Abstract
Background
Adequate pain control after open esophagectomy is associated with reduced complications, earlier recovery and higher patient satisfaction. While further developing surgical procedures like robot-assisted minimally invasive esophagectomy (RAMIE) it is relevant to adapt postoperative pain management. The primary question of this observational survey was whether one of the two standard treatments, thoracic epidural analgesia (TEA) or intravenous patient-controlled analgesia (PCA), is superior for pain control after RAMIE as the optimal pain management for these patients still remains unclear. Use of additional analgesics, changes in forced expiratory volume in 1 s (FEV1), postoperative complications and duration of intensive care and hospital stay were also analyzed.
Methods
This prospective observational pilot study analyzed 50 patients undergoing RAMIE (postoperative PCA with piritramide or TEA using bupivacaine; each n = 25). Patient reported pain using the numeric rating scale score and differences in FEV1 using a micro spirometer were measured at postoperative day 1, 3 and 7. Additional data of secondary endpoints were collected from patient charts.
Results
Key demographics, comorbidity, clinical and operative variables were equivalently distributed. Patients receiving TEA had lower pain scores and a longer-lasting pain relief. Moreover, TEA was an independent predictive variable for reduced length of hospital stay (HR -3.560 (95% CI: −6.838 to −0.282), p = 0.034).
Conclusions
Although RAMIE leads to reduced surgical trauma, a less invasive pain therapy with PCA appears to be inferior compared to TEA in case of sufficient postoperative analgesia and length of hospital stay. According to the results of this observational pilot study analgesia with TEA provided better and longer-lasting pain relief compared to PCA. Further randomized controlled trials should be conducted to evaluate the optimal postoperative analgesic treatment for RAMIE.
Keywords: Robotic surgery, Esophagectomy, Patient-controlled analgesia, Epidural anesthesia, Pain-management
1. Introduction
Esophageal cancer is the 6th leading cause of cancer related death worldwide with estimated 400,000 new cases annually [1]. A transthoracic esophagectomy with abdominal and thoracic lymph node dissection is the cornerstone of the multimodal treatment concept with curative intent for patients suffering of esophageal cancer or cancer of the gastroesophageal junction [[2], [3], [4]].
In order to improve short-term postoperative outcome by reducing surgical trauma minimally invasive esophagectomy (MIE) was developed [5]. Until now, there are some randomized controlled trials (RCT) which compare minimally invasive esophagectomy (MIE) [6,7], hybrid esophagectomy [8,9] or robot-assisted minimally invasive esophagectomy (RAMIE) [10] to open transthoracic esophagectomy (OTE). Aforementioned RCTs all show a benefit of minimally invasive esophagectomy over OTE considering postoperative complications and quality of life. In our center we were able to validate the results of the mentioned studies [11,12].
Besides the surgical technique, appropriate postoperative pain management is essential to enhance recovery after OTE or MIE [13,14]. Thoracic epidural analgesia (TEA) still represents the gold standard of postoperative pain management in patients undergoing OTE [15,16]. In addition to sufficient analgesia, the commonly known advantages of TEA are positive effects on postoperative pulmonary morbidity, faster recovery, earlier restitution of bowel function and a reduced systemic stress response due to sympathetic blockade [[17], [18], [19], [20], [21]]. Regarding method-related complications and disadvantages a higher failure rate due to inability to place the catheter or secondary dislocation resulting in insufficient analgesia have to be taken into account [22]. By contrast, the systemic opioid-based intravenous (iv) patient-controlled analgesia (PCA) with piritramide or morphine is a widely used convenient and less invasive method [23]. Alternative pain management concepts such as intercostal or paravertebral blockade were implemented increasingly in MIE as well, so far without proven superiority of one of them compared to TEA [24,25]. Up to now the question remains, which peri- and postoperative analgesia regimen is the optimal pain management in patients undergoing robot-assisted minimally invasive esophagectomy (RAMIE) for esophageal cancer, as this approach causes even less surgical trauma to the patient and has shown to lower postoperative pain scores and opioid consumption compared to OTE [26,27].
Currently there are no prospective studies, which compare the two standard clinical treatment options TEA and PCA in patients undergoing RAMIE. The present pilot study investigates whether PCA is equally effective compared to TEA in this case. The additional use of analgesics, respiratory parameters, length of stay at intensive care unit (ICU) and hospital as well as postoperative complications are also analyzed.
2. Methods
This study was approved by the local ethics committee (Rhineland-Palatinate, Germany; registration number 837.512.17 (11346); German trial register DRKS00019220). A prospective single-center observational pilot study was conducted for patients subjected to RAMIE as a surgical treatment of esophageal cancer to investigate two common standard analgesic treatment options over 7 months.
Inclusion criteria were elective RAMIE as a treatment for esophageal cancer, written informed consent, age ≥18 years and American Society of Anesthesiologists classification (ASA) status ≤3. Exclusion criteria were age ≤18 years, chronic pain syndrome, preoperative use of opioids, polyneuropathy, psychological disorders e.g. depression and ASA status ≥4.
The patients received either TEA or PCA due to their informed consent of anesthesia given at the consultation by an anesthesiologist, who was not involved in the study design and performance. If the patient has consented to both regimes, the procedure was chosen by the anesthesiologist, who was also independent regarding the study project. Based on the recommendations of the local ethics committee, the patients were not randomized to the procedures to ensure their option of the analgesic regime as long as possible and hence the patients’ comfort.
2.1. Anesthetic management
Induction of anesthesia was performed intravenously with sufentanil (0.3–0.6 μg/kg), propofol (1.5–2 mg/kg) and rocuronium (0.9–1 mg/kg). Additional boluses of sufentanil (0,1–0,2 μg/kg) were administered as needed. All patients were intubated with a double-lumen endotracheal tube for single-lung ventilation. Patients obtaining TEA received an epidural catheter (27 G catheter Perifix®, B.Braun, Melsungen, Germany) using an 18 G Tuohy needle. The epidural catheter was placed at thoracic level 7/8 or 8/9 interspace. The epidural space was identified by using the midline approach and the loss of resistance technique with normal saline. Epidural block analgesia was induced with 10 ml of bupivacaine 0,125% followed by 5–10 ml bupivacaine 0,125% every hour during surgery. PCA allocated patients received 3–5 mg piritramide as an iv bolus injection before the end of surgery and 1 g metamizole was administered to every patient in both groups as a short-term iv infusion before the end of surgery.
2.2. Postoperative pain management
In the TEA group a continuous infusion of bupivacaine 0,125% and fentanyl 0,0002% with an infusion rate of 5–10 ml/h in the first 24 h postoperatively (patient-controlled bolus of 3 ml, lock-out time 20 min) was administered (CADD Legacy® PCA pumps, Smiths Medical Inc., Minneapolis, USA). Hereafter, the regimen was changed to single bupivacaine 0,125% with a continuous infusion rate of 5–10 ml/h and patient-controlled bolus application (bolus 3 ml, lock-out time 20 min). PCA was started immediately in the PCA group after admission on ICU. The device was programmed to deliver piritramide iv (bolus 1.5 mg, lockout time 10 min and a restricted dose of 30 mg/4 h) on demand (Perfusor ® Space Syringe Pump, B.Braun, Melsungen, Germany). In addition, all patients of both groups received 5 g of metamizole in 24 h via continuous iv infusion starting soon after admission to ICU.
All patients were visited twice a day by an anesthesiologist during their hospital stay and pain medication was adjusted as needed. The numeric rating scale (NRS) was used to document and measure the pain level. It comprises one item and represents the numbers 0 (no pain at all) to 10 (worst imaginable pain). Pain medication was adjusted by the following concept, if the NRS score was ≥4 during rest:
-
•
TEA: a bolus of 5–8 ml 1% lidocaine was administered. If analgesia remains inadequate, the epidural catheter was disconnected and we switched to a systemic analgesia regimen.
-
•
PCA: repeated iv boluses of 3–5 mg piritramide were applicated. If necessary, a higher iv bolus of piritramide via the PCA was coded (bolus 2.5 mg, lockout time 10 min, restricted dose of 40 mg/4 h).
30 min after administration of rescue medication, the NRS score was re-assessed. With NRS ≥4, the dose of pain medication was adjusted. In addition to the routine pain management assessments carried out twice a day by the resident of the department for pain therapy, pain scores at rest, during elevation of the right arm (right-sided placement of single chest tube), while sitting up and during maximum inspiration were evaluated at POD1, POD3 and POD7. Removal of the epidural catheter was on POD3 or 4 after a withdrawal trial. Upon removal of the catheter, patients received up to 4 g metamizole per day iv and piritramide 15 mg subcutaneously on demand. The same treatment was provided to patients in the PCA group as soon as they presented with a requirement of less than 30 mg piritramide iv in 24 h, which was usually at POD3 or 4. Moreover, the intraoperative placed right-sided chest-tube was removed routinely at POD3 or 4 in both groups after the amount of fluid was less than 300 ml/24 h.
2.3. Postoperative management
All patients were extubated in the operating theater and were admitted to the intermediate (IMC) or intensive care unit (ICU) hereafter. After spending at least 12 h in IMC/ICU hemodynamical and respiratory stable patients were discharged towards the surgical ward. No feeding tubes were placed. All patients were placed on a nil per os routine for the first 3 days postoperatively. In absence of clinical signs of anastomotic insufficiency, patients started with sips of water and the oral intake was gradually increased to solid food. Postoperative esophageal swallow tests were not routinely performed. There was no enhanced recovery program.
2.4. Outcome measures
The primary endpoint was postoperative pain assessed by NRS (0–10) at POD1, POD3 and POD7 after RAMIE. Secondary outcome parameters were use of additional analgesia or side effects of the analgesic therapies (hypotension, paresthesia, nausea or vomiting), differences in forced expiratory volume in 1 s (FEV1) at POD1, POD3 and POD7 corrected for preoperative FEV1 (flows measured with COPD 6, Vitalograph Inc., United Kingdom), duration of intensive care and hospital stay. Postoperative complications were graded according to the modified Clavien Dindo scale (MCDC) or Esophagectomy Complications Consensus Group (ECCG) definitions [28,29].
2.5. Statistical analysis
Sample size was calculated a priori for Wilcoxon-Mann-Whitney test for two groups. 24 participants would be required to find a significant correlation of r = 0.85 at α = 0.05 (2-tailed) and a power of 80%.
All analyses were performed according to the intention-to-treat (ITT) principle. To evaluate significance of differences between groups, the X2 test was used as appropriate for categorical variables and the Student T-test and nonparametric Mann-Whitney U test for continuous variables. Differences over time, pain scores and FEV1 results between and within treatment groups were assessed using linear mixed-effects models adjusted for the baseline value. For the association between risk factors and hospital stay univariable and multivariable regression analysis were performed. All reported P-values were 2 sided. Significance level was set at 0.05.
3. Results
Among the 55 screened patients undergoing RAMIE as a treatment of esophageal cancer from April 2018 to December 2019 51 meet the inclusion criteria. One patient did not receive surgery due to withdrawal of consent for surgery. Finally, 50 out of 51 eligible patients gave informed consent of whom 25 patients received TEA and 25 patients received PCA for postoperative analgesia. In every group 3 patients (12%) were female, and the median age was 70 (48–85) years in the PCA and 63 (48–80) years in the TEA group. There were no differences in baseline characteristics between groups (Table 1). In the PCA group, there were 19 adenocarcinoma and 6 squamous cell carcinoma. 20 adenocarcinoma and 5 squamous cell carcinoma occurred in the TEA group. Detailed information about cancer classification is available in the supplements.
Table 1.
Patient demographics and tumor characteristics (n = 50).
| PCA (n = 25) | TEA (n = 25) | P-value | |
|---|---|---|---|
| Age (y) [median (minimum – maximum)] | 70 (48–85) | 63 (48–80) | .14 |
| Gender [n (%)] | >.99 | ||
| Male | 22 (88) | 22 (88) | |
| Female | 3 (3) | 3 (3) | |
| BMI (kg/m2) [median (minimum – maximum)] | 25 (15–46) | 25 (18–32) | .99 |
| Comorbidity [n (%)] | |||
| No comorbidity | 7 (28) | 10 (40) | .37 |
| Vascular | 8 (32) | 11 (44) | .38 |
| Cardiac | 5 (20) | 2 (8) | .22 |
| Diabetes | 5 (20) | 5 (20) | >99 |
| Pulmonary | 6 (24) | 3 (12) | .27 |
| Oncologic | 2 (8) | 3 (12) | .64 |
| Previous abdominal operation | 7 (28) | 6 (24) | .75 |
| Neurologic | 0 (0) | 2 (8) | .15 |
| ASA score [n (%)] | .34 | ||
| 2 | 13 (52) | 13 (52) | |
| 3 | 12 (48) | 12 (48) | |
| Tumor location [n (%)] | .93 | ||
| Middle esophageal | 4 (16) | 4 (16) | |
| Distal esophageal | 17 (68) | 16 (64) | |
| GEJ | 4 (16) | 5 (20) | |
| Tumor type [n (%)] | .22 | ||
| Adenocarcinoma | 19 (76) | 20 (80) | |
| Squamous cell carcinoma | 6 (24) | 5 (20) | |
| Operation type [n (%)] | .73 | ||
| Ivor-Lewis | 25 (100) | 24 (96) | |
| McKeown | 0 (0) | 1 (4) | |
| Neoadjuvant treatment [n (%)] | .11 | ||
| No therapy | 6 (24) | 1 (4) | |
| Chemotherapy | 7 (28) | 11 (44) | |
| Chemoradiotherapy | 12 (48) | 13 (52) | |
Table 1: PCA = patient-controlled analgesia, BMI = body mass index, ASA = American Society of Anesthesiologists, TEA = thoracic epidural analgesia.
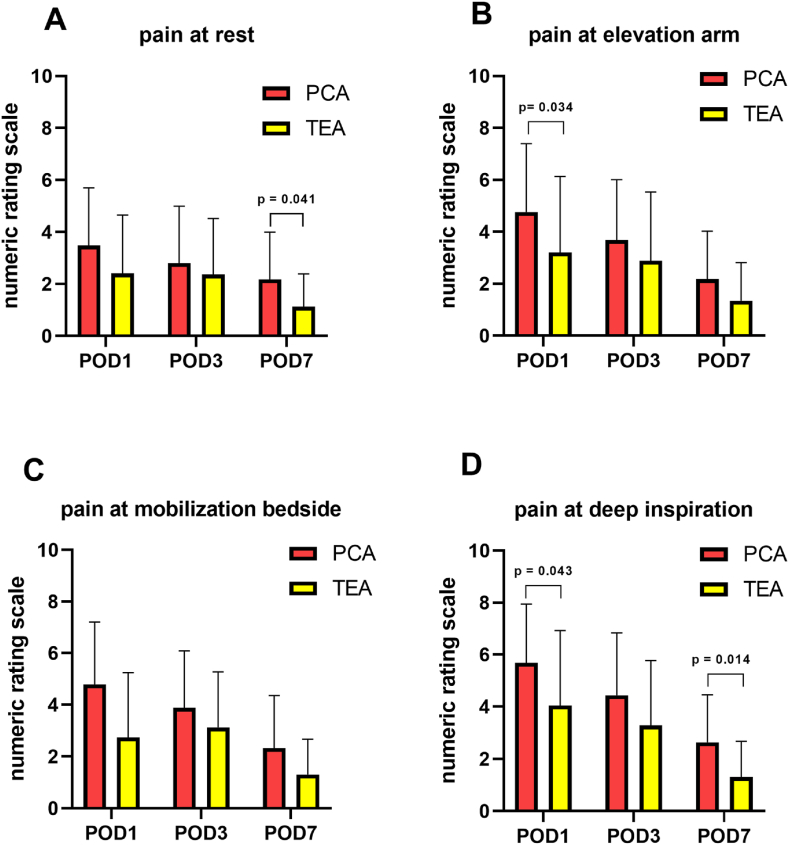
Patients with TEA had lower median pain scores at arm movement (elevation to 90°) at POD1 (TEA 2.0, PCA 4.0, p = 0.034), lower median pain scores at deep inspiration at POD1 (TEA 4.0, PCA 5.0, p = 0.043) and POD7 (TEA 1.0, PCA 2.5, p = 0.014) as well as lower median pain scores at POD7 at rest (TEA 1.0, PCA 1.5, p = 0.041; Fig. 1, Table 2) assessed by NRS. Furthermore, fewer patients in the TEA group received additional analgesia with metamizole (POD1 TEA n = 19, PCA n = 23, p = 0.123 and POD3 TEA n = 15, PCA n = 23, p = 0.008; Table 2).
Fig. 1.
Evaluated pain levels at different tasks and postoperative days
Fig. 1: Pain scores evaluated by numeric rating scale at rest, elevation of the right arm, bedside mobilization and at deep inspiration on POD1, 3 and 7 in PCA and TEA group (each group n = 25; mean +SD; A - D). Patients with TEA had lower median pain scores at arm movement (elevation to 90°) at POD1 (TEA 2.0, PCA 4.0, p = 0.034, B), lower median pain scores at deep inspiration at POD1 (TEA 4.0, PCA 5.0, p = 0.043, D) and POD7 (TEA 1.0, PCA 2.5, p = 0.014, D) as well as lower median pain scores at POD7 at rest (TEA 1.0, PCA 1.5, p = 0.041, A). PCA = patient-controlled analgesia, TEA = thoracic epidural analgesia, POD = postoperative day.
Table 2.
Postoperative results for patients undergoing RAMIE.
| PCA (n = 25) | TEA (n = 25) | P-value | |
|---|---|---|---|
| Pain rest | |||
| NRS POD1 | 3.00 (0–7) | 1.00 (0–7) | .09 |
| NRS POD3 | 3.00 (0–10) | 2.00 (0–8) | .42 |
| NRS POD7 | 1.50 (0–7) | 1.00 (0–4) | .041 |
| Pain elevation arm | |||
| NRS POD1 | 4.00 (0–10) | 2.00 (0–10) | .03 |
| NRS POD3 | 4.00 (0–10) | 2.00 (0–8) | .14 |
| NRS POD7 | 1.00 (0–7) | 1.00 (0–4) | .12 |
| Pain mobilization bed side | |||
| NRS POD1 | 4.50 (1–10) | 2.00 (0–9) | .1 |
| NRS POD3 | 3.00 (1–10) | 2.00 (0–8) | .24 |
| NRS POD7 | 1.00 (0–7) | 1.00 (0–4) | .09 |
| Pain deep inspiration | |||
| NRS POD1 | 5.00 (1–8) | 4.00 (0–9) | .04 |
| NRS POD3 | 4.50 (1–10) | 3.00 (0–9) | .11 |
| NRS POD7 | 2.50 (0–7) | 1.00 (0–5) | .014 |
| FEV1 (l/s) | |||
| POD1 | 1.16 | 1.05 | .81 |
| POD3 | 1.14 | 1.29 | .47 |
| POD7 | 1.65 | 1.92 | .1 |
| Bupivacaine 0.125% in ml cumulative | |||
| POD1 | 176 (84–371) | ||
| POD3 | 521 (176–889) | ||
| POD7 | 569 (176–1518) | ||
| Piritramide iv in mg cumulative | |||
| POD1 | 32 (3–70) | 0 (0–22) | |
| POD3 | 39.25 (3–168) | 0 (0–110) | |
| POD7 | 39.25 (3–324) | 0 (0–225) | |
| Piritramide sc in mg cumulative | |||
| POD1 | 0 (0–15) | 0 (0–15) | |
| POD3 | 0 (0–15) | 0 (0–45) | |
| POD7 | 0 (0–45) | 15 (0–112.5) | |
| Metamizole iv [n (%)] | |||
| POD1 | 23 (92) | 19 (76) | .12 |
| POD3 | 23 (92) | 15 (60) | .008 |
| POD7 | 16 (64) | 13 (54) | .48 |
| Side effects [n (%)] | |||
| Sedation | 0 | 0 | |
| Nausea/Vomiting | 0 | 0 | |
| Motor blockade | 1 (4) | ||
| Catheter displacement | 4 (16) | ||
| Use of rescue medication | 4 (16) | 4 (16) | |
| Altered analgesia (TEA → PCA) | 3 (12) | ||
Table 2: Data are presented as median (min – max). PCA = patient-controlled analgesia, TEA = thoracic epidural analgesia, NRS = numeric rating scale, POD = postoperative day, FEV1 = forced expiratory volume in 1 s.
There were no differences in median FEV1 at POD1 (TEA 1.05, PCA 1.16, p = 0.808), at POD3 (TEA 1.29, PCA 1.44, p = 0.473) and at POD7 (TEA 1.92, PCA 1.65, p = 0.104; Table 2).
Rescue medication was applied 4 times in the TEA group (2 after unnoticed disconnection of the catheter) and 7 times in the PCA group (twice in 3 patients). One patient in the TEA group developed a Bromage motor blockade with a score of III on the evening of surgery which was subsiding after reduction of the infusion rate (Table 2).
Median day of TEA removal was at POD4. In 3 out of 25 patients (12%) of the TEA group, the catheter was dislocated intraoperatively or could not be placed preoperatively. These patients received a PCA instead. One patient (4%) of the TEA group had a dislocation or blockage of the epidural catheter on POD2 and received the standard treatment equal to the patients after planned catheter removal. According to the intention to treat principle, all aforementioned patients were analyzed in the TEA group.
There were no differences in postoperative complications between groups. However, there was a difference in postoperative hospital stay in favor of patients receiving TEA (10 days) compared to patients who received PCA (13 days) (p = 0.034; Table 3). Multivariable regression analysis showed that TEA was an independent predictive variable for reduced length of hospital stay after RAMIE (p = 0.034; Table 4).
Table 3.
Postoperative complications after esophagectomy.
| PCA (n (%)) | TEA (n (%)) | P-value | |
|---|---|---|---|
| Postoperative complications | 12 (48) | 7 (28) | .15 |
| Anastomotic leakage | 2 (8) | 0 (0) | .49 |
| Pulmonary complications | 6 (24) | 4 (16) | .48 |
| Pneumonia | 3 (12) | 3 (12) | >.99 |
| RLN paralysis | 1 (4) | 1 (4) | >.99 |
| Chyle leakage | 3 (12) | 0 (0) | .07 |
| Cardiac complications | 3 (12) | 1 (4) | .3 |
| Atrial fibrillation | 3 (12) | 1 (4) | .3 |
| Wound infection | 1 (4) | 0 (0) | .3 |
| Delayed gastric emptying | 1 (4) | 0 (0) | .3 |
| Urinary tract infection | 1 (4) | 1 (4) | >.99 |
| Hospital stay in days [Median] | 13 | 10 | .03 |
| ICU stay in days [Median] | 1 | 1 | .09 |
| Readmission ICU | 3 (12) | 1 (4) | .3 |
| Readmission hospital in 30 days | 4 (16) | 1 (4) | .16 |
| IHM 30 days | 0 (0) | 1 (4) | .3 |
| IHM 90 days | 0 (0) | 1 (4) | .3 |
Table 3: PCA = patient-controlled analgesia, TEA = thoracic epidural analgesia, RLN = Recurrent laryngeal nerve, IHM = in-hospital mortality, ICU = intensive care unit.
Table 4.
Univariable and multivariable analysis of the association between risk factors and hospital stay for esophagectomy patients.
|
Characteristic |
Unadjusted HR (95% CI), univariable | P-value | Unadjusted HR (95% CI), multivariable | P-value |
|---|---|---|---|---|
| Overall complications | −0.912 (-5.881–4.056) | 0.713 | ||
| Anastomotic insufficiency | −1.320 (-10.199–7.560) | 0.766 | ||
| Pulmonary complications | 0.580 (-5.441–6.602) | 0.847 | ||
| Cardiac complications | −1.903 (-8.700–4.894) | 0.575 | ||
| Length of hospital stay PCA versus TEA |
−3.457 (-7.107–-0.193) | 0.063 | −3.560 (-6.838–-0.282) | .03 |
Table 4: HR = hazard ratio, CI = confidence interval.
4. Discussion
This is the first prospective study comparing TEA to PCA regarding postoperative pain management in patients undergoing RAMIE for treatment of esophageal cancer, designed as an observational pilot study. TEA was associated with lower median pain scores without any motor blockade. Thus, mobilization of the patient was not affected by TEA. Furthermore, TEA was independently associated with reduced length of hospital stay compared to patients receiving PCA after RAMIE.
TEA remains the gold standard of perioperative analgesia in case of open esophagectomy [30,31]. Regarding MIE the best analgesic regimen is ambiguous. Supporting the less invasive technique of RAMIE there are several observational studies showing lower postoperative pain scores and lower opioid consumption after RAMIE compared to OTE [26,27]. Some data attribute beneficial effects to PCA compared to TEA and paravertebral blocks (PVB), others to PVB compared to TEA [25,30,32,33]. However, enhanced recovery programs following open or video-assisted thoracoscopic surgery still recommend TEA as the preferred analgesic regimen [34,35]. Regarding thoracotomies in general PVB seemed to be comparable to TEA with no difference in analgesic efficiency or major complications like hematomas but with a lower incidence of hypotension [36]. In case of robot-assisted thoracic surgery (RATS) there is some data showing lower pain scores and less use of rescue medication using TEA in comparison to PCA combined with intercostal nerve block [37]. Unknown is the optimal analgesic approach for patients undergoing RAMIE for esophageal cancer so far. Considering the robotic technique involves much less surgical trauma, PCA as the less invasive analgesic regimen was hypothesized to be sufficient for pain relief. One meta-analysis investigated postoperative pain management after OTE and MIE with mainly open surgical approaches (n = 8) including 5 randomized trials and 5 cohort studies [25]. This meta-analysis determined no differences in postoperative pain relief between TEA and PCA, temporary observed for 48 h. Only two included studies monitored length of hospital stay (LOS) without any differences [25].
Interestingly, in the present pilot study TEA was proven superior to PCA with better postoperative pain scores and shorter length of hospital stay (Table 2, Table 3). The positive effect of TEA on the length of hospital stay could be explained by better and earlier mobilization due to adequate pain control. As part of a multimodal concept, pain therapy especially with the use of regional anesthesia is a key component to enhanced recovery protocols [35]. A positive influence on pain memory due to a prevented memorized painful event may be a fundamental mechanism [38,39]. Hypotension is an unfavorable concomitant of TEA accompanied by the potentially increased risk of anastomotic insufficiency [25]. The present data did not show any differences in anastomotic insufficiency or other complications between groups (Table 3).
The main limitations of this prospective pilot study are the small sample size and the observational single-center design. Furthermore, the observation period was short and no statement on the long-term outcome could be given. The group size follows the calculation of the local statistical institute and the observational design is based on recommendation of the local ethics committee. As this study was performed as an initial pilot study, the provided information is of great value having the mentioned limitations in mind.
With latter techniques available such as paravertebral analgesia, one could argue what the best method may be in this patient population. Retrospective case series suggest that paravertebral analgesia is associated with less technical failure and reduced incidence of hypotensive events, both could promote postoperative recovery [40,41]. Based on these retrospective results, a prospective multicenter randomized controlled trial comparing paravertebral and epidural analgesia regarding perioperative outcomes and treatment costs in patients undergoing MIE is performed in the Netherlands with expected results by 2023 [42]. Until illuminating insights are forthcoming, TEA should be the favored option based on the current results and literature.
5. Conclusions
According to this prospective observational pilot study TEA seems to be more effective in providing pain relief for patients undergoing RAMIE compared to PCA although RAMIE causes less surgical damage and in turn likely less pain than MIE. TEA provided better postoperative analgesia despite less additional analgesic co-medication. Furthermore, TEA was independently associated with a shorter length of hospital stay compared to PCA. Based on results of this pilot study, TEA should be taken in consideration as the preferred analgesic treatment option for patients undergoing RAMIE as treatment of esophageal cancer until randomized controlled trials prove otherwise.
Author contribution statement
Ann Kristin Rosner; Eva-Verena Griemert: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Pieter C. van der Sluis: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Lena Meyer: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data. Eva Wittenmeier: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data. Kristin Engelhard; Peter P. Grimminger: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This paper contains parts of the doctoral dissertation of Lena Meyer and the professorial dissertation (Habilitation) of Ann Kristin Rosner. No funding has to be declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e13842.
Contributor Information
Ann Kristin Rosner, Email: annkristin.rosner@gmail.com.
Eva-Verena Griemert, Email: ev.griemert@uni-mainz.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gupta B., Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur. J. Cancer Prev. 2017;26:107–118. doi: 10.1097/CEJ.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 2.Omloo J.M., Lagarde S.M., Hulscher J.B., Reitsma J.B., Fockens P., van Dekken H., Ten Kate F.J., Obertop H., Tilanus H.W., van Lanschot J.J. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann. Surg. 2007;246:992–1000. doi: 10.1097/SLA.0b013e31815c4037. discussion 1000-1001. 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- 3.Burmeister B.H., Smithers B.M., Gebski V., Fitzgerald L., Simes R.J., Devitt P., Ackland S., Gotley D.C., Joseph D., Millar J., et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 4.Mariette C., Piessen G., Triboulet J.P. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545–553. doi: 10.1016/S1470-2045(07)70172-9. [DOI] [PubMed] [Google Scholar]
- 5.Luketich J.D., Alvelo-Rivera M., Buenaventura P.O., Christie N.A., McCaughan J.S., Litle V.R., Schauer P.R., Close J.M., Fernando H.C. Minimally invasive esophagectomy: outcomes in 222 patients. Ann. Surg. 2003;238:486–494. doi: 10.1097/01.sla.0000089858.40725.68. ; discussion 494-485. 10.1097/01.sla.0000089858.40725.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biere S.S., van Berge Henegouwen M.I., Maas K.W., Bonavina L., Rosman C., Garcia J.R., Gisbertz S.S., Klinkenbijl J.H., Hollmann M.W., de Lange E.S., et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 7.Straatman J., van der Wielen N., Cuesta M.A., Daams F., Roig Garcia J., Bonavina L., Rosman C., van Berge Henegouwen M.I., Gisbertz S.S., van der Peet D.L. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann. Surg. 2017;266:232–236. doi: 10.1097/SLA.0000000000002171. [DOI] [PubMed] [Google Scholar]
- 8.Mariette C., Markar S., Dabakuyo-Yonli T.S., Meunier B., Pezet D., Collet D., D'Journo X.B., Brigand C., Perniceni T., Carrere N., et al. Health-related quality of life following hybrid minimally invasive versus open esophagectomy for patients with esophageal cancer, analysis of a multicenter, open-label, randomized phase III controlled trial: the MIRO trial. Ann. Surg. 2020;271:1023–1029. doi: 10.1097/SLA.0000000000003559. [DOI] [PubMed] [Google Scholar]
- 9.Mariette C., Markar S.R., Dabakuyo-Yonli T.S., Meunier B., Pezet D., Collet D., D'Journo X.B., Brigand C., Perniceni T., Carrere N., et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N. Engl. J. Med. 2019;380:152–162. doi: 10.1056/NEJMoa1805101. [DOI] [PubMed] [Google Scholar]
- 10.van der Sluis P.C., van der Horst S., May A.M., Schippers C., Brosens L.A.A., Joore H.C.A., Kroese C.C., Haj Mohammad N., Mook S., Vleggaar F.P., et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann. Surg. 2019;269:621–630. doi: 10.1097/SLA.0000000000003031. [DOI] [PubMed] [Google Scholar]
- 11.Grimminger P.P., Hadzijusufovic E., Babic B., van der Sluis P.C., Lang H. Innovative fully robotic 4-arm Ivor Lewis esophagectomy for esophageal cancer (RAMIE4) Dis. Esophagus. 2020;33 doi: 10.1093/dote/doz015. [DOI] [PubMed] [Google Scholar]
- 12.van der Sluis P.C., Tagkalos E., Hadzijusufovic E., Babic B., Uzun E., van Hillegersberg R., Lang H., Grimminger P.P. Robot-assisted minimally invasive esophagectomy with intrathoracic anastomosis (ivor lewis): promising results in 100 consecutive patients (the European experience) J. Gastrointest. Surg. 2020 doi: 10.1007/s11605-019-04510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson J., Sabanathan S., Mearns A.J., Evans C.S., Bembridge J., Fairbrass M. Efficacy of pre-emptive analgesia and continuous extrapleural intercostal nerve block on post-thoracotomy pain and pulmonary mechanics. J. Cardiovasc. Surg. 1994;35:219–228. [PubMed] [Google Scholar]
- 14.Seesing M.F.J., Kingma B.F., Weijs T.J., Ruurda J.P., van Hillegersberg R. Reducing pulmonary complications after esophagectomy for cancer. J. Thorac. Dis. 2019;11:S794–S798. doi: 10.21037/jtd.2018.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W., Li Y., Huang Q., Ye S., Rong T. Short and long-term outcomes of epidural or intravenous analgesia after esophagectomy: a propensity-matched cohort study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschalk A., Cohen S.P., Yang S., Ochroch E.A. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104:594–600. doi: 10.1097/00000542-200603000-00027. [DOI] [PubMed] [Google Scholar]
- 17.Ballantyne J.C., Carr D.B., deFerranti S., Suarez T., Lau J., Chalmers T.C., Angelillo I.F., Mosteller F. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth. Analg. 1998;86:598–612. doi: 10.1097/00000539-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Guay J. The benefits of adding epidural analgesia to general anesthesia: a metaanalysis. J. Anesth. 2006;20:335–340. doi: 10.1007/s00540-006-0423-8. [DOI] [PubMed] [Google Scholar]
- 19.Guay J., Nishimori M., Kopp S.L. Epidural local anesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting, and pain after abdominal surgery: a cochrane review. Anesth. Analg. 2016;123:1591–1602. doi: 10.1213/ANE.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S., Wu X., Guo H., Ma L. Thoracic epidural anesthesia improves outcomes in patients undergoing cardiac surgery: meta-analysis of randomized controlled trials. Eur. J. Med. Res. 2015;20:25. doi: 10.1186/s40001-015-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olausson K., Magnusdottir H., Lurje L., Wennerblom B., Emanuelsson H., Ricksten S.E. Anti-ischemic and anti-anginal effects of thoracic epidural anesthesia versus those of conventional medical therapy in the treatment of severe refractory unstable angina pectoris. Circulation. 1997;96:2178–2182. doi: 10.1161/01.cir.96.7.2178. [DOI] [PubMed] [Google Scholar]
- 22.Kingma B.F., Visser E., Marsman M., Ruurda J.P., van Hillegersberg R. Epidural analgesia after minimally invasive esophagectomy: efficacy and complication profile. Dis. Esophagus. 2019;32 doi: 10.1093/dote/doy116. [DOI] [PubMed] [Google Scholar]
- 23.McNicol E.D., Ferguson M.C., Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst. Rev. 2015:CD003348. doi: 10.1002/14651858.CD003348.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borggreve A.S., Kingma B.F., Domrachev S.A., Koshkin M.A., Ruurda J.P., van Hillegersberg R., Takeda F.R., Goense L. Surgical treatment of esophageal cancer in the era of multimodality management. Ann. N. Y. Acad. Sci. 2018;1434:192–209. doi: 10.1111/nyas.13677. [DOI] [PubMed] [Google Scholar]
- 25.Visser E., Marsman M., van Rossum P.S.N., Cheong E., Al-Naimi K., van Klei W.A., Ruurda J.P., van Hillegersberg R. Postoperative pain management after esophagectomy: a systematic review and meta-analysis. Dis. Esophagus. 2017;30:1–11. doi: 10.1093/dote/dox052. [DOI] [PubMed] [Google Scholar]
- 26.Hoelzen J.P., Sander K.J., Sesia M., Roy D., Rijcken E., Schnabel A., Struecker B., Juratli M.A., Pascher A. Robotic-assisted esophagectomy leads to significant reduction in postoperative acute pain: a retrospective clinical trial. Ann. Surg Oncol. 2022;29:7498–7509. doi: 10.1245/s10434-022-12200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vimolratana M., Sarkaria I.S., Goldman D.A., Rizk N.P., Tan K.S., Bains M.S., Adusumilli P.S., Sihag S., Isbell J.M., Huang J., et al. Two-Year quality of life outcomes after robotic-assisted minimally invasive and open esophagectomy. Ann. Thorac. Surg. 2021;112:880–889. doi: 10.1016/j.athoracsur.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934. (ae) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low D.E., Alderson D., Cecconello I., Chang A.C., Darling G.E., D'Journo X.B., Griffin S.M., Holscher A.H., Hofstetter W.L., Jobe B.A., et al. International Consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications Consensus group (ECCG) Ann. Surg. 2015;262:286–294. doi: 10.1097/SLA.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 30.Durkin C., Schisler T., Lohser J. Current trends in anesthesia for esophagectomy. Curr. Opin. Anaesthesiol. 2017;30:30–35. doi: 10.1097/ACO.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 31.Feltracco P., Bortolato A., Barbieri S., Michieletto E., Serra E., Ruol A., Merigliano S., Ori C. Perioperative benefit and outcome of thoracic epidural in esophageal surgery: a clinical review. Dis. Esophagus. 2018;31 doi: 10.1093/dote/dox135. [DOI] [PubMed] [Google Scholar]
- 32.Tankard K.A., Brovman E.Y., Allen K., Urman R.D. The effect of regional anesthesia on outcomes after minimally invasive ivor lewis esophagectomy. J. Cardiothorac. Vasc. Anesth. 2020;34:3052–3058. doi: 10.1053/j.jvca.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 33.Hargrave J., Capdeville M. Minimally invasive esophagectomy in the modern era: is regional anesthesia still the answer? J. Cardiothorac. Vasc. Anesth. 2020;34:3059–3062. doi: 10.1053/j.jvca.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Carli F., Kehlet H., Baldini G., Steel A., McRae K., Slinger P., Hemmerling T., Salinas F., Neal J.M. Evidence basis for regional anesthesia in multidisciplinary fast-track surgical care pathways. Reg. Anesth. Pain Med. 2011;36:63–72. doi: 10.1097/AAP.0b013e31820307f7. [DOI] [PubMed] [Google Scholar]
- 35.McIsaac D.I., Cole E.T., McCartney C.J. Impact of including regional anaesthesia in enhanced recovery protocols: a scoping review. Br. J. Anaesth. 2015;115(Suppl 2):ii46–56. doi: 10.1093/bja/aev376. [DOI] [PubMed] [Google Scholar]
- 36.Yeung J.H., Gates S., Naidu B.V., Wilson M.J., Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst. Rev. 2016;2:CD009121. doi: 10.1002/14651858.CD009121.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawagoe I., Hayashida M., Satoh D., Kochiyama T., Fukuda M., Kishii J. Postoperative analgesia in patients undergoing robot-assisted thoracic surgery: a comparison between thoracic epidural analgesia and intercostal nerve block combined with intravenous patient-controlled analgesia. Ann. Palliat. Med. 2021;10:1985–1993. doi: 10.21037/apm-20-1607. [DOI] [PubMed] [Google Scholar]
- 38.Richebe P., Capdevila X., Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. 2018;129:590–607. doi: 10.1097/ALN.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 39.Rivat C., Bollag L., Richebe P. Mechanisms of regional anaesthesia protection against hyperalgesia and pain chronicization. Curr. Opin. Anaesthesiol. 2013;26:621–625. doi: 10.1097/01.aco.0000432511.08070.de. [DOI] [PubMed] [Google Scholar]
- 40.Donohoe C.L., Phillips A.W., Flynn E., Donnison C., Taylor C.L., Sinclair R.C.F., Saunders D., Immanuel A., Griffin S.M. Multimodal analgesia using intrathecal diamorphine, and paravertebral and rectus sheath catheters are as effective as thoracic epidural for analgesia post-open two-phase esophagectomy within an enhanced recovery program. Dis. Esophagus. 2018;31 doi: 10.1093/dote/doy006. [DOI] [PubMed] [Google Scholar]
- 41.Phillips S., Dedic-Hagan J., Baxter D.F., Van der Wall H., Falk G.L. A novel technique of paravertebral thoracic and preperitoneal analgesia enhances early recovery after oesophagectomy. World J. Surg. 2018;42:1787–1791. doi: 10.1007/s00268-017-4369-9. [DOI] [PubMed] [Google Scholar]
- 42.Kingma B.F., Eshuis W.J., de Groot E.M., Feenstra M.L., Ruurda J.P., Gisbertz S.S., Ten Hoope W., Marsman M., Hermanides J., Hollmann M.W., et al. Paravertebral catheter versus EPidural analgesia in Minimally invasive Esophageal resectioN: a randomized controlled multicenter trial (PEPMEN trial) BMC Cancer. 2020;20:142. doi: 10.1186/s12885-020-6585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.