Abstract
A few studies concerning hypercoagulable states have sufficiently been reported in patients with acute cerebral infarction (ACI), as ACI is generally considered to be caused by platelet activation. Clot waveform analyses (CWA) for activated partial thromboplastin time (APTT) and small amount of tissue factor FIX activation assay (sTF/FIXa) were examined in 108 patients with ACI, 61 patients without ACI, and 20 healthy volunteers. CWA-APTT and CWA-sTF/FIXa showed that the peak heights were significantly higher in ACI patients without anticoagulant therapy than in healthy volunteers. Absorbance exceeding 78.1 mm on the 1st DPH in the CWA-sTF/FIXa showed the highest odds ratio for ACI. The peak heights were significantly lower in the CWA-sTF/FIXa of ACI patients receiving argatroban therapy than in those of ACI patients without anticoagulant therapy. CWA can suggest a hypercoagulable state in ACI patients and may be useful for monitoring the need for anticoagulant therapy.
Keywords: CWA, APTT, sTF/FIXa, acute cerebral infarction, hypercoagulable state
Introduction
Approximately 70% (9.5 million people) of strokes are ischemic strokes (acute cerebral infarction, [ACI]).1,2 ACI is classified into the following entities: cardioembolic ACI,3 atherosclerotic ACI,4 or lacunar ACI.5 As ACI patients still have high mortality, they require prompt treatment with antiplatelet agents (APA),1,6 direct anti-thrombin agents,8 direct oral anticoagulants (DOACs),9 unfractionated heparin (UFH), or warfarin. In Japan, most patients with atherosclerotic ACI are generally treated with argatroban.8 Cases of atherosclerotic or lacunar ACI or transient ischemic attack (TIA) are treated with aspirin or other APAs,1,6 as platelet activation may play an important role in atherosclerosis.7 Although the elevation of soluble C-type lectin-like receptor 2 (sCLEC-2) as a platelet activation marker was reported in patients with ACI,10 few biomarkers of a hypercoagulable state have been reported in ACI patients.
Hypercoagulability with increased tissue factor (TF) or microparticles was proposed as a risk factor for thrombosis in patients with cancer.11 The activation of the coagulation system induced by TF may cause VTE in cancer patients through tumor growth and metastasis.12 Platelet activation was also proposed as a mechanism underlying thrombosis.13 Although elevated soluble fibrin (SF) and D-dimer levels are useful for detecting fibrin formation in patients with thrombosis,14,15 there are few routine tests available for hypercoagulability or thrombosis.
The routine-activated partial thromboplastin time (APTT) and prothrombin time are useful for detecting bleeding tendency but are not adequate for evaluating hypercoagulability. A clot waveform analysis (CWA) including APTT and small amount of TF-induced FIX activation assay (sTF/FIXa) was recently developed; the APTT is useful for analyzing hemostatic abnormalities and monitoring anticoagulant therapy.16–18 In addition, sTF/FIXa using platelet-rich plasma (PRP) can evaluate hemostatic abnormalities, including those involving platelets.18 CWA-APTT and CWA-sTF/FIXa assays can be performed using a routine full auto-blood coagulation analyzer and APTT and PT reagents. These costs are similar to those of routine APTT and PT assays.
In the present study, the hypercoagulable state was examined in 108 ACI patients and 61 patients without ACI using a CWA-APTT or CWA-sTF/FIXa assay and the effects of anticoagulant therapies were also examined.
Materials and Methods
Hemostatic abnormalities based on 155 samples in 108 ACI patients (median age, 75.0 years old: 25th-75th percentile, 65.0-82.0 years old, and 36 females and 72 males) who were admitted to Mie Prefectural General Medical Center from September 1, 2020, to April 30, 2022 were examined using a CWA. CWA examinations were also performed in 61 patients without thrombotic complications including ACI and a prothrombin time-international normalized ratio ≤1.05 (median age, 60.0 years old: 25th-75th percentile, 46.8-71.3 years old; 26 females and 35 males, and 35 with chronic hepatitis, 6 with myeloproliferative neoplasms, 6 with anemia, 5 with digestive diseases, 5 with carriers of hepatitis type B virus, and 4 with other diseases) and 20 healthy volunteers. Treatments for ACI were started on day 1 of admission, and blood sampling was performed on days 2, 7, and 15 of admission. ACI was diagnosed using clinical symptoms, physical examinations, the medical history, and computed tomography or magnetic resonance imaging findings. The study protocol (2019-K9) was approved by the Human Ethics Review Committee of Mie Prefectural General Medical Center, and informed consent was obtained from each participant. This study was carried out in accordance with the principles of the Declaration of Helsinki.
PRP was prepared by centrifugation at 900 rpm for 15 min, and platelet-poor plasma (PPP) was prepared by centrifugation at 3000 rpm for 15 min.18 The APTT was measured using PPP and APTT-SP® (Instrumentation Laboratory, Bedford, MA, USA) with an ACL-TOP® (Instrumentation Laboratory), as previously reported.16,19 The sTF/FIXa assay was measured using 2000-fold diluted HemosIL RecombiPlasTin 2G (TF concentration <0.1 pg/ml; Instrumentation Laboratory) and PRP. A CWA was performed as follows; three curves were expressed on the monitor of the ACL-TOP® system.16 The fibrin formation (FF) curve corresponded to the changes in the absorbance observed while measuring the APTT. The first derivative peak (first DP) curve corresponded to the coagulation velocity. The second derivative peak (2nd DP) curve corresponded to the coagulation acceleration. The height and time of the FF, 1st DP, and 2nd DP curves were called the FFH and FFT, 1st DPH and 1st DPT, and 2nd DPH and 2nd DPT, respectively.
Statistical Analyses
The data are expressed as the median (range). The significance of differences between groups was examined using the Mann-Whitney U-test. The cut-off values, determined as the point at which the sensitivity curve and specificity curve intersected, were examined by a receiver operating characteristic (ROC) analysis. P values of <.05 were considered to indicate statistical significance. All statistical analyses were performed using the Stat-Flex software program (Version 7; Artec Co., Ltd, Osaka, Japan).
Results
Treatments for ACI
Out of the 108 patients, 51 with lacunar ACI or atherosclerotic ACI at 48 h or longer after the onset, were treated with APAs of aspirin® (Bayer Yakuhin, Ltd, Osaka, Japan), clopidogrel® (Pfizer Inc, Tokyo, Japan), or cilostazol (Sawai Pharmaceutical Co., Ltd, Osaka, Japan), 29 patients with atherosclerotic ACI within 48 h of the onset were treated with the direct anti-thrombin agents argatroban® (Alfresa Pharma Corporation, Osaka, Japan) and APA; 19 with cardioembolic ACI were treated with DOACs of rivaroxaban® (Bayer Yakuhin, Ltd), apixaban® (Bristol Myers Squibb, Tokyo, Japan), and edoxaban® (Daiichi Sankyo Co, Ltd, Tokyo, Japan); 5 with cardioembolic ACI were treated with unfractionated heparin (UFH Novo Nordisk Japan, Tokyo, Japan) about 10,000 units/day without dose adjustment by APTT; and 4 with cardioembolic ACI were treated with warfarin® (Eisai's hhc concept, Tokyo, Japan) (Table 1). Most cardioembolic patients had already taken DOAC or warfarin, and about 10% of patients with lacunar ACI or atherosclerotic ACI had already received antiplatelet agents, with these treatments continued after the onset of ACI. Treatment with double antiplatelets was performed in 19 patients in the APA group and 13 in the APA + argatroban group. There were no significant differences in the age, platelet numbers or fibrinogen levels among the five treatment groups, but the APTT and PT were significantly longer in the argatroban and APA treatment group, DOAC treatment group, UFH treatment group and warfarin treatment group than in the APA treatment group (Table 1). There were no significant differences in any data among days 2, 7, and 15.
Table 1.
Patients with ACI.
| Therapy | APA | Argatroban + APA | DOAC | UFH | Warfarin |
|---|---|---|---|---|---|
| Patients (n) | 51 | 29 | 19 | 5 | 4 |
| Age (years) | 75.0 (64.0-79.8) |
70.0 (58.0-78.3) |
79.0 (73.3-83.0) |
83.0 (80.0-84.0) |
82.5# (73.0-84.0) |
| Sex (females:males) | 17:34 | 6:23 | 6:13 | 4:1 | 3:1 |
| Samples number | 69 | 45 | 28 | 7 | 6 |
| ACI type | Lacunar or atherosclerotic-a | Atherosclerotic-w | Cardioembolic ACI | ||
| PLT (×1010/μl) | 22.0 (19.2-28.3) |
21.8 (19.6-25.5) |
20.3 (15.0-26.8) |
21.1 (19.1-27.8) |
27.5 (CNC) |
| APTT (s) | 30.0 (28.0-32.0) |
44.0*** (33.3-52.0) |
35.0*** (32.0-39.0) |
39.0* (30.5-72.8) |
37.0** (CNC) |
| PT-INR | 0.98 (0.94-1.03) |
1.10*** (1.04-1.16) |
1.09*** (1.04-1.14) |
1.11** (1.07-1.18) |
2.29*** (CNC) |
| D-dimer (μg/ml) | 0.90 (0.50-1.68) | 0.50 (0.50-1.05) * | 1.10 (0.58-2.03) | 3.30 (CNC) * | 1.45 (CNC) |
| Fibrinogen (mg/dl) | 329 (298-427) | 326 (299-359) | 343 (281-433) | 456 (331-520) | 350 (CNC) |
Data are expressed as the median (25th-75th percentile); #, range. ACI, acute cerebral infarction; APA, antiplatelet agent; DOAC, direct oral anticoagulant; UFH, unfractionated heparin; CNC, cannot be calculated; PLT, platelet count; PT-INR, prothrombin time-international normalized ratio; atherosclerotic-a, atherosclerotic: at 48 h after the onset; atherosclerotic-w, atherosclerotic within 48 h of the onset.
***P < .001; **P < .01; *P < .05 compared with the APA group.
Examining the Hypercoagulability in ACI Patients Treated Without Anticoagulants (APA Group)
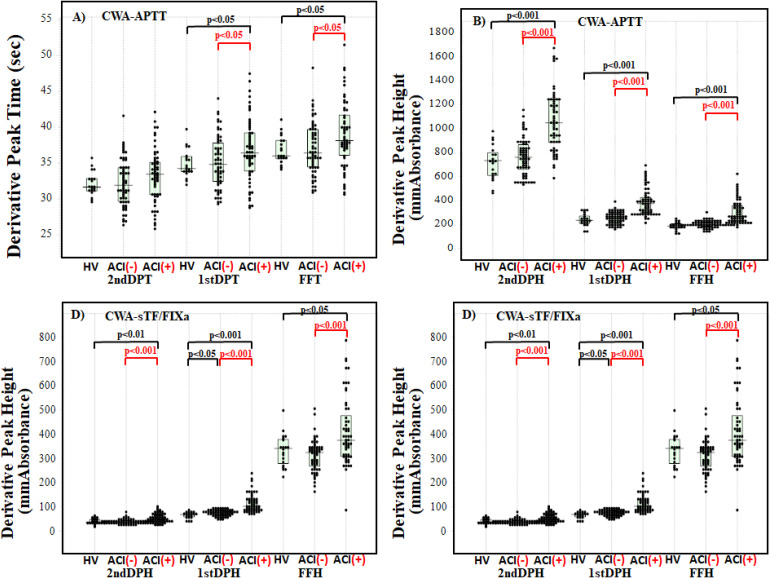
Regarding the CWA-APTT between healthy volunteers and ACI patients treated with APAs without anticoagulants, the 1st DPT and FFT were significantly longer (P < .05, respectively) in ACI patients than healthy volunteers (Figure 1A), and the 2nd DPH, 1st DPH, and FFH were significantly higher (P < .001, respectively) in ACI patients than in healthy volunteers (Figure 1B). Regarding the CWA-FIXa between healthy volunteers and ACI patients treated with APA without anticoagulants, the 2nd DPT, 1st DPT, and FFT were significantly shorter (P < .001, P < .01, and P < .05, respectively) in ACI patients than in healthy volunteers (Figure 1C) and the 2nd DPH, 1st DPH, and FFH were significantly higher (P < .01, P < .001, and P < .05, respectively) in ACI patients than in healthy volunteers (Figure 1D).
Figure 1.
CWA-sTF/FIXa and APTT in acute cerebral infarction (ACI) patients without anticoagulant therapy, control group without ACI, and healthy volunteers. Derivative peak times of CWA-APTT (A); derivative peak heights of CWA-APTT (B); derivative peak times of CWA-sTF/FIXa (C); derivative peak heights of CWA-sTF/FIXa (d). APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor-induced FIX activation; FFT, fibrin formation time; FFH, fibrin formation height; 1stDPT, first derivative peak time; 1stDPH, first derivative peak height; second DPT, second derivative peak time; second DPH, second derivative peak height; HV, healthy volunteer; ACI (-), control group without ACI; ACI (+), ACI patients.
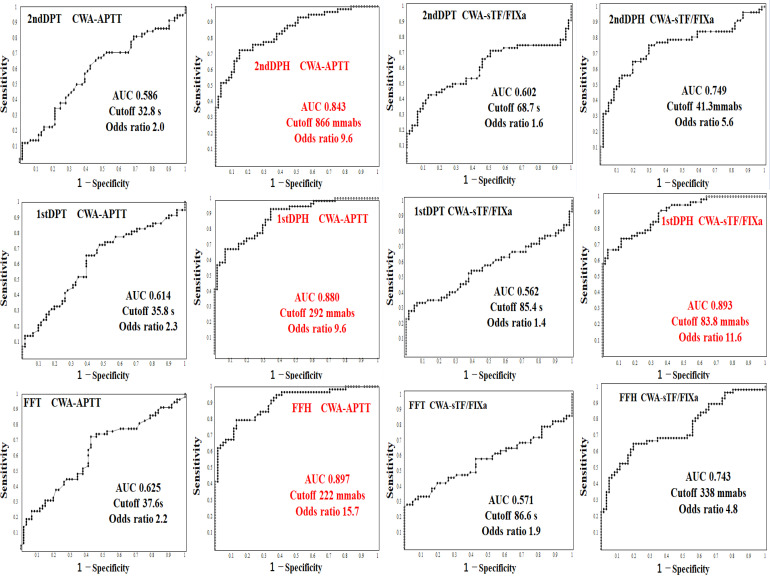
Results of an ROC Analysis for Diagnosing Hypercoagulability in the APA Group of ACI Patients Versus Patients Without ACI Using a CWA-APTT or CWA-sTF/FIXa
Regarding the ROC analysis of the CWA-APTT or sTF/FIXa for diagnosing ACI patients without anticoagulants versus control patients without thrombotic complications and anticoagulants (Table 2), the CWA-APTT showed that the AUC was low at the peak times but high at the peak heights. The 1st DPH of CWA-sTF/FIXa showed that the AUC and odds ratio were the highest among many parameters of CWA-APTT or CWA-sTF/FIXa. The cutoff value was 78.1 mm for the absorbance of the 1st DPH in the CWA-sTF/FIXa (Figure 2).
Table 2.
Results of a Receiver Operating Characteristic Analysis of the CWA-APTT or sTF/FIXa for Diagnosing ACI Without Anticoagulants Versus Control Group Without ACI.
| AUC | Cut-off value | Sensitivity (%) | Odds ratio | ||
|---|---|---|---|---|---|
| CWA-APTT | 2nd DPT | 0.586 | 32.8 s | 58.8 | 2.04 |
| 1st DPT | 0.614 | 35.8 s | 60.7 | 2.35 | |
| FFT | 0.625 | 37.6 s | 59.0 | 2.19 | |
| 2nd DPH | 0.843 | 866 mm Abs | 75.9 | 9.64 | |
| 1st DPH | 0.880 | 292 mm Abs | 75.4 | 9.62 | |
| FFH | 0.897 | 222 mm Abs | 79.3 | 15.7 | |
| sTF/FIXa | 2nd DPT | 0.602 | 68.7 s | 55.7 | 1.56 |
| 1st DPT | 0.562 | 85.4 s | 56.2 | 1.41 | |
| FFT | 0.571 | 86.6 s | 57.4 | 1.86 | |
| 2nd DPH | 0.749 | 41.3 mm Abs | 70.5 | 5.62 | |
| 1st DPH | 0.893 | 83.8 mm Abs | 77.2 | 10.5 | |
| FFH | 0.743 | 338 mm Abs | 66.7 | 4.78 |
ACI, acute cerebral infarction; CWA, clot waveform analysis; APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor-induced factor IX activation; second DPT, second derivative peak time; first DPT, first derivative peak time; FFT, fibrin formation time; second DPH, second derivative peak height; first DPH, first derivative peak height; FFT, fibrin formation height; mm Abs, mm absorbance.
Figure 2.
A receiver operating characteristic analysis regarding the diagnosis of acute cerebral infarction ACI without anticoagulant versus control group without ACI using 1stDPT (A) and 1stDPH (B) of CWA-APTT and 1stDPT (C) and 1stDPH (D) of CWA-sTF/FIXa. CWA, clot waveform analysis; APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor-induced FIX activation; 1stDPT, first derivative peak time; 1stDPH, first derivative peak height; second DPT, second derivative peak time; second DPH, second derivative peak height.
A Comparison of the Anticoagulant Effects in Each Groups Versus APA Group Using a CWA-APTT
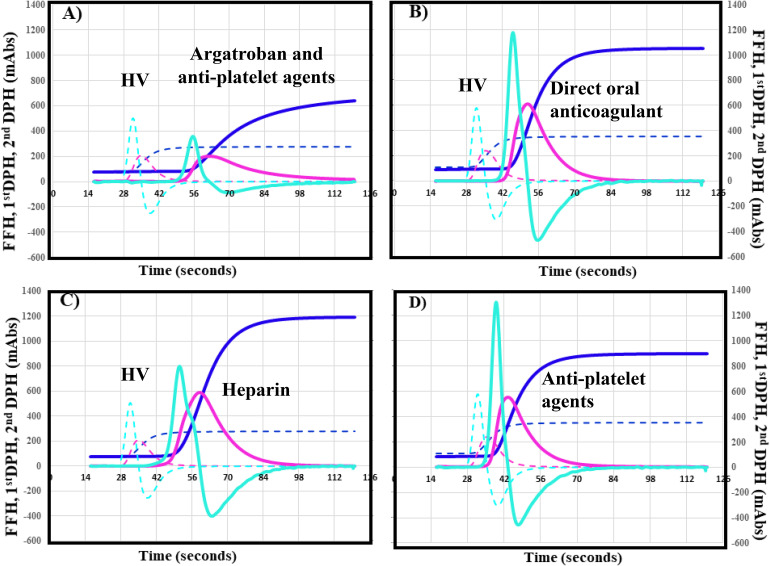
The CWA-APTT among ACI patients treated with argatroban and APAs (Figure 3A), DOACs (Figure 3B), UFH (Figure 3C), and warfarin were compared to the values in ACI patients with APAs alone (Figure 2D). Data were obtained on day 2 in the Argatroban + APA group and at days 2, 7, and 15 in the APA, DOAC, UFH, and warfarin groups. The 2nd DPT, 1st DPT, and FFT were significantly longer in the argatroban and APA group (all P < .001), UFH group (all P < .01), and warfarin group (all P < .01) than in the APA group (Table 3). There were no significant differences in the peak times between the DOAC group and APA group. The 2nd DPH and first DPH were significantly lower in the argatroban and APA group (all P < .001) and warfarin group (all P < .001) than in the APA group. There were no significant differences in the 1st DPH and FFH between the DOAC or UFH group and the APA group.
Figure 3.
CWA-APTT in patients with acute cerebral infarction treated with argatroban and anti-platelet agents (A), direct oral anticoagulant (B), heparin (C), or anti-platelet agents (D). CWA, clot wave form; APTT, activated partial thromboplastin time; navy line, fibrin formation curve; FFH, fibrin formation height; pink line, first derivative curve (velocity); 1stDPH, first derivative peak height; light blue, second derivative curve (acceleration); second DPH, second derivative peak height; solid line, patient; dotted line, healthy volunteer. The peak time of (A)-(D) is longer for the solid line (patient) than for the dotted line (healthy volunteer). The peak height of (A) is lower and that of (B)-(D) higher for the solid line (patient) than for the dotted line (healthy volunteer).
Table 3.
The Clot Waveform Analysis-Activated Partial Thromboplastin Time among Patients with ACI Given various Treatments.
| Therapy | APA | Argatroban + APA | DOAC | UFH | Warfarin |
|---|---|---|---|---|---|
| 2nd DPT (s) |
33.5 (30.6-35.1) |
38.1*** (35.4-48.2) |
34.4 (33.4-36.6) |
43.1** (36.5-77.0) |
40.2** (36.6-47.5) |
| 2nd DPH (mm Abs) |
1049 (884-1241) |
757*** (513-947) |
924** (788-1032) |
820* (456-1024) |
624*** (387-691) |
| 1st DPT (s) |
36.4 (33.9-39.2) |
42.5*** (38.6-51.8) |
37.9 (36.6-40.8) |
46.1** (40.8-86.1) |
43.6** (40.3-50.7) |
| 1st DPH (mm Abs) |
374 (292-426) |
287*** (232-344) |
315 (281-388) |
356 (310-427) |
229*** (194-238) |
| FFT (s) |
38.2 (36.1-41.7) |
44.8*** (40.5-54.8) |
40.1* (38.9-43.9) |
49.5** (43.0-87.3) |
50.7** (43.7-60.0) |
| FFH (mm Abs) |
268 (228-357) |
254 (221-296) |
272 (235-335) |
371 (315-398) |
334 (251-355) |
Data are expressed as the median (25-75 percentile); Blood samples were obtained at day 2 in Argatroban + APA group, or days 2, 7, and 15 in APA, DOAC, UFH, and warfarin groups. ACI, acute cerebral infarction; CWA, clot wave form; APA, antiplatelet agent; DOAC, direct oral anticoagulants; UFH, unfractionated heparin; second DPT, second derivative peak time; first DPT, first derivative peak time; FFT, fibrin formation time; second DPH, second derivative peak height; first DPH, first derivative peak height; FFT, fibrin formation height; mm Abs, mm absorbance.
***P < .001; **P < .01; *P < .05 in compared with APA group.
A Comparison of the Anticoagulant Effects in Each Groups Versus APA Group Using a CWA-sTF/FIXa
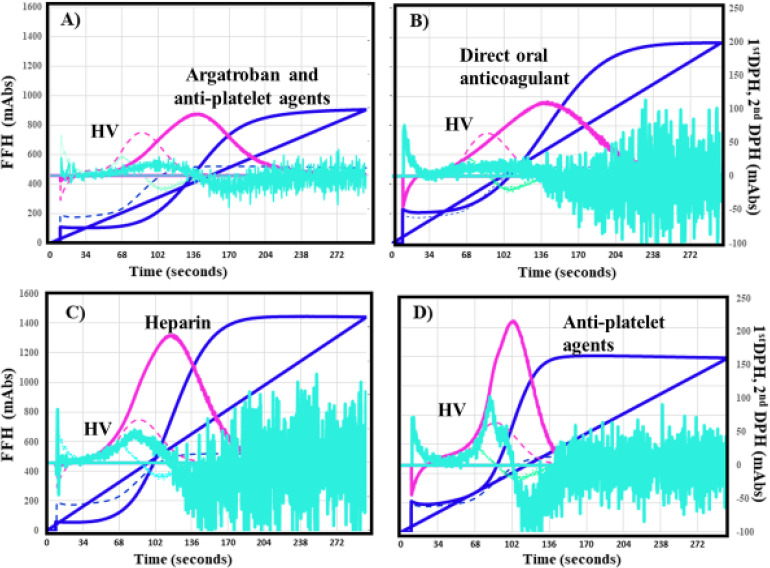
The CWA-sTF/FIXa showed significantly longer peak times were in the argatroban and APA group (Figure 4A), DOAC group (Figure 4B), and warfarin group than in the APA group (Figure 4D) (Table 4). There were no significant differences in the peak times between the UFH group (Figure 4C) and APA group. The 2nd DPH and first DPH were significantly lower in the argatroban and APAs group and DOAC group than in the APA group. There were no significant differences in the 1st DPH and FFH between the UFH or warfarin group and the APA group.
Figure 4.
CWA-sTF/FIXa in patients with acute cerebral infarction treated with argatroban and anti-platelet agents (A), direct oral anticoagulant (B), heparin (C), or anti-platelet agents (D). CWA, clot wave form; stf/FIXa, small amount of tissue factor-induced FIX activation; navy line, fibrin formation curve; FFH, fibrin formation height; pink line, first derivative curve (velocity); 1stDPH, first derivative peak height; light blue, second derivative curve (acceleration); second DPH, second derivative peak height; solid line, patient; dotted line, healthy volunteer. The peak times of (A) and (B) are longer for the solid line (patient) than for the dotted line (healthy volunteer). The peak height of (A) and (B) is slightly and of that of (B)-(D) higher for the solid line (patient) than for the dotted line (healthy volunteer).
Table 4.
Results of a Clot Waveform Analysis-Small Amount of Tissue Factor-Induced Factor IX Activation Assay (sTF/FIXa) among Patients with ACI Given various Treatments.
| Therapy | APA | Argatroban + APA | DOAC | UFH | Warfarin |
|---|---|---|---|---|---|
| 2nd DPT (s) |
67.3 (56.4-76.3) |
78.6** (64.7-97.3) |
78.5** (70.4-96.0) |
67.5 (64.0-83.8) |
109** (82.4-168.1) |
| 2nd DPH (mm Abs) |
49.1 (40.8-65.8) |
36.5*** (20.9-53.4) |
31.2** (21.1-54.8) |
56.7 (41.7-59.9) |
20.9* (18.4-52.3) |
| 1st DPT (s) |
86.7 (80.0-97.3) |
99.8*** (84.8-127) |
105*** (89.1-125) |
102 (85.8-117) |
145** (94.2-178) |
| 1st DPH (mm Abs) |
102 (86.3-129) |
89.4** (71.3-103) |
78.5** (54.5-117) |
112 (88.8-166) |
74.1 (67.3-134) |
| FFT (s) |
88.3 (80.8-98.5) |
100.0** (83.0-124) |
110*** (93.6-131) |
104 (87.4-115) |
146* (91.4-175) |
| FFH (mm Abs) |
374 (308-475) |
354 (304-413) |
395 (322-440) |
455 (388-518) |
391 (329-495) |
Data are expressed as the median (25-75 percentile); Blood samples were obtained at day 2 in Argatroban + APA group, or days 2, 7, and 15 in APA, DOAC, UFH, and warfarin groups. ACI, acute cerebral infarction; CWA, clot wave form; APA, anti-platelet agent; DOAC, direct oral anticoagulant; UFH, unfractionated heparin; second DPT, second derivative peak time; first DPT, first derivative peak time; FFT, fibrin formation time; second DPH, second derivative peak height; first DPH, first derivative peak height; FFT, fibrin formation height; mm Abs, mm absorbance.
***P < .001; **P < .01; *P < .05 in compared with the APA group.
Discussion
As a limitation of this study, blood sampling in ACI patients was performed on the second day, after the start of ACI treatment, instead of on arival to the hospital. Therefore, the hypercoagulability was examined in ACI patients without anticoagulants. This analysis was performed regardless of the effect of APAs. ACI is usually treated with anti-thrombotic agents, such as direct anti-thrombin agents, DOACs, warfarin or UFH, or APAs. However, hypercoagulability has not been evaluated well and hemostatic abnormalities are not monitored by routine testing as APTT or PT without CWA. Regarding the CWA-clotting time of APTT or sTF/FIXa, the peak time is usually shorter and peak height is higher in a hypercoagulable state than in a normal state.16 Although the CWA-APTT showed that the peak heights were significantly higher in ACI patients than in healthy volunteers or patients without ACI in our study, the peak times were longer in ACI patients than in healthy volunteers or patients without ACI; conversely, in the CWA-sTF/FIXa, the peak heights were significantly higher and the peak times were significantly shorter in ACI patients than in healthy volunteers or patients without ACI, suggesting that the CWA-sTF/FIXa is more useful than the CWA-APTT.
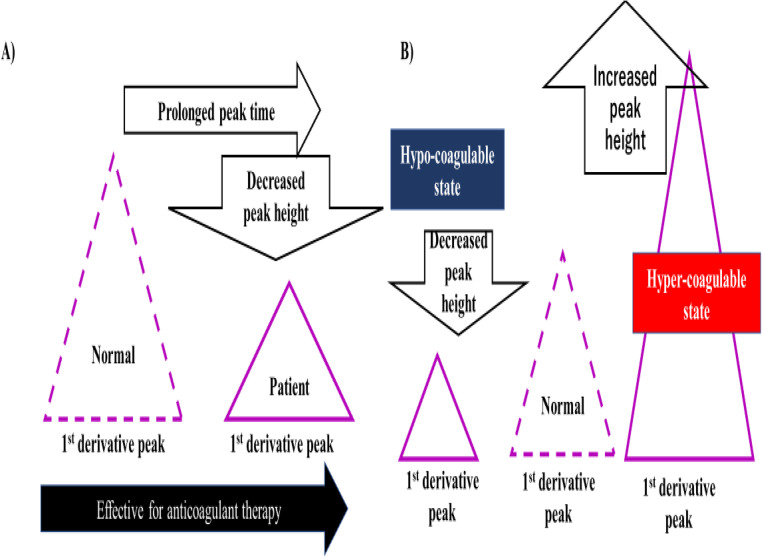
The CWA-sTF/FIXa showed extremely high peak heights in patients with cancer associated with thrombosis,20 suggesting that for evaluating hypercoagulability, CWA-APTT or CWA-sTF/FIXa using the peak heights is more useful than that using the peak times. The peak height of the CWA-APTT was also reported to be useful in cases of hemophilia.21,22 ACI patients without anticoagulant therapies are considered hypercoagulable. In addition, the evidence from this study supports the establishment of a hypercoagulable state to prevent the onset of thrombosis (Figure 5).
Regarding the effects of antiplatelet agents on the CWA-APTT or CWA-sTF/FIXa (Figure 5), their administration did not decrease the peak heights, suggesting that these clotting assays cannot evaluate the effects of antiplatelet agents. However, the strongest inhibition of the CWA-APTT and CWA-sTF/FIXa was observed in patients treated with argatroban, and mild inhibition of the CWA-APTT and moderate inhibition of the CWA-sTF/FIXa were observed in patients treated with DOACs. These findings suggest that the evalution of the effects of argatroban should be performed using the CWA-APTT, while the evalution of the effects from DOACs should be performed using the CWA-sTF/FIXa. The in vitro analysis of the differences between direct thrombin inhibitors and direct Xa inhibitors was reported.23 The peak height of the CWA-APTT or CWA-sTF/FIXa was not sufficiently decreased in patients treated with UFH, suggesting that the dose of heparin might be low compared with the treatment of pulmonary embolism, as the mechanism of underlying the onset for ACI was previously considered to involve activation of platelets and not coagulation.10,24 Our findings suggest that not only platelet activation but also a hypercoagulable state exist in ACI patients, so anti-thrombin agents may be useful for improving the hypercoagulability in ACI patients. Argatroban was also reported to improve the hematological coagulation abnormalities.8,25,26 However, the relationship between hypercoagulability and a poor outcome in ACI has not been established.
Figure 5.
Effectiveness of anticoagulant therapy (A) and a hypo-coagulable state and hyper-coagulable state (B) on a clot waveform analysis.
The CWA-APTT and CWA-sTF/FIXa were able to visualize the clotting reaction and showed a peak time that was similar to the findings of routine APTT or PT and a peak height that reflected hyper- or hypo-coagulability as well as the thrombin generation test or a thromboelastogram.27,28 In addition, the CWA-APTT and CWA-sTF/FIXa were able to be performed as easily and cheaply as routine assays in the general laboratory.
In conclusion, the increased 1st DPH of the CWA-APTT or CWA-sTF/FIXa indicated hypercoagulability in ACI patients without anticoagulants and this abnormality on the CWA disappeared in ACI patients treated with direct anti-thrombin therapy. Before the onset, the presence of a hypercoagulable state can be diagnosed with CWA-sTF/FIXa, and the onset of thrombosis can be prevented.
This research was funded by a grant-in-aid (H30-015) from the Ministry of Health, Labor and Welfare of Japan.
Acknowledgments
We would like to thank the physicians and the laboratory technicians (Chief technicians, Shinya Hiromori) for measuring the laboratory biomarkers at Mie Prefectural General Medical Center Hospital. We also thank Mrs Yumi Sakano and Hiroko Nishii for CWA.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CWA was partially supported by Instrumentation Laboratory Japan. The authors declare no other conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Health, Labor and Welfare of Japan (grant number H30-015).
ORCID iD: Hideo Wada https://orcid.org/0000-0001-9021-8633
References
- 1.Phipps MS, Cronin CA. Management of acute ischemic stroke. Br Med J. 2020;368:l6983. [DOI] [PubMed] [Google Scholar]
- 2.World Stroke Organization (WSO). Global stroke fact sheet. https://www.world-stroke.org/.
- 3.Strandberg M, Mustonen P, Taina M, et al. Etiology, diagnostics and treatment of cardiogenic stroke. Duodecim. 2016;132(18):1625‐1633. [PubMed] [Google Scholar]
- 4.Ministrini S, Carbone F, Montecucco F. Updating concepts on atherosclerotic inflammation: From pathophysiology to treatment. Eur J Clin Invest. 2020:51(5):e13467. [DOI] [PubMed] [Google Scholar]
- 5.Chokesuwattanaskul A, Cheungpasitporn W, Thongprayoon C, et al. Impact of circadian blood pressure pattern on silent cerebral small vessel disease: A systematic review and meta-analysis. J Am Heart Assoc. 2020;9(12):e016299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amarenco P, Denison H, Evans SR, et al. Ticagrelor added to aspirin in acute ischemic stroke or transient ischemic attack in prevention of disabling stroke: A randomized clinical trial. JAMA Neurol. 2020;78(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pircher J, Engelmann B, Massberg S, et al. Platelet-neutrophil crosstalk in atherothrombosis. Thromb Haemost. 2019;119(8):1274‐1282. [DOI] [PubMed] [Google Scholar]
- 8.Huang P, He XY, Xu M. Effect of argatroban injection on clinical efficacy in patients with acute cerebral infarction: Preliminary findings. Eur Neurol. 2021;84(1):38‐42. [DOI] [PubMed] [Google Scholar]
- 9.Macha K, Marsch A, Siedler G, et al. Cerebral ischemia in patients on direct oral anticoagulants. Stroke. 2019;50(4):873‐879. [DOI] [PubMed] [Google Scholar]
- 10.Nishigaki A, Ichikawa Y, Ezaki E, et al. Soluble C-type lectin-like receptor 2 elevation in patients with acute cerebral infarction. J Clin Med. 2021;10(15):3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130(13):1499‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma BK, Flick MJ, Palumbo JS. Cancer-associated thrombosis: A two-way street. Semin Thromb Hemost. 2019;45(6):559‐568. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki-Inoue K. Platelets and cancer-associated thrombosis: Focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. 2019;134(22):1912‐1918. [DOI] [PubMed] [Google Scholar]
- 14.Maestre A, Trujillo-Santos J, Visoná A, et al. D-dimer levels and 90-day outcome in patients with acute pulmonary embolism with or without cancer. Thromb Res. 2014;133(3):384‐389. [DOI] [PubMed] [Google Scholar]
- 15.Gerotziafas GT, Mahé I, Lefkou E, et al. Overview of risk assessment models for venous thromboembolism in ambulatory patients with cancer. Thromb Res. 2020;191(Suppl 1):S50‐S57. [DOI] [PubMed] [Google Scholar]
- 16.Wada H, Matsumoto T, Ohishi K, et al. Update on the clot waveform analysis. Clin Appl Thromb Hemost. 2020:1076029620912027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa M, Tone S, Wada H, et al. The evaluation of hemostatic abnormalities using a CWA-small amount tissue factor induced FIX activation assay in major orthopedic surgery patients. Clin Appl Thromb Hemost. 2021:1076029620976913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada H, Shiraki K, Matsumoto T, et al. Effects of platelet and phospholipids on clot formation activated by a small amount of tissue factor. Thromb Res. 2020;193:146‐153. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Wada H, Fujimoto N, et al. An evaluation of the activated partial thromboplastin time waveform. Clin Appl Thromb Hemost. 2018;24(5):764‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi M, Wada H, Fukui S, et al. A clot waveform analysis showing a hypercoagulable state in patients with malignant neoplasms. J Clin Med. 2021;10(22):5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa S, Nogami K, Shimonishi N, et al. Prediction of the haemostatic effects of bypassing therapy using comprehensive coagulation assays in emicizumab prophylaxis-treated haemophilia A patients with inhibitors. Br J Haematol. 2020;190(5):727‐735. [DOI] [PubMed] [Google Scholar]
- 22.Takeyama M, Furukawa S, Onishi T, et al. Heterogeneous coagulant potential of emicizumab in neonatal factor VIII-deficient plasma. Pediatr Blood Cancer. 2022;69(7):e29731. [DOI] [PubMed] [Google Scholar]
- 23.Wakui M, Fujimori Y, Katagiri H, et al. Assessment of in vitro effects of direct thrombin inhibitors and activated factor X inhibitors through clot waveform analysis. J Clin Pathol. 2019;72(3):244‐250. [DOI] [PubMed] [Google Scholar]
- 24.Wan H, Han W, Wu Z, et al. Whole blood dynamic platelet aggregation counting and 1-year clinical outcomes in patients with coronary heart diseases treated with clopidogrel. Platelets. 2021;32(7):968‐974. [DOI] [PubMed] [Google Scholar]
- 25.Huang P, He XY, Xu M. Effect of argatroban injection on clinical efficacy in patients with acute cerebral infarction: Preliminary findings. Eur Neurol. 2021;84(1):38‐42. [DOI] [PubMed] [Google Scholar]
- 26.Urabe T, Tanaka R, Noda K, et al. Anticoagulant therapy with a selective thrombin inhibitor for acute cerebral infarction: Usefulness of coagulation markers for evaluation of efficacy. J Thromb Thrombolysis. 2002;13(3):155‐160. [DOI] [PubMed] [Google Scholar]
- 27.Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62(5):699‐707. [DOI] [PubMed] [Google Scholar]
- 28.Konstantinidi A, Sokou R, Parastatidou S, et al. Clinical application of hromboelastography/thromboelastometry (TEG/TEM) in the neonatal population: A narrative review. Semin Thromb Hemost. 2019;45(5):449‐457. [DOI] [PubMed] [Google Scholar]