Abstract
Purpose
Little is known about whether sugar intake is a risk factor for myopia, and the influence of glycemic control remains unclear, with inconsistent results reported. This study aimed to clarify this uncertainty by evaluating the link between multiple glycemic traits and myopia.
Methods
We employed a two-sample Mendelian randomization (MR) design using summary statistics from independent genome-wide association studies. A total of six glycemic traits, including adiponectin, body mass index, fasting blood glucose, fasting insulin, hemoglobin A1c (HbA1c), and proinsulin levels, were used as exposures, and myopia was used as the outcome. The inverse-variance-weighted (IVW) method was the main applied analytic tool and was complemented with comprehensive sensitivity analyses.
Results
Out of the six glycemic traits studied, we found that adiponectin was significantly associated with myopia. The genetically predicted level of adiponectin was consistently negatively associated with myopia incidence: IVW (odds ratio [OR] = 0.990; P = 2.66 × 10−3), MR Egger (OR = 0.983; P = 3.47 × 10−3), weighted median method (OR = 0.989; P = 0.01), and weighted mode method (OR = 0.987; P = 0.01). Evidence from all sensitivity analyses further supported these associations. In addition, a higher HbA1c level was associated with a greater risk of myopia: IVW (OR = 1.022; P = 3.06 × 10−5).
Conclusions
Genetic evidence shows that low adiponectin levels and high HbA1c are associated with an increased risk of myopia. Given that physical activity and sugar intake are controllable variables in blood glycemia treatment, these findings provide new insights into potential strategies to delay myopia onset.
Keywords: myopia, glycemic traits, adiponectin, HbA1c, mendelian randomization, genome-wide association study, sugar control
Myopia is the most common ocular disorder, and its prevalence has dramatically increased over the past few decades.1 It is estimated that nearly half of the world population will be myopic by 2050,2 posing a major global public health concern. There is a growing need to develop novel approaches to enhance therapeutic management to delay the incidence of myopia.3 This realization emphasizes the importance of identifying risk factors for developing myopia.
Myopia is a multifactorial disease associated with genetic and environmental factors.4 Results from a few studies on the role of sugar intake in modulating the incidence of myopia vary widely.5,6 Some studies have demonstrated that hyperglycemia leads to a myopic shift.7,8 Patients with diabetes mellitus had a significantly higher prevalence of myopia than those without diabetes mellitus.7,8 Another study found no significant difference in the refractive errors of their diabetic and control groups.9,10 This discrepancy among previous studies might be due to biases or confounders inherent in observational epidemiological studies, such as small sample sizes, heterogeneity in demographic characteristics, reverse causation, and selection bias.
Mendelian randomization (MR) is an analytic method that uses reported genetic variants of exposure to estimate their causal contribution to disease outcomes of interest.11 Compared to traditional observational studies, MR is less susceptible to the influence of confounding factors or reverse causation.11,12 Here, we describe this MR study to gain new insights into the pathogenesis of myopia. For the first time, to the best of our knowledge, the causal impact of multiple glycemic traits on the risk of myopia was systematically evaluated.
Methods
Study Design
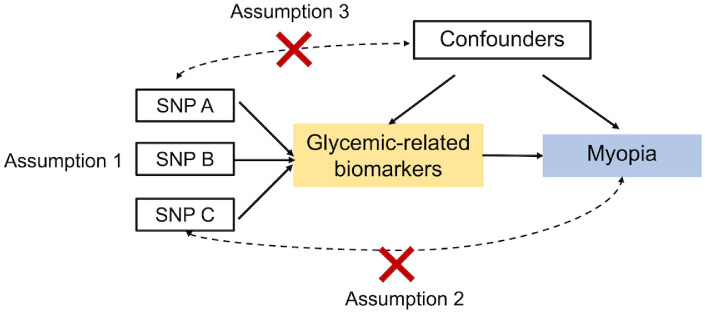
We employed a two-sample MR design to investigate the effect of exposures on myopia outcome using genome-wide association study (GWAS) summary statistics of independent studies. A total of six glycemic traits—adiponectin, body mass index (BMI), fasting blood glucose, fasting insulin, hemoglobin A1c (HbA1c), and proinsulin levels—were used as exposures, and myopia was defined as the outcome. The MR design relies on three assumptions (Fig. 1).13 First, the genetic instruments must be robustly associated with the alleged biomarker of interest. Second, the genetic instruments must be associated with the outcome only via the exposure and not via a different biological pathway independent of the exposure. Third, the genetic instruments must not be associated with any confounders of the exposure–outcome relationship. Multiple testing was performed using the Bonferroni correction, and the significant P value was <8.3 × 10−3 (= 0.05/6).
Figure 1.
Diagram of MR principles and assumptions. Assumption 1: The genetic instruments must be robustly associated with the alleged biomarker of interest. Assumption 2: The genetic instruments must be associated with the outcome only via the exposure and not via a different biological pathway independent of the exposure. Assumption 3: The genetic instruments are not associated with any confounders of the exposure–outcome relationship.
GWAS Summary Statistics for Glycemic Traits and Myopia
GWAS summary datasets of glycemic traits were obtained from the MRC Integrative Epidemiology Unit (IEU) OpenGWAS database (https://gwas.mrcieu.ac.uk/).14 Consumption of high-sugar diets is linked to obesity, insulin resistance, and hyperglycemia.15,16 Thus, hyperglycemia (HbA1c and fasting glucose), insulin resistance (fasting insulin levels and adiponectin), β-cell dysfunction (fasting proinsulin levels), and the obesity-related trait BMI were selected as components of glycemic traits. These six glycemic traits from different GWASs were analyzed as exposures in this study, including adiponectin (dataset ieu-a-1; n = 39,883),17 BMI (ebi-a-GCST006368; n = 315,347),18 fasting blood glucose (ebi-a-GCST005186; n = 58,074),19 fasting insulin (ieu-b-116; n = 108,557),20 HbA1c (ieu-b-104; n = 46,368),21 and proinsulin levels (ebi-a-GCST001212; n = 10,701).15 Detailed information about each trait is summarized in Table 1. Myopia data integrated by the MRC IEU (ukb-b-6353; “Phenotype: Reason for glasses/contact lenses: For short-sightedness, i.e., only or mainly for distance viewing such as driving, cinema etc, [called ‘myopia’]”) was used as an outcome, with a total of 460,536 participants of European ethnicity. We selected only genetic variants with genome-wide significance (P < 5 × 10−8) for MR analysis.
Table 1.
Description of GWAS Summary Statistics for Glycemic Traits
| Traits | Accession Number* | Sample Sizes | Number of SNPs | Population Ethnicity | Study |
|---|---|---|---|---|---|
| Adiponectin | ieu-a-1 | 39,883 | 2,675,209 | Mixed | Dastani et al.17 |
| BMI | ebi-a-GCST006368 | 315,347 | 27,854,527 | European | Hoffmann et al.18 |
| Fasting blood glucose | ebi-a-GCST005186 | 58,074 | 2,599,409 | European | Manning et al.19 |
| Fasting insulin | ieu-b-116 | 108,557 | 64,421 | European | Scott et al.20 |
| HbA1c | ieu-b-104 | 46,368 | 2,529,804 | European | Soranzo et al.21 |
| Proinsulin levels | ebi-a-GCST001212 | 10,701 | 2,479,861 | European | Strawbridge et al.15 |
GWAS summary datasets of glycemic traits are from the MRC IEU OpenGWAS database (https://gwas.mrcieu.ac.uk/).
MR Analysis and Sensitivity Analysis
MR analysis between the exposures (adiponectin, BMI, fasting blood glucose, fasting insulin, HbA1c, and proinsulin levels) and myopia was performed using the TwoSampleMR v0.5.5 R package (R Foundation for Statistical Computing, Vienna, Austria).22 The following standards were applied in the selection of genetic instruments for each glycemic trait: (1) P < 5 × 10−8 for each glycemic trait; (2) linkage disequilibrium r2 < 0.001; and (3) linkage disequilibrium distance > 10,000 kb. In this study, the inverse-variance weighted (IVW) method23 was the main method used to estimate associations between glycemic traits and myopia. For sensitivity analysis, three additional approaches based on the TwoSampleMR R package were used, including MR-Egger regression,24 weighted median method,25 and weighted mode method,22 which allowed for the presence of horizontal pleiotropy. Therefore, MR results were considered meaningful if the IVW method identified an association (P < 0.0083) and all four MR methods had effects in the same direction.
To further assess the robustness of these identified associations, the impact was assessed for potential horizontal pleiotropy; comprehensive sensitivity using the MR pleiotropy residual sum and outlier (MR-PRESSO) test,26 Egger intercept calculation,24 leave-one-out analysis,22 heterogeneity tests,27 and the Steiger test.28
Results
Effect of Adiponectin on Myopia
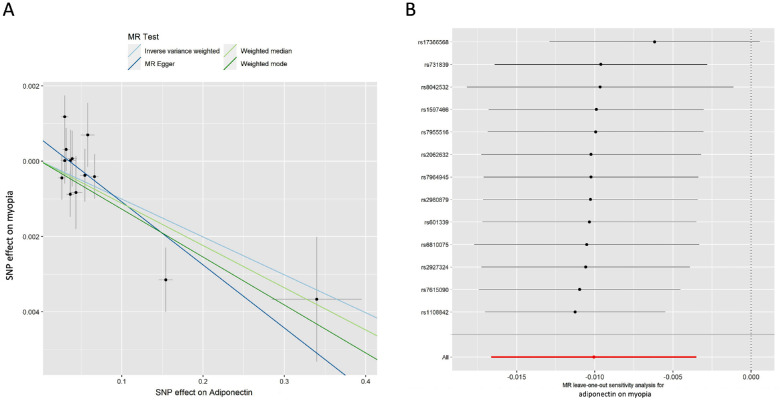
Out of the six glycemic traits, the genetically predicted level of adiponectin was found to be significantly and inversely associated with the incidence of myopia (Table 2): IVW (odds ratio [OR] = 0.990; 95% confidence interval [CI], 0.984–0.997; P = 2.66 × 10−3), MR Egger (OR = 0.983; 95% CI, 0.975–0.992; P = 3.47 × 10–3), weighted median method (OR = 0.989; 95% CI, 0.980–0.997; P = 0.01), and weighted mode method (OR = 0.987; 95% CI, 0.979–0.996; P = 0.01). The consistent direction of the adiponectin level impact suggests that it has a protective role against myopia (Fig. 2A). Figure 2A shows scatterplots of the MR analyses revealing the effect sizes of associations between adiponectin and myopia. Results from IVW and MR Egger remained significant even after Bonferroni correction (P < 0.0083).
Table 2.
Effect of Glycemic Traits on Myopia
| Exposures | SNPs | Methods | OR | 95% CI | P * |
|---|---|---|---|---|---|
| Adiponectin | 13 | Inverse variance weighted | 0.990 | 0.984–0.997 | 2.66 × 10−3 |
| MR Egger | 0.983 | 0.975–0.992 | 3.47 × 10−3 | ||
| Weighted median | 0.989 | 0.980–0.997 | 0.01 | ||
| Weighted mode | 0.987 | 0.979–0.996 | 0.01 | ||
| BMI | 145 | Inverse variance weighted | 0.987 | 0.980–0.994 | 5.98 × 10−4 |
| MR Egger | 1.009 | 0.988–1.031 | 0.36 | ||
| Weighted median | 0.992 | 0.984–1.001 | 0.08 | ||
| Weighted mode | 0.992 | 0.970–1.014 | 0.51 | ||
| Fasting blood glucose | 22 | Inverse variance weighted | 1.007 | 0.995–1.019 | 0.28 |
| MR Egger | 0.986 | 0.963–1.009 | 0.25 | ||
| Weighted median | 1.007 | 0.995–1.019 | 0.24 | ||
| Weighted mode | 0.998 | 0.987–1.009 | 0.73 | ||
| Fasting insulin | 14 | Inverse variance weighted | 1.007 | 0.971–1.044 | 0.70 |
| MR Egger | 0.810 | 0.690–0.951 | 0.02 | ||
| Weighted median | 1.015 | 0.979–1.052 | 0.43 | ||
| Weighted mode | 1.068 | 0.995–1.147 | 0.09 | ||
| HbA1c | 213 | Inverse variance weighted | 1.022 | 1.011–1.032 | 3.06 × 10−5 |
| MR Egger | 1.033 | 1.002–1.066 | 0.04 | ||
| Weighted median | 1.012 | 1.001–1.024 | 0.03 | ||
| Weighted mode | 1.008 | 0.981–1.036 | 0.57 | ||
| Proinsulin levels | 8 | Inverse variance weighted | 1.006 | 0.996–1.015 | 0.24 |
| MR Egger | 0.995 | 0.974–1.018 | 0.69 | ||
| Weighted median | 1.005 | 0.996–1.013 | 0.28 | ||
| Weighted mode | 0.999 | 0.990–1.010 | 0.99 |
Significance was defined as P < 8.3 × 10−3.
Figure 2.
MR analysis and leave-one-out analysis of the causal effect of adiponectin on myopia. (A) Scatterplots for MR analyses of the causal effect of adiponectin on myopia. The slope of each line corresponds to the estimated MR effect per method. (B) Leave-one-out analysis of the causal effect of adiponectin level on myopia. Each black point represents the IVW MR method applied to estimate the causal effect of adiponectin level on myopia, excluding that particular variant from the analysis. The red point represents the IVW estimate using all SNPs.
To assess the robustness of the causal association between adiponectin levels and myopia, we next conducted comprehensive sensitivity analyses. The Egger intercept was close to zero (intercept < 0.001) with P > 0.05, indicating no evidence of directional horizontal pleiotropy effects. Additionally, the MR-PRESSO test demonstrated that no horizontal pleiotropic outliers were distorting these results, with global test P > 0.05 (P = 0.225). The heterogeneity test also confirmed a lack of significant heterogeneities for both IVW and MR Egger models (P > 0.05). Leave-one-out analysis was also performed, and no outlier was observed (Fig. 2B). We also tested the reverse model by estimating the effect of myopia on adiponectin levels. Notably, no associations were observed (Supplementary Table S1). To calculate the explained variance of instrumented single nucleotide polymorphisms (SNPs) on exposure and outcome, we conducted the Steiger test on each SNP. Remarkably, our findings revealed that the variance in exposure was significantly greater than in the outcome (Supplementary Table S2). Taken together, these results corroborate a causal link between low adiponectin and the incidence of myopia.
Effect of HbA1c on Myopia
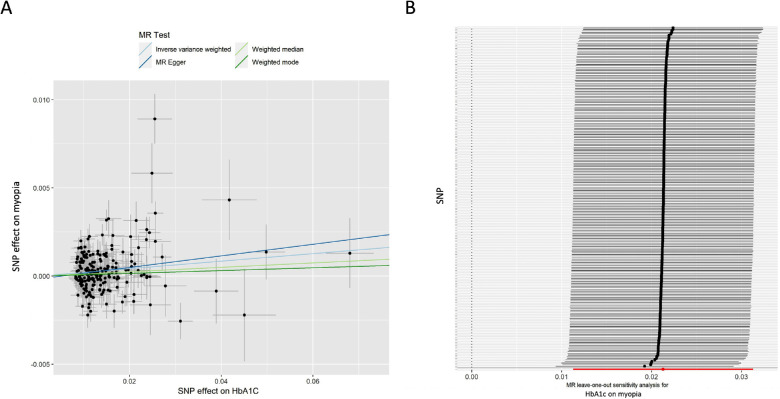
In addition, associations were observed between the HbA1c level and myopia. The IVW approach showed that higher HbA1c levels were strongly associated with an increased risk of myopia (OR = 1.022; 95% CI, 1.011–1.032; P = 3.06 × 10−5) (Table 2, Fig. 3A). Consistent MR analysis results were found using the other three MR methods, including MR Egger (OR = 1.033; 95% CI, 1.002–1.066; P = 0.04), weighted median (OR = 1.012; 95% CI, 1.001–1.024; P = 0.03), and weighted mode (OR = 1.008; 95% CI, 0.981–1.036; P = 0.57).
Figure 3.
MR analysis and leave-one-out analysis of the causal effect of HbA1c on myopia. (A) Scatterplots for MR analyses of the causal effect of HbA1c level on myopia. The slope of each line corresponds to the estimated MR effect per method. (B) Leave-one-out analysis of the causal effect of HbA1c level on myopia. Each black point represents the IVW MR method applied to estimate the causal effect of HbA1c level on myopia, excluding that particular variant from the analysis. The red point represents the IVW estimate using all SNPs. Detailed information on SNPs is displayed in Supplementary Table S4.
Leave-one-out analysis showed no outliers (Fig. 3B). Calculation of the Egger intercept suggested no evidence of directional horizontal pleiotropy effects (intercept < 0.001; P > 0.05). The MR-PRESSO test showed that the P values of raw MR analyses and outlier-corrected MR analyses were 4.46 × 10−5 and 7.40 × 10−4, respectively. The distortion test showed no significant difference (P = 0.097). The heterogeneity test indicated the presence of heterogeneities (P < 0.05). Thus, we performed IVW analysis with a multiplicative random effects model, which also showed a strong association (OR = 1.021; 95% CI, 1.011–1.031; P = 2.62 × 10−5). Analysis of the reverse model by estimating the effect of myopia on HbA1c revealed no associations (Supplementary Table S1). The Steiger test was performed for each SNP, and the results revealed that the variance in the exposure was significantly greater than in the myopia outcome (Supplementary Table S3). Altogether, our initial results are supported by further evidence from all sensitivity analyses. According to a strict standard (IVW P < 8.3 × 10−3 and all four MR approaches should show consistent directions), no significant associations were observed between any other glycemic traits and myopia.
Discussion
Myopia is a multifactorial condition that involves both genetic and environmental factors. However, little is known about whether sugar intake is a risk factor for myopia, which is complicated by a lack of agreement among the findings of different studies. Our study used the MR method and provided evidence to support a causal relationship between lower adiponectin levels and a higher risk of myopia. Moreover, our findings suggest that higher levels of HbA1c increase the risk of myopia.
Metabolic syndrome, a cluster of metabolic abnormalities that includes obesity, hyperglycemia, hypertension, and dyslipidemia, has become more common in children and adolescents in recent years due to less physically demanding lifestyles and increased caloric intake.29 Adiponectin, a protein hormone primarily produced and secreted by adipocytes, plays an essential role in modulating serum glucose and lipid metabolism,30 which is negatively correlated with insulin resistance, obesity, type 2 diabetes, and cardiovascular diseases.31,32 Notably, our current results suggest that adiponectin is a protective factor for myopia, suggesting that increasing adiponectin levels reduces the risk of myopia. In this study, we found that higher levels of HbA1c were associated with an increased risk of myopia, but there was no significant effect of fasting blood glucose levels. The discordant relationship between genetically predicted HbA1c levels and fasting glucose levels has been reported in a previous study.33 It could be partly explained by two reasons. First, HbA1c can reflect the average glucose levels over the preceding 2 to 3 months.34 Previous studies have shown a significant discordance in the diagnosis of diabetes between HbA1c and fasting blood glucose.35,36 Second, valid genetic instruments for HbA1c levels (n = 213) are as much as 10 times higher than fasting glucose levels (n = 22). These findings suggest that, in clinical practice, a strict glycemic control strategy that encompasses regular exercise and a healthy diet is necessary to reduce the risk of myopia.
These intriguing results prompt questions about how and why levels of adiponectin and chronic blood glycemia affect myopia. Some considered possibilities include a myopic shift due to a change in the refractive index of the crystalline lens; however, such a condition can only explain transient refraction shifts.37 Here, we speculate at a theoretical level that the myopic shift is influenced by the choroidal blood perfusion. Jo et al.38 demonstrated that choroid thickness is significantly increased after intensive diabetes control (from 226 ± 56 μm to 215 ± 52 μm; P < 0.05), indicating that HbA1c level negatively correlates with choroidal thickness. Such an increase in choroidal thickness has been linked to increased choroidal blood flow and oxygenation of the adjacent scleral tissue in guinea pigs.39,40
This study focuses primarily on myopia and provides additional insights into the causal roles of serum biomarkers, particularly adiponectin. One major strength of this study is that we used the MR method to evaluate large-scale datasets with standard protocols. Compared with traditional studies, MR methodology is less likely to be affected by confounding factors or biases from reverse causation. However, some limitations of the study should be noted. First, as this study was based on participants of European ancestry, the degree to which our findings can be generalized to other ethnic groups requires further investigation. Second, this study was based on a cross-sectional design; therefore, long-term follow-up studies are needed to understand the impact of hyperglycemia and low adiponectin level on myopia risk and progression over time. Third, the effects of blood sugar control on the incidence of myopia have not yet been experimentally studied, and further laboratory investigation is needed. Nevertheless, our study shows that some biomarkers of diabetes can have predictive value in assessing the possibility of myopia onset, which has important research and clinical implications.
In conclusion, our study identified a causal link between low adiponectin levels and an increased risk of myopia. Moreover, higher HbA1c levels also increase the risk of myopia. These observations, applied in clinical practice, suggest that improved myopia control may be achieved by implementing a strict glycemic control strategy that includes regular exercise and a healthy diet.
Supplementary Material
Acknowledgments
The authors thank Peter Reinach, PhD, College of Optometry, State University of New York, for his help in editing and his guidance in improving our manuscript. All of the data generated are presented in this article, and the data analyzed as part of this study can be accessed through the data repositories listed in the references.
Supported by grants from the National Natural Science Foundation of China (82201229) and the Major Science and Technology Special Project of Wenzhou (2018ZY018).
Disclosure: F.-F. Li, None; M.-C. Zhu, None; Y.-L. Shao, None; F. Lu, None; Q.-Y. Yi, None; X.-F. Huang, None
References
- 1. Yam JC, Tang SM, Kam KW, et al.. High prevalence of myopia in children and their parents in Hong Kong Chinese Population: the Hong Kong Children Eye Study. Acta Ophthalmol. 2020; 98(5): e639–e648. [DOI] [PubMed] [Google Scholar]
- 2. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123(5): 1036–1042. [DOI] [PubMed] [Google Scholar]
- 3. Morgan IG, Wu P-C, Ostrin LA, et al.. IMI risk factors for myopia. Invest Ophthalmol Vis Sci. 2021; 62(5): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morgan IG, Ohno-Matsui K, Saw S-M. Myopia. Lancet. 2012; 379(9827): 1739–1748. [DOI] [PubMed] [Google Scholar]
- 5. Lim LS, Gazzard G, Low Y-L, et al.. Dietary factors, myopia, and axial dimensions in children. Ophthalmology. 2010; 117(5): 993–997.e4. [DOI] [PubMed] [Google Scholar]
- 6. Li M, Tan C-S, Foo L-L, et al.. Dietary intake and associations with myopia in Singapore children. Ophthalmic Physiol Opt. 2022; 42(2): 319–326. [DOI] [PubMed] [Google Scholar]
- 7. Rani PK, Raman R, Rachapalli SR, Kulothungan V, Kumaramanickavel G, Sharma T. Prevalence of refractive errors and associated risk factors in subjects with type 2 diabetes mellitus SN-DREAMS, report 18. Ophthalmology. 2010; 117(6): 1155–1162. [DOI] [PubMed] [Google Scholar]
- 8. Lin HT, Zheng C-M, Fang Y-A, et al.. Prevalence and risk factors for myopia in Taiwanese diabetes mellitus patients: a multicenter case-control study in Taiwan. Sci Rep. 2021; 11(1): 8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adnan X, Suheimat M, Efron N, et al.. Biometry of eyes in type 1 diabetes. Biomed Opt Express. 2015; 6(3): 702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geloneck MM, Forbes BJ, Shaffer J, Ying G-S, Binenbaum G. Ocular complications in children with diabetes mellitus. Ophthalmology. 2015; 122(12): 2457–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018; 362: k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pingault JB, O'Reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, Dudbridge F. Using genetic data to strengthen causal inference in observational research. Nat Rev Genet. 2018; 19(9): 566–580. [DOI] [PubMed] [Google Scholar]
- 13. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008; 27(8): 1133–1163. [DOI] [PubMed] [Google Scholar]
- 14. Elsworth B, Lyon M, Alexander T, et al.. The MRC IEU OpenGWAS data infrastructure. Available at: https://www.biorxiv.org/content/10.1101/2020.08.10.244293v1. Accessed February 17, 2023.
- 15. Strawbridge RJ, Dupuis J, Prokopenko I, et al.. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011; 60(10): 2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morimoto RI, Cuervo AM. Proteostasis and the aging proteome in health and disease. J Gerontol A Biol Sci Med Sci. 2014; 69(suppl 1): S33–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dastani Z, Hivert M-F, Timpson N, et al.. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012; 8(3): e1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann TJ, Choquet H, Yin J, et al.. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics. 2018; 210(2): 499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manning AK, Hivert M-F, Scott RA, et al.. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012; 44(6): 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scott RA, Lagou V, Welch RP, et al.. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012; 44(9): 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soranzo N, Sanna S, Wheeler E, et al.. Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010; 59(12): 3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hemani G, Zheng J, Elsworth B, et al.. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018; 7: e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013; 37(7): 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015; 44(2): 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016; 40(4): 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018; 50(8): 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corlin L, Ruan M, Tsilidis KK, et al.. Two-sample Mendelian randomization analysis of associations between periodontal disease and risk of cancer. JNCI Cancer Spectr. 2021; 5(3): pkab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017; 13(11): e1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017; 92(2): 251–265. [DOI] [PubMed] [Google Scholar]
- 30. Yamauchi T, Minokoshi KY, Ito Y, et al.. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8(11): 1288–1295. [DOI] [PubMed] [Google Scholar]
- 31. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006; 116(7): 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nielsen MB, Çolak Y, Benn M, Nordestgaard BG. Low plasma adiponectin in risk of type 2 diabetes: observational analysis and one- and two-sample Mendelian randomization analyses in 756,219 individuals. Diabetes. 2021; 70(11): 2694–2705. [DOI] [PubMed] [Google Scholar]
- 33. Georgakis MK, Harshfield EL, Malik R, et al.. Diabetes mellitus, glycemic traits, and cerebrovascular disease: a Mendelian randomization study. Neurology. 2021; 96(13): e1732–e1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldstein DE, Little RR, Lorenz RA, et al.. Tests of glycemia in diabetes. Diabetes Care. 2004; 27(7): 176–173. [DOI] [PubMed] [Google Scholar]
- 35. Ho-Pham LT, Nguyen UDT, Tran TX, Nguyen TV. Discordance in the diagnosis of diabetes: comparison between HbA1c and fasting plasma glucose. PLoS One. 2017; 12(8): e0182192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abdul Murad NA, Abdullah N, Kamaruddin MA, et al.. Discordance between fasting plasma glucose (FPG) and HbA1c in diagnosing diabetes and pre-diabetes in the Malaysian cohort. J ASEAN Fed Endocr Soc. 2021; 36(2): 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feldman-Billard S, Dupas B. Eye disorders other than diabetic retinopathy in patients with diabetes. Diabetes Metab. 2021; 47(6): 101279. [DOI] [PubMed] [Google Scholar]
- 38. Jo Y, Ikuno Y, Iwamoto R, Okita K, Nishida K. Choroidal thickness changes after diabetes type 2 and blood pressure control in a hospitalized situation. Retina. 2014; 34(6): 1190–1198. [DOI] [PubMed] [Google Scholar]
- 39. Zhang S, Zhang G, Zhou X, et al.. Changes in choroidal thickness and choroidal blood perfusion in guinea pig myopia. Invest Ophthalmol Vis Sci. 2019; 60(8): 3074–3083. [DOI] [PubMed] [Google Scholar]
- 40. Zhou X, Zhang S, Zhang G, et al.. Increased choroidal blood perfusion can inhibit form deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2020; 61(13): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.