Introduction
Angioimmunoblastic T-cell lymphoma (AITL) is an aggressive peripheral T-cell lymphoma. Clinically, AITL presents in older individuals with B-symptoms, such as drenching night sweats or fever, and generalized lymphadenopathy.1 Skin rash occurs in close to 50% of cases.2 We report a case of AITL presenting as generalized pruritus with lymphadenopathy developing in the setting of dupilumab therapy.
Case
A 56-year-old man with a history of asthma, diabetes, and hypertension presented in January 2022 for generalized pruritus. The pruritus had been ongoing for months and the patient described affected areas as “all over the body”. Patient denied a history of atopic dermatitis. Patient also denied any recent travel and the use of new soaps, laundry detergents, or other topical products nor had the patient started any new oral medication in the past 6 months. Physical examination demonstrated non-specific macular erythema and excoriations on the torso and all 4 extremities (Fig 1). Patient was prescribed cetirizine 20 mg twice daily and triamcinolone 0.1% cream to be applied twice daily for 2 to 3 weeks. Laboratory work-up to assess for a systemic etiology was ordered including a basic metabolic panel, liver function tests, complete blood count with differential, and thyroid stimulating hormone. Laboratory results were unremarkable. The patient was instructed to complete age-appropriate cancer screenings and had a normal prostate-specific antigen and a negative fecal occult blood test.
Fig 1.
Cutaneous manifestations of angioimmunoblastic T-cell lymphoma. At presentation, patient with generalized pruritis and a non-specific macular erythema and excoriations on the torso.
In February 2022, the patient reported no improvement and returned for further management. A skin biopsy was performed and showed mild spongiotic dermatitis (Fig 2). Additional work-up included a frontal and lateral chest x-ray which showed no abnormalities. The patient was prescribed prednisone starting at 0.75 mg/kg with instructions to taper off over 3 weeks.
Fig 2.
Skin punch biopsy of the upper back. Histology showed mild spongiotic dermatitis. 20× (A) 40× (B).
The patient followed up after completing treatment with prednisone and reported transient improvement with recurrent, severe pruritus noted upon discontinuation of prednisone. Given the patient’s severe symptoms, lack of response to topical and systemic steroids and pathology results demonstrating spongiotic dermatitis a decision was made to initiate treatment with dupilumab. The patient received a 600 mg loading dose of dupilumab in March 2022 followed by 300 mg subcutaneous injections every other week thereafter. In April 2022, 1 month after starting dupilumab, the patient reported “lumps in the neck” and new onset of night sweats. Physical examination revealed tender left cervical lymphadenopathy and a computed tomography scan of the neck demonstrated innumerable lymph nodes bilaterally many of which were enlarged. The largest lymph node in the left submandibular region measured 2.4 cm × 1.5 cm transaxially. At this time, the patient also reported 30 pounds of weight loss over 4 months which was initially attributed to lifestyle changes. The patient was instructed to stop dupilumab and, in May 2022, the patient underwent an excisional biopsy of an enlarged lymph node leading to a diagnosis of AITL. A subsequent bone marrow biopsy showed multiple atypical lymphohistiocytic aggregates supporting a diagnosis of AITL. The malignant cells were cluster of differentiation (CD)3+, CD4+, and negative for CD8, B cell markers, and CD30 (Figs 3 and 4). The patient is currently managed by medical oncology on a chemotherapy regimen of cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone with plan for future stem cell transplant.
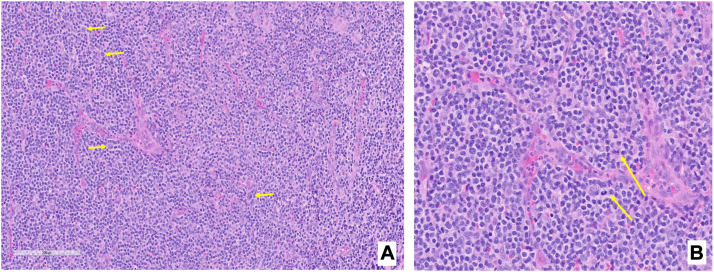
Fig 3.
Bone marrow biopsy. A, Hematoxylin and eosin stain showing arborizing blood vessel surrounded by medium sized atypical lymphocytes with clear cytoplasm and irregular nuclear contours; occasional larger cells also present (yellow arrows). B, H&E stain at higher magnification showing arborizing blood vessel surrounded by atypical lymphocytes with irregular nuclear contours and atypical mitotic figure (yellow arrows).
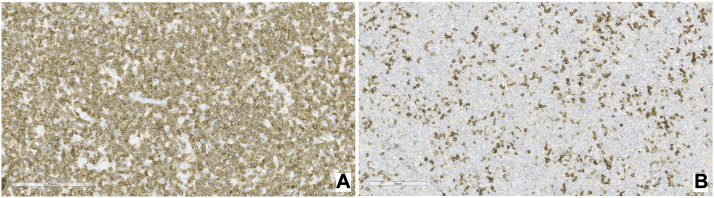
Fig 4.
Bone marrow biopsy immunohistochemistry. A, CD3 stains. B, Programmed Death Receptor-1 stains.
Discussion
AITL is an aggressive subtype of peripheral T-cell lymphoma consisting of a monoclonal proliferation of CD3+ and CD4+ follicular helper T cells. It accounts for almost 2% of all non-Hodgkin’s lymphomas. AITL most commonly occurs in the sixth or seventh decade of life and presents clinically with B-symptoms, such as fever, drenching night sweats or unintentional weight loss, generalized lymphadenopathy, hepatosplenomegaly, and pruritus. A non-specific morbilliform or exanthematous eruption develops in approximately half of all patients with AITL.1-3 At the time of presentation, our patient reported no systemic symptoms and had no palpable lymphadenopathy. In patients with AITL, anemia and hypergammaglobulinemia are commonly reported lab abnormalities.3 Our patient, however, had a normal hemoglobin and was informed that if he did not improve with dupilumab that additional laboratory work-up, including serum protein electrophoresis and peripheral blood flow cytometry, would be warranted.
This case is unique because the patient developed clinically evident lymphadenopathy after initiation of dupilumab, which appeared to unmask the patient’s AITL. Dupilumab is an food and drug administration-approved treatment for moderate to severe atopic dermatitis. It is an IgG4 monoclonal antibody that targets the IL-4 receptor α chain, thereby blocking the signal of IL-4 and IL-13, type 2 cytokines that play a role in pathogenesis of atopic dermatitis. Cases of cutaneous T-cell lymphoma (CTCL) have been well documented to occur following dupilumab initiation.4 CTCL cases that are unmasked by dupilumab are diagnosed on average 8 months following initiation of therapy with dupilumab. Most patients presenting with CTCL triggered by dupilumab are diagnosed with more advanced disease and it is thought that dupilumab causes rapid worsening in CTCL via immune dysregulation with a Th2-dominant immune profile.5 To our knowledge, AITL occurring in the setting of dupilumab therapy has not been previously reported. Notably, AITL is a significantly more aggressive disease compared to CTCL. The overall 5-year survival rate of AITL is 33% whereas 5-year survival for mycosis fungoides is 88%.1,5
Although patients with AITL may present with a rash, skin biopsy is often of limited utility for establishing a diagnosis of AITL. Diagnosis depends on histologic examination of lymph nodes from an excisional biopsy which highlights the importance of a complete lymph node exam in all patients presenting with generalized pruritus of unknown etiology.1 If patients have an inconclusive exam, computed tomography scan can be considered as part of a full systemic investigation prior to starting a medication like dupilumab. Initial skin biopsies of patients with CTCL in the setting of dupilumab often resemble inflammatory skin diseases due to a predominance of reactive lymphocytes and relative lack of cytologically atypical lymphocytes leading to a delay in diagnosis.1 Therefore, worsening atopic dermatitis despite initiation of dupilumab should raise concerns for a broader differential that includes AITL as well as CTCL and should prompt additional systemic investigations.
Conflicts of interest
Dr Mehlis and a first-degree relative report a relationship with Sanofi and Regeneron that includes equity or stocks. Choo and Drs Akinyemi, Cibull, and Waldinger have no conflicts of interest to declare.
Footnotes
Funding sources: Dr Mehlis and a first-degree relative report a relationship with Sanofi and Regeneron that includes funding grants.
IRB approval status: Not applicable.
Patient consent: Patient gave consent for their photographs and medical information to be published in print and online. Consent forms available upon request.
References
- 1.Federico M., Rudiger T., Bellei M., et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31(2):240–246. doi: 10.1200/JCO.2011.37.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botros N., Cerroni L., Shawwa A. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37(4):274–283. doi: 10.1097/DAD.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 3.Dogan A., Attygalle A.D., Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematol. 2003;121:681–691. doi: 10.1046/j.1365-2141.2003.04335.x. [DOI] [PubMed] [Google Scholar]
- 4.Espinosa M.L., Nguyen M.T., Aguirre A.S., et al. Progression of cutaneous T-cell lymphoma after dupilumab: case review of 7 patients. J Am Acad Dermatol. 2020;83(1):197–199. doi: 10.1016/j.jaad.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park A., Wong L., Lang A., Kraus C., Anderson N., Elsensohn A. Cutaneous T-cell lymphoma following dupilumab use: a systematic review. Int J Dermatol. 2022;61:1–15. doi: 10.1111/ijd.16388. [DOI] [PubMed] [Google Scholar]