Abstract
Cefiderocol is a novel injectable siderophore cephalosporin that hijacks the bacterial iron transport machinery to facilitate cell entry and achieve high periplasmic concentrations. It has broad in vitro activity against gram-negative bacteria, including multidrug-resistant (MDR) organisms such as carbapenem-resistant Enterobacterales, carbapenem-resistant Pseudomonas aeruginosa, and Acinetobacter baumannii. It was approved by the US Food and Drug Administration for the treatment of complicated urinary tract infections and nosocomial pneumonia based on clinical trials that demonstrated noninferiority to comparators. In this review, we summarize the available in vitro and clinical data, including recent evidence from 2 phase 3 clinical trials (APEKS-NP and CREDIBLE-CR), and discuss the place of cefiderocol in the clinician’s armamentarium against MDR gram-negative infections.
Keywords: cefiderocol, multidrug resistant, gram, negative bacteria, carbapenem resistant Enterobacterales, extended spectrum beta, lactamase producing (ESBL)
Cefiderocol, a siderophore cephalosporin, demonstrates in vitro activity against multidrug-resistant gram-negative bacteria and is approved for complicated urinary tract infections and nosocomial pneumonia caused by susceptible organisms. We review data supporting its clinical use and discuss its role in therapy.
The World Health Organization has declared antimicrobial resistance a top global public health threat [1]. The Centers for Disease Control and Prevention estimates that at least 2.8 million infections due to multidrug-resistant organisms (MDROs) occur in the United States annually, resulting in more than 35 000 deaths [2]. Treatment choices are limited for MDRO infections, particularly for those caused by carbapenem-resistant Enterobacterales, extended-spectrum beta-lactamase–producing Enterobacterales, carbapenem-resistant Pseudomonas aeruginosa, and Acinetobacter baumannii. Carbapenem-sparing antibiotics are critically needed as resistance to this antibiotic class is increasingly common. Cefiderocol is a novel injectable siderophore cephalosporin with broad-spectrum in vitro activity that was specifically developed as a treatment option for challenging MDRO infections [3].
CHEMISTRY AND MECHANISM OF ACTION
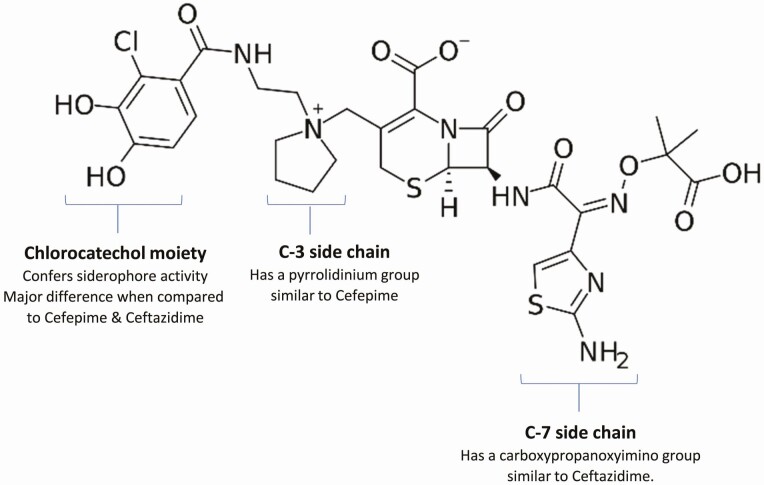
Cefiderocol contains a cephalosporin core with 2 side chains similar to those of ceftazidime and cefepime (Figure 1) [4–6]. The C-7 side chain has an aminothiazole ring and a carboxypropyl-oxyimino group (Figure 1), both of which improve transport across the outer membrane of gram-negative bacteria and confer stability against hydrolysis by several beta-lactamases. The C-3 side chain has a pyrrolidinium group that enhances its water solubility by conferring zwitterionic properties.
Figure 1.
Cefiderocol’s structural components responsible for antibacterial activity. *Image source: https://en.wikipedia.org/wiki/Cefiderocol#/media/File:Cefiderocol.svg. I, the copyright holder of this work, release this work into the public domain. This applies worldwide. In some countries this may not be legally possible; if so: I grant anyone the right to use this work for any purpose, without any conditions, unless such conditions are required by law.
The catechol moiety on the C-3 position distinguishes cefiderocol from cefepime and ceftazidime and functions as a siderophore, which chelates extracellular iron, forming a cefiderocol-ferric complex. As a consequence, while cefiderocol, like other beta-lactams, transits the outer cell membrane by passive diffusion through porins, it is also actively transported into organism by its iron uptake system [4, 5]. Once cefiderocol is transported into the periplasmic space, it dissociates from the iron and binds penicillin-binding proteins (PBP), primarily PBP3, to inhibit peptidoglycan synthesis, causing cell death.
The potent activity of cefiderocol against MDR gram-negative bacteria is due, at least in part, to its ability to overcome resistance caused by porin changes and achieve high concentrations in the periplasmic space via its active transport. Other factors include its high affinity for PBP3, its relative indifference to increased efflux pump expression [4, 7], and its resistance to hydrolysis by many beta-lactamases, including most serine carbapenemases and some metallo beta-lactamases, likely due to its C-3 side chain [4, 8]. Cefiderocol lacks significant activity against gram-positive organisms and anaerobes (the latter of which have lesser dependence on siderophore-iron transport systems for growth) [7, 9].
ANTIMICROBIAL ACTIVITY IN VITRO
The proposed cefiderocol minimum inhibitory concentration (MIC) breakpoints per the Clinical and Laboratory Standards Institute (CLSI), US Food and Drug Administration (FDA), and European Committee on Antimicrobial Susceptibility Testing (EUCAST) are listed in Table 1. CLSI published investigational MIC breakpoints for research purposes. The FDA released more conservative breakpoints when it first approved cefiderocol for complicated urinary tract infections (cUTIs) in November 2019; these were subsequently revised in September 2020 based on clinical data from the CREDIBLE-CR [10] and APEKS-NP [11] trials. A CLSI breakpoint update is anticipated in 2021; some advocate for use of FDA and EUCAST breakpoints for clinical care in the interim [9].
Table 1.
Cefiderocol Minimum Inhibitory Concentration Breakpoints Per the Clinical and Laboratory Standards Institute, US Food and Drug Administration, and European Committee on Antimicrobial Susceptibility Testing
| Organism | Clinical and Laboratory Standards Institute MIC Investigational Breakpoints (µg/mL)a | US Food and Drug Administration MIC Breakpoints (µg/mL)b | European Committee on Antimicrobial Susceptibility Testing MIC Breakpoints (µg/mL)c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | S | I | R | |
| Enterobacterales | <4 | 8 | >16 | <4 | 8 | >16 | <2 | -- | >2 |
| Pseudomonas aeruginosa | <4 | 8 | >16 | <1 | 2 | >4 | <2 | -- | >2 |
| Acinetobacter baumannii | <4 | 8 | >16 | <1 | 2 | >4 | NA | NA | NA |
| Stenotrophomonas maltophilia | <4 | 8 | >16 | NA | NA | NA | NA | NA | NA |
Investigational.
MICs are determined by broth microdilution using iron-depleted and cation-adjusted Mueller-Hinton broth.
Abbreviations: I, Intermediate; MIC, minimum inhibitory concentration; NA, Not applicable; R, Resistant; S, Sensitive.
aClinical and Laboratory Standards Institute (CLSI) investigational MIC breakpoints are intended for research purposes and should not be used for routine clinical care purposes. CLSI breakpoints published June 2018; update anticipated 2021.
bUS Food and Drug Administration breakpoints updated September 2020.
cEuropean Committee on Antimicrobial Susceptibility Testing breakpoints published April 2020.
Two large studies, SIDERO 2014 [12] and SIDERO 2015 [13], established that cefiderocol exhibits potent in vitro activity against a broad spectrum of gram-negative bacilli, including meropenem-nonsusceptible isolates of the Enterobacteriaceae family, P. aeruginosa, and A. baumannii. Cumulatively, more than 99% of the more than 18 000 gram-negative bacilli isolates tested showed a cefiderocol MIC <4 µg/mL. In addition, SIDERO 2015 [13] further demonstrated that cefiderocol retained potent in vitro activity against both Enterobacteriaceae and Pseudomonas isolates that were nonsusceptible to ceftazidime-avibactam and ceftolozone-tazobactam, all with MIC90 values <4 µg/mL.
Cefiderocol additionally exhibits in vitro activity against Stenotrophomonas maltophilia, despite its intrinsic beta-lactam resistance (L1 metallo-beta-lactamase and L2 serine-beta-lactamase) [12, 13]. A limited amount of data suggest that cefiderocol may be active against some Burkholderia cepacia isolates [12, 13].
Cefiderocol Resistance Stratified by Beta-Lactamases
Cefiderocol’s in vitro activity against MDR Enterobacterales, P. aeruginosa, and A. baumannii has been further characterized according to the Ambler classification of beta-lactamases. Within the Enterobacterales order, resistance did not differ by species [14]. Overall, cefiderocol retains potent activity against Enterobacterales, Pseudomonas, and Acinetobacter isolates that produce serine-beta-lactamases, cephalosporinases, and oxacillinases. However, although cefiderocol retains activity in the presence of most metallo-beta-lactamases, in vitro data are concerning for reduced potency against New Delhi Metallo-β-Lactamase (NDM)-producing isolates (Table 2).
Table 2.
In Vitro Cefiderocol Activity Against Gram-Negative Bacilli With Differing Resistance Mechanisms
| Reference | Resistance Mechanism | Isolate Number | Range | MIC50 | MIC90 |
|---|---|---|---|---|---|
| Enterobacterales | |||||
| Ambler class A – serine-beta-lactamases | |||||
| Kohira et al [16] | ESBL | 92 | <0.125–4 | 0.125 | 0.5 |
| Mushtaq et al [14]* | ESBL + porin loss | 26 | 0.125–32 | 2 | 8 |
| Kazmierczak et al [12] | KPC | 75 | 0.03–4 | 1 | 2 |
| Kohira et al [16] | KPC | 47 | <0.125–4 | 0.125 | 0.5 |
| Mushtaq et al [14] | KPC | 56 | <0.03–8 | 0.25 | 2 |
| Jacobs et al [15]* | KPC-2 | 355 | <0.03–32 | 1 | 8 |
| Delgado-Valverde et al [39] | ST512/KPC-3 | 25 | 0.25–4 | 2 | 4 |
| Delgado-Valverde et al [39] | ST258/KPC-3 | 25 | 0.06–4 | 2 | 2 |
| Jacobs et al [15] | KPC-3 | 380 | <0.03–64 | 0.25 | 2 |
| Ambler class B – metallo-beta-lactamases | |||||
| Kazmierczak et al [12] | VIM | 27 | 0.12–4 | 1 | 4 |
| Kohira et al [16] | VIM | 12 | <0.125–16 | 0.125 | 0.25 |
| Mushtaq et al [14] | VIM | 47 | <0.03–8 | 0.5 | 4 |
| Kanazawa et al [40] | IMP-6 | 82 | <0.03–2 | 0.06 | 1 |
| Kohira et al [16] | IMP | 8 | <0.125–16 | – | – |
| Mushtaq et al [14] | IMP | 15 | <0.03–4 | 0.25 | 2 |
| Kazmierczak et al [12]* | NDM | 12 | 1–8 | 4 | 8 |
| Kohira et al [16]* | NDM | 49 | <0.125–>16 | 1 | 16 |
| Jacobs et al [15]* | NDM | 28 | 0.25–>64 | 2 | 8 |
| Mushtaq et al [14]* | NDM | 61 | 0.25–32 | 4 | 8 |
| Ambler class C – cephalosporinases | |||||
| Mushtaq et al [14] | AmpC + porin loss | 25 | 0.06–2 | 0.5 | 2 |
| Ambler class D – oxacillinases | |||||
| Delgado-Valverde et al [39] | OXA-48 | 3 | 0.06–0.5 | – | – |
| Kazmierczak et al [12] | OXA-48-like | 32 | 0.03–4 | 0.5 | 4 |
| Mushtaq et al [14] | OXA-48-like | 56 | <0.03–8 | 0.25 | 2 |
| Jacobs et al [15] | OXA-48-like | 7 | <0.03–1 | 0.25 | 1 |
| Combination resistance mechanisms | |||||
| Delgado-Valverde et al [39] | ST11/OXA-48 + CTX-M-15 | 25 | <0.03–4 | 0.25 | 2 |
| Delgado-Valverde et al [39] | ST15/OXA-48 + CTX-M-15 | 25 | <0.03–4 | 0.25 | 4 |
| Delgado-Valverde et al [39] | ST392/OXA-48 + CTX-M-15 | 4 | 0.06–1 | – | – |
| Kazmierczak et al [12] | Carbapenemase negative, meropenem resistant | 13 | 0.008–4 | 0.12 | 2 |
| Pseudomonas aeruginosa | |||||
| Ambler class A – serine-beta-lactamases | |||||
| Kazmierczak et al [12] | GES | 4 | 0.12–0.25 | – | – |
| Mushtaq et al [14] | GES | 20 | 0.06–4 | 0.25 | 2 |
| Mushtaq et al [14]* | PER | 15 | 0.06–16 | 1 | 16 |
| Mushtaq et al [14] | VEB | 10 | 0.5–8 | – | – |
| Ambler class B – metallo-beta-lactamases | |||||
| Kazmierczak et al [12] | VIM | 26 | 0.008–2 | 0.25 | 2 |
| Mushtaq et al [14] | VIM | 30 | <0.03–>128 | 0.25 | 1 |
| Kazmierczak et al [12] | IMP | 4 | 1–2 | – | – |
| Mushtaq et al [14]* | IMP | 25 | 0.06–16 | 0.25 | 8 |
| Mushtaq et al [14]* | NDM | 11 | 1–>128 | 4 | 16 |
| Kazmierczak et al [12] | Carbapenemase-negative, meropenem non-susceptible | 319 | <0.002–4 | 0.12 | 0.5 |
| Jacobs et al [15] | Carbapenem-resistant NOS | 27 | <0.03–1 | 0.25 | 0.5 |
| Acinetobacter baumannii | |||||
| Ambler class A – serine-beta-lactamases | |||||
| Kazmierczak et al [12] | GES-type | 7 | 0.25–8 | – | – |
| Ambler class B – metallo-beta-lactamases | |||||
| Kazmierczak et al [12] | NDM | 2 | 1–1 | – | – |
| Mushtaq et al [14]* | NDM | 20 | 1–>128 | 2 | 16 |
| Ambler class D – oxacillinases | |||||
| Delgado-Valverde et al [39] | OXA-23 | 25 | 0.06–1 | 0.25 | 0.5 |
| Kazmierczak et al [12] | OXA-23 | 543 | <0.002–16 | 0.12 | 1 |
| Mushtaq et al [14]* | OXA-23 | 41 | 0.06–>128 | 0.25 | 16 |
| Delgado-Valverde et al [39]* | OXA-24 | 25 | 0.5–16 | 2 | 16 |
| Kazmierczak et al [12] | OXA-24 | 124 | 0.004–64 | 0.12 | 1 |
| Mushtaq et al [14] | OXA-51 | 19 | 0.06–16 | 0.125 | 0.5 |
| Kazmierczak et al [12] | OXA-58 | 14 | 0.06–1 | 0.06 | 1 |
| Delgado-Valverde et al [39] | ST2/OXA-58 | 25 | 0.06–0.5 | 0.125 | 0.5 |
| Delgado-Valverde et al [39] | ST745/OXA-58 | 5 | 0.06–0.25 | – | – |
| Mushtaq et al [14] | OXA-58 | 10 | 0.06–>128 | – | – |
| Mushtaq et al [14] | OXA-24/40 | 9 | 0.25–4 | ||
| Kazmierczak et al [12]* | Carbapenemase-negative, meropenem non-susceptible | 86 | 0.008–8 | 0.25 | 2 |
| Jacobs et al [15] | Carbapenem-resistant NOS | 101 | <0.03–>63 | 0.25 | 1 |
MICs are determined by broth microdilution using iron-depleted and cation-adjusted Mueller-Hinton broth.
MIC50, MIC at which cefiderocol inhibits growth of 50% of tested isolates.
MIC90, MIC at which cefiderocol inhibits growth of 90% of tested isolates.
MIC50 and MIC90 not determined if <10 isolates.
*MIC 90 exceeding either FDA or EUCAST breakpoint, whichever is higher.
Abbreviations: CTX-M, group of class A extended-spectrum β-lactamases; ESBL, extended-spectrum beta-lactamase; GES, Guiana extended spectrum β-lactamase; IMP, imipenem-hydrolyzing β-lactamase;
KPC, Klebsiella pneumoniae carbapenemase; MIC, minimum inhibitory concentration; NDM, New Delhi metallo β-lactamase; NOS, not otherwise specified; OXA, oxacillin-hydrolyzing β-lactamases; PER: extended-spectrum β-lactamase first discovered in strains of P. aeruginosa; ST, sequence type; VEB, Vietnamese extended-spectrum β-lactamase; VIM, Verona integron-encoded metallo-β-lactamase.
Data describing cefiderocol’s activity in the presence of NDM enzymes are primarily limited to isolates from the Enterobacterales order (Table 2). In these studies, cefiderocol MICs were variable, and a substantial portion of these NDM-producing isolates were cefiderocol-nonsusceptible [12, 14–16] (Table 2). Similarly, more limited data of NDM-producing Pseudomonas and Acinetobacter isolates demonstrate variable in vitro cefiderocol activity with wide MIC ranges (Table 2). These data suggest that susceptibility testing will be important before use of cefiderocol as a single agent to treat infections due to NDM-producing organisms.
In vitro testing data pooled from 6 studies suggest cefiderocol does retain activity against most Enterobacterales, Pseudomonas, and Acinetobacter isolates producing non-NDM beta-lactamase enzymes (Table 2). However, there are reports of reduced cefiderocol potency without a clear pattern across Ambler class or beta-lactamase enzyme type. For instance, among Enterobacterales isolates producing serine-beta-lactamases, there is a wide distribution of MIC ranges. Furthermore, a large study showed variable cefiderocol potency against Enterobacterales within and between differing Klebsiella pneumoniae carbapenemase (KPC) enzyme subtypes (Table 2) [15]. These inconsistent resistance patterns within and among beta-lactamases suggest cefiderocol resistance is not exclusively determined by the presence of beta-lactamase enzymes.
Despite limited data, studies have started to shed light on mechanisms of cefiderocol resistance. Although it seems cefiderocol resistance is not caused by the presence of beta-lactamases alone (potential exception being NDM-producing organisms), some studies suggest that cefiderocol activity may be potentiated by the addition of beta-lactamase inhibitors [14, 17]. These data suggest cefiderocol resistance is likely mediated by a combination of resistance mechanisms, and the addition of beta-lactamase inhibitors may be sufficient to restore the drug’s activity against some of these mechanisms. Other proposed mechanisms of cefiderocol resistance include mutations in iron transport channels, which inhibit cefiderocol’s novel mechanism of entry into bacteria [7]. Unlike other gram-negative bacilli, cefiderocol resistance does not seem to be mediated by porin and efflux mutations [7]. To further elucidate nuances of cefiderocol resistance, future studies should pay particular attention to NDM enzymes and combinations of resistance mechanisms, including beta-lactamases and mutations in iron transport.
Considerations for Clinical Laboratory In Vitro Testing
Both broth microdilution (using iron-depleted media) and agar disc diffusion methods have been approved and validated for evaluation of cefiderocol’s in vitro activity. Broth microdilution allows for determination of MICs, whereas agar disc diffusion does not allow precise determination of the amount of antimicrobial agent diffused into agar. However, agar disc diffusion is likely the most practical method for clinical laboratories to adopt until cefiderocol testing can be included on commercially available automated antimicrobial susceptibility testing panels [9].
PHARMACOKINETICS AND PHARMACODYNAMICS
Animal Studies
Similar to other cephalosporins, the activity of cefiderocol is best described by time-dependent killing [18], which is enhanced when cefiderocol is administered as an extended 3-hour infusion compared with a 1-hour infusion [19]. Humanized exposures of cefiderocol in murine thighs (dosed at an equivalent of 2 g intravenous every 8 hours, 3-hour infusion) induced bacterial stasis or >1 log10 reduction in bacterial colony-forming units in the majority of gram-negative bacilli with a MIC <4 µg/mL; however, the probability of target attainment was significantly reduced against isolates with MIC >8 µg/mL [20, 21]. These in vivo studies demonstrate the effectiveness of cefiderocol and support consideration of an MIC of 4 µg/mL as the susceptibility breakpoint for Enterobacteriaceae, Pseudomonas, Acinetobacter, and Stenotrophomonas. Additionally, in vivo studies show repeat doses of cefiderocol were not associated with the development of resistance [22], but results of these animal studies need correlation with clinical trial data.
Human Studies
Cefiderocol displays linear kinetics. It is primarily renally excreted and does not undergo significant hepatic metabolism [23, 24]. Dose adjustments are required for renal impairment but not for hepatic impairment [25].
Early human pharmacokinetic studies support the use of cefiderocol for treatment of pulmonary, urinary tract, and possibly bloodstream infections. Phase 1 studies show cefiderocol achieves intrapulmonary concentrations similar to plasma concentrations [26]. Phase 2 studies in cUTI and uncomplicated pyelonephritis show modestly increased drug clearance with infection, but plasma levels of cefiderocol remained adequate to treat cUTIs [27]. The largest study used plasma cefiderocol concentrations collected from 516 patients, including healthy volunteers and those enrolled in clinical trials [10, 11, 28], to develop a population pharmacokinetics model. Renal function was the most significant variable in the model. Analysis using the model showed a >95% probability of target attainment (75% time above MIC) for gram-negative isolates with MICs <4 regardless of infection site. The model predicted a >90% probability of target attainment (100% time above MIC) for isolates with MICs <4 for pneumonia and cUTI but dropped to 85% for bloodstream infection (BSI) [29]. No data are yet available on cerebrospinal fluid penetration.
DOSAGE AND ADMINISTRATION
Recommended cefiderocol dosing for patients with normal renal function is 2 g administered intravenously over 3 hours every 8 hours. Dosing recommendations based on renal function are described in Table 3. Intermittent hemodialysis can remove up to 60% of cefiderocol, so there may be a role for administering a supplemental dose of cefiderocol following hemodialysis [25, 30, 31]. Presently, there are no recommendations for weight-based dosing in obesity and no data regarding cerebral spinal fluid penetration. With these sophisticated dosing regimens and minimal pharmacokinetic data in disease states, therapeutic drug monitoring may be helpful.
Table 3.
Cefiderocol Dose Regimens Based on Renal Function
| Renal Function | Renal Function Estimates | Dose Regimen |
|---|---|---|
| Augmenteda | CG-CLCR, >120 mL/min | 2 g every 6 hours, 3-hour infusion |
| Normal | MDRD-eGFR, 90 to <120 mL/min/1.73 m2 | 2 g every 8 hours, 3-hour infusion |
| Mild impairment | MDRD-eGFR, 60 to <90 mL/min/1.73 m2 | 2 g every 8 hours, 3-hour infusion |
| Moderate impairment | MDRD-eGFR, 30 to <60 mL/min/1.73 m2 | 1.5 g every 8 hours, 3-hour infusion |
| Severe impairment | MDRD-eGFR, 15 to <30 mL/min/1.73 m2 | 1 g every 8 hours, 3-hour infusion |
| End-stage renal disease | MDRD-eGFR, <15 mL/min/1.73 m2 | 0.75 g every 12 hours, 3-hour infusion |
| Requiring intermittent hemodialysis | 0.75 g every 12 hours, 3-hour infusion, with consideration for supplemental (third) dose of 0.75 g administered after hemodialysis on dialysis days [25] | |
| Requiring continuous renal replacement therapy | Dosed based on effluent flow rate | |
| ≤2 L/hour | 1.5 g every 12 hours | |
| 2.1 to 3 L/hour | 2 g every 12 hours | |
| 3.1 to 4 L/hour | 1.5 g every 8 hours | |
| ≥4.1 L/hour | 2 g every 8 hours |
Abbreviations: CG-CLCR, Cockcroft-Gault creatinine clearance; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
aPatients with hypermetabolic states due to sepsis [31].
CLINICAL EXPERIENCE
Complicated Urinary Tract Infection
The APEKS-cUTI study [28] was a phase 2, multicenter, double-blind, parallel-group noninferiority trial that evaluated cefiderocol vs imipenem-cilastatin for the treatment of cUTI. Patients were randomized to receive 1-hour intravenous infusions of cefiderocol (2 g) or imipenem-cilastatin (1 g) every 8 hours for 7–14 days. The primary efficacy end point was the composite of clinical and microbiological response 7 days (±2 days) after the end of antibiotic treatment.
A total of 452 patients were randomized to receive cefiderocol (n = 303) and imipenem-cilastatin (n = 149). Of these, 252 in the cefiderocol group and 119 in the imipenem-cilastatin group were included in the modified intention-to-treat (mITT) population. The most common baseline uropathogens were Escherichia coli and Klebsiella pneumoniae, and close to half were pan-susceptible (cefiderocol n = 117 of 252, 46%; imipenem-cilastatin n = 60 of 119, 50%.) Cefiderocol was deemed noninferior to impenem-cilastatin as the primary efficacy end point was achieved by 73% (n = 183) in the cefiderocol group and 55% (n = 65) in the imipenem-cilastatin group with an adjusted treatment difference of 18.58% (95% confidence interval [CI], 8.23% to 28.92%). The clinical response rates were similar between the treatment groups (the preset lower bound to conclude noninferiority was 15%). However, microbiological response at test of cure was higher in the cefiderocol arm (73% vs 56%; difference 17.25%; 6.92% to 27.58%). Sensitivity analyses of composite and microbiological outcomes were consistent with those of the mITT cohort.
Nosocomial Pneumonia
The APEKS-NP study [11] was a phase 3, randomized, double-blind, multicenter, noninferiority trial that compared cefiderocol vs high-dose, extended infusion meropenem for adults with hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), or healthcare-associated pneumonia (HCAP) due to gram-negative pathogens. Patients were randomized 1:1 to receive 3-hour intravenous infusions of either cefiderocol 2 g or meropenem 2 g every 8 hours for 7–14 days. All patients also received open-label intravenous linezolid (600 mg every 12 hours) for at least 5 days. The primary end point was all-cause mortality at day 14 in the mITT population.
A total of 300 patients were randomized to receive cefiderocol (n = 148) or meropenem (n = 152). Of these, 145 in the cefiderocol group and 147 in the meropenem group were included in the mITT population (all patients who received at least 1 study drug dose, excluding those with gram-positive monomicrobial infections). Cefiderocol was noninferior to high-dose extended infusion meropenem as the primary outcome (all-cause mortality at day 14) was similar in the 2 groups (12.4% cefiderocol vs 11.6% meropenem; adjusted difference, 0.8%; 95% CI, –6.6% to 8.2%) based on a 12.5% noninferiority margin. The proportion of patients with clinical cure and microbiological eradication at test of cure was similar in both groups. The most common pathogen was K. pneumoniae followed by P. aeruginosa and A. baumannii. Predefined subgroup analyses showed more deaths in the cefiderocol arm in patients with HCAP (9 vs 2 deaths), but mortality was similar in both arms in all other subgroups. Sensitivity analyses for the all-cause mortality at days 14 and 28 in the mITT population were similar to the analyses of the microbiologically evaluable per-protocol population (all patients in the mITT population who followed the study protocol and had a positive culture for gram-negative bacilli).
Severe Carbapenem-Resistant Infections
The CREDIBLE-CR study [10] differs from the previous 2 studies in its design and study population. This phase 3, randomized, open-label, multicenter, descriptive study assessed cefiderocol vs clinician-directed best available therapy (BAT) in adults with serious carbapenem-resistant (CR) gram-negative infections. Investigators included patients hospitalized with nosocomial pneumonia (HAP, VAP, HCAP), BSI, cUTI, or sepsis not otherwise specified (NOS) caused by a suspected or proven carbapenem-resistant gram-negative pathogen. Participants were assigned 2:1 to receive either a 3-hour intravenous infusion of cefiderocol 2 g every 8 hours or BAT for an expected 7–14 days (could be extended to 21 days per clinician discretion). For patients with nosocomial pneumonia, BSI, or sepsis NOS, the primary end point was clinical cure after treatment completion (7 days ± 2 days after end of therapy). The primary end point for patients with cUTI was microbiological eradication at test of cure.
A total of 152 patients were randomized to receive cefiderocol (n = 101) or BAT (n = 51). The most common diagnosis upon enrollment was nosocomial pneumonia (n = 67, 45%) followed by BSI/sepsis NOS (n = 47, 31%) and cUTI (n = 36, 24%). The primary analysis included 118 patients (cefiderocol n = 80, BAT n = 38) who had a confirmed carbapenem-resistant infection. In the cefiderocol group, 83% (66 of 80) of patients received monotherapy, while in the BAT arm, 71% (27 of 38) received combination therapy (the majority of which were colistin-based regimens). The most common carbapenem-resistant pathogens were A. baumannii, K. pneumoniae, and P. aeruginosa (cefiderocol MIC90 of 1 µg/mL, 4 µg/mL, and 2 µg/mL, respectively).
Clinical cure at test of cure was similar in each group for those with nosocomial pneumonia (cefiderocol 50% [20 of 40], 95% CI, 33.8% to 66.2%; BAT 53% [10 of 19], 95% CI, 28.9% to 75.6%) and BSI or sepsis NOS (cefiderocol 43% [10 of 23], 95% CI, 23.2% to 65.5%; BAT 43% [6 of 14], 95% CI, 17.7% to 71.1%). For patients with cUTI, microbiological eradication at test of cure was 53% (9 of 17, 95% CI, 27.8% to 77.0%) in the cefiderocol group and 20% (1 of 5, 95% CI, 0.5% to 71.6%) in the BAT group.
More patients died in the cefiderocol arm compared with those treated with BAT (33.7% [34 of 101] vs 18.3% [9 of 49]). Post hoc all-cause mortality in the cefiderocol arm was higher at day 28 (difference 6.4%; 95% CI, –8.6% to 19.2%) and at day 49 (difference 13.3%; 95% CI, –2.5% to 27.8%). Exploratory analyses also found that the mortality rate differed by underlying infection and infecting organism. A study adjudication committee attributed more deaths in the cefiderocol arm to treatment failure compared with the BAT arm (15.8% [16 of 101] vs 8.2% [4 of 49]), most of which occurred within 15 days of study initiation [32]; the remainder of the deaths were attributed to underlying comorbidities. Most of the treatment failure deaths in the cefiderocol arm occurred in patients with Acinetobacter infections (13 of 16) compared with only 1 death (1 of 4) in the BAT arm. Fifteen patients who received cefiderocol had evidence suggesting treatment emergent in vitro resistance, with a 4-fold increase in cefiderocol MIC from baseline; 10 of these patients experienced treatment failure.
The FDA published its analysis of the CREDIBLE data [10] and described aspects of the study design that limit its interpretation, including the open-label design, small sample size, imbalance between study groups, and limited descriptive analytic plan [32]. Notably, FDA approval of cefiderocol is based solely on APEKS-cUTI [28] and APEKS-NP [11] data, which did not show increased mortality in patients who received cefiderocol except for the HCAP subgroup in the APEKS-NP trial. Regardless, the FDA recommends that clinicians closely monitor patients who receive cefiderocol for evidence of treatment failure. Of note, the European Medical Agency, the European Union’s pharmaceutical governing body, has issued a pathogen-focused approval based, in part, on CREDIBLE trial data, authorizing cefiderocol use for aerobic gram-negative infections in patients with limited treatment options [3, 33].
Cases Reports and Case Series
Limited case reports demonstrate variable success of off-label use of cefiderocol. Although CREDIBLE data are concerning for treatment failure with the use of cefiderocol for Carbapenem-resistant (CR) Acinetobacter, case reports illustrate mixed success in treating extensively resistant Acinetobacter infections including orthopedic infections [34–37].
Safety and Adverse Events
Phase 1 studies in healthy humans demonstrated cefiderocol was safe and well tolerated [23]. In phase 2 and 3 clinical trials, adverse events were reported at similar rates in the cefiderocol arm and in the comparator arm, and the majority were mild or moderate in severity (Table 4). The most common adverse events reported across these trials were nausea, diarrhea, rash, elevated liver function tests, and hypokalemia. There were no reported laboratory abnormalities in these studies to suggest cefiderocol impacted patients’ iron hemostasis.
Table 4.
Adverse Events Reported in Clinical Trials
| APEKS-NP, % | APEKS-cUTI, % | CREDIBLE-CR, % | ||||
|---|---|---|---|---|---|---|
| Adverse Event | Cefiderocol (n = 148) | Meropenem (n = 150) | Cefiderocol (n = 300) | Imipenem-cilastam (n = 148) | Cefiderocol (n = 101) | Best Available Therapy (n = 49) |
| All TEAEs | 88 | 86 | 41 | 51 | 91 | 96 |
| Mild | 22 | 25 | NR | NR | 23 | 18 |
| Moderate | 28 | 31 | NR | NR | 26 | 33 |
| Severe | 38 | 30 | NR | NR | 43 | 45 |
| TEAEs | Reported >5% in either group | Reported >2% in either group | Reported >5% in either group | |||
| Diarrhea | 9 | 9 | 4 | 6 | 19 | 12 |
| Constipation | 5 | 4 | 3 | 4 | 8 | 6 |
| Nausea | NR | NR | 2 | 4 | 7 | 4 |
| Vomiting | NR | NR | 2 | 1 | 13 | 14 |
| Headache | NR | NR | 2 | 5 | NR | NR |
| Infusion site pain | NR | NR | 3 | 3 | NR | NR |
| Rash | 1 | 0 | NR | NR | 3 | 8 |
| Hypokalemia | 11 | 15 | 2 | 3 | 9 | 14 |
| Elevated LFTs | 2.7 | 6.7 | 8 | 2 | ||
| TEAE leading to drug discontinuation | 8 | 9 | 2 | 2 | 3 | 4 |
| Drug-related TEAEs | 9 | 11 | 9 | 11 | 15 | 22 |
| Serious adverse events | 36 | 30 | 5 | 8 | 50 | 47 |
| Clostridium difficile–associated disease | 3 | 3 | <1 | 3 | 1 | 0 |
Numbers of patients included in the safety analysis of each study.
Abbreviations: LFT, Liver function test; NR, not reported; TEAE, treatment emergent adverse events.
The FDA approval includes a warning of an increased risk of all-cause mortality for treatment of carbapenem-resistant gram-negative bacterial infections based on data from the CREDIBLE trial (see above) [10]. It also includes warnings applicable to other beta-lactams including the risk of Clostridioides difficile–associated diarrhea, cephalosporin-associated risk of potentiating seizure activity (similar to cefepime), and hypersensitivity reactions. Cefiderocol’s R1 side chain is exactly the same as that of ceftazidime and aztreonam and is similar to that of ceftaroline. These similarities confer potential cross-reactivity of allergic reactions, and clinicians should be especially cognizant of these similarities when prescribing alternate beta-lactams in patients with allergies [38].
Place in Therapy and Formulary Considerations
Cefiderocol’s role in treating MDR gram-negative infections is still uncertain. Although its in vitro spectrum of activity is promising, cefiderocol’s clinical trial performance thus far has only demonstrated results similar to comparators (namely, carbapenems) at a greater cost (Table 5). Although unexplained, the increased mortality observed in the CREDIBLE trial casts doubt on cefiderocol’s role in treating carbapenem-resistant A. baumanii and perhaps other nonfermenting gram-negative bacilli infections unless no other viable option exists. The lack of official CLSI breakpoints and challenges in susceptibility testing also hinder its clinical use. Until more data become available, cefiderocol will most likely be reserved for second-line or salvage therapy in selected MDR gram-negative infections.
Table 5.
Comparison of Agents for Treatment of Drug-Resistance Gram-Negative Infections
| In Vitro Activity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Enterobacterales | ||||||||
| Agent | Ambler Class A | Ambler Class B | Ambler Class D | Pseudomonas aeruginosa | Acinetobacter baumannii | Stenotrophomonas maltophilia | US Food and Drug Administration–Approved Indications | Daily Cost for Normal Renal Function |
| Cefiderocol | Yes | Yes | Partial | Yes | Yes | Yes | cUTI/AP, HABP/VABP | $1320.00 |
| Ceftazidime-avibactam | Yes | No | Yes | Yes | No | No | cUTI/AP, cIAI, HABP/VABP | $1291.71 |
| Ceftolozane-tazobactam | No | No | No | Yes | No | No | cUTI/AP, cIAI | $450.78 |
| Meropenem-vaborbactam | Yes | No | No | Yes | No | No | cUTI/AP | $1283.04 |
| Imipenem-cilastatin-relebactam | Yes | No | No | Yes | No | No | cUTI/AP, cIAI | $1322.56 |
Dosing: Cefiderocol: 2 g intravenous (IV) every 8 hours. Ceftazidime-avibactam: 2.5 g (ceftazidime 2 g/avibactam 0.5 g) IV every 8 hours. Ceftolozane-tazobactam: 1.5 g (ceftolozane 1 g/tazobactam 0.5 g) IV every 8 hours. Meropenem-vaborbactam: 4 g (meropenem 2 g/vaborbactam 2 g) IV every 8 hours. Imipenem-cilastatin-relebactam: 1.25 g (imipenem 500 mg/cilastatin 500 mg/relebactam 250 mg) IV every 6 hours.
Abbreviations: AP, acute pyelonephritis; cIAI, complicated intraabdominal infection; cUTI, complicated urinary tract infection; HABP, hospital-acquired bacterial pneumonia; VABP, ventilator-associated bacterial pneumonia.
Conclusions
Cefiderocol is a novel injectable siderophore cephalosporin with broad in vitro activity against gram-negative bacteria, including carbapenem-resistant pathogens. It is FDA approved for the treatment of cUTIs and nosocomial pneumonia, requires renally adjusted dosing, and appears to be fairly well tolerated. Early data suggest cefiderocol is a promising alternative agent for some carbapenem-resistant infections, but its role in the management of carbapenem-resistant Pseudomonas and Acinetobacter infections is uncertain. Pathogen-specific trials would help to refine cefiderocol’s place in therapy.
Notes
Acknowledgments. The authors thank Dr Stan Deresinski for his careful review of this manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Sharon Ong’uti, Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, California, USA.
Mary Czech, Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, California, USA.
Elizabeth Robilotti, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Marisa Holubar, Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, California, USA.
References
- 1. World Health Organization. Ten threats to global health in 2019. 2019. Available at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed 22 September 2021.
- 2. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States 2019. 2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 22 September 2021.
- 3. Echols R, Ariyasu M, Nagata TD. Pathogen-focused clinical development to address unmet medical need: cefiderocol targeting carbapenem resistance. Clin Infect Dis 2019; 69:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis 2019; 69:538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhanel GG, Golden AR, Zelenitsky S, et al. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant gram-negative bacilli. Drugs 2019; 79:271–89. [DOI] [PubMed] [Google Scholar]
- 6. Wu JY, Srinivas P, Pogue JM. Cefiderocol: a novel agent for the management of multidrug-resistant gram-negative organisms. Infect Dis Ther 2020; 9:17–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito A, Sato T, Ota M, et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacteria. Antimicrob Agents Chemother 2018; 62:: e01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giacobbe DR, Ciacco E, Girmenia C, et al. ; Italian Study Group on Resistant Infections of the Italian Society of Anti-infective Therapy . Evaluating cefiderocol in the treatment of multidrug-resistant gram-negative bacilli: a review of the emerging data. Infect Drug Resist 2020; 13:4697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simner PJ, Patel R. Cefiderocol antimicrobial susceptibility testing considerations: the Achilles’ heel of the trojan horse? J Clin Microbiol 2020; 59:e00951-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2020; 21:226–40. [DOI] [PubMed] [Google Scholar]
- 11. Wunderink RG, Matsunaga Y, Ariyasu M, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2021; 21:213–25. [DOI] [PubMed] [Google Scholar]
- 12. Kazmierczak KM, Tsuji M, Wise MG, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents 2019; 53:177–84. [DOI] [PubMed] [Google Scholar]
- 13. Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. In vitro activity of cefiderocol, a siderophore cephalosporin, against gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015-2016: SIDERO-WT-2015. Int J Antimicrob Agents 2019; 53:456–66. [DOI] [PubMed] [Google Scholar]
- 14. Mushtaq S, Sadouki M, Vickers A, Livermore DM, Woodford N. In vitro activity of cefiderocol, a siderophore cephalosporin, against multidrug-resistant gram-negative bacteria. Antimicrob Agents Chemother 2020; 64:e01582-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobs MR, Abdelhamed AM, Good CE, et al. ARGONAUT-I: activity of cefiderocol (S-649266), a siderophore cephalosporin, against gram-negative bacteria, including carbapenem-resistant nonfermenters and Enterobacteriaceae with defined extended-spectrum beta-lactamases and carbapenemases. Antimicrob Agents Chemother 2019; 63:e01801-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kohira N, West J, Ito A, et al. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 2016; 60:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kohira N, Hackel MA, Ishioka Y, et al. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J Glob Antimicrob Resist 2020; 22:738–41. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura R, Ito-Horiyama T, Takemura M, et al. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother 2019; 63:e02031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsumoto S, Singley CM, Hoover J, et al. Efficacy of cefiderocol against carbapenem-resistant gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother 2017; 61:e00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 2017; 61:e01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen IH, Kidd JM, Abdelraouf K, Nicolau DP. Comparative in vivo antibacterial activity of human-simulated exposures of cefiderocol and ceftazidime against Stenotrophomonas maltophilia in the murine thigh model. Antimicrob Agents Chemother 2019; 63:e01558-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stainton SM, Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of humanized cefiderocol exposures over 72 hours against a diverse group of gram-negative isolates in the neutropenic murine thigh infection model. Antimicrob Agents Chemother 2019; 63:e01040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother 2018; 62:e02163-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyazaki S, Katsube T, Shen H, Tomek C, Narukawa Y. Metabolism, excretion, and pharmacokinetics of [14 C]-cefiderocol (S-649266), a siderophore cephalosporin, in healthy subjects following intravenous administration. J Clin Pharmacol 2019; 59:958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katsube T, Wajima T, Ishibashi T, Ferreira JCA, Echols R. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother 2017; 61:e01381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katsube T, Saisho Y, Shimada J, Furuie H. Intrapulmonary pharmacokinetics of cefiderocol, a novel siderophore cephalosporin, in healthy adult subjects. J Antimicrob Chemother 2019; 74:1971–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawaguchi N, Katsube T, Echols R, Wajima T. Population pharmacokinetic analysis of cefiderocol, a parenteral siderophore cephalosporin, in healthy subjects, subjects with various degrees of renal function, and patients with complicated urinary tract infection or acute uncomplicated pyelonephritis. Antimicrob Agents Chemother 2018; 62:e01391-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Portsmouth S, van Veenhuyzen D, Echols R, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2018; 18:1319–28. [DOI] [PubMed] [Google Scholar]
- 29. Kawaguchi N, Katsube T, Echols R, Wajima T. Population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses of cefiderocol, a parenteral siderophore cephalosporin, in patients with pneumonia, bloodstream infection/sepsis, or complicated urinary tract infection. Antimicrob Agents Chemother 2020; 65:e01437-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C. Cefiderocol, a siderophore cephalosporin for gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol 2017; 57:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fetroja (Cefiderocol). Osaka, Japan: Shionogi & Co., Ltd.. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209445s000lbl.pdf. [Google Scholar]
- 32. Naseer S, Weinstein EA, Rubin DB, et al. US Food and Drug Administration (FDA): benefit-risk considerations for cefiderocol (Fetroja(R)). Clin Infect Dis 2020; 72:e1103-11. [DOI] [PubMed] [Google Scholar]
- 33. Fetcroja. Summary of product characteristics [product information, labeling, package leaflet]. Shionogi & Co., Ltd. Available at: https://www.ema.europa.eu/en/documents/product-information/fetcroja-epar-product-information_en.pdf. Accessed 22 September 2021.
- 34. Falcone M, Tiseo G, Nicastro M, et al. Cefiderocol as rescue therapy for Acinetobacter baumannii and other carbapenem-resistant gram-negative infections in ICU patients. Clin Infect Dis 2020; 72:2021–4. [DOI] [PubMed] [Google Scholar]
- 35. Oliva A, Ceccarelli G, De Angelis M, et al. Cefiderocol for compassionate use in the treatment of complicated infections caused by extensively and pan-resistant Acinetobacter baumannii. J Glob Antimicrob Resist 2020; 23:292–6. [DOI] [PubMed] [Google Scholar]
- 36. Dagher M, Ruffin F, Marshall S, et al. Case report: successful rescue therapy of extensively drug-resistant Acinetobacter baumannii osteomyelitis with cefiderocol. Open Forum Infect Dis 2020: 7:ofaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zingg S, Nicoletti GJ, Kuster S, et al. Cefiderocol for extensively drug-resistant gram-negative bacterial infections: real-world experience from a case series and review of the literature. Open Forum Infect Dis 2020; 7:ofaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaudhry SB, Veve MP, Wagner JL. Cephalosporins: a focus on side chains and beta-lactam cross-reactivity. Pharmacy 2019; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Delgado-Valverde M, Conejo MDC, Serrano L, Fernández-Cuenca F, Pascual Á. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother 2020; 75:1840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kanazawa S, Sato T, Kohira N, Ito-Horiyama T, Tsuji M, Yamano Y. Susceptibility of imipenem-susceptible but meropenem-resistant blaimp-6-carrying Enterobacteriaceae to various antibacterials, including the siderophore cephalosporin cefiderocol. Antimicrob Agents Chemother 2017; 61:e00576-17. [DOI] [PMC free article] [PubMed] [Google Scholar]