Summary
In November 2021, Omicron BA.1, containing a raft of new spike mutations, emerged and quickly spread globally. Intense selection pressure to escape the antibody response produced by vaccines or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection then led to a rapid succession of Omicron sub-lineages with waves of BA.2 and then BA.4/5 infection. Recently, many variants have emerged such as BQ.1 and XBB, which carry up to 8 additional receptor-binding domain (RBD) amino acid substitutions compared with BA.2. We describe a panel of 25 potent monoclonal antibodies (mAbs) generated from vaccinees suffering BA.2 breakthrough infections. Epitope mapping shows potent mAb binding shifting to 3 clusters, 2 corresponding to early-pandemic binding hotspots. The RBD mutations in recent variants map close to these binding sites and knock out or severely knock down neutralization activity of all but 1 potent mAb. This recent mAb escape corresponds with large falls in neutralization titer of vaccine or BA.1, BA.2, or BA.4/5 immune serum.
Keywords: SARS-CoV-2, BA.2, variant, mutation, RBD, antibodies, binding site, breakthrough, neutralizing, structure, COVID-19
Graphical abstract
Highlights
-
•
25 potent RBD mAbs generated from vaccinees suffering BA.2 breakthrough infections
-
•
Potent mAbs bind 3 clusters, 2 correspond to early-pandemic binding hotspots
-
•
RBD mutations in recent variants map close to these binding sites
-
•
Neutralization activity of all but 1 potent mAb is reduced
Dijokaite-Guraliuc et al. analyze potently neutralizing antibodies from vaccinated individuals with BA.2 breakthrough infections. The antibodies bind 3 sites on the receptor-binding domain, 2 of which are in common with early-pandemic antibodies. Mutations in more recent variants map closely to these sites, leading to reduced neutralization in all but one mAb.
Introduction
There have been billions of infections and millions of deaths since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan in late 2019. Since its zoonotic jump, SARS-CoV-2 has been under intense selective pressure to adapt to its new human host and evade the immune response.1 The SARS-CoV-2 RNA polymerase is intrinsically error prone, and it has been estimated that every single-nucleotide change in the SARS-CoV-2 RNA genome will be generated in an infected individual every day.2 Large-scale genomic sequencing efforts in a number of countries have charted progressive evolution of SARS-CoV-2 (https://www.cogconsortium.uk; https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html).
The spike protein (S) mediates cell entry by binding the angiotensin-converting enzyme 2 (ACE2) cellular receptor,3 and many neutralizing antibodies bind S.4,5,6,7 Mutations in S can therefore impart two important selective advantages: firstly, mutations to the ACE2-binding surface in the receptor-binding domain (RBD) can increase affinity for ACE2, potentially increasing viral transmissibility.8,9,10,11 Secondly, mutations at the site of interaction of neutralizing antibody on S may reduce neutralizing titers, allowing reinfection or breakthrough of vaccines.12 Structure function analyses of panels of human monoclonal antibodies (mAbs) generated from convalescent SARS-CoV-2 cases have been performed by a number of laboratories, giving insight into the mechanisms of viral neutralization.4,6,7,13,14 Most potently neutralizing antibodies bind on, or near, the ACE2 interaction surface on the RBD to prevent interaction with ACE2. A second group, exemplified by mAb S309, binds close to the N-linked glycan at residue 343, does not block interaction with ACE2, and may destabilize the S trimer.15 Note that bebtelovimab (Ly-CoV1404) competes with S309 yet blocks ACE2 binding.16 A third site of binding of potent mAbs is to the N-terminal domain (NTD) in S15,17; these mAbs do not antagonize ACE2 binding, and the mechanism of neutralization is not yet clear, although steric hindrance of ACE2 interaction at the cell surface has been proposed.5 A total of five epitopes were defined for early-pandemic RBD-binding mAbs based on a torso analogy and were defined as left shoulder, right shoulder, neck, right flank, and left flank, with the latter two corresponding to less potently neutralizing antibodies.4
Early during the pandemic, when population immunity was low, S mutations such as N501Y in Alpha, which increases affinity for ACE2,10 were likely driven by selection for increased transmissibility,18 while those occurring on the background of strong herd immunity from vaccines and infection are likely selected for their ability to escape the neutralizing antibody response. SARS-CoV-2 executed a large sequence jump to Omicron BA.1, which carries 30 substitutions plus the deletions of 6 and an insertion of 3 residues compared with the ancestral Wuhan spike sequence, leading to a large antigenic distance from preceding strains.13,19 Most mutations were clustered in the RBD and NTD and led to large falls or knockout of neutralizing titers of sera from vaccinees or following SARS-CoV-2 infection.12 In addition, neutralizing activity of several therapeutic mAbs was reduced or knocked out against BA.112,20,21.
Omicron emerged as at least 3 distinct lineages in November 2021. BA.1 caused the first Omicron wave, closely followed by a combination of BA.1 sub-lineages BA.1.1 and BA.2 in early 2021 (https://cov-lineages.org/lineage_list, https://nextstrain.org/nextclade/sars-cov-2).22 BA.2 became globally dominant and continues to spawn a succession of variants: first BA.2.12.1,23 followed by BA.4 and BA.5,24 with BA.5 being the most widespread strain globally in June 2022 (https://covspectrum.org/explore/United%20Kingdom/AllSamples/Past6M).
Following BA.5, several new trends were observed in the evolution of Omicron: (1) the emergence of “second-generation” BA.2 variants (including derivatives of BA.5)—variants with long phylogenetic branch lengths, multiple antigenic mutations, and a lack of genetic intermediates, for example BA.2.75, BJ.1, BS.1, BA.2.10.4, and BA.2.3.20,19 and (2) antigenic drift, seen both in BA.524 and within these second-generation BA.2 lineages, notably BQ.1 and BA.2.75 (https://nextstrain.org/nextclade/sars-cov-2/21L) (Figures 1A–1C). Finally, recombination between two of these second-generation variants (BJ.1 and BM.1.1.1) has produced XBB (Figure 1C). Many of these variants show a large degree of convergent evolution in known antigenic RBD residues, and mutations lie in areas that may threaten the binding of neutralizing antibodies, leading to escape from protection afforded by vaccine or previous SARS-CoV-2 infection, including prior Omicron infection. These second-generation BA.2 variants are now dominant globally, with BQ.1 alone accounting for 50% of infections as of December 27, 2022 (https://cov-spectrum.org/explore/World/AllSamples/Past6M) and XBB.1.5 (XBB.1 + F486P currently expanding rapidly in North America; https://cov-spectrum.org/explore/North%20America/AllSamples/Past6M).
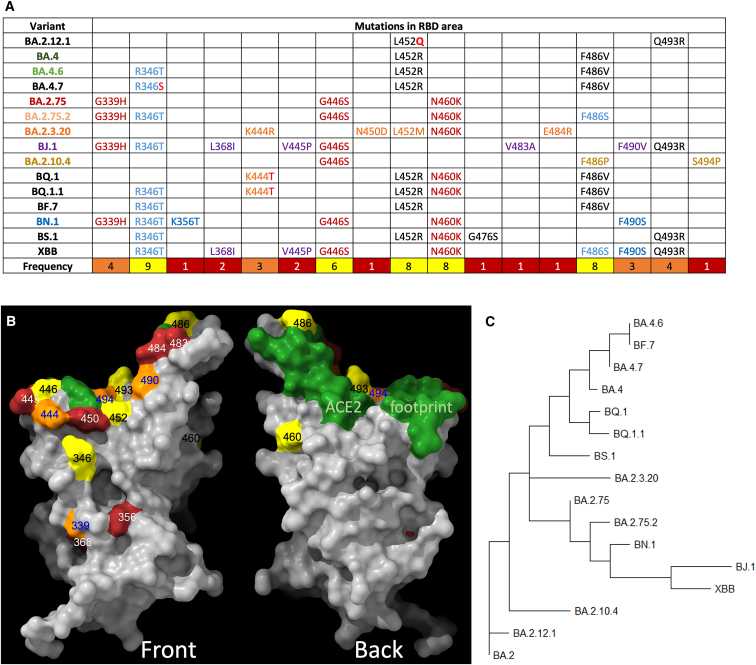
Figure 1.
Phylogeny of BA.2 sub-variants
(A) Alignments of mutated RBD amino acid substitutions; these are mutations formed on the BA.2 background, i.e., they are in addition to mutations found in BA.2.
(B) Amino acid substitutions present in sub-variants positioned on the RBD surface. Coloring is according to the frequency count for the change in the various sub-variants, shown in (A). The ACE2 footprint is shown in green.
(C) Phylogenetic tree of a selection of BA.2/4/5 sub-lineages.
In this report, we generate a panel of 25 potent mAbs (IC50 < 100 ng/mL) from vaccinees suffering breakthrough Omicron BA.2 infections. We have determined structures for complexes of four of these antibodies by X-ray crystallography and cryoelectron microscopy (cryo-EM) analysis. To generalize the structural information to all 25 antibodies, we used biolayer interferometry (BLI) competition measurements and prior structures to create an interaction map of the antibody panel; potent mAbs bind to three distinct patches on the RBD, one of which is quite extended and reaches between two of the five epitopes seen in early-pandemic responses, while the other two correspond to diminished responses at hotspots for potent antibody binding in early-pandemic responses. This corresponds to a refocusing of the response, similar to that seen for BA.1.13 The positions of mutations in the new BA.2 sub-lineages strongly overlap with the positions of antibody binding.25 Pseudoviral neutralization assays confirmed knockout or severe reduction in neutralization titers for all but one of the antibody panels by one or more variants. There were also large falls in serum neutralization titers from vaccinees or from Omicron-infected individuals. Finally, all BA.2 mAbs showed potent neutralization of an early-pandemic SARS-CoV-2 strain and are likely derived from the memory B cell response to vaccination. Reliance on the recall response may over time narrow the polyclonality of neutralizing antibodies causing increased focusing on a smaller number of epitopes.
Results
Emerging BA.2, BA.4, and BA.5 sub-lineages
At present, a number of lineages are growing rapidly from within both the BA.2 and BA.5 branches. Most striking is the large degree of convergent evolution, particularly at antigenic RBD positions such as 346, 444, 452, 460, 486, 490, 493, and 494. These lineages include examples from the BA.4/5 branches (which contain L452R, F486V, and the reversion R493Q), such as BA.4.6 and BF.7 (R346T); BA.4.7 (R346S); BQ.1 (K444T, N460K); and BQ.1.1 (R346T, K444T, N460K) and from the BA.2.75 branch (which contains G339H, G446S, N460K, and the reversion R493Q), such as BA.2.75.2 (R346T and F486S and BA.2.75 mutations); BN.1 (also known as [aka] BA.2.75.5.1 with R346T, K356T, F490S, and BA.2.75 mutations); and BM.1.1.1 (aka BA.2.75.3.1.1.1 with R346T, F486S, F490S, and BA.2.75 mutations). There are also examples of several other second-generation BA.2 variant lines such as BJ.1 (aka BA.2.10.1.1; G339H, R346T, L368I, V445P, G446S, V483A, and F490V); BA.2.10.4 (G446S, F486P, S494P, and the R493Q reversion); BS.1 (aka BA.2.3.2.1; R346T, L452R, N460K, G476S, and the Q493R reversion); BA.2.3.20 (K444R, N450D, L452M, N460K, E484R, and the Q493R reversion); and finally a BJ.1 × BM.1.1.1 (aka BA.2.75.3.1.1.1) recombinant, XBB (which, relative to BA.2, contains R346T, L368I, V445P, G446S, N460K, F486S, F490S, and the Q493R reversion). These sequence changes are compiled in Figure 1A and Table S1, and the distribution of the mutations on the surface of the RBD is shown in Figure 1B. The rooted phylogenetic tree26 (https://nextstrain.org/nextclade/sars-cov-2) shown in Figure 1C provides a helpful grouping of the lineages.
Outside the RBD, the degree of convergent evolution is lesser but still present. Many of the second-generation BA.2 variant lineages contain deletions or mutations in the NTD, often similar to those seen in the variants of concern (VOC), for example Δ∼144 in BJ.1, BS.1, and BA.2.10.4 (previously seen in Alpha and BA.1) and NSP12 G671S in BJ.1, XBB, and BA.2.10.4 (previously seen in Delta).
Generation of mAbs from vaccinees suffering BA.2 breakthrough infections
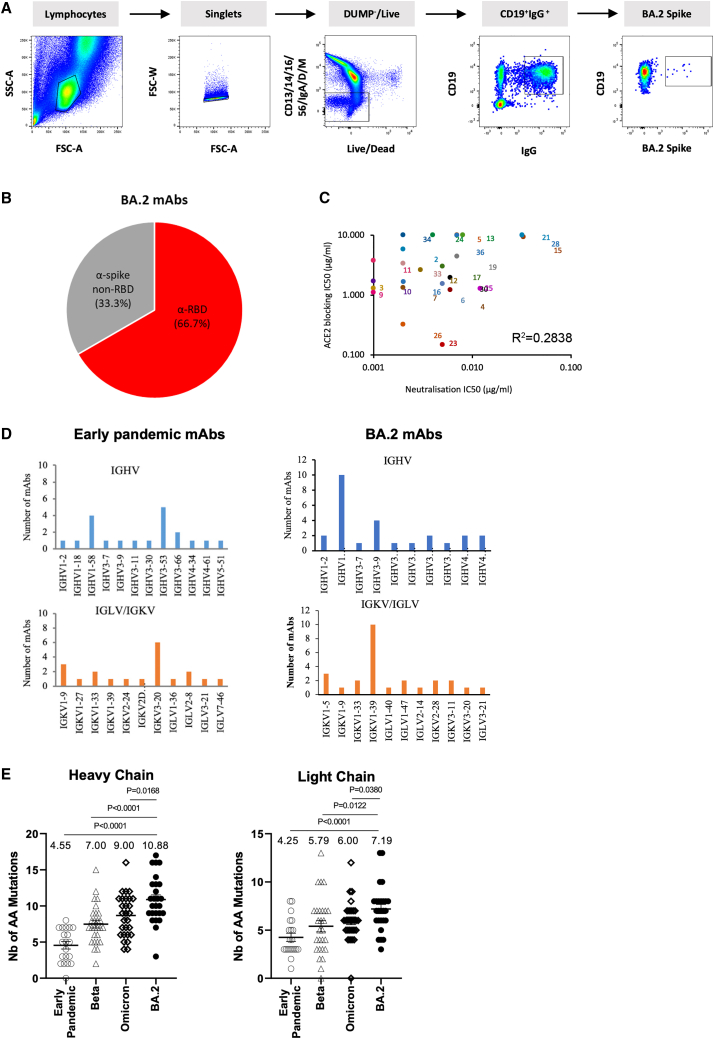
Blood was obtained from 7 volunteers, all triple vaccinated >24 days (median 29) from PCR- (n = 1) or lateral flow test-confirmed (n = 6) SARS-CoV-2 infection, during the BA.2 wave in April 2022 (https://cov-spectrum.org/explore/United%20Kingdom/AllSamples/from=2022-04-01&to=2022-04-30/variants?&). To select samples for antibody production, we first performed focus reduction neutralization assays (FRNTs) on BA.2 virus. Three samples with the highest titers were selected for mAb production, and single immunoglobulin G (IgG)-positive B cells were stained and sorted using BA.2 S trimer (Figure 2A). Following a degenerate PCR reaction, heavy and light chains (HC and LCs) were assembled into an expression vector using Gibson assembly, and the products were expressed by transient transfection. Supernatants were tested for reactivity to full-length BA.2 S or BA.2 RBD; in common with the panel of mAbs generated following Omicron BA.1 infection,13 a high proportion (67%) of mAbs recognized the BA.2 RBD (Figure 2B). Supernatants were also tested in BA.2 neutralization assays, and those showing potent neutralization (FRNT50 < 100 ng/mL) were selected for further study. From 672 sorted cells, 383 antibodies were recovered, leading to the generation of 25 potent mAbs.
Figure 2.
Generation of BA.2 mAbs
(A) Sorting of BA.2-specific B cells.
(B) Proportion of BA.2 mAbs binding to RBD.
(C) ACE2 receptor blocking activity of mAbs.
(D) Gene usage in 25 potent BA.2 mAbs compared with potent antibodies produced following infection with early-pandemic virus, which have been previously reported.4
(E) Number of somatic mutations found in BA.2 mAbs compared with sets previously recovered from early-pandemic, Beta, and Omicron (BA.1) infections, which have been reported previously.4,13,14
We tested the ability of mAbs to block ACE2 interaction with S (Figure 2C); a number of mAbs (BA.2–5, 13, 15, 21, 24, 34, 36) showed little or no ACE2-blocking function. The BA.2 mAbs are somewhat less able to inhibit ACE2 binding than similarly potent early-pandemic mAbs.4 Heavy- and light-chain gene usage is shown, compared with the gene usage we found in mAbs generated from early-pandemic infection, in Figure 2D and Table S2. 10/25 mAbs belonged to the IGHV1-69 gene family, a further expansion from that found within the Omicron BA.1 panel of mAbs (6/28) previously generated.13 The public gene family IGHV3-53/66, which has been found on multiple occasions in SARS-CoV-2, was less prominent in the BA.2 set (3/25) than in the BA.1 set (9/28).
Somatic mutation in the BA.2 mAbs was greater than we have seen in any previous set of SARS-CoV-2 mAbs and was especially significant compared with the early-pandemic set (mean number of mutations: 10.9 vs. 4.6 and 7.2 vs. 4.3 for the light and heavy chains, respectively) (Figure 2E).
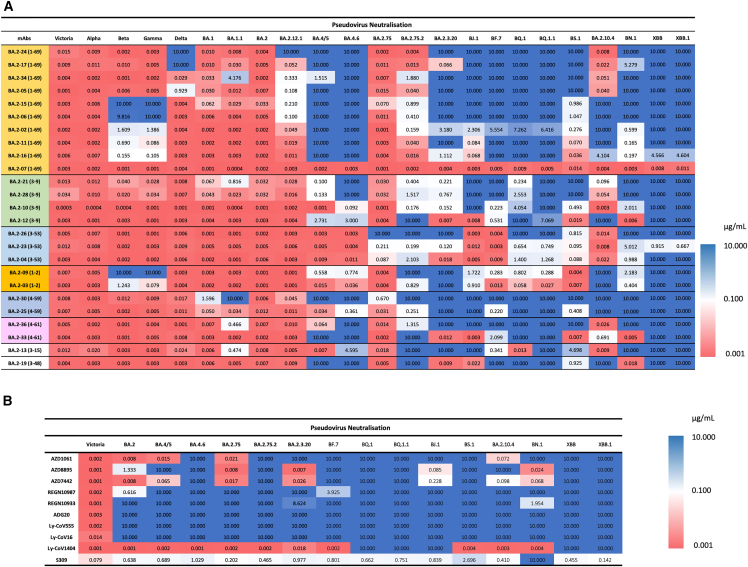
Neutralization assays show that all potent BA.2 mAbs cross-react with early-pandemic virus Victoria (IC50 < 100 ng/mL) (Figure 3). In contrast, from a previous set of mAbs isolated following primary Beta infection, only 3/18 (16.7%) of the potent Beta neutralizers (IC50 < 100 ng/mL) were able to neutralize Victoria, suggesting that the BA.2 mAbs are likely derived from memory B cells induced by vaccination.14
Figure 3.
Heatmap of IC50 neutralization titers
(A) BA.2 mAb panel.
(B) Commercial mAbs. Pseudoviral neutralization IC50 titers for indicated mAb against a panel of pseudoviruses expressing variant S sequences. All assays have been done at least twice. Commercial mAbs against Victoria, BA.2, BA.2.75, BA.4/5, and BA.4.6 previously reported are included for comparison.24,27,28
See also Figures S3 and S4.
Epitope mapping of the mAb panel
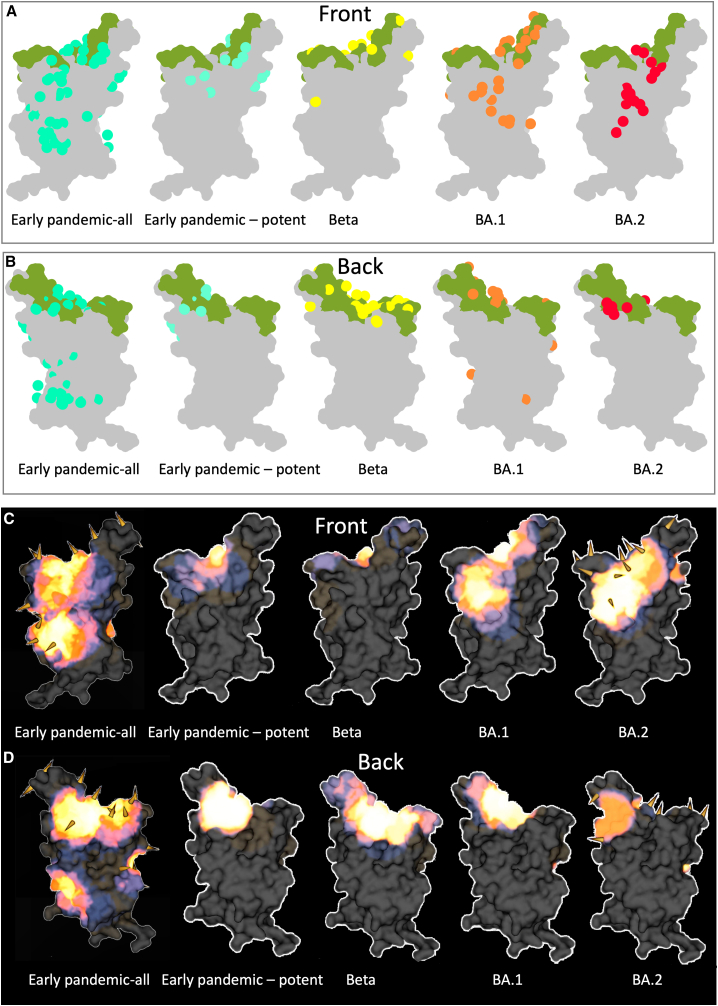
We performed pairwise BLI competition measurements on the 25 potent RBD-binding BA.2 mAbs and several pre-pandemic and BA.1 mAbs of known binding position (Figures 4A and S1A) to obtain a 3D map of the binding positions of antibodies on the RBD4 (Figure 4B). To understand if antibody responses have changed as SARS-CoV-2 has evolved, we show in Figures 5A and 5B the antibody-binding sites on the RBD surface for four sets of responses following the pandemic, and Figures 5C and 5D and Videos S1, S2, S3, and S4 display these as heatmaps to indicate the concentration of antibody across the RBD surface.
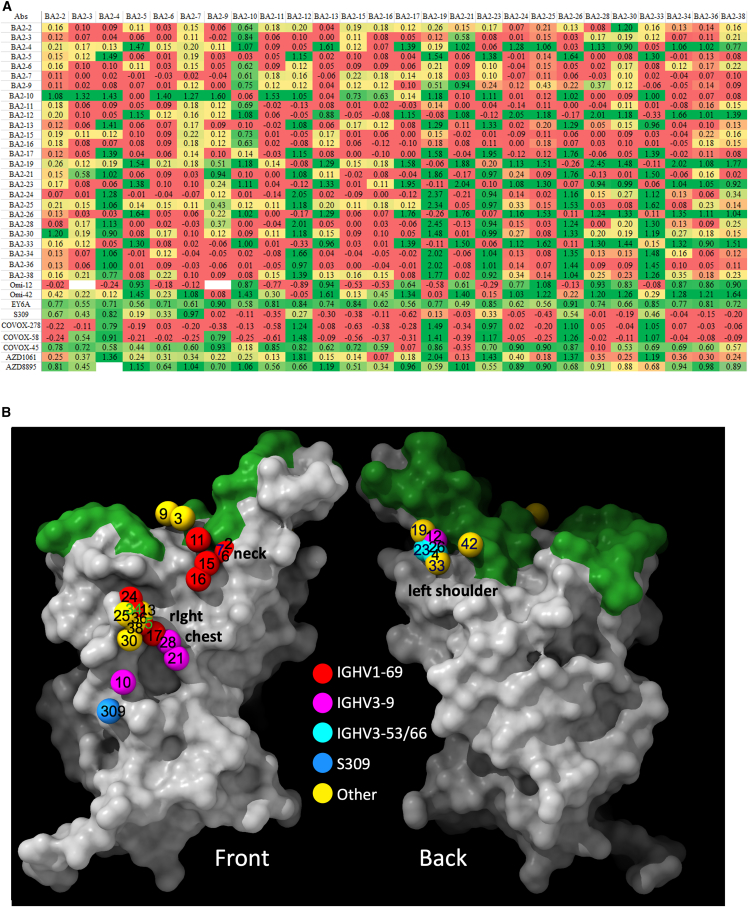
Figure 4.
Result of BLI competition mapping of BA.2 mAbs
(A) Competition ratio results (using Mabscape; see STAR Methods). Numbers close to 0 represent complete competition between pairs of antibodies, and numbers close to 1 mean no competition. Cells are colored as red, yellow, and green, with the values ranging from 0 to 1. Antibodies with known structures (Omi-12, Omi-42, EY6A, S309, COVOX-45, COVOX-58, COVOX-278, AZD1061, and AZD8895) were used as references.
(B) RBD surface representation with ACE2-binding site in green and balls corresponding to center of gravity of mapped potent BA.2 mAbs colored according to variable gene usage.
See also Figure S1.
Figure 5.
Comparison of mAb binding to RBD
(A and B) Front and back views of Mabscape antibody maps from early-pandemic, Beta, BA.1, and BA.2 antibody panels. Early pandemic all represents the full set of antibodies, irrespective of neutralization potency, and all other panels show potent mAbs (IC50 < 100 ng/mL). The RBDs are shown surface rendered (gray) with the ACE2 footprint in green.
(C and D) Heatmaps of surface occupation of RBD by early-pandemic, Beta, BA.1, and BA.2 antibody panels by iron heat colors (black > blue > red > orange > yellow > white hot) according to the relative level of antibody contact, calculated for each surface vertex as the number of antibodies within a 10 Å radius. BA.1 mutations are shown by the spikes. Only the early-pandemic-all panels and the sub-variant substitutions discussed here are shown mapped onto BA.2. Early-pandemic, Beta, and BA.1 data are taken from Dejnirattisai et al.,4 Nutalai et al.,13 Liu et al.14
See also Figure S1.
article
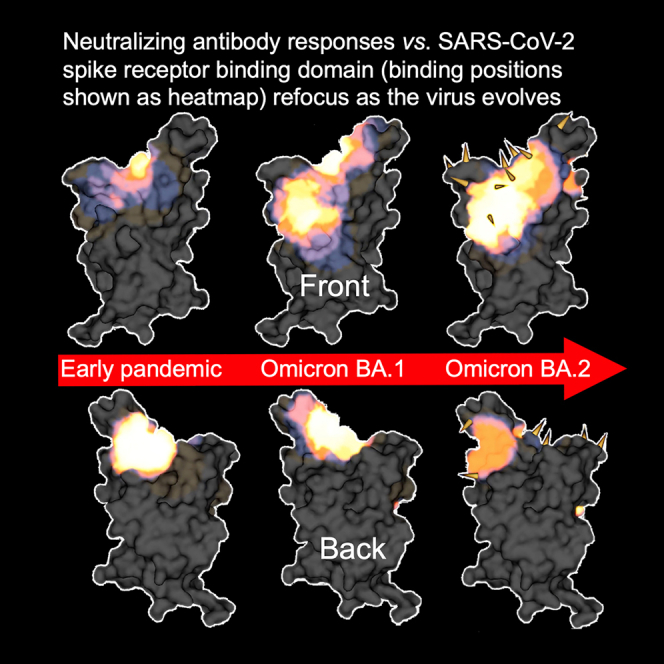
The BA.2 mAbs segregate into 3 adjacent clusters, two of which (left shoulder and neck in our anatomical definition of RBD topology4) are hotspots for the binding of potent mAbs against early-pandemic virus,4,14 while the third cluster, which we term the right chest, spans between the neck and right flank epitopes in early-pandemic responses (note that in the early responses, right flank binders were not found to be potent [IC50 > 100 ng/mL]). In contrast, in early-pandemic and Beta responses, we find that potent antibodies are clustered into the three regions closest to the ACE2-binding site: the right shoulder, the neck, and the left shoulder. Beta responses are somewhat more evenly distributed, probably because of the significant reduction of the IGHV3-53/66 so-called public responses to the left shoulder epitope, which dominate early-pandemic responses (Figures 5C and 5D). Overall, the three BA.2 clusters are very similar to the three clusters found in the BA.1 mAb set13 (Figures 5C and 5D).
The right chest cluster, the most populous in BA.2, has been characterized by binding of the BA.1 IGHV1-69 gene family antibodies.13 However, for BA.2, only 4 of the 10 IGHV1-69 antibodies bind here (5, 17, 24, 34), in addition to 3/4 IGHV3-9 antibodies (10, 21, 28), which lie a little below the IGHV1-69 antibodies (Figure 4). The second cluster, neck, harbors the remaining five BA.2 IGHV1-69 antibodies. This cluster also matches a cluster of BA.1 antibodies.13 The final cluster, left shoulder, includes the remaining IGHV3-9 antibody, BA.2-12, and the 3 IGHV3-53/3–66 antibodies (Figure 4). This cluster is diminished for BA.2 due to the relative paucity of effective IGHV3-53/66 antibodies. Note that in Figure 4, 42 refers to mAb Omi-42 isolated from a BA.1-infected case, which also binds more medially at the back of the RBD and is the only BA.1 mAb able to resist all the variants described in this article.
The relationship between the three BA.2 binding hotspots and the mutations in the recent variants is shown on the heatmap in Figures 5C and 5D. There is a strong correlation between the hotspots of antibody binding and the locations of mutations. In summary, Omicron antibodies show a shift in focus of the antibody response, with BA.1 and BA.2 mAb panels converging on similar areas but with BA.2 somewhat more skewed from the early-pandemic response (Figures 5A–5D).
Structural analysis
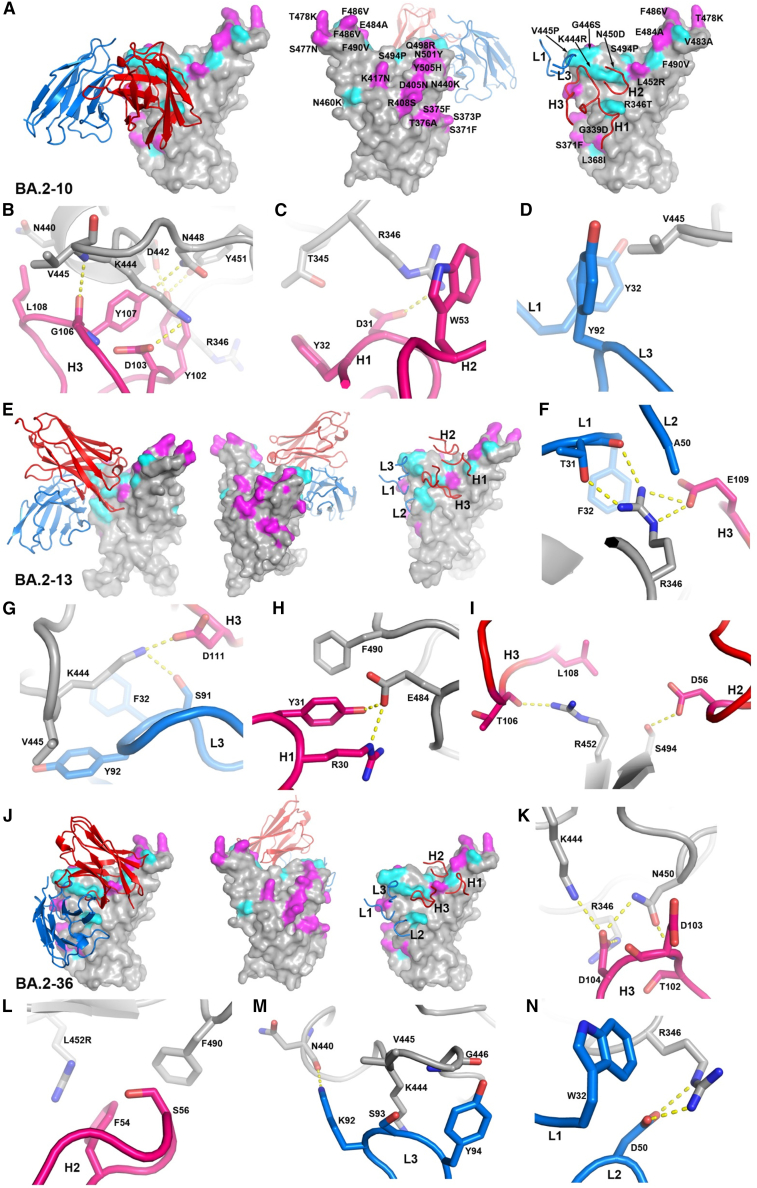
To obtain more detailed insight into selected BA.2 mAbs, we determined the cryo-EM structure of the complex of the Delta-RBD with BA.2-23 and BA.2-36, along with the early-pandemic mAb-454 and EY6A29 and also X-ray structures of the Delta RBD with BA.2-10 and EY6A, the Delta-RBD with BA.2-13 and C1 nanobody, and the Delta-RBD with BA.2-36 (Figures 6, S1B–S1D, and S2; Table S3).
Figure 6.
Structures of Delta-RBD complexes with BA.2-10, BA.2-13, and BA.2-36
(A) Binding position and orientation of BA.2-10 viewed from the front (left panel) and back (middle panel) of the RBD, and positions of the CDRs that have contact with the RBD (right panel). Only Vh (red) and Vl (blue) domains of the Fab are shown as ribbons for clarity. RBD is drawn as gray surface representation with BA.4 mutation sites highlighted in magenta, and the additional mutation sites of all variants shown in Figure 1A are shown in cyan.
(B–D) Details of BA.2-10 and RBD interactions. The side chains of the RBD and Fab Vh and Vl are shown as gray, red, and blue sticks, respectively. The yellow broken bonds represent hydrogen bonds or salt bridges.
(E and J) Complex of (E) Delta-RBD/BA.2-13 and (J) Delta-RBD/BA.2-36. The drawing style and color scheme are as in (A).
(F–I and K–N) Contact details between RBD and BA.2-13 and RBD and BA.2-36, respectively. The drawing style and color scheme are as in (B)–(D). Antibodies bind both Delta and BA.2 RBDs well; however, mutations from early pandemic to Delta are T478K and L452R (in BA.2, the T478K change is present, while L452R is not). Additional changes in BA.2 are G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, and Y505H.
See also Figures S1 and S2 and Table S3.
BA.2-10 (IGHV3-9) binds at the right chest region of the RBD as expected from the competition mapping. It makes extensive interactions via complementarity-determining regions (CDRs) H3, L3, and L1 with residue V445 of the RBD and with R346 via CDRs H1 and H2, explaining its reduced neutralization of variants containing the R346T mutation (e.g., BA.4.6 and BA.2.75.2) and complete knockout by the BJ.1 variant, which contains both the R346T and V445P mutations (Figures 6A–6D).
BA.2-13 (IGHV3-15) also binds to the right chest region but higher and more toward the midline of the RBD than BA.2-10 and in a very different orientation so that its LC contact area on the RBD largely overlaps with the footprint of BA.2-10 HC. However, the contact area of BA.2-10 CDR-H3 overlaps the CDR-H3 contacts of BA.2-13. BA.2-13 interacts with RBD residues 346, 444–445, 450, and 452 (Figures 6E–6I). Variants containing the R346T mutation either seriously reduce or completely knock out neutralization of BA.2-13. BA.2.3.20, which contains K444R, N450D, and L452M mutations, also completely knocks out the activity of BA.2-13. Although BA.2-13 contacts RBD residues 452 and 484, it is not sensitive to mutations L452R or E484K, as it fully neutralizes Beta, Gamma, and Delta variants.
BA.2-36 (IGHV4-61) binds to the right chest region in a similar position and orientation to BA.2-13 (Figures 6J–6N). It also contacts RBD residues 346, 444, 450, and 452, explaining its similar variant neutralization profile to BA.2-13, with neutralization seriously reduced or completely knocked out for variants containing the R346T mutation and by BA.2.3.20.
BA.2-23 is an IGHV3-53 family antibody and binds the left shoulder in the expected position. Note that most potent IGHV3-53/3-66 antibodies bind in essentially the same way (although a second binding mode has also been observed),30 and yet they vary in their sensitivity to mutations, as we have discussed before.13 For the recent variants, a key mutation is residue 486, where the change from F to V has a negative effect and the change to the even smaller and polar S side chain is more deleterious (Figures S2 and S3; Table S1). The N460K mutation also impacts the binding of this heavy-chain family. Since IGHV3-53/3-66 form a major public response in SARS-CoV-2 infection, it is likely that BA.2.75.2 has acquired F486S and N460K to evade them (Table S1).
Widespread escape from mAb neutralization
To test the resilience of the BA.2 mAbs to newly described BA.2 variants, we constructed a panel of pseudoviruses expressing S gene sequences for the variants and measured neutralization using BA.2 as comparison (Figures 3A and S3A).
On BA.4, the activity of 11/25 mAbs was knocked out, and only 10 retained IC50 titers ≤100 ng/mL. Activity of 8/10 IGHV1-69 mAbs was knocked out completely on BA.4, likely due to mutations 452, 486, and 493 (reversion) on the neck and left shoulder of the RBD (Figure 1B). Activity of 3 further mAbs was lost with the addition of the R346T mutation in BA.4.6 including the 2 IGHV3-9 mAbs BA.2-21 and BA.2-28, which map to the chest, overlapping residue 346 (Figure 1B).
BA.2.75 had a minor effect on neutralization, with only BA.2-26 (IGHV3-66) showing near knockout of activity. The effect of BA.2.75.2 was much more marked: the activity of 7/25 mAbs was completely lost, with only 6/25 mAbs retaining IC50 values <100 ng/mL, and the 2 IGHV3-53 mAbs BA.2-04 and BA.2-26 were knocked out, showing that BA.2.75.2 has evolved to evade this very common public response.7 The mutation F486S is a radical change and, together with N460K, is likely responsible for these effects, mapping close to the affected mAb (Figures 1B and 4).
BA.2.3.20, which has a number of mutations across the top of the shoulders, neck, and at the back of the RBD (Figures 1A and 1B), also caused large disruption: 13/25 mAbs were knocked out, with only 6/25 retaining IC50 activity at <100 ng/mL. Finally, for BJ.1, which has a different set of mutations across the top of the RBD, the activity of 13/25 mAbs was completely knocked out, with only 9/25 retaining IC50 activity at <100 ng/mL.
The activity of most mAbs was reduced or completely knocked out against BQ.1, BQ.1.1, XBB, and XBB.1, while the activity of some mAbs was preserved on BN.1 and BA.2.10.4. Overall, it appears that mutational change in SARS-CoV-2 since the emergence of BA.2 has led to mutations that knock out or severely reduce activity of almost all BA.2-directed mAbs; only BA.2-04, BA.2-07, and BA.2-23 did not suffer a complete knockout of activity with any of the pseudoviruses tested, and only BA.2-07 maintained full activity (Figures 3A and S3).
Finally, we returned to a panel of mAbs made following BA.1 infection13 and found that there was a similar attrition of mAb activity with the new variants (Figures S3B and S4), with XBB leading to the most extreme escape. Activity of all 9 IGHV3-53/66 mAbs was reduced >100-fold with complete knockout of activity in 5/9 by BA.2.75.2. Only a single mAb, Omi-42, was unaffected by all variants. Omi-42 is unusual, as it binds at the back of the left shoulder of the RBD (Figure 4, right panel)13 in a region that has not yet been targeted for mutation by the set of newly emerging BA.2 variants, perhaps because of the relative rarity of antibodies binding in this region (Figures 1B and 4).
Escape from therapeutic mAbs
We tested the activity of a panel of mAbs that have been developed for clinical use.31,32,33,34 Many of these have already been severely impacted by a number of variants. Activity of all mAbs was knocked out by one or more variants including Ly-CoV1404, which was inactive on BJ.1 and XBB probably due largely to the V445P mutation (Figures S3C and S2I).16 LY-CoV1404 binds at the side of the right shoulder of the RBD, making extensive interactions with residues K444 and V445 (PDB: 7MMO; Figures S2G–S2I), and the K444T mutation in BQ.1 likely knocks out activity of LY-CoV1404.
Severe knockdown of serum neutralization titers
In a final series of experiments, we tested neutralization on serum collected 28 days following a third dose of Pfizer BNT162b2 vaccine35 and in cases infected with BA.1, BA.2, and BA.4/5 (the characteristics of these subjects are described in the STAR Methods).
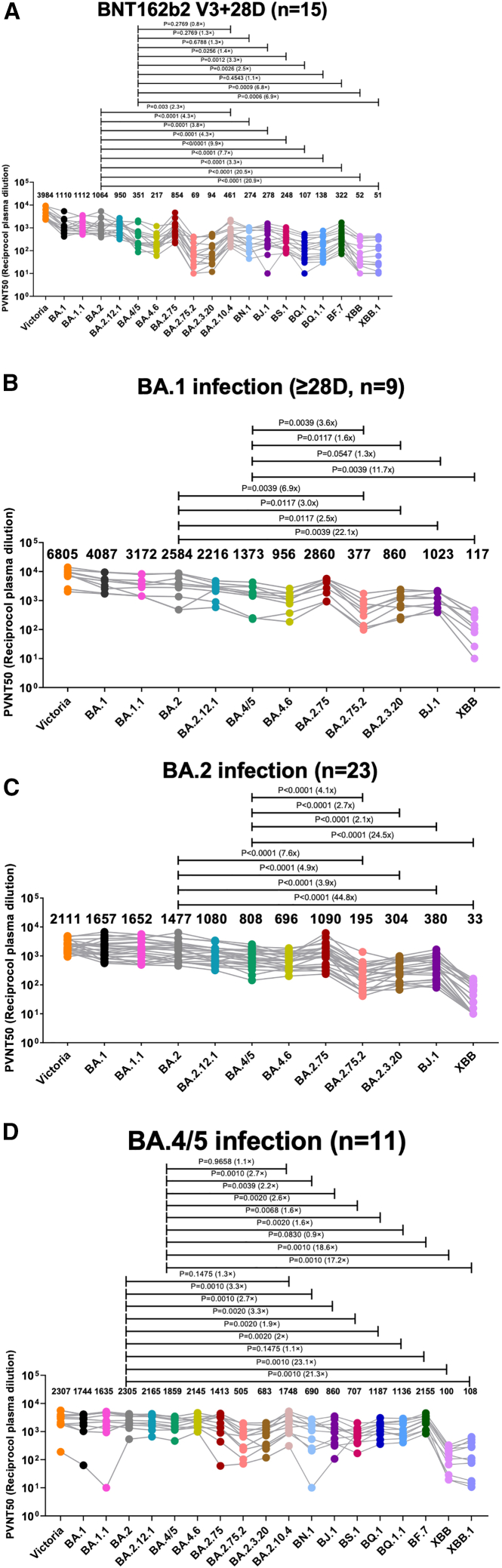
For serum obtained 28 days following BNT162b vaccination, neuralization titers to BA.2.75.2 and XBB showed large reductions (Figure 7A), 16.6- and 20.9-fold (p < 0.0001), respectively, compared with BA.2. In contrast to BA.2.75.2, BA.2.75 showed only a modest reduction compared with BA.2. There were also large reductions in titers to BQ.1 and BA.2.3.20, 11.4- and 7.7- fold, respectively (p ≤ 0.0001) compared with BA.2. For BJ.1, titers were reduced 3.6-fold compared with BA.2 (p < 0.0001), but reductions were less marked compared with BA.2.75.2 and BA.2.3.20.
Figure 7.
Serum neutralization IC50 titers (fold dilution) of lentivirus pseudotyped with the S gene of the indicated BA.2 sub-lineages
(A and B) Serum obtained 28 days following the third dose of BNT162b2 vaccine (n = 15) or following infection with (B) BA.1 (n = 10 all vaccinated) taken 28–55 days following diagnosis median 41.5.
(C and D) BA.2 (n = 23 all vaccinated) taken 12–43 days following diagnosis median 29 or (D) BA.4/5 (n = 11 all but one vaccinated) taken 23–48 days following diagnosis median 38 days. Geometric mean titers are shown above each column. The single unvaccinated serum shows the lowest reactivity to BA.4/5 in (D). The Wilcoxon matched-pairs signed rank test (C and D) and Mann-Whitney test were used and two-tailed p values calculated. Data for BNT162b2-vaccinated sera and BA.1 infection sera against Victoria, BA.1, BA.1.1, BA.2, BA.4/5, BA.4.6, BA.2.75, and BA.2.12.1 previously reported are included for comparison.23,24,27,28 All assays have been done with the number of biological replicates indicated in the brackets.
Next, we tested serum taken following BA.1, BA.2, or BA.4/5 infection; all were vaccine breakthrough infections, barring one unvaccinated case of BA.4 (Figures 7B–7D). In all cases, new variant Omicron lineages showed reduced neutralization titers compared with BA.2 and BA.4/5, but the reductions were generally less compared with those seen with BNT162b2 vaccine serum, presumably due to the higher levels of antibody to the Omicron lineage found in these sera. The reduction in neutralization of XBB, which showed the greatest escape from BA.2 mAbs described above, was particularly marked with 22-, 45-, and 21-fold reduction in titers compared with BA.2 using BA.1, BA.2, and BA.4/5 serum, respectively.
Overall, in line with the observations on the set of mAbs described above, there were large reductions in neutralization titers against most BA.2 sub-lineages, particularly BA.2.75.2, BA.2.3.20, BQ.1, and XBB, suggesting that they have been selected to escape pre-existing immunity to vaccines or earlier waves of SARS-CoV-2 infection. Titers will be expected to wane considerably from those shown here for samples taken quite soon after vaccination or infection, meaning the newly emerging BA.2 variants may fuel future waves of SARS-CoV-2 infection; indeed, BQ.1/BQ.1.1 and XBB/XBB.1/XBB.1.5 are becoming the dominant Omicron sub-lineages in several regions (https://cov-spectrum.org/explore/World/AllSamples/Past6M).
Discussion
Before the emergence of Omicron, RBD mutations were small in number: 1 in Alpha, 2 in Delta, and 3 in Beta and Gamma. These may in part have been selected for increased ACE2 receptor binding, although they also led to a degree of antibody evasion, which is most pronounced for Beta.9,10,36,37 The arrival and rapid spread of Omicron BA.1 in November 2021 surprised many in the field, as it contained so many mutations, particularly in the RBD and NTD.
It is likely that evolution of SARS-CoV-2 Omicron is now primarily driven by extreme pressure to escape antibody responses in vaccinated and/or naturally infected individuals, with compensatory mutations to maintain or increase ACE2 affinity. There has been a succession of changes to the RBD with evidence for convergent evolution. Now that the majority of the population is either vaccinated or has been exposed, herd immunity is exerting huge pressure for immune escape. Many mutations are occurring on the edge of the ACE2-binding footprint to throw off potent antibodies that block ACE2.3 The RBD shows a remarkable degree of plasticity, seemingly able to accommodate wide-ranging changes while still maintaining sufficient affinity for ACE2, similar to that shown for the human seasonal coronavirus 229E.38 Taking all the new Omicron variants (Figure 1A), the ∼200 residue RBD bears 29 mutated residues compared with the early-pandemic virus; this extraordinary level of variation, focused on the antigenic sites and leading to such effective antigenic escape, would conventionally lead to these viruses being classified as different serotypes.39 What is even more remarkable, and what may be of some concern, is that this level of variation is focused on the part of the virus whose job it is to attach to cells and initiate infection, raising the possibility of inadvertent receptor switching,40 which would have completely unpredictable results (already seen to date in a far more limited sense with variation altering the host range of the virus).41 The plasticity of the RBD at and around the small ACE2-binding site means that rather than being a conserved Achilles heel for SARS-CoV-2, it is proving to be quite the opposite, giving considerable opportunity to throw off huge chunks of the antibody response through a succession of mutations.
We find, by analysis of a panel of antibodies generated from vaccinees suffering BA.2 breakthrough infections, that the focusing of potently neutralizing antibodies has shifted from that observed in early-pandemic responses and resembles that reported for BA.1.13 BA.2 antibodies cluster tightly in two of the three regions characteristic of potent early-pandemic responses, although mutations have knocked out many of the so-called “public” responses, including gene families IGHV3-53/66, diminishing the potent left shoulder responses. There is a third more extensive binding patch at the front of the RBD, the right chest, which spans between the right flank and neck regions identified in the early-pandemic responses, extending down almost as far as the S309 therapeutic antibody on the right flank. S309 does not compete with ACE2, and a general feature is that many of the potent BA.2 antibodies are much further from the ACE2-binding site and, as a result, are less strongly competitive with ACE2 than early-pandemic antibodies (Figure 2C). In contrast to the potent BA.2 chest binders identified here, the early-pandemic right flank antibodies, which bind nearby, were generally weakly neutralizing. These shifts in binding patterns are seen in the heatmaps of antibody binding between antibody responses produced successively through the pandemic (Figures 5A–5D).
The changed pattern of potent antibody binding is likely because almost all antibodies seem to be derived from responses induced by vaccination (and sometimes also SARS-CoV-2 infection). In support of this, every member of our set of potent antibodies to BA.2 potently cross-reacted with Wuhan-related strains and was likely originally induced by vaccination, since all donors were triple vaccinated. It is not clear whether the cross-reacting antibodies were fully matured before BA.2 infection or whether affinity maturation of lower affinity clones occurred in response to BA.2 infection. The panel probably contains a mix of both, and the strong representation of weaker neutralizing right flank antibodies in the early responses suggests that the chest binders that overlap these may have been matured to higher affinity and selected because this portion of the RBD has been subjected to less mutational change in BA.1 and BA.2.
The change away from antibodies binding more directly on the ACE2 footprint is highlighted by the substantial role in early responses played by public gene families such as IGHV3-53/66,7,42 IGHV1-69, IGHV3-9, and IGHV1-58.13 Thus, in this article, the BA.2 IGHV1-69 mAbs are almost all knocked out by BA.4. Finally, the few members of the most frequently discovered gene family of all, IGHV3-53/66, that are active against BA.2 are severely affected by the F486S mutation in BA.75.2, as are the larger number of IGHV3-53/66 mAbs isolated following BA.1 infection. The refocusing is therefore explained by the evolution of the virus to the point of BA.1 and BA.2; however, there is no reason to think that residues on the right chest are required to be conserved. Indeed, the mutations in the recent sub-lineages are found on the edge or outside of the ACE2 epitope, deployed so as to effectively escape all three of the hotspots we see in BA.1 and BA.2 responses.
In summary, continuous attrition of the serological response and reliance on vaccine-induced clones to repopulate it is likely to reduce the memory pool and may limit the refocusing of the response required in the face of antigenic escape, increasing vulnerability. It is telling that the mutations to BA.2 described here target the regions used to bind 25 potent mAbs, rendering all but BA.2-07 compromised for at least one variant.
The situation is the same for the commercial mAbs, which largely bind to conserved hotspots, leading to failure of all therapeutic candidates to at least one variant. New approaches may be required to discover potent mAbs that have unusual binding sites that are not under such selective pressure if a pipeline of therapeutic/prophylactic candidates is to be maintained.
Now that herd immunity from vaccination and/or natural infection is high, there is intense pressure to escape from the antibody response, meaning it is likely we will see successive waves of infection as the virus continues to evolve. However, background immunity, perhaps led by the T cell response, which is less sensitive to mutations, will probably maintain protection against severe disease for the majority. Further monitoring of the antibody response throughout successive waves will tell us whether there is increasing attrition of polyclonality. However, as observed for BA.1, somatic mutation will probably enable the repair of some antibodies13; for instance, it seems unlikely that this is the end of the road for neutralization using the VH3-53/66- or VH1-69-binding sites.
Finally, many people are now receiving bivalent vaccine boosters containing the spike of both Wuhan and one Omicron BA.1 or BA.4/5. The data presented here, where all BA.2 mAbs cross-react with early-pandemic viruses, suggests that the Omicron component may mount a BA.1 or BA.4/5 response using B cell clones recalled from the original vaccine response rather than eliciting a primary response to BA.1 or BA.4. Finally, if recall responses are largely responsible for the population of responses on reinfection, is it possible that the structure of the response to reinfection is in part molded by the sequence of the priming virus or the vaccine reflecting original antigenic sin or imprinting?
Limitations of the study
The neutralization assays presented here are performed in vitro and may underestimate in vivo neutralization where antibody-dependent cell-mediated cytotoxicity and complement will be present.
Consortia
The members of the OPTIC consortium are Christopher Conlon, Alexandra Deeks, John Frater, Siobhan Gardiner, Anni Jämsén, Katie Jeffery, Tom Malone, Eloise Phillips, Barbara Kronsteiner-Dobramysl, Priyanka Abraham, Sagida Bibi, Teresa Lambe, Stephanie Longet, Tom Tipton, Miles Carrol, and Lizzie Stafford.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Fab | Dejnirattisai et al.4 | N/A |
| IgG | Dejnirattisai et al.4 and Liu et al.4,14 | N/A |
| Human anti-NP (mAb 206) | Dejnirattisai et al.4 | N/A |
| Regeneron mAbs | AstraZeneca | Cat#REGN10933 and REGN10987 |
| AstraZeneca mAbs | AstraZeneca | Cat#AZD1061, AZD8895 and AZD7442 |
| Vir mAbs | Adagio | Cat#S309 |
| Lilly mAbs | Adagio | Cat#Ly-CoV555, Ly-CoV16 and Ly-CoV1404 |
| Adagio mAbs | Adagio | Cat#ADG10, ADG20 and ADG30 |
| Omicron antibodies | Nutalai et al.13 | N/A |
| Bacterial, virus strains, and yeast | ||
| DH5α bacteria | InVitrogen | Cat#18263012 |
| Biological samples | ||
| Serum from Pfizer-vaccinated individuals | University of Oxford | N/A |
| Serum from AstraZeneca-Oxford-vaccinated individuals | University of Oxford | N/A |
| Plasma from SARS-CoV-2 patients | John Radcliffe Hospital in Oxford UK, South Africa, and FIOCRUZ (WHO) Brazil | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| His-tagged SARS-CoV-2 RBD | Dejnirattisai et al.4 | N/A |
| His-tagged Avi-tagged SARS-CoV-2/BA.2.75 RBD | This paper | N/A |
| His-tagged SARS-CoV-2/BA.2 + R493Q RBD | This paper | N/A |
| His-tagged SARS-CoV-2/BA.2 RBD | Nutalai et al.13 | N/A |
| His-tagged SARS-CoV-2/BA.4/5 RBD | Tuekprakhon et al.24 | N/A |
| His-tagged SARS-CoV-2/Alpha RBD | Supasa et al.10 | N/A |
| Human ACE2-hIgG1Fc | Liu et al.14 | N/A |
| Phosphate buffered saline tablets | Sigma-Aldrich | Cat#P4417 |
| Dulbecco’s Modified Eagle Medium, high glucose | Sigma-Aldrich | Cat#D5796 |
| Dulbecco’s Modified Eagle Medium, low glucose | Sigma-Aldrich | Cat#D6046 |
| FreeStyle™ 293 Expression Medium | Gibco | Cat#12338018 |
| L-Glutamine–Penicillin–Streptomycin solution | Sigma-Aldrich | Cat#G1146 |
| GlutaMAX™ Supplement | Gibco | Cat#35050061 |
| UltraDOMA PF Protein-free Medium | Lonza | Cat#12–727F |
| Opti-MEM™ | Gibco | Cat#11058021 |
| Fetal Bovine Serum | Gibco | Cat#12676029 |
| Strep-Tactin®XT | IBA Lifesciences | Cat#2-1206-025 |
| HEPES | Melford | Cat#34587–39108 |
| LB broth | Fisher Scientific UK | Cat#51577–51656 |
| Trypsin-EDTA | Gibco | Cat#2259288 |
| TrypLE™ Express Enzyme | Gibco | Cat#12604013 |
| L-Glutamine 200 mM (100X) | Gibco | Cat#2036885 |
| Isopropyl β-d-1-thiogalactopyranoside | Meridian Bioscience | Cat#BIO-37036 |
| Kanamycin | Melford | Cat#K22000 |
| Ampicillin | Sigma-Aldrich | Cat#PHR2838 |
| Agarose | Sigma-Aldrich | Cat#A2929 |
| SYBR™ Safe DNA Gel Stain | Fisher Scientific UK | Cat#S33102 |
| QIAprep Spin Miniprep Kit | Qiagen | Cat#27106X4 |
| QIAquick® PCR & Gel Cleanup Kit | Qiagen | Cat#28704 |
| Phusion™ High-Fidelity DNA Polymerase | Fisher Scientific UK | Cat#F530S |
| Bright-Glo™ Luciferase Assay System | Promega | Cat#E2620 |
| HIV1 p24 ELISA Kit | Abcam | Cat#ab218268 |
| NaCl | Sigma-Aldrich | Cat#S9888 |
| Sensor Chip Protein A | Cytiva | Cat#29127555 |
| Biotin CAPture Kit, Series S | Cytiva | CAT#28920234 |
| HBS-EP+ Buffer 10× | Cytiva | Cat# BR100669 |
| Regeneration Solution (glycine-HCl pH 1.7) | Cytiva | Cat# BR100838 |
| Deposited data | ||
| Crystal structures of: SARS-CoV-2 Delta-RBD/BA.2–36 and SARS-CoV-2 Delta-RBD/EY6A/BA.2–10 and SARS-CoV-2 Delta RBD/BA.2–13/C1 | This paper | PDB: 8BBO, PDB:8BBN, PDB:8C3V |
| CryoEM structure of: SARS-CoV-2 Delta-RBD/BA.2–23/BA.2–36/EY6A/Fab-45 | This paper | PDB:8BCZ, EMDB:EMD-15971 |
| Experimental models: Cell lines | ||
| HEK293 cells | ATCC | Cat#CRL-3216 |
| Expi293F™ Cells | Gibco, | Cat#A14527 |
| HEK293T/17 cells | ATCC | Cat#CRL-11268™ |
| HEK293T cells | ATCC | Cat#CRL-11268 |
| Vero CCL-81 cells | ATCC | Cat#CCL-81 |
| VeroE6/TMPRSS2 cells | NIBSC | Ref. no. 100978 |
| Recombinant DNA | ||
| Vector: pHLsec | Aricescu et al.43 | N/A |
| Vector: pNEO | Aricescu et al.43 | N/A |
| Vector: pHLsec-SARS-CoV-2 spike of Omicron | Nutalai et al.13 | N/A |
| Vector: pOPINTTGneo-BAP-SARS-CoV-2 RBD of BA.2.75 | This paper | N/A |
| Vector: pNEO-SARS-CoV-2 RBD of BA.2 | Nutalai et al.13 | N/A |
| Vector: pNEO-SARS-CoV-2 RBD of BA.4/5 | Tuekprakhon et al.24 | N/A |
| Vector: pNEO-SARS-CoV-2 RBD of BA.2 + R493Q | This paper | N/A |
| Vector: pNEO-SARS-CoV-2 RBD of Alpha | Supasa et al.10 | N/A |
| Vector: pCMV-VSV-G | Stewart et al.44 | Addgene plasmid # 8454 |
| pHR-SIN-ACE2 | Alain Townsend, Oxford | N/A |
| Vector: pOPING-ET | Nettleship et al.45 | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of Victoria strain (S247R) | Liu et al.14 | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.1 strain (A67V, Δ69–70, T95I, G142D/Δ143-145, Δ211/L212I, ins214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F) | Nutalai et al.13 | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.1.1 strain (A67V, Δ69–70, T95I, G142D/Δ143-145, Δ211/L212I, ins214EPE, G339D, R346K, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F) | Nutalai et al.13 | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.2 strain (T19I, Δ24–26, A27S, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K) | Nutalai et al.13 | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.2.12.1 strain (T19I, Δ24–26, A27S, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, L452Q, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, S704L, N764K, D796Y, Q954H, N969K) | Nutalai et al.13 | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.4/5 strain (T19I, Δ24–26, A27S, Δ69-70, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, L452R, S477N, T478K, E484A, F486V, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K) | Tuekprakhon et al.24 | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.2.75 strain (T19I, Δ24–26, A27S, G142D, K147E, W152R, F157L, I210V, V213G, G257S, D339H, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, G446S, N460K, S477N, T478K, E484A, R493Q, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K) | This paper | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.2 + D339H strain (T19I, Δ24–26, A27S, G142D, V213G, D339H, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K) | This paper | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.2 + R493Q strain (T19I, Δ24–26, A27S, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, S477N, T478K, E484A, R493Q, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K) | This paper | N/A |
| Vector: pcDNA-SARS-CoV-2 spike of BA.2 + G446S strain (T19I, Δ24–26, A27S, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K) | This paper | N/A |
| Vector: human IgG1 heavy chain | German Cancer Research Center, Heidelberg, Germany (H. Wardemann | N/A |
| Vector: human lambda light chain | German Cancer Research Center, Heidelberg, Germany (H. Wardemann | N/A |
| Vector: human kappa light chain | German Cancer Research Center, Heidelberg, Germany (H. Wardemann | N/A |
| Vector: Human Fab | University of Oxford | N/A |
| Vector: pJYDC1 | Adgene | ID: 162,458 |
| TM149 BirA pDisplay | University of Oxford, NDM (C. Siebold) | N/A |
| Software and algorithms | ||
| COOT | Emsley et al.46 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Xia2-dials | Winter et al.47 | https://xia2.github.io/parameters.html |
| PHENIX | Liebschner et al.48 | https://www.phenix-online.org/ |
| PyMOL | Warren DeLano, Schrodinger | https://pymol.org/ |
| Data Acquisition Software 11.1.0.11 | Fortebio | https://www.fortebio.com/products/octet-systems-software |
| Data Analysis Software HT 11.1.0.25 | Fortebio | https://www.fortebio.com/products/octet-systems-software |
| CryoSPARC v4.1.2 | Structura Biotechnology Inc. | https://cryosparc.com/ |
| SerialEM (version 3.8.0 beta) | https://bio3d.colorado.edu/SerialEM/ | N/A |
| EPU | Thermo Fisher | https://www.thermofisher.com/uk/en/home/electron-microscopy/products/software-em-3d-vis/epu-software.html |
| Prism 9.0 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Yeast display titration curve fitting were done by the standard non-cooperative Hill equation, fitted by nonlinear least-squares regression with two additional parameters using Python 3.7 | Zahradnik et al.11 | N/A |
| IBM SPSS Software 27 | IBM | https://www.ibm.com |
| Mabscape | This paper |
https://github.com/helenginn/mabscape https://snapcraft.io/mabscape |
| Biacore T200 Evaluation Software 3.1 | Cytiva | www.cytivalifesciences.com |
| Other | ||
| X-ray data were collected at beamline I03, Diamond Light Source, under proposal ib27009 for COVID-19 rapid access | This paper | https://www.diamond.ac.uk/covid-19/for-scientists/rapid-access.html |
| Cryo-EM data were collected at OPIC, Division of Structural Biology, University of Oxford | This paper | https://www.opic.ox.ac.uk |
| TALON® Superflow Metal Affinity Resin | Clontech | Cat#635668 |
| HiLoad® 16/600 Superdex® 200 pg | Cytiva | Cat#28-9893-35 |
| Superdex 200 increase 10/300 GL column | Cytiva | Cat#28990944 |
| HisTrap nickel HP 5-mL column | Cytiva | Cat#17524802 |
| HiTrap Heparin HT 5-mL column | Cytiva | Cat#17040703 |
| Amine Reactive Second-Generation (AR2G) Biosensors | Fortebio | Cat#18–5092 |
| Octet RED96e | Fortebio | https://www.fortebio.com/products/label-free-bli-detection/8-channel-octet-systems |
| Buffer exchange system “QuixStand” | GE Healthcare | Cat#56-4107-78 |
| Cartesian dispensing system | Genomic solutions | Cat#MIC4000 |
| Hydra-96 | Robbins Scientific | Cat#Hydra-96 |
| 96-well crystallization plate | Greiner bio-one | Cat#E20113NN |
| Crystallization Imaging System | Formulatrix | Cat#RI-1000 |
| Sonics vibra-cell vcx500 sonicator | VWR | Cat#432–0137 |
| Biacore T200 | Cytiva | https://www.cytivalifesciences.com/en/us/shop/protein-analysis/spr-label-free-analysis/systems/biacore-t200-p-05644 |
Resource availability
Lead contact
Resources, reagents and further information requirement should be forwarded to and will be responded by the lead contact, David I Stuart (dave@strubi.ox.ac.uk).
Materials availability
Reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Experimental model and subject details
Bacterial strains and cell culture
Vero (ATCC CCL-81) and VeroE6/TMPRSS2 cells were cultured at 37°C in Dulbecco’s Modified Eagle medium (DMEM) high glucose (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS), 2 mM GlutaMAX (Gibco, 35050061) and 100 U/ml of penicillin–streptomycin. Human mAbs were expressed in HEK293T cells cultured in FreeStyle 293 Expression Medium (Cat# 12338018, Gibco) at 37°C with 5% CO2. HEK293T (ATCC CRL-11268) cells were cultured in DMEM high glucose (Sigma-Aldrich) supplemented with 10% FBS, 1% 100X Mem Neaa (Gibco) and 1% 100X L-Glutamine (Gibco) at 37°C with 5% CO2. To express RBD, RBD variants and ACE2, HEK293T cells were cultured in DMEM high glucose (Sigma) supplemented with 2% FBS, 1% 100X Mem Neaa and 1% 100X L-Glutamine at 37°C for transfection. BA.2 RBD were expressed in HEK293T (ATCC CRL-11268) cells cultured in FreeStyle 293 Expression Medium (Cat# 12338018, Gibco) at 37°C with 5% CO2. E.coli DH5α bacteria were used for transformation and large-scale preparation of plasmids. A single colony was picked and cultured in LB broth at 37 °C at 200 rpm in a shaker overnight.
Sera from BA.1 infected cases, study subjects
Following informed consent, individuals with omicron BA.1 were co-enrolled into the ISARIC/WHO Clinical Characterisation Protocol for Severe Emerging Infections [Oxford REC C, ref. 13/SC/0149] and the “Innate and adaptive immunity against SARS-CoV-2 in healthcare worker family and household members” protocol affiliated to the Gastro-intestinal illness in Oxford: COVID sub study [Sheffield REC, ref. 16/YH/0247] further approved by the University of Oxford Central University Research Ethics Committee. Diagnosis was confirmed through reporting of symptoms consistent with COVID-19 or a positive contact of a known Omicron case, and a test positive for SARS-CoV-2 using reverse transcriptase polymerase chain reaction (RT-PCR) from an upper respiratory tract (nose/throat) swab tested in accredited laboratories and lineage sequence confirmed through national reference laboratories. A blood sample was taken following consent at least 10 days after PCR test confirmation. Clinical information including severity of disease (mild, severe or critical infection according to recommendations from the World Health Organisation) and times between symptom onset and sampling and age of participant was captured for all individuals at the time of sampling (see Table S4).
Sera and PBMC from BA.2 infected study subjects
Following informed consent, healthcare workers with BA.2 infection were co-enrolled under the Sheffield Biobank study (STHObs) (18/YH/0441) during March 2022 (median 22 March, BA.2 sequence was confirmed for all samples taken before 22 March). All individuals had PCR-confirmed symptomatic disease and sequence confirmed BA.2 infection through national UKHSA sequencing data. A blood sample was taken following consent at least 12 days after PCR test confirmation. Clinical information including vaccination history, times between symptom onset and sampling and age of participant was captured for all individuals at the time of sampling (see Table S4).
Sera from BA.4/5 infected cases, study subjects
Following informed consent, individuals with omicron BA.4 or BA.5 were co-enrolled into one or more of the following three studies: the ISARIC/WHO Clinical Characterisation Protocol for Severe Emerging Infections [Oxford REC C, ref. 13/SC/0149], the “Innate and adaptive immunity against SARS-CoV-2 in healthcare worker family and household members” protocol (approved by the University of Oxford Central University Research Ethics Committee), or the Gastro-intestinal illness in Oxford: COVID sub study [Sheffield REC, ref. 16/YH/0247]. Diagnosis was confirmed through reporting of symptoms consistent with COVID-19, hospital presentation, and a test positive for SARS-CoV-2 using reverse transcriptase polymerase chain reaction (RT-PCR) from an upper respiratory tract (nose/throat) swab tested in accredited laboratories and lineage sequence confirmed through national reference laboratories in the United Kingdom. A blood sample was taken following consent at least 14 days after PCR test confirmation. Clinical information including severity of disease (mild, severe or critical infection according to recommendations from the World Health Organisation) and times between symptom onset and sampling and age of participant was captured for all individuals at the time of sampling (see Table S4).
Sera from Pfizer vaccinees
Pfizer vaccine serum was obtained from volunteers who had received three doses of the BNT162b2 vaccine. Vaccinees were Health Care Workers, based at Oxford University Hospitals NHS Foundation Trust, not known to have prior infection with SARS-CoV-2 and were enrolled in the OPTIC Study as part of the Oxford Translational Gastrointestinal Unit GI Biobank Study 16/YH/0247 [research ethics committee (REC) at Yorkshire & The Humber – Sheffield] which has been amended for this purpose on 8 June 2020. The study was conducted according to the principles of the Declaration of Helsinki (2008) and the International Conference on Harmonization (ICH) Good Clinical Practice (GCP) guidelines. Written informed consent was obtained for all participants enrolled in the study. Participants were sampled approximately 28 days (range 25–56) after receiving a third “booster dose of BNT162B2 vaccine. The mean age of vaccinees was 37 years (range 22–66), 21 male and 35 female.
Method details
Isolation of BA.2 S-specific single B cells by FACS
BA.2 S-specific single B cell sorting was performed as previously described.4 Briefly, PBMC were stained with LIVE/DEAD Fixable Aqua dye (Invitrogen) followed by recombinant trimeric S-twin-Strep of BA.2. Cells were then incubated with CD3-FITC, CD14-FITC, CD16-FITC, CD56-FITC, IgM-FITC, IgA-FITC, IgD-FITC, IgG-BV786 and CD19-BUV395, along with Strep-MAB-DY549 to stain the twin strep tag of the S protein. IgG+ memory B cells were gated as CD19+, IgG+, CD3-, CD14-, CD56-, CD16-, IgM-, IgA- and IgD-, and S+ was further selected and single cells were sorted into 96-well PCR plates with 10 μL of catching buffer (Tris, Nuclease free-H2O and RNase inhibitor). Plates were briefly centrifuged at 2000ⅹg for 1 min and left on dry ice before being stored at −80°C.
Cloning and expression of BA.2 S-specific human mAbs
BA.2 S-specific human mAbs were cloned and expressed as described previously.4 Briefly, genes for Ig IGHV, Ig Vκ and Ig Vλ were recovered from positive wells by RT-PCR. Genes encoding Ig IGHV, Ig Vκ and Ig Vλ were then amplified using Nested-PCR by a cocktail of primers specific to human IgG. PCR products of HC and LCs were ligated into the expression vectors of human IgG1 or immunoglobulin κ-chain or λ-chain by Gibson assembly.49 For mAb expression, plasmids encoding HCs and LCs were co-transfected by PEI-transfection into a HEK293T cell line, and supernatants containing mAbs were collected and filtered 4–5 days after transfection, and the supernatants were further characterized or purified.
ACE2 binding inhibition assay by ELISA
MAXISORP immunoplates were coated with 5 μg/mL of purified ACE2-His protein overnight at 4°C and then blocked by 2% BSA in PBS. Meanwhile, mAbs were serially diluted and mixed with 2.5 μg/mL of recombinant BA.1 trimeric S-twin-Strep. Antibody-S protein mixtures were incubated at 37°C for 1 h. After incubation, the mixtures were transferred into the ACE2-coated plates and incubated for 1 h at 37°C. After wash, StrepMAB-Classic (2-1507-001, iba) was diluted at 0.2 μg/mL by 2% BSA and used as primary antibody followed by Goat anti-mouse IgG-AP (#A16093, Invitrogen) at 1:2000 dilution. The reaction was developed by adding PNPP substrate and stopped with NaOH. The absorbance was measured at 405nm. The ACE2/S binding inhibition was calculated by comparing to the antibody-free control well. IC50 was determined using the Probit program from the SPSS package.
Pseudovirus plasmid construction and lentiviral particle production
Pseudotyped lentiviruses expressing SARS-CoV-2 S proteins from ancestral strain (Victoria, S247R), BA.2 and BA.4/5 were constructed as described previously.13,24,37,50 We applied the same method to construct BA.2.75, BA.2.75.2, BA.2.3.20, BA.2.10.4, BJ.1, BS.1, BN.1, XBB, and XBB.1 by adding more mutations into the BA.2 construct. To generate BA.2.75, we added K147E, W152R, F157L, I210V, G275S, G446S and N460K into BA.2 backbone, also changed 339D in BA.2 S into 339H, and reversed 493R in BA.2 to 493Q as in the ancestral strain. To create BA.2.75.2, we added R346T, F486S and D1199N into BA.2.75 backbone. To create BA.2.3.20, we introduced M153T, N164K, H245N, G257D, K444R, N450D, L452M, and N460K, as well we changed L452R in BA.2 into L452M, and reversed 493R in BA.2 to 493Q as in the ancestral strain. BA.2.10.4 was generated by introducing W64R, G446S, F486P, R493Q, S494P and P1143L into BA.2 backbone, as well as deleting aa142-144 and 243–244. To generate BJ.1, we introduced V83A, deletion at position 145, H146Q, Q183E, R346T, L368I, V445P, G446S, V483A, F490V and S1003I, in addition we changed 213G in BA.2 into 213E and also changed 339D in BA.2 S into 339H in BA.2 backbone. To create BS.1, we added aa144 deletion, G257V, R346T, L452R, G476S, N460K, F486S, F490S, and Q493H reversion into BA.2 backbone. To generate BN.1, the BA.2.75 vector was used as backbone with addition of R346T, K357T and F490S. To construct XBB, we added V83A, aa144 deletion, H146Q, Q183E, V213E, R346T, L368I, V445P, G446S, N460K, F486S, and F490S into BA.2 backbone and also changed 339D in BA.2 to 339H and reversed 493Q to H. XBB.1 was constructed by adding G252V into XBB. The same method was used to construct BF.7, BA4.6, BQ.1 and BQ.1.1 with new RBD mutations from BA.2 lineages. To create BF.7, R346T was added into BA.4 backbone. To generate BA.4.6, we introduced R346T and N658S into BA.4 backbone. BQ.1 was constructed by introducing K444T and N460K into BA.4 backbone, while BQ.1.1 was to add R346T into BQ.1 construct. The resulting pcDNA3.1 plasmid carrying S gene was used for generating pseudoviral particles together with the lentiviral packaging vector and transfer vector encoding luciferase reporter. All the constructs were sequence confirmed.
Pseudoviral neutralization test
The pseudoviral neutralization test has been described previously.37 Briefly, the neutralizing activity of potent monoclonal antibodies generated from donors who had recovered from BA.1 and BA.2 infections were tested against Victoria, BA.2, BA.2.75, BA.2.75.2, BA.2.3.20, BJ.1, BA.4/5, BA.4.6, BA.4.7 and chimeric BA.4. Four-fold serial diluted mAbs were incubated with pseudoviral particles at 37°C, 5% CO2 for 1 h. Stable HEK293T/17 cells expressing human ACE2 were then added to the mixture at 1.5 × 104 cells/well. 48 h post infection, culture supernatants were removed and 50 μL of 1:2 Bright-Glo TM Luciferase assay system (Promega, USA) in 1 × PBS was added to each well. The reaction was incubated at room temperature for 5 min and firefly luciferase activity was measured using CLARIOstar (BMG Labtech, Ortenberg, Germany). The percentage neutralization was calculated relative to the control. Probit analysis was used to estimate the dilution that inhibited half maximum pseudotyped lentivirus infection (PVNT50). To determine the neutralizing activity of convalescent plasma/serum samples or vaccine sera, 3-fold serial dilutions of samples were incubated with pseudoviral particles for 1 h and the same strategy as for mAbs was applied.
RBD production for structural analysis
Delta RBD was cloned into pNEO vector as described previously.37 Protein production was as described by Zhou et al.36 Briefly, plasmids encoding RBD were transiently expressed in HEK293T (ATCC CRL-11268) cells. The conditioned medium was dialyzed and purified with a 5-mL HisTrap nickel column (GE Healthcare) and further polished using a Superdex 75 HiLoad 16/60 gel filtration column (GE Healthcare).
IgG mAbs and Fabs production
AstraZeneca and Regeneron antibodies were provided by AstraZeneca, Vir, Lilly and Adagio antibodies were provided by Adagio, LY-CoV1404 was provided by LifeArc. For the in-house antibodies, heavy and light chains of the indicated antibodies were transiently transfected into 293T cells and antibody purified from supernatant on protein A as previously described.13 Fabs were digested from purified IgGs with papain using a Pierce Fab Preparation Kit (Thermo Fisher), following the manufacturer’s protocol.
Competition assays of anti-Omicron BA.2 RBD mAbs
Competition assays of anti-Omicron BA.2 RBD mAbs were performed on an Octet Red 96e machine (Sartorius) using Octet Anti-HIS (HIS2) Biosensors (Sartorius). His-tagged Omicron BA.2 RBD dissolved in the running buffer (10 mM HEPES, pH 7.4 and 150 mM NaCl) was used as the ligand and was first immobilized onto the biosensors. The biosensors were then washed with the running buffer to remove unbound RBD. Each biosensor was dipped into different saturating mAbs (Ab1) to saturate the bound RBD, except one biosensor was dipped into running buffer in this step, acting as the reference. Then all biosensors were washed with the running buffer again and dipped into wells containing the same competing antibody (Ab2). The y axis values of signals of different saturating antibodies in this step were divided by the value of the reference channel to get ratio results of different Ab1-Ab2 pairs. Ratio results close to 0 indicated total competition while 1 indicated no competition.
Antibody mapping to RBD surface
All BA.2 antibodies and several antibodies with previously solved structures (mAb-45, -58, −278, EY6A AZD8895, AZD1061)4 were used in a competition assay prepared for antibody mapping to the RBD surface. Antibody mapping was carried out using Mabscape4 and cluster4x.51 Mid-point positions of mAb-45, -58, −278, EY6A AZD8895, AZD10614 were calculated from crystal structures and used to seed the analysis in 1000 Monte Carlo runs, whereas several known structural positions were not included in the analysis and used as a cross-check. A total of 178 Monte Carlo runs formed a single cluster with the lowest score and these were used to calculate average positions for BA.2 antibodies.
Crystallization, X-ray data collection and structure determination
Purified Delta RBD was deglycosylated with Endo F1 and combined with Fabs EY6A and BA2-10 in a 1:1:1 M ratio with a final concentration of 7 mg mL−1 and combined with Fab BA2-36 in a 1:1 M ratio with a final concentration of 13 mg mL−1. The samples were incubated at room temperature for 30 min. Initial screening of crystals was set up in Crystalquick 96-well X plates (Greiner Bio-One) with a Cartesian Robot using the nanoliter sitting-drop vapor-diffusion method, with 100 nL of protein plus 100 nL of reservoir in each drop, as previously described.52 Good crystals of Delta RBD/EY6A/BA2-10 complex were formed in Hampton Research PEGRx condition 2–18, containing 10% (v/v) 2-propanol, 0.1 M BICINE, pH 8.5 and 30% (w/v) PEG 1500. Crystals of Delta RBD/BA.2–13/C1 nanobody complex were grown in the condition containing 0.1 M BICINE pH 8.5 and 15% (w/v) PEG 1,500. Good crystals of the Delta RBD/BA2-36 complex were formed in Hampton Research PEGRx condition 2–31, containing 2% (v/v) PEG400, 0.1M imidazole pH7.0 and 24% (w/v) PEG MME 5000.
Crystals were mounted in loops and dipped in solution containing 25% glycerol and 75% mother liquor for a second before being frozen in liquid nitrogen. Diffraction data of Delta RBD/EY6A/BA2-10 and Delta RBD/BA2-36 were collected at 100 K at beamline I03 of Diamond Light Source, UK, using the automated queue system that allows unattended automated data collection (https://www.diamond.ac.uk/Instruments/Mx/I03/I03-Manual/Unattended-Data-Collections.html). 3600 diffraction images of 0.1° each were collected from a single crystal of the Delta-RBD/BA.2–10/EY6A complex. 7200 diffraction images were collected from two crystals for Delta-RBD/BA.2–36 complex. 360° of diffraction data for Delta RBD/BA.2–13/C1 were collected at beamline I04. Data were automatically processed with Xia2-dials.53,47 Each of the structures was determined using molecular replacement with Phaser54 and a model of our previously determined RBD/Fab structures that has maximum sequence identity with the current structure.4,13,14 Model rebuilding used COOT46 and refinement Phenix.48
Data collection and structure refinement statistics are given in Table S3. Structural comparisons used SHP55 and figures were prepared with PyMOL (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC).
Cryo-EM structure determination
For Delta-RBD/BA.2–23/BA.2–36/EY6A/mAb-4, delta RBD was incubated with a 1.1 M excess of each Fab on ice for ca. 10 min before application of a 3 μL aliquot of this complex mixture to a freshly glow-discharged (35 s, high with a Plasma Cleaner PDC-002-CE, Harrick Plasma) Quantifoil 2/1 300 mesh grids. Excess liquid was removed by blotting for 6 s with a force of −1 using vitrobot filter paper (grade 595, Ted Pella Inc.) at 4.5°C, 100% reported humidity before plunge freezing into liquid ethane using a Vitrobot Mark IV (Thermo Fisher).
Movies, 20,535 in total, were collected on a Titan Krios operating at 300 kV equipped with a Falcon-IV Selectris at 130 kX magnification, corresponding to a calibrated pixel size of 0.7303 Å2 with a total dose of 50 e−/Å2 using EPU software (ThermoFisher scientific) and defocus range of 0.8–2.6 μm in EER format.
Data were pre-processed on-the-fly in the cryoSPARC live interface, using initial 2D classes from blob-picked particles a template for template picking.56 3,529,798, picked particles, were then 2D classified into 250 classes in cryoSPARC v3.3.2 ‘static’ version, and 335,851 particles that were not obviously ‘junk’ were further classified, resulting in 29,589 particles in classes representing a variety of views. These were then used as input for ab-initio 3D reconstruction to three classes before heterogeneous refinement using the resulting volume set. One class, containing 167,492 particles, clearly commensurate with an RBD decorated with four Fabs, was then refined using non-uniform refinement before unbinning and further refinement to a final reported resolution of 2.9 Å resolution (−93 reported global b-factor).
Phylogenetic tree
The phylogenetic tree was generated by pruning the Nextclade reference tree (https://nextstrain.org/nextclade/sars-cov-2/21L) which contains one sequence per Pango lineage. The tree was generated with a Snakemake workflow using the Augur toolchain. The workflow is available at: https://github.com/neherlab/nextclade_data_workflows/tree/27bf7e0b4f62cbbbc9a8ac96db1587cd76b3ae10.
The topology of the tree was constrained using the Usher tree that incorporates nearly all sequences available through GISAID.26 The tree was pruned using a BioPython script and visualized using Figtree.
Quantification and statistical analysis
Statistical analyses are reported in the results and figure legends. Neutralization was measured on pseudovirus. The percentage reduction was calculated and IC50 determined using the probit program from the SPSS package. The Wilcoxon matched-pairs signed rank test was used for the analysis and two-tailed p values were calculated on geometric mean values.
Acknowledgments
This work was supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (CIFMS), China (grant number: 2018-I2M-2-002), to D.I.S. and G.R.S. We are also grateful for support from Schmidt Futures, the Red Avenue Foundation, and the Oak Foundation. H.M.E.D. and J.R. are supported by Wellcome (203141/Z/16/Z and 101122/Z/13/Z respectively) and D.I.S. and E.E.F. by the UKRI MRC (MR/N00065X/1). D.I.S. and G.R.S. are Jenner Investigators. This is a contribution from the UK Instruct-ERIC Centre. A.J.M. is an NIHR-supported Academic Clinical Lecturer. This work is supported by the Oxford Biomedical Research Centre and an Oxfordshire Health Services Research Committee grant to A.J.M. The Wellcome Center for Human Genetics is supported by the Wellcome Trust (grant 090532/Z/09/Z). The computational aspects of this research were supported by the Wellcome Trust Core Award grant number 203141/Z/16/Z and the NIHR Oxford BRC and we acknowledge the Wellcome Human Genetics Flow Cytometry Facility. Electron microscopy provision was provided through the OPIC electron microscopy facility, a UK Instruct-ERIC Centre, which was founded by a Wellcome JIF award (060208/Z/00/Z) and is supported by a Wellcome equipment grant (093305/Z/10/Z). This work was supported by the UK Department of Health and Social Care as part of the PITCH (Protective Immunity from T cells to Covid-19 in Health workers) consortium, the UK Coronavirus Immunology consortium (UK-CIC), and the Huo Family Foundation. We acknowledge the Diamond Light Source for time on Beamline I03 under proposal lb27009 for COVID-19 Rapid Access. E.B. and P.K. are NIHR Senior Investigators, and P.K. is funded by WT222426/Z/21/Z and NIH (U19 I082360). S.J.D. is funded by an NIHR Global Research Professorship (NIHR300791). The Sheffield Teaching Hospitals Observational Study of Patients with Pulmonary Hypertension, Cardiovascular and Other Respiratory Diseases (STH-ObS) was supported by the British Heart Foundation (PG/11/116/29288). We gratefully acknowledge financial support from the UK Department of Health via the Sheffield NIHR Clinical Research Facility award to the Sheffield Teaching Hospitals Foundation NHS Trust. T.P.P. is funded by the G2P-UK National Virology Consortium funded by the MRC (MR/W005611/1).
Author contributions
A.D.-G., R.D., C.L., R.N., P.S., M.S., G.R.S., and J.M. generated and characterized mAbs. A.D.-G., R.D., C.L., and R.N. generated pseudoviruses and performed neutralization tests. D.Z., J.R., N.G.P., M.A.W., and D.R.H. prepared the crystals and enabled and performed X-ray data collection. H.M.E.D. performed cryo-EM analyses. J.R., E.E.F., H.M.E.D., and D.I.S. analyzed the structural results. D.Z. and J.H. prepared the RBDs. D.Z. performed BLI competition assays. N.T. provided materials. T.P.P. and C.R. assisted with analysis of new BA.2 sub-lineages, C.R. made the phylogenetic tree. H.M.G. wrote Mabscape and performed mapping and cluster analysis, including sequence and antigenic space analyses. T.I.d.S., M.P., T.A.H.N., H.H., A.J.M., D.S., T.G.R., P.K., E.B., and S.J.D. assisted with patient samples and vaccine trials in the OPTIC Healthcare Worker and the Sheffield STH-Obs studies. E.B., S.J.D., and P.K. conceived the study of vaccinated healthcare workers and oversaw study and sample collection/processing. G.R.S., and D.I.S. conceived the study and wrote the initial manuscript draft with other authors providing editorial comments. All authors read and approved the manuscript.
Declaration of interests
G.R.S. sits on the GSK Vaccines Scientific Advisory Board, consults for AstraZeneca, and is a founding member of RQ Biotechnology. D.I.S. consults for AstraZeneca. Oxford University holds intellectual property related to SARS-CoV-2 mAbs discovered in G.R.S.’s laboratory. S.J.D. is a scientific advisor to the Scottish Parliament on COVID-19.
Published: March 7, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112271.
Contributor Information
Elizabeth E. Fry, Email: liz@strubi.ox.ac.uk.
Juthathip Mongkolsapaya, Email: juthathip.mongkolsapaya@well.ox.ac.uk.
Jingshan Ren, Email: ren@strubi.ox.ac.uk.
David I. Stuart, Email: dave@strubi.ox.ac.uk.
Gavin R. Screaton, Email: gavin.screaton@medsci.ox.ac.uk.
OPTIC consortium:
Christopher Conlon, Alexandra Deeks, John Frater, Siobhan Gardiner, Anni Jämsén, Katie Jeffery, Tom Malone, Eloise Phillips, Barbara Kronsteiner-Dobramysl, Priyanka Abraham, Sagida Bibi, Teresa Lambe, Stephanie Longet, Tom Tipton, Miles Carrol, and Lizzie Stafford
Supplemental information
and supplemental references
Data and code availability
-
•
Coordinates are deposited in the PDB: Delta-RBD/BA.2-36, PDB:8BBO. Delta-RBD/EY6A/BA.2-10, PDB:8BBN. Delta-RBD/BA.2-13/C1, PDB:8C3V. Delta-RBD/BA.2-23/BA.2-36/EY6A/Fab-45, PDB:8BCZ and EMDB:EMD-15971.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
- 1.Domingo E. Mechanisms of viral emergence. Vet. Res. 2010;41:38. doi: 10.1051/vetres/2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sender R., Bar-On Y.M., Gleizer S., Bernshtein B., Flamholz A., Phillips R., Milo R. The total number and mass of SARS-CoV-2 virions. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2024815118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 4.Dejnirattisai W., Zhou D., Ginn H.M., Duyvesteyn H.M.E., Supasa P., Case J.B., Zhao Y., Walter T.S., Mentzer A.J., Liu C., et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell. 2021;184:2183–2200.e22. doi: 10.1016/j.cell.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerutti G., Guo Y., Zhou T., Gorman J., Lee M., Rapp M., Reddem E.R., Yu J., Bahna F., Bimela J., et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe. 2021;29:819–833.e7. doi: 10.1016/j.chom.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes C.O., Jette C.A., Abernathy M.E., Dam K.M.A., Esswein S.R., Gristick H.B., Malyutin A.G., Sharaf N.G., Huey-Tubman K.E., Lee Y.E., et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan M., Liu H., Wu N.C., Lee C.C.D., Zhu X., Zhao F., Huang D., Yu W., Hua Y., Tien H., et al. Structural basis of a shared antibody response to SARS-CoV-2. Science. 2020;369:1119–1123. doi: 10.1126/science.abd2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gobeil S.M.C., Janowska K., McDowell S., Mansouri K., Parks R., Stalls V., Kopp M.F., Manne K., Li D., Wiehe K., et al. Effect of natural mutations of SARS-CoV-2 on spike structure, conformation, and antigenicity. Science. 2021;373 doi: 10.1126/science.abi6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejnirattisai W., Zhou D., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184:2939–2954.e9. doi: 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Nutalai R., Tuekprakhon A., et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201–2211.e7. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zahradník J., Marciano S., Shemesh M., Zoler E., Harari D., Chiaravalli J., Meyer B., Rudich Y., Li C., Marton I., et al. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat. Microbiol. 2021;6:1188–1198. doi: 10.1038/s41564-021-00954-4. [DOI] [PubMed] [Google Scholar]
- 12.Dejnirattisai W., Huo J., Zhou D., Zahradník J., Supasa P., Liu C., Duyvesteyn H.M.E., Ginn H.M., Mentzer A.J., Tuekprakhon A., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484.e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nutalai R., Zhou D., Tuekprakhon A., Ginn H.M., Supasa P., Liu C., Huo J., Mentzer A.J., Duyvesteyn H.M.E., Dijokaite-Guraliuc A., et al. Potent cross-reactive antibodies following Omicron breakthrough in vaccinees. Cell. 2022;185:2116–2131.e18. doi: 10.1016/j.cell.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C., Zhou D., Nutalai R., Duyvestyn H., Tuekprakhon A., Ginn H., Dejnirattisai W., Supasa P., Mentzer A., Wang B., et al. The Beta mAb response underscores the antigenic distance to other SARS-CoV-2 variants Cell. Host and Microbe. 2021;30:53–68. doi: 10.1016/j.chom.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 16.Westendorf K., Žentelis S., Wang L., Foster D., Vaillancourt P., Wiggin M., Lovett E., van der Lee R., Hendle J., Pustilnik A., et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Liu J., Plante K.S., Plante J.A., Xie X., Zhang X., Ku Z., An Z., Scharton D., Schindewolf C., et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature. 2022;602:294–299. doi: 10.1038/s41586-021-04245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Straten K., Guerra D., van Gils M.J., Bontjer I., Caniels T.G., van Willigen H.D.G., Wynberg E., Poniman M., Burger J.A., Bouhuijs J.H., et al. Antigenic cartography using sera from sequence-confirmed SARS-CoV-2 variants of concern infections reveals antigenic divergence of Omicron. Immunity. 2022;55:1725–1731.e4. doi: 10.1016/j.immuni.2022.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., San J.E., Cromer D., Scheepers C., Amoako D.G., et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCallum M., Czudnochowski N., Rosen L.E., Zepeda S.K., Bowen J.E., Walls A.C., Hauser K., Joshi A., Stewart C., Dillen J.R., et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022;375:864–868. doi: 10.1126/science.abn8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia S., Wang L., Zhu Y., Lu L., Jiang S. Origin, virological features, immune evasion and intervention of SARS-CoV-2 Omicron sublineages. Signal Transduct. Target. Ther. 2022;7:241. doi: 10.1038/s41392-022-01105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huo J., Dijokaite-Guraliuc A., Nutalai R., Das R., Zhou D., Mentzer A., Fry E., Mongkolsapaya J., Ren J., Stuart D., Screaton G. Humoral responses against SARS-CoV-2 Omicron BA.2.11, BA.2.12.1 and BA.2.13 from vaccine and BA.1 serum. Cell Discovery. 2022;8:119. doi: 10.1038/s41421-022-00482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., Zhou D., Ginn H.M., Selvaraj M., Liu C., Mentzer A.J., Supasa P., Duyvesteyn H.M.E., et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422–2433.e13. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starr T.N., Greaney A.J., Hilton S.K., Ellis D., Crawford K.H.D., Dingens A.S., Navarro M.J., Bowen J.E., Tortorici M.A., Walls A.C., et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182:1295–1310.e20. doi: 10.1016/j.cell.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turakhia Y., Thornlow B., Hinrichs A.S., De Maio N., Gozashti L., Lanfear R., Haussler D., Corbett-Detig R. Ultrafast Sample placement on Existing tRees (UShER) enables real-time phylogenetics for the SARS-CoV-2 pandemic. Nat. Genet. 2021;53:809–816. doi: 10.1038/s41588-021-00862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dijokaite-Guraliuc A., Das R., Nutalai R., Zhou D., Mentzer A.J., Liu C., Supasa P., Dunachie S.J., Lambe T., Fry E.E., et al. Antigenic characterization of SARS-CoV-2 Omicron subvariant BA.4.6. Cell Discov. 2022;8:127. doi: 10.1038/s41421-022-00493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo J., Dijokaite-Guraliuc A., Liu C., Zhou D., Ginn H.M., Das R., Supasa P., Selvaraj M., Nutalai R., Tuekprakhon A., et al. A delicate balance between antibody evasion and ACE2 affinity for Omicron BA.2.75. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2022.111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D., Duyvesteyn H.M.E., Chen C.P., Huang C.G., Chen T.H., Shih S.R., Lin Y.C., Cheng C.Y., Cheng S.H., Huang Y.C., et al. Structural basis for the neutralization of SARS-CoV-2 by an antibody from a convalescent patient. Nat. Struct. Mol. Biol. 2020;27:950–958. doi: 10.1038/s41594-020-0480-y. [DOI] [PubMed] [Google Scholar]
- 30.Yuan M., Huang D., Lee C.C.D., Wu N.C., Jackson A.M., Zhu X., Liu H., Peng L., van Gils M.J., Sanders R.W., et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. Science. 2021;373:818–823. doi: 10.1126/science.abh1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong J., Zost S., Greaney A., Starr T.N., Dingens A.S., Chen E.C., Chen R., Case B., Sutton R., Gilchuk P., et al. Genetic and structural basis for recognition of SARS-CoV-2 spike protein by a two-antibody cocktail. bioRxiv. 2021 doi: 10.1101/2021.01.27.428529. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan M., Zhu X., He W.T., Zhou P., Kaku C.I., Capozzola T., Zhu C.Y., Yu X., Liu H., Yu W., et al. A broad and potent neutralization epitope in SARS-related coronaviruses. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2205784119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y., Ho M. Emerging antibody-based therapeutics against SARS-CoV-2 during the global pandemic. Antib. Ther. 2020;3:246–256. doi: 10.1093/abt/tbaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou D., Dejnirattisai W., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., Nutalai R., Zhou D., Mentzer A.J., Zhao Y., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eguia R.T., Crawford K.H.D., Stevens-Ayers T., Kelnhofer-Millevolte L., Greninger A.L., Englund J.A., Boeckh M.J., Bloom J.D. A human coronavirus evolves antigenically to escape antibody immunity. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon-Loriere E., Schwartz O. Towards SARS-CoV-2 serotypes? Nat. Rev. Microbiol. 2022;20:187–188. doi: 10.1038/s41579-022-00708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q., Ye S.B., Zhou Z.J., Li J.Y., Lv J.Z., Hu B., Yuan S., Qiu Y., Ge X.Y. Key mutations on spike protein altering ACE2 receptor utilization and potentially expanding host range of emerging SARS-CoV-2 variants. J. Med. Virol. 2023;95:e28116. doi: 10.1002/jmv.28116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thakur N., Gallo G., Newman J., Peacock T.P., Biasetti L., Hall C.N., Wright E., Barclay W., Bailey D. SARS-CoV-2 variants of concern alpha, beta, gamma and delta have extended ACE2 receptor host ranges. J. Gen. Virol. 2022;103 doi: 10.1099/jgv.0.001735. [DOI] [PubMed] [Google Scholar]
- 42.He P., Liu B., Gao X., Yan Q., Pei R., Sun J., Chen Q., Hou R., Li Z., Zhang Y., et al. SARS-CoV-2 Delta and Omicron variants evade population antibody response by mutations in a single spike epitope. Nat. Microbiol. 2022;7:1635–1649. doi: 10.1038/s41564-022-01235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aricescu A.R., Lu W., Jones E.Y. (2006). A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 62, 1243–50. 10.1107/S0907444906029799. [DOI] [PubMed]
- 44.Stewart S.A., Dykxhoorn D.M., Palliser D., Mizuno H., Yu E.Y., An D.S., Sabatini D.M., Chen I.S., Hahn W.C., Sharp P.A., Weinberg R.A., Novina C.D. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nettleship Joanne, Ren Jingshan, Rahman Nahid, Berrow Nick, Hatherley Deborah, Barclay A., Owens Raymond.A pipeline for the production of antibody fragments for structural studies using transient expression in HEK 293T cells. Protein expression and purification. 2008;62:83–89. doi: 10.1016/j.pep.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winter G., Waterman D.G., Parkhurst J.M., Brewster A.S., Gildea R.J., Gerstel M., Fuentes-Montero L., Vollmar M., Michels-Clark T., Young I.D., et al. DIALS: implementation and evaluation of a new integration package. Acta Crystallogr. D Struct. Biol. 2018;74:85–97. doi: 10.1107/S2059798317017235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebschner D., Afonine P.V., Baker M.L., Bunkóczi G., Chen V.B., Croll T.I., Hintze B., Hung L.W., Jain S., McCoy A.J., et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 2019;75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibson D.G. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011;498:349–361. doi: 10.1016/B978-0-12-385120-8.00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M., et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ginn H.M. Pre-clustering data sets using cluster4x improves the signal-to-noise ratio of high-throughput crystallography drug-screening analysis. Acta Crystallogr. D Struct. Biol. 2020;76:1134–1144. doi: 10.1107/S2059798320012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter T.S., Diprose J., Brown J., Pickford M., Owens R.J., Stuart D.I., Harlos K. A procedure for setting up high-throughput nanolitre crystallization experiments. I. Protocol design and validation. J. Appl. Crystallogr. 2003;36:308–314. doi: 10.1107/S0021889803001997. [DOI] [Google Scholar]
- 53.Winter G. xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr. 2010;43:186–190. [Google Scholar]
- 54.McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuart D.I., Levine M., Muirhead H., Stammers D.K. Crystal structure of cat muscle pyruvate kinase at a resolution of 2.6 A. J. Mol. Biol. 1979;134:109–142. doi: 10.1016/0022-2836(79)90416-9. [DOI] [PubMed] [Google Scholar]
- 56.Punjani A., Rubinstein J.L., Fleet D.J., Brubaker M.A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
article
and supplemental references
Data Availability Statement
-
•
Coordinates are deposited in the PDB: Delta-RBD/BA.2-36, PDB:8BBO. Delta-RBD/EY6A/BA.2-10, PDB:8BBN. Delta-RBD/BA.2-13/C1, PDB:8C3V. Delta-RBD/BA.2-23/BA.2-36/EY6A/Fab-45, PDB:8BCZ and EMDB:EMD-15971.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.