Abstract
Imperata cylindrica is a globally distributed plant known for its antiepileptic attributes, but there is a scarcity of robust evidence for its efficacy. The study investigated neuroprotective attributes of Imperata cylindrica root extract on neuropathological features of epilepsy in a Drosophila melanogaster mutant model of epilepsy. It was conducted on 10-day-old (at the initiation of study) male post-eclosion bang-senseless paralytic Drosophila (parabss1) involved acute (1-3 h) and chronic (6-18 days) experiments; n = 50 flies per group (convulsions tests); n = 100 flies per group (learning/memory tests and histological examination). Administrations were done in 1 g standard fly food, per os. The mutant flies of study (parabss1) showed marked age-dependent progressive brain neurodegeneration and axonal degeneration, significant (P < 0.05) bang sensitivity and convulsions, and cognitive deficits due to up-regulation of the paralytic gene in our mutants. The neuropathological findings were significantly (P < 0.05) alleviated in dose and duration-dependent fashions to near normal/normal after acute and chronic treatment with extract similar to sodium valproate. Therefore, para is expressed in neurons of brain tissues in our mutant flies to bring about epilepsy phenotypes and behaviors of the current juvenile and old-adult mutant D. melanogaster models of epilepsy. The herb exerts neuroprotection by anticonvulsant and antiepileptogenic mechanisms in mutant D. melanogaster due to plant flavonoids, polyphenols, and chromones (1 and 2) which exert antioxidative and receptor or voltage-gated sodium ion channels’ inhibitory properties, and thus causing reduced inflammation and apoptosis, increased tissue repair, and improved cell biology in the brain of mutant flies. The methanol root extract provides anticonvulsant and antiepileptogenic medicinal values which protect epileptic D. melanogaster. Therefore, the herb should be advanced for more experimental and clinical studies to confirm its efficacy in treating epilepsy.
Keywords: cognition disorder, drosophila melanogaster brain, epilepsy, imperata cylindrica, neuroprotection, seizure characteristics
Introduction
Epilepsy is a chronic noncommunicable brain (neurological) disorder associated with excessive neuronal activity and unprovoked seizures referred to as ‘epileptic seizures’ and sensory-motor deficits that cause neurobiological, cognitive, psychological, and social consequences to the patient.1-4 It is one of the most common neurological disorders worldwide,1-5 is among the most common chronic brain diseases, and affects people of all ages. Around 50 million people or more worldwide have epilepsy, with almost 80% of them living in low- and middle-income countries.2-4 The global prevalence of active epilepsy is 0.64%, with a lifetime prevalence of 0.76% and an incidence of 61.4 per 100,000 person-years. The prevalence of the disease increases with age and is higher in people who are socially deprived. 6 Africa is estimated to contribute about 20–23% of the global cases, and the prevalence of epilepsy in Uganda is estimated to be ranging between 0.22 and 13 per 1000 population, with an estimated incidence of 156 cases per 100,000 each year.7,8
Genetic epilepsy arises from mutations in one or multiple genes and can occur in non-ion and ion channel genes. 9 Of huge significance are the voltage-gated sodium ion channels (VGSCs) which are transporters of ions facilitating the depolarization of action potentials and transmission of signals in neurons. The most common types of epilepsy occurring in humans are the genetic forms due to genetic disorder (s) in the VGSCs of the brain (brain sodium channelopathies).9-11 Studies involving animal models with genetic forms of epilepsy due to brain sodium channelopathies are vital for a better understanding of the pathophysiology of neuropathological features of epilepsy associated with these channelopathies. This knowledge can be utilized to develop newer effective therapies for the genetic epilepsies that arise due to channelopathies. 11
Drosophila melanogaster is a good model for studying neurological disease pathways and for utilization during drug discovery for neurological diseases including epilepsy mainly because the flies and humans have similar basic biology, physiology, and neurological properties, with 75% of disease-causing human genes being conserved in the flies. 12 In D. melanogaster flies the VGSCs are mainly coded by a single gene called the paralytic (para) gene.13,14 A mutation in the para gene (overexpression of para) in the neuronal brain tissues of bang-senseless (bss) mutant epileptic Drosophila models (parabss) gives rise to epileptic neuropathological features in the flies that are similar to those in epileptic mammals. This mutation induces a reduction in seizure stimulation threshold in parabss which makes the flies more susceptible to getting seizures following electrical, mechanical, or physical stimulation of the animals.15,16 In addition, gene mutations or alterations due to the para gene in D. melanogaster fly models are associated with defective metabolism and viability in neurons of these flies leading to defective neurophysiology and behavior.17,18 Again, the gene defects are associated with extensive neurodegeneration in the brain of the flies 17 as well as an impairment of sensory-motor activities, and neurobiological, and cognitive deficits in the animals.19,20 Because almost 67% of human disease genes are conserved in Drosophila, 21 the defects and deficits observed in the epileptic fly models (referred to as ‘para neuropathology’) are related to the ‘epileptic syndrome’ in people with genetic epilepsy that is characterized by brain sclerosis, convulsions, cognitive deficits, motor deficits, etc.21-25
Some epileptic patients (almost a third of them) do not get the expected relief from seizures and other neuropathological features of epilepsy after administration with the current anti-epileptic treatments,10,26 also, the anti-epileptic drugs are associated with numerous severe side-effects causing discomfort, drug non-compliance, and drug resistance in the patients.16,27,28 Other challenges faced by epileptic patients include the inadequate number of neurologists and the lack of good healthcare facilities for epilepsy patients in developing countries.29,30 Therefore, herbal extracts are an important source for the development of novel complementary and alternative antiepileptic medicines both in developing and developed countries.30-33
Imperata cylindrica (L.) P. Beauv (Poaceae) is native to tropical regions of Africa, tropical and subtropical zones, southwestern Asia, and some parts of the U.S.A,34,35 is an important neuromedicinal plant in Africa, Asia, and elsewhere, and could be a good source for the development and discovery of novel complementary and alternative antiepileptic medicines globally due to its widespread utilization in treating epilepsy in people with epilepsy.34-36 Phytochemicals from the herb such as the 2-(2-Phenylethyl) and 5-Hydroxy-2-(2-phenylethyl) chromones have been found to possess neuroprotective potential on glutamate-induced neurotoxicity in rat cerebral cortical cells,37,38 additionally plant-based nonenzymatic antioxidants mainly phenols, flavonoids, chromones, and glycosides provide neuroprotective attributes against brain cell damage through their strong antioxidant potentials that improve brain cell biology,37,39 provide anticonvulsant and antiepileptogenic attributes, 40 and alleviate cognitive deficits 41 of animals by the antioxidation mechanism which controls oxidative stress markers in the brain of animals.39-41
Although it is widely used, there is a lack of strong evidence for the efficacy and safety of Imperata cylindrica or I. cylindrica as an anticonvulsant in folk medicine in Africa, Asia etc,35,36,42 therefore, information on evidence-based scrutiny of antiepileptic/anticonvulsant efficacy and safety of this commonly used neuromedicinal (antiepileptic) herb is crucial for establishing targeted and effective community-based intervention policies and programs during the management of epilepsy.32,43,44
The study aimed to establish the neuroprotective effects of phytochemicals and changes in convulsion, cognitive, and histological parameters in brain tissues caused by Imperata cylindrica root extract on the neuropathological features of Drosophila melanogaster mutant models of epilepsy.
Materials and Methods
Plant Materials
Fresh plants of Imperata cylindrica were harvested in Ishaka-Bushenyi Municipality, Bushenyi District, western Uganda (GPS coordinates; Latitude: −0.53828 S 0°32′17.79583″; Longitude: 30.14546 E 30°8′43.64405″), and identified and authenticated by a botanist at the Department of botany, Mbarara University of Science and Technology (voucher No. P. Beauv /11/2020). The plants were cleaned and washed with distilled water to remove all foreign matter and the roots were collected and made into pieces and dried under shade. The methanol root extract was prepared using standard methods.45-47 Briefly, the dried roots were ground using a grinding machine into fine powders that were kept in air-tight glass containers. The methanolic extract of the plant was prepared by soaking 100 g of the powdered sample in 1000 mL of 70% methanol in an Erlenmeyer conical flask (Corning® CLS431145, Darmstadt, Germany). This was placed on an orbital shaker incubator (Amerex®, Gyromax 727, NY, USA) to allow complete mixing (24 h, 100 rpm, 30°C). The debris was removed by centrifugation (CYAN CL008N, USA; 4000 rpm, 15 min) to obtain a fine extract that was then filtered using Whatman number 1 filter papers (Whatman® qualitative filter paper, Grade 1, WHA1001325, Darmstadt, Germany). The extract was concentrated to dryness using a rotary evaporator (Büchi® Rotavapor® R-210, Büchi 23011V010, Darmstadt, Germany) at 53°C to evaporate off the methanol, followed by a concentration to dryness at 53°C using an electric oven (GallenKamp BS300, London, England). The remaining dry concentrated extract was weighed and stored in universal bottles put in a freezer at 4°C (Samsung RF22N9781SR, UPC: 887276259277, Brooklyn, NY, USA) till utilization.45-47
Animals
The study was conducted on the male adult or post-eclosion D. melanogaster flies at 10 days of age at the time of study initiation following previous studies on bang-senseless (bss) D. melanogaster models (parabss) and novel anti-epileptic treatments.15,16 The Drosophila models of the bang sensitive (BS) family used in the study were the bang senseless or bss1 mutants of the paralytic gene (parabss1),48,49 and the wild-type strains used as controls were Oregon-R or OrR and Canton-Special (CS).49-51 Male flies were used as a previous laboratory study has shown differences between female and male bss Drosophila because the parabss is located on the X chromosome, and heterozygous parabss/+ female Drosophila have significantly decreased recovery times. 52 Only animals that underwent seizures were included in the study during behavioral (convulsion and learning/memory tests) and histological assessments. 49
The bang-sensitive mutant flies (parabss1) obtained from Prof. Richard Baines laboratory (University of Manchester, UK)48,49 were used, and the wild-type flies ie, OrR (BDSC stock #25211) and CS (BDSC stock #64349), were obtained from Bloomington Drosophila Stock Center, Indiana University, U.S.A, and all the stocks were delivered to the Institute of Biomedical Research for culturing into sufficient stock colonies. The bss paralytic (para) D. melanogaster mutant flies used in the study have a gain-of-function mutation in the para sodium channel gene located at the para locus in neurons of the mutant flies.52-54
The flies were cultured on a standard yeast/cornmeal/agar diet (distilled water, 6.65% cornmeal, 7.15% dextrose, 5% yeast, 0.66% industrial agar supplemented with 3.4 mL/L propionic acid, and 2.2% nipagin) for Drosophila flies following previous methods,55,56 (Supplementary file 1). The flies were kept in incubators at 25°C, 65% humidity, and on a 12-h light/dark cycle (Fly incubator with programmable day/night cycle; Powers Scientific Inc., S33SD, USA). Flies were transferred to fresh plastic fly vials (Genesee Scientific, 32-116, USA), every 3 days, and fly density was kept to 10 flies per plastic fly vial (Genesee Scientific, 32-116, USA).
Experimental Design and Flies’ Treatment
The study was conducted for 1, 2, 3 h, and 6, 12, and 18 days for acute and chronic experiments respectively based on previous studies.52,57,58 The preliminary acute toxicity tests on D. melanogaster from our laboratory using a previous method, 59 found the lethal concentration 50 (LC50) of I. cylindrica P. Beauv methanol root extract (acute oral exposure for 24 h in 20-day-old post-eclosion Drosophila) to be 3 g/mL of extract in the medium, and doses below 2 g/mL produced no lethality. Mutant animals treated with the extract solution appeared healthy and showed reduced seizure-like behavior compared to the untreated mutant controls. Therefore, we decided to expand the assessments to lower extract concentrations (0.6, 0.8, and 1 g/mL) and (0.0125, 0.025, and 0.05 g/mL) in our acute and chronic experiments respectively. The doses of sodium valproate or SV (0.3 and 0.15 mg/mL, p.o) that were used in standard control groups of the study were based on previous studies that have shown them to provide significant anticonvulsant, anti-epileptic activities, and alleviative control of bss behaviour in epileptic D. melanogaster models eg parabss.15,60,61
The initial experiments assayed the bang-sensitivity and convulsion characteristics of our mutant flies, and also evaluated the extract doses that provide anticonvulsant potential on the mutant flies of the study following short-term and prolonged treatments. To do this, juvenile adult flies (10 days) were anesthetized using CO2 anesthesia (Fly CO2 anesthesia setup; Genesee Scientific, 59-114/54-104M, USA), sexing was performed on a CO2 perfused pad to separate flies into females and males (dissecting stereo microscope; Leica Microsystems, Leica M60 CMO), and the males were randomly assigned to plastic fly vials (Genesee Scientific, 32-116, USA), with 10 flies each. Male Animals were subjected to a vortex stimulus to induce seizures,62,63 seizing males were separated from non-seizing males and the former were then randomly assigned to the different groups (negative/mutant control, standard control, and low and high-dose extract tests groups), and the wild-type flies were used in the normal control groups, Table 1.
Table 1.
Study Design on Treatment Groups Of Bang-Sensitivity and Convulsion Tests.
| Group | Study Group | Treatment | Number of Flies |
|---|---|---|---|
| Acute experiments | |||
| I | Mutant and normal controls | 1 g of standard fly food without extract, per os (p.o) for 2 h (hrs.) | 50 flies per fly genotype, total = 150 flies per group |
| II | Low-dose extract test | 0.6 g/mL extract mixed directly in 1g of standard fly food p.o for 2 h. | 50 per genotype, total = 150 |
| III | Moderate-dose extract test | 0.8 g/mL extract mixed directly in 1g of standard fly food p.o for 2 h. | 50 per genotype, total = 150 |
| IV | High-dose extract test | 1 g/ml extract mixed directly in 1g of standard fly food p.o for 2 h. | 50 per fly genotype, total = 150 |
| V | Standard control | 0.3 mg/mL sodium valproate (SV) mixed directly in 1g standard fly food p.o for 2 h. | 50 per fly genotype, total = 150 |
| Chronic experiments | |||
| VI | Mutant and normal controls | 1 g of standard fly food without extract, p.o for 12 days | 50 per fly genotype, total = 150 |
| VII | Low-dose extract test | 0.0125 g/mL extract mixed directly in 1g of standard fly food p.o for 12 days | 50 per fly genotype, total = 150 |
| VIII | Moderate-dose extract test | 0.025 g/mL extract mixed directly in 1g of standard fly food p.o for 12 days | 50 per fly genotype, total = 150 |
| IX | High-dose extract test | 0.05 g/mL extract mixed directly in 1g of standard fly food p.o for 12 days | 50 per fly genotype, total = 150 |
| X | Standard control | 0.15 mg/mL SV mixed directly in 1g standard fly food p.o for 12 days | 50 per fly genotype, total = 150 |
Then, during the subsequent learning, memory, and histological tests, juvenile adult flies (10 days) were also anesthetized using CO2 anesthesia with Fly CO2 anesthesia setup (Genesee Scientific, 59-114/54-104M, USA), separated on a CO2 perfused pad into females and males (dissecting stereo microscope; Leica Microsystems, Leica M60 CMO), and the males were randomly assigned to plastic fly vials (Genesee Scientific, 32-116, USA), with 10 flies each. The flies were then tested for bang sensitivity (seizures) and the animals that underwent seizures were then randomly assigned to the different groups (negative/mutant control, standard control, and extract test groups), while wild-type flies were used in the normal control group, Table 2.
Table 2.
Study Design on Treatment Groups of Learning, Memory, and Histological Tests.
| Group | Study Group | Treatment | Number of Flies |
|---|---|---|---|
| Acute experiments | |||
| XI | Mutant and normal controls | 1 g of standard fly food without extract, p.o for 1, 2, or 3 h. | 100 flies per genotype, total = 100 × 3 = 300 flies per sub-group in this group |
| XII | Extract test | 0.8 g/mL of extract mixed directly in 1g of standard fly food p.o for 1, 2, 3 h. | 100 per genotype, total = 300 per sub-group |
| XIII | Standard control | 0.3 mg/mL SV mixed directly in 1g standard fly food p.o for 1, 2, 3 h. | 100 per genotype, total = 300 per sub-group |
| Chronic experiments | |||
| XIV | Mutant and normal controls | 1 g of standard fly food without extract, p.o for 6, 12, or 12 days | 100 per genotype, total = 300 per sub-group |
| XV | Extract test | 0.025 g/mL extract mixed directly in 1g of standard fly food p.o for 6, 12, and 18 days | 100 per genotype, total = 300 per sub-group |
| XVI | Standard control | 0.15 mg/mL SV mixed directly in 1g standard fly food p.o for 6, 12, and 18 days | 100 per genotype, total = 300 per sub-group |
Drug Preparation and Administration
I. cylindrica root extract and sodium valproate were administered by mixing them with standard cornmeal fly food (Supplementary file 1), and extract and drug solutions were prepared fresh prior to each experiment. The experiment does are expressed in g of extract per mL of fly food and mg of sodium valproate per mL of fly food. For extract doses (0.6, 0.8, 1, 0.0125, 0.025, and 0.05 g/mL) the dry concentrated root extract was dissolved in distilled water in a beaker at room temperature and warm food (50°C) was added and mixed before aliquoting approximately 1.5 mL per testing vial. For sodium valproate, one uncoated 100 mg tablet (Agog Pharma Ltd, India) was crushed, 0.3 mg or 0.15 mg were suspended in 1 mL distilled water each, and the drug suspension was then mixed with 5 mL of warm food to produce low and high sodium valproate concentrations respectively; SV 0.3 mg/mL and 0.15 mg.
Administration of the extract and standard control drug were done following previous methods.46,49,64,65 For acute study models, 10-day-old flies in replicates of 10 flies per plastic fly vial (Genesee Scientific, 32-116, USA), were starved for 15 h to induce appetite, after which they were fed 1 g of standard yeast/cornmeal/agar media without extract (negative or mutant controls and wild-type controls for acute study), or 1 g of standard media mixed directly with 0.6 g/mL, 0.8 g/mL, or 1 g/mL extract (extract tests for acute study), or 1 g of standard media mixed with 0.3 mg/mL SV (standard controls for acute study), and for the acute tests, the flies were allowed to feed for 1, 2, or 3 h (each treatment was tested for 1-3 h) before being transferred into individual empty plastic fly vials (Genesee Scientific, 32-116, USA) that were capped with a cotton plug. The flies were allowed to sit undisturbed for 20 min before tests were conducted.46,49,64,65 For chronic experiments, 10-day-old flies at the start of experiments in replicates of 10 flies per plastic fly vial (Genesee Scientific, 32-116, USA), were fed 1 g of standard yeast/cornmeal/agar media without extract (mutant/negative controls and wild-type controls for chronic study) or 1 g of standard media mixed directly with 0.0125 g/mL, 0.025 g/mL or 0.05 g/mL extract (extract tests for chronic study), or 1 g of standard media mixed with 0.15 mg/mL SV (standard controls for chronic study), and the flies were transferred to fresh plastic fly vials (Genesee Scientific, 32-116, USA) every 3 days. The flies for chronic studies were allowed to feed for 6, 12, or 18 days (each treatment was tested for 6-18 days) before being transferred into individual empty plastic fly vials (Genesee Scientific, 32-116, USA) which were capped with cotton plug, and the flies were left to sit undisturbed for 20 min after which the tests were conducted.46,49,64,65
Analytical Procedures
Phytochemical Analysis of I. cylindrica Root Extract
Phytochemical analysis of the methanol root extract of the plant was performed in triplicates in the Ethnobotany Laboratory, Department of Biological Sciences, Makerere University in Uganda. Qualitative analysis of the main bioactive compounds of the plant (alkaloids, saponins, tannins, cardiac glycosides, flavonoids, phenols, terpenoids, etc)34,35,66 was carried out using standard qualitative analytical methods described previously.67,68 Quantitative analyses of the isolated compounds of the crude extract of the herb were carried out following standard spectrophotometric analytical methods (PerkinElmer's Lambda 35 UV/Vis, USA) described previously.69-75
Saponin Analysis. The levels of saponins were measured by the vanillin–sulfuric acid method described by Shiau et al, 74 at absorbance (O.D) 535 nm, expressed as milligrams of saponin per gram of dry extract.
Cardiac Glycoside Analysis. Amounts of glycosides in the powdered sample were determined using the Baljet's test described previously, 75 and the O.D of the colored complex was determined spectrophotometrically at 495 nm, expressed as milligrams of glycoside per gram of dry extract.
Flavonoid Analysis. The flavonoid levels were analyzed using the aluminum chloride test using a method described previously,69,72 at O.D = 420 nm expressed as milligrams of flavonoid per gram of dry extract.
Polyphenol Analysis. The polyphenol contents were assessed using the Folin-Ciocalteu method described previously,69,71 at a spectrophotometer O.D of 730 nm, and the result was expressed as milligrams of phenol per gram of dry extract.
Tannin analysis. The tannin levels were determined using the vanillin-hydrochloric acid test by employing a method by Morrison et al 73 as suggested by Piana, 69 at O.D = 500 nm. The data were expressed as milligrams of tannin per gram of dry extract.
Alkaloid Analysis. The contents of alkaloids were analyzed using Dragendorff's test described previously,69,76 at O.D = 435 nm. The data were expressed as milligrams of alkaloids per gram of each fraction.
Chromone Analysis. The amounts of two chromone compounds ie, 5-hydroxy-2-(2-phenylethyl) chromone (1) and 5-hydroxy-2-[2-(2hydroxyphenyl)ethyl]chromone (2) were evaluated using high-performance liquid chromatography (HPLC) technique following the reflux extraction method described previously. 77 Briefly, standard solutions (1 mg/mL of standards) were prepared by diluting stock solutions with acetonitrile (ACN) to make 2, 4, 6, 8, and 10 µg/mL of 1 and 2.5, 5, 10, 15 µg/mL of 2, and quantification of the chromones in the herb was performed using high-performance liquid chromatography (HPLC) following the reflux extraction method as follows: 10 g of plant powder was weighed and refluxed with 70% methanol for 3 h at 45°C, the extract obtained was filtered through Whatman number 1 filter papers (Whatman® qualitative filter paper, Grade 1, WHA1001325, Darmstadt, Germany) and the solvent removed in vacuo. After preparing the methanol extract 2.5 mg were dissolved in 1 mL of ACN for HPLC analysis (injection volume, 20 µL, flow rate, 1 mL/min, temperature, 22- 30°C, detection at 330 nm, and acetic acid was used to reduce the tailing). Separation was done on a C18 column with a mobile phase of 0.5% (v/v) AR grade acetic acid (CDH, India) in distilled water (A), and ACN (B) under the best gradient elution determined to give a stable baseline peak and resolution of 1 and 2. 1 and 2 were identified by analysing peaks at corresponding retention times by spiking with standards of the same corresponding compounds isolated and characterized earlier from I. cylindrica into methanol extract. 77 The amounts of 1 and 2 in the sample were quantified using standard spectrophotometric analytical methods (PerkinElmer's Lambda 35 UV/Vis, USA). The data were expressed as milligrams of chromones per gram of each fraction.
In Vivo Anticonvulsant and Antiepileptic Effects of I. cylindrica Root Extract on Mutant Flies
Determination of Bang-sensitivity Behavior and Seizure Parameters of Flies
To determine the bang sensitivity and seizure characteristics of the flies, we subjected the mutant flies (parabss1) and wild-type controls (OrR and CS) in different groups to mechanical stress, then observed for the occurrence of bang sensitivity and measured the time taken during convulsions, paralysis, to recovery from the paralysis.62,63 Bang sensitivity and convulsion parameters (mean convulsion time, CT; mean paralysis time, PT; and mean recovery time, RT, were assessed following previous methods.49,62,63,78 Briefly, on the day of the behavioral assessment, each fly was transferred into an empty plastic cylindrical 10 mL vial capped with a cotton plug, the fly was mechanically stimulated by placing an inverted vial on a bench-top vortex, then vortexed at high speed (maximum speed of 3200 rpm for 10 s, at room temperature of 22- 30°C) with a vortex mixer (Benchmark Scientific, BMK-BV1000, USA). The durations spent during convulsions, paralysis, and recovery for each animal to right itself (recover) were recorded as CT, PT, and RT respectively (QIANZICAI Stopwatch 382 ®). CT is the time taken while the fly is experiencing a convulsion (seizures, shaking, and lying on its back); paralysis is the second phase after a convulsion; PT is the time between the end of a convulsion and recovery; recovery is when the fly is standing and exhibiting normal mobility or flight; RT is the time taken by a fly that exhibits bang-sensitive behavior to recover completely from the convulsion.49,62,63,78 The mean of the convulsion, paralysis, and recovery times (CT, PT, and RT) of the 50 flies in each group were calculated as follows:
Determination of learning and Memory Pass Rates of Flies
The learning and memory assays were performed at 22–30°C using the Aversive Phototaxic Suppression (APS) assay previously described57,79 with a modified T-maze set-up.
Learning Assay. On the day of the behavioral assessments (learning and memory tests), flies were anaesthetized using CO2 anesthesia with Fly CO2 anesthesia setup (Genesee Scientific, 59-114/54-104M, USA), secluded from various treatment groups in groups of 10 (ten) flies per plastic fly vial (Genesee Scientific, 32-116, USA), and in 10 replicates (n = 100 flies in each experimental group). The secluded flies were positioned in an empty polystyrene vial with a water-dampened filter paper for six hours to induce starvation for ensuring a good perception of the flies to the aversive taste and to allow complete recovery before the conduction of the assay. Our modified T-maze was designed by attaching a lighted chamber (15mL plastic centrifuge tube connected to a light source) and a dark chamber (15mL plastic centrifuge tube wrapped in aluminum foil) on each side of the center column using adapter connectors. Quinine solution (1 μM, 180 μL) was added to filter paper using a 200 μL pipetter and placed in the light chamber. Then each fly was trained 10 times to the APS assay to enable learning of the aversive quinine taste stimulus as described previously.57,79 Immediately after the training of the fly for APS, ten test trials were conducted for each fly, and in each test trial, the light was switched on, the fly was pushed into the dark chamber using a small paint brush, then 10 s allowed to pass before the trained fly was left to move to the lighted chamber containing the aversive (quinine) stimulus. Failure of the fly to move to the lighted vial or chamber within 10 s was recorded as “pass” (equivalent to “task learned through reinforcement”). For each fly, the average ‘pass’ over the ten consecutive test trials was recorded as PC0, this was the learning indicator (learning pass) value of that fly.57,79 The percent learning pass rate of the 100 flies in each group was calculated as follows:
Memory Pass rate. After the initial learning tests and PC0 recordings, the flies from each experimental group were put back into their original food vials (plastic fly vials; Genesee Scientific, 32-116, USA) and kept for six hours. The memory-retaining capability of each fly was assessed on the same flies that were used in the learning tests. Memory-retaining capacity was recorded 6 h after the initial training session of the flies in the learning experiments. To do this, each fly was again subjected to ten (10) test trials as described in the learning assay.57,79 The number of times out of the10 test trials that each fly evaded the lighted chamber within 10 s indicated the memory ‘pass’ for that fly and was recorded as PC6 (6 h after the initial training), and this was the indicator (memory pass) value of short-term memory of the fly.57,79 The percent memory pass rate of the 100 flies in each group was calculated as follows:
Histopathological Examination of Brain Tissues of Flies
Histopathological tests were performed using standard histological methods as previously described.78,80-82 Briefly, on the day of histological assessments, Drosophila melanogaster flies were anesthetized with CO2 (Fly CO2 anesthesia setup; Genesee Scientific, 59-114/54-104M, USA), and placed on a CO2 perfused pad for collecting. The flies were decapitated under a dissecting stereo microscope (Leica Microsystems, Leica M60 CMO), and the heads were fixed in 10% neutral buffered formalin (NBF) fixative at room temperature (22- 30°C) for 24 h, after which tissues were processed using routine histology techniques by dehydrating with graded alcohols, clearing with xylene, and embedding into paraffin wax using an automated tissue processor (Histokinette-SLEE MAINZ, MTP type). Every tissue was randomly sectioned into six, 6 μm-thick transverse histological sections using a rotary microtome (SLEE MAINZ, CUT4062), and the sections were then placed on slides and stained with hematoxylin and eosin (H &E) and combined Luxol fast blue (LFB) and Nissl (Klüver's) staining techniques following standard protocols.78,80-82 The stained sections were mounted in mounting media and qualitative histological examination of the sections was done and photographed with a light microscope (Nikon Eclipse Ci-L Upright Microscope, New York, USA), at a magnification of 200x or 400x, digital camera (Nikon DS-Fi1c Digital Camera, New York, USA), and imaging software (Nikon NIS- NIS-Elements F Ver4.60.00 Imaging software), for image analysis and documentation.
A qualitative examination of the tissues was done using previously described methods64,83-85 where brain neurodegeneration was categorized as normal, moderate, or severe based on the size and frequency of brain vacuolations in H and E stained-sections following previous methods.84,85 The non-myelinating Schwann cells in mammals are comparable to the ‘ensheathing’ glial cells within the CNS of Drosophila which encase axons and neuropil of the flies,86,87 therefore, LFB in Klüver LFB stain was used to demonstrate axonal degeneration of neurons rather than axonal demyelination. 88 The nature of the Nissl substance and nerve tracts (axons) were demonstrated using the Klüver LFB-stained tissues following previous methods,64,83 where the nerve tracts were shown by blue color and the Nissl substance was shown by magenta (violet) colour. A weak LFB stain (light blue patchy areas) indicated axonal degeneration of nerve tracts, while a weak cresyl echt violet stain (light violet) indicated abnormalities in Nissl substance,64,83 supplementary file 2.
Statistical Analysis
The data was recorded and organized into an excel spreadsheet (version 219), then imported into Graph Pad Prism Version 6 software for statistical analysis. Data were subjected to one-way analysis of variance (ANOVA) and two-way ANOVA for convulsion and learning/memory parameters respectively and Tukey's multiple comparison tests were used to determine sources of variation, and significant differences (P < 0.05) at a 95% confidence interval were indicated with different superscript letters (a, b, c, d, e, f, and g). The information was expressed as mean ± SD and presented in graphs and tables. For easy graphical visualization and representation of brain histopathological findings of the flies, histological data were also graphically represented using the GraphPad Prism software, and the grouped interleaved bars were used to represent the general brain morphology, and grouped stacked bar graphs were employed to represent the brain axonal morphology of flies.
Results
Phytochemical Composition of I. cylindrica Root Extract
Phytochemical analysis of the methanol I. cylindrica root extract showed the availability of saponins, flavonoids, tannins, cardiac glycosides, polyphenols, alkaloids, and chromone derivatives (1 and 2). The standard spectrophotometric analytical methods showed that the levels of phytochemicals in the methanol extract were in the order flavonoids > polyphenols > chromones > saponins > tannins > cardiac glycosides > alkaloids. Therefore, flavonoids, polyphenols, and chromones were in the highest concentration in the methanol extract of our herb, ( Table 3 ).
Table 3.
Total Phytochemical Content in the Methanol Root Extract of I. cylindrica.
| Phytochemical compound | Content (mg/g Extract) |
|---|---|
| Tannins | 1.5 ± 0.06 |
| Polyphenols | 20.17 ± 1.00 |
| Flavonoids | 80.3 ± 0.10 |
| Cardiac glycosides | 1.06 ± 0.03 |
| Saponins | 8.17 ± 0.02 |
| Alkaloids | 0.9 ± 0.03 |
| Chromone (1) | 5.8 ± 0.01 |
| Chromone (2) | 3.8 ± 0.12 |
Values represent means ± standard deviations (SD) for triplicate experiments.
Juvenile-Adult and Old-Adult bss Paralytic Mutant Flies Portray Acute and Chronic Bang-Sensitivity and Seizure Phenotypes
Bang-sensitive assays revealed that the wild-type control flies (OrR and CS) were unaffected by mechanical stress (did not experience bang-sensitivity), while the mutant control flies of acute and chronic studies in the same groups (I and VI) displayed bang-sensitivity. This indicates that juvenile adults (10-day-old post-eclosion) and old-adults (16-28-day-old post-eclosion) mutant flies show acute and chronic bang-sensitivity, unlike the wild-type controls which show normal phenotypes (Figure 1A).
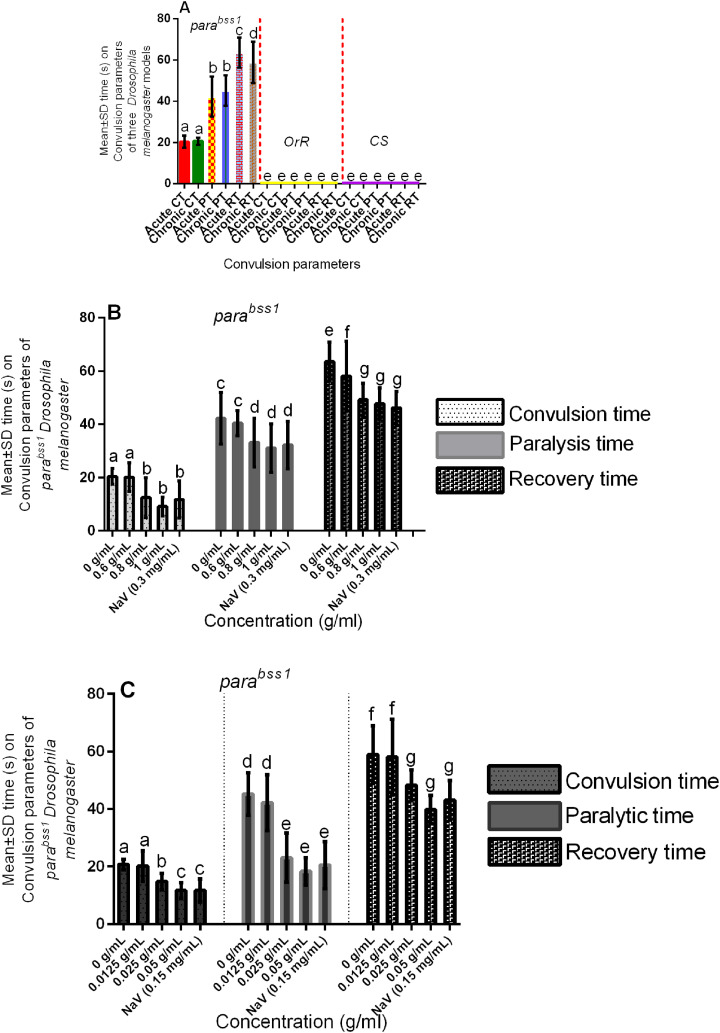
Figure 1.
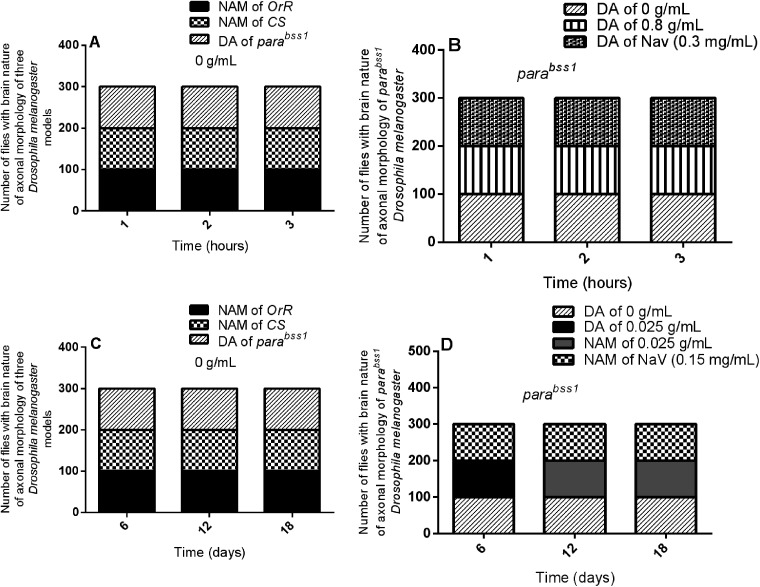
Acute and chronic effects of methanol I. cylindrica root extract on bang-sensitivity and seizure phenotypes of male juvenile-adults (10-day-old) and old-adults (22-day-old) bang-sensitive mutant Drosophila (parabss1). A. Acute and chronic seizure parameters (CT, PT, and RT) of juvenile-adults and old-adults; mean-time (y-axis) plotted for the three parameters. B. Acute (2-h) extract treatment alleviates acute seizures of juvenile adults. C. Chronic (12-day) extract treatment alleviates chronic seizures of old adults; mean time (y-axis) plotted against different treatment groups. OrR and CS = wild-type controls; parabss1 = mutant (test) model. Each value is expressed as mean ± SD; n = 50. Different letters (a, b, c, d, e, f, and g) meant significant differences among groups, (p < 0.05). Abbreviations: BSS = bang-senseless, CS = Canton-Special, CT = mean convulsion time, NaV = sodium valproate, OrR = Oregon R, Para = Paralytic, PT = mean paralysis time, RT = mean recovery time. Juvenile-adult and old-adult bss mutant flies showed bang-sensitivity and seizure phenotypes unlike the wild-type controls (A). The acute bang-sensitivity and seizure phenotypes of mutant flies were alleviated by acute (2 h) extract feeding in a dose-dependent pattern similar to SV (B). The chronic bang-sensitivity and seizures of mutant flies were alleviated by chronic (12 days) extract feeding similar to SV (D).
The bss paralytic mutant Drosophila (parabss1) were used in our subsequent convulsion experiments to determine their convulsion characteristics (convulsion, paralysis, and recovery times), and the wild-type controls that showed neither bang-sensitivity nor convulsion phenotypes were only used for purposes of controlling the experiments. Bang-sensitivity tests revealed that the mean CT, PT, and RT of juvenile-adult mutant controls in group I of the acute experiments were 20.46 s, 42.26 s, and 63.6 s respectively, and the average CT, PT, and RT of the old-adult mutant controls in group VI of the chronic tests were 20.74 s, 45.24 s, and 58.98 s respectively, with no significant (P > 0.05) difference between the acute and chronic CT and PT, but with significant (P < 0.05) difference between the acute and chronic RT ( Figure 1A and Supplementary file 3: Table 1).
I. cylindrica Root Extract Controlled Acute and Chronic Bang-Sensitivity and Convulsion Phenotypes of bss Paralytic Mutant Flies Following Short-Term and Prolonged Treatments
The mean CT, PT, and RT of the untreated mutant control or negative control group (group I) were significantly (P < 0.05) elevated compared to the moderate and high-dose extract test groups (groups III and IV) in acute experiments, with no significant (P > 0.05) difference between SV group (group V) compared to the moderate and high-dose extract test groups, but with significant (P < 0.05) difference between the low-dose group (II) compared to SV, moderate and high-dose extract test groups (III, IV, and V), (Figure 1B, Table 4, and Supplementary file 3: Table 2). The results indicate that short-term ingestion of the extract similar to SV protects against acute seizures of the mutants in a dose-dependent fashion, with 0.8 mg/ml and 1 g/mL extract, and 0.3 mg/mL SV being effective seizure-controlling doses compared to 0.6 g/mL extract.
Table 4.
Multiple Comparisons of Acute and Chronic Effects of Methanol I. cylindrica Root Extract on Acute and Chronic Seizure Parameters of Male Juvenile-Adult (10-day-old) and old-Adult (22-day-old) Bang-Sensitive Mutant Drosophila (parabss1), n = 50.
| Tukey's Multiple Comparisons Tests | Convulsion Time | Paralysis Time | Recovery Time |
|---|---|---|---|
| Adjusted P-Value | |||
| Acute (2 h) | |||
| 0 g/ml versus 0.6 g/ml | 0.9036 | 0.8300 | 0.0035 |
| 0 g/ml versus 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| 0 g/ml versus 1 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| 0 g/ml versus NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| 0.6 g/ml versus 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| 0.6 g/ml versus 1 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| 0.6 g/ml versus NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| 0.8 g/ml versus 1 g/ml | 0.2786 | 0.7451 | 0.8718 |
| 0.8 g/ml versus NaV (0.3 mg/ml) | 0.9036 | 0.9036 | 0.3478 |
| 1 g/ml versus NaV (0.3 mg/ml) | 0.5746 | 0.9036 | 0.8718 |
| Chronic (day 12) | |||
| 0 g/ml versus 0.0125 g/ml | > 0.9999 | 0.7297 | > 0.9999 |
| 0 g/ml versus 0.025 g/ml | 0.0026 | < 0.0001 | < 0.0001 |
| 0 g/ml versus 0.05 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| 0 g/ml versus NaV (0.15 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| 0.0125 g/ml versus 0.025 g/ml | 0.0128 | < 0.0001 | < 0.0001 |
| 0.0125 g/ml versus 0.05 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| 0.0125 g/ml versus NaV (0.15 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| 0.025 g/ml versus 0.05 g/ml | 0.6704 | 0.0491 | < 0.0001 |
| 0.025 g/ml versus NaV (0.15 mg/ml) | 0.6704 | 0.8816 | 0.0192 |
| 0.05 g/ml versus NaV (0.15 mg/ml) | > 0.9999 | 0.9646 | 0.5974 |
p < 0.05 meant significant differences among the groups. Abbreviations: BSS = bang-senseless, CT = mean convulsion time, NaV = sodium valproate, Para = Paralytic, PT = mean paralysis time, RT = mean recovery time.
The mean CT, PT, and RT of the untreated mutant controls (group VI) were significantly (P < 0.05) elevated compared to the moderate and high-dose extract test groups (groups VIII and IX) in chronic experiments, with no significant (P > 0.05) difference between SV group (X) compared to the moderate and high-dose extract test groups (VIII and IX), but with significant (P < 0.05) difference between the low-dose group (VII) compared to SV group, moderate and high-dose extract test groups (VIII, IX, and X), (Figure 1C, Table 4, and Supplementary file 3: Table 2). The results indicate that prolonged ingestion of the extract similar to SV protects against chronic seizures of the mutants in a dose-dependent fashion, with 0.025 mg/mL and 0.050 g/mL extract, and 0.15 mg/mL SV being effective seizure-controlling doses compared to 0.0125 g/mL extract.
Juvenile-Adult and Old-Adult bss Paralytic Mutant Flies have Acute and Chronic Learning and Memory Impairments
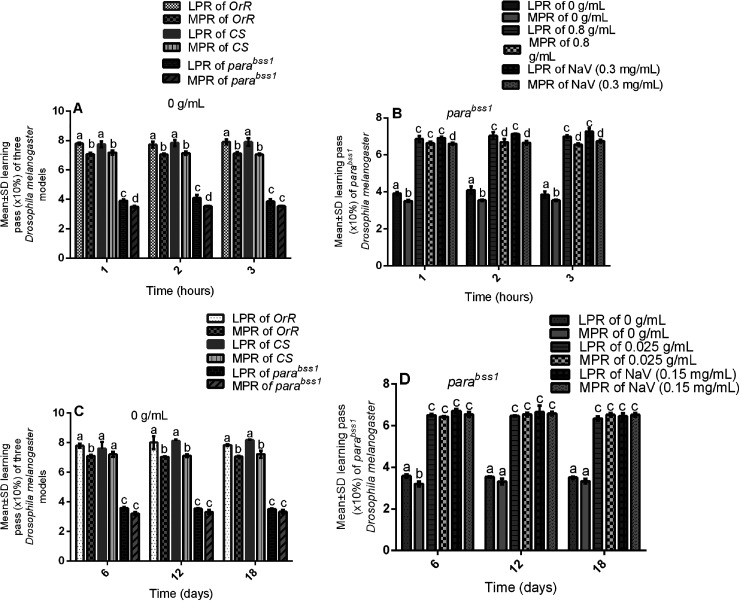
APS assay revealed the average learning and memory pass rates of wild-type controls as being78% and 71% respectively and were significantly (P < 0.05) higher than the learning and memory pass rates of the mutant controls (40% and 35% respectively) in group XI of the acute tests, with no significant (P > 0.05) difference in the average learning and memory pass rates of the mutant flies at 1, 2, or 3 h within the mutant controls of group XI (Figure 2A, Table 5 and Supplementary file 3: Table 3). This indicates that juvenile-adult (10-day-old) mutant flies show acute non-progressive deficits in learning and memory performance compared to the wild-type control group in the acute phases of the study.
Figure 2.
Acute and chronic effects of methanol I. cylindrica root extract on acute and chronic learning and memory deficits of male juvenile-adults (10-day-old) and old-adults (16–28-day-old) bang-sensitive mutant Drosophila (parabss1). A. Acute learning and memory deficits of juvenile-adult bang-sensitive mutant Drosophila. B. Acute (1–3-h) extract treatment alleviates acute learning and memory deficits of juvenile-adult mutant flies; mean learning and memory pass rate for mutant flies (y-axis) plotted against time (hours) with respect to the concentration of extract. C. Chronic learning and memory deficits in old-adult bang-sensitive mutant Drosophila; mean learning and memory pass rate (y-axis) plotted against time (days). D. Chronic (6–18-day) extract treatment alleviates chronic learning and memory deficits in old-adult mutant flies; mean learning and memory pass rate for mutant flies (y-axis) plotted against time (days) with respect to the concentration of extract; mean learning and memory pass rates (y-axis) plotted against time with respect to different treatment groups. OrR and CS = wild-type controls; parabss1 = mutant (test) model. Each value is expressed as mean ± SD; n = 100. Different letters (a, b, c, and d) meant significant differences among groups, (p < 0.05). Abbreviations: BSS = bang-senseless, CS = Canton-Special, LPR = learning pass rate, MPR = memory pass rate, NaV = sodium valproate, OrR = Oregon R, Para = Paralytic. Juvenile-adult and old-adult bss mutant flies showed impaired learning and memory function compared to the wild-type controls with normal learning and memory function (A & C). The acute learning and memory functions of the mutant flies were rescued by acute (1–3 h) extract feeding in a duration-independent pattern similar to SV (B). The chronic learning and memory functions of the mutant flies were rescued by chronic (6–18 days) extract feeding in a duration-independent pattern similar to SV (D).
Table 5.
Multiple Comparisons on Acute and Chronic Learning and Memory Deficits of Male Juvenile-Adults (10-day-old) and Old-Adults (16–28-day-old) Bang-Sensitive Mutant and Wild-Type Control Drosophila at 0 g/ml. n = 100.
| Tukey's Multiple Comparisons Tests | Learning and Memory Pass Rate | ||
|---|---|---|---|
| Adjusted P-Value | |||
| Acute Treatment | Time | ||
| 1hr | 2hrs | 3hrs | |
| LPR of OrR versus MPR of OrR | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of OrR versus LPR of CS | 0.9948 | 0.9322 | > 0.9999 |
| LPR of OrR versus MPR of CS | < 0.0001 | 0.0003 | < 0.0001 |
| LPR of OrR versus LPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of OrR versus MPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of OrR versus LPR of CS | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of OrR versus MPR of CS | 0.9322 | 0.9681 | 0.9968 |
| MPR of OrR versus LPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of OrR versus MPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of CS versus MPR of CS | 0.0005 | < 0.0001 | < 0.0001 |
| LPR of CS versus LPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of CS versus MPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of CS versus LPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of CS versus MPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR parabss1vs. MPR of parabss1 | 0.0163 | 0.0005 | 0.1057 |
| Chronic treatment | Day 6 | Day 12 | Day 18 |
| LPR of OrR versus MPR of OrR | 0.0006 | < 0.0001 | 0.0001 |
| LPR of OrR versus LPR of CS | 0.7608 | 0.9767 | 0.2656 |
| LPR of OrR versus MPR of CS | 0.0050 | < 0.0001 | 0.0036 |
| LPR of OrR versus LPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of OrR versus MPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of OrR versus LPR of CS | 0.0252 | < 0.0001 | < 0.0001 |
| MPR of OrR versus MPR of CS | 0.9767 | 0.9963 | 0.8794 |
| MPR of OrR versus LPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of OrR versus MPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of CS versus MPR of CS | 0.1341 | < 0.0001 | < 0.0001 |
| LPR of CS versus LPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of CS versus MPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of CS versus LPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of CS versus MPR of parabss1 | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of parabss1vs. MPR of parabss1 | 0.1707 | 0.6895 | 0.8794 |
OrR and CS = wild-type controls; parabss1 = mutant (test) model. p < 0.05 meant significant differences among the groups. Abbreviations: BSS = bang-senseless, CS = Canton-Special, LPR = learning pass rate, MPR = memory pass rate, NaV = sodium valproate, OrR = Oregon R, Para = Paralytic.
Also, the assay revealed that the average learning and memory pass rates of wild-type controls (78% and 71% respectively) were significantly (P < 0.05) higher than that of the mutant controls (35% and 33% respectively) in the group XIV of the chronic tests, with no significant (P > 0.05) difference in the mean learning and memory pass rates of the mutant flies at 6, 12, or 18 days within the mutant controls of group XIV (Figure 2C, Table 5 and Supplementary file 3: Table 4). This indicates that old-adult (16-28-day old) mutant flies show chronic non-progressive deficits in learning and memory performance compared to the wild-type control group in the chronic phases of the study.
I. cylindrica Root Extract Controlled Acute and Chronic Learning and Memory Impairments of bss Paralytic Mutant Flies Following Short-Term and Prolonged Treatments
The average learning and memory pass rates of untreated mutant controls or negative controls (group XI) were significantly (P < 0.05) decreased compared to the extract test group (XII) of acute experiments, with no significant (P > 0.05) difference between the SV group (XIII) and extract test group (XII), and with no significant (P > 0.05) difference of learning and memory pass rates after 1, 2, or 3 h of treatment in the extract test group (XV), (Figure 2B, Table 6 and Supplementary file 3: Table 3). The results indicate that short-term ingestion of 0.8 g/mL extract (similar to 0.3 mg/mL SV) alleviates the acute learning and memory deficits of the mutant flies in a time-independent fashion during the acute phase of the study.
Table 6.
Multiple Comparisons on the Acute and Chronic Effect of Methanol I. cylindrica Root Extract on Acute and Chronic Learning and Memory Deficits of Male Juvenile-Adult (10-day-old) and old-Adult (16–28-day-old) Bang-Sensitive Mutant Drosophila (parabss1). n = 100.
| Tukey's Multiple Comparisons Tests | Learning and Memory Pass Rate | ||
|---|---|---|---|
| Adjusted P-Value | |||
| Acute Treatment | Time | ||
| 1hr | 2hrs | 3hrs | |
| LPR of 0 g/ml versus MPR of 0 g/ml | 0.0024 | < 0.0001 | 0.0300 |
| LPR of 0 g/ml versus LPR of 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of 0 g/ml versus MPR of 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of 0 g/ml versus LPR of NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of 0 g/ml versus MPR of NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of 0 g/ml versus LPR of 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of 0 g/ml versus MPR of 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of 0 g/ml versus LPR of NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of 0 g/ml versus MPR of NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of 0.8 g/ml versus MPR of 0.8 g/ml | 0.2315 | 0.0107 | 0.0014 |
| LPR of 0.8 g/ml versus LPR of NaV (0.3 mg/ml) | 0.9925 | 0.9313 | 0.0735 |
| LPR of 0.8 g/ml versus MPR of NaV (0.3 mg/ml) | 0.1394 | 0.0040 | 0.1627 |
| MPR of 0.8 g/ml versus LPR of NaV (0.3 mg/ml) | 0.0735 | 0.0007 | < 0.0001 |
| MPR of 0.8 g/ml versus MPR of NaV (0.3 mg/ml) | 0.9998 | 0.9992 | 0.4221 |
| LPR of NaV (0.3 mg/ml) versus MPR of NaV (0.3 mg/ml) | 0.0394 | 0.0003 | < 0.0001 |
| Chronic treatment | Day 6 | Day 12 | Day 18 |
| LPR of 0 g/ml versus MPR of 0 g/ml | 0.0042 | 0.1907 | 0.4882 |
| LPR of 0 g/ml versus LPR of 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of 0 g/ml versus MPR of 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of 0 g/ml versus LPR of NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of 0 g/ml versus MPR of NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of 0 g/ml versus LPR of 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of 0 g/ml versus MPR of 0.8 g/ml | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of 0 g/ml versus LPR of NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| MPR of 0 g/ml versus MPR of NaV (0.3 mg/ml) | < 0.0001 | < 0.0001 | < 0.0001 |
| LPR of 0.8 g/ml versus MPR of 0.8 g/ml | 0.9669 | 0.9669 | 0.3718 |
| LPR of 0.8 g/ml versus LPR of NaV (0.3 mg/ml) | 0.2715 | 0.3718 | 0.8389 |
| LPR of 0.8 g/ml versus MPR of NaV (0.3 mg/ml) | 0.9986 | 0.8389 | 0.3718 |
| MPR of 0.8 g/ml versus LPR of NaV (0.3 mg/ml) | 0.0543 | 0.8389 | 0.9669 |
| MPR of 0.8 g/ml versus MPR of NaV (0.3 mg/ml) | 0.8389 | 0.9986 | > 0.9999 |
| LPR of NaV (0.3 mg/ml) versus MPR of NaV (0.3 mg/ml) | 0.4882 | 0.9669 | 0.9669 |
p < 0.05 meant significant differences among groups. Abbreviations: BSS = bang-senseless, CS = Canton-Special, LPR = learning pass rate, MPR = memory pass rate, NaV = sodium valproate, OrR = Oregon R, Para = Paralytic.
The mean learning and memory pass rates of untreated mutant controls (group XIV) were significantly (P < 0.05) decreased compared to the extract test group (XV) in chronic tests, with no significant (P > 0.05) difference between the SV group (XVI) and extract test group (XV), and with no significant (P > 0.05) difference of learning and memory pass rates following 6, 12, or 18 days of treatment in the extract test group (XV), (Figure 2D, Table 6 and Supplementary file 3: Table 4). The results indicate that prolonged ingestion of 0.025 g/mL extract (similar to 0.15 mg/mL SV) protects against the chronic learning and memory deficits of the mutant flies in a duration-independent pattern during the chronic phases of the study
The acute and chronic APS assays in mutant and wild-type control groups showed significantly (P < 0.05) higher average learning pass rates compared to the corresponding average memory pass rates within each experimental group (Figure 2A-D, Table 5, and Table 6). This indicates that in general terms, the learning performance of flies is higher than their memory performance in both acute and chronic phases of the study, this disparity is probably due to the method used to obtain the two parameters.
Juvenile-Adult and Old-Adult bss Paralytic Mutant Flies Show Acute and Chronic Brain Neurodegeneration
Histopathological examination of H & E-stained brain tissues found no significant brain lesions (focal brain vacuolations ˂ 3 µm in diameter) in wild-type controls of group XI, while mutant (negative) controls of the group (XI) showed moderate brain neurodegeneration (multifocal vacuolations, 3-5 µm in diameter) in acute tests, with a similar nature of brain histopathology in mutant controls of group XI at 1, 2, or 3 h (Figure 3A and 4, and Supplementary file 3: Table 5). This indicates that juvenile-adult (10-day-old) mutant flies show acute non-progressive brain neurodegeneration compared to the juvenile-adult wild-type controls which depict normal general brain histology in the acute phases of the study.
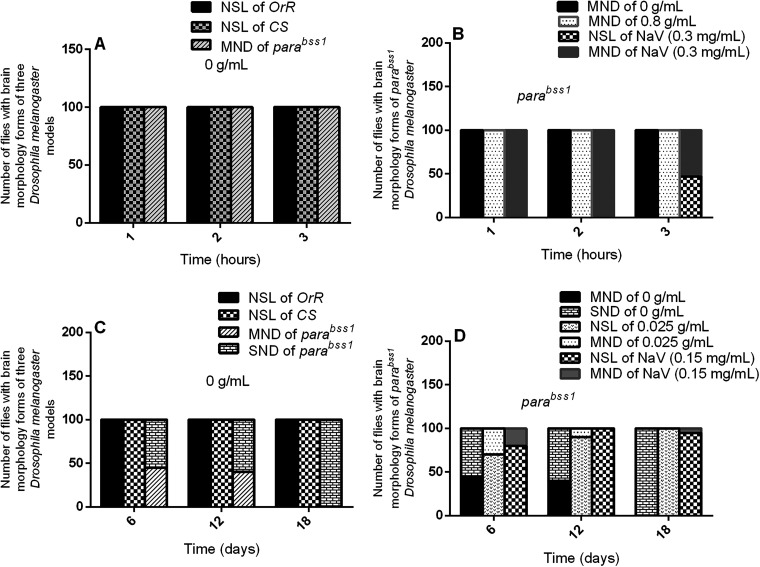
Figure 3.
Acute and chronic effects of methanol I. cylindrica root extract on acute and chronic brain neurodegeneration in male juvenile-adult (10-day-old) and old-adult (16–28-day-old) bang-sensitive mutant Drosophila (parabss1). A. Acute brain neurodegeneration of juvenile-adult bang-sensitive mutant Drosophila. B. Effect of acute (1–3-h) extract treatment on acute brain neurodegeneration of juvenile-adult mutant flies. C. Chronic neurodegeneration of old-adult bang-sensitive mutant Drosophila. D. Effect of chronic (6–18-day) extract treatment on chronic brain neurodegeneration of old-adult mutant flies. The number of flies having each type of brain morphology (y-axis) plotted against time concerning different treatment groups; n = 100. OrR and CS = wild-type controls; parabss1 = mutant (test) model. Abbreviations: BSS = bang-senseless, CS = Canton-Special, NaV = sodium valproate, NSL = no significant lesion, MND = moderate neurodegeneration, OrR = Oregon R, Para = Paralytic, SND = severe neurodegeneration. 100% of juvenile-adult (10-day-old) bss mutant flies showed moderate brain neurodegeneration versus the wild-type controls of the same age with normal brain morphology/NSL (A). The acute brain neurodegeneration of mutants was not alleviated by acute extract feeding versus alleviation by acute feeding with SV (B). 100% of old-adult (16–28-day-old) bss mutant flies showed moderate-severe brain neurodegeneration versus the wild-type controls of the same age with NSL (C). The chronic brain neurodegeneration of mutants was rescued in 100% of mutant flies by chronic (18 days) extract feeding similar to feeding with SV (D).
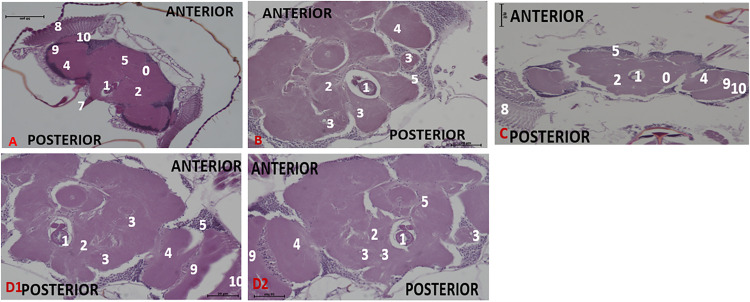
Figure 4.
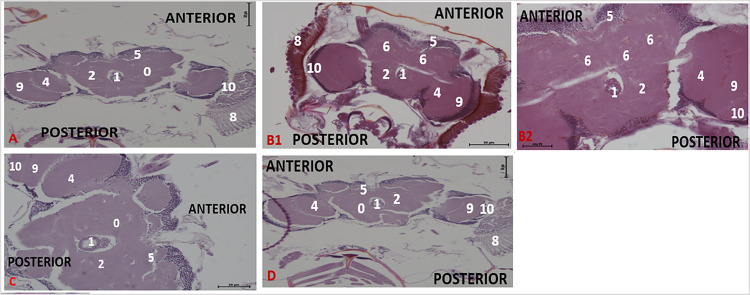
Effect of acute treatment with methanol I. cylindrica root extract on acute brain neurodegeneration of male juvenile-adults (10-day-old) bang-sensitive mutant Drosophila (parabss1). n = 100, (H&E; transverse sections at level of mid-brain, x200 or x400). Bar, 20 µm or 50 µm. A. Wild-type control, x200. B. Mutant control, x400. C. Standard control, x200. D. Methanol extract test, x200 (D1), x400 (D2). Categories of brain neurodegeneration: NSL (vacuolations <3 µm in diameter) = Normal; MND, (vacuolations 3–5 µm in diameter); SND, (vacuolations >5 µm in diameter). OrR and CS = wild-type controls; parabss1 = mutant (test) model. 0 = No significant lesion in the neuropil, 1= Oesophagus; 2= Central brain; 3= Moderate vacuolations in neuropil; 4= Optic lobe; 5= Cell bodies; 7= Ventral nerve cord; 8= Compound eyes; 9= Medulla; 10= Lamina. Abbreviations: BSS = bang-senseless, CS = Canton-Special, NaV = sodium valproate, NSL = no significant lesion, MND = moderate neurodegeneration, OrR = Oregon R, Para = Paralytic, SND = severe neurodegeneration. 100% of juvenile-adult (10-day-old) bss mutant flies showed moderate brain neurodegeneration (B) versus wild-type controls of the same age with NSL (A). The acute brain neurodegeneration of mutants was not alleviated by acute extract feeding (D) versus alleviation by acute feeding with SV (C). See the bar graphs in Figure 3 above for a summary.
Histopathological examination of H & E-stained brain tissues showed no significant brain lesions (focal brain vacuolations ˂ 3 µm in diameter) in wild-type controls of group XIV, while mutant controls of the group (XIV) showed age and duration-dependent progressive brain neurodegeneration during the chronic phase of the study, as follows: 45% of animals showed moderate brain neurodegeneration and 55% of them showed severe brain neurodegeneration on day 6; 40%, moderate brain neurodegeneration and 60%, severe brain neurodegeneration on day 12; and 100%, severe brain neurodegeneration on day 18 (Figure 3C and 5, and Supplementary file 3: Table 6). This indicates that old-adult (16-28-day old) mutant flies show chronic age-dependent progressive brain neurodegeneration, unlike old-adult wild-type controls that depict normal general brain histology during chronic phases of the study.
Figure 5.
Effect of chronic treatment with methanol I. cylindrica root extract on chronic brain neurodegeneration of male old-adult (16-28-day-old) bang-sensitive mutant Drosophila (parabss1). n = 100, (H&E; transverse sections at level of mid-brain, x200 or x400). Bar, 20 µm or 50 µm. A. Wild-type control, x200. B. Mutant control, x200 (B1), x400 (B2). C. Standard control, x400. D. Methanol extract test, x200. Categories of brain neurodegeneration: NSL (vacuolations <3µm in diameter) = Normal; MND, (vacuolations 3-5µm in diameter); SND, (vacuolations >5µm in diameter) = brain histopathology. OrR and CS = wild-type controls; parabss1 = mutant (test) model. 0 = No significant lesion in the neuropil, 1 = Oesophagus; 2 = Central brain; 4 = Optic lobe; 5 = Cell bodies; 6 = Moderate to severe vacuolations in neuropil; 7 = Ventral nerve cord; 8 = Compound eyes; 9 = Medulla; 10 = Lamina. Abbreviations: BSS = bang-senseless, CS = Canton-Special, NaV = sodium valproate, NSL = no significant lesion, MND = moderate neurodegeneration, OrR = Oregon R, Para = Paralytic, SND = severe neurodegeneration. 100% of old-adult (16-28-day-old) bss mutant flies showed brain neurodegeneration of moderate or severe type (B) versus the wild-type controls of the same age with NSL (A). The chronic brain neurodegeneration of mutants was rescued in 100% of mutant flies by chronic (18 days) extract feeding (D) in a similar way to SV feeding (C). See the bar graphs in Figure 3 for a summary.
I. cylindrica Root Extract Lessened Brain Neurodegeneration of bss Paralytic Mutant Flies Following Prolonged but not Short-Term Treatment
The acute brain histopathological features (with H & E-stained tissues) of untreated mutant controls (group XI) were similar to the extract test group (XII) in acute tests, with a marked difference between histopathological findings of SV group (XIII) and extract test group (XII), ie, SV group showed improved brain histology from moderate brain neurodegeneration to normal brain forms in 47% of the animals after 3 h of treatment with SV (Figure 3B and 4, and Supplementary file 3: Table 5). Therefore, short-term ingestion of 0.8 g/mL extract (unlike SV) does not reduce the acute brain neurodegeneration of juvenile-adult mutant flies during the acute phase of the study.
The brain histopathological features (with H & E stain) of untreated mutant controls (group XIV) were markedly defective compared to the extract test group (XV) in the chronic phase of the study. The extract test group (XV) showed a noticeable alleviation of brain neurodegeneration in a duration-dependent fashion as follows: 70% of animals, showed no significant brain lesions, and 30%, had moderate brain neurodegeneration on day 6; 90%, had no significant brain lesions, and 10%, moderate brain neurodegeneration on day 12; 100%, no significant brain lesions on day 18. SV group (XVI) showed similar brain histological features as the extract test group (XV) during the chronic phases of the study as follows: 80% of animals, no significant brain lesions and 20% moderate brain neurodegeneration on day 6; 100%, no significant brain lesions on day 12; 95% no significant brain lesions and 5% of the animals regressed to moderate brain neurodegeneration on day 18, (Figure 3D and 5, and Supplementary file 3: Table 6). The results indicate that prolonged ingestion of 0.025 g/mL extract (like SV) alleviates chronic brain neurodegeneration of old-adult mutant flies in a duration-dependent fashion during the chronic phase of the study. Also, prolonged administration with SV (beyond 12 days) may be detrimental to the improved brain histology of the mutant flies.
Juvenile-Adult and Old-Adult bss Paralytic Mutant Flies Portray Acute and Chronic Brain Axonal Degeneration
Histopathological examination of axonal morphology and Nissl substance on Klüver Luxol Fast Blue-stained tissues found no significant lesions in axons and Nissl substance of wild-type controls of group XI, while mutant controls of the group (XI) showed axonal degeneration in the brain but with normal Nissl substance during the acute phase of the study, and with no differences in axonal morphology and Nissl substance of mutant flies at 1, 2, or 3 h within mutant control group (XI), (Figure 6A and 7, and Supplementary file 3: Table 5). This indicates that juvenile-adult (10-day-old) mutant flies show acute non-progressive axonal degeneration in the brain, compared to the juvenile-adult wild-type controls that depict normal axonal morphology in the acute phase of the study.
Figure 6.
Acute and chronic effects of methanol I. cylindrica root extract on acute and chronic axonal degeneration in the brain of male juvenile adult (10-day-old) and old-adult (16–28-day-old) bang-sensitive mutant Drosophila (parabss1). A. Acute axonal degeneration in the brain of juvenile-adult bang-sensitive mutant Drosophila. B. Effect of acute (1–3-h) extract treatment on acute axonal degeneration in the brain of juvenile-adult mutant flies. C. Chronic axonal degeneration in the brain of old-adult bang-sensitive mutant Drosophila. D. Effect of chronic (6–18-day) extract treatment on chronic axonal degeneration in the brain of old-adult mutant flies. The number of flies with each type of brain axonal morphology (y-axis) plotted against time for different treatment groups; n = 100. OrR and CS = wild-type controls; parabss1 = mutant (test) model. Abbreviations: BSS = bang-senseless, CS = Canton-Special, DA = degenerated axons, NAM = normal axonal morphology, NaV = sodium valproate, OrR = Oregon R, Para = Paralytic. 100% of juvenile-adult (10-day-old) bss mutant flies showed axonal degeneration in the brain versus wild-type controls of the same age with normal brain axonal morphology (A). Acute brain axonal degeneration of mutants was neither alleviated by short-term extract feeding nor short-term feeding with SV (B). 100% of old-adult (16–28-day-old) bss mutant flies showed brain axonal degeneration versus wild-type controls of the same age with normal brain axonal morphology (C). Chronic axonal degeneration of mutants was rescued in 100% of mutant flies by prolonged (6–18 days) extract feeding, similar to feeding with SV (D).
Figure 7.
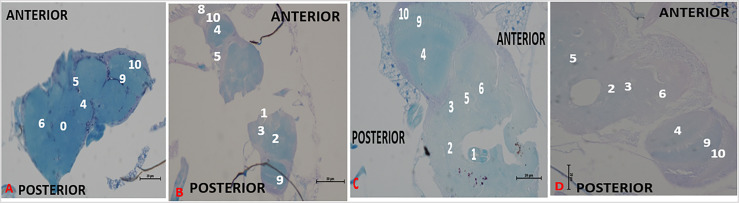
Effect of acute treatment with methanol I. cylindrica root extract on acute axonal degeneration in the brain of male juvenile-adult (10-day-old) bang-sensitive mutant Drosophila (parabss1). n = 100, (Klüver Luxol Fast Blue; transverse sections, x200 or x400). Bar, 20 µm or 50 µm. A low-intensity patchy Klüver LFB stain indicates axonal degeneration/lesion in the Nissl substance. Axons are depicted in blue colour and Nissl substance is shown in magenta/ violet colour. A. Wild-type control, x400 in peripheral neuropil. B. Mutant control, x200 at the level of mid-brain. C. Standard control, x400 at the level of mid-brain. D. Methanol extract test, x400 at the level of mid-brain. NAM, a consistent LFB stain (no light blue patchy areas) = Normal morphology of axons; DA (weak LFB stain/light blue patchy areas) = axonal degeneration. OrR and CS = wild-type controls; parabss1 = mutant (test) model. 0 = No significant lesion in axonal structure, 1 = Oesophagus; 2 = Central brain; 3 = Axonal degeneration; 4 = Optic lobe; 5 = Cell bodies; 6 = No significant lesion in Nissl substance; 8 = Compound eyes; 9 = Medulla; 10 = Lamina. Abbreviations: BSS = bang-senseless, CS = Canton-Special, DA = degenerated axons, NAM = normal axonal morphology, NaV = sodium valproate, OrR = Oregon R, Para = Paralytic. 100% of juvenile-adult (10-day-old) bss mutant flies showed axonal degeneration in the brain (B) versus wild-type controls of the same age with normal axonal morphology (A). Acute axonal degeneration of mutants was neither alleviated by acute extract feeding (D) nor acute feeding with SV (C). See the bar graphs in Figure 6 above for a summary.
Histopathological examination of Klüver Luxol Fast Blue-stained tissues for axonal morphology and Nissl substance showed no significant lesions in axons and Nissl substance of wild-type controls in group XIV, however, the mutant controls in the group (XIV) showed brain axonal degeneration but with normal Nissl substance during the chronic phase of the study, and with no marked differences in nature of axon morphonology and Nissl substance of mutant flies at 6, 12, or 18 days within the mutant controls (group XIV), Figure 6C and 8, and Supplementary file 3: Table 6). This indicates that old-adult (16-28-day old) mutant flies show chronic age-independent non-progressive brain axonal degeneration compared to old-adult wild-type controls which depict the normal axonal structure and Nissl substance in chronic phases of the study.
Figure 8.
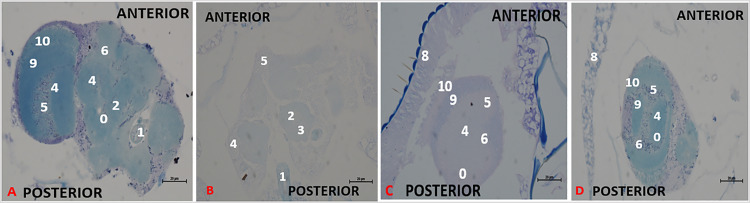
Effect of chronic treatment with methanol I. cylindrica root extract on chronic axonal degeneration in the brain of male old-adult (16-28-day-old) bang-sensitive mutant Drosophila (parabss1). n = 100, (Klüver Luxol Fast Blue; transverse sections, x200 or x400). Bar, 20 µm or 50 µm. A low-intensity patchy Klüver LFB stain indicates axonal degeneration/lesion in the Nissl substance. Axons are depicted in blue colour and Nissl substance is shown in magenta/ violet colour. A. Wild-type control, x400 at the level of mid-brain. B. Mutant control, x400 at the level of mid-brain. C. Standard control, x400 in peripheral neuropil. D. Methanol extract test, x400 in peripheral neuropil. NAM, a consistent LFB stain (no light blue patchy areas) = Normal morphology of axons = Normal; DA (weak LFB stain/light blue patchy areas) = axonal degeneration. OrR and CS = wild-type controls; parabss1 = mutant (test) model. 0 = No significant lesion in axonal structure, 1 = Oesophagus; 2 = Central brain; 3 = Axonal degeneration; 4 = Optic lobe; 5 = Cell bodies; 6 = No significant lesion in Nissl substance; 8 = Compound eyes; 9 = Medulla; 10 = Lamina. Abbreviations: BSS = bang-senseless, CS = Canton-Special, DA = degenerated axons, NAM = normal axonal morphology, NaV = sodium valproate, OrR = Oregon R, Para = Paralytic. 100% of old-adult (16-28-day-old) bss mutant flies showed axonal degeneration in the brain (B) versus wild-type controls of the same age with normal axonal morphology (A). Chronic axonal degeneration of mutants was rescued in 100% of mutant flies by prolonged (6-18 days) extract feeding (C) in a similar way to SV feeding (D). See the bar graphs in Figure 6 for a summary.
I. cylindrica Root Extract Alleviated Brain Axonal Degeneration of bss Paralytic Mutant Flies Following Prolonged but Not Short-Term Treatment
The acute brain axonal degeneration seen with Kluvers LFB-stained tissues of untreated mutant controls (group XI) was similar to the extract test group (XII) of the acute study, with no differences between SV group (XIII) and extract test group (XII), (Figure 6B and 7, and Supplementary file 3: Table 5), indicating that short-term ingestion of 0.8 g/mL extract (like 0.3 mg/mL SV) did not alleviate the acute brain axonal degeneration of juvenile-adult mutant flies during the acute phase of the study.
The chronic axonal degeneration was found in untreated mutant controls of the chronic study (group XIV), whereas the extract test group (XII) of the chronic study showed normal axonal morphology in 100% of treated mutant animals after days 6, 12, or 18 of treatment, and similar changes in axonal morphology were observed with the SV group (XVI), (Figure 6D and 8, and Supplementary file 3: Table 6), indicates that prolonged ingestion of 0.025 g/mL extract (like 0.15 mg/mL SV) alleviates the chronic brain axonal degeneration of old-adult mutant flies in a duration-independent pattern during the chronic phase of the study.
Discussion
The number of epilepsy cases has increased tremendously within the past few years and between 2019 and 2020 around 50 million people had the condition globally and almost 80% of these live in low- and middle-income countries.2,3 Imperata cylindrica (L.) P. Beauv (Poaceae) is a famous neuromedicinal, anticonvulsant, and antiepileptic herb in traditional therapy due to its receptor inhibition and antioxidant properties,32,34-38 however, to the best of our knowledge little is known regarding the neuroprotective efficacy of I. cylindrica as an antiepileptic herb and few studies have demonstrated the neuroprotective potential of the plant on glutamate-induced neurotoxicity in rat cerebral cortical cells.37,38 The study demonstrated that phytochemicals of I. cylindrica root extract had neuroprotective roles on convulsions, cognitive deficits, and brain histopathology of Drosophila melanogaster bang senseless mutant models of epilepsy. This is vital because little is known about the neuroprotective role of the phytochemicals,35,36,42 and in this study, the plant phytochemicals are thought to have upregulated endogenous antioxidants and inhibited receptor or voltage-gated sodium ion channels (VGSCs) which are linked to increased antioxidant status, decreased inflammation and apoptosis, increased tissue repair, and improved cell biology in the brain of the mutant flies faced with epilepsy-like neuropathological features.
The high contents of phytochemicals isolated from the methanol Imperata cylindrica (L.) P. Beauv (Poaceae) root extract (flavonoids, polyphenols, chromones 1 and 2, saponins, tannins, cardiac glycosides, and alkaloids) are similar to those isolated previously from ethanolic, methanolic, and aqueous root and rhizome extracts of Imperata cylindrica (Poaceae or Gramineae), but previous data also demonstrated lignans, coumarins, and reducing sugars in the plant.34,35,66 Plant antioxidants have alleviative properties and can be utilised in the control and treatment of neurodegenerative diseases89,90 and epilepsy phenotypes91,92 due to genetic mutations in humans and lower organisms such as D. melanogaster, rodents, zebrafish (Danio rerio), and nematodes (C. elegans).89,90 Polyphenols, flavonoids, and chromones were in the highest concentration in the extract which concurs with previous studies involving ethanolic and methanolic extracts of rhizomes of I. cylindrica Beauv. (Gramineae) var major, (Nees) C. E. Hubb.37,93 and these have strong antioxidant properties.94,95 Therefore, the herb can be utilized in the development of therapeutic molecules47,96-100 due to the neuroprotective attributes of flavonoids, polyphenols, and chromones that have been demonstrated in brain cells of rodents37-39 via antioxidant and receptor inhibition mechanisms37-39 and these mechanisms are linked to antiseizure, antiepileptogenic, and cognitive deficit alleviation attributes in rodents with neurological disorders.40,41
The current study has demonstrated that bang-senseless (bss) D. melanogaster mutant models (parabss) depict marked acute and chronic abnormal brain phenotypes (age-dependent progressive brain neurodegeneration and axonal degeneration) and significant neuropathological symptoms (acute and chronic convulsions and cognitive deficits or learning and memory deficits) in juvenile and old-adults arising from a gain-of-function point mutation in a para sodium channel gene at the para locus (up-regulates and increases the activity of the gene) in neurons of the current mutant D. melanogaster models of epilepsy (parabss1), unlike the wild-type controls (Oregon R and Canton-Special) which showed normal brain morphology, cognitive functions, and without bang sensitive and convulsion behaviors. Therefore, the abnormal brain phenotype and neuropathological features that were demonstrated in the mutant flies (parabss1), validate a mutation in the para gene (overexpression of para) in the neurons of brain tissues of juvenile and old-adult D. melanogaster bss mutants15,16 and is similar to the expression of a para gene demonstrated in neuronal brain tissues of adult D. melanogaster when UAS-para is driven by ELAV-GAL4 driver,101-104 and the expression of the mutant genes has been linked to human-like brain neurodegenerative disorders in Drosophila.101,103,105,106
Altered gene expression and tissue-specific expression of specific neurodegenerative mutant genes such as epilepsy mutant genes21,107 in D. melanogaster cause neuropathological phenotypes of the neurodegenerative disorder (in our case, epilepsy)21,108-110. The tissue-specific expression of mutant genes and transgenes in neurons has been linked to neuropathological phenotypes such as neurodegeneration, defects in mean lifespan, and locomotion in D. melanogaster models of neurodegenerative diseases and epilepsy.101-106,108,109,111 The expression of tissue-specific mutants and transgenes provides robust and precise models with which to study human neurodegenerative diseases, epilepsy, and possible preventive and curative treatments for neurological disorders.21,108-110
The neuropathological findings of the current study concur with previous studies that demonstrated bang-sensitive behaviour, convulsions in mutant paralytic D. melanogaster models of seizures overexpressing para gene (parabss1) due to a mutation of the gene15,16 and resulting reduction of seizure-stimulation threshold arising from increased expression of the VGSCs in the neurons of the mutants.15,16 The impaired learning and memory functions demonstrated in the current parabss1 mutant Drosophila compared to the wild-type controls are similar to the cognitive phenotypes previously shown in the model (parabss1),15-17 and concur with other findings that have shown cognitive deficits in Drosophila models of neurodegeneration (ELAV-GAL4 > UAS-GFP and ELAV-GAL4 > UAS-TAU). 57 Cognitive deficits in bang-sensitive paralytic D. melanogaster are attributable to a mutation of the para gene in neurons of the flies which disrupts the metabolic pathways and neuronal activities leading to bang-sensitive and seizure behaviors that originate in the brain of the flies (epileptogenic zone),15,53,83,112 and the seizures and their effects spread to secondary brain centers including the motor centers and learning and memory centers in mushroom bodies (cognitive centers) hence distorting the learning and memory functions (cognitive deficits) in the brain of D. melanogaster. 113 The study demonstrated that in general, the current parabss1 mutants have less magnitudes of memory function than the corresponding levels of learning function in all the study groups, the observed general differences in the two parameters come from the method (APS assay) that was employed to assess the parameters, ie, memory tests were performed 6 h after performance of the learning tests without re-training the flies to the task learnt. 57
The current parabss1 mutants show progressive age-related brain neurodegeneration (neurovacuolation) and axonal degeneration concurring with previous studies on bss and excitability Drosophila (parabss, jus, eas, tko, ses B) that found age-related brain neurodegeneration due to gene mutations in neurons of the flies.22,114,115 The axonal degeneration found in the current mutant models is typical of axonal-type neurodegenerative disorder (axonopathy) found in Drosophila models of human genetic neurodegenerative disease,116-118 and axonal degeneration is a cause of disease pathogenesis, disability, and loss of function in the CNS during epilepsy.119-121 It is characterized by a reduction in the density and number of nerve tracts as demonstrated previously in D. melanogaster bang-sensitive mutants (parabss, ses B, eas, jus, tko), 115 and is due to a mutation of the para gene at the para locus in neurons of bss para D. melanogaster models of epilepsy which induces defective nerve fibers.20,83,84,115,122 Similarly, mammalian forms of genetic epilepsy due to gene mutations in VGSC β subunit genes (SCN1B-SCN4B) and α subunit genes (SCN1A, SCN2A, SCN3A, and SCN8A) in the brain have been linked to neurodegeneration and pathology found in epilepsy.9-11,123
The neurodegeneration (degeneration of nerve tracts) in the brain of the bang senseless paralytic flies distorts electrical activity and conduction of impulses,124-126 contributes to the development of seizure and epileptic phenotypes (bang-sensitivity, convulsions, learning, and memory deficits) observed in the brain and peripheral parts of the current mutants, features of which are consistent with previous studies.124-126 In conclusion, the mutant flies of the study (parabss1) display fascinating epileptic phenotypes and behaviors similar to mutants of the BS paralytic family such as sdaiso,7,8 easPC, 80 and tko25t mutants, as well as other Drosophila models of human seizures10,15,17,22,114,115 ie, progressive age-related brain neurodegeneration and axonal degeneration of the mutant flies, are linked to bang-sensitive behavior, seizures, and defective cognitive functions, consistent with CNS neurodegenerative and axonopathy features of bss paralytic D. melanogaster seizure models.10,17,83,114,124-127
To the best of our knowledge, the study is the first of its kind to demonstrate the staining affinity of CNS axons for combined Luxol fast blue (LFB) and Nissl (Klüver's) stain in Drosophila models indicating that Drosophila neurons and axonal sheaths could be containing biological compounds mimicking the phospholipids or lipoproteins of the myelin sheath and Schwann cells of myelinated neurons. However, Drosophila axons are non-myelinated and not surrounded by Schwann cells, 117 instead, they are encased in ‘ensheathing glial cells’ within the CNS of Drosophila , 87 and although they are non-myelinated and not surrounded by Schwann cells,117,128,129 the current data concurs with previous studies which demonstrated Schwann-like (Drosophila) or Schwann cells (mammals) to be existing in unmyelinated axons in form of grooves but not surrounding the axons, closely interdependent with the axons to provide structural integrity and function of the axons.117,130-132 Also, Schwann cells have been reported to secrete neurotrophic factors and myelin-related proteins.130,133 The non-myelinating Schwann cells of mammals are comparable in function to the ‘ensheathing’ and ‘wrapping’ Drosophila glial cells of the CNS and peripheral nervous system of Drosophila respectively and are responsible for encasing axons and neuropil of the flies.86,87,134 Future studies should focus on the utility of combined Luxol Fast Blue and Nissl (Klüver's) staining technique in experimental examination of the basic neuronal structure in the CNS of Drosophila to establish the staining properties of the nervous tissues of Drosophila.
Acute and chronic extract treatment significantly reduced the neuropathological features of the mutant flies (convulsions, learning and memory deficits, and brain histopathology) in the extract test animals and the standard control group treated with SV in duration and dose-dependent fashions compared to the mutant (negative) control group, and the values of the parameters (CT, PT, RT, learning/memory pass rate, brain, and axonal neurodegeneration) of the extract test and standard control groups were similar to (not significantly different from) the wild-type control group indicating that our I. cylindrica extract induces significant neuroprotective activities (alleviates the neuropathological features) in the nervous tissue of the mutant flies, with a similar neuroprotective capacity to the standard control drug of our study (SV).
However, the acute and chronic age-related brain neurodegeneration and axonal degeneration of the mutant flies were alleviated by prolonged (6-18 days) extract treatment but not by short-term (1-3 h) treatment and the standard antiepileptic (SV) depicted better alleviative potential on the brain histopathology than the plant extract since the former modulated the brain histological defects of the mutant flies following both short-term (3 h) and prolonged (6-12 days) treatments compared to the extract which showed modulatory potential only after prolonged treatment (6-18 days). The standard antiepileptic showed a side-effect on brain morphology since further feeding with SV (18 days) caused brain histopathological features in the previously improved brain histology of the mutant flies indicating that although antiepileptic molecules have marked beneficial roles, they may cause detrimental side effects on the CNS, especially in cases of chronic antiepileptic therapy.135,136
The neuroprotective attributes of the current herb (I. cylindrica) are consistent with previous findings that demonstrated the phytochemicals of the herb to be possessing neuroprotective attributes on the neuropathology of rat brain cells37,38 and is in tandem with previous studies that suggest plant-based nonenzymatic antioxidants notably phenols, flavonoids, chromones, and glycosides to be contributors neuroprotection on brain cell damage by the provision of significant antioxidant activities (antioxidation mechanism controls oxidative stress markers in the brain of animals) that are linked to improved brain cell biology,37,39 thereby inducing anticonvulsant and antiepileptogenic pathways,40,137 and alleviate cognitive deficits of animals. 41 The antiepileptic similarity of the herb compared to standard anticonvulsant (SV) indicates that the extract similar to standard antiepileptics can alleviate the bang-sensitive behaviour of Drosophila models of epilepsy by improving brain cell biology, seizure-control, and cognitive-function modulatory pathways in concurrence with previous findings.15,61,127
Again, the potential of our extract to modulate the brain histopathology of the mutant flies is in tandem with a review by Trojnar et al, (2002) who demonstrated novel standard antiepileptics (lamotrigine, tiagabine, and topiramate) as having neuroprotective effects on CNS morphology of epileptic animals, 138 and concurs with previous studies that reported similar neuroprotectant potential in antioxidant compounds and antiepileptic drugs on epileptic animals.137,139 However, our findings contradict with findings of Qiao et al, (2000) where Vigabatrin demyelinated the brain of epileptic rats 140 indicating that antiepileptic molecules have side effects. 135 The differences and similarities between the current data and those by Trojnar et al, (2002), Qiao et al, (2000), and Cavanna et al, (2010) are striking but not surprising since antiepileptics can depict neuroprotective, neutral, or detrimental CNS activities in epileptic models.135,138,140
Oxidative stress has been strongly related to the pathogenesis of neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease (PD), and epilepsy.115,141-146 Bang-sensitive Drosophila models of epilepsy are vital models for oxidative stress-related neurological defects, and the abnormal phenotypes in the models such as parabss, jus, eas, and tko are attenuated by feeding with an antioxidant. 115 In addition, attenuation and rescue of neurological defects by natural antioxidants have been reported in Drosophila models of epilepsy 143 and Drosophila is an emerging model organism for plant-derived antioxidants.147-149 Most importantly, the potential role of natural antioxidants from plant extracts as neuroprotectants for treating and preventing neurodegenerative diseases and epilepsy has been shown in vitro, in vivo, and in clinical settings.89,137,150 In addition, hyperactivity of the VGCs due to mutation (s) in the para gene (gain-of-function mutation in the para sodium channel gene at the para locus) in neuronal brain tissues of bang-sensitive (BS) Drosophila models of epilepsy is strongly linked to the neuropathological features of epilepsy (brain histopathology, seizures, and cognitive deficits).15,16
The brain has very high metabolic activities and is the most aerobically active organ in the body, the organ is very susceptible to oxidative stress, causing oxidative-induced brain injuries and neuronal hyperexcitability, 139 such as in bang-sensitive Drosophila and the defects can be rescued via the antioxidant pathways (feeding with neuroprotectant antioxidants),115,139 and by treating with molecules that inhibit the VGSCs.151,152 Therebefore, the proposed mechanisms by which I. cylindrica provides its neuroprotective attributes are the ‘antioxidant’ and ‘receptor inhibition’ mechanisms115,139,151,152 arising from the main phytochemicals of the plant (polyphenols, flavonoids, and chromones), these provide important biological activities within the brain cells including the improvement of antioxidant status, tissue repair, decreased inflammation and apoptosis, improvement of brain cell biology,37,39 and binding and inhibition of VGSCs and receptors (eg, 5-HT2B) by chromones 1 and 2.37,38,153 These in turn result in the alleviation of brain histopathology, seizure control, and alleviation of cognitive deficits in our mutant D. melanogaster model of epilepsy. This concurs with previous studies that have shown novel therapeutic strategies including ‘natural antioxidant therapy’ using plant extracts to be able to prevent epileptogenesis, modify epileptic phenotypes, and attenuate the associated neurological deficits and defects in epileptic animals.89,90,154 Also, similar findings from a previous study showed attenuation of neuromotor deficits by natural antioxidants of a plant (Decalepis hamiltonii) root extract in a transgenic Drosophila model of Parkinson's disease. 155 The current findings concur with previous data where antioxidants promoted brain regeneration and axonal growth and repair in animal models of seizure disorders through the inhibition of oxidative stress and promotion of antioxidant pathways156,157 and by epigenetic modulation. 158 These regenerative potentials are linked to the rescue of the defective brain electrophysiology, improvement of brain metabolic pathways, and establishment of improved conduction of impulses in the motor brain centers and mushroom bodies of Drosophila brains,83,159 which causes cessation of seizures and improves cognitive functions in the flies. 160
Given the weight of the flies tested (1 mg per fly) and that they can consume between 20–100 µg a day and twice this amount when starved for 14–17 h, 161 the doses used can be extrapolated to the human context eg 0.8 g/mL utilized in acute therapy, and 0.025 g/mL used in chronic therapy correspond to 16 g/kg and 0.5 g/kg respectively when projected to a human adult.
The current study had some limitations: it did not focus on the evaluation of the neuroprotective molecular mechanisms via the antioxidant signaling and receptor binding inhibition mechanisms, did not emphasize the gender-related differences associated with the abnormal phenotypes and effect of the herb on the phenotypes of the mutant flies, and only focused on the effect of the herb on a genetic form of epilepsy while leaving out acquired/induced convulsions like the antimuscarinic and pentylenetetrazole kindling models that are often studied137,162; a prospective study will be conducted to assess the practical attributes of antioxidant and receptor inhibition pathways related to the neuroprotective effects of I. cylindrica root extract using bss Drosophila, other genetic models and kindling models of convulsions.
Conclusion
The juvenile-adult and old-adult mutant flies (parabss1) show age-related progressive brain neurodegeneration and axonal degeneration and associated acute and chronic seizure and cognitive deficit phenotypes. The methanol root extract of I. cylindrica in a similar magnitude to sodium valproate exerts neuroprotective properties on the neuropathological features of the mutant flies by proposed antioxidant and receptor inhibition mechanisms arising from the main phytochemicals of the herb (Polyphenols, flavonoids, and chromones 1 and 2) which increase the antioxidant status and tissue repair, decrease inflammation and apoptosis, improve brain cell biology and cause inhibition of VGSCs and receptors in the brain cells of the mutant flies. This results in the improvement of brain histology, seizure control, and improvement of cognitive functions in our mutant D. melanogaster model of epilepsy.
Therefore, the methanolic extract contains high amounts of non-enzymatic antioxidants (phenols, flavonoids, and chromones 1 and 2) and non-nitrogenous ligands for receptor and VGSC inhibition (chromones 1 and 2) reflecting huge antioxidant, receptor binding, and inhibition, and neuroprotective powers of I. cylindrica root extract in genetic cases of epilepsy associated with a gain-of-function point mutation in the para sodium channel gene at the para locus in parabss1 mutant lines. Thus, we recommend more experimental and clinical studies regarding this extract in treating genetic forms of epilepsy.
Supplemental Material
Supplemental material, sj-docx-1-chp-10.1177_2515690X231160191 for Attenuation of Seizures, Cognitive Deficits, and Brain Histopathology by Phytochemicals of Imperata cylindrica (L.) P. Beauv (Poaceae) in Acute and Chronic Mutant Drosophila melanogaster Epilepsy Models by Fred Ssempijja, Samuel Sunday Dare, Edmund E. M. Bukenya, Keneth Iceland Kasozi, Ritah Kenganzi, Edgar Mario Fernandez and Marta Vicente-Crespo in Journal of Evidence-Based Integrative Medicine
Supplemental material, sj-docx-2-chp-10.1177_2515690X231160191 for Attenuation of Seizures, Cognitive Deficits, and Brain Histopathology by Phytochemicals of Imperata cylindrica (L.) P. Beauv (Poaceae) in Acute and Chronic Mutant Drosophila melanogaster Epilepsy Models by Fred Ssempijja, Samuel Sunday Dare, Edmund E. M. Bukenya, Keneth Iceland Kasozi, Ritah Kenganzi, Edgar Mario Fernandez and Marta Vicente-Crespo in Journal of Evidence-Based Integrative Medicine
Supplemental material, sj-docx-3-chp-10.1177_2515690X231160191 for Attenuation of Seizures, Cognitive Deficits, and Brain Histopathology by Phytochemicals of Imperata cylindrica (L.) P. Beauv (Poaceae) in Acute and Chronic Mutant Drosophila melanogaster Epilepsy Models by Fred Ssempijja, Samuel Sunday Dare, Edmund E. M. Bukenya, Keneth Iceland Kasozi, Ritah Kenganzi, Edgar Mario Fernandez and Marta Vicente-Crespo in Journal of Evidence-Based Integrative Medicine
Acknowledgements
We thank the members of DrosAfrica, TReND (Teaching and Research in Natural Sciences for Development in Africa), and 4Sayansi for the helpful donation of laboratory equipment used in the present study at the Institute of Biomedical Research (IBR), Kampala International University, Western Campus, Bushenyi, Uganda, as well as for the technical support provided through conduction of workshops on the use of Drosophila models in Biomedical research in support of the study. We thank fellow members of our laboratory for their advice and helpful discussions throughout the project, Richard Baines and Bloomington Drosophila Stock Center, U.S.A for providing the mutant (parabss1) and wild-type (OrR and CS) fly stocks respectively.
Abbreviations
- BS
Bang-sensitive
- BSS
Bang senseless
- CS
Canton-Special
- CNS
Central nervous system
- OrR
Oregon R
- Para
Paralytic
- SV
Sodium valproate
- VGSC
Voltage-gated sodium ion channel.
Correction (September 2023): :Figures 5, 7 and 8 have been updated since its original publication.
Footnotes
Authors’ Note: The datasets used and/or analyzed during the current study and the Supplemental materials are available at: https://figshare.com/s/b484daeacf410f8fb9ea.
Authors' Contributions: Fred Ssempijja: conceptualization, Principal Investigator, methodology, data collection, validation, data analysis and interpretation, writing—original draft preparation, and writing—review and editing. Samuel Sunday Dare: data analysis and interpretation, writing—review and editing, and supervision of this study. Edmund E. M. Bukenya: validation, writing—review and editing, and supervision of this study. Keneth Iceland Kasozi: validation, data analysis and interpretation, writing—original draft preparation, and writing—review and editing. Ritah Kenganzi: methodology, data collection, validation, writing—original draft preparation, and writing—review and editing. Edgar Mario Fernandez: conceptualization, methodology, and writing—review and editing. Marta Vicente-Crespo: conceptualization, methodology, validation, writing—review and editing, and supervision of this study. All authors have read and agreed to publish the version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The experimental protocol was approved by the Institutional Review Ethics Committee of Kampala International University, Western Campus, Uganda (study protocol code: MSC-ANA-001/142-DU), and carried out following the guidelines given by the Uganda Council for Higher Education (UCHE).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Not applicable
ORCID iDs: Fred Ssempijja https://orcid.org/0000-0003-1849-7185
Keneth Iceland Kasozi https://orcid.org/0000-0002-5763-7964
Trial Registration: Not applicable
Supplemental Material: Supplemental materials for this article are available online at: https://figshare.com/s/b484daeacf410f8fb9ea
References
- 1.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE Official report: A practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-482. doi: 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 2.WHO. Epilepsy. World Health Organization. Published 2019. Accessed July 14, 2021. https://www.who.int/health-topics/epilepsy#tab=tab_1
- 3.WHO. Epilepsy. World Health Organization. Published 2020. Accessed July 14, 2021. https://www.who.int/health-topics/epilepsy#tab=tab_1
- 4.WHO. Epilepsy. World Health Organization. Published 2022. Accessed January 7, 2022. https://www.who.int/news-room/fact-sheets/detail/epilepsy
- 5.Reynolds EH. The ILAE/IBE/WHO global campaign against epilepsy: Bringing epilepsy “out of the shadows.”. Epilepsy Behav. 2000;1(4):S3-S8. doi: 10.1006/ebeh.2000.0104 [DOI] [PubMed] [Google Scholar]
- 6.Beghi E, Giussani G. Aging and the epidemiology of epilepsy. Neuroepidemiology. 2018;51(3-4):216-223. doi: 10.1159/000493484 [DOI] [PubMed] [Google Scholar]
- 7.Duggan M. Epilepsy and its effects on children and families in rural Uganda. Afr Health Sci. 2013;13(3):613-623. doi: 10.4314/ahs.v13i3.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ba-Diop A, Marin B, Druet-Cabanac M, Ngoungou EB, Newton CR, Preux P-M. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13(10):1029-1044. doi: 10.1016/S1474-4422(14)70114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ademuwagun IA, Rotimi SO, Syrbe S, Ajamma YU, Adebiyi E. Voltage gated sodium channel genes in epilepsy: Mutations, functional studies, and treatment dimensions. Front Neurol. 2021;12(387). doi: 10.3389/fneur.2021.600050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlett IC, Rusan ZM, Parker L, Tanouye MA. Drosophila as a model for intractable epilepsy: Gilgamesh suppresses seizures in para bss1 heterozygote flies. G3 Genes|Genomes|Genetics. 2013;3(8):1399-1407. doi: 10.1534/g3.113.006130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaman T, Helbig I, Božović IB, et al. Correction. Ann Neurol. 2019;85(6):948-948. doi: 10.1002/ana.25485 [DOI] [PubMed] [Google Scholar]
- 12.Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery.Pharmacol Rev 2011;63(2):411-436. doi: 10.1124/pr.110.003293.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravenscroft TA, Janssens J, Lee P-T, et al. Drosophila voltage-gated sodium channels are only expressed in active neurons and are localized to distal axonal initial segment-like domains. J Neurosci. 2020;40(42):7999-8024. doi: 10.1523/JNEUROSCI.0142-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piggott BJ, Peters CJ, He Y, et al. Paralytic, the Drosophila voltage-gated sodium channel, regulates proliferation of neural progenitors. Genes Dev. 2019;33(23-24):1739-1750. doi: 10.1101/gad.330597.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker L, Padilla M, Du Y, Dong K, Tanouye MA. Drosophila as a model for epilepsy: Bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics. 2011;187(2):523-534. doi: 10.1534/genetics.110.123299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunliffe VT, Baines RA, Giachello CNG, et al. Epilepsy research methods update: Understanding the causes of epileptic seizures and identifying new treatments using non-mammalian model organisms. Seizure. 2015;24(44-51). doi: 10.1016/j.seizure.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 17.Michno K, van de Hoef D, Wu H, Boulianne GL. Modeling age-related diseases in Drosophila: Can this fly? Curr Top Dev Biol. 2005;71(05):199-223. doi: 10.1016/S0070-2153(05)71006-1 [DOI] [PubMed] [Google Scholar]
- 18.Kaas GA, Kasuya J, Lansdon P, et al. Lithium-Responsive seizure-like hyperexcitability is caused by a mutation in the Drosophila voltage-gated sodium channel gene paralytic. eneuro. 2016;3(5):ENEURO.0221-16.2016. doi: 10.1523/ENEURO.0221-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Voet M, Nijhof B, Oortveld MAW, Schenck A. Drosophila models of early onset cognitive disorders and their clinical applications. Neurosci Biobehav Rev. 2014;46(P2):326-342. doi: 10.1016/j.neubiorev.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 20.Kroll JR, Saras A, Tanouye MA. Drosophila sodium channel mutations: Contributions to seizure-susceptibility. Exp Neurol. 2015;274(Pt A):80-87. doi: 10.1016/j.expneurol.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasko P, Lüthy K. Investigating rare and ultrarare epilepsy syndromes with Drosophila models. Fac Rev. 2021;10(10). doi: 10.12703/r/10-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Wu C-F. Genetic modifications of seizure susceptibility and expression by altered excitability in Drosophila Na + and K + channel mutants. J Neurophysiol. 2006;96(5):2465-2478. doi: 10.1152/jn.00499.2006 [DOI] [PubMed] [Google Scholar]
- 23.Löscher W, Schmidt D. New horizons in the development of antiepileptic drugs: Innovative strategies. Epilepsy Res. 2006;69(3):183-272. doi: 10.1016/j.eplepsyres.2006.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paemka L, Mahajan VB, Ehaideb SN, et al. Seizures are regulated by ubiquitin-specific peptidase 9 X-linked (USP9X), a De-ubiquitinase. PLOS Genet. 2015;11(3):e1005022. Frankel WN, ed. doi: 10.1371/journal.pgen.1005022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varesio C, Provenzi L, Donetti Dontin S, et al. Pathways to quality of life in adolescents with genetic generalized epilepsy: The role of seizure features and affective symptoms. Epilepsy Behav. 2020;109(107115). doi: 10.1016/j.yebeh.2020.107115 [DOI] [PubMed] [Google Scholar]
- 26.Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust. 2018;208(5):226-233. doi: 10.5694/mja17.00951 [DOI] [PubMed] [Google Scholar]
- 27.Kwan P, Brodie MJ. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet. 2001;357(9251):216-222. doi: 10.1016/S0140-6736(00)03600-X [DOI] [PubMed] [Google Scholar]
- 28.Reid CA, Phillips AM, Petrou S. HCN Channelopathies: Pathophysiology in genetic epilepsy and therapeutic implications. Br J Pharmacol. 2012;165(1):49-56. doi: 10.1111/j.1476-5381.2011.01507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel UK, Malik P, DeMasi M, Lunagariya A, Jani VB. Multidisciplinary Approach and Outcomes of Tele-neurology: A Review. Cureus. 2019;11(4):e4410. doi: 10.7759/cureus.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danesi MA, Adetunji JB. Use of alternative medicine by patients with epilepsy: A survey of 265 epileptic patients in a developing country. Epilepsia. 1994;35(2):344-351. doi: 10.1111/j.1528-1157.1994.tb02442.x [DOI] [PubMed] [Google Scholar]
- 31.Easterford K, Clough P, Comish S, Lawton L, Duncan S. The use of complementary medicines and alternative practitioners in a cohort of patients with epilepsy. Epilepsy Behav. 2005;6(1):59-62. doi: 10.1016/j.yebeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Ge T, Pan Z, Leng Y, Lv J, Li B. The effects of herbal medicine on epilepsy. Oncotarget. 2017;8(29):48385-48397. doi: 10.18632/oncotarget.16801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur J, Famta P, Famta M, et al. Potential anti-epileptic phytoconstituents: An updated review. J Ethnopharmacol. 2021;268(113565). doi: 10.1016/j.jep.2020.113565 [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Zhang B-F, Yang L, Chou G-X, Wang Z-T. Two new chromones and a new flavone glycoside from Imperata cylindrica. Chin J Nat Med. 2014;11(1):77-80. doi: 10.3724/SP.J.1009.2013.00077 [DOI] [Google Scholar]
- 35.Jung Y-K, Shin D. Imperata cylindrica: A review of phytochemistry, pharmacology, and industrial applications. Molecules. 2021;26(5):1454. doi: 10.3390/molecules26051454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan AW, Khan A, Shah SMM, Ullah A, Faheem M, Saleem M. An updated list of neuromedicinal plants of Pakistan, their uses, and phytochemistry. Evidence-Based Complement Altern Med. 2019;2019(6191505):1-27. doi: 10.1155/2019/6191505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon JS, Lee MK, Sung SH, Kim YC. Neuroprotective 2-(2-Phenylethyl)chromones of Imperata c ylindrica. J Nat Prod. 2006;69(2):290-291. doi: 10.1021/np0503808 [DOI] [PubMed] [Google Scholar]
- 38.Williams DA, Zaidi SA, Zhang Y. 5-Hydroxy-2-(2-phenylethyl)chromone (5-HPEC): A novel non-nitrogenous ligand for 5-HT2B receptor. Bioorg Med Chem Lett. 2014;24(6):1489-1492. doi: 10.1016/j.bmcl.2014.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altinoz E, Erdemli M, Gul M, et al. Neuroprotection against CCl 4 induced brain damage with crocin in Wistar rats. Biotech Histochem. 2018;93(8):623-631. doi: 10.1080/10520295.2018.1519725 [DOI] [PubMed] [Google Scholar]
- 40.Azikiwe C, Siminialayi I, Brambaifa N, Amazu L, Enye J, Ezeani M. Anticonvulsant activity of the fractionated extract of Crinum jagus bulbs in experimental animals. Asian Pacific J Trop Dis. 2012;2(SUPPL.1):S446-S452. doi: 10.1016/S2222-1808(12)60201-1 [DOI] [Google Scholar]
- 41.Mir NT, Saleem U, Anwar F, et al. Lawsonia Inermis markedly improves cognitive functions in animal models and modulate oxidative stress markers in the brain. Medicina (B Aires). 2019;55(5):192. doi: 10.3390/medicina55050192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuete V, Sandjo LP, Wiench B, Efferth T. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J Ethnopharmacol. 2013;149(1):245-253. doi: 10.1016/j.jep.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 43.Auditeau E, Chassagne F, Bourdy G, et al. Herbal medicine for epilepsy seizures in Asia, Africa and Latin America: A systematic review. J Ethnopharmacol. 2019;234(December 2018):119-153. doi: 10.1016/j.jep.2018.12.049 [DOI] [PubMed] [Google Scholar]
- 44.Aghdash SN. Herbal medicine in the treatment of epilepsy. Curr Drug Targets. 2021;22(3):356-367. doi: 10.2174/1389450121999201001152221 [DOI] [PubMed] [Google Scholar]
- 45.Solanki R, Nagori BP. New method for extracting phytoconstituents from plants. Int J Biomed Adv Res. 2012;3(10):770-774. doi: 10.7439/ijbar.v3i10.779 [DOI] [Google Scholar]
- 46.Benil Pb, Rani S, Kim YO, et al. Prophylactic efficacy of Boerhavia diffusa L. Aqueous extract in toluene induced reproductive and developmental toxicity in Drosophila melanogaster. J Infect Public Health. 2020;13(2):177-185. doi: 10.1016/j.jiph.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 47.Lalthanpuii PB, Lalchhandama K. Phytochemical analysis and in vitro anthelmintic activity of Imperata cylindrica underground parts. BMC Complement Med Ther. 2020;20(1):332. doi: 10.1186/s12906-020-03125-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nara M, Richard B. Drosophila melanogaster in the study of epilepsy. 1st ed. Vol. 20. Taylor & Francis; 2008. Accessed January 10, 2022. https://pubmed.ncbi.nlm.nih.gov/18309791/ [Google Scholar]
- 49.Dare SS, Merlo E, Rodriguez Curt J, Ekanem PE, Hu N, Berni J. Drosophila parabss flies as a screening model for traditional medicine: Anticonvulsant effects of Annona senegalensis. Front Neurol. 2021;11(January):1-14. doi: 10.3389/fneur.2020.606919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wesley ER, Hawley RS, Billmyre KK. Genetic background impacts the timing of synaptonemal complex breakdown in Drosophila melanogaster. Chromosoma. 2020;129(3-4):243-254. doi: 10.1007/s00412-020-00742-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tapia A, Giachello CN, Palomino-Schätzlein M, Baines RA, Galindo MI. Generation and characterization of the Drosophila melanogaster paralytic gene knock-out as a model for dravet syndrome. Life. 2021;11(11):1261. doi: 10.3390/life11111261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin W-H, Giachello CNG, Baines RA. Seizure control through genetic and pharmacological manipulation of Pumilio in Drosophila : A key component of neuronal homeostasis. Dis Model Mech. 2017;10(2):141-150. doi: 10.1242/dmm.027045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edelsparre AH, Vesterberg A, Lim JH, Anwari M, Fitzpatrick MJ. Alleles underlying larval foraging behaviour influence adult dispersal in nature. Ecol Lett. 2014;17(3):333-339. Thrall P, ed. doi: 10.1111/ele.12234. [DOI] [PubMed] [Google Scholar]
- 54.Liu G, Tan FH, Lau SA, et al. Lactic acid bacteria feeding reversed the malformed eye structures and ameliorated gut microbiota profiles of Drosophila melanogaster Alzheimer’s Disease model. J Appl Microbiol. 2020;132(4):3155-3167. doi:10.1111/jam.14773 [DOI] [PubMed] [Google Scholar]
- 55.Thurmond J, Goodman JL, Strelets VB, et al. Flybase 2. 0 : The next generation.Nucleic Acids Res 2019;47(October 2018):759-765. doi: 10.1093/nar/gky1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hudry B, de Goeij E, Mineo A, et al. Sex differences in intestinal carbohydrate metabolism promote food intake and sperm maturation. Cell. 2019;178(4):901-918.e16. doi: 10.1016/j.cell.2019.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali YO, Escala W, Ruan K, Zhai RG. Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. J Vis Exp. 2011;49(2504):1-5. doi: 10.3791/2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takai A, Yamaguchi M, Yoshida H, Chiyonobu T. Investigating developmental and epileptic encephalopathy using Drosophila melanogaster. Int J Mol Sci. 2020;21(17):6442. doi: 10.3390/ijms21176442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mihajilov-Krstev T, Jovanović B, Jović J, et al. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 2014;80(18):1698-1705. doi: 10.1055/s-0034-1383182 [DOI] [PubMed] [Google Scholar]
- 60.Song J, Tanouye MA. From bench to drug: Human seizure modeling using Drosophila. Prog Neurobiol. 2008;84(2):182-191. doi: 10.1016/j.pneurobio.2007.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds ER, Stauffer EA, Feeney L, Rojahn E, Jacobs B, McKeever C. Treatment with the antiepileptic drugs phenytoin and gabapentin ameliorates seizure and paralysis of Drosophila bang-sensitive mutants. J Neurobiol. 2004;58(4):503-513. doi: 10.1002/neu.10297 [DOI] [PubMed] [Google Scholar]
- 62.Arias AM, Dahmann C. In: Dahmann C, ed. Drosophila: Methods and Protocols. 1st ed. Humana Press Inc.; 2008:1-25. https://www.springer.com/gp/book/9781588298171#aboutBook [Google Scholar]
- 63.Parker L, Howlett IC, Rusan ZM, Tanouye MA. Seizure and epilepsy: Studies of seizure disorders in Drosophila. Int Rev Neurobiol. 2011;99(1-21). Elsevier Inc. doi: 10.1016/B978-0-12-387003-2.00001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abolaji AO, Kamdem JP, Farombi EO, et al. Drosophila melanogaster as a promising model organism in toxicological studies. Arch Basic Appl Med. 2013;1(1):33-38. http://archivesbam.com/ojs/index.php/abam/article/view/13/41 [Google Scholar]
- 65.Stone B, Burke B, Pathakamuri J, Coleman J, Kuebler D. A low-cost method for analyzing seizure-like activity and movement in Drosophila. J Vis Exp. 2014;84(e51460):1-9. doi: 10.3791/51460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohamed GA, Abdel-Lateff A, Fouad MA, Ibrahim SR, Elkhayat ES, Okino T. Chemical composition and hepato-protective activity of Imperata cylindrica beauv. Pharmacogn Mag. 2009;5(17):28. https://www.phcog.com/article.asp?issn=0973-1296;year=2009;volume=5;issue=17;spage=28;epage=36;aulast=Mohamed [Google Scholar]
- 67.Trease GE, Evans WC. Textbook of Pharmacognosy. 12th ed. Balliers Tindall; 1983. [Google Scholar]
- 68.Oke-Altuntas F, Demirci MA, Demirtas I, Yaglioglu AS, Behcet L. Phytochemical screening, antiproliferative and antioxidant properties of Various extracts from endemic Origanum acutidens. Comb Chem High Throughput Screen. 2018;21(4):281-291. doi: 10.2174/1386207321666180416154404 [DOI] [PubMed] [Google Scholar]
- 69.Piana M, Boligon AA, Brum TFD, et al. Phytochemical analysis and antioxidant capacity ofTabernaemontana catharinensis A. DC. Fruits and branches. An Acad Bras Cienc. 2014;86(2):881-888. doi: 10.1590/0001-3765201420120020 [DOI] [PubMed] [Google Scholar]
- 70.Shaik S, Singh N, Nicholas A. Comparison of the selected secondary metabolite content present in the cancer-bush Lessertia (Sutherlandia) frutescens L. Extracts. African J Tradit Complement Altern Med. 2011;8(4):429-434. doi: 10.4314/ajtcam.v8i4.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chandra S, Gonzalez de Mejia E. Polyphenolic compounds, antioxidant capacity, and quinone reductase activity of an aqueous extract of Ardisia compressa in comparison to Mate (Ilex paraguariensis) and green (Camellia sinensis). Teas. J Agric Food Chem. 2004;52(11):3583-3589. doi: 10.1021/jf0352632 [DOI] [PubMed] [Google Scholar]
- 72.Woisky RG, Salatino A. Analysis of propolis: Some parameters and procedures for chemical quality control. J Apic Res. 1998;37(2):99-105. doi: 10.1080/00218839.1998.11100961 [DOI] [Google Scholar]
- 73.Morrison IM, Asiedu EA, Stuchbury T, Powell AA. Determination of lignin and tannin contents of cowpea seed coats. Ann Bot. 1995;76(3):287-290. doi: 10.1006/anbo.1995.1097 [DOI] [Google Scholar]
- 74.Shiau IL, Shih TL, Wang YN, et al. Quantification for saponin from a soapberry (Sapindus mukorossi gaertn) in cleaning products by a chromatographic and two colorimetric assays. J Fac Agric Kyushu Univ. 2009;54(1):215-221. [Google Scholar]
- 75.Morsy N. Phytochemical analysis of biologically active constituents of medicinal plants. Main Gr Chem. 2014;13(1):7-21. doi: 10.3233/MGC-130117 [DOI] [Google Scholar]
- 76.Sreevidya N, Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with dragendorff’s reagent in plant materials. J AOAC Int. 2003;86(6):1124-1127. doi: 10.1093/jaoac/86.6.1124 [DOI] [PubMed] [Google Scholar]
- 77.Gautam R, Srivastava A, Jachak SM. Determination of chromones in Dysophylla stellata by HPLC: Method development, validation and comparison of different extraction methods. Nat Prod Commun. 2010;5(4):555-558. http://www.ncbi.nlm.nih.gov/pubmed/20433071 [PubMed] [Google Scholar]
- 78.Burman JL, Itsara LS, Kayser E-B, et al. A Drosophila model of mitochondrial disease caused by a complex I mutation that uncouples proton pumping from electron transfer. Dis Model Mech. 2014;7(10):1165-1174. doi: 10.1242/dmm.015321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malik BR, Hodge JJL. Drosophila adult olfactory shock learning. J Vis Exp. 2014;90(e50107):3-7. doi: 10.3791/50107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb Protoc. 2008;2008(6):pdb.prot4986-pdb.prot4986. doi: 10.1101/pdb.prot4986 [DOI] [PubMed] [Google Scholar]
- 81.Burke D. A modification for the combined staining of cells and fibers in the nervous system. Am J Med Technol. 1968;34(11):667-670. [PubMed] [Google Scholar]
- 82.Slaoui M, Fiette L. In: Gautier J-C, ed. Drug Safety Evaluation. Vol 691. Humana Press; 2011. doi: 10.1007/978-1-60761-849-2 [DOI] [Google Scholar]
- 83.Fergestad T, Olson L, Patel KP, Miller R, Palladino MJ, Ganetzky B. Neuropathology in Drosophila mutants with increased seizure susceptibility. Genetics. 2008;178(2):947-956. doi: 10.1534/genetics.107.082115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fergestad T, Ganetzky B, Palladino MJ. Neuropathology in Drosophila membrane excitability mutants. Genetics. 2006;172(2):1031-1042. doi: 10.1534/genetics.105.050625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fergestad T, Bostwick B, Ganetzky B. Metabolic disruption in Drosophila bang-sensitive seizure mutants. Genetics. 2006;1364(July):1357-1364. doi: 10.1534/genetics.106.057463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matzat T, Sieglitz F, Kottmeier R, Babatz F, Engelen D, Klämbt C. Axonal wrapping in the Drosophila PNS is controlled by glia-derived neuregulin homolog vein. Development. 2015;142(7):1336-1345. doi: 10.1242/dev.116616 [DOI] [PubMed] [Google Scholar]
- 87.Sasse S, Neuert H, Klämbt C. Differentiation of Drosophila glial cells. Wiley Interdiscip Rev Dev Biol. 2015;4(6):623-636. doi: 10.1002/wdev.198 [DOI] [PubMed] [Google Scholar]
- 88.Sajadi E, Raoofi A, Abdi S, Azimi H, Abdollahifar M-A. The modified method of luxol fast blue for paraffin-embedded myelin sheath staining. Int J Morphol. 2020;38(5):1197-1200. doi: 10.4067/S0717-95022020000501197 [DOI] [Google Scholar]
- 89.Pohl F, Kong Thoo Lin P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules. 2018;23(12):3283. doi: 10.3390/molecules23123283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis Model Mech. 2017;10(5):499-502. doi: 10.1242/dmm.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Golden TR, Patel M. Catalytic antioxidants and neurodegeneration. Antioxid Redox Signal. 2009;11(3):555-569. doi: 10.1089/ars.2008.2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ambrogini P, Torquato P, Bartolini D, et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim Biophys Acta - Mol Basis Dis. 2019;1865(6):1098-1112. doi: 10.1016/j.bbadis.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 93.Liu R, Chen S, Ren G, Shao F, Huang H. Phenolic compounds from roots of Imperata cylindrica var. major. Phenolic Compd from Roots Imp Cylind var major. 2013;5(3):240-243. doi:10.3969 [Google Scholar]
- 94.Mbarki S, Alimi H, Bouzenna H, Elfeki A, Hfaiedh N. Phytochemical study and protective effect of Trigonella foenum graecum (fenugreek seeds) against carbon tetrachloride-induced toxicity in liver and kidney of male rat. Biomed Pharmacother. 2017;88(19-26). doi: 10.1016/j.biopha.2016.12.078 [DOI] [PubMed] [Google Scholar]
- 95.Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37(2):153-161. doi: 10.1046/j.1365-2621.2002.00552.x [DOI] [Google Scholar]
- 96.An H-J, Nugroho A, Song B-M, Park H-J. Isoeugenin, a novel nitric oxide synthase inhibitor isolated from the rhizomes of Imperata cylindrica. Molecules. 2015;20(12):21336-21345. doi: 10.3390/molecules201219767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cerdeira AL, Cantrell CL, Dayan FE, Byrd JD, Duke SO. Tabanone, a new phytotoxic constituent of cogongrass (Imperata cylindrica). Weed Sci. 2012;60(2):212-218. doi: 10.1614/WS-D-11-00160.1 [DOI] [Google Scholar]
- 98.Matsunaga K, Shibuya M, Ohizumi Y. Graminone B, a novel lignan with vasodilative activity from Imperata cylindrica. J Nat Prod. 1994;57(12):1734-1736. doi: 10.1021/np50114a020 [DOI] [PubMed] [Google Scholar]
- 99.Matsunaga K, Shibuya M, Ohizumi Y. Imperanene, a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica. J Nat Prod. 1995;58(1):138-139. doi: 10.1021/np50115a022 [DOI] [PubMed] [Google Scholar]
- 100.Ma J, Sun H, Liu H, et al. Hepatoprotective glycosides from the rhizomes of Imperata cylindrical. J Asian Nat Prod Res. 2018;20(5):451-459. doi: 10.1080/10286020.2018.1471065 [DOI] [PubMed] [Google Scholar]
- 101.Sarkar A, Mohideen SS. Pathogenic tauR406 W induced neurodegeneration and memory deficits rescued by HDAC6 null mutation in Drosophila melanogaster. Alzheimer’s Dement. 2021;17(S2):e049554. doi: 10.1002/alz.058020 [DOI] [Google Scholar]
- 102.Khyati, Malik I, Agrawal N, Kumar V. Melatonin and curcumin reestablish disturbed circadian gene expressions and restore locomotion ability and eclosion behavior in Drosophila model of Huntington’s disease. Chronobiol Int. 2021;38(1):61-78. doi: 10.1080/07420528.2020.1842752 [DOI] [PubMed] [Google Scholar]
- 103.Lv F, Yang X, Cui C, Su C. Exogenous expression of Drp1 plays neuroprotective roles in the Alzheimer’s disease in the Aβ42 transgenic Drosophila model. PLoS One. 2017;12(5):e0176183. Reddy H, ed. doi: 10.1371/journal.pone.0176183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomita J, Ueno T, Mitsuyoshi M, Kume S, Kume K. The NMDA receptor promotes sleep in the fruit fly, drosophila melanogaster. PLoS One. 2015;10(5):e0128101. Brembs B, ed. doi: 10.1371/journal.pone.0128101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh P, Chowdhuri DK. Modulation of sestrin confers protection to Cr(VI) induced neuronal cell death in drosophila melanogaster. Chemosphere. 2018;191(302-314). doi: 10.1016/j.chemosphere.2017.10.037 [DOI] [PubMed] [Google Scholar]
- 106.Ishola IO, Afolayan O, Odutola IO, Faniyan O, Adeyemi OO. Therapeutic potential of hesperidin in Parkinson’s disease with dementia: Inhibition of alpha synuclein and amyloid beta in Drosophila melanogaster. Niger J Physiol Sci. 2021;36(1):43-48. Accessed January 7, 2022. http://www.ncbi.nlm.nih.gov/pubmed/34987244. [PubMed] [Google Scholar]
- 107.Reijnders MRF, Janowski R, Alvi M, et al. PURA Syndrome: Clinical delineation and genotype-phenotype study in 32 individuals with review of published literature. J Med Genet. 2018;55(2):104-113. doi: 10.1136/jmedgenet-2017-104946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merzetti EM, Staveley BE. Identifying potential PARIS homologs in D. melanogaster. Genet Mol Res. 2016;15(4):10.4238/gmr15048934. doi: 10.4238/gmr15048934 [DOI] [PubMed] [Google Scholar]
- 109.Merzetti EM, Staveley BE. Spargel, the PGC-1α homologue, in models of Parkinson disease in Drosophila melanogaster. BMC Neurosci. 2015;16(1):70. doi: 10.1186/s12868-015-0210-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee D, Zheng X, Shigemori K, et al. Expression of mutant CHMP2B linked to neurodegeneration in humans disrupts circadian rhythms in Drosophila. FASEB BioAdvances. 2019;1(8):511-520. doi: 10.1096/fba.2019-00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Straub J, Gregor A, Sauerer T, et al. Genetic interaction screen for severe neurodevelopmental disorders reveals a functional link between Ube3a and Mef2 in Drosophila melanogaster. Sci Rep. 2020;10(1):1204. doi: 10.1038/s41598-020-58182-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ashburner M. In: Ashburner M, Kent G, Golic A, Hawley RS, eds. Drosophila. A Laboratory Handbook. Vol 1. illustrate. Cold Spring Harbor Laboratory Press; 1989. https://books.google.co.ug/books/about/Drosophila_A_laboratory_handbook.html?id=Sc-dGjsID7wC&redir_esc=y [Google Scholar]
- 113.Chen K-F, Crowther DC. Functional genomics in Drosophila models of human disease. Brief Funct Genomics. 2012;11(5):405-415. doi: 10.1093/bfgp/els038 [DOI] [PubMed] [Google Scholar]
- 114.Lee S, Bang SM, Lee JW, Cho KS. Evaluation of traditional medicines for neurodegenerative diseases using drosophila models. Evidence-Based Complement Altern Med. 2014;2014(1-14). doi: 10.1155/2014/967462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reynolds ER. Shortened lifespan and other age-related defects in bang sensitive mutants of drosophila melanogaster. G3 Genes|Genomes|Genetics. 2018;8(12):3953-3960. doi: 10.1534/g3.118.200610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bilen J, Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu Rev Genet. 2005;39(1):153-171. doi: 10.1146/annurev.genet.39.110304.095804 [DOI] [PubMed] [Google Scholar]
- 117.Yamaguchi M, Takashima H. Drosophila charcot-marie-tooth disease models. Adv Exp Med Biol. 2018;1076(97-117). doi: 10.1007/978-981-13-0529-0_7 [DOI] [PubMed] [Google Scholar]
- 118.Chung H, Wangler MF, Marcogliese PC, et al. Loss- or gain-of-function mutations in ACOX1 cause axonal loss via different mechanisms. Neuron. 2020;106(4):589-606.e6. doi: 10.1016/j.neuron.2020.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krauss R, Bosanac T, Devraj R, Engber T, Hughes RO. Axons matter: The promise of treating neurodegenerative disorders by targeting SARM1-mediated axonal degeneration. Trends Pharmacol Sci. 2020;41(4):281-293. doi: 10.1016/j.tips.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 120.Li R, Liu Y, Liu H, Li J. Ketogenic diets and protective mechanisms in epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J Food Biochem. 2020;44(3):e13140. doi: 10.1111/jfbc.13140 [DOI] [PubMed] [Google Scholar]
- 121.Farrell JS, Wolff MD, Teskey GC. Neurodegeneration and pathology in epilepsy: Clinical and basic perspectives. Adv Neurobiol. 2017;15(317-334). Springer New York LLC; doi: 10.1007/978-3-319-57193-5_12 [DOI] [PubMed] [Google Scholar]
- 122.Radlicz C, Chambers A, Olis E, Kuebler D. The addition of a lipid-rich dietary supplement eliminates seizure-like activity and paralysis in the drosophila bang sensitive mutants. Epilepsy Res. 2019;155(106153). doi: 10.1016/j.eplepsyres.2019.106153 [DOI] [PubMed] [Google Scholar]
- 123.Bouza AA, Isom LL. Voltage-Gated sodium channel β subunits and their related diseases. Handb Exp Pharmacol. 2017;246(423-450). Springer New York LLC; doi: 10.1007/164_2017_48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kretzschmar D, Hasan G, Sharma S, Heisenberg M, Benzer S. The Swiss cheese mutant causes glial hyperwrapping and brain degeneration in drosophila. J Neurosci. 1997;17(19):7425-7432. doi: 10.1523/JNEUROSCI.17-19-07425.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cahoy JD, Emery B, Kaushal A, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci. 2008;28(1):264-278. doi: 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jeibmann A, Paulus W. Drosophila melanogaster as a model organism of brain diseases. Int J Mol Sci. 2009;10(2):407-440. doi: 10.3390/ijms10020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lucey BP, Leahy A, Rosas R, Shaw PJ. A new model to study sleep deprivation-induced seizure. Sleep. 2015;38(5):777-785. doi: 10.5665/sleep.4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kadas D, Duch C, Consoulas C. Postnatal increases in axonal conduction velocity of an identified drosophila interneuron require fast sodium, L-type calcium and shaker potassium channels. eneuro. 2019;6(4):ENEURO.0181-19.2019. doi: 10.1523/ENEURO.0181-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muller B, Kistner U, Veh R, et al. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J Neurosci. 1995;15(3):2354-2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Naruse K. Schwann cells as crucial players in diabetic neuropathy. In: Advances in Experimental Medicine and Biology. 2019;1190(345-356). Springer; doi: 10.1007/978-981-32-9636-7_22 [DOI] [PubMed] [Google Scholar]
- 131.Easy Biology Class. Myelinated vs Unmyelinated Nerve Fibres. Easy Biology Class. Published 2021. Accessed July 28, 2021. https://www.easybiologyclass.com/difference-between-myelinated-and-unmyelinated-neurons/
- 132.Sharghi-Namini S, Turmaine M, Meier C, et al. The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J Neurosci. 2006;26(23):6364-6376. doi: 10.1523/JNEUROSCI.0157-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mizisin AP. Mechanisms of diabetic neuropathy. Handb Clin Neurol. 2014;126:401-428. Elsevier B.V.; doi: 10.1016/B978-0-444-53480-4.00029-1. [DOI] [PubMed] [Google Scholar]
- 134.Nave K-A. Myelination and support of axonal integrity by glia. Nature. 2010;468(7321):244-252. doi: 10.1038/nature09614 [DOI] [PubMed] [Google Scholar]
- 135.Cavanna AE, Ali F, Rickards HE, McCorry D. Behavioral and cognitive effects of anti-epileptic drugs. Discov Med. 2010;9(45):138-144. http://www.ncbi.nlm.nih.gov/pubmed/20193640 [PubMed] [Google Scholar]
- 136.Meador KJ, Baker GA, Ph D, et al. NIH Public access. N Engl J Med. 2009;360(16):1597-1605. doi: 10.1056/NEJMoa0803531.Cognitive [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ergul Erkec O, Yunusoglu O, Huyut Z. Evaluation of repeated ghrelin administration on seizures, oxidative stress and neurochemical parameters in pentyleneterazole induced kindling in rats. Int J Neurosci. Published online August 28, 2022;2022(1-9). doi: 10.1080/00207454.2022.2107516 [DOI] [PubMed] [Google Scholar]
- 138.Trojnar MK, Małek R, Chrościńska M, Nowak S, Błaszczyk B, Czuczwar SJ. Neuroprotective effects of antiepileptic drugs. Pol J Pharmacol. 2002;54(6):557-566. http://www.ncbi.nlm.nih.gov/pubmed/12866709 [PubMed] [Google Scholar]
- 139.Geronzi U, Lotti F, Grosso S. Oxidative stress in epilepsy. Expert Rev Neurother. 2018;18(5):427-434. doi: 10.1080/14737175.2018.1465410 [DOI] [PubMed] [Google Scholar]
- 140.Qiao M, Malisza KL, Bigio MR, Kozlowski P, Seshia SS, Tuor UI. Effect of long-term vigabatrin administration on the immature rat brain. Epilepsia. 2000;41(6):655-665. doi: 10.1111/j.1528-1157.2000.tb00225.x [DOI] [PubMed] [Google Scholar]
- 141.Menzies FM, Yenisetti SC, Min K-T. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15(17):1578-1582. doi: 10.1016/j.cub.2005.07.036 [DOI] [PubMed] [Google Scholar]
- 142.Lavara-Culebras E, Paricio N. Drosophila DJ-1 mutants are sensitive to oxidative stress and show reduced lifespan and motor deficits. Gene. 2007;400(1-2):158-165. doi: 10.1016/j.gene.2007.06.013 [DOI] [PubMed] [Google Scholar]
- 143.Lüthy K, Mei D, Fischer B, et al. TBC1D24-TLDc-related Epilepsy exercise-induced dystonia: Rescue by antioxidants in a disease model. Brain. 2019;142(8):2319-2335. doi: 10.1093/brain/awz175 [DOI] [PubMed] [Google Scholar]
- 144.Khatoon R, Rasheed MZ, Rawat M, Alam MM, Tabassum H, Parvez S. Effect of melatonin on Aβ42 induced changes in the mitochondrial function related to Alzheimer’s disease in Drosophila melanogaster. Neurosci Lett. 2019;711(134376). doi: 10.1016/j.neulet.2019.134376 [DOI] [PubMed] [Google Scholar]
- 145.Johnson SL, Iannucci J, Seeram NP, Grammas P. Inhibiting thrombin improves motor function and decreases oxidative stress in the LRRK2 transgenic Drosophila melanogaster model of Parkinson’s disease. Biochem Biophys Res Commun. 2020;527(2):532-538. doi: 10.1016/j.bbrc.2020.04.068 [DOI] [PubMed] [Google Scholar]
- 146.Uzunhan TA, Uyanik B. Disrupted oxidative stress resistance: A homozygous mutation in the catalytic (TLDc) domain of TBC1D24 gene associated with epileptic encephalopathy. Clin Neurol Neurosurg. 2020;196(106080). doi: 10.1016/j.clineuro.2020.106080 [DOI] [PubMed] [Google Scholar]
- 147.Dweck HKM, Ebrahim SAM, Farhan A, Hansson BS, Stensmyr MC. Olfactory proxy detection of dietary antioxidants in Drosophila. Curr Biol. 2015;25(4):455-466. doi: 10.1016/j.cub.2014.11.062 [DOI] [PubMed] [Google Scholar]
- 148.Jajoo A, Donlon C, Shnayder S, Levin M, McVey M. Sertraline induces DNA damage and cellular toxicity in Drosophila that can be ameliorated by antioxidants. Sci Rep. 2020;10(1):4512. doi: 10.1038/s41598-020-61362-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yi Y, Xu W, Fan Y, Wang H-X. Drosophila as an emerging model organism for studies of food-derived antioxidants. Food Res Int. 2021;143(110307). doi: 10.1016/j.foodres.2021.110307 [DOI] [PubMed] [Google Scholar]
- 150.Yang N, Guan Q-W, Chen F-H, et al. Antioxidants targeting mitochondrial oxidative stress: Promising neuroprotectants for epilepsy. Cárdenas-Rodríguez N, ed. Oxid Med Cell Longev. 2020;2020(1-14). doi: 10.1155/2020/6687185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bao G-S, Wang W-A, Wang T-Z, et al. Overexpression of human MRP1 in neurons causes resistance to antiepileptic drugs in Drosophila seizure mutants. J Neurogenet. 2011;25(4):201-206. doi: 10.3109/01677063.2011.620662 [DOI] [PubMed] [Google Scholar]
- 152.Wang Y-W, Yang C-T, Gong C-L, et al. Inhibition of voltage-gated Na+ channels by hinokiol in neuronal cells. Pharmacol Reports. 2015;67(6):1049-1054. doi: 10.1016/j.pharep.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 153.Kim M, Truss M, Pagare PP, Essandoh MA, Zhang Y, Williams DA. Structure activity relationship exploration of 5-hydroxy-2-(3-phenylpropyl)chromones as a unique 5-HT2B receptor antagonist scaffold. Bioorg Med Chem Lett. 2020;30(21):127511. doi: 10.1016/j.bmcl.2020.127511 [DOI] [PubMed] [Google Scholar]
- 154.Shekh-Ahmad T, Lieb A, Kovac S, et al. Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy. Redox Biol. 2019;26(101278). doi: 10.1016/j.redox.2019.101278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jahromi SR, Haddadi M, Shivanandappa T, Ramesh SR. Attenuation of neuromotor deficits by natural antioxidants of Decalepis hamiltonii in transgenic Drosophila model of Parkinson’s disease. Neuroscience. 2015;293(136-150). doi: 10.1016/j.neuroscience.2015.02.048 [DOI] [PubMed] [Google Scholar]
- 156.Wang H, Zheng Z, Han W, et al. Metformin promotes axon regeneration after spinal cord injury through inhibiting oxidative stress and stabilizing microtubule. Oxid Med Cell Longev. 2020;2020(1-20). doi: 10.1155/2020/9741369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zeng J, Chen Y, Ding R, et al. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J Neuroinflammation. 2017;14(1):119. doi: 10.1186/s12974-017-0895-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hong JY, Davaa G, Yoo H, Hong K, Hyun JK. Ascorbic acid promotes functional restoration after spinal cord injury partly by epigenetic modulation. Cells. 2020;9(5):1310. doi: 10.3390/cells9051310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stilwell GE, Saraswati S, Littleton JT, Chouinard SW. Development of a Drosophila seizure model for in vivo high-throughput drug screening. Eur J Neurosci. 2006;24(8):2211-2222. doi: 10.1111/j.1460-9568.2006.05075.x [DOI] [PubMed] [Google Scholar]
- 160.Yi J, Zhang L, Tang B, et al. Sodium valproate alleviates neurodegeneration in SCA3 / MJD via suppressing apoptosis and rescuing the hypoacetylation levels of histone H3 and H4. PLoS One. 2013;8(1):2-11. doi: 10.1371/journal.pone.0054792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Markow TA. The secret lives of Drosophila flies. Elife. 2015;4(JUNE):1-10. doi: 10.7554/eLife.06793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Büget B, Türkmen AZ, Allahverdiyev O, Enginar N. Antimuscarinic-induced convulsions in fasted animals after food intake: Evaluation of the effects of levetiracetam, topiramate and different doses of atropine. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(1):57-62. doi: 10.1007/S00210-015-1175-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-chp-10.1177_2515690X231160191 for Attenuation of Seizures, Cognitive Deficits, and Brain Histopathology by Phytochemicals of Imperata cylindrica (L.) P. Beauv (Poaceae) in Acute and Chronic Mutant Drosophila melanogaster Epilepsy Models by Fred Ssempijja, Samuel Sunday Dare, Edmund E. M. Bukenya, Keneth Iceland Kasozi, Ritah Kenganzi, Edgar Mario Fernandez and Marta Vicente-Crespo in Journal of Evidence-Based Integrative Medicine
Supplemental material, sj-docx-2-chp-10.1177_2515690X231160191 for Attenuation of Seizures, Cognitive Deficits, and Brain Histopathology by Phytochemicals of Imperata cylindrica (L.) P. Beauv (Poaceae) in Acute and Chronic Mutant Drosophila melanogaster Epilepsy Models by Fred Ssempijja, Samuel Sunday Dare, Edmund E. M. Bukenya, Keneth Iceland Kasozi, Ritah Kenganzi, Edgar Mario Fernandez and Marta Vicente-Crespo in Journal of Evidence-Based Integrative Medicine
Supplemental material, sj-docx-3-chp-10.1177_2515690X231160191 for Attenuation of Seizures, Cognitive Deficits, and Brain Histopathology by Phytochemicals of Imperata cylindrica (L.) P. Beauv (Poaceae) in Acute and Chronic Mutant Drosophila melanogaster Epilepsy Models by Fred Ssempijja, Samuel Sunday Dare, Edmund E. M. Bukenya, Keneth Iceland Kasozi, Ritah Kenganzi, Edgar Mario Fernandez and Marta Vicente-Crespo in Journal of Evidence-Based Integrative Medicine