Abstract
A 67-year-old man with metastatic prostate cancer was treated with leuprorelin and enzalutamide, but presented radiographic progression after 1 year. Although docetaxel chemotherapy was initiated, liver metastasis appeared with elevation of nerve-specific enolase in serum. Pathological findings of needle biopsy of lymph node metastasis in the right inguinal region showed neuroendocrine carcinoma. FoundationOne CDx® using a biopsy sample of the prostate at initial diagnosis detected the BRCA1 mutation (deletion of intron 3–7), but BRACAnalysis® test revealed no BRCA mutation in germline. Then, olaparib treatment was initiated, resulting in remarkable remission of tumors, but comorbidity with interstitial pneumonia. This case suggested that olaparib could be effective for neuroendocrine prostate cancer with BRCA1 gene mutation, but may cause interstitial pneumonia.
Keywords: BRCA1 mutation, Neuroendocrine prostate cancer, Olaparib
Introduction
Neuroendocrine prostate cancer (NEPC) rarely emerges after hormonal therapy for prostate cancer [1]. NEPC is characterized by poor response to castration, and increased potential of progression, resulting in lethality [2]. There is no standard therapy for NEPC, although it is often treated with etoposide and cisplatin chemotherapy, based on the treatment for small cell lung cancer [3]. Olaparib is a poly ADP ribose polymerase (PARP) inhibitor, that is effective for BRCA1- or BRCA2-mutated prostate cancer [4]. We report a case of NEPC with BRCA1 mutation treated with olaparib.
Case report
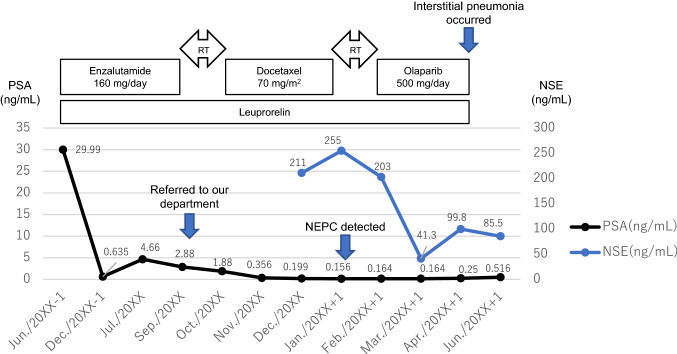
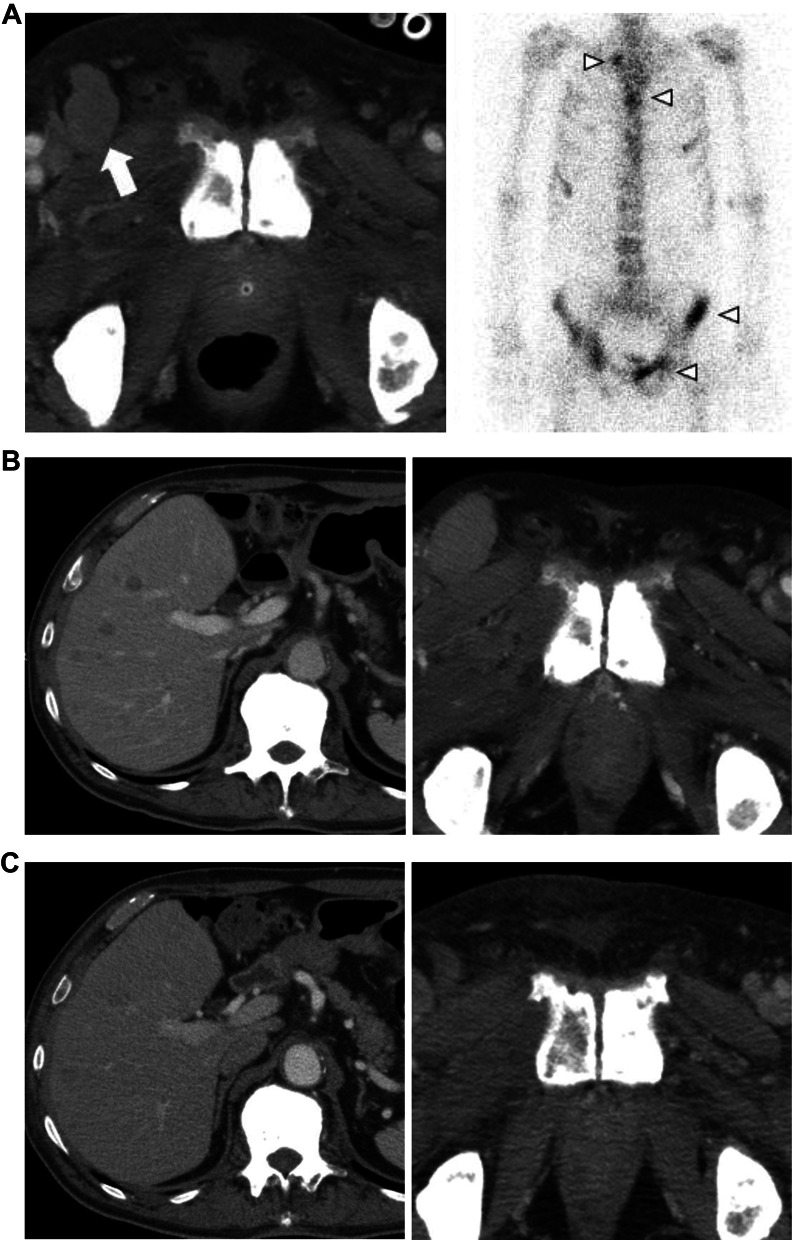
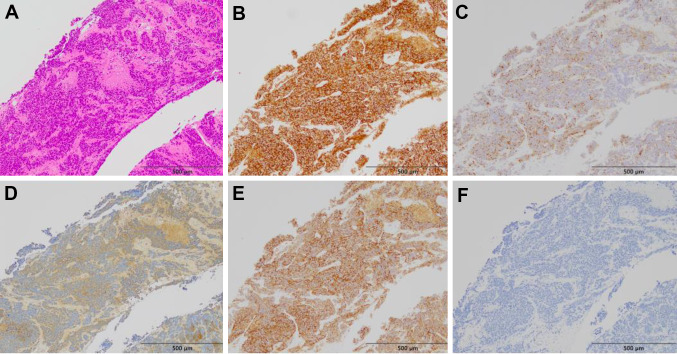
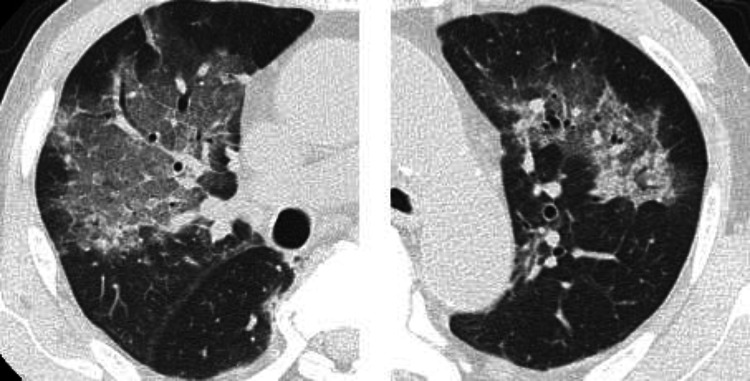
A 67-year-old man consulted a urologist, complaining of urinary hesitancy. The value of prostate-specific antigen (PSA) was 29.99 ng/mL, and the patient underwent a transperineal prostate biopsy that revealed adenocarcinoma of the prostate with the highest Gleason score of 5 + 5. Serum levels of nerve-specific enolase (NSE) and pro-gastrin-releasing peptide (proGRP) and immunostaining of biopsy specimen for neuroendocrine markers were not measured. A computed tomography (CT) scan and bone scintigraphy showed multiple bone and lymph node metastases. Then, the patient began a combination therapy using leuprorelin and enzalutamide (Fig. 1). After 1 year, although PSA levels declined to 2.88 ng/mL, a CT scan showed enlargement of multiple lymph nodes including inguinal region and bone metastases (Fig. 2A). At that time, the patient was referred to our department, and began subsequent treatment with docetaxel (70 mg/m2) plus prednisolone (10 mg/day) (Fig. 1). Moreover, a spinal metastasis compressed the spinal cord and caused bilateral leg paresis, which was treated with palliative radiotherapy (RT, 29 Gy/13Fr) to Th3, Th 12 and pelvis. After four cycles of docetaxel chemotherapy, a CT scan showed multiple liver metastases and further enlargement of lymph node metastases (Fig. 2B). NSE in serum was elevated to 211 ng/mL, although the PSA level declined to 0.19 ng/mL and serum proGRP level was within the normal range (53.5 pg/mL). Consistently, pathological findings of needle biopsy of lymph node metastasis in the right inguinal region showed neuroendocrine carcinoma (Fig. 3). Also, because of pain, palliative RT (29 Gy/13Fr) to L3 and right inguinal region was performed. Furthermore, FoundationOne CDx® (Foundation Medicine, Inc., Cambridge, MA, USA) using a biopsy sample of the prostate at initial diagnosis detected the BRCA1 mutation (deletion of intron 3–7) in addition to the AKT1 mutation, FUBP1-DFFA fusion, PTEN loss, and RB1 mutation. Subsequent BRACAnalysis® (Myriad Genetic Laboratories, Inc., Salt Lake City, UT, USA) test revealed no BRCA mutation in germline, indicating that his BRCA1 mutation was of somatic origin. Accordingly, olaparib treatment was initiated at a reduced dose of 500 mg daily because there was severe anemia at treatment initiation. After 1 month, the serum NSE level declined to 41.3 ng/mL, and PSA and proGRP remained within normal levels. Consistently, the CT scan showed a partial response with shrinkage of multiple metastases in lymph nodes and liver (Fig. 2C). No adverse event was observed at 1 month. However, 2 months later the patient presented with high-grade fever and dyspnea. Although the serum KL-6 level was within the normal range (246 U/mL), the CT scan showed a ground glass shadow in bilateral lung fields (Fig. 4). Accordingly, interstitial pneumonia accompanied was diagnosed and treated with steroid pulse therapy, which led to the discontinuation olaparib. Although the pneumonia improved after intensive treatment, the patient’s general condition worsened, and palliative and supportive care was administered. The patient died of prostate cancer 5 months after the initiation of olaparib treatment.
Fig. 1.
Clinical course of this case. Prostate-specific antigen (PSA) and nerve-specific enolase (NSE) measurement levels correspond to the timeline showing therapy
Fig. 2.
A A CT scan when the patient was referred to our hospital shows inguinal lymph node metastases (white arrow), and bone scintigraphy also detected multiple bone metastases (arrowheads). B A CT scan after four cycles of docetaxel chemotherapy shows multiple liver metastases (left) and further enlargement of lymph node metastases (right). C A CT scan 1 month after olaparib initiation; downsize in the liver (left) and lymph node (right) metastases
Fig. 3.
Pathological findings of a lymph node metastasis in the right inguinal region. Microscopic finding shows atypical cells having hyperchromic and enlarged nuclei with tumor necrosis (A). Immunohistochemically, the tumor cells stained positive for synaptophysin (B), chromogranin A (C), NSE (D), and CD56 (E), and negative for PSA (F)
Fig. 4.
A CT scan shows a ground glass shadow in the right superior lobe and left inferior lobe
Discussion
Prostate cancer is the most frequent cancer in the Japanese male population. According to a survey by the National Cancer Center Japan, approximately 90,000 men are diagnosed with prostate cancer which causes over 12,000 deaths every year in Japan [5]. Since prostate cancer is initially an androgen driven disease [6], androgen deprivation therapy combined with androgen receptor (AR) axis targeted therapy is the standard treatment for metastatic prostate cancer [7]. However, continuation of this treatment possibly leads to resistance to castration [8]. Although most castration-resistant prostate cancers remain dependent on the AR signaling pathway, some acquire a histological transformation characterized by AR-negative, poorly differentiated small cells with neuroendocrine morphology [9]. It has been reported that 10–17% of cases with metastatic castration-resistant prostate cancer (mCRPC) acquire neuroendocrine features, while de novo NEPC is extremely rare (less than 2%) [1, 10]. Interestingly, recent researches on genomic alterations revealed that inactivation of RB1, TP53 and PTEN were commonly observed in NEPC [3]. Consistently, prostate tumor in this case carried PTEN loss and RB1 mutation. Furthermore, coexisting of genomic alterations (PTEN loss and AKT1 mutation) in PI-3-Kinase pathway were observed in the tumor, which is rare because this coexistence was not observed in The Cancer Genome Atlas Research [11]. Therefore, extremely augmented PI-3-Kinase pathway by double genomic alterations might be a driver of neuroendocrine differentiation in this case. NEPC is characterized by a poor prognosis; the median survival time from the diagnosis of NEPC is approximately 1.5 years [12]. Therapy for prostate cancer with neuroendocrine differentiation is controversial. Platinum-based chemotherapy is commonly administrated to patients with pure small cell carcinoma based on the treatment of small cell lung cancer, whose response is limited [3]. Although second-line treatment for NEPC is unestablished, few studies focused on NEPC have been reported [13].
Previous studies have revealed various types of genomic mutations related to prostate cancer [14–17]. Among them, BRCA1, BRCA2, and ATM are the most well-characterized genes involved in homologous recombination repair (HRR), with 13% of patients with mCRPC harboring somatic or germline BRCA2 alterations [18]. Regarding NEPC, Beltran et al. reported 29% of BRCA2 mutations with 8% of identifiable biallelic alterations [19]. Consistently, Symonds et al. have reported a higher prevalence of BRCA2 mutation in NEPC (26%), compared to those without NEPC histology (9%) [20]. HRR or non-homologous end joining repairs a DNA double-strand break. If there are loss-of-function alterations in HRR-related genes, only the non-homologous end joining repairs the double-strand breaks, with frequent repair errors that lead to cancer progression [21]. PARP enzymes repair DNA single-strand breaks, thus PARP inhibition leaves single-strand breaks unrepaired, causing the accumulation of double-strand breaks after DNA replication. As non-homologous end joining mainly repairs double-strand breaks in tumors with mutations of HRR-related genes, most of them cannot be efficiently repaired, eventually leading to cancer cell death [22–24]. The PROfound trial showed longer radiographic progression-free survival and overall survival with olaparib treatment than with androgen receptor (AR) axis targeted therapy (median radiographic progression-free survival, 7.4 months vs. 3.6 months; median overall survival, 19.1 months vs. 14.7 months) [4, 25]. The most common adverse events of any grade were anemia or nausea in the olaparib cohort, and fatigue or asthenia in the control cohort, while no interstitial pneumonia was reported [4]. However, the PROfound trial did not include NEPC patients, and then the efficacy of olaparib for NEPC with the BRCA mutation has not been well characterized.
To our knowledge, this is the first case report of NEPC with somatic BRCA1 mutation treated with a PARP inhibitor as the first therapy after the detection of neuroendocrine differentiation. Pandya et al. reported a case of NEPC with germline BRCA2 mutation with a complete response to platinum-based chemotherapy, but a limited disease control duration by maintenance treatment with olaparib [26]. Additionally, Turina et al. reported a case of somatic BRCA2-altered NEPC successfully treated by olaparib as a maintenance therapy [27]. Wu et al. reported a case of germline BRCA1-mutated NEPC, who showed partial tumor response over 2.5 months with olaparib and rapid tumor progression with subsequent combination chemotherapy with etoposide and cisplatin after treatment failure with olaparib [28]. Thus, it was suggested that NEPC with BRCA1 or BRCA2 mutation could be managed using olaparib, as supported by this case. However, more reports on the outcomes in patients with NEPC with HRR-related gene mutations are needed.
In addition, this case experienced interstitial pneumonia that emerged after olaparib treatment. A case of olaparib-related interstitial pneumonia after a 6-week treatment was reported in a patient with breast cancer with BRCA1 deleterious mutation [29]. Furthermore, 107 cases (1.7%) of interstitial lung disease were reported among 6402 reports of olaparib in the public version of the U.S. Food and Drug Administration Adverse Event Reporting System [30]. Thus, although drug-induced interstitial pneumonia during olaparib treatment is rare, this disease should be recognized and carefully observed, especially during the early treatment phase. In this case, radiotherapy to thoracic vertebrae was performed before olaparib treatment, which might affect the occurrence of interstitial pneumonia although further report is required.
Conclusion
NEPC is an aggressive subtype of prostate cancer, and there is scarce evidence of adequate treatment. This case suggested that olaparib could be effective for NEPC with HRR-related gene mutations as in other advanced prostate cancers, but may cause interstitial pneumonia.
Acknowledgements
None.
Funding
None.
Data availability
The data in this report are available on request from the corresponding author.
Declarations
Conflict of interest
Masaki Shiota received honoraria from Janssen Pharmaceutical, AstraZeneca, Astellas Pharma, and Sanofi. Masatoshi Eto received honoraria from Takeda Pharmaceutical, and Janssen Pharmaceutical, and research funding support from Sanofi, Bayer Yakuhin, Astellas Pharma, and Takeda Pharmaceutical.
Ethical approval
This study was approved by the Institutional Ethics Committee (Approval No. 2021–123).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nadal R, Schweizer M, Kryvenko ON, et al. Small cell carcinoma of the prostate. Nat Rev Urol. 2014;11(4):213–219. doi: 10.1038/nrurol.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HT, Yao YH, Li BG, et al. Neuroendocrine prostate cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis—a systematic review and pooled analysis. J Clin Oncol. 2014;32(30):3383–3390. doi: 10.1200/JCO.2013.54.3553. [DOI] [PubMed] [Google Scholar]
- 3.Okasho K, Ogawa O, Akamatsu S. Narrative review of challenges in the management of advanced neuroendocrine prostate cancer. Transl Androl Urol. 2021;10(10):3953–3962. doi: 10.21037/tau-20-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 5.National cancer center Japan: Projected Cancer Statistics. https://ganjoho.jp/reg_stat/statistics/stat/short_pred_en.html [Accessed on 21 Sep. 2022]
- 6.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 7.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29(27):3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 8.Galletti G, Leach BI, Lam L, et al. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev. 2017;57:16–27. doi: 10.1016/j.ctrv.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Vlachostergious PJ, Puca L, Beltran H. Emerging variants of castration-resistant prostate cancer. Curr Oncol Rep. 2017;19(5):32. doi: 10.1007/s11912-017-0593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36(24):2492–2503. doi: 10.1200/JCO.2017.77.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate Part I A clinicopathologic study of 20 cases. Cancer. 1987;59(10):1803–1809. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y, Beltran H. Clinical and biological features of neuroendocrine prostate cancer. Curr Oncol Rep. 2021;23(2):15. doi: 10.1007/s11912-020-01003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper CS, Eeles R, Wedge DC, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat Genet. 2015;47(4):367–372. doi: 10.1038/ng.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutros PC, Fraser M, Harding NJ, et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat Genet. 2015;47(7):736–745. doi: 10.1038/ng.3315. [DOI] [PubMed] [Google Scholar]
- 16.Fraser M, Sabelnykova VY, Yamaguchi TN, et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature. 2017;541(7637):359–364. doi: 10.1038/nature20788. [DOI] [PubMed] [Google Scholar]
- 17.Ku SY, Gleave ME, Beltran H. Towards precision oncology in advanced prostate cancer. Nat Rev Urol. 2019;16(11):645–654. doi: 10.1038/s41585-019-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltran H, Oromendia C, Danila DC, et al. A phase II trial of the Aurora Kinase A inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin Cancer Res. 2019;25(1):43–51. doi: 10.1158/1078-0432.CCR-18-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Symonds L, Konnick E, Vakar-Lopez F, et al. BRCA2 alternations in neuroendocrine/small-cell carcinoma prostate cancer: a case series. JCO Precis Oncol. 2022;6:e2200091. doi: 10.1200/PO.22.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano R, Castro E, Aragón IM, et al. Genetic aberrations in DNA repair pathways: a cornerstone of precision oncology in prostate cancer. Br J Cancer. 2021;124(3):552–563. doi: 10.1038/s41416-020-01114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momozawa Y, Iwasaki Y, Parsons MT, et al. Germline pathogenic variants of 11 breast cancer genes in 7051 Japanese patients and 11,241 controls. Nat Commun. 2018;9(1):4083. doi: 10.1038/s41467-018-06581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enomoto T, Aoki D, Hattori K, et al. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: CHARacterizing the cross-sectionaL approach to Ovarian cancer geneTic TEsting of BRCA (CHARLOTTE) Int J Gynecol Cancer. 2019;29(6):1043–1049. doi: 10.1136/ijgc-2019-000384. [DOI] [PubMed] [Google Scholar]
- 24.Momozawa Y, Iwasaki Y, Hirata M, et al. Germline pathogenic variants in 7636 Japanese patients with prostate cancer and 12 366 controls. J Natl Cancer Inst. 2020;112(4):369–376. doi: 10.1093/jnci/djz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain M, Mateo J, Fizazi K, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 26.Pandya D, Shah M, Kaplan F, et al. Treatment-emergent neuroendocrine prostate cancer with a germline BRCA2 mutation: identification of a candidate reversion mutation associated with platinum/PARP-inhibitor resistance. Cold Spring Harb Mol Case Stud. 2021;7(1):a005801. doi: 10.1101/mcs.a005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turina CB, Coleman DJ, Thomas GV, et al. Molecular testing identifies determinants of exceptional response and guides precision therapy in a patient with lethal, treatment-emergent neuroendocrine prostate cancer. Cureus. 2019;11(7):e5197. doi: 10.7759/cureus.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Gao Y, Dou X, et al. Metastatic castration-resistant prostate cancer with neuroendocrine transformation and BRCA 1 germ-line mutation: a case report and literature review. Onco Targets Ther. 2020;13:8049–8054. doi: 10.2147/OTT.S264347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H, Oshino T, Hagio K, et al. Interstitial pneumonia caused by olaparib during treatment of breast cancer-a case report. Gan To Kagaku Ryoho. 2020;47(9):1351–1353. [PubMed] [Google Scholar]
- 30.Shu Y, He X, Liu Y, et al. A real-world disproportionality analysis of olaparib: data mining of the public version of FDA adverse event reporting system. Epidemiology. 2022;14:789–802. doi: 10.2147/CLEP.S365513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this report are available on request from the corresponding author.