Abstract
Simplified drug regimens may improve retention in care for persons with chronic diseases. In April 2013, South Africa adopted a once-daily single-pill human immunodeficiency virus (HIV) treatment regimen as the standard of care, replacing a multiple-pill regimen. Because the regimens had similar biological efficacy, the shift to single-pill therapy offered a real-world test of the impact of simplified drug-delivery mechanisms on patient behavior. Using a quasi-experimental regression discontinuity design, we assessed retention in care among patients starting HIV treatment just before and just after the guideline change. The study included 4,484 patients starting treatment at a large public sector clinic in Johannesburg, South Africa. The share of patients prescribed a single-pill regimen increased by over 40 percentage points between March and April 2013. Initiating treatment after the policy change was associated with 11.7–percentage-points’ higher retention at 12 months (95% confidence interval: −2.2, 29.4). Findings were robust to different measures of retention, different bandwidths, and different statistical models. Patients starting treatment early in HIV infection—a key population in the test-and-treat era—experienced the greatest improvements in retention from single-pill regimens.
Keywords: fixed-dose combination treatment, HIV, instrumental variables, regression discontinuity, retention in care, single-tablet treatment, South Africa
Abbreviations
- ART
antiretroviral therapy
- CACE
complier average causal effect
- CD4
cluster of differentiation 4
- CI
confidence interval
- FDC
fixed-dose combination
- HIV
human immunodeficiency virus
- ITT
intention-to-treat
- MSE
mean squared error
- RDD
regression discontinuity design
- WHO
World Health Organization
Management of human immunodeficiency virus (HIV) infection requires long-term adherence to daily medication use, similar to many other chronic conditions. Patients with poor adherence to and retention in HIV treatment are at increased risk for drug resistance (1, 2), hospitalization (3, 4), transmission (5), and mortality (6, 7). Regimen complexity may be an important barrier to treatment adherence and retention (8). Early treatment regimens involved multiple pills taken multiple times per day. Fixed-dose combinations (FDCs) combining 3 antiretroviral medications into a single daily pill were designed to lower pill-taking burdens (9) and improve adherence and retention (10).
South Africa has the largest HIV treatment program in the world, with 4 million people on antiretroviral therapy (ART) in 2019 (11). However, just half of patients who start therapy remain in care at 5 years (12). Beginning on April 1, 2013, South Africa offered FDC treatment for all HIV patients starting therapy in the country’s public facilities (13). FDCs were subsequently offered to patients already established on first-line treatment (14). Dr. Ashraf Coovadia of the South African National AIDS Council highlighted the motivations for the FDC guideline change in an interview (emphasis added):
The drugs that are combined in the FDC tablet are neither new nor superior to the individual drugs that we have been using. The difference is that they are more convenient to take in this form. Added to this convenience is the ability to take it only once per day. We hope and expect that adherence will become better as a result of having only one pill to take (15).
Because the biological efficacies of the FDC and multiple-pill regimens were similar, the rapid introduction of FDCs offered a test of the hypothesis that simplified regimens can improve patient outcomes even without changes in the underlying therapeutic value of a drug.
Although taking multiple pills may seem like a small inconvenience for life-saving therapy, evidence from the behavioral economics literature suggests that small, nonmonetary “hassle” costs can have substantial effects on behavior (16–18). The challenges of multiple pill regimens may also interact with other barriers to adherence and retention, including poor mental health, substance use, inflexible work hours, migration, lack of social support, poverty, and stigma (19–21). Simplified regimens are likely to have the greatest impact on behavior for people who are not already strongly motivated to be in HIV treatment. For example, whereas ART is a matter of life and death for people with advanced HIV disease, “hassle” costs may be more salient for patients starting ART early in HIV infection, when the therapeutic benefits of ART are smaller (22, 23). Because ART virtually eliminates HIV transmission, HIV test-and-treat policies have sought to increase ART uptake, adherence, and retention early in HIV infection in order to reduce population incidence of HIV.
Using a quasi-experimental regression discontinuity design (RDD), we sought to estimate the causal effect of initiating FDC treatment, as compared with multiple pills, on clinical retention in a large public-sector HIV clinic in South Africa. Clinical trials in North America, Europe, and Australia have shown improved adherence to single-pill regimens as compared with multiple-pill regimens (9, 24, 25), and these results have been confirmed in observational studies (26–28); but few studies have evaluated causal effects of these regimens on retention in real-world, nontrial settings in sub-Saharan Africa (29). Understanding the value of single-pill FDC regimens in real-world settings has implications for treatment guidelines and investments in pharmaceutical innovation to reduce regimen complexity—for HIV as well as for other manageable chronic conditions.
METHODS
Data and study population
The study population included treatment-naive adult patients (ages 16 years or older) initiating first-line ART from September 1, 2011 to August 31, 2014, at Themba Lethu Clinic, a large outpatient public-sector HIV treatment clinic in Johannesburg, South Africa (30). Information on patient demographic characteristics, laboratory results, prescriptions, visit history, clinical conditions, and follow-up status (in care, died, lost to follow-up, or transferred) was captured at each clinical encounter in an electronic medical record called TherapyEdge-HIV (Advanced Biological Laboratories S.A./TherapyEdge Inc., Luxembourg City, Luxembourg). After initiating ART, patients had medical follow-up visits at months 1, 3, 6, and 12, and annually thereafter. Viral loads were measured at 6 and 12 months and annually thereafter, until 2013, when the 12-month measure was eliminated. Patients returned to the clinic to pick up ART medications monthly for the first 6–12 months of treatment and every 2 months thereafter, once stable (30).
Pharmacy dispensing data were recorded in a separate electronic system and were available for September 2012–October 2014. Data on prescription date, regimen, brand name, and dosing instructions were used to determine the patient’s first treatment regimen. Beginning on April 1, 2013, single-tablet FDCs of tenofovir/emtricitabine/efavirenz were recommended as first-line treatment (14), replacing a prior regimen which included 3 pills, once per day, of tenofovir, lamivudine or emtricitabine, and efavirenz or nevirapine (13).
All patients were followed up for at least 1 year. Throughout the study period, patients were eligible for ART if they had 1) a cluster of differentiation 4 (CD4)-positive (CD4+) cell count less than or equal to 350 cells/μL or 2) a CD4+ cell count greater than 350 cells/μL and World Health Organization (WHO) stage 4 disease (31). Eligibility was extended to patients with WHO stage 3 disease on April 13, 2013. Because this change coincided with the FDC policy, we excluded patients with CD4+ cell counts greater than 350 cells/μL and WHO stage 3 disease (n = 17 patients), who would have been ART-eligible only in the postpolicy period. We additionally excluded pregnant women and patients with tuberculosis (32, 33) and patients whose regimen could not be ascertained from pharmacy data (n = 125).
Study design
We performed a regression discontinuity analysis (34, 35) to assess whether starting ART in the FDC era affected retention in treatment. We compared outcomes among patients who initiated ART immediately before the guideline change and those who initiated it immediately afterward. Under the assumption that dates of ART initiation are as-good-as-randomly assigned, patients initiating ART before and after the policy change were similar, on average, with respect to observed and unobserved characteristics, similar to a randomized trial. Differences at the threshold are interpretable as intention-to-treat (ITT) effects of the policy change. The policy change can also be used as an instrumental variable to estimate the effect of starting FDC vis-à-vis multiple pills.
Exposure and outcome assessment
Our primary measure of attrition was lapse in care within the first year of treatment, defined as any ≥4-month period with no clinical visits or ART pickups. This included short-term gaps as well as deaths, losses to follow-up, and transfers for patients with final visits within the first 12 months of treatment (with follow-up until 16 months to detect the 4-month gap). To assess robustness to different definitions of retention, we also examined risks of not being in care 1 year after initiation (no visits at 12–16 months), long-term attrition (≥3-year absence from care starting in the first year), and no 6-month viral load testing (4–10 months) as a laboratory-based proxy for retention in ART. For all outcomes, mortality was included as loss to follow-up.
Our primary exposure was whether the patient starting ART was prescribed an FDC or multiple-pill regimen (“regimen type”). Regimen type was not universally documented in clinical notes (52% missing) or pharmacy records (5% missing). We classified patients as starting FDC if either source indicated an FDC regimen. We assumed that patients who initiated ART prior to the September 2012 availability of pharmacy data were prescribed multiple pills, since FDCs were not yet available. We used date of ART initiation as the assignment variable in the RDD, with patients starting on April 1, 2013, or later exposed to the new guidelines.
Statistical analysis
We estimated the association between starting ART after the FDC policy change and retention in care in regression discontinuity models, following RDD best practices. We modeled the relationship between the assignment variable (date of initiation) and outcomes using local linear regression models, allowing for an intercept shift at the threshold (April 1, 2013) and separate slopes on either side of the threshold. We limited our analyses to patients who started ART within a bandwidth around the threshold and used a triangular kernel to place greater weight on observations closer to the threshold (36, 37). We selected the bandwidth using a data-driven algorithm that minimizes the mean squared error (MSE) of the RDD treatment effect estimator, balancing the fit of the model (less bias with smaller bandwidths) against precision (lower variance with larger bandwidths) (37–39). Point estimates and robust bias-corrected 95% confidence intervals (CIs) were calculated using the “rdrobust” command in Stata (StataCorp LLC, College Station, Texas) (39). In sensitivity analyses, we reran our analyses using bandwidths of 50% and 200% of the MSE-optimal bandwidth and with a rectangular kernel. Linear probability models offer an intuitive risk-difference interpretation and perform well when the predicted probability is not close to 0 or 1. We used logistic regression models in sensitivity analyses.
RDDs yield valid causal inferences if patients starting ART just before/after April 2013 are truly similar. Causal inference may be jeopardized if ART starting dates were manipulated—for example, if select patients were deliberately “held back” to initiate ART after the policy change. We assessed for systematic manipulation using the McCrary density test (40), comparing the number of patients starting ART just before the policy change with those starting ART just after the policy change. We also assessed the similarity of patients starting ART just before/after the policy change with respect to measured covariates. Assessing similarity in baseline covariates at the threshold serves the same purpose as a balance table in a randomized clinical trial, building confidence that the treatment was in fact as-good-as-randomly assigned. We also evaluated the potential for bias due to missingness in pharmacy data used to classify regimen type, comparing completeness of dispensing records before and after the policy change. In addition, we assessed for changes in dosing that could have led to mismeasurement of retention on different regimens.
Our primary analysis assessed the ITT effect of starting treatment after the policy change. We note that this ITT effect was diluted by the presence of patients who started multiple-pill regimens even after the policy change (“never takers”) and a small share of patients who started FDC ahead of the policy change (“always takers”). Because these patients’ treatment regimens were not affected by their ART starting date, we expected no difference in attrition for these groups. In order to estimate the causal effect of regimen type actually prescribed, we fitted “fuzzy” RDD models, using the April 2013 policy change as an instrument for whether the patient started FDC. Under additional assumptions of excludability and monotonicity (see the Web Appendix, available at https://doi.org/10.1093/aje/kwac006), this analysis estimates a complier average causal effect (CACE)—that is, the causal effect of being prescribed FDC on attrition among “compliers,” those patients who were prescribed FDC because of the guideline change. We used the “rdrobust, fuzzy()” routine in Stata (37) to estimate the CACE, with a single MSE-optimal bandwidth jointly selected for the combined model, accounting for bias and precision in both the first-stage and ITT estimates. We report robust, bias-corrected 95% CIs for the CACE (39, 41).
We hypothesized that the impact of FDC on retention would be larger for treatment initiators who were in better health. We therefore stratified our analyses by baseline patient health: CD4+ cell count (0–199 cells/μL or ≥200 cells/μL), WHO clinical stage (stage 1 or 2, representing early-stage HIV disease, vs. stage 3 or 4, representing later-stage HIV disease), and anemia status (hemoglobin concentration <13 g/dL for men and <11.5 g/dL for women). We also stratified by sex (male/female) and age at treatment initiation (16–29, 30–39, 40–49, or ≥50 years).
RESULTS
Cohort characteristics
The study population included 4,626 patients who initiated first-line ART between September 2011 and August 2014, prior to exclusion of patients with CD4+ cell counts greater than 350 cells/μL and WHO stage 3 disease, pregnant women, and patients with tuberculosis (n = 142). After exclusions, our analysis included 4,484 patients, of whom 1,121 started ART within 180 days of the guideline change. Around the time of the policy change (±180 days), the population of patients initiating treatment at Themba Lethu was 43% male, with a mean age of 38.5 (standard deviation, 9.9) years and a mean CD4+ cell count of 192.8 (standard deviation, 162.2) cells/μL. Forty-five percent of patients were anemic, and 14% presented with WHO stage 3 or 4 disease (Table 1). Of 1,281 patients initiating a single-treatment FDC regimen, 98.6% were prescribed tenofovir/emtricitabine/efavirenz. Of the 3,203 patients prescribed multiple-pill regimens, treatments included tenofovir/lamivudine/efavirenz (79.0%), stavudine/lamivudine/efavirenz (12.6%), tenofovir/lamivudine/nevirapine (4.6%), tenofovir/emtricitabine/efavirenz (2.8%), and stavudine/lamivudine/nevirapine (1.0%).
Table 1.
Baseline Characteristics of Patients Initiating Antiretroviral Therapy for HIV Infection at Themba Lethu Clinic ≤180 Days Before and After the Policy Change Recommending Use of Fixed-Dose Combination Treatment Regimens, Johannesburg, South Africa, 2011–2014
|
Total
(n = 1,121) |
≤180 Days Before Policy Change (n = 598) | ≤180 Days After Policy Change (n = 523) | Difference Associated With Policy Change a | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
Baseline Characteristic
or ART Regimen |
No. | % | No. | % | No. | % |
RDD
Estimate, PPb |
95% CI |
Bandwidth
c
,
days |
| Male sex | 487 | 43.4 | 252 | 42.1 | 235 | 44.9 | 2.2 | −10.5, 17.5 | ±216 |
| Age at treatment initiation, yearsd | 38.5 (9.9) | 38.6 (9.5) | 38.5 (10.3) | −1.8 | −5.1, 1.3 | ±171 | |||
| CD4+ cell count at baseline, cells/μLd | 192.8 (162.2) | 196.2 (163.1) | 188.8 (161.2) | −25.6 | −99.0, 36.2 | ±135 | |||
| Anemia at baseline | 500 | 44.6 | 270 | 45.2 | 230 | 44.0 | 8.9 | −7.2, 25.0 | ±149 |
| WHO stage ≥3 | 155 | 13.8 | 81 | 13.5 | 74 | 14.1 | 3.6 | −7.9, 15.6 | ±142 |
| Received FDC regimen | 463 | 41.3 | 15 | 2.5 | 448 | 85.7 | 41.8 | 19.3, 57.3 | ±70 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; FDC, fixed-dose combination; HIV, human immunodeficiency virus; MSE, mean squared error; PP, percentage points; RDD, regression discontinuity design; WHO, World Health Organization.
a The bandwidth is the window of data around the threshold that was used to generate the RDD estimate. For each outcome, an MSE-optimal bandwidth was computed using the “rdrobust” command in Stata (37).
b Values presented are PP unless otherwise indicated.
c Calculated using local linear regression with an MSE-optimal bandwidth, a triangular kernel, and robust, bias-corrected 95% CIs. The model included separate slopes on either side of the threshold and a shift in intercept at the threshold, which is the risk difference associated with the policy.
d Values are expressed as mean (standard deviation) and difference in mean values at the time of the policy change.
Initiation of patients on a single-tablet FDC regimen
We observed a clear shift from multiple-pill regimens to single-pill regimens after April 1, 2013 (Figure 1, Web Figure 1, Table 1), with the policy leading to an immediate 41.8–percentage-point (95% CI: 19.3, 57.3) increase in the share of patients starting FDC and further increases thereafter. Overall, FDC was prescribed to 2.5% of patients in the 6 months before the policy change and 85.7% of patients in the 6 months after the policy change (Table 1), an increase of 83.2 percentage points in the percentage of patients starting FDC.
Figure 1.
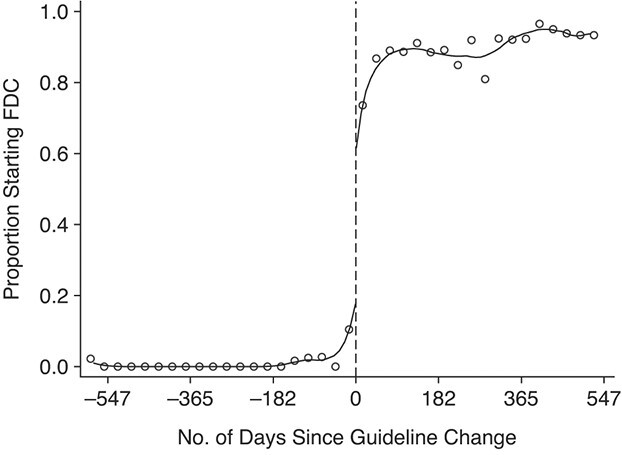
Monthly proportion of all patients initiating standard first-line antiretroviral therapy at Themba Lethu Clinic who were prescribed a single-pill regimen, Johannesburg, South Africa, 2011–2014. Dots represent monthly percentages; lines represent local linear regression models with a 70-day bandwidth and a triangular kernel. FDC, fixed-dose combination.
Evidence for the validity of the design
The McCrary density test found no evidence that ART starting dates were manipulated (Web Figure 2). Regression discontinuity models revealed that patients starting ART just before and just after the guideline change were similar with regard to baseline covariates, consistent with the quasi-random assignment of ART starting dates in the neighborhood around the threshold (Table 1, Web Figure 3). A comparison of monthly treatment type distribution (FDC vs. multiple pills) from clinic records versus pharmacy data showed similar distributions over time from the 2 data sources (Web Figure 4), suggesting that completeness of pharmacy records did not differ by regimen type. Further, we observed no notable change in the number of pharmacy pickups among patients remaining in care (Web Figure 5), implying that the quantity of daily doses dispensed at one pickup was the same for multiple-pill and FDC regimens. These findings support causal attribution of differences in outcomes at the threshold to the FDC policy.
Effects of the FDC policy change on retention in care
Table 2 presents ITT estimates of the impact of the FDC policy change on patient retention. Our primary outcome—the number of patients experiencing a ≥4-month gap in care—dropped from 38.3% to 26.5% (−11.7 percentage points; 95% CI: −29.4, 2.2) among patients starting treatment before the policy change versus after the policy change. Absence from care at 1 year decreased from 31.0% to 19.4% (−11.6 percentage points; 95% CI: −28.1, 1.0), long-term attrition by 1 year decreased from 29.4% to 16.8% (−12.6 percentage points; 95% CI: −28.2, −0.8), and noncompliance with 6-month viral load monitoring decreased from 34.4% to 19.0% (−15.5 percentage points; 95% CI: −28.9, −2.5). Plots of our primary outcome (Figure 2) and secondary outcomes (Figure 3) illustrate the differences in attrition with the policy change.
Table 2.
Regression Discontinuity Results for Attrition Outcomes Associated With the April 1, 2013, Switch to Fixed-Dose Combination Treatment as Standard First-Line Antiretroviral Therapy for HIV at Themba Lethu Clinic, Johannesburg, South Africa, 2011–2014
| Intention-to-Treat Effect a | Complier Average Causal Effect b | |||||||
|---|---|---|---|---|---|---|---|---|
| Predicted % | ||||||||
| Outcome | Bandwidth c , days | Just Before April 1, 2013 | Just After April 1, 2013 | RD | 95% CI | Bandwidth c , days | RD | 95% CI |
| ≥4-month gap in care during first year | ±147.0 | 38.3 | 26.5 | −11.7 | −29.4, 2.2 | ±129.6 | −21.7 | −54.7, 2.1 |
| Absent from care at 1 year | ±143.5 | 31.0 | 19.4 | −11.6 | −28.1, 1.0 | ±130.4 | −19.9 | −49.7, 1.9 |
| Long-term attrition by 1 year | ±146.3 | 29.4 | 16.8 | −12.6 | −28.2, −0.8 | ±127.4 | −21.6 | −51.1, −0.1 |
| No 6-month viral load monitoring | ±193.3 | 34.4 | 19.0 | −15.5 | −28.9, −2.5 | ±119.2 | −27.7 | −58.8, −0.7 |
Abbreviations: CI, confidence interval; ITT, intention-to-treat; MSE, mean squared error; RD, risk difference.
a ITT effects were estimated using the “rdrobust” command in Stata (37), with an MSE-optimal bandwidth based on a triangular kernel. The 95% CIs are robust, bias-adjusted CIs.
b The complier average causal effect was estimated with the “rdrobust, fuzzy()” command in Stata (37), using the same MSE-optimal bandwidth for the first- and second-stage equations. The corresponding ITT effect and first-stage models are displayed in Web Table 1.
c The bandwidth is the window of data around the threshold that was used to generate the regression discontinuity design estimate. For each outcome, an MSE-optimal bandwidth was computed using the “rdrobust” command in Stata (37).
Figure 2.
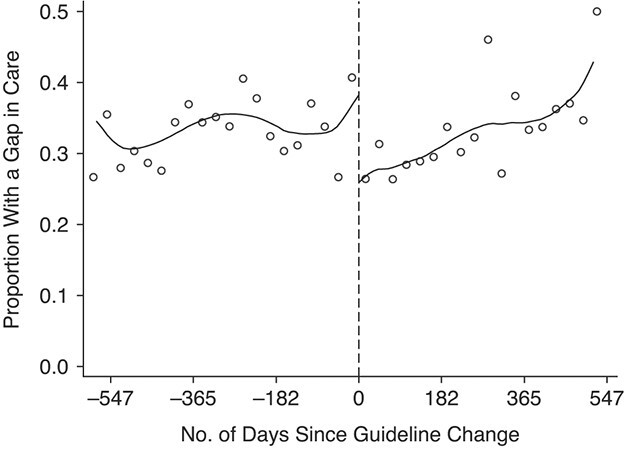
Proportion of patients with a 4-month gap in care among patients starting antiretroviral therapy before and after the switch to a single-pill regimen, Themba Lethu Clinic, Johannesburg, South Africa, 2011–2014. Dots represent monthly percentages; lines represent local linear regression models with a bandwidth of 147.0 days and a triangular kernel.
Figure 3.
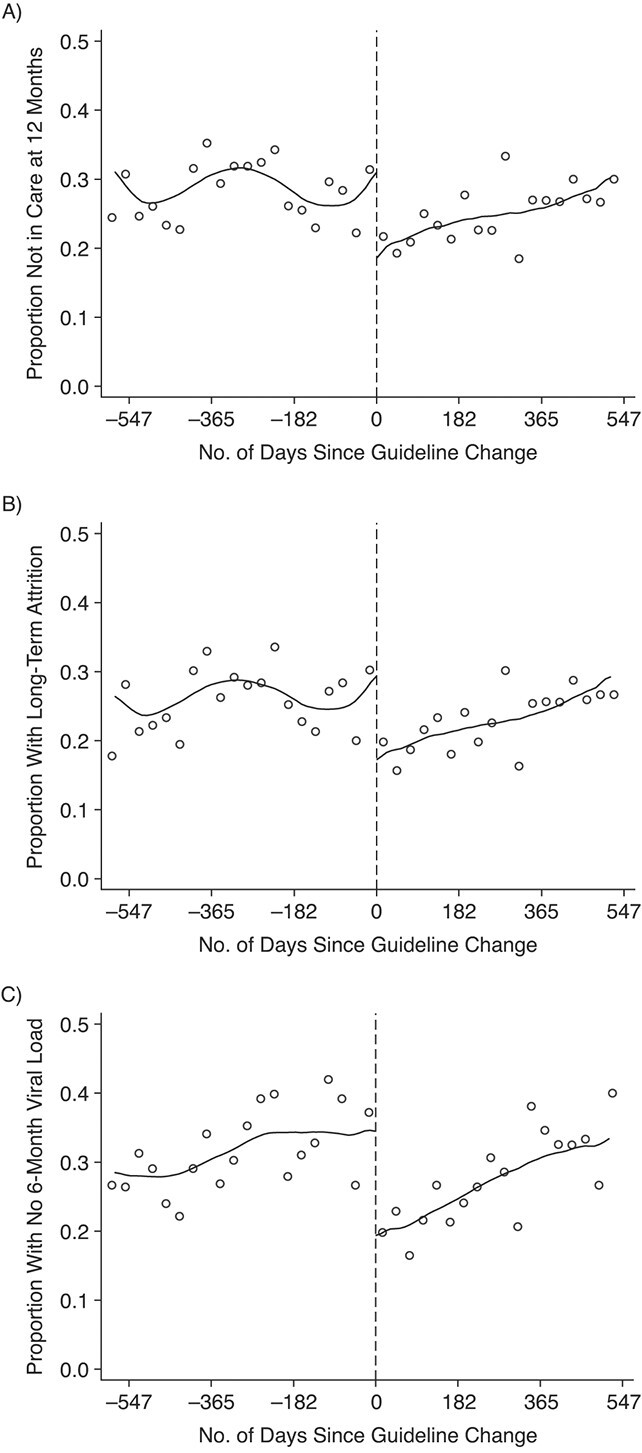
Secondary attrition outcomes among patients who initiated antiretroviral therapy at Themba Lethu Clinic in Johannesburg, South Africa, 2011–2014. A) Absence from care at 1 year; B) long-term attrition by 1 year; C) failure to have a 6-month viral load measurement. Dots represent monthly percentages; lines represent local linear regression models with bandwidths of 143.5 days (A), 146.3 days (B), and 193.3 days (C) and a triangular kernel.
Effects of starting FDC among “compliers”
The ITT effect of the policy change underestimates the effect of being prescribed FDC on retention in care, because not all patients were prescribed FDC after the policy change. We estimated CACEs by scaling the ITT by the share of patients who were prescribed FDC because of the policy. Our CACE estimates revealed that being prescribed an FDC instead of multiple pills led to a 21.7–percentage-point decrease in 4-month gaps in care (95% CI: −54.7, 2.1), a 19.9–percentage-point decrease in absence from care at 1 year (95% CI: −49.7, 1.9), a 21.6–percentage-point decrease in long-term attrition by 1 year (95% CI: −51.1, −0.1), and a 27.7–percentage-point decrease in missed 6-month viral load tests (95% CI: −58.8, −0.7) (Table 2, Web Table 1).
Subgroup analyses
We then stratified our CACE analyses to understand the effect of FDCs on attrition in clinic subpopulations (Table 3). Percentage-point reductions in attrition were largest for patients with higher CD4+ cell counts (≥200 cells/μL) (percentage-point change = −56.7, 95% CI: −122.5, −14.2), patients with early clinical disease (WHO stage 1 or 2) (percentage-point change = −23.2, 95% CI: −53.7, −5.1), nonanemic patients (percentage-point change = −26.2, 95% CI: −67.4, 2.1), and women (percentage-point change = −26.2, 95% CI: −62.9, −2.8).
Table 3.
Stratified Regression Discontinuity Design Estimates of the Effect of Single-Pill Fixed-Dose Combination Treatment for HIV (Compared With Multiple Pills) on the Risk of a 4-Month Gap in Care Within the First Year of Treatment, 2011–2014
| Complier Average Causal Effect a | |||
|---|---|---|---|
| Stratifying Variable | Bandwidth b , days | RD | 95% CI |
| Overall | ±129.6 | −21.7 | −54.7, 2.1 |
| Sex | |||
| Male | ±128.2 | −3.4 | −45.3, 35.7 |
| Female | ±174.3 | −26.2 | −62.9, −2.8 |
| Age, years | |||
| 16–29 | ±191.1 | −23.8 | −66.7, 21.2 |
| 30–39 | ±264.0 | −14.1 | −47.1, 9.3 |
| 40–49 | ±125.9 | −18.5 | −72.8, 28.0 |
| ≥50 | ±171.7 | −19.5 | −88.8, −1.6 |
| Anemia | |||
| Yes | ±222.0 | −6.3 | −32.7, 20.8 |
| No | ±156.3 | −26.2 | −67.4, 2.1 |
| WHO stage | |||
| 3 or 4 | ±141.8 | 15.9 | −66.2, 100.8 |
| 1 or 2 | ±166.6 | −23.2 | −53.7, −5.1 |
| CD4+ cell count, cells/μL | |||
| 0–199 | ±238.7 | 5.8 | −16.5, 28.7 |
| ≥200 | ±135.4 | −56.7 | −122.5, −14.2 |
Abbreviations: CD4, cluster of differentiation 4; CI, confidence interval; MSE, mean squared error; RD, risk difference; WHO, World Health Organization.
a The complier average causal effect was estimated using the “rdrobust, fuzzy()” command in Stata (37), with a triangular kernel and the same MSE-optimal bandwidth for the first- and second-stage equations. The 95% CIs are robust, bias-adjusted CIs.
b The bandwidth is the window of data around the threshold that was used to generate the regression discontinuity design estimate. For each outcome, an MSE-optimal bandwidth was computed using the “rdrobust” command in Stata (37).
Sensitivity analyses
Results were robust to changes in the bandwidth (Web Table 2) and kernel and to the use of logistic regression in lieu of the linear model (Web Figure 6).
DISCUSSION
We investigated the impact of “1 pill, once-a-day” HIV treatment on patient retention in South Africa’s public-sector HIV program. Using an RDD, we exploited the rapid shift from multiple-pill regimens to single-pill FDC for new patients. We estimated that 1-year attrition was 11.7 percentage points lower (38.3% vs. 26.5%) among patients who initiated ART after the introduction of FDC. Among “compliers,” patients whose regimen type was determined by the policy change, starting FDC reduced attrition by 21.7 percentage points. Although the 95% confidence intervals indicated a wide range of possible parameter values, our point estimates were consistent across different measures, including measures relying on different underlying data sources (laboratory results vs. clinic visits), and were robust to different bandwidths and regression specifications.
Prior studies have compared adherence to single-tablet regimens versus multiple-tablet regimens, with most investigators reporting higher adherence for single-tablet regimens (9, 24–26, 28, 29, 42–46). However, the majority of these studies were conducted in North America and Western Europe, with limited generalizability to sub-Saharan Africa, and most did not assess retention as an outcome. To our knowledge, this was the first study to evaluate real-world retention impacts of single-pill ART in sub-Saharan Africa using a causally robust RDD study design (47–50).
Our subgroup analyses revealed strong associations between starting a single-pill FDC and retention among healthier patients—that is, those with a CD4+ cell count greater than or equal to 200 cells/μL, no anemia, and no stage 3 or 4 HIV illness—and no association among sicker patients. Patients who have not yet experienced advanced HIV illness may lack motivation to be on ART (23). Our data suggested that the reduction in “hassle” costs associated with FDC had the greatest impact on ART retention in this population. With countries seeking to expand ART coverage among healthy patients via test-and-treat policies, eliminating hassle costs such as regimen complexity may play an even more important role in supporting adherence and retention going forward.
We also found effect modification by sex, with large estimated effects among women and no estimated effect among men. Men face many barriers to HIV testing and care-seeking (51, 52) and tend to seek care later in disease progression (53). Whereas men often delay care-seeking until they experience HIV-related symptoms, women are often diagnosed with HIV and started on ART as part of routine reproductive health care. Because many women enter HIV care without ever actually seeking out HIV care, women may be more likely than men to be “on the fence” about treatment and therefore more likely to be impacted by a small reduction in hassle costs. We note that this interpretation is not inconsistent with the high rates of attrition documented among men (54), which could indicate that men face other large obstacles to staying on ART that are not addressed by simplified drug regimens.
Our findings have implications for the therapeutic management of HIV and other chronic diseases. First, our results suggest that simplified regimens have benefits for patients, complementing recent evidence that less toxic regimens also improve retention (49). Messaging on the relative convenience and tolerability of modern ART regimens might increase treatment uptake as South Africa strives to end the HIV epidemic. Second, as our study illustrates, simplified regimens can be scaled up very quickly through changes in guidelines and centralized procurement, without requiring changes in patient or health-care provider behavior. In this, FDC contrasts with other retention interventions such as case management and adherence clubs. Third, our findings suggest that pharmaceutical innovations to simplify complex drug regimens could have substantial public health benefit.
Our study had several limitations. First, we were unable to determine why FDCs improved retention. Greater patient satisfaction (55) and improved quality of life (56) have been attributed to FDCs in prior studies. The most commonly prescribed FDC and the leading multipill regimen at the time had similar biological efficacy and similar risks of side effects (57). Thus, it is likely that the observed increase in retention was related to the lower pill burden rather than to any change in the efficacy or tolerability of treatment. Second, FDC was rolled out at the same time ART eligibility was extended to patients with stage 3 illness and CD4+ cell counts greater than 350 cells/μL. All such patients were excluded from the analysis. However, increased facility congestion could have contributed to the continuous rise in background attrition rates during the study period. Third, while we are unaware of other contemporaneous changes to clinical procedures, our results might be biased if the introduction of FDC led to an overall focus on improving initiation procedures for patients starting on the new regimen. Fourth, as with all clinical cohorts observed through routine data, gaps in record-keeping could have led us to underestimate retention. Our use of multiple definitions of retention provides confidence that our results were not sensitive to 1 specific definition. Although overall retention may have been underestimated, there is no reason to believe that there would be different patterns of missingness in clinical records for patients starting just before the FDC policy implementation date versus just after the FDC policy date. Patients starting just before/after the policy change had follow-up that overlapped nearly completely and experienced nearly identical conditions at the clinic; the only difference was that patients starting ART just after the policy change were much more likely to start FDC. Fifth, while we found reduced attrition among patients starting single-pill ART, it is unknown whether our results would be generalizable to patients who were already established on multiple-pill regimens and were switched to FDC.
In summary, starting patients on “1 pill, once-a-day” ART increased retention in HIV care at a large public-sector clinic in South Africa. Simplified treatment regimens can improve the real-world management of chronic diseases in low-resource settings.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, Boston University, Boston, Massachusetts, United States (Jacob Bor, Sheryl A. Kluberg, Matthew P. Fox); Department of Global Health, School of Public Health, Boston University, Boston, Massachusetts, United States (Jacob Bor, Lawrence Long, Matthew P. Fox); Health Economics and Epidemiology Research Office, Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa (Jacob Bor, Denise Evans, Kamban Hirasen, Mhairi Maskew, Lawrence Long, Matthew P. Fox); and Department of Biostatistics, School of Public Health, Boston University, Boston, Massachusetts, United States (Michael P. LaValley).
J.B. and S.A.K. contributed equally to this article.
This work was funded by the National Institutes of Health (grants K01-MH105320, R01-HD084233, and R01-AI152149 to J.B.).
Replication data and the Stata.do file are available on GitHub (58).
We thank participants in the 2018 Population Health Science Research Workshop (Boston University) for feedback on an earlier draft of this article.
Ethical approval for this analysis was granted by the Human Research Ethics Committee of the University of the Witwatersrand and by the Institutional Review Board of Boston University.
The contents of this article are the responsibility of the authors and do not necessarily reflect the views of the US government. The funders played no role in the study design; the collection, analysis, and interpretation of the data; manuscript preparation; or the decision to publish.
Conflict of interest: none declared.
REFERENCES
- 1. Gardner EM, Hullsiek KH, Telzak EE, et al. Antiretroviral medication adherence and class-specific resistance in a large prospective clinical trial. AIDS. 2010;24(3):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gardner EM, Burman WJ, Steiner JF, et al. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS. 2009;23(9):1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sax PE, Meyers JL, Mugavero M, et al. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7(2):e31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nachega JB, Leisegang R, Bishai D, et al. Association of antiretroviral therapy adherence and health care costs. Ann Intern Med. 2010;152(1):18–25. [DOI] [PubMed] [Google Scholar]
- 5. Anglemyer A, Rutherford GW, Horvath T, et al. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev. 2013;4(4):CD009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giordano TP, Gifford AL, White AC, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–1499. [DOI] [PubMed] [Google Scholar]
- 7. Mugavero MJ, Lin H-Y, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nachega JB, Mugavero MJ, Zeier M, et al. Treatment simplification in HIV-infected adults as a strategy to prevent toxicity, improve adherence, quality of life and decrease healthcare costs. Patient Prefer Adherence. 2011;5:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nachega JB, Parienti J-J, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58(9):1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laurent C, Kouanfack C, Koulla-Shiro S, et al. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. Lancet. 2004;364(9428):29–34. [DOI] [PubMed] [Google Scholar]
- 11. Joint United Nations Programme on HIV/AIDS . Global AIDS Update 2019—Communities at the Centre. Geneva, Switzerland: Joint United Nations Program on HIV/AIDS; 2019. https://www.unaids.org/sites/default/files/media_asset/2019-global-AIDS-update_en.pdf. Accessed October 26, 2021. [Google Scholar]
- 12. South African National AIDS Council . Republic of South Africa 2016 Global AIDS Response Progress Report (Quantitative Report). Pretoria, South Africa: South African National AIDS Council; 2015. https://sanac.org.za/global-aids-response-progress-report/. Accessed October 26, 2021. [Google Scholar]
- 13. South African National Department of Health. The South African Antiretroviral Treatment Guidelines 2013. Pretoria, South Africa: South African National Department of Health; 2013. https://sahivsoc.org/Files/2013%20ART%20Treatment%20Guidelines%20Final%2025%20March%202013%20corrected.pdf. Accessed October 26, 2021.
- 14. South African National Department of Health. Revised Anti-Retroviral Treatment Guideline: Update for Frontline Clinical Health Professionals. Pretoria, South Africa: South African National Department of Health; 2013. https://sahivsoc.org/Files/FDC%20Training%20Manual%2014%20March%202013(1).pdf. Accessed October 26, 2021.
- 15. Coovadia A. How FDCs will impact on PMTCT—Q & A with Professor Ashraf Coovadia. Pretoria, South Africa: South African National AIDS Council; 2013. https://sanac.org.za/how-fdcs-will-impact-on-pmtct-q-a-with-professor-ashraf-coovadia/. Accessed October 26, 2021. [Google Scholar]
- 16. Bertrand M, Mullainathan S, Shafir E. Behavioral economics and marketing in aid of decision making among the poor. J Public Policy Mark. 2006;25(1):8–23. [Google Scholar]
- 17. Baicker K, Congdon WJ, Mullainathan S. Health insurance coverage and take-up: lessons from behavioral economics. Milbank Q. 2012;90(1):107–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aizer A. Public health insurance, program take-up, and child health. Rev Econ Stat. 2007;89(3):400–415. [Google Scholar]
- 19. Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Society. 2013;16(3 suppl 2):18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hodgson I, Plummer ML, Konopka SN, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS One. 2014;9(11):e111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendershot CS, Stoner SA, Pantalone DW, et al. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bor J, Chiu C, Ahmed S, et al. Failure to initiate HIV treatment in patients with high CD4 counts: evidence from demographic surveillance in rural KwaZulu-Natal. Trop Med Int Health. 2018;23(2):206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed S, Autrey J, Katz IT, et al. Why do people living with HIV not initiate treatment? A systematic review of qualitative evidence from low- and middle-income countries. Soc Sci Med. 2018;213:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clay PG, Nag S, Graham CM, et al. Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine (Baltimore). 2015;94(42):e1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramjan R, Calmy A, Vitoria M, et al. Systematic review and meta-analysis: patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health. 2014;19(5):501–513. [DOI] [PubMed] [Google Scholar]
- 26. Bangsberg DR, Ragland K, Monk A, et al. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. AIDS. 2010;24(18):2835–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65(5):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US Medicaid population with HIV. BMJ Open. 2013;3(8):e003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirasen K, Evans D, Maskew M, et al. The right combination—treatment outcomes among HIV-positive patients initiating first-line fixed-dose antiretroviral therapy in a public sector HIV clinic in Johannesburg, South Africa. Clin Epidemiol. 2018;10:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fox MP, Maskew M, MacPhail AP, et al. Cohort profile: the Themba Lethu clinical cohort, Johannesburg, South Africa. Int J Epidemiol. 2013;42(2):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Office of the Deputy President, Republic of South Africa . Statement on the meeting of the South African National AIDS Council (SANAC). https://www.gov.za/statement-meeting-south-african-national-aids-council-sanac. Published 2011. Accessed October 26, 2021.
- 32. Kaplan R, Orrell C, Zwane E, et al. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22(13):1679–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clouse K, Pettifor AE, Maskew M, et al. Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary health care clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2013;62(2):e39–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee DS, Lemieux T. Regression discontinuity designs in economics. J Econ Lit. 2010;48(2):281–355. [Google Scholar]
- 35. Bor J, Moscoe E, Mutevedzi P, et al. Regression discontinuity designs in epidemiology: causal inference without randomized trials. Epidemiology. 2014;25(5):729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan J, Gijbels I. Local Polynomial Modelling and Its Applications. London, United Kingdom: Chapman & Hall Ltd.; 1996. [Google Scholar]
- 37. Calonico S, Cattaneo MD, Titiunik R. Robust nonparametric confidence intervals for regression-discontinuity designs. Econometrica. 2014;82(6):2295–2326. [Google Scholar]
- 38. Imbens GW, Kalyanaraman K. Optimal bandwidth choice for the regression discontinuity estimator. Rev Econ Stud. 2011;79(3):933–959. [Google Scholar]
- 39. Calonico S, Cattaneo MD, Farrell MH, et al. rdrobust: software for regression-discontinuity designs. Stata J. 2017;17(2):372–404. [Google Scholar]
- 40. McCrary J. Manipulation of the running variable in the regression discontinuity design: a density test. J Econom. 2008;142(2):698–714. [Google Scholar]
- 41. Cattaneo MD, Idrobo N, Titiunik R. A Practical Introduction to Regression Discontinuity Designs: Foundations. Cambridge, United Kingdom: Cambridge University Press; 2019. [Google Scholar]
- 42. Drozd DR, Saag MS, Westfall AO, et al. Comparative effectiveness of single versus multiple tablet antiretroviral therapy regimens in clinical HIV practice. Medicine (Baltimore). 2017;96(14):e6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sterrantino G, Santoro L, Bartolozzi D, et al. Self-reported adherence supports patient preference for the single tablet regimen (STR) in the current cART era. Patient Prefer Adherence. 2012;6:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Airoldi M, Zaccarelli M, Bisi L, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence. 2010;4:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buscher A, Hartman C, Kallen MA, et al. Impact of antiretroviral dosing frequency and pill burden on adherence among newly diagnosed, antiretroviral-naive HIV patients. Int J STD AIDS. 2012;23(5):351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raffi F, Yazdanpanah Y, Fagnani F, et al. Persistence and adherence to single-tablet regimens in HIV treatment: a cohort study from the French National Healthcare Insurance Database. J Antimicrob Chemother. 2015;70(7):2121–2128. [DOI] [PubMed] [Google Scholar]
- 47. Mody A, Sikazwe I, Czaicki NL, et al. Estimating the real-world effects of expanding antiretroviral treatment eligibility: evidence from a regression discontinuity analysis in Zambia. PLoS Med. 2018;15(6):e1002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bor J, Fox MP, Rosen S, et al. Treatment eligibility and retention in clinical HIV care: a regression discontinuity study in South Africa. PLoS Med. 2017;14(11):e1002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brennan AT, Bor J, Davies M-A, et al. Medication side effects and retention in HIV treatment: a regression discontinuity study of tenofovir implementation in South Africa and Zambia. Am J Epidemiol. 2018;187(9):1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tymejczyk O, Brazier E, Yiannoutsos CT, et al. Changes in rapid HIV treatment initiation after national “treat all” policy adoption in 6 sub-Saharan African countries: regression discontinuity analysis. PLoS Med. 2019;16(6):e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fitzgerald M, Collumbien M, Hosegood V. “No one can ask me ‘Why do you take that stuff?’”: men’s experiences of antiretroviral treatment in South Africa. AIDS Care. 2010;22(3):355–360. [DOI] [PubMed] [Google Scholar]
- 52. Chikovore J, Gillespie N, McGrath N, et al. Men, masculinity, and engagement with treatment as prevention in KwaZulu-Natal. South Africa. AIDS Care. 2016;28(suppl 3):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carmona S, Bor J, Nattey C, et al. Persistent high burden of advanced HIV disease among patients seeking care in South Africa’s national HIV program: data from a nationwide laboratory cohort. Clin Infect Dis. 2018;66(suppl 2):S111–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cornell M, Schomaker M, Garone DB, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9(9):e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hodder SL, Mounzer K, Dejesus E, et al. Patient-reported outcomes in virologically suppressed, HIV-1-infected subjects after switching to a simplified, single-tablet regimen of efavirenz, emtricitabine, and tenofovir DF. AIDS Patient Care STDs. 2010;24(2):87–96. [DOI] [PubMed] [Google Scholar]
- 56. Aldir I, Horta A, Serrado M. Single-tablet regimens in HIV: does it really make a difference? Curr Med Res Opin. 2014;30(1):89–97. [DOI] [PubMed] [Google Scholar]
- 57. Ford N, Vitoria M, Doherty M, et al. Candidates for inclusion in a universal antiretroviral regimen: are lamivudine and emtricitabine interchangeable? Curr Opin HIV AIDS. 2017;12(4):334–338. [DOI] [PubMed] [Google Scholar]
- 58. Bor J. FDC. https://github.com/jacobbor/FDC.git. Published January 10, 2022. Accessed January 10, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


