Abstract
Disturbed insulin receptor (InsR) trafficking is associated with impaired insulin signaling and the development of diabetes. Sphingosine kinase (SphK), including SphK1 and SphK2, is a key enzyme of sphingolipid metabolism, which has been implicated in the regulation of membrane trafficking. More recently, we have reported that SphK2 is a key regulator of hepatic insulin signaling and glucose homeostasis. However, the role of SphK in InsR trafficking is still undefined. Huh7 cells were treated with specific SphK1 and SphK2 inhibitors or SphK1- and SphK2-specific small interfering RNA (siRNA) in the presence or absence of insulin. Flow cytometry and immunofluorescence assays were carried out to investigate the role of SphK in InsR trafficking. InsR endocytosis, recycling, and insulin signaling were analyzed. Inhibition of SphK2, but not SphK1, by either specific pharmaceutic inhibitors or siRNA, significantly suppressed InsR endocytosis and recycling following insulin stimulation. Consequently, the insulin-stimulated Akt activation was significantly attenuated by SphK2 inhibition in hepatocytes. Moreover, the effect of SphK2 on InsR trafficking was mediated via the clathrin-dependent mechanism. Thus, our results show that SphK2 is able to regulate InsR trafficking. These findings suggest that SphK2 may impinge on hepatic insulin signaling by regulating InsR trafficking, providing further mechanistic evidence that SphK2 could serve as a potential intervention target against insulin resistance and T2D (type 2 diabetes).
Keywords: Sphingosine kinase, insulin receptor, receptor trafficking, insulin signaling
Impact Statement
We have recently reported that sphingosine kinase 2 (SphK2) plays a key role in the regulation of hepatic insulin signaling and glucose homeostasis. In this study, we provided compelling evidence showing, for the first time, that SphK2 is able to regulate InsR trafficking, suggesting a new mechanism for the effect of SphK2 on insulin signaling. Our work not only provides new mechanistic evidence for the role of SphK2 in insulin signaling but also suggests a potential intervention target for antidiabetic treatment.
Introduction
Insulin receptor (InsR) trafficking is essential to the transmission of insulin signaling. Upon insulin binding, InsR is activated within the plasma membrane and then internalized rapidly into the early endosome, which is critical for the transduction and amplification of insulin signaling.1–4 After dissociation from insulin, the receptor either recycles back to the plasma membrane or moves to the lysosome for degradation.5,6 InsR internalization can occur via clathrin-mediated endocytosis, which is important for intracellular insulin signaling.7,8 Disturbed InsR trafficking and impaired insulin signaling are a key molecular basis for the development of type 2 diabetes (T2D). 9 Thus, understanding of the regulatory mechanisms of InsR trafficking is urgently required for creating a new way to antidiabetes.
Sphingolipids and cholesterol coexist as critical integral components of the plasma membrane, playing an important physiological role in the maintenance of normal cell structure and function. In addition to the well-documented role of cholesterol,10,11 accumulating evidence has suggested an important role for sphingolipids and their metabolic enzymes in endocytic membrane trafficking.11–14 Sphingosine kinase (SphK), which catalyzes the conversion of sphingosine to sphingosine-1-phosphate (S1P), is a key enzyme in sphingolipid metabolism. There are two isoforms of SphK – SphK1 and SphK2. Several lines of evidence have suggested a critical role of SphK in regulating insulin signaling. For instance, enforced overexpression of either SphK1 or SphK2 have improved glucose metabolism and ameliorated insulin resistance in various animal models.15–17 More recently, we have reported that hepatocyte-specific deletion of SphK2 (SphK2-LKO) led to impaired hepatic insulin signaling and glucose intolerance in mice. 18 However, the detailed molecular mechanism underlying the effect of SphK2 on hepatic insulin signaling is needed to be further elucidated. Interestingly, the importance of sphingosine and its conversion to S1P by SphK in endocytic membrane trafficking have been documented. 19 Therefore, it is intriguing to test whether SphK affects InsR trafficking, whereby it regulates insulin signaling in hepatocytes.
Materials and methods
Cell culture, transfection, and treatment
Huh7 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 1% penicillin and streptomycin in a humidified atmosphere with 5% CO2 at 37 °C. The transient transfection of siRNA (GenePharma, China) and the C-terminal green fluorescent protein (GFP)–tagged human InsR plasmid (InsR-GFP; Addgene, Watertown, MA, USA) into Huh7 cells was performed with the Lipofectamine 3000 reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. After siRNA transfection, with or without pretreatment with the indicated chemicals, cells were serum starved and stimulated with 10 nM insulin for the indicated time.
InsR internalization and recycling assays by flow cytometry
Flow cytometry was used to measure cell surface expression levels of InsR using the antibodies that specifically recognizes the α-subunit of InsR (Abcam, UK) as previously described.20,21 Briefly, Huh7 cells transiently transfected with siRNA or treated with the SphK2-specific inhibitor ABC294640 were serum starved, stimulated with or without 10 nM insulin at 37 °C for the indicated time, and then chilled on ice. Some of the cells were again incubated at 37 °C for the indicated time without insulin and were then placed on ice. After several washes with ice-cold phosphate-buffered saline (PBS), the cells were fixed with 2% paraformaldehyde (PFA) for 30 min on ice without permeabilization. Cells were again washed with PBS, incubated with InsRα antibodies for 1 h at 4 °C and were washed in ice-cold PBS. Then, cells were incubated with Alexa Fluor 488–conjugated secondary antibodies for 40 min on ice in the dark. After two more washes with ice-cold PBS, the cell surface receptor levels were analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). The basal cell fluorescence intensity was measured in cells stained with the second antibodies alone. The percentage of remaining surface InsR at various times (normalized to that at time zero) was determined. A reduction in cell surface fluorescence represents the internalization of the indicated receptors, and an elevation represents the recycling of InsR. When indicated, cells were pretreated with 80 µM dynasore (Dyn; Sigma-Aldrich, St. Louis, MO, USA) for 2 h prior to insulin stimulation.
Immunofluorescence and live cell imaging
Human InsR-GFP transfected cells grown on glass coverslips were transfected with siRNA or treated with SphK1-specific inhibitor PF543 (1 µM) or the SphK2-specific inhibitor ABC294640 (10 µM). Cells were then serum starved and stimulated with 10 nM insulin for the indicated times at 37 °C. The cells were placed on ice, washed with ice-cold PBS, and fixed with 4% PFA on ice for 30 min. The cells were then permeabilized with 0.1% Triton X-100 at room temperature for 10 min. After washing with PBS, cells were incubated with 5% BSA for 1 h at room temperature and incubated with anti-InsRβ antibody (1:100) in 3% bovine serum albumin (BSA) overnight at 4 °C. Following several washes with PBS, cells were treated with a fluorescent secondary antibody (Invitrogen) for 1 h, washed with PBS and then mounted with mounting medium containing DAPI (Molecular Probes, Eugene, OR, USA). The slides were visualized with confocal microscopy. For live cell imaging, the human InsR-GFP transfected cells were grown in chambered slides, and images were obtained before and after stimulation with insulin using the Delta Vision system.
Western blotting
Western blotting assays were performed as previously described. 18 Anti-SphK2 antibodies were purchased from ProteinTech (Rosemont, IL, USA), and anti-SphK1, anti-p-Akt, and anti-Akt were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-actin was purchased from Sigma.
Statistical analysis
The data are shown as the mean value ± standard error of mean (SEM). The statistical differences were evaluated by a two-tailed Student’s t-test. A P value ⩽ 0.05 was considered statistically significant.
Results
Inhibition of SphK2, but not of SphK1, disrupts InsR endocytosis
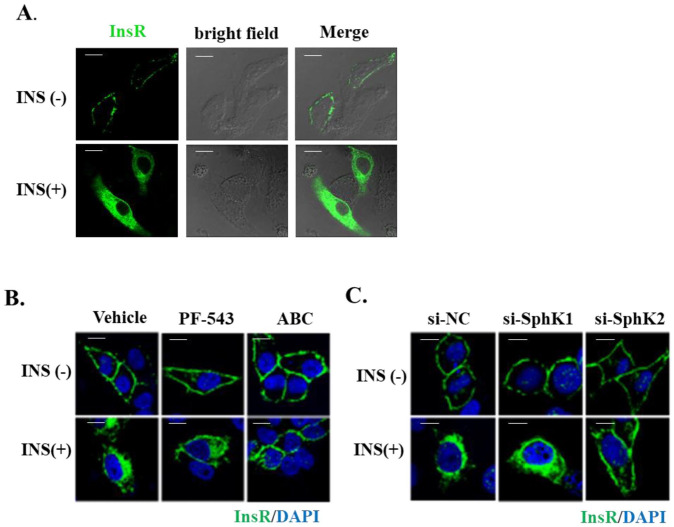
To visualize insulin-induced internalization of InsR, an immunofluorescence assay was performed in Huh7 hepatocytes transfected with InsR-GFP, the distribution of InsR in plasma membrane and the cytosol was monitored following insulin stimulation. As expected, InsR was predominantly localized in plasma membrane of the hepatocytes under basal conditions. Upon insulin stimulation, the amount of InsR at the cell membrane was markedly reduced, and the majority of InsR was relocated into the cytosol (Figure 1(A)), indicating InsR internalization occurred. To address whether SphK is involved in InsR endocytosis, the InsR-GFP-transfected Huh7 cells were treated with the SphK1-specific inhibitor PF543 (1 µM) or the SphK2-specific inhibitor ABC294640 (10 µM) or vehicle alone for 24 h followed by insulin stimulation. In line with the aforementioned observations, insulin stimulated a marked increase in the cytosolic InsR in the vehicle-treated control cells. While the PF543-pretreated cells responded to insulin in the same manner as the control cells, the amount of endocytosed InsR was significantly reduced in the cells pretreated with ABC294640, compared with that in control cells (Figure 1(B)). In keeping with these findings, the cells transfected with SphK2-specific siRNA (si-SphK2) resulted in a significant reduction of the insulin-induced InsR endocytosis, compared to control siRNA (si-NC) and SphK1-specific siRNA (si-SphK1) transfected cells (Figure 1(C)). The specificity of these siRNAs was demonstrated by their inability to inhibit the alternative isoform of SphK as shown in supplemental material. Taken together, the data indicated that SphK2, but not SphK1, is critically involved in the regulation of InsR endocytosis.
Figure 1.
SphK2 inhibition impairs InsR endocytosis. (A) Huh7 cells were transfected with GFP-tagged InsR and treated with or without insulin (10 nM, 10 min) followed by confocal cell imaging analysis. (B) Huh7 cells were pretreated with vehicle, PF543 (1 µM) or ABC294640 (10 µM), and (C) transfected with siRNAs targeting SphK1 and SphK2 or control siRNA as indicated, followed by insulin (10 nM, 10 min) stimulation. The treated cells were stained with DAPI (blue) and anti-InsRβ (green) antibodies. Representative photographs are presented. Bar is 10 µM. (A color version of this figure is available in the online journal.)
SphK2 inhibition perturbs InsR trafficking kinetics
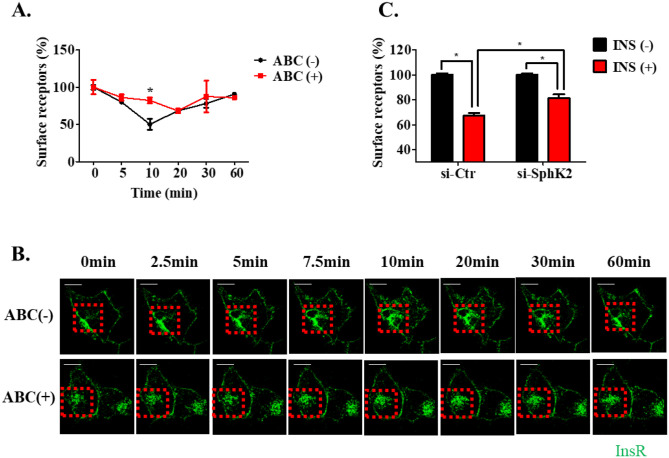
To further characterize the effect of SphK2 in modulating InsR trafficking, we conducted a flow cytometry–based analysis. In the presence of insulin, the amount of membrane-specific InsR was decreased with a peak at 10 min, and then it steadily increased, which reflected InsR recycling. Interestingly, there was a reduction in the percentage of internalized InsR in ABC294640 pretreated cells at 10 min, and the percentage reached the lowest point in terms of the membrane-specific InsR level at 20 min (Figure 2(A)), compared to that in controls, demonstrating that SphK2 inhibition led to the perturbed and delayed InsR endocytosis SphK. These observations were further supported by live cell imaging analysis, in which we monitored InsR trafficking following insulin stimulation over a 60-min time course. As shown in Figure 2(B), InsR internalization and recycling were significantly attenuated in ABC294640 treated cells, compared with the control cells. Consistent with this, a reduction in InsR endocytosis was also observed in SphK2 knockdown cells under insulin stimulation (Figure 2(C)). Collectively, these results demonstrated that SphK2 inhibition disturbs InsR trafficking kinetics in hepatocytes.
Figure 2.
The kinetics of InsR internalization and recycling. (A) Cell surface InsR expression was examined by flow cytometry analysis in Huh7 cells upon insulin (10 nM) stimulation at various times (0, 5, 10, 20, 30, and 60 min) in the presence or absence of ABC294640 (10 µM, 24 h). (B) InsR-GFP expressing Huh7 cells were pretreated with or without 10 µM ABC294640 (ABC) for 24 h. Live cell imaging was performed during stimulation with insulin (10 nM). Bar is 10 µM. (C) Cell surface InsR expression upon insulin (10 nM) stimulation for 10 min in the presence or absence of the indicated siRNA transfection was examined by flow cytometry. The data are presented as a percentage of the remaining cell surface fluorescence, for which a reduction represents the internalization of receptors and an elevation represents the recycling of receptors. Data represent the mean value ± SEM of three independent experiments. (A color version of this figure is available in the online journal.)
*P < 0.05, compared to the control at each time point.
SphK2 regulated InsR endocytosis via a clathrin-dependent pathway
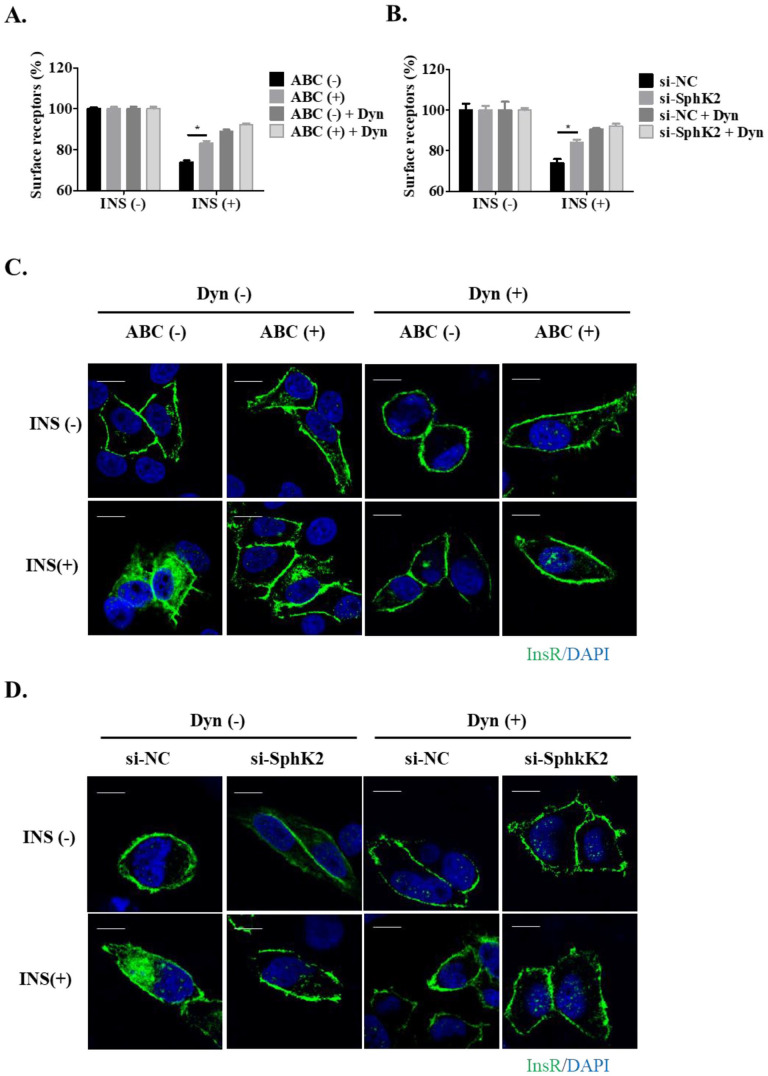
InsR internalization is chiefly mediated through a clathrin-dependent pathway. 7 Dynamin plays a key role in the clathrin-dependent endocytosis. We found that the SphK2 inhibition–induced suppression of InsR endocytosis by either ABC294640 or si-SphK2 was significantly abolished in the cells pretreatment with Dyn, a specific inhibitor of dynamin, compared with that in control cells under insulin stimulation (Figure 3(A) and (B)). Likewise, confocal cell imaging also showed that pretreatment with Dyn ameliorated the differences in the degree of InsR endocytosis between the control and SphK2 suppressed cells (Figure 3(C) and (D)), suggesting that the effect of SphK2 in InsR endocytosis is clathrin-dependent.
Figure 3.
SphK2 regulates InsR endocytosis in a clathrin-dependent manner. (A) Huh7 cells were pretreated with 10 µM ABC294640 or vehicle and (B) transfected with si-SphK2 or control siRNA. Then, the cells were treated with or without dynasore (Dyn) followed by insulin stimulation (10 nM, 10 min). Bar is 10 µM. The percentage of the remaining cell surface fluorescence was determined by flow cytometry (mean value ± SEM; *P < 0.05; n = 3). (C) InsR-GFP expressing Huh7 cells were treated with 10 µM ABC294640 or vehicle and (D) transfected with si-SphK2 or control siRNA. Confocal cell imaging was then conducted on the cells treated with or without dynasore (Dyn) followed by insulin stimulation. Representative photographs are presented. Bar is 10 µM. (A color version of this figure is available in the online journal.)
SphK2 suppression impairs insulin signaling
Having shown the effect of SphK2 on hepatic InsR trafficking, we reasoned that SphK2 may affect insulin signaling in hepatocytes. Thus, we examined levels of Akt phosphorylation, a pivotal signaling event of insulin’s action in hepatocytes. In keeping with our previous report, 18 the inhibition of SphK2 by either the SphK inhibitor ABC294640 or its siRNA significantly attenuated insulin-induced phosphorylation of Akt (Figure 4(A) and (B)), further verifying that SphK2 suppression impairs the action of insulin in hepatocytes.
Figure 4.
The effect of SphK2 on insulin-induced Akt activation. (A) Huh7 cells were pretreated with 10 µM ABC294640 or vehicle, and (B) transfected with si-SphK2 or control siRNA, followed by stimulation with or without insulin (10 nM) for 10 min. Western blotting analysis of p-Akt, total Akt, and actin was performed. The change in the p-Akt/total Akt ratio after insulin treatment was shown in the right bar charts. The data are expressed as the mean value ± SEM of three independent experiments.
*P < 0.05 versus control.
Discussion
In our previous study, we have uncovered an important role for SphK2 in the regulation of hepatic insulin signaling and glucose homeostasis. 18 We have found that hepatocyte-specific deletion of SphK2 resulted in insulin resistance and glucose intolerance in mice. The SphK2 deficient hepatocytes were evidently resistant to insulin-mediated suppression of gluconeogenesis and glucose production via inhibition of the PI3K–Akt signaling pathway. 18 In an attempt to understand the mechanisms underlying the effect of SphK2 in hepatic insulin signaling, this study was focused on the examination of InsR trafficking in hepatocytes.
InsR is a transmembrane receptor that contains four subunits, including two α- and two β-subunits in its extracellular and intracellular domain, respectively. 22 Upon insulin binding to InsR, the receptors are internalized as a complex with insulin, exerting their biological functions on the responsive target cells. Once the defect in this process exists, insulin resistance occurs. Indeed, the decreased InsR internalization is often presented in obese and diabetic subjects, whereas metformin can improve the insulin–InsR complex trafficking. 23
In the present work, we have provided the first evidence that SphK2 is critically involved in the regulation of InsR trafficking. Inhibition of SphK2 by either siRNA or pharmacological inhibitor resulted in a significant reduction in InsR endocytosis and impaired insulin signaling (i.e. Akt phosphorylation) in hepatic cells. We applied the established methods to probe InsR endocytosis using flow cytometry and immunofluorescence assays.20,21,24 We found that insulin induced rapid internalization of InsR into the cytosol within 5 min, reaching a peak steady state at 10 min, after which InsR began to recycle to the plasma membrane (Figure 2(A)). This is consistent with previous reports showing that InsR endocytosis peaked at 10 min after insulin stimulation, and then InsR recycled to the plasma membrane in rat hepatocytes.20,25 Notably, inhibition of SphK2 by ABC294640 treatment led to less translocation of InsR from plasma membrane to the cytosol (Figure 2(A)). A similar inhibitory effect on insulin-induced InsR endocytosis was further confirmed in the hepatocytes, where SphK2 was downregulated by siRNA-mediated SphK knockdown (Figure 2(B)). However, inhibition of SphK1 by either its specific inhibitor or siRNA had no effect on InsR endocytosis, indicating a specific role for SphK2. The subtype selectivity of SphK in the regulation of InsR in hepatocytes may be attributed to SphK2 being the main SphK isoenzyme in hepatocytes. 26
Endocytosis is a precise and dynamic flow process. InsR at the cell membrane can go in one of two different directions after endocytosis. InsR either recycles back to the plasma membrane or moves to the lysosome for degradation. 6 Within a short period of time, most of the InsR returns to the cell membrane, and only a small portion of the InsR will be degraded after endocytosis.27,28 Interestingly, as shown in live cell imaging (Figure 2(B)), SphK2 inhibition led to delayed recycling of InsR, indicating that SphK2 has dual effects on both internalization and recycling of InsR. It is worth mentioned that whether the effect of SphK2 in the regulation of receptor endocytosis and recycling is InsR-specific or is applicable to other receptors remains to be elucidated. We did have a try to use primary hepatocytes for this study; however, it was failed because of very low transfecting efficiency of the InsR-GFP plasmid that we used. Further studies in primary hepatocytes will be performed in the future.
Endocytosis is believed to occur often in different manners for different receptors, while the same receptor may undergo different forms of endocytosis. Although InsR endocytosis has been demonstrated to take place via different endocytic pathways in different cells, the clathrin-dependent internalization is the major pathway.7,8 As such, we sought to determine whether the SphK2-mediated regulation of InsR trafficking is clathrin-dependent. To this end, we disrupted clathrin-dependent endocytosis using a specific clathrin inhibitor, Dyn, in the hepatocytes. The findings that Dyn significantly abolished the effect of SphK2 on InsR endocytosis (Figure 3) suggest the effect of SphK dependent on the clathrin-mediated pathway. It was also noted that Dyn did not completely block InsR endocytosis, suggesting that clathrin-independent pathways, such as the lipid raft–dependent InsR endocytosis, may exist in hepatocytes. Indeed, studies have shown that InsR can be internalized through a caveolae-dependent pathway.29–31 Considering the fact that sphingolipids are key structural lipids of the cell rafts, 32 and growing evidences indicates that functional role of SphK are mainly dependent on subcellular localization,33,34 further studies are needed to clarify whether the different effect of SphK on insulin signaling in hepatocytes is dependent on its specific subcellular localization, whether SphK2 affects the lipid raft–mediated InsR internalization. However, given the fact that SphK2 is often located in the nuclear under basal conditions, how does it regulate the membrane-located InsR traffic needs more detailed investigation in the future.
The finding that SphK2 inhibition impairs InsR trafficking provides a mechanistic insight into the effect of SphK2 in the regulation of hepatic insulin signaling and glucose homeostasis as we have recently reported. 18 We have previously reported that SphK2-LKO mice exhibit impaired glucose homeostasis and insulin responsiveness on both normal diet and high-fat diet (HFD) conditions. SphK2-LKO upregulates gluconeogenic genes and downregulates glucose disposal genes, leading to an increase in hepatic glucose production. In keeping with previous findings, inhibition of SphK2 by the selective inhibitors or siRNA-mediated SphK2 knockdown in hepatocytes significantly suppresses insulin-induced Akt activation (Figure 4). Interestingly, our previous study has demonstrated that sphingosine accumulation is critically attributable to the defect of insulin signaling in SphK2-deficient hepatocytes. 18 Therefore, further studies are warranted to elucidate whether the effect of SphK2 on InsR trafficking is also associated with its substrate sphingosine.
In conclusion, our data for the first time illustrated an important role of SphK2 in hepatic InsR trafficking, which may account for its effect in the regulation of insulin signaling and metabolic homeostasis in hepatocytes. By further elucidating the role of SphK2 in insulin resistance and diabetes, the study could identify a potential intervention target for antidiabetic treatment.
Supplemental Material
Supplemental material, sj-docx-1-ebm-10.1177_15353702221131886 for Sphingosine kinase 2 regulates insulin receptor trafficking in hepatocytes by Gulibositan Aji, Sheng Jiang, Halmurat Obulkasim, Zhiqiang Lu, Wei Wang and Pu Xia in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. GA and SJ performed the experiments and analyzed the data. ZL and WW gave the technical assistance. GA and PX wrote the manuscript. PX conceived and designed the experiments. HO gave critical advice in design and statistical analysis of experiments, and also helped in the revising process.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (grant no. 81561128014 and 81870559 to PX) and the Fudan Distinguished Professorship (to PX). This work was also supported by the fund project in the State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia (grant no. SKL-HIDCA-2019-15 to SJ).
ORCID iD: Gulibositan Aji  https://orcid.org/0000-0002-7185-8210
https://orcid.org/0000-0002-7185-8210
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Backer JM, Kahn CR, White MF. Tyrosine phosphorylation of the insulin receptor during insulin-stimulated internalization in rat hepatoma cells. J Biol Chem 1989;264:1694–701 [PubMed] [Google Scholar]
- 2. Knutson VP. Cellular trafficking and processing of the insulin receptor. FASEB J 1991;5:2130–8 [DOI] [PubMed] [Google Scholar]
- 3. Bevan AP, Burgess JW, Drake PG, Shaver A, Bergeron JJ, Posner BI. Selective activation of the rat hepatic endosomal insulin receptor kinase. Role for the endosome in insulin signaling. J Biol Chem 1995;270: 10784–91 [DOI] [PubMed] [Google Scholar]
- 4. Balbis A, Baquiran G, Dumas V, Posner BI. Effect of inhibiting vacuolar acidification on insulin signaling in hepatocytes. J Biol Chem 2004;279: 12777–85 [DOI] [PubMed] [Google Scholar]
- 5. Marshall S, Olefsky JM. Effects of lysosomotropic agents on insulin interactions with adipocytes. Evidence for a lysosomal pathway for insulin processing and degradation. J Biol Chem 1979;254:10153–60 [PubMed] [Google Scholar]
- 6. Foti M, Moukil MA, Dudognon P, Carpentier JL. Insulin and IGF-1 receptor trafficking and signalling. Novartis Found Symp 2004;262:125–41; discussion 141–7, 265–8 [PubMed] [Google Scholar]
- 7. Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb Perspect Biol 2013;5:a017459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceresa BP, Kao AW, Santeler SR, Pessin JE. Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways. Mol Cell Biol 1998;18:3862–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 2014; 6:a009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breslow DK, Weissman JS. Membranes in balance: mechanisms of sphingolipid homeostasis. Mol Cell 2010;40:267–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hannich JT, Umebayashi K, Riezman H. Distribution and functions of sterols and sphingolipids. Cold Spring Harb Perspect Biol 2011;3:a004762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thiele C, Hannah MJ, Fahrenholz F, Huttner WB. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat Cell Biol 2000;2:42–9 [DOI] [PubMed] [Google Scholar]
- 13. Rohrbough J, Rushton E, Palanker L, Woodruff E, Matthies HJG, Acharya U, Acharya JK, Broadie K. Ceramidase regulates synaptic vesicle exocytosis and trafficking. J Neurosci 2004;24:7789–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yonamine I, Bamba T, Nirala NK, Jesmin N, Kosakowska-Cholody T, Nagashima K, Fukusaki E, Acharya JK, Acharya U. Sphingosine kinases and their metabolites modulate endolysosomal trafficking in photoreceptors. J Cell Biol 2011;192:557–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma MM, Chen JL, Wang GG, Wang H, Lu Y, Li JF, Yi J, Yuan YJ, Zhang QW, Mi J, Wang LS, Duan HF, Wu CT. Sphingosine kinase 1 participates in insulin signalling and regulates glucose metabolism and homeostasis in KK/Ay diabetic mice. Diabetologia 2007;50:891–900 [DOI] [PubMed] [Google Scholar]
- 16. Lee SY, Hong IK, Kim BR, Shim SM, Sung Lee J, Lee HY, Soo Choi C, Kim BK, Park TS. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology 2015;62:135–46 [DOI] [PubMed] [Google Scholar]
- 17. Qi YF, Wang W, Song ZY, Aji G, Liu XT, Xia P. Role of sphingosine kinase in type 2 diabetes mellitus. Front Endocrinol 2021;11:627076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aji G, Huang Y, Ng ML, Wang W, Lan T, Li M, Li Y, Chen Q, Li R, Yan S, Tran C, Burchfield JG, Couttas TA, Chen J, Chung LH, Liu D, Wadham C, Hogg PJ, Gao X, Vadas MA, Gamble JR, Don AS, Xia P, Qi Y. Regulation of hepatic insulin signaling and glucose homeostasis by sphingosine kinase 2. Proc Natl Acad Sci USA 2020;39:24434–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goñi FM, Sot J, Alonso A. Biophysical properties of sphingosine, ceramides and other simple sphingolipids. Biochem Soc Trans 2014;42: 1401–8 [DOI] [PubMed] [Google Scholar]
- 20. Tseng LT, Lin CL, Tzen KY, Chang SC, Chang MF. LMBD1 protein serves as a specific adaptor for insulin receptor internalization. J Biol Chem 2013;288:32424–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiang B, Yu GH, Guo J, Chen L, Hu W, Pei G, Ma L. Heterologous activation of protein kinase C stimulates phosphorylation of delta-opioid receptor at serine 344, resulting in beta-arrestin- and clathrin-mediated receptor internalization. J Biol Chem 2001;276:4709–16 [DOI] [PubMed] [Google Scholar]
- 22. Ward CW, Lawrence MC, Streltsov VA, Adams TE, McKern NM. The insulin and EGF receptor structures: new insights into ligand-induced receptor activation. Trends Biochem Sci 2007;32:129–37 [DOI] [PubMed] [Google Scholar]
- 23. Benzi L, Trischitta V, Ciccarone A, Cecchetti P, Brunetti A, Squatrito S, Marchetti P, Vigneri R, Navalesi R. Improvement with metformin in insulin internalization and processing in monocytes from NIDDM patients. Diabetes 1990;39:844–9 [DOI] [PubMed] [Google Scholar]
- 24. Choi E, Zhang XL, Xing C, Yu HT. Mitotic checkpoint regulators control insulin signaling and metabolic homeostasis. Cell 2016;166:567–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levy JR, Olefsky JM. The trafficking and processing of insulin and insulin receptors in cultured rat hepatocytes. Endocrinology 1987;121:2075–86 [DOI] [PubMed] [Google Scholar]
- 26. Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem 2000;275:19513–20 [DOI] [PubMed] [Google Scholar]
- 27. Marshall S, Green A, Olefsky JM. Evidence for recycling of insulin receptors in isolated rat adipocytes. J Biol Chem 1981;256:11464–70 [PubMed] [Google Scholar]
- 28. Marshall S. Kinetics of insulin receptor internalization and recycling in adipocytes. Shunting of receptors to a degradative pathway by inhibitors of recycling. J Biol Chem 1985;260:4136–44 [PubMed] [Google Scholar]
- 29. Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Strålfors P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J 1999;13:1961–71 [PubMed] [Google Scholar]
- 30. Foti M, Porcheron G, Fournier M, Maeder C, Carpentier JL. The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 2007;104:1242–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fagerholm S, Ortegren U, Karlsson M, Ruishalme I, Strålfors P. Rapid insulin-dependent endocytosis of the insulin receptor by caveolae in primary adipocytes. PLoS ONE 2009;4:e5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000;1:31–9 [DOI] [PubMed] [Google Scholar]
- 33. Siow D, Wattenberg B. The compartmentalization and translocation of the sphingosine kinases: mechanisms and functions in cell signaling and sphingolipid metabolism. Crit Rev Biochem Mol Biol 2011;46:365–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leclercq TM, Pitson SM. Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life 2006;58:467–72 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ebm-10.1177_15353702221131886 for Sphingosine kinase 2 regulates insulin receptor trafficking in hepatocytes by Gulibositan Aji, Sheng Jiang, Halmurat Obulkasim, Zhiqiang Lu, Wei Wang and Pu Xia in Experimental Biology and Medicine