Abstract
Background
The worldwide prevalence of eczema has continued to rise over the past decades. This has led to the emphasis on the association between air pollution and eczema. This study investigated the relationship between daily exposure to air pollution and the number of eczema outpatient visits in Guangzhou with the overarching goal of providing novel insights on the interventions for eczema aggravation and prevention.
Methods
Daily air pollution data, meteorological data, and the number of eczema outpatients were obtained from 18 January 2013 to 31 December 2018 in Guangzhou. A generalized additive model with Poisson distribution was used to assess the association between the number of eczema outpatient visits and short-term exposure to PM2.5 and PM10. In addition, the association of PM2.5 and PM10 by age (<65 years, ≥65 years) and gender was evaluated.
Results
A total of 293,343 eczema outpatient visits were recorded. The obtained results indicated that a 10 μg/m3 increase of the same day/lag 1 day/lag 2 days PM2.5 was associated with increments of 2.33%, 1.81%, and 0.95% in eczema outpatient risk, respectively. On the other hand, a 10 μg/m3 increase of PM10 was associated with eczema outpatients risk increments of 1.97%, 1.65%, and 0.98% respectively. Furthermore, the associations of PM on the increment of eczema were similar in the male and female groups. Results obtained after age stratified analyses indicated that the strongest positive association between PM2.5 exposure and eczema was observed at lag 0 day with the percent changes being 4.72% and 3.34% in <12 years old, ≥12 and <65 years old, and ≥65 years old groups, respectively.
Conclusion
Short-term exposure to PM2.5 and PM10 increases the number of eczema outpatients, especially among children and the elderly. The relationship between air quality trends and hospital resource arrangement should be paid attention to by hospital managers which may aid in disease prevention and lower the health burden.
Keywords: short-term exposure, particulate matter, PM2.5, PM10, air pollution, eczema, outpatients
Background
An eczema is a group of common inflammatory skin diseases. Patients may develop different skin lesions and relapsing pruritus in the different stages, thereby causing a considerable physical and psychological burden on the patients (1–3). The worldwide prevalence of eczema has been rising continuously over the past decades (4, 5). The available clinical treatments only improve the symptoms as opposed to curing the disease (5). Therefore, the lack of a cure for eczema highlights the importance of prevention. A previous study has reported that the identification of the underlying modifiable risk factors for eczema including environmental stimuli plays a decisive role in the prophylactic management of the disease (6).
In addition to the genetic background, environment, dietary habits, lifestyle, and societal status have also been associated with the pathogenic condition of skin diseases (7, 8). Air pollution, including particulate matter (PM), nitrogen dioxide (NO2), and sulfur dioxide (SO2), is one of the environmental stimuli (9). Recently, our team has revealed the tight association between exposure to NO2 with eczema incidence and outpatient visits (10). In addition to NO2, accumulating scientific evidence has reported the associations of PM with the development of eczema and dermatitis (11, 12). PM is naturally produced from salt and dust while the majority of PM is derived from anthropogenic activities of fossil fuel combustion, mostly contributed by the consumption of fuel by vehicles (13). PM is a complicated mixture with alternative concentrations, particle diameter, and chemical properties in various spaces and times (14). According to the particles' aerodynamic diameter, PM can be categorized into PM0.1 (particle diameter ≤ 0.1 μm), PM2.5 (particle diameter ≤ 2.5 μm), and PM10 (particle diameter ≤ 10 μm) (15).
Recently, the article revealed the aggravation of PM in children's atopic dermatitis in South Korea but did not mention adult and elderly patients (16). The skin manifestations of patients with AD of different ages are different. Therefore, we studied the effects of PM on dermatitis in children, adults, and the elderly to reveal the effect of PM on patients with AD in different age categories is consistent. We expect PM to have similar effects in children as in previous studies, as well as in the elderly. Some studies showed PM enables to inducement of skin inflammation and deteriorates inflammatory skin disorder symptoms, which may attribute to the mechanisms of oxidative stress or programmed cell death. However, the exact and complete mechanisms of skin disease induced by PM are still calling for exploration.
Moreover, a meta-analysis (17) included 13 clinical studies, a total of 72,000 participants, aiming to demonstrate the effects of PM2.5 and PM10 on human skin diseases including atopic dermatitis, eczema, and skin aging. Short-term effects have been estimated that increases of 10 μg/m3 in PM2.5 and PM10 were associated with a 1.60% (0.45–2.82) and 1.01% (0.08–2.05) increase in total skin disease risk, respectively. In addition, the study has demonstrated that long-term exposure to PM2.5 upwards of 47.09 μg/m3 and PM10 upwards of 26.04 μg/m3 would adversely affect human skin. However, this study failed to reveal the effect of PM on eczema specially and up to now, evidence of the association between PM and eczema is still lacking.
Guangzhou, with a population density of 2,059 people/km2 is the 3rd most densely populated city in China in 2019 (https://www.askci.com/news/data/hongguan/20200730/1441261166838.shtml). A previous study reported that PM has become one of the main sources of air pollution in Guangzhou due to rapid industrial development and advanced transportation (18). Therefore, this study investigated the association between daily air pollution exposure and the number of outpatients with eczema in Guangzhou. In addition, the previously mentioned associations were also evaluated in different ages, and gender subgroups. The main aim of the study was to identify the potential environmental risk factors and to provide novel insights into the interventions for eczema aggravation and prevention.
Materials and methods
Study setting
Guangzhou, the capital city of Guangdong province, is one of the developed cities in China with an approximate population of 14.9 million in 2018. The city, located at 23°10′N, 113°18′E, has a typical subtropical humid-monsoon climate with an average annual temperature and rainfall of 22°C and 1,500–2,000 mm, respectively (http://tjj.gz.gov.cn/tjdt/content/post_5727607.html). In addition, Guangzhou is the top-ranking commercial and manufacturing city in China. Therefore, the problem of air pollution in the city as a result of economic advancement should not be underestimated. In 2019, the average concentrations of PM2.5, PM10, NO2, SO2, and O3 in Guangzhou were 30 ug/m3, 53 ug/m3, 45 ug/m3, 7 ug/m3, and 178 ug/m3, respectively1, while in Haikou, China, the average concentrations were lower as 17 ug/m3, 32 ug/m3, 13 ug/m3, 5 ug/m3, and 144 ug/m3 respectively (http://www.haikou.gov.cn/xxgk/szfbjxxgk/tjxx/hjzk/202006/t20200630_1519119.html).
Data sources
Patient data
The Dermatology Hospital of Southern Medical University, located in Tianhe district, Guangzhou, is a large-scale dermatology specialty and third-class hospital in South China. The hospital has the largest number of eczema outpatient visits in Guangzhou (http://www.gdskin.com/ShowClass.aspx?ID=704). This study enrolled outpatients who were diagnosed with eczema coded as L30.902 according to the World Health Organization's International Classification of Diseases [the 10th version (ICD-10)] in the hospital from 18 January 2013 to 31 December 2018. If recurrent eczema occurred within 21 days of a previous outpatient visit, it was regarded as a single eczema event.
Air pollution and meteorological data
Air quality monitoring data was obtained from the public sharing system of the Guangzhou Environmental Monitoring Center (http://sthjj.gz.gov.cn/infoindex.html). Data recorded in the system was obtained from 11 national air quality monitor stations in Guangzhou and the daily average air quality data was used for our analyses. Daily average concentrations of PM2.5, PM10, NO2, SO2, and O3 during the period ranging from 18 January 2013 to 31 December 2018 were also collected. Daily meteorological data, including mean humidity and temperature, were obtained from the China Meteorological Data Sharing Service System based on data from different meteorological stations in Guangzhou and used for analyses. Both air pollution and meteorology data followed the quality control programs which are mandated by the Chinese government.
Statistical analyses
A generalized additive model (GAM) with Poisson distribution was used to assess the association between eczema outpatient visits and short-term exposure to PM2.5 and PM10 (19). Lag 0–3 days were used to explore the cumulative exposure and displacement associations of PM and other pollutants (20). This study used a single pollutant model to examine the association between PM and eczema admission. In addition, we built a two-pollutant model to control the potential confounding effects of other pollutants (21). These models can be represented as follows:
where μt represents the expected number of eczema outpatients on day t; α is the intercept; Xit represents the concentrations of pollutants (PM2.5, PM10, NO2, SO2, and O3) on day t, i = 1 or i = 2 represents single pollutant model or two-pollutant model, respectively; and βi stands for the coefficient of Xi. A natural cubic spline function (NS) with 10, 3, and 3 degrees of freedom (df) was used to capture the non-linear relationships of time trends, temperature, and humidity (22). In our model, the day of week (DOWt) was set in the form of categorical variables, while γ represents the effect of DOWt on eczema outpatients.
Age and gender stratified analyses using potential individual-level effect modifiers were also conducted. This study used the above basic models to examine outcomes by stratification of age (<65 years, ≥65 years) and gender for the purpose of exploring the potential modification.
In addition, the dfs for temperature, humidity, and time used in the above models were assessed using the Akaike information criterion for quasi-Poisson (Q-AIC). The minimum value of Q-AIC represented the best goodness and the optimum of dfs. In order to check the robustness of our modeling strategies, sensitivity analyses were done by changing the df for temperature (2–4), humidity (2–4), and calendar time (9–11 per year) to control time trends (23). R software version 4.0.2 was used to conduct all the analyses and a P < 0.05 was considered to be statistically significant in all statistical analyses.
Results
Data description
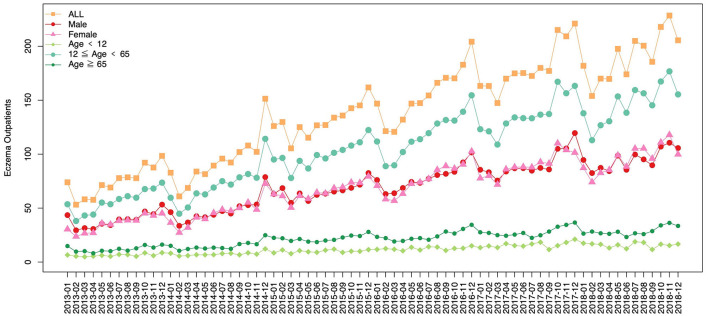
In this study, a total of 293,343 eczema outpatients were recorded in the Dermatology Hospital of Southern Medical University from 18 January 2013 to 31 December 2018. Table 1 shows the descriptive statistics of daily eczema outpatients, air pollutants, and meteorological variables. The average number of daily eczema outpatients in the hospital was 190 in 2018, while the average concentrations of air pollutants including PM2.5, PM10, NO2, SO2, and O3 were 34.7 μg/m3, 55.7 μg/m3, 48.1 μg/m3, 9.6 μg/m3, and 50.7 μg/m3, respectively. In addition, the annual mean temperature and humidity were 22.3°C and 81.7%, respectively. With the exception of temperature (F value = 0.313, P = 0.905), the annual average values of all other variables were significantly different from 2013 to 2018. Furthermore, there was an increased trend of eczema outpatient visits. Figure 1 is presenting the time trend of eczema events overall and by sex and age groups during the study period.
Table 1.
Daily outpatients of eczema, meteorological factors and air pollutants in Guangzhou (2013/1/18–2018/12/31).
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | F | P | |
|---|---|---|---|---|---|---|---|---|
| Eczema outpatients | 75.261 ± 23.840 | 93.688 ± 31.839 | 131.406 ± 37.233 | 155.644 ± 43.374 | 180.638 ± 48.581 | 190.845 ± 48.430 | 478.389 | <0.001 |
| Temperaturea (°C) | 22.160 ± 5.585 | 21.768 ± 6.580 | 22.229 ± 5.927 | 22.019 ± 6.395 | 22.136 ± 5.837 | 22.255 ± 6.177 | 0.313 | 0.905 |
| Humidity* (%) | 81.296 ± 11.546 | 78.448 ± 10.628 | 78.057 ± 8.907 | 81.991 ± 9.698 | 80.942 ± 11.373 | 81.685 ± 10.203 | 9.472 | <0.001 |
| (mg/m3) | 50.165 ± 27.241 | 49.350 ± 24.731 | 38.958 ± 21.112 | 34.163 ± 17.220 | 35.033 ± 19.055 | 34.663 ± 21.135 | 40.575 | <0.001 |
| (mg/m3) | 72.139 ± 34.403 | 67.476 ± 30.655 | 60.636 ± 28.122 | 54.739 ± 25.081 | 56.388 ± 27.007 | 55.658 ± 27.538 | 21.298 | <0.001 |
| (mg/m3) | 51.237 ± 21.476 | 44.093 ± 18.826 | 45.188 ± 17.063 | 43.716 ± 18.109 | 49.664 ± 19.595 | 48.064 ± 19.605 | 9.315 | <0.001 |
| (mg/m3) | 20.798 ± 8.447 | 20.953 ± 15.474 | 12.998 ± 5.216 | 11.954 ± 3.783 | 11.739 ± 3.645 | 9.603 ± 3.262 | 135.621 | <0.001 |
| (mg/m3) | 61.797 ± 36.736 | 57.796 ± 33.119 | 47.715 ± 24.465 | 47.175 ± 24.994 | 50.253 ± 27.124 | 50.678 ± 25.623 | 14.398 | <0.001 |
F = 0.313, P = 0.905.
P <0.001.
Figure 1.
A line plot presenting the time trend of eczema events (monthly average) overall and by sex and age groups.
Obtained results indicated that the correlation coefficients between PM2.5 and temperature, humidity, PM10, NO2, SO2, and O3 were 0.32, 0.22, 0.94, 0.73, 0.60, and 0.31, respectively. PM2.5 was negatively correlated with temperature and humidity and positively correlated with PM10, NO2, SO2, and O3. On the other hand, the correlation coefficients between PM10 and temperature, humidity, PM2.5, NO2, SO2, and O3 were 0.24, 0.26, 0.94, 0.80, 0.44, and 0.35, respectively. Similarly, PM10 was negatively correlated with temperature and humidity and positively correlated with PM2.5, NO2, SO2, and O3. The highest Pearson correlations were observed between PM2.5 and PM10 in Table 2.
Table 2.
The correlation coefficients between PM2.5, PM10, and other pollutants.
| Temperature | Humidity | PM2.5 | O3 | PM10 | SO2 | NO2 | |
|---|---|---|---|---|---|---|---|
| Temperature | – | 0.31** | −0.32** | 0.20** | −0.24** | −0.15** | −0.27** |
| Humidity | – | – | −0.22** | −0.41** | −0.26** | −0.17** | 0.02 |
| PM2.5 | – | – | – | 0.31** | 0.94** | 0.60** | 0.73** |
| O3 | – | – | – | – | 0.35** | 0.26** | 0.11** |
| PM10 | – | – | – | – | – | 0.44** | 0.80** |
| SO2 | – | – | – | – | – | – | 0.27** |
P < 0.0001.
Distributed lag non-linear model analyses
The smallest Q-AIC of 27,558.6 was obtained with per year df of 3, 3, 10 for temperature, humidity, and time, respectively, in Table 3. This result formed the basis for the distributing lag non-linear model analyses which followed.
Table 3.
Results of Akaike information criterion for quasi-Poisson (Q-AIC).
| Time, DF (/year) | Temperature, DF | Humidity, DF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 8 | 3 | 27„687.49 | 27,698.63 | 27„710.13 | 27,713.52 | 27„720.51 | 27,730.71 | 27,733.56 | 27,735.89 |
| 4 | 27,697.89 | 27,709.01 | 27,720.50 | 27,723.89 | 27,730.41 | 27,740.75 | 27,743.74 | 27,745.83 | |
| 5 | 27,705.25 | 27,716.44 | 27,727.74 | 27,731.32 | 27,737.59 | 27,747.87 | 27,750.27 | 27,752.44 | |
| 6 | 27,714.62 | 27,725.84 | 27,737.11 | 27,740.58 | 27,746.86 | 27,757.19 | 27,759.62 | 27,761.81 | |
| 7 | 27,718.72 | 27,729.97 | 27,741.24 | 27,744.36 | 27,750.68 | 27,761.08 | 27,763.70 | 27,766.01 | |
| 8 | 27,694.57 | 27,705.30 | 27,716.62 | 27,719.05 | 27,725.94 | 27,736.22 | 27,738.77 | 27,742.45 | |
| 9 | 27,695.71 | 27,706.24 | 27,717.44 | 27,719.90 | 27,726.16 | 27,737.02 | 27,739.44 | 27,742.62 | |
| 10 | 27,701.47 | 27,711.98 | 27,722.74 | 27,725.05 | 27,731.92 | 27,743.25 | 27,745.65 | 27,748.14 | |
| 9 | 3 | 27,650.09 | 27,661.39 | 27,672.00 | 27,673.68 | 27,679.41 | 27,688.31 | 27,692.51 | 27,695.53 |
| 4 | 27,661.41 | 27,672.71 | 27,683.41 | 27,685.04 | 27,690.90 | 27,699.79 | 27,704.04 | 27,707.11 | |
| 5 | 27,669.24 | 27,680.59 | 27,691.06 | 27,692.94 | 27,698.59 | 27,707.45 | 27,711.32 | 27,714.42 | |
| 6 | 27,678.54 | 27,689.91 | 27,700.32 | 27,702.12 | 27,707.80 | 27,716.70 | 27,720.56 | 27,723.69 | |
| 7 | 27,683.88 | 27,695.26 | 27,705.69 | 27,707.15 | 27,712.83 | 27,721.83 | 27,725.83 | 27,729.02 | |
| 8 | 27,661.06 | 27,672.12 | 27,682.52 | 27,683.34 | 27,689.60 | 27,698.53 | 27,702.70 | 27,707.03 | |
| 9 | 27,663.26 | 27,674.19 | 27,684.39 | 27,685.27 | 27,690.84 | 27,700.41 | 27,704.45 | 27,708.37 | |
| 10 | 27,669.28 | 27,680.20 | 27,689.80 | 27,690.48 | 27,696.72 | 27,706.81 | 27,710.80 | 27,714.25 | |
| 10 | 3 | 27,558.60 | 27,570.00 | 27,579.86 | 27,582.72 | 27,586.32 | 27,595.13 | 27,599.11 | 27,601.70 |
| 4 | 27,564.76 | 27,576.08 | 27,586.21 | 27,589.03 | 27,593.53 | 27,602.16 | 27,606.28 | 27,609.24 | |
| 5 | 27,570.71 | 27,582.11 | 27,591.93 | 27,594.91 | 27,599.01 | 27,607.66 | 27,611.29 | 27,614.29 | |
| 6 | 27,579.87 | 27,591.27 | 27,600.96 | 27,603.89 | 27,608.17 | 27,616.79 | 27,620.38 | 27,623.46 | |
| 7 | 27,587.79 | 27,599.20 | 27,608.96 | 27,611.69 | 27,615.91 | 27,624.67 | 27,628.44 | 27,631.55 | |
| 8 | 27,570.61 | 27,581.98 | 27,591.66 | 27,593.79 | 27,598.67 | 27,607.49 | 27,611.45 | 27,615.53 | |
| 9 | 27,573.03 | 27,584.35 | 27,593.81 | 27,595.89 | 27,600.09 | 27,609.48 | 27,613.28 | 27,617.01 | |
| 10 | 27,579.26 | 27,590.61 | 27,599.42 | 27,601.30 | 27,606.12 | 27,615.98 | 27,619.68 | 27,622.92 | |
Results obtained from the single pollutant model indicated that a per 10 g/m3 increase of PM2.5 was associated with a 2.33% (95%CI: 2.06%, 2.60%, P < 0.001), 1.81% (95%CI: 1.53%, 2.09%, P < 0.001), 0.95% (95%CI: 0.68%, 1.23%, P < 0.001), and 0.29% (95%CI: 0.29%, 0.56%, P = 0.274) increase in the number of daily eczema outpatients at lag 0–3 days, respectively. In addition, a per 10 g/m3 increase of PM10 was interrelated to a 1.97% (95%CI: 1.77%, 2.17%, P < 0.001), 1.65% (95%CI: 1.45%, 1.86%, P < 0.001), 0.98% (95%CI: 0.78%, 1.18%, P < 0.001), and 0.52% (95%CI: 0.32%, 0.71%, P = 0.008) increase in the number of daily eczema outpatients at lag 0–3 days, respectively, in Table 4.
Table 4.
The RR and percent change of eczema risk associated with 10 mg/m3 increase of PM2.5 and PM10.
| Pollutant (per 10 mg/m3) | Lag day | RR | PC (%) | P |
|---|---|---|---|---|
| PM2.5 | Lag 0 | 1.0233 (1.0206–1.0260) | 2.33 (2.06–2.6) | <0.001 |
| Lag 1 | 1.0181 (1.0153–1.0209) | 1.81 (1.53–2.09) | <0.001 | |
| Lag 2 | 1.0095 (1.0068–1.0123) | 0.95 (0.68–1.23) | <0.001 | |
| Lag 3 | 1.0029 (1.0003–1.0056) | 0.29 (0.03–0.56) | 0.274 | |
| PM10 | Lag 0 | 1.0197 (1.0177–1.0217) | 1.97 (1.77–2.17) | <0.001 |
| Lag 1 | 1.0165 (1.0145–1.0186) | 1.65 (1.45–1.86) | <0.001 | |
| Lag 2 | 1.0098 (1.0078–1.0118) | 0.98 (0.78–1.18) | <0.001 | |
| Lag 3 | 1.0052 (1.0032–1.0071) | 0.52 (0.32–0.71) | 0.008 |
RR, risk ratio; PC, percentage change.
On the other hand, results obtained from the two-pollutant model analyses indicated that a per 10 g/m3 increase of PM2.5 adjusting for PM10, NO2, SO2, and O3 was related to a 1.29% (95%CI: 1.13%, 1.45%), 2.01% (95%CI: 1.80%, 2.23%), 2.54% (95%CI: 2.23%, 2.85%), and 1.46% (95%CI: 1.29%, 1.60%) increase of eczema outpatient visits at lag 0–3 days, respectively. In addition, per 10 g/m3 increase of PM10 increased eczema outpatient visits by 1.75% (95%CI: 1.58%, 1.92%), 2.34% (95%CI: 2.10%, 2.58%), and 1.4% (95%CI: 1.25%, 1.54%) at lag 0–3 days, respectively, after adjusting for NO2, SO2, and O3 in Table 5.
Table 5.
The RR and percent change of eczema risk associated with 10 mg/m3 increase of PM2.5 and PM10 under two-pollutant model.
| Pollutant (per 10 mg/m3) | Model type | RR | PC (%) |
|---|---|---|---|
| PM2.5 + PM10 | 1.0129 (1.0113–1.0145) | 1.29 (1.13–1.45) | |
| PM2.5 + NO2 | 1.0201 (1.018–1.0223) | 2.01 (1.8–2.23) | |
| PM2.5 + SO2 | 1.0254 (1.0223–1.0285) | 2.54 (2.23–2.85) | |
| PM2.5 + O3 | 1.0146 (1.0129–1.0163) | 1.46 (1.29–1.63) | |
| PM10 + NO2 | 1.0175 (1.0158–1.0192) | 1.75 (1.58–1.92) | |
| PM10 + SO2 | 1.0234 (1.021–1.0258) | 2.34 (2.1–2.58) | |
| PM10 + O3 | 1.014 (1.0125–1.0154) | 1.4 (1.25–1.54) |
*P < 0.001.
RR, risk ratio; PC, percentage change.
Stratified analyses
Results obtained after gender stratified analyses indicated that both in the male and female group, a 10 μg/m3 increase of PM2.5 or PM10 concentrations was associated with an increased risk of eczema outpatient visits at lag 0–3 days, respectively. Gradual weakening trends could also be observed at a lag of 1–3 days in Table 6. Both in the male and female groups, the strongest associations between PM2.5 [2.32%, (95%CI: 2.03%, 2.61%, P < 0.001) vs. 2.31% (95%CI: 2.01%, 2.61%, P < 0.001)] and PM10 [1.97% (95%CI: 1.76%, 2.18%, P < 0.001) vs. 1.97%, (95%CI: 1.75%, 2.19%, P < 0.001)] on lag 0 were figured out. Age stratified analyses results indicated that the strongest positive association between PM2.5 or PM10 exposure and eczema were also observed at lag 0 day with the percent changes being [4.72% (95%CI, 4.18%, 5.28%, P < 0.001) vs. 3.74% (95%CI, 3.34%, 4.14%, P < 0.001)], [1.92% (95%CI: 1.65%, 2.19%, P < 0.001) vs. 1.66% (95%CI, 1.47%, 1.86%, P < 0.001)] and [3.34% (95%CI: 2.90%, 3.78%, P < 0.001) vs. 2.55%, (95%CI: 2.24%, 2.87%, P < 0.001)] in the <12 years old, ≥12 and <65 years old, and ≥65 years old groups, respectively. Moreover, Table 7 showed the similar associations of PM2.5 and PM10 on the number of eczema outpatients in both the two-pollutant model and the single pollutant model.
Table 6.
Stratification analysis of the percent change of eczema risk associated with 10 mg/m3 increase of PM2.5 and PM10.
| Pollutant (per 10 mg/m3) | Lag day | Male | Female | <12 | 12–64 | ≥65 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC (%) | P | PC (%) | P | PC (%) | P | PC (%) | P | PC (%) | P | ||
| PM2.5 | Lag 0 | 2.32 (2.03–2.61) | <0.001 | 2.31 (2.01–2.61) | <0.001 | 4.72 (4.18–5.28) | <0.001 | 1.92 (1.65–2.19) | <0.001 | 3.34 (2.9–3.78) | <0.001 |
| Lag 1 | 1.77 (1.47–2.07) | <0.001 | 1.82 (1.51–2.13) | <0.001 | 3.11 (2.54–3.68) | <0.001 | 1.59 (1.31–1.87) | <0.001 | 2.27 (1.82–2.73) | <0.001 | |
| Lag 2 | 0.9 (0.61–1.19) | 0.002 | 0.99 (0.68–1.29) | 0.001 | 0.91 (0.36–1.46) | 0.096 | 0.84 (0.57–1.12) | 0.002 | 1.59 (1.14–2.03) | <0.001 | |
| Lag 3 | 0.16 (-0.13–0.44) | 0.579 | 0.41 (0.12–0.71) | 0.160 | −0.74 (−1.26-−0.22) | 0.159 | 0.3 (0.03–0.56) | 0.26 | 1.15 (0.72–1.59) | 0.008 | |
| PM10 | Lag 0 | 1.97 (1.76–2.18) | <0.001 | 1.97 (1.75–2.19) | <0.001 | 3.74 (3.34–4.14) | <0.001 | 1.66 (1.47–1.86) | <0.001 | 2.55 (2.24–2.87) | <0.001 |
| Lag 1 | 1.6 (1.38–1.81) | <0.001 | 1.71 (1.48–1.93) | <0.001 | 2.81 (2.4–3.23) | <0.001 | 1.46 (1.26–1.66) | <0.001 | 1.9 (1.57–2.23) | <0.001 | |
| Lag 2 | 0.94 (0.73–1.15) | <0.001 | 1.01 (0.79–1.23) | <0.001 | 1.16 (0.77–1.56) | 0.003 | 0.88 (0.69–1.08) | <0.001 | 1.27 (0.95–1.59) | <0.001 | |
| Lag 3 | 0.39 (0.18–0.6) | 0.059 | 0.65 (0.43–0.86) | 0.002 | −0.27 (-0.64–0.12) | 0.485 | 0.53 (0.33–0.72) | 0.006 | 0.99 (0.68–1.3) | 0.002 | |
PC, percentage change.
Table 7.
Stratification analysis of the percent change of eczema risk associated with 10 mg/m3 increase of PM2.5 and PM10 under two-pollutant model.
| Pollutant (per 10 mg/m3) | Model type | Male | Female | <12 | 12–64 | ≥65 |
|---|---|---|---|---|---|---|
|
PM10* |
PM2.5 + PM10 | 1.24 (1.06–1.41) | 1.34 (1.17–1.52) | 1.86 (1.54–2.18) | 1.13 (0.97–1.3) | 1.81 (1.55–2.07) |
| PM2.5 + NO2 | 1.96 (1.73–2.19) | 2.04 (1.81–2.28) | 2.65 (2.22–3.08) | 1.79 (1.58–2.01) | 2.67 (2.32–3.02) | |
| PM2.5 + SO2 | 2.46 (2.12–2.79) | 2.6 (2.25–2.94) | 3.71 (3.09–4.34) | 2.22 (1.91–2.54) | 3.65 (3.15–4.16) | |
| PM2.5 + O3 | 1.41 (1.22–1.59) | 1.52 (1.33–1.71) | 2.55 (2.2–2.89) | 1.26 (1.09–1.43) | 2.02 (1.74–2.3) | |
| PM2.5 + NO2 | 1.69 (1.51–1.88) | 1.8 (1.61–1.99) | 2.35 (2–2.7) | 1.57 (1.4–1.74) | 2.19 (1.91–2.47) | |
| PM2.5 + SO2 | 2.25 (2–2.51) | 2.42 (2.15–2.68) | 3.38 (2.9–3.87) | 2.07 (1.84–2.31) | 3.03 (2.64–3.41) | |
| PM2.5 + O3 | 1.34 (1.19–1.5) | 1.46 (1.3–1.62) | 2.33 (2.03–2.62) | 1.22 (1.07–1.37) | 1.82 (1.58–2.06) |
*P < 0.001.
Sensitivity analyses
The obtained results after sensitivity analyses done in this study showed that the association estimates of PM2.5 and PM10 were not materially influenced by changing the df for the smooth function of the time trend indicating the credibility of our results in Table 8.
Table 8.
The RR and percent change of eczema risk associated with 10 mg/m3 increase of PM2.5 and PM10 with different degrees of freedom.
| Temperature, DF | Humidity, DF | Time, DF (/year) | PM 2.5 | PM 10 | ||
|---|---|---|---|---|---|---|
| RR * | PC (%) * | RR * | PC (%) * | |||
| 2 | 2 | 9 | 1.026 (1.0222–1.0298) | 2.6 (2.22–2.98) | 1.0238 (1.0211–1.0265) | 2.38 (2.11–2.65) |
| 2 | 2 | 10 | 1.0257 (1.0218–1.0295) | 2.57 (2.18–2.95) | 1.0238 (1.0211–1.0266) | 2.38 (2.11–2.66) |
| 2 | 2 | 11 | 1.0246 (1.0207–1.0285) | 2.46 (2.07–2.85) | 1.0234 (1.0206–1.0262) | 2.34 (2.06–2.62) |
| 2 | 3 | 9 | 1.026 (1.0222–1.0298) | 2.6 (2.22–2.98) | 1.0238 (1.0211–1.0265) | 2.38 (2.11–2.65) |
| 2 | 3 | 10 | 1.0257 (1.0218–1.0295) | 2.57 (2.18–2.95) | 1.0238 (1.021–1.0265) | 2.38 (2.1–2.65) |
| 2 | 3 | 11 | 1.0246 (1.0207–1.0285) | 2.46 (2.07–2.85) | 1.0234 (1.0206–1.0262) | 2.34 (2.06–2.62) |
| 2 | 4 | 9 | 1.026 (1.0222–1.0299) | 2.6 (2.22–2.99) | 1.0238 (1.0211–1.0265) | 2.38 (2.11–2.65) |
| 2 | 4 | 10 | 1.0257 (1.0218–1.0295) | 2.57 (2.18–2.95) | 1.0238 (1.0211–1.0265) | 2.38 (2.11–2.65) |
| 2 | 4 | 11 | 1.0246 (1.0207–1.0285) | 2.46 (2.07–2.85) | 1.0233 (1.0205–1.0262) | 2.33 (2.05–2.62) |
| 3 | 2 | 9 | 1.0262 (1.0224–1.0301) | 2.62 (2.24–3.01) | 1.024 (1.0213–1.0267) | 2.4 (2.13–2.67) |
| 3 | 2 | 10 | 1.0259 (1.0221–1.0298) | 2.59 (2.21–2.98) | 1.0241 (1.0213–1.0268) | 2.41 (2.13–2.68) |
| 3 | 2 | 11 | 1.0249 (1.021–1.0288) | 2.49 (2.1–2.88) | 1.0237 (1.0209–1.0265) | 2.37 (2.09–2.65) |
| 3 | 3 | 9 | 1.0262 (1.0224–1.0301) | 2.62 (2.24–3.01) | 1.024 (1.0213–1.0267) | 2.4 (2.13–2.67) |
| 3 | 3 | 10 | 1.0259 (1.022–1.0298) | 2.59 (2.2–2.98) | 1.0241 (1.0213–1.0268) | 2.41 (2.13–2.68) |
| 3 | 3 | 11 | 1.0248 (1.0209–1.0288) | 2.48 (2.09–2.88) | 1.0236 (1.0208–1.0264) | 2.36 (2.08–2.64) |
| 3 | 4 | 9 | 1.0262 (1.0224–1.0301) | 2.62 (2.24–3.01) | 1.024 (1.0213–1.0267) | 2.4 (2.13–2.67) |
| 3 | 4 | 10 | 1.0259 (1.022–1.0298) | 2.59 (2.2–2.98) | 1.0241 (1.0213–1.0268) | 2.41 (2.13–2.68) |
| 3 | 4 | 11 | 1.0248 (1.0209–1.0288) | 2.48 (2.09–2.88) | 1.0236 (1.0208–1.0264) | 2.36 (2.08–2.64) |
| 4 | 2 | 9 | 1.0264 (1.0226–1.0303) | 2.64 (2.26–3.03) | 1.0242 (1.0214–1.0269) | 2.42 (2.14–2.69) |
| 4 | 2 | 10 | 1.0264 (1.0225–1.0303) | 2.64 (2.25–3.03) | 1.0244 (1.0217–1.0272) | 2.44 (2.17–2.72) |
| 4 | 2 | 11 | 1.0254 (1.0214–1.0293) | 2.54 (2.14–2.93) | 1.024 (1.0212–1.0268) | 2.4 (2.12–2.68) |
| 4 | 3 | 9 | 1.0264 (1.0225–1.0302) | 2.64 (2.25–3.02) | 1.0242 (1.0214–1.0269) | 2.42 (2.14–2.69) |
| 4 | 3 | 10 | 1.0263 (1.0225–1.0302) | 2.63 (2.25–3.02) | 1.0244 (1.0216–1.0272) | 2.44 (2.16–2.72) |
| 4 | 3 | 11 | 1.0253 (1.0214–1.0293) | 2.53 (2.14–2.93) | 1.024 (1.0211–1.0268) | 2.4 (2.11–2.68) |
| 4 | 4 | 9 | 1.0264 (1.0225–1.0302) | 2.64 (2.25–3.02) | 1.0242 (1.0214–1.0269) | 2.42 (2.14–2.69) |
| 4 | 4 | 10 | 1.0263 (1.0225–1.0302) | 2.63 (2.25–3.02) | 1.0244 (1.0216–1.0272) | 2.44 (2.16–2.72) |
| 4 | 4 | 11 | 1.0253 (1.0214–1.0293) | 2.53 (2.14–2.93) | 1.024 (1.0211–1.0268) | 2.4 (2.11–2.68) |
P < 0.001.
RR, risk ratio; PC, percentage change; DF, degrees of freedom.
Discussion
This study investigated the correlations between meteorological and environmental factors with the number of eczema outpatient visits in Guangzhou, a central city of south China located in the flourishing Pearl River Delta area. In total, 293,343 cases of eczema were analyzed using PM2.5 and PM10. The number of outpatient visits has been widely used in environmental epidemiology studies to estimate the influence of air pollutants on eczema. The confounders including seasonal changes and long-term trends can be modified using the distributed lag non-linear model (24–26). The obtained results suggested that the increasing concentrations of air pollutants were significantly associated with the rising number of eczema cases. The combined results of PM with other pollutants did not change significantly with PM alone. To date, no more research has revealed the effects of different combinations of pollutants on eczema. The effects of single pollutants on eczema still have different results in different regions, such as previous studies have revealed that O3 and NO2 were not associated with increased patient visits for AD but our team has revealed that NO2 exposure increases eczema outpatient (10, 27). The effect of pollutants on eczema is still inconclusive, thus, the effect of combined pollutants has not been concluded. Generally, the identification of vulnerable groups is of great significance for public health prevention. The existing evidence has limitations in revealing whether the associations of air pollution on eczema differ in population subgroups. Therefore, we stratified the eczema cases into three groups including <12, ≥12 and <65, and ≥65 years old. The obtained results indicated that children (<12) and the elderly (≥65) were more vulnerable to the adverse effects of short-term exposure to PM. The skin, which is the largest organ in the human body, contains four different barrier components including physical, chemical, microbial, and immunological barriers (28). The physical barrier, which mainly consists of the stratum corneum, can exert the protection function against the penetration of pathogens, allergens, and several other exogenous air pollutants such as PM, NO2, and SO2 (29, 30). A healthy skin barrier plays an essential role in preventing damage caused by stimuli including air pollutants. The vulnerable groups can easily suffer from eczema after short-term exposure to PM due to the immature and aging skin barrier in children and the elderly, respectively. Undeveloped sebaceous glands, skin infections, and local immune dysregulation are the main reasons for epidermal barrier abnormalities in children (31–33). On the other hand, epidermal dysfunction, compromised permeability homeostasis, reduced stratum corneum hydration, and elevated skin surface pH are the aggressive factors for the elderly (33, 34). Therefore, this study suggests that children and the elderly should avoid staying outdoors frequently to prevent PM exposure, which will potentially decrease the risk of eczema.
Previous studies conducted in Changsha, Shanghai, and Chengdu, representative cities with high urbanization in China, have reported that multiple air pollutants in both cities had the potential of increasing the incidence and prevalence of eczema (35–38). In Changsha, early childhood eczema is associated with exposure to air pollutants during both the preconceptional and perinatal periods (37, 38). In Chengdu, western China, the combined effect of NO2, SO2, and PM10 showed that the percentage change in daily outpatient visits for eczema increased (36). The Beijing study reported a significant positive correlation between the two pollutant models, but the impact was lower than the single pollutant model (39). The conclusions from both studies are consistent with the results obtained in this study. In addition, Dong et al. suggested that raising public awareness is crucial for constantly improving air quality. This is because PM is not only associated with cardiovascular and respiratory issues but also has a positive connection with the occurrence of skin diseases in China (40). Although a decreasing tendency in the levels of PM concentrations was observed from 2013 in the main regions in China including the Beijing-Tianjin-Hubei region, Yangtze River Delta, and Pearl River Delta, the recorded concentration levels still exceeded the limits recommended by the World Health Organization and Chinese authorities (41). With the acceleration of economic and industrial development, air pollution will most likely be an inevitable issue, particularly in developing countries. Although the increased risk we found is very small, the results of studies in some cities in China have found that the increase in PM will make AD more prone to the onset. If the concentration of PM can be reduced and the awareness of protection can be improved and promoted, it can prevent a large number of patients from relapse and shorten their sick time which will reduce a lot of health expenditure and burden.
However, the mechanisms of how PM induces and aggravates eczema in humans have not yet been elucidated. It is likely that PM induces the disruption of the epidermis by modulating the structural proteins including the small proline-rich (SPRR) family, occludens-1 (ZO-1), keratins, filaggrin, and claudin-1 (42–48), thereby resulting in both increased epithelial and endothelial barrier permeability (49). Moreover, a previous study reported that the penetration of PM was observed in both intact and barrier-disrupted skin in vivo, resulting in inflammatory responses (50). Current scientific opinions on the down-stream mechanisms through which air pollutants affect skin health mainly include: (1) elevation of oxidative stress via exogenous and endogenous reactive oxygen species (ROS) generation, which is a series of highly reactive chemical substances. Numerous pieces of evidence have suggested that PM can induce direct and indirect ROS formation, resulting in lipid, protein, and DNA damage (14, 51, 52); (2) activation of the aryl hydrocarbon receptor (AhR), which further limits cell proliferation and implicates in skin senescence (53); (3) activation of the inflammatory cascade in the skin and impairing of the immunological barrier (42, 46, 54); (4) activation of toll-like receptors, NF-κB, and MAPK signaling pathways (55–59); and (5) induction of apoptosis and autophagy (60, 61). In addition, SO2 can enhance the production of active oxygen and further reduce the content of antioxidants in the skin (62). Moreover, our previous study revealed that outpatient visits for patients with eczema were positively associated with short-term exposure to ambient NO2 (10). NO2 will damage the skin integrity which conducts a higher risk of exposure to allergic substances (63). Currently, the clinical management strategies for eczema mainly include the administration of moisturizers, topical corticosteroids, and calcineurin inhibitors, representing an anti-allergen and anti-inflammation strategy. In addition, to protect the skin, respiratory exposure should be noticed because respiratory exposure induces a higher risk of food allergy which will increase higher risk of eczema in children (64). Since the skin and reparatory is the most important defense barrier against environmental contaminants, future research should elucidate the clear mechanisms of how air pollutants aggravate eczema in humans with the overarching goal of providing recommendations for improving skin protection against air pollution.
To the best of our knowledge, this is the first study that has uncovered the associations and lagged effects between exposure to environmental factors and the number of eczema outpatients in Guangzhou, southern China. However, the study has several potential limitations. First, the study used data on the number of eczema outpatient visits to estimate the association of exposure to PM rather than using authentic physiological records or measurements. In addition, the clinical cases were only selected from one hospital. Although the hospital selection bias might be inevitable, the Dermatology Hospital of Southern Medical University is one of the largest and most representative dermatological departments among the local hospitals. Therefore, it can be used to reflect the trend of eczema outpatient visits from the whole region. Second, the data on the exposure of individuals to the environmental factors and the residual confounding factors (e.g., smoking, alcoholic consumption, dietary habits, and physical activities) were unavailable. Smoking, drinking, lack of exercise, etc. will lead to the recurrence of AD and prolonged attack time. Therefore, the lack of these data will make the data biased. Future research needs to collect more detailed personal data to avoid biased results. Furthermore, the outdoor average concentrations of PM2.5, PM10, NO2, SO2, and O3 were recorded as the average obtained from the different fixed monitoring stations, which may raise errors in documented measurements. Third, the study does not include detailed information on the severity of eczema and the differentiation of initial onset or relapsing cases, which may lead to bias while interpreting the results. The inaccurate grouping may result in biased results due to the severity and time of onset of the patient. Therefore, in the follow-up study, we will further take into account detailed information such as the age of onset of eczema, the time of onset, and the severity of the patient to further analyze from more dimensions. However, our study still offers considerable evidence on the relationship between environmental factors and the prevalence of eczema, thereby offering further motivations to investigate the mechanistic linkages between air pollutants and eczema, as well as proposing highlights for promoting the environmentally friendly actions which will lead to the reduction of air pollution for a better skin condition.
Conclusion
This study has contributed to the limited scientific evidence which suggests that PM2.5 and PM10 may induce and aggravate eczema, especially among children and the elderly. The relationship between air quality trends and hospital resource arrangement should be paid attention to by hospital managers which may aid in disease prevention and lower the health burden.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Dermatology Hospital, Southern Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
SS and JC designed the study. JZ, LF, and DJ analyzed the data. YY drafted the manuscript. BS and YC contributed to the data collection. All authors critically reviewed and approved the manuscript.
Funding Statement
This project was supported by the Natural Science Foundation of Guangdong Province (grant number 2018030310559), the Guangdong Province Medical Science and Technology Research Foundation (grant numbers C2020061 and C2020063), and Department of Science and Technology of Guangdong Province (grant number 2020A1414010213).
Abbreviations
PM, particulate matter; GAM, generalized additive model; ICD-10, International Classification of Diseases, the 10th version; DOW, day of week; Q-AIC, Akaike information criterion for quasi-Poisson; SPRR, small proline-rich; ROS, reactive oxygen species; AhR, aryl hydrocarbon receptor.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Deckers I, McLean S, Linssen S, Mommers M, van Schayck C, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PLoS ONE. (2012) 7:e39803. 10.1371/journal.pone.0039803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. (2015) 2015:8–16. 10.1159/000370220 [DOI] [PubMed] [Google Scholar]
- 3.Karimkhani C, Dellavalle R, Coffeng L, Flohr C, Hay R, Langan S, et al. Global skin disease morbidity and mortality: an update from the global burden of disease study 2013. JAMA Dermatol. (2017) 153:406–12. 10.1001/jamadermatol.2016.5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrendt H, Alessandrini F, Buters J, Krämer U, Koren H, Ring J. Environmental pollution and allergy: historical aspects. Chem Immunol Allergy. (2014) 100:268–77. 10.1159/000359918 [DOI] [PubMed] [Google Scholar]
- 5.Chong M, Fonacier L. Treatment of eczema: corticosteroids and beyond. Clin Rev Allergy Immunol. (2016) 51:249–62. 10.1007/s12016-015-8486-7 [DOI] [PubMed] [Google Scholar]
- 6.Blome C, Radtke M, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis: disease burden, measurement, and treatment benefit. Am J Clin Dermatol. (2016) 17:163–9. 10.1007/s40257-015-0171-3 [DOI] [PubMed] [Google Scholar]
- 7.Chang W, Lee C, Hirota T, Wang L, Doi S, Miyatake A, et al. ORAI1 genetic polymorphisms associated with the susceptibility of atopic dermatitis in Japanese and Taiwanese populations. PLoS ONE. (2012) 7:e29387. 10.1371/journal.pone.0029387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apfelbacher C, Diepgen T, Schmitt J. Determinants of eczema: population-based cross-sectional study in Germany. Allergy. (2011) 66:206–13. 10.1111/j.1398-9995.2010.02464.x [DOI] [PubMed] [Google Scholar]
- 9.Nikolopoulou M, Kleissl J, Linden P, Lykoudis S. Pedestrians' perception of environmental stimuli through field surveys: focus on particulate pollution. Sci Total Environ. (2011) 409:2493–502. 10.1016/j.scitotenv.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Jing D, Lu Q, Shen S. NO exposure increases eczema outpatient visits in Guangzhou, China: an indication for hospital management. BMC Public Health. (2021) 21:506. 10.1186/s12889-021-10549-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantor R, Silverberg J. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. (2017) 13:15–26. 10.1080/1744666X.2016.1212660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabashima K, Otsuka A, Nomura T. Linking air pollution to atopic dermatitis. Nat Immunol. (2016) 18:5–6. 10.1038/ni.3615 [DOI] [PubMed] [Google Scholar]
- 13.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. (2008) 151:362–7. 10.1016/j.envpol.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 14.Dijkhoff I, Drasler B, Karakocak B, Petri-Fink A, Valacchi G, Eeman M, et al. Impact of airborne particulate matter on skin: a systematic review from epidemiology to in vitro studies. Part Fibre Toxicol. (2020) 17:35. 10.1186/s12989-020-00366-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsten C, Melén E. Air pollution, genetics, and allergy: an update. Curr Opin Allergy Clin Immunol. (2012) 12:455–60. 10.1097/ACI.0b013e328357cc55 [DOI] [PubMed] [Google Scholar]
- 16.Oh I, Lee J, Ahn K, Kim J, Kim Y, Sun Sim C, et al. Association between particulate matter concentration and symptoms of atopic dermatitis in children living in an industrial urban area of South Korea. Environ Res. (2018) 160:462–8. 10.1016/j.envres.2017.10.030 [DOI] [PubMed] [Google Scholar]
- 17.Ngoc L, Park D, Lee Y, Lee Y. Systematic review and meta-analysis of human skin diseases due to particulate matter. Int J Environ Res Public Health. (2017) 14:1458. 10.3390/ijerph14121458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo P, Wang Y, Feng W, Wu J, Fu C, Deng H, et al. Ambient air pollution and risk for ischemic stroke: a short-term exposure assessment in south China. Int J Environ Res Public Health. (2017) 14:1091. 10.3390/ijerph14091091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravindra K, Rattan P, Mor S, Aggarwal A. Generalized additive models: Building evidence of air pollution, climate change and human health. Environ Int. (2019) 132:104987. 10.1016/j.envint.2019.104987 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz J. Harvesting and long term exposure effects in the relation between air pollution and mortality. Am J Epidemiol. (2000) 151:440–8. 10.1093/oxfordjournals.aje.a010228 [DOI] [PubMed] [Google Scholar]
- 21.Shen S, Li X, Yuan C, Huang Q, Liu D, Ma S, et al. Association of short-term exposure to sulfur dioxide and hospitalization for ischemic and hemorrhagic stroke in Guangzhou, China. BMC Public Health. (2020) 20:263. 10.1186/s12889-020-8354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou M, Wang L, Liu T, Zhang Y, Lin H, Luo Y, et al. Health impact of the 2008 cold spell on mortality in subtropical China: the climate and health impact national assessment study (CHINAs). Environ Health. (2014) 13:60. 10.1186/1476-069X-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin P, Chen R, Wang L, Liu C, Niu Y, Wang W, et al. The added effects of heatwaves on cause-specific mortality: a nationwide analysis in 272 Chinese cities. Environ Int. (2018) 121:898–905. 10.1016/j.envint.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 24.Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol. (2013) 42:1187–95. 10.1093/ije/dyt092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhadwal G, Albrecht L, Gniadecki R, Poulin Y, Yeung J, Hong C, et al. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. Section IV: treatment options for the management of atopic dermatitis. J Cutan Med Surg. (2018) 22:21S–9S. 10.1177/1203475418805721 [DOI] [PubMed] [Google Scholar]
- 26.Chow S, Seow C, Dizon M, Godse K, Foong H, Chan V, et al. A clinician's reference guide for the management of atopic dermatitis in Asians. Asia Pac Allergy. (2018) 8:e41. 10.5415/apallergy.2018.8.e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park T, Park S, Cho M, Kim S. Associations of particulate matter with atopic dermatitis and chronic inflammatory skin diseases in South Korea. Clin Exp Dermatol. (2022) 47:325–34. 10.1111/ced.14910 [DOI] [PubMed] [Google Scholar]
- 28.Grice E, Segre J. The skin microbiome. Nat Rev Microbiol. (2011) 9:244–53. 10.1038/nrmicro2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheuplein R, Blank I. Permeability of the skin. Physiol Rev. (1971) 51:702–47. 10.1152/physrev.1971.51.4.702 [DOI] [PubMed] [Google Scholar]
- 30.Madison K. Barrier function of the skin: “la raison d'être” of the epidermis. J Invest Dermatol. (2003) 121:231–41. 10.1046/j.1523-1747.2003.12359.x [DOI] [PubMed] [Google Scholar]
- 31.Gupta J, Grube E, Ericksen M, Stevenson M, Lucky A, Sheth A, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. (2008) 121:725–30.e2. 10.1016/j.jaci.2007.12.1161 [DOI] [PubMed] [Google Scholar]
- 32.Cork M, Danby S, Vasilopoulos Y, Hadgraft J, Lane M, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. (2009) 129:1892–908. 10.1038/jid.2009.133 [DOI] [PubMed] [Google Scholar]
- 33.Ramos-e-Silva M, Boza J, Cestari T. Effects of age (neonates and elderly) on skin barrier function. Clin Dermatol. (2012) 30:274–6. 10.1016/j.clindermatol.2011.08.024 [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Man M, Li T, Elias P, Mauro T. Aging-associated alterations in epidermal function and their clinical significance. Aging. (2020) 12:5551–65. 10.18632/aging.102946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, Yang Y, Chen R, Kan H, Song W, Tan J, et al. Ambient air pollution, meteorological factors and outpatient visits for eczema in Shanghai, China: a time-series analysis. Int J Environ Res Public Health. (2016) 13:e1106. 10.3390/ijerph13111106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li A, Fan L, Xie L, Ren Y, Li L. Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south-western China: a time series analysis. J Eur Acad Dermatol Venereol. (2018) 32:486–94. 10.1111/jdv.14730 [DOI] [PubMed] [Google Scholar]
- 37.Deng Q, Lu C, Li Y, Sundell J, Norbäck D. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. (2016) 150:119–27. 10.1016/j.envres.2016.05.050 [DOI] [PubMed] [Google Scholar]
- 38.Lu C, Deng L, Ou C, Yuan H, Chen X, Deng Q. Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children. J Dermatol Sci. (2017) 85:85–95. 10.1016/j.jdermsci.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 39.Guo Q, Liang F, Tian L, Schikowski T, Liu W, Pan X. Ambient air pollution and the hospital outpatient visits for eczema and dermatitis in Beijing: a time-stratified case-crossover analysis. Environ Sci Process Impacts. (2019) 21:163–73. 10.1039/C8EM00494C [DOI] [PubMed] [Google Scholar]
- 40.Dong Y, Liao L, Li L, Yi F, Meng H, He Y, et al. Skin inflammation induced by ambient particulate matter in China. Sci Total Environ. (2019) 682:364–73. 10.1016/j.scitotenv.2019.05.155 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Wang J, Hart JE, Laden F, Zhao C, Li T, et al. National scale spatiotemporal land-use regression model for PM2.5, PM10 and NO2 concentration in China. Atmos Environ. (2018) 192:48–54. 10.1016/j.atmosenv.2018.08.046 [DOI] [Google Scholar]
- 42.Kim H, Bae I, Son E, Park J, Cha N, Na H, et al. Transcriptome analysis of airborne PM-induced detrimental effects on human keratinocytes. Toxicol Lett. (2017) 273:26–35. 10.1016/j.toxlet.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 43.Bae J, Choi H, Shin D, Na H, Park N, Kim J, et al. Fine particulate matter (PM2.5) inhibits ciliogenesis by increasing SPRR3 expression via c-Jun activation in RPE cells and skin keratinocytes. Sci Rep. (2019) 9:3994. 10.1038/s41598-019-40670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehmann A, Blank F, Baum O, Gehr P, Rothen-Rutishauser B. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part Fibre Toxicol. (2009) 6:26. 10.1186/1743-8977-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan T, Wang P, Aljuffali I, Huang C, Lee C, Fang J. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J Dermatol Sci. (2015) 78:51–60. 10.1016/j.jdermsci.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 46.Jin S, Li Z, Choi E, Lee S, Kim Y, Seo E, et al. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J Dermatol Sci. (2018). 10.1016/j.jdermsci.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 47.Lee C, Lin Z, Hu S, Chiang Y, Hsu L, Lin Y, et al. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci Rep. (2016) 6:27995. 10.1038/srep27995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao R, Guo Z, Zhang R, Deng C, Xu J, Dong W, et al. Nasal epithelial barrier disruption by particulate matter ≤ 2.5 μm via tight junction protein degradation. J Appl Toxicol. (2018) 38:678–87. 10.1002/jat.3573 [DOI] [PubMed] [Google Scholar]
- 49.Wang T, Wang L, Moreno-Vinasco L, Lang G, Siegler J, Mathew B, et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol. (2012) 9:35. 10.1186/1743-8977-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali M, Sayeski P, Safavi A, Lyles M, Bernstein K. Janus kinase 2 (Jak2) must be catalytically active to associate with the AT1 receptor in response to angiotensin II. Biochem Biophys Res Commun. (1998) 249:672–7. 10.1006/bbrc.1998.9054 [DOI] [PubMed] [Google Scholar]
- 51.Øvrevik J, Refsnes M, Låg M, Holme J, Schwarze P. Activation of Proinflammatory Responses in cells of the airway mucosa by particulate matter: oxidant- and non-oxidant-mediated triggering mechanisms. Biomolecules. (2015) 5:1399–440. 10.3390/biom5031399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang T, Lakey P, Weber R, Shiraiwa M. Oxidative potential of particulate matter and generation of reactive oxygen species in epithelial lining fluid. Environ Sci Technol. (2019) 53:12784–92. 10.1021/acs.est.9b03823 [DOI] [PubMed] [Google Scholar]
- 53.Ryu Y, Kang K, Piao M, Ahn M, Yi J, Bossis G, et al. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp Mol Med. (2019) 51:1–14. 10.1038/s12276-019-0305-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ushio H, Nohara K, Fujimaki H. Effect of environmental pollutants on the production of pro-inflammatory cytokines by normal human dermal keratinocytes. Toxicol Lett. (1999) 105:17–24. 10.1016/S0378-4274(98)00379-8 [DOI] [PubMed] [Google Scholar]
- 55.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. (2006) 117:979–87; quiz 88. 10.1016/j.jaci.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 56.Lebre M, van der Aar A, van Baarsen L, van Capel T, Schuitemaker J, Kapsenberg M, et al. Human keratinocytes express functional toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. (2007) 127:331–41. 10.1038/sj.jid.5700530 [DOI] [PubMed] [Google Scholar]
- 57.Liu T, Zhang L, Joo D, Sun S. NF-κB signaling in inflammation. Signal Transd Targeted Therapy. (2017) 2:e23. 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith W, Garavito R, DeWitt D. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and−2. J Biol Chem. (1996) 271:33157–60. 10.1074/jbc.271.52.33157 [DOI] [PubMed] [Google Scholar]
- 59.Kawai T, Akira S, TLR. signaling. Cell Death Differ. (2006) 13:816–25. 10.1038/sj.cdd.4401850 [DOI] [PubMed] [Google Scholar]
- 60.Zhen A, Piao M, Hyun Y, Kang K, Madushan Fernando P, Cho S, et al. Diphlorethohydroxycarmalol attenuates fine particulate matter-induced subcellular skin dysfunction. Marine Drugs. (2019) 17:95. 10.3390/md17020095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piao M, Kang K, Zhen A, Fernando P, Ahn M, Koh Y, et al. Particulate matter 2.5 mediates cutaneous cellular injury by inducing mitochondria-associated endoplasmic reticulum stress: protective effects of ginsenoside Rb1. Antioxidants. (2019) 8:e383. 10.3390/antiox8090383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mancebo S, Wang S. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol. (2015) 29:2326–32. 10.1111/jdv.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schikowski T, Krutmann J. Air pollution (particulate matter and nitrogen dioxide) and skin aging. Hautarzt. (2019) 70:158–62. 10.1007/s00105-018-4338-8 [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Lu C, Li Y, Norbäck D, Murthy P, Sram R, et al. Early-life exposure to air pollution associated with food allergy in children: Implications for 'one allergy' concept. Environ Res. (2023) 216:114713. 10.1016/j.envres.2022.114713 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.