Abstract
Background
With nearly one-third of patients with major depressive disorder being resistant to available antidepressants, there is a need to develop new treatments for this population. Stellate ganglion block (SGB) is a procedure used to block sympathetic input to the central autonomic system; it has been administered to treat several conditions, including pain. Recently, indications for SGB have extended and the potential benefits for psychiatric disorders are under investigation.
Methods
The Local Injection For Treating Mood Disorders (LIFT-MOOD) study investigated the feasibility of a trial of 2 right-sided injections of bupivacaine 0.5% (7 mL) at the stellate ganglion in participants with treatment-resistant depression (TRD) using a randomized, placebo-controlled, pilot trial. Ten participants were randomized in a 1:1 allocation to receive active treatment or placebo (saline). Primary feasibility outcomes included recruitment rate, withdrawal, adherence, missing data, and adverse events. As a secondary, exploratory objective, we explored the efficacy of SGB in improving symptoms of depression by calculating the change in scores from baseline to follow-up on day 42 for each treatment group.
Results
The recruitment rate was reasonable and sufficient, retention and adherence were high, missing data were low, and adverse events were mild and temporary. Both treatment groups demonstrated decreases in Montgomery-Åsberg Depression Rating Scale scores, compared to baseline, by the end of the study.
Conclusion
This study supports the feasibility of a confirmatory trial of SGB in participants with TRD. Conclusions regarding efficacy cannot be made based on this preliminary study due to the small number of participants who completed active treatment. Larger-scale randomized controlled trials with long-term follow-ups and alternate sham procedures are needed to assess the efficacy and duration of symptom improvement with the use of SGB in TRD.
Keywords: treatment-resistant depression, depression, stellate ganglion block, sympathetic block, stellate ganglion, anesthesia
Introduction
Psychiatric disorders are responsible for almost one-quarter of the global burden of diseases, with major depressive disorder (MDD) being the foremost contributor.1 Due to the heterogeneous nature of MDD and its associated neurobiology, nearly one-third of patients remain resistant to available antidepressants.2 New treatments and medications are being developed; however, despite the effective initial outcomes, some interventions do not show significantly more benefits than placebo in phase II and III trials.3 The repurposing approach entails recognizing novel off-target medication effects, developing the drug for a new indication where the secondary target is pertinent, and determining the applicability of a known drug to a new disease.4
Pain and depression have similar symptoms such as attentional biases, adverse mood effects, and an increased tendency to apply associations with negative content.5 Additionally, among subjects with chronic pain, poor functioning and quality of life are common,6 which are also reported in epidemiological and clinical studies of MDD.7 The relationship between chronic pain and depression, besides the shared biomarkers,8 suggests that similar treatments can work for both disorders.9 Indeed, many treatments for pain conditions, such as neuropathic pain, have their primary indication as treatments for depression, including tricyclic antidepressants and selective serotonin-norepinephrine reuptake inhibitors.10 Sympathetic blocks have been utilized for pain relief since the early 1900s.11 The inputs from the sympathetic ganglia have been implicated in the pathophysiology of various conditions, including but not limited to complex regional pain syndrome and cancer pain of different origins.12 The stellate ganglion block (SGB) is an effective method to temporarily block sympathetic input to the central autonomic network in the brain, head, face, and arm.13 The effect of sympathetic blocks in these conditions usually lasts longer than the duration of the local anesthetic used, suggesting that blocked sympathetic transmission interrupts maladaptive feedback circuits and decreases central hyperexcitability.14
With the ability to alter inputs from the peripheral nervous system to the central nervous system effectively, indications for the SGB have been explored for conditions beyond pain13,15 (eg, cardiac and pulmonary conditions).16,17 The potential benefits of SGBs for psychiatric disorders, such as the anxiety associated with post-traumatic stress disorder (PTSD), are under investigation.14,18,19 Right-sided SGB can mitigate anxiety symptoms in PTSD by impacting the central autonomic network (including the amygdala, insular cortex, and hypothalamus) and the cardiovascular system.13 Besides anxiety, SGB has resulted in improvement in depression scores of patients with PTSD19 and has been used as an adjuvant to treatment in patients who have not fully responded to conventional therapies.12
The first reported use of SGB for psychiatric disorders occurred with the treatment of depression in 1947.20 In 1990, SGB was subsequently applied to the treatment of PTSD21, and to date, numerous studies have demonstrated promising outcomes for the condition.12,22 A recent review investigating the utility of SGB for PTSD, depression, and anxiety reported a lack of available evidence supporting the use of SGB to treat depression independent of PTSD.23 For instance, a 2014 presentation by Alkire et al24 provided evidence for the use of SGB in treating PTSD and depression symptoms. Thus, there is a need for trials assessing the efficacy of SGBs for MDD. A recently published preclinical study on 48 rodents demonstrated that an SGB exerted antidepressant-like effects on stress-induced depressive behaviors.25 Although this study has significant limitations, it bolsters the impetus to direct research toward critically assessing the utility of SGBs for depression. Since the therapeutic effect of SGB for depression, as a new potential intervention, has not been previously studied in a controlled trial in humans, our objective was to explore if SGB can be utilized in patients presenting with treatment-resistant depression (TRD). The current study, Local Injection For Treating Mood Disorders (LIFT-MOOD), aimed to evaluate the feasibility of a trial of bupivacaine injection in patients with TRD by estimating the rates of recruitment, withdrawal, adherence, missing data, and the number of adverse events. The secondary objective of LIFT-MOOD was to explore the efficacy of bupivacaine injection for depressive symptoms as an exploratory outcome. We hypothesized that this study would support the feasibility of a confirmatory trial of SGB in TRD.
Methods
Participants
The LIFT-MOOD study was conducted at St. Michael's Hospital, Unity Health Toronto. Recruitment began in June 2021, with randomization completed by February 2022. All participants were outpatients screened for study eligibility at the Centre for Depression and Suicide Studies at St. Michael's Hospital. Participants were included if they were between 18 and 65 years of age, had a diagnosis of MDD without psychotic features according to the Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition criteria, were in a major depressive episode as confirmed by the Mini International Neuropsychiatric Interview,26 and had a score of 18 or greater on the Hamilton Depression Rating Scale (HAMD).27 According to the definition of TRD, all participants had to have failed at least 2 trials of antidepressant therapy of an adequate dose and duration during the current depressive episode.28 Data regarding antidepressant history and current medication use were collected using the Antidepressant Treatment History Form.29 Participants were required to maintain their current antidepressant regimen 28 days before the screening visit and during the study. Highly effective or double-barrier contraceptive methods were used for women of childbearing potential.
Participants presenting with comorbidity of neuropsychological disorders (eg, PTSD, dementia) or with a diagnosis of depression secondary to medical illnesses were excluded. Participants were also excluded if they presented with acute suicidality or underwent electroconvulsive therapy within the current depressive episode. The full list of inclusion and exclusion criteria is presented in Supplemental Materials (Table S1). A research coordinator carried out all screening assessments. The Research Ethics Board of St. Michael's Hospital, Unity Health Toronto, approved the study (20-301). The trial is registered at http://clinicaltrials.gov (NCT04727229). All participants provided written informed consent after fully reviewing the study protocol and prior to participating.
Study Design
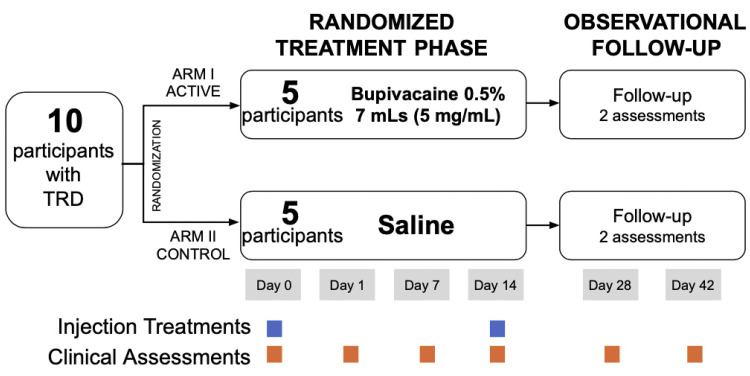
A double-blind, randomized, placebo-controlled pilot trial was conducted. Following baseline assessments, participants were randomized in a 1:1 allocation to either the active treatment or placebo (saline) group. An online random number generator was used to produce an allocation sequence list with random permuted blocks.30 The allocation list was sent to a research staff member from Research Pharmacy at St. Michael's Hospital who prepared the allocation packages containing either the study drug or placebo in an obscure paper bag. The physician opened the package once the participant entered the procedure room and subsequently prepared it for injection. Injections took place on days 0 and 14, with an additional 2 follow-up sessions on days 28 and 42 (Figure 1).
Figure 1.
Study design. TRD, treatment-resistant depression.
Dose and Drug Administration
The active intervention was bupivacaine 0.5% 7 mL (5 mg/mL) that was injected at the stellate ganglion on the right side of the neck. For both the active treatment and placebo arms, real-time ultrasonography was used. A 22G intravenous was started in the right hand while the participants were positioned supine on a stretcher, with their heads slightly turned away from the injection site. A preliminary scan was conducted using a high-frequency linear ultrasound probe to examine the anatomy and ensure no anatomic variability. Once the positioning of the stellate ganglion was confirmed, the skin was cleansed with a chlorhexidine solution, and a fenestrated drape was applied. The C6 tubercle and critical neurovascular structures were identified using ultrasound. Once optimal imaging was obtained, the skin was topicalized with lidocaine 1% 3 mL. A 40-mm echogenic needle was used with an in-plane approach, where the needle was advanced until its tip was identified anterolateral to the longus colli muscle, deep to the prevertebral fascia, and superficial to the fascia of the longus colli muscle. Once confirmed with hydrodissection and after negative aspiration, a solution of either bupivacaine 0.5% 7 mL (5 mg/mL) or saline 7 mL was injected, depending on the allocation assignment.
All participants were monitored in the same fashion post-procedure. Temperature was measured serially to look for a change from pre- to post-intervention. Participants were also monitored for symptoms of Horner's syndrome to confirm successful blockade. This information was not disclosed to the participant or the assessment team. The participant was discharged after 60 min of observation. For participant safety, the interventional team was unblinded. Their interactions with the participants were scripted and limited to the 2 interventions.
Study Outcomes Assessment
The primary outcome measures were the feasibility metrics, including the rates of recruitment, withdrawal, adherence, missing data, and the number of adverse events. Adverse events were assessed at each visit by a study nurse or research personnel. Secondary outcomes were continuous measures of change in depressive symptoms, anxiety, and quality of life from baseline to day 42 (last follow-up visit) as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS),31 Generalized Anxiety Disorder 7-item (GAD-7),32 Quick Inventory of Depressive Symptomatology - Self Report (QIDS-SR),33 and Quality-of-Life Enjoyment and Satisfaction (Q-LES-Q).34
Statistical Analyses
Tabulation and analysis were performed after all study-related data were collected, and the follow-up of all study subjects was complete. Descriptive statistics were used to report baseline characteristics, including frequencies and percentages for categorical variables and mean and standard deviation (SD), median and interquartile range (IQR), and range for continuous variables.
Primary feasibility outcomes were summarized using frequencies and percentages by treatment group. The recruitment rate was calculated based on the final number of randomized individuals divided by all individuals assessed for eligibility. The withdrawal rate was calculated using the number of randomized participants as the denominator, while the rates of adherence and missing data were calculated based on the number of participants who did not withdraw at the visit. Adverse events were reported for each treatment group at each visit.
Main efficacy outcomes were based on the change in the MADRS total score from day 0 to day 42 and were calculated as the difference between scores at these 2 time points for each treatment group. Other efficacy outcomes included scores obtained from all administered scales and were reported using mean, SD, median, and IQR obtained at each visit. Changes in the scores over time were visualized using spaghetti plots. Data were analyzed using R (version 4.2.0).
Results
The participant flow is illustrated in the CONSORT diagram presented in Supplemental Materials (Figure S1). Eighty-four participants were screened prior to eligibility assessment and 56 were assessed for eligibility. Of these 56, 46 were excluded because they did not meet inclusion criteria (n = 5), declined to participate (n = 23), were unable to be contacted (n = 4), or were not approached as full enrollment was neared (n = 14).
Ten eligible participants were randomized to either treatment group (n = 5 to SGB injection and n = 5 to placebo injection). Of the 10 participants randomized, 4 (40%) were females and 6 (60%) were males. The median age was 38 years old (IQR 38-48) in the SGB injection group and 53 years old (IQR 51-59) in the placebo injection group. The median of the total HAMD score at baseline was 20 (IQR 19-21) in the SGB injection group and 21 (IQR 20-23) in the placebo injection group (Table 1).
Table 1.
Baseline Characteristics of the Study Sample.
| Variable | SGB Injection | Placebo Injection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean/ N (%) | SD | Min | IQR | Median | Max | N | Mean/ N (%) | SD | Min | IQR | Median | Max | |
| Sex | 5 | 5 | ||||||||||||
| Female | 1 | 20% | 3 | 60% | ||||||||||
| Male | 4 | 80% | 2 | 40% | ||||||||||
| Age (years) | 5 | 43.2 | 9.8 | 34 | 38-48 | 38 | 58 | 5 | 51.6 | 10.7 | 34 | 51–59 | 53 | 61 |
| Weight (kg) | 5 | 69.6 | 6.6 | 59 | 68–74.8 | 71 | 75 | 5 | 83.4 | 22.6 | 51 | 77–104 | 79 | 106 |
| Height (cm) | 5 | 170.6 | 10.4 | 155 | 167–175 | 173 | 183 | 5 | 169.4 | 14.6 | 145 | 170–178 | 171 | 183 |
| HAMD score | 5 | 21 | 3.5 | 18 | 19–21 | 20 | 27 | 5 | 22.2 | 4.2 | 18 | 20–23 | 21 | 29 |
| Antidepressant use | ||||||||||||||
| SSRI | 0 | 0% | 1 | 20% | ||||||||||
| SNRI | 1 | 20% | 1 | 20% | ||||||||||
| TCA | 0 | 0% | 2 | 40% | ||||||||||
| MAOI | 1 | 20% | 0 | 0% | ||||||||||
| Atypical antidepressant | 2 | 40% | 0 | 0% | ||||||||||
| Atypical antipsychotic | 0 | 0% | 1 | 20% | ||||||||||
| Other | 3 | 60% | 1 | 20% | ||||||||||
| Comorbidities | ||||||||||||||
| Panic Disorder | 1 | 20% | 3 | 60% | ||||||||||
| Agoraphobia | 1 | 20% | 2 | 40% | ||||||||||
| Social phobia | 0 | 0% | 3 | 60% | ||||||||||
| Generalized Anxiety Disorder | 2 | 40% | 3 | 60% | ||||||||||
| Obsessive-compulsive Disorder | 0 | 0% | 1 | 20% | ||||||||||
| Anorexia | 0 | 0% | 1 | 20% | ||||||||||
| Bulimia | 0 | 0% | 0 | 0% | ||||||||||
Abbreviations: SGB, stellate ganglion block; IQR, interquartile range; HAMD, Hamilton Depression Rating Scale; SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin-norepinephrine reuptake inhibitors; TCA, tricyclic antidepressants; MAOI, monoamine oxidase inhibitors.
Primary Feasibility Outcomes
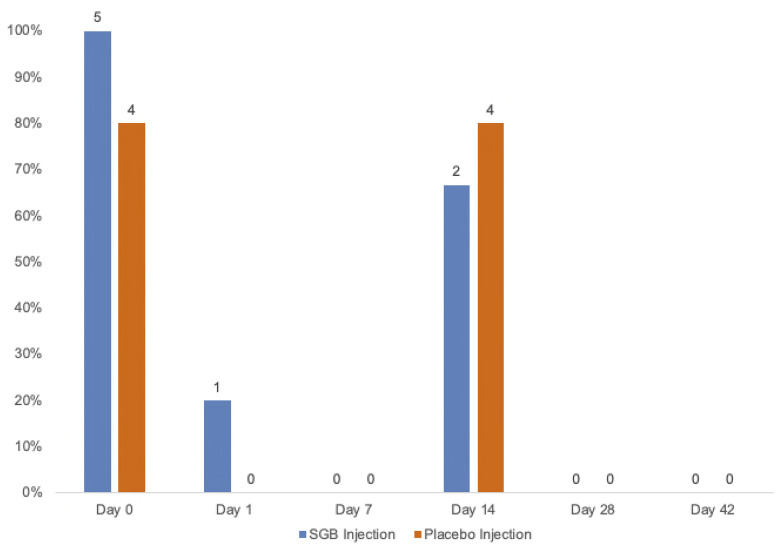
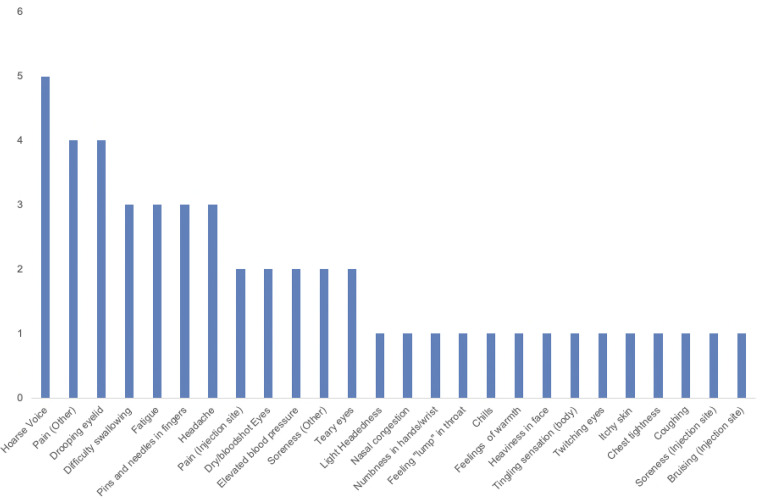
The overall recruitment rate was 18%, and the lost-to-follow-up rate was 0%. Two participants (40%) in the SGB injection group were withdrawn, 1 on day 1 due to a personal emergency that was unrelated to the study and 1 on day 7 due to a decline in mood. No participants were withdrawn from the placebo group. Adherence to study visits and to study medication were 100% for both groups. Of the entire dataset, data from 1 individual in the SGB injection group were missing for the QIDS-SR, GAD-7, and Q-LES-Q scores on day 28. This equates to 33% of data missing for those questionnaires in the SGB injection group on day 28. The number of participants who experienced at least 1 adverse event ranged from 0% (n = 0) to 80% (n = 4) in the placebo injection group and 0% (n = 0) to 100% (n = 5) in the SGB injection group over the course of the trial (Figure 2 and Table 2). Participants most commonly developed hoarse voice, followed by pain in body parts other than the injection site (eg, jaw, lower back, and neck) and drooping eyelid (Figure 3). Both groups showed no adverse events by day 28.
Figure 2.
Adverse event occurrences by group with percent and frequency counts. SGB, stellate ganglion block.
Table 2.
Adverse Events at Day 0 to Day 42 for Each Study Participant.
| Group (ID #) | Day 0 | Day 1 | Day 7 | Day 14 | Day 28 | Day 42 |
|---|---|---|---|---|---|---|
| SGB Injection | ||||||
| 1 | Dry and red eyes, hoarse voice, difficulty swallowing, light-headedness, fatigue | - | - | Hoarse voice, difficulty swallowing | - | - |
| 3 | Hoarse voice, drooping eyelid, pain/numbness (jaw), pain (low back) difficulty swallowing, nasal congestion | - | - | Hoarse voice, drooping eyelid, lump in throat feeling, pain (neck/shoulder), difficulty swallowing, nasal congestion, tearing eyes | - | - |
| 5 | Drooping eyelid, red eyes, tingling (body) | - | NA | NA | NA | NA |
| 6 | Elevated pre- and post-intervention blood pressure, hoarse voice, pain (neck/shoulder), pain (right arm), tingling (right arm), difficulty swallowing | Elevated blood pressure, pain (neck/shoulder/chest), difficulty swallowing, twitching eye | - | NA | NA | NA |
| 9 | Drooping eyelid, drowsiness, headache, tearing eyes | - | - | - | - | - |
| Placebo Injection | ||||||
| 2 | - | - | - | Tingling (hand/fingers) | - | - |
| 4 | Headache, tingling (fingers), chills/hot flashes, drowsy, pain (hip) | - | - | Pain (injection site), hoarse voice, chills, headache, heaviness in face, numbness (hands/wrist), soreness (neck) | - | - |
| 7 | Elevated pre-intervention blood pressure, bruising/soreness (injection site) | - | - | Bruising/soreness (injection site) | - | - |
| 8 | Hoarse voice, drooping eyelid, tingling (fingers), pain (injection site), itchy skin, pain/numbness (jaw) | - | - | Chest tightness, coughing, headache | - | - |
| 10 | Soreness (legs) | - | - | - | - | - |
Abbreviation: SGB, stellate ganglion block.
Figure 3.
Frequency of specific adverse events across all participants.
Efficacy Outcomes
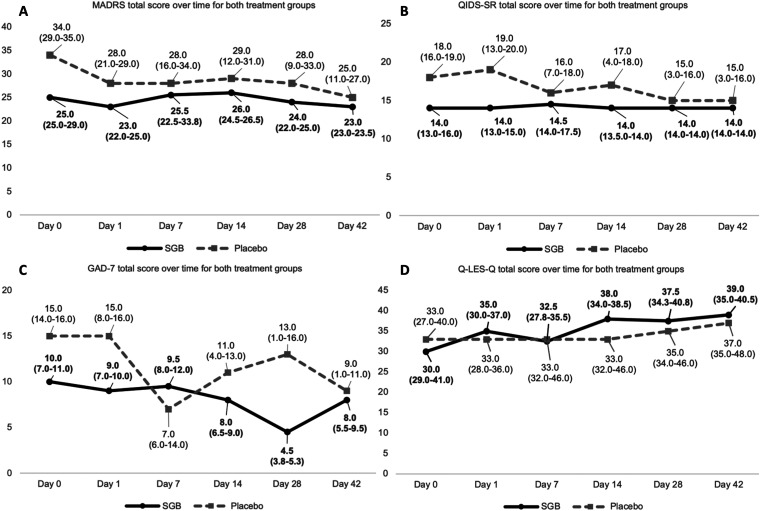
Median baseline MADRS total score was 25 (IQR 25-29) in the SGB injection group and 34 (IQR 29-35) in the placebo injection group. Median change in total MADRS score from day 0 to day 42 was −1 (IQR −1.5-0.5) in the SGB injection group and −4 (IQR −13 to −4) in the placebo injection group. Table 3 presents MADRS scores across the study duration for each participant. See Figure 4 and Table 4 for the trajectories of scores for other clinical scales.
Table 3.
MADRS Scores at Day 0 to Day 42 for Each Study Participant.
| SGB Injection | Placebo Injection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID # | Day 0 | Day 1 | Day 7 | Day 14 | Day 28 | Day 42 | ID # | Day 0 | Day 1 | Day 7 | Day 14 | Day 28 | Day 42 |
| 1 | 25 | 22 | 23 | 26 | 20 | 24 | 2 | 15 | 15 | 16 | 12 | 9 | 11 |
| 3 | 25 | 25 | 28 | 27 | 24 | 23 | 4 | 40 | 36 | 37 | 42 | 33 | 27 |
| 5 | 29 | 23 | NA | NA | NA | NA | 7 | 29 | 28 | 28 | 29 | 28 | 25 |
| 6 | 45 | 45 | 51 | NA | NA | NA | 8 | 34 | 21 | 10 | 10 | 2 | 3 |
| 9 | 21 | 21 | 21 | 23 | 26 | 23 | 10 | 35 | 29 | 34 | 31 | 37 | 31 |
Abbreviations: MADRS, Montgomery-Åsberg Depression Rating Scale; SGB, stellate ganglion block.
Figure 4.
Total scores of scales (A) MADRS, (B) QIDS-SR, (C) GAD-7, and (D) Q-LES-Q for both treatment groups. MADRS, Montgomery-Åsberg Depression Rating Scale; QIDS-SR, Quick Inventory of Depressive Symptomatology – Self Report; GAD-7, Generalized Anxiety Disorder 7-item; Q-LES-Q, Quality-of-Life Enjoyment and Satisfaction; SGB, stellate ganglion block.
Table 4.
Changes in Clinical Scales From Day 0 to Day 42 by Treatment Group.
| Scale | SGB Injection | Placebo Injection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Median | IQR | N | Mean | SD | Median | IQR | |
| MADRS | 3 | −0.3 | 2.1 | −1 | −1.5 to 0.5 | 5 | −11.1 | 11.7 | −4 | −13 to −4 |
| QIDS-SR | 3 | 1 | 1 | 1 | 0.5 to 1.5 | 5 | −6.4 | 6.1 | −6 | −9 to −3 |
| GAD-7 | 3 | 0 | 3.6 | 1 | −1.5 to 2 | 5 | −5.5 | 4.7 | −4 | −7 to −3 |
| Q-LES-Q | 3 | −0.3 | 1.5 | 0 | −1 to 0.5 | 5 | 7.6 | 6 | 8 | 4 to 8 |
Abbreviations: SGB, stellate ganglion block; IQR, interquartile range; MADRS, Montgomery-Åsberg Depression Rating Scale; QIDS-SR, Quick Inventory of Depressive Symptomatology – Self Report; GAD-7, Generalized Anxiety Disorder 7-item; Q-LES-Q, Quality-of-Life Enjoyment and Satisfaction.
Discussion
The LIFT-MOOD study conducted a randomized, placebo-controlled, parallel-arm pilot trial to investigate the feasibility of an SGB trial in participants with TRD. Primary feasibility outcomes included the rates of recruitment, withdrawal, adherence, missing data, and adverse events. The results of this study propose that a future trial utilizing 2 injections of bupivacaine 0.5% (7 mL) at the stellate ganglion might be feasible in TRD as the recruitment rate was reasonable and sufficient, retention and adherence were high, missing data were low, and adverse events were mild and temporary.
To our knowledge, this is the first randomized controlled trial (RCT) to date examining the use of SGB in depression. A recent systematic review conducted by our group synthesized preliminary evidence from clinical trials and case reports that broadly utilized SGB in treating psychiatric disorders; however, this was largely based on studies conducted on PTSD samples.12 The reviewed studies most commonly administered 1 or 2 SGBs using 0.5% of 7 mL solution,12 aligning with the treatment parameters of the current study, with the only exception being the type of anesthetic used (ie, ropivacaine vs bupivacaine). Where assessed, feasibility results of the reviewed studies demonstrated that participants were satisfied with the procedure, completion rates were typically high, and adverse events were temporary and minor (eg, cough, soreness, redness); these results mainly originated from studies administering ropivacaine.12
In the present study, an 18% recruitment rate was deemed reasonable and sufficient in reaching the target sample size of 10 participants. Moreover, the eligibility criteria for this study did not appear to restrict the number of recruited participants. Although a large portion of potential participants was deemed ineligible at screening, failed to meet the specified eligibility criteria at enrollment, or were unable to be contacted/not approached, only a subset declined to participate. Five eligible participants consented and ultimately decided not to participate in the study as they changed their mind or sought out other treatment. This could arise as procedures such as SGB may appear overwhelming to participants with needle phobias. Peterson et al35 have expressed concern regarding the invasiveness of SGB being a barrier to treatment based on patient preference, despite previous reports of post-treatment patient satisfaction.36 Past randomized trials of SGB for pain37 and PTSD18 have randomized upwards of 100 participants, demonstrating the potential for larger samples and higher recruitment rates in TRD.
In further support of feasibility, our results indicate that retention and adherence were high (80% and 100%, respectively) and missing data were low (0% to 33% of eligible participants). Only 1 participant was withdrawn due to reasons potentially related to the study (ie, a decline in mood). Taken together, this suggests that, once enrolled, participants may have generally tolerated the procedure. This is supported by a trial by McLean36 in PTSD which reported that 100% of participants were satisfied with SGB and would recommend the procedure to a friend, while 95% of participants were willing to undergo as many repeated procedures as necessary based on the tolerability and minimal discomfort. High rates of treatment completion are also supported by an RCT in PTSD which reported that 12% of the 42 randomized participants discontinued treatment due to adverse events, withdrawal, or removal of consent.19 This is relatively low considering that it has been suggested that dropout rates are typically between 20% and 40% in veterans who begin pharmacotherapy or psychotherapy treatment through an RCT.38 To improve rates of missing data, future SGB trials in this population may consider administering clinical scales in alternative ways as participants may be hesitant to respond over the phone, as in the current study.
The SGB has been used since 1925, and very few side effects have been reported.14 A previous study on the safety of SGB in PTSD noted that a majority of participants deemed the side effects of the procedure to be equal to, or less than, those of conventional PTSD treatment,36 which often includes antidepressant pharmacotherapy.39 The observed symptoms associated with Horner's syndrome (drooping eyelid, red or tearing eyes, difficulty swallowing, hoarse voice, nasal congestion) in the SGB injection group can be considered an expected effect of the blockade, which confirms the block is successful. Consistent with the findings from a previous study administering SGB for PTSD,40 there were no significant adverse events that required hospital admission or emergency intervention. Moreover, most adverse events reported here were mild, except for pain in the neck/shoulder (left side) and chest (near the armpit) on day 1. Both were moderate and reported by 1 of the participants in the active treatment group. All mild and moderate adverse events were temporary, and none were reported on day 28. In fact, almost all adverse events were resolved on the day of, or the day after, injection. Some reported adverse events also align with previous studies applying SGB to participants with various pain and psychiatric disorders (eg, pain and soreness, hoarseness, headache, light-headedness, numbness, and coughing)12,41 and represent a direct consequence of blocking the sympathetic chain. The ultrasound-guided approach utilized here allows for improved safety of the injection by direct visualization of vascular and soft tissue structures as opposed to fluoroscopy, as well as minimization of undesired effects, such as recurrent laryngeal nerve palsy, by permitting the use of smaller volumes of injectate.42 Notably, one participant in the placebo injection group experienced drooping eyelid following treatment. This could have occurred due to the mechanical stimulation of our placebo group (saline injection) along the cervical sympathetic trunk on the function of the sympathetic nervous system. In fact, an early case series that administered 10 mL of saline around the stellate ganglion produced the appearance of Horner's syndrome, of which drooping eyelid is an expected result.43 Thus, it was suggested that, while saline has been used as a placebo in SGB procedures, saline itself may result in signs of sympathetic blockade.43 As such, further research using alternative methods for placebo administration is needed to clearly delineate the effects of active SGB versus placebo.
As a secondary, exploratory objective, we explored the efficacy of SGB in improving symptoms of depression, relative to baseline, by follow-up at day 42. Although both treatment groups demonstrated decreases in MADRS scores by the end of the study, conclusions regarding efficacy cannot be made based on this preliminary study. Notably, the sample size utilized in this pilot study was small with only 3 participants completing active treatment, and there were possible sex differences in the active and placebo groups (male- and female-dominated, respectively). Moreover, injecting saline at the stellate ganglion may have produced physiological effects, which could have influenced the outcome of the sham procedure.18 Thus, more research is needed to determine the efficacy of SGB in TRD and the results of the current study should instead be used to support the feasibility of a trial of bupivacaine injection in patients with TRD. Indeed, Thabane et al44 recommend that pilot studies remain focused on assessing feasibility, rather than treatment effects, due to small sample sizes and lack of adequate power. Results from an early case series suggest that temporarily blocking sympathetic impulses to the brain via SGB was associated with heightened affect and indirect reports of satisfaction, though both were qualitative.20 Although early research has demonstrated the positive effects of SGB on mood in participants with psychosis45 and depression,20 further investigation with larger TRD samples and alternate sham procedures is encouraged.
As proposed in PTSD, SGB may exert its effects by decreasing levels of norepinephrine and regulating the sympathetic nervous system.46 Since MDD is associated with chronic stress and sympathetic nervous system activation, SGB might also be beneficial for MDD based on its ability to attenuate sympathetic flow.47 Research has shown that specific symptoms of PTSD which overlap with MDD (eg, irritability; sleep, memory, and concentration difficulties48) improve following SGB.49,50 A study in rodents provided evidence for a reduction in depression-like behaviors induced by chronic stress following SGB.25 An increase in noradrenaline and adrenaline was also found to be ameliorated, which perhaps reflects the effects of treatment on the autonomic nervous system and hypothalamic–pituitary–adrenal axis.25 Moreover, the stellate ganglion has been shown to be anatomically connected to the amygdala, a brain region implicated in MDD due to its abnormal activity and functional connectivity,51 indicating that the 2 structures may interact.52,53 SGB, therefore, has the potential to be promising in MDD based on the proposition that it acts as a down regulator of sympathetic nervous system activity.12
Strengths and Limitations
The strength of the current pilot study is that it is the first, to our knowledge, to use an RCT design to investigate SGB in a new clinical population—participants with TRD. Although other investigations into SGB in psychiatric disorders tend to be case reports or use nonrandomized open-label designs,12 we are aware of only 2 other RCTs administering SGB in PTSD.18,19 In preparation for larger trials, it should be considered that the current study's procedures were conducted at St. Michael's Hospital, Unity Health Toronto, within the chronic pain clinic which already administers SGB. Therefore, all the required equipment for the study were readily available, and no specialized training of personnel was necessary.
Limitations of the current study include the small sample size and resulting age and sex imbalances across treatment groups. Despite the long-standing history of the use of SGB in neurological disorders54 and emerging evidence for use in PTSD,22 pilot studies with small sample sizes, such as the current one, are necessary to determine the viability of this treatment in other clinical populations. Although outcome assessors were blinded, physicians and nurses performing the procedure were unable to be blinded due to the foreseeable emergence of symptoms of Horner's syndrome and to ensure participant safety—this is a limitation also reported by previous literature.18 Interactions of the treating personnel with participants were, therefore, scripted and limited to the 2 interventions, similar to how this limitation was mitigated in a prior trial.18 Providers also told participants that Horner's syndrome might be a possible result of the procedure rather than an effect of the study drug to ensure participants remained blinded to treatment allocation; participants were debriefed about this at the end of the study. However, maintaining the blindness of outcome assessors presented a challenge as the study staff were required to measure adverse events that were sometimes known to be associated with active treatment. Outcome assessors maintained the mentality that these adverse events may be a result of placebo treatment as well. For future studies, it is recommended that nonblinded research staff, separate from the outcome assessors, collect adverse events. There are limitations associated with using identical active and placebo procedures, with the sole exception being the injectate used. Research using an alternative method in PTSD has suggested that injecting saline close to the stellate ganglion could interfere with nerve transmission due to physical effects.18 Thus, the lack of a true sham intervention that does not alter the cervical sympathetic trunk precludes conclusions regarding efficacy from being drawn as the saline injections performed here might have been responsible for the observed reductions in MADRS scores for the placebo group.
Future Directions and Conclusion
The results of this study support the feasibility of a confirmatory trial administering 2 right-sided injections of bupivacaine 0.5% (7 mL) at the stellate ganglion in participants with TRD. Our findings suggest that the recruitment rate was reasonable and sufficient, retention and adherence were high, missing data were low, and adverse events were mild and temporary. Future studies should employ sham procedures used by Rae Olmsted et al18 (ie, injection of 1-2 mL of saline into the musculature anterolateral to the C6 anterior tubercle) to prevent saline-induced physiological alterations and related adverse events. Larger-scale RCTs with long-term follow-ups are also needed to determine the efficacy of SGB in treating TRD, as well as the duration of symptom improvement, as research in other disorders suggests that a single SGB injection can produce effects within 5 min and last a minimum of up to 1 month.12 Future studies may also administer SGB in conjunction with psychotherapy for TRD. Research administering SGB as an adjunctive treatment for patients with PTSD actively receiving psychotherapy shows that this may be feasible and results in clinically meaningful improvement.55
Supplemental Material
Supplemental material, sj-docx-1-css-10.1177_24705470231160315 for Local Injection for Treating Mood Disorders (LIFT-MOOD): A Pilot Feasibility RCT of Stellate Ganglion Block for Treatment-Resistant Depression by David Sussman, Vanessa K Tassone, Fatemeh Gholamali Nezhad, Michelle Wu, Fathima Adamsahib, Gabriella F Mattina, Janneth Pazmino-Canizares, Ilya Demchenko, Hyejung Jung, Wendy Lou, Karim S Ladha and Venkat Bhat in Chronic Stress
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KSL is supported in part by Merit Awards from the Department of Anesthesiology and Pain Medicine at the University of Toronto and is a co-principal investigator of a study funded by Shoppers Drug Mart. VB is supported by an Academic Scholar Award from the University of Toronto Department of Psychiatry and has received research support from the Canadian Institutes of Health Research, Brain & Behavior Foundation, Ontario Ministry of Health Innovation Funds, Royal College of Physicians and Surgeons of Canada, Department of National Defence (Government of Canada), New Frontiers in Research Fund, Associated Medical Services Inc. Healthcare, American Foundation for Suicide Prevention, Roche Canada, Novartis, and Eisai.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the St. Michael's Hospital Medical Services Association Anesthesia AFP fund.
ORCID iDs: Vanessa K Tassone https://orcid.org/0000-0002-9208-8809
Ilya Demchenko https://orcid.org/0000-0001-7876-4981
Venkat Bhat https://orcid.org/0000-0002-8768-1173
Supplemental Material: Supplemental material for this article is available online.
References
- 1.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392(10159): 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry 2014; 59(7): 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai H, Yonezawa K, Tani H, Mimura M, Bauer M, Uchida H. Novel antidepressants in the pipeline (phase II and III): a systematic review of the US clinical trials registry. Pharmacopsychiatry 2022; 55(04): 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gately S, West R. Novel therapeutics with enhanced biological activity generated by the strategic introduction of silicon isosteres into known drug scaffolds. Drug Dev Res 2007; 68(4): 156–163. [Google Scholar]
- 5.Sitges C, García-Herrera M, Pericás M, Collado D, Truyols M, Montoya P. Abnormal brain processing of affective and sensory pain descriptors in chronic pain patients. J Affect Disord 2007; 104(1–3): 73–82. [DOI] [PubMed] [Google Scholar]
- 6.Laursen BS, Bajaj P, Olesen AS, Delmar C, Arendt-Nielsen L. Health related quality of life and quantitative pain measurement in females with chronic non-malignant pain. Eur J Pain 2005; 9(3): 267–275. [DOI] [PubMed] [Google Scholar]
- 7.Hansson L. Quality of life in depression and anxiety. Int Rev Psychiatry 2002; 14(3): 185–189. [Google Scholar]
- 8.Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987; 31(1): 1–21. [DOI] [PubMed] [Google Scholar]
- 9.Abdallah CG, Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress 2017; 1: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciaramella A. Psychopharmacology of chronic pain. Handb Clin Neurol 2019; 165(3): 317–337. [DOI] [PubMed] [Google Scholar]
- 11.Jänig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol 2003; 2(11): 687–697. [DOI] [PubMed] [Google Scholar]
- 12.Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress 2021; 5: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvaney SW, Lynch JH, Kotwal RS. Clinical guidelines for stellate ganglion block to treat anxiety associated with posttraumatic stress disorder. J Spec Oper Med 2015; 15(2): 79–85. [DOI] [PubMed] [Google Scholar]
- 14.Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep 2015; 17(8): 63. [DOI] [PubMed] [Google Scholar]
- 15.Moore DC. Stellate ganglion block: techniques, indications, uses [internet]. Thomas.1954. 280 p.
- 16.Fudim M, Boortz-Marx R, Ganesh A, et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias: a systematic review and meta-analysis. J Cardiovasc Electrophysiol 2017; 28(12): 1460–1467. [DOI] [PubMed] [Google Scholar]
- 17.Parris WCV, Lin S, Frist W. Use of stellate ganglion blocks for chronic chest pain associated with primary pulmonary hypertension. Anesth Analg 1988; 67(10): 993–995. [PubMed] [Google Scholar]
- 18.Rae Olmsted KL, Bartoszek M, Mulvaney S, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry 2020; 77(2): 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanling SR, Hickey A, Lesnik I, et al. Stellate ganglion block for the treatment of posttraumatic stress disorder: a randomized, double-blind, controlled trial. Reg Anesth Pain Med 2016; 41(4): 494–500. [DOI] [PubMed] [Google Scholar]
- 20.Karnosh LJ, Gardner WJ. The effects of bilateral stellate ganglion block on mental depression: report of 3 cases. Cleve Clin J Med 1947; 14(3): 133–138. [DOI] [PubMed] [Google Scholar]
- 21.Lebovits AH, Yarmush J, Lefkowitz M. Reflex sympathetic dystrophy and posttraumatic stress disorder: multidisciplinary evaluation and treatment. Clin J Pain 1990; 6(2): 153–157. [DOI] [PubMed] [Google Scholar]
- 22.Summers MR, Nevin RL. Stellate ganglion block in the treatment of post-traumatic stress disorder: a review of historical and recent literature. Pain Pract 2017; 17(4): 546–553. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Can J Health Technol 2021; 1(3). [PubMed] [Google Scholar]
- 24.Alkire MT, Hollifield M, Khoshsar R, et al. The Anesthesiology Annual Meeting: Long Beach VA Healthcare System, Long Beach, California; 2014.
- 25.Wang W, Shi W, Qian H, Deng X, Wang T, Li W. Stellate ganglion block attenuates chronic stress induced depression in rats. Kavushansky A, editor. PLoS One. 2017; 12(8): e0183995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3): 189–198. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23(1): 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 2003; 53(8): 649–659. [DOI] [PubMed] [Google Scholar]
- 29.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry 2001; 62 (Suppl 16): 10–17. [PubMed] [Google Scholar]
- 30.https://sealedenvelope.com Sealed Envelope [Internet].
- 31.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134(4): 382–389. [DOI] [PubMed] [Google Scholar]
- 32.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166(10): 1092. [DOI] [PubMed] [Google Scholar]
- 33.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54(5): 573–583. [DOI] [PubMed] [Google Scholar]
- 34.Stevanovic D. Quality of life enjoyment and satisfaction questionnaire-short form for quality of life assessments in clinical practice: a psychometric study: Q-LES-Q-SF in clinical practice. J Psychiatr Ment Health Nurs 2011; 18(8): 744–750. [DOI] [PubMed] [Google Scholar]
- 35.Peterson K, Bourne D, Anderson J, Mackey K, Helfand M. Evidence brief: effectiveness of stellate ganglion block for treatment of posttraumatic stress disorder (PTSD). VA Evid Synth Program Evid Briefs 2017; 22. [PubMed] [Google Scholar]
- 36.McLean B. Safety and patient acceptability of stellate ganglion blockade as a treatment adjunct for combat-related post-traumatic stress disorder: a quality assurance initiative. Cureus. 2015 Sep 10 [cited 2022 Jul 19]; Available from: http://www.cureus.com/articles/2958-safety-and-patient-acceptability-of-stellateganglion-blockade-as-a-treatment-adjunct-for-combat-related-post-traumatic-stress-disorder-a-quality-assuranceinitiative [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoo Y, Lee C soon, Kim YC, Moon JY, Finlayson RJ. A randomized comparison between 4, 6 and 8 mL of local anesthetic for ultrasound-guided stellate ganglion block. J Clin Med 2019; 8(9): 1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoge CW. Interventions for war-related posttraumatic stress disorder: meeting veterans where they are. JAMA [Internet]. 2011; 306(5). 549–551. [DOI] [PubMed] [Google Scholar]
- 39.Albucher RC, Liberzon I. Psychopharmacological treatment in PTSD: a critical review. J Psychiatr Res 2002; 36(6): 355–367. [DOI] [PubMed] [Google Scholar]
- 40.Lipov EG, Jacobs R, Springer S, Candido KD, Knezevic NN. Utility of cervical sympathetic block in treating post-traumatic stress disorder in multiple cohorts: a retrospective analysis. Pain Physician 2022; 25: 77–85. [PubMed] [Google Scholar]
- 41.Goel V, Patwardhan AM, Ibrahim M, Howe CL, Schultz DM, Shankar H. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med 2019; 44(6): 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narouze S. Ultrasound-Guided stellate ganglion block: safety and efficacy. Curr Pain Headache Rep 2014; 18(6): 424. [DOI] [PubMed] [Google Scholar]
- 43.Benzon HT, Linde HW, Hawes DD, Brunner EA. Stellate ganglion block using physiologic saline solution. Anesthesiol Phila 1980; 52(6): 511–512. [DOI] [PubMed] [Google Scholar]
- 44.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010; 10(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haber J. Stellate ganglion infiltration in organic psychoses of late life. Am J Psychiatry 1955; 111(10): 751–755. [DOI] [PubMed] [Google Scholar]
- 46.Lipov EG, Joshi JR, Sanders S, Slavin KV. A unifying theory linking the prolonged efficacy of the stellate ganglion block for the treatment of chronic regional pain syndrome (CRPS), hot flashes, and posttraumatic stress disorder (PTSD). Med Hypotheses 2009; 72(6): 657–661. [DOI] [PubMed] [Google Scholar]
- 47.Won E, Kim YK. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr Neuropharmacol 2016; 14(7): 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bleich A, Koslowsky M, Dolev A, Lerer B. Post-traumatic stress disorder and depression: an analysis of comorbidity. Br J Psychiatry 1997; 170(5): 479–482. [DOI] [PubMed] [Google Scholar]
- 49.Lynch JH, Mulvaney SW, Kim EH, de Leeuw JB, Schroeder MJ, Kane SF. Effect of stellate ganglion block on specific symptom clusters for treatment of post-traumatic stress disorder. Mil Med 2016; 181(9): 1135–1141. [DOI] [PubMed] [Google Scholar]
- 50.Lipov EG, Navaie M, Brown PR, Hickey AH, Stedje-Larsen ET, McLay RN. Stellate ganglion block improves refractory post-traumatic stress disorder and associated memory dysfunction: a case report and systematic literature review. Mil Med 2013; 178(2): e260–e264. [DOI] [PubMed] [Google Scholar]
- 51.Tassone VK, Demchenko I, Salvo J, et al. Contrasting the amygdala activity and functional connectivity profile between antidepressant-free participants with major depressive disorder and healthy controls: a systematic review of comparative fMRI studies. Psychiatry Res Neuroimaging 2022; 325: 111517. [DOI] [PubMed] [Google Scholar]
- 52.Lipov EG, Lipov S, Joshi JR, Santucci VD, Slavin KV, Beck Vigue SG. Stellate ganglion block may relieve hot flashes by interrupting the sympathetic nervous system. Med Hypotheses 2007; 69(4): 758–763. [DOI] [PubMed] [Google Scholar]
- 53.Westerhaus MJ, Loewy AD. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res 2001; 903(1–2): 117–127. [DOI] [PubMed] [Google Scholar]
- 54.Risteen WA, Volpitto PP. Role of stellate ganglion block in certain neurologic disorders. South Med J 1946; 39: 431–435. [DOI] [PubMed] [Google Scholar]
- 55.Navaie M, Keefe MS, Hickey AH, McLay RN, Ritchie EC, Abdi S. Use of stellate ganglion block for refractory post-traumatic stress disorder: a review of published cases. J Anesth Clin Res 2014; 5(403): 1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-css-10.1177_24705470231160315 for Local Injection for Treating Mood Disorders (LIFT-MOOD): A Pilot Feasibility RCT of Stellate Ganglion Block for Treatment-Resistant Depression by David Sussman, Vanessa K Tassone, Fatemeh Gholamali Nezhad, Michelle Wu, Fathima Adamsahib, Gabriella F Mattina, Janneth Pazmino-Canizares, Ilya Demchenko, Hyejung Jung, Wendy Lou, Karim S Ladha and Venkat Bhat in Chronic Stress