Abstract
Purpose
To review and represent three different mandibular reconstruction modalities following surgical treatment of massive mandibular fibrous Dysplasia (MMFD).
Methods
The present retrospective case series study was conducted on 24 patients who had MMFD and treated via resection and immediate reconstruction at Al-Azhar University Hospitals, Egypt. Patients were divided into three groups according to the grafting procedure. Group I patients were grafted with iliac bone graft (IBG), group II patients were grafted with IBG and bone marrow aspirate concentrate (BMAC), while group III patients were grafted with free vascularized fibula graft (FVFG). Postoperative clinical and radiographic assessments were performed immediately, at 6 months, 12 months and 2 years to evaluate lesion recurrence and bone graft resorption. Other study variables included assessment of postoperative wound dehiscence, infection rate, amount of edema, and facial bone contour.
Results
The parameters of the clinical analysis showed non-statistically significant differences among all groups. Postoperative wound healing was clinically uneventful in all groups, except for two cases of wound dehiscence in group I (8.3%) and one case in group III (4.2%). Most patients had appropriate postoperative facial contour, and adequate facial symmetry. The radiographic measurements revealed a highly statistically significant difference between group I and II at 12 months, and two years, without any statistically significant difference between group II and III.
Conclusion
MMFD surgical defect should be repaired for function and cosmetics aims especially in young adult patients. The findings of the present study have shown that when compared to traditional IBG alone or FVFG, the use of autogenous IBG with BMAC injection produces a favorable outcome with few difficulties.
Keywords: Fibrous dysplasia, Iliac crest, IBG s, BMAC, Free vascularized fibula graft
Introduction
Fibrous dysplasia (FD) is considered developmental hamartomatous fibro-osseous bony disorder of unknown etiology. It may represent developmental arrest in a benign fibro-osseous proliferation which lacks the ability to complete differentiation. A mutation occurring in the gene coding of alpha subunits of the stimulatory G protein GS in the guanine nucleotide binding alpha simulating (GNAS) has been proposed to cause monostotic and polystotic conditions. Disease onset is characteristic for the first and second decades and site predominance is for the maxilla. Mandibular cases are represented frequently as case reports in the literatures [1–3].
FD are divided into about 70% in the form of monostatic and 30% as polystatic. Albright syndrome is characterized by association of FD and precocious puberty and skin pigmentation with some forms of endocrine abnormalities [4]. In the jaw bone all forms had been seen. Malignant transformation were rare about 0.5% for monostatic form and about 4% in Albright syndrome. Differential diagnosis of maxillofacial FD include Paget's disease, ossifying or cementifying lesions of the jaw. It has specific radiographic ground glass appearance on the selected computed tomography (CT) [5–7].
Although FD is slowly progressive disorder of the bone, there are some concomitant reported complications due to replacement of cancellous bone by dysplastic fibrous woven immature tissues. Definitive diagnosis usually obtained through biopsy [8–10].
For avoidance of high recurrence rate, surgical radical resection of the lesion is recommended as showed by Valentini et al. [11]. Treatment of mandibular fibrous dysplasia (MFD) depends mainly on the age, associated complications, extended size of the lesion and site. Surgical corrections recommended rebuilding of resected part of bone to avoid its relapse. The complicated 3 dimensional anatomy of the skull and face, however, makes it difficult to recontour this region [12–15].
Different reconstructive modalities for immediate mandibular reconstruction specifically in the teenagers and young adults' patients are available nowadays as vascularized advanced microvascular surgery, and non-vascularized autogenous bone grafts [16, 17]. But, there are several drawbacks for non-vascularized bone grafts such as donor site complications, as well as rapid resorption of bone graft along time [18, 19]. Recently advanced adjunctive use of PRP, stem cells, bone marrow aspirate and other regenerative procedures aid in rapid healing and functional rehabilitation of the jaw bone [1, 20, 21].
Despite of versatility of different immediate reconstructive modalities of mandibular defects, there are still no consensus regarding recommended post reconstructive option of the massive MFD radical resection [22, 23]. The present study was designed to review and assess three different modalities for reconstruction of mandible after surgical radical resection of massive mandibular fibrous Dysplasia (MFD).
Methods
Study Design and Setting
The present study included 24 patients (15 males and 9 female) with age ranging from 12 to 18 years who had massive mandibular fibrous dysplasia and treated via segmental radical resection and immediate reconstruction.
They were collected from those attending the outpatient Oral and Maxillofacial clinics at Faculty of Dental Medicine, Al-Azhar University, Assiut branch over a period of five years (April 2015–April 2020). The choice of the reconstructive procedure was discussed with the patients and their guardians and determined according to lesion size and the patients preferences. Informed consent was obtained from all participants before surgery and commitment to complete the two years follow-up period was confirmed. The present study was approved from the institutional ethical Committee of Assiut Branch, Al-Azhar University (AUAREC20200715-4) and conducted in complete accordance with Helsinki Declaration of research ethics.
Patient Selection and Inclusion Criteria
Massive mandibular fibrous dysplasia based on size of bone defects more than 2.5 cm3 and aesthetic affection.
Absence of any syndromic features or osseous malformations of the face in the extended family based on skeletal imaging.
Absence of any associated endocrine abnormality based on the preoperative performed hormonal laboratory testing.
Preoperative radiographic presentation of fibrous dysplasia as poorly defined ovoid areas as a “ground glass” appearance.
Confirmed preoperative biopsy of the fibrous dysplasia.
Medically fit without any blood diseases or other systemic disorders.
Patients Grouping
Patients with severe fibrous mandibular dysplasia were assorted into three groups according to their performed reconstruction technique:
Group I: Includes patients grafted with non-vascularized iliac crest bone graft (IBG).
Group II: Included patients grafted with iliac crest bone graft injected with bone marrow aspirate concentrate (BMAC) at surgical site.
Group III: Included patients grafted with free vascularized fibula graft (FVFG).
Preoperative Preparations
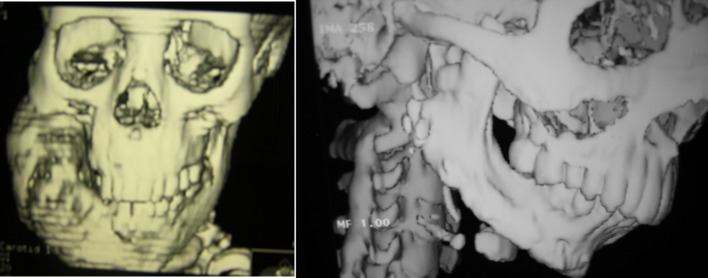
For each participant, preoperative patient history, intraoral, and extra oral clinical examination was collected (Fig. 1). Completed radiographic analysis using OPGs (Orthopantomograph) and CT scan was thoroughly studied (Fig. 2). For all participants, Incisional surgical biopsy was performed to confirm diagnosis of fibrous dysplasia. The 3D CT stereolithographic model was reconstructed and the Titanium reconstructive bone plate was adapted and contoured on it. The extent of the lesion has been graded according to HCL classification by Jewer and modified by Boyd et al. [24].
Fig. 1.
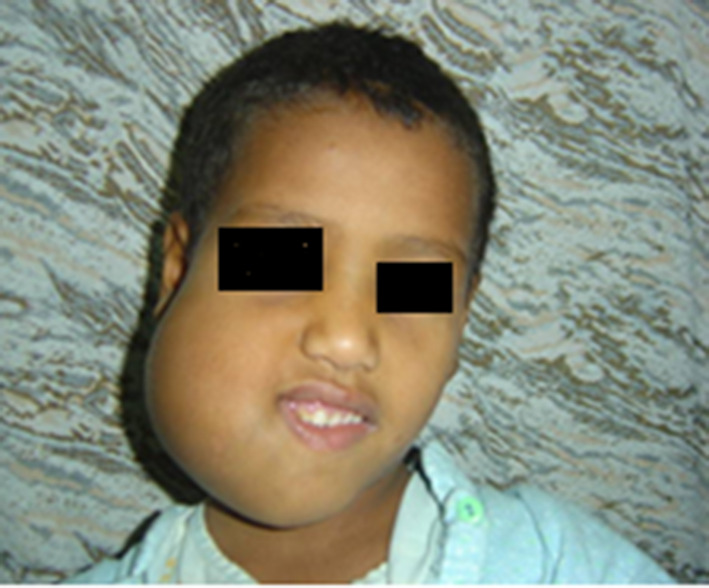
Preoperative clinical photograph for a patient with massive monostatic fibrous dysplasia at the right ramus area of the mandible
Fig. 2.
Reconstructed computed tomogram photograph showing: a Preoperative massive monostatic fibrous dysplasia at the right ramus area of mandible extending to the inferior border, b one year postoperative reconstructed mandible with iliac crest bone graft
Surgical operation
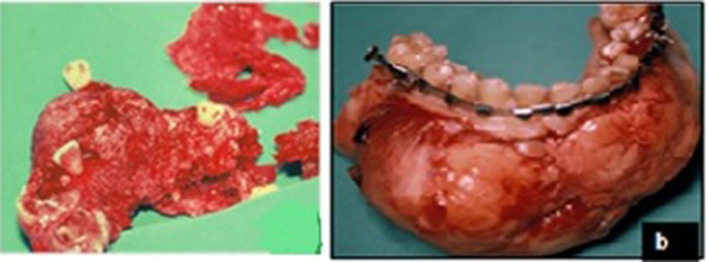
Segmental mandibular resection with insertion of titanium reconstruction bone plate was performed with iliac crest bone grafting alone in group I or with BMA injection as in group II simultaneously (Fig. 3). While the vascularized fibula graft was used in group III for bone grafting. Surgical procedure for harvesting of free vascularized fibular graft performed as outlined in detail by Robert M Laughlin and Christopher Harris et al. [25]. Pressure bandage dressing has been applied in all cases. All intraoperative procedures were conducted by the same experienced surgical team (Fig. 4).
Fig. 3.
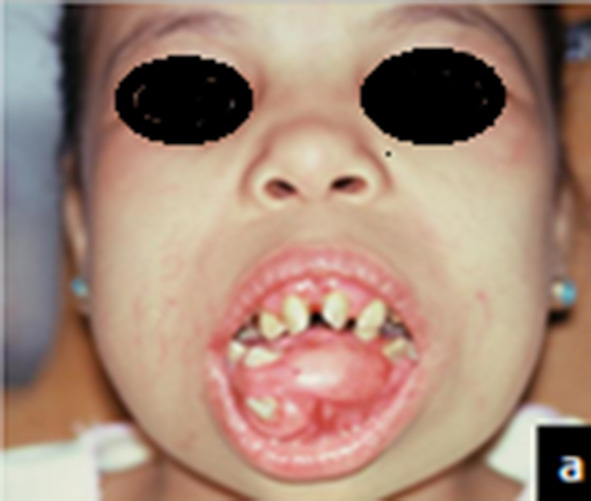
Preoperative clinical photograph for patients with massive monostatic fibrous dysplasia at the anterior area of mandible
Fig. 4.
Intraoperative clinical photograph showing lesions after resection
Bone Marrow Aspiration
Anterior iliac Bone marrow aspirates was harvested using bone marrow aspirating needle (Islam STD Aspirate Needle Set 100 × 2.55 MM. Downs international, B. Braun medical Ltd. England) in the following steps according to Chahla et al. [21].
After swabbing of anterior iliac crest region by Betadine solution, a sterile Islam marrow aspirating needle was inserted through the skin, after stretching it opposite to the anterior crest of the ilium. Continues passage of needle until cortex penetration started with half circular rotary movement and minimal pressure to avoid bending of the needle. When needle reached to cancellous marrow spaces, the trocher was removed and 10ml sterile plastic syringe was loaded to the aspirating part of needle. After aspiration, needle was withdrawn leaving only skin puncture. Collection of 60 mL of bone marrow aspirate and centrifuge according to Chahla et al. [21] protocol, to obtain approximately 6 mL BMAC sample. The injection was carried out at a reconstruction site following incision closure as slowly as possible to stop potential washout.
Postoperative Care and Follow-up Protocol
According to standardized maxillofacial surgery regimens in our institute, enhanced recovery after surgery protocol has been applied to all patients. All patients remain in the ICU for one day, then stay in the inpatient unit for another five days prior to discharge. For 10 days after surgery, analgesics, anti-inflammatory medications, antibiotics, and antiseptic mouthwash were used. One week after surgery, the skin sutures were eliminated and all patients underwent physiotherapy.
Pressure bandage dressing was applied in all cases. All patient after reconstruction were followed up for two years. Patients wound examined by two investigators immediately after surgery using intraoral inspection and palpation to examine complete incision closure. Classification of the wound dehiscence was present or not (yes or no) without grading. First day cleaning and irrigation with normal saline were performed and wound dehiscence was reported if it is present.
The patients were closely observed daily in the first week and then asked for follow-up at 2nd, 3rd and 4th weeks and again, at 6 months, 12 months and 2 years to evaluate recurrence of lesion and bone graft resorption. Study variables included assessment of postoperative wound dehiscence, infection rate, amount of edema, contour of the facial bone symmetry and recurrence of the lesion.
After year year form reconstruction bone plate removed and dental implant inserted in the newly formed bone. Fixed or removable prosthodontic were placed after four month from dental implant insertion. The clinical peri-implant soft tissue and radiographic osseointegration and stability of the implants were monitored till the end of the follow-up periods (Fig. 5).
Fig. 5.
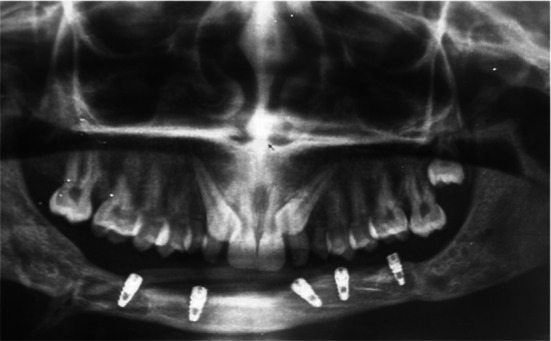
Postoperative OPG photograph showing fibula bone graft reconstructed the anterior mandible and implant rehabilitation after one year
Radiographic Evaluation
Radiographic follow-up was performed at the following intervals via digital panoramic radiograph at Immediate, 3, 6, 12 and 24 months postoperatively to determine improvement in radiodensitometric bone changes at the bone graft site.
The same electronically operated panoramic machine (Orthophos 3, Sirona, Dental systems GmbH fabrirkstr.31D-64626 Bensheim, Germany) imaged all radiographs for each patient, the exposure parameters for all patients were considered to be 70 kv and 10 A for 15 s.
Radiographic Measurements
Each panoramic radiograph was assessed for bone graft stabilization, bone graft union with natural bone, new bone regeneration, and bone graft resorption rate. The Digora (Soredex) specially crafted software was used for manipulation of the digital radiographic images. The mean gray value of the region of interest (ROI) marked on each digital image was calculated using the following steps:
Point A was chosen at the bone graft site, and that point's pixel density was determined on a scale from 0 to 255 based on its radio-opacity, where the most radiopaque is 255. The totally black regions (totally radiolucent) were given zero scale.
A second point (point B) of ROI was chosen and measured at the sound bone. The difference was calculated between these two points. In order to standardize the location of the point of interest under investigation, the exact coordinates (X and Y coordinates) determined for each of points A and B for each case and replicated during the radiography follow-up.
Data Analysis
All data was gathered in Excel sheet and then transferred to version 16 SPSS file. Categorical variables were analyzed for comparison using the Crosstab chi-square test between the study groups. ANOVA test was used to compare continuous homogeneous data and Multiple Comparisons Post-Hoc analysis using Bonferroni test was performed at a confidence interval of 95% for distinction between mean values. The P-value was set to or below 0.05.
Results
All patients included in this study had massive mandibular fibrous dysplasia [16 monostotic type (66.5%) and 8 polyostotic type (33.5%)]. Both the preoperative and the final postoperative histopathological examinations confirmed benign nature of the lesions without any malignancy. The duration of the lesions ranged from 6 to 36 months (Table 1). Duration of surgery ranged from 3 to 5 h for group I, 4 to 5 h for group II, and 6 to 9 h for group III.
Table 1.
Descriptive and statistical analysis of the preoperative study variables of all groups
| Study variables | Variables categories | Total | Group I | Group II | Group III | P-value |
|---|---|---|---|---|---|---|
| No | No | 24 | 7 (29.2%) | 8 (33.3%) | 9 (37.5%) | |
| Age range (12–18) | Mean | 13.5 | 13.1429 | 14.0000 | 13.3333 | 0.73 |
| ST/Dev | ± 2.16 | ± 2.26779 | ± 2.13809 | ± 2.29129 | ||
| Gender | Male | 15 (62.5%) | 5 (20.8%) | 5 (20.8%) | 5 (20.8%) | 0.81 |
| Female | 9 (37.5%) | 2 (8.3%) | 3 (12.5%) | 4 (16.7%) | ||
| Fibrous dysplasia type | Monostotic type | 16 (66.5%) | 5 (20.8%) | 4 (16.7%) | 7 (29.1%) | 0.93 |
| Polyostotic type | 8 (33.5%) | 3 (12.5%) | 3 (12.5%) | 2 (8.3%) | ||
| Side | R | 11 (45.8%) | 4 (16.7%) | 3 (12.5%) | 4 (16.7%) | 0.88 |
| Left | 7 (29.2%) | 2 (8.3%) | 3 (12.5%) | 2 (8.3%) | ||
| Ant region | 6(25%) | 1 (4.2%) | 2 (8.3%) | 3 (12.5%) | ||
| Size | L | 15 (62.5%) | 5 (21.7%) | 5 (21.7%) | 5 (21.7%) | 0.93 |
| LC | 4 (16.7%) | 1 (4.3%) | 2 (8.7%) | 1 (4.3%) | ||
| LCL | 4 (16.7%) | 1 (4.3%) | 1 (4.3%) | 2 (8.7%) | ||
| C | 1 (4.2%) | 1 (4.2%) | ||||
| Facial bone contour | Normal | 5 (20.8%) | 2 (8.3%) | 1 (4.2%) | 2 (8.3%) | 0.74 |
| Asymmetry | 19 (79.2%) | 5 (20.8%) | 7 (29.2%) | 7 (29.2%) |
All patients showed uneventful wound healing except for two cases (8.3%) in group I and one case (4.2%) in group III who showed postoperative wound dehiscence at the incision site, which treated with drug prescription, vigorous saline irrigation and debridement plus patient emphasis on good oral care. The injection site and BMA site in group II showed non eventful side effects or complications. 70.8% of the lesions involved one side of the mandible.
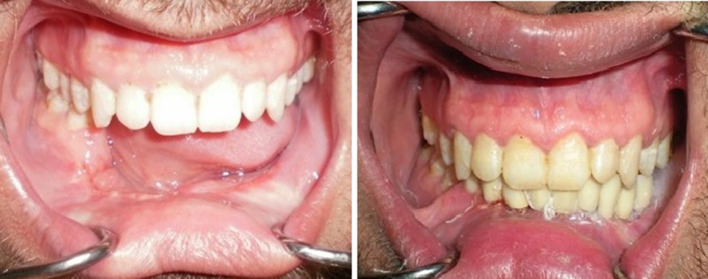
There was no recurrence of the mandibular lesions after 2 years follow-up in all groups. The results showed that all patients had adequate facial contour, proper facial symmetry postoperatively. The free iliac crest graft and free fibula graft were well taken (Fig. 6). Chi-Square test for comparison of the study group's categorical variables revealed a non-statistically significant difference between all dependent variables (Table 2).
Fig. 6.
Postoperative intraoral clinical photographs showing the reconstructed mandible rehabilitation with fixed implant supported restorations
Table 2.
Postoperative study variables statistical analysis of all groups
| Study variables | Variables categories | Total | Group I | Group II | Group III | P-value |
|---|---|---|---|---|---|---|
| No | No | 24 | 7 (29.2%) | 8 (33.3%) | 9 (37.5%) | |
| Lesion recurrence | 0 | 0 | 0 | 0 | ||
| Postop wound dehiscence | Yes | 3 (12.5%) | 2 (8.3%) | 0.0% | 1 (4.2%) | 0.25 |
| No | 21 (87.5%) | 6 (25.0%) | 8 (33.3%) | 7 (33.3%) | ||
| Infection | Yes | 3 (12.5%) | 2 (8.3%) | 0.0% | 1 (4.2%) | 0.98 |
| No | 21 (87.5%) | 6 (25.0%) | 8 (33.3%) | 7 (29.2%) | ||
| Edema | Mild | 19 (79.2%) | 4 (16.7%) | 8 (33.3%) | 7 (29.2%) | 0.36 |
| Moderate | 4 (16.5%) | 3 (12.5%) | 0.0% | 1 (4.2%) | ||
| Severe | 1 (4.3%) | 1 (4.3%) | 0.0% | 0.0% | ||
| Hematoma | Yes | 5 (20.8%) | 3 (12.5%) | 0.0% | 2 (8.5%) | 0.77 |
| No | 19 (79.2%) | 4 (16.7%) | 8 (33.3%) | 7 (29.2%) | ||
| Bone resorption | Yes | 10 (41.7%) | 4 (16.7%) | 2 (8.3%) | 4 (16.7%) | 0.31 |
| No | 14 (58.3%) | 3 (12.5%) | 7 (29.2%) | 4 (16.7%) | ||
| Immediate | 54.85 ± 8.33 | 59.56 ± 3.80 | 58.49 ± 6.11 | 0.33 | ||
| Bone densitometric change analysis | 6 months | 45.69 ± 9.87 | 43.43 ± 2.12 | 47.02 ± 6.20 | 0.54 | |
| 12 months | 25.65 ± 5.36 | 13.51 ± 2.95 | 20.98 ± 2.62 | 0.001 | ||
| Two years | 18.73 ± 2.69 | 10.92 ± 1.85 | 17.14 ± 2.95 | 0.001 |
Difference in age between groups was not statistically important. Group III showed less occurrence of recipient site postoperative edema and hematoma while these variations between groups were non-statistically different. Donor site complications have shown a tendency to be higher in the fibula flap group, including pain and edema and hematoma.
ANOVA test found that the measurements of bone density showed a statistically significant difference in one and two years of follow-up periods between groups as group II showed the least densitometric mean changes from the normal bony side.
Discussion
Reconstructive modalities for the defects in mandibular radical resection have been developed and significantly improved over the last few years. Reconstructive modalities for fibrous dysplasia still lacked consensus or solid evidence although some recommended guidelines have been advocated by Javaid et al. [23]. Any reconstructive decision should enhance the aesthetics and function of the patient [26].
The surgical management of these massive bone deformities is controversial because the degree of FD deformity varies between children and adults. Furthermore, aggressively large lesions that did not respond to recontouring or curettage due to significant expansion of the lesion and the fear of malignant changes may necessitate radical resection and graft reconstruction [23, 27], which provided more effective results, particularly in monostotic types like the current cases. We chose to do radical excision in accordance with the recommendations for major lesion management [23, 28, 29], which was thoroughly addressed with the patient's family.
Furthermore, there is no available cortex to leave at the inferior border of the mandible after curettage in aggressive massive fibrous dysplasia, increasing the risk of mandibular fracture and necessitating further mandibular reconstruction even in young growing patients, as is the case in the management of other benign lesions such as ameloblastoma. In FD lesions, we do not aim for resection; however, we must preserve the continuity and contouring of the flail mandible after the lesion is removed.
In the current clinical report, we reviewed all extensive mandibular fibrous dysplasia lesions at our department treated with radical excision and simultaneous reconstruction with three different modalities. BMA use can be of benefit in promoting healing in cases of large bone defect [23, 30, 31]. In accordance with this recommendation, the present study was conducted to review and evaluate the efficacy of IBG s with BMAC injection in bone defect versus IBG alone and free fibular grafting following surgical treatment of massive mandibular fibrous Dysplasia (MMFD).
In the present series, MFDs were painless in all cases although it was massive and aggressive. FD's aggressiveness typically predominates in the polystotic form and is estimated to range from 0.9 to 4%.[32] There was no recurrence or malignant transformation in all cases presented till the end in follow-up periods and this may be attributed to early surgical radical resection and reconstruction. There was no gender difference between groups affected by MFD and this coincide with what Lynda et al [22] had confirmed.
While mandibular fibrous dysplasia is rare, the majority of cases included in the present study were monostatic type (66.5%). This occurrence is consistent with the study by Ameli et al. [33] and Guruprasad et al. [34] which concluded that the monostatic form of fibrous dysplasia is the most common, comprising 70% of cases, and most likely to quiescent at puberty.
The present series showed that rate and amount of bone resorption following free non-vascularized fibular bone grafting was not statistically significantly different from NVIBG in contrast with comparable results from Szpindor [35] and Lenzen et al. [36] studies which reported that more than 50% of the bone grafts resorbed in patients with immediate reconstruction. Nevertheless, group II showed substantially better results with respect to bone quality in 1 and 2 years follow-up periods and this can be explained by the regenerative effect of BMA on IBG after immediate mandibular reconstruction that accelerates bone healing and decreases the risk of postoperative wound dehiscence and enhance reepithelialization. This finding was consistent with Pittenger et al. [37], Quarto et al. [38], and Chahla et al. [21] results although different techniques for stem cell preparation were applied. Furthermore, two cases of wound dehiscence in group I (8.3%) and one case in group III (4.2%) are consistent with previous research, which found that intraoral wound dehiscence occurs in 10% of mandibular fracture patients, regardless of age or gender. When a cautious and competent surgical technique is adopted and with the aid of regenerative adjunctive methods, this complication could be prevented and if does occur, conservative management is enough as used in the present cases [39].
Group II demonstrated an improvement in bone regenerative potential resulting in detectable homogenous union between the edges of resected site and the autogenous bone graft. The load on the condyle which was more noted in fibular bone graft group (Group III) also decreased in group I and II. This findings support the use of IBG adjunctive with BMAC in cases requiring reconstruction after aggressive mandibular fibrous dysplasia resection instead of fibular graft [1].
Indications and outcomes of the different modalities i.e.,: IBG, IBG with BMAC, and VFG are widely considered for use in mandibular reconstruction. For mandibular angle or body defects or even defects requiring greater soft tissue bulk for the intraoral lining, the iliac crest is the first alternative. The fibula flap is best when the bony length is required, for example in subtotal or complete mandibulectomy, although it is accompanied by the most morbidities at the donor site [40].
In addition, some patients refused the option of fibular grafting and preferred another method of regeneration, while at the same time allowing them the opportunity to complete functional rehabilitation and improved osseointegration which may also be enhanced with the use of BMAC in conjunction with cortico-cancellous bone grafting comparable to the compact fibular graft which requires readability of facilities for free vascular fibula flap.
Postoperative radiographic assessment of six months showed evidence of newly bone formation with decreased density and arrangement of bone trabeculae with superiority for group II compared to group I. Although, at this time, the group III displayed adequate normal bone formation and well-arranged trabecular bone, bone maturation in group II was more evident at 12 months postoperatively than other groups and the density was close to that of the normal adjacent bone and this findings is important especially in young age group patients for rehabilitation with fixed implantation osteosynthesis.
Other researchers have recently used these regenerative methods alone without any bony augmentation to regenerate the bony defects as described by Hatem Al-Ahmady et al. [31], in 2018 however this technique requires more time for functional bone rehabilitation after regenerative bone formation and is still not suitable for large massive MFD lesions.
Hence, because of the non-significant difference between the studied groups over time, IBG with BMA injection could be a reasonable alternative with comparative densitometric bony results to the free vascularized fibula. This is in contrast to Feingold et al. [27] who said that reconstruction of microvascular free flaps has a strong role to play in handling giant FD involving the mandible after segmental excision.
The treatment of MFD in young adults may necessitate significant surgical intervention, and each case is unique because many strategies may be used. In the present case series reports we represent different reconstructive modalities for management of a benign disorder that requires radical resection of the mandible with the existing limitation of a small sample size which involved only one institution. Furthermore, further studies are still needed to determine the long-term survival of osseointegration implants in different types of grafts.
Conclusion
Mandibular defect should be repaired for function and cosmetics aims after surgical treatment of massive fibrous dysplasia especially in young adult patients. Given the limited number of patients, the findings of the present study have shown that the use of autogenous iliac bone graft with bone marrow aspirate concentrate injection has been shown to deliver a good result with few complications comparable to the use of IBG alone or vascularized free fibular graft. Future research should focus on developing standardized treatment guidelines for MDF.
Acknowledgment
None.
Funding
No funding received.
Declarations
Conflict of interest
All authors declared that they have no conflict of interest.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the present study
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdel Aziz Baiomy Abdullah Baiomy, Email: abdelazizbaiomy@azhar.edu.eg.
Shadia Abdelhameed Elsayed, Email: Shadiaelsayed@azhar.edu.eg, Email: shadiaelsayed25@gmail.com.
References
- 1.Caridad JJM, Platas F. Fibrous dysplasia of the mandible: Surgical treatment with platelet-rich plasma and a corticocancellous iliac crest graft—report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 2008;105:e12–e18. doi: 10.1016/j.tripleo.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Couturier A, Aumaître O, Gilain L, et al. Craniofacial fibrous dysplasia: a 10-case series. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134:229–235. doi: 10.1016/j.anorl.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Galuppo B, Piazza DMB, Macedo AP, et al. Monostotic fibrous dysplasia: case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;129:e72. doi: 10.1016/j.oooo.2019.06.287. [DOI] [Google Scholar]
- 4.Béquignon E, Cardinne C, Lachiver X, et al. Craniofacial fibrous dysplasia surgery: a functional approach. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130:215–220. doi: 10.1016/j.anorl.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Hoshi M, Matsumoto S, Manabe J, Tanizawa T, Shigemitsu T, Izawa N, et al. Malignant change secondary to fibrous dysplasia. Int J Clin Oncol. 2006;11:229–35. doi: 10.1007/s10147-006-0559-4. [DOI] [PubMed] [Google Scholar]
- 6.Albright F, Butler AM, Hampton AOSP. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females. N Engl JMed. 1937;216:727–46. doi: 10.1056/NEJM193704292161701. [DOI] [Google Scholar]
- 7.Lietman SA, Ding C, Levine MA. A highly sensitive polymerase chain reaction method detects activating mutations of the GNAS gene in peripheral blood cells in Mccune–Albright syndrome or isolated fibrous dysplasia. J Bone Jt Surg Ser A. 2005;87:2489–2494. doi: 10.2106/JBJS.E.00160. [DOI] [PubMed] [Google Scholar]
- 8.Yetiser S, Gonul E, Tosun F, Tasar MHY. Monostotic cra-niofacial fibrous dysplasia: the Turkish experience. J Craniofac Surg. 2006;17:62–7. doi: 10.1097/01.scs.0000186459.29967.e7. [DOI] [PubMed] [Google Scholar]
- 9.Ralston SH. Pathogenesis of Paget’s disease of bone. Bone. 2008;43:819–25. doi: 10.1016/j.bone.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Reymond J, Podsiadło M, Wyskiel M. Fibrous dysplasia situated in maxilla–diagnostic and treatment difficulties illustrated with case report. Otolaryngol Pol Pol Otolaryngol. 2006;60:79–84. [PubMed] [Google Scholar]
- 11.Valentini V, Cassoni A, Marianetti TM, Terenzi V, Fadda MTI. Craniomaxillofacial fibrous dysplasia: conservative treatment or rad-ical surgery? A retrospective study on 68 patients. Plast Reconstr Surg. 2009;123:653–60. doi: 10.1097/PRS.0b013e318196bbbe. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Liang L, Gu B, et al. A retrospective study on craniofacial fibrous dysplasia: preoperative serum alkaline phosphatase as a prognostic marker? J Cranio-Maxillofacial Surg. 2013;41:644–647. doi: 10.1016/j.jcms.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Temerek AT, Ali S, Shehab MF. Computer guided resection and reconstruction of intra-osseous zygomatic hemangioma: case report and systematic review of literature. Int J Surg Case Rep. 2020;66:240–256. doi: 10.1016/j.ijscr.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tekin AF, Ünal ÖF, Göksel S, Özcan İ. Clinical and radiological evaluation of cherubism: a rare case report. Radiol Case Rep. 2020;15:416–419. doi: 10.1016/j.radcr.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nwabueze NN, Shinn JR, Owen S, et al. Reconstructive septorhinoplasty in radiation-induced reactive bone dysplasia. Otolaryngol Case Rep. 2019;13:100131. doi: 10.1016/j.xocr.2019.100131. [DOI] [Google Scholar]
- 16.Khalifa GA, Abd El Moniem NA, Elsayed SAE, Qadry Y. Segmental mirroring: does it eliminate the need for intraoperative readjustment of the virtually pre-bent reconstruction plates and is it economically valuable? J Oral Maxillofac Surg. 2016;74:621–630. doi: 10.1016/j.joms.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Yang C, Fang B, et al. Treatment of hemimandibular fibrous dysplasia with radical excision and immediate reconstruction with free double costochondral graft. J Oral Maxillofac Surg. 2010;68:2000–2004. doi: 10.1016/j.joms.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Foster RD, Anthony JP, Sharma A, et al. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21:66–71. doi: 10.1002/(SICI)1097-0347(199901)21:1<66::AID-HED9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Pogrel MA, Podlesh S, Anthony JP, et al. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55:1200–6. doi: 10.1016/S0278-2391(97)90165-8. [DOI] [PubMed] [Google Scholar]
- 20.Jager M, Herten M, Fochtmann U, et al. Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res. 2011;29:173–80. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
- 21.Chahla J, Mannava S, Cinque M, Geeslin A, Codina DLR. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6:e441–e445. doi: 10.1016/j.eats.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bile-Gui LN, Ahoury J, Kabas RM. Imaging of advanced craniofacial fibrous dysplasia associated with McCune–Albright syndrome: a case report. Eur J Radiol Open. 2020;7:100208. doi: 10.1016/j.ejro.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javaid MK, Boyce A, Appelman-Dijkstra N, et al. Best practice management guidelines for fibrous dysplasia/McCune–Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14:1–17. doi: 10.1186/s13023-019-1102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewer DD, Boyd JB, Manktelow RT, et al. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plast Reconstr Surg. 1989;84:404–5. doi: 10.1097/00006534-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin RM, Harris CM (2015) Free vascularized fibula and graft harvest. In: Atlas of operative oral and maxillofacial surgery. Wiley, pp 505–510
- 26.Rau LH, Reinheimer A, Meurer MI, et al. Fibrous dysplasia with secondary aneurysmal bone cyst—a rare case report and literature review. Oral Maxillofac Surg. 2019;23:101–107. doi: 10.1007/s10006-019-00741-w. [DOI] [PubMed] [Google Scholar]
- 27.Stanton RP, Ippolito E, Springfield D, Lindaman L, Shlomo Wientroub AL. The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis. 2012;7:1–9. doi: 10.1186/1750-1172-7-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji T, Tanaka S, Kishino M, Kogo M. Fibrous dysplasia of mandible-Report of a case presenting with a rare clinical course. J Oral Maxillofac Surg Med Pathol. 2014;26:483–487. doi: 10.1016/j.ajoms.2013.04.007. [DOI] [Google Scholar]
- 29.Singh P, Shergil A, Solomon M, Carnelio S. Osseous dysplasia: an aggressive swelling at an uncommon site. J Oral Maxillofac Surg Med Pathol. 2016;28:264–266. doi: 10.1016/j.ajoms.2015.09.006. [DOI] [Google Scholar]
- 30.Mossaad AM, Ahmady HH Al, Ghanem WH, et al (2021) The use of dual energy X-ray bone density scan in assessment of alveolar cleft grafting using bone marrow stem cells concentrate/platelet-rich fibrin regenerative technique. J Craniofac Surg [DOI] [PubMed]
- 31.Al-Ahmady HH, Abd Elazeem AF, Bellah Ahmed NE, et al. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with nano hydroxyapatite, and platelet-rich fibrin: reporting a novel strategy for alveolar cleft bone regeneration. J Cranio-Maxillofacial Surg. 2018;46:1593–1600. doi: 10.1016/j.jcms.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 32.Ippolito E, Caterini R, Farsetti PPV. Surgical treatment of fibrous dysplasia of bone in McCune–Albright syndrome. J Pediatr Endocrinol Metab. 2002;15:939–44. [PubMed] [Google Scholar]
- 33.Ameli NO, Rahmat HAK. Monostotc fbrous dysplasia of the cranial bones: report of fourteen cases. Neurosurg Rev. 1981;4:71–7. doi: 10.1007/BF01837749. [DOI] [PubMed] [Google Scholar]
- 34.Guruprasad Y, Chauhan D. Craniofacial fibrous dysplasia - A review of current management techniques. Chron Young Sci. 2012;3:106. doi: 10.4103/2229-5186.98672. [DOI] [Google Scholar]
- 35.Szpindor E. Evaluation of the usefulness of autogenic bone grafts in reconstruction of the mandible. Ann Acad Med Stetin. 1995;41:155–69. [PubMed] [Google Scholar]
- 36.Lenzen C, Meiss ABH. Augmentation of the extremely atrophied maxilla and mandible by autologous calvarial bone transplantation. Mund Kiefer Gesichtschir. 1999;3:S40–2. doi: 10.1007/PL00014514. [DOI] [PubMed] [Google Scholar]
- 37.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 38.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 39.Elsayed SA, Abdullah AAB, Dar-Odeh N, Altaweel AA. Intraoral wound dehiscence after open reduction internal fixation of mandibular fractures: a retrospective cohort study. Wounds A Compend Clin Res Pract. 2021;33:60–64. [PubMed] [Google Scholar]
- 40.Lonie S, Herle P, Paddle A, et al. Mandibular reconstruction: meta-analysis of iliac- versus fibula-free flaps. ANZ J Surg. 2016;86:337–342. doi: 10.1111/ans.13274. [DOI] [PubMed] [Google Scholar]