Abstract
The tyrosine kinase ZAP-70 has been implicated as a critical intermediary between T-cell antigen receptor (TCR) stimulation and Erk activation on the basis of the ability of dominant negative ZAP-70 to inhibit TCR-stimulated Erk activation, and the reported inability of anti-CD3 antibodies to activate Erk in ZAP-70-negative Jurkat cells. However, Erk is activated in T cells receiving a partial agonist signal, despite failing to activate ZAP-70. This discrepancy led us to reanalyze the ZAP-70-negative Jurkat T-cell line P116 for its ability to support Erk activation in response to TCR/CD3 stimulation. Erk was activated by CD3 cross-linking in P116 cells. However, this response required a higher concentration of anti-CD3 antibody and was delayed and transient compared to that in Jurkat T cells. Activation of Raf-1 and MEK-1 was coincident with Erk activation. Remarkably, the time course of Ras activation was comparable in the two cell lines, despite proceeding in the absence of LAT tyrosine phosphorylation in the P116 cells. CD3 stimulation of P116 cells also induced tyrosine phosphorylation of phospholipase C-γ1 (PLCγ1) and increased the intracellular Ca2+ concentration. Protein kinase C (PKC) inhibitors blocked CD3-stimulated Erk activation in P116 cells, while parental Jurkat cells were refractory to PKC inhibition. The physiologic relevance of these signaling events is further supported by the finding of PLCγ1 tyrosine phosphorylation, Erk activation, and CD69 upregulation in P116 cells on stimulation with superantigen and antigen-presenting cells. These results demonstrate the existence of two pathways leading to TCR-stimulated Erk activation in Jurkat T cells: a ZAP-70-independent pathway requiring PKC and a ZAP-70-dependent pathway that is PKC independent.
Signals generated on engagement of the T-cell antigen receptor (TCR) are critical in the regulation of T-lymphocyte function. TCR signal transduction is mediated proximally by multiple tyrosine kinases, which act in concert to activate a diverse array of signaling molecules (6, 10, 35, 55–57, 64). Key among these downstream effectors are the enzymes phospholipase C-γ1 (PLCγ1) and the extracellular-signal-regulated kinase (Erk), both of which need to be activated in order for TCR engagement to result in T cell activation. Activated PLCγ1 catalyzes the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) to inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). The former product regulates the levels of intracellular Ca2+, while the latter is an activator of the classical (cPKC: α, βI, βII and γ), and novel (nPKC: δ, ɛ, η and θ) isoforms of protein kinase C (PKC) and of Ras-GRP (25). Erk is a proline-directed serine/threonine kinase that can phosphorylate and regulate multiple downstream effectors, including p90RSK and the transcription factor Elk-1.
The nature of the intervening steps between TCR stimulation and activation of these enzymes has begun to be elucidated, but our understanding of this process remains incomplete. Considerable evidence points to a required Lck/Fyn-catalyzed tyrosine phosphorylation of the CD3 and TCRζ chains, with the resultant TCR recruitment and activation of the protein tyrosine kinase (PTK) ZAP-70, which then phosphorylates two of its substrates, SLP-76 and LAT, on key tyrosine residues (10, 35, 56, 57, 64). These last two proteins serve as linker molecules. They have no intrinsic enzymatic activity but, when tyrosine phosphorylated, function by appropriately colocalizing other signaling molecules. SLP-76 is cytosolic, while the majority of LAT partitions to the lipid rafts by virtue of posttranslational palmitoylation proximal to the endofacial side of its transmembrane domain. When phosphorylated, LAT binds directly to PLCγ1, Grb2, Grap, and Gads, effectively localizing these molecules and their associated proteins (including phosphatidylinositol 3-kinase, SOS, c-Cbl, Vav, SLP-76, and Itk) to the lipid rafts of the plasma membrane. This event is thought to be required for PLCγ1 tyrosine phosphorylation and activation, as well as the activation of Erk. It has been proposed that the LAT-assembled complex colocalizes PLCγ1 with the activated PTK (possibly Itk) that phosphorylates and activates it and that this process requires Gads-bound SLP-76 (35, 56, 64). Additionally, LAT association positions PLCγ1 near its substrate, PI-4,5-P2, potentially increasing the rate of PI-4,5-P2 hydrolysis.
Precisely how the formation of the LAT-associated signaling complex leads to Erk activation is unclear. Erk activation proceeds primarily through the sequential activation of Ras, Raf-1, and MEK. It has been suggested that Ras is activated in TCR-stimulated T cells via recruitment of Grb2-associated SOS, a guanine nucleotide exchange factor for Ras, to the plasma membrane by virtue of the capability of the SH2 domains of Grb2 to bind to membrane-resident, tyrosine-phosphorylated proteins such as LAT (10, 35, 56, 64). This is analogous to what has been observed for Ras activation mediated by the engagement of growth factor receptors (37). However, other mechanisms of Ras activation have also been found in T cells. One mechanism involves activation of PKC (6), which can activate Raf-1 directly (7, 26, 32, 53), and another involves Ras-GRP, which is expressed at high levels in lymphocytes, and was recently identified as a phorbol ester-activated (and presumably DAG-activated) guanine nucleotide exchange factor for Ras (15, 29, 51). Ras-GRP is required for normal thymocyte development and is activated in response to TCR engagement (14, 16). Therefore, multiple signaling pathways probably exist for connecting TCR engagement to Ras, and subsequently Erk, activation.
Given the reported requirement for LAT and SLP-76 in TCR-initiated activation of PLCγ1 and Erk and the demonstrated importance of ZAP-70-mediated tyrosine phosphorylation in supporting the function of these linker molecules (10, 35, 56, 64), it would be expected that ZAP-70 would also be required for PLCγ1 activation and Erk activation in TCR-stimulated T cells. Indeed, a previous study found that overexpression of dominant negative forms of ZAP-70 could block the activation of nuclear factor of activated T cells (NF-AT) (an event distal to PLCγ1 and Erk activation) in Jurkat T cells (38). In addition, it has been reported that ZAP-70-negative mutants of the Jurkat T-cell line fail to flux Ca2+ or activate Erk in response to anti-CD3 monoclonal antibody (MAb) (59, 60). However, increased Erk activation (8, 9) and Ca2+ mobilization (4, 28, 47) have been measured in T-cell clones and naive CD4+ peripheral T cells stimulated under partial-agonist conditions, conditions that do not support the activation of ZAP-70 (4, 28, 31, 46). In an attempt to reconcile these apparently discrepant observations, we have reexamined the signaling pathways leading to Ca2+ mobilization and Erk activation in TCR-stimulated, ZAP-70-negative P116 Jurkat T cells.
MATERIALS AND METHODS
Cells.
The wild-type E6.1 subline of Jurkat T cells (20, 41) and the P116, J14, JCaM1, and JCaM2 somatic mutants of E6.1 Jurkat, which have deficient expression of ZAP-70, SLP-76, Lck, and LAT, respectively, have been described previously (18, 49, 60, 61). P116 cells were a gift of R.T. Abraham (Duke University, Raleigh, NC). The J14-v-29 (vector-transfected) and J14-s-11 (SLP-76-transfected) cells were a gift of A. Weiss (University of California, San Franciso, Calif.). The JCaM1 cells were purchased from the American Tissue Culture Collection, and the Lck-transfected JCaM1 cells were a gift of David B. Straus (University of Chicago, Chicago, Ill.). The JCaM2.5 and LAT-transfected JCaM2 cells (JCaM2.5B3) were a gift of A. Weiss and L. E. Samelson (National Cancer Institute, Bethesda, Md.). These cells were maintained in RPMI 1640 medium supplemented with 7.5% fetal bovine serum (HyClone), 2 mM l-glutamine, and 10 μg of ciprofloxacin hydrochloride (Bayer) per ml. The parental and mutant Jurkat T-cell lines were periodically tested by flow cytometry to confirm equivalent expression levels of CD3 and by Western blotting to confirm the appropriate expression level of the signaling molecules under investigation. In particular, the P116 cells were routinely tested to ensure that ZAP-70 was not being expressed in these cells. The LG2 B lymphoblastoid cell line that expresses HLA-DR1 and B7.1 was kindly provided by A. Sette (Epimmune, San Diego, Calif.) and was maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 1× MEM nonessential amino acids (GIBCO), penicillin (10 IU), and streptomycin (100 μg/ml) (Wisent, Montreal, Quebec, Canada).
Antibodies and other reagents.
The human CD3-ɛ-specific MAbs OKT3 (immunoglobulin G2A [IgG2a]) and UCHT-1 (IgG1), were a custom ascites (Biodesign International) and purchased from Pharmingen, respectively. The MAbs to antiphosphotyrosine (4G10) and PLCγ1 were obtained from UBI. Rabbit polyclonal antibody to human phosphorylated, activated Erk was purchased from Promega or StressGen. MAb to phosphorylated, activated Erk and rabbit polyclonal antibody to phosphorylated, activated MEK were purchased from Cell Signaling. Anti-pan Ras antibody was purchased from CalBiochem. MAb (for immunoprecipitation) and rabbit antisera (for blotting) against Raf-1 were obtained from BD Transduction Laboratories and Santa Cruz Biotechnology, respectively. Horseradish peroxidase-conjugated sheep anti-mouse and donkey anti-rabbit IgGs were purchased from Amersham. Horseradish peroxidase-conjugated goat anti-rabbit IgG was from Bio-Rad. Anti-TCR Vβ8-phycoarythrinP and anti-CD69-fluorescein isothiocyanate were from Pharmingen. Partially purified staphylococcal enterotoxin E (SEE) was purchased from Toxin Technologies, Inc. (Sarasota, Fla.). Purified, full-length MEK (Raf-1 substrate) was from Santa Cruz Biotechnology. The fusion protein containing the full-length sequence of Grb2 fused to the C terminus of glutathione S-transferase (GST) was purified from Escherichia coli cultures transformed with the plasmid pGEX-2TK-Grb2 (a gift of G. A. Koretzky, University of Pennsylvania, Philadelphia, Pa.) as previously described (43). The bacterial expression plasmid encoding the Ras binding domain of Raf-1 fused to GST was a gift of S. J. Taylor (Cornell University, Ithaca, N.Y.). The NF-AT-luciferase reporter construct (36) was a gift of G. R. Crabtree.
Cellular stimulation, immunoprecipitation, affinity precipitation, and immunoblot analyses.
Cells were washed and resuspended in RPMI 1640 at 108 cells/ml. Cell aliquots were preincubated at 37°C for 5 min prior to the addition of stimulus. OKT3 or UCHT-1 was used at the concentrations and for the durations indicated in the figures. Where indicated, the cells were also preincubated for 15 min at 37°C with the PKC inhibitors Gö6850, Gö6976, or Ro-31-8220 or the tyrosine kinase inhibitor PP-1. Inhibitors were purchased from Calbiochem. Anti-CD3 stimulation was terminated by the addition of 5 volumes of 4°C lysis buffer [20 mM HEPES (pH 7.4), 1% Triton X-100, 50 mM β-glycerophosphate, 2 mM EGTA, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 10% glycerol, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 100 μg of 4-(2-aminoethyl)-benzenesulfonyl fluoride) per ml]. After a 30-min incubation on ice, whole-cell lysates (WCL) were prepared by a 10-min centrifugation at 4°C and 21,000 × g. The lysates were either directly analyzed by Western blotting or subjected to immunoprecipitation or affinity precipitation with GST-Grb2 followed by immunoblotting as previously described (43). Immunoblots were developed by enhanced chemiluminescence using the Renaissance horseradish peroxidase substrate (Dupont NEN). Where indicated, the immunoblots were quantitated by densitometric analysis using Scion Image (Scion Corp.)
Ras and Raf-1 activation assays.
The Ras activation assay was carried out as previously described (50). In brief, Jurkat lysates were incubated with a glutathione agarose-immobilized GST fusion protein containing the Ras binding domain (RBD) of Raf-1. The fusion protein does not bind to Ras-GDP but binds with high affinity to Ras-GTP. The amount of Ras detected in immunoblots of the GST–Raf-1–RBD affinity precipitations is a measure of Ras activation (GTP binding). The Raf-1 activation assay was carried out as previously described (3) and measures the ability of immunoprecipitated Raf-1 to phosphorylate purified MEK protein in an immune complex kinase assay, where Raf-1-phosphorylated MEK-1 is detected by immunoblotting with an antibody specific for phosphorylated MEK-1 (Ser-217 and Ser-221).
Intracellular free Ca2+ measurements.
Jurkat and P116 cells were loaded with the Ca2+ indicator dye, Indo-1-AM (Molecular Probes) essentially as described previously (60), and stimulated with UCHT-1 (various concentrations), pervanadate (100 μM Na3VO4, 300 μM H2O2) or Ionomycin (1 μg/ml). A 2-ml volume of cells at a density of 5 × 105 cells/ml were placed online into the mixing chamber of a FACStar Plus flow cytometer (Becton Dickinson). Baseline measurements were collected for 1 min, 50 μl of 40× stimulant was added, and measurement was continued for an additional 5 min. Stimulus-induced changes in the intracellular Ca2+ concentration were determined over time by monitoring the fluorescence emission ratio of the Ca2+-bound versus free form of indo-1 at 405 and 495 nm, respectively; data were analyzed and plots were generated using MultiTime Kinetic software (Phoenix Flow Systems).
NF-AT reporter assay.
The NF-AT luciferase reporter gene was transfected into Jurkat and P116 cells as previously described (42). The cells were maintained overnight in complete medium before being stimulated with increasing concentrations of the anti-CD3 UCHT1 or phorbol myristate acetate PMA (50 ng/ml) for 6 hours at 37°C. After stimulation, the cells were washed twice in phosphate-buffered saline, lysed, and analyzed by the luciferase assay system (Promega) with an Autolumat LB953 luminometer (Perkin-Elmer).
Superantigen stimulation.
For stimulation with SEE, T cells were washed and resuspended in RPMI 1640 at 108 cells/ml. After equilibration at 37°C for 10 min, aliquots of 107 cells were incubated for 30 min at 37°C for the indicated times with 2 × 106 LG2 cells previously loaded with the indicated amount of SEE. SEE stimulation was terminated by the addition at 4°C of phosphate-buffered saline–0.4 mM EDTA–0.4 mM sodium orthovanadate. The cells were lysed with lysis buffer (10 mM Tris-HCl [pH 7.5], 1% Triton X-100, 5 mM EDTA, 1 mM sodium orthovanadate, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 25 μM p-nitrophenyl-p'-guanidinobenzoate) for 30 min at 4°C. Whole-cell lysates were prepared by a 10-min centrifugation at 4°C and 21,000 × g and analyzed by Western blotting, either directly or after immunoprecipitation with anti-PLCγ1 MAb. Immunoblots were developed by enhanced chemiluminescence using the BM chemiluminescence substrate (Roche).
RESULTS
CD3 cross-linking activates Erk and MEK-1 in the absence of ZAP-70 expression.
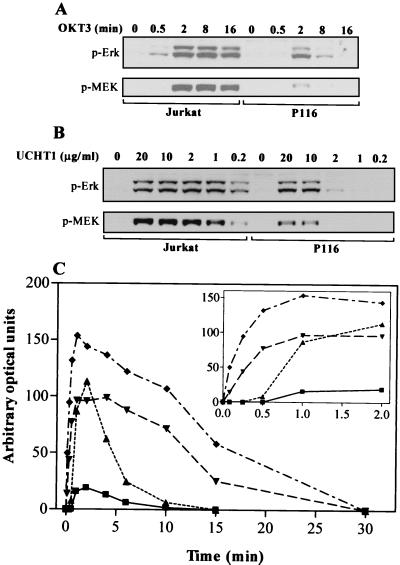
Previously we reported that the activation of Erk in Jurkat T cells stimulated with anti-CD3 MAb does not necessarily require the PTK ZAP-70. This conclusion was drawn from the observation that Erk2 activation, after 3 min of stimulation with OKT3, was largely comparable in both ZAP-70-negative P116 cells and in the parental, ZAP-70-replete, Jurkat T-cell line (21). This observation was unexpected, since overexpression of dominant negative ZAP-70 blocks Erk activation in Jurkat T cells (38), and previous studies have reported that P116 cells fail to flux Ca2+ or activate Erk in response to anti-CD3 MAb (59, 60). In light of these discrepancies, we undertook to more thoroughly examine the role played by ZAP-70 in Erk activation and the mechanisms that might underlie ZAP-70-independent Erk activation in Jurkat T cells. A kinetic analysis of Erk activation, as measured by immunoblotting for the dually phosphorylated, activated forms of Erk1 and Erk2, found that Erk activation in response to CD3 cross-linking was delayed and transient in the P116 cells compared to the wild-type Jurkat cells (Fig. 1A). In the parental Jurkat cells, Erk activation was seen as early as 30 s and persisted at high levels from 2 to 16 min. However, in P116 cells Erk activation was not seen until 2 min after stimulation and declined rapidly thereafter, approaching baseline by 8 min. Quantitation by densitometric analysis of the anti-phospho-Erk immunoblots from a similar experiment with a more extensive time course more clearly illustrates the delayed and transient nature of Erk activation in P116 cells (Fig 1C). Activation of Erk1 and Erk2 was very rapid in CD3-stimulated Jurkat T cells, with measurable increases in Erk phosphorylation as early as 5 s after stimulation (inset in Fig. 1C). Appreciable Erk1 and Erk2 activation in CD3-stimulated P116 cells was not detected until 1 min after stimulation. Erk1 activation was very weak in P116 cells. Identical results were obtained with OKT3 and UCHT1 (anti-CD3 MAbs of IgG2a and IgG1 isotypes, respectively) (data not shown). Isotype-matched control MAbs had no effect on Erk activation (data not shown), indicating that Erk activation in the P116 T cells was not due to nonspecific effects of the high concentration of antibody.
FIG. 1.
CD3 cross-linking induces Erk and MEK-1 phosphorylation in P116 T cells. (A) Parental Jurkat and ZAP-70-negative P116 T cells were stimulated by CD3 cross-linking at 37°C with OKT3 ascites (1:50) for 0, 0.5, 2, 8, or 16 min. WCL proteins were resolved by electrophoresis on 4 to 12% NuPAGE gradient gels run in morpholinepropanesulfonic acid (MOPS) buffer, transferred to nitrocellulose, and immunoblotted for dually phosphorylated, activated Erk. Phospho-Erk1 is the upper band, and phospho-Erk2 is the lower band. Membranes were stripped and then blotted for phosphorylated MEK-1. (B) Jurkat and P116 T cells were stimulated by CD3 cross-linking at 37°C for 3 min with purified UCHT1 MAb. The concentration of UCHT1 was varied from 0.2 to 20 μg/ml. Erk and MEK-1 phosphorylation were measured as in panel A. (C) As for panel Δ, except that the time course encompassed 0-, 0.08-, 0.25-, 0.5-, 1-, 2-, 4-, 6-, 10-, 15-, and 30-min stimulations with OKT3 ascites (1:50). The data from the phospho-Erk immunoblot were quantitated by densitometric analysis using Scion Image software. Symbols: ⧫, Jurkat Erk2; ▾, Jurkat Erk1; ▴, P116 Erk2; ■, P116 Erk1. The inset is a replot of the first six time points using an expanded scale on the x axis.
In addition to examining the kinetics of Erk activation in these two cell lines, we examined the dose response for Erk activation by 3 min of stimulation with anti-CD3 MAb (UCHT1). Erk activation in the P116 cells required approximately 10-fold more UCHT1 than was required for comparable Erk activation in the parental Jurkat cells (Fig. 1B). Notably, while 1 μg of anti-CD3 MAb per ml was sufficient to induce maximal Erk activation in the parental Jurkat T cells, this concentration was completely ineffective in stimulating Erk activation in P116 cells.
Erk phosphorylation and activation proceeds via the phosphorylation and activation of MEK-1, which directly phosphorylates the regulatory threonine and tyrosine residues of Erk. MEK-1 phosphorylation in response to CD3 stimulation in parental and P116 Jurkat T cells was measured by immunoblotting WCLs with a polyclonal antibody specific for phosphorylated MEK-1 (Fig. 1). In both cell lines, CD3-stimulated MEK-1 phosphorylation proceeded via a time course and dose response that was identical to Erk phosphorylation. This is consistent with Erk phosphorylation in both the ZAP-70-negative and -replete Jurkat T-cell lines proceeding through the phosphorylation and activation of MEK-1 by Raf-1.
Activation of Raf-1 and Ras in the absence of ZAP-70 expression.
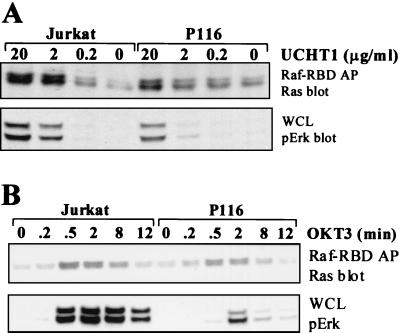
Anti-CD3 MAb-stimulated activation of Raf-1 kinase activity was measured via an immune complex kinase assay for Raf-1 purified from either parental or P116 Jurkat T cells (Fig. 2A). Raf-1 activation was observed in both cell lines and, as was the case for Erk and MEK-1 phosphorylation, required higher concentrations of anti-CD3 MAb to elicit activation of Raf-1 in the ZAP-70-negative P116 cells. We also examined the time course of Raf-1 activation in the parental and P116 Jurkat T cells (Fig. 2B). In both cell lines, the time course of Raf-1 kinase activation correlated well with the time course of Erk and MEK-1 phosphorylation stimulated by anti-CD3 MAb. These data provide additional evidence that CD3-stimulated Erk activation is likely to be proceeding via Raf-1 and MEK-1 in both the ZAP-70-replete and -deficient Jurkat T cells.
FIG. 2.
CD3 cross-linking activates Raf-1 in P116 T cells. (A) Jurkat and P116 T cells were stimulated by CD3 cross-linking at 37°C for 2 min with purified UCHT1 MAb. The concentration of UCHT1 was varied from 0.2 to 20 μg/ml. Raf-1 was immunoprecipitated from WCLs and subjected to an in vitro immune complex kinase assay as described in Materials and Methods (top panel). A Raf-1 immunoblot of the Raf-1 immunoprecipitates is shown (second panel). Erk and MEK-1 phosphorylation in WCLs were measured as in Fig. 1 (third and bottom panels). (B) As for panel A, except that 20 μg/of UCHT1 per ml was used and the time of stimulation was varied from 0 to 16 min.
The biochemical events that regulate Raf-1 activation are complex and incompletely understood (33); however, Ras is recognized as a common upstream regulator of Raf-1. Ras becomes activated when GTP is bound to its guanine nucleotide binding site. Once Ras is activated, a high-affinity binding site for Raf-1 is exposed, permitting Raf-1 recruitment to the plasma membrane, an event that facilitates the activation of Raf-1. To assess if Ras becomes activated on CD3 stimulation of ZAP-70-negative Jurkat T cells, increasing concentrations of anti-CD3 MAb were used to stimulate the parental and P116 Jurkat T-cell lines (Fig 3A). The level of GTP-bound Ras was measured by its ability to coprecipitate with a GST fusion protein containing the Ras binding domain of Raf-1. Ras activation was observed in both cell lines. As with Erk and MEK phosphorylation, Ras activation was seen in the P116 cells only above 10 μg of anti-CD3 MAb per ml, while much lower concentrations of anti-CD3 were effective for Ras activation in the parental Jurkat T cells. We also examined the time course of Ras activation in these two cell lines in response to stimulation with a high concentration of anti-CD3 MAb (Fig 3B). Activated Ras could be detected as early as 2 min poststimulation and persisted to 8 min poststimulation in both cell lines. Notably, the time course for Ras activation was very similar in both the ZAP-70-negative and -replete Jurkat T-cell lines, even though activation of Raf-1, MEK-1, and Erk showed markedly delayed activation in the P116 cells.
FIG. 3.
CD3 cross-linking activates Ras in P116 T cells. (A) Jurkat and P116 T cells were stimulated by CD3 cross-linking at 37°C for 2 min with purified UCHT1 MAb. The concentration of UCHT1 was varied from 0.2 to 20 μg/ml. Activated (GTP-bound) Ras was purified on the Ras binding domain of Raf-1 (Raf-1–RBD) as described in Materials and Methods. Raf-1–RBD-associated proteins were resolved by electrophoresis on 4 to 12% NuPAGE gradient gels run in morpholinaethanesulfonic acid (MES) buffer, transferred to nitrocellulose, and immunoblotted for Ras (top). Erk phosphorylation in WCLs was measured as in Fig. 1 (bottom). (B) As for panel A, except that OKT3 ascites (1:50) was used and the time of stimulation was varied from 0 to 12 min.
Failure of SLP-76 and LAT tyrosine phosphorylation on T-cell stimulation with high concentrations of anti-CD3 MAb.
While the above data show that Erk activation can be initiated by CD3 cross-linking in Jurkat T cells in the absence of ZAP-70, the data just as clearly indicate that there are pronounced differences in the strength of the signal required to initiate Erk activation, as well as in the kinetics of Erk activation, depending on the expression status of ZAP-70. This suggests that different signaling mechanisms may be linking TCR/CD3 cross-linking to Erk activation in the ZAP-70-negative and -replete cell lines above the level of either Ras or Raf-1 activation. In Jurkat T cells, TCR-stimulated, ZAP-70-mediated tyrosine phosphorylation of the adapter proteins SLP-76 and LAT has been correlated with efficient activation of Ras and signaling events downstream of Ras. Likewise, Jurkat T cells deficient in SLP-76 or LAT expression have been reported to be defective in these pathways (18, 61, 63). We examined whether the high concentration of stimulatory anti-CD3 MAb used in our studies is sufficient to stimulate appreciable LAT and SLP-76 tyrosine phosphorylation in ZAP-70-negative P116 T cells. A fusion protein containing full-length Grb2 fused to GST was used to concurrently precipitate SLP-76 and LAT (5, 34, 44) from Jurkat and P116 T cells stimulated at 37°C with 10 μg of UCHT1 per ml for various times between 0 and 30 min (Fig. 4A). While tyrosine phosphorylation of SLP-76 and LAT was readily detectable within 1 min of stimulation in the parental Jurkat T cells, in the P116 cells LAT phosphorylation was undetectable and SLP-76 phosphorylation could be detected only just above background in the 2-min-stimulated sample. This suggests that Erk activation in P116 cells is occurring independently of LAT and SLP-76 tyrosine phosphorylation. Similar results were obtained in SLP-76 and LAT immunoprecipitations (data not shown).
FIG. 4.
SLP-76 and LAT are not required for Erk activation in CD3-stimulated P116 cells. (A) Jurkat and P116 T cells were stimulated by CD3 cross-linking at 37°C with 10 μg of UCHT1 per ml for 0, 0.25, 0.75, 2, 8, 15, or 30 min. WCLs were precleared with 20 μg of GST bound to glutathione-agarose. Grb2-associating proteins were precipitated from the cleared lysates with 20 μg of glutathione-agarose-immobilized GST-Grb2. The extent of tyrosine phosphorylation of associated proteins was determined by antiphosphotyrosine (4G10) immunoblotting. Shown are the 76- and 36-kDa proteins, which correspond to SLP-76 and LAT. (B) SLP-76-deficient (J14-v-29) and SLP-76-reconstituted (J14-76-11) Jurkat T-cell lines were stimulated with OKT3 ascites (1:50) for 0, 0.25, 0.75, 2, 8, 15, or 30 min at 37°C, and Erk activation was measured as described in the legend to Fig. 1. (C) LAT-deficient (JCaM2.5) and LAT-reconstituted (JCaM2.5.B3) Jurkat T-cell lines were analyzed as in panel B.
T-cell stimulation with high concentrations of anti-CD3 MAb induces Erk activation in the absence of SLP-76 or LAT.
One would predict that if CD3-stimulated Erk activation can occur in Jurkat T-cell mutants in the absence of ZAP-70 expression and in the absence of significant LAT and SLP-76 tyrosine phosphorylation, the same CD3-stimulated Erk activation pattern should also be detectable in Jurkat T cells that fail to express LAT (JCaM2.5 cells) or SLP-76 (J14 cells). Indeed, stimulation of these cell lines with OKT3 for various times from 15s to 30 min resulted in Erk activation that was delayed and transient compared to that in cell lines in which the expression of these proteins had been restored (Figs. 4B and C).
Kinetics and dose response of PLCγ1 phosphorylation coincides with Erk activation.
While there has been considerable interest in the potential of Grb2-SOS as a mediator of Ras activation, there is some evidence indicating that TCR-stimulated Ras activation may be mediated by PLCγ1 (6, 16, 25), whose activity has been correlated with its degree of tyrosine phosphorylation (17, 39, 57). Therefore, in consideration of the possibility that PLCγ1 activation is responsible for anti-CD3 MAb-induced activation of Erk in P116 T cells, PLCγ1 was immunoprecipitated from OKT3-stimulated P116 and Jurkat T-cell lines and the degree of tyrosine phosphorylation of PLCγ1 was measured by antiphosphotyrosine immunoblotting (Fig. 5A). Comparable amounts of PLCγ1 were recovered in each of the immunoprecipitates. While PLC-γ1 tyrosine phosphorylation could be readily detected in Jurkat T cells after 2 min of CD3 cross-linking with as low as 1 μg of UCHT1 per ml, 10 times as much UCHT1 was required to obtain a comparable level of PLC-γ1 phosphorylation in the P116 cells. Notably, the dose dependence for UCHT1 in the activation of Erk correlates well with the dose dependence for PLCγ1 tyrosine phosphorylation. Examination of the kinetics of PLCγ1 tyrosine phosphorylation after CD3 stimulation of these cells found that PLCγ1 tyrosine phosphorylation, like Erk activation, was delayed and transient in P116 cells compared to parental Jurkat T cells (Fig. 5B). Interestingly, PLCγ1 tyrosine phosphorylation slightly preceded Erk activation in both cell lines. Together, these data are consistent with the possibility of PLCγ1 activation being upstream of Erk activation in the CD3-stimulated P116 cells.
FIG. 5.
CD3 cross-linking induces PLCγ1 tyrosine phosphorylation in P116 T cells. (A) Jurkat and P116 T cells were stimulated by CD3 cross-linking for 2 min at 37°C with the indicated concentration of purified UCHT1 MAb. PLCγ1 was immunoprecipitated from WCLs and immunoblotted for phosphotyrosine with the 4G10 MAb (top). The membrane was then stripped and blotted for PLCγ1 (bottom). The presence of active Erk in the WCLs (middle) was measured as described in the legend to Fig. 1. (B) Jurkat and P116 T cells were stimulated by CD3 cross-linking at 37°C with OKT3 ascites (1:50) for the indicated times. WCLs were analyzed as in panel A.
Distinct calcium mobilization pattern in P116 cells on CD3 cross-linking.
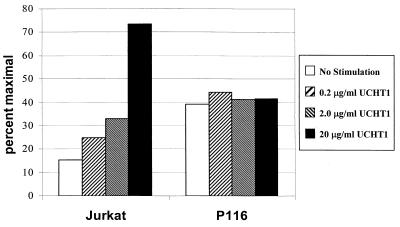
To assess if the transient and low-level PLCγ1 tyrosine phosphorylation seen in anti-CD3 MAb-stimulated P116 cells is associated with activation of PLCγ1, we monitored changes of intracellular calcium ([Ca2+]i) in Indo-1-AM-loaded Jurkat and P116 cells upon CD3 stimulation (Fig. 6). Figure 6A reflects changes in [Ca2+]i with time, while Fig. 6B shows the maximum mean channel response, a measure of the peak Ca2+ response. Anti-CD3 MAb initiated a dose-dependent increase in [Ca2+]i in both cell lines, although P116 cells required substantially more MAb to initiate a response. It should be noted that the P116 data in Fig. 6A are plotted on an exaggerated scale compared to the Jurkat data in order to better visualize the Ca2+ response in the P116 cells and that the stimulatory MAb dosage is 1 log unit higher in the P116 samples. Even saturating levels of anti-CD3 MAb (20 μg/ml) engendered submaximal increases in [Ca2+]i in P116 cells, taking substantially longer to reach peak values and reaching only 45% of the peak response elicited in parental Jurkat T cells stimulated with a 10-fold-lower concentration of MAb. As reported previously, pervanadate stimulation gives the same maximal response in P116 cells but is delayed in onset compared to the situation in parental Jurkat T cells (60). Equivalent loading of Indo-1-AM into each cell line was indicated by the comparable response of both cell lines to administration of calcium ionophore (Fig 6B). An isotype-matched control MAb had no effect on [Ca2+]i in either cell type (data not shown). These data are consistent with the induction of a transient and weak activation of PLCγ1 in the ZAP-70-deficient P116 cells on CD3 stimulation.
FIG. 6.
CD3 cross-linking induces Ca2+ flux in P116 T cells. Jurkat and P116 T cells were loaded with Indo-1-AM. Baseline fluorescence ratio (405 nm to 495 nm) was measured for 1 min prior to the administration of indicated stimulus, and readings of the fluorescence ratio were acquired for an additional 5 min. (A) Change in fluorescence ratio as a function of time. Concentrations refer to purified UCHT1 MAb. The y-axis scale, reflecting changes in fluorescence ratio, runs from 0 to 160 for Jurkat cells and 0 to 100 for P116 cells. (B) Plot of maximum mean channel response observed during a 6-min run versus anti-CD3 MAb concentration. Solid bars, P116 cells; open bars, parental Jurkat cells. “n.d”, data points that were not determined.
Differential sensitivity of Erk activation to PKC inhibitors in anti-CD3 MAb-stimulated P116 and parental Jurkat T cells.
PKC (classical and novel isoforms) is also activated in response to PLCγ1 activation. PKC has the capacity to indirectly activate Erk through its stimulatory effects on Raf-1 (7, 26, 32, 53). Furthermore, it has been previously shown that TCR-mediated activation of the Ras/Raf-1/MEK/Erk cascade is partially sensitive to inhibition of PKC activity (6). On the basis of the ability of CD3 stimulation of P116 cells to induce tyrosine phosphorylation of PLCγ1 and mobilization of intracellular Ca2+, we hypothesized that PLCγ1-driven PKC activation might be responsible for the Erk activation observed in these cells. To ascertain the contribution of PKC activity to Erk activation in P116 versus parental Jurkat T cells, we examined the ability of three widely used pharmacologic inhibitors of PKC to disrupt Erk activation in response to either anti-CD3 MAb cross-linking or stimulation with the phorbol ester PMA (Fig. 7). Two of the inhibitors, Gö6850 (also known as BIM-1 or GF 109203X) and Ro-31-8220 (also known as BIM-IX), display little selectivity between the classical and novel classes of PKC isozymes, while the third inhibitor, Gö6976, is specific for the classical PKC isozymes. Looking first at PMA-stimulated Erk activation, very similar patterns were seen in P116 cells and parental Jurkat cells. Both cell lines exhibited a dose-dependent loss of PMA-induced Erk activation when pretreated with the pan-PKC inhibitors Gö6850 and Ro-31-8220, while the classical-PKC-specific inhibitor Gö6976 had little effect. In contrast, CD3-stimulated Erk activation in the two cell lines showed very different susceptibilities to the inhibition of PKC activity. UCHT1-stimulated parental Jurkat cells were refractory to all but the highest doses (30 μM) of Gö6850 and Ro-31-8220 used and, even then, were only partially affected. UCHT1-stimulated P116 cells, on the other hand, gave a pattern of inhibition that was nearly indistinguishable from that of PMA-stimulated Jurkat and P116 cells. Neither UCHT1-stimulated P116 nor Jurkat cells were particularly sensitive to Erk inhibition by the cPKC-specific inhibitor Gö6976 (the apparent decrement in the Erk signal in 10 and 30 μM Gö6976-treated P116 cells was not a reproducible observation). These data suggest that the Erk activation observed in the CD3-stimulated ZAP-70-negative P116 cells occurs via a mechanism entirely dependent on the activation of one or more isozymes of the nPKCs, while in the ZAP-70-replete cells, PKC plays only a small role in CD3-stimulated Erk activation.
FIG. 7.
Anti-CD3 MAb-stimulated Erk activation is PKC dependent in P116 cells but not in parental Jurkat T cells. Jurkat cells and P116 cells at 6 × 107 cells/ml were preincubated for 15 min at 37°C with the indicated concentration of PKC inhibitors, prior to stimulation at 37°C with either 10 μg of UCHT1 per ml for 3 min or 50 ng of PMA per ml. for 15 min. Erk activation was measured in these cells as described in the legend to Fig. 1. The first lane in each panel is an unstimulated, vehicle control.
Non-Lck protein tyrosine kinase activity is required for ZAP-70-independent Erk activation.
If Erk activation in P116 cells is a consequence of PLCγ1 activation subsequent to its tyrosine phosphorylation, then CD3-stimulated Erk activation in P116 cells should be subject to inhibition by PTK inhibitors. PP-1 is a potent PTK inhibitor that is selective for Src family PTKs (22). Erk activation induced by CD3-cross-linking was inhibited in both Jurkat and P116 cells with a similar sensitivity to PP-1 (≥30 μM) (Fig. 8). Herbimycin (a broader-specificity PTK inhibitor) (54) also blocked Erk activation comparably in both cell lines above 3 μM (data not shown). However, PMA-mediated Erk activation, which bypasses the requirement for PTK activity, is insensitive to either PTK inhibitor. These data indicate the involvement of another PTK, possibly a Src family member, in the process of anti-CD3 MAb-stimulated, ZAP-70-independent Erk activation and are consistent with a role for PLCγ1 tyrosine phosphorylation and activation in Erk activation in these cells.
FIG. 8.
Anti-CD3 MAb-stimulated Erk activation in P116 cells can be blocked by PP-1. Jurkat cells and P116 cells at 6 × 107 cells/ml were preincubated for 15 min at 37°C with the indicated concentration of PP-1, prior to stimulation at 37°C with either OKT3 ascites (1:50) for 3 min or 50 ng of PMA per ml for 15 min. Erk activation was measured as described in the legend to Fig. 1.
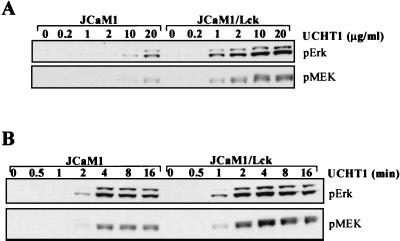
As a test of whether the PTK activity required for signaling in the absence of ZAP-70 comes from Lck, we examined the Lck-negative JCaM1 cell line, which does not activate ZAP-70 in response to CD3 cross-linking (13), for its ability to support the phosphorylation of MEK and Erk in response to CD3 stimulation. UCHT1-stimulated JCaM1 cells require MAb concentrations of 10 μg/ml or above to elicit detectable phosphorylation of MEK-1 and Erk; however, JCaM1 cells in which Lck expression is restored (JCaM1/Lck) respond to concentrations of UCHT1 as low as 1 μg/ml (Fig. 9A). As was seen in the ZAP-70-negative cells, the time course of Erk and MEK-1 phosphorylation is delayed in the absence of Lck expression (Fig. 9B). In other experiments, Erk phosphorylation was also observed to be transient, although this is not evident in this experiment. These data argue that Lck is dispensable for activation of the Raf-1/MEK-1/Erk kinase cascade in response to high-concentration anti-CD3 MAb stimulation of Jurkat T cells in the absence of ZAP-70 expression (P116) or activation (JCaM1).
FIG. 9.
High-concentration anti-CD3 MAb stimulates Erk activation in Lck-negative Jurkat. (A) JCaM1 and JCaM1/Lck T cells were stimulated by CD3 cross-linking for 2 min at 37°C with the indicated concentration of purified UCHT1 MAb. Erk and MEK-1 phosphorylation in WCLs were measured as in Fig. 1. (B) As for panel A, except that 20 μg of UCHT1 per ml was used and the time of stimulation was varied between 0 and 16 min.
Failure to activate the NF-AT transcriptional reporter in CD3-stimulated P116 T cells.
Given the ability of CD3 stimulation to induce Erk activation and Ca2+ mobilization in ZAP-70-negative P116 T cells, we also assessed the ability of CD3 stimulation to induce transcriptional activation of the luciferase reporter construct containing the triplicated NF-AT binding sites of the interleukin-2 promoter region driving the transcription of luciferase (Fig. 10). The parental Jurkat cells showed a dose-dependent increase in reporter activity in response to CD3 stimulation, while the P116 cells were unresponsive. The apparent increase in the constitutive level of NF-AT activity in the P116 cells is due to the relatively poor response of these cells to the 50 ng of PMA per ml used as the maximal stimulus for normalizing the results.
FIG. 10.
CD3 cross-linking enhances NF-AT transcriptional activity in Jurkat cells but not P116 cells. Parental Jurkat cells and P116 cells were transfected with the luciferase reporter construct driven by NF-AT. These cells were subsequently stimulated with the indicated dosages of UCHT1 or 50 ng of PMA per ml (defined as the maximal response) for 6 h before being analyzed for luciferase activity. The results shown are expressed as the percentage of the maximal response to PMA.
Superantigen induces PLCγ1 tyrosine phosphorylation, Erk activation, and CD69 upregulation in the absence of ZAP-70 expression.
The high degree of TCR engagement implicit in the use of the high levels of anti-CD3 MAb is reminiscent of the conditions that can exist after challenge of peripheral T cells with superantigen (SAg). The staphylococcal enterotoxins are prototypic SAg, that stimulate T cells in a manner specific for the Vβ region of the TCR (23, 27). SAgs have also been causally implicated in many diseases, including rheumatoid arthritis, diabetes mellitus, and toxic shock syndrome. Activation of T cells by staphylococcal enterotoxins leads to rapid proliferation followed by apoptosis or anergy. Since Jurkat cells express TCR Vα1/Vβ8.1, we stimulated these cells with SEE in the context of HLA-DR1-expressing antigen-presenting cells (APCs).
APCs, LG2 B cells, pulsed with increasing amounts of SEE were used to stimulate ZAP-70-negative and -replete Jurkat T cells, and the induction of PLCγ1 tyrosine phosphorylation and Erk phosphorylation was assessed (Fig. 11A). P116 cells did phosphorylate PLCγ1 and Erk in response to SEE-pulsed APCs, although the response was considerably weaker than that which was elicited in ZAP-70-replete Jurkat T cells at all concentrations of SEE analyzed. Analysis of the time course of PLCγ1 tyrosine phosphorylation in response to SEE-pulsed APC stimulation of the ZAP-70-negative and -replete Jurkat T-cell lines indicated that P116 and the parental Jurkat T cells exhibit markedly different kinetics (Fig. 11B, top panel). In both cell lines, maximal phosphorylation of PLCγ1 was very rapid, being seen within 30 s. However, while PLCγ1 remained strongly tyrosine phosphorylated out to 30 min poststimulation in the parental Jurkat T cells, PLCγ1 tyrosine phosphorylation was transient in the P116 cells, falling steadily after the peak at 30 s and returning to baseline by 10 min. In contrast, the kinetics of Erk activation was comparable in the two cell lines, with rapid and prolonged phosphorylation being induced on stimulation with SEE-pulsed APCs (Fig. 11B, third panel). As was the case throughout the study, ZAP-70 was undetectable in the P116 cells but was readily detectable in the parental Jurkat T cells.
FIG. 11.
SAg induces PLCγ1 and Erk phosphorylation in P116 T cells. (A) Parental E6.1 Jurkat and ZAP-70-negative P116 T cells were stimulated at 37°C for 2 min by APCs loaded with the indicated amount of SEE. Cell lysates (10,000,000 T-cell and 2,000,000 LG2 cell equivalents) were subjected to immunoprecipitation with anti-PLCγ-1, transferred to a polyvinylidene difluoride membrane, and immunoblotted for phosphotyrosine (top). The membrane was then stripped and blotted for PLCγ1 (middle). Lanes: APCs; PLCγ-1 immunoprecipitate from 2,000,000 LG2 cell equivalents; Beads, immunoprecipitating antibody-coated beads alone without cell lysate. WCL proteins (450,000 T-cell and 90,000 LG2 cell equivalents) were resolved by electrophoresis on 8% polyacrylamide gels, transferred to a polyvinylidene difluoride membrane, and immunoblotted for dually phosphorylated, activated Erk (bottom). Lane APCs, WCL from LG2 cells (90,000 cell equivalents) alone. (B) Parental Jurkat and ZAP-70-negative P116 T cells were stimulated with APCs loaded with 300 ng of SEE per ml at 37°C for 0.5, 2, 5, 10, or 30 min. Tyrosine phosphorylation of PLCγ1 and activation of Erk in the cells were measured as in panel A. The membrane loaded with WCLs was also immunoblotted for ZAP-70.
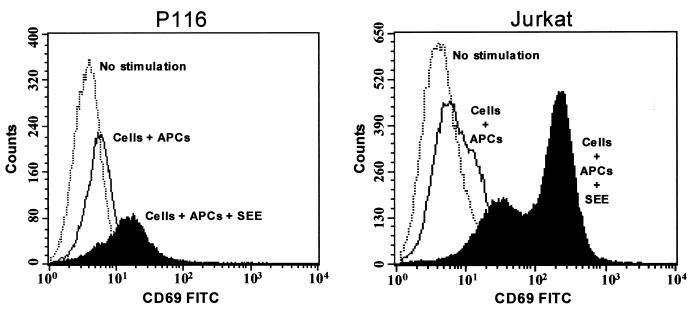
To determine if the prolonged Erk activation that could be induced in the P116 cells in response to SEE-pulsed APC stimulation can support distal signaling events, the ability of SEE-pulsed APC stimulation to upregulate CD69 in Jurkat T cells was assessed. While SEE-pulsed APC-stimulated CD69 upregulation in P116 cells was inferior to that stimulated in ZAP-70-replete Jurkat T cells, there was nonetheless a marked increase in CD69 expression that was not seen in the absence of SEE (Fig. 12).
FIG. 12.
SAg induces surface expression of CD69 in P116 T cells. Parental Jurkat cells and ZAP-70-negative P116 T cells were stimulated at 37°C for 15 h by APCs loaded with 300 ng of SEE per ml, and CD69 expression was analyzed by FACS on TCR-positive cells. FITC, Fluorescein isothiocyanate.
DISCUSSION
Previously, in the course of experiments that examined the requirement for ZAP-70 in T cells responding to hydrogen peroxide treatment, we made the unexpected observation that although ZAP-70 was required for Erk activation in response to hydrogen peroxide, it was not necessarily required for TCR-stimulated Erk activation (21). In the present study we have attempted to determine the mechanism by which Erk becomes activated after TCR/CD3 stimulation of ZAP-70-negative Jurkat T cells by carrying out more detailed analyses of the kinetics and dose response of Erk activation in these cells, as well as by examining the status of various signaling molecules believed to be intermediate between TCR engagement and Erk activation. Additionally, to explore the potential physiologic relevance of this signaling pathway, we have examined the ability of superantigen-pulsed APCs to activate signaling in ZAP-70-negative Jurkat T cells.
In addition to confirming our previous observation of CD3-stimulated Erk activation in ZAP-70-negative Jurkat T cells, our results indicate that PLCγ1, PKC, Ras, Raf-1, and MEK-1 are being activated in these cells in response to CD3 stimulation. Notably, though, much higher concentrations of anti-CD3 MAb were required to elicit a response in the P116 T cells than were required to elicit a comparable response in the ZAP-70-replete Jurkat T cells. This finding may help to explain why previous studies with the P116 cells did not detect the activation of these signaling molecules following TCR/CD3 stimulation (59, 60). These previous studies examined responses to only a single, comparatively low concentration of anti-CD3 MAb (1 μg/ml). In the present study, no signaling could be detected in P116 cells after stimulation with 1 μg of anti-CD3 MAb per ml, even though this concentration of MAb was sufficient to induce maximal Erk activation in the parental Jurkat T cells. In addition to the requirement of P116 cells for a higher concentration of anti-CD3 MAb for induction of signaling, signaling in these cells was significantly delayed and transient compared to that in parental Jurkat T cells. This narrow kinetic window also helps to explain the failure of earlier studies to observe signaling in P116 cells in response to CD3 stimulation (59, 60). The possibility that the P116 cell line had regained the capacity to express ZAP-70 or Syk or that the cell line had become contaminated with parental Jurkat T cells was ruled out by Western blotting for ZAP-70 and Syk (data not shown). Likewise, CD3 expression levels were equal in all cell lines (reference 24 and data not shown).
In all experiments, there was a strong positive correlation for the timing and dose dependency of phosphorylation and activation between Raf-1, MEK-1, and Erk following CD3 stimulation of both the ZAP-70-negative and -replete Jurkat T cells, although clearly the pattern of activation was different between the two cell lines. This suggests that Raf-1 and MEK-1 are intermediate signaling components between TCR/CD3 engagement and Erk activation in both cell lines and is consistent with previous reports identifying Raf-1 and MEK-1 as being upstream of TCR-stimulated Erk activation in Jurkat T cells (6). The MEK-1 inhibitor PD98059 was also able to inhibit CD3-stimulated Erk activation in both cell lines (data not shown), further supporting this supposition.
Despite generally weaker Raf-1 and MEK-1 activation in the P116 cells, the degree of Erk2 activation, as measured by the strength of signal detected on immunoblots of dually phosphorylated Erk2 present in cellular lysates (40), was generally comparable at the peak time point (usually 2 to 3 min poststimulation) between Jurkat and P116 cells. This presumably reflects the catalytic nature of MEK-1-mediated Erk2 activation and indicates that a relatively small amount of activated MEK-1 can support maximal activation of the Erk2 pool. Erk1 activation showed considerably more variability, and Erk1 activation was often much weaker in P116 cells, even at the time point of maximum stimulation. The reason for this is unknown, but it may indicate different requirements for full activation of these two isozymes.
The results of the Ras activation assays with the ZAP-70-negative and -replete Jurkat T-cell lines are interesting in a number of respects. In keeping with the results for the other signaling molecules investigated, Ras was activated in the P116 cells only in response to anti-CD3 MAb in the range of 10 to 20 μg/ml; however, unlike for Raf-1, MEK-1, Erk, and PLCγ1, there was no marked difference in the time course of Ras activation in the P116 cells compared to the parental Jurkat T cells. Thus, despite the failure to initiate LAT tyrosine phosphorylation in the CD3-stimulated P116 cells, Ras activation appears to be proceeding normally in these cells. Precisely how Ras is being activated under these conditions remains to be established but may involve the activation of Ras-GRP as a consequence of DAG production by activated PLCγ1 (15, 29, 51). Additionally there is an apparent discrepancy between the time courses of Ras activation and Raf-1/MEK-1/Erk cascade activation in the CD3-stimulated P116 cells. Notably, while Ras activation peaked in both parental Jurkat and P116 cells at 30 s following stimulation with soluble anti-CD3 MAb, Erk activation was nearly maximal in wild-type Jurkat at this time whereas no Erk activation could be detected at this time in the P116 cells. While other explanations are also tenable, these results suggest the existence of additional signaling events that are required in efficiently translating Ras activation into the activation of the Raf-1, as has been suggested previously (58). Our data suggest that ZAP-70 plays an important role in this process.
The distinct magnitude, kinetics, and dose response of Raf-1/MEK-1/Erk cascade activation in P116 cells stimulated by CD3 cross-linking compared to that in the parental Jurkat T cells suggests the existence of an alternative Raf-1 activation pathway that can be initiated in response to TCR stimulation in the absence of ZAP-70. Notably, Raf-1/MEK-1/Erk cascade activation in these cells is temporally correlated with tyrosine phosphorylation of PLCγ1 and increases in [Ca2+]i, indicating that activation of PLCγ1, with its attendant production of IP3 and DAG, is coincident with activation of Raf-1, MEK-1, and Erk. Unfortunately, attempts to directly test the importance of PLCγ1 in CD3-stimulated Raf-1/MEK-1/Erk cascade activation in P116 cells were uninformative, since in our hands the reported PLCγ1 pharmacologic inhibitor U73122 had no effect on PLCγ1 activity at concentrations that were not overtly toxic to the cells.
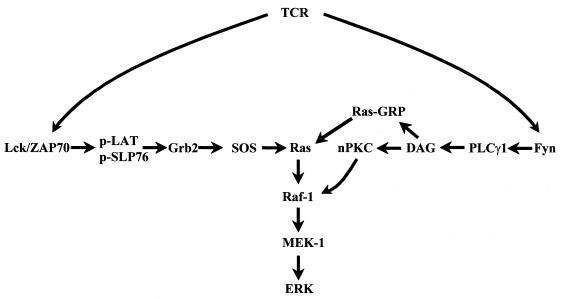
Nonetheless, the correlation between activation of the Raf-1/MEK-1/Erk cascade and PLCγ1 tyrosine phosphorylation suggests some possible mechanisms by which Erk activation may be occurring in CD3-stimulated P116 T cells. Two signaling pathways have been recognized in T cells that can support Erk activation in response to increases in the concentration of Ca2+ and DAG (Fig. 13). The first is based on the ability of PKC to activate Raf-1 (7, 26, 32, 53). The second pathway relies on the recently discovered guanine nucleotide exchange factor for Ras, Ras-GRP, which is believed to be activated by the binding of DAG to its C1 domain (15, 16, 51). Support for the idea that the first pathway plays an important role in Erk activation in CD3-stimulated P116 cells is provided by the sensitivity of Erk activation in these cells to the panspecific PKC inhibitors Gö6850 and Ro-31-8220. The inability of Gö6976, a classical-PKC-selective inhibitor, to block Erk activation in P116 cells suggest that the implicated PKC is a members of the “novel” class of PKC isozymes. This result is consistent with previous results showing that transient transfection with PKC-ɛ (novel class) could strongly support transactivation of NF-AT and AP-1 in Jurkat T cells while PKC-α (classical class) was considerably less effective (19). Which novel PKC mediates this signaling pathway remains to be established, although it is unlikely that this enzyme is PKC-θ, since ZAP-70 expression is required for PKC-θ activation in Jurkat T cells (24) and since PKC-θ activation requires the activation of both TCR/CD3 and CD28 (11). Consistent with previously published results, in the ZAP-70-replete Jurkat cells, Erk activation was largely insensitive to PKC inhibition (6). Additional studies are required to determine if Ras-GRP is also contributing to the activation of the Raf-1/MEK-1/Erk cascade in P116 cells. Although the ability of the PKC inhibitors to block Erk activation in CD3-stimulated P116 cells shows that Ras-GRP activation is not sufficient to stimulate Erk activation, DAG-mediated activation of Ras-GRP may be required for the activation of Ras observed in these cells.
FIG. 13.
Potential pathways leading to Erk activation following TCR stimulation. The figure schematically depicts two pathways by which TCR stimulation can lead to Erk activation. The pathway on the left is proposed to mediate ZAP-70-dependent Erk activation, while the pathway on the right is proposed to mediate Erk activation in the absence of active ZAP-70.
Anti-CD3 MAb signaling to Erk in the P116 cells required a signal generated by an Src family PTK, as evidenced by the ability of the Src family-selective PTK inhibitor PP-1 to block Erk activation in these cells. Unlike the parental Jurkat T cells, this signal seems unlikely to be delivered by Lck in the P116 cells, since Lck does not appear to play an important role in the ZAP-70-independent activation of the Raf-1/MEK-1/Erk cascade, on the basis that Erk could also be activated following CD3 stimulation in the Lck-negative Jurkat T-cell line JCaM1, with a dose dependency and time course similar to those observed in the ZAP-70-negative P116 cells. Fyn would seem to be a logical candidate for providing the requisite Src family signal, since Fyn is also rapidly activated following TCR/CD3 stimulation (12, 52) and has been implicated in TCR/CD3-stimulated PLCγ1 activation (1, 48, 62).
Consistent with the ability of Fyn to signal PLCγ1 and Erk activation following TCR stimulation, Denny et al. (13) have recently demonstrated that increasing the expression level of Fyn in the Lck-negative Jurkat T-cell line JCaM1 enabled signaling to multiple pathways in response to TCR/CD3 stimulation. In particular, TCR/CD3-stimulated PLCγ1 tyrosine phosphorylation, Ca2+ mobilization, Ras activation, and Erk activation were all enabled, via a ZAP-70-independent signaling pathway (13). Notably, as with the ZAP-70-negative signaling pathway that we report here, this signaling pathway was also found to be independent of LAT tyrosine phosphorylation. It seems likely that the increased expression level of Fyn in these cells has shifted the dose-response function of the signaling pathway to TCR stimulation, such that responses which previously required high levels of anti-TCR/CD3 MAb (this study) or superantigen stimulation (62) can now be stimulated with lower concentrations of soluble antibody (13). Whether increasing the expression level of Fyn in the P116 cells would permit a signaling response to lower concentrations of anti-CD3 MAb is the subject of current investigations.
In an effort to probe the physiologic relevance of a ZAP-70-independent signaling pathway that is revealed only at high levels of anti-CD3 MAb, we also tested whether SAg-pulsed APCs, which provide a potent stimulus via the TCR, could deliver activating signals to Erk in P116 cells. SEE-pulsed LG2 B cells rapidly stimulated PLCγ1 tyrosine phosphorylation and Erk activation in P116 cells, demonstrating the competency of these cells to respond to TCR stimulation via SAg. Indeed, it is interesting that Erk activation was often observed to be as good (strength of signal and duration) in SEE-stimulated P116 cells as it was in ZAP-70-replete Jurkat T cells. SEE stimulation of P116 cells also resulted in upregulation of CD69 surface expression. The differences in the duration of Erk activation and downstream signaling events (NF-AT activation or CD69 upregulation) in response to anti-CD3 MAb and SEE-pulsed LG2 B cells are likely to be due to the additional costimulatory signals provided by the APCs that are lacking during stimulation with soluble MAb to CD3, although we have not ruled out the possibility that the two stimulatory agents are activating Erk via different pathways.
In considering situations in which a mature peripheral T cell would be likely to be stimulated via TCR engagement in the absence of a signal from ZAP-70 (or Syk) activity, two scenarios seem plausible: (i) stimulation by partial-agonist- or antagonist-altered peptide ligands, and (ii) maintenance of peripheral T cell tolerance in previously tolerized T cells. It has been well established, for both T-cell clones and CD4+ peripheral T cells, that engagement of the TCR with a conservatively modified major histocompatibility complex-antigenic peptide ligand or in the absence of CD4 costimulation can result in a partial engagement of the TCR-associated signaling machinery (30, 45). A hallmark of these partial-agonist systems is that these stimuli do not activate ZAP-70, despite their ability to stimulate TCR-mediated Ca2+ flux (4, 28, 47) and Erk activation (8, 9). It seemed likely, therefore, that there should exist conditions under which the TCR of Jurkat T cells could be induced to stimulate Ca2+ mobilization and Erk activation in the absence of ZAP-70 activation. This hypothesis is borne out by the data presented here. Interestingly, the increase in [Ca2+]i that was observed in P116 cells stimulated with anti-CD3 MAb was substantially weaker than that observed in the parental Jurkat T cells, even when 100 times more stimulatory antibody was used. This is reminiscent of what has been observed for Ca2+ flux in partial-agonist-stimulated cells (4, 28, 47). Likewise, partial-agonist-stimulated Erk activation has been reported to be significantly more transient than full-agonist-stimulated Erk activation in T-cell clones (8, 9), which is consistent with our observations in anti-CD3 MAb-stimulated P116 T cells.
The biochemical mechanism by which peripheral T cells become tolerized or anergized is incompletely understood, and even less is known about how the anergic state is maintained in these cells. It seems clear, though, that maintenance of peripheral T-cell anergy is an active process, since removal of the tolerizing antigen often results in release of anergy. On becoming anergized, T cells elevate their Fyn expression and/or activity levels three- to fourfold while simultaneously reducing the Lck expression level by 50 to 90% (2). Anergized T cells also exhibit pronounced defects in ZAP-70 activation and show decreased SLP-76 and LAT tyrosine phosphorylation in response to TCR stimulation. Thus, anergized T cells increase their Fyn signaling capacity while simultaneously reducing their Lck signaling capacity, thereby favoring signaling pathways that bypasses ZAP-70 activation. Presumably these alterations are important in the process of initiating and maintaining tolerance in these cells. We would like to suggest that the signaling pathway that we have uncovered in the ZAP-70-negative and Lck-negative Jurkat T cells in response to high-dose anti-CD3 MAb and that we and others (62) have demonstrated in these cells in response to superantigen stimulation is representative of the signaling pathway delivered by the TCR in anergized cells and may be responsible for the maintenance of anergy in these cells.
In summary, we find, contrary to previously published results, that the ZAP-70-negative Jurkat T-cell line P116 is able to generate signals in response to TCR/CD3 cross-linking that result in Ca2+ mobilization and Erk activation. This finding provides further evidence for the idea that T cells can generate both ZAP-70-dependent and -independent signaling pathways. We also provide evidence to suggest that the pathways leading to Erk activation are qualitatively different in the presence and absence of ZAP-70 signaling, with the ZAP-70-independent pathway being completely dependent on PKC activation and ZAP-70-dependent Erk activation being independent of PKC activity.
ACKNOWLEDGMENTS
We thank R. T. Abraham, G. R. Crabtree, G. A. Koretzky, L. E. Samelson, A. Sette, D. B. Straus, S. J. Taylor, and A. Weiss for their generous gifts of reagents. We are also grateful to F. J. Chrest and R. Wersto of the Flow Cytometry Laboratory for their skillful assistance with the intracellular calcium measurements. We also thank P. Schwartzberg and D. McVicar for many insightful conversations.
Work at the Madrenas laboratory was funded by the Canadian Institutes of Health Research and the Ontario Research and Development Challenge Fund. G.C. is an ORDCF postdoctoral fellow, and J.M. holds a Canada Research Chair in Transplantation and Immunobiology.
REFERENCES
- 1.Appleby M W, Gross J A, Cooke M P, Levin S D, Qian X, Perlmutter R M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 2.Appleman L J, Tzachanis D, Grader-Beck T, van Puijenbroek A A, Boussiotis V A. Helper T cell anergy: from biochemistry to cancer pathophysiology and therapeutics. J Mol Med. 1901;78:673–683. doi: 10.1007/s001090000180. [DOI] [PubMed] [Google Scholar]
- 3.Bondzi C, Grant S, Krystal G W. A novel assay for the measurement of Raf-1 kinase activity. Oncogene. 2000;19:5030–5033. doi: 10.1038/sj.onc.1203862. [DOI] [PubMed] [Google Scholar]
- 4.Boutin Y, Leitenberg D, Tao X, Bottomly K. Distinct biochemical signals characterize agonist- and altered peptide ligand-induced differentiation of naive CD4+ T cells into Th1 and Th2 subsets. J Immunol. 1997;159:5802–5809. [PubMed] [Google Scholar]
- 5.Buday L, Egan S E, Rodriguez V P, Cantrell D A, Downward J. A complex of Grb2 adapter protein, Sos exchange factor, and a 36-kDa membrane-bound tyrosine phosphoprotein is implicated in ras activation in T cells. J Biol Chem. 1994;269:9019–9023. [PubMed] [Google Scholar]
- 6.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;74:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 7.Carroll M P, May W S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J Biol Chem. 1994;269:1249–1256. [PubMed] [Google Scholar]
- 8.Chau L A, Bluestone J A, Madrenas J. Dissociation of intracellular signaling pathways in response to partial agonist ligands of the T cell receptor. J Exp Med. 1998;187:1699–1709. doi: 10.1084/jem.187.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chau L A, Madrenas J. Phospho-LAT-independent activation of the ras-mitogen-activated protein kinase pathway: a differential recruitment model of TCR partial agonist signaling. J Immunol. 1999;163:1853–1858. [PubMed] [Google Scholar]
- 10.Clements J L, Boerth N J, Lee J R, Koretzky G A. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu Rev Immunol. 1999;17:89–108. doi: 10.1146/annurev.immunol.17.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc Natl Acad Sci USA. 2000;97:3394–3399. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva A J, Yamamoto M, Zalvan C H, Rudd C E. Engagement of the TcR/CD3 complex stimulates p59fyn(T) activity: detection of associated proteins at 72 and 120-130 kD. Mol Immunol. 1992;29:1417–1425. doi: 10.1016/0161-5890(92)90215-j. [DOI] [PubMed] [Google Scholar]
- 13.Denny M F, Patai B, Straus D B. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol Cell Biol. 2000;20:1426–1435. doi: 10.1128/mcb.20.4.1426-1435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dower N A, Stang S L, Bottorff D A, Ebinu J O, Dickie P, Ostergaard H L, Stone J C. RasGRP essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 15.Ebinu J O, Bottorff D A, Chan E Y, Stang S L, Dunn R J, Stone J C. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 16.Ebinu J O, Stang S L, Teixeira C, Bottorff D A, Hooton J, Blumberg P M, Barry M, Bleakley R C, Ostergaard H L, Stone J C. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 17.Exton J H. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 18.Finco T S, Kadlecek T, Zhang W, Samelson L E, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 19.Genot E M, Parker P J, Cantrell D A. Analysis of the role of protein kinase C-alpha, -epsilon, and -zeta in T cell activation. J Biol Chem. 1995;270:9833–9839. doi: 10.1074/jbc.270.17.9833. [DOI] [PubMed] [Google Scholar]
- 20.Gillis S, Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980;152:1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith C E, Zhang W, Wange R L. ZAP-70-dependent and -independent activation of Erk in Jurkat T cells. Differences in signaling induced by H2O2 and CD3 cross-linking. J Biol Chem. 1998;273:10771–10776. doi: 10.1074/jbc.273.17.10771. [DOI] [PubMed] [Google Scholar]
- 22.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 23.Herman A, Kappler J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 24.Herndon T M, Shan X, Tsokos G C, Wange R L. ZAP-70 and SLP-76 Regulate PKC-θ and NF-B Activation in Response to Engagement of CD3 and CD28. J Immunol. 2001;166:5654–5664. doi: 10.4049/jimmunol.166.9.5654. [DOI] [PubMed] [Google Scholar]
- 25.Kazanietz M G. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol ester tumor promoters. Mol Carcinog. 2000;28:5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp U R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 27.Kotzin B L, Leung D Y, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 28.Leitenberg D, Boutin Y, Constant S, Bottomly K. CD4 regulation of TCR signaling and T cell differentiation following stimulation with peptides of different affinities for the TCR. J Immunol. 1998;161:1194–1203. [PubMed] [Google Scholar]
- 29.Lorenzo P S, Beheshti M, Pettit G R, Stone J C, Blumberg P M. The guanine nucleotide exchange factor RasGRP is a high-affinity target for diacylglycerol and phorbol esters. Mol Pharmacol. 2000;57:840–846. [PubMed] [Google Scholar]
- 30.Madrenas J. Differential signalling by variant ligands of the T cell receptor and the kinetic model of T cell activation. Life Sci. 1999;64:717–731. doi: 10.1016/s0024-3205(98)00381-6. [DOI] [PubMed] [Google Scholar]
- 31.Madrenas J, Wange R L, Wang J L, Isakov N, Samelson L E, Germain R N. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 32.Marais R, Light Y, Mason C, Paterson H, Olson M F, Marshall C J. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 33.Morrison D K, Cutler R E. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 34.Motto D G, Ross S E, Jackman J K, Sun Q, Olson A L, Findell P R, Koretzky G A. In vivo association of Grb2 with pp116, a substrate of the T cell antigen receptor-activated protein tyrosine kinase. J Biol Chem. 1994;269:21608–21613. [PubMed] [Google Scholar]
- 35.Myung P S, Boerthe N J, Koretzky G A. Adapter proteins in lymphocyte antigen-receptor signaling. Curr Opin Immunol. 2000;12:256–266. doi: 10.1016/s0952-7915(00)00085-6. [DOI] [PubMed] [Google Scholar]
- 36.Northrop J P, Ullman K S, Crabtree G R. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993;268:2917–2923. [PubMed] [Google Scholar]
- 37.Pawson T, Saxton T M. Signaling networks—do all roads lead to the same genes? Cell. 1999;97:675–678. doi: 10.1016/s0092-8674(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 38.Qian D, Mollenauer M N, Weiss A. Dominant-negative zeta-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebecchi M J, Pentyala S N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 40.Robbins D J, Zhen E, Owaki H, Vanderbilt C A, Ebert D, Geppert T D, Cobb M H. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 41.Schneider U, Schwenk H U, Bornkamm G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977;19:521–526. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 42.Shan X, Czar M J, Bunnell S C, Liu P, Liu Y, Schwartzberg P L, Wange R L. Deficiency of PTEN in jurkat T cells causes constitutive localization of itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shan X, Wange R L. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J Biol Chem. 1999;274:29323–29330. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 44.Sieh M, Batzer A, Schlessinger J, Weiss A. GRB2 and phospholipase C-gamma 1 associate with a 36- to 38-kilodalton phosphotyrosine protein after T-cell receptor stimulation. Mol Cell Biol. 1994;14:4435–4442. doi: 10.1128/mcb.14.7.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sloan-Lancaster J, Allen P M. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. :1–27. [DOI] [PubMed] [Google Scholar]
- 46.Sloan-Lancaster J, Shaw A S, Rothbard J B, Allen P M. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 47.Sloan-Lancaster J, Steinberg T H, Allen P M. Selective activation of the calcium signaling pathway by altered peptide ligands. J Exp Med. 1996;184:1525–1530. doi: 10.1084/jem.184.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein P L, Lee H M, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 49.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 50.Taylor S J, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 51.Tognon C E, Kirk H E, Passmore L A, Whitehead I P, Der C J, Kay R J. Regulation of RasGRP via a phorbol ester-responsive C1 domain. Mol Cell Biol. 1998;18:6995–7008. doi: 10.1128/mcb.18.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsygankov A Y, Broker B M, Fargnoli J, Ledbetter J A, Bolen J B. Activation of tyrosine kinase p60fyn following T cell antigen receptor cross-linking. J Biol Chem. 1992;267:18259–18262. [PubMed] [Google Scholar]
- 53.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 54.Uehara Y, Murakami Y, Mizuno S, Kawai S. Inhibition of transforming activity of tyrosine kinase oncogenes by herbimycin A. Virology. 1988;164:294–298. doi: 10.1016/0042-6822(88)90649-6. [DOI] [PubMed] [Google Scholar]
- 55.van Leeuwen J E, Samelson L E. T cell antigen-receptor signal transduction. Curr Opin Immunol. 1999;11:242–248. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 56.Wange, R. L. 2000 LAT, the linker for activation of t cells: a bridge between t cell specific and general signaling pathways. Science's STKE. [Online.] http://www.stke.org/cgi/content/full/OC_sigtmas;2000/63/rel. [DOI] [PubMed]
- 57.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 58.Whitehurst C E, Owaki H, Bruder J T, Rapp U R, Geppert T D. The MEK kinase activity of the catalytic domain of RAF-1 is regulated independently of Ras binding in T cells. J Biol Chem. 1995;270:5594–5599. doi: 10.1074/jbc.270.10.5594. [DOI] [PubMed] [Google Scholar]
- 59.Williams B L, Irvin B J, Sutor S L, Chini C C, Yacyshyn E, Bubeck W J, Dalton M, Chan A C, Abraham R T. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation. EMBO J. 1999;18:1832–1844. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams B L, Schreiber K L, Zhang W, Wange R L, Samelson L E, Leibson P J, Abraham R T. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 62.Yamasaki S, Tachibana M, Shinohara N, Iwashima M. Lck-independent triggering of T-cell antigen receptor signal transduction by staphylococcal enterotoxins. J Biol Chem. 1997;272:14787–14791. doi: 10.1074/jbc.272.23.14787. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, Irvin B J, Trible R P, Abraham R T, Samelson L E. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W, Samelson L E. The role of membrane-associated adaptors in T cell receptor signalling. Semin Immunol. 2000;12:35–41. doi: 10.1006/smim.2000.0205. [DOI] [PubMed] [Google Scholar]