Abstract
Background:
Trastuzumab and chemotherapy is the standard first-line treatment in human epidermal growth factor receptor 2 (HER2)-positive advanced gastro-oesophageal cancer. The objective was to develop a predictive model for overall survival (OS) and progression-free survival (PFS) in patients treated with trastuzumab.
Methods:
Patients with HER2-positive advanced gastro-oesophageal adenocarcinoma (AGA) from the Spanish Society of Medical Oncology (SEOM)-AGAMENON registry and treated first line with trastuzumab and chemotherapy between 2008 and 2021 were included. The model was externally validated in an independent series (The Christie NHS Foundation Trust, Manchester, UK).
Results:
In all, 737 patients were recruited (AGAMENON-SEOM, n = 654; Manchester, n = 83). Median PFS and OS in the training cohort were 7.76 [95% confidence interval (CI), 7.13–8.25] and 14.0 months (95% CI, 13.0–14.9), respectively. Six covariates were significantly associated with OS: neutrophil-to-lymphocyte ratio, Eastern Cooperative Oncology Group performance status, Lauren subtype, HER2 expression, histological grade and tumour burden. The AGAMENON-HER2 model demonstrated adequate calibration and fair discriminatory ability with a c-index for corrected PFS/OS of 0.606 (95% CI, 0.578–0.636) and 0.623 (95% CI, 0.594–0.655), respectively. In the validation cohort, the model is well calibrated, with a c-index of 0.650 and 0.683 for PFS and OS, respectively.
Conclusion:
The AGAMENON-HER2 prognostic tool stratifies HER2-positive AGA patients receiving trastuzumab and chemotherapy according to their estimated survival endpoints.
Keywords: gastric cancer, HER2-positive, nomogram, oesophageal cancer, survival, trastuzumab
Introduction
Globally, gastroesophageal cancer is the fourth most common cancer and represents 8.7% of all cancers. In 2020, there were an estimated 1,700,000 new cases. Likewise, it is the second cause of cancer mortality, accounting for 13.2% with 1,312,869 estimated deaths in 2020.1 The Cancer Genome Atlas divides gastric cancer into four subtypes that may serve as a valuable adjunct to histopathology.2 Tumors with chromosomal instability are the most frequently ocurring subtype (50%), mainly in neoplasms located in the gastroesophageal junction (GEJ)/cardia, with intestinal histology, and the presence of various chromosomal abnormalities actionable by receptor tyrosine kinase-directed therapies. Amplification or overexpression of human epidermal growth factor receptor 2 (HER2) is one of the most frequent alterations (9–36%) and serves as a predictive biomarker.3,4 In 2010, the ToGA phase III randomised clinical trial (RCT) demonstrated the benefit of adding an anti-HER2 antibody (trastuzumab) to a backbone of six cycles of cisplatin and fluoropyrimidine in HER2-positive (HER2+) for unresectable, recurrent or metastatic gastro-oesophageal and gastric adenocarcinoma.5 Patients receiving trastuzumab had increased overall survival (OS), 13.8 versus 11.0 months [hazard ratio, 0.74, 95% confidence interval (CI), 0.60–0.91)], and delayed deterioration in quality of life.6 In an exploratory analysis, median OS reached 16 months in the subgroup with immunohistochemistry (IHC) 2+/fluorescence in situ hybridisation (FISH) + or IHC 3+.5 Reassuringly, this has been confirmed in the real-world setting where rates of testing for HER2 in gastric cancer patients have been shown to correlate with improved OS.4 In the near future, it is likely that other HER2-directed therapies will be incorporated into the therapeutic arsenal (e.g. margetuximab, trastuzumab–deruxtecan).7–9 The addition of trastuzumab and chemotherapy to an immune checkpoint inhibitor, the anti-programmed cell death 1 (PD-1) antibody pembrolizumab, has shown favourable preliminary results in the phase III RCT Keynote 811.10
In this context of expanding therapeutic options, a predictive tool for the benefit of trastuzumab-based regimens could be useful to select the most appropriate treatment for each patient, help understand the variation in prognosis of patients with HER2+ advanced gastro-oesophageal adenocarcinoma (AGA), identify which patient subgroups have poorer OS expectations, and stratify patients in future clinical trials. Thus, multivariable models may complement validated biomarkers in the development of risk-based therapeutic strategies. However, despite the recognition that these neoplasms form an entity with particular clinical-biological and therapeutic characteristics,11 specific models of HER2+ AGA are scarce.12–14 This limits the understanding of prognostic heterogeneity, as models that do not consider HER2 status perform worse.15,16 With this premise, we have used the AGAMENON-Spanish Society of Medical Oncology (SEOM) registry (NCT04958720) to develop a predictive model of progression-free survival (PFS) and OS specific to patients with a HER2+ AGA who received trastuzumab as first-line therapy. The model has been externally validated in a cohort at the Christie Hospital in Manchester.
Method
Patients and study design
The model was designed in a training cohort that included patients from the AGAMENON oesophageal and gastric cancer registry managed by the SEOM in which 42 Spanish university hospitals participate. The characteristics of this registry, quality criteria, methods and data collection criteria have been described previously.17–20 To comprehend the representativeness of the data, Supplemental Annex Table 1 contains a description of all the centres that are participating in the registry.
Patients eligible for this study were adults (age ⩾18 years), diagnosed with locally advanced or metastatic unresectable adenocarcinoma of the distal oesophagus, GEJ and stomach, HER2+ who received at least one cycle of chemotherapy and trastuzumab as first-line treatment. HER2 status was studied locally at the centres and defined as IHC 3+ or IHC 2+/FISH+. Subjects who had completed perioperative or adjuvant systemic treatment in the previous 6 months and those with HER2+ cancers that were not treated with first-line trastuzumab were excluded.
Data were collected from medical records or directly from the patient by medical oncologists experienced in treating AGA and trained to meet the requirements of the study via an online platform. This tool includes real-time alerts to avoid inconsistencies, unjustified missing values and errors, and regular telephone and online monitoring is performed. Patient-reported outcome measures were not collected.
Results were externally validated in a separate cohort from The Christie NHS Foundation Trust (Manchester, UK), which included consecutive patients who met the same eligibility criteria.
Objectives and variables of interest
The objective was to develop and externally validate a multivariable model to stratify patients with HER2+ AGA according to PFS/OS. Outcome variables were PFS and OS defined as the time in months between initiation of first-line chemotherapy and progression or death, respectively, censoring subjects alive at last follow-up.
Candidate predictors were selected after an exhaustive literature search, and after consultation with experts from the participating centres. No data-driven method was used in the final selection of variables.21 All covariates had to be available at the start of treatment (e.g. the primary tumour surgery variable was only considered when subjects had been exposed before the start of first-line treatment). The covariates considered in this model were age, Eastern Cooperative Oncology Group performance status (ECOG PS; ⩾2 versus 0–1), primary tumour location (oesophagus, GEJ, stomach), HER2 expression level (IHC 2+/FISH+ versus IHC 3+), Lauren subtype (intestinal versus diffuse and mixed), signet ring cells, histological grade (1, 2, versus 3), overall tumour burden (stratified into four categories, Table 1), neutrophil-to-lymphocyte ratio (NLR; non-linear, continuous), albumin, carcinoembryonic antigen, CEA (non-linear, continuous), primary tumour surgery, chemotherapy regimen (anthracycline-based triplets, carboplatin–5-fluorouracil, carboplatin–capecitabine, cisplatin–5-fluorouracil, docetaxel-containing regimens, 5-fluorouracil/oxaliplatin, capecitabine/oxaliplatin, capecitabine/cisplatin, others). Criteria to stratify the overall tumour burden (Table 1) have been used previously by our group.22 The OS analyses stratified by this variable on the entire cohort, as well as the Manchester series, suggest that these criteria are valid (Supplemental Annex Figure 1).
Table 1.
Definitions of overall tumour burden.
| Tumour burden | Definition |
|---|---|
| Low | • 1 single organ involved with 1 or 2 lesions or mild ascites/microscopic peritoneal dissemination with no other sites |
| Moderate | • 1 single involved organ with 3–5 lesions • 2 involved organs with <3 lesions per organ |
| High | • 1 single organ involved with >5 lesions • 3 or more involved organs |
| Very high | • Baseline sum of total diameters >15 cm • Moderate or severe ascites • Diffuse peritoneal metastases with nodules >2–3 cm • CNS metastases • 3 or more bone metastases • Liver tumour burden >50% |
Mild ascites was defined as not clinically evident, diagnosed with CT or ultrasound.
CNS, central nervous system; CT, computed tomography.
Statistics
Correlation of predictors with OS was assessed using Somers’ Dxy rank correlations. Missing data were imputed using predictive mean matching.23 None of the predictors were found to have more than 20% missing data. Redundancy analysis was performed with flexible parametric additive models to remove any covariates that could be predicted by the remaining variables.21 We used a log-normal accelerated failure time (AFT) model, as the aim was to model the mean time to PFS/OS.24 We tested the adequacy of the log-normal parametric model by calculating the Kaplan–Meier estimate of the distribution of the residuals versus the theoretical gaussian. In AFT models, survival times are multiplied by a constant effect under this formulation so that the exponentiated coefficients, exp(β), are called time ratios (TRs). A TR of more than 1 for the covariate implies that the covariate slows down or lengthens the time to the event, while a TR of less than 1 indicates that an event is more likely to occur earlier. Thus, the regression coefficient of a binary predictor equal to log(0.5) means that the median time to event is halved in its presence. CIs of 90%, 95% and 99% are reported. Time is expressed in months. Non-linear effects were modelled using restricted cubic splines. In terms of sample size, the number of predictors was chosen so that there were at least 15 events per covariate.21 To obtain a simplified, more convenient model, a version of fast backward elimination on factors was used, using a method based on Lawless and Singhal.25 The simplified model was represented as a nomogram and online calculator; calibration curves were plotted and discrimination was assessed using Harrell’s C-index, which takes into account right-censored data. In the derivation series, the c-index was corrected for bias by bootstrap. Using the linear predictor, the same measures of model performance were obtained in the validation cohort. Analyses were performed with R version 4.01,26 including the Hmisc and rms libraries.27,28
Results
Patients and treatments
A total of 737 patients treated between 2008 and 2021 were consecutively enrolled – 654 from the training cohort of the AGAMENON-SEOM registry and 83 from the external validation subset. Baseline characteristics are illustrated in Table 2. Demographics and therapies are comparable in both cohorts, except for a higher percentage of GEJ adenocarcinomas in the Christie Hospital series (21% versus 53%), more tumours with very high tumour burden in the AGAMENON-SEOM cohort (41% versus 13%), and a more homogeneous treatment pattern with a predominance of ToGA regimens (cisplatin/capecitabine in 93%) in the Christie Hospital series versus a greater predilection for oxaliplatin-based doublets (n = 301, 46%) in AGAMENON-SEOM.
Table 2.
Baseline characteristics.
| AGAMENON-HER2 training cohort | Christie hospital cohort | |
|---|---|---|
| N (%) | N (%) | |
| Age, median (range) | 65 (22–87) | 67 (27–85) |
| Sex, male | 510 (77.9) | 70 (84.3) |
| HER2+ | ||
| IHC 3+ | 470 (71.8) | 54 (65.0) |
| IHC 2+ and FISH+ | 184 (28.1) | 29 (34.9) |
| ECOG PS | ||
| 0 | 158 (24.1) | 25 (30.1) |
| 1 | 396 (60.5) | 42 (50.6) |
| 2 | 91 (13.9) | 15 (18.0) |
| 3 | 0 | 1 (1.2) |
| Lauren subtype | ||
| Intestinal | 393 (60.0) | 59 (71.0) |
| Diffuse | 95 (14.5) | 16 (19.2) |
| Mixed | 33 (5.0) | – |
| Not available | 124 (18.9) | 8 (9.6) |
| Signet-ring cells | 85 (12.9) | 5 (6.0) |
| Histological grade | ||
| 1 | 124 (18.9) | – |
| 2 | 219 (33.4) | 42 (50.60) |
| 3 | 164 (25.0) | 28 (33.7) |
| Not available | 138 (21.1) | 13 (15.6) |
| Surgery of primary tumour | 116 (17.7) | 9 (10.8) |
| Primary tumour site | ||
| Oesophagus | 88 (13.4) | 21 (25.3) |
| Stomach | 421 (64.3) | 18 (21.6) |
| GEJ | 136 (20.7) | 44 (53.0) |
| Number of metastatic sites (organs involved), >2 | 194 (29.6) | 30 (36.4) |
| Site of metastases | ||
| Peritoneum | 196 (29.9) | 11 (13.2) |
| Lung | 153 (23.3) | 21 (25.3) |
| Liver | 352 (53.8) | 37 (44.5) |
| Ascites | 93 (14.2) | 1 (1.2) |
| Bone | 61 (9.3) | 10 (12.0) |
| Non-regional lymph nodes | 329 (50.3) | 40 (48.1) |
| Liver tumour burden | ||
| No | 290 (44.3) | 46 (55.4) |
| <25% | 168 (25.6) | 25 (30.1) |
| 25–50% | 98 (14.9) | 10 (12.0) |
| 51–75% | 64 (9.7) | 2 (2.4) |
| >75% | 25 (3.8) | – |
| Overall tumour burden | ||
| Low | 82 (12.5) | 17 (20.4) |
| Moderate | 116 (17.7) | 22 (26.5) |
| High | 190 (29.0) | 33 (39.7) |
| Very high | 266 (40.6) | 11 (13.2) |
| CEA, median (range) | 9.5 (0–36001) | |
| Albumin | ||
| Normal (>35 g/dL) | 429 (65.5) | 79 (95.1) |
| 30–35 g/dL | 100 (15.2) | 3 (3.6) |
| <30 g/dL | 54 (8.2) | 1 (1.2) |
| Not available | 62 (9.4) | – |
| NLR, median (range) | 3.4 (0.1–29.3) | 4.3 (1.2–42.2) |
| Chemotherapy regimens | ||
| Anthracycline-based triplets | 28 (4.2) | 0 |
| Carboplatin–5-fluorouracil | 32 (4.8) | 0 |
| Carboplatin–capecitabine | 0 | 5 (6.0) |
| Cisplatin–5-fluorouracil | 92 (14.0) | 0 |
| Docetaxel-containing regimens | 15 (2.2) | 0 |
| FOLFOX | 110 (16.8) | 0 |
| Other | 18 (2.7) | 1 (1.2) |
| CAPOX | 191 (29.2) | 0 |
| XP | 168 (25.6) | 77 (92.7) |
| Total | 654 (100) | 83 (100) |
The criteria for overall tumour burden are specified in Table 1.
CAPOX, capecitabine/oxaliplatin; CEA, carcinoembryonic antigen; FOLFOX, 5-fluorouracil/oxaliplatin; GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; N, number of patients; NLR, neutrophil-to-lymphocyte ratio; XP, capecitabine/cisplatin.
Eighty-two percent (82%) of the subjects (538/654) in the derivation sample had synchronous metastases since the time of diagnosis, whereas the rest had undergone surgery with curative intent and relapsed later on.
Outcomes
In the training cohort (AGAMENON-SEOM), 580 progression events and 549 deaths were recorded after a median follow-up in living patients of 62.8 months (95% CI, 49.1–86.0). Median PFS was 7.7 months (95% CI, 7.1–8.2), and median OS was 14.0 months (95% CI, 13.0–14.9). Survival data stratified by regimen are summarised in Supplemental Annex Table 2, with comparable results except for carboplatin-based regimens. At the time of analysis, 93% (n = 609) had discontinued first-line treatment. Patients received a median of six cycles of platinum chemotherapy (range, 1–14), and eight cycles of a fluoropyrimidine. The median duration of trastuzumab treatment was 7.6 months (95% CI, 7.1–8.3).
In the Christie Hospital test cohort, 55/83 death events were detected, after a median follow-up in living subjects of 38.6 months (95% CI, 26.2–not reached). The median PFS was 8.1 months (95% CI, 7.1–11.3) and OS 12.8 months (95% CI, 10.3–20.4). Patients received chemotherapy for a median of five cycles (range, 1–6), and trastuzumab therapy was maintained for a median of 6.3 months (range, 1–74).
Development of predictive models for PFS and OS
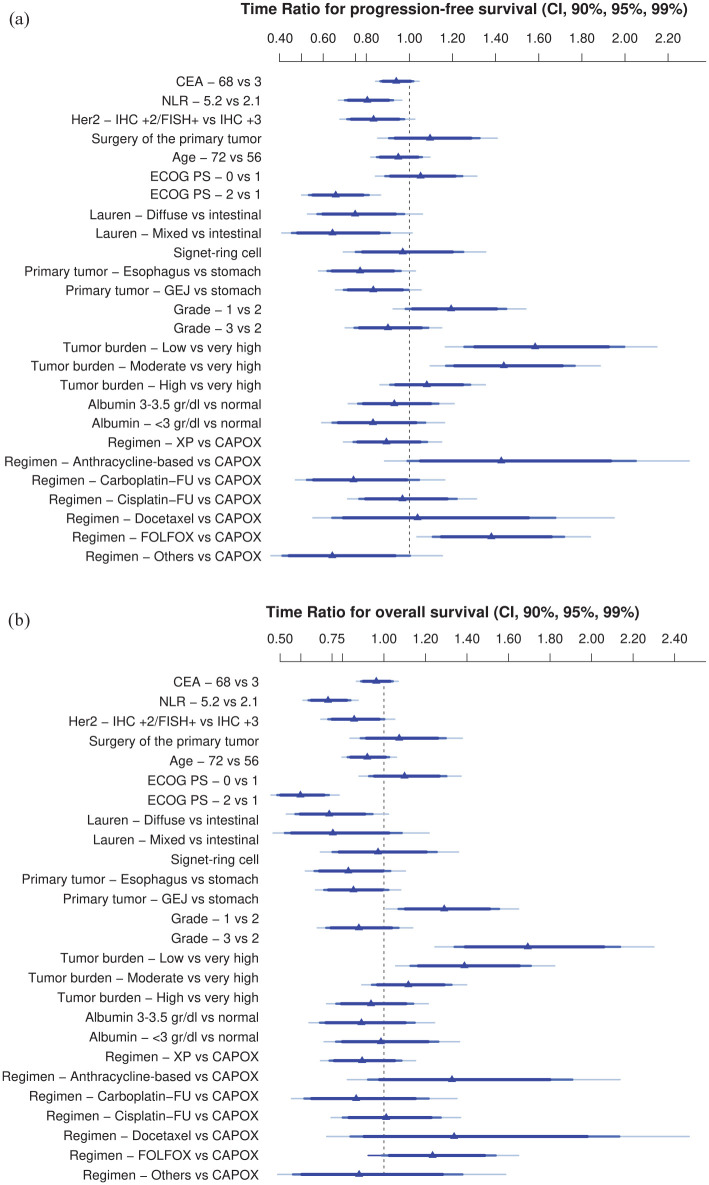
AFT models to predict PFS and OS were fitted with all candidate covariates. The exponentiated coefficients (TRs) are shown in Figure 1 and Table 3. For continuous variables, the contrasts show interquartile effects. The full model with all coefficients is shown in Supplemental Annex Table 3.
Figure 1.
Effect of AGAMENON-HER2 covariates on PFS (a) and OS (b).
Adjusted TRs are derived from a multivariable log-normal AFT model and represent its exponentiated coefficients (Table 3). Interpretation of the adjusted TRs: TR > 1 means that an increase in the value of the covariate is associated with longer survival. TR < 1 means that an increase in the value of the covariate is associated with shorter survival. The criteria for overall tumour burden are specified in Table 1.
AFT, accelerated failure time; CAPOX, capecitabine/oxaliplatin; CEA, carcinoembryonic antigen; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridisation; FOLFOX, 5-fluorouracil/oxaliplatin; GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor-2; IHC, immunohistochemistry; OS, overall survival; PFS, progression-free survival; TRs, time ratios; XP, capecitabine/cisplatin.
Table 3.
AFT models to predict survival-based endpoints.
| Variables | TR for PFS (95% CI) | TR for OS (95% CI) |
|---|---|---|
| CEA, 68 versus 3 | 0.93 (0.86–1.01) | 0.96 (0.88–1.04) |
| NLR, 5.2 versus 2.1 | 0.80 (0.70–0.92) | 0.73 (0.63–0.83) |
| ECOG PS, 0 versus 1 | 1.05 (0.88–1.24) | 1.09 (0.92–1.30) |
| ECOG PS, 2 versus 0 | 0.65 (0.53–0.81) | 0.59 (0.48–0.73) |
| Lauren subtype, diffuse versus intestinal | 0.74 (0.57–0.97) | 0.73 (0.57–0.94) |
| Lauren subtype, mixed versus intestinal | 0.64 (0.45–0.90) | 0.75 (0.52–1.08) |
| HER2, IHC +2/FISH+ versus IHC +3 | 0.83 (0.71–0.97) | 0.85 (0.73–1.00) |
| Signet-ring cells | 0.96 (0.75–1.25) | 0.97 (0.75–1.25) |
| Surgery of the primary tumour | 1.09 (0.90–1.32) | 1.07 (0.88–1.29) |
| Location, GEJ versus stomach | 0.83 (0.69–0.99) | 0.85 (0.71–1.03) |
| Location, oesophagus versus stomach | 0.77 (0.61–0.95) | 0.82 (0.66–1.03) |
| Histological grade, 3 versus 2 | 0.90 (0.74–1.08) | 0.87 (0.72–1.07) |
| Histological grade, 1 versus 2 | 1.19 (0.98–1.44) | 1.29 (1.07–1.55) |
| Age, 72 versus 56 | 0.94 (0.84–1.05) | 0.82 (0.69–1.02) |
| Tumour burden, low versus very high | 1.58 (1.25–1.99) | 1.69 (1.34–2.13) |
| Tumour burden, moderate versus very high | 1.43 (1.16–1.76) | 1.38 (1.12–1.70) |
| Tumour burden, high versus very high | 1.08 (0.91–1.28) | 1.11 (0.94–1.32) |
| Albumin 3–3.5 g/dL versus normal | 0.92 (0.76–1.13) | 0.93 (0.77–1.14) |
| Albumin <3 g/dL versus normal | 0.83 (0.64–1.07) | 0.89 (0.69–1.14) |
| Anthracycline-based regimens versus CAPOX | 1.42 (0.99–2.05) | 1.32 (0.92–1.90) |
| Carboplatin–5FU versus CAPOX | 0.74 (0.52–1.04) | 0.87 (0.61–1.22) |
| Cisplatin–5FU versus CAPOX | 0.96 (0.76–1.21) | 1.01 (0.80–1.27) |
| Docetaxel-containing regimens versus CAPOX | 1.03 (0.64–1.67) | 1.33 (0.83–2.13) |
| FOLFOX versus CAPOX | 1.38 (1.10–1.71) | 1.23 (0.99–1.53) |
| Others versus CAPOX | 0.64 (0.41–1.00) | 0.88 (0.56–1.37) |
| XP versus CAPOX | 0.89 (0.73–1.08) | 0.89 (0.73–1.08) |
The criteria for overall tumour burden are specified in Table 1. Interpretation of adjusted TRs: TR > 1 means that an increase in the value of the covariate is associated with longer survival; TR < 1 means that an increase in the value of the covariate is associated with shorter survival. Adjusted TRs are derived from a multivariable log-normal AFT models and represent its exponentiated coefficients.
5FU, 5-fluorouracil; AFT, accelerated failure time; CAPOX, capecitabine/oxaliplatin; CEA, carcinoembryonic antigen; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridisation; FOLFOX, 5-fluorouracil/oxaliplatin; GEJ, gastroesophageal junction; IHC, immunohistochemistry; OS, overall survival; PFS, progression-free survival; TR, time ratio; XP, capecitabine/cisplatin.
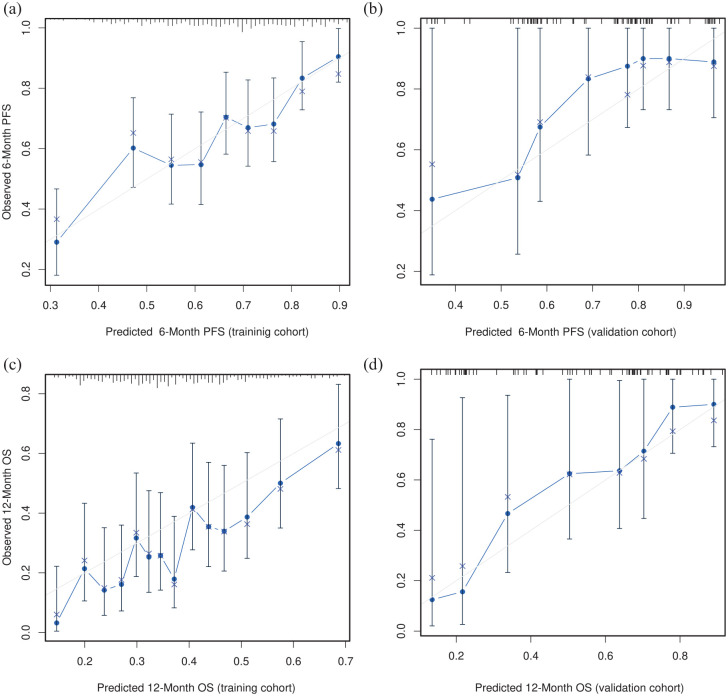
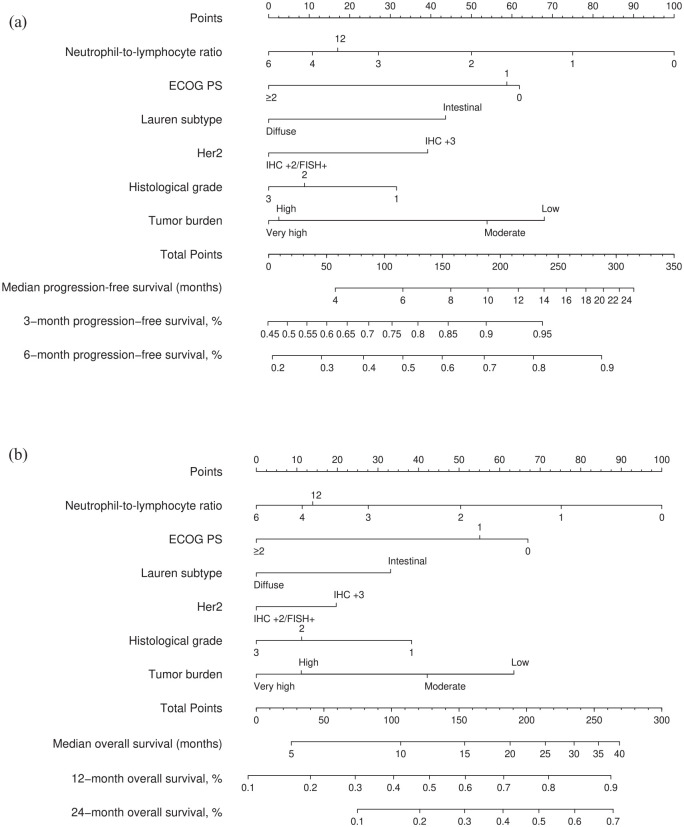
The log-normal parametric survival time model adequately fitted the data to the most relevant prognostic factors (Supplemental Annex Figure 2). The PFS model has a bias-corrected c-index of 0.606 (95% CI, 0.578–0.636) and is well calibrated (Figure 2(a)). The OS model has a bias-corrected c-index of 0.623 (95% CI, 0.594–0.655), and is well calibrated at various time points (Figure 2(c) and Supplemental Annex Figure 3). As these models are too complex for use in clinical practice, they were simplified using Lawless and Singhal’s factor reduction algorithm. The simplified models contain six predictors (ECOG PS, HER2 status, Lauren subtype, histological grade, overall tumour burden and NLR). These covariates have the highest correlation with OS in the Somers’ Dxy rank correlations so that omission of the remaining variables does not substantially affect the discrimination indices (Supplemental Annex Figure 4). The NLR showed a clear non-linear behaviour with worse prognosis at higher values, up to a value of 8 (see Supplemental Annex Figure 5). None of the outliers (NLR > 8) were due to errors, steroid use or obvious infections. The discrimination indices in the simplified model (0.610 and 0.635 for PFS and OS, respectively) are comparable to those of the complete model. The simplified models for PFS and OS have been graphically represented as nomograms (Figure 3(a) and (b)), and a web-based calculator has been designed to access the predictions conveniently (https://www.prognostictools.es/her2/inicio.aspx). The underlying equations are specified in Supplemental Annex Figure 6.
Figure 2.
Calibration curves in the training cohort for PFS and OS. The term ‘predicted’ means the probability of PFS/OS at fixed points of time, whereas observed refers to the Kaplan–Meier survival estimate stratified by intervals. (a) 6-month PFS in the AGAMENON-SEOM cohort. (b) 6-Month PFS in the Christie Hospital cohort. (c) 12-month OS in the AGAMENON-SEOM cohort. (d) 12-month OS in the Christie Hospital cohort.
OS, overall survival; PFS, progression-free survival.
Figure 3.
AGAMENON-HER2 nomograms for PFS (a) and OS (b).
ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridisation; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; OS, overall survival; PFS, progression-free survival.
External validation
The AGAMENON-HER2 nomograms were validated in an independent series. In this external validation cohort, the models retained adequate calibration (Figure 2(b) and (d) shows the calibration plots for the PFS and OS predictor models, respectively). The models allow for adequate discrimination with a c-index of 0.650 and 0.683 for PFS and OS, respectively. In the testing cohort, the NLR variable shows the same non-linear trend seen in the training cohort (Supplemental Annex Table 4).
Discussion
This study describes the development and external validation of a model predictive of survival-based endpoints in individuals with AGA treated with trastuzumab-based therapy. This tool was needed because of the significant variation in outcomes seen in AGA patients treated with HER2-directed therapy.13 To our knowledge, the AGAMENON-HER2 model is the first tool developed to make individualised predictions and stratify subjects with AGA treated with HER2-targeted therapies and chemotherapy. The model has been externally validated in a sample of consecutive patients, achieving adequate calibration and discrimination capacity. The resulting models assign an estimate of PFS and OS based on the additive effect of key clinicopathological variables in these patients.
All the covariates used are known, and feature in other models for prognosis in HER2-negative AGA.13 The contribution of NLR is known in AGA29 and other advanced malignancies, reflecting the presence of a systemic proinflammatory state.30,31 It should be noted that the effect on survival-based endpoints is non-linear, with a threshold between 6 and 8, beyond which successive increases are not associated with worse prognosis. The non-linear association with various endpoints has been described in oncology and non-oncology patients in various settings,19,32,33 but does not occur in all studies.30 The impact of tumour histology on prognosis is another aspect to consider. Most HER2+ AGA usually have a well or moderately differentiated intestinal histological subtype.11 However, about 19% of HER2+ neoplasms in our series (both AGAMENON-SEOM and Christie hospital) have a diffuse histological component. The presence of diffuse histology and poor differentiation of HER2+ AGA, although infrequent, has been documented and consistently associated with a worse prognosis.34 As there are no definitive criteria for classification our model does not consider intratumoural heterogeneity of HER2 expression a known prognostic factor and incorporation of this in future developments would be important.35,36 In our series, IHC 2+/FISH+ tumours had worse PFS/OS, in line with the rest of the literature.37–40 A novel variable in our model is the incorporation of overall tumour burden. In the absence of pre-established criteria, we resorted to a classification previously defined by our group.22 The observed results from our current and previous studies, including the capacity to stratify the participants in the Manchester series, suggest that these criteria are valid.
Regarding the external validity of our nomogram, there are several key points to consider. First, the use of alternative regimens to the ToGA trial is common in this and other contemporary series, which may subtly modify the survival results of HER2-directed therapy.19,41 To our knowledge, the contribution of the chemotherapy backbone has not been evaluated in any comparative research. However, this impact is not expected to be relevant in a recommended setting of using platinum- and fluoropyrimidine-based chemotherapy combinations, as is the case in more than 95% of patients in our cohorts. Second, the optimal duration of chemotherapy and maintenance with trastuzumab-associated fluoropyrimidine, a strategy used in patients in this series, remain uncertain.42 In the ToGA trial, chemotherapy was administered for six cycles, with trastuzumab maintained thereafter until progression.5 In phase II clinical trials not stratified by HER2-status, there appears to be no substantial difference between stop & go or maintenance strategies.43,44 However, a previous analysis of the AGAMENON-SEOM registry suggested that maintenance with fluoropyrimidines and trastuzumab might be useful when the chemotherapy backbone was FOLFOX.19,42 Third, although the ToGA trial did not include them, the benefit of anti-HER2 therapies has been extrapolated to distal oesophageal adenocarcinoma and is supported by retrospective data analyses.45,46 As a real-world prospective data collection, our database incorporates the diversity seen in clinical practice supporting the validity of our nomogram in a broad range of settings. This is confirmed by the similar performance of our model in the AGAMENON cohort and the Christie NHS Foundation Trust validation cohort despite the diversity in tumour subtypes and chemotherapy regimen. Fourth, given that the model was developed on a sample of Western patients, its external validation in broader populations (e.g. Asian) must be confirmed. Indeed, the results observed here are in line with the findings of trials conducted in our setting,47 although discreetly lower than those reported in other studies performed in predominantly Asian countries.41
Our study has several limitations, the most obvious being its retrospective nature, with the obvious risk of bias. Although the data have been reviewed and are consistent across two independent series, they do not entirely exclude the possibility of systematic errors. Recent studies have demonstrated the existence of remarkable molecular heterogeneity within HER2-positive tumours, which could substantially influence the outcome of HER2-targeting therapies.48–55 The introduction of new HER2-targeted therapies may influence the prognosis of these patients. The development of future HER2-testing strategies (i.e. next-generation sequencing, liquid biopsy) could affect the applicability of the nomogram. While the model captures the most general predictors, it is necessary to be aware of infrequent risk factors that could have a decisive influence on the evolution of some patients. In particular, the criteria for classifying the overall tumour burden are empirical and need further validation, although a sensitivity analysis suggests that the classification method is promising. Moreover, the model has been established with a sample of patients treated with trastuzumab and chemotherapy, so its validity should be confirmed with the addition of PD-1 targeting drugs and/or new anti-HER2 therapies.7–9,56 This model captures a mix of covariates associated with general functional status, proinflammatory levels, histopathological diversity and tumour burden, so we believe it will continue to be useful as regimens for HER2-positive AGA evolve. While other tumour inflammatory signature-related parameters, as well as immune-related prognostic biomarkers (e.g. programmed cell death ligand 1 pathway, tumour infiltrating lymphocytes, interleukins) have demonstrated an emerging prognostic role, these determinations are not yet available for most of the cases included in this registry. Regardless, it may prove difficult to uncouple the possible prognostic effect of these biomarkers from the use of immunotherapy in the future.57 The c-index of this model (0.650), while relatively low, is deemed acceptable within the context of a well-calibrated model that accurately predicts the probability of failure at specific time points.58 Although the data have been validated in an independent series, the reader must bear in mind the uncertainty in a relatively small sample when estimating performance indices. Finally, the reader should be aware that our analysis does not provide insight into the per se benefit of trastuzumab-based chemotherapy, although the model is able to identify subgroups with poor survival expectations, which should be a priority in clinical research for a more personalised approach. As for applicability, this model can in no way be used to choose treatment strategies in first line other than the ones used in the derivation series, with all participants receiving trastuzumab and polychemotherapy.
In conclusion, the AGAMENON-SEOM-HER2 evidence-based prognostic tool is able to predict survival-based endpoints in patients with HER2+ AGA receiving trastuzumab-based treatment. This nomogram may be useful when stratifying patients with HER2+ AGA in future trials. Furthermore, evidence-based knowledge about prognosis could be useful as part of the discussion that entails decision-making in daily clinical practice during the course of anti-HER2 therapy (conversations with the patient about initiating drugs, assessing a clinical trial, multidisciplinary committee deliberations regarding locoregional therapies, how long to administer the therapy, response evaluation timing, ICU management in individuals having a better prognosis, etc.).
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-4-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-5-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors would like to thank the investigators of the AGAMENON study, Sofía Carmona Mantilla for her contribution with authorships, and Natalia Cateriano, Miguel Vaquero, IRICOM S.A. for supporting the registry website. The AGAMENON-SEOM registry of advanced gastroesophageal adenocarcinoma pertains to the Spanish Society of Medical Oncology (SEOM).
Footnotes
ORCID iDs: Nieves Martínez Lago  https://orcid.org/0000-0002-0408-6871
https://orcid.org/0000-0002-0408-6871
Vilma Pacheco Barcia  https://orcid.org/0000-0003-0141-1306
https://orcid.org/0000-0003-0141-1306
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Paula Jimenez-Fonseca, Medical Oncology Department, Hospital Universitario Central de Asturias, ISPA, Oviedo, Spain.
Victoria Foy, Department of Medical Oncology, Christie Hospital, Manchester, UK.
Sophie Raby, Department of Medical Oncology, Christie Hospital, Manchester, UK.
Alberto Carmona-Bayonas, Medical Oncology Department, Hospital Universitario Morales Meseguer, Calle Marqués de los Vélez, s/n, Murcia 30007, Spain.
Lola Macía-Rivas, Pharmacy Department, Hospital Universitario Central de Asturias, Oviedo, Spain.
Virginia Arrazubi, Medical Oncology Department, Complejo Hospitalario de Navarra, IdiSNA (Navarra Institute for Health Research), Pamplona, Spain.
Diego Cacho Lavin, Medical Oncology Department, Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain.
Raquel Hernandez San Gil, Medical Oncology Department, Hospital Universitario de Canarias, Tenerife, Spain.
Ana Custodio, Medical Oncology Department, Hospital Universitario La Paz, CIBERONC CB16/12/00398, Madrid, Spain.
Juana María Cano, Medical Oncology Department, Hospital General Universitario de Ciudad Real, Ciudad Real, Spain.
Ana Fernández Montes, Medical Oncology Department, Complejo Hospitalario de Orense, Orense, Spain.
Oriol Mirallas, Medical Oncology Department, Hospital Universitario Vall d’Hebron, Vall Hebron Institute of Oncology (VHIO), Barcelona, Spain.
Ismael Macias Declara, Medical Oncology Department, Hospital Universitario Parc Tauli, Sabadell, Spain.
Rosario Vidal Tocino, Medical Oncology Department, Complejo Asistencial Universitario de Salamanca-IBSAL, Salamanca, Spain.
Laura Visa, Medical Oncology Department, Hospital Universitario El Mar, Barcelona, Spain.
María Luisa Limón, Medical Oncology Department, Hospital Universitario Virgen del Rocío, Sevilla, Spain.
Paola Pimentel, Medical Oncology Department, Hospital General Universitario Santa Lucía, Cartagena, Spain.
Nieves Martínez Lago, Medical Oncology Department, Complejo Hospitalario Universitario de A Coruña, A Coruña, Spain.
Tamara Sauri, Medical Oncology Department, Hospital Clinic, IDIBAPS, Barcelona, Spain.
Marta Martín Richard, Medical Oncology Department, Catalan Institute of Oncology, Barcelona, Spain.
Monserrat Mangas, Medical Oncology Department, Hospital Galdakao-Usansolo, Usansolo, Spain.
Mireia Gil Raga, Medical Oncology Department, Hospital General Universitario de Valencia, Valencia, Spain.
Aitana Calvo, Medical Oncology Department, Hospital Universitario Gregorio Marañón, Madrid, Spain.
Pablo Reguera, Medical Oncology Department, Hospital Universitario Ramón y Cajal, Madrid, Spain.
Mónica Granja, Medical Oncology Department, Hospital Universitario Clínico San Carlos, Madrid, Spain.
Alfonso Martín Carnicero, Medical Oncology Department, Hospital San Pedro, Logroño, Spain.
Carolina Hernández Pérez, Medical Oncology Department, Hospital Universitario Nuestra Señora de the Candelaria, Santa Cruz de Tenerife, Spain.
Paula Cerdá, Medical Oncology Department, Hospital Universitario Santa Creu i Sant Pau, Barcelona, Spain.
Lucía Gomez Gonzalez, Medical Oncology Department, Hospital General Universitario de Alicante, Alicante, Spain.
Francisco Garcia Navalon, Medical Oncology Department, Hospital Universitario Son Llatzer, Mallorca, Spain.
Vilma Pacheco Barcia, Medical Oncology Department, Hospital Universitario de Torrejón, Madrid, Spain.
David Gutierrez Abad, Medical Oncology Department, Hospital Universitario de Fuenlabrada, Madrid, Spain.
Maribel Ruiz Martín, Medical Oncology Department, Hospital Universitario Rio Carrión de Zamora, Zamora, Spain.
Jamie Weaver, Department of Medical Oncology, Christie Hospital/University of Manchester, Manchester, UK.
Wasat Mansoor, Department of Medical Oncology, Christie Hospital/University of Manchester, Manchester, UK.
Javier Gallego, Medical Oncology Department, Hospital General Universitario of Elche, Elche, Spain.
Declarations
Ethics approval and consent to participate: This study was performed in accordance with the ethical standards of the Good Clinical Practice guidelines and the Declaration of Helsinki and its subsequent amendments. This observational, non-interventional trial was approved by the Research Ethics Committee of the Principality of Asturias and by the Spanish Agency of Medicines and Medical Devices (AEMPS) (EFP-AGA-2014-01). All patients signed written informed consent.
Consent for publication: Informed consent and approval by the competent national authorities includes permission for publication and dissemination of the data.
Author contribution(s): Paula Jimenez-Fonseca: Conceptualisation; Data curation; Formal analysis; Investigation; Project administration.
Victoria Foy: Validation; Writing – review & editing.
Sophie Raby: Validation; Writing – review & editing.
Alberto Carmona-Bayonas: Conceptualisation; Formal analysis; Investigation; Methodology; Software; Validation; Visualisation; Writing – original draft; Writing – review & editing.
Lola Macía-Rivas: Conceptualisation.
Virginia Arrazubi: Conceptualisation; Writing – original draft; Writing – review & editing.
Diego Cacho Lavin: Conceptualisation.
Raquel Hernandez San Gil: Conceptualisation.
Ana Custodio: Investigation; Validation.
Juana María Cano: Conceptualisation.
Ana Fernández Montes: Investigation.
Oriol Mirallas: Conceptualisation.
Ismael Macias Declara: Investigation; Methodology.
Rosario Vidal Tocino: Investigation.
Laura Visa: Investigation.
María Luisa Limón: Investigation.
Paola Pimentel: Investigation.
Nieves Martínez Lago: Investigation.
Tamara Sauri: Investigation.
Marta Martín Richard: Investigation.
Monserrat Mangas: Investigation.
Mireia Gil Raga: Conceptualisation.
Aitana Calvo: Investigation.
Pablo Reguera: Conceptualisation.
Mónica Granja: Investigation.
Alfonso Martín Carnicero: Investigation.
Carolina Hernández Pérez: Investigation.
Paula Cerdá: Investigation.
Lucía Gomez Gonzalez: Investigation.
Francisco Garcia Navalon: Investigation.
Vilma Pacheco Barcia: Investigation.
David Gutierrez Abad: Investigation.
Maribel Ruiz Martín: Investigation.
Jamie Weaver: Investigation.
Wasat Mansoor: Investigation; Validation.
Javier Gallego: Conceptualisation; Funding acquisition; Investigation; Methodology; Project administration.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The database is available through a centralised web platform: http://www.agamenonstudy.com/. The details of analyses used in the current study are available from the first author or corresponding author upon request.
Code availability: The R Code is available upon request to the authors.
References
- 1. SEOM. Cifras del cáncer en España [Internet], https://seom.org/seomcms/images/stories/recursos/Cifras_del_cancer_2020.pdf (2020, accessed December 2020)
- 2. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deng N, Goh LK, Wang H, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012; 61: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiménez-Fonseca P, Carmona-Bayonas A, Sánchez Lorenzo ML, et al. Prognostic significance of performing universal HER2 testing in cases of advanced gastric cancer. Gastric Cancer 2017; 20: 465–474. [DOI] [PubMed] [Google Scholar]
- 5. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697. [DOI] [PubMed] [Google Scholar]
- 6. Satoh T, Bang Y-J, Gotovkin EA, et al. Quality of life in the trastuzumab for gastric cancer trial. Oncologist 2014; 19: 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Cutsem E, Di Bartolomeo M, Smyth E, et al. LBA55 primary analysis of a phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable or metastatic gastric or gastroesophageal junction (GEJ) cancer who progressed on or after a trastuzu. Ann Oncol 2021; 32: S1332. [Google Scholar]
- 8. Shitara K, Seraj J, Franke FA, et al. 1436TiP trastuzumab deruxtecan (T-DXd) in patients (Pts) with HER2-positive gastric cancer (GC) or gastroesophageal junction (GEJ) adenocarcinoma who have progressed on or after a trastuzumab-containing regimen (DESTINY-gastric04, DG-04): a randomized pha. Ann Oncol 2021; 32: S1073. [Google Scholar]
- 9. Chung HC, Bang Y-J, Fuchs CS, et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Futur Oncol 2021; 17: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janjigian YY, Kawazoe A, Yanez PE, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: initial findings of the global phase 3 KEYNOTE-811 study. 2021 ASCO Annual Meeting, Wolters Kluwer Health, 2021. [Google Scholar]
- 11. Wang H-B, Liao X-F, Zhang J. Clinicopathological factors associated with HER2-positive gastric cancer: a meta-analysis. Medicine (Baltimore) 2017; 96: e8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marshall S, Wakatsuki T, Takahari D, et al. Prognostic factors in patients with advanced HER2-positive gastric cancer treated with trastuzumab-based chemotherapy: a cohort study. J Gastrointest Cancer. Epub ahead of print April 2022. DOI: 10.1007/s12029-022-00815-1. [DOI] [PubMed] [Google Scholar]
- 13. Custodio A, Carmona-Bayonas A, Jiménez-Fonseca P, et al. Nomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumab. Br J Cancer 2017; 116: 1526–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Narita Y, Kadowaki S, Oze I, et al. Establishment and validation of prognostic nomograms in first-line metastatic gastric cancer patients. J Gastrointest Oncol 2018; 9: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takahari D, Boku N, Mizusawa J, et al. Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912. Oncologist 2014; 19: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004; 22: 2395–2403. [DOI] [PubMed] [Google Scholar]
- 17. Carmona-Bayonas A, Jiménez-Fonseca P, Garrido M, et al. Multistate models: accurate and dynamic methods to improve predictions of thrombotic risk in patients with cancer. Thromb Haemost 2019; 119: 1849–1859. [DOI] [PubMed] [Google Scholar]
- 18. Cotes Sanchís A, Gallego J, Hernandez R, et al. Second-line treatment in advanced gastric cancer: data from the Spanish AGAMENON registry. PLoS One 2020; 15: e0235848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jimenez-Fonseca P, Carmona-Bayonas A, Martinez-Torron A, et al. External validity of clinical trials with diverse trastuzumab-based chemotherapy regimens in advanced gastroesophageal adenocarcinoma: data from the AGAMENON-SEOM registry. Ther Adv Med Oncol 2021; 13: 17588359211019672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carmona-Bayonas A, Jiménez-Fonseca P, Gallego J, et al. Causal considerations can inform the interpretation of surprising associations in medical registries. Cancer Invest 2022; 40: 1–13. [DOI] [PubMed] [Google Scholar]
- 21. Harrell F. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd ed. New York: Springer, 2015. [Google Scholar]
- 22. Gallego-Plazas J, Arias-Martinez A, Arrazubi V. Sex and gender disparities in patients with advanced gastroesophageal adenocarcinoma: data from AGAMENON-SEOM registry. ESMO Open; 2000; 7(3): 100514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris TP, White IR, Royston P. Tuning multiple imputation by predictive mean matching and local residual draws. BMC Med Res Methodol 2014; 14: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orbe J, Ferreira E, Núñez-Antón V. Comparing proportional hazards and accelerated failure time models for survival analysis. Stat Med 2002; 21: 3493–3510. [DOI] [PubMed] [Google Scholar]
- 25. Lawless JF, Singhal K. Efficient screening of nonnormal regression models. Biometrics 1978; 34: 318–327. [Google Scholar]
- 26. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 27. Harrell F, Jr, Frank E, Maintaner Frank E. Package ‘rms’ [Internet], http://cran.r-project.org/web/packages/rms/index.html (2015, accessed January 2020)
- 28. Harrell F., Jr. Package ‘Hmisc’ [Internet], https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf (2015, accessed January 2023). Harrell FE, Jr, Dupont MC. The Hmisc package. R package version. 2006, p. 3.
- 29. Park J-H, Yeo JH, Kim YS, et al. Predictive roles of HER2 gene amplification and neutrophil-to-lymphocyte ratio on survival in HER2-positive advanced gastric cancer treated with trastuzumab-based chemotherapy. Am J Clin Oncol 2021; 44: 232–238. [DOI] [PubMed] [Google Scholar]
- 30. Carmona-Bayonas A, Jiménez-Fonseca P, Lamarca Á, et al. Prediction of progression-free survival in patients with advanced, well-differentiated, neuroendocrine tumors being treated with a somatostatin analog: the getne-trasgu study. J Clin Oncol 2019; 37: 2571–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernández Montes A, Carmona-Bayonas A, Jimenez-Fonseca P, et al. Prediction of survival in patients with advanced, refractory colorectal cancer in treatment with trifluridine/tipiracil: real-world vs clinical trial data. Sci Rep 2021; 11: 14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawano T, Sasaki T, Gon Y, et al. High neutrophil/lymphocyte ratio at cancer diagnosis predicts incidence of stroke in cancer patients. Brain Commun 2021; 3: fcab071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Yuan M, Ma Y, et al. The admission (neutrophil+ monocyte)/lymphocyte ratio is an independent predictor for in-hospital mortality in patients with acute myocardial infarction. Front Cardiovasc Med 2022; 9: 870176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu C, Liu Y, Jiang D, et al. Poor efficacy response to trastuzumab therapy in advanced gastric cancer with homogeneous HER2 positive and non-intestinal type. Oncotarget 2017; 8: 33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaito A, Kuwata T, Tokunaga M, et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J Clin Cases 2019; 7: 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wakatsuki T, Yamamoto N, Sano T, et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J Gastroenterol 2018; 53: 1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bang K, Cheon J, Park YS, et al. Association between HER2 heterogeneity and clinical outcomes of HER2-positive gastric cancer patients treated with trastuzumab. Gastric Cancer 2022; 25: 794–803. [DOI] [PubMed] [Google Scholar]
- 38. Ock C-Y, Lee K-W, Kim JW, et al. Optimal patient selection for trastuzumab treatment in HER2-positive advanced gastric cancer. Clin Cancer Res 2015; 21: 2520–2529. [DOI] [PubMed] [Google Scholar]
- 39. Gomez-Martin C, Plaza JC, Pazo-Cid R, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol 2013; 31: 4445–4452. [DOI] [PubMed] [Google Scholar]
- 40. Xuan Q, Ji H, Tao X, et al. Quantitative assessment of HER2 amplification in HER2-positive breast cancer: its association with clinical outcomes. Breast Cancer Res Treat 2015; 150: 581–588. [DOI] [PubMed] [Google Scholar]
- 41. ter Veer E, Creemers A, de Waal L, et al. Comparing cytotoxic backbones for first-line trastuzumab-containing regimens in human epidermal growth factor receptor 2-positive advanced oesophagogastric cancer: a meta-analysis. Int J Cancer 2018; 143: 438–448. [DOI] [PubMed] [Google Scholar]
- 42. Viúdez A, Carmona-Bayonas A, Gallego J, et al. Optimal duration of first-line chemotherapy for advanced gastric cancer: data from the AGAMENON registry. Clin Transl Oncol 2019; 22: 734–750. [DOI] [PubMed] [Google Scholar]
- 43. Park SR, Kim M-J, Nam B-H, et al. A randomised phase II study of continuous versus stop-and-go S-1 plus oxaliplatin following disease stabilisation in first-line chemotherapy in patients with metastatic gastric cancer. Eur J Cancer 2017; 83: 32–42. [DOI] [PubMed] [Google Scholar]
- 44. Li W, Zhao X, Wang H, et al. Maintenance treatment of Uracil and Tegafur (UFT) in responders following first-line fluorouracil-based chemotherapy in metastatic gastric cancer: a randomized phase II study. Oncotarget 2017; 8: 37826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alvarez-Manceñido F, Jimenez-Fonseca P, Carmona-Bayonas A, et al. Is advanced esophageal adenocarcinoma a distinct entity from intestinal subtype gastric cancer? Data from the AGAMENON-SEOM Registry. Gastric Cancer 2021; 24: 926–936. [DOI] [PubMed] [Google Scholar]
- 46. Plum PS, Gebauer F, Krämer M, et al. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer 2019; 19: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grávalos C, Gómez-Martín C, Rivera F, et al. Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol 2011; 13: 179–184. [DOI] [PubMed] [Google Scholar]
- 48. Nakamura N, Kaida D, Tomita Y, et al. Intra-tumoral FGFR2 expression predicts prognosis and chemotherapy response in advanced HER2-positive gastric cancer patients. Cancer Diagn Progn 2022; 2: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang S, Zhao Y, Song Y, et al. ERBB2D16 expression in HER2 positive gastric cancer is associated with resistance to trastuzumab. Front Oncol 2022; 12: 855308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shimozaki K, Shinozaki E, Yamamoto N, et al. KRAS mutation as a predictor of insufficient trastuzumab efficacy and poor prognosis in HER2-positive advanced gastric cancer. J Cancer Res Clin Oncol. Epub ahead of print April 2022. DOI: 10.1007/s00432-022-03966-7. [DOI] [PubMed] [Google Scholar]
- 51. Lian J, Zhang G, Zhang Y, et al. PD-L1 and HER2 expression in gastric adenocarcinoma and their prognostic significance. Dig Liver Dis 2022; 54: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 52. Cho H, Ryu M-H, Lee HE, et al. Prognostic value of natural killer cell activity for patients with HER2+ advanced gastric cancer treated with first-line fluoropyrimidine–platinum doublet plus trastuzumab. Cancer Immunol Immunother 2022; 71: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bermúdez A, Arranz-Salas I, Mercado S, et al. Her2-positive and microsatellite instability status in gastric cancer—clinicopathological implications. Diagnostics 2021; 11: 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang J, Wang S, Sun J, et al. Expression of c-MET, EGFR and HER-2 in gastric adenocarcinoma tissue and its relationship with clinicopathological characteristics. Am J Transl Res 2021; 13: 10856. [PMC free article] [PubMed] [Google Scholar]
- 55. Yokoyama D, Hisamori S, Deguchi Y, et al. PTEN is a predictive biomarker of trastuzumab resistance and prognostic factor in HER2-overexpressing gastroesophageal adenocarcinoma. Sci Rep 2021; 11: 9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu M, Meng X, Lu Y, et al. Efficacy and safety of camrelizumab in combination with trastuzumab and chemotherapy as the first-line treatment for patients with HER2-positive advanced gastric cancer. J Gastrointest Oncol 2022; 13: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang C, Liu T, Wang J, et al. Development and verification of an immune-related gene prognostic index for gastric cancer. Sci Rep 2022; 12: 15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med 1999; 130: 515–524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-4-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-5-tam-10.1177_17588359231157641 for The AGAMENON-SEOM model for prediction of survival in patients with advanced HER2-positive oesophagogastric adenocarcinoma receiving first-line trastuzumab-based therapy by Paula Jimenez-Fonseca, Victoria Foy, Sophie Raby, Alberto Carmona-Bayonas, Lola Macía-Rivas, Virginia Arrazubi, Diego Cacho Lavin, Raquel Hernandez San Gil, Ana Custodio, Juana María Cano, Ana Fernández Montes, Oriol Mirallas, Ismael Macias Declara, Rosario Vidal Tocino, Laura Visa, María Luisa Limón, Paola Pimentel, Nieves Martínez Lago, Tamara Sauri, Marta Martín Richard, Monserrat Mangas, Mireia Gil Raga, Aitana Calvo, Pablo Reguera, Mónica Granja, Alfonso Martín Carnicero, Carolina Hernández Pérez, Paula Cerdá, Lucía Gomez Gonzalez, Francisco Garcia Navalon, Vilma Pacheco Barcia, David Gutierrez Abad, Maribel Ruiz Martín, Jamie Weaver, Wasat Mansoor and Javier Gallego in Therapeutic Advances in Medical Oncology