Abstract
Vinblastine and other microtubule-damaging agents, such as nocodazole and paclitaxel, cause cell cycle arrest at the G2/M transition and promote apoptosis in eukaryotic cells. The roles of these drugs in disrupting microtubule dynamics and causing cell cycle arrest are well characterized. However, the mechanisms by which these agents promote apoptosis are poorly understood. We disrupted the MEKK1 kinase domain in chicken bursal B-cell line DT40 by homologous recombination and have shown that it is essential for both vinblastine-mediated apoptosis and vinblastine-mediated c-Jun N-terminal protein kinase activation. In addition, our data indicate that vinblastine-mediated apoptosis in DT40 cells requires new protein synthesis but does not require G2/M arrest, suggesting that vinblastine-mediated cell cycle arrest and apoptosis are two independent processes.
Eukaryotic cells treated with microtubule-disrupting agents such as vinblastine, nocodazole, and paclitaxel undergo cell cycle arrest at the G2/M transition (19, 25). Because of their inhibitory effect on proliferation, these drugs are used in the treatment of many types of cancer. Cell cycle arrest at the G2/M transition after treatment with these drugs is initiated by the G2/M checkpoint in response to the disruption of the mitotic spindle apparatus (4, 13), which is in part composed of microtubules. At high concentrations, vinblastine and nocodazole disrupt the mitotic spindle apparatus by binding to tubulin monomers and preventing microtubule polymerization (8, 19, 30). Paclitaxel, on the other hand, binds to microtubules and stabilizes them, preventing the dynamic instability of microtubules needed for normal functioning of the mitotic spindle apparatus (8, 19, 30).
In addition to cell cycle arrest, treatment of eukaryotic cells with antimicrotubule agents also causes apoptosis. Apoptosis in response to these agents is not simply a consequence of G2/M arrest but appears to require phosphoregulatory signaling mechanisms (9, 14, 24, 26, 28, 32). The c-Jun N-terminal protein kinase (JNK) pathway is activated after treatment with microtubule-disrupting agents (29, 33) and has been shown to phosphorylate the antiapoptotic protein Bcl-2 in response to paclitaxel and vinblastine (9, 24, 26, 28, 32). Overexpression of dominant-negative versions of kinases in the JNK pathway has been shown to abolish Bcl-2 phosphorylation in response to both paclitaxel and vinblastine treatment (9, 24, 32). Although there has been controversy as to whether Bcl-2 phosphorylation is protective or proapoptotic, most mutational analyses of Bcl-2 indicate that Bcl-2 phosphorylation inactivates Bcl-2 (9, 21, 32). This suggests a model for paclitaxel-mediated apoptosis whereby the JNK pathway phosphorylates Bcl-2, rendering the cell susceptible to apoptosis. The JNK pathway or other pathways may also promote cell death by activating the transcription of proapoptotic genes or repressing the transcription of antiapoptotic genes, since treatment of cells with antimicrotubule drugs has been shown to modulate p53, Bak, Bcl-X, Bcl-2, and Bax expression (3, 18, 22, 23, 27). However, involvement of the JNK pathway in apoptosis, mediated by antimicrotubule agents, has not been conclusively demonstrated, since most of the studies supporting this conclusion depended upon protein overexpression.
MEKK1, a mitogen-activated protein kinase kinase kinase implicated in JNK signaling, is a 196-kDa protein that is activated by chemoattractants such as formyl-Met-Leu-Phe in neutrophils (2), epidermal growth factor in epithelial cells (10), and antigen ligation to immunoglobulin E (IgE) receptor FcɛRI in mast cells (17). Overexpression studies indicate that MEKK1 may be involved in the induction of apoptosis. Treatment of cells with genotoxic agents and prolonged suspension of adherent cells, which both result in apoptosis, have been shown to cause caspase-dependent cleavage of MEKK1 and activation of its kinase activity (5, 7, 12). Overexpression of MEKK1 cleavage products has been shown to induce apoptosis, while overexpression of kinase-inactive MEKK1 or cleavage-resistant forms of MEKK1 inhibits apoptosis (5, 12). These data strongly suggest that MEKK1 is involved in both genotoxin-induced cell death and cell death induced by the prolonged suspension of adherent cells (anoikis).
A recent study with human leukemia HL-60 cells also suggests that MEKK1 is involved in antimicrotubule drug-mediated apoptosis. Overexpression of dominant-negative MEKK1 in these cells prevented paclitaxel-induced JNK activation, Bcl-2 phosphorylation, and apoptosis (24). These data and evidence for a role for MEKK1 in genotoxin-mediated apoptosis and anoikis strongly suggest that MEKK1 may be a component of a signal transduction cascade that promotes antimicrotubule drug-induced cell death. However, recent studies with mekk1−/− mouse ES cells have demonstrated that MEKK1 partially protects against nocodazole-induced apoptosis (34). These conflicting results raise the question of whether MEKK1 plays a proapoptotic or protective role in microtubule disruption-induced apoptosis. Using a somatic cell knockout approach, we demonstrated that DT40 chicken bursal B cells deficient in MEKK1 are defective in both vinblastine-mediated JNK activation and apoptosis. Cycloheximide treatment of DT40 cells prevented vinblastine-mediated cell death, indicating that vinblastine-mediated cell death requires new protein synthesis. We also showed that vinblastine-mediated apoptosis does not require G2/M arrest, since arrest at the G1/S transition prior to vinblastine treatment does not protect cells from vinblastine-mediated apoptosis. Gene disruption of MEKK1 protected DT40 cells arrested at G1/S from vinblastine-mediated apoptosis, suggesting that MEKK1 promotes apoptosis through a mechanism unrelated to the G2/M checkpoint. These data indicate a critical role for MEKK1 and new protein synthesis in vinblastine-mediated apoptosis and suggest that vinblastine-mediated G2/M arrest and vinblastine-mediated apoptosis occur independently of each other.
MATERIALS AND METHODS
Isolation of mekk1 genomic clones and construction of mekk1 targeting constructs.
Genomic clones of mekk1 were isolated from a λGEM11 Leghorn chicken genomic library by standard methods. An XhoI fragment of approximately 18 kb from a genomic clone designated MEKK1 was subcloned into pBluescript SK and restriction mapped. The location of the kinase domain within MEKK1 was determined by Southern blotting using the cDNA fragment 20E11, which contains the kinase domain. The SmaI-EcoRI fragment at the 3′ end of MEKK1 was removed by truncation. The EcoRI-HpaI fragment, which contains the kinase domain, was removed and replaced with the linker SphI-SacII-XbaI-BamHI. The EcoRI site and the HpaI site were disrupted as a consequence of linker ligation into the construct. For targeting construct pMEKK1LΔSB, a 2.5-kb neomycin resistance cassette was subcloned at the introduced BamHI site. For targeting construct pMEKK1LΔS, more genomic sequence was eliminated by removal of a 2-kb SphI fragment. The Neor cassette was then subcloned at the introduced BamHI site.
Cell lines and transfection.
DT40 cell lines were maintained in DT40 medium (RPMI 1640 medium containing 10% fetal bovine serum, 1% chicken serum, 50 μM 2-mercaptoethanol, 50 U of penicillin per ml, and 50 μg of streptomycin per ml) at a density of 0.5 × 106 to 1.5 × 106 cells/ml. In the transfections of targeting constructs, DT40 cells in 0.5 ml of medium were transferred to a 0.4-cm electroporation cuvette and pulsed at 550 V and 25 μF in the presence of 30 μg of the pMEKK1LΔSB or the pMEKK1LΔS targeting construct linearized with KpnI. Electroporated DT40 cells were then resuspended in 20 ml of DT40 medium and plated on two 96-well plates at 5 × 104 cells/well. The transfected cells were selected with 2 mg of G418 per ml (active concentration; Mediatech, Inc.).
Southern blot hybridization.
DT40 or mekk1−/− cells (1 × 107) were lysed in 300 μl of lysis buffer (100 mM Tris [pH 8.5], 5 mM EDTA, 0.2% sodium dodecyl sulfate [SDS], 200 mM NaCl, 100 μg of proteinase K per ml) overnight at 55°C. Saturated NaCl solution (90 μl) was added to each tube, and the pellet was spun down. Genomic DNA was ethanol precipitated from the supernatant and washed with 70% ethanol. Genomic DNA (20 μg) for each sample was run on a 0.7% agarose gel. The gel was transferred to nylon. Fragment NE and FR probes were purified from a 1× TAE–0.7% agarose gel by using the Geneclean II kit (Qbiogene, Inc.). Each fragment (50 ng) was labeled with [α-32P]dCTP by using the Prime-It RmT random primer labeling kit (Stratagene). Nylon filters were hybridized in FBI buffer (1.5× SSPE [1× SSPE is 0.15 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA at pH 7.4], 7% SDS, 100 μg of salmon sperm DNA per ml, 10% [wt/vol] polyethylene glycol 8000) at 65°C overnight. Nylon filters were successively washed in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS, 0.5× SSC–0.1% SDS, and 0.2× SSC–0.1% SDS for 30 min at 65°C and then exposed to X-ray film.
Western blot analysis.
For MEKK1 Western blotting, 107 DT40 or mekk1−/− cells were spun down and lysed in 150 μl of 1× SDS loading dye (50 mM Tris [pH 6.8], 2% SDS, 0.1% bromophenol blue, 10% glycerol, 5% 2-mercaptoethanol). Samples were sonicated, boiled for 5 min, and run on an SDS–7% polyacrylamide gel electrophoresis (PAGE) separating gel. The SDS-PAGE gel was transferred to nitrocellulose by using a Bio-Rad Trans-Blot semidry transfer apparatus. The nitrocellulose membrane was incubated with a 1:1,000 dilution of polyclonal rabbit anti-MEKK1 serum (PharMingen International), followed with a 1:3,000 dilution of goat anti-rabbit IgG-horseradish paroxidase. For the JNK Western blot assay, 25 μg of total protein for each sample was loaded on an SDS–10% PAGE separating gel. The blot was incubated with a 1:1,000 dilution of an anti-JNK (C17) antibody (Santa Cruz Biotechnology, Inc.), followed with a 1:3,000 dilution of anti-goat IgG-horseradish peroxidase (Santa Cruz Biotechnology, Inc.).
JNK in vitro kinase assays.
DT40 or mekk1−/− cells (1.5 × 107) were incubated in DT40 medium alone (30 min), anti-IgM (15 min), 50 ng of phorbol myristate acetate (PMA) per ml–250 ng of ionomycin per ml (30 min), 1 μg of nocodazole per ml (30 min), 1 μM paclitaxel (30 min), and 1 μM vinblastine sulfate (30 min) at 37°C. Cells were washed once with ice-cold phosphate-buffered saline and lysed in 800 μl of modified radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Igepal CA-630, 0.25% sodium deoxycholate, 1 mM EGTA, 5 mM NaF, 1 μg of leupeptin per ml, 1 μg of aprotinin per ml, 1 mM sodium vanadate, 1 mM phenylmethyl sulfonyl fluoride) on ice for 30 min with periodic agitation. The total protein concentration of each cytoplasmic extract was determined by using the Bio-Rad protein assay. For each sample, 300 μg of total protein was used in each JNK in vitro kinase assay, which was performed as previously described (16). To quantitate JNK in vitro kinase assay results, gels were scanned by using the Molecular Dynamics STORM 860 PhosphorImager, and integrated band intensities were obtained by using Molecular Dynamics ImageQuant software. Local average background correction was used to correct for the background, and volume values were used to calculate fold activation over untreated cells.
Analysis of cytotoxicity and apoptosis.
The CytoTox nonradioactive cytotoxicity assay kit from Promega was used for all cytotoxicity assays. Wild-type DT40 or mekk1−/− cells (5 × 104 in each well) were plated on 96-well plates. Experiments were performed in accordance with the Promega protocol, except that 20 μl of supernatant or medium samples diluted to 50 μl with phosphate-buffered saline were used in the assay instead of 50 μl of undiluted samples. All experiments were performed in triplicate. A492 readings were taken on a plate reader. Percent cytotoxicity was calculated as follows: % cytotoxicity = 100 × (A492 of treated cell − A492 of medium alone)/(A492 after complete lysis − A492 of medium alone).
In DNA laddering assays, 7.5 × 106 DT40 or mekk1−/− cells at a density of 5 × 105/ml were treated for the indicated times with 1 μM vinblastine sulfate, 12.5 μM etoposide, 4 mM thymidine, 50 μg of cycloheximide per ml, or combinations of these agents and then processed as previously described (15). In the caspase 3 activity assay, 2 × 107 cells treated for the indicated times with 1 μM vinblastine sulfate were lysed in 50 μl of ice-cold cell lysis buffer containing 50 mM HEPES (pH 7.4), 100 mM NaCl, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 1 mM dithiothreitol, and 0.1 mM EDTA, flash frozen in an ethanol-dry ice bath, and stored at −70°C before use. Caspase 3 activity assay experiments were performed as described in the Calbiochem protocol for colorimetric caspase 3 substrate I (Ac-DEVD-ρNA). A405 readings were taken on a plate reader at 10-min intervals.
Analysis of cell cycle.
For each experimental sample, 106 DT40 or MC2 cells at a density of 5 × 105/ml were treated for the indicated times with 1 μM vinblastine sulfate. Cells were then spun down and resuspended in 4°C hypotonic DNA staining buffer (3.4 mM sodium citrate, 0.3% Triton X-100, 150 μM propidium iodide, 20 μg of RNase A per ml). Samples were incubated at 4°C for 30 min to 1 h, followed by cell cycle analysis on a FACScalibur (Becton Dickinson, Inc.) flow cytometer.
RESULTS
Establishment of mekk1−/− cell lines in DT40 cells.
We isolated a 1.2-kb cDNA that was homologous to the mammalian MEKK1 kinase domain from a concanavalin A-stimulated chicken T-cell cDNA library in vector pCDNA3. Additional sequence was isolated through the PCR amplification of more 5′ sequence from the T-cell cDNA library. Amino acid sequence comparisons between the putative chicken MEKK1 kinase domain and the human and mouse MEKK1 kinase domains (residues 1221 to 1493 of the mouse sequence) indicated that the isolated sequence is approximately 97% identical to the mammalian MEKK1 kinase domain. In contrast, the putative chicken MEKK1 kinase domain is approximately 40% identical to human MEKK2 and MEKK3, indicating that it is indeed an MEKK1 homolog. A genomic sequence was subsequently isolated that contained kinase domain sequence.
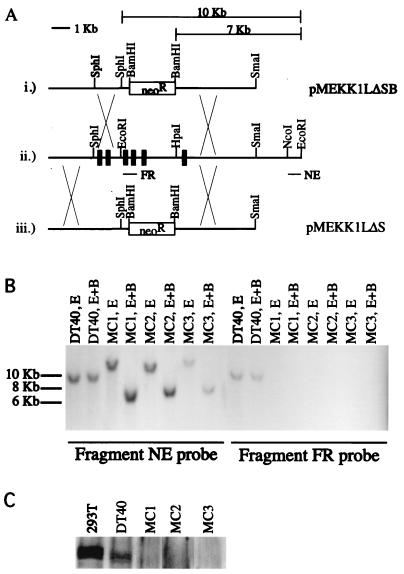
To determine the role of MEKK1 in bursal B-cell line DT40, the mekk1 locus was disrupted by homologous recombination. DT40 cells were transfected with either the pMEKK1LΔSB or the pMEKK1LΔS targeting construct (Fig. 1A). These targeting constructs disrupt the C-terminal MEKK1 kinase domain. The pMEKK1LΔSB and pMEKK1LΔS targeting constructs remove a region of MEKK1 homologous to amino acids 1206 to 1447 and 1312 to 1447 of human MEKK1, respectively. Transfected cells were plated on 96-well plates, and G418-resistant clones were isolated. Disruption of the mekk1 locus in each of the individual clones was assessed by genomic Southern blotting using fragment NE as a probe (Fig. 1A).
FIG. 1.
Targeting constructs for disruption of the MEKK1 kinase domain and confirmation of gene disruption. (A) (i) For targeting construct pMEKK1LΔSB, the EcoRI-HpaI fragment, which contains the kinase domain (see ii), was removed and replaced with the linker SphI-SacII-XbaI-BamHI. The EcoRI and HpaI sites were both disrupted as a result of linker ligation. A 2.5-kb neomycin resistance cassette was then subcloned into the BamHI site. (ii) Partial restriction map of the mekk1 genomic region containing the MEKK1 kinase domain. FR is a PCR product spanning two exons, and its sequence is contained within the region deleted in the two targeting constructs shown in i and iii. NE is an NcoI-EcoRI fragment that was used in the initial genomic Southern screen for gene disruption at the mekk1 locus. (iii) Targeting construct pMEKK1LΔS is similar to pMEKK1LΔSB, except that the SphI fragment 5′ of the Neo cassette has been removed. The 10-kb bar above the targeting construct indicates the size of the EcoRI fragment at the endogenous mekk1 locus (see ii). The 7-kb bar above the targeting constructs indicates the size of the EcoRI-BamHI fragment at the mekk1 locus disrupted with the pMEKK1LΔSB or pMEKK1LΔS targeting construct. (B) DNAs from wild-type DT40 cells (20 μg) and MEKK1 knockout cell lines MC1, MC2, and MC3 were digested with EcoRI (E) and EcoRI-BamHI (E+B) and run on a 0.7% agarose gel in duplicate. The gel was transferred to a nylon filter, which was subsequently cut to obtain two duplicate filters. One filter was hybridized with fragment NE, and the other filter was hybridized with fragment FR. (C) Whole-cell extracts were prepared for 293T cells, wild-type DT40 cells, and MEKK1 knockout cell lines as described in Materials and Methods. Each extract (30 μl) was loaded. The Western blot was probed with an anti-MEKK1 antibody.
MEKK1-positive clone 1 (MC1) and MC3 were generated by using pMEKK1LΔSB, while MC2 was generated by using pMEKK1LΔS. Southern blotting results for MC1, MC2, and MC3 indicated that mekk1 is hemizygous in DT40 cells. Isolated clones showed only the expected 7-kb BamHI-EcoRI knockout band, as well as a >10-kb EcoRI knockout band, after one round of G418 selection (Fig. 1B). The 10-kb wild-type band is missing from all of the positive clones tested (Fig. 1B and data not shown). To confirm that the mekk1 locus is hemizygous, additional Southern blots were performed by using fragment FR, which is derived from the region deleted in the MEKK1 targeting constructs (Fig. 1A). Fragment FR hybridized to the expected 10-kb BamHI-EcoRI and 10-kb EcoRI bands in the wild-type DT40 DNA control lanes but did not hybridize in the knockout genomic DNA lanes (Fig. 1B), indicating that DT40 cells contain only one copy of the mekk1 gene and that this copy was disrupted in one round of homologous recombination.
To determine whether gene disruption of the MEKK1 kinase domain affects the MEKK1 protein, Western blots were performed by using an antibody against the C terminus of human MEKK1. Wild-type DT40 and control 293T extracts showed a triplet of approximately 196 kDa that was not present in the MC1, MC2, and MC3 extracts (Fig. 1C), indicating that expression of at least the MEKK1 kinase domain had been abolished in these knockout cell lines. Northern blot results obtained by using fragment FR as a probe corroborated the Western blotting data (data not shown).
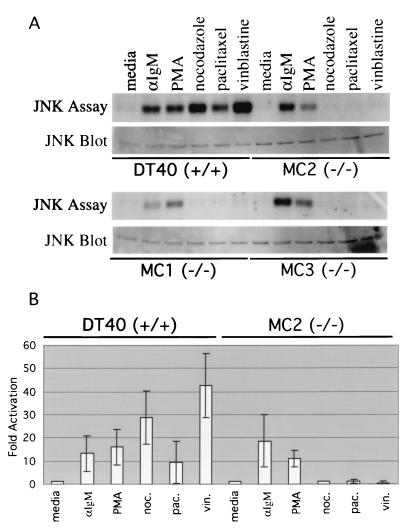
JNK activation in DT40 cells after stimulation with microtubule-disrupting agents is abolished in mekk1−/− clones.
MEKK1 is a mitogen-activated protein kinase kinase kinase in the JNK cascade. Disruption of mekk1 by homologous recombination should therefore abolish JNK activation for a subset of JNK-activating stimuli. To determine whether the mekk1−/− DT40 clones have a defect in JNK activation, in vitro JNK kinase assays were performed. Stimulation of wild-type DT40 cells with the microtubule-disrupting agents nocodazole, vinblastine, and paclitaxel caused a marked increase in JNK activity (Fig. 2A). In contrast, JNK activity after treatment with microtubule-disrupting agents was completely abolished in mekk1−/− DT40 cell lines MC1, MC2, and MC3 (Fig. 2A). Anti-JNK Western blots showed that roughly equivalent amounts of JNK were present in the extracts used for immunoprecipitation (Fig. 2A). The same defect was seen in all of the other mekk1−/− cell lines tested (data not shown). Results from three separate in vitro JNK kinase assays were quantified, and average values were determined. The defect in JNK activation in mekk1−/− cells is specific for microtubule-disrupting agents, since JNK activation following PMA-ionomycin and anti-IgM stimulation was unaffected (Fig. 2A and B). MEKK1 appears to function specifically in antimicrotubule drug-induced JNK activation, since mekk1 kinase domain disruption had no effect on vinblastine- or PMA-ionomycin-induced NF-κB, p38, and ERK activation (data not shown).
FIG. 2.
JNK activation in DT40 cells after stimulation with microtubule-disrupting agents is abolished in mekk1−/− cell lines MC1, MC2, and MC3. (A) DT40, MC1, MC2, and MC3 cells were incubated in DT40 medium alone (30 min), anti-IgM (15 min), 50 ng of PMA per ml–250 ng of ionomycin per ml (30 min), 1 μg of nocodazole (noc.) per ml (30 min), 1 μM paclitaxel (pac.) (30 min), and 1 μM vinblastine sulfate (vin.) (30 min) at 37°C. Cells were subsequently lysed in modified radioimmunoprecipitation assay buffer, and 300 μg of total protein from each extract was used in a JNK in vitro kinase assay as described in Materials and Methods. For the JNK Western blot assay, 25 μg of total protein from each extract was subjected to SDS-PAGE. The filter was incubated with an anti-JNK (C17) antibody. (B) Three separate JNK in vitro kinase assays were performed, and results were quantitated as described in Materials and Methods. Results are expressed as fold activation over untreated cells. Average values are shown.
Cell death induced by microtubule-disrupting agents is abrogated in mekk1−/− cell lines.
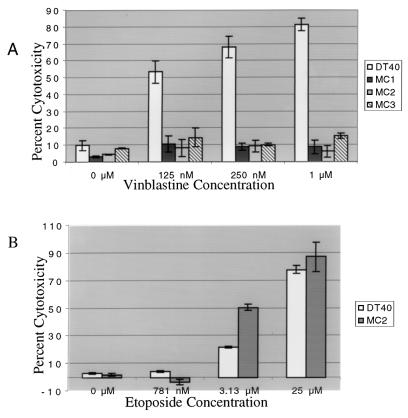
Since the JNK pathway has been implicated in apoptosis induced by microtubule-disrupting agents, mekk1−/− cell lines were tested in cytotoxicity assays after exposure to vinblastine and nocodazole. In cytotoxicity assays, increasing concentrations of vinblastine caused increasing levels of cytotoxicity in wild-type DT40 cells (Fig. 3A). In mekk1−/− cell lines MC1, MC2, and MC3, however, increasing concentrations of vinblastine had no effect on cytotoxicity (Fig. 3A). Identical results were obtained by using nocodazole (data not shown). This defect in cell death is specific for the cytotoxicity induced by microtubule-disrupting agents, since cytotoxicity in response to etoposide (Fig. 3B) and doxorubicin (data not shown) was unimpaired in the MC2 cell line.
FIG. 3.
Cell death induced by microtubule-disrupting agents is abrogated in mekk1−/− cell lines. The CytoTox nonradioactive cytotoxicity assay kit from Promega was used for all cytotoxicity assays. Wild-type DT40, MC1, MC2, and MC3 cells (5 × 104 per well) were plated on a 96-well plate. (A) In the vinblastine dose-response experiment, vinblastine was titrated into the wells at concentrations of 0 to 1 μM. (B) In the etoposide dose-response experiment, etoposide was titrated into the wells at concentrations of 781 nM, 3.13 μM, and 25 μM. Plates for both dose-response experiments were incubated for approximately 16 h at 37°C. The cytotoxicity assay was performed as described in Materials and Methods.
The difference in vinblastine-mediated cytotoxicity between wild-type and mekk1−/− DT40 cells is due to a defect in vinblastine-mediated apoptosis.
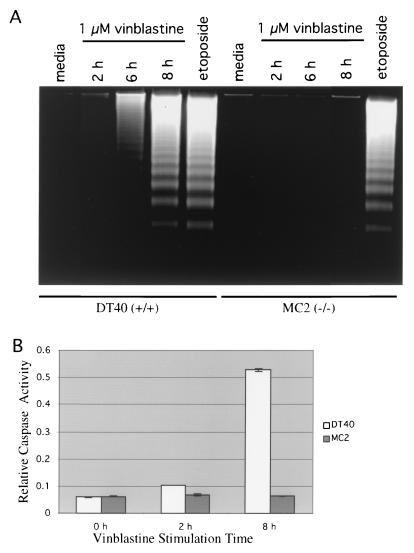
To determine whether the difference in vinblastine-mediated cytotoxicity between wild-type and mekk1−/− cells is due to an apoptosis defect in the mekk1 knockout cell lines, apoptosis assays were performed. In DNA laddering assays, wild-type DT40 cells exhibited DNA laddering indicative of apoptosis after 6 and 8 h of vinblastine treatment (Fig. 4A). MC2 cells showed no DNA laddering after 2, 6, or 8 h of vinblastine treatment (Fig. 4A). In contrast, etoposide induced apoptosis equally well in wild-type DT40 cells and MC2 cells (Fig. 4A).
FIG. 4.
The difference in vinblastine-mediated cytotoxicity between wild-type and mekk1−/− DT40 cells is due to a defect in vinblastine-mediated apoptosis. (A) In DNA laddering assays, 7.5 × 106 DT40 or MC2 cells were treated for 2, 6, or 8 h with 1 μM vinblastine sulfate, for 3.5 h with 12.5 μM etoposide, or for 8 h with DT40 medium alone. DNAs from these samples were prepared as described in Materials and Methods. The equivalent of 3.75 × 106 cells of each sample was run on a 1% agarose gel. (B) In the caspase 3 activity assay, 2 × 107 cells were treated for 2 or 8 h with 1 μM vinblastine sulfate or for 8 h with DT40 medium alone (0 h) and then lysed in 50 μl of ice-cold cell lysis buffer. Extracts were incubated at 37°C with the caspase 3 substrate Ac-DEVD-ρNA. A405 readings were taken on a plate reader at 10-min intervals. A405 readings (relative caspase activity) at 120 min are shown.
These results were recapitulated in caspase 3 activity assays. Extracts prepared from DT40 cells treated for 8 h with vinblastine showed a marked increase in caspase 3 activity in comparison with untreated cell extracts (Fig. 4B). On the other hand, extracts prepared from MC2 cells treated for 8 h with vinblastine showed no increase in caspase 3 activity compared with untreated MC2 cells (Fig. 4B). These data indicate that MEKK1 promotes apoptosis through a caspase 3-dependent signal transduction pathway.
mekk1-null DT40 cells remain arrested at G2/M, but wild-type DT40 cells undergo apoptosis after 8 h of vinblastine treatment.
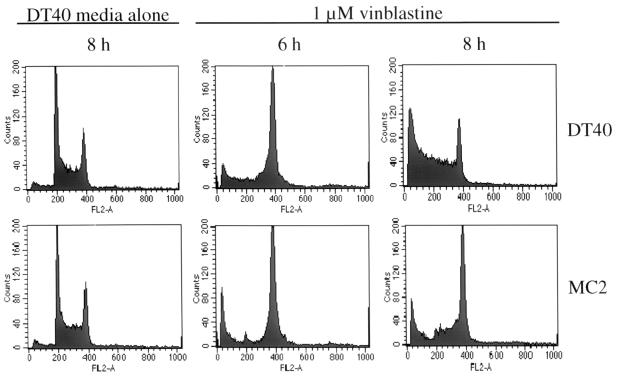
Microtubule-disrupting agents cause cell cycle arrest at M phase. To determine whether there is a difference in the cell cycle profiles of vinblastine-treated DT40 and MC2 cells that might explain the apoptosis defect in MC2 cells, the cell cycle profiles of wild-type DT40 and MC2 cells were taken after 2 to 24 h of vinblastine treatment. Untreated DT40 and MC2 cells had similar cell cycle profiles (Fig. 5). In both wild-type DT40 and MC2 cells, vinblastine treatment resulted in accumulation of cells at the G2/M transition after 6 h of treatment (Fig. 5), indicating that MEKK1 is not required for vinblastine-mediated cell cycle arrest at the G2/M transition. The increase in the size of the sub-G1 peak (Fig. 5 and 6A) and the decrease in the percentage of wild-type DT40 cells in G2/M phase suggested that the wild-type DT40 cells started to undergo apoptosis by 8 h of vinblastine treatment. Unlike wild-type DT40 cells, vinblastine-treated MC2 cells remained blocked at the G2/M transition for 8 to 24 h, with a much less pronounced decrease in cell viability (Fig. 5 and 6A). These results indicate that abrogation of vinblastine-mediated apoptosis by mekk1 gene disruption does not occur through modulation of cell cycle progression and that vinblastine-mediated apoptosis is not simply a consequence of prolonged G2/M arrest.
FIG. 5.
mekk1-null DT40 cells remain arrested at G2/M after prolonged vinblastine treatment, but wild-type DT40 cells undergo apoptosis after 8 h of vinblastine treatment. DT40 or MC2 cells (106) were treated for 6 or 8 h with 1 μM vinblastine sulfate or for 8 h with DT40 medium alone. Cells were stained in hypotonic DNA staining buffer and analyzed as described in Materials and Methods.
FIG. 6.
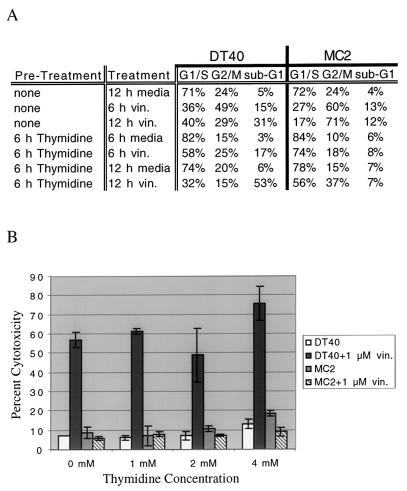
Vinblastine (vin.)-mediated cell death in DT40 cells does not depend on G2/M arrest. (A) DT40 or MC2 cells (106) were either pretreated with 4 mM thymidine (6 h) or left untreated (6 h). After the initial 6-h incubation, 1 μM vinblastine was added to designated samples. The treated cells were further incubated for 6 or 12 h, and cell cycle analysis was performed as described in Materials and Methods. Treatments were staggered over an 18-h period so that all samples could be processed at the same timepoint. (B) DT40 or MC2 cells (5 × 104) were plated in each well of a 96-well plate. Two duplicate sets of thymidine titrations were performed for both DT40 and MC2 cells at thymidine concentrations of 0, 1, 2, and 4 μM. Plates (96 wells) were incubated for 6 h at 37°C. Vinblastine was then added at a concentration of 1 μM into one set of thymidine titrations for both DT40 and MC2 cells, and the cells were further incubated for 12 h. A cytotoxicity assay was performed on treated cells as described in Materials and Methods.
Vinblastine-mediated cell death in DT40 cells does not depend on G2/M arrest.
It has been postulated that G2/M arrest is required for microtubule-disrupting agent-mediated apoptosis (32). To test this hypothesis, we decided to block DT40 cells at G1 or S phase prior to vinblastine treatment. Cell cycle analysis was first performed to determine if thymidine and l-mimosine can block DT40 cells at S phase and G1 phase, respectively. Cell cycle analysis indicated that over 80% of DT40 cells were arrested at G1/S following 6 to 18 h of treatment with 4 mM thymidine (Fig. 6A).
Addition of 1 μM vinblastine to DT40 cells pretreated for 6 h with 4 mM thymidine resulted in an increase in the sub-G1 fraction (apoptotic cells) to over 50% of the total cells after 12 h of treatment, similar to DT40 cells treated with 1 μM vinblastine alone. Cytotoxicity assays were subsequently performed on DT40 cells treated with thymidine, vinblastine, or thymidine plus vinblastine. DT40 cells treated with thymidine alone exhibited low levels of cytotoxicity that increased slightly with increasing thymidine concentrations (Fig. 6B). DT40 cells treated with 1 μM vinblastine alone exhibited approximately 60% cytotoxicity (Fig. 6B). Arrest of DT40 cells at G1/S with thymidine prior to addition of 1 μM vinblastine had no effect on vinblastine-mediated cytotoxicity (Fig. 6B), indicating that G2/M arrest is not required for vinblastine-mediated apoptosis. MEKK1 deficiency was still able to protect MC2 cells blocked at G1/S with thymidine from vinblastine-mediated apoptosis (Fig. 6B), suggesting that MEKK1 acts independently of the G2/M checkpoint to promote vinblastine-mediated apoptosis. These results were recapitulated with the G1 phase blocker l-mimosine (data not shown).
Vinblastine-mediated cell death in DT40 cells is dependent on new protein synthesis.
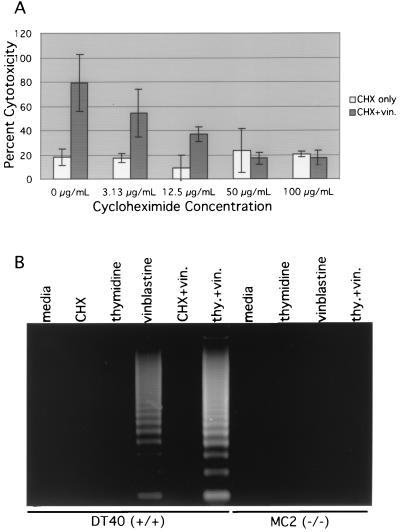
If the role of MEKK1 in vinblastine-mediated apoptosis is to activate the transcription of genes necessary for apoptosis, blocking of transcription or new protein synthesis in vinblastine-treated DT40 cells should attenuate the apoptotic response. To investigate this possibility, vinblastine-treated DT40 cells were treated with increasing concentrations of cycloheximide. DT40 cells treated with various concentrations of cycloheximide alone exhibited a percentage of cytotoxicity comparable to that of untreated cells (Fig. 7A), and DT40 cells treated with vinblastine alone exhibited approximately 80% cytotoxicity (Fig. 7A). In contrast, vinblastine-treated cells exhibited a decreasing percentage of cytotoxicity with increasing concentrations of cycloheximide (Fig. 7A). At a cycloheximide concentration of 50 μg/ml, vinblastine-treated DT40 cells exhibited a percentage of cytotoxicity equivalent to that of DT40 cells treated with 50 μg of cycloheximide per ml alone (Fig. 7A). Cycloheximide inhibited vinblastine-induced DNA laddering (Fig. 7B) and caspase 3 activity (data not shown) in DT40 cells, indicating that it specifically inhibited apoptosis. These data indicate that new protein synthesis, initiated by a MEKK1-dependent pathway or other pathways, is required for vinblastine-mediated apoptosis.
FIG. 7.
Vinblastine (vin.)-mediated cell death in DT40 cells is dependent on new protein synthesis. (A) DT40 cells (5 × 104) were plated in each well of a 96-well plate. Two duplicate sets of cycloheximide (CHX) titrations were performed at cycloheximide concentrations of 0, 3.13, 12.5, 50, and 100 μg/ml. In one set of cycloheximide titrations, vinblastine was added at a concentration of 1 μM. The CytoTox nonradioactive cytotoxicity assay was performed as described in Materials and Methods. Shown are the effects of increasing concentrations of cycloheximide on DT40 cells alone and on DT40 cells treated with 1 μM vinblastine. (B) In the DNA laddering assay, 7.5 × 106 DT40 or MC2 cells were treated as follows. For vinblastine stimulations, cells were stimulated at a concentration of 1 μM (8 h). For thymidine (thy.) experiments, cells were pretreated for 6 h in 4 mM thymidine with or without subsequent addition of 1 μM vinblastine (8 h). For cycloheximide experiments, cells were treated at a concentration of 50 μg/ml (8 h). Treatments were staggered over a 14-h period so that all samples could be processed at the same time.
DISCUSSION
Antimicrotubule agents cause both G2/M arrest and apoptosis in eukaryotic cells. Because G2/M arrest precedes apoptosis (19, 25), it has been thought that antimicrotubule agent-induced apoptosis requires G2/M arrest. Previous studies have shown that Bcl-2 is inactivated at G2/M through JNK-dependent phosphorylation (32). Based on these observations, it has been postulated that antimicrotubule agents kill cells by arresting them at G2/M, thus keeping them in a state of prolonged sensitization to apoptosis through the phosphorylation-dependent inactivation of Bcl-2. However, a requirement for G2/M arrest in antimicrotubule agent-mediated apoptosis has not been conclusively demonstrated.
In this study, we showed that vinblastine-mediated apoptosis in DT40 cells does not depend on G2/M arrest. DT40 cells arrested at G1/S by thymidine or l-mimosine treatment are still sensitive to vinblastine-mediated apoptosis to the same extent as G2/M-arrested cells. In addition, we showed that new protein synthesis is required for vinblastine-mediated apoptosis. Cycloheximide (50 μg/ml) completely suppressed vinblastine-mediated cytotoxicity in DT40 cells. These data lend support to a model in which one or more signaling pathways, independent of G2/M arrest, promote antimicrotubule drug-induced apoptosis by activating the transcription of proapoptotic genes.
We demonstrated that MEKK1 is a component of a signaling pathway that is necessary for nocodazole- and vinblastine-mediated cell death. The results of our gene disruption experiments indicate that MEKK1 is required for vinblastine- and nocodazole-mediated apoptosis but not for etoposide- and doxorubicin-mediated apoptosis. mekk1 gene disruption had no effect on cell cycle progression and vinblastine-mediated G2/M arrest, indicating that MEKK1 is specifically involved in vinblastine-mediated apoptosis. We also showed that mekk1 gene disruption protects DT40 cells arrested at G1/S from vinblastine-mediated apoptosis, suggesting that MEKK1 promotes apoptosis independently of vinblastine-mediated G2/M arrest. However, our data contradict the results of an independently produced MEKK1 knockout. In these studies, mekk1 gene disruption sensitizes ES cells to nocodazole-mediated apoptosis (34). The discrepancy between our results and results from ES cell studies indicates that different cell types have different mechanisms for antimicrotubule drug-mediated apoptosis. Indeed, the JNK pathway, of which MEKK1 is a component, has been implicated in both cell survival and apoptosis (6). It is possible that DT40 cells require MEKK1 for antimicrotubule drug-mediated apoptosis, while ES cells are partially protected from antimicrotubule drug-mediated apoptosis by MEKK1.
Consistent with results from two independently generated MEKK1 knockouts, we also demonstrated that mekk1 gene disruption prevents vinblastine-, paclitaxel-, and nocodazole-mediated JNK activation but has no effect on p38, ERK, and NF-κB activation in response to these stimuli (31, 34, 35). The JNK activation defect is specific for antimicrotubule drug stimulation, since PMA-ionomycin-mediated JNK activation was not affected by mekk1 gene disruption. Moreover, we showed that MEKK1 is not required for anti-IgM-mediated JNK activation.
The current study does not address the question of which signaling pathways downstream of MEKK1 are involved in apoptosis in response to treatment with antimicrotubule agents. Based on the fact that JNK is specifically activated by MEKK1 during microtubule disruption, JNK is likely to be involved in MEKK1-mediated apoptosis. One possible mechanism by which MEKK1 mediates apoptosis in response to antimicrotubule agents is the JNK-dependent phosphorylation and concomitant inactivation of Bcl-2. Previous studies have shown that Bcl-2 is phosphorylated in response to microtubule disorganization and that overexpression of dominant-negative MEKK1 or JNK1 inhibits paclitaxel-induced Bcl-2 phosphorylation and apoptosis (9, 14, 24, 27, 28, 32). The phosphorylation sites have been mapped to the serine residues at positions 70 and 87 of human Bcl-2, and mutated Bcl-2 with alanine substitutions at these phosphorylation sites shows an enhanced ability to protect against apoptosis (32). We compared the human and chicken Bcl-2 amino acid sequences and found that these two human Bcl-2 phosphorylation sites are not conserved in chicken Bcl-2. Nevertheless, this result does not preclude the possibility that chicken Bcl-2 is phosphorylated at alternate sites.
Another possible mechanism by which MEKK1 promotes apoptosis in DT40 cells is transcriptional up-regulation of proapoptotic genes. We showed that both MEKK1 and new protein synthesis play a critical role in vinblastine-induced apoptosis. This hypothesis is further supported by data from prostate cancer cells, in which overexpression of a dominant-active version of MEKK1 activates the transcription of androgen receptor-regulated genes and promotes apoptosis (1). Moreover, apoptosis in T-lymphocytes in response to stress stimuli occurs through the JNK-dependent transcriptional up-regulation of Fas ligand and overexpression of dominant-negative MEKK1 inhibits these events (11, 20). In addition, increased expression of proapoptotic molecules such as p53 and Bax and decreased expression of antiapoptotic molecules such as Bcl-x and Bcl-2 in response to paclitaxel treatment have also been observed (3, 18, 22, 23, 27). These observations strongly suggest a role for MEKK1 in the transcriptional up-regulation of proapoptotic genes in response to antimicrotubule agents. However, the exact mechanisms for MEKK1-mediated apoptosis remain to be elucidated.
Antimicrotubule agents represent a major class of chemotherapeutic drugs. The demonstration of MEKK1 as an essential kinase involved in antimicrotubule agent-mediated apoptosis should provide valuable information for further improvements in cancer chemotherapy. One may screen for MEKK1-specific small molecules to enhance MEKK1-mediated apoptosis in cancer cells without affecting normal cell proliferation. In addition, down-regulation of MEKK1 or kinases downstream of MEKK1 may represent a potential mechanism for the development of chemoresistance.
ACKNOWLEDGMENTS
This work was supported by National Institute of General Medical Sciences grant GM57559 and by The Leukemia & Lymphoma Society. R. Kwan was supported by National Cancer Institute National Research Service Award grant CA09056.
We thank Paul Dempsey for assistance with flow cytometry. We thank C. Sawyers and members of the Cheng laboratory for useful discussions and critical review of the manuscript.
REFERENCES
- 1.Abreu-Martin M T, Chari A, Palladino A A, Craft N A, Sawyers C L. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol. 1999;19:5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avdi N J, Winston B W, Russel M, Young S K, Johnson G L, Worthen G S. Activation of MEKK by formyl-methionyl-leucyl-phenylalanine in human neutrophils. Mapping pathways for mitogen-activated protein kinase activation. J Biol Chem. 1996;271:33598–33606. doi: 10.1074/jbc.271.52.33598. [DOI] [PubMed] [Google Scholar]
- 3.Blagosklonny M V, Schulte T W, Nguyen P, Mimnaugh E G, Trepel J, Neckers L. Taxol induction of p21WAF1 and p53 requires c-raf-1. Cancer Res. 1995;55:4623–4626. [PubMed] [Google Scholar]
- 4.Burke D J. Complexity in the spindle checkpoint. Curr Opin Genet Dev. 2000;10:26–31. doi: 10.1016/s0959-437x(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 5.Cardone M H, Salvesen G S, Widmann C, Johnson G, Frisch S M. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 6.Davis R J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 7.Deak J C, Cross J V, Lewis M, Qian Y, Parrott L A, Distelhorst C W, Templeton D J. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc Natl Acad Sci USA. 1998;95:5595–5600. doi: 10.1073/pnas.95.10.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downing K H. Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Annu Rev Cell Dev Biol. 2000;16:89–111. doi: 10.1146/annurev.cellbio.16.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde W A, Chambers T C. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–29985. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- 10.Fanger G R, Johnson N L, Johnson G L. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faris M, Latinis K M, Kempiak S J, Koretzky G A, Nel A. Stress-induced Fas ligand expression in T cells is mediated through a MEK kinase 1-regulated response element in the Fas ligand promoter. Mol Cell Biol. 1998;18:5414–5424. doi: 10.1128/mcb.18.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson S, Widmann C, Johnson G L. Differential involvement of MEK kinase 1 (MEKK1) in the induction of apoptosis in response to microtubule-targeted drugs versus DNA damaging agents. J Biol Chem. 1999;274:10916–10922. doi: 10.1074/jbc.274.16.10916. [DOI] [PubMed] [Google Scholar]
- 13.Gimenez-Abian J F, Clarke D J. Checkpoints controlling mitosis. Bioessays. 2000;22:351–363. doi: 10.1002/(SICI)1521-1878(200004)22:4<351::AID-BIES5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Haldar S, Jena N, Croce C M. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann M, Lorenz H, Voll R, Grunke M, Woith W, Kalden J. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka T, Oshiba A, Sakata N, Terada N, Johnson G L, Gelfand E W. Aggregation of the FcRI on mast cells stimulates c-Jun amino-terminal kinase activity. J Biol Chem. 1996;271:12762–12766. doi: 10.1074/jbc.271.22.12762. [DOI] [PubMed] [Google Scholar]
- 18.Jones N A, Turner J, McIlwrath A J, Brown R, Dive C. Cisplatin and paclitaxel-induced apoptosis of ovarian carcinoma cells and the relationship between bax and bak up-regulation and the functional status of p53. Mol Pharmacol. 1998;53:819–826. [PubMed] [Google Scholar]
- 19.Jordan M A, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10:123–130. doi: 10.1016/s0955-0674(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 20.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell Biol. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 21.Ling Y, Tornos C, Perez-Soler R. Phosphorylation of bcl-2 is a marker of M phase events and not a determinant of apoptosis. J Biol Chem. 1998;273:18984–18991. doi: 10.1074/jbc.273.30.18984. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Y, Stein C A. Taxol and estramustine-induced modulation of human prostate cancer cell apoptosis via alteration in bcl-xL and bak expression. Clin Cancer Res. 1997;3:2039–2046. [PubMed] [Google Scholar]
- 23.Moos P J, Fitzpatrick F A. Taxane-mediated gene induction is independent of microtubule stabilization: induction of transcription regulators and enzymes that modulate inflammation and apoptosis. Proc Natl Acad Sci USA. 1998;95:3896–3901. doi: 10.1073/pnas.95.7.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiah S, Chuang S, Kuo M. Involvement of Asp-Glu-Val-Asp-directed, caspase-mediated mitogen-activated protein kinase kinase 1 cleavage, c-Jun N-terminal kinase activation, and subsequent Bcl-2 phosphorylation for paclitaxel-induced apoptosis in HL-60 cells. Mol Pharmacol. 2001;59:254–262. doi: 10.1124/mol.59.2.254. [DOI] [PubMed] [Google Scholar]
- 25.Sorger P K, Dobles M, Tournebize R, Hyman A A. Coupling cell division and cell death to microtubule dynamics. Curr Opin Cell Biol. 1997;9:807–814. doi: 10.1016/s0955-0674(97)80081-6. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava R K, Srivastava A R, Korsmeyer S J, Nesterova M, Cho-Chung Y S, Longo D L. Involvement of microtubules in the regulation of Bcl-2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol Cell Biol. 1998;18:3509–3517. doi: 10.1128/mcb.18.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L G, Liu X M, Kreis W, Budman D R. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review. Cancer Chemother Pharmacol. 1999;44:355–361. doi: 10.1007/s002800050989. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Popp D M, Wang H, Saitoh M, Mural J G, Henley D C, Ichijo H, Wimalasena J. Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK)-dependent and -independent pathways in ovarian cancer cells. J Biol Chem. 1999;274:8208–8216. doi: 10.1074/jbc.274.12.8208. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Wang H, Ichijo H, Paraskevi G, Foster J S, Fojo T, Wimalasena J. Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J Biol Chem. 1998;273:4928–4936. doi: 10.1074/jbc.273.9.4928. [DOI] [PubMed] [Google Scholar]
- 30.Wilson L, Jordan M A. Pharmacological probes of microtubule function. In: Hyams J S, Lloyd C W, editors. Microtubules. New York, N.Y: Wiley-Liss, Inc.; 1994. pp. 59–83. [Google Scholar]
- 31.Xia Y, Makris C, Su B, Li E, Yang J, Nemerow G R, Karin M. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc Natl Acad Sci USA. 2000;97:5243–5248. doi: 10.1073/pnas.97.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto K, Ichijo H, Korsmeyer S J. Bcl-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yujiri T, Fanger G R, Garrington T P, Schlesinger T K, Gibson S, Johnson G. MEK kinase 1 (MEKK1) transduces c-Jun NH2-terminal kinase activation in response to changes in the microtubule cytoskeleton. J Biol Chem. 1999;274:12605–12610. doi: 10.1074/jbc.274.18.12605. [DOI] [PubMed] [Google Scholar]
- 34.Yujiri T, Sather S, Fanger G R, Johnson G L. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 35.Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, Fanger G R, Henson P, Johnson G L. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-κB activation. Proc Natl Acad Sci USA. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]