Abstract
It has been reported that residents of low–socioeconomic-status (SES) neighborhoods have a higher risk of developing cardiovascular disease (CVD). However, most of the previous studies focused on 1-time measurement of neighborhood SES in middle-to-older adulthood and lacked demographic diversity to allow for comparisons across different race/ethnicity and sex groups. We examined neighborhood SES in childhood and young, middle, and older adulthood in association with CVD risk among Black and White men and women in the Atherosclerosis Risk in Communities Study (1996–2019). We found that lower neighborhood SES in young, middle, and older adulthood, but not in childhood, was associated with a higher risk of CVD later in life. When compared with the highest quartile, the lowest quartile of neighborhood SES in young, middle, and older adulthood was associated with 18% (hazard ratio (HR) = 1.18, 95% confidence interval (CI): 1.02, 1.36), 21% (HR = 1.21, 95% CI: 1.04, 1.39), and 12% (HR = 1.12, 95% CI: 0.99, 1.26) increases in the hazard of total CVD, respectively. The association between lower neighborhood SES in older adulthood and higher CVD hazard was particularly strong among Black women. Our study findings support the role of neighborhood SES in cardiovascular health in both Black and White adults.
Keywords: cardiovascular disease, health disparities, life course, neighborhood socioeconomic status
Abbreviation
- ARIC
Atherosclerosis Risk in Communities
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- LCSES
Life Course Socioeconomic Status, Social Context and Cardiovascular Disease
- SES
socioeconomic status
Social determinants play a prominent role in cardiovascular health (1, 2). Neighborhood environment is a crucial aspect of social determinants of health, and lower neighborhood socioeconomic status (SES) has been linked with increased cardiovascular disease (CVD) risk. Previous studies suggest that residents of low-SES neighborhoods, as compared with high-SES neighborhoods, have a higher risk of coronary heart disease (CHD) (3–6), stroke (7–9), CVD mortality (10–12), and overall CVD (13, 14) and are more likely to experience delays in diagnosis and hospital admissions (15) and to receive less-than-optimal treatments (16, 17), all of which contribute to higher burdens of CVD in disadvantaged communities.
Both exposure to neighborhood SES and the relationship between neighborhood SES and CVD may change over the life course, but most studies measure neighborhood SES at only a single time point, often when study participants are in middle-to-older adulthood (3–14). Several studies have suggested that early-life socioeconomic disadvantage, including low neighborhood SES, may be associated with higher risk of CVD (18–20) or its risk factors, such as hypertension and obesity (21, 22), independently of neighborhood SES in adulthood. However, limited research has focused on neighborhood SES at different life stages and CVD risks later in life. Such investigations may help us understand the dynamic relationship between neighborhood SES and CVD and assess the cumulative health effects of life-course socioeconomic disadvantage.
In the United States, CVD risks and mortality are higher among Black Americans than among White individuals (23).
Because of historical and structural factors, when compared with their White counterparts, Black Americans, even those in the middle-to-upper classes, are more likely to live in low-SES neighborhoods with persisting poverty and disinvestment (24–26). Several studies examined neighborhood SES in relation to CVD risk in Black Americans specifically (7, 9, 12, 13) and reported mixed findings: Some reported an inverse association between neighborhood SES and CVD (12, 13), while others reported null-to-weak (9, 10) or even positive (7) associations. In addition, an earlier study suggested sex differences in the relationship between neighborhood disadvantage and risk of CVD among Black residents, with the association appearing stronger in Black women than in Black men (13). However, none of these studies examined neighborhood SES at different life stages.
To address the aforementioned research gaps, we examined neighborhood SES in childhood and young, middle, and older adulthood, as well as neighborhood SES patterns across life stages, in relation to incident CVD over 16 years of follow-up among Black and White men and women in the Atherosclerosis Risk in Communities Study (ARIC) Study. We hypothesized that lower neighborhood SES would be associated with higher risk of CVD in both racial/ethnic groups.
METHODS
Study population
The ARIC Study is a multicenter prospective cohort study that was established in 1987 by recruiting adults aged 45–64 years from 4 US communities (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis, Minnesota) (27, 28). Cohort members were contacted yearly to complete annual surveys on hospitalizations and general health. In addition, multiple follow-up visits were conducted from baseline through visit 8 (2020–2021). Between 2001 and 2002, participants additionally reported their residential addresses at different life stages as part of an ARIC ancillary study, the Life Course SES, Social Context and Cardiovascular Disease (LCSES) Study (29, 30). Of the ARIC visits with information on current residential address, visit 4 (1996–1999) occurred most closely in time with the LCSES Study, when neighborhood exposure at earlier life stages was assessed. Thus, we used visit 4 addresses to measure neighborhood SES in older adulthood and considered visit 4 as the baseline of the current analysis. Web Figure 1 (available at https://doi.org/10.1093/aje/kwac070) presents the timeline of the ARIC Study.
Of the 15,792 participants examined at ARIC baseline (1987–1989), 11,656 participated in visit 4. Of these, we excluded 1,804 who developed CVD by visit 4 and 27 who did not self-identify as either Black or White. We also excluded Black participants in Washington County, Maryland, and Minneapolis, Minnesota, because of small numbers (n = 33). Our analytical sample included 9,692 participants (2,165 Black and 7,527 White). The study protocol was approved by the institutional review boards at all participating centers, and all study participants provided written informed consent.
Assessment of life-course neighborhood SES
At visit 4, study participants reported their current residential address, which was considered their address in older adulthood (median age at visit 4, 62 years; range, 52–75). In the LCSES Study, participants were asked to report their addresses in the following age periods: approximately age 10 years (defined as childhood), approximately age 30 years (young adulthood), and age 40–50 years (middle adulthood). All addresses were geocoded and linked to US Census data. Neighborhood SES in older adulthood was assessed using data from the 2000 Census at the census tract level. Neighborhood SES in middle and young adulthood was assessed on the basis of the most proximate Census (1960–1980) at the census tract level. Childhood neighborhood SES was based on the most proximate Census data (1930–1950) at the county level, the smallest geographic unit with aggregated data for all participating areas during this period. Validation studies found high accuracy and repeatability of geocodes in the ARIC Study (31–33).
Analytical details about the derivation of neighborhood SES measures at different life stages have been published previously (30, 34). Briefly, composite measures of neighborhood SES were derived by summarizing z scores for multiple Census variables representing neighborhood-level education, occupation, income, home ownership, and property value for different life stages. We also calculated average life-course neighborhood SES as follows:
 |
where 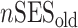 ,
, 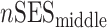 ,
, 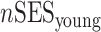 , and
, and 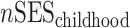 represent neighborhood SES at different life stages and
represent neighborhood SES at different life stages and 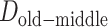 ,
,  , and
, and 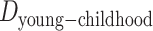 represent the difference (time gap, in years) between the midpoints of 2 different life stages. Specifically, we used ages 10 and 30 years as the midpoints for childhood and young adulthood, respectively, because participants were asked to report their address information at approximately these ages for these life stages. For middle adulthood, we used age 45 years because it represents the midpoint of the age period (40–50 years) for which address information was collected. For older adulthood, we used the median age at baseline (visit 4).
represent the difference (time gap, in years) between the midpoints of 2 different life stages. Specifically, we used ages 10 and 30 years as the midpoints for childhood and young adulthood, respectively, because participants were asked to report their address information at approximately these ages for these life stages. For middle adulthood, we used age 45 years because it represents the midpoint of the age period (40–50 years) for which address information was collected. For older adulthood, we used the median age at baseline (visit 4). 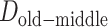 ,
, 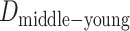 , and
, and 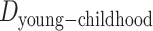 are 17 years (age 62 years – age 45 years), 15 years (age 45 years – age 30 years), and 20 years (age 30 years – age 10 years), respectively.
are 17 years (age 62 years – age 45 years), 15 years (age 45 years – age 30 years), and 20 years (age 30 years – age 10 years), respectively.
Because White and Black Americans on average reside in vastly different neighborhoods (35), we calculated race/ethnicity-specific quartiles for neighborhood SES at different life stages. We also derived variables for long-term neighborhood SES patterns across life stages based on the race/ethnicity-specific median split of neighborhood SES at each stage. For example, neighborhood SES patterns between middle and older adulthood included 4 categories: low-high or improvement (below the median in middle adulthood and at or above the median in older adulthood), high-low or decline, low-low or stable low, and high-high or stable high. We estimated long-term neighborhood SES patterns for 3 periods: middle-to-older adulthood, young-to-middle adulthood, and childhood to young adulthood.
Outcome assessments
We examined the following incident cardiovascular events as the outcomes: CHD, heart failure, and stroke. The follow-up for CVD outcomes extended through December 31, 2019, for Washington County, Forsyth County, and Minneapolis, while the end of follow-up for Jackson County was December 31, 2017, because of incomplete hospital records in one large Jackson hospital in 2018 and 2019. Details on clinical surveillance and outcome ascertainment in ARIC have been previously published (36, 37). In brief, fatal or nonfatal incident CHD cases were adjudicated by an endpoints committee and included the first occurrence of hospitalized definite or probable myocardial infarction, definite fatal CHD, or silent myocardial infarction based on Minnesota-coded serial electrocardiographic changes over ARIC visits. Incident heart failure included the first occurrence of a hospitalization with an International Classification of Diseases, Ninth Revision, discharge diagnosis code of 428 before 2005; after 2005, heart failure cases were centrally adjudicated by an endpoints committee. Incident stroke included the first occurrence of definite or probable stroke according to National Survey of Stroke criteria. We also derived a variable for overall CVD outcome by combining the 3 types of events.
Covariates
At ARIC visit 4, study participants reported their sociodemographic characteristics, lifestyle factors, and medical histories. Anthropometric and blood pressure measurements were obtained by trained study staff. Participants also donated fasting blood samples, from which glucose and high- and low-density lipoprotein cholesterol concentrations were estimated. Self-rated health was reported in annual surveys.
Statistical analysis
The percentage of missingness in neighborhood SES measures ranged from 7% (older adulthood) to 36% (young adulthood). The higher rate of missingness for younger adulthood was due to both a lower proportion of reported addresses successfully geocoded during this life stage and incomplete census tract coverage in the 1960s (32, 33). To address missing data, we used multiple imputation by chained equations (38), including the following variables: all neighborhood SES variables, age, race/ethnicity, sex, education, annual household income, marital status, clinical center, body mass index, alcohol use, smoking, high- and low-density lipoprotein cholesterol levels, hypertension, diabetes, and self-rated health. Ten imputed data sets were created. We present mean values and standard deviations for continuous variables that were normally distributed, median values and interquartile ranges for continuous variables that were skewed, and percentages for categorical variables. Descriptive statistics (Table 1) were calculated on the basis of the original data set without imputation.
Table 1.
Characteristics of Study Participants, by Race/Ethnicity, in the Atherosclerosis Risk in Communities Study, 1996–1999
| Quartile of Neighborhood SES in Older Adulthood a | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black Participants (n = 1,960) | White Participants (n = 7,050) | |||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||||||||
| Characteristic | Median (IQR) | % | Median (IQR) | % | Median (IQR) | % | Median (IQR) | % | Median (IQR) | % | Median (IQR) | % | Median (IQR) | % | Median (IQR) | % |
| Neighborhood SES z scoreb | −14.3 to −9.3 | −9.2 to −6.6 | −6.6 to −4.7 | −4.7 to 12.5 | −11.7 to −0.5 | −0.5 to 1.5 | 1.6 to 4.9 | 4.9 to 15.8 | ||||||||
| Age, years | 61 (58–67) | 62 (57–67) | 60 (57–65) | 59 (56–63) | 63 (58–68) | 63 (58–68) | 62 (58–68) | 62 (57–67) | ||||||||
| Female sex | 67.5 | 69.8 | 65.1 | 61.3 | 58.6 | 56.9 | 56.4 | 55.3 | ||||||||
| College graduation or more | 20.6 | 28.1 | 45.6 | 55.5 | 23.4 | 31.5 | 43.9 | 65.1 | ||||||||
| Annual family income ≥$25,000 | 22.7 | 30.2 | 50.8 | 65.0 | 64.8 | 73.5 | 80.8 | 91.0 | ||||||||
| Married | 46.7 | 46.7 | 64.5 | 65.8 | 79.5 | 82.9 | 82.9 | 83.4 | ||||||||
| Current smoker | 19.7 | 22.2 | 16.3 | 19.3 | 15.6 | 13.0 | 12.9 | 13.0 | ||||||||
| Current alcohol drinker | 22.0 | 24.2 | 27.8 | 35.5 | 42.2 | 42.5 | 38.0 | 32.4 | ||||||||
| Body mass indexc | 30.2 (26.6–34.6) | 30.2 (26.5–34.6) | 29.1 (26.2–32.8) | 29.3 (26.2–33.0) | 27.7 (24.8–31.3) | 27.9 (25.0–32.0) | 27.0 (24.2–30.6) | 26.9 (24.1–30.2) | ||||||||
| Fasting glucose level, mmol/L | 5.9 (5.3–6.8) | 5.7 (5.3–6.8) | 5.7 (5.3–6.5) | 5.7 (5.3–6.2) | 5.55 (5.2–6.1) | 5.5 (5.2–6.0) | 5.4 (5.2–5.9) | 5.4 (5.1–5.9) | ||||||||
| HDL cholesterol level, mmol/L | 1.3 (1.1–1.6) | 1.3 (1.1–1.6) | 1.3 (1.1–1.7) | 1.3 (1.1–1.6) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | 1.2 (1.0–1.6) | 1.3 (1.0–1.6) | ||||||||
| LDL cholesterol level, mmol/L | 3.1 (2.6–3.8) | 3.1 (2.6–3.7) | 3.2 (2.5–3.8) | 3.2 (2.6–3.8) | 3.2 (2.7–3.8) | 3.1 (2.6–3.7) | 3.2 (2.6–3.7) | 3.1 (2.6–3.6) | ||||||||
| Excellent self-rated health | 21.2 | 17.6 | 25.6 | 35.6 | 18.9 | 23.9 | 26.5 | 30.7 | ||||||||
| Hypertensiond | 64.6 | 66.8 | 64.3 | 57.4 | 32.6 | 32.4 | 29.5 | 24.8 | ||||||||
| Diabetese | 27.5 | 28.7 | 21.6 | 19.4 | 13.7 | 12.7 | 10.9 | 9.7 | ||||||||
Abbreviations: HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; Q, quartile; SD, standard deviation; SES, socioeconomic status.
a Calculated using data without imputation (n = 9,010).
b Values are expressed as range.
c Calculated as weight (kg)/height (m)2.
d Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of medication to treat hypertension.
e Diabetes was defined as fasting blood glucose concentration ≥126 mg/dL, self-reported diagnosis of diabetes, or use of medication to treat diabetes.
We calculated incidence rates using Poisson regression and hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox regression. Results from the 10 imputed data sets were summarized using PROC MIANALYZE (SAS Institute, Inc., Cary, North Carolina). We considered 2 models: model 1, the model adjusting only for age and sex, and model 2, the full model adjusting for potential confounders, which were defined as factors that could influence both the likelihood of living in certain neighborhoods and CVD incidence but were not mediators of the associations. These factors included age, sex, race/ethnicity (not controlled in race/ethnicity-specific analysis), marital status, education, annual household income, and study center. We calculated P values for trend by modeling quartiles of neighborhood SES variables numerically (1, 2, 3, 4) and P values for interaction using a likelihood ratio test comparing models with and without a cross-product term. All analyses were performed using SAS, version 9.4.
RESULTS
Baseline individual-level study characteristics according to neighborhood SES for Black and White participants are presented in Table 1. For both racial/ethnic groups, neighborhood SES was associated positively with formal education, family income, and self-rated excellent health and inversely with the prevalence of hypertension and diabetes. Black residents of neighborhoods in the lower SES quartiles (quartiles 1 and 2) were also less likely to be married when compared with their counterparts in higher quartiles. Moreover, White residents in the first quartile of neighborhood SES had lower high-density lipoprotein cholesterol levels and higher low-density lipoprotein cholesterol levels than those in quartiles 2–4. Finally, when compared with lower quartiles, the highest quartile of neighborhood SES was associated with a higher prevalence of alcohol drinking among Black participants, but a lower prevalence among White participants.
Over the course of a mean 16.4 (standard deviation, 6.7) years of follow-up, there were 2,950 incident CVD events (448 among Black women, 280 among Black men, 1,154 among White women, and 1,068 among White men). Results from minimally adjusted models suggested that being in the lowest neighborhood SES quartile, as compared with the highest, in older, middle, and young adulthood was associated with an increased risk of total CVD (model 1, Table 2). After adjustment for additional sociodemographic factors and individual-level SES characteristics, the associations were attenuated in magnitude but the trend across quartiles remained similar (model 2, Table 2). When compared with the highest quartiles (denoted Q), the lowest quartiles of neighborhood SES in older, middle, and young adulthood were associated with 12% (HRQ1 vs. Q4 = 1.12 (95% CI: 0.99, 1.26); P for trend = 0.03), 21% (HRQ1 vs. Q4 = 1.21 (95% CI: 1.04, 1.39); P for trend = 0.02), and 18% (HRQ1 vs. Q4 = 1.18 (95% CI: 1.02, 1.36); P for trend = 0.02) increases in the risk of total CVD, respectively. Associations for individual CVD outcomes differed. For example, neighborhood SES in older and middle adulthood showed a stronger association with heart failure than with CHD. Childhood neighborhood SES was not associated with total CVD or CVD subtypes. Finally, lower average life-course neighborhood SES was associated with higher risks of total CVD (HRQ1 vs. Q4 = 1.17 (95% CI: 1.01, 1.35); P for trend = 0.04) and heart failure (HRQ1 vs. Q4 = 1.19 (95% CI: 1.00, 1.42); P for trend = 0.04).
Table 2.
Associations Between Life-Course Neighborhood Socioeconomic Status and Incident Cardiovascular Disease Among 9,692 Participants in the Atherosclerosis Risk in Communities Study, 1996–2019
| Total Cardiovascular Disease (n = 2,950) | Heart Failure (n = 1,931) | Coronary Heart Disease (n = 1,182) | Stroke (n = 788) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 b | Model 2 c | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||||
|
Life Stage
a
and Quartile of Neighborhood SES |
HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Older adulthood | ||||||||||||||||
| Q1 | 1.37 | 1.24, 1.53 | 1.12 | 0.99, 1.26 | 1.51 | 1.31, 1.73 | 1.19 | 1.03, 1.39 | 1.29 | 1.09, 1.53 | 1.03 | 0.84, 1.26 | 1.47 | 1.19, 1.81 | 1.26 | 0.99, 1.61 |
| Q2 | 1.25 | 1.12, 1.39 | 1.08 | 0.96, 1.21 | 1.26 | 1.10, 1.44 | 1.06 | 0.92, 1.23 | 1.29 | 1.09, 1.53 | 1.09 | 0.90, 1.31 | 1.27 | 1.02, 1.57 | 1.13 | 0.89, 1.42 |
| Q3 | 1.12 | 0.38, 3.30 | 0.98 | 0.87, 1.11 | 1.09 | 0.95, 1.26 | 0.95 | 0.82, 1.10 | 1.09 | 0.92, 1.30 | 0.96 | 0.80, 1.15 | 1.30 | 1.05, 1.61 | 1.15 | 0.92, 1.44 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | <0.001 | 0.03 | <0.001 | 0.007 | 0.003 | 0.49 | 0.003 | 0.10 | ||||||||
| Middle adulthood | ||||||||||||||||
| Q1 | 1.44 | 1.28, 1.62 | 1.21 | 1.04, 1.39 | 1.56 | 1.35, 1.80 | 1.28 | 1.08, 1.53 | 1.38 | 1.15, 1.65 | 1.11 | 0.90, 1.38 | 1.33 | 1.05, 1.68 | 1.19 | 0.90, 1.56 |
| Q2 | 1.19 | 1.06, 1.33 | 1.04 | 0.92, 1.18 | 1.31 | 1.13, 1.51 | 1.13 | 0.97, 1.32 | 1.21 | 1.01, 1.45 | 1.03 | 0.84, 1.26 | 1.02 | 0.82, 1.28 | 0.91 | 0.72, 1.15 |
| Q3 | 1.12 | 0.99, 1.26 | 1.03 | 0.91, 1.16 | 1.13 | 0.97, 1.32 | 1.04 | 0.89, 1.21 | 1.18 | 0.98, 1.42 | 1.06 | 0.88, 1.29 | 1.09 | 0.86, 1.38 | 1.00 | 0.79, 1.28 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | <0.001 | 0.02 | <0.001 | 0.006 | 0.002 | 0.41 | 0.03 | 0.29 | ||||||||
| Young adulthood | ||||||||||||||||
| Q1 | 1.33 | 1.17, 1.51 | 1.18 | 1.02, 1.36 | 1.30 | 1.10, 1.54 | 1.13 | 0.94, 1.35 | 1.34 | 1.12, 1.60 | 1.18 | 0.97, 1.43 | 1.33 | 1.02, 1.75 | 1.23 | 0.92, 1.66 |
| Q2 | 1.25 | 1.08, 1.45 | 1.12 | 0.95, 1.32 | 1.23 | 1.04, 1.46 | 1.08 | 0.90, 1.30 | 1.28 | 1.05, 1.56 | 1.14 | 0.93, 1.41 | 1.20 | 0.91, 1.58 | 1.14 | 0.82, 1.59 |
| Q3 | 1.09 | 0.94, 1.26 | 1.02 | 0.88, 1.19 | 1.04 | 0.86, 1.26 | 0.97 | 0.80, 1.17 | 1.12 | 0.91, 1.38 | 1.05 | 0.85, 1.30 | 1.08 | 0.84, 1.40 | 1.03 | 0.77, 1.38 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | 0.0001 | 0.02 | 0.001 | 0.09 | 0.002 | 0.07 | 0.04 | 0.15 | ||||||||
| Childhood | ||||||||||||||||
| Q1 | 1.05 | 0.93, 1.17 | 0.92 | 0.81, 1.04 | 1.13 | 0.98, 1.30 | 0.96 | 0.82, 1.12 | 0.97 | 0.82, 1.16 | 0.86 | 0.71, 1.04 | 0.90 | 0.72, 1.11 | 0.80 | 0.63, 1.01 |
| Q2 | 1.08 | 0.96, 1.22 | 1.02 | 0.90, 1.15 | 1.11 | 0.95, 1.29 | 1.02 | 0.87, 1.19 | 1.01 | 0.84, 1.21 | 0.96 | 0.79, 1.16 | 1.05 | 0.83, 1.32 | 0.99 | 0.77, 1.26 |
| Q3 | 1.03 | 0.92, 1.16 | 0.92 | 0.80, 1.04 | 1.10 | 0.95, 1.27 | 0.93 | 0.79, 1.09 | 0.96 | 0.80, 1.16 | 0.87 | 0.71, 1.06 | 0.95 | 0.76, 1.20 | 0.85 | 0.67, 1.09 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | 0.41 | 0.53 | 0.15 | 0.99 | 0.89 | 0.28 | 0.44 | 0.19 | ||||||||
| Average life course | ||||||||||||||||
| Q1 | 1.42 | 1.26, 1.60 | 1.17 | 1.01, 1.35 | 1.50 | 1.29, 1.74 | 1.19 | 1.00, 1.42 | 1.33 | 1.11, 1.59 | 1.07 | 0.87, 1.32 | 1.50 | 1.18, 1.90 | 1.31 | 0.99, 1.73 |
| Q2 | 1.21 | 1.08, 1.37 | 1.04 | 0.92, 1.19 | 1.24 | 1.06, 1.45 | 1.04 | 0.88, 1.24 | 1.25 | 1.04, 1.50 | 1.06 | 0.87, 1.30 | 1.09 | 0.85, 1.39 | 0.96 | 0.73, 1.25 |
| Q3 | 1.10 | 0.98, 1.29 | 1.01 | 0.89, 1.15 | 1.08 | 0.93, 1.27 | 0.99 | 0.84, 1.16 | 1.08 | 0.88, 1.31 | 0.98 | 0.80, 1.20 | 1.29 | 1.02, 1.64 | 1.18 | 0.93, 1.51 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | <0.0001 | 0.04 | <0.0001 | 0.04 | 0.002 | 0.41 | 0.01 | 0.19 | ||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; Q, quartile; SES, socioeconomic status.
a Older adulthood: baseline (median age, 62 years); middle adulthood: age 40–50 years; young adulthood: age 30 years; childhood: age 10 years. Average life-course neighborhood SES was calculated as the mean of neighborhood SES at each life stage, weighted by the duration of each stage.
b Results were adjusted for age (years; continuous) and sex (male or female).
c Results were adjusted for age (years; continuous), sex (male or female), race/ethnicity (Black or White), marital status (married, widowed, divorced, separated, or never married), education (less than high school, high school or vocational school, or college or higher), annual household income (<$25,000, $25,000–$49,999, $50,000–$74,999, or ≥$75,000), and study center (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; or the suburbs of Minneapolis, Minnesota).
When compared with White participants, Black participants had a higher incidence of total CVD, heart failure, and stroke in all quartiles of life-course neighborhood SES (Figure 1). Among both racial/ethnic groups, the incidence of total CVD and heart failure appeared to decrease with increased neighborhood SES (Figure 1). In analysis stratified by race and sex (Table 3), we found that associations between total CVD and neighborhood SES in older and middle adulthood, as well as average life-course neighborhood SES, appeared the strongest among Black women, while the results among other race-sex groups were weaker or null. Specifically, Black women in the lowest quartiles of neighborhood SES in older and middle adulthood and average life-course neighborhood SES had 58% (HRQ1 vs. Q4 = 1.58, 95% CI: 1.15, 2.17), 42% (HRQ1 vs. Q4 = 1.42, 95% CI: 1.05, 1.91), and 37% (HRQ1 vs. Q4 = 1.37, 95% CI: 1.00, 1.88) higher risks of total CVD when compared with those in the highest quartiles. Similarly, stronger results among Black women were also observed for CVD subtypes (Web Tables 1–3). For example, the lowest neighborhood SES in older adulthood was associated with 85% and 90% increases in risk of heart failure and stroke, respectively. Finally, among Black men, lower childhood neighborhood SES was associated with lower risks of total CVD (HRQ1 vs. Q4 = 0.61 (95% CI: 0.41, 0.91); P for trend = 0.02) (Table 3), heart failure (HRQ1 vs. Q4 = 0.59 (95% CI: 0.35, 0.99); P for trend = 0.05) (Web Table 1), and stroke (HRQ1 vs. Q4 = 0.44 (95% CI: 0.20, 0.99); P for trend = 0.08) (Web Table 3). However, such a pattern was not observed among other race-sex groups.
Figure 1.
Adjusted incidence rates (per 1,000 person-years) of A) total cardiovascular disease, B) heart failure, C) coronary heart disease, and D) stroke according to quartiles (Q) of average life-course neighborhood socioeconomic status (SES), overall (n = 9,692) and in the White (n = 7,527) and Black (n = 2,165) populations of the Atherosclerosis Risk in Communities Study, 1996–2019. Incidence rates were adjusted for age (years; continuous), sex (male or female), race/ethnicity (Black or White; not adjusted in race/ethnicity-specific results), marital status (married, widowed, divorced, separated, or never married), education (less than high school, high school or vocational school, or college or higher), annual household income (<$25,000, $25,000–$49,999, $50,000–$74,999, or ≥$75,000), and study center (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; or the suburbs of Minneapolis, Minnesota). Average life-course neighborhood SES was calculated as the mean of neighborhood SES in older, middle, and young adulthood and in childhood, weighted by the duration of each life stage. Bars, 95% confidence intervals.
Table 3.
Associationsa Between Life-Course Neighborhood Socioeconomic Status and Total Incident Cardiovascular Disease, by Race/Ethnicity and Sex, in the Atherosclerosis Risk in Communities Study, 1996–2019
| Risk of Cardiovascular Disease | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black Participants | White Participants | |||||||||||
|
Overall
(n = 728) |
Black Women
(n = 448) |
Black Men
(n = 280) |
Overall
(n = 2,222) |
White Women
(n = 1,154) |
White Men
(n = 1,068) |
|||||||
| Life Stage and Quartile of Neighborhood SES b | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Older adulthood | ||||||||||||
| Q1 | 1.30 | 1.02, 1.66 | 1.58 | 1.15, 2.17 | 1.00 | 0.66, 1.50 | 1.07 | 0.93, 1.24 | 1.16 | 0.95, 1.42 | 0.98 | 0.80, 1.21 |
| Q2 | 1.17 | 0.91, 1.50 | 1.42 | 1.03, 1.96 | 0.86 | 0.55, 1.35 | 1.06 | 0.93, 1.21 | 1.17 | 0.97, 1.41 | 0.95 | 0.78, 1.15 |
| Q3 | 1.08 | 0.85, 1.38 | 1.10 | 0.80, 1.52 | 1.09 | 0.76, 1.56 | 0.96 | 0.83, 1.10 | 1.05 | 0.87, 1.27 | 0.86 | 0.71, 1.04 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | 0.03 | 0.003 | 0.71 | 0.15 | 0.11 | 0.75 | ||||||
| P for interaction (SES × sex)c | 0.009 | 0.11 | ||||||||||
| P for interaction (SES × race/ethnicity)d | 0.24 | |||||||||||
| Middle adulthood | ||||||||||||
| Q1 | 1.32 | 1.03, 1.68 | 1.42 | 1.05, 1.91 | 1.21 | 0.82, 1.78 | 1.14 | 0.96, 1.36 | 1.13 | 0.88, 1.45 | 1.14 | 0.90, 1.44 |
| Q2 | 1.05 | 0.83, 1.33 | 1.11 | 0.83, 1.50 | 0.98 | 0.67, 1.43 | 1.02 | 0.88, 1.18 | 1.01 | 0.81, 1.25 | 1.01 | 0.82, 1.26 |
| Q3 | 0.98 | 0.77, 1.25 | 0.97 | 0.71, 1.33 | 0.98 | 0.69, 1.40 | 1.04 | 0.90, 1.19 | 1.03 | 0.83, 1.28 | 1.03 | 0.84, 1.25 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | 0.03 | 0.02 | 0.37 | 0.17 | 0.34 | 0.33 | ||||||
| P for interaction (SES × sex) | 0.21 | 0.40 | ||||||||||
| P for interaction (SES × race/ethnicity) | 0.23 | |||||||||||
| Young adulthood | ||||||||||||
| Q1 | 1.21 | 0.92, 1.60 | 1.19 | 0.82, 1.72 | 1.28 | 0.87, 1.88 | 1.16 | 0.99, 1.36 | 1.10 | 0.88, 1.39 | 1.21 | 0.99, 1.49 |
| Q2 | 1.09 | 0.78, 1.51 | 1.15 | 0.78, 1.69 | 1.00 | 0.62, 1.63 | 1.13 | 0.97, 1.32 | 1.11 | 0.89, 1.40 | 1.14 | 0.93, 1.40 |
| Q3 | 0.94 | 0.72, 1.22 | 0.93 | 0.66, 1.31 | 0.96 | 0.64, 1.43 | 1.05 | 0.90, 1.24 | 1.01 | 0.79, 1.28 | 1.10 | 0.90, 1.35 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | 0.16 | 0.22 | 0.29 | 0.05 | 0.28 | 0.08 | ||||||
| P for interaction (SES × sex) | 0.89 | 0.97 | ||||||||||
| P for interaction (SES × race/ethnicity) | 0.61 | |||||||||||
| Childhood | ||||||||||||
| Q1 | 0.88 | 0.69, 1.12 | 1.08 | 0.78, 1.48 | 0.61 | 0.41, 0.91 | 0.94 | 0.82, 1.08 | 1.00 | 0.82, 1.22 | 0.88 | 0.72, 1.07 |
| Q2 | 1.00 | 0.79, 1.27 | 1.14 | 0.83, 1.56 | 0.82 | 0.56, 1.19 | 1.03 | 0.89, 1.19 | 1.02 | 0.83, 1.26 | 1.04 | 0.85, 1.26 |
| Q3 | 0.90 | 0.68, 1.19 | 0.95 | 0.66, 1.36 | 0.83 | 0.54, 1.28 | 0.94 | 0.81, 1.08 | 1.04 | 0.85, 1.26 | 0.85 | 0.69, 1.05 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | 0.52 | 0.30 | 0.02 | 0.66 | 0.91 | 0.65 | ||||||
| P for interaction (SES × sex) | 0.01 | 0.67 | ||||||||||
| P for interaction (SES × race/ethnicity) | 0.57 | |||||||||||
| Average life course | ||||||||||||
| Q1 | 1.19 | 0.91, 1.55 | 1.37 | 1.00, 1.88 | 1.00 | 0.66, 1.51 | 1.15 | 0.97, 1.36 | 1.13 | 0.89, 1.43 | 1.15 | 0.90, 1.48 |
| Q2 | 0.97 | 0.76, 1.23 | 1.10 | 0.80, 1.50 | 0.82 | 0.57, 1.19 | 1.07 | 0.91, 1.25 | 1.08 | 0.86, 1.35 | 1.03 | 0.84, 1.28 |
| Q3 | 0.97 | 0.74, 1.27 | 1.01 | 0.70, 1.44 | 0.94 | 0.64, 1.40 | 1.03 | 0.89, 1.19 | 1.00 | 0.81, 1.24 | 1.04 | 0.83, 1.30 |
| Q4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for trend | 0.17 | 0.04 | 0.84 | 0.12 | 0.25 | 0.29 | ||||||
| P for interaction (SES × sex) | 0.06 | 0.44 | ||||||||||
| P for interaction (SES × race/ethnicity) | 0.74 | |||||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; Q, quartile; SES, socioeconomic status.
a Results were adjusted for age (years; continuous), sex (male or female), marital status (married, widowed, divorced, separated, or never married), education (less than high school, high school or vocational school, or college or higher), annual household income (<$25,000, $25,000–$49,999, $50,000–$74,999, or ≥$75,000), and study center (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; or the suburbs of Minneapolis, Minnesota).
b Older adulthood: baseline (median age, 62 years); middle adulthood: age 40–50 years; young adulthood: age 30 years; childhood: age 10 years. Average life-course neighborhood SES was calculated as the mean of neighborhood SES at each life stage, weighted by the duration of each stage.
c P value for the interaction term between neighborhood SES variables and sex in each racial/ethnic group.
d P value for the interaction term between neighborhood SES variables and race/ethnicity in the overall sample.
We examined patterns of change in neighborhood SES across life stages in relation to CVD in the overall population (Table 4) and among race-sex groups (Table 5). In general, when compared with the stable-high groups of neighborhood SES, the stable-low groups consistently showed higher risks for total CVD and heart failure, while the results for the neighborhood SES decline and improvement groups were mixed (Table 4). Although we observed a suggestive trend for an increased risk associated with neighborhood SES decline (high-low vs. high-high) and a decreased risk with neighborhood improvement (low-high vs. low-low), most of the results were not statistically significant. In stratified analysis (Table 5), the results were generally stronger among Black women. For example, when compared with those in the stable-high group, Black women residing in neighborhoods with a stable-low or declining neighborhood SES from middle adulthood to older adulthood had 47% (HRQ1 vs. Q4 = 1.47, 95% CI: 1.16, 1.85) and 59% (HRQ1 vs. Q4 = 1.59, 95% CI: 1.08, 2.35) increased risks of total CVD when compared with their counterparts living in stable-high neighborhoods.
Table 4.
Associations Between Neighborhood Socioeconomic Status Patterns Across Life Stages and Incident Cardiovascular Disease Among 9,692 Participants in the Atherosclerosis Risk in Communities Study, 1996–2019
| Total Cardiovascular Disease | Heart Failure | Coronary Heart Disease | Stroke | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 b | Model 2 c | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||||
| Quartile of Neighborhood SES Over the Life Course a | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Middle-to-older adulthood | ||||||||||||||||
| Low-low | 1.31 | 1.20, 1.43 | 1.14 | 1.03, 1.26 | 1.44 | 1.30, 1.61 | 1.23 | 1.09, 1.40 | 1.28 | 1.11, 1.47 | 1.08 | 0.92, 1.28 | 1.19 | 1.01, 1.40 | 0.94 | 0.78, 1.14 |
| Low-high | 1.17 | 1.03, 1.33 | 1.08 | 0.95, 1.23 | 1.25 | 1.07, 1.47 | 1.16 | 0.99, 1.37 | 1.22 | 1.00, 1.50 | 1.11 | 0.90, 1.37 | 0.92 | 0.71, 1.18 | 0.81 | 0.63, 1.05 |
| High-low | 1.21 | 1.06, 1.38 | 1.14 | 0.99, 1.31 | 1.26 | 1.06, 1.49 | 1.19 | 0.99, 1.41 | 1.37 | 1.12, 1.68 | 1.26 | 1.03, 1.54 | 1.05 | 0.81, 1.36 | 0.91 | 0.69, 1.19 |
| High-high | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Young-to-middle adulthood | ||||||||||||||||
| Low-low | 1.35 | 1.23, 1.49 | 1.17 | 1.05, 1.31 | 1.46 | 1.29, 1.65 | 1.24 | 1.08, 1.42 | 1.31 | 1.13, 1.52 | 1.11 | 0.94, 1.32 | 1.26 | 1.03, 1.53 | 0.89 | 0.71, 1.11 |
| Low-high | 1.18 | 1.03, 1.35 | 1.1 | 0.96, 1.27 | 1.21 | 1.01, 1.44 | 1.12 | 0.93, 1.34 | 1.17 | 0.95, 1.45 | 1.11 | 0.90, 1.37 | 1.19 | 0.88, 1.61 | 0.98 | 0.76, 1.25 |
| High-low | 1.18 | 1.02, 1.35 | 1.04 | 0.90, 1.20 | 1.34 | 1.14, 1.59 | 1.16 | 0.97, 1.38 | 1.1 | 0.88, 1.37 | 0.97 | 0.77, 1.22 | 1.02 | 0.77, 1.34 | 0.81 | 0.60, 1.08 |
| High-high | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Childhood to young adulthood | ||||||||||||||||
| Low-low | 1.26 | 1.12, 1.42 | 1.14 | 1.01, 1.30 | 1.31 | 1.12, 1.52 | 1.16 | 0.99, 1.37 | 1.23 | 1.04, 1.45 | 1.1 | 0.93, 1.31 | 1.19 | 0.94, 1.50 | 0.91 | 0.70, 1.18 |
| Low-high | 1.06 | 0.93, 1.21 | 1.01 | 0.89, 1.16 | 1.11 | 0.91, 1.35 | 1.05 | 0.86, 1.28 | 1.03 | 0.86, 1.24 | 0.99 | 0.82, 1.19 | 0.91 | 0.72, 1.16 | 0.79 | 0.59, 1.05 |
| High-low | 1.27 | 1.10, 1.47 | 1.14 | 0.98, 1.32 | 1.31 | 1.06, 1.62 | 1.14 | 0.92, 1.43 | 1.27 | 1.07, 1.52 | 1.14 | 0.95, 1.37 | 1.17 | 0.91, 1.50 | 0.96 | 0.76, 1.22 |
| High-high | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
Abbreviations: CI, confidence interval; HR, hazard ratio; SES, socioeconomic status.
a Low and high neighborhood SES were defined on the basis of the median neighborhood SES at each life stage. Older adulthood: baseline (median age, 62 years); middle adulthood: age 40–50 years; young adulthood: age 30 years; childhood: age 10 years.
b Results were adjusted for age (years; continuous) and sex (male or female).
c Results were adjusted for age (years; continuous), sex (male or female), race/ethnicity (Black or White), marital status (married, widowed, divorced, separated, or never married), education (less than high school, high school or vocational school, or college or higher), annual household income (<$25,000, $25,000–$49,999, $50,000–$74,999, or ≥$75,000), and study center (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; or the suburbs of Minneapolis, Minnesota).
Table 5.
Associationsa Between Neighborhood Socioeconomic Status Patterns Across Life Stages and Total Incident Cardiovascular Disease, by Race/Ethnicity and Sex, in the Atherosclerosis Risk in Communities Study, 1996–2019
| Total Cardiovascular Disease Risk | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black Participants | White Participants | |||||||||||
|
Overall
(n = 728) |
Black Women
(n = 448) |
Black Men
(n = 280) |
Overall
(n = 2,222) |
White Women
(n = 1,154) |
White Men
(n = 1,068) |
|||||||
| Quartile of Neighborhood SES Over the Life Course b | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Middle-to-older adulthood | ||||||||||||
| Low-low | 1.24 | 1.03, 1.48 | 1.47 | 1.16, 1.85 | 0.98 | 0.72, 1.32 | 1.09 | 0.97, 1.23 | 1.11 | 0.94, 1.31 | 1.07 | 0.89, 1.28 |
| Low-high | 1.25 | 0.95, 1.65 | 1.22 | 0.83, 1.79 | 1.33 | 0.90, 1.96 | 1.01 | 0.86, 1.18 | 1.04 | 0.83, 1.30 | 0.97 | 0.78, 1.22 |
| High-low | 1.26 | 0.93, 1.71 | 1.59 | 1.08, 2.35 | 0.83 | 0.48, 1.46 | 1.12 | 0.96, 1.31 | 1.23 | 0.99, 1.52 | 1.00 | 0.79, 1.26 |
| High-high | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for interaction (SES × sex)c | 0.04 | 0.33 | ||||||||||
| P for interaction (SES × race/ethnicity)d | 0.27 | |||||||||||
| Young-to-middle adulthood | ||||||||||||
| Low-low | 1.30 | 1.02, 1.67 | 1.40 | 1.05, 1.87 | 1.20 | 0.81, 1.77 | 1.11 | 0.98, 1.27 | 1.10 | 0.92, 1.31 | 1.12 | 0.93, 1.34 |
| Low-high | 1.07 | 0.82, 1.40 | 1.13 | 0.79, 1.61 | 0.99 | 0.65, 1.49 | 1.12 | 0.96, 1.30 | 1.08 | 0.86, 1.36 | 1.14 | 0.92, 1.42 |
| High-low | 1.07 | 0.83, 1.39 | 1.19 | 0.88, 1.62 | 0.92 | 0.60, 1.40 | 1.01 | 0.85, 1.20 | 0.98 | 0.77, 1.24 | 1.04 | 0.83, 1.31 |
| High-high | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for interaction (SES × sex) | 0.37 | 0.59 | ||||||||||
| P for interaction (SES × race/ethnicity) | 0.38 | |||||||||||
| Childhood to young adulthood | ||||||||||||
| Low-low | 1.16 | 0.89, 1.51 | 1.4 | 1.02, 1.93 | 0.90 | 0.61, 1.33 | 1.13 | 0.97, 1.30 | 1.09 | 0.89, 1.34 | 1.15 | 0.95, 1.40 |
| Low-high | 0.94 | 0.72, 1.23 | 1.19 | 0.87, 1.62 | 0.66 | 0.43, 1.03 | 1.03 | 0.87, 1.21 | 1.00 | 0.80, 1.25 | 1.06 | 0.86, 1.30 |
| High-low | 1.13 | 0.79, 1.61 | 1.26 | 0.87, 1.83 | 1.00 | 0.60, 1.67 | 1.13 | 0.96, 1.34 | 1.12 | 0.89, 1.41 | 1.13 | 0.91, 1.40 |
| High-high | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| P for interaction (SES × sex) | 0.04 | 0.79 | ||||||||||
| P for interaction (SES × race/ethnicity) | 0.91 | |||||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; SES, socioeconomic status.
a Results were adjusted for age (years; continuous), sex (male or female), marital status (married, widowed, divorced, separated, or never married), education (less than high school, high school or vocational school, or college or higher), annual household income (<$25,000, $25,000–$49,999, $50,000–$74,999, or ≥$75,000), and study center (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; or the suburbs of Minneapolis, Minnesota).
b Older adulthood: baseline (median age, 62 years); middle adulthood: age 40–50 years; young adulthood: age 30 years; childhood: age 10 years. Average life-course neighborhood SES was calculated as the mean of neighborhood SES at each life stage, weighted by the duration of each stage.
c P value for the interaction term between neighborhood SES variables and sex in each racial/ethnic group.
d P value for the interaction term between neighborhood SES variables and race/ethnicity in the overall sample.
DISCUSSION
In this large cohort of residents of 4 geographically defined areas in the United States, we found that those living in neighborhoods with low SES during various stages in adulthood, but not in childhood, were more likely to develop CVD later in life than study participants living in high–neighborhood-SES communities. We also detected differences across race/ethnicity and sex, such that the associations between neighborhood SES in older and middle adulthood and CVD risk were particularly strong among Black women.
The inverse association between neighborhood SES in adulthood and CVD is consistent with previous findings from numerous large cohort studies. For example, in an earlier investigation in the ARIC cohort with shorter follow-up (mean = 9.1 years), when compared with the highest tertile, the lowest tertile of baseline neighborhood SES was associated with 70% (HRQ1 vs. Q4 = 1.7, 95% CI: 1.3, 2.3) and 40% (HRQ1 vs. Q4 = 1.4, 95% CI: 0.9, 2.0) increases in incident CHD in White and Black study participants, respectively (3). Similarly, a study that included 3.7 million Swedish adults aged 35–74 years who were free of prevalent CHD suggested that those living in neighborhoods with high socioeconomic deprivation, as compared with low socioeconomic deprivation (1 standard deviation above the mean), were more likely to develop CHD (odds ratios were 1.28 (95% CI: 1.24, 1.33) for women and 1.21 (95% CI: 1.18, 1.24) for men) over 5 years (1996–2000) (5). In the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study, the lowest quartile of neighborhood SES was associated with a 25% increase in stroke risk, with borderline statistical significance (HR = 1.25 (95% CI: 0.99, 1.56); P for trend = 0.085) (9). A similar inverse association between neighborhood SES and stroke was observed in a large Japanese cohort of middle-aged to older adults with long follow-up (approximately 16 years) (8). Finally, in the NIH-AARP Diet and Health Study, men and women residing in neighborhoods with the lowest quintile of neighborhood SES were 33% and 18% more likely to die from CVD, respectively, when compared with their counterparts residing in neighborhoods in the highest quintile (11). The consistency in findings across studies that focused on a variety of CVD outcomes and diverse populations suggests that neighborhood SES is probably a fundamental contributor to CVD disparities in the United States and other high-income countries. Our investigation extended previous research by showing that neighborhood SES in young, middle, and older adulthood was similarly associated with CVD risk, suggesting that the processes that link neighborhood disadvantage with CVD may begin early in adulthood. Therefore, any interventions aimed at improving neighborhood conditions to reduce CVD burden may benefit populations with a wide age range.
Our analysis revealed no association between neighborhood SES in childhood and incidence of CVD in adulthood. In contrast, many previous studies suggested that early life is an important stage and that childhood socioeconomic conditions may have a unique and lasting impact on disease risk in adulthood: In a systematic review including 24 prospective and 11 case-control studies on childhood SES and adult CVD outcomes, Galobardes et al. (39) concluded that more adverse socioeconomic conditions during childhood were a risk factor for incident CHD, stroke, and overall CVD risk and mortality. However, all of the studies measured childhood SES at the individual or family level (mostly by father’s occupation), and there are few investigations of childhood neighborhood conditions and cardiovascular health. One example is a recent analysis based on data from the New England Family Study, which found that high neighborhood SES at birth and in adulthood was associated with a favorable cardiovascular risk profile (i.e., lower blood pressure and body mass index), while neighborhood SES in childhood (mean age = 7.1 years) was not associated with CVD risk factors in adulthood (18). In contrast, in a study conducted in Finland, Kivimäki et al. (40) reported that childhood neighborhood disadvantage was associated with multiple cardiometabolic risk factors, including obesity, hypertension, and diabetes, by middle age.
The lack of association between childhood neighborhood SES and CVD risk in older adulthood in our study may be explained by several factors. First, recalled childhood neighborhood SES may not be accurate. Unlike adulthood neighborhood SES, which was measured at the census-tract level, childhood neighborhood SES was measured at the county level. Misclassification of neighborhood SES is thus possible, as this larger geographic unit does not reflect neighborhood exposures. Moreover, it is challenging to recall early-life residential locations precisely, which may also lead to errors in the assignment of census data (32). Taken together, these issues suggest that our measure of childhood neighborhood SES may have been subject to exposure misclassification. Second, it has been proposed that childhood neighborhood SES may influence CVD risk by shaping socioeconomic trajectories later in life (41), and thus may not be an independent risk factor for CVD. However, we found no evidence suggesting an association between childhood neighborhood SES and CVD risk even without adjusting for adulthood education and income, implying that the null findings cannot be explained by this reason alone. Third, it has been proposed that children in low-SES neighborhoods, particularly Black girls, may avoid spending time in their home neighborhood because of safety concerns (42), and therefore neighborhood measurement based on home addresses may not accurately reflect actual neighborhood exposure. Finally, it is also possible that family SES plays a more important role than neighborhood SES in childhood development and has a larger impact on health trajectories in adulthood. Given that there is currently very limited research on childhood neighborhood SES and CVD, we encourage investigators in future studies to examine whether and how neighborhood conditions in childhood may influence cardiovascular health.
In race/ethnicity- and sex-specific analysis, we found that the association between neighborhood SES in older and middle adulthood and CVD risk was particularly strong among Black women. This finding is consistent with results from the Jackson Heart Study, a cohort study of over 4,000 African Americans, in which Barber et al. (13) also reported a sex difference in the association between neighborhood SES and total CVD risk: Each standard-deviation increase in neighborhood socioeconomic disadvantage was associated with a 25% increase in CVD incidence in women (HR = 1.25, 95% CI: 1.05, 1.45) but not in men (HR = 1.08, 95% CI: 0.82, 1.41). In the Black Women’s Health Study, the lowest quartile of neighborhood SES was associated with a 40% increase in CVD mortality (12), and the effect estimate was higher than in predominantly White cohorts. These results, together with ours, suggest that neighborhood conditions in older and middle adulthood may be a particularly strong predictor of cardiovascular health outcomes among Black women. It has been proposed that individual characteristics may play an important role in modifying the association between neighborhood environment and health outcomes, because of differences in exposure patterns, relative socioeconomic standing in communities, perception about neighborhood conditions, and resources and abilities to take advantage of services in high-SES neighborhoods and/or cope with challenging environments (43, 44). For CVD, although there have been few studies examining its association with neighborhood SES according to both race/ethnicity and sex, it has been reported that the association may be particularly strong among low-SES individuals (45). Our findings extend current evidence and highlight the importance of considering individual-level factors as modulators of the association between neighborhood SES and health.
Our analysis focusing on life-course patterns of neighborhood SES found that chronic exposure to low neighborhood SES was a consistent predictor of high risk of CVD. In contrast, the association between changes in neighborhood SES (e.g., the low-high and high-low groups) and CVD was weaker and less consistent. In a previous analysis in the NIH-AARP Diet and Health Study, we found that declining neighborhood SES was associated with a higher risk of CVD, while improving neighborhood SES was associated with a lower risk (46). However, the study also found that such associations were only apparent for relatively large changes in neighborhood SES (i.e., a reduction or increase in neighborhood SES ranking by 20 percentile points or more). In the current analysis, we were not able to examine larger changes in neighborhood conditions because of a limited sample size. Studies with larger sample sizes, more dynamic neighborhood exposures, and longer follow-up periods are needed to further clarify the relationship between change in neighborhood SES and CVD risk.
Our study had several strengths. First, we assessed neighborhood SES during 4 life stages, from childhood to older adulthood, which allowed us to assess the 4 stages’ distinctive relationships with CVD and to examine the associations for cumulative neighborhood SES exposures and changes in neighborhood SES across different life stages. Second, the large sample size and long follow-up enabled us to examine potential differences in the associations for each race-sex group and for different CVD subtypes. Our study also had several limitations. Neighborhood SES in middle and young adulthood and childhood was assessed on the basis of recall of address information, which may be subject to recall bias and open to exposure misclassification. As noted above, information on neighborhood SES in childhood could be assessed only at the county level, and lack of more granular measures may have contributed to the null findings for childhood SES. Moreover, for administrative reasons, follow-up for CVD outcomes at the Jackson, Mississippi, study site was paused in 2017, and we were not able to include more recent CVD events in the analysis as was done for other study sites. In addition, Black participants were clustered within study sites, with the majority of Black cohort members recruited from Jackson, Mississippi. Thus, the racial/ethnic differences we observed in this study may have been partially driven by regional differences. Studies using widely representative samples are needed to further examine race/ethnicity-specific associations between neighborhood SES and CVD. In addition, our study sample was not representative of the entire Black and White population of the United States, and the findings may not be generalizable to populations that differ from the study sample. Finally, we focused only on neighborhood SES measures and did not have information on other neighborhood factors, such as geographic racial discrimination and disparities in neighborhood health environment, that may have contributed to the observed associations between neighborhood SES and CVD, as well as potential differences among race-sex groups. In future studies, researchers should systematically investigate specific neighborhood attributes and pathways underlying the potential health effects of neighborhood environment in Black and White populations.
In summary, our findings from this large biracial cohort add to the growing literature supporting a role of neighborhood SES in various stages of adulthood and cardiovascular health. Our study also suggests that older Black women living in low-SES neighborhoods may be at especially high risk for developing cardiovascular events, such as heart failure and stroke. In future studies aimed at improving neighborhood conditions to reduce health disparities, investigators should consider the impact of such programs on individuals with different sociodemographic characteristics.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Human Genetics and Environmental Health, School of Public Health, University of Texas Health Science Center at Houston, Houston, Texas, United States (Qian Xiao); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina, United States (Gerardo Heiss, Anna Kucharska-Newton, Ganga Bey, Shelly-Ann M. Love, Eric A. Whitsel); Department of Epidemiology, College of Public Health, University of Kentucky, Lexington, Kentucky, United States (Anna Kucharska-Newton); and Department of Medicine, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States (Eric A. Whitsel).
The Atherosclerosis Risk in Communities (ARIC) Study is supported by the National Heart, Lung, and Blood Institute (contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I).
We thank the staff and participants of the ARIC Study for their important contributions.
Information about access to ARIC data can be found on the study’s website (https://sites.cscc.unc.edu/aric/).
Conflict of interest: none declared.
REFERENCES
- 1. Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):873–898. [DOI] [PubMed] [Google Scholar]
- 2. Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 4. Sundquist K, Winkleby M, Ahlen H, et al. Neighborhood socioeconomic environment and incidence of coronary heart disease: a follow-up study of 25,319 women and men in Sweden. Am J Epidemiol. 2004;159(7):655–662. [DOI] [PubMed] [Google Scholar]
- 5. Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. Am J Prev Med. 2007;32(2):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaix B, Rosvall M, Merlo J. Neighborhood socioeconomic deprivation and residential instability: effects on incidence of ischemic heart disease and survival after myocardial infarction. Epidemiology. 2007;18(1):104–111. [DOI] [PubMed] [Google Scholar]
- 7. Brown AF, Liang LJ, Vassar SD, et al. Neighborhood disadvantage and ischemic stroke: the Cardiovascular Health Study (CHS). Stroke. 2011;42(12):3363–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Honjo K, Iso H, Nakaya T, et al. Impact of neighborhood socioeconomic conditions on the risk of stroke in Japan. J Epidemiol. 2015;25(3):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howard VJ, McClure LA, Kleindorfer DO, et al. Neighborhood socioeconomic index and stroke incidence in a national cohort of blacks and whites. Neurology. 2016;87(22):2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borrell LN, Diez Roux AV, Rose K, et al. Neighbourhood characteristics and mortality in the Atherosclerosis Risk in Communities Study. Int J Epidemiol. 2004;33(2):398–407. [DOI] [PubMed] [Google Scholar]
- 11. Major JM, Doubeni CA, Freedman ND, et al. Neighborhood socioeconomic deprivation and mortality: NIH-AARP Diet and Health Study. PLoS One. 2010;5(11):e15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bethea TN, Palmer JR, Rosenberg L, et al. Neighborhood socioeconomic status in relation to all-cause, cancer, and cardiovascular mortality in the Black Women’s Health Study. Ethn Dis. 2016;26(2):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barber S, Hickson DA, Wang X, et al. Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among African American adults in the Jackson Heart Study. Am J Public Health. 2016;106(12):2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hussein M, Diez Roux AV, Mujahid MS, et al. Unequal exposure or unequal vulnerability? Contributions of neighborhood conditions and cardiovascular risk factors to socioeconomic inequality in incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2018;187(7):1424–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foraker RE, Rose KM, McGinn AP, et al. Neighborhood income, health insurance, and prehospital delay for myocardial infarction: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 2008;168(17):1874–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foraker RE, Rose KM, Whitsel EA, et al. Neighborhood socioeconomic status, Medicaid coverage and medical management of myocardial infarction: Atherosclerosis Risk in Communities (ARIC) community surveillance. BMC Public Health. 2010;10:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rose KM, Foraker RE, Heiss G, et al. Neighborhood socioeconomic and racial disparities in angiography and coronary revascularization: the ARIC Surveillance Study. Ann Epidemiol. 2012;22(9):623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jimenez MP, Wellenius GA, Subramanian SV, et al. Longitudinal associations of neighborhood socioeconomic status with cardiovascular risk factors: a 46-year follow-up study. Soc Sci Med. 2019;241:112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walsemann KM, Goosby BJ, Farr D. Life course SES and cardiovascular risk: heterogeneity across race/ethnicity and gender. Soc Sci Med. 2016;152:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lippert AM, Evans CR, Razak F, et al. Associations of continuity and change in early neighborhood poverty with adult cardiometabolic biomarkers in the United States: results from the National Longitudinal Study of Adolescent to Adult Health, 1995–2008. Am J Epidemiol. 2017;185(9):765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alvarado SE. The indelible weight of place: childhood neighborhood disadvantage, timing of exposure, and obesity across adulthood. Health Place. 2019;58:102159. [DOI] [PubMed] [Google Scholar]
- 23. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 24. Iceland J, Sharpe C, Steinmetz E. Class differences in African American residential patterns in US metropolitan areas: 1990–2000. Soc Sci Res. 2005;34(1):252–266. [Google Scholar]
- 25. Charles CZ. The dynamics of racial residential segregation. Annu Rev Sociol. 2003;29(1):167–207. [Google Scholar]
- 26. Sharkey P. Stuck in Place: Urban Neighborhoods and the End of Progress Toward Racial Equality. Chicago, IL: University of Chicago Press; 2013. [Google Scholar]
- 27. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 28. Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk in Communities) Study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pollitt RA, Kaufman JS, Rose KM, et al. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Community Health. 2008;62(6):484–491. [DOI] [PubMed] [Google Scholar]
- 30. Carson AP, Rose KM, Catellier DJ, et al. Cumulative socioeconomic status across the life course and subclinical atherosclerosis. Ann Epidemiol. 2007;17(4):296–303. [DOI] [PubMed] [Google Scholar]
- 31. Whitsel EA, Rose KM, Wood JL, et al. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160(10):1023–1029. [DOI] [PubMed] [Google Scholar]
- 32. Whitsel EA, Quibrera PM, Smith RL, et al. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov. 2006;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rose KM, Wood JL, Knowles S, et al. Historical measures of social context in life course studies: retrospective linkage of addresses to decennial censuses. Int J Health Geogr. 2004;3(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diez-Roux AV, Kiefe CI, Jacobs DR Jr, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11(6):395–405. [DOI] [PubMed] [Google Scholar]
- 35. Sampson RJ. Racial stratification and the durable tangle of neighborhood inequality. Ann Am Acad Political Soc Sci. 2009;621(1):260–280. [Google Scholar]
- 36. White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. [DOI] [PubMed] [Google Scholar]
- 37. Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. [DOI] [PubMed] [Google Scholar]
- 38. Azur MJ, Stuart EA, Frangakis C, et al. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16(2):91–104. [DOI] [PubMed] [Google Scholar]
- 40. Kivimäki M, Vahtera J, Tabák AG, et al. Neighbourhood socioeconomic disadvantage, risk factors, and diabetes from childhood to middle age in the Young Finns Study: a cohort study. Lancet Public Health. 2018;3(8):e365–e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 42. Clampet-Lundquist S, Kling JR, Edin K, et al. Moving teenagers out of high-risk neighborhoods: how girls fare better than boys. AJS. 2011;116(4):1154–1189. [DOI] [PubMed] [Google Scholar]
- 43. Winkleby M, Cubbin C, Ahn D. Effect of cross-level interaction between individual and neighborhood socioeconomic status on adult mortality rates. Am J Public Health. 2006;96(12):2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stafford M, Cummins S, Macintyre S, et al. Gender differences in the associations between health and neighbourhood environment. Soc Sci Med. 2005;60(8):1681–1692. [DOI] [PubMed] [Google Scholar]
- 45. Boylan JM, Robert SA. Neighborhood SES is particularly important to the cardiovascular health of low SES individuals. Soc Sci Med. 2017;188:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiao Q, Berrigan D, Powell-Wiley TM, et al. Ten-year change in neighborhood socioeconomic deprivation and rates of total, cardiovascular disease, and cancer mortality in older US adults. Am J Epidemiol. 2018;187(12):2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



