Abstract
The SMRT corepressor complex participates in transcriptional repression by a diverse array of vertebrate transcription factors. The ability to recruit SMRT appears to play a crucial role in leukemogenesis by the PML-retinoic acid receptor α (RARα) oncoprotein, an aberrant nuclear hormone receptor implicated in human acute promyelocytic leukemia (APL). Arsenite induces clinical remission of APL through a incompletely understood mechanism. We report here that arsenite is a potent inhibitor of the interaction of SMRT with its transcription factor partners, including PML-RARα. Arsenite operates, in part, through a mitogen-activated protein (MAP) kinase cascade culminating in phosphorylation of the SMRT protein, dissociation of SMRT from its nuclear receptor partners, and a relocalization of SMRT out of the nucleus into the cytoplasm of the cell. Conversely, inhibition of this MAP kinase cascade attenuates the effects of arsenite on APL cells. Our results implicate SMRT as an important biological target for the actions of arsenite in both normal and neoplastic cells.
Nuclear hormone receptors are hormone-regulated transcription factors that bind to cognate hormone, bind to specific DNA sequences, and regulate the expression of adjacent target genes (3, 38, 39, 61). A wide variety of nuclear hormone receptors have been identified that mediate cellular responses to an assortment of different hormone ligands, including thyroid hormone, retinoids, steroids, vitamin D3, and a number of lipid metabolites. As a consequence, nuclear hormone receptors play key roles in many aspects of metazoan development, differentiation, and homeostasis (3, 38, 39, 61).
Many nuclear receptors are bipolar in function and are able to either repress or activate expression of target genes. Repression is conferred through the ability of nuclear receptors to recruit a complex of auxiliary proteins, designated corepressors, that mediate the molecular events necessary for transcriptional silencing (7, 10, 22, 54, 60, 66). The corepressor protein SMRT and its paralog, N-CoR, play a particularly important role in this process by serving as the principal point of contact of the corepressor complex with the nuclear receptors (6, 12, 21, 31, 50, 51, 67). Conversely, transcriptional activation is associated with release of SMRT/N-CoR from the nuclear receptor, followed by acquisition of a novel set of coactivator proteins (7, 10, 22, 25, 49, 54, 60, 66). Corepressors and coactivators regulate transcription through multiple mechanisms, including modifications of the chromatin template and interactions with the general transcriptional machinery (1, 24, 27, 32, 46, 65).
Thyroid hormone receptors (T3Rs) and retinoic acid receptors (RARs) typically bind to corepressors in the absence of hormone; on addition of hormone agonists, these nuclear receptors physically release from the corepressors and recruit coactivators (23, 33, 44, 47). Intriguingly, however, nonligand signal transduction pathways also play important roles in modulating the interaction of nuclear receptors with corepressors and coactivators. Particularly notable is the ability of protein kinase signaling pathways, such as those represented by the epidermal growth factor (EGF) receptor or by protein kinase A, to interfere with the SMRT-nuclear receptor interaction and to counteract transcriptional repression (19, 30, 62). Activation of the EGF receptor, for example, virtually abolishes the ability of SMRT to interact with T3Rs and eliminates T3R-mediated repression, even in the absence of thyroid hormone (19). The inhibitory effects of EGF receptor signaling on SMRT function are also observed with RARs and are mediated, at least in part, through a mitogen-activated protein (MAP) kinase cascade that culminates in phosphorylation of the SMRT protein, dissociation of SMRT from the nuclear receptor partner, and a relocalization of SMRT out of the nucleus and into the cytoplasm of the cell (20).
Aberrations in the interaction of nuclear receptors with corepressors can result in endocrine and neoplastic disorders. For example, human acute promyelocytic leukemia (APL) is associated with chromosomal translocations that fuse ectopic open reading frames to the DNA and hormone-binding domains of RARα (8, 11, 29, 41, 45). The most common form of translocation in APL results in the synthesis of a PML (promyelocytic leukemia)-RARα chimeric polypeptide. The PML-RARα chimera requires significantly higher retinoid concentrations to release from corepressor than does the wild-type RARα (15–17, 34, 35, 42). This defect in retinoid signaling plays an important role in generating the leukemic phenotype, and treatment of PML-RARα leukemic cells with supraphysiological levels of retinoic acid leads to release of corepressor from the PML-RARα and differentiation of the leukemic cell (15–17, 18, 35).
The ability of high concentrations of retinoic acid to induce differentiation in PML-RARα leukemias has been employed clinically to treat human APL (8, 11, 29, 41, 45). Recently, it has been recognized that arsenic trioxide acts synergistically with retinoic acid to induce long-term remissions in APL and can be effective in retinoid-resistant cases of APL (5, 14, 28, 53, 58). The precise molecular mechanisms behind the effects of arsenite in APL cells are not fully understood but are thought to involve both an apoptotic and a differentiation response (4, 14, 26, 28, 52, 59, 63, 70). Notably, arsenic trioxide is a strong inducer of MAP kinase signaling cascades in many cell contexts (2, 9, 36, 37, 40, 48). Given the ability of growth factor receptors to abolish SMRT function through a MAP kinase cascade (20), we tested whether arsenic trioxide treatment might affect SMRT function in a similar fashion. We report here that treatment with arsenic trioxide results in a profound inhibition in the ability of SMRT to interact with its transcription factor partners, such as T3Rs and RARs. At least one component of this inhibition of SMRT function appears to be mediated by an arsenite-mediated activation of a MAP kinase cascade, resulting in hyperphosphorylation of the corepressor and an alteration in its subcellular distribution. Notably, arsenite induces a similar and rapid inhibition of the ability of SMRT to interact with the PML-RARα oncoprotein in APL cells, and the ability of SMRT to induce differentiation and apoptosis in these cells is antagonized by inhibitors of MAP kinase cascade signaling. We propose that the effects of arsenite on normal cells and in APL are, at least in part, conferred through a MAP kinase cascade-mediated inhibition of corepressor function.
MATERIALS AND METHODS
Plasmid constructs.
The pCMV-SMRT-C vectors were constructed by inserting EcoRI fragments from the previously described pSG-5 TRAC-1 constructs into a pCR3.1 vector (Invitrogen, Carlsbad, Calif.) (50). Construction of the mammalian two-hybrid vectors for various SMRT and T3Rα derivatives was as previously described (19, 20, 64). The pSG5-GAL4-activation domain (AD)-RARα and retinoid X receptor α (RXRα) vectors were constructed by inserting EcoRV and XhoI fragments, generated by PCR, into the pSG5-GAL4-AD vector. The green fluorescent protein (GFP)-SMRT vector was constructed by inserting the BsrGI-to-EcoRI(blunt) fragment from pSG5-SMRT into the BsrGI-HindIII(blunt) sites on a CMV-GFPTYGBH vector (20). Expression vectors for full-length mitogen-elevated kinase kinase 1 (MEKK-1) and mitogen-elevated kinase 1 (MEK-1) expression plasmids were obtained from Chris Jamieson (University of California, San Francisco).
Transient transfections.
CV-1 cells transfections were performed by a Lipofectin-mediated method using the general protocol recommended by the manufacturer (GIBCO/BRL Life Technologies, Rockville, Md.). Approximately 4 × 105 cells were transfected with 50 ng of pSG5-T3Rα or pSG5-v-erb A plasmid (designated T3R-vA), 100 ng of pCMV-lacZ or pCH110 as an internal control, and 100 ng of a ptk-luc-TRE reporter, together with expression vectors for the various signal transducers tested here (equal quantities of the equivalent empty vectors were substituted where appropriate) (20). The cells were transferred to serum-free Dulbecco's modified Eagle's medium (DMEM) 24 h after transfection and were harvested 24 h later. The luciferase and β-galactosidase assays were performed as previously described, and the relative luciferase activity (expressed as the ratio of luciferase to β-galactosidase activity) was determined (20).
Mammalian two-hybrid assays.
Exponentially growing CV-1 cells (7 × 104 cells per well in 12-well culture plates) were transiently transfected by the Lipofectin method using 25 ng of the appropriate pSG5-GAL4DBD vector, 100 ng of the appropriate pSG5-GAL4AD vector, 100 ng of the pGL2-GAL4(17-mer)-luciferase reporter, 100 ng of the pCMV-lacZ internal control, and an appropriate expression vector for the various signal transducers tested here (or equal quantities of the equivalent empty vector where appropriate) (19, 20). The cells were transferred to serum-free DMEM 24 h after transfection and were harvested an additional 24 h later. The luciferase and β-galactosidase assays were performed as previously described, and the relative luciferase activity (expressed as the ratio of luciferase to β-galactosidase activity) was determined for each sample (19, 20).
Immunoblotting.
CV-1 cells (7 × 104 per well) were transfected with the appropriate expression vectors as indicated for each experiment, harvested 48 h after transfection by scraping, and lysed by mixing the cells with sodium dodecyl sulfate (SDS)-electrophoresis sample buffer. The lysates were then sonicated to reduce viscosity, boiled for 5 min, and loaded into an SDS–7.5% polyacrylamide–0.3% bis-acrylamide gel. After electrophoresis, the proteins were electrophoretically transferred to a nitrocellulose membrane. The membrane was incubated in blocking buffer (2.5% nonfat dry milk in TBST [0.1% Tween 20, 150 mM NaCl, 10 mM Tris-Cl; pH 7.6]) for 1 h and then incubated with appropriate primary antibodies (diluted in 2.5% bovine serum albumin in TBST) for 1 h. The membranes were next washed extensively with TBST and incubated with appropriate secondary antibodies (either horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antibody, diluted 1:2,000; Affinity BioReagents, Golden, Colo.). After extensive washing with TBST, the chemiluminescent Western detection system was employed for visualization of the immunoreactive proteins as specified by the manufacturer (NEN Life Science, Boston, Mass.).
Phosphorylation-dephosphorylation assay.
CV-1 cells (2.5 × 105) were transfected with the pCMV-SMRT-C and arsenite or with the MEKK-1 expression vector, as indicated for the individual experiments. The cells were harvested 48 h later by scraping and centrifugation in 150 μl of whole-cell extraction buffer (25 mM HEPES [pH 7.5], 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1% Triton X-100, 0.1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and complete protease inhibitor cocktail [Boehringer-Mannheim, Mannheim, Germany]). Lysates were then incubated in the presence or absence of 0.5 U of calf intestinal alkaline phosphatase (New England Biolabs [NEB], Beverly, Mass.) for 30 min at 30°C. The incubations were terminated by mixing the samples with SDS sample buffer; the samples were boiled for 5 min, loaded into an SDS–7.5% polyacrylamide–0.3% bis acrylamide gel, and subjected to electrophoresis and immunoblotting as described above.
Coimmunoprecipitation assay.
Approximately 107 NB-4 cells were treated with either 1 μM all-trans retinoic acid or 0.1 or 1 μM As2O3 for various times. Whole-cell lysates were prepared by gently sonicating the cells in 1 ml of immunoprecipitation (IP) buffer (1× phosphate-buffered saline, 1 mM EDTA, 1.5 mg of iodoacetamide per ml, 100 μM Na3VO4, 0.5% Triton X-100, 20 mM β-glycerolphosphate, 0.2 mM phenylmethylsulfonyl fluoride, and 1× complete protease inhibitor cocktail). After clarification by a 15-min centrifugation in a microcentrifuge at 4°C, the resulting supernatant was incubated for an additional 3 h at 4°C with either 2.5 μl of anti-SMRT antibody (PA1-843, diluted 1:400; Affinity Bioreagent), 2.5 μl of anti-RARα antibody, or 10 μl of anti-PML antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). Fifteen microliters of either protein A-Sepharose (as a 50% slurry in IP buffer; Sigma Chemical Co., St. Louis, Mo.) or protein G-Sepharose was then added, and the samples were incubated with continuous mixing for 4 h more at 4°C. The protein A-Sepharose matrix was extensively washed with IP buffer, and any proteins remaining bound to it were eluted with SDS sample buffer and detected by Western analysis.
Laser-scanning confocal microscopy.
Approximately 8 × 104 CV-1 cells were seeded in a chambered cover glass cell culture system (Nalge-Nunc, Rochester, N.Y.). The cells were then transfected with the pCMV-GFP-SMRT vector together with MEKK-1 expression vector (or an equivalent empty vector as a control) using the Effectene procedure (Qiagen, Hilden, Germany). A day after transfection the cells were transferred into serum-free DMEM containing 15 μM As2O3 and were incubated for an additional 24 h. The subcellular location of the GFP-SMRT fusion polypeptide was visualized using a Leica TCS-SP UV/Ar/Kr laser-scanning confocal microscope, employing excitation at 488 nm and detection at 500 to 540 nm. The effect of arsenite on the subcellular location of TFIIB, employed as a control, was analyzed in a similar fashion but using an indirect immunofluorescence protocol: CV-1 cells were treated with arsenite for 24 h (or left untreated) as described above, washed, fixed with 3% formaldehyde, permeabilized with 0.5% Triton X-100 in 1× PBS, and visualized using anti-TFIIB antiserum (sc-225; Santa Cruz Biotechnology) and rhodamine-conjugated anti-rabbit immunoglobulin G (Cappel, West Chester, Pa.).
Analysis of level of phosphorylated MEK-1/2.
CV-1 cells were grown in a 37°C humidified atmosphere containing 5% CO2 in DMEM supplemented with 10% fetal bovine serum. Cells were maintained in serum-free medium for at least 16 h prior to treatment with As2O3. As2O3 was added to the medium to a final concentration of 20 μM. Cells were harvested by adding hot SDS sample buffer, and the amount of phosphorylated MEK-1/2 was examined by Western analysis using a PhosphoPlus MEK-1/2 (Ser217/221) antibody kit (NEB).
Analysis of cell surface marker, apoptosis, and DNA content.
A fluorescence-activated cell sorting (FACS) flow cytometer was calibrated with CaliBRITE beads prior to use (Becton Dickinson, Mountain View, Calif.). The amount of myeloid cell surface marker CD11c on the NB-4 cells was determined by flow cytometry analysis using phycoerythrin-conjugated antibody from Becton Dickinson. The percent positive cells was quantified by FACScan analyzer (CellQuest program; Becton Dickinson). A terminal deoxynucleotidyl transferase labeling (TUNEL) reaction was performed on the NB-4 cells according to the manufacturer's instructions (Roche Molecular Biochemical Company, Indianapolis, Ind.) to measure apoptosis-associated DNA fragmentation. Cells were analyzed by flow cytometry using a FACScan. All data were collected and analyzed by CellQuest software. Cells were also analyzed for DNA content by flow cytometry analysis of propidium iodide-stained nuclei.
RESULTS
Arsenic trioxide strongly and specifically interferes with the ability of SMRT to interact with nuclear receptors.
We wished to determine if arsenic trioxide could alter the ability of SMRT to interact with its nuclear receptor partners. To address this question, we first employed a mammalian two-hybrid assay. For this assay, a GAL4DBD-SMRT fusion construct, a GAL4-AD-T3R fusion construct, and a luciferase reporter bearing GAL4(17-mer) binding sites were introduced separately or in combination into CV-1 cells. A strong activation of the GAL(17-mer) luciferase reporter was observed when all three constructs were introduced together, reflecting the ability of the SMRT and T3R sequences to interact and thereby reconstitute a functional GAL4 transcription factor (Fig. 1A). This two-hybrid assay appeared to be a valid measure of the SMRT-T3R interaction: (i) each of the fusion constructs was transcriptionally inactive when introduced separately; (ii) T3 thyroid hormone abolishes the T3R interaction with SMRT both in vitro and in the mammalian two-hybrid system; (iii) mutants of T3R (e.g., P156R) or other nuclear receptors, such as vitamin D3 receptor, that do not interact with SMRT in vitro do not interact in the two-hybrid system; (iv) unrelated proteins or SMRT mutants (SMRT ΔRID) that fail to interact with T3R in vitro do not interact with T3R in the two-hybrid system; and (v) mutants of T3R that bind SMRT in a hormone-independent fashion in vitro (e.g., T3R-vA) exhibit a hormone-independent interaction with SMRT in the two-hybrid assay (Fig. 1A).
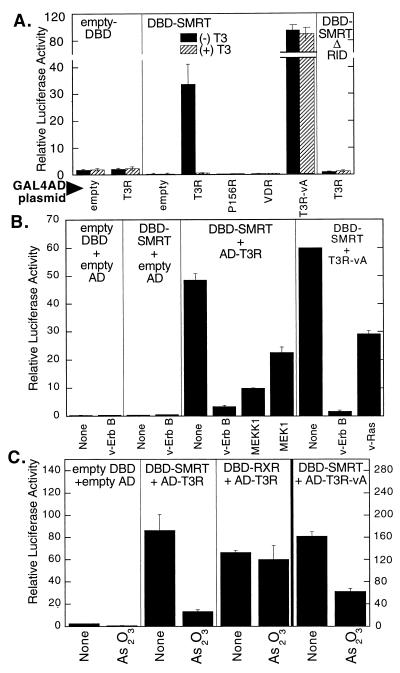
FIG. 1.
Effect of kinase signaling and As2O3 on the interaction between T3Rα and SMRT. (A) Effect of hormone on SMRT interaction with nuclear hormone receptors in a mammalian two-hybrid assay. A pSG5 plasmid containing either GAL4DBD alone (empty DBD), a GAL4DBD fused with the receptor interaction domain of SMRT (amino acids 751 to 1495; DBD-SMRT), or a GAL4DBD fused with a region of SMRT lacking the receptor interaction domain (amino acids 751 to 1074, DBD-SMRT ΔRID) were cotransfected into CV-1 cells with a GAL4(17-mer) thymidine kinase promoter-luciferase reporter and a pSG5-GAL4AD construct by a calcium phosphate precipitation method. Cotransfected GAL4AD constructs are indicated below the panels (empty, GAL4AD domain alone; T3R, GAL4-AD fused with T3Rα ligand-binding domain; P156R, GAL4-AD fused with a T3Rα construct with a mutation that disrupts SMRT association; T3R-vA, GAL4-AD fused with the analogous region of the v-Erb A mutant form of T3Rα, v-Erb A; VDR, GAL4-AD fused with a vitamin D3 receptor ligand-binding domain). The cells were incubated in the absence or presence of 1 μM cognate hormone (T3), the cells were harvested, and luciferase activity was determined relative to β-galactosidase activity of pCH110 plasmid introduced as an internal control. Data are averages and standard deviations from duplicate experiments. (B) Inhibition of two-hybrid SMRT-T3Rα interaction by v-Erb B, MEKK-1, and MEK-1. A pSG5 plasmid expressing a GAL4DBD alone (empty DBD) or a GAL4DBD fused with the receptor interaction domain of SMRT (DBD-SMRT) was transiently cotransfected into CV-1 cells by Lipofectin, with either an empty pSG5-GAL4AD vector, the pSG5-GAL4AD fused to the ligand-binding domain of T3Rα, or T3R-vA. In addition, the cells were cotransfected with 100 ng of expression plasmid for either v-Erb B, v-Ras, a full-length MEKK-1, or a MEK-1 clone, as indicated at the bottom. The cells were incubated in the absence of T3, and the luciferase activity was determined and normalized to β-galactosidase activity for each sample. (C) Inhibition of the mammalian two-hybrid interaction between SMRT and T3Rα by As2O3. The protocol for panel B was repeated, but in the absence or presence or absence of 20 μM As2O3, as indicated at the bottom. A control two-hybrid interaction between a DBD-SMRT construct and an AD-T3Rα construct was assayed under the same conditions to detect any nonspecific effects of arsenite on the two-hybrid system (see the text). The scale for the v-Erb A interaction with SMRT (right axis) differs from that for the other three panels (left axis).
As reported previously (19, 20), the ability of SMRT to interact with T3R in the mammalian two-hybrid assay was severely compromised by cointroduction of v-Erb B, a constitutively activated derivative of the EGF receptor (Fig. 1B). The inhibitory effect of v-Erb B on the SMRT-T3R interaction was also observed for the T3R-vA mutant, demonstrating that the inhibitory actions of v-Erb B do not require ligand binding by the nuclear hormone receptor (Fig. 1B). We have shown that these inhibitory effects are mediated by a MAP kinase cascade that operates downstream of the EGF receptor/v-Erb B protein, resulting in phosphorylation of the SMRT corepressor (20). The individual components of this kinase cascade, such as Ras, MEKK-1, and MEK-1, can substitute for v-Erb B in this regard and when introduced into the CV-1 cells also inhibited the SMRT-T3R interaction (Fig. 1B).
Notably, treatment of the CV-1 cells with low levels of arsenic trioxide resulted in a similar and potent inhibition of the two-hybrid interaction between SMRT and T3R (Fig. 1C). To exclude the possibility that arsenic was inhibiting the expression, stability, or function of the GAL4DBD or GAL4AD fusion, rather than interfering with the SMRT-T3R interaction itself, we assayed the effects of arsenic trioxide on the two-hybrid interaction between T3R and RXRs; RXRs are heterodimer partners for T3Rs, and the two receptor classes can physically associate in vitro and in vivo (3, 38). T3R exhibited a strong interaction with RXR in our mammalian two-hybrid system which was unaltered by arsenic trioxide treatment, in clear contrast to the arsenite-mediated inhibition observed for the T3R/SMRT interaction (Fig. 1C); note that the expression vector backbones, promoters, Kozak sequences, GAL4DBD and AD sequences, and reporter vectors were the same for both assays. Similarly, arsenic trioxide had little or no effect on expression of a β-galactosidase reporter lacking GAL4-binding sites and employed as an internal control, indicating that the effects we observed under these conditions were not simply due to a generic arsenite-mediated toxicity or an overall inhibition of transcription or translation (data not shown). An inhibition of the SMRT-T3R interaction by arsenite was also observed for the T3R-vA mutant, further suggesting that the effects of arsenite did not require ligand binding by the T3R (Fig. 1C). We conclude that the interaction between SMRT and T3R is inhibited by arsenic trioxide in a manner at least superficially resembling the inhibitory effects of the EGF receptor/MAP kinase cascade pathway.
Activation of the EGF receptor/MAP kinase cascade interferes not only with the interaction of SMRT with T3R but also with the interaction of SMRT with a variety of its other transcription factor partners, including RARs and PLZF, a nonreceptor transcriptional repressor (19, 20). Paralleling its effect on T3R, arsenic trioxide strongly interfered with the mammalian two-hybrid interaction between SMRT and RARα but not with the control two-hybrid interaction between RXR and RARα (Fig. 2A). The ability of SMRT to interact with PLZF in the two-hybrid system was also inhibited by arsenite treatment (Fig. 2B). We conclude that arsenic trioxide treatment, in common with MAP kinase cascade signaling, interferes with the ability of SMRT to interact with an assortment of receptor and nonreceptor transcription factors.
FIG. 2.
Effect of As2O3 on the interaction between SMRT and RARα and between SMRT and PLZF. (A) Inhibition of the mammalian two-hybrid interaction between SMRT and RARα by As2O3. A protocol similar to that for Fig. 1C was used, but with pSG5-GAL4AD fused to the ligand-binding domain of RARα in place of the T3R construct. The cells were left untreated or treated with 15 μM As2O3 or with all-trans retinoic acid (ATRA). (B) Inhibition of the interaction between SMRT and PLZF by As2O3. The same protocol was used, but with pSG5-GAL4AD fused to the PLZF open reading frame in the absence or presence of 15 μM As2O3. Data are averages and standard deviations of duplicate experiments.
The inhibitory effects of arsenic trioxide on SMRT function in CV-1 cells are mediated, in part, through activation of a MAP kinase cascade and result in phosphorylation of the corepressor.
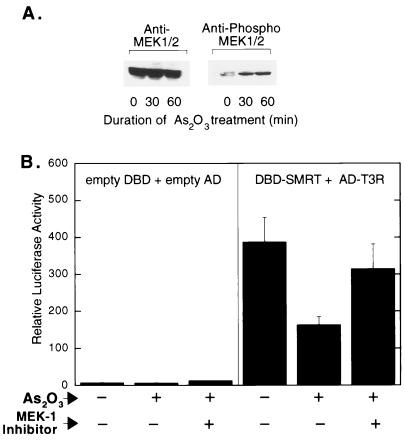
In many cell types, arsenic trioxide acts as a strong inducer of MAP kinase cascade signaling (2, 9, 36, 37, 40, 48). Given the overall similarity between the inhibitory effects of arsenic trioxide and the inhibitory effects of MEKK-1 or MEK-1, we next explored whether the effects of arsenic trioxide on SMRT function were, in fact mediated through this (or an analogous) MAP kinase cascade. Consistent with this hypothesis, arsenic trioxide treatment resulted in a rapid increase in the phosphorylation state of MEK-1/2, suggestive of an activation of MAP kinase cascade signaling in these cells (Fig. 3A). We have reported that chemical inhibitors of MEK-1/2 counteract the effects of MAP kinase cascade signaling and stabilize the SMRT-nuclear receptor interaction (20). Significantly, the inhibitory effects of arsenite in the SMRT two-hybrid assay were also counteracted by MEK-1/2 inhibitors, such as PD98059 (Fig. 3B), whereas MEK-1/2 inhibitors had little or no effect on the SMRT-T3R two-hybrid interaction in the absence of arsenite (reference 20 and data not shown) and only a very small effect on reporter gene expression in the empty-vector system (Fig. 3B). These results suggest that the arsenite antagonizes SMRT function, at least in part, through a MAP kinase cascade involving MEK-1 or a comparable MAP kinase kinase intermediate.
FIG. 3.
Effect of As2O3 treatment on phosphorylation of MEK-1/2 (A) and effect of a MEK-1 inhibitor on SMRT-T3Rα interaction (B). (A) CV-1 cells were treated with As2O3 for the specified times. The cells were subsequently lysed, and the extracts were resolved by SDS-PAGE and analyzed by immunoblotting using antibody specific for bulk MEK-1/2 or for phosphorylated MEK-1/2 (Ser217/221). (B) The effect of As2O3 on the SMRT-T3Rα two-hybrid interaction was tested as described for Fig. 1C, but in the absence or presence of 5 μM PD98059, as indicated at the bottom.
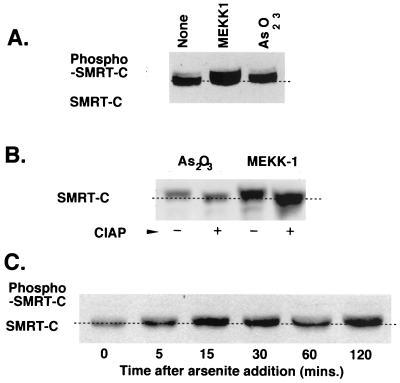
SMRT is a substrate for phosphorylation by several kinases operating within the MAP kinase cascade, and these phosphorylation events are manifested as a change in the electrophoretic mobility of SMRT and a redistribution in the subcellular location of SMRT, as well as a loss in the ability of SMRT to interact with its transcription factor partners (20). We therefore examined whether SMRT was indeed phosphorylated in response to arsenic trioxide, as revealed by a change in its mobility on SDS-polyacrylamide gel electrophoresis (PAGE). Treatment of CV-1 cells with arsenic trioxide led to a reproducible decrease in the electrophoretic mobility of SMRT in a manner characteristic of phosphorylation (Fig. 4A). This arsenite-mediated change in the electrophoretic properties of SMRT was comparable to that observed in response to activation of the MAP kinase cascade by introduction of MEKK-1 (Fig. 4A) and, consistent with this SMRT modification representing phosphorylation, was reversed by treatment with calf intestine alkaline phosphatase (Fig. 4B). This presumptive phosphorylation was detectable within 15 to 30 min of arsenite addition (Fig. 4C), paralleling the rapid increase in MEK-1/2 phosphorylation seen in Fig. 3A. Although treatment with high arsenite concentrations decreased the overall level of SMRT protein expression in the CV-1 cells (data not shown), this decrease was not typically observed at the arsenite concentrations employed in these experiments; conversely, the introduction of MEKK-1, but not MEK-1, increased the levels of SMRT protein (e.g., Fig. 4A and B).
FIG. 4.
Phosphorylation of SMRT induced by As2O3. (A) SMRT immunoblot of CV-1 cells after MEKK-1 and As2O3 treatments. pCMV-SMRT-C (a construct limited to expressing amino acids 751 to 1495 of SMRT) was introduced into the CV-1 cells together with an empty vector (None) or a MEKK-1 expression vector; alternatively, the cells were treated with 20 μM As2O3. Whole-cell lysates were prepared from the cells, and the extract was analyzed by Western blotting using antibody specific for SMRT protein. (B) Phosphatase treatment of SMRT reverses the change in electrophoretic mobility induced by arsenite. CV-1 cells were transfected together with pCMV-SMRT-C (amino acids 751 to 1495) and either empty vector or the MEKK-1 expression vector. After As2O3 treatment as in panel A, whole-cell lysates were prepared and were incubated without or with 1 U of calf intestinal alkaline phosphatase (CIAP) for 30 min. (C) SMRT shifts in mobility rapidly after arsenite treatment. The experiment used for panel A was repeated, with samples taken and analyzed at the time points listed.
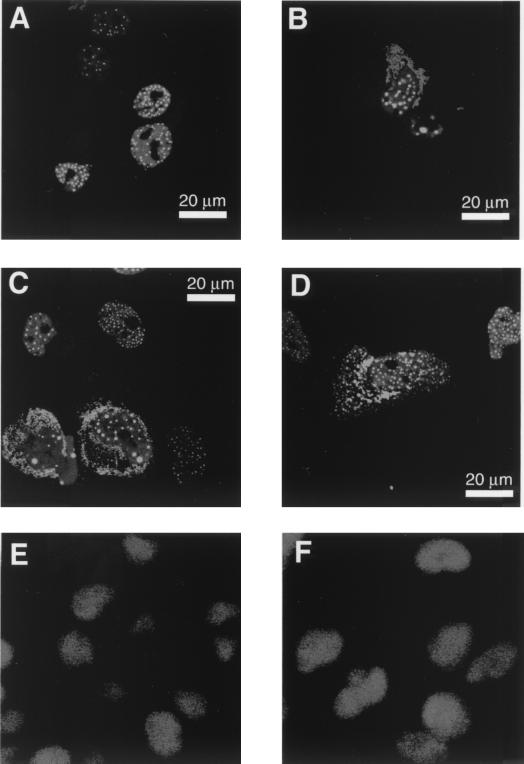
We next examined the effects of arsenite on the subcellular location of SMRT by use of a GFP-SMRT fusion construct. In agreement with prior identifications of SMRT as a nuclear protein, the GFP-SMRT protein was located virtually exclusively in the nucleus of unstimulated, transfected cells, forming a punctate pattern of fluorescent spots superimposed on a diffuse fluorescent nucleoplasm (Fig. 5A) (13, 20, 57). Cointroduction of a MEKK-1 expression vector into these cells led to a change in the GFP-SMRT pattern, manifested as a coalescence of the punctate spots into a smaller number of larger dots per nucleus and, in many of these cells, a shift of the GFP-SMRT out of the nucleus and into a perinuclear or cytoplasmic distribution (Fig. 5B). Notably, arsenic trioxide treatment resulted in a similar change in GFP-SMRT localization, with a reduction in the number but an increase in the overall size of the fluorescent nuclear dots and/or a shift to a more perinuclear or cytoplasmic distribution (Fig. 5C and D). Approximately 59% of the fluorescent-positive, arsenite-treated cells displayed this redistribution of GFP-SMRT (both responsive and nonresponsive cells are illustrated in Fig. 5C and D), whereas virtually all of the CV-1 cells transfected with MEKK-1 underwent GFP-SMRT relocalization. We do not know the molecular basis for this heterogeneity in the CV-1 cell response to arsenite but presume that it reflects some corresponding heterogeneity in the metabolic state of these cells. The overall appearance of the nuclei, and the subcellular localization of an unrelated nuclear protein, TFIIB, did not change in response to arsenite (Fig. 5E and F and data not shown), indicating that the redistribution of SMRT was a specific response to arsenic trioxide and was not due to an overall loss of nuclear integrity. Taken as a whole, our results support our hypothesis that the inhibitory effects of arsenic trioxide on SMRT are mediated, at least in part, through a MAP kinase cascade that results in phosphorylation and redistribution of the corepressor.
FIG. 5.
Effect of As2O3 treatment on subcellular location of SMRT proteins. CV-1 cells were transfected with GFP-SMRT alone, cotransfected with MEKK-1, or treated with 15 μM As2O3 after transfection. The subcellular location of the GFP-SMRT was subsequently visualized by confocal fluorescent microscopy. As a control experiment, the subcellular location of TFIIB under the same conditions was analyzed by immunofluorescence. (A) GFP-SMRT alone; (B) GFP-SMRT plus MEKK-1 expression vector; (C and D) GFP-SMRT plus As2O3 treatment; (E) TFIIB without As2O3 treatment; (F) TFIIB after treatment with 15 μM As2O3.
Arsenite can induce both apoptosis and an incomplete differentiation response in APL-derived NB-4 cells.
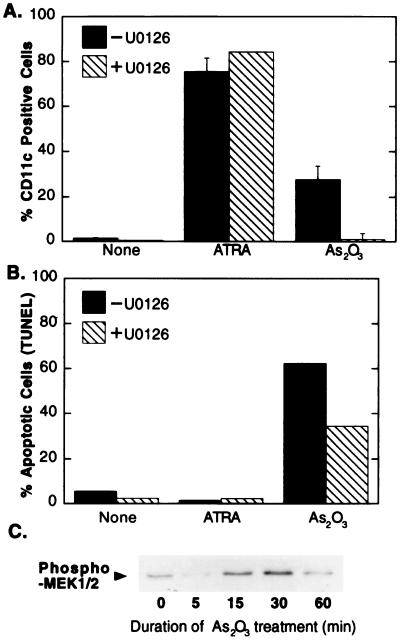
The ability of arsenite to antagonize SMRT function in CV-1 cells led us to examine the effects of arsenite in APLs. We employed NB-4 cells, which are derived from a human APL and which synthesize the PML-RARα chimeric oncoprotein. NB-4 cells can be induced to differentiate by treatment with high levels of all-trans retinoic acid, and this response is thought to be mediated by a hormone-mediated release of corepressor from the PML-RARα protein (15–17, 35). Consistent with these prior observations, addition of all-trans retinoic acid to our NB-4 cell cultures resulted in a strong differentiation response which could be detected using FACS analysis with an antibody directed against CD-11c, an early differentiation marker (Fig. 6A), or a colorimetric assay for leukocyte alkaline phosphatase, a late differentiation marker (data not shown) (4, 14). In contrast to these robust differentiation responses, all-trans retinoic acid treatment induced little or no apoptosis in the NB-4 cells, as determined by TUNEL assay (Fig. 6B).
FIG. 6.
Apoptosis and differentiation of NB-4 cells upon As2O3 treatment. (A) Effect of As2O3 and a MEK-1 inhibitor on the differentiation of NB-4 cells. NB-4 cells were left untreated (None), treated with all-trans retinoic acid (ATRA), or treated with As2O3 in the presence or absence of the MEK-1 inhibitor U0126 for 72 h. Cells were subjected to flow cytometry after staining with phycoerythrin-conjugated anti-CD11c antibody. (B) Effect of As2O3 and MEK-1 inhibitor on the apoptosis of NB-4 cells. NB-4 cells were treated with a 1 μM concentration of either ATRA or As2O3 in the presence or absence of the MEK-1 inhibitor U0126 for 72 h. Cells were subjected to flow cytometry after TUNEL reaction. (C) Induction of MEK-1 signaling by arsenite in NB-4 cells treated with As2O3. The cells were lysed, and the lysate was analyzed by SDS-PAGE and immunoblotting using anti-phospho-MEK-1/2 (Ser217/221) antibody as for Fig. 3A.
Unlike all-trans retinoic acid, arsenic trioxide has been reported to induce a dual response in NB-4 cells, exhibiting elements both of differentiation and apoptosis (4, 14, 26, 28, 52, 59, 63, 70). Consistent with these studies, treatment of our NB-4 cells with arsenite resulted in both a substantial increase in the number of cells expressing the CD-11c early differentiation marker (Fig. 6A) and a strong apoptotic response, with over 60% of the NB-4 cell population becoming positive in the TUNEL assay (Fig. 6B). The arsenite-mediated differentiation response was somewhat weaker than that observed with all-trans retinoic acid treatment (e.g., Fig. 6A) and appeared to be restricted to early differentiation: unlike all-trans retinoic acid, arsenite treatment did not increase expression of the late differentiation marker leukocytic alkaline phosphatase in these cells (data not shown). Combined treatment with all-trans retinoic acid and arsenite resulted in no alteration in the differentiation or apoptotic responses beyond that observed with these compounds when tested individually (data not shown).
The effects of arsenic trioxide on APL cell differentiation and apoptosis are mediated, in part, through a MEK-1 intermediate and may operate by disrupting the interaction between SMRT and the PML-RARα oncoprotein.
PML-RARα-mediated leukemogenesis is closely linked to the ability of PML-RARα to bind corepressor and to act as a dominant negative repressor of transcription; release of corepressor from PML-RARα in response to supraphysiological concentrations of all-trans retinoic acid results in differentiation of the APL cells and remission of disease (15–17, 35). Might the ability of arsenite to induce APL cell differentiation reflect a corresponding ability of arsenite to inhibit the interaction of SMRT with PML-RARα? We first tested whether the effects of arsenite on the NB-4 cells involve an activation of a MAP kinase cascade similar to that observed in CV-1 cells. Consistent with this hypothesis, arsenic trioxide induced a transient increase in MAP kinase cascade signaling in the NB-4 cells, as demonstrated by an increase in phosphorylation of MEK-1/2 (Fig. 6C); MEK-1/2 phosphorylation in NB-4 cells peaked somewhat more rapidly than that in CV-1 cells and then returned to baseline (Fig. 3), presumably reflecting differences in signal induction and attenuation in the two different cell lines. Is this activation of this MAP kinase cascade involved in mediating the effects of arsenite on NB-4 cell differentiation and apoptosis? Indeed, treatment of the NB-4 cells with the MEK-1/2 inhibitor U0126 significantly impaired the ability of arsenite to induce differentiation and, to a lesser degree, apoptosis in these cells (Fig. 6A and B). In contrast, U0126 did not inhibit the differentiation of NB-4 cells in response to all-trans retinoic acid treatment (Fig. 6A).
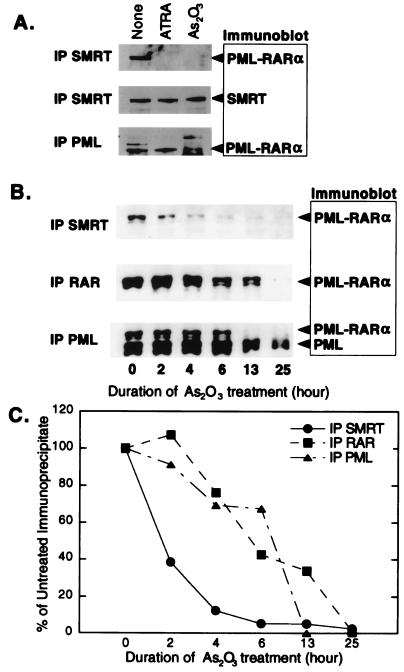
We next examined whether arsenic trioxide treatment of NB-4 cells results in an inhibition of the interaction of SMRT with PML-RARα. The NB-4 cell line cannot be efficiently transfected with exogenous DNA, precluding use of a two-hybrid interaction system. We therefore employed a coimmunoprecipitation technique. We exposed NB-4 cells to a mock treatment, to arsenic trioxide, and to retinoic acid. We then lysed the cells, immunoprecipitated the lysates with SMRT-directed antisera, and analyzed the immunoprecipitates by Western analysis using antibodies to PML-RARα. In the absence of treatment, PML-RARα was efficiently coprecipitated with SMRT, indicative of a stable interaction between these two proteins (Fig. 7A, top). Notably, treatment of the NB-4 cells with either all-trans retinoic acid or with arsenite resulted in disruption of this PML-RARα–SMRT interaction, manifested as a loss of PML-RARα from the SMRT immunoprecipitate (Fig. 7A, top).
FIG. 7.
Effect of As2O3 on the physical association of PML-RARα with SMRT protein in NB-4 cells. (A) Physical association of SMRT protein with PML-RARα in NB-4 cells. NB-4 cells were treated with all-trans retinoic acid (ATRA) or As2O3 for 24 h. Whole-cell extracts were prepared and subjected to immunoprecipitation with SMRT or PML antibody. The immunoblots were subsequently probed with the antibodies indicated on the right. (B) Loss of the physical association of SMRT with PML-RARα occurs before degradation of PML-RARα protein in NB-4 cells. NB-4 cells were treated with 0.1 μM As2O3. Whole-cell extracts were prepared at the indicated times and subjected to immunoprecipitation with SMRT, RARα, or PML antibody. The immunoprecipitates were then analyzed by SDS-PAGE, and the immunoblot was probed with anti-PML antibodies. The positions of SMRT, PML-RARα, and the product of the untranslocated PML locus are indicated. (C) Quantification of data in panel B.
Arsenic trioxide treatment has been reported to induce degradation of PML-RARα in NB-4 cells (4, 14, 43, 52, 63, 69). To test whether degradation of either PML-RARα or SMRT could account for the loss of coimmunoprecipitation of these two proteins, we modified our immunoprecipitation-blotting procedure to examine the effects of arsenite and all-trans retinoic acid on the overall levels of PML-RARα and of SMRT in the NB-4 cells. Neither arsenite nor all-trans retinoic acid altered the overall levels of SMRT protein under the conditions employed here (Fig. 7A, middle). However, the bulk levels of PML-RARα in these cells did decline after extensive arsenite treatment (Fig. 7A, bottom). We therefore performed a more detailed time course experiment, comparing the effects of arsenite on the interaction of SMRT with PML-RARα to the effects of arsenite on PML-RARα stability (Fig. 7B and C). Consistent with prior studies, we detected a decline in the overall level of PML-RARα in the NB-4 cells beginning at 4 h of arsenite treatment and resulting in substantial loss of this protein by 25 h. Nonetheless, the arsenite-induced dissociation of SMRT from PML-RARα occurred extremely rapidly and clearly preceded the degradation of the PML-RARα protein (Fig. 7B and C; compare the amount of PML-RARα coprecipitated by the anti-SMRT antibodies to the overall levels of PML-RARα detected by anti-PML and anti-RAR antibodies). The overall levels of SMRT were stable at these intermediate time points (data not shown). Our results therefore indicate that arsenic trioxide treatment induces the release of SMRT from PML-RARα in these cells prior to degradation of the PML-RARα and without notable degradation of SMRT.
DISCUSSION
Arsenic trioxide is a potent inhibitor of SMRT function.
The ability of nuclear receptors to modulate gene expression is mediated through the recruitment of auxiliary proteins, referred to as corepressors and coactivators (7, 10, 22, 54, 60, 66). The interactions of nuclear receptors with these auxiliary proteins are tightly regulated and play a central role in determining the transcriptional properties, positive or negative, displayed by a given receptor. Hormone ligand is one important modulator of this corepressor-coactivator exchange; binding of cognate hormone is thought to induce a specific change in the conformation of the nuclear receptors that leads to a release of the corepressor complex and an acquisition of the coactivator complex (23, 33, 44, 47). However, the association of these auxiliary proteins with nuclear receptors can also be regulated in a ligand-independent manner through the effects of protein kinases (19, 62). We have previously shown that SMRT is phosphorylated in response to a MAP kinase cascade that is initiated by the EGF receptor and propagated through the kinase hierarchy by MEKK-1 and MEK-1 (20). As a result of these phosphorylation events, the ability of SMRT to interact with T3Rs, RARs, or PLZF is severely impaired.
Arsenic trioxide displays pleiotropic effects in many biological systems. At high concentrations, arsenic trioxide is toxic. At lower concentrations, however, arsenic trioxide can act as a carcinogen (55) or, as described here, can function conversely as a chemotherapeutic in the treatment of APL. It is this capacity of arsenite to induce remission in APL, together with the known ability of arsenite to up-regulate MAP kinases in cells, that led us to examine whether arsenic trioxide might mediate some of its effects through an inhibition of SMRT function. As we report here, arsenic trioxide treatment transiently up-regulates MAP kinase cascade signaling in CV-1 and NB-4 cells and results in inhibition of the ability of SMRT to interact with nuclear receptors and with nonreceptor transcription factors, such as PLZF. This arsenite-mediated inhibition of SMRT function is among the most potent we have observed. These effects of arsenite appear to be specific for the SMRT-transcription factor interaction and are not due to a general arsenic-mediated toxicity or an overall inhibition of transcription or translation.
Consistent with the proposal that at least some of the effects of arsenite are mediated through activation of a MAP kinase cascade, the SMRT protein was hyperphosphorylated and altered in its subcellular distribution in response to arsenite treatment in a fashion analogous to that observed in response to the introduction of an activated EGF receptor, MEKK-1 or MEK-1; conversely, chemical inhibitors of MAP kinase cascade signaling counteracted the effects of arsenite on SMRT function. It remains unclear, however, precisely how arsenite leads to an increase in MAP kinase cascade signaling in our system. Two targets of arsenite identified previously, the EGF receptor itself and JNK phosphatases (2, 9), do not appear to play a role in the phenomena reported here (unpublished data). It is possible that a different growth factor receptor or a component of the MAP kinase cascade itself in CV-1 and NB-4 cells may interact with and be stimulated by arsenite (e.g., see references 9, 36, 37, 40, and 48), thereby accounting for the up-regulation of MAP kinase signaling we observe. Alternatively, arsenic may operate by targeting the activity of an as-yet-undetermined phosphatase in these cells, thereby elevating the phosphorylation state, and enzymatic activity, of one or more components of the MAP kinase cascade.
Arsenite results in dissociation of SMRT from PML-RARα and induces a mixed differentiation and apoptotic response in APL-derived cells that is counteracted by inhibitors of MEK-1 signaling.
APL in humans is associated with chromosomal translocations that lead to the synthesis of aberrant x-RAR chimeric proteins (8, 11, 29, 41, 45). These x-RAR chimeras retain the ability to bind to retinoid-responsive target genes but are impaired in hormone-mediated corepressor release, and they are thought to function as dominant negative inhibitors of normal retinoid signaling. Consistent with this proposal, supraphysiological levels of retinoic acid that induce release of corepressor from PML-RARα also induce differentiation in PML-RARα APL cells in culture and result in clinical remission in APL patients bearing the PML-RARα translocation (10, 15–17, 34, 35, 42).
Arsenic trioxide serves as an important adjuvant to retinoic acid-mediated differentiation therapy, and arsenic trioxide can induce remission in cases of recurrent APL that are resistant to the effects of retinoids (reviewed in references 56 and 68). The palliative effects of arsenic trioxide are, in part, attributed to a strong, arsenite-induced apoptotic response, an effect that we could reproduce in our own experiments with cultured NB-4 cells. However, we also observed in the NB-4 cell population a distinct, arsenite-induced differentiation response. This differentiation response was manifested as a substantial increase in the number of cells displaying a CD11c epitope, an early marker of macrophage/monocyte differentiation, but not as an increase in the expression of leukocyte alkaline phosphatase, a terminal granulocyte differentiation marker. A similar abortive differentiation response in arsenite-treated NB-4 cells has been reported previously (e.g., see references 4 and 14). Intriguingly, many of the NB-4 cells that display the apoptotic response to arsenite represent a distinct population from those that display the differentiation response (S. H. Hong and M. L. Privalsky, unpublished observations). It is possible that arsenite-induced differentiation precedes apoptosis and that once the cells enter into apoptosis they lose the CD11c marker. Alternatively, these two pathways may be alternative responses to arsenite, with some of the NB-4 cells entering apoptosis and others entering differentiation.
An abnormally stable association with SMRT corepressor helps maintain the oncogenic state in APL (8, 15–17, 18, 35). Arsenite destabilizes the ability of SMRT to interact with nuclear receptors in CV-1 cells through a non-ligand-based pathway involving a MAP kinase cascade. Does arsenite induce a similar inhibition of the SMRT interaction with PML-RARα in APL cells, and might this arsenite-mediated release of SMRT initiate the abortive differentiation phenotype? Consistent with this hypothesis, we determined that arsenite treatment induces MAP kinase signaling in the NB-4 cells, reflected as a transient increase in the phosphorylation level, and presumably activity, of MEK-1. This increase in MEK-1 activity by arsenite is paralleled by a rapid disruption of the interaction of SMRT with PML-RARα, causing a loss of corepressor from the PML-RARα oncoprotein similar to that observed in response to retinoic acid treatment. Conversely, the ability of arsenite to induce differentiation in NB-4 cells is blocked by U0126, a specific inhibitor of the MAP kinase cascade. The inhibitory effects of U0126 were specific for differentiation mediated by arsenite; U0126 did not alter the ability of NB-4 cells to differentiate in response to retinoic acid.
We therefore propose that arsenite induces differentiation in NB-4 cells, at least in part, through a MAP kinase cascade, leading to inhibition of SMRT function. This model may help explain why differentiation of NB-4 cells in response to arsenite is inefficient and incomplete compared to the more vigorous differentiation seen in response to retinoic acid. Arsenite induces only release of corepressor from PML-RARα, whereas retinoic acid induces both corepressor release and coactivator acquisition. Thus, whereas the effects of arsenite treatment may be limited to reversing PML-RARα-mediated repression, producing modest increases in the expression of differentiation-related target genes, retinoic acid would both reverse repression and induce transcriptional activation of these target genes to levels substantially above basal levels. It should be noted, however, that the effects of arsenite on NB-4 cell differentiation are complex and may involve mechanisms in addition to the phosphorylation of SMRT noted here. For example, arsenite leads to the SUMO modification of PML and of PML-RARα, the reformation of nuclear bodies, and an eventual loss of detectable PML-RARα protein (4, 14, 43, 52, 59, 63, 69). It is also interesting that the U0126 MEK-1 inhibitor not only blocked the partial differentiation phenotype induced in NB-4 cells by arsenic trioxide but also blocked the apoptotic response mediated by the same treatment. Precisely which of these differentiation and proapoptotic events are related to the arsenite-mediated inhibition of SMRT association by PML-RARα, and which are independent outcomes, remains to be determined by future experiments. In addition, many cells express both SMRT and its paralog, N-CoR. RARα and PML-RARα have been reported to interact with SMRT in preference to N-CoR (23, 33, 44, 47), leading us to focus our present studies on the former. Nonetheless, MEKK-1 signaling can inhibit the interaction of N-CoR with its nuclear receptor partners in a manner similar to what we have reported for SMRT (unpublished observations), and N-CoR may also prove to be a target of arsenite inhibition in these, or in other, cell lines.
Arsenite and cancer.
Arsenic is an element that occurs in nature in many forms and exerts many pleiotropic effects on biological systems. In addition to its widely recognized, acutely toxic actions, arsenic produces many chronic effects on organisms. Somewhat paradoxically, in light of its antineoplastic effects in APL, arsenic trioxide is also known to be a powerful tumor promoter in human beings, and arsenic-mediated oncogenicity represents a significant health issue both for personnel in the chemical industry and for the human population as a whole as a result of arsenic contamination of ground water (55). Arsenic operates in biological systems through multiple mechanisms. Based on the results presented here, we suggest that a previously unrecognized effect of arsenic, the ability of arsenite to inhibit corepressor function, must be added to the mechanisms by which arsenic can perturb biological systems and should be considered in interpreting the toxic, oncogenic, and antineoplastic effects of the trivalent metalloids.
ACKNOWLEDGMENTS
We thank Christina Jamieson and Fred Schaufele for generously providing molecular clones, Donna Lagarias for expert advice and generous assistance with the FACS analysis, and Valentina Taryanik for dedicated technical assistance.
This work was supported by Public Health Service/NIH grants R01 DK-53528 and R37 CA-53394.
REFERENCES
- 1.Ayer D E. Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol. 1999;9:193–198. doi: 10.1016/s0962-8924(99)01536-6. [DOI] [PubMed] [Google Scholar]
- 2.Cavigelli M, Li W W, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- 3.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 4.Chen G Q, Shi X G, Tang W, Xiong S M, Zhu J, Cai X, Han Z G, Ni J H, Shi G Y, Jia P M, Liu M M, He K L, Niu C, Ma J, Zhang P, Zhang T D, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang Z Y, de The H, Chen S J, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 5.Chen G Q, Zhu J, Shi X G, Ni J H, Zhong H J, Si G Y, Jin X L, Tang W, Li X S, Xong S M, Shen Z X, Sun G L, Ma J, Zhang P, Zhang T D, Gazin C, Naoe T, Chen S J, Wang Z Y, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 6.Chen J D, Evans R M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen J D, Li H. Coactivation and corepression in transcriptional regulation by steroid/nuclear hormone receptors. Crit Rev Eukaryot Gene Expr. 1998;8:169–190. doi: 10.1615/critreveukargeneexpr.v8.i2.40. [DOI] [PubMed] [Google Scholar]
- 8.Chen S J, Wang Z Y, Chen Z. Acute promyelocytic leukemia: from clinic to molecular biology. Stem Cells. 1995;13:22–31. doi: 10.1002/stem.5530130104. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Martindale J L, Holbrook N J, Liu Y. Tumor promoter arsenite activates extracellular signal-regulated kinase through a signaling pathway mediated by epidermal growth factor receptor and Shc. Mol Cell Biol. 1998;18:5178–5188. doi: 10.1128/mcb.18.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collingwood T N, Urnov F D, Wolffe A P. Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription. J Mol Endocrinol. 1999;23:255–275. doi: 10.1677/jme.0.0230255. [DOI] [PubMed] [Google Scholar]
- 11.de Thé H. Altered retinoic acid receptors. FASEB J. 1996;10:955–960. doi: 10.1096/fasebj.10.9.8801177. [DOI] [PubMed] [Google Scholar]
- 12.Downes M, Burke L J, Bailey P J, Muscat G E. Two receptor interaction domains in the corepressor, N-CoR/RIP13, are required for an efficient interaction with Rev-erbA alpha and RVR: physical association is dependent on the E region of the orphan receptors. Nucleic Acids Res. 1996;24:4379–4386. doi: 10.1093/nar/24.22.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downes M, Ordentlich P, Kao H Y, Alvarez J G, Evans R M. Identification of a nuclear domain with deacetylase activity. Proc Natl Acad Sci USA. 2000;97:10330–10335. doi: 10.1073/pnas.97.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannì M, Koken M H, Chelbi-Alix M K, Benoit G, Lanotte M, Chen Z, de Thé H. Combined arsenic and retinoic acid treatment enhances differentiation and apoptosis in arsenic-resistant NB4 cells. Blood. 1998;91:4300–4310. [PubMed] [Google Scholar]
- 15.Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara F F, Zamir I, Seiser C, Lazar M A, Minucci S, Pelicci P G. Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature. 1998;391:815–818. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- 16.Guidez F, Ivins S, Zhu J, Söderström M, Waxman S, Zelent A. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARα underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. 1998;91:2634–2642. [PubMed] [Google Scholar]
- 17.He L Z, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A, Pandolfi P P. Distinct interactions of PML-RARα and PLZF-RARα with co-repressors determine differential responses to RA in APL. Nat Genet. 1998;18:126–135. doi: 10.1038/ng0298-126. [DOI] [PubMed] [Google Scholar]
- 18.Hong S H, David G, Wong C W, Dejean A, Privalsky M L. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor alpha (RARα) and PLZF-RARα oncoproteins associated with acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:9028–9033. doi: 10.1073/pnas.94.17.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong S H, Wong C W, Privalsky M L. Signaling by tyrosine kinases negatively regulates the interaction between transcription factors and SMRT (silencing mediator of retinoic acid and thyroid hormone receptor) corepressor. Mol Endocrinol. 1998;12:1161–1171. doi: 10.1210/mend.12.8.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong S-K, Privalsky M L. SMRT corepressor is regulated by a MEK-1 kinase pathway: inhibition of corepressor function is associated with SMRT phosphorylation and nuclear export. Mol Cell Biol. 2000;20:6612–6625. doi: 10.1128/mcb.20.17.6612-6625.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hörlein A J, Näär A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass C K, Rosenfeld M G. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz K B, Jackson T A, Bain D L, Richer J K, Takimoto G S, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Lazar M A. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–96. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- 24.Huang E Y, Zhang J S, Miska E A, Guenther M G, Kouzarides T, Lazar M A. Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Gene Dev. 2000;14:45–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Ito M, Yuan C X, Malik S, Gu W, Fondell J D, Yamamura S, Fu Z Y, Zhang X, Qin J, Roeder R G. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol Cell. 1999;3:361–370. doi: 10.1016/s1097-2765(00)80463-3. [DOI] [PubMed] [Google Scholar]
- 26.Jing Y, Dai J, Chalmers-Redman R M, Tatton W G, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 27.Kao H Y, Downes M, Ordentlich P, Evans R M. Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Gene Dev. 2000;14:55–66. [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamura K, Yoshida H, Ohno R, Naoe T. Toxic effects of arsenic (As3+) and other metal ions on acute promyelocytic leukemia cells. Int J Hematol. 1997;65:179–185. doi: 10.1016/s0925-5710(96)00547-6. [DOI] [PubMed] [Google Scholar]
- 29.Lavau C, Jansen J, Dejean A. The t(15;17) translocation in acute promyelocytic leukemia. Pathol Biol (Paris) 1995;43:188–196. [PubMed] [Google Scholar]
- 30.Lavinsky R M, Jepsen K, Heinzel T, Torchia J, Mullen T M, Schiff R, Del-Rio A L, Ricote M, Ngo S, Gemsch J, Hilsenbeck S G, Osborne C K, Glass C K, Rosenfeld M G, Rose D W. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA. 1998;95:2920–2925. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Leo C, Schroen D J, Chen J D. Characterization of receptor interaction and transcriptional repression by the corepressor SMRT. Mol Endocrinol. 1997;11:2025–2037. doi: 10.1210/mend.11.13.0028. [DOI] [PubMed] [Google Scholar]
- 32.Li J W, Wang J, Wang J X, Nawaz Z, Liu J M, Qin J, Wong J M. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 2000;19:4342–4350. doi: 10.1093/emboj/19.16.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin B C, Hong S H, Krig S, Yoh S M, Privalsky M L. A conformational switch in nuclear hormone receptors is involved in coupling hormone binding to corepressor release. Mol Cell Biol. 1997;17:6131–6138. doi: 10.1128/mcb.17.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin R J, Evans R M. Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol Cell. 2000;5:821–830. doi: 10.1016/s1097-2765(00)80322-6. [DOI] [PubMed] [Google Scholar]
- 35.Lin R J, Nagy L, Inoue S, Shao W, Miller W H, Jr, Evans R M. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature. 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Guyton K Z, Gorospe M, Xu Q, Lee J C, Holbrook N J. Differential activation of ERK, JNK/SAPK and P38/CSBP/RK map kinase family members during the cellular response to arsenite. Free Radic Biol Med. 1996;21:771–781. doi: 10.1016/0891-5849(96)00176-1. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig S, Hoffmeyer A, Goebeler M, Kilian K, Häfner H, Neufeld B, Han J, Rapp U R. The stress inducer arsenite activates mitogen-activated protein kinases extracellular signal-regulated kinases 1 and 2 via a MAPK kinase 6/p38-dependent pathway. J Biol Chem. 1998;273:1917–1922. doi: 10.1074/jbc.273.4.1917. [DOI] [PubMed] [Google Scholar]
- 38.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier C A. Regulation of gene expression by nuclear hormone receptors. J Recept Signal Transduct Res. 1997;17:319–335. doi: 10.3109/10799899709036612. [DOI] [PubMed] [Google Scholar]
- 40.Meier R, Rouse J, Cuenda A, Nebreda A R, Cohen P. Cellular stresses and cytokines activate multiple mitogen-activated-protein kinase kinase homologues in PC12 and KB cells. Eur J Biochem. 1996;236:796–805. doi: 10.1111/j.1432-1033.1996.00796.x. [DOI] [PubMed] [Google Scholar]
- 41.Minucci S, Cioce M, Maccarana M, Pelicci P G. The APL-associated fusion proteins. Haematologica. 1999;84:70–71. [PubMed] [Google Scholar]
- 42.Minucci S, Maccarana M, Cioce M, De Luca P, Gelmetti V, Segalla S, Di Croce L, Giavara S, Matteucci C, Gobbi A, Bianchini A, Colombo E, Schiavoni I, Badaracco G, Hu X, Lazar M A, Landsberger N, Nervi C, Pelicci P G. Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol Cell. 2000;5:811–820. doi: 10.1016/s1097-2765(00)80321-4. [DOI] [PubMed] [Google Scholar]
- 43.Müller S, Matunis M J, Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 1998;17:61–70. doi: 10.1093/emboj/17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy L, Kao H Y, Love J D, Li C, Banayo E, Gooch J T, Krishna V, Chatterjee K, Evans R M, Schwabe J W. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999;13:3209–3216. doi: 10.1101/gad.13.24.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandolfi P P. PML, PLZF and NPM genes in the molecular pathogenesis of acute promyelocytic leukemia. Haematologica. 1996;81:472–482. [PubMed] [Google Scholar]
- 46.Pazin M J, Kadonaga J T. What's up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 47.Perissi V, Staszewski L M, McInerney E M, Kurokawa R, Krones A, Rose D W, Lambert M H, Milburn M V, Glass C K, Rosenfeld M G. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13:3198–3208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porter A C, Fanger G R, Vaillancourt R R. Signal transduction pathways regulated by arsenate and arsenite. Oncogene. 1999;18:7794–7802. doi: 10.1038/sj.onc.1203214. [DOI] [PubMed] [Google Scholar]
- 49.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Näär A M, Erdjument-Bromage H, Tempst P, Freedman L P. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 50.Sande S, Privalsky M L. Identification of TRACs (T3 receptor-associating cofactors), a family of cofactors that associate with, and modulate the activity of, nuclear hormone receptors. Mol Endocrinol. 1996;10:813–825. doi: 10.1210/mend.10.7.8813722. [DOI] [PubMed] [Google Scholar]
- 51.Seol W, Mahon M J, Lee Y K, Moore D D. Two receptor interacting domains in the nuclear hormone receptor corepressor RIP13/N-CoR. Mol Endocrinol. 1996;10:1646–1655. doi: 10.1210/mend.10.12.8961273. [DOI] [PubMed] [Google Scholar]
- 52.Shao W, Fanelli M, Ferrara F F, Riccioni R, Rosenauer A, Davison K, Lamph W W, Waxman S, Pelicci P G, Lo Coco F, Avvisati G, Testa U, Peschle C, Gambacorti-Passerini C, Nervi C, Miller W H., Jr Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90:124–133. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- 53.Shen Z X, Chen G Q, Ni J H, Li X S, Xiong S M, Qiu Q Y, Zhu J, Tang W, Sun G L, Yang K Q, Chen Y, Zhou L, Fang Z W, Wang Y T, Ma J, Zhang P, Zhang T D, Chen S J, Chen Z, Wang Z Y. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 54.Shibata H, Spencer T E, Oñate S A, Jenster G, Tsai S Y, Tsai M J, O'Malley B W. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–164. [PubMed] [Google Scholar]
- 55.Simeonova P P, Luster M I. Mechanisms of arsenic carcinogenicity: genetic or epigenetic mechanisms? J Environ Pathol Toxicol Oncol. 2000;19:281–286. [PubMed] [Google Scholar]
- 56.Slack J L. Biology and treatment of acute progranulocytic leukemia. Curr Opin Hematol. 1999;6:236–240. doi: 10.1097/00062752-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Söderström M, Vo A, Heinzel T, Lavinsky R M, Yang W M, Seto E, Peterson D A, Rosenfeld M G, Glass C K. Differential effects of nuclear receptor corepressor (N-CoR) expression levels on retinoic acid receptor-mediated repression support the existence of dynamically regulated corepressor complexes. Mol Endocrinol. 1997;11:682–692. doi: 10.1210/mend.11.6.0018. [DOI] [PubMed] [Google Scholar]
- 58.Soignet S L, Maslak P, Wang Z G, Jhanwar S, Calleja E, Dardashti L J, Corso D, DeBlasio A, Gabrilove J, Scheinberg D A, Pandolfi P P, Warrell R P., Jr Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 59.Sternsdorf T, Puccetti E, Jensen K, Hoelzer D, Will H, Ottmann O G, Ruthardt M. PIC-1/SUMO-1-modified PML-retinoic acid receptor alpha mediates arsenic trioxide-induced apoptosis in acute promyelocytic leukemia. Mol Cell Biol. 1999;19:5170–5178. doi: 10.1128/mcb.19.7.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torchia J, Glass C, Rosenfeld M G. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 61.Tsai M J, O'Malley B W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 62.Wagner B L, Norris J D, Knotts T A, Weigel N L, McDonnell D P. The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol Cell Biol. 1998;18:1369–1378. doi: 10.1128/mcb.18.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z G, Rivi R, Delva L, König A, Scheinberg D A, Gambacorti-Passerini C, Gabrilove J L, Warrell R P, Jr, Pandolfi P P. Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARα independent manner. Blood. 1998;92:1497–1504. [PubMed] [Google Scholar]
- 64.Wong C W, Privalsky M L. Transcriptional silencing is defined by isoform- and heterodimer-specific interactions between nuclear hormone receptors and corepressors. Mol Cell Biol. 1998;18:5724–5733. doi: 10.1128/mcb.18.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Workman J L, Kingston R E. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 66.Xu L, Glass C K, Rosenfeld M G. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 67.Zamir I, Harding H P, Atkins G B, Hörlein A, Glass C K, Rosenfeld M G, Lazar M A. A nuclear hormone receptor corepressor mediates transcriptional silencing by receptors with distinct repression domains. Mol Cell Biol. 1996;16:5458–5465. doi: 10.1128/mcb.16.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang P. The use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia. J Biol Regul Homeost Agents. 1999;13:195–200. [PubMed] [Google Scholar]
- 69.Zhu J, Koken M H, Quignon F, Chelbi-Alix M K, Degos L, Wang Z Y, Chen Z, de Thé H. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:3978–3983. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X H, Shen Y L, Jing Y K, Cai X, Jia P M, Huang Y, Tang W, Shi G Y, Sun Y P, Dai J, Wang Z Y, Chen S J, Zhang T D, Waxman S, Chen Z, Chen G Q. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–778. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]