(See the Major Article by Edwards et al on pages 1928–36.)
Recurrent vulvovaginal candidiasis (RVVC) is a debilitating disease of immunologically normal women. The major causative agent of RVVC is Candida albicans. Although RVVC episodes respond to antifungal therapy, the ongoing need for therapy poses the risk of selecting for antifungal resistance. There is no validated therapy to prevent recurrence. In this issue of Clinical Infectious Diseases Edwards et al [1] describe the results of a phase 2 clinical trial testing a C. albicans vaccine in patients with RVVC. The results show that the vaccine was safe and well tolerated. Remarkably, vaccinated women also experienced a reduction in symptoms. Given that therapy for RVVC is unsatisfactory, fungal diseases are an ever-increasing clinical problem, and there are no licensed vaccines against fungal pathogens, this report of a safe vaccine associated with some protection against disease is a significant advance. Although the results of early clinical trials always warrant caution, this report is a major development for the fungal vaccine field.
The NDV-3A vaccine is composed of recombinant Als3 antigen formulated with aluminum hydroxide as an adjuvant. Als stands for agglutinin-like sequence, and Als3 is one glycoprotein in this family [2]. Als3 has protean functions in C. albicans pathogenesis functioning in invasion, adhesion, and biofilm formation [3]. The NDV-3A vaccine elicits antibodies to Als3 that promote killing of C. albicans by neutrophils and appears to mediate protection by inducing both B- and T-cell responses [4]. A monoclonal antibody to Als3 mediates direct fungicidal activity against C. albicans [5].
This study is remarkable for its innovation. It is the first vaccine composed of a purified recombinant fungal antigen tested in humans. It is also the first vaccine targeting a commensal organism that reduces symptoms without eradicating the microbe. In addition, it is a therapeutic vaccine, because its target population is women already infected with C. albicans. This point is of particular interest because most vaccines mediate protection by eliciting immune responses that prevent the targeted microbe from establishing itself in the host. There are currently only 2 licensed vaccines that are “therapeutic” in the sense that they are administered to people who already harbor the microbe they target. These vaccines are for the prevention of rabies and zoster. In the case of rabies, the vaccine is effective because it elicits an immune response that controls the virus before it reaches the brain. In the case of zoster, the vaccine is effective because it boosts immunity against varicella virus and prevents its reactivation.
To understand the fundamental innovation in the design of NDV-3A it is important to review the pathogenesis of VVC. The current view of VCC pathogenesis posits that the disease is the result of an overexuberant host inflammatory response to fungal antigens in the vagina [6]. This view puts vaginal candidiasis more in the realm of allergic inflammation than a locally invasive fungal disease. As such, the success of the NDV-3A vaccine is remarkable and highly innovative, because it may reduce the likelihood of RVVC by altering the host inflammatory response to Candida, rather than mediating fungal clearance. The finding that the vaccine is safe is of major importance. At present, there is no evidence that NDV-3A-elicited immunity affected other body surfaces colonized with C. albicans.
Administered systemically, the NDV-3A vaccine mediates protection at a mucosal site. This is reminiscent of a novel herpes vaccine that mediates mucosal immunity after systemic vaccination in experimental herpes simplex [7]. The herpes vaccine mediates viral elimination via antibody immunity, namely, antibody-dependent cellular cytotoxicity, rather than viral neutralization. Although the mechanism of action of the NDV-3A vaccine in humans remains to be determined, it is likely that it too controls symptomatic VVC via antibody immunity. Therefore, assuming the pathogenesis of VVC involves overexuberant vaginal mucosal inflammation; vaccine-elicited antibodies are likely to mediate protection by controlling the inflammatory response. Certain immunoglobulin Gs dampen inflammation by engagement of the inhibitory Fc receptor, whereas others can reduce inflammation by triggering microbial uptake and apoptosis. These mechanisms of antibody action require Fc receptors. There are also direct mechanisms of antibody action that may dampen inflammation. Bacterial and fungal antibodies to capsular polysaccharides can directly affect the biology of the microbe they target [8]. Given that fungal burdens did not differ significantly in vaccinated and control participants, it is unlikely vaccine-mediated immunity involved fungal elimination. Therefore, it is interesting to speculate that binding of certain NDV3-elicted antibodies to Als3 may alter C. albicans biology in a manner that alters expression of antigens that trigger inflammation. In support of this hypothesis, a monoclonal antibody to Als3 (C7) mediated direct antifungal effects [5]. Although C7 induced fungal killing, other mechanisms of direct antibody effects, including immune modulation, may occur. Thus, NDV-3A could work via antibodies that mediate a reduction in the inflammatory response to Als3 without a significant effect on the fungal burden. In this regard, NDV-3A may be a landmark human vaccine that mediates protection by altering the immune response at a mucosal body site without affecting the microbial burden.
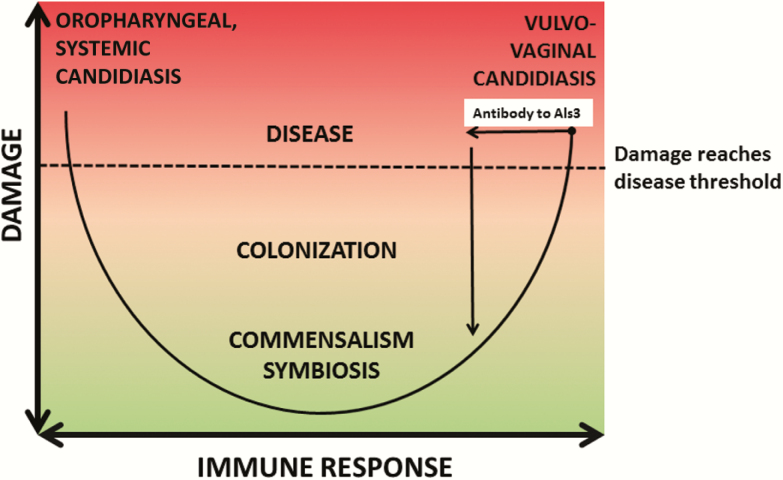
When the pathogenesis of candidiasis is viewed through the lens of the “damage-response framework” (DRF) of microbial pathogenesis [9], it is apparent that disease can occur at the extremes of the immune response (Figure 1). In settings of impaired immunity such as the immunosuppression of AIDS or breaches of integument following intravenous line placement, oropharyngeal or systemic candidiasis, respectively, may occur. The DRF depicts the outcome of host-microbe interaction with a parabola, whereby the Y-axis shows damage as a function of the host response on the X-axis, ranging from weak on the left to strong on the right [10]. The aforementioned Candida-associated disease states fall on the left-hand side of the parabola. In contrast, VVC, which occurs in immunologically normal women who exhibit an inappropriately overexuberant or strong immune response to candida, falls on the right-hand side of the parabola. Thus, the NDV-3A vaccine may mediate its protective effect in immunized individuals by moving the outcome of their interaction with candida in the vaginal mucosa from the right-hand to the left-hand side of the parabola, thereby reducing damage. Notably, an overexuberant immune response may be protective in the setting of systemic candidiasis by enhancing fungal clearance via inflammation. As such, a vaccine to prevent systemic candidiasis in patients without the ability to generate a sufficient immune response may need to promote, rather than dampen inflammation. This might call for antibodies and/or other immune mediators that enhance fungal killing and induce fungal elimination. Nonetheless, if antibodies to Als3 can exert direct effects on C. albicans, it is possible the NDV-3A vaccine will also be useful for other types of candidiasis, particularly in patients with defects in cellular immunity that may impair antibody action, although this must await further clinical trials.
Figure 1.
Proposal for how the NDV-3A vaccine may mediate protection from the view of the damage-response (DRF) of microbial pathogenesis. The DRF posits that disease occurs in the setting of disproportionately, or inappropriately, weak or strong immune responses. According to the DRF, the vaccine may work by eliciting an antibody response that reduces the vaginal immune response to Candida. This would result in a reduction in the inflammatory response, which in would in turn move the outcome of host-microbe interaction to the left, resulting in reduced damage, fewer clinical symptoms, and fewer recurrences of vulvovaginal candidiasis.
The success of the NDV-3A vaccine should encourage development of vaccines for other commensal organisms that are also associated with states of disease. This vaccine sets an important precedent, namely, that microbial clearance/elimination may not be necessary to prevent diseases that occur at/on mucosal surfaces. This may be relevant to the design of vaccines against Staphylococcus spp. and bacterial causes of gingivitis. Finally, during these times of attacks on science, it is worth noting that the NDV-3A vaccine is a product of basic science research. The discovery of Als3 came from basic studies of C. albicans biology and led to hypothesis testing in preclinical animal models of disease, which produced insights that led to a vaccine that addresses a difficult clinical problem. In short, the NDV-3A is a scientific triumph that holds promise for alleviating human suffering.
Note
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Arturo Casadevall, Department of Molecular Microbiology and Immunology of the Johns Hopkins School of Public Health, Baltimore, Maryland.
Liise-anne Pirofski, Division of Infectious Diseases, Department of Medicine, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, New York.
References
- 1. Edwards JE, Jr., Schwartz MM, Schmidt CSet al. Fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis; a phase 2 randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2018; 66:1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet 1998; 33:451–9. [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell 2011; 10:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ibrahim AS, Luo G, Gebremariam Tet al. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine 2013; 31:5549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brena S, Omaetxebarría MJ, Elguezabal N, Cabezas J, Moragues MD, Pontón J. Fungicidal monoclonal antibody C7 binds to Candida albicans Als3. Infect Immun 2007; 75:3680–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fidel PL, Jr. History and update on host defense against vaginal candidiasis. Am J Reprod Immunol 2007; 57:2–12. [DOI] [PubMed] [Google Scholar]
- 7. Petro C, Gonzalez PA, Cheshenko Net al. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casadevall A, Pirofski LA. A new synthesis for antibody-mediated immunity. Nat Immunol 2012; 13:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 2003; 1:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jabra-Rizk MA, Kong EF, Tsui Cet al. Candida albicans pathogenesis: fitting within the host-microbe damage response framework. Infect Immun 2016; 84:2724–39. [DOI] [PMC free article] [PubMed] [Google Scholar]