Abstract
The situs ambiguous or heterotaxy syndrome is a type of syndrome that involves multiple visceral abnormalities, vascular ones and associated with left isomerism. Malformation of gastroenterologic system includes polysplenia (segmented spleen or multiple splenules), agenesis (partial or complete) of the dorsal pancreas and anomalous of the inferior vena cava implantation. Here, we describe and show the anatomy of a patient with left side inferior vena cava, situs ambiguous (complete common mesentery), polysplenia, and short pancreas. We also discuss about the embryologic process and the implications of these anomalies during gynecologic, digestive, and liver surgeries.
Keywords: Situs ambiguous, Polysplenia, Left side IVC, Anatomic variations
Introduction
The normal arrangement of the organs is called situs solitus. When all the organs are in a mirror image of the normal anatomy with a complete rotation of the midline it is called situs inversus. Intermediate situation are termed situs ambiguous or heterotaxy syndrome. Polysplenia - heterotaxy syndrome (or situs ambiguous) is a rare disorder describe by Helwing in 1929 [1,2]. This syndrome is a subtype of heterotaxy syndrome which involves multiple visceral and vascular abnormalities associated with situs ambiguous characterized with left isomerism [3,4].
Here, we describe the anatomy of a patient with left side inferior vena cava (IVC), situs ambiguous (complete common mesentery), polysplenia and short pancreas.
Case description
Here, we report a case of a 65 year old woman referred for postmenopausal bleeding.
Endometrial biopsy and pelvic magnetic resonance imaging showed a serous adenocarcinoma of the endometrium, stage Fédération Internationale de Gynécologie et d'Obstétrique IB.
At the preoperative workup computerized tomography scan a disseminated peritoneal carcinomatosis with mesentery involvement was diagnosed.
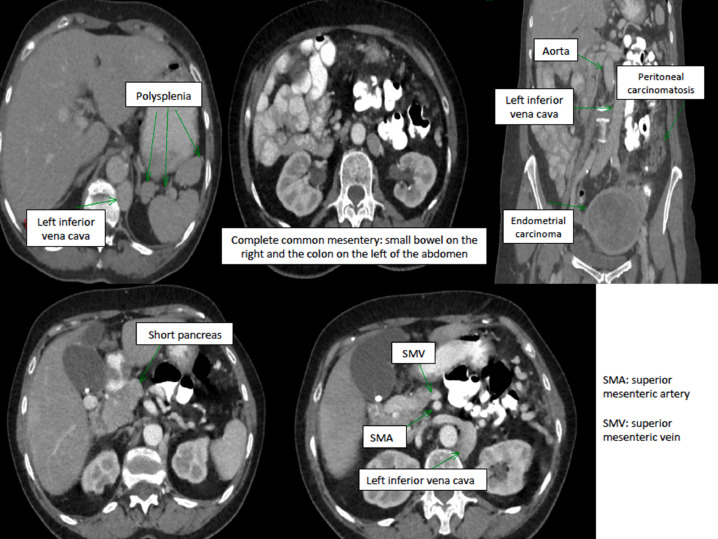
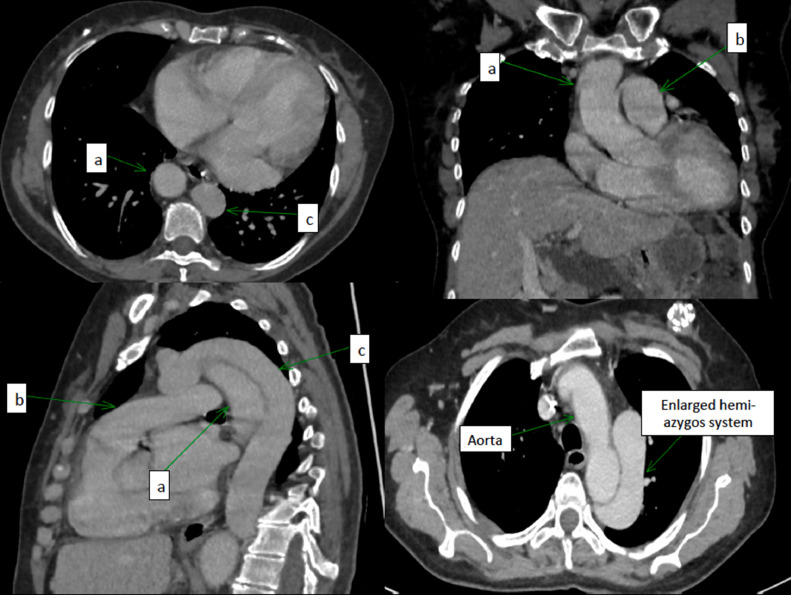
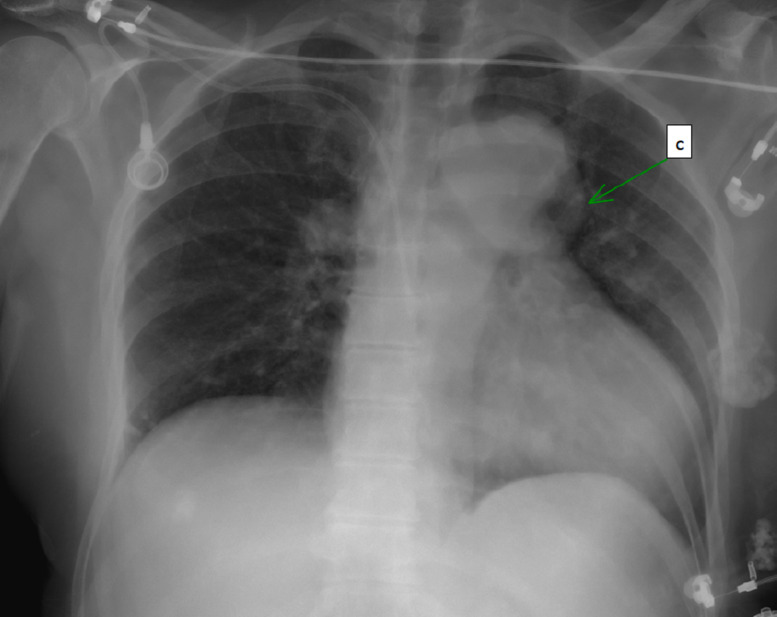
Several anatomic anomalies were detected in computerized tomography (Figs. 1 and 2) and in Chest radiograph (Fig. 3):
Fig. 1.
Abdominal anatomic anomalies detected in CT- scan.
Fig. 2.
Chest anatomic anomalies detected in CT. (A) Aorta; (B) pulmonary artery; (c) enlarged hemi-azygos system.
Fig. 3.
Chest radiograph. C= enlarged hemi-azygos system.
Vascular anomalies
-
-
Right IVC agenesis in intra-hepatic level. Left IVC, which is continued intrathoracically by the hemi-azygos system and which drains on the lower right face of the right atrium after a journey in the upper mediastinum. The IVC remains on the left of the aorta until the level of iliac bifurcation.
Digestive anomalies
-
-
Situs ambiguous- heterotaxy: Complete common mesentery with small bowel on the right, angle of Treitz on the right, colon on the left entirely. The superior mesenteric artery was behind and in contact with the superior mesenteric vein.
-
-
Polysplenia with 2 spleens with an accessory splenic nodule
-
-
Short pancreas
Decision to undergo neoadjuvant chemotherapy. After 4 cycles of carboplatinun and paclitaxel a significative regression of the carcinomatosis was confirmed in a diagnostic laparoscopy and Ca 125 normalized. Decision for interval debulking surgery that was performed with complete cytoreduction. The treatment was completed with 2 cycles of chemotherapy after surgery.
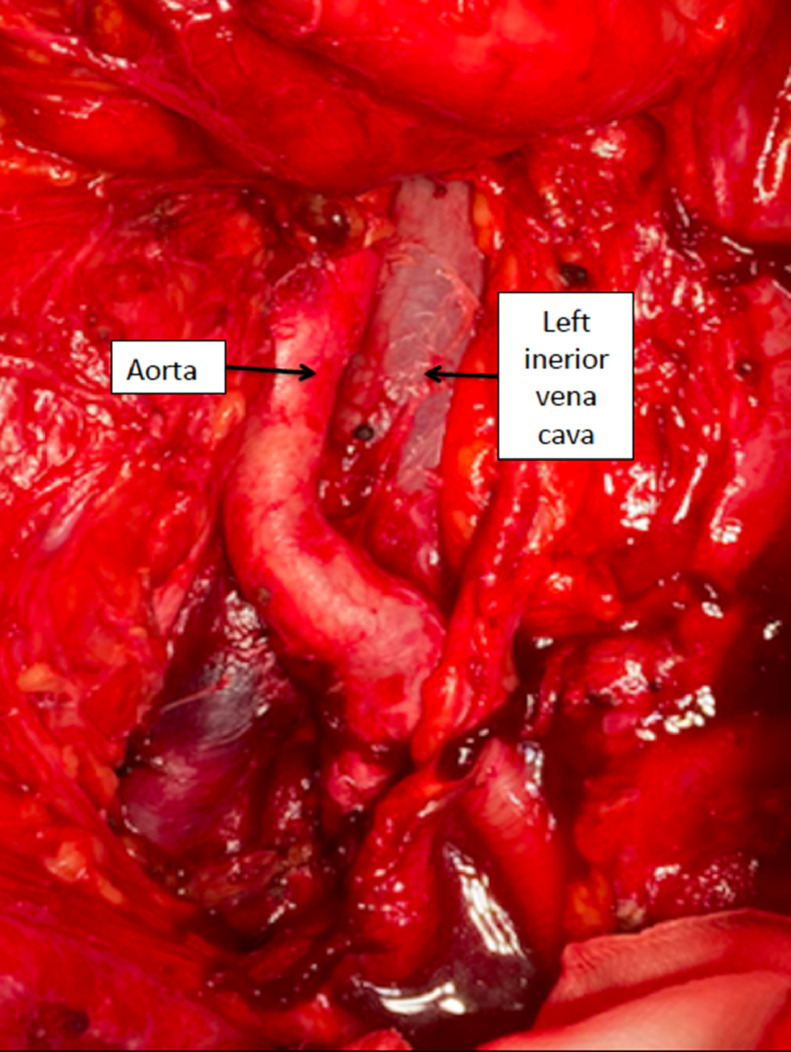
During the surgery we performed para-aortic lymphadenectomy which dissection showed the vascular abdominal anomalies (Fig. 4).
Fig. 4.
Left IVC with aorta moved to the right side of the abdomen.
Discussion
Human embryonic development is characterized as a highly ordered and nonrandom process that leads to an evident left-right asymmetry [5]. During embryogenesis, different processes are activated with the purpose of generating the organs that make up the human anatomy, as well as their correct positioning [6]. Asymmetry during embryonic development is determined by the ciliary dynamics of the primitive node. There are 2 types of monocilia in the primitive node: mobile and immobile. The firsts are in the center of the node and the seconds in the periphery, theses ones have calcium channels (Ca2+) Polycystin 2. Ciliary movement is clockwise, and this action generates the first right-left asymmetry of the organism. This ciliary dynamic generates an increase in Ca2+ entry through calcium channels (Ca2+) Polycystin 2 and the consequent activation of the Nodal gene. This is the signal that sets in motion the mechanisms that culminate in the asymmetric distribution of organs in the human anatomy [7]. The correct accomplishment of this process culminates with the normal development and positioning of the organs in the thorax and abdomen, called situs solitus. In this scenario the spleen, stomach, aorta, and cardiac apex with the bilobed lung are located on the left side while the liver, gall bladder, IVC and the trilobed lung are located on the right side. The most frequent malformation associated with an alteration of this process involves the anomalous position of the organs in the body rotated around the midline as a mirror image of the situs solitus and is called situs inversus. It has a prevalence of 0.01% and it is associated with 5%-10% of structural heart defects [8].
On the other hand, situs ambiguous or heterotaxia is defined as a clinical situation in which there is an alteration in the usual left-right distribution of the organs without a fixed or specific pattern. It is a very rare situation with an associated incidence of 0.0001% [9]. It can be divided into 2 categories according to the presence or absence of spleen:
-
-
Situs ambiguous with asplenia or right atrial isomerism (RAI); absence of spleen.
-
-
Situs ambiguous with polysplenia or left atrial isomerism (LAI); multiple splenules.
Characteristically, patients with RAI usually have a bilateral superior vena cava (SVC) and absence of coronary sinus. They often associate bilateral right atrial appendages, bilateral sinoatrial nodes, atrial septal defects, and a single atrioventricular (AV) valve or atresia. Due to the absence of the left atrium, the pulmonary veins are often associated with anomalous drainage, most often infracardiac to the hepatic/portal system or supracardiac to the SVC. On the other hand, they are also usually associated with a defect of the common AV canal, which generates a hemodynamic imbalance that functionally generates a single ventricle with a high incidence of outflow obstruction to the pulmonary territory [10,11]. Extracardiac manifestations include the presence of a symmetrical liver, midline stomach with associated intestinal malrotation, trilobed lungs and bilateral hypoarterial bronchi [12].
Patients with LAI, like our patient, characteristically have an interrupted IVC that drains into the azygos system through the liver, thence into the SVC and finally into the heart. Most often, they present with bilateral left atrial appendages, an ectopic atrial rhythm due to the absence of sinus node, pulmonary veins often drain directly into the atria, although not necessarily through a common central confluence, and AV septal defects resulting in a functional single ventricle anatomy with functional single ventricle, with outflow abnormalities including double outlet right ventricle and coarctation. Pulmonary outflow obstruction is much less common [10,12]. The most characteristic extracardiac manifestation is polysplenia, although they also associate a bilobed liver with variable position of the stomach on the right side with small bowel malrotation/obstruction, bilateral bilobed lungs, and bilateral hypoarterial bronchi [11]. And up to 10% of these patients will have associated congenital hepatobiliary malformations [13]. In relation to polysplenia, despite the above, in these patients it is neither constant nor necessary to diagnose the syndrome; recent publications confirm the possibility of these patients having a single polylobulated spleen or even a normal spleen [14].
One of the most important clinical differences between the 2 syndromes is their impact on hemodynamics. Patients affected with RAI syndrome usually have cyanosis, since almost all the supply to the pulmonary circulation depends on the ductus arteriosus and drainage to the pulmonary circulation is usually severely compromised; the mortality rate during the first year of life in these patients is 80%-90% [15]. On the other hand, those affected by LAI syndrome, in whom the alteration of the related structures of the pulmonary circulation is not so severe, most newborns will not have cyanosis associated with lower mortality rates during the pediatric phase [9].
For all the above, prenatal diagnosis of this syndrome in either of its 2 most frequent subtypes, is fundamental. The literature shows that this diagnosis is possible even when cardiac defects are not detectable [16]. Ultrasonographically, the most frequently described defects are: AV canal, double-outlet right ventricle, transposition of the great arteries, atrial septal defect, ventricular septal defect, malpositioned stomach, malpositioned liver, biliary cystic malformations, and polysplenia/asplenia [12].
As all malformation syndromes, the etiopathogenesis is multifactorial, a series of risk factors have been described that favor its development [12] and a series of genes related to its pathogenesis have been identified [7]. The most important of these are:
-
-
Cocaine use in the first trimester
-
-
Family history of congenital heart defects.
- -
-
-
Mutations in the ZIC3, NODAL, CFC1, ACVR2B, LEFTY2, CITED2, and GDF1 genes have been associated with the development of heterotaxia. The inheritance pattern is varied and all are related to growth factor beta3 synthesis [7].
Fetal mortality in patients with situs ambiguous is around 6%-10% [12]. The subgroup with RAI is the one with the worst prognosis, with a mortality rate in the first year of life of 80%-90% [15]. Death during this period is mainly due to heart block because of malformations at this level. The only risk factor for fetal death identified is bradycardia [19,20]. Regarding the malformations associated with heterotaxia syndrome, those most closely related to prognosis are cardiac malformations and the presence-absence of biliary atresia. The overall mortality associated with these patients is 85% in those with RAI and 50% in those with LAI [12].
The treatment of these patients must therefore be personalized and is determined by the types of malformations associated with them. In relation to cardiac malformations, in most cases, it is palliative in nature since total reconstruction of the normal anatomy is not possible in most cases. The surgical techniques used are varied, the most frequent being the Fontan technique, in any of its variants, but sometimes cardiac transplantation is necessary [10,13,21]. As for extracardiac congenital malformations, they should be carefully evaluated to determine the normal rotation of the intestinal bundle, the state of the biliary tract and splenic functionality. Depending on the findings, 1 surgical treatment or another should be considered, and sometimes liver transplantation is indicated [22].
In the case we present, the patient had not been previously diagnosed with the malformation syndrome she presented, since due to the absence of cardiac malformations and the venous drainage of the inferior vena cava, the hemodynamic alterations were nonexistent.
The preoperative diagnosis of these anomalies is important in digestive and gynecologic surgeries. In gynecologic oncological surgeries, vascular anomalies are important to consider if para-aortic lymphadenectomy is necessary, especially to avoid left renal vein or inferior mesenteric artery injuries. In cases of peritoneal carcinomasis, digestive resections may be necessary, as well as hepatic resection or mobilization of the liver for diaphragmatic stripping. In these cases, prior knowledge of the anatomic variations is essential to avoid complications.
Patient consent
Written informed consent for the publication of this case report was obtained from the patient's family member.
Ethical approval
Not applicable.
Authors' contributions
R.M.M. wrote manuscript text and supervision. D.K. wrote manuscript text and literature review. V.V. Supervision. M.K. Prepared figures. J.C.T. wrote manuscript text.
Availability of data and materials
Not applicable.
Footnotes
Funding: None.
Competing Interests: The authors declare do not have conflict of interests.
References
- 1.Rasool F, Mirza B. Polysplenia syndrome associated with situs inversus abdominus and type I jejunal atresia. APSP J Case Rep. 2011;2:18. [PMC free article] [PubMed] [Google Scholar]
- 2.El Mortaji H, Elatiqi K, El Hammaoui H, Alj S. Polysplenia syndrome with situs ambiguous, common mesentery, and IVC interruption discovered incidentally in an adult. Radiol Case Rep. 2019;14(9):1072–1075. doi: 10.1016/j.radcr.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapa S, Gleeson FC, Vege SS. Dorsal pancreas agenesis and polysplenia/heterotaxy syndrome: a novel association with aortic coarctation and a review of the literature. JOP. 2007;8(4):433–437. [PubMed] [Google Scholar]
- 4.Suthar T, Banker H, Shah M, Thakkar G. Splenic infarct with polysplenia syndrome and situs inversus. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abut E, Arman A, Güveli H, Bölükbaş C, Kendir T, Dalay R, et al. Malposition of internal organs: a case of situs ambiguous anomaly in an adult. Turk J Gastroenterol. 2003;14:151‑5. [PubMed] [Google Scholar]
- 6.Lambert TE, Kuller J, Small M, Rhee E, Barker P. Abnormalities of fetal situs: an overview and literature review. Obstet Gynecol Surv. 2016;71:33‑8. doi: 10.1097/OGX.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 7.Anwar A, Jubin J, Raza S, Mirza ZK. A rare case of recurrent pneumonia in heterotaxy syndrome, polysplenia/left isomerism. Cureus. 2021;13(10):e19055. doi: 10.7759/cureus.19055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marta MJ, Falcão LM, Saavedra JA, Ravara L. A case of complete situs inversus. Rev Port Cardiol. 2003;22:91‑104. [PubMed] [Google Scholar]
- 9.Joshi BM, Singh S, Kumar A, Sandhu MS, Rana D. Situs ambiguous anomaly during laparoscopic cholecystectomy in an adult female. Niger J Surg. 2020;26(1):72–77. doi: 10.4103/njs.NJS_47_183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiraishi I, Ichikawa H. Human heterotaxy syndrome—from molecular genetics to clinical features, management, and prognosis. Circ J. 2012;76:2066–2075. doi: 10.1253/circj.cj-12-0957. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MS, Anderson RH, Cohen MI, Atz AM, Fogel M, Gruber PJ, et al. Controversies, genetics, diagnostic assessment, and outcomes relating to the heterotaxy syndrome. Cardiol Young. 2007;17(2):29–43. doi: 10.1017/S104795110700114X. [DOI] [PubMed] [Google Scholar]
- 12.Lambert TE, Kuller J, Small M, Rhee E, Barker P. Abnormalities of fetal situs: an overview and literature review. Obstet Gynecol Surv. 2016;71(1):33–38. doi: 10.1097/OGX.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ. Heterotaxy syndrome. Korean Circ J. 2011;41(5):227–232. doi: 10.4070/kcj.2011.41.5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath J, Brueckner M. Cilia are at the heart of vertebrate leftright asymmetry. Curr Opin Genet Dev. 2003;13:385–392. doi: 10.1016/s0959-437x(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 15.Balan A, Lazoura O, Padley SP, Rubens M, Nicol ED. Atrial isomerism: a pictorial review. J Cardiovasc Comput Tomogr. 2012;6:127‑36. doi: 10.1016/j.jcct.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Lin JH, Chang CI, Wang JK, Wu MH, Shyu MK, Lee CN, et al. Intrauterine diagnosis of heterotaxy syndrome. Am Heart J. 2002;143(6):1002–1008. doi: 10.1067/mhj.2002.122873. [DOI] [PubMed] [Google Scholar]
- 17.Belmont JW, Mohapatra B, Towbin JA, Ware SM. Molecular genetics of heterotaxy syndromes. Curr Opin Cardiol. 2004;19(3):216–220. doi: 10.1097/00001573-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Frías JL, Frías JP, Frías PA, Martínez-Frías ML. Infrequently studied congenital anomalies as clues to the diagnosis of maternal diabetes mellitus. Am J Med Genet A. 2007;143:2904–2909. doi: 10.1002/ajmg.a.32071. [DOI] [PubMed] [Google Scholar]
- 19.Escobar-Diaz MC, Friedman K, Salem Y, Marx GR, Kalish BT, Lafranchi T, et al. Perinatal and infant outcomes of prenatal diagnosis of heterotaxy syndrome (asplenia and polysplenia) Am J Cardiol. 2014;114(4):612–617. doi: 10.1016/j.amjcard.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MS, Schultz AH, Tian ZY, Donaghue DD, Weinberg PM, Gaynor JW, et al. Heterotaxy syndrome with functional single ventricle: does prenatal diagnosis improve survival? Ann Thorac Surg. 2006;82(5):1629–1636. doi: 10.1016/j.athoracsur.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 21.de Leval MR. The Fontan circulation: a challenge to William Harvey? Nat Clin Pract Cardiovasc Med. 2005;2:202–208. doi: 10.1038/ncpcardio0157. [DOI] [PubMed] [Google Scholar]
- 22.Kothari SS. Non-cardiac issues in patients with heterotaxy syndrome. Ann Pediatr Cardiol. 2014;7:187–192. doi: 10.4103/0974-2069.140834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.