Abstract
Purpose
To report two cases of deep anterior lamellar keratoplasty (DALK) rejection occurring in association with SARS-CoV-2 vaccination.
Methods
Two patients with prior history of DALK developed immunologic rejection after SARS-CoV-2 vaccination. The first patient, a 15-year-old girl, had a stromal and sub-epithelial rejection nine days after receiving the first dose of the SARS-CoV-2 vaccine BBV152(COVAXINTM, Bharat Biotech, India). The second patient, an 18-year-old male, had a stromal rejection 13 days after receiving the second dose of the ChAdOx1 SARS-CoV-2 vaccine (COVISHIELDTM, Serum Institute of India, India).
Results
Both patients received frequent topical corticosteroids. The first patient recovered within four weeks and the second patient recovered within two weeks of initiating therapy. Both patients experienced complete resolution of corneal edema and had improvement in their visual acuity.
Conclusions
DALK rejection is a rare but distinct possibility in patients following SARS-CoV-2 immunization. Further studies are required before establishing clear guidelines regarding risk, follow-up and treatment strategies in such a scenario.
Keywords: DALK rejection, SARS-CoV-2 vaccination.
Introduction
Although the cornea is an immune-privileged site, graft failure remains a possibility, with allogeneic rejection being its most frequent cause. Deep Anterior Lamellar Keratoplasty (DALK), by preserving the host Descemet's Membrane-endothelial complex, reduces the risk of rejection when compared to Penetrating Keratoplasty(PK).1,2 However, epithelial, sub-epithelial and stromal rejections are still possible.3 Though rejection in DALK is almost always reversible with appropriate and timely treatment,4 it can cause visual acuity deterioration due to sequelae such as sub-epithelial scarring, stromal haze, neovascularization and lipid keratopathy.
The SARS-CoV-2 pandemic has led to massive vaccination worldwide and multiple case reports of corneal graft rejection post vaccination against SARS cov-2 have been reported. We hereby report two possible cases of DALK rejection after administration of SARS-CoV-2 vaccine.
Case 1
A 15-year-old girl presented for a regular follow up for her RE, 9 days after receiving the first dose of the SARS-CoV-2 vaccine BBV152 (COVAXINTM, Bharat Biotech, India). She had 2 loose sutures removed 15 days prior to the visit. Neovascularisation was noted around the loose sutures but there were no signs of rejection such as graft edema or sub-epithelial infiltrates. After the removal of sutures, treatment with fluorometholone eye drops four times daily and dexamethasone eye ointment thrice daily was initiated.
At presentation, her best-corrected visual acuity (BCVA) in the RE was 6/18, which was comparable to the vision recorded 15 days before, but the patient had noticed a slight worsening in her vision. Intraocular pressure was 12 mm Hg. Slit-lamp biomicroscopy revealed minimal ciliary injection, diffuse stromal haze with minimal edema within the graft, significant interface vascularization, subepithelial infiltrates and few stromal folds indicating sub-epithelial and stromal graft rejection. There were no keratic precipitates or anterior chamber reaction. Fourteen sutures were intact, and the graft host junction was well apposed. Systemic reactions were absent. Ocular history included DALK (uneventful manual dissection with ‘groove and peel’ technique) performed five months before in her RE due to keratoconus. The LE, which had undergone collagen cross linking for keratoconus 1 year earlier, was unremarkable.
Fundus examination was normal in both eyes. Anterior segment Optical Coherence Tomography (OCT) (RTVue, Optovue, USA) confirmed a series of morphological changes including corneal thickening, sub-epithelial hyper-reflective spots indicating sub-epithelial Infiltrates but the DM-endothelial complex was normal in morphology.The OCT pachymetry showed diffuse corneal thickening (530 μm) compared to last visit (465 μm). The patient was started on hourly topical prednisolone acetate (1%), cycloplegics and lubricants.
Over the next four weeks, the rejection reversed completely as the patient noted improved clarity in her vision, though the BCVA in RE did not improve beyond 6/18. The central corneal thickness reduced to 490 μm and the sub-epithelial infiltrates and graft haze completely resolved.The steroids were tapered, and the patient was asked to follow up in 6 weeks.
Case 2
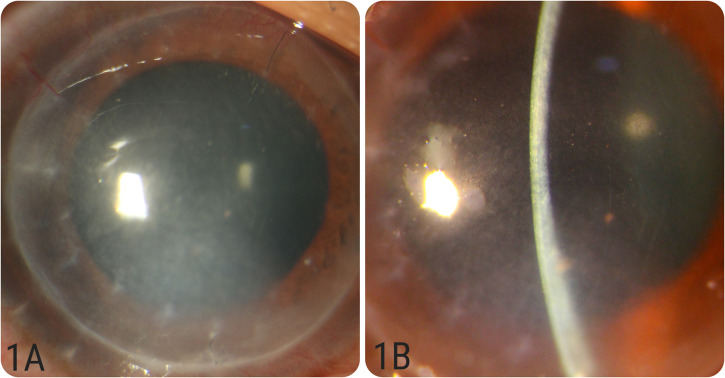
An 18-year-old male presented to our clinic with complaints of diminution of vision, redness and watering in his RE, 13 days after receiving the second dose of the ChAdOx1 SARS-CoV-2 vaccine (COVISHIELDTM, Serum Institute of India, India). Systemic reactions were absent. Ocular history included uneventful DALK using manual dissection with ‘groove and peel’ technique performed 42 months earlier for his right eye as a treatment for keratoconus. At presentation, his BCVA in the RE was 6/60 and he was not on any steroid medication. Intraocular pressure was 12 mm Hg. Slit-lamp biomicroscopy revealed ciliary injection, diffuse stromal haze and stromal edema within the graft indicating stromal graft rejection (Figure 1A and 1B). There were few endothelial pigments, but no significant anterior chamber reaction and the epithelium was intact throughout. The LE, which also had keratoconus, was unremarkable. Fundus examination was normal in both eyes. Anterior segment OCT confirmed the stromal edema and the central corneal thickness was noted to be 630 μm. The patient was started on hourly topical Prednisolone acetate (1%), cycloplegics and lubricants.
Figure 1.
(A&B): Diffuse and slit images of case number 2 showing acute stromal graft rejection with stromal edema, haze and some endothelial pigments.
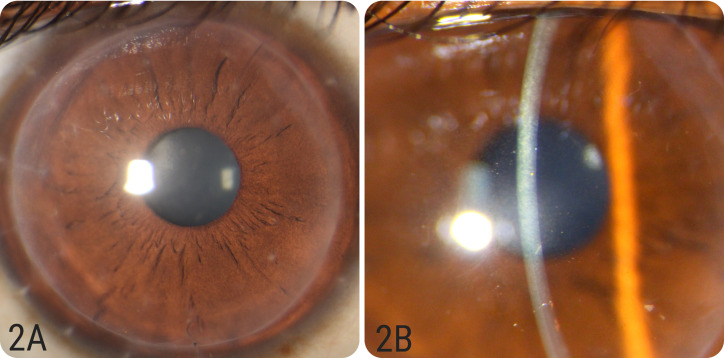
On his follow up two weeks later, the stromal rejection episode was noted to have completely resolved and the graft was clear with no haze or edema (Figure 2A and 2B). No neovascularization was noted.The RE BCVA was 6/9 and IOP was 12 mm Hg. Central corneal thickness reduced to 475 μm. The steroids were tapered, and the patient was asked to follow up in six weeks.
Figure 2.
(A&B): Diffuse and slit images of case number 2 showing reversal of rejection episode after 2 weeks of medical therapy with clear and compact graft.
Discussion
Though DALK, in theory and largely also in reported literature, seems to be fairly resistant to vaccine induced rejection, our cases show a rare but distinct possibility of the same. Fujimoto et al did a retrospective chart analysis of all patients who underwent rejection after vaccination (SARS-CoV-2 mRNA vaccine BNT162b2) at their center, and out of 7 patients that had an episode of rejection; none of the patients had undergone DALK.5
Shah et al recently described four cases of acute corneal transplant rejection occurring in association mRNA-1273 vaccination for SARS-CoV-2 where two patients had history of undergoing penetrating keratoplasty and two patients had history of undergoing Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) each.6
Mercedes et al have also described 5 cases-2 patients with DSAEK who presented with acute corneal graft rejection after their first dose of mRNA (BNT162) vaccine and 3 patients with penetrating keratoplasty who presented with acute graft rejection—2 patients after their second dose of adenovirus vector (AZD1222) vaccine and 1 patient after first dose of mRNA (BNT162) vaccine.7
Hamilton et al have reported the only case of DALK rejection post influenza vaccination where a 33-year-old patient developed stromal rejection in the eye which had received Anterior Lamellar Transplant for keratoconus 31 months prior to the rejection episode.8
Although it is difficult to demonstrate the direct causality between SARS-CoV-2 vaccine and corneal graft rejection, a temporal association between the two events can be made considering the criteria described in the specific guidelines for Causality Assessment of an Adverse Event Following Immunization—AEFI.9 It is important to note that local immune tolerance had been maintained till the time of the particular episodes of rejection considering no evidence of any such episodes previously. Also, the 9-day and the 13-day lag between vaccine administration and corneal graft rejection that we observed falls within the window of increased risk described in the specific guidelines (AEFI). And finally, there were no other “qualifying factors’’ such as exposure to drugs, allergens, previous such episodes or any other medical condition that could have contributed to the rejection episode in the second case. However, in the first case, loose sutures were noted on the previous visit with superficial vascularization at the graft-host junction which could have been the trigger for the rejection episode. Moreover, the indication of surgery in both the cases reported was keratoconus, which, as an indication, has one of the best rates of graft survival.10
Although the mechanisms that lead to corneal transplant rejection have not been fully understood, the activation and dysregulation of the immune system following the vaccine plays an important role. SARS-CoV-2 vaccines have been shown to induce SARS-CoV-2 neutralizing antibodies and elicit strong Th1-biased CD4 + responses in humans. CD4+ Th1 cells have been shown to be the main mediators of corneal graft rejection. Overall, corneal rejections after immunization are not a new phenomenon. Among the etiological mechanisms hypothesized earlier, local inflammation owing to the deposition of immune complexes, pro-inflammatory action of interferon-gamma released at the systemic level, and production of effector T cells that cross-react with allo-major histocompatibility complex antigens of the corneal button seem to be the most prominent causes of allograft rejection.11 Future studies should aim to enhance a better understanding of these hypotheses related to the SARS-CoV-2 vaccines.
Various authors, while reporting corneal allograft rejection post SARS-CoV-2 vaccination, have suggested an ethical conundrum regarding how to educate such patients regarding the risks and benefits of vaccination and its possible adverse effect.
Considering the lifesaving effect of SARS-CoV-2 vaccination on the individual and overall benefits to the community at large12 along with the relatively good outcomes in terms of reversing allograft rejection with medical therapy alone,4 vaccination should not be discouraged in such patients. We suggest that the patients can be notified about the risk-benefit ratio in such a setting and encouraged to follow up with the operating surgeon immediately after the vaccination and be kept under close watch. It may be prudent to even step up topical steroid therapy before undergoing vaccination in such cases.13
Future studies are needed to study the mechanisms underlying potential allogeneic transplant failure after immunization against SARS-CoV-2 and to establish the epidemiological features of this complication.
Footnotes
Conflict of interest: None
Financial interest: None
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article
ORCID iD: Samip Mehta https://orcid.org/0000-0003-4703-1966
References
- 1.Guilbert E, Bullet J, Sandali Oet al. et al. Long-term rejection incidence and reversibility after penetrating and lamellar keratoplasty. Am J Ophthalmol 2013; 155: 560–569.e2. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Tzekov R, Li W, et al. Deep anterior lamellar keratoplasty versus penetrating keratoplasty: a meta-analysis of randomized controlled trials. Cornea 2016; 35: 169–174. [DOI] [PubMed] [Google Scholar]
- 3.Gonzales A, Price MO, Feng MT, et al. Immunologic rejection episodes after deep anterior lamellar keratoplasty: incidence and risk factors. Cornea 2017; 36: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 4.Olson EA, Tu EY, Basti S. Stromal rejection following deep anterior lamellar keratoplasty: implications for postoperative care. Cornea 2012; 31: 969–973. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto H, Kiryu J. Incidence of corneal transplant rejection following BNT162b2 SARS-CoV-2 messenger RNA vaccination. Journal of Ophthalmology and Research 2021; 04. [Google Scholar]
- 6.Shah AP, Dzhaber D, Kenyon KR, et al. Acute corneal transplant rejection after COVID-19 vaccination. Cornea 2022 Jan 1; 41: 121–124. [DOI] [PubMed] [Google Scholar]
- 7.Molero-Senosiain M, Houben I, Savant S, et al. Five cases of corneal graft rejection after recent COVID-19 vaccinations and a review of the literature. Cornea 2022; 41: 669–672. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton A, Massera R, Maloof A. Stromal rejection in a deep anterior lamellar keratoplasty following influenza vaccination. Clin Exp Ophthalmol 2015; 43: 838–839. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification. 2nd ed. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 10.Kubaloglu A, Sari E, Unal M, et al. Long-Term results of deep anterior lamellar keratoplasty for the treatment of keratoconus. Am J Ophthalmol 2011; 151: 760–767.e1. [DOI] [PubMed] [Google Scholar]
- 11.Steinemann T, Koffler B, Jennings C. Corneal allograft rejection following immunization. Am J Ophthalmol 1988; 106: 575–578. [DOI] [PubMed] [Google Scholar]
- 12.Meslé M, Brown J, Mook P, et al. Estimated number of deaths directly averted in people 60 years and older as a result of COVID-19 vaccination in the WHO European Region, December 2020 to November 2021. Eurosurveillance 2021; 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin U, Muik A, Vogler I, et al. BNT162b2 Vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021; 595: 572–577. [DOI] [PubMed] [Google Scholar]