Abstract
Purpose
The Histolog® Scanner (SamanTree Medical SA, Lausanne, Switzerland) is a large field-of-view confocal laser scanning microscope designed to allow intraoperative margin assessment by the production of histological images ready for assessment in the operating room. We evaluated the feasibility and the performance of the Histolog® Scanner (HS) to correctly identify infiltrated margins in clinical practice of lumpectomy specimens. It was extrapolated if the utilization of the HS has the potential to reduce infiltrated margins and therefore reduce re-operation rates in patients undergoing breast conserving surgery (BCS) due to a primarily diagnosed breast cancer including ductal carcinoma in situ.
Methods
This is a single-center, prospective, non-interventional, diagnostic pilot study including 50 consecutive patients receiving BCS. The complete surface of the specimen was scanned using the HS intraoperatively. The surgery and the intraoperative margin assessment of the specimen was performed according to the clinical routine consisting of conventional specimen radiography as well as the clinical impression of the surgeon. Three surgeons and an experienced pathologist assessed the scans produced by the HS for cancer cells on the surface. The potential of the HS to correctly identify involved margins was compared to the results of the conventional specimen radiography alone as well as the clinical routine. The histopathological report served as the gold standard.
Results
50 specimens corresponding to 300 surfaces were scanned by the HS. The mean sensitivity of the surgeons to identify involved margins with the HS was 37.5% ± 5.6%, the specificity was 75.2% ± 13.0%. The assessment of resection margins by the pathologist resulted in a sensitivity of 37.5% and a specificity of 81.0%, while the local clinical routine resulted in a sensitivity of 37.5% and a specificity of 78.2%.
Conclusion
Acquisition of high-resolution histological images using the HS was feasible in clinical practice. Sensitivity and specificity were comparable to clinical routine. With more specific training and experience on image interpretation and acquisition, the HS may have the potential to enable more accuracy in the margin assessment of BCS specimens.
Keywords: Breast cancer, Breast conserving surgery, Margin assessment, Confocal microscopy, Fresh tissue imaging, Conventional specimen radiography
Highlights
-
•
The Histolog®Scanner is a microscope for intraoperative margin assessment.
-
•
High-resolution histological images can be obtained in the operation room.
-
•
Intraoperative margin assessment in breast conserving surgery was performed.
-
•
This is the largest study using the Histolog®Scanner.
-
•
Sensitivity and Specificity were comparable to clinical routine.
Abbreviations
- AGO
German Working Group of Gynecological Oncology
- BCS
Breast conserving surgery
- BCT
Breast conserving therapy
- CI
Clinical impression
- CSR
Conventional specimen radiography
- DCIS
Ductal carcinoma in situ
- HS
Histolog®Scanner
- ILC
Invasive lobular carcinoma
- NPV
Negative predictive value
- NST
Non special type
- PO
Proportion of overall agreement
- PPV
Positive predictive value
- SD
Standard deviation
- 95% CI
95% confidence interval
1. Introduction
Breast cancer is the most common cancer in women with a lifetime probability of 12.3% [1]. Following the introduction of comprehensive mammography screenings in many countries in the last decades, the incidence of early-stage breast cancer has steadily increased. This frequently positions primary breast conserving therapy (BCT), defined as the combination of breast conserving surgery (BCS) and adjuvant radiation, as first choice treatment option. BCT is associated with improved overall survival compared to mastectomy in women with early-stage, node-negative breast cancer [[2], [3], [4]]. Furthermore, BCT is associated with higher patient satisfaction with improved aesthetic outcomes, while the risk of postoperative complications is reduced simultaneously [[5], [6], [7]]. BCS aims to remove all cancerous tissue to obtain clear resections margins, while at the same time removing as little healthy tissue as possible [8]. Current guidelines define a resection margin for invasive carcinoma as clear when no tumor cells are detectable on the resected surface, also called “no ink on tumor”. For ductal carcinoma in situ (DCIS) clear resection margin of > 2 mm is recommended, although individual approaches with consideration of the patient's age and tumor extent are possible [ [9,10]]. The final margin status is assessed by the pathologist after a few days following histopathological processing. Infiltrated margins are a known risk factor for local recurrence [11]. Therefore, 17–35% of BCS procedures result in a secondary re-resection due to margin involvement causing additional anxiety for the patients, increased risks for perioperative complications, an unfavorable cosmetic result, as well as additional costs [ [12,13]].
Although being proven as a critical component of BCT, intraoperative margin assessment techniques during the index procedure are currently lacking standardization. There are several methods available, e.g. intraoperative ultrasound, breast imprint and scrape cytology, frozen section analysis or Margin Probe, all with varying results [[14], [15], [16], [17], [18]]. Conventional specimen radiography (CSR) using two plane mammography and/or ultrasound is the current standard method established in Germany, as recommended by the German Working Group of Gynecological Oncology (AGO) Breast Committees guidelines [ [19,20]].
This study aimed to evaluate a confocal imaging device for fresh ex-vivo tissue surface imaging (Histolog®Scanner, SamanTree Medical SA) for its ability to detect infiltrated margins. The Histolog®Scanner (HS) is a digital microscopy scanner that produces images of the superficial cell layer of fresh tissue specimen.
We investigated if the HS is applicable in clinical routine as a tool for intra-operative decision-making regarding margin assessment. As a part of the evaluation for the intraoperative use of HS, it is of interest to compare the HS use with routine intraoperative assessments at our center which consists of combined CSR and gross surgical inspection. Furthermore, we assessed if reduction of positive margins and re-operation rates can potentially be achieved using this device.
2. Methods
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local ethics committee (reference number S-609/2020) and written informed consent was obtained from each patient.
2.1. Patient population
A total of 50 patients who received primary BCS due to a histologically confirmed malignant breast lesion between September 2020 and January 2021 were included. Exclusion criteria were neoadjuvant chemotherapy, presurgical radiotherapy of the target breast, previous breast surgeries of the target breast, or multicentricity of the breast lesion. Patients who did not receive CSR due to palpability of the target lesion were excluded. Patients with palpable lesions who received CSR despite palpability as part of the clinical routine were included. All histological subtypes including carcinoma in situ were included.
2.2. Standard surgical procedures
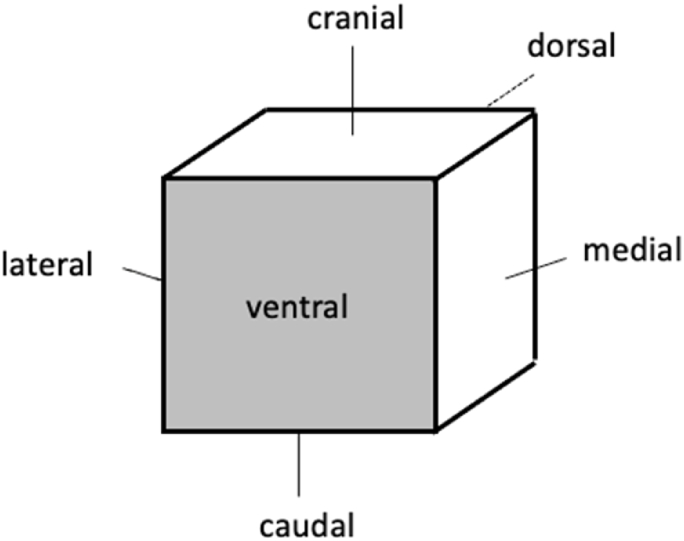
To facilitate excision of non-palpable lesions, all patients underwent ultrasound- or mammography-guided wire localization before surgery. Following excision predominantly conducted with electric surgical knives, the tumorous tissue was immediately marked by sutures on three distinct surfaces (cranial, lateral, medial) by the surgeon to ensure reproducibility of specimen orientation. Including the corresponding opposite surfaces, there were overall six surfaces per specimen (cranial, caudal, lateral, medial, ventral, and dorsal, Fig. 1).
Fig. 1.
Simplified explanation of a specimen's orientation.
The specimen was sent to the breast unit where the intraoperative specimen radiography was performed. The radiographs were reviewed for the presence of the malignant lesion close to the resection margin by a single experienced examiner who was in charge for the radiography assessment of that day in clinical routine. If infiltration of the resection margin could not be ruled out, a margin was considered positive. A re-excision was recommended if the examiner considered one or more of the resection margins to be infiltrated, but it was up to the surgeon's decision if a re-excision was to be performed. If, based on the CSR, a re-excision was recommended in a direction in which clinically a resection was not possible (e.g. dorsal dissection was already performed down to the fascia of the pectoralis muscle), the surgeon decided to what extent further surgery should be performed. At the same time, the surgeon could decide based on his or her intraoperative clinical impression (CI) or palpation to perform a re-excision in a direction in which no recommendation was made by the CSR. Therefore, the surgeon's CI by gross inspection also served as a margin assessment tool and may have led to re-excisions during surgery even if the CSR did not suggest further excisions.
2.3. Use of the Histolog®Scanner
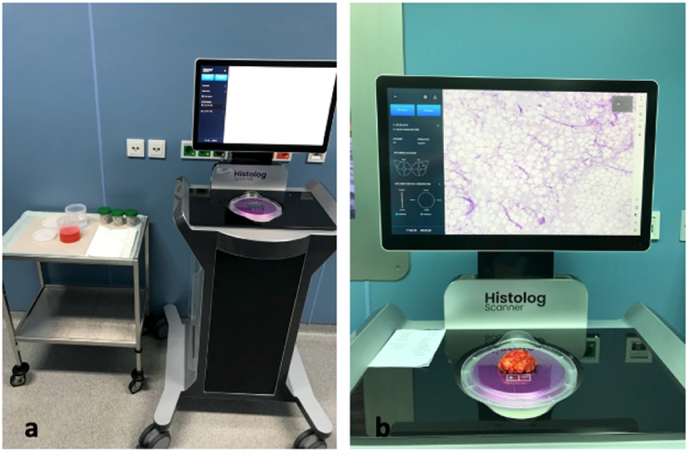
After the performance of CSR all six surfaces of the specimen were scanned by the HS in the operating room directly after wound closure (Fig. 2a). Every specimen was stained in Histolog®Dip (SamanTree Medical SA, Lausanne, Switzerland) containing fluorescence. After rinsing off superfluous dip with saline, the specimen was positioned on a Histolog®Dish (SamanTree Medical SA, Lausanne, Switzerland), previously mounted on the HS. To acquire an image, specimen surface in contact with the optical interface of the HS was digitized and the resulting image was visualized on a display monitor (Fig. 2b). Every specimen was visualized by a minimum of six scans. Up to two scans were taken of a surface if the surface area of a specimen was bigger than the optical interface. All images were stored digitally on an external hard drive until assessed. The fluorescence staining as well as the scanning of the surfaces were performed by a trained study team member.
Fig. 2.
Histolog®Scanner (HS) setting in the operating room
a setting of the HS in the operation room. b acquisition of a digital histopathological image of one surface of a specimen.
In this pilot study, the images were reviewed after finishing the surgery due to the non-interventional study design. Considering the exploratory character of this study, no clinical consequences were drawn based on the HS scans.
Since the three breast surgeons had no previous experience with confocal imaging, they received training data sets consisting of reference images to be able to differentiate between cancerous and physiological breast tissue, before they performed the image assessment. The training data sets contained HS images with NST and ILC breast cancer as well as DCIS. It was performed on a web-based platform and was completed within 2 h.
Then, the study scans were presented anonymously and in a random order to the surgeons as well as to a pathologist experienced in confocal imaging. The observer had to evaluate every scan for its quality (good versus limited versus no assessment possible). After that the tissue pictured on the scan was assessed for invasive cancer or in situ carcinoma. The observer had to decide based on the scan provided by the HS if a potential re-excision is recommended or not.
The duration from scanning the specimen to produce a histological image were measured to evaluate the necessary time expenditure.
2.4. Histopathological examination
After the use of the HS all excised tissue were directly sent to pathology for histopathological assessment functioning as reference standard.
The histopathologic examination included gross and microscopic inspection of all resection margins. According to the AJCC Cancer Staging Manual, the presence or absence of residual tumor was classified as R0 (no residual tumor), R1 (microscopic residual tumor), or Rx (presence of residual tumor cannot be assessed). All margins assessed as R1, or Rx were handled as positive in our study. For our study, R0 was defined as “no ink on tumor” for both invasive and non-invasive carcinoma, since the HS does not have the potential to evaluate non-superficial cell layers. Therefore, R1 was defined as cancer cells on the superficial cell layer. A DCIS with a safety margin of 1 mm would have resulted in a Rx situation in our clinical standard but in the context of this study it would be defined as R0. The pathologist was unaware of the findings of the CSR as well as the HS.
2.5. Statistical analysis
The statistical analysis is of descriptive nature. The patient characteristics were tabulated using the measures of empirical distributions such as mean with standard deviation (SD) as well as 95% confidence intervals (95%CI) depending on the level of measurement for continuous outcomes and absolute and relative frequencies for categorical outcomes. Statistical analysis was performed using R (Version 1.0.136 – © 2009–2016 RStudio, Inc).
3. Results
3.1. Patient and tumor characteristics
Fifty consecutive patients undergoing BCS were included. Mean age was 60.9 years ±10.6 years. Twenty-nine (58%) of all breast lesions were in the left breast, 21 (42%) in the right breast of the patient. Of all 50 patients 43 (86%) had an invasive cancer, of these 34 patients had concomitant in-situ carcinoma, while 7 (14%) patients had in-situ carcinoma alone. The clinical T classification is specified in Table 1. All 50 patients received preoperative wire-marking of the target lesion: 46 (92%) guided by ultrasound and 4 (8%) guided by mammography.
Table 1.
Clinical T classification.
| Clinical T classification | Number of patientsa | |
|---|---|---|
| is | 7 (14) | |
| 1 | 29 (58) | |
| 1a | 3 (6) | |
| 1b | 9 (18) | |
| 1c | 17 (34) | |
| 2 | 12 (24) | |
| 3 | 1 (2) | |
| 4 | 1 (2) | |
Values are absolute frequencies. Relative frequencies are given as percentages in parentheses. Percentages are rounded.
3.2. Histopathological examination of the main specimen
Considering the margin status before the performance of CSR and consecutive targeted re-excision, 42 (14.0%) of all 300 margins showed infiltration (R1) in the histopathological examination, 6 (2.0%) showed an unclear margin (Rx). This translates to 23 (46%) patients with an infiltrated margin and 4 (8%) patients with an unclear margin on case level, respectively (Table 2).
Table 2.
Histopathological margin assessment before performance of targeted re-excisions.
| a on margin level | |
|---|---|
| Number of surfacesa | |
| R0 | 252 (84) |
| R1 | 42 (14) |
| Rx |
6 (2) |
| b on case level | |
| Number of patientsa |
|
| R0 | 23 (46) |
| R1 | 23 (46) |
| Rx | 4 (8) |
Values are absolute frequencies. Relative frequencies are given as percentages in parentheses. Percentages are rounded.
3.3. CSR and clinical routine
CSR was performed in all 50 cases; therefore 300 margins were evaluated.
46 (15.3%) margins were considered positive, while 254 (84.7%) margins were considered negative by CSR. Of these, 13 (4.3%) were assessed correctly as positive comparing to the final histopathological examination. 33 (11.0%) margins were considered falsely as positive. 219 (73.0%) margins were correctly considered negative, while 35 (11.7%) were histologically infiltrated although being stated negative by CSR. This results in a sensitivity of 27.1% (95%CI: 15.3–41.9%) a specificity of 86.9% (95%CI: 82.1–90.8%) a positive predictive value (PPV) of 28.3% (95%CI: 18.3–40.9%) and a negative predictive value (NPV) of 86.2% (95%CI: 84.0–88.2%). The proportion of overall agreement (PO) was 77.3% (95%CI: 72.2–82.0%).
Considering the standard clinical routine consisting of CSR and CI, the results were as following: sensitivity 37.5% (95%CI: 24.0–52.7%), specificity 78.2% (95%CI: 72.6–83.1%), PPV 24.7% (95%CI: 17.5–33.6%), NPV 86.8% (95%CI: 83.9–89.2%), and PO 71.7% (95%CI: 66.2–76.7%).
3.4. Histolog®Scanner assessment
HS was used in all 50 cases; thus 300 surfaces were scanned. Overall, 320 images were obtained from the HS (Fig. 3, Fig. 4). Mean time taken for scanning was 14.7 ± 5.2 min per specimen.
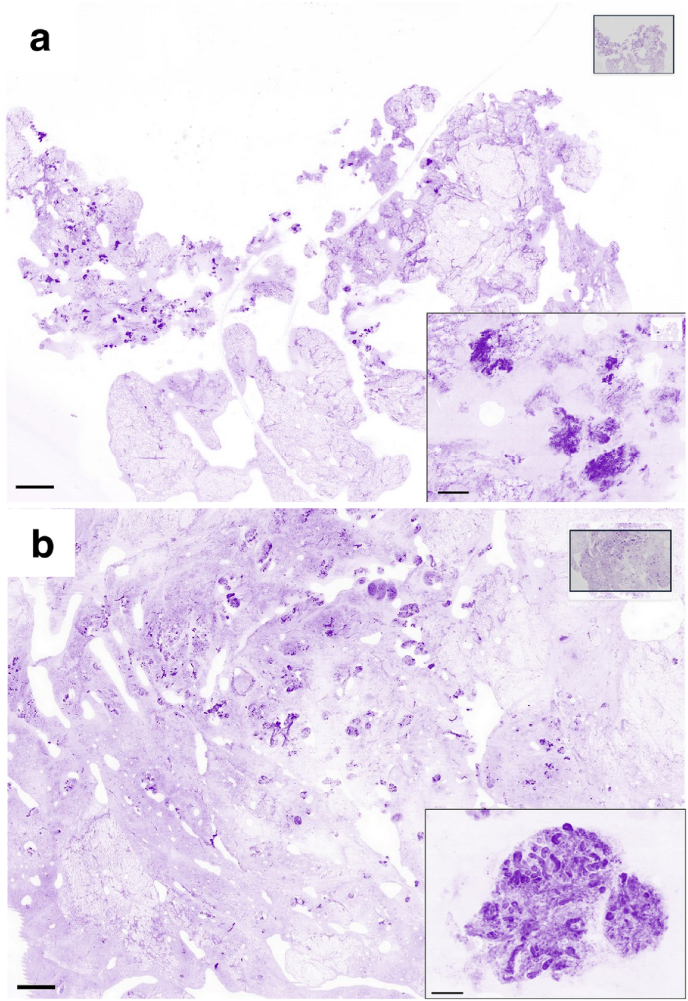
Fig. 3.
Images produced by the Histolog®Scanner of non-cancerous breast lumpectomy margins
a Surface of the lumpectomy is presenting artifacts affecting overall image quality and limiting proportion of the tissue in contact with the sensor. These artifacts are supposedly due to use of electric knife and to a superficial drying of the specimen surface. Insert: high magnification of the artifacts that are masking cellular details useful for identification. b Surface of the lumpectomy is presenting no artifacts with good image quality and good contact. Insert: high magnification of a benign lobule, where acini and small ducts could be appreciated. Scale bars in the main images and in the inserts represent 2 mm and 250 μm respectively.
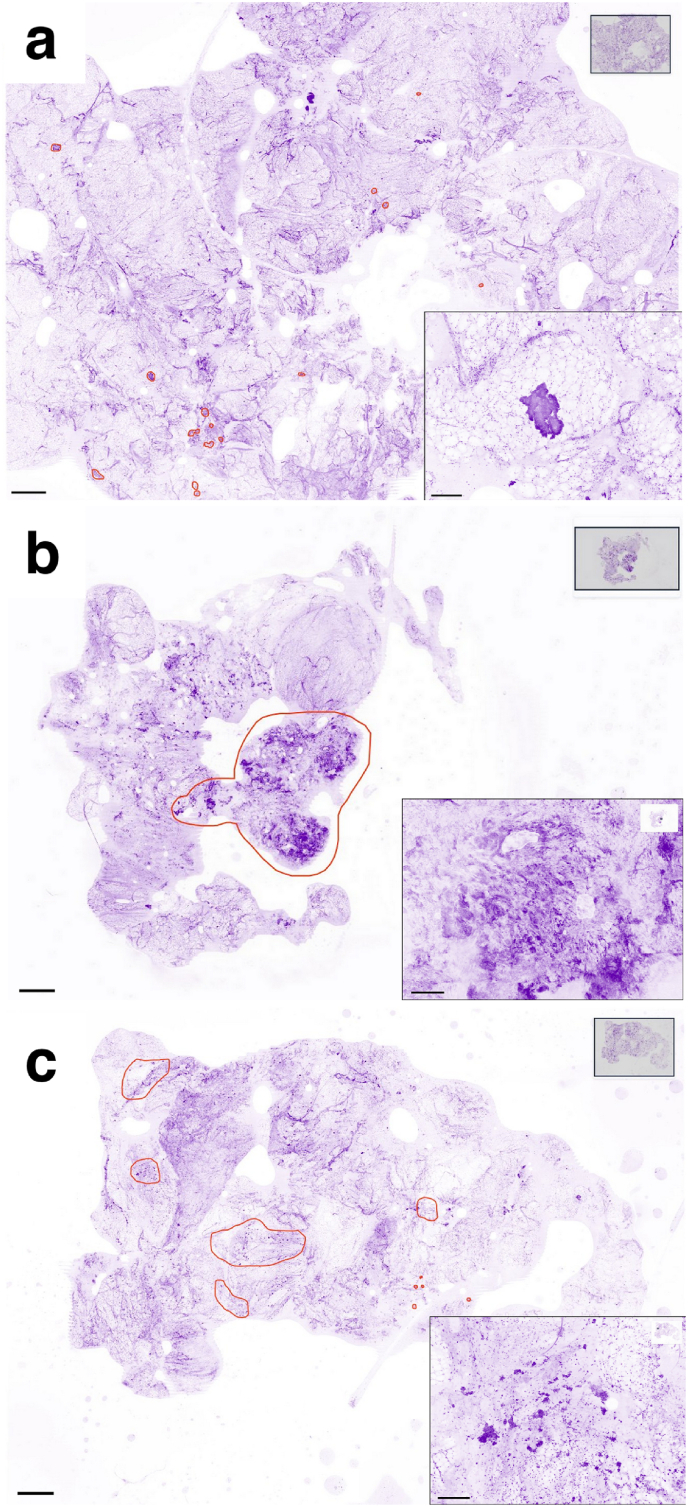
Fig. 4.
Images produced by the Histolog®Scanner of cancerous breast lumpectomy margins
Main images are showing cancerous areas encircled in red color: cancerous areas are often easily detected because they appear darker than both fibrous stroma and adipose tissue, due to a higher content of nuclei. Inserts: high magnification of the lesions. a ductal carcinoma in situ: one solid nodule with well-defined border is recognizable in the fat, composed of neoplastic cells with enlarged nuclei. b, c invasive carcinoma: normal lobular architecture is not present, whereas a nodule composed of haphazardly distributed cords and large aggregates of neoplastic cells is shown (b), and small nests of neoplastic cells infiltrating into connective tissue are recognizable (c) Scale bars in the main images and in the inserts represent 2 mm and 250 μm respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In 0.9%, 2.4%, and 0.9% of scans, respectively, HS image quality was graded poor quality by the three surgeons leading to no adequate assessment.
The margin assessment by the three observers resulted in a mean sensitivity of 37.5% ± 5.6%, a specificity of 75.2% ± 13.0%, with a PPV of 24.2% ± 6.0%, and NPV of 86.2% ± 1.2%. The mean PO was 69.2% ± 10.0%. The values for the respective observers are displayed in Table 3. All scans obtained by the HS were also assessed by the pathologist. HS image quality was rated poor in 4% of all cases and limited in the majority of the other cases (Fig. 3).
Table 3.
A Comparison of sensitivity, specificity, PPV, NPV and PO.
| Sensitivity | Specificity | PPV | NPV | PO | |
|---|---|---|---|---|---|
| Clinical routine (CSR + CI) | 37.5% (18 of 48) | 78.2% (197 of 252) | 24.7% (18 of 73) | 86.8% (197 of 227) | 71.7% (215 of 300) |
| CSR | 27.1% (13 of 48) | 86.9% (219 of 252) | 28.3% (13 of 46) | 86.2% (219 of 254) | 77.3% (232 of 300) |
| Observer 1 | 43.8% (21 of 48) | 60.3% (152 of 252) | 17.4% (21 of 121) | 84.9% (152 of 179) | 57.7% (173 of 300) |
| Observer 2 | 33.3% (16 of 48) | 84.1% (212 of 252) | 28.6% (16 of 56) | 86.9% (212 of 244) | 76.0% (228 of 300) |
| Observer 3 | 35.4% (17 of 48) | 81.3% (205 of 252) | 26.6% (17 of 64) | 86.9% (205 of 236) | 74.0% (223 of 300) |
| Pathologist | 37.5% (18 of 48) | 81.0% (204 of 252) | 27.3% (18 of 66) | 87.2% (204 of 234) | 74.0% (222 of 300) |
Absolute frequencies are given in parentheses.
CSR: conventional specimen radiography; CI: clinical impression of the surgeon by gross inspection; PPV: positive predictive value; NVP: negative predictive value; PO: proportion of overall agreement.
Margin assessment by the pathologist resulted in a sensitivity of 37.5% (95%CI: 24.0–52.7%), a specificity of 81.0% (95%CI: 75.6–85.6%), a PPV of 27.3% (95%CI: 19.4–36.9%), a NPV of 87.2% (95%CI: 84.4–89.5%), and a PO of 74.0% (95%CI: 68.7–78.9%) (Table 3).
3.5. Targeted Re-Excisions
Based on the recommendations of CSR and the surgeon's CI, a total of 64 re-excisions were performed on the 50 study patients. 17 patients did not require any re-excision, while 33 patients received at least one targeted re-excision. Of these, 22 (34.4%) were recommended by CSR only, 24 (37.5%) depended on CI only. Re-excisions in 18 directions (28.1%) depended on CSR as well as CI.
3.6. Final histopathological examination including targeted re-excisions
Of all 50 patients 43 had an invasive cancer in the final histopathological examination, while 7 patients had an in-situ carcinoma. Most of the invasive cancers were categorized as non-special type (NST, 35), 4 were invasive-lobular (ILC) and in 2 specimens both NST and ILC were detected. The pathological T classification and the histological tumor type are presented in Table 4.
Table 4.
Pathological data.
| Pathological T classification | Number of patientsa | |
|---|---|---|
| is | 7 (14) | |
| 1 | 30 (60) | |
| 1mi | 1 (2) | |
| 1a | 2 (4) | |
| 1b | 10 (20) | |
| 1c | 17 (34) | |
| 2 | 13 (26) | |
| Histological type | ||
| In situ | 7 (14) | |
| Invasive | 43 (86) | |
| NST | 35 (68) | ||
|---|---|---|---|
| + in situ | 28 (56) | ||
| - in situ | 7 (14) | ||
| ILC | 4 (8) | ||
| + in situ | 1 (2) | ||
| - in situ | 3 (6) | ||
| NST + ILC | 2 (4) | ||
| + in situ | 0 (0) | ||
| - in situ | 2 (4) | ||
| others | 2 (4) | ||
| + in situ | 1 (2) | ||
| - in situ | 1 (2) | ||
NST: nonspecial type; ILC: invasive lobular carcinoma.
Values are absolute frequencies. Relative frequencies are given as percentages in parentheses. Percentages are rounded.
Considering all primary re-excisions performed during the surgery, overall, 35 (11.7%) margins showed infiltration (R1) in the final histopathological examination and 4 (1.3%) margins were assessed as unclear (Rx). The margin assessment for each orientation is presented in Table 5a. Of all infiltrated margins, 23 (65.7%) were infiltrated by in-situ carcinoma, 10 (28.6%) by NST, 1 (2.9%) by ILC, and 1 (2.9%) by a micropapillary subtype. Of all unclear margins, 3 (75%) were associated to in-situ carcinoma, and 1 (25%) to NST (Table 5b). This translates to 17 (34%) patients with an infiltrated and 2 (4%) patients with an unclear margin on case level, respectively (Table 5c).
Table 5.
Histopathological margin assessment after targeted re-excisions.
| a on specimen level | |||||||
|---|---|---|---|---|---|---|---|
| medial | lateral | cranial | caudal | frontal | dorsal | total | |
| R0 | 45 | 43 | 44 | 44 | 42 | 44 | 261 (87.0%) |
| R1 | 4 (3)a | 6 (4) | 5 (1) | 5 (4) | 8 (6) | 7 (5) | 35 (11.6%) |
| Rx | 1 (0) | 1 (1) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 4 (1.3%) |
|
b on specimen level, divided according to histological subtypes | ||||||
|---|---|---|---|---|---|---|
| NST | ILC | NST + ILC | DCIS | Other | total | |
| R0 | 190 | 21 | 10 | 30 | 10 | 261 (87.0%) |
| R1 | 10 | 1 | 0 | 23 | 1 | 35 (11.6%) |
| Rx | 1 | 0 | 0 | 3 | 0 | 4 (1.3%) |
|
c on case level | |
|---|---|
| Number of patientsb | |
| R0 | 31 (62) |
| R1 | 17 (34) |
| Rx | 2 (4) |
The number of in-situ carcinoma is given in parentheses.
Values are absolute frequencies. Relative frequencies are given as percentages in parentheses. Percentages are rounded.
3.7. Margin assessment after the implementation of the Histolog®Scanner
If every evaluation as positive hypothetically resulted in a re-excision and every re-excision resulted in a clear margin, the following margin status would have been achieved after implementation of the HS in this study cohort: on average, a R0-status could be achieved in 9 additional surfaces (Table 6a). At the same time, on average 58.8 false positive assessments would have led to unnecessary resections of healthy tissue in directions were already a R0 situation was achieved in the main specimen. On a case-by-case level 4 to 8 patients would have profited from the implementation of the HS regarding the necessity of secondary re-excisions (Table 6b).
Table 6.
Margin status after implementing the Histolog® Scanner.
|
a on margin level | |||||
|---|---|---|---|---|---|
| Margin statusa | Observer 1 | Observer 2 | Observer 3 | Pathologist | |
| R0 | 261 | 273 (+12)b | 268 (+7) | 269 (+8) | 270 (+9) |
| R1 | 35 | 23 (- 12) | 29 (- 6) | 28 (- 7) | 25 (- 10) |
| Rx | 4 | 4 ( ± 0) | 3 (- 1) | 3 (- 1) | 5 (+1) |
|
b on case level | |||||
|---|---|---|---|---|---|
| Margin statusa | Observer 1 | Observer 2 | Observer 3 | Pathologist | |
| R0 | 23 | 31 (+8)b | 30 (+7) | 30 (+7) | 27 (+4) |
| R1 | 23 | 17 (- 6) | 18 (- 5) | 18 (- 5) | 19 (- 4) |
| Rx | 4 | 2 (- 2) | 2 (- 2) | 2 (- 2) | 4 ( ± 0) |
Margin status before performance of targeted re-excisions.
The changes in R-status compared to the status before performance of targeted re-excisions are given in parenthesis.
4. Discussion
Reducing the risk of local recurrence by clear resection margins while allowing preservation of as much healthy tissue as possible is the main challenge for surgeons performing BCS. Current techniques of intraoperative margin assessment still have limited efficacy since up to a third of patients undergoing BCS receives multiple surgeries [21].
In this prospective non-interventional pilot study, a novel CE-marked device for fresh tissue imaging was evaluated for the margin assessment of lumpectomies. In previous studies the HS was used and evaluated as an on-site method for assessment of core-cut biopsies in breast cancer diagnostics. In this context assessment with the HS corresponded in up to 95% of the core-cut biopsy to the histopathological examination [22]. Another single center study by Sandor et al. including 40 patients undergoing breast conserving surgery using the HS showed a sensitivity of 30.7% and 53.8% when assessments were performed by breast surgeons and a pathologist, respectively, while specificity was 85.1% and 85.2%, respectively [23]. In our study, sensitivity ranged between 33.3% and 43.8% whereas specificity ranged between 60.3% and 84.1%. Compared to CSR alone, the HS showed higher detection rate with a gain of sensitivity of 6.2%–16.7% while specificity was 2.8%–26.6% lower. In the standard workflow, the addition of CI to the CSR for margin assessment improved the detection rate. Since the HS was used retrospectively, it was not possible to assess the effect of combining it with CI. The use of the HS in combination with CI should therefore be evaluated in further studies. With a mean scanning time of 14.7 min the scanning time required may also be short enough to provide real-time feedback to the surgeon in the operating room to allow immediate decision-making regarding margin assessment. Especially when lumpectomies are combined with axillary surgery the HS can be operated while the surgeon performs sentinel lymph node biopsies or axillary dissection.
The imaging of full lumpectomy specimens with large field-of-view confocal laser scanning microscopy is an innovative approach for the margin assessment. It requires to manipulate and place the specimen above the imaging window by the user. This placement necessitates training, especially for specimens with less cubic shape to ensure that all surfaces have been imaged and maintain false negative rate as low as possible. Especially specimens with a larger size than the optical interface of the HS can be more difficult to assess since they require longer imaging time (two scans may be needed to image one margin) and are more prone to artifacts due to difficulties in specimen positioning leading to more sources of false assessments. At our center, lumpectomy is predominantly conducted with electric surgical knives, which stiffen the specimen and leave cauterization on the surfaces limiting the readability of the images. This could be one reason for the limited image quality reported by the pathologist. In addition, the workflow applied in this observational study included sending the specimen to CSR prior to confocal imaging. This results in the drying out of the surface and may have negatively impacted the overall image quality for cancer detection, as previously reported for conjunctival tumors that needed to be kept into saline solution during any waiting time prior imaging to ensure correct confocal image quality [24].
Cancer detection rates in HS could also be improved by an additional understanding of cancerous patterns in lumpectomy margins when visualized in HS images. This study was one of the first to image such margins with the HS so there was a lack of reference images. The present study has generated new images that can be used in further studies as a training material to achieve better recognition of cancer-positive margins. Due to the fresh tissue imaging and unconventional staining this might also be necessary for experienced pathologist.
Further studies are needed after adjusted training to evaluate the role of the HS in clinical routine.
5. Conclusion
The current study is the largest study to evaluate the use of the HS in lumpectomies. This device supports intraoperative margin assessment in patients undergoing BCS to reduce re-excision rates by detecting infiltrated margins on-site. The present study demonstrated feasibility of the HS in 50 consecutive cases in clinical routine. This evaluation of the HS is already showing similar detection rates for breast cancer compared to the intraoperative standard of care. This rate is expected to be improved with additional training material and further studies are required to demonstrate.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local ethics committee (Medical Faculty Heidelberg, reference number S-609/2020). Written informed consent was obtained from each patient.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Prof. Dr. Michael Golatta reports financial support was provided by SamanTree Medical SA. Prof. Michael Lux reports financial support was provided by SamanTree Medical SA.
Acknowledgement
Prof. Dr. Michael Golatta and Prof. Michael Lux received financial support by SamanTree Medical SA. Saman Tree Medical SA was funded by the European Union's Horizon 2020 research and innovation program (No 823284).
References
- 1.Rojas K., Stuckey A. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol. 2016;59:651–67210. doi: 10.1097/GRF.0000000000000239. 1097/grf.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 2.Hofvind S., Holen Å., Aas T., Roman M., Sebuødegård S., Akslen L.A. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol. 2015;41:1417–142210. doi: 10.1016/j.ejso.2015.07.002. 1016/j.ejso.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Lagendijk M., van Maaren M.C., Saadatmand S., Strobbe L.J.A., Poortmans P.M.P., Koppert L.B., Tilanus-Linthorst M.M.A., Siesling S. Breast conserving therapy and mastectomy revisited: breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer. 2018;142:165–17510. doi: 10.1002/ijc.31034. 1002/ijc.31034. [DOI] [PubMed] [Google Scholar]
- 4.Hwang E.S., Lichtensztajn D.Y., Gomez S.L., Fowble B., Clarke C.A. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013;119:1402–141110. doi: 10.1002/cncr.27795. 1002/cncr.27795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg S.M., Dominici L.S., Gelber S., Poorvu P.D., Ruddy K.J., Wong J.S., Tamimi R.M., Schapira L., Come S., Peppercorn J.M., Borges V.F., Partridge A.H. Association of breast cancer surgery with quality of life and psychosocial well-being in young breast cancer survivors. JAMA Surg. 2020;155:1035–104210. doi: 10.1001/jamasurg.2020.3325. 1001/jamasurg.2020.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen K., Pan Z., Zhu L., Hu T., Peng M., Jia W., Su F., Li S., Song E. Comparison of breast-conserving surgery and mastectomy in early breast cancer using observational data revisited: a propensity score-matched analysis. Sci China Life Sci. 2018;61:1528–153610. doi: 10.1007/s11427-018-9396-x. 1007/s11427-018-9396-x. [DOI] [PubMed] [Google Scholar]
- 7.Heil J., Holl S., Golatta M., Rauch G., Rom J., Marmé F., Gebauer G., Sohn C. Aesthetic and functional results after breast conserving surgery as correlates of quality of life measured by a German version of the Breast Cancer Treatment Outcome Scale (BCTOS) Breast. 2010;19:470–47410. doi: 10.1016/j.breast.2010.05.004. 1016/j.breast.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Foersterling E., Golatta M., Hennigs A., Schulz S., Rauch G., Schott S., Domschke C., Schuetz F., Sohn C., Heil J. Predictors of early poor aesthetic outcome after breast-conserving surgery in patients with breast cancer: initial results of a prospective cohort study at a single institution. J Surg Oncol. 2014;110:801–80610. doi: 10.1002/jso.23733. 1002/jso.23733. [DOI] [PubMed] [Google Scholar]
- 9.Kelly B.N., Kantor O., Tang R., Coopey S.B., Smith B.L., Lanahan C.R., Korotkin J.E., Specht M.C. Similar rates of residual disease in patients with DCIS within 2 mm of lumpectomy margin regardless of the presence of invasive carcinoma. Breast Cancer Res Treat. 2020 doi: 10.1007/s10549-020-06026-1. 10.1007/s10549-020-06026-110.1007/s10549-020-06026-1. [DOI] [PubMed] [Google Scholar]
- 10.Moran M.S., Schnitt S.J., Giuliano A.E., Harris J.R., Khan S.A., Horton J., Klimberg S., Chavez-MacGregor M., Freedman G., Houssami N., Johnson P.L., Morrow M. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21:704–71610. doi: 10.1245/s10434-014-3481-4. 1245/s10434-014-3481-4. [DOI] [PubMed] [Google Scholar]
- 11.Hennigs A., Fuchs V., Sinn H.P., Riedel F., Rauch G., Smetanay K., Golatta M., Domschke C., Schuetz F., Schneeweiss A., Sohn C., Heil J. Do patients after reexcision due to involved or close margins have the same risk of local recurrence as those after one-step breast-conserving surgery? Ann Surg Oncol. 2016;23:1831–183710. doi: 10.1245/s10434-015-5067-1. 1245/s10434-015-5067-1. [DOI] [PubMed] [Google Scholar]
- 12.Baliski C., Hughes L., Bakos B. Lowering Re-excision rates after breast-conserving surgery: unraveling the intersection between surgeon case volumes and techniques. Ann Surg Oncol. 2021;28:894–90110. doi: 10.1245/s10434-020-08731-z. 1245/s10434-020-08731-z. [DOI] [PubMed] [Google Scholar]
- 13.Wilke L.G., Czechura T., Wang C., Lapin B., Liederbach E., Winchester D.P., Yao K. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004-2010. JAMA Surg. 2014;149:1296–130510. doi: 10.1001/jamasurg.2014.926. 1001/jamasurg.2014.926. [DOI] [PubMed] [Google Scholar]
- 14.Rubio I.T., Esgueva-Colmenarejo A., Espinosa-Bravo M., Salazar J.P., Miranda I., Peg V. Intraoperative ultrasound-guided lumpectomy versus mammographic wire localization for breast cancer patients after neoadjuvant treatment. Ann Surg Oncol. 2016;23:38–4310. doi: 10.1245/s10434-015-4935-z. 1245/s10434-015-4935-z. [DOI] [PubMed] [Google Scholar]
- 15.Muttalib M., Tai C.C., Briant-Evans T., Maheswaran I., Livni N., Shousha S., Sinnett H.D. Intra-operative assessment of excision margins using breast imprint and scrape cytology. Breast. 2005;14:42–5010. doi: 10.1016/j.breast.2004.10.002. 1016/j.breast.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Osako T., Nishimura R., Nishiyama Y., Okumura Y., Tashima R., Nakano M., Fujisue M., Toyozumi Y., Arima N. Efficacy of intraoperative entire-circumferential frozen section analysis of lumpectomy margins during breast-conserving surgery for breast cancer. Int J Clin Oncol. 2015;20:1093–110110. doi: 10.1007/s10147-015-0827-2. 1007/s10147-015-0827-2. [DOI] [PubMed] [Google Scholar]
- 17.Thill M., Dittmer C., Baumann K., Friedrichs K., Ju Blohmer. MarginProbe®--final results of the German post-market study in breast conserving surgery of ductal carcinoma in situ. Breast. 2014;23:94–9610. doi: 10.1016/j.breast.2013.11.002. 1016/j.breast.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Schnabel F., Boolbol S.K., Gittleman M., Karni T., Tafra L., Feldman S., Police A., Friedman N.B., Karlan S., Holmes D., Willey S.C., Carmon M., Fernandez K., Akbari S., Harness J., Guerra L., Frazier T., Lane K., Simmons R.M., Estabrook A., Allweis T. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21:1589–159510. doi: 10.1245/s10434-014-3602-0. 1245/s10434-014-3602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AGO . 2016. Diagnostik und Therapie früher und fortgeschrittener Mammakarzinome. [Google Scholar]
- 20.Funk A., Heil J., Harcos A., Gomez C., Stieber A., Junkermann H., Hennigs A., Rauch G., Sinn H.P., Riedel F., Schäfgen B., Hug S., Maier A., Blumenstein M., Domschke C., Schott S., Wallwiener M., Rom J., Schütz F., Sohn C., Golatta M. Efficacy of intraoperative specimen radiography as margin assessment tool in breast conserving surgery. Breast Cancer Res Treat. 2020;179:425–43310. doi: 10.1007/s10549-019-05476-6. 1007/s10549-019-05476-6. [DOI] [PubMed] [Google Scholar]
- 21.Kuritzky A., Reyna C., McGuire K.P., Sun W., DeSnyder S.M., Aubry S., Nayyar A., Strassle P., Hunt K.K., Zhou J.M., Lee M.C. Evaluation of 2014 margin guidelines on re-excision and recurrence rates after breast conserving surgery: a multi-institution retrospective study. Breast. 2020;51:29–3310. doi: 10.1016/j.breast.2020.02.013. 1016/j.breast.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elfgen C., Papassotiropoulos B., Varga Z., Moskovszky L., Nap M., Güth U., Baege A., Amann E., Chiesa F., Tausch C. Comparative analysis of confocal microscopy on fresh breast core needle biopsies and conventional histology. Diagn Pathol. 2019;14:5810. doi: 10.1186/s13000-019-0835-z. 1186/s13000-019-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandor M.F., Schwalbach B., Hofmann V., Istrate S.E., Schuller Z., Ionescu E., Heimann S., Ragazzi M., Lux M.P. Imaging of lumpectomy surface with large field-of-view confocal laser scanning microscope for intraoperative margin assessment - POLARHIS study. Breast. 2022;66:118–12510. doi: 10.1016/j.breast.2022.10.003. 1016/j.breast.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iovieno A., Longo C., De Luca M., Piana S., Fontana L., Ragazzi M. Fluorescence confocal microscopy for ex vivo diagnosis of conjunctival tumors: a pilot study. Am J Ophthalmol. 2016;168:207–21610. doi: 10.1016/j.ajo.2016.06.001. 1016/j.ajo.2016.06.001. [DOI] [PubMed] [Google Scholar]