Abstract
Objective
To observe the expression of Runt-Related Transcription Factors 2 (RUNX2) and Alkaline Phosphatase (ALP) markers in osteoblast cell cultures exposed to Polymethylmethacrylate (PMMA) combined with hydroxyapatite (HAp) material to improve osteointegration of bone implants.
Methods
Sample of PMMA and HAp materials with a mixture of PMMA with HAp made from limestone as natural source which processed through Balai Besar Keramik (HApBBK) in the first group and a mixture of PMMA with HAp made from bovine bone which processed through Good Manufacturing Practice (HApGMP) in the second group. Twenty-four fetal rat calvarie osteoblast cell cultures were randomly divided into 6 groups: 7- and 14-day control group, 7 and 14 days PMMA-HApGMP group, 7 and 14 days PMMA-HApBBK group. The expression of RUNX2 and ALP was seen by immunocytochemical examination.
Result
The one-way ANOVA with a significance value of 0.000 (p < 0.05). There was an increase in RUNX2 and ALP expressions on both PMMA-HApBBK and PMMA-HApGMP groups on days 7 and 14 in osteoblast cell cultures.
Conclusion
The PMMA-HApBBK and PMMA-HApGMP showed an increase in the RUNX2 and ALP expression in osteoblast cell cultures which indicates a potential increase of osseointegration of bone implants.
Keywords: ALP, Bone graft, Dental implant, Hydroxyapatite, Polymethylmethacrylate, RUNX2
Graphical abstract
1. Introduction
One of the most common diseases in the oral cavity is periodontal disease. Periodontitis, or periodontal tissue damage, occurs when gingivitis is left untreated leading to bone loss and destruction of the periodontal ligament which can cause tooth loss.1 Moreover, aging, is also a non-negligible factor associated with an increase in the number of missing teeth.2
The condition of tooth loss if not treated can result in impaired chewing function, esthetics and phonetics that will affect the quality of life. The replacement of missing teeth in dentistry can be achieved with a variety of treatments and materials. Dental implants have made a major contribution to the world of dentistry because of their breakthrough in replacing missing teeth with a high success rate.3 Dental implants provide good retention of dentures, have natural teeth-like characteristics, and are aesthetically pleasing and comfortable.4
The material choice for a particular implant application will be a general consideration to fill a lot of different functions that are specifically required in the fabrication of an implant design. Despite the diversity of needs for the creation of an implant, one aspect that is always a major concern is the tissue response around the implant area because of its contact with the implant materials. This aspect holds a key role because, at the beginning of the installation, the implant will be identified as foreign material by the tissue.5
One of the materials that have been widely used as a substitute for dental implants is Polymethylmethacrylate (PMMA). PMMA is a very well-known ceramic material in the medical field as a bone replacement material.6 PMMA has several properties that are less than optimal in its interaction with bone tissue, so the addition of other ingredients such as hydroxyapatite (HAp), aims to improve biocompatibility, regeneration process, and osteointegration as a bone implant. The addendum of HAp to conventional PMMA-based bone cement can produce reinforced bioceramic polymers with better biological and mechanical properties. The polymeric phase of PMMA/HAp composites can also provide a means of chemically binding other bioactive molecules that have been shown to stimulate the function of osteoblast and promote the formation of bone.7
There are so many sources used in the industry to make HAp, either it is from a natural source or synthetic. The natural source of HAp can be sourced from eggshells, fish bones, bovine bones, limestone, and so on. In Indonesia, there were two big sources of HAp production, which were made from bovine bone and processed through Good Manufacturing Practice by Tissue Bank (HApGMP) and from limestone was collected from the deposition of animal skeletons or exoskeleton, foraminifera or algae, so that it contains a lot of calcium carbonate (CaCO3) which processed by Balai Besar Keramik (HApBBK).8,9
The metabolism of bone tissue at its contact between the implant and bone is known as osseointegration. Osseiointegration is one of the most important factors that contribute to the success of dental implants.10 Osseointegration has also become a standard in the application of implant materials and their clinical effectiveness. The process it requires the activation of regulatory pathways that can affect osteoblastogenesis, promote osteoblast differentiation and maturation, as well as repair or regenerate the bone.11 In addition, osseointegration relies on the basic principles of bone regeneration and the osteoconductivity of the biomaterials used.12
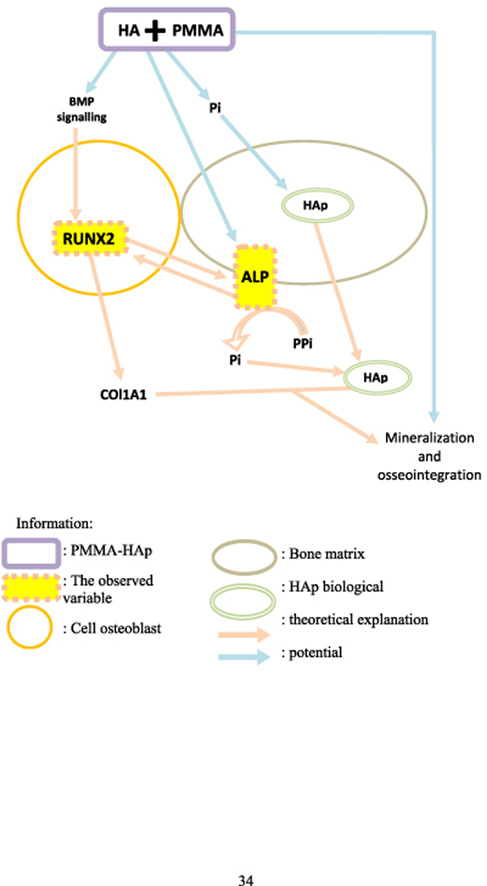
The osteoblasts are osteogenic cells derived from mesenchymal stem cells, induced to differentiate into mature osteoblasts by Runt-Related Transcription Factors 2 (RUNX2) as a major regulator of osteoblastogenesis and modulate local concentrations of calcification inhibitors (inorganic phosphates) via Alkaline Phosphatase (ALP).13 Moreover, the RUNX2 expression was found to be increased, followed by Col-I and ALP. These two bone regeneration markers have important roles in osteoblast proliferation.14
Furthermore, ALP expression is an early indicator of osteogenesis,15 involved and functions in the early stages of the mineralization process by osteoblasts.16,17 In the mineralization stage, HAp crystals penetrate the vesicle membrane matrix and extend into the extracellular space. This extension of HAp into the extracellular space requires adequate calcium and phosphate concentrations outside the vesicle matrix. The extracellular fluid in the growth plate matrix contains sufficient calcium and Pi to form new HAp crystals, which proves that ALP plays an important role in the mineralization process.17,18
Moreover, the calcium ions from HAp would be responded by the transmembrane Calcium Sensing Receptors (CaSR). These transmembrane receptors convey information from the extracellular matrix to the intracellular compartment by involving the Extracellular Signal-Regulated Kinase (ERK) pathway via Protein Kinase C (PKC) and Phospholipase C (PLC) pathways. The ERK signaling pathway further induces the RUNX2 gene.19 RUNX2 further activates the expression of the important osteogenesis marker ALP.14,20, 21, 22
So far, little is known about the osteoblast's cellular response to PMMA/HAp materials. Moreover, the specific response of osteoblasts exposed to PMMA/HAp was expressed by RUNX2 and ALP markers. Based on this background, this study aimed to observe the expression of RUNX2 and ALP markers in osteoblast cell cultures exposed to PMMA-HApBBK and PMMA-HApGMP materials.
2. Methods
2.1. Study design
The study was an experimental randomized post-test only control group design, the protocol was approved by ethical clearances No.612/HRECC.FODM/XI/2021 (Date approval: September 30, 2021) from the Faculty of Dentistry Research Ethics Commission, Airlangga University, Surabaya, Indonesia.
The study was conducted on 24 osteoblast cell cultures from fetal rat calvarie exposed to PMMA-HApBBK and PMMA-HApGMP at CDAST University of Jember which were divided into groups, including the control group on day 7 (C7), and day 14 (C14), the 7th-day treatment group PMMA-HApBBK (BBK7), the 14th-day PMMA-HApBBK treatment group (BBK14), the 7th-day treatment group PMMA-HApGMP (GMP7), and the 14th-day PMMA-HApGMP treatment group (GMP14). Immunocytochemistry staining was carried out on the preparations using anti-RUNX2 and anti-ALP monoclonal antibodies.
2.2. Preparations and sterilization of PMMA-HAp
PMMA was from Cemex® System Genta (Tecres- Italy) and mixed with HAp from limestone as natural source which processed through Balai Besar Keramik Indonesia (HApBBK) and from bovine bone which processed through Good Manufacturing Practice from Tissue Bank of the Dr. Soetomo, Indonesia (HApGMP), with a planetarium mixer in a ratio of 80: 20 for 3 h. For each mixture of 0.1gr PMMA/HAp, 0.016 ml of monomer is added so that the ratio of PMMA: HAp becomes 83.8: 16.2. The mixture of PMMA and HAp is stirred using a cement spatula on a dappen glass for 1–1.5 min until it reaches a dough stage consistency. The mixture of PMMA and HAp was molded using a nylon mold with a height of 2 mm and a diameter of 1 mm, waiting for setting time for 5–10 min, then removed from the mold, and the prints were uniformed with a weight of 0.003 mg. Samples were washed with PBS for three repetitions, followed by immersion in 70% ethanol for 2 h, followed by radiation with UV light for 2 h.23
2.3. Osteoblast cells culture
Osteoblasts isolated from a 19-day-old fetal rat calvarie. Pretreatment of calvariae for 10 min, at 37 °C with type II. Osteoblasts were released from the calvariae twice for 10 min and twice for 20 min. Osteoblasts were left overnight to 25,000 cells/cm2 on culture plates. Osteoblasts were removed, using 0.05% trypsin and 0.53 mm EDTA in a buffered saline solution. Osteoblasts were grown at 36,000 cells/cm2 density. Osteoblasts grown in alpha-Minimum Essential Medium were added with 10% fetal bovine serum, 0.1% gentamicin, 0.5% Fungizone, 1% L-glutamine, and 0.5% non-essential amino acids. Every three days, the media was supplemented with b-glycerophosphate (3 mm) and ascorbic acid (50 mg/ml).
2.4. Testing and immunochemistry
Synthetic materials PMMA-HApBBK and PMMA-HApGMP have soaked overnight in Osteoblast Growth Medium and fetal bovine serum (FBS-specific media); osteoblast cell culture was applied to both groups and incubated at 37 °C and 5% CO2. Cell preparations on days 7 and 14 of the incubation period, then washed with PBS, applied 3% H2O2 (15 min), washed under running water three times (5 s), given primary antibody 2 μg/100 μL (2 h), secondary antibodies (1 h), washed under running water 3 x (5 s), Strep Avidin Horse Predise (40 s), then washed with PBS and aquades, application of Diamino Benzoade (10 s) with a ratio of buffer: substrate = 20:1 to brownish color, rinsed using distilled water three times (5 s), washing and drying, 1–2 drops of entelan, after drying covered with a cover glass and reading with a microscope.
2.5. Results analysis with image J
Quantitative observations of RUNX2 and ALP expression were carried out using immunohistochemistry under a light microscope with 100x, 400x, and 1000× magnifications with three fields of view, then the results were calculated using the image tool (image J) and then recorded. This technique is a technique for determining the location of antigens (target proteins) in tissues or cells using the principle of the antigen-antibody reaction.
The photo analyze of quantitative results was conducted using the Image J program. After getting the research results, the photo was taken using an electron microscope to get a high-resolution photo. Then, open the image-J program, and perform the inversion process on the photo/image analyzed. After that, do a split colour, so that the photo will be divided into three base colours automatically by this system command. Furthermore, perform image analysis by converting the split results to 32 bits. This can be achieved by changing the image menu to 32-bit mode. The results of the analysis are the average light colour luminescence (representation of a brown colour from DAB staining) in all areas of the photo, and the value is called arbitrary units (au).24
2.6. Statistical analysis
The study results were calculated with the mean and standard deviation using the SPSS version 25. The Shapiro-Wilk Test was conducted to determine the distribution of the data. The differences between the one-way ANOVA and posthoc test Tukey HSD (p < 0.05).
3. Results
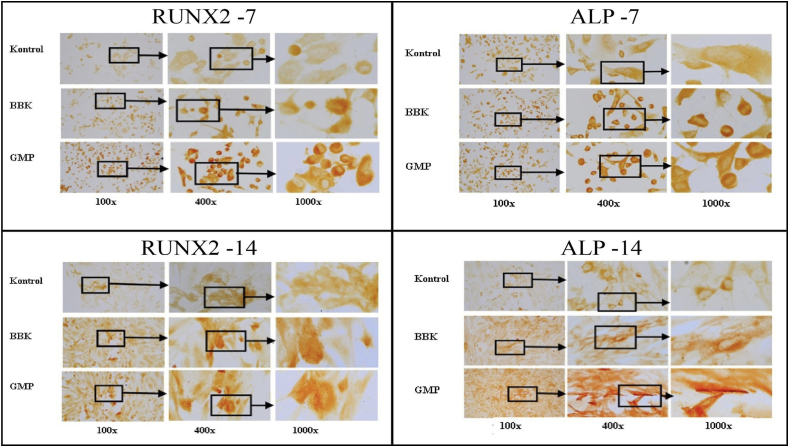
Quantitative observations of RUNX and ALP markers by osteoblast cells exposed to PMMA-HApBBK and PMMA-HApGMP were seen from the average light colour luminescence (representation of a brown colour or DAB staining) in all areas of the photo with the result of an arbitrary unit (au) value. The au value indicates the marker expression being viewed.24
A higher au value indicates more RUNX2 and ALP expression in osteoblast cells after PMMA-HApBBK and PMMA-HApGMP samples were given, while the lower au value indicates that there is less RUNX2 and ALP expression in osteoblast cells after PMMA-HApBBK and PMMA-HApGMP. Therefore, marker expression indirectly indicates an increase in osseointegration potential Fig. 1.
Fig. 1.
Immunocytochemistry staining under a light microscope with 100x, 400x, and 1000× magnifications (Counterclockwise: RUN×2 and ALP on day 7 and ALP and RUNX2 on day 14).
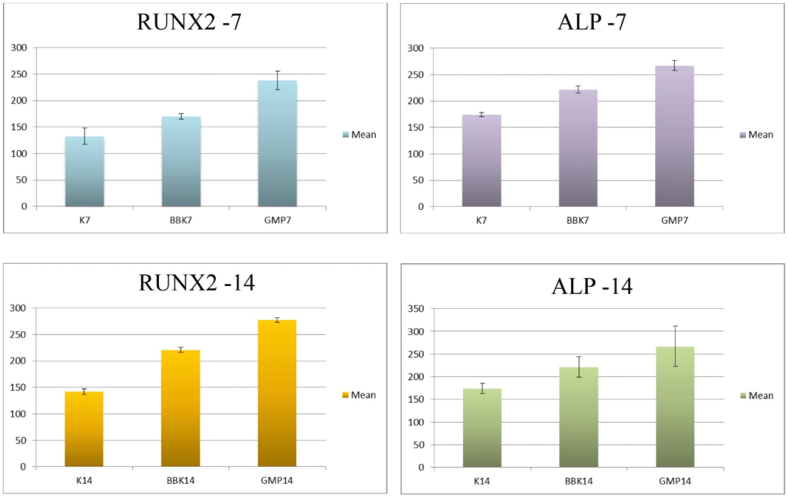
RUNX2 expression significantly differed in mean value on the 7th day between the control, PMMA-HApBBK, and PMMA-HApGMP groups (p < 0.000). On the 7th and 14th day, the highest mean score was obtained from the PMMA-HApGMP group Fig. 2. RUNX2 expression in all groups had a significant difference in the mean value Table 2.
Fig. 2.
Comparison graph of RUNX2 and ALP expression on day 7 and 14, between control, PMMA-HApBBK, and PMMA-HApGMP groups.
Table 2.
The ANOVA test of RUNX2 expression.
| Mean Difference | p-value | ||
|---|---|---|---|
| C7 | HApBBK7 | 37,298,761* | 0,003 |
| C7 | HApGMP7 | 105,521,602* | 0,000 |
| HApGMP7 | HApBBK7 | 68,222,841* | 0,000 |
| HApBBK14 | C14 | 79,029,483* | 0,000 |
| HApGMP14 | C14 | 135,598,895* | 0,000 |
| HApGMP14 | HApBBK14 | 56,569,412* | 0,000 |
*p < 0.05 = there is a significant difference.
Based on the results of the ANOVA test, the mean value of ALP expression was significant on the 7th day between the control group and PMMA-HApBBK (p < 0.0035). and between the control group and PMMA-HApGMP (p < 0.0004). The mean value of PMMA-HApGMP was higher than PMMA-HApBBK, but no significant difference was found between the two groups.
On the 14th day, the ANOVA test results showed the highest value in the PMMA-HApGMP group Fig. 2. A significant difference in the mean value of ALP expression was found between the control group and PMMA-HApGMP (p < 0.001), while between the control group and PMMA-HApBBK (p < 0.062) and between the PMMA-HApBBK group and PMMA-HApGMP (p < 0.074) there was no significant difference.
Based on the results of reading the average value of the two markers, the PMMA-HApGMP group achieved the highest value, on the 7th and 14th days, followed by the PMMA-HApBBK treatment group Fig. 2. The increase in the value of the PMMA-HApGMP group on both markers on the 7th and 14th day had a significant difference from the value in the control group Table 1, Table 2.
Table 1.
The ANOVA test of ALP expression.
| Mean Difference | p-value | ||
|---|---|---|---|
| C7 | HApBBK7 | 13,356,956* | 0,035 |
| C7 | HApGMP7 | 19,217,853* | 0,004 |
| HApGMP7 | HApBBK7 | 5,860,897 | 0,443 |
| HApBBK14 | C14 | 47,373,227 | 0,062 |
| HApGMP14 | C14 | 92,804,494* | 0,001 |
| HApGMP14 | HApBBK14 | 45,431,267 | 0,074 |
*p < 0.05 = there is a significant difference.
Furthermore, the increase in the expression value between the two treatment groups on the 7th and 14th day had a significant difference in the RUNX2 marker, with the highest value obtained by the PMMA-HApGMP group p < 0.000, p < 0.000 respectively Table 1. Whereas in the ALP marker, the two treatment groups on the 7th and 14th days have insignificant differences, with the highest value obtained by the PMMA-HApGMP group p < 0.443, p < 0.074 respectively Table 2.
Almost all treatment groups had significantly higher scores compared to the control group Table 1, Table 2, Fig. 2. A non-significant increase in the mean value between the expression of the PMMA-HApBBK group and the control group was only found in the ALP marker on day 14 p < 0.062. These results indicate that both the PMMA-HApBBK and PMMA-HApGMP groups can increase the RUNX2 and ALP expression in osteoblast cells.
4. Discussion
4.1. Role of PMMA-HAp on RUNX2 and ALP markers
The HAp is an osteoconductive material that enables PMMA-HAp to bind chemically by its bioactive molecules to stimulate osteoblast function. HAp is an ideal platform for new bone to grow and provides excellent osseointegration characteristics.24 Meanwhile, direct attachment of HAp to the bone will prevent fibrous tissue formation and lead to bone formation. This will allow the invasion by connective tissue from the surrounding bone to HAp, which will later harden (ossify) and retain its original characteristics.25
Calcium ions from HAp would be responded to by the transmembrane CaSR. These transmembrane receptors convey information from the extracellular matrix to the intracellular compartment by involving the ERK pathway via PKC and PLC pathways. The ERK signaling pathway further induces the RUNX2 gene.19 RUNX2 further activates the expression of the important osteogenesis marker ALP.14,20, 21, 22
4.2. RUNX2 and ALP expression
RUNX2 expression is linear to the increasing bone regeneration.26 Research conducted by Chen21 revealed that the highest RUNX2 expression was found on days 7 and 10. Moreover, research by Mohammadi et al.27 stated that RUNX2 expression reached its highest on days 7 and 14. According to Bose et al.,22 the RUNX2 expression ratio had a gradual increase from day 5–15. This is consistent with the results of this research, where there was an increase in the RUNX2 expression value on days 7–14, with a significant increase in value between the control and the PMMA-HApBBK and PMMA-HApGMP groups.
ALP expression is an early indicator of osteogenesis,15 involved and functions in the early stages of the mineralization process by osteoblasts.16,17 In the mineralization stage, HAp crystals penetrate the vesicle membrane matrix and extend into the extracellular space. This extension of HAp into the extracellular space requires adequate calcium and phosphate concentrations outside the vesicle matrix. The extracellular fluid in the growth plate matrix contains sufficient calcium and Pi to form new HAp crystals, which proves that ALP plays an important role in the mineralization process.17,18 Chen et al.20 revealed that a significant increase in ALP activity occurred on days 7 and 10, with the highest value on day 7. Moreover, accordance with the research of Mohammadi et al.27 which revealed that the highest ALP activity was on the 7th and 14th, while Wahab et al.28 observed a significant increase in ALP activity on days 7–14.
These findings are in line with the results of this research where on days 7 and 14 there was an increase in the value of ALP expression in both the PMMA-HApBBK and PMMA-HApGMP groups compared to the control group. Furthermore, PMMA-HApBBK and PMMA-HApGMP groups had significantly higher scores than the control group. Meanwhile, the PMMA-HApGMP group achieved the highest mean score compared to all groups, with or without significant differences compared to other groups.
This can be caused by a lot of factors affecting the osteoblast cells and material interaction. First, the pore size and porosity of the material can affect cell attachment efficiency, which consequently impacts cell seeding density, cell migration, and cell distribution. The HAp is a porous bioceramic that allows the growth of capillaries and other blood vessels.29 These factors have been shown to influence osteogenic differentiation through changes in cell signaling distance. In addition, porosity and pore size significantly influence the mechanical strength of the graft material, as well as affect the scaffold's ability to promote osteoconduction and vascularization in-vivo studies .30
Apart from all the differences in the increase in values between the treatment groups, the results showed that both the PMMA-HApBBK and PMMA-HApGMP groups could increase the RUNX2 and ALP expression that was exposed to osteoblast cells. In addition, both treatment groups had a significant increase in RUNX2 and ALP expression values concerning expression values compared to the control group.
In this study, the number of cells was not counted to see the potential effect of the material on cells cellularly. In addition, the interaction of biological potential with the mechanical strength of PMMA-HApBBK and PMMA-HApGMP materials has not been carried out, considering the need to understand various aspects in these areas to create a bone graft material that is suitable for various clinical applications. Further research is also needed on the potential of PMMA-HApBBK and PMMA-HApGMP as bone graft alternative materials in many forms (scaffold, powder graft, implant, etc.).
5. Conclusion
Based on the results of this research, it was found that the expression of RUNX2 and ALP was increased in osteoblast cells exposed to PMMA-HApBBK and PMMA-HApGMP. The increase in the expression of these markers indicates a potential increase of osseointegration by PMMA-HApBBK and PMMA-HApGMP materials of bone implants.
Source of funding
This study was not-for-profit sector and didn't receive any specific grant from agencies funding public or commercial.
Ethical approval
The research protocol was approved by ethical clearances No.612/HRECC.FODM/XI/2021 (Date approval: September 30, 2021) from the Faculty of Dentistry Research Ethics Commission, Airlangga University, Surabaya, Indonesia.
Authors contributions
CD, CP, and BK carried out the designer of the study, conducted, supervised the research, and collected the data. EF validated and visualized the data. The data were organized, analyzed, interpreted, and revised in the article by KEW and MA. All authors have approved and critically reviewed the final draft and are accountable for the similarity index and content of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Chiquita Prahasanti, Email: chiquita-p-s@fkg.unair.ac.id.
Mohammed Aljunaid, Email: mohammed.aljunaid90@gmail.com.
References
- 1.Malathi Selvam. Obesity and its role in periodontal disease, A review. Int J Sci Res Rev. 2013;2(4):126–135. [Google Scholar]
- 2.Darjanki C.M., Perdana S., Purwaningsih Y., Palupi R. Relationship between age in patiets with dental and oral health with quality of life. Eur J Mol Clin Med. 2020;7(10):1375–1387. [Google Scholar]
- 3.Warreth A., Ibieyou N., O'Leary R.B., Cremonese M., Abdulrahim M. Dental implants: an overview. Dent Update. 2017;44(7):596–620. doi: 10.12968/DENU.2017.44.7.596. [DOI] [Google Scholar]
- 4.Han J., Dong J., Zhao H., Ma Y., Yang S., Ma Y. Impact of different implant materials on osteoblast activity after oral implantation. Int J Clin Exp Med. 2019;12(5):5389–5396. [Google Scholar]
- 5.Ananth H., Kundapur V., Mohammed H.S., Anand M., Amarnath G.S., Mankar S. A review on biomaterials in dental implantology. Int J Biomed Sci. 2015;11(3):113. [PMC free article] [PubMed] [Google Scholar]
- 6.Komang-Agung I.S., Hydravianto L., Sindrawati O., William P.S. Effect of polymethylmethacrylate-hydroxyapatite composites on callus formation and compressive strength in goat vertebral body. Malaysian Orthop J. 2018;12(3):6. doi: 10.5704/MOJ.1811.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moursi A.M., Winnard A.V., Winnard P.L., Lannutti J.J., Seghi R.R. Enhanced osteoblast response to a polymethylmethacrylate–hydroxyapatite composite. Biomaterials. 2002;23(1):133–144. doi: 10.1016/S0142-9612(01)00088-6. [DOI] [PubMed] [Google Scholar]
- 8.Wahyudi K., Edwin F., Sofyaningsih N. Sintesis dan karakterisasi bone ash sintetik Dari bahan alam. J Keramik dan Gelas Indones. 2016;25(2):46–58. [Google Scholar]
- 9.Pridanti, Ayu Krisnynda, Cahyaraeni Firlana, et al. Characteristics and cytotoxicity of hydroxyapatite from Padalarang–cirebon limestone as bone grafting candidate. Biochem Cell Arch. 2020;20(2):4727–4731. [Google Scholar]
- 10.Jiang Y., Yuan Y., Xiong Y., et al. Low-intensity pulsed ultrasound improves osseointegration of dental implant in mice by inducing local neuronal production of αCGRP. Arch Oral Biol. 2020;115 doi: 10.1016/j.archoralbio.2020.104736. [DOI] [PubMed] [Google Scholar]
- 11.Hayes J.S., Khan I.M., Archer C.W., Richards R.G. The role of surface microtopography in the modulation of osteoblast differentiation. Eur Cell Mater. 2010;20:98–108. doi: 10.22203/ecm.v020a09. [DOI] [PubMed] [Google Scholar]
- 12.Gruber R., Bosshardt D.D. Dental implantology and implants - tissue interface. Stem Cell Biol Tissue Eng Dent Sci. Published online January. 2015;1:735–747. [Google Scholar]
- 13.Nakamura T., Nakamura-Takahashi A., Kasahara M., Yamaguchi A., Azuma T. Tissue-nonspecific alkaline phosphatase promotes the osteogenic differentiation of osteoprogenitor cells. Biochem Biophys Res Commun. 2020;524(3):702–709. doi: 10.1016/j.bbrc.2020.01.136. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B., Xu H., Gao Y., et al. Promoting osteoblast proliferation on polymer bone substitutes with bone-like structure by combining hydroxyapatite and bioactive glass. Mater Sci Eng C. 2019;96:1–9. doi: 10.1016/J.MSEC.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 15.De Witte T.M., Fratila-Apachitei L.E., Zadpoor A.A., Peppas N.A. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater. 2018;5(4):197–211. doi: 10.1093/RB/RBY013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tirachaimongkol C., Pothacharoen P., Reichart P.A., Khongkhunthian P. Relation between the stability of dental implants and two biological markers during the healing period: a prospective clinical study. Int J Implant Dent. 2016;2(1):1–11. doi: 10.1186/s40729-016-0058-y. 2016 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caetano-Lopes J., Canhão H., Fonseca J.E. Osteoblasts and bone formation. Acta Reum Port. 2007;32(2):103–110. doi: 10.1016/s0169-6009(08)80210-3. [DOI] [PubMed] [Google Scholar]
- 18.Halling Linder C., Ek-Rylander B., Krumpel M., et al. Bone alkaline phosphatase and tartrate-resistant acid phosphatase: potential Co-regulators of bone mineralization. Calcif Tissue Int. 2017;101(1):92–101. doi: 10.1007/s00223-017-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vimalraj S. Alkaline phosphatase: structure, expression and its function in bone mineralization. Gene. 2020;754 doi: 10.1016/J.GENE.2020.144855. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Huang Z., Li X., et al. In vitro biocompatibility and osteoblast differentiation of an injectable chitosan/nano-hydroxyapatite/collagen scaffold. J Nanomater. 2012;2012:1–6. doi: 10.1155/2012/401084. [DOI] [Google Scholar]
- 21.Florencio-Silva R., Sasso G.R.D.S., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res Int. 2015;2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bose S., Vahabzadeh S., Banerjee D., Ke D. Enhanced osteogenic protein expression on human osteoblast-osteoclast co-culture system using doped hydroxyapatite plasma coatings for orthopedic and dental applications. Mater Today Commun. 2019;21 [Google Scholar]
- 23.Putri T.S., Hayashi K., Ishikawa K. Fabrication of three-dimensional interconnected porous blocks composed of robust carbonate apatite frameworks. Ceram Int. 2020;46(12):20045–20049. doi: 10.1016/j.ceramint.2020.05.076. [DOI] [Google Scholar]
- 24.Lunde A., Glover J.C. A versatile toolbox for semi-automatic cell-by-cell object-based colocalization analysis. Sci Rep. 2020;10(1):1–26. doi: 10.1038/s41598-020-75835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaramillo Carlos D., Rivera Jairo A., Echavarría Alejandro, O’byrne Johan, Congote Diego, Restrepo Luis F. Osteoconductive and osseointegration properties of a commercial hydroxyapatite compared to a synthetic product. Rev Colombiana Ciencias Pecuarias. 2010;23(4) [Google Scholar]
- 26.Prahasanti C., Ulfah N., Kusuma I., et al. Transforming growth factor-β1 and runt-related transcription factor 2 as markers of osteogenesis in stem cells from human exfoliated deciduous teeth enriched bone grafting. Contemp Clin Dent. 2018;9(4):574. doi: 10.4103/ccd.ccd_609_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi M., Alibolandi M., Abnous K., Salmasi Z., Jaafari M.R., Ramezani M. Fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomed Nanotechnol Biol Med. 2018;14(7):1987–1997. doi: 10.1016/j.nano.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Wahab R.M.A., Abdullah N., Ariffin S.H.Z., Abdullah C.A.C., Yazid F. Effects of the sintering process on nacre-derived hydroxyapatite scaffolds for bone engineering. Mol. 2020;25:3129. doi: 10.3390/molecules25143129. 2020;25(14):3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prahasanti C., Krismariono A., Takanamita R., et al. Enhancement of osteogenesis using a combination of hydroxyapatite and stem cells from exfoliated deciduous teeth. J Int Dent Med Res. 2020;13(2):508–512. [Google Scholar]
- 30.Maté Sánchez de Val J.E., Calvo-Guirado J.L., Gómez-Moreno G., Pérez-Albacete Martínez C., Mazón P., De Aza P.N. Influence of hydroxyapatite granule size, porosity, and crystallinity on tissue reaction in vivo. Part A: synthesis, characterization of the materials, and SEM analysis. Clin Oral Implants Res. 2016;27(11):1331–1338. doi: 10.1111/clr.12722. [DOI] [PubMed] [Google Scholar]