar]
- 159. McDowell G, Coutie W, Shaw C, Buchanan KD, Struthers AD, Nicholls DP. The effect of the neutral endopeptidase inhibitor drug, candoxatril, on circulating levels of two of the most potent vasoactive peptides. Br J Clin Pharmacol 43: 329–332, 1997. doi: 10.1046/j.1365-2125.1997.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ludwig R, Feindt J, Lucius R, Petersen A, Mentlein R. Metabolism of neuropeptide Y and calcitonin gene-related peptide by cultivated neurons and glial cells. Brain Res Mol Brain Res 37: 181–191, 1996. doi: 10.1016/0169-328X(95)00312-G. [DOI] [PubMed] [Google Scholar]
- 161. Le Grevès P, Nyberg F, Hökfelt T, Terenius L. Calcitonin gene-related peptide is metabolized by an endopeptidase hydrolyzing substance P. Regul Pept 25: 277–286, 1989. doi: 10.1016/0167-0115(89)90176-6. [DOI] [PubMed] [Google Scholar]
- 162. Katayama M, Nadel JA, Bunnett NW, Di Maria GU, Haxhiu M, Borson DB. Catabolism of calcitonin gene-related peptide and substance P by neutral endopeptidase. Peptides 12: 563–567, 1991. doi: 10.1016/0196-9781(91)90102-U. [DOI] [PubMed] [Google Scholar]
- 163. Davies D, Medeiros MS, Keen J, Turner AJ, Haynes LW. Eosinophil chemotactic peptide sequences in rat alpha-CGRP. Activation of a novel trophic action by neutral endopeptidase 24.11. Ann N Y Acad Sci 657: 405–411, 1992. doi: 10.1111/j.1749-6632.1992.tb22786.x. [DOI] [PubMed] [Google Scholar]
- 164. Chen JJ, Barber LA, Dymshitz J, Vasko MR. Peptidase inhibitors improve recovery of substance P and calcitonin gene-related peptide release from rat spinal cord slices. Peptides 17: 31–37, 1996. doi: 10.1016/0196-9781(95)02091-8. [DOI] [PubMed] [Google Scholar]
- 165. Padilla BE, Cottrell GS, Roosterman D, Pikios S, Muller L, Steinhoff M, Bunnett NW. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins. J Cell Biol 179: 981–997, 2007. doi: 10.1083/jcb.200704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sams-Nielsen A, Orskov C, Jansen-Olesen I. Pharmacological evidence for CGRP uptake into perivascular capsaicin sensitive nerve terminals. Br J Pharmacol 132: 1145–1153, 2001. doi: 10.1038/sj.bjp.0703910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Braslis KG, Shulkes A, Fletcher DR, Hardy KJ. Pharmacokinetics and organ-specific metabolism of calcitonin gene-related peptide in sheep. J Endocrinol 118: 25–31, 1988. doi: 10.1677/joe.0.1180025. [DOI] [PubMed] [Google Scholar]
- 168. Rubinstein C, Fletcher DR, Shulkes A, Hardy KJ. The role of the kidney in the clearance of calcitonin gene related peptide (CGRP). Aust NZ J Surg 64: 266–269, 1994. doi: 10.1111/j.1445-2197.1994.tb02199.x. [DOI] [PubMed] [Google Scholar]
- 169. Fernandez-Patron C, Stewart KG, Zhang Y, Koivunen E, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ Res 87: 670–676, 2000. doi: 10.1161/01.RES.87.8.670. [DOI] [PubMed] [Google Scholar]
- 170. Eftekhari S, Salvatore CA, Gaspar RC, Roberts R, O’Malley S, Zeng Z, Edvinsson L. Localization of CGRP receptor components, CGRP, and receptor binding sites in human and rhesus cerebellar cortex. Cerebellum 12: 937–949, 2013. doi: 10.1007/s12311-013-0509-4. [DOI] [PubMed] [Google Scholar]
- 171. Eftekhari S, Edvinsson L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci 12: 112, 2011. doi: 10.1186/1471-2202-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Tschopp FA, Henke H, Petermann JB, Tobler PH, Janzer R, Hökfelt T, Lundberg JM, Cuello C, Fischer JA. Calcitonin gene-related peptide and its binding sites in the human central nervous system and pituitary. Proc Natl Acad Sci USA 82: 248–252, 1985. doi: 10.1073/pnas.82.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Tschopp FA, Tobler PH, Fischer JA. Calcitonin gene-related peptide in the human thyroid, pituitary and brain. Mol Cell Endocrinol 36: 53–57, 1984. doi: 10.1016/0303-7207(84)90084-4. [DOI] [PubMed] [Google Scholar]
- 174. Smith D, Hill RG, Edvinsson L, Longmore J. An immunocytochemical investigation of human trigeminal nucleus caudalis: CGRP, substance P and 5-HT1D-receptor immunoreactivities are expressed by trigeminal sensory fibres. Cephalalgia 22: 424–431, 2002. doi: 10.1046/j.1468-2982.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- 175. Uddman R, Tajti J, Hou M, Sundler F, Edvinsson L. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia 22: 112–116, 2002. doi: 10.1046/j.1468-2982.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- 176. Eftekhari S, Gaspar RC, Roberts R, Chen TB, Zeng Z, Villarreal S, Edvinsson L, Salvatore CA. Localization of CGRP receptor components and receptor binding sites in rhesus monkey brainstem: a detailed study using in situ hybridization, immunofluorescence, and autoradiography. J Comp Neurol 524: 90–118, 2016. doi: 10.1002/cne.23828. [DOI] [PubMed] [Google Scholar]
- 177. Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 1600: 93–109, 2015. doi: 10.1016/j.brainres.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 178. Skofitsch G, Jacobowitz DM. Autoradiographic distribution of 125I calcitonin gene-related peptide binding sites in the rat central nervous system. Peptides 6: 975–986, 1985. doi: 10.1016/0196-9781(85)90331-6. [DOI] [PubMed] [Google Scholar]
- 179. Inagaki S, Kito S, Kubota Y, Girgis S, Hillyard CJ, MacIntyre I. Autoradiographic localization of calcitonin gene-related peptide binding sites in human and rat brains. Brain Res 374: 287–298, 1986. doi: 10.1016/0006-8993(86)90423-3. [DOI] [PubMed] [Google Scholar]
- 180. Sexton PM, McKenzie JS, Mendelsohn FA. Evidence for a new subclass of calcitonin/calcitonin gene-related peptide binding site in rat brain. Neurochem Int 12: 323–335, 1988. doi: 10.1016/0197-0186(88)90171-4. [DOI] [PubMed] [Google Scholar]
- 181. Sexton PM, McKenzie JS, Mason RT, Moseley JM, Martin TJ, Mendelsohn FA. Localization of binding sites for calcitonin gene-related peptide in rat brain by in vitro autoradiography. Neuroscience 19: 1235–1245, 1986. doi: 10.1016/0306-4522(86)90137-5. [DOI] [PubMed] [Google Scholar]
- 182. van Rossum D, Ménard DP, Chang JK, Quirion R. Comparative affinities of human adrenomedullin for 125I-labelled human alpha calcitonin gene related peptide ([125I]hCGRP alpha) and 125I-labelled Bolton-Hunter rat amylin ([125I]BHrAMY) specific binding sites in the rat brain. Can J Physiol Pharmacol 73: 1084–1088, 1995. doi: 10.1139/y95-155. [DOI] [PubMed] [Google Scholar]
- 183. Li XF, Kinsey-Jones JS, Bowe JE, Wilkinson ES, Brain SD, Lightman SL, O’Byrne KT. A role for the medial preoptic area in CGRP-induced suppression of pulsatile LH secretion in the female rat. Stress 12: 259–267, 2009. doi: 10.1080/10253890802379922. [DOI] [PubMed] [Google Scholar]
- 184. Pozo-Rosich P, Storer RJ, Charbit AR, Goadsby PJ. Periaqueductal gray calcitonin gene-related peptide modulates trigeminovascular neurons. Cephalalgia 35: 1298–1307, 2015. doi: 10.1177/0333102415576723. [DOI] [PubMed] [Google Scholar]
- 185. Lu YC, Chen YZ, Wei YY, He XT, Li X, Hu W, Yanagawa Y, Wang W, Wu SX, Dong YL. Neurochemical properties of the synapses between the parabrachial nucleus-derived CGRP-positive axonal terminals and the GABAergic neurons in the lateral capsular division of central nucleus of amygdala. Mol Neurobiol 51: 105–118, 2015. doi: 10.1007/s12035-014-8713-x. [DOI] [PubMed] [Google Scholar]
- 186. Ji Y, Rizk A, Voulalas P, Aljohani H, Akerman S, Dussor G, Keller A, Masri R. Sex differences in the expression of calcitonin gene-related peptide receptor components in the spinal trigeminal nucleus. Neurobiol Pain 6: 100031, 2019. doi: 10.1016/j.ynpai.2019.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Filiz A, Tepe N, Eftekhari S, Boran HE, Dilekoz E, Edvinsson L, Bolay H. CGRP receptor antagonist MK-8825 attenuates cortical spreading depression induced pain behavior. Cephalalgia 39: 354–365, 2019. doi: 10.1177/0333102417735845. [DOI] [PubMed] [Google Scholar]
- 188. Chen R, Wu X, Jiang L, Zhang Y. Single-cell RNA-Seq reveals hypothalamic cell diversity. Cell Rep 18: 3227–3241, 2017. doi: 10.1016/j.celrep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Li N, Fang CY, Wang ZZ, Wang YL, Wang FB, Gao E, Zhang GX. Expression of calcitonin gene-related peptide type 1 receptor mRNA and their activity-modifying proteins in the rat nucleus accumbens. Neurosci Lett 362: 146–149, 2004. doi: 10.1016/j.neulet.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 190. Oliver KR, Wainwright A, Heavens RP, Hill RG, Sirinathsinghji DJ. Distribution of novel CGRP1 receptor and adrenomedullin receptor mRNAs in the rat central nervous system. Brain Res Mol Brain Res 57: 149–154, 1998. doi: 10.1016/S0169-328X(98)00052-7. [DOI] [PubMed] [Google Scholar]
- 191. Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE, Berman NE. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache 51: 674–692, 2011. doi: 10.1111/j.1526-4610.2011.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Summ O, Charbit AR, Andreou AP, Goadsby PJ. Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain 133: 2540–2548, 2010. doi: 10.1093/brain/awq224. [DOI] [PubMed] [Google Scholar]
- 193. Warfvinge K, Edvinsson L. Distribution of CGRP and CGRP receptor components in the rat brain. Cephalalgia 39: 342–353, 2019. doi: 10.1177/0333102417728873. [DOI] [PubMed] [Google Scholar]
- 194. Miller S, Liu H, Warfvinge K, Shi L, Dovlatyan M, Xu C, Edvinsson L. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 328: 165–183, 2016. doi: 10.1016/j.neuroscience.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 195. Reiner DJ, Mietlicki-Baase EG, Olivos DR, McGrath LE, Zimmer DJ, Koch-Laskowski K, Krawczyk J, Turner CA, Noble EE, Hahn JD, Schmidt HD, Kanoski SE, Hayes MR. Amylin acts in the lateral dorsal tegmental nucleus to regulate energy balance through gamma-aminobutyric acid signaling. Biol Psychiatry 82: 828–838, 2017. doi: 10.1016/j.biopsych.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Baisley SK, Bremer QZ, Bakshi VP, Baldo BA. Antipsychotic-like actions of the satiety peptide, amylin, in ventral striatal regions marked by overlapping calcitonin receptor and RAMP-1 gene expression. J Neurosci 34: 4318–4325, 2014. doi: 10.1523/JNEUROSCI.2260-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Barth SW, Riediger T, Lutz TA, Rechkemmer G. Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res 997: 97–102, 2004. doi: 10.1016/j.brainres.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 198. Liberini CG, Boyle CN, Cifani C, Venniro M, Hope BT, Lutz TA. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur J Neurosci 43: 653–661, 2016. doi: 10.1111/ejn.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Oliver KR, Kane SA, Salvatore CA, Mallee JJ, Kinsey AM, Koblan KS, Keyvan-Fouladi N, Heavens RP, Wainwright A, Jacobson M, Dickerson IM, Hill RG. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur J Neurosci 14: 618–628, 2001. doi: 10.1046/j.0953-816x.2001.01688.x. [DOI] [PubMed] [Google Scholar]
- 200. Ueda T, Ugawa S, Saishin Y, Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res Mol Brain Res 93: 36–45, 2001. doi: 10.1016/S0169-328X(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 201. Sheward WJ, Lutz EM, Harmar AJ. The expression of the calcitonin receptor gene in the brain and pituitary gland of the rat. Neurosci Lett 181: 31–34, 1994. doi: 10.1016/0304-3940(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 202. Assadullah, Ieda N, Kawai N, Ishii H, Ihara K, Inoue N, Uenoyama Y, Tsukamura H. Co-expression of the calcitonin receptor gene in the hypothalamic kisspeptin neurons in female rats. Reprod Med Biol 17: 164–172, 2018. doi: 10.1002/rmb2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Doi M, Murai I, Kunisue S, Setsu G, Uchio N, Tanaka R, Kobayashi S, Shimatani H, Hayashi H, Chao HW, Nakagawa Y, Takahashi Y, Hotta Y, Yasunaga J, Matsuoka M, Hastings MH, Kiyonari H, Okamura H. Gpr176 is a Gz-linked orphan G-protein-coupled receptor that sets the pace of circadian behaviour. Nat Commun 7: 10583, 2016. doi: 10.1038/ncomms10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Hendrikse ER, Rees TA, Tasma Z, Le Foll C, Lutz TA, Siow A, Wookey PJ, Walker CS, Hay DL. Calcitonin receptor antibody validation and expression in the rodent brain. Cephalalgia 42: 815–826, 2022. doi: 10.1177/03331024221084029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Becskei C, Riediger T, Zünd D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res 1030: 221–233, 2004. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 206. Coester B, Koester-Hegmann C, Lutz TA, Le Foll C. Amylin/calcitonin receptor-mediated signaling in POMC neurons influences energy balance and locomotor activity in chow-fed male mice. Diabetes 69: 1110–1125, 2020. doi: 10.2337/db19-0849. [DOI] [PubMed] [Google Scholar]
- 207. Huang X, Yang J, Chang JK, Dun NJ. Amylin suppresses acetic acid-induced visceral pain and spinal c-fos expression in the mouse. Neuroscience 165: 1429–1438, 2010. doi: 10.1016/j.neuroscience.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Nakamoto H, Soeda Y, Takami S, Minami M, Satoh M. Localization of calcitonin receptor mRNA in the mouse brain: coexistence with serotonin transporter mRNA. Brain Res Mol Brain Res 76: 93–102, 2000. doi: 10.1016/S0169-328X(99)00335-6. [DOI] [PubMed] [Google Scholar]
- 209. Nashawi H, Gustafson TJ, Mietlicki-Baase EG. Palatable food access impacts expression of amylin receptor components in the mesocorticolimbic system. Exp Physiol 105: 1012–1024, 2020. doi: 10.1113/EP088356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Tolcos M, Tikellis C, Rees S, Cooper M, Wookey P. Ontogeny of calcitonin receptor mRNA and protein in the developing central nervous system of the rat. J Comp Neurol 456: 29–38, 2003. doi: 10.1002/cne.10478. [DOI] [PubMed] [Google Scholar]
- 211. Bower RL, Eftekhari S, Waldvogel HJ, Faull RL, Tajti J, Edvinsson L, Hay DL, Walker CS. Mapping the calcitonin receptor in human brain stem. Am J Physiol Regul Integr Comp Physiol 310: R788–R793, 2016. doi: 10.1152/ajpregu.00539.2015. [DOI] [PubMed] [Google Scholar]
- 212. Chance WT, Balasubramaniam A, Zhang FS, Wimalawansa SJ, Fischer JE. Anorexia following the intrahypothalamic administration of amylin. Brain Res 539: 352–354, 1991. doi: 10.1016/0006-8993(91)91644-G. [DOI] [PubMed] [Google Scholar]
- 213. D’Este L, Casini A, Wimalawansa SJ, Renda TG. Immunohistochemical localization of amylin in rat brainstem. Peptides 21: 1743–1749, 2000. doi: 10.1016/S0196-9781(00)00325-9. [DOI] [PubMed] [Google Scholar]
- 214. Szabó ER, Cservenák M, Dobolyi A. Amylin is a novel neuropeptide with potential maternal functions in the rat. FASEB J 26: 272–281, 2012. doi: 10.1096/fj.11-191841. [DOI] [PubMed] [Google Scholar]
- 215. Dobolyi A. Central amylin expression and its induction in rat dams. J Neurochem 111: 1490–1500, 2009. doi: 10.1111/j.1471-4159.2009.06422.x. [DOI] [PubMed] [Google Scholar]
- 216. Ferrier GJ, Pierson AM, Jones PM, Bloom SR, Girgis SI, Legon S. Expression of the rat amylin (IAPP/DAP) gene. J Mol Endocrinol 3: R1–R4, 1989. doi: 10.1677/jme.0.003R001. [DOI] [PubMed] [Google Scholar]
- 217. Li Z, Kelly L, Heiman M, Greengard P, Friedman JM. Hypothalamic amylin acts in concert with leptin to regulate food intake. Cell Metab 22: 1059–1067, 2015. doi: 10.1016/j.cmet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 218. Skofitsch G, Wimalawansa SJ, Jacobowitz DM, Gubisch W. Comparative immunohistochemical distribution of amylin-like and calcitonin gene related peptide like immunoreactivity in the rat central nervous system. Can J Physiol Pharmacol 73: 945–956, 1995. doi: 10.1139/y95-131. [DOI] [PubMed] [Google Scholar]
- 219. Martinez-Valbuena I, Valenti-Azcarate R, Amat-Villegas I, Riverol M, Marcilla I, de Andrea CE, Sánchez-Arias JA, Del Mar Carmona-Abellan M, Marti G, Erro ME, Martínez-Vila E, Tuñon MT, Luquin MR. Amylin as a potential link between type 2 diabetes and Alzheimer disease. Ann Neurol 86: 539–551, 2019. doi: 10.1002/ana.25570. [DOI] [PubMed] [Google Scholar]
- 220. Rodrigues EC, Grawenhoff J, Baumann SJ, Lorenzon N, Maurer SP. Mammalian neuronal mRNA transport complexes: the few knowns and the many unknowns. Front Integr Neurosci 15: 692948, 2021. doi: 10.3389/fnint.2021.692948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Rees TA, Hendrikse ER, Hay DL, Walker CS. Beyond CGRP: the calcitonin peptide family as targets for migraine and pain. Br J Pharmacol 179: 381–399, 2022. doi: 10.1111/bph.15605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Rees TA, Hay DL, Walker CS. Amylin antibodies frequently display cross-reactivity with CGRP: characterization of eight amylin antibodies. Am J Physiol Regul Integr Comp Physiol 320: R697–R703, 2021. doi: 10.1152/ajpregu.00338.2020. [DOI] [PubMed] [Google Scholar]
- 223. Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, Rimm DL, Rodriguez H, Hiltke T, Snyder M, Yamamoto T. A proposal for validation of antibodies. Nat Methods 13: 823–827, 2016. doi: 10.1038/nmeth.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Shiers SI, Sankaranarayanan I, Jeevakumar V, Cervantes A, Reese JC, Price TJ. Convergence of peptidergic and non-peptidergic protein markers in the human dorsal root ganglion and spinal dorsal horn. J Comp Neurol 529: 2771–2788, 2021. doi: 10.1002/cne.25122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Shiers S, Klein RM, Price TJ. Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain 161: 2410–2424, 2020. doi: 10.1097/j.pain.0000000000001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Lee Y, Kawai Y, Shiosaka S, Takami K, Kiyama H, Hillyard CJ, Girgis S, MacIntyre I, Emson PC, Tohyama M. Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat: immunohistochemical analysis. Brain Res 330: 194–196, 1985. doi: 10.1016/0006-8993(85)90027-7. [DOI] [PubMed] [Google Scholar]
- 227. Lee Y, Takami K, Kawai Y, Girgis S, Hillyard CJ, MacIntyre I, Emson PC, Tohyama M. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience 15: 1227–1237, 1985. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- 228. Mason RT, Peterfreund RA, Sawchenko PE, Corrigan AZ, Rivier JE, Vale WW. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature 308: 653–655, 1984. doi: 10.1038/308653a0. [DOI] [PubMed] [Google Scholar]
- 229. Kruger L, Sternini C, Brecha NC, Mantyh PW. Distribution of calcitonin gene-related peptide immunoreactivity in relation to the rat central somatosensory projection. J Comp Neurol 273: 149–162, 1988. doi: 10.1002/cne.902730203. [DOI] [PubMed] [Google Scholar]
- 230. Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide: detailed immunohistochemical distribution in the central nervous system. Peptides 6: 721–745, 1985. doi: 10.1016/0196-9781(85)90178-0. [DOI] [PubMed] [Google Scholar]
- 231. Réthelyi M, Metz CB, Lund PK. Distribution of neurons expressing calcitonin gene-related peptide mRNAs in the brain stem, spinal cord and dorsal root ganglia of rat and guinea-pig. Neuroscience 29: 225–239, 1989. doi: 10.1016/0306-4522(89)90345-X. [DOI] [PubMed] [Google Scholar]
- 232. Schütz B, Mauer D, Salmon AM, Changeux JP, Zimmer A. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J Comp Neurol 476: 32–43, 2004. doi: 10.1002/cne.20211. [DOI] [PubMed] [Google Scholar]
- 233. Noguchi K, Senba E, Morita Y, Sato M, Tohyama M. Alpha-CGRP and beta-CGRP mRNAs are differentially regulated in the rat spinal cord and dorsal root ganglion. Brain Res Mol Brain Res 7: 299–304, 1990. doi: 10.1016/0169-328X(90)90080-W. [DOI] [PubMed] [Google Scholar]
- 234. Noguchi K, Senba E, Morita Y, Sato M, Tohyama M. Co-expression of alpha-CGRP and beta-CGRP mRNAs in the rat dorsal root ganglion cells. Neurosci Lett 108: 1–5, 1990. doi: 10.1016/0304-3940(90)90696-7. [DOI] [PubMed] [Google Scholar]
- 235. Xiang Q, Tao JS, Li JJ, Tian RB, Li XH. Changes in dorsal root ganglion CGRP expression in mouse pinch nerve injury model: modulation by somatostatin type-2 receptor. J Chem Neuroanat 121: 102086, 2022. doi: 10.1016/j.jchemneu.2022.102086. [DOI] [PubMed] [Google Scholar]
- 236. Tie-Jun SS, Xu Z, Hökfelt T. The expression of calcitonin gene-related peptide in dorsal horn neurons of the mouse lumbar spinal cord. Neuroreport 12: 739–743, 2001. doi: 10.1097/00001756-200103260-00025. [DOI] [PubMed] [Google Scholar]
- 237. Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 490: 239–255, 2005. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- 238. Skofitsch G, Jacobowitz DM. Quantitative distribution of calcitonin gene-related peptide in the rat central nervous system. Peptides 6: 1069–1073, 1985. doi: 10.1016/0196-9781(85)90429-2. [DOI] [PubMed] [Google Scholar]
- 239. Copray JC, Ter Horst GJ, Liem RS, van Willigen JD. Neurotransmitters and neuropeptides within the mesencephalic trigeminal nucleus of the rat: an immunohistochemical analysis. Neuroscience 37: 399–411, 1990. doi: 10.1016/0306-4522(90)90410-6. [DOI] [PubMed] [Google Scholar]
- 240. Kawai Y, Takami K, Shiosaka S, Emson PC, Hillyard CJ, Girgis S, MacIntyre I, Tohyama M. Topographic localization of calcitonin gene-related peptide in the rat brain: an immunohistochemical analysis. Neuroscience 15: 747–763, 1985. doi: 10.1016/0306-4522(85)90076-4. [DOI] [PubMed] [Google Scholar]
- 241. Peltier AC, Bishop GA. The site of origin of calcitonin gene-related peptide-like immunoreactive afferents to the inferior olivary complex of the mouse. Neurosci Res 34: 177–186, 1999. doi: 10.1016/S0168-0102(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 242. Kruger L, Mantyh PW, Sternini C, Brecha NC, Mantyh CR. Calcitonin gene-related peptide (CGRP) in the rat central nervous system: patterns of immunoreactivity and receptor binding sites. Brain Res 463: 223–244, 1988. doi: 10.1016/0006-8993(88)90395-2. [DOI] [PubMed] [Google Scholar]
- 243. Smith GS, Savery D, Marden C, López Costa JJ, Averill S, Priestley JV, Rattray M. Distribution of messenger RNAs encoding enkephalin, substance P, somatostatin, galanin, vasoactive intestinal polypeptide, neuropeptide Y, and calcitonin gene-related peptide in the midbrain periaqueductal grey in the rat. J Comp Neurol 350: 23–40, 1994. doi: 10.1002/cne.903500103. [DOI] [PubMed] [Google Scholar]
- 244. Kresse A, Jacobowitz DM, Skofitsch G. Detailed mapping of CGRP mRNA expression in the rat central nervous system: comparison with previous immunocytochemical findings. Brain Res Bull 36: 261–274, 1995. doi: 10.1016/0361-9230(94)00201-B. [DOI] [PubMed] [Google Scholar]
- 245. Nemoto T, Konno A, Chiba T. Synaptic contact of neuropeptide-and amine-containing axons on parasympathetic preganglionic neurons in the superior salivatory nucleus of the rat. Brain Res 685: 33–45, 1995. doi: 10.1016/0006-8993(95)00409-J. [DOI] [PubMed] [Google Scholar]
- 246. Warfvinge K, Edvinsson L, Pickering DS, Sheykhzade M. The presence of calcitonin gene-related peptide and its receptors in rat, pig and human brain: species differences in calcitonin gene-related peptide pharmacology. Pharmacology 104: 332–341, 2019. doi: 10.1159/000502471. [DOI] [PubMed] [Google Scholar]
- 247. Yasui Y, Saper CB, Cechetto DF. Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat. J Comp Neurol 308: 293–310, 1991. doi: 10.1002/cne.903080212. [DOI] [PubMed] [Google Scholar]
- 248. Sowers LP, Wang M, Rea BJ, Taugher RJ, Kuburas A, Kim Y, Wemmie JA, Walker CS, Hay DL, Russo AF. Stimulation of posterior thalamic nuclei induces photophobic behavior in mice. Headache 60: 1961–1981, 2020. doi: 10.1111/head.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Yasui Y, Saper CB, Cechetto DF. Calcitonin gene-related peptide immunoreactivity in the visceral sensory cortex, thalamus, and related pathways in the rat. J Comp Neurol 290: 487–501, 1989.doi: 10.1002/cne.902900404. [DOI] [PubMed] [Google Scholar]
- 250. Noseda R, Kainz V, Borsook D, Burstein R. Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One 9: e103929, 2014. doi: 10.1371/journal.pone.0103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide coexists with substance P in capsaicin sensitive neurons and sensory ganglia of the rat. Peptides 6: 747–754, 1985. doi: 10.1016/0196-9781(85)90179-2. [DOI] [PubMed] [Google Scholar]
- 252. McCulloch J, Uddman R, Kingman TA, Edvinsson L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci USA 83: 5731–5735, 1986. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett 62: 131–136, 1985. doi: 10.1016/0304-3940(85)90296-4. [DOI] [PubMed] [Google Scholar]
- 254. Lopes DM, Denk F, McMahon SB. The molecular fingerprint of dorsal root and trigeminal ganglion neurons. Front Mol Neurosci 10: 304, 2017. doi: 10.3389/fnmol.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkühler J. Modification of classical neurochemical markers in identified primary afferent neurons with Aβ-, Aδ-, and C-fibers after chronic constriction injury in mice. J Comp Neurol 502: 325–336, 2007. doi: 10.1002/cne.21311. [DOI] [PubMed] [Google Scholar]
- 256. Messlinger K, Balcziak LK, Russo AF. Cross-talk signaling in the trigeminal ganglion: role of neuropeptides and other mediators. J Neural Transm (Vienna) 127: 431–444, 2020. doi: 10.1007/s00702-020-02161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Törnwall J, Konttinen YT, Uusitalo H. Immunohistochemical distribution of calcitonin gene-related peptide in nerve fibres of the anterior buccal gland of the rat. Histochem J 28: 1–5, 1996. doi: 10.1007/BF02331421. [DOI] [PubMed] [Google Scholar]
- 258. Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, et al. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci 4: 3101–3111, 1984. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Frederiksen SD, Warfvinge K, Ohlsson L, Edvinsson L. Expression of pituitary adenylate cyclase-activating peptide, calcitonin gene-related peptide and headache targets in the trigeminal ganglia of rats and humans. Neuroscience 393: 319–332, 2018. doi: 10.1016/j.neuroscience.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 260. Tajti J, Kuris A, Vécsei L, Xu CB, Edvinsson L. Organ culture of the trigeminal ganglion induces enhanced expression of calcitonin gene-related peptide via activation of extracellular signal-regulated protein kinase 1/2. Cephalalgia 31: 95–105, 2011. doi: 10.1177/0333102410382796. [DOI] [PubMed] [Google Scholar]
- 261. Raddant AC, Russo AF. Reactive oxygen species induce procalcitonin expression in trigeminal ganglia glia. Headache 54: 472–484, 2014. doi: 10.1111/head.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Kogelman LJ, Elgaard-Christensen R, Olesen J, Jansen-Olesen I, Hansen TF. Transcriptomic profiling of trigeminal nucleus caudalis and spinal cord dorsal horn. Brain Res 1692: 23–33, 2018. doi: 10.1016/j.brainres.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 263. Bhatt DK, Gupta S, Ploug KB, Jansen-Olesen I, Olesen J. mRNA distribution of CGRP and its receptor components in the trigeminovascular system and other pain related structures in rat brain, and effect of intracerebroventricular administration of CGRP on Fos expression in the TNC. Neurosci Lett 559: 99–104, 2014. doi: 10.1016/j.neulet.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 264. Gimeno-Ferrer F, Eitner A, Bauer R, Lehmenkühler A, Edenhofer ML, Kress M, Schaible HG, Richter F. From spreading depolarization to epilepsy with neuroinflammation: the role of CGRP in cortex. Exp Neurol 356: 114152, 2022. doi: 10.1016/j.expneurol.2022.114152. [DOI] [PubMed] [Google Scholar]
- 265. Löken LS, Braz JM, Etlin A, Sadeghi M, Bernstein M, Jewell M, Steyert M, Kuhn J, Hamel K, Llewellyn-Smith IJ, Basbaum A. Contribution of dorsal horn CGRP-expressing interneurons to mechanical sensitivity. Elife 10: e59751, 2021. doi: 10.7554/eLife.59751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Gibson SJ, Polak JM, Giaid A, Hamid QA, Kar S, Jones PM, et al. Calcitonin gene-related peptide messenger RNA is expressed in sensory neurones of the dorsal root ganglia and also in spinal motoneurones in man and rat. Neurosci Lett 91: 283–288, 1988. doi: 10.1016/0304-3940(88)90694-5. [DOI] [PubMed] [Google Scholar]
- 267. Goodman EC, Iversen LL. Calcitonin gene-related peptide: novel neuropeptide. Life Sci 38: 2169–2178, 1986. doi: 10.1016/0024-3205(86)90568-0. [DOI] [PubMed] [Google Scholar]
- 268. Manteniotis S, Lehmann R, Flegel C, Vogel F, Hofreuter A, Schreiner BS, Altmüller J, Becker C, Schöbel N, Hatt H, Gisselmann G. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PLoS One 8: e79523, 2013. doi: 10.1371/journal.pone.0079523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Flegel C, Schöbel N, Altmüller J, Becker C, Tannapfel A, Hatt H, Gisselmann G. RNA-Seq analysis of human trigeminal and dorsal root ganglia with a focus on chemoreceptors. PLoS One 10: e0128951, 2015. doi: 10.1371/journal.pone.0128951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Sandercock DA, Coe JE, Di Giminiani P, Edwards SA. Determination of stable reference genes for RT-qPCR expression data in mechanistic pain studies on pig dorsal root ganglia and spinal cord. Res Vet Sci 114: 493–501, 2017. doi: 10.1016/j.rvsc.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, Lam T, Kim JY, Kim TH, Zhang MQ, Dussor G, Price TJ. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 159: 1325–1345, 2018. doi: 10.1097/j.pain.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Petermann JB, Born W, Chang JY, Fischer JA. Identification in the human central nervous system, pituitary, and thyroid of a novel calcitonin gene-related peptide, and partial amino acid sequence in the spinal cord. J Biol Chem 262: 542–545, 1987. doi: 10.1016/S0021-9258(19)75816-5. [DOI] [PubMed] [Google Scholar]
- 273. Hendrikse ER, Bower RL, Hay DL, Walker CS. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 39: 403–419, 2019. doi: 10.1177/0333102418765787. [DOI] [PubMed] [Google Scholar]
- 274. de Lacalle S, Saper CB. Calcitonin gene-related peptide-like immunoreactivity marks putative visceral sensory pathways in human brain. Neuroscience 100: 115–130, 2000. doi: 10.1016/S0306-4522(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 275. Yang Y, Ozawa H, Lu H, Yuri K, Hayashi S, Nihonyanagi K, Kawata M. Immunocytochemical analysis of sex differences in calcitonin gene-related peptide in the rat dorsal root ganglion, with special reference to estrogen and its receptor. Brain Res 791: 35–42, 1998. doi: 10.1016/S0006-8993(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 276. Aggarwal M, Puri V, Puri S. Effects of estrogen on the serotonergic system and calcitonin gene-related peptide in trigeminal ganglia of rats. Ann Neurosci 19: 151–157, 2012. doi: 10.5214/ans.0972.7531.190403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Kuzawińska O, Lis K, Cudna A, Bałkowiec-Iskra E. Gender differences in the neurochemical response of trigeminal ganglion neurons to peripheral inflammation in mice. Acta Neurobiol Exp (Wars) 74: 227–232, 2014. [DOI] [PubMed] [Google Scholar]
- 278. Herbison AE, Spratt DP. Sexually dimorphic expression of calcitonin gene-related peptide (CGRP) mRNA in rat medial preoptic nucleus. Brain Res Mol Brain Res 34: 143–148, 1995. doi: 10.1016/0169-328X(95)00144-H. [DOI] [PubMed] [Google Scholar]
- 279. Herbison AE. Identification of a sexually dimorphic neural population immunoreactive for calcitonin gene-related peptide (CGRP) in the rat medial preoptic area. Brain Res 591: 289–295, 1992. doi: 10.1016/0006-8993(92)91710-V. [DOI] [PubMed] [Google Scholar]
- 280. Leclercq P, Herbison AE. Sexually dimorphic expression of calcitonin gene-related peptide (CGRP) immunoreactivity by rat mediobasal hypothalamic neurons. J Comp Neurol 367: 444–453, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 281. van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev 21: 649–678, 1997. doi: 10.1016/S0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 282. Chakravarty P, Suthar TP, Coppock HA, Nicholl CG, Bloom SR, Legon S, Smith DM. CGRP and adrenomedullin binding correlates with transcript levels for calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) in rat tissues. Br J Pharmacol 130: 189–195, 2000.doi: 10.1038/sj.bjp.0702975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Flühmann B, Muff R, Hunziker W, Fischer JA, Born W. A human orphan calcitonin receptor-like structure. Biochem Biophys Res Commun 206: 341–347, 1995. doi: 10.1006/bbrc.1995.1047. [DOI] [PubMed] [Google Scholar]
- 284. Aiyar N, Rand K, Elshourbagy NA, Zeng Z, Adamou JE, Bergsma DJ, Li Y. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J Biol Chem 271: 11325–11329, 1996. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- 285. Njuki F, Nicholl CG, Howard A, Mak JCW, Barnes PJ, Girgis SI, Legon S. A new calcitonin-receptor-like sequence in rat pulmonary blood vessels. Clin Sci (Lond) 85: 385–388, 1993. doi: 10.1042/cs0850385. [DOI] [PubMed] [Google Scholar]
- 286. McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393: 333–339, 1998. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 287. Garelja ML, Bower RL, Brimble MA, Chand S, Harris PW, Jamaluddin MA, Petersen J, Siow A, Walker CS, Hay DL. Pharmacological characterisation of mouse calcitonin and calcitonin receptor-like receptors reveals differences compared with human receptors. Br J Pharmacol 179: 416–434, 2022. doi: 10.1111/bph.15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Hay DL, Poyner DR, Quirion R; International Union of Pharmacology. International Union of Pharmacology. LXIX. Status of the calcitonin gene-related peptide subtype 2 receptor. Pharmacol Rev 60: 143–145, 2008. doi: 10.1124/pr.108.00372. [DOI] [PubMed] [Google Scholar]
- 289. Hay DL. What makes a CGRP2 receptor? Clin Exp Pharmacol Physiol 34: 963–971, 2007. doi: 10.1111/j.1440-1681.2007.04703.x. [DOI] [PubMed] [Google Scholar]
- 290. Rorabaugh BR, Scofield MA, Smith DD, Jeffries WB, Abel PW. Functional calcitonin gene-related peptide subtype 2 receptors in porcine coronary arteries are identified as calcitonin gene-related peptide subtype 1 receptors by radioligand binding and reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther 299: 1086–1094, 2001. [PubMed] [Google Scholar]
- 291. Wisskirchen FM, Marshall I. CGRP2 receptor in the internal anal sphincter of the rat: implications for CGRP receptor classification. Br J Pharmacol 130: 464–470, 2000. doi: 10.1038/sj.bjp.0703315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Wisskirchen FM, Doyle PM, Gough SL, Harris CJ, Marshall I. Conformational restraints revealing bioactive beta-bend structures for halpha CGRP8-37 at the CGRP2 receptor of the rat prostatic vas deferens. Br J Pharmacol 126: 1163–1170, 1999. doi: 10.1038/sj.bjp.0702432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Dumont Y, Fournier A, St-Pierre S, Quirion R. A potent and selective CGRP2 agonist, [Cys(Et)2,7]hCGRP alpha: comparison in prototypical CGRP1 and CGRP2 in vitro bioassays. Can J Physiol Pharmacol 75: 671–676, 1997. [PubMed] [Google Scholar]
- 294. Longmore J, Hogg JE, Hutson PH, Hill RG. Effects of two truncated forms of human calcitonin-gene related peptide: implications for receptor classification. Eur J Pharmacol 265: 53–59, 1994. doi: 10.1016/0014-2999(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 295. Dennis T, Fournier A, Cadieux A, Pomerleau F, Jolicoeur FB, St Pierre S, Quirion R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J Pharmacol Exp Ther 254: 123–128, 1990. [PubMed] [Google Scholar]
- 296. Muff R, Leuthäuser K, Bühlmann N, Foord SM, Fischer JA, Born W. Receptor activity modifying proteins regulate the activity of a calcitonin gene-related peptide receptor in rabbit aortic endothelial cells. FEBS Lett 441: 366–368, 1998. doi: 10.1016/S0014-5793(98)01587-7. [DOI] [PubMed] [Google Scholar]
- 297. Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, Main MJ, Foord SM, Sexton PM. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol 56: 235–242, 1999. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 298. Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 275: 31438–31443, 2000. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- 299. Hay DL, Christopoulos G, Christopoulos A, Poyner DR, Sexton PM. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol Pharmacol 67: 1655–1665, 2005. doi: 10.1124/mol.104.008615. [DOI] [PubMed] [Google Scholar]
- 300. Furness SG, Wootten D, Christopoulos A, Sexton PM. Consequences of splice variation on Secretin family G protein-coupled receptor function. Br J Pharmacol 166: 98–109, 2012. doi: 10.1111/j.1476-5381.2011.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Dal Maso E, Just R, Hick C, Christopoulos A, Sexton PM, Wootten D, Furness SG. Characterization of signalling and regulation of common calcitonin receptor splice variants and polymorphisms. Biochem Pharmacol 148: 111–129, 2018. doi: 10.1016/j.bcp.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 302. Qi T, Dong M, Watkins HA, Wootten D, Miller LJ, Hay DL. Receptor activity-modifying protein-dependent impairment of calcitonin receptor splice variant Delta(1-47)hCT((a)) function. Br J Pharmacol 168: 644–657, 2013. doi: 10.1111/j.1476-5381.2012.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: a comparison of effects of adrenomedullin22-52, CGRP8-37 and BIBN4096BS. Br J Pharmacol 140: 477–486, 2003. doi: 10.1038/sj.bjp.0705472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Husmann K, Born W, Fischer JA, Muff R. Three receptor-activity-modifying proteins define calcitonin gene-related peptide or adrenomedullin selectivity of the mouse calcitonin-like receptor in COS-7 cells. Biochem Pharmacol 66: 2107–2115, 2003. doi: 10.1016/j.bcp.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 305. Bailey RJ, Walker CS, Ferner AH, Loomes KM, Prijic G, Halim A, Whiting L, Phillips AR, Hay DL. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br J Pharmacol 166: 151–167, 2012. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Halim A, Hay DL. The role of glutamic acid 73 in adrenomedullin interactions with rodent AM2 receptors. Peptides 36: 137–141, 2012. doi: 10.1016/j.peptides.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 307. Edvinsson L, Edvinsson JC, Haanes KA. Biological and small molecule strategies in migraine therapy with relation to the calcitonin gene-related peptide family of peptides. Br J Pharmacol 179: 371–380, 2022. doi: 10.1111/bph.15669. [DOI] [PubMed] [Google Scholar]
- 308. Kenakin T, Morgan P, Lutz M. On the importance of the “antagonist assumption” to how receptors express themselves. Biochem Pharmacol 50: 17–26, 1995. doi: 10.1016/0006-2952(95)00137-O. [DOI] [PubMed] [Google Scholar]
- 309. Chiba T, Yamaguchi A, Yamatani T, Nakamura A, Morishita T, Inui T, Fukase M, Noda T, Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37). Am J Physiol Endocrinol Metab 256: E331–E335, 1989. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- 310. Bailey RJ, Hay DL. Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides 27: 1367–1375, 2006. doi: 10.1016/j.peptides.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 311. Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Determinants of 1-piperidinecarboxamide, N-[2-[[5-amino-l-[[4-(4-pyridinyl)-l-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-d ibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazoliny l) (BIBN4096BS) affinity for calcitonin gene-related peptide and amylin receptors–the role of receptor activity modifying protein 1. Mol Pharmacol 70: 1984–1991, 2006. doi: 10.1124/mol.106.027953. [DOI] [PubMed] [Google Scholar]
- 312. Hay DL, Howitt SG, Conner AC, Doods H, Schindler M, Poyner DR. A comparison of the actions of BIBN4096BS and CGRP8-37 on CGRP and adrenomedullin receptors expressed on SK-N-MC, L6, Col 29 and Rat 2 cells. Br J Pharmacol 137: 80–86, 2002. doi: 10.1038/sj.bjp.0704844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Bohn KJ, Li B, Huang X, Mason BN, Wattiez AS, Kuburas A, Walker CS, Yang P, Yu J, Heinz BA, Johnson KW, Russo AF. CGRP receptor activity in mice with global expression of human receptor activity modifying protein 1. Br J Pharmacol 174: 1826–1840, 2017. doi: 10.1111/bph.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Gingell JJ, Burns ER, Hay DL. Activity of pramlintide, rat and human amylin but not Abeta1-42 at human amylin receptors. Endocrinology 155: 21–26, 2014. doi: 10.1210/en.2013-1658. [DOI] [PubMed] [Google Scholar]
- 315. Jamaluddin A, Chuang CL, Williams ET, Siow A, Yang SH, Harris PW, Petersen JS, Bower RL, Chand S, Brimble MA, Walker CS, Hay DL, Loomes KM. Lipidated calcitonin gene-related peptide (CGRP) peptide antagonists retain CGRP receptor activity and attenuate CGRP action in vivo. Front Pharmacol 13: 832589, 2022. doi: 10.3389/fphar.2022.832589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Srinivasan K, Kozminski K, Zhang Y, Wisniewski K, Kohout T, Wisniewska H, Harris G, Lindstrom B, Hargrove D. Pharmacological, pharmacokinetic, pharmacodynamic and physicochemical characterization of FE 205030: a potent, fast acting, injectable CGRP receptor antagonist for the treatment of acute episodic migraine. J Pharm Sci 111: 247–261, 2022. doi: 10.1016/j.xphs.2021.06.034. [DOI] [PubMed] [Google Scholar]
- 317. Garelja ML, Walker CS, Hay DL. CGRP receptor antagonists for migraine. Are they also AMY1 receptor antagonists? Br J Pharmacol 179: 454–459, 2022. doi: 10.1111/bph.15585. [DOI] [PubMed] [Google Scholar]
- 318. Dos Santos JB, da Silva MR. Small molecule CGRP receptor antagonists for the preventive treatment of migraine: a review. Eur J Pharmacol 922: 174902, 2022. doi: 10.1016/j.ejphar.2022.174902. [DOI] [PubMed] [Google Scholar]
- 319. Hargreaves R, Olesen J. Calcitonin gene-related peptide modulators—the history and renaissance of a new migraine drug class. Headache 59: 951–970, 2019. doi: 10.1111/head.13510. [DOI] [PubMed] [Google Scholar]
- 320. Bucknell SJ, Ator MA, Brown AJ, Brown J, Cansfield AD, Cansfield JE, Christopher JA, Congreve M, Cseke G, Deflorian F, Jones CR, Mason JS, O’Brien MA, Ott GR, Pickworth M, Southall SM. Structure-based drug discovery of N-((R)-3-(7-methyl-1H-indazol-5-yl)-1-oxo-1-(((S)-1-oxo-3-(piperidin-4-yl)-1-(4- (pyridin-4-yl)piperazin-1-yl)propan-2-yl)amino)propan-2-yl)-2′-oxo-1′,2′-dihydrosp iro[piperidine-4,4′-pyrido[2,3-d][1,3]oxazine]-1-carboxamide (HTL22562): a calcitonin gene-related peptide receptor antagonist for acute treatment of migraine. J Med Chem 63: 7906–7920, 2020. doi: 10.1021/acs.jmedchem.0c01003. [DOI] [PubMed] [Google Scholar]
- 321. Cansfield AD, Ator MA, Banerjee J, Bestwick M, Bortolato A, Brown GA, Brown J, Butkovic K, Cansfield JE, Christopher JA, Congreve M, Cseke G, Deflorian F, Dugan B, Hunjadi MP, Hutinec A, Inturi TK, Landek G, Mason J, O'Brien A, Ott GR, Rupcic R, Saxty G, Southall SM, Zadravec R, Watson SP. Novel macrocyclic antagonists of the calcitonin gene-related peptide receptor: design, realization, and structural characterization of protein-ligand complexes. ACS Chem Neurosci 13: 751–765, 2022. doi: 10.1021/acschemneuro.1c00696. [DOI] [PubMed] [Google Scholar]
- 322. von Mentzer B, Russo AF, Zhang Z, Kuburas A, Killoran PM, D’Aloisio V, Nizic L, Capel V, Kendall DA, Coxon CR, Hutcheon GA. A CGRP receptor antagonist peptide formulated for nasal administration to treat migraine. J Pharm Pharmacol 72: 1352–1360, 2020. doi: 10.1111/jphp.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Moore EL, Burgey CS, Paone DV, Shaw AW, Tang YS, Kane SA, Salvatore CA. Examining the binding properties of MK-0974: a CGRP receptor antagonist for the acute treatment of migraine. Eur J Pharmacol 602: 250–254, 2009. doi: 10.1016/j.ejphar.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 324. Taylor CK, Smith DD, Hulce M, Abel PW. Pharmacological characterization of novel alpha-Calcitonin Gene-Related Peptide (CGRP) receptor peptide antagonists that are selective for human CGRP receptors. J Pharmacol Exp Ther 319: 749–757, 2006. doi: 10.1124/jpet.106.108316. [DOI] [PubMed] [Google Scholar]
- 325. Choksi T, Hay DL, Legon S, Poyner DR, Hagner S, Bloom SR, Smith DM. Comparison of the expression of calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) with CGRP and adrenomedullin binding in cell lines. Br J Pharmacol 136: 784–792, 2002. doi: 10.1038/sj.bjp.0704761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Mallee JJ, Salvatore CA, LeBourdelles B, Oliver KR, Longmore J, Koblan KS, Kane SA. Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J Biol Chem 277: 14294–14298, 2002. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- 327. Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol 129: 420–423, 2000. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Moore E, Fraley ME, Bell IM, Burgey CS, White RB, Li CC, Regan CP, Danziger A, Stranieri Michener M, Hostetler E, Banerjee P, Salvatore C. Characterization of ubrogepant: a potent and selective antagonist of the human calcitonin gene related peptide receptor. J Pharmacol Exp Ther 373: 160–166, 2020. doi: 10.1124/jpet.119.261065. [DOI] [PubMed] [Google Scholar]
- 329. Moore EL, Salvatore CA. Targeting a family B GPCR/RAMP receptor complex: CGRP receptor antagonists and migraine. Br J Pharmacol 166: 66–78, 2012. doi: 10.1111/j.1476-5381.2011.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Salvatore CA, Moore EL, Calamari A, Cook JJ, Michener MS, O’Malley S, Miller PJ, Sur C, Williams DL Jr, Zeng Z, Danziger A, Lynch JJ, Regan CP, Fay JF, Tang YS, Li CC, Pudvah NT, White RB, Bell IM, Gallicchio SN, Graham SL, Selnick HG, Vacca JP, Kane SA. Pharmacological properties of MK-3207, a potent and orally active calcitonin gene-related peptide receptor antagonist. J Pharmacol Exp Ther 333: 152–160, 2010. doi: 10.1124/jpet.109.163816. [DOI] [PubMed] [Google Scholar]
- 331. Salvatore CA, Hershey JC, Corcoran HA, Fay JF, Johnston VK, Moore EL, Mosser SD, Burgey CS, Paone DV, Shaw AW, Graham SL, Vacca JP, Williams TM, Koblan KS, Kane SA. Pharmacological characterization of MK-0974 [N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther 324: 416–421, 2008. doi: 10.1124/jpet.107.130344. [DOI] [PubMed] [Google Scholar]
- 332. Miller PS, Barwell J, Poyner DR, Wigglesworth MJ, Garland SL, Donnelly D. Non-peptidic antagonists of the CGRP receptor, BIBN4096BS and MK-0974, interact with the calcitonin receptor-like receptor via methionine-42 and RAMP1 via tryptophan-74. Biochem Biophys Res Commun 391: 437–442, 2010. doi: 10.1016/j.bbrc.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Pan KS, Siow A, Hay DL, Walker CS. Antagonism of CGRP signaling by rimegepant at two receptors. Front Pharmacol 11: 1240, 2020. doi: 10.3389/fphar.2020.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Shi L, Lehto SG, Zhu DX, Sun H, Zhang J, Smith BP, Immke DC, Wild KD, Xu C. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J Pharmacol Exp Ther 356: 223–231, 2016. doi: 10.1124/jpet.115.227793. [DOI] [PubMed] [Google Scholar]
- 335. Bhakta M, Vuong T, Taura T, Wilson DS, Stratton JR, Mackenzie KD. Migraine therapeutics differentially modulate the CGRP pathway. Cephalalgia 41: 499–514, 2021. doi: 10.1177/0333102420983282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Hage La Cour S, Juhler K, Kogelman LJ, Olesen J, Klærke DA, Kristensen DM, Jansen-Olesen I. Characterization of erenumab and rimegepant on calcitonin gene-related peptide induced responses in Xenopus laevis oocytes expressing the calcitonin gene-related peptide receptor and the amylin-1 receptor. J Headache Pain 23: 59, 2022. doi: 10.1186/s10194-022-01425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Chaturvedula PV, Mercer SE, Pin SS, Thalody G, Xu C, Conway CM, Keavy D, Signor L, Cantor GH, Mathias N, Moench P, Denton R, Macci R, Schartman R, Whiterock V, Davis C, Macor JE, Dubowchik GM. Discovery of (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxamide (BMS-742413): a potent human CGRP antagonist with superior safety profile for the treatment of migraine through intranasal delivery. Bioorg Med Chem Lett 23: 3157–3161, 2013. doi: 10.1016/j.bmcl.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 338. Garces F, Mohr C, Zhang L, Huang CS, Chen Q, King C, Xu C, Wang Z. Molecular insight into recognition of the CGRPR complex by migraine prevention therapy aimovig (erenumab). Cell Rep 30: 1714–1723.e6, 2020. doi: 10.1016/j.celrep.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 339. Johnson KW, Li X, Huang X, Heinz BA, Yu J, Li B. Characterization of transit rates in the large intestine of mice following treatment with a CGRP antibody, CGRP receptor antibody, and small molecule CGRP receptor antagonists. Headache 62: 848–857, 2022. doi: 10.1111/head.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Xu C, Bussiere J. Assessing migraine therapeutics. Cephalalgia 41: 1402–1403, 2021. doi: 10.1177/03331024211021569. [DOI] [PubMed] [Google Scholar]
- 341. Mackenzie KD, Stratton JR. Response to letter to the editor: Assessing migraine therapeutics. Cephalalgia 41: 1404–1406, 2021. doi: 10.1177/03331024211021563. [DOI] [PubMed] [Google Scholar]
- 342. Liang YL, Khoshouei M, Deganutti G, Glukhova A, Koole C, Peat TS, Radjainia M, Plitzko JM, Baumeister W, Miller LJ, Hay DL, Christopoulos A, Reynolds CA, Wootten D, Sexton PM. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature 561: 492–497, 2018. doi: 10.1038/s41586-018-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Booe JM, Walker CS, Barwell J, Kuteyi G, Simms J, Jamaluddin MA, Warner ML, Bill RM, Harris PW, Brimble MA, Poyner DR, Hay DL, Pioszak AA. Structural basis for receptor activity-modifying protein-dependent selective peptide recognition by a G protein-coupled receptor. Mol Cell 58: 1040–1052, 2015. doi: 10.1016/j.molcel.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Josephs TM, Belousoff MJ, Liang YL, Piper SJ, Cao J, Garama DJ, Leach K, Gregory KJ, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. Structure and dynamics of the CGRP receptor in apo and peptide-bound forms. Science 372: eabf7258, 2021. doi: 10.1126/science.abf7258. [DOI] [PubMed] [Google Scholar]
- 345. Pioszak AA, Hay DL. RAMPs as allosteric modulators of the calcitonin and calcitonin-like class B G protein-coupled receptors. Adv Pharmacol 88: 115–141, 2020. doi: 10.1016/bs.apha.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Hay DL, Harris PW, Kowalczyk R, Brimble MA, Rathbone DL, Barwell J, Conner AC, Poyner DR. Structure-activity relationships of the N-terminus of calcitonin gene-related peptide: key roles of alanine-5 and threonine-6 in receptor activation. Br J Pharmacol 171: 415–426, 2014. doi: 10.1111/bph.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Watkins HA, Rathbone DL, Barwell J, Hay DL, Poyner DR. Structure-activity relationships for alpha-calcitonin gene-related peptide. Br J Pharmacol 170: 1308–1322, 2013. doi: 10.1111/bph.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Garelja ML, Au M, Brimble MA, Gingell JJ, Hendrikse ER, Lovell A, Prodan N, Sexton PM, Siow A, Walker CS, Watkins HA, Williams GM, Wootten D, Yang SH, Harris PW, Hay DL. Molecular mechanisms of class B GPCR activation: insights from adrenomedullin receptors. ACS Pharmacol Transl Sci 3: 246–262, 2020. doi: 10.1021/acsptsci.9b00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Moad HE, Pioszak AA. Selective CGRP and adrenomedullin peptide binding by tethered RAMP-calcitonin receptor-like receptor extracellular domain fusion proteins. Protein Sci 22: 1775–1785, 2013. doi: 10.1002/pro.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Woolley MJ, Reynolds CA, Simms J, Walker CS, Mobarec JC, Garelja ML, Conner AC, Poyner DR, Hay DL. Receptor activity-modifying protein dependent and independent activation mechanisms in the coupling of calcitonin gene-related peptide and adrenomedullin receptors to Gs. Biochem Pharmacol 142: 96–110, 2017. doi: 10.1016/j.bcp.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Woolley MJ, Simms J, Mobarec JC, Reynolds CA, Poyner DR, Conner AC. Understanding the molecular functions of the second extracellular loop (ECL2) of the calcitonin gene-related peptide (CGRP) receptor using a comprehensive mutagenesis approach. Mol Cell Endocrinol 454: 39–49, 2017. doi: 10.1016/j.mce.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 352. Woolley MJ, Watkins HA, Taddese B, Karakullukcu ZG, Barwell J, Smith KJ, Hay DL, Poyner DR, Reynolds CA, Conner AC. The role of ECL2 in CGRP receptor activation: a combined modelling and experimental approach. J R Soc Interface 10: 20130589, 2013. doi: 10.1098/rsif.2013.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Vohra S, Taddese B, Conner AC, Poyner DR, Hay DL, Barwell J, Reeves PJ, Upton GJ, Reynolds CA. Similarity between class A and class B G-protein-coupled receptors exemplified through calcitonin gene-related peptide receptor modelling and mutagenesis studies. J R Soc Interface 10: 20120846, 2013. doi: 10.1098/rsif.2012.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. ter Haar E, Koth CM, Abdul-Manan N, Swenson L, Coll JT, Lippke JA, Lepre CA, Garcia-Guzman M, Moore JM. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure 18: 1083–1093, 2010. doi: 10.1016/j.str.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 355. Leung L, Liao S, Wu C. To probe the binding interactions between Two FDA approved migraine drugs (ubrogepant and rimegepant) and calcitonin-gene related peptide receptor (CGRPR) using molecular dynamics simulations. ACS Chem Neurosci 12: 2629–2642, 2021. doi: 10.1021/acschemneuro.1c00135. [DOI] [PubMed] [Google Scholar]
- 356. Walker CS, Conner AC, Poyner DR, Hay DL. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol Sci 31: 476–483, 2010. doi: 10.1016/j.tips.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 357. Walker CS, Hay DL. CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol 170: 1293–1307, 2013. doi: 10.1111/bph.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Routledge SJ, Simms J, Clark A, Yeung HY, Wigglesworth MJ, Dickerson IM, Kitchen P, Ladds G, Poyner DR. Receptor component protein, an endogenous allosteric modulator of family B G protein coupled receptors. Biochim Biophys Acta Biomembr 1862: 183174, 2020. doi: 10.1016/j.bbamem.2019.183174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Bower RL, Yule L, Rees TA, Deganutti G, Hendrikse ER, Harris PW, Kowalczyk R, Ridgway Z, Wong AG, Swierkula K, Raleigh DP, Pioszak AA, Brimble MA, Reynolds CA, Walker CS, Hay DL. Molecular signature for receptor engagement in the metabolic peptide hormone amylin. ACS Pharmacol Transl Sci 1: 32–49, 2018. doi: 10.1021/acsptsci.8b00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Weston C, Winfield I, Harris M, Hodgson R, Shah A, Dowell SJ, Mobarec JC, Woodlock DA, Reynolds CA, Poyner DR, Watkins HA, Ladds G. Receptor activity-modifying protein-directed G protein signaling specificity for the calcitonin gene-related peptide family of receptors. J Biol Chem 291: 21925–21944, 2016. doi: 10.1074/jbc.M116.751362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Fletcher MM, Keov P, Truong TT, Mennen G, Hick CA, Zhao P, Furness SG, Kruse T, Clausen TR, Wootten D, Sexton PM. AM833 is a novel agonist of calcitonin family G protein-coupled receptors: pharmacological comparison with six selective and nonselective agonists. J Pharmacol Exp Ther 377: 417–440, 2021. doi: 10.1124/jpet.121.000567. [DOI] [PubMed] [Google Scholar]
- 362. Udawela M, Christopoulos G, Morfis M, Tilakaratne N, Christopoulos A, Sexton PM. The effects of C-terminal truncation of receptor activity modifying proteins on the induction of amylin receptor phenotype from human CTb receptors. Regul Pept 145: 65–71, 2008. doi: 10.1016/j.regpep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 363. Morfis M, Tilakaratne N, Furness SG, Christopoulos G, Werry TD, Christopoulos A, Sexton PM. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology 149: 5423–5431, 2008. doi: 10.1210/en.2007-1735. [DOI] [PubMed] [Google Scholar]
- 364. Udawela M, Christopoulos G, Morfis M, Christopoulos A, Ye S, Tilakaratne N, Sexton PM. A critical role for the short intracellular C terminus in receptor activity-modifying protein function. Mol Pharmacol 70: 1750–1760, 2006. doi: 10.1124/mol.106.024257. [DOI] [PubMed] [Google Scholar]
- 365. Fabbretti E, D’Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci 26: 6163–6171, 2006. doi: 10.1523/JNEUROSCI.0647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Clark AJ, Mullooly N, Safitri D, Harris M, de Vries T, MaassenVanDenBrink A, Poyner DR, Gianni D, Wigglesworth M, Ladds G. CGRP, adrenomedullin and adrenomedullin 2 display endogenous GPCR agonist bias in primary human cardiovascular cells. Commun Biol 776: 4, 2021. doi: 10.1038/s42003-021-02293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Yarwood RE, Imlach WL, Lieu T, Veldhuis NA, Jensen DD, Klein Herenbrink C, Aurelio L, Cai Z, Christie MJ, Poole DP, Porter CJ, McLean P, Hicks GA, Geppetti P, Halls ML, Canals M, Bunnett NW. Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc Natl Acad Sci USA 114: 12309–12314, 2017. doi: 10.1073/pnas.1706656114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Gingell JJ, Rees TA, Hendrikse ER, Siow A, Rennison D, Scotter J, Harris PW, Brimble MA, Walker CS, Hay DL. Distinct patterns of internalization of different calcitonin gene-related peptide receptors. ACS Pharmacol Transl Sci 3: 296–304, 2020. doi: 10.1021/acsptsci.9b00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Gingell JJ, Hendrikse ER, Hay DL. New insights into the regulation of CGRP-family receptors. Trends Pharmacol Sci 40: 71–83, 2019. doi: 10.1016/j.tips.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 370. Cottrell GS. CGRP receptor signalling pathways. In: Calcitonin Gene-Related Peptide (CGRP) Mechanisms: Focus on Migraine, edited by Brain SD, Geppetti P.. Cham, Switzerland: Springer International Publishing, 2019. [Google Scholar]
- 371. De Logu F, Nassini R, Hegron A, Landini L, Jensen DD, Latorre R, Ding J, Marini M, Souza Monteiro de Araujo D, Ramírez-Garcia P, Whittaker M, Retamal J, Titiz M, Innocenti A, Davis TP, Veldhuis N, Schmidt BL, Bunnett NW, Geppetti P. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat Commun 13: 646, 2022. doi: 10.1038/s41467-022-28204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. McNeish AJ, Roux BT, Aylett SB, Van Den Brink AM, Cottrell GS. Endosomal proteolysis regulates calcitonin gene-related peptide responses in mesenteric arteries. Br J Pharmacol 167: 1679–1690, 2012. doi: 10.1111/j.1476-5381.2012.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Thomsen AR, Jensen DD, Hicks GA, Bunnett NW. Therapeutic targeting of endosomal G-protein-coupled receptors. Trends Pharmacol Sci 39: 879–891, 2018. doi: 10.1016/j.tips.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Manoukian R, Sun H, Miller S, Shi D, Chan B, Xu C. Effects of monoclonal antagonist antibodies on calcitonin gene-related peptide receptor function and trafficking. J Headache Pain 20: 44, 2019. doi: 10.1186/s10194-019-0992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Van Rossum D, Ménard DP, Fournier A, St-Pierre S, Quirion R. Binding profile of a selective calcitonin gene-related peptide (CGRP) receptor antagonist ligand, [125I-Tyr]hCGRP8-37, in rat brain and peripheral tissues. J Pharmacol Exp Ther 269: 846–853, 1994. [PubMed] [Google Scholar]
- 376. Yashpal K, Kar S, Dennis T, Quirion R. Quantitative autoradiographic distribution of calcitonin gene-related peptide (hCGRP alpha) binding sites in the rat and monkey spinal cord. J Comp Neurol 322: 224–232, 1992. doi: 10.1002/cne.903220208. [DOI] [PubMed] [Google Scholar]
- 377. Dotti-Sigrist S, Born W, Fischer JA. Identification of a receptor for calcitonin gene-related peptides I and II in human cerebellum. Biochem Biophys Res Commun 151: 1081–1087, 1988. doi: 10.1016/S0006-291X(88)80476-5. [DOI] [PubMed] [Google Scholar]
- 378. Henke H, Sigrist S, Lang W, Schneider J, Fischer JA. Comparison of binding sites for the calcitonin gene-related peptides I and II in man. Brain Res 410: 404–408, 1987. doi: 10.1016/0006-8993(87)90348-9. [DOI] [PubMed] [Google Scholar]
- 379. Henke H, Tschopp FA, Fischer JA. Distinct binding sites for calcitonin gene-related peptide and salmon calcitonin in rat central nervous system. Brain Res 360: 165–171, 1985. doi: 10.1016/0006-8993(85)91232-6. [DOI] [PubMed] [Google Scholar]
- 380. Flühmann B, Lauber M, Lichtensteiger W, Fischer JA, Born W. Tissue-specific mRNA expression of a calcitonin receptor-like receptor during fetal and postnatal development. Brain Res 774: 184–192, 1997. doi: 10.1016/S0006-8993(97)81702-7. [DOI] [PubMed] [Google Scholar]
- 381. Holland PR, Saengjaroentham C, Vila-Pueyo M. The role of the brainstem in migraine: Potential brainstem effects of CGRP and CGRP receptor activation in animal models. Cephalalgia 39: 390–402, 2019. doi: 10.1177/0333102418756863. [DOI] [PubMed] [Google Scholar]
- 382. Edvinsson L, Grell AS, Warfvinge K. Expression of the CGRP family of neuropeptides and their receptors in the trigeminal ganglion. J Mol Neurosci 70: 930–944, 2020. doi: 10.1007/s12031-020-01493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Barbash S, Lorenzen E, Persson T, Huber T, Sakmar TP. GPCRs globally coevolved with receptor activity-modifying proteins, RAMPs. Proc Natl Acad Sci USA 114: 12015–12020, 2017. doi: 10.1073/pnas.1713074114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Hay DL, Pioszak AA. Receptor activity-modifying proteins (RAMPs): new insights and roles. Annu Rev Pharmacol Toxicol 56: 469–487, 2016. doi: 10.1146/annurev-pharmtox-010715-103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Argunhan F, Thapa D, Aubdool AA, Carlini E, Arkless K, Hendrikse ER, de Sousa Valente J, Kodji X, Barrett B, Ricciardi CA, Gnudi L, Hay DL, Brain SD. Calcitonin gene-related peptide protects against cardiovascular dysfunction independently of nitric oxide in vivo. Hypertension 77: 1178–1190, 2021. doi: 10.1161/HYPERTENSIONAHA.120.14851. [DOI] [PubMed] [Google Scholar]
- 386. Coester B, Foll CL, Lutz TA. Viral depletion of calcitonin receptors in the area postrema: a proof-of-concept study. Physiol Behav 223: 112992, 2020. doi: 10.1016/j.physbeh.2020.112992. [DOI] [PubMed] [Google Scholar]
- 387. Goda T, Doi M, Umezaki Y, Murai I, Shimatani H, Chu ML, Nguyen VH, Okamura H, Hamada FN. Calcitonin receptors are ancient modulators for rhythms of preferential temperature in insects and body temperature in mammals. Genes Dev 32: 140–155, 2018. doi: 10.1101/gad.307884.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Pagani F, Guidobono F, Netti C, Re F, Pecile A. Age-related increase in CGRP binding site densities in rat cerebellum. Pharmacol Res 21, Suppl 1: 105–106, 1989. doi: 10.1016/S1043-6618(89)80074-X. [DOI] [PubMed] [Google Scholar]
- 389. Guidobono F, Netti C, Bettica P, Sibilia V, Pagani F, Cazzamalli E, Pecile A. Effects of age on binding sites for calcitonin gene-related peptide in the rat central nervous system. Neurosci Lett 102: 20–26, 1989. doi: 10.1016/0304-3940(89)90301-7. [DOI] [PubMed] [Google Scholar]
- 390. Sohn I, Sheykhzade M, Edvinsson L, Sams A. The effects of CGRP in vascular tissue—classical vasodilation, shadowed effects and systemic dilemmas. Eur J Pharmacol 881: 173205, 2020. doi: 10.1016/j.ejphar.2020.173205. [DOI] [PubMed] [Google Scholar]
- 391. Benarroch EE. CGRP: sensory neuropeptide with multiple neurologic implications. Neurology 77: 281–287, 2011. doi: 10.1212/WNL.0b013e31822550e2. [DOI] [PubMed] [Google Scholar]
- 392. Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 6: 573–582, 2010. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 393. Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med 13: e36, 2011. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol 92: 2859–2866, 2004. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 395. Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol 194: 451–491, 2009. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- 396. Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 158: 543–559, 2017. doi: 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Campos CA, Bowen AJ, Roman CW, Palmiter RD. Encoding of danger by parabrachial CGRP neurons. Nature 555: 617–622, 2018. doi: 10.1038/nature25511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol 8: 89–99, 2012. doi: 10.3988/jcn.2012.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Marvizón JC, Pérez OA, Song B, Chen W, Bunnett NW, Grady EF, Todd AJ. Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic alpha2C receptors. Neuroscience 148: 250–265, 2007. doi: 10.1016/j.neuroscience.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Gu XL, Yu LC. The colocalization of CGRP receptor and AMPA receptor in the spinal dorsal horn neuron of rat: a morphological and electrophysiological study. Neurosci Lett 414: 237–241, 2007. doi: 10.1016/j.neulet.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 401. Marquez de Prado B, Hammond DL, Russo AF. Genetic enhancement of calcitonin gene-related peptide-induced central sensitization to mechanical stimuli in mice. J Pain 10: 992–1000, 2009. doi: 10.1016/j.jpain.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Rogoz K, Andersen HH, Kullander K, Lagerström MC. Glutamate, substance P, and calcitonin gene-related peptide cooperate in inflammation-induced heat hyperalgesia. Mol Pharmacol 85: 322–334, 2014. doi: 10.1124/mol.113.089532. [DOI] [PubMed] [Google Scholar]
- 403. Rogoz K, Andersen HH, Lagerström MC, Kullander K. Multimodal use of calcitonin gene-related peptide and substance P in itch and acute pain uncovered by the elimination of vesicular glutamate transporter 2 from transient receptor potential cation channel subfamily V member 1 neurons. J Neurosci 34: 14055–14068, 2014. doi: 10.1523/JNEUROSCI.1722-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol 142: 1171–1181, 2004. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Fischer MJ, Koulchitsky S, Messlinger K. The nonpeptide calcitonin gene-related peptide receptor antagonist BIBN4096BS lowers the activity of neurons with meningeal input in the rat spinal trigeminal nucleus. J Neurosci 25: 5877–5883, 2005. doi: 10.1523/JNEUROSCI.0869-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Sixt ML, Messlinger K, Fischer MJ. Calcitonin gene-related peptide receptor antagonist olcegepant acts in the spinal trigeminal nucleus. Brain 132: 3134–3141, 2009. doi: 10.1093/brain/awp168. [DOI] [PubMed] [Google Scholar]
- 407. Zheng F, Nixdorf-Bergweiler BE, van Brederode J, Alzheimer C, Messlinger K. Excitatory effects of calcitonin gene-related peptide (CGRP) on superficial Sp5C neurons in mouse medullary slices. Int J Mol Sci 22: 3794, 2021. doi: 10.3390/ijms22073794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Messlinger K. Migraine: where and how does the pain originate? Exp Brain Res 196: 179–193, 2009. doi: 10.1007/s00221-009-1756-y. [DOI] [PubMed] [Google Scholar]
- 409. Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain 14: 1289–1303, 2013. doi: 10.1016/j.jpain.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 410. Younis S, Hougaard A, Noseda R, Ashina M. Current understanding of thalamic structure and function in migraine. Cephalalgia 39: 1675–1682, 2019. doi: 10.1177/0333102418791595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 68: 81–91, 2010. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 79: 964–982, 1998. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 413. Liu Y, Broman J, Zhang M, Edvinsson L. Brainstem and thalamic projections from a craniovascular sensory nervous centre in the rostral cervical spinal dorsal horn of rats. Cephalalgia 29: 935–948, 2009. doi: 10.1111/j.1468-2982.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- 414. Noseda R, Bernstein CA, Nir RR, Lee AJ, Fulton AB, Bertisch SM, Hovaguimian A, Cestari DM, Saavedra-Walker R, Borsook D, Doran BL, Buettner C, Burstein R. Migraine photophobia originating in cone-driven retinal pathways. Brain 139: 1971–1986, 2016. doi: 10.1093/brain/aww119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci 13: 239–245, 2010. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Maleki N, Becerra L, Upadhyay J, Burstein R, Borsook D. Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: implications for photophobia. Hum Brain Mapp 33: 75–88, 2012. doi: 10.1002/hbm.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Li XH, Matsuura T, Liu RH, Xue M, Zhuo M. Calcitonin gene-related peptide potentiated the excitatory transmission and network propagation in the anterior cingulate cortex of adult mice. Mol Pain 15: 1744806919832718, 2019. doi: 10.1177/1744806919832718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Jaggi AS, Singh N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res 1381: 187–201, 2011. doi: 10.1016/j.brainres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 419. Hou KS, Wang LL, Wang HB, Fu FH, Yu LC. Role of calcitonin gene-related peptide in nociceptive modulation in anterior cingulate cortex of naive rats and rats with inflammatory pain. Front Pharmacol 11: 928, 2020. doi: 10.3389/fphar.2020.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Qiu S, Zhang M, Liu Y, Guo Y, Zhao H, Song Q, Zhao M, Huganir RL, Luo J, Xu H, Zhuo M. GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J Neurosci 34: 13505–13515, 2014. doi: 10.1523/JNEUROSCI.1431-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Zhuo M. Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience 338: 220–229, 2016. doi: 10.1016/j.neuroscience.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 422. Liu Y, Chen QY, Lee JH, Li XH, Yu S, Zhuo M. Cortical potentiation induced by calcitonin gene-related peptide (CGRP) in the insular cortex of adult mice. Mol Brain 13: 36, 2020. doi: 10.1186/s13041-020-00580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Huang Y, Brodda-Jansen G, Lundeberg T, Yu LC. Anti-nociceptive effects of calcitonin gene-related peptide in nucleus raphe magnus of rats: an effect attenuated by naloxone. Brain Res 873: 54–59, 2000. doi: 10.1016/S0006-8993(00)02473-2. [DOI] [PubMed] [Google Scholar]
- 424. Charbit AR, Akerman S, Holland PR, Goadsby PJ. Neurons of the dopaminergic/calcitonin gene-related peptide A11 cell group modulate neuronal firing in the trigeminocervical complex: an electrophysiological and immunohistochemical study. J Neurosci 29: 12532–12541, 2009. doi: 10.1523/JNEUROSCI.2887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Burstein R, Jakubowski M. Neural substrate of depression during migraine. Neurol Sci 30, Suppl 1: S27–S31, 2009. doi: 10.1007/s10072-009-0061-7. [DOI] [PubMed] [Google Scholar]
- 426. May A, Burstein R. Hypothalamic regulation of headache and migraine. Cephalalgia 39: 1710–1719, 2019. doi: 10.1177/0333102419867280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Charbit AR, Akerman S, Goadsby PJ. Dopamine: what’s new in migraine? Curr Opin Neurol 23: 275–281, 2010. doi: 10.1097/WCO.0b013e3283378d5c. [DOI] [PubMed] [Google Scholar]
- 428. Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain 6: 10, 2010. doi: 10.1186/1744-8069-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci 31: 1802–1810, 2011. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 10: 221–234, 2004. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 431. Okutsu Y, Takahashi Y, Nagase M, Shinohara K, Ikeda R, Kato F. Potentiation of NMDA receptor-mediated synaptic transmission at the parabrachial-central amygdala synapses by CGRP in mice. Mol Pain 13: 1744806917709201, 2017. doi: 10.1177/1744806917709201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Presto P, Neugebauer V. Sex Differences in CGRP regulation and function in the amygdala in a rat model of neuropathic pain. Front Mol Neurosci 15: 928587, 2022. doi: 10.3389/fnmol.2022.928587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Sink KS, Chung A, Ressler KJ, Davis M, Walker DL. Anxiogenic effects of CGRP within the BNST may be mediated by CRF acting at BNST CRFR1 receptors. Behav Brain Res 243: 286–293, 2013. doi: 10.1016/j.bbr.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Dhillo WS, Small CJ, Jethwa PH, Russell SH, Gardiner JV, Bewick GA, Seth A, Murphy KG, Ghatei MA, Bloom SR. Paraventricular nucleus administration of calcitonin gene-related peptide inhibits food intake and stimulates the hypothalamo-pituitary-adrenal axis. Endocrinology 144: 1420–1425, 2003. doi: 10.1210/en.2002-220902. [DOI] [PubMed] [Google Scholar]
- 435. Uddin O, Anderson M, Smith J, Masri R, Keller A. Parabrachial complex processes dura inputs through a direct trigeminal ganglion-to-parabrachial connection. Neurobiol Pain 9: 100060, 2021. doi: 10.1016/j.ynpai.2021.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci 41: 280–293, 2018. doi: 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Chen JY, Campos CA, Jarvie BC, Palmiter RD. Parabrachial CGRP neurons establish and sustain aversive taste memories. Neuron 100: 891–899.e5, 2018. doi: 10.1016/j.neuron.2018.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Campos CA, Bowen AJ, Han S, Wisse BE, Palmiter RD, Schwartz MW. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat Neurosci 20: 934–942, 2017. doi: 10.1038/nn.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci 35: 4582–4586, 2015. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Sabatini PV, Frikke-Schmidt H, Arthurs J, Gordian D, Patel A, Rupp AC, Adams JM, Wang J, Beck Jørgensen S, Olson DP, Palmiter RD, Myers MG Jr, Seeley RJ. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc Natl Acad Sci USA 118: e2021357118, 2021. doi: 10.1073/pnas.2021357118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci 29: 8798–8804, 2009. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci 32: 15439–15449, 2012. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology 58: 156–165, 2010. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Mason BN, Kaiser EA, Kuburas A, Loomis MM, Latham JA, Garcia-Martinez LF, Russo AF. Induction of migraine-like photophobic behavior in mice by both peripheral and central CGRP mechanisms. J Neurosci 37: 204–216, 2017. doi: 10.1523/JNEUROSCI.2967-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Hanani M, Spray DC. Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci 21: 485–498, 2020. doi: 10.1038/s41583-020-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain 7: 94, 2011. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Reddington M, Priller J, Treichel J, Haas C, Kreutzberg GW. Astrocytes and microglia as potential targets for calcitonin gene related peptide in the central nervous system. Can J Physiol Pharmacol 73: 1047–1049, 1995. doi: 10.1139/y95-148. [DOI] [PubMed] [Google Scholar]
- 448. Ceruti S, Villa G, Fumagalli M, Colombo L, Magni G, Zanardelli M, Fabbretti E, Verderio C, van den Maagdenberg AM, Nistri A, Abbracchio MP. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 knock-in mice: implications for basic mechanisms of migraine pain. J Neurosci 31: 3638–3649, 2011. doi: 10.1523/JNEUROSCI.6440-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol 37: 83–90, 2008. doi: 10.1007/s12035-008-8020-5. [DOI] [PubMed] [Google Scholar]
- 450. Simonetti M, Giniatullin R, Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J Biol Chem 283: 18743–18752, 2008. doi: 10.1074/jbc.M800296200. [DOI] [PubMed] [Google Scholar]
- 451. Fischer M, Wille G, Klien S, Shanib H, Holle D, Gaul C, Broessner G. Brain-derived neurotrophic factor in primary headaches. J Headache Pain 13: 469–475, 2012. doi: 10.1007/s10194-012-0454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Haanes KA, Labastida-Ramírez A, Blixt FW, Rubio-Beltrán E, Dirven CM, Danser AH, Edvinsson L, MaassenVanDenBrink A. Exploration of purinergic receptors as potential anti-migraine targets using established pre-clinical migraine models. Cephalalgia 39: 1421–1434, 2019. doi: 10.1177/0333102419851810. [DOI] [PubMed] [Google Scholar]
- 453. Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 407: 1015–1017, 2000. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- 454. Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache 47: 1008–1023, 2007. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Vause CV, Durham PL. Calcitonin gene-related peptide differentially regulates gene and protein expression in trigeminal glia cells: findings from array analysis. Neurosci Lett 473: 163–167, 2010. doi: 10.1016/j.neulet.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain 5: 43, 2009. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Dieterle A, Fischer MJ, Link AS, Neuhuber WL, Messlinger K. Increase in CGRP- and nNOS-immunoreactive neurons in the rat trigeminal ganglion after infusion of an NO donor. Cephalalgia 31: 31–42, 2011. doi: 10.1177/0333102410375725. [DOI] [PubMed] [Google Scholar]
- 458. Seiler K, Nusser JI, Lennerz JK, Neuhuber WL, Messlinger K. Changes in calcitonin gene-related peptide (CGRP) receptor component and nitric oxide receptor (sGC) immunoreactivity in rat trigeminal ganglion following glyceroltrinitrate pretreatment. J Headache Pain 14: 74, 2013. doi: 10.1186/1129-2377-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Bonnet C, Hao J, Osorio N, Donnet A, Penalba V, Ruel J, Delmas P. Maladaptive activation of Nav1.9 channels by nitric oxide causes triptan-induced medication overuse headache. Nat Commun 10: 4253, 2019. doi: 10.1038/s41467-019-12197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Eberhardt M, Dux M, Namer B, Miljkovic J, Cordasic N, Will C, Kichko TI, de la Roche J, Fischer M, Suárez SA, Bikiel D, Dorsch K, Leffler A, Babes A, Lampert A, Lennerz JK, Jacobi J, Martí MA, Doctorovich F, Högestätt ED, Zygmunt PM, Ivanovic-Burmazovic I, Messlinger K, Reeh P, Filipovic MR. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun 5: 4381, 2014. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Dux M, Will C, Vogler B, Filipovic MR, Messlinger K. Meningeal blood flow is controlled by H2 S-NO crosstalk activating a HNO-TRPA1-CGRP signalling pathway. Br J Pharmacol 173: 431–445, 2016. doi: 10.1111/bph.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84: 903–934, 2004. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 463. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94: 1099–1142, 2014. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Argunhan F, Brain SD. The vascular-dependent and -independent actions of calcitonin gene-related peptide in cardiovascular disease. Front Physiol 13: 833645, 2022. doi: 10.3389/fphys.2022.833645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Skaria T, Vogel J. The neuropeptide alpha-calcitonin gene-related peptide as the mediator of beneficial effects of exercise in the cardiovascular system. Front Physiol 13: 825992, 2022. doi: 10.3389/fphys.2022.825992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Kumar A, Williamson M, Hess A, DiPette DJ, Potts JD. Alpha-calcitonin gene related peptide: new therapeutic strategies for the treatment and prevention of cardiovascular disease and migraine. Front Physiol 13: 826122, 2022. doi: 10.3389/fphys.2022.826122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. MaassenVanDenBrink A, Meijer J, Villalón CM, Ferrari MD. Wiping out CGRP: potential cardiovascular risks. Trends Pharmacol Sci 37: 779–788, 2016. doi: 10.1016/j.tips.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 468. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 14: 338–350, 2018. doi: 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 469. Brain SD, Tippins JR, Morris HR, MacIntyre I, Williams TJ. Potent vasodilator activity of calcitonin gene-related peptide in human skin. J Invest Dermatol 87: 533–536, 1986. doi: 10.1111/1523-1747.ep12455620. [DOI] [PubMed] [Google Scholar]
- 470. Ando K, Pegram BL, Frohlich ED. Hemodynamic effects of calcitonin gene-related peptide in spontaneously hypertensive rats. Am J Physiol Integr Comp Physiol 258: R425–R429, 1990. doi: 10.1152/ajpregu.1990.258.2.R425. [DOI] [PubMed] [Google Scholar]
- 471. Gardiner SM, Compton AM, Kemp PA, Bennett T, Bose C, Foulkes R, Hughes B. Antagonistic effect of human alpha-calcitonin gene-related peptide (8-37) on regional hemodynamic actions of rat islet amyloid polypeptide in conscious Long-Evans rats. Diabetes 40: 948–951, 1991. doi: 10.2337/diab.40.8.948. [DOI] [PubMed] [Google Scholar]
- 472. Gardiner SM, Compton AM, Bennett T. Regional haemodynamic effects of human alpha- and beta-calcitonin gene-related peptide in conscious Wistar rats. Br J Pharmacol 98: 1225–1232, 1989. doi: 10.1111/j.1476-5381.1989.tb12668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Beglinger C, Born W, Münch R, Kurtz A, Gutzwiller JP, Jäger K, Fischer JA. Distinct hemodynamic and gastric effects of human CGRP I and II in man. Peptides 12: 1347–1351, 1991. doi: 10.1016/0196-9781(91)90218-E. [DOI] [PubMed] [Google Scholar]
- 474. Smillie SJ, Brain SD. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides 45: 93–104, 2011. doi: 10.1016/j.npep.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 475. Goadsby PJ, Dodick DW, Ailani J, Trugman JM, Finnegan M, Lu K, Szegedi A. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol 19: 727–737, 2020. doi: 10.1016/S1474-4422(20)30234-9. [DOI] [PubMed] [Google Scholar]
- 476. Dodick DW, Tepper SJ, Ailani J, Pannacciulli N, Navetta MS, Loop B, Zhang F, Khodavirdi AC, Mann A, Abdrabboh A, Kalim J. Risk of hypertension in erenumab-treated patients with migraine: analyses of clinical trial and postmarketing data. Headache 61: 1411–1420, 2021. doi: 10.1111/head.14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477. Loder EW, Burch RC. Who should try new antibody treatments for migraine? JAMA Neurol 75: 1039–1040, 2018. doi: 10.1001/jamaneurol.2018.1268. [DOI] [PubMed] [Google Scholar]
- 478. Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltrán E, Vigneri S, Edvinsson L, Maassen Van Den Brink A; European Headache Federation School of Advanced Studies (EHF-SAS). Blocking CGRP in migraine patients—a review of pros and cons. J Headache Pain 18: 96, 2017. doi: 10.1186/s10194-017-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Diener HC. CGRP-targeted drugs for migraine: still many uncertainties. Lancet Neurol 21: 209–210, 2022. doi: 10.1016/S1474-4422(21)00468-3. [DOI] [PubMed] [Google Scholar]
- 480. Al-Hassany L, Goadsby PJ, Danser AH, MaassenVanDenBrink A. Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol 21: 284–294, 2022. doi: 10.1016/S1474-4422(21)00409-9. [DOI] [PubMed] [Google Scholar]
- 481. de Boer I, MaassenVanDenBrink A, Terwindt GM. The potential danger of blocking CGRP for treating migraine in CADASIL patients. Cephalalgia 40: 1676–1678, 2020. doi: 10.1177/0333102420941814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Aradi S, Kaiser E, Cucchiara B. Ischemic stroke associated with calcitonin gene-related peptide inhibitor therapy for migraine: a case report. J Stroke Cerebrovasc Dis 28: 104286, 2019. doi: 10.1016/j.jstrokecerebrovasdis.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 483. Saely S, Croteau D, Jawidzik L, Brinker A, Kortepeter C. Hypertension: a new safety risk for patients treated with erenumab. Headache 61: 202–208, 2021. doi: 10.1111/head.14051. [DOI] [PubMed] [Google Scholar]
- 484. Al-Hassany L, Van Den Brink AM. Targeting CGRP in migraine: a matter of choice and dose. Lancet Neurol 19: 712–713, 2020. doi: 10.1016/S1474-4422(20)30282-9. [DOI] [PubMed] [Google Scholar]
- 485. Smillie SJ, King R, Kodji X, Outzen E, Pozsgai G, Fernandes E, Marshall N, de Winter P, Heads RJ, Dessapt-Baradez C, Gnudi L, Sams A, Shah AM, Siow RC, Brain SD. An ongoing role of alpha-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension 63: 1056–1062, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02517. [DOI] [PubMed] [Google Scholar]
- 486. Aubdool AA, Thakore P, Argunhan F, Smillie SJ, Schnelle M, Srivastava S, Alawi KM, Wilde E, Mitchell J, Farrell-Dillon K, Richards DA, Maltese G, Siow RC, Nandi M, Clark JE, Shah AM, Sams A, Brain SD. A novel alpha-calcitonin gene-related peptide analogue protects against end-organ damage in experimental hypertension, cardiac hypertrophy, and heart failure. Circulation 136: 367–383, 2017. doi: 10.1161/CIRCULATIONAHA.117.028388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Bentsen S, Sams A, Hasbak P, Edvinsson L, Kjaer A, Ripa RS. Myocardial perfusion recovery induced by an alpha-calcitonin gene-related peptide analogue. J Nucl Cardiol 29: 2090–2099, 2022. doi: 10.1007/s12350-021-02678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Jacobs B, Dussor G. Neurovascular contributions to migraine: moving beyond vasodilation. Neuroscience 338: 130–144, 2016. doi: 10.1016/j.neuroscience.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489. Zhang Z, Dickerson IM, Russo AF. Calcitonin gene-related peptide receptor activation by receptor activity-modifying protein-1 gene transfer to vascular smooth muscle cells. Endocrinology 147: 1932–1940, 2006. doi: 10.1210/en.2005-0918. [DOI] [PubMed] [Google Scholar]
- 490. Moreira LM, Takawale A, Hulsurkar M, Menassa DA, Antanaviciute A, Lahiri SK, et al. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature 587: 460–465, 2020. doi: 10.1038/s41586-020-2890-8. [DOI] [PubMed] [Google Scholar]
- 491. Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, Deutsch G, Sun X. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360: eaan8546, 2018. doi: 10.1126/science.aan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Voisin T, Bouvier A, Chiu IM. Neuro-immune interactions in allergic diseases: novel targets for therapeutics. Int Immunol 29: 247–261, 2017. doi: 10.1093/intimm/dxx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Springer J, Geppetti P, Fischer A, Groneberg DA. Calcitonin gene-related peptide as inflammatory mediator. Pulm Pharmacol Ther 16: 121–130, 2003. doi: 10.1016/S1094-5539(03)00049-X. [DOI] [PubMed] [Google Scholar]
- 494. Keith IM, Pelto-Huikko M, Schalling M, Hökfelt T. Calcitonin gene-related peptide and its mRNA in pulmonary neuroendocrine cells and ganglia. Histochemistry 96: 311–315, 1991. doi: 10.1007/BF00271351. [DOI] [PubMed] [Google Scholar]
- 495. Xie W, Fisher JT, Lynch TJ, Luo M, Evans TI, Neff TL, Zhou W, Zhang Y, Ou Y, Bunnett NW, Russo AF, Goodheart MJ, Parekh KR, Liu X, Engelhardt JF. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J Clin Invest 121: 3144–3158, 2011. doi: 10.1172/JCI41857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496. Atanasova KR, Reznikov LR. Neuropeptides in asthma, chronic obstructive pulmonary disease and cystic fibrosis. Respir Res 19: 149, 2018. doi: 10.1186/s12931-018-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Tjen ALS, Ekman R, Lippton H, Cary J, Keith I. CGRP and somatostatin modulate chronic hypoxic pulmonary hypertension. Am J Physiol Heart Circ Physiol 263: H681–H690, 1992. doi: 10.1152/ajpheart.1992.263.3.H681. . [DOI] [PubMed] [Google Scholar]
- 498. Champion HC, Bivalacqua TJ, Toyoda K, Heistad DD, Hyman AL, Kadowitz PJ. In vivo gene transfer of prepro-calcitonin gene-related peptide to the lung attenuates chronic hypoxia-induced pulmonary hypertension in the mouse. Circulation 101: 923–930, 2000. doi: 10.1161/01.CIR.101.8.923. [DOI] [PubMed] [Google Scholar]
- 499. Shivaraju M, Chitta UK, Grange RM, Jain IH, Capen D, Liao L, Xu J, Ichinose F, Zapol WM, Mootha VK, Rajagopal J. Airway stem cells sense hypoxia and differentiate into protective solitary neuroendocrine cells. Science 371: 52–57, 2021. doi: 10.1126/science.aba0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Xu J, Xu L, Sui P, Chen J, Moya EA, Hume P, Janssen WJ, Duran JM, Thistlethwaite P, Carlin A, Gulleman P, Banaschewski B, Goldy MK, Yuan JX, Malhotra A, Pryhuber G, Crotty-Alexander L, Deutsch G, Young LR, Sun X. Excess neuropeptides in lung signal through endothelial cells to impair gas exchange. Dev Cell 57: 839–853.e6, 2022. doi: 10.1016/j.devcel.2022.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Assas BM, Pennock JI, Miyan JA. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front Neurosci 8: 23, 2014. doi: 10.3389/fnins.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Broome CS, Miyan JA. Neuropeptide control of bone marrow neutrophil production. A key axis for neuroimmunomodulation. Ann NY Acad Sci 917: 424–434, 2000. doi: 10.1111/j.1749-6632.2000.tb05407.x. [DOI] [PubMed] [Google Scholar]
- 503. Shepherd AJ, Downing JE, Miyan JA. Without nerves, immunology remains incomplete -in vivo veritas. Immunology 116: 145–163, 2005. doi: 10.1111/j.1365-2567.2005.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Morrison JF, Dhanasekaran S, Howarth FC. Neuropeptides in the rat corpus cavernosum and seminal vesicle: effects of age and two types of diabetes. Auton Neurosci 146: 76–80, 2009. doi: 10.1016/j.autneu.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 505. De Winter BY, Bredenoord AJ, Van Nassauw L, De Man JG, De Schepper HU, Timmermans JP, Pelckmans PA. Involvement of afferent neurons in the pathogenesis of endotoxin-induced ileus in mice: role of CGRP and TRPV1 receptors. Eur J Pharmacol 615: 177–184, 2009. doi: 10.1016/j.ejphar.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 506. Mikami N, Watanabe K, Hashimoto N, Miyagi Y, Sueda K, Fukada S, Yamamoto H, Tsujikawa K. Calcitonin gene-related peptide enhances experimental autoimmune encephalomyelitis by promoting Th17-cell functions. Int Immunol 24: 681–691, 2012. doi: 10.1093/intimm/dxs075. [DOI] [PubMed] [Google Scholar]
- 507. Smith AS, Smid SD. Impaired capsaicin and neurokinin-evoked colonic motility in inflammatory bowel disease. J Gastroenterol Hepatol 20: 697–704, 2005. doi: 10.1111/j.1440-1746.2005.03759.x. [DOI] [PubMed] [Google Scholar]
- 508. Reinshagen M, Flämig G, Ernst S, Geerling I, Wong H, Walsh JH, Eysselein VE, Adler G. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. J Pharmacol Exp Ther 286: 657–661, 1998. doi: 10.1111/j.1440-1746.2005.03759.x. [DOI] [PubMed] [Google Scholar]
- 509. Engel MA, Becker C, Reeh PW, Neurath MF. Role of sensory neurons in colitis: increasing evidence for a neuroimmune link in the gut. Inflamm Bowel Dis 17: 1030–1033, 2011. doi: 10.1002/ibd.21422. [DOI] [PubMed] [Google Scholar]
- 510. Thompson BJ, Washington MK, Kurre U, Singh M, Rula EY, Emeson RB. Protective roles of alpha-calcitonin and beta-calcitonin gene-related peptide in spontaneous and experimentally induced colitis. Dig Dis Sci 53: 229–241, 2008. doi: 10.1007/s10620-007-9848-7. [DOI] [PubMed] [Google Scholar]
- 511. Kim YJ, Granstein RD. Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain Behav Immun Health 18: 100361, 2021. doi: 10.1016/j.bbih.2021.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Dong J, He Y, Zhang X, Wang L, Sun T, Zhang M, Liang Y, Qi M. Calcitonin gene-related peptide regulates the growth of epidermal stem cells in vitro. Peptides 31: 1860–1865, 2010. doi: 10.1016/j.peptides.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 513. Roggenkamp D, Köpnick S, Stäb F, Wenck H, Schmelz M, Neufang G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol 133: 1620–1628, 2013. doi: 10.1038/jid.2012.464. [DOI] [PubMed] [Google Scholar]
- 514. Levy D, Labastida-Ramirez A, MaassenVanDenBrink A. Current understanding of meningeal and cerebral vascular function underlying migraine headache. Cephalalgia 39: 1606–1622, 2019. doi: 10.1177/0333102418771350. [DOI] [PubMed] [Google Scholar]
- 515. Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101: 412–420.e3, 2019. doi: 10.1016/j.neuron.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. Pedersen SH, la Cour SH, Calloe K, Hauser F, Olesen J, Klaerke DA, Jansen-Olesen I. PACAP-38 and PACAP(6-38) degranulate rat meningeal mast cells via the orphan MrgB3-receptor. Front Cell Neurosci 13: 114, 2019. doi: 10.3389/fncel.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. McIlvried LA, Cruz JA, Borghesi LA, Gold MS. Sex-, stress-, and sympathetic post-ganglionic-dependent changes in identity and proportions of immune cells in the dura. Cephalalgia 37: 36–48, 2017. doi: 10.1177/0333102416637832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. Schain AJ, Melo-Carrillo A, Borsook D, Grutzendler J, Strassman AM, Burstein R. Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann Neurol 83: 508–521, 2018. doi: 10.1002/ana.25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Zhang J, Czerpaniak K, Huang L, Liu X, Cloud ME, Unsinger J, Hotchkiss RS, Li D, Cao YQ. Low-dose interleukin-2 reverses behavioral sensitization in multiple mouse models of headache disorders. Pain 161: 1381–1398, 2020. doi: 10.1097/j.pain.0000000000001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Schafflick D, Wolbert J, Heming M, Thomas C, Hartlehnert M, Börsch AL, Ricci A, Martín-Salamanca S, Li X, Lu IN, Pawlak M, Minnerup J, Strecker JK, Seidenbecher T, Meuth SG, Hidalgo A, Liesz A, Wiendl H, Meyer Zu Horste G. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat Neurosci 24: 1225–1234, 2021. doi: 10.1038/s41593-021-00880-y. [DOI] [PubMed] [Google Scholar]
- 521. Umeda Y, Takamiya M, Yoshizaki H, Arisawa M. Inhibition of mitogen-stimulated T lymphocyte proliferation by calcitonin gene-related peptide. Biochem Biophys Res Commun 154: 227–235, 1988. doi: 10.1016/0006-291X(88)90674-2. [DOI] [PubMed] [Google Scholar]
- 522. Bomsel M, Ganor Y. Calcitonin gene-related peptide induces HIV-1 proteasomal degradation in mucosal Langerhans cells. J Virol 91: e01205–e01217, 2017. doi: 10.1128/JVI.01205-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523. Ganor Y, Drillet-Dangeard AS, Bomsel M. Calcitonin gene-related peptide inhibits human immunodeficiency type 1 transmission by Langerhans cells via an autocrine/paracrine feedback mechanism. Acta Physiol (Oxf) 213: 432–441, 2015. doi: 10.1111/apha.12366. [DOI] [PubMed] [Google Scholar]
- 524. Ganor Y, Drillet-Dangeard AS, Lopalco L, Tudor D, Tambussi G, Delongchamps NB, Zerbib M, Bomsel M. Calcitonin gene-related peptide inhibits Langerhans cell-mediated HIV-1 transmission. J Exp Med 210: 2161–2170, 2013. doi: 10.1084/jem.20122349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Mullins MW, Ciallella J, Rangnekar V, McGillis JP. Characterization of a calcitonin gene-related peptide (CGRP) receptor on mouse bone marrow cells. Regul Pept 49: 65–72, 1993. doi: 10.1016/0167-0115(93)90385-L. [DOI] [PubMed] [Google Scholar]
- 526. Suekane A, Saito Y, Nakahata S, Ichikawa T, Ogoh H, Tsujikawa K, Morishita K. CGRP-CRLR/RAMP1 signal is important for stress-induced hematopoiesis. Sci Rep 9: 429, 2019. doi: 10.1038/s41598-018-36796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Gao X, Zhang D, Xu C, Li H, Caron KM, Frenette PS. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589: 591–596, 2021. doi: 10.1038/s41586-020-03057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Xu C, Gulinello M, Frenette PS. Nociceptors protect sickle cell disease mice from vaso-occlusive episodes and chronic organ damage. J Exp Med 218: e20200065, 2021. doi: 10.1084/jem.20200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Ray JC, Allen P, Bacsi A, Bosco JJ, Chen L, Eller M, Kua H, Lim LL, Matharu MS, Monif M, Ruttledge M, Stark RJ, Hutton EJ. Inflammatory complications of CGRP monoclonal antibodies: a case series. J Headache Pain 22: 121, 2021. doi: 10.1186/s10194-021-01330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Lima WG, Marques-Oliveira GH, da Silva TM, Chaves VE. Role of calcitonin gene-related peptide in energy metabolism. Endocrine 58: 3–13, 2017. doi: 10.1007/s12020-017-1404-4. [DOI] [PubMed] [Google Scholar]
- 531. Sanford D, Luong L, Gabalski A, Oh S, Vu JP, Pisegna JR, Germano P. An intraperitoneal treatment with calcitonin gene-related peptide (CGRP) regulates appetite, energy intake/expenditure, and metabolism. J Mol Neurosci 67: 28–37, 2019. doi: 10.1007/s12031-018-1202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Nilsson C, Hansen TK, Rosenquist C, Hartmann B, Kodra JT, Lau JF, Clausen TR, Raun K, Sams A. Long acting analogue of the calcitonin gene-related peptide induces positive metabolic effects and secretion of the glucagon-like peptide-1. Eur J Pharmacol 773: 24–31, 2016. doi: 10.1016/j.ejphar.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 533. Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides 19: 309–317, 1998. doi: 10.1016/S0196-9781(97)00292-1. [DOI] [PubMed] [Google Scholar]
- 534. Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E. Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides 19: 1533–1540, 1998. doi: 10.1016/S0196-9781(98)00114-4. [DOI] [PubMed] [Google Scholar]
- 535. Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E. Evidence for a physiological role of central calcitonin gene-related peptide (CGRP) receptors in the control of food intake in rats. Neurosci Lett 230: 159–162, 1997. doi: 10.1016/S0304-3940(97)00503-X. [DOI] [PubMed] [Google Scholar]
- 536. Liu T, Kamiyoshi A, Sakurai T, Ichikawa-Shindo Y, Kawate H, Yang L, Tanaka M, Xian X, Imai A, Zhai L, Hirabayashi K, Dai K, Tanimura K, Liu T, Cui N, Igarashi K, Yamauchi A, Shindo T. Endogenous calcitonin gene-related peptide regulates lipid metabolism and energy homeostasis in male mice. Endocrinology 158: 1194–1206, 2017. doi: 10.1210/en.2016-1510. [DOI] [PubMed] [Google Scholar]
- 537. Walker CS, Li X, Whiting L, Glyn-Jones S, Zhang S, Hickey AJ, Sewell MA, Ruggiero K, Phillips AR, Kraegen EW, Hay DL, Cooper GJ, Loomes KM. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology 151: 4257–4269, 2010. doi: 10.1210/en.2010-0284. [DOI] [PubMed] [Google Scholar]
- 538. Bartelt A, Jeschke A, Müller B, Gaziano I, Morales M, Yorgan T, Heckt T, Heine M, Gagel RF, Emeson RB, Amling M, Niemeier A, Heeren J, Schinke T, Keller J. Differential effects of Calca-derived peptides in male mice with diet-induced obesity. PLoS One 12: e0180547, 2017. doi: 10.1371/journal.pone.0180547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Makwana K, Chodavarapu H, Morones N, Chi J, Barr W, Novinbakht E, Wang Y, Nguyen PT, Jovanovic P, Cohen P, Riera CE. Sensory neurons expressing calcitonin gene-related peptide alpha regulate adaptive thermogenesis and diet-induced obesity. Mol Metab 45: 101161, 2021. doi: 10.1016/j.molmet.2021.101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Halloran J, Lalande A, Zang M, Chodavarapu H, Riera CE. Monoclonal therapy against calcitonin gene-related peptide lowers hyperglycemia and adiposity in type 2 diabetes mouse models. Metabol Open 8: 100060, 2020. doi: 10.1016/j.metop.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 157: 1023–1036, 2014. doi: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 542. Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP neurons control meal termination. Cell Metab 23: 811–820, 2016. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun 7: 11905, 2016. doi: 10.1038/ncomms11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Mora M, Marchi M, Polak JM, Gibson SJ, Cornelio F. Calcitonin gene-related peptide immunoreactivity at the human neuromuscular junction. Brain Res 492: 404–407, 1989. doi: 10.1016/0006-8993(89)90930-X. [DOI] [PubMed] [Google Scholar]
- 545. Csillik B, Tajti L, Kovács T, Kukla E, Rakic P, Knyihár-Csillik E. Distribution of calcitonin gene-related peptide in vertebrate neuromuscular junctions: relationship to the acetylcholine receptor. J Histochem Cytochem 41: 1547–1555, 1993. doi: 10.1177/41.10.8245413. [DOI] [PubMed] [Google Scholar]
- 546. Lu B, Fu WM, Greengard P, Poo MM. Calcitonin gene-related peptide potentiates synaptic responses at developing neuromuscular junction. Nature 363: 76–79, 1993. doi: 10.1038/363076a0. [DOI] [PubMed] [Google Scholar]
- 547. Sakaguchi M, Inaishi Y, Kashihara Y, Kuno M. Release of calcitonin gene-related peptide from nerve terminals in rat skeletal muscle. J Physiol 434: 257–270, 1991. doi: 10.1113/jphysiol.1991.sp018468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548. Uchida S, Yamamoto H, Iio S, Matsumoto N, Wang XB, Yonehara N, Imai Y, Inoki R, Yoshida H. Release of calcitonin gene-related peptide-like immunoreactive substance from neuromuscular junction by nerve excitation and its action on striated muscle. J Neurochem 54: 1000–1003, 1990. doi: 10.1111/j.1471-4159.1990.tb02349.x. [DOI] [PubMed] [Google Scholar]
- 549. New HV, Mudge AW. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. Nature 323: 809–811, 1986. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- 550. Aracil-Marco A, Sarabia JM, Pastor D, Guillén S, López-Grueso R, Gallar J, Moya-Ramón M. Acute increase in blood alphaCGRP at maximal exercise and its association to cardiorespiratory fitness, carbohydrate oxidation and work performed: an exploratory study in young men. Biology (Basel) 10: 783, 2021. doi: 10.3390/biology10080783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551. Parnow A, Gharakhanlou R, Gorginkaraji Z, Rajabi S, Eslami R, Hedayati M, Mahdian R. Effects of endurance and resistance training on calcitonin gene-related Peptide and acetylcholine receptor at slow and fast twitch skeletal muscles and sciatic nerve in male wistar rats. Int J Pept 2012: 962651, 2012. doi: 10.1155/2012/962651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552. Danaher RN, Loomes KM, Leonard BL, Whiting L, Hay DL, Xu LY, Kraegen EW, Phillips AR, Cooper GJ. Evidence that alpha-calcitonin gene-related peptide is a neurohormone that controls systemic lipid availability and utilization. Endocrinology 149: 154–160, 2008. doi: 10.1210/en.2007-0583. [DOI] [PubMed] [Google Scholar]
- 553. Walker CS, Hay DL, Fitzpatrick SM, Cooper GJ, Loomes KM. alpha-Calcitonin gene related peptide (alpha-CGRP) mediated lipid mobilization in 3T3-L1 adipocytes. Peptides 58: 14–19, 2014. doi: 10.1016/j.peptides.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 554. Aveseh M, Koushkie-Jahromi M, Nemati J, Esmaeili-Mahani S. Serum calcitonin gene-related peptide facilitates adipose tissue lipolysis during exercise via PIPLC/IP3 pathways. Endocrine 61: 462–472, 2018. doi: 10.1007/s12020-018-1640-2. [DOI] [PubMed] [Google Scholar]
- 555. Martins-Oliveira M, Tavares I, Goadsby PJ. Was it something I ate? Understanding the bidirectional interaction of migraine and appetite neural circuits. Brain Res 1770: 147629, 2021. doi: 10.1016/j.brainres.2021.147629. [DOI] [PubMed] [Google Scholar]
- 556. Rosenfeld MG, Amara SG, Evans RM. Alternative RNA processing: determining neuronal phenotype. Science 225: 1315–1320, 1984. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- 557. Evangelista S. CGRP. In: Handbook of Biologically Active Peptides, edited by Kastin AJ. Boston, MA: Academic Press, 2013, p. 1204–1209. [Google Scholar]
- 558. Somasundaram C, Diz DI, Coleman T, Bukoski RD. Adventitial neuronal somata. J Vasc Res 43: 278–288, 2006. doi: 10.1159/000092765. [DOI] [PubMed] [Google Scholar]
- 559. Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr Opin Pharmacol 7: 563–569, 2007. doi: 10.1016/j.coph.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Evangelista S. Role of calcitonin gene-related peptide in gastric mucosal defence and healing. Curr Pharm Des 15: 3571–3576, 2009. doi: 10.2174/138161209789207024. [DOI] [PubMed] [Google Scholar]
- 561. Engel MA, Khalil M, Mueller-Tribbensee SM, Becker C, Neuhuber WL, Neurath MF, Reeh PW. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J Gastroenterol 47: 256–265, 2012. doi: 10.1007/s00535-011-0495-6. [DOI] [PubMed] [Google Scholar]
- 562. Engel MA, Khalil M, Siklosi N, Mueller-Tribbensee SM, Neuhuber WL, Neurath MF, Becker C, Reeh PW. Opposite effects of substance P and calcitonin gene-related peptide in oxazolone colitis. Dig Liver Dis 44: 24–29, 2012. doi: 10.1016/j.dld.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 563. Holzer P, Holzer-Petsche U. Constipation caused by anti-calcitonin gene-related peptide migraine therapeutics explained by antagonism of calcitonin gene-related peptide’s motor-stimulating and prosecretory function in the intestine. Front Physiol 12: 820006, 2021. doi: 10.3389/fphys.2021.820006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. Clifton MS, Hoy JJ, Chang J, Idumalla PS, Fakhruddin H, Grady EF, Dada S, Corvera CU, Bhargava A. Role of calcitonin receptor-like receptor in colonic motility and inflammation. Am J Physiol Gastrointest Liver Physiol 293: G36–G44, 2007. doi: 10.1152/ajpgi.00464.2006. [DOI] [PubMed] [Google Scholar]
- 565. Holzer P. Implications of tachykinins and calcitonin gene-related peptide in inflammatory bowel disease. Digestion 59: 269–283, 1998. doi: 10.1159/000007504. [DOI] [PubMed] [Google Scholar]
- 566. Manela FD, Ren J, Gao J, McGuigan JE, Harty RF. Calcitonin gene-related peptide modulates acid-mediated regulation of somatostatin and gastrin release from rat antrum. Gastroenterology 109: 701–706, 1995. doi: 10.1016/0016-5085(95)90376-3. [DOI] [PubMed] [Google Scholar]
- 567. L’Heureux MC, St-Pierre S, Trudel L, Plourde V, Lepage R, Poitras P. Digestive motor effects and vascular actions of CGRP in dog are expressed by different receptor subtypes. Peptides 21: 425–430, 2000. doi: 10.1016/S0196-9781(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 568. Holzer P, Barthó L, Matusák O, Bauer V. Calcitonin gene-related peptide action on intestinal circular muscle. Am J Physiol Gastrointest Liver Physiol 256: G546–G552, 1989. doi: 10.1152/ajpgi.1989.256.3.G546. [DOI] [PubMed] [Google Scholar]
- 569. Ono T, Nagao M, Imoto H, Watanabe K, Tanaka N, Motoi F, Naitoh T, Unno M. Effects of calcitonin gene-related peptide on colonic motility and defecation in conscious dogs. J Gastrointest Surg 22: 2097–2103, 2018. doi: 10.1007/s11605-018-3858-y. [DOI] [PubMed] [Google Scholar]
- 570. Chen FX, Yu YB, Yuan XM, Zuo XL, Li YQ. Brain-derived neurotrophic factor enhances the contraction of intestinal muscle strips induced by SP and CGRP in mice. Regul Pept 178: 86–94, 2012. doi: 10.1016/j.regpep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 571. Bartho L, Benko R, Holzer-Petsche U, Holzer P, Undi S, Wolf M. Role of extrinsic afferent neurons in gastrointestinal motility. Eur Rev Med Pharmacol Sci 12: 21–31, 2008. [PubMed] [Google Scholar]
- 572. Grider JR. CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. Am J Physiol Gastrointest Liver Physiol 266: G1139–G1145, 1994. doi: 10.1152/ajpgi.1994.266.6.G1139. [DOI] [PubMed] [Google Scholar]
- 573. Hasler WL. Small intestinal motility. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. Burlington, MA: Academic Press, 2006, p. 935–964. [Google Scholar]
- 574. Deligianni CI, Mitsikostas DD, Ashina M. Safety and tolerability evaluation of erenumab for the preventive treatment of migraine. Expert Opin Drug Saf 20: 867–876, 2021. doi: 10.1080/14740338.2021.1933941. [DOI] [PubMed] [Google Scholar]
- 575. Ailani J, Lipton RB, Goadsby PJ, Guo H, Miceli R, Severt L, Finnegan M, Trugman JM; ADVANCE Study Group. Atogepant for the preventive treatment of migraine. N Engl J Med 385: 695–706, 2021. doi: 10.1056/NEJMoa2035908. [DOI] [PubMed] [Google Scholar]
- 576. Kudrow D, Nguyen L, Semler J, Stroud C, Samaan K, Hoban DB, Wietecha L, Hsu HA, Pearlman E. A phase IV clinical trial of gastrointestinal motility in adult patients with migraine before and after initiation of a calcitonin gene-related peptide ligand (galcanezumab) or receptor (erenumab) antagonist. Headache 62: 1164–1176, 2022. doi: 10.1111/head.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577. Kaiser EA, Rea BJ, Kuburas A, Kovacevich BR, Garcia-Martinez LF, Recober A, Russo AF. Anti-CGRP antibodies block CGRP-induced diarrhea in mice. Neuropeptides 64: 95–99, 2017. doi: 10.1016/j.npep.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578. Falkenberg K, Bjerg HR, Olesen J. Two-hour CGRP infusion causes gastrointestinal hyperactivity: possible relevance for CGRP antibody treatment. Headache 60: 929–937, 2020. doi: 10.1111/head.13795. [DOI] [PubMed] [Google Scholar]
- 579. Yamatani T, Kadowaki S, Chiba T, Abe H, Chihara K, Fukase M, Fujita T. Calcitonin gene-related peptide stimulates somatostatin release from isolated perfused rat stomach. Endocrinology 118: 2144–2145, 1986. doi: 10.1210/endo-118-5-2144. [DOI] [PubMed] [Google Scholar]
- 580. Ren J, Dunn ST, Tang Y, Wang Y, Gao J, Brewer K, Harty RF. Effects of calcitonin gene-related peptide on somatostatin and gastrin gene expression in rat antrum. Regul Pept 73: 75–82, 1998. doi: 10.1016/S0167-0115(97)01039-2. [DOI] [PubMed] [Google Scholar]
- 581. Kawashima K, Ishihara S, Karim Rumi MA, Moriyama N, Kazumori H, Suetsugu H, Sato H, Fukuda R, Adachi K, Shibata M, Onodera S, Chiba T, Kinoshita Y. Localization of calcitonin gene-related peptide receptors in rat gastric mucosa. Peptides 23: 955–966, 2002. doi: 10.1016/S0196-9781(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 582. Beglinger C, Born W, Hildebrand P, Ensinck JW, Burkhardt F, Fischer JA, Gyr K. Calcitonin gene-related peptides I and II and calcitonin: distinct effects on gastric acid secretion in humans. Gastroenterology 95: 958–965, 1988. doi: 10.1016/0016-5085(88)90169-2. [DOI] [PubMed] [Google Scholar]
- 583. Cox HM, Ferrar JA, Cuthbert AW. Effects of alpha- and beta-calcitonin gene-related peptides upon ion transport in rat descending colon. Br J Pharmacol 97: 996–998, 1989. doi: 10.1111/j.1476-5381.1989.tb12553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584. McCulloch CR, Cooke HJ. Human alpha-calcitonin gene-related peptide influences colonic secretion by acting on myenteric neurons. Regul Pept 24: 87–96, 1989. doi: 10.1016/0167-0115(89)90214-0. [DOI] [PubMed] [Google Scholar]
- 585. Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neuroscience 25: 181–193, 1988. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- 586. Le TL, Grell AS, Sheykhzade M, Warfvinge K, Edvinsson L, Sams A. CGRP in rat mesenteric artery and vein—receptor expression, CGRP presence and potential roles. Eur J Pharmacol 875: 173033, 2020. doi: 10.1016/j.ejphar.2020.173033. [DOI] [PubMed] [Google Scholar]
- 587. Holzer P, Wachter C, Jocic M, Heinemann A. Vascular bed-dependent roles of the peptide CGRP and nitric oxide in acid-evoked hyperaemia of the rat stomach. J Physiol 480: 575–585, 1994. doi: 10.1113/jphysiol.1994.sp020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Ohno T, Hattori Y, Komine R, Ae T, Mizuguchi S, Arai K, Saeki T, Suzuki T, Hosono K, Hayashi I, Oh-Hashi Y, Kurihara Y, Kurihara H, Amagase K, Okabe S, Saigenji K, Majima M. Roles of calcitonin gene-related peptide in maintenance of gastric mucosal integrity and in enhancement of ulcer healing and angiogenesis. Gastroenterology 134: 215–225, 2008. doi: 10.1053/j.gastro.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 589. Szolcsányi J, Barthó L. Capsaicin-sensitive afferents and their role in gastroprotection: an update. J Physiol Paris 95: 181–188, 2001. doi: 10.1016/S0928-4257(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 590. Tache Y. Brainstem neuropeptides and vagal protection of the gastric mucosal against injury: role of prostaglandins, nitric oxide and calcitonin-gene related peptide in capsaicin afferents. Curr Med Chem 19: 35–42, 2012. doi: 10.2174/092986712803414097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591. Arzani M, Jahromi SR, Ghorbani Z, Vahabizad F, Martelletti P, Ghaemi A, Sacco S, Togha M; School of Advanced Studies of the European Headache Federation (EHF-SAS). Gut-brain Axis and migraine headache: a comprehensive review. J Headache Pain 21: 15, 2020. doi: 10.1186/s10194-020-1078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592. Wei P, Keller C, Li L. Neuropeptides in gut-brain axis and their influence on host immunity and stress. Comput Struct Biotechnol J 18: 843–851, 2020. doi: 10.1016/j.csbj.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593. Lai NY, Musser MA, Pinho-Ribeiro FA, Baral P, Jacobson A, Ma P, Potts DE, Chen Z, Paik D, Soualhi S, Yan Y, Misra A, Goldstein K, Lagomarsino VN, Nordstrom A, Sivanathan KN, Wallrapp A, Kuchroo VK, Nowarski R, Starnbach MN, Shi H, Surana NK, An D, Wu C, Huh JR, Rao M, Chiu IM. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate Salmonella host defense. Cell 180: 33–49.e22, 2020. doi: 10.1016/j.cell.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594. Chen Y, Mu J, Zhu M, Mukherjee A, Zhang H. Transient receptor potential channels and inflammatory bowel disease. Front Immunol 11: 180, 2020. doi: 10.3389/fimmu.2020.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595. Yang D, Jacobson A, Meerschaert KA, Sifakis JJ, Wu M, Chen X, Yang T, Zhou Y, Anekal PV, Rucker RA, Sharma D, Sontheimer-Phelps A, Wu GS, Deng L, Anderson MD, Choi S, Neel D, Lee N, Kasper DL, Jabri B, Huh JR, Johansson M, Thiagarajah JR, Riesenfeld SJ, Chiu IM. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell 185: 4190–4205.e25, 2022. doi: 10.1016/j.cell.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596. Chang CL, Hsu SY. Roles of CLR/RAMP receptor signaling in reproduction and development. Curr Protein Pept Sci 14: 393–406, 2013. doi: 10.2174/13892037113149990056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597. Lenhart PM, Caron KM. Adrenomedullin and pregnancy: perspectives from animal models to humans. Trends Endocrinol Metab 23: 524–532, 2012. doi: 10.1016/j.tem.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598. Stevenson JC, Macdonald DW, Warren RC, Booker MW, Whitehead MI. Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed) 293: 1329–1330, 1986. doi: 10.1136/bmj.293.6558.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599. Dong YL, Vegiraju S, Gangula PR, Kondapaka SB, Wimalawansa SJ, Yallampalli C. Expression and regulation of calcitonin gene-related peptide receptor in rat placentas. Biol Reprod 67: 1321–1326, 2002. doi: 10.1093/biolreprod/67.4.1321. [DOI] [PubMed] [Google Scholar]
- 600. Gangula PR, Zhao H, Supowit S, Wimalawansa S, DiPette D, Yallampalli C. Pregnancy and steroid hormones enhance the vasodilation responses to CGRP in rats. Am J Physiol Heart Circ Physiol 276: H284–H288, 1999. doi: 10.1152/ajpheart.1999.276.1.H284. [DOI] [PubMed] [Google Scholar]
- 601. Gangula PR, Thota C, Wimalawansa SJ, Bukoski RD, Yallampalli C. Mechanisms involved in calcitonin gene-related peptide-induced relaxation in pregnant rat uterine artery. Biol Reprod 69: 1635–1641, 2003. doi: 10.1095/biolreprod.103.016725. [DOI] [PubMed] [Google Scholar]
- 602. Chauhan M, Betancourt A, Balakrishnan M, Mishra A, Espinosa J, Shamshirsaz AA, Fox K, Belfort M, Yallampalli C. Calcitonin gene related peptide, adrenomedullin, and adrenomedullin 2 function in uterine artery during human pregnancy. Endocrinology 163: bqab204, 2022. doi: 10.1210/endocr/bqab204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603. Yallampalli C, Wimalawansa SJ. Calcitonin gene-related peptide (CGRP) is a mediator of vascular adaptations during hypertension in pregnancy. Trends Endocrinol Metab 9: 113–117, 1998. doi: 10.1016/S1043-2760(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 604. Dong YL, Green KE, Vegiragu S, Hankins GD, Martin E, Chauhan M, Thota C, Yallampalli C. Evidence for decreased calcitonin gene-related peptide (CGRP) receptors and compromised responsiveness to CGRP of fetoplacental vessels in preeclamptic pregnancies. J Clin Endocrinol Metab 90: 2336–2343, 2005. doi: 10.1210/jc.2004-1481. [DOI] [PubMed] [Google Scholar]
- 605. Gangula PR, Dong YL, Wimalawansa SJ, Yallampalli C. Infusion of pregnant rats with calcitonin gene-related peptide (CGRP)8-37, a CGRP receptor antagonist, increases blood pressure and fetal mortality and decreases fetal growth. Biol Reprod 67: 624–629, 2002. doi: 10.1095/biolreprod67.2.624. [DOI] [PubMed] [Google Scholar]
- 606. Vig SJ, Garza J, Tao Y. The use of erenumab for migraine prophylaxis during pregnancy: a case report and narrative review. Headache 62: 1256–1263, 2022. doi: 10.1111/head.14305. [DOI] [PubMed] [Google Scholar]
- 607. Bussiere JL, Davies R, Dean C, Xu C, Kim KH, Vargas HM, Chellman GJ, Balasubramanian G, Rubio-Beltran E, MaassenVanDenBrink A, Monticello TM. Nonclinical safety evaluation of erenumab, a CGRP receptor inhibitor for the prevention of migraine. Regul Toxicol Pharmacol 106: 224–238, 2019. doi: 10.1016/j.yrtph.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 608. McNeill DL, Papka RE, Harris CH. CGRP immunoreactivity and NADPH-diaphorase in afferent nerves of the rat penis. Peptides 13: 1239–1246, 1992. doi: 10.1016/0196-9781(92)90035-2. [DOI] [PubMed] [Google Scholar]
- 609. Lissbrant E, Collin O, Bergh A. Localization and effects of calcitonin gene-related peptide in the testicular vasculature of the rat. J Androl 18: 385–392, 1997. [PubMed] [Google Scholar]
- 610. Leung AY, Leung PY, Cheng-Chew SB, Wong PY. The role of calcitonin gene-related peptide in the regulation of anion secretion by the rat and human epididymis. J Endocrinol 133: 259–268, 1992. doi: 10.1677/joe.0.1330259. [DOI] [PubMed] [Google Scholar]
- 611. Champion HC, Wang R, Santiago JA, Murphy WA, Coy DH, Kadowitz PJ, Hellstrom WJ. Comparison of responses to adrenomedullin and calcitonin gene-related peptide in the feline erection model. J Androl 18: 513–521, 1997. [PubMed] [Google Scholar]
- 612. Truss MC, Becker AJ, Thon WF, Kuczyk M, Djamilian MH, Stief CG, Jonas U. Intracavernous calcitonin gene-related peptide plus prostaglandin E1: possible alternative to penile implants in selected patients. Eur Urol 26: 40–45, 1994. doi: 10.1159/000475340. [DOI] [PubMed] [Google Scholar]
- 613. Ashina M. Migraine. N Engl J Med 383: 1866–1876, 2020. doi: 10.1056/NEJMra1915327. [DOI] [PubMed] [Google Scholar]
- 614.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38: 1–211, 2018. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 615. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci 35: 6619–6629, 2015. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616. Brennan KC, Pietrobon D. A systems neuroscience approach to migraine. Neuron 97: 1004–1021, 2018. doi: 10.1016/j.neuron.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617. Dussor G. New discoveries in migraine mechanisms and therapeutic targets. Curr Opin Physiol 11: 116–124, 2019. doi: 10.1016/j.cophys.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618. Villalón CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther 124: 309–323, 2009. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 619. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extra-cerebral circulation of humans during migraine headache. Ann Neurol 28: 183–187, 1990. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 620. Bellamy JL, Cady RK, Durham PL. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache 46: 24–33, 2006. doi: 10.1111/j.1526-4610.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 621. Kamm K, Straube A, Ruscheweyh R. Calcitonin gene-related peptide levels in tear fluid are elevated in migraine patients compared to healthy controls. Cephalalgia 39: 1535–1543, 2019. doi: 10.1177/0333102419856640. [DOI] [PubMed] [Google Scholar]
- 622. Cernuda-Morollón E, Larrosa D, Ramón C, Vega J, Martínez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 81: 1191–1196, 2013. doi: 10.1212/WNL.0b013e3182a6cb72. [DOI] [PubMed] [Google Scholar]
- 623. Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache 49: 1258–1266, 2009. doi: 10.1111/j.1526-4610.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- 624. Cernuda-Morollón E, Ramón C, Martínez-Camblor P, Serrano-Pertierra E, Larrosa D, Pascual J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain 156: 820–824, 2015. doi: 10.1097/j.pain.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 625. Alpuente A, Gallardo VJ, Asskour L, Caronna E, Torres-Ferrus M, Pozo-Rosich P. Salivary CGRP can monitor the different migraine phases: CGRP (in)dependent attacks. Cephalalgia 42: 186–196, 2022. doi: 10.1177/03331024211040467. [DOI] [PubMed] [Google Scholar]
- 626. Messlinger K. The chicken and egg problem: CGRP release due to trigeminal activation or vice versa? Cephalalgia 42: 183–185, 2022. doi: 10.1177/03331024211042360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627. Sarchielli P, Alberti A, Floridi A, Gallai V. Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology 57: 132–134, 2001. doi: 10.1212/WNL.57.1.132. [DOI] [PubMed] [Google Scholar]
- 628. Gallai V, Alberti A, Gallai B, Coppola F, Floridi A, Sarchielli P. Glutamate and nitric oxide pathway in chronic daily headache: evidence from cerebrospinal fluid. Cephalalgia 23: 166–174, 2003. doi: 10.1046/j.1468-2982.2003.00552.x. [DOI] [PubMed] [Google Scholar]
- 629. Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 20: 907–918, 2000. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 630. Hoffmann J, Wecker S, Neeb L, Dirnagl U, Reuter U. Primary trigeminal afferents are the main source for stimulus-induced CGRP release into jugular vein blood and CSF. Cephalalgia 32: 659–667, 2012. doi: 10.1177/0333102412447701. [DOI] [PubMed] [Google Scholar]
- 631. Friberg L, Olesen J, Olsen TS, Karle A, Ekman R, Fahrenkrug J. Absence of vasoactive peptide release from brain to cerebral circulation during onset of migraine with aura. Cephalalgia 14: 47–54, 1994. doi: 10.1046/j.1468-2982.1994.1401047.x. [DOI] [PubMed] [Google Scholar]
- 632. Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol 58: 561–568, 2005. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- 633. Gupta R, Ahmed T, Banerjee B, Bhatia M. Plasma calcitonin gene-related peptide concentration is comparable to control group among migraineurs and tension type headache subjects during inter-ictal period. J Headache Pain 10: 161–166, 2009. doi: 10.1007/s10194-009-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634. Lee MJ, Lee SY, Cho S, Kang ES, Chung CS. Feasibility of serum CGRP measurement as a biomarker of chronic migraine: a critical reappraisal. J Headache Pain 19: 53, 2018. doi: 10.1186/s10194-018-0883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635. van Dongen RM, Zielman R, Noga M, Dekkers OM, Hankemeier T, van den Maagdenberg AM, Terwindt GM, Ferrari MD. Migraine biomarkers in cerebrospinal fluid: a systematic review and meta-analysis. Cephalalgia 37: 49–63, 2017. doi: 10.1177/0333102415625614. [DOI] [PubMed] [Google Scholar]
- 636. Ferroni P, Barbanti P. Biomarkers in migraine headache: prognostic and therapeutic implications. Curr Med Chem 26: 6188–6190, 2019. doi: 10.2174/092986732634191112142448. [DOI] [PubMed] [Google Scholar]
- 637. Tfelt-Hansen P, Le H. Calcitonin gene-related peptide in blood: is it increased in the external jugular vein during migraine and cluster headache? A review. J Headache Pain 10: 137–143, 2009. doi: 10.1007/s10194-009-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638. Frederiksen SD, Bekker-Nielsen Dunbar M, Snoer AH, Deen M, Edvinsson L. Serotonin and neuropeptides in blood from episodic and chronic migraine and cluster headache patients in case-control and case-crossover settings: a systematic review and meta-analysis. Headache 60: 1132–1164, 2020. doi: 10.1111/head.13802. [DOI] [PubMed] [Google Scholar]
- 639. Tesfay B, Karlsson WK, Moreno RD, Hay DL, Hougaard A. Is calcitonin gene-related peptide a reliable biochemical marker of migraine? Curr Opin Neurol 35: 343–352, 2022. doi: 10.1097/WCO.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 640. Ashina M, Terwindt GM, Al-Karagholi MA, de Boer I, Lee MJ, Hay DL, Schulte LH, Hadjikhani N, Sinclair AJ, Ashina H, Schwedt TJ, Goadsby PJ. Migraine: disease characterisation, biomarkers, and precision medicine. Lancet 397: 1496–1504, 2021. doi: 10.1016/S0140-6736(20)32162-0. [DOI] [PubMed] [Google Scholar]
- 641. Messlinger K, Vogler B, Kuhn A, Sertel-Nakajima J, Frank F, Broessner G. CGRP measurements in human plasma—a methodological study. Cephalalgia 41: 1359–1373, 2021. doi: 10.1177/03331024211024161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642. Valdemarsson S, Edvinsson L, Hedner P, Ekman R. Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Invest 50: 385–388, 1990. doi: 10.3109/00365519009091595. [DOI] [PubMed] [Google Scholar]
- 643. Fanciullacci M, Alessandri M, Figini M, Geppetti P, Michelacci S. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain 60: 119–123, 1995. doi: 10.1016/0304-3959(94)00097-X. [DOI] [PubMed] [Google Scholar]
- 644. Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 22: 54–61, 2002. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 645. Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning PV, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol 69: 635–645, 2011. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- 646. Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 30: 1179–1186, 2010. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 647. Guo S, Vollesen AL, Olesen J, Ashina M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain 157: 2773–2781, 2016. doi: 10.1097/j.pain.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 648. Christensen CE, Younis S, Deen M, Khan S, Ghanizada H, Ashina M. Migraine induction with calcitonin gene-related peptide in patients from erenumab trials. J Headache Pain 19: 105, 2018. doi: 10.1186/s10194-018-0927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649. Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther 77: 202–213, 2005. doi: 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 650. Edvinsson ML, Edvinsson L. Comparison of CGRP and NO responses in the human peripheral microcirculation of migraine and control subjects. Cephalalgia 28: 563–566, 2008. doi: 10.1111/j.1468-2982.2008.01558.x. [DOI] [PubMed] [Google Scholar]
- 651. Hansen JM, Thomsen LL, Olesen J, Ashina M. Calcitonin gene-related peptide does not cause migraine attacks in patients with familial hemiplegic migraine. Headache 51: 544–553, 2011. doi: 10.1111/j.1526-4610.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- 652. Hansen JM, Thomsen LL, Olesen J, Ashina M. Calcitonin gene-related peptide does not cause the familial hemiplegic migraine phenotype. Neurology 71: 841–847, 2008. doi: 10.1212/01.wnl.0000325482.64106.3f. [DOI] [PubMed] [Google Scholar]
- 653. de Vries T, Villalón CM, MaassenVanDenBrink A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther 211: 107528, 2020. doi: 10.1016/j.pharmthera.2020.107528. [DOI] [PubMed] [Google Scholar]
- 654. Ho TW, Connor KM, Zhang Y, Pearlman E, Koppenhaver J, Fan X, Lines C, Edvinsson L, Goadsby PJ, Michelson D. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 83: 958–966, 2014. doi: 10.1212/WNL.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 655. Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM; BIBN 4096 BS Clinical Proof of Concept Study Group. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350: 1104–1110, 2004. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 656. Ailani J, McAllister P, Winner PK, Chakhava G, Krog Josiassen M, Lindsten A, Sperling B, Ettrup A, Cady R. Rapid resolution of migraine symptoms after initiating the preventive treatment eptinezumab during a migraine attack: results from the randomized RELIEF trial. BMC Neurol 22: 205, 2022. doi: 10.1186/s12883-022-02714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657. Russo AF. CGRP-based migraine therapeutics: how might they work, why so safe, and what next? ACS Pharmacol Transl Sci 2: 2–8, 2019. doi: 10.1021/acsptsci.8b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658. Al-Hassany L, Haas J, Piccininni M, Kurth T, Maassen Van Den Brink A, Rohmann JL. Giving researchers a headache—sex and gender differences in migraine. Front Neurol 11: 549038, 2020. doi: 10.3389/fneur.2020.549038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659. Krause DN, Warfvinge K, Haanes KA, Edvinsson L. Hormonal influences in migraine - interactions of oestrogen, oxytocin and CGRP. Nat Rev Neurol 17: 621–633, 2021. doi: 10.1038/s41582-021-00544-2. [DOI] [PubMed] [Google Scholar]
- 660. Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J Neurosci 39: 4323–4331, 2019. doi: 10.1523/JNEUROSCI.0364-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661. Rea BJ, Wattiez AS, Waite JS, Castonguay WC, Schmidt CM, Fairbanks AM, Robertson BR, Brown CJ, Mason BN, Moldovan-Loomis MC, Garcia-Martinez LF, Poolman P, Ledolter J, Kardon RH, Sowers LP, Russo AF. Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: implications for migraine. Pain 159: 2306–2317, 2018. doi: 10.1097/j.pain.0000000000001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662. Araya EI, Turnes JM, Barroso AR, Chichorro JG. Contribution of intraganglionic CGRP to migraine-like responses in male and female rats. Cephalalgia 40: 689–700, 2020. doi: 10.1177/0333102419896539. [DOI] [PubMed] [Google Scholar]
- 663. Edvinsson L. CGRP blockers in migraine therapy: where do they act? Br J Pharmacol 155: 967–969, 2008. doi: 10.1038/bjp.2008.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664. Tfelt-Hansen P, Olesen J. Possible site of action of CGRP antagonists in migraine. Cephalalgia 31: 748–750, 2011. doi: 10.1177/0333102411398403. [DOI] [PubMed] [Google Scholar]
- 665. Loder E. Triptan therapy in migraine. N Engl J Med 363: 63–70, 2010. doi: 10.1056/NEJMct0910887. [DOI] [PubMed] [Google Scholar]
- 666. Edvinsson L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol 80: 193–199, 2015. doi: 10.1111/bcp.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667. Edvinsson L, Tfelt-Hansen P. The blood-brain barrier in migraine treatment. Cephalalgia 28: 1245–1258, 2008. doi: 10.1111/j.1468-2982.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 668. Lundblad C, Haanes KA, Grände G, Edvinsson L. Experimental inflammation following dural application of complete Freund’s adjuvant or inflammatory soup does not alter brain and trigeminal microvascular passage. J Headache Pain 16: 91, 2015. doi: 10.1186/s10194-015-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669. Johnson KW, Morin SM, Wroblewski VJ, Johnson MP. Peripheral and central nervous system distribution of the CGRP neutralizing antibody [125I] galcanezumab in male rats. Cephalalgia 39: 1241–1248, 2019. doi: 10.1177/0333102419844711. [DOI] [PubMed] [Google Scholar]
- 670. Noseda R, Schain AJ, Melo-Carrillo A, Tien J, Stratton J, Mai F, Strassman AM, Burstein R. Fluorescently-labeled fremanezumab is distributed to sensory and autonomic ganglia and the dura but not to the brain of rats with uncompromised blood brain barrier. Cephalalgia 40: 229–240, 2020. doi: 10.1177/0333102419896760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671. Casillo F, Sebastianelli G, Di Renzo A, Cioffi E, Parisi V, Di Lorenzo C, Serrao M, Coppola G. The monoclonal CGRP-receptor blocking antibody erenumab has different effects on brainstem and cortical sensory-evoked responses. Cephalalgia 42: 1236–1245, 2022. doi: 10.1177/03331024221103811. [DOI] [PubMed] [Google Scholar]
- 672. Basedau H, Sturm LM, Mehnert J, Peng KP, Schellong M, May A. Migraine monoclonal antibodies against CGRP change brain activity depending on ligand or receptor target—an fMRI study. Elife 11: e77146, 2022. doi: 10.7554/eLife.77146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673. Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci 4: 386–398, 2003. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 674. Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol 15: 483–490, 2019. doi: 10.1038/s41582-019-0216-y. [DOI] [PubMed] [Google Scholar]
- 675. Edvinsson JC, Warfvinge K, Krause DN, Blixt FW, Sheykhzade M, Edvinsson L, Haanes KA. C-fibers may modulate adjacent Aδ-fibers through axon-axon CGRP signaling at nodes of Ranvier in the trigeminal system. J Headache Pain 20: 105, 2019. doi: 10.1186/s10194-019-1055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676. Liu Y, Broman J, Edvinsson L. Central projections of the sensory innervation of the rat middle meningeal artery. Brain Res 1208: 103–110, 2008. doi: 10.1016/j.brainres.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 677. Liu Y, Broman J, Edvinsson L. Central projections of sensory innervation of the rat superior sagittal sinus. Neuroscience 129: 431–437, 2004. doi: 10.1016/j.neuroscience.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 678. Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 8: 136–142, 2002. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 679. Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol 18: 795–804, 2019. doi: 10.1016/S1474-4422(19)30185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680. Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 69: 855–865, 2011. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681. Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci 30: 8807–8814, 2010. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682. Charles A, Nwaobi SE, Goadsby P. Inflammation in migraine…or not…: a critical evaluation of the evidence. Headache 61: 1575–1578, 2021. doi: 10.1111/head.14224. [DOI] [PubMed] [Google Scholar]
- 683. Levy D, Burstein R. The vascular theory of migraine: leave it or love it? Ann Neurol 69: 600–601, 2011. doi: 10.1002/ana.22422. [DOI] [PubMed] [Google Scholar]
- 684. Goadsby PJ. The vascular theory of migraine—a great story wrecked by the facts. Brain 132: 6–7, 2009. doi: 10.1093/brain/awn321. [DOI] [PubMed] [Google Scholar]
- 685. Brennan KC, Charles A. An update on the blood vessel in migraine. Curr Opin Neurol 23: 266–274, 2010. doi: 10.1097/WCO.0b013e32833821c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686. Schytz HW, Schoonman GG, Ashina M. What have we learnt from triggering migraine? Curr Opin Neurol 23: 259–265, 2010. doi: 10.1097/WCO.0b013e328337b884. [DOI] [PubMed] [Google Scholar]
- 687. Ashina M. Vascular changes have a primary role in migraine. Cephalalgia 32: 428–430, 2012. doi: 10.1177/0333102412438978. [DOI] [PubMed] [Google Scholar]
- 688. Charles A. Migraine is not primarily a vascular disorder. Cephalalgia 32: 431–432, 2012. doi: 10.1177/0333102412441717. [DOI] [PubMed] [Google Scholar]
- 689. Frederiksen SD, Haanes KA, Warfvinge K, Edvinsson L. Perivascular neurotransmitters: Regulation of cerebral blood flow and role in primary headaches. J Cereb Blood Flow Metab 39: 610–632, 2019. doi: 10.1177/0271678X17747188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690. Al-Karagholi MA, Ghanizada H, Waldorff Nielsen CA, Skandarioon C, Snellman J, Lopez-Lopez C, Hansen JM, Ashina M. Opening of BKCa channels causes migraine attacks: a new downstream target for the treatment of migraine. Pain 162: 2512–2520, 2021. doi: 10.1097/j.pain.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 691. Al-Karagholi MA, Hansen JM, Guo S, Olesen J, Ashina M. Opening of ATP-sensitive potassium channels causes migraine attacks: a new target for the treatment of migraine. Brain 142: 2644–2654, 2019. doi: 10.1093/brain/awz199. [DOI] [PubMed] [Google Scholar]
- 692. Mason BN, Russo AF. Vascular contributions to migraine: time to revisit? Front Cell Neurosci 12: 233, 2018. doi: 10.3389/fncel.2018.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693. Mason BN, Wattiez AS, Balcziak LK, Kuburas A, Kutschke WJ, Russo AF. Vascular actions of peripheral CGRP in migraine-like photophobia in mice. Cephalalgia 40: 1585–1604, 2020. doi: 10.1177/0333102420949173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694. Goadsby PJ. Can we develop neurally acting drugs for the treatment of migraine? Nat Rev Drug Discov 4: 741–750, 2005. doi: 10.1038/nrd1822. [DOI] [PubMed] [Google Scholar]
- 695. Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol 8: 679–690, 2009. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 696. Tfelt-Hansen PC. Verisimilitude (or “truthlikeness”) as an alternative to pro and cons: migraine and cluster headache mechanisms. J Headache Pain 11: 379–389, 2010. doi: 10.1007/s10194-010-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697. Chiarugi A. A Popperian view on anti-CGRP biologics in migraine. Headache 59: 1855–1860, 2019. doi: 10.1111/head.13695. [DOI] [PubMed] [Google Scholar]
- 698. Le Prell CG, Hughes LF, Dolan DF, Bledsoe SC Jr.. Effects of calcitonin-gene-related-peptide on auditory nerve activity. Front Cell Dev Biol 9: 752963, 2021. doi: 10.3389/fcell.2021.752963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699. Maison SF, Emeson RB, Adams JC, Luebke AE, Liberman MC. Loss of alpha CGRP reduces sound-evoked activity in the cochlear nerve. J Neurophysiol 90: 2941–2949, 2003. doi: 10.1152/jn.00596.2003. [DOI] [PubMed] [Google Scholar]
- 700. Allen PD, Luebke AE. Reflex modification audiometry reveals dual roles for olivocochlear neurotransmission. Front Cell Neurosci 11: 361, 2017. doi: 10.3389/fncel.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701. Wattiez AS, Castonguay WC, Gaul OJ, Waite JS, Schmidt CM, Reis AS, Rea BJ, Sowers LP, Cintrón-Pérez CJ, Vázquez-Rosa E, Pieper AA, Russo AF. Different forms of traumatic brain injuries cause different tactile hypersensitivity profiles. Pain 162: 1163–1175, 2021. doi: 10.1097/j.pain.0000000000002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702. Finger TE, Böttger B. Peripheral peptidergic fibers of the trigeminal nerve in the olfactory bulb of the rat. J Comp Neurol 334: 117–124, 1993. doi: 10.1002/cne.903340110. [DOI] [PubMed] [Google Scholar]
- 703. Luebke AE, Holt JC, Jordan PM, Wong YS, Caldwell JS, Cullen KE. Loss of alpha-calcitonin gene-related peptide (alphaCGRP) reduces the efficacy of the vestibulo-ocular reflex (VOR). J Neurosci 34: 10453–10458, 2014. doi: 10.1523/JNEUROSCI.3336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704. Jones SM, Vijayakumar S, Dow SA, Holt JC, Jordan PM, Luebke AE. Loss of alpha-calcitonin gene-related peptide (alphaCGRP) reduces otolith activation timing dynamics and impairs balance. Front Mol Neurosci 11: 289, 2018. doi: 10.3389/fnmol.2018.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705. Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol 9: 637–644, 2013. doi: 10.1038/nrneurol.2013.192. [DOI] [PubMed] [Google Scholar]
- 706. Levy D. Migraine pain and nociceptor activation–where do we stand? Headache 50: 909–916, 2010. doi: 10.1111/j.1526-4610.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 707. Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol 515: 331–348, 2009. doi: 10.1002/cne.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708. Close LN, Eftekhari S, Wang M, Charles AC, Russo AF. Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia 39: 428–434, 2019. doi: 10.1177/0333102418774299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709. Tozzi A, de Iure A, Di Filippo M, Costa C, Caproni S, Pisani A, Bonsi P, Picconi B, Cupini LM, Materazzi S, Geppetti P, Sarchielli P, Calabresi P. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci USA 109: 18985–18990, 2012. doi: 10.1073/pnas.1215435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710. Wang Y, Li Y, Wang M. Involvement of CGRP receptors in retinal spreading depression. Pharmacol Rep 68: 935–938, 2016. doi: 10.1016/j.pharep.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 711. Wang MY, Jiang LW, Wang Y, Bu F. Both anti-CGRP and anti-CALCRL antibodies suppress cortical spreading depression. Cephalalgia 37: 292–293, 2017. [Google Scholar]
- 712. Eftekhari S, Kechechyan G, Faas G, Charles A. The CGRP receptor antagonist olcegepant modulates cortical spreading depression in vivo. Cephalalgia 37: 295, 2017. [Google Scholar]
- 713. Matteo E, Pensato U, Favoni V, Giannini G, Pierangeli G, Cevoli S. Do anti-CGRP drugs have a role in migraine aura therapy? J Neurol 268: 2273–2274, 2021. doi: 10.1007/s00415-021-10546-1. [DOI] [PubMed] [Google Scholar]
- 714. Tang C, Unekawa M, Kitagawa S, Takizawa T, Kayama Y, Nakahara J, Shibata M. Cortical spreading depolarisation-induced facial hyperalgesia, photophobia and hypomotility are ameliorated by sumatriptan and olcegepant. Sci Rep 10: 11408, 2020. doi: 10.1038/s41598-020-67948-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715. Melo-Carrillo A, Noseda R, Nir RR, Schain AJ, Stratton J, Strassman AM, Burstein R. Selective inhibition of trigeminovascular neurons by fremanezumab: a humanized monoclonal anti-CGRP antibody. J Neurosci 37: 7149–7163, 2017. doi: 10.1523/JNEUROSCI.0576-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716. Schain AJ, Melo-Carrillo A, Stratton J, Strassman AM, Burstein R. CSD-induced arterial dilatation and plasma protein extravasation are unaffected by fremanezumab: implications for CGRP’s role in migraine with aura. J Neurosci 39: 6001–6011, 2019. doi: 10.1523/JNEUROSCI.0232-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717. Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol 99: 2035–2047, 2008. doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718. Kim KJ, Ramiro Diaz J, Iddings JA, Filosa JA. Vasculo-neuronal coupling: retrograde vascular communication to brain neurons. J Neurosci 36: 12624–12639, 2016. doi: 10.1523/JNEUROSCI.1300-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719. Hinzman JM, Andaluz N, Shutter LA, Okonkwo DO, Pahl C, Strong AJ, Dreier JP, Hartings JA. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain 137: 2960–2972, 2014. doi: 10.1093/brain/awu241. [DOI] [PubMed] [Google Scholar]
- 720. Viggiano A, Viggiano E, Valentino I, Monda M, Viggiano A, De Luca B. Cortical spreading depression affects reactive oxygen species production. Brain Res 1368: 11–18, 2011. doi: 10.1016/j.brainres.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 721. Shatillo A, Koroleva K, Giniatullina R, Naumenko N, Slastnikova AA, Aliev RR, Bart G, Atalay M, Gu C, Khazipov R, Davletov B, Grohn O, Giniatullin R. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience 253: 341–349, 2013. doi: 10.1016/j.neuroscience.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 722. Wang Y, Tye AE, Zhao J, Ma D, Raddant AC, Bu F, Spector BL, Winslow NK, Wang M, Russo AF. Induction of calcitonin gene-related peptide expression in rats by cortical spreading depression. Cephalalgia 39: 333–341, 2019. doi: 10.1177/0333102416678388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723. Gormley P, Anttila V, Winsvold BS, Palta P, Esko T, Pers TH, et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 48: 856–866, 2016. doi: 10.1038/ng.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724. van den Maagdenberg AM, Nyholt DR, Anttila V. Novel hypotheses emerging from GWAS in migraine? J Headache Pain 20: 5, 2019. doi: 10.1186/s10194-018-0956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725. Hautakangas H, Winsvold BS, Ruotsalainen SE, Bjornsdottir G, Harder AV, Kogelman LJ, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles (Preprint). medRxiv 21249647, 2021. doi: 10.1101/2021.01.20.21249647. [DOI] [PMC free article] [PubMed]
- 726. Hautakangas H, Winsvold BS, Ruotsalainen SE, Bjornsdottir G, Harder AV, Kogelman LJ, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet 54: 152–160, 2022. doi: 10.1038/s41588-021-00990-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727. Sutherland HG, Griffiths LR. Genetics of migraine: insights into the molecular basis of migraine disorders. Headache 57: 537–569, 2017. doi: 10.1111/head.13053. [DOI] [PubMed] [Google Scholar]
- 728. Gervil M, Ulrich V, Kaprio J, Olesen J, Russell MB. The relative role of genetic and environmental factors in migraine without aura. Neurology 53: 995–999, 1999. doi: 10.1212/WNL.53.5.995. [DOI] [PubMed] [Google Scholar]
- 729. Mulder EJ, Van Baal C, Gaist D, Kallela M, Kaprio J, Svensson DA, Nyholt DR, Martin NG, MacGregor AJ, Cherkas LF, Boomsma DI, Palotie A. Genetic and environmental influences on migraine: a twin study across six countries. Twin Res 6: 422–431, 2003. doi: 10.1375/136905203770326420. [DOI] [PubMed] [Google Scholar]
- 730. Eising E, Datson NA, van den Maagdenberg AM, Ferrari MD. Epigenetic mechanisms in migraine: a promising avenue? BMC Med 11: 26, 2013. doi: 10.1186/1741-7015-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731. Scuteri D, Corasaniti MT, Tonin P, Nicotera P, Bagetta G. Role of CGRP pathway polymorphisms in migraine: a systematic review and impact on CGRP mAbs migraine therapy. J Headache Pain 22: 87, 2021. doi: 10.1186/s10194-021-01295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732. Choquet H, Yin J, Jacobson AS, Horton BH, Hoffmann TJ, Jorgenson E, Avins AL, Pressman AR. New and sex-specific migraine susceptibility loci identified from a multiethnic genome-wide meta-analysis. Commun Biol 4: 864, 2021. doi: 10.1038/s42003-021-02356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733. Dodick DW, Goadsby PJ, Lucas C, Jensen R, Bardos JN, Martinez JM, Zhou C, Aurora SK, Yang JY, Conley RR, Oakes T. Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: Results from 3-month double-blind treatment. Cephalalgia 40: 935–948, 2020. doi: 10.1177/0333102420905321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734. Goadsby PJ, Dodick DW, Leone M, Bardos JN, Oakes TM, Millen BA, Zhou C, Dowsett SA, Aurora SK, Ahn AH, Yang JY, Conley RR, Martinez JM. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med 381: 132–141, 2019. doi: 10.1056/NEJMoa1813440. [DOI] [PubMed] [Google Scholar]
- 735. Kopruszinski CM, Turnes JM, Swiokla J, Weinstein TJ, Schwedt TJ, Dodick DW, Anderson T, Navratilova E, Porreca F. CGRP monoclonal antibody prevents the loss of diffuse noxious inhibitory controls (DNIC) in a mouse model of post-traumatic headache. Cephalalgia 41: 749–759, 2021. doi: 10.1177/0333102420981688. [DOI] [PubMed] [Google Scholar]
- 736. Mitsikostas DD, Moskowitz MA. Making headway—a role for CGRP in post-traumatic headache. Nat Rev Neurol 17: 133–134, 2021. doi: 10.1038/s41582-020-00431-2. [DOI] [PubMed] [Google Scholar]
- 737. Ashina H, Iljazi A, Al-Khazali HM, Christensen CE, Amin FM, Ashina M, Schytz HW. Hypersensitivity to calcitonin gene-related peptide in post-traumatic headache. Ann Neurol 88: 1220–1228, 2020. doi: 10.1002/ana.25915. [DOI] [PubMed] [Google Scholar]
- 738. Ashina H, Al-Khazali HM, Iljazi A, Ashina S, Jørgensen NR, Amin FM, Ashina M, Schytz HW. Low plasma levels of calcitonin gene-related peptide in persistent post-traumatic headache attributed to mild traumatic brain injury. Cephalalgia 40: 1276–1282, 2020. doi: 10.1177/0333102420941115. [DOI] [PubMed] [Google Scholar]
- 739. Bree D, Stratton J, Levy D. Increased severity of closed head injury or repetitive subconcussive head impacts enhances post-traumatic headache-like behaviors in a rat model. Cephalalgia 40: 1224–1239, 2020. doi: 10.1177/0333102420937664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740. Ashina H, Iljazi A, Al-Khazali HM, Eigenbrodt AK, Larsen EL, Andersen AM, Hansen KJ, Bräuner KB, Mørch-Jessen T, Chaudhry B, Antic S, Christensen CE, Ashina M, Amin FM, Schytz HW. Efficacy, tolerability, and safety of erenumab for the preventive treatment of persistent post-traumatic headache attributed to mild traumatic brain injury: an open-label study. J Headache Pain 21: 62, 2020. doi: 10.1186/s10194-020-01136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741. Bree D, Mackenzie K, Stratton J, Levy D. Enhanced post-traumatic headache-like behaviors and diminished contribution of peripheral CGRP in female rats following a mild closed head injury. Cephalalgia 40: 748–760, 2020. doi: 10.1177/0333102420907597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742. Navratilova E, Rau J, Oyarzo J, Tien J, Mackenzie K, Stratton J, Remeniuk B, Schwedt T, Anderson T, Dodick D, Porreca F. CGRP-dependent and independent mechanisms of acute and persistent post-traumatic headache following mild traumatic brain injury in mice. Cephalalgia 39: 1762–1775, 2019. doi: 10.1177/0333102419877662. [DOI] [PubMed] [Google Scholar]
- 743. Bree D, Levy D. Development of CGRP-dependent pain and headache related behaviours in a rat model of concussion: implications for mechanisms of post-traumatic headache. Cephalalgia 38: 246–258, 2018. doi: 10.1177/0333102416681571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744. Krymchantowski AV. Combining therapies for the treatment of migraine: is there a role? Expert Rev Neurother 5: 145–147, 2005. doi: 10.1586/14737175.5.2.145. [DOI] [PubMed] [Google Scholar]
- 745. Silberstein SD, Dodick D, Freitag F, Pearlman SH, Hahn SR, Scher AI, Lipton RB. Pharmacological approaches to managing migraine and associated comorbidities—clinical considerations for monotherapy versus polytherapy. Headache 47: 585–599, 2007. doi: 10.1111/j.1526-4610.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 746. Jakate A, Blumenfeld AM, Boinpally R, Butler M, Borbridge L, Contreras-De Lama J, McGeeney D, Periclou A, Lipton RB. Pharmacokinetics and safety of ubrogepant when coadministered with calcitonin gene-related peptide-targeted monoclonal antibody migraine preventives in participants with migraine: a randomized phase 1b drug-drug interaction study. Headache 61: 642–652, 2021. doi: 10.1111/head.14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747. Berman G, Croop R, Kudrow D, Halverson P, Lovegren M, Thiry AC, Conway CM, Coric V, Lipton RB. Safety of rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache 60: 1734–1742, 2020. doi: 10.1111/head.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748. Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real-world patient experience with erenumab for the preventive treatment of migraine. Headache 60: 2014–2025, 2020. doi: 10.1111/head.13951. [DOI] [PubMed] [Google Scholar]
- 749. Dapkutė A, Vainauškienė J, Ryliskienė K. Patient-reported outcomes of migraine treatment with erenumab: results from a national patient survey. Neurol Sci 43: 3305–3312, 2022. doi: 10.1007/s10072-021-05861-4. [DOI] [PubMed] [Google Scholar]
- 750. Ailani J, Kuruppu DK, Rettiganti M, Oakes T, Schroeder K, Wietecha L, Port M, Blumenfeld AM. Does “wearing off” of efficacy occur in galcanezumab-treated patients at the end of the monthly treatment cycle? Post hoc analyses of four phase III randomized trials. Headache 62: 198–207, 2022. doi: 10.1111/head.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751. Vernieri F, Brunelli N, Messina R, Costa CM, Colombo B, Torelli P, Quintana S, Cevoli S, Favoni V, d’Onofrio F, Egeo G, Rao R, Filippi M, Barbanti P, Altamura C. Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study. J Headache Pain 22: 154, 2021. doi: 10.1186/s10194-021-01363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752. Chiang CC, Arca KN, Dunn RB, Girardo ME, Quillen JK, Dodick DW, Starling AJ. Real-world efficacy, tolerability, and safety of ubrogepant. Headache 61: 620–627, 2021. doi: 10.1111/head.14062. [DOI] [PubMed] [Google Scholar]
- 753. Salem-Abdou H, Simonyan D, Puymirat J. Identification of predictors of response to Erenumab in a cohort of patients with migraine. Cephalalgia Reports 4: 251581632110266, 2021. doi: 10.1177/25158163211026646. [DOI] [Google Scholar]
- 754. Ernstsen C, Christensen SL, Rasmussen RH, Nielsen BS, Jansen-Olesen I, Olesen J, Kristensen DM. The PACAP pathway is independent of CGRP in mouse models of migraine: possible new drug target? Brain 145: 2450–2460, 2022. doi: 10.1093/brain/awac040. [DOI] [PubMed] [Google Scholar]
- 755. Kim MS, Kim BY, Saghetlians A, Zhang X, Okida T, Kim SY. Anti-nociceptive effects of dual neuropeptide antagonist therapy in mouse model of neuropathic and inflammatory pain. Korean J Pain 35: 173–182, 2022. doi: 10.3344/kjp.2022.35.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756. Cohen F, Armand C, Lipton RB, Vollbracht S. Efficacy and tolerability of calcitonin gene-related peptide-targeted monoclonal antibody medications as add-on therapy to onabotulinumtoxinA in patients with chronic migraine. Pain Med 22: 1857–1863, 2021. doi: 10.1093/pm/pnab093. [DOI] [PubMed] [Google Scholar]
- 757. Blumenfeld AM, Frishberg BM, Schim JD, Iannone A, Schneider G, Yedigarova L, Manack Adams A. Real-world evidence for control of chronic migraine patients receiving CGRP monoclonal antibody therapy added to onabotulinumtoxinA: a retrospective chart review. Pain Ther 10: 809–826, 2021. doi: 10.1007/s40122-021-00264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758. Melo-Carrillo A, Strassman AM, Schain AJ, Adams AM, Brin MF, Burstein R. Combined onabotulinumtoxinA/atogepant treatment blocks activation/sensitization of high-threshold and wide-dynamic range neurons. Cephalalgia 41: 17–32, 2021. doi: 10.1177/0333102420970507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759. Mechtler L, Saikali N, McVige J, Hughes O, Traut A, Adams AM. Real-world evidence for the safety and efficacy of CGRP monoclonal antibody therapy added to onabotulinumtoxinA treatment for migraine prevention in adult patients with chronic migraine. Front Neurol 12: 788159, 2021. doi: 10.3389/fneur.2021.788159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760. Ozudogru SN, Bartell JW, Yuan H, Digre KB, Baggaley SK. The effect of adding calcitonin gene-related peptide monoclonal antibodies to onabotulinum toxin a therapy on headache burden: a retrospective observational case series. Headache 60: 1442–1443, 2020. doi: 10.1111/head.13839. [DOI] [Google Scholar]
- 761. Pellesi L, Do TP, Ashina H, Ashina M, Burstein R. Dual therapy with anti-CGRP monoclonal antibodies and botulinum toxin for migraine prevention: is there a rationale? Headache 60: 1056–1065, 2020. doi: 10.1111/head.13843. [DOI] [PubMed] [Google Scholar]
- 762. Mullin K, Kudrow D, Croop R, Lovegren M, Conway CM, Coric V, Lipton RB. Potential for treatment benefit of small molecule CGRP receptor antagonist plus monoclonal antibody in migraine therapy. Neurology 94: e2121–e2125, 2020. doi: 10.1212/WNL.0000000000008944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763. Hay DL, Walker CS. CGRP and its receptors. Headache 57: 625–636, 2017. doi: 10.1111/head.13064. [DOI] [PubMed] [Google Scholar]
- 764. De Matteis E, Affaitati G, Frattale I, Caponnetto V, Pistoia F, Giamberardino MA, Sacco S, Ornello R. Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci 42: 3297–3303, 2021. doi: 10.1007/s10072-020-05022-z. [DOI] [PubMed] [Google Scholar]
- 765. Raffaelli B, Terhart M, Overeem LH, Mecklenburg J, Neeb L, Steinicke M, Reuter U. Migraine evolution after the cessation of CGRP(-receptor) antibody prophylaxis: a prospective, longitudinal cohort study. Cephalalgia 42: 326–334, 2022. doi: 10.1177/03331024211046617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766. Moreno-Ajona D, Villar-Martínez MD, Goadsby PJ. New generation gepants: migraine acute and preventive medications. J Clin Med 11: 1656, 2022. doi: 10.3390/jcm11061656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767. Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia 39: 445–458, 2019. doi: 10.1177/0333102418821662. [DOI] [PubMed] [Google Scholar]
- 768. Irimia P, Martínez-Valbuena I, Mínguez-Olaondo A, Domínguez-Vivero C, Sánchez-Arias JA, Martínez-Vila E, Luquin MR, Leira R. Interictal amylin levels in chronic migraine patients: a case-control study. Cephalalgia 41: 604–612, 2021. doi: 10.1177/0333102420977106. [DOI] [PubMed] [Google Scholar]
- 769. Zhang C, Kaye JA, Cai Z, Wang Y, Prescott SL, Liberles SD. Area postrema cell types that mediate nausea-associated behaviors. Neuron 109: 461–472.e5, 2021. doi: 10.1016/j.neuron.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770. Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 132: 16–25, 2009. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- 771. Ashina M, Martelletti P. Pituitary adenylate-cyclase-activating polypeptide (PACAP): another novel target for treatment of primary headaches? J Headache Pain 19: 33, 2018. doi: 10.1186/s10194-018-0860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772. Edvinsson L, Tajti J, Szalárdy L, Vécsei L. PACAP and its role in primary headaches. J Headache Pain 19: 21, 2018. doi: 10.1186/s10194-018-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773. Kaiser EA, Russo AF. CGRP and migraine: could PACAP play a role too? Neuropeptides 47: 451–461, 2013. doi: 10.1016/j.npep.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774. Rustichelli C, Lo Castro F, Baraldi C, Ferrari A. Targeting pituitary adenylate cyclase-activating polypeptide (PACAP) with monoclonal antibodies in migraine prevention: a brief review. Expert Opin Investig Drugs 29: 1269–1275, 2020. doi: 10.1080/13543784.2020.1811966. [DOI] [PubMed] [Google Scholar]
- 775. Kuburas A, Mason BN, Hing B, Wattiez AS, Reis AS, Sowers LP, Moldovan Loomis C, Garcia-Martinez LF, Russo AF. PACAP induces light aversion in mice by an inheritable mechanism independent of CGRP. J Neurosci 41: 4697–4715, 2021. doi: 10.1523/JNEUROSCI.2200-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776. Hoffmann J, Supronsinchai W, Akerman S, Andreou AP, Winrow CJ, Renger J, Hargreaves R, Goadsby PJ. Evidence for orexinergic mechanisms in migraine. Neurobiol Dis 74: 137–143, 2015. doi: 10.1016/j.nbd.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 777. Strother LC, Srikiatkhachorn A, Supronsinchai W. Targeted orexin and hypothalamic neuropeptides for migraine. Neurotherapeutics 15: 377–390, 2018. doi: 10.1007/s13311-017-0602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778. Tzabazis A, Kori S, Mechanic J, Miller J, Pascual C, Manering N, Carson D, Klukinov M, Spierings E, Jacobs D, Cuellar J, Frey WH 2nd, Hanson L, Angst M, Yeomans DC. Oxytocin and migraine headache. Headache 57: 64–75, 2017. doi: 10.1111/head.13082. [DOI] [PubMed] [Google Scholar]
- 779. Hoffmann J, Charles A. Glutamate and its receptors as therapeutic targets for migraine. Neurotherapeutics 15: 361–370, 2018. doi: 10.1007/s13311-018-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs 74: 1165–1176, 2014. doi: 10.1007/s40265-014-0262-0. [DOI] [PubMed] [Google Scholar]
- 781. Hassler SN, Ahmad FB, Burgos-Vega CC, Boitano S, Vagner J, Price TJ, Dussor G. Protease activated receptor 2 (PAR2) activation causes migraine-like pain behaviors in mice. Cephalalgia 39: 111–122, 2019. doi: 10.1177/0333102418779548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782. Kopruszinski CM, Thornton P, Arnold J, Newton P, Lowne D, Navratilova E, Swiokla J, Dodick DW, Dobson C, Gurrell I, Chessell IP, Porreca F. Characterization and preclinical evaluation of a protease activated receptor 2 (PAR2) monoclonal antibody as a preventive therapy for migraine. Cephalalgia 40: 1535–1550, 2020. doi: 10.1177/0333102420966581. [DOI] [PubMed] [Google Scholar]
- 783. Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M. Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain 18: 34, 2017. doi: 10.1186/s10194-017-0741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784. Chuinsiri N, Edwards D, Telezhkin V, Nile CJ, Van der Cruyssen F, Durham J. Exploring the roles of neuropeptides in trigeminal neuropathic pain: a systematic review and narrative synthesis of animal studies. Arch Oral Biol 130: 105247, 2021. doi: 10.1016/j.archoralbio.2021.105247. [DOI] [PubMed] [Google Scholar]
- 785. Parascandolo E, Levinson K, Rizzoli P, Sharon R. Efficacy of erenumab in the treatment of trigeminal neuralgia: a retrospective case series. Neurol Clin Pract 11: 227–231, 2021. doi: 10.1212/CPJ.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786. Benschop RJ, Collins EC, Darling RJ, Allan BW, Leung D, Conner EM, Nelson J, Gaynor B, Xu J, Wang XF, Lynch RA, Li B, McCarty D, Nisenbaum ES, Oskins JL, Lin C, Johnson KW, Chambers MG. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage 22: 578–585, 2014. doi: 10.1016/j.joca.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 787. Bowler KE, Worsley MA, Broad L, Sher E, Benschop R, Johnson K, Yates JM, Robinson PP, Boissonade FM. Evidence for anti-inflammatory and putative analgesic effects of a monoclonal antibody to calcitonin gene-related peptide. Neuroscience 228: 271–282, 2013. doi: 10.1016/j.neuroscience.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 788. Bulling DG, Kelly D, Bond S, McQueen DS, Seckl JR. Adjuvant-induced joint inflammation causes very rapid transcription of beta-preprotachykinin and alpha-CGRP genes in innervating sensory ganglia. J Neurochem 77: 372–382, 2001. doi: 10.1046/j.1471-4159.2001.00175.x. [DOI] [PubMed] [Google Scholar]
- 789. Hirsch S, Corradini L, Just S, Arndt K, Doods H. The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain 154: 700–707, 2013. doi: 10.1016/j.pain.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 790. Uchida K, Takano S, Takata K, Mukai M, Koyama T, Ohashi Y, Saito H, Takaso M, Miyagi M, Inoue G. Differential synovial CGRP/RAMP1 expression in men and women with knee osteoarthritis. Cureus 13: e15483, 2021. doi: 10.7759/cureus.15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791. Jin Y, Smith C, Monteith D, Brown R, Camporeale A, McNearney TA, Deeg MA, Raddad E, Xiao N, de la Peña A, Kivitz AJ, Schnitzer TJ. CGRP blockade by galcanezumab was not associated with reductions in signs and symptoms of knee osteoarthritis in a randomized clinical trial. Osteoarthritis Cartilage 26: 1609–1618, 2018. doi: 10.1016/j.joca.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 792. White S, Marquez de Prado B, Russo AF, Hammond DL. Heat hyperalgesia and mechanical hypersensitivity induced by calcitonin gene-related peptide in a mouse model of neurofibromatosis. PLoS One 9: e106767, 2014. doi: 10.1371/journal.pone.0106767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793. Akerman S, Romero-Reyes M. Preclinical studies investigating the neural mechanisms involved in the co-morbidity of migraine and temporomandibular disorders: the role of CGRP. Br J Pharmacol 177: 5555–5568, 2020. doi: 10.1111/bph.15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794. Hay DL, Poyner DR. Calcitonin gene-related peptide, adrenomedullin and flushing. Maturitas 64: 104–108, 2009. doi: 10.1016/j.maturitas.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 795. Wilhelms DB, Dock H, Brito HO, Pettersson E, Stojakovic A, Zajdel J, Engblom D, Theodorsson E, Hammar ML, Spetz Holm AE. CGRP is critical for hot flushes in ovariectomized mice. Front Pharmacol 9: 1452, 2018. doi: 10.3389/fphar.2018.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796. Holmes AD, Steinhoff M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp Dermatol 26: 659–667, 2017. doi: 10.1111/exd.13143. [DOI] [PubMed] [Google Scholar]
- 797. Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc 15: 33–39, 2011. doi: 10.1038/jidsymp.2011.8. [DOI] [PubMed] [Google Scholar]
- 798. Helfrich YR, Maier LE, Cui Y, Fisher GJ, Chubb H, Fligiel S, Sachs D, Varani J, Voorhees J. Clinical, histologic, and molecular analysis of differences between erythematotelangiectatic rosacea and telangiectatic photoaging. JAMA Dermatol 151: 825–836, 2015. doi: 10.1001/jamadermatol.2014.4728. [DOI] [PubMed] [Google Scholar]
- 799. Bunker CB, Terenghi G, Springall DR, Polak JM, Dowd PM. Deficiency of calcitonin gene-related peptide in Raynaud’s phenomenon. Lancet 336: 1530–1533, 1990. doi: 10.1016/0140-6736(90)93307-B. [DOI] [PubMed] [Google Scholar]
- 800. Terenghi G, Bunker CB, Liu YF, Springall DR, Cowen T, Dowd PM, Polak JM. Image analysis quantification of peptide-immunoreactive nerves in the skin of patients with Raynaud’s phenomenon and systemic sclerosis. J Pathol 164: 245–252, 1991. doi: 10.1002/path.1711640310. [DOI] [PubMed] [Google Scholar]
- 801. Shawket S, Dickerson C, Hazleman B, Brown MJ. Selective suprasensitivity to calcitonin-gene-related peptide in the hands in Raynaud’s phenomenon. Lancet 2: 1354–1357, 1989. doi: 10.1016/s0140-6736(89)91966-1. [DOI] [PubMed] [Google Scholar]
- 802. Brain SD, Petty RG, Lewis JD, Williams TJ. Cutaneous blood flow responses in the forearms of Raynaud's patients induced by local cooling and intradermal injections of CGRP and histamine. Br J Clin Pharmacol 30: 853–859, 1990. doi: 10.1111/j.1365-2125.1990.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803. Bunker CB, Goldsmith PC, Leslie TA, Hayes N, Foreman JC, Dowd PM. Calcitonin gene-related peptide, endothelin-1, the cutaneous microvasculature and Raynaud’s phenomenon. Br J Dermatol 134: 399–406, 1996. [PubMed] [Google Scholar]
- 804. Shawket S, Dickerson C, Hazleman B, Brown MJ. Prolonged effect of CGRP in Raynaud’s patients: a double-blind randomised comparison with prostacyclin. Br J Clin Pharmacol 32: 209–213, 1991. doi: 10.1111/j.1365-2125.1991.tb03883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805. Bunker CB, Reavley C, O’Shaughnessy DJ, Dowd PM. Calcitonin gene-related peptide in treatment of severe peripheral vascular insufficiency in Raynaud’s phenomenon. Lancet 342: 80–83, 1993. doi: 10.1016/0140-6736(93)91286-U. [DOI] [PubMed] [Google Scholar]
- 806. Manickam AH, Buture A, Tomkins E, Ruttledge M. Raynaud’s phenomenon secondary to erenumab in a patient with chronic migraine. Clin Case Rep 9: e04625, 2021. doi: 10.1002/ccr3.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807. Breen ID, Brumfiel CM, Patel MH, Butterfield RJ, VanderPluym JH, Griffing L, Pittelkow MR, Mangold AR. Evaluation of the safety of calcitonin gene-related peptide antagonists for migraine treatment among adults with Raynaud phenomenon. JAMA Netw Open 4: e217934, 2021. doi: 10.1001/jamanetworkopen.2021.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808. Evans RW. Raynaud’s phenomenon associated with calcitonin gene-related peptide monoclonal antibody antagonists. Headache 59: 1360–1364, 2019. doi: 10.1111/head.13596. [DOI] [PubMed] [Google Scholar]
- 809. Gérard AO, Merino D, Van Obberghen EK, Rocher F, Destere A, Lantéri-Minet M, Drici MD. Calcitonin gene-related peptide-targeting drugs and Raynaud’s phenomenon: a real-world potential safety signal from the WHO pharmacovigilance database. J Headache Pain 23: 53, 2022. doi: 10.1186/s10194-022-01424-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810. Jean EE, Good O, Rico JM, Rossi HL, Herbert DR. Neuroimmune regulatory networks of the airway mucosa in allergic inflammatory disease. J Leukoc Biol 111: 209–221, 2022. doi: 10.1002/JLB.3RU0121-023R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811. Springer J, Amadesi S, Trevisani M, Harrison S, Dinh QT, McGregor GP, Fischer A, Geppetti P, Groneberg DA. Effects of alpha calcitonin gene-related peptide in human bronchial smooth muscle and pulmonary artery. Regul Pept 118: 127–134, 2004. doi: 10.1016/j.regpep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 812. Lange M, Enkhbaatar P, Traber DL, Cox RA, Jacob S, Mathew BP, Hamahata A, Traber LD, Herndon DN, Hawkins HK. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J Appl Physiol (1985) 107: 176–184, 2009. doi: 10.1152/japplphysiol.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813. Aoki-Nagase T, Nagase T, Oh-Hashi Y, Kurihara Y, Yamaguchi Y, Yamamoto H, Nagata T, Kurihara H, Ouchi Y. Calcitonin gene-related peptide mediates acid-induced lung injury in mice. Respirology 12: 807–813, 2007. doi: 10.1111/j.1440-1843.2007.01172.x. [DOI] [PubMed] [Google Scholar]
- 814. McFarland AJ, Yousuf MS, Shiers S, Price TJ. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep 6: e885, 2021. doi: 10.1097/PR9.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815. Rocha-Filho PA, Magalhães JE. Headache associated with COVID-19: frequency, characteristics and association with anosmia and ageusia. Cephalalgia 40: 1443–1451, 2020. doi: 10.1177/0333102420966770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816. Uygun Ö, Ertaş M, Ekizoğlu E, Bolay H, Özge A, Kocasoy Orhan E, Çağatay AA, Baykan B. Headache characteristics in COVID-19 pandemic-a survey study. J Headache Pain 21: 121, 2020. doi: 10.1186/s10194-020-01188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817. Souza DD, Shivde S, Awatare P, Avati A, John SK, Badachi S, Nadig R, Sarma G, Mathew T. Headaches associated with acute SARS-CoV-2 infection: a prospective cross-sectional study. SAGE Open Med 9: 20503121211050227, 2021. doi: 10.1177/20503121211050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818. Bolay H, Karadas Ö, Oztürk B, Sonkaya R, Tasdelen B, Bulut TD, Gülbahar Ö, Özge A, Baykan B. HMGB1, NLRP3, IL-6 and ACE2 levels are elevated in COVID-19 with headache: a window to the infection-related headache mechanism. J Headache Pain 22: 94, 2021. doi: 10.1186/s10194-021-01306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819. Kursun O, Yemisci M, van den Maagdenberg AM, Karatas H. Migraine and neuroinflammation: the inflammasome perspective. J Headache Pain 22: 55, 2021. doi: 10.1186/s10194-021-01271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820. Trigo J, García-Azorín D, Sierra-Mencía Á, Tamayo-Velasco Á, Martínez-Paz P, Tamayo E, Guerrero AL, Gonzalo-Benito H. Cytokine and interleukin profile in patients with headache and COVID-19: A pilot, CASE-control, study on 104 patients. J Headache Pain 22: 51, 2021. doi: 10.1186/s10194-021-01268-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821. Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24: 168–175, 2021. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 822. Messlinger K, Neuhuber W, May A. Activation of the trigeminal system as a likely target of SARS-CoV-2 may contribute to anosmia in COVID-19. Cephalalgia 42: 176–180, 2022. doi: 10.1177/03331024211036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823. Frasnelli J, Schuster B, Hummel T. Interactions between olfaction and the trigeminal system: what can be learned from olfactory loss. Cereb Cortex 17: 2268–2275, 2007. doi: 10.1093/cercor/bhl135. [DOI] [PubMed] [Google Scholar]
- 824. Daiber P, Genovese F, Schriever VA, Hummel T, Möhrlen F, Frings S. Neuropeptide receptors provide a signalling pathway for trigeminal modulation of olfactory transduction. Eur J Neurosci 37: 572–582, 2013. doi: 10.1111/ejn.12066. [DOI] [PubMed] [Google Scholar]
- 825. Genovese F, Bauersachs HG, Grässer I, Kupke J, Magin L, Daiber P, Nakajima J, Möhrlen F, Messlinger K, Frings S. Possible role of calcitonin gene-related peptide in trigeminal modulation of glomerular microcircuits of the rodent olfactory bulb. Eur J Neurosci 45: 587–600, 2017. doi: 10.1111/ejn.13490. [DOI] [PubMed] [Google Scholar]
- 826. Robertson CE. Could CGRP antagonists be helpful in the fight against COVID-19? Headache 60: 1450–1452, 2020. doi: 10.1111/head.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827. Skaria T, Wälchli T, Vogel J. CGRP receptor antagonism in COVID-19: potential cardiopulmonary adverse effects. Trends Mol Med 27: 7–10, 2021. doi: 10.1016/j.molmed.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828. Ailani J, Kaiser EA, Mathew PG, McAllister P, Russo AF, Vélez C, Ramajo AP, Abdrabboh A, Xu C, Rasmussen S, Tepper SJ. Role of calcitonin gene-related peptide on the gastrointestinal symptoms of migraine-clinical considerations: a narrative review. Neurology 99: 841–853, 2022. doi: 10.1212/WNL.0000000000200899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829. Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 41: 646–657, 2001. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 830. Aamodt AH, Stovner LJ, Hagen K, Zwart JA. Comorbidity of headache and gastrointestinal complaints. The Head-HUNT Study. Cephalalgia 28: 144–151, 2008. doi: 10.1111/j.1468-2982.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- 831. Aurora SK, Shrewsbury SB, Ray S, Hindiyeh N, Nguyen L. A link between gastrointestinal disorders and migraine: insights into the gut-brain connection. Headache 61: 576–589, 2021. doi: 10.1111/head.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832. Gazerani P. A bidirectional view of migraine and diet relationship. Neuropsychiatr Dis Treat 17: 435–451, 2021. doi: 10.2147/NDT.S282565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833. Noor-Mohammadi E, Ligon CO, Mackenzie K, Stratton J, Shnider S, Greenwood-Van Meerveld B. A monoclonal anti-calcitonin gene-related peptide antibody decreases stress-induced colonic hypersensitivity. J Pharmacol Exp Ther 379: 270–279, 2021. doi: 10.1124/jpet.121.000731. [DOI] [PubMed] [Google Scholar]
- 834. Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet 394: 1765–1774, 2019. doi: 10.1016/S0140-6736(19)32504-8. [DOI] [PubMed] [Google Scholar]
- 835. Schafmayer C, Harrison JW, Buch S, Lange C, Reichert MC, Hofer P, et al. Genome-wide association analysis of diverticular disease points towards neuromuscular, connective tissue and epithelial pathomechanisms. Gut 68: 854–865, 2019. doi: 10.1136/gutjnl-2018-317619. [DOI] [PubMed] [Google Scholar]
- 836. Riera CE. Can monoclonal antibodies against CGRP offer new treatment options for type 2 diabetes? J Diabetes Clin Res 2: 114–118, 2020. doi: 10.33696/diabetes.1.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837. Baker B, Schaeffler B, Hirman J, Hompesch M, Pederson S, Smith J. Tolerability of eptinezumab in overweight, obese or type 1 diabetes patients. Endocrinol Diabetes Metab 4: e00217, 2021. doi: 10.1002/edm2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]