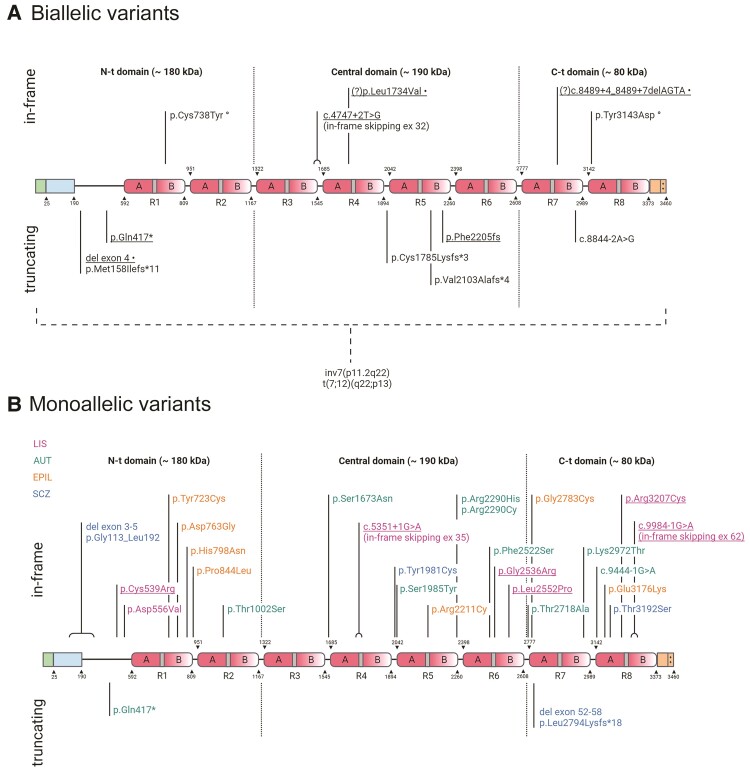
Figure 1.
RELN protein cartoon showing pathogenic and probably pathogenic variants. Protein diagrams show the domain organization of RELN with two in vivo proteolysis sites shown as dotted lines highlighting N-terminal (N-t), Central and C-terminal (C-t) domains. The known boundaries of each domain or other functional region are indicated above or below the diagram and identified by numbers corresponding to the human RELN sequence (Uniprot no. P78509). RELN repeats are numbered (R1–R8) and their composition is marked with sub-repeats A and B separated by EGF-like domains (grey). The N-terminal region contains a signal peptide (positions 0–25 in green) and F-spondin-like domain (positions 25–190 in blue) followed by a unique sequence region (positions 190–502). The C-terminal region (3373–3460 in orange) ends with a stretch of 33 amino acids rich in basic residues (++). (A) Biallelic variants in RELN. Thirteen variants are shown, including six reported in this study (underlined). Compound heterozygous variants in one individual are marked with symbols following the description of the variant; (?) indicates two VUS identified in cis in one individual, both with potential disease relevance. Two structural chromosome rearrangements disrupting RELN are indicated below the dashed bracket. (B) Monoallelic variants in RELN. Twenty-seven variants are shown, including six reported in this study (underlined). The colour legend for associated phenotypes is also shown beneath the diagram; AUT = autism; EPIL = lateral temporal epilepsy; SCZ = schizophrenia. Created with BioRender.com.