Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) is an advanced endoscopic procedure that might lead to severe adverse events. Post-ERCP pancreatitis (PEP) is the most common post-procedural complication, which is related to significant mortality and increasing healthcare costs. Up to now, the prevalent approach to prevent PEP consisted of employing pharmacological and technical expedients that have been shown to improve post-ERCP outcomes, such as the administration of rectal nonsteroidal anti-inflammatory drugs, aggressive intravenous hydration, and the placement of a pancreatic stent. However, it has been reported that PEP originates from a more complex interaction of procedural and patient-related factors. Appropriate ERCP training has a pivotal role in PEP prevention strategy, and it is not a chance that a low PEP rate is universally considered one of the most relevant indicators of proficiency in ERCP. Scant data on the acquisition of skills during the ERCP training are currently available, although some efforts have been recently done to shorten the learning curve by way of simulation-based training and demonstrate competency by meeting technical requirements as well as adopting skill evaluation scales. Besides, the identification of adequate indications for ERCP and accurate pre-procedural risk stratification of patients might help to reduce PEP occurrence regardless of the endoscopist’s technical abilities, and generally preserve safety in ERCP. This review aims at delineating current preventive strategies and highlighting novel perspectives for a safer ERCP focusing on the prevention of PEP.
Keywords: Artificial Intelligence (D001185), Cholangiopancreatography, Endoscopic Retrograde (D002760), Education (D004493), Machine Learning (D000069550), Medical (D004501), Pancreatitis (D010195), prevention and control (Q000517), Risk Factors (D012307)
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is an advanced endoscopic technique. Given the expansion of less invasive imaging such as computed tomography, magnetic resonance cholangiopancreatography (MRCP), and endoscopic ultrasound (EUS), today ERCP is mostly considered to treat rather than diagnose biliopancreatic disorders. However, therapeutic ERCP is technically demanding, and it is associated with significant morbidity and mortality.1
Post-ERCP pancreatitis (PEP) is the most common serious adverse event, with an incidence ranging between 4% and 10%, and a mortality rate that may reach 0.7%.2 In high-risk patients, PEP might occur in up to 15% of the cases.3 PEP also represents a major socioeconomic burden; it is estimated that the annual cost of PEP in the United States reaches 200 million USD.4 Other less frequent ERCP-related adverse events are post-sphincterotomy bleeding, cholangitis and/or cholecystitis, and perforation.5
Given the morbidity, mortality, and costs associated with PEP, the prevention of this post-procedural adverse event is of paramount importance. Despite several efforts to reduce the incidence of PEP, only a few preventive measures have proven to be effective in clinical practice. An appropriate patient selection, procedural strategies such as pancreatic stent placement, the administration of rectal nonsteroidal anti-inflammatory drugs (NSAIDs), and aggressive hydration with Lactated Ringer are today considered effective in reducing the risk of PEP.
Strategies to train and assess competency in this demanding endoscopic technique are needed to minimize the potential contribution of the trainee experience in the occurrence of ERCP-related adverse events. Furthermore, accurate preoperative stratification of patients could allow optimal allocation of expertise and perioperative management. Together with intraprocedural technical expedients and pharmacoprevention, these are the most important areas of investigation for safe ERCP.
This perspective review aims at examining current paradigms and discuss novel strategies in training, risk stratification, and technical approaches for safe ERCP.
A focus on training in ERCP: A paradigm shift is upon us
Several studies have demonstrated that ERCP is a highly operator-dependent procedure that requires appropriate competency before independent practice. Therefore, intensive training is needed to achieve technical success and to improve safety.6,7 Training programs should start after the achievement of an adequate proficiency level in gastrointestinal endoscopy.8 Then, the first goal of an ERCP training program is ensuring that firm cognitive skills are acquired.9,10 Second, ERCP has several technical aspects that a trainee should master at the end of the training: the first proficiency level in ERCP includes an appropriate scope maneuvering and orientation, selective cannulation of the common bile duct (CBD), and/or main pancreatic duct (MPD), and successful sphincterotomy, stone clearance, stent placement.11
State-of-the-art methods of training in ERCP: Who trains the trainer?
ERCP training requires time, commitment, and solid preparation from mentors. As standardized ERCP teaching programs are still lacking, parallelly there are no guidelines on how endoscopists should teach the procedure.12 European Society of Gastrointestinal Endoscopy (ESGE) Position Statement on the ERCP curriculum recommends a minimum 3-year independent practice before starting mentoring ERCP trainees, strongly suggesting undertaking a specific course to reach skill development as a teacher.8 In this setting, institutional commitment is essential, as much as trainers should be conscious of the importance of individual competence to instruct future ERCP endoscopists.13
State-of-the-art methods of training in ERCP: Simulation-based models
Aiming at preventing serious short- and long-term complications and offering an appropriate education to ERCP trainees, initial simulation-based training could guide a fellow to achieve technical skills and self-confidence with the endoscopic devices before shifting to a hands-on practice on patients. This setting includes in vivo and ex vivo animal models, mechanical simulators, and computer-based/virtual reality simulators.14,15
Live animal models represent the most realistic endoscopic simulators, despite some differences from human anatomy.15 Due to their safety, low cost, and high availability, the most frequent in vivo and ex vivo animal models are swine, so far.16,17 Nevertheless, ethical limitations should always be taken into account, reserving porcine models only for a restricted group of trainees that may truly benefit from this kind of training.18
Mechanical simulators are physical models designed to mimic human anatomic structures. ERCP mechanical simulators include the X-Vision system,19 ERCP Mechanical Simulator (EMS),20 and the Boškoski-Costamagna ERCP Trainer.21 These models are usually made of plastic molds that attempt to represent the papillary orifice and allow the practice of selective ductal cannulation and endoscopic sphincterotomy. To our knowledge, the Boškoski-Costamagna ERCP Trainer is the first mechanical model that has been independently validated, showing good construct and face validity22 (Figures 1 and 2).
Figure 1.
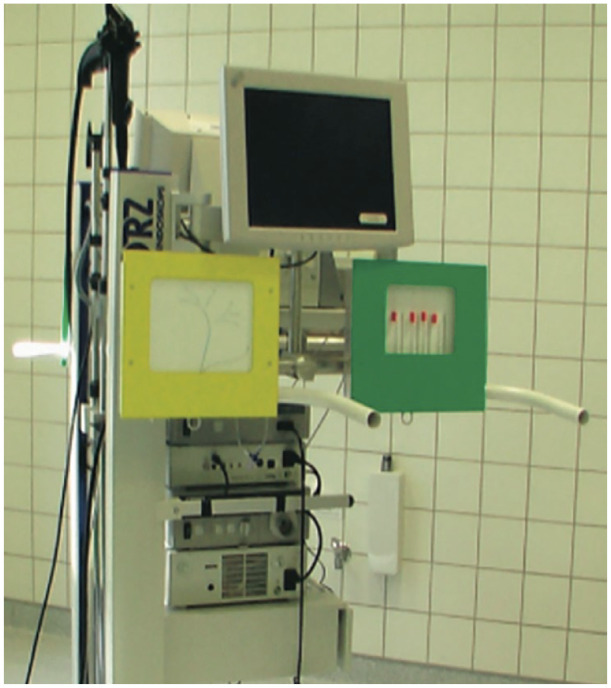
The X-Vision ERCP Training System simulator. Reproduced from Gallo et al.23
Figure 2.

The Boškoski-Costamagna ERCP trainer (Cook Medical, Limerick Ireland).
The GastroIntestinaI (GI)-Mentor II from Simbionix24,25 and the AccuTouch/Endo Virtual Reality (VR) from CAE Healthcare25 are the existing computer-based simulators (Figure 3). These simulation models are integrated systems consisting of mechanical parts and software able to provide various scenarios of endoscopic training, ranging from basic procedures to more complex situations such as emergency endoscopic interventions. Compared with the mechanical models, they do not need human supervision, given their ability to record trainees’ technical progress and to provide immediate, objective feedback.26 The main disadvantage of computer-based simulators is their high cost.
Figure 3.
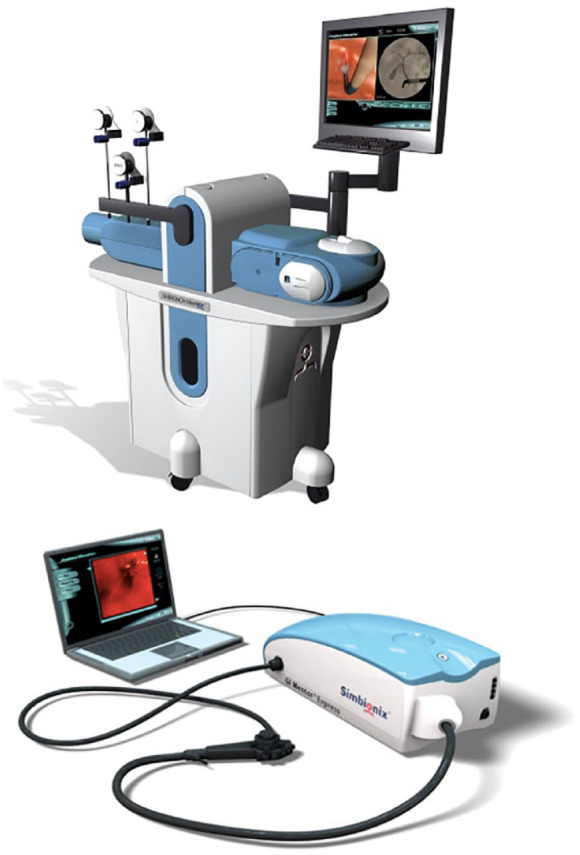
The Simbionix GastroIntestinal (GI)-mentor simulator. Courtesy of OKB medical limited (Chichester, UK).
Despite the increasing use of any kind of simulator, standardized, evidence-based strategies that incorporate a simulated setting into ERCP training are still lacking.27 A single-center experience on a year-long flexible endoscopy training, made of both theoretical teaching and simulation-based practice, documented an overall improvement of endoscopic skills of trainees.28 Indeed, the use of simulation-based training before switching to hands-on practice could help ERCP fellows to acquire new abilities, accelerate the learning curve, and consequently minimize the development of ERCP-related adverse events when endoscopic practice is done on real patients.
State-of-the-art methods of training in ERCP: Competency-based education
Even though ERCP is one of the most technically demanding endoscopic procedures, universally accepted metrics of proficiency are still lacking. Competence is assessed by trainers’ subjective opinion while supervising trainees’ work and improvements, and great variability of worldwide ERCP training programs and individual learning curves has been documented.11 First, there is not a global consensus on how long the ERCP training program should last. Gastrointestinal endoscopy societies suggest a minimum duration of 12 consecutive months, assuming that trainees should previously have achieved an acceptable level of basic endoscopy.29 On the other hand, some studies tried to measure trainee competency on the basis of a minimum number of ERCPs performed during training.7,30,31 Current guidelines by the American Society of Gastrointestinal Endoscopy set this threshold at 200 procedures per trainee at the end of the training program,32 while the ESGE set the threshold at 300.8 However, it is now well established that there is a discrepancy between the required minimum level of competency and the threshold of procedures suggested by guidelines, which most of fellows do not reach during ERCP training.33
Current data support a new paradigm shift, from an apprenticeship, volume-based model to a competency-based approach, that emphasizes the importance of reaching some predefined outcomes instead of assessment scores, focuses on skills rather than knowledge, and promotes a major engagement of masters.34
Nevertheless, where do we stand in the evaluation of proficiency in ERCP?
Assessing proficiency in ERCP: Meeting technical endpoints
A well-structured education in ERCP should present accurate instructional methods, assess ability milestones toward proficiency, and adopt specific assessment tools along the learning path. Few studies evaluated the learning curve and tried to measure the competence of ERCP trainees among several worldwide teaching programs.11,35 CBD selective cannulation is one of the most complicated technical phases of ERCP and a CBD cannulation rate ranging from 80% to 90% has been considered a measure of proficiency for a long time.36,37 In a prospective study evaluating the learning curves of 15 trainees, Ekkelenkamp et al.35 demonstrated an increased unassisted cannulation rate of CBDs after 200 ERCPs conducted on both patients with a native duodenal papilla and subjects who had already undergone sphincterotomy (from 36% at baseline to 85%, p < 0.001). An overall CBD cannulation rate of at least 80%, proposed as a measure of competence, was reached only by 2 out of 15 trainees.35 Wani et al.38 examined the learning curves of ERCP trainees coming from five different American training centers, highlighting a great variability in the number of successful procedures completed during the training programs; moreover, using a cumulative sum analysis, none of the training centers reached the threshold for competence in cannulation of native papilla after a 12-month training period. Additional ERCP performance measures have been reported in a recent systematic review,39 and later adopted in the ESGE Position Statement on the ERCP curriculum to define the basic level of trainee competency. These are the following: selective native papilla cannulation rate of at least 80%, complete stone clearance in at least 85% of subjects, and successful stent positioning in case of distal biliary strictures in at least 90% of patients.8 Moreover, ESGE considers PEP as the most pertinent indicator of complication rate: proficiency in ERCP also requires an overall PEP rate below 10%.8
Assessing proficiency in ERCP: Skills evaluation scales
Recent scientific evidence suggests the use of assessment tools to document competence in ERCP training.11,35 These forms aim at facilitating both the trainee and his supervisor to identify all skill deficiencies and hence allowing to fix the cognitive and technical gaps, to arise trainees’ insight into the quality of ERCPs, and build a personal projection of the overtime improvement during the training period.10 Over the last decade, three self-assessment forms have been constructed,35,40,41 but only two of them have gained validation, being therefore recommended by ESGE8: ‘The EUS and ERCP Skills Assessment Tool’ (TEESAT) and/or the ‘Direct Observation of Procedural Skills’ (DOPS) in the assessment of fellow’s learning curve.40,41 Besides, ERCP trainees are invited to regularly record all the endoscopy cases, and the degree of their supervisor support: this logbook may represent a real picture of the competency developed by the trainee.8
State-of-the-art methods of training in ERCP: There is still a long way to go
Despite the slow change taking place, well-defined proficiency thresholds in ERCP training deriving from a competency-based education model are not universally accepted by the scientific community. Furthermore, few studies investigated how trainee involvement may influence ERCP-related clinical outcomes. Recently, Voiosu et al.42 conducted a prospective, multicenter, observational study in which the participation of trainees did not seem to affect the technical success and adverse events rate of ERCP. In particular, no difference was found in the incidence of technical failure (7.6% versus 6.3%, p = 0.31) or adverse events (14.7% versus 14.6%, p = 0.99) between the trainee group and the control group. However, major limitations of the study include its observational nature, the lack of a standardized intraprocedural trainee involvement, and the concentration of high-risk ERCP interventions in the group without the participation of trainees.42
An in-depth exploration of new metrics of proficiency based on a trainee’s learning curve rather than procedural thresholds is still needed to embrace an upgraded and evidence-based strategy in ERCP training. Moreover, future research should investigate the association between trainee participation and ERCP-related adverse events as PEP, preferably through a large prospective, multicenter trial.
Patient selection and risk stratification in the prevention of PEP
Not all biliary cannulations are the same, and so is the risk of developing adverse events following ERCP. A careful evaluation of all patients before undergoing ERCP is essential to provide the most appropriate peri-procedural management, and, consequently, to prevent any post-ERCP complications.
In the last three decades, numerous retrospective and prospective studies have explored several risk factors for PEP. Two recent systematic reviews conducted, respectively, on 32.381 and 54.889 individuals, indicate suspected sphincter of Oddi dysfunction (SOD), female sex, and previous episodes of pancreatitis or PEP as definite patient-related risk factors for PEP.43,44 In a prospective study including 996 subjects, younger patients, namely those less than 35 years old, had a greater risk to develop PEP.45 In addition, ESGE suggests as likely patient-related risk factors non-dilated extrahepatic bile duct, absence of chronic pancreatitis, normal serum bilirubin, and end-stage renal disease.46
Although research appeared to advance in the recognition of the potential patient-related conditions predisposing to PEP occurrence, data variability makes it challenging to stratify patients as high risk, moderate risk, or low risk for PEP. Our focus will be on the appropriate selection of candidates for ERCP, and the recently developed prediction models for PEP.
Patient selection before ERCP
Undeniably, the proper selection of patients undergoing ERCP might represent the very first step in the prevention strategy of PEP. In 2006, Peter B. Cotton analyzed a series of 59 ERCP-related lawsuits, highlighting that the principal accusation was that ERCP did not meet an appropriate indication.47 Since the utilization of ERCP as a diagnostic procedure has declined in favor of other less invasive and accurate diagnostic tools, such as MRCP and EUS, and ERCP is currently performed for strictly therapeutic reasons, endoscopists should acquire the cognitive skills necessary for the correct selection of ERCP candidates by identifying the good indications for this procedure. In general, ERCP should be considered whenever patients with a biliopancreatic disease need a therapeutic intervention upon an individual benefit–risk assessment. Appropriate indications for ERCP are biliary obstruction as a result of symptomatic choledocholithiasis, pancreatic cancer, unresectable cholangiocarcinoma, indeterminate or benign biliary strictures, as well as bile duct injury after cholecystectomy and liver transplantation, and symptomatic pancreatic strictures as it occurs in chronic pancreatitis.48
Nevertheless, there are some cases in which the indications for ERCP are not always clear. In fact, it has been recently highlighted that it is questionable whether this procedure should be reserved also for patients with silent CBD stones, defined as the absence of abdominal pain, nausea and vomiting, and abnormal liver function tests. A few retrospective studies reported that the incidence of PEP in subjects with asymptomatic choledocholithiasis was over 10% (12.5–20.8%) when compared with symptomatic CBD stones (1.5–6.9%, p < 0.05),49–52 and at multivariate analysis, the presence of silent CBD stones in patients undergoing ERCP was found to be an independent risk factor for PEP.50,51 These data are apparently in contrast with current guidelines, that recommend ERCP even in patients with asymptomatic choledocholithiasis with low-quality evidence.53,54 Prospective studies comparing the reliability and safety of the wait-and-see to the therapeutic approach of silent CBD stones are needed to establish a more suitable strategy for this clinical setting.
SOD was historically considered a risk factor for PEP, but the real existence of the disease was recently disavowed from the EPISOD study.55 Long-term outcomes of this study including a randomized sham-controlled trial and a non-randomized protocol highlighted that a sham procedure in patients with suspected SOD type III – for example, post-cholecystectomy pain without abnormal liver function test results and non-dilated CBD – was not inferior when compared to the active treatment, that is, sphincterotomy.55 Therefore, these results suggest against performing ERCP for this particular context.
Risk stratification models for PEP
Risk stratification is defined as a process for systematically categorizing patients based on data reflecting their health status, lifestyle, and medical history.56 This method is increasingly perceived as helpful in clinical decision-making, by providing risk-stratified care management.
In 2002, Friedland et al.57 first developed a risk stratification model aiming at predicting the risk of pancreatitis in subjects undergoing ERCP. The authors identified multiple preoperative and perioperative factors that could predict the development of PEP: pain during the procedure, cannulation of the pancreatic duct, a previous history of PEP, and the number of cannulation attempts were the four significant variables provided by multivariate analysis. A simple scoring system was created from the results of the multivariate analysis, and three risk groups were identified: a low-risk group (⩽4 points), a medium-risk group (5–8 points), and a high-risk group (⩾9 points), with a probability to develop post-procedural pancreatitis of 1.9%, 6.9%, and 28%, respectively.57 This scoring system was also applied to two categories of patients already at high risk of PEP, those with a suspected SOD, and those undergoing cannulation of minor papilla,57,58 showing a good performance in predicting post-procedural pancreatitis.57 Unfortunately, this prediction model did not meet any validation, but it pointed the right way forward.
A turning point was represented by a Scandinavian work that constructed and validated a morphologic classification of the duodenal native major papilla, whose appearance is associated with bile duct cannulation complexity.59 Namely, small papilla – type 2 – [52%; 95% confidence interval (CI), 45–59%] and protruding or pendulous papilla – type 3 – (48%; 95% CI, 42–53%) showed a significantly higher complexity in the phase of biliary cannulation when compared with regular papilla – type 1 (36%; 95% CI, 33–40%). Even though PEP was not significantly associated with the different ampulla types, the frequency of this complication was increased in case of difficult cannulation (p < 0.05), setting the basis for the implementation of the well-known patient-related risk factors for this fearsome complication.59 Indeed, Zheng et al.60 rapidly seized the opportunity to create a novel risk prediction model including the morphological features of the native papillary orifice among the predictive factors for PEP. In particular, patients were stratified into three risk categories through a scoring system based on gastrectomy history, high serum albumin, villous type of papillary orifice, nodular type of papillary orifice, pancreatic guidewire passages, and pre-cut sphincterotomy as risk factors, and high serum direct bilirubin, CBD stone, and high operator experience as protective factors. Although the probability of PEP development significantly correlated with the degree of risk in the three stratified groups (the probability of PEP was 6.1%, 17.0%, and 37.5% in patients with low-, moderate-, and high-risk scores, respectively, p < 0.05), a multicenter study with larger sample size is still needed to assess and validate the ability of the scoring system to predict PEP.60
Recently, two different studies proposed similar clinical scoring systems that allow stratifying patients at low and high risk of developing pancreatitis after they have been treated with ERCP61,62 The principal identified risk factors are a personal history of PEP,62 native papilla,61,62 difficult cannulation,61,62 pancreatic injection,61,62 pancreatic and biliary intraductal ultrasonography,62 and absence of pancreatic stents.61 Both studies validated their stratification models using a bootstrapping resampling. Chiba et al.61, that applied a propensity score analysis for an internal validation, showed that their prediction model had an optimism-corrected area under the curve of 0.81 (95% CI, 0.77–0.86), whereas Fujita et al.62 using a simple addition of integer scores, reported an area under the curve on the validation set of 0.791, reaching a value similar to the performance in the training set (0.799).
Despite the growing interest in the development of feasible PEP prediction models, several limitations are common in the abovementioned studies. First, some methodological weaknesses, such as a small sample size and retrospective analysis are recognizable. Moreover, a great part of them are single tertiary center studies, without external validation, that are focused on both pre-procedural and peri-procedural variables.57,60,61 Pre-procedural risk stratification is in fact advisable to facilitate health cost containment and to minimize unnecessary admissions. To our knowledge, only one Korean study pertained to developing a pre-ERCP risk prediction model for PEP. The scoring system included younger age (⩽65 years), female sex, previous acute pancreatitis, and malignant biliary obstruction as independent risk factors for the development of PEP.63 Although the incidence of PEP in the validation cohort was greater in the high-risk group (6.9%) when compared to the low- (2.2%) and the moderate-risk groups (3.8%), the difference was not statistically significant (p > 0.05).63
Cutting-edge tools for predicting PEP: When machine learning comes into play
Over the last decades, a great number of studies have focused on understanding the risk factors for PEP, and the way to incorporate them into clinical decision-making. Nevertheless, the abovementioned risk prediction models are generally developed using multivariate regression models, not considering the synergetic effect between the different risk factors for PEP,64 and showing a suboptimal predictive performance. Studies developing and validating prediction models using artificial intelligence (AI) and machine learning (ML) are gaining increasing success in the healthcare community, because of their excellent performance and greater accuracy in the prediction of outcomes. A recent conference paper presented the first data of an international, multicenter, prospective cohort study that applied ML techniques in the development of two different models for the prediction of PEP, respectively, based on gradient boosting and logistic regression.65 Preliminary results of this study reported that relevant variables included in the analysis were mostly pre-procedural factors, such as total bilirubin level, body mass index, age, units of alcohol drunk per day, and previous ERCP with sphincterotomy.65 Interestingly, the gradient boosting-based model showed a significantly better performance when compared to the logistic regression-based one,65 raising awareness that the application of ML for risk stratification would lead to the development of more reliable and accurate models for the prediction of PEP.
Technical expedients and pharmacological measures in the prevention of PEP
Several technical issues have been reported as factors that impact the risk of PEP. These are difficult cannulation, need for pre-cut sphincterotomy, endoscopic papillary balloon dilation, pancreatic duct contrast injection, and self-expanding metal stent placement.66 Moreover, the pathophysiology of PEP development is still not clearly understood, though the occurrence of chemical, mechanical, or thermal injuries on the pancreatic acini seems to be the first step for the inflammatory cascade activation and the systemic cytokines release.67 The putative involvement of these pathogenic mechanisms has guided the development of different intra- and post-procedural approaches to reduce the incidence of PEP, and the reported preventive strategies might be divided into technical and pharmacological measures.
Technical approaches
As abovementioned, difficult cannulation is reported to be a risk factor for PEP development.3 Wire-guided biliary cannulation technique is recommended to facilitate the bile duct cannulation avoiding the unintentional injection of contrast medium into the pancreatic duct and reducing the risk of hydrostatic and chemical injury into the pancreatic tissue.68,69 A meta-analysis of nine randomized clinical trials including 2583 patients reported that the guidewire-assisted cannulation technique was associated with significantly higher success in primary cannulation (risk difference) (RD) - 0.07; 95% confidence interval (CI), 0.03–0.12; l2 = 12%) and a lower incidence of PEP (RD - 0.03; 95% CI, 0.01–0.05; l2 = 45%) when compared with the conventional contrast medium-assisted cannulation accidentally passed into the pancreas.70
The electrosurgical current used to perform the endoscopic sphincterotomy is involved in the development of thermal injuries to pancreatic parenchyma and, consequently, PEP. Several studies evaluated the differences between pure cutting and blended current in the involvement of PEP occurrence, leading to controversial results.71,72 Some authors reported an increased risk of adverse events when a blended current was used for sphincterotomy when compared with a pure-cut current.71 Conversely, no differences between blended current and pure-cut in the risk of PEP were documented in a subsequent study, demonstrating that pure-cut current was associated with a mildly increased risk of bleeding.72 Finally, in a Bayesian network meta-analysis of nine studies comparing different electrocautery modes (blended-cut, pure-cut, Endocut, and pure-cut followed by blended-cut), no statistically significant differences in the risk of PEP development were showed.73
One of the most promising technical options to reduce the occurrence of PEP is the decompression of the pancreatic duct system placing a stent in the MPD. It is well established that this expedient improves the drainage of the pancreatic juice and reduces hydrostatic injury.74 Sofuni et al.75 performed a multicenter, randomized, controlled trial including 426 patients undergoing ERCP to assess the incidence of PEP after pancreatic stent positioning. In all, 213 patients received a pancreatic stent placement and showed a lower incidence of PEP when compared with those patients not receiving the pancreatic stent (7.9% versus 15.2%, p = 0.021). Moreover, they reported that pancreatography, procedure time >30 min, sampling of pancreatic tissue, intraductal ultrasonography, and difficulty of cannulation were additional risk factors associated with an increased incidence of PEP, therefore justifying the positioning of a prophylactic pancreatic stent in high-risk patients.75 The use of small plastic stents is recommended to reduce the risk of pancreatic duct injuries associated with larger pancreatic stents and increase the possibility of spontaneous stent migration to avoid additional endoscopic procedure.66 Nevertheless, it has been shown that the size 5-French (Fr) stent is superior to the 3-Fr stent in preventing PEP, even better when pancreatic stents are provided with a duodenal flange or pigtail, due to the reduced risk of intraductal migration and a subsequent facilitated spontaneous dislodgement.76,77 Despite the current ESGE recommendation of using prophylactic pancreatic stents in selected patients with a high risk of developing PEP (e.g. unintentional guidewire insertion and/or contrast opacification of the pancreatic duct, double-guidewire cannulation),46 a recent meta-analysis including 10 randomized controlled trials on 1239 patients with pancreatic stent positioning documented that it was an efficient preventive approach even for average-risk subjects when compared to placebo (average-risk patients: relative risk (RR) = 0.07, 95% CI, 0.002–0.58, high-risk patients: RR = 0.20, 95% CI, 0.051–0.56).78 At present, ESGE recommends against the routinely prophylactic pancreatic stent positioning in all patients undergoing ERCP, because of a greater risk of pancreatitis in case of intraprocedural failed pancreatic stenting or subsequent endoscopic removal of retained stents.46 However, this does not happen very frequently, and, over recent years, novel helicoidally shaped biodegradable stents have been developed to overcome the abovementioned issues related to pancreatic conventional stents.79,80 Undoubtedly, the most valuable advantages of biodegradable stents are the avoidance of a later ERCP to remove them and the permanence inside the pancreatic duct for a sufficient period of time before degradation to guarantee pancreatic drainage.81 Whether the degradation of pancreatic stents could lead to the presence of stent fragments into a normal pancreatic duct with subsequent sequelae is not known; therefore, randomized controlled trials comparing outcomes of pancreatic biodegradable stents versus conventional stents positioning are warranted to define the best setting in which using this novel device. Moreover, high costs of biodegradable stents is a limitation to their use in clinical practice (Figure 4).
Figure 4.
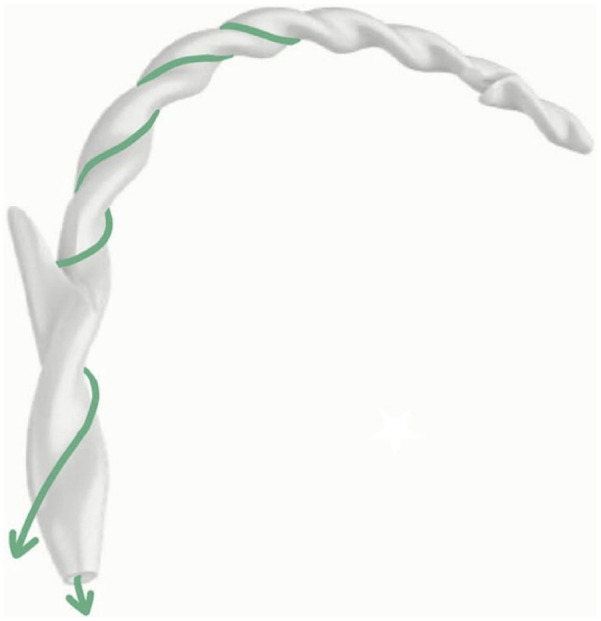
The novel helicoid-shaped biliary and pancreatic biodegradable stent (Archimedes stent; Amg International GmbH, Winsen, Germany). Reproduced from Anderloni et al.79
Finally, poor data on the more appropriate duration of EPBD to prevent the occurrence of PEP are available, though all studies suggest for a prolonged EPBD time. A randomized controlled trial including 170 patients with choledocholithiasis undergoing EPBD found that 5-min dilation was associated with a lower incidence of PEP when compared with 1-min EPBD (15.1% versus 4.8%, p = 0.038).82 This has been explained as a result of a complete loosening of the papillary sphincter, leading to less difficult cannulation and stone extraction.82 Another study reported that PEP occurrence was significantly higher in case of <3-min duration of EPBD when compared to the 3- to 5-min dilation (13.3% versus 3.1%, p = 0.032).83 Moreover, a large randomized controlled trial including 1920 consecutive patients with choledocholithiasis highlighted that an EPBD time of 30 s after endoscopic sphincterotomy had a lower risk of PEP when compared to a 300-s dilation, suggesting for a very short duration of EPBD in the case of combined endoscopic papilla dilation and sphincterotomy.84
Despite not being mentioned in the majority of the papers and guidelines, the quality of the radiological equipment can impact the rate of PEP since a good quality of fluoroscopy can permit a more careful and detailed control of the cannulation maneuvers.
Pharmacological approaches
Rectally administered NSAIDs are the only drug approved and recommended for the prevention of PEP and represent the first pharmacological approach to reduce the risk of this worrisome complication. A meta-analysis of 19 studies conducted on 5031 patients undergoing ERCP highlighted that NSAIDs administration was associated with a lower risk of PEP (RR = 0.54, 95% CI, 0.45–0.64, I2 = 40.4%) when compared with the control group.85 The route of administration of NSAIDs seems to be crucial in PEP prevention: given that the rectal administration showed significant efficacy, it is currently the only recommended route.86
Another evidence-based pharmacological strategy is periprocedural hydration. Aggressive hydration with Lactated Ringer (3 cc/kg/h during the procedure, 20 cc/kg bolus after the procedure, and 3 cc/kg/h for 8 h after the procedure) has been associated with a lower incidence of PEP when compared with standard hydration (1.5 cc/kg/h during and for 8 h after the procedure).87 In a randomized, controlled double-blind clinical trial including 150 patients, Shaygan-Nejad et al.88 reported that aggressive hydration was associated with a lower incidence of PEP if compared with standard hydration in patients undergoing ERCP without the prophylactic administration of rectal NSAIDs (5.5% versus 22.7%, p value = 0.002). Furthermore, hyperamylasemia and pancreatic pain occurred more likely in the standard hydration group (44% versus 22.7% and 37.3% versus 5.3%, respectively).88 This type of prophylaxis, despite being effective, is difficult to manage due to the complex administration with different regimens and volumes in the pre-, intra-, and post-ERCP phases. Aggressive hydration can be easier to apply in centers where ERCPs are performed together with the anesthesiologist.
Several other drugs have been evaluated to find new pharmacological strategies to prevent PEP. Nitrates are shown to relax the biliary and pancreatic sphincters, and might theoretically facilitate biliary cannulation and reduce pancreatic outflow obstruction after the procedure.89 In a meta-analysis of 11 trials including 1814 subjects undergoing ERCP, the overall risk of PEP was significantly lower in the nitrates group if compared with placebo (odds ratio: 0.56, 95% CI, 0.40–0.79; p = 0.001).90 Modulators of pancreatic secretions, such as somatostatin and its analog, octreotide, have been widely evaluated in the prevention of PEP, even though results are controversial. A meta-analysis of 11 studies conducted on 2869 patients documented no benefits in administrating somatostatin as a short-term infusion (RR = 1.40, 95% CI, 0.93–2.12; p = 0.11), but a slight benefit when administrated as a bolus or a long-term injection (RR = 0.25, 95% CI, 0.13–0.47, p < 0.0001; RR = 0.44, 95% CI, 0.27–0.71, p = 0.0008).91 In another meta-analysis of 15 studies, the administration of octreotide showed no efficacy in PEP prevention.92 Several other drugs, such as gabexate mesylate, allopurinol, heparin, and corticosteroids, topical epinephrine spray, have been evaluated to prevent the occurrence of PEP, however, the results are sparse and their use is currently not recommended.93–96
Upcoming developments
Despite the improvement of preventive strategies for the development of PEP, this complication still presents a high incidence and relevant mortality. Since PEP could result from a combination of multiple mechanisms, a comprehensive approach to its prevention should be employed by ERCP operators. Our review focused on three areas of preventive strategies: adequate training in ERCP, appropriate patient selection and risk stratification, and intraprocedural and pharmacological expedients. Even though certain progress in these domains has been made, current preventive approaches seem to lower the risk of PEP without eliminating it. The application of AI might have a promising role in the whole strategy of PEP prevention. AI implementation could provide reliable predictive models to appropriately select ERCP candidates and help both trainees and experts to better face some crucial steps during ERCP, such as accessing the bile duct or pancreatic stent positioning. AI is indeed providing a rapid shift paradigm in medicine. In such a risky but essential endoscopic procedure, AI-based methods might improve therapeutic and prognostic models in ERCP leading to safer clinical management. Further studies are needed to expand this innovative area of research.
Acknowledgments
None.
Footnotes
ORCID iD: Ivo Boškoski  https://orcid.org/0000-0001-8194-2670
https://orcid.org/0000-0001-8194-2670
Contributor Information
Federica Borrelli de Andreis, First Department of Internal Medicine, Fondazione IRCCS San Matteo Hospital, University of Pavia, Pavia, Italy; Gastroenterology Unit, Istituti Clinici Maugeri, University of Pavia, Pavia, Italy; Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Pietro Mascagni, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Institute of Image-Guided Surgery, IHU-Strasbourg, France.
Tommaso Schepis, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Fabia Attili, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Andrea Tringali, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Guido Costamagna, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Centre for Endoscopic Research Therapeutics and Training (CERTT), Università Cattolica Del Sacro Cuore di Roma, Roma, Italy.
Ivo Boškoski, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Largo A. Gemelli, Rome, 00168, Italy; IHU Strasbourg 1, Place de l’Hopital 67091 Strasbourg Cedex, France; Centre for Endoscopic Research Therapeutics and Training (CERTT), Università Cattolica Del Sacro Cuore di Roma, Roma, Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Federica Borrelli de Andreis: Conceptualization; Writing – original draft.
Pietro Mascagni: Conceptualization; Writing – review & editing.
Tommaso Schepis: Writing – original draft.
Fabia Attili: Supervision; Visualization.
Andrea Tringali: Supervision; Visualization.
Guido Costamagna: Conceptualization; Supervision; Visualization.
Ivo Boškoski: Conceptualization; Supervision; Visualization.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
IB is a consultant for Apollo Endosurgery, Cook Medical, BostonScientific, and Nitinotes. GC is a consultant for Cook Medical, Boston Scientific, and Olympus. AT is a consultant for Olympus and Boston Scientific. All the other authors have nothing to declare.
Availability of data and materials: Not applicable.
References
- 1. Cohen S, Bacon BR, Berlin JA, et al. National institutes of health state-of-the-science conference statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc 2002; 56: 803–809. [DOI] [PubMed] [Google Scholar]
- 2. Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc 2015; 81: 143.e9–149.e9. [DOI] [PubMed] [Google Scholar]
- 3. Wang AY, Strand DS, Shami VM. Prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: medications and techniques. Clin Gastroenterol Hepatol 2016; 14: 1521.e3–1532.e3. [DOI] [PubMed] [Google Scholar]
- 4. Elmunzer BJ. Prevention of ERCP-induced pancreatitis. Pancreapedia: The Exocrine Pancreas Knowledge Base. Epub ahead of print June 2015. DOI: 10.3998/panc.2015.19. [DOI] [Google Scholar]
- 5. Talukdar R. Complications of ERCP. Best Pract Res Clin Gastroenterol 2016; 30: 793–805. [DOI] [PubMed] [Google Scholar]
- 6. Sivak MV. Trained in ERCP. Gastrointest Endosc 2003; 58: 412–414. [DOI] [PubMed] [Google Scholar]
- 7. Jowell PS, Baillie J, Branch MS, et al. Quantitative assessment of procedural competence. A prospective study of training in endoscopic retrograde cholangiopancreatography. Ann Intern Med 1996; 125: 983–989. [DOI] [PubMed] [Google Scholar]
- 8. Johnson G, Webster G, Boškoski I, et al. Curriculum for ERCP and endoscopic ultrasound training in Europe: European society of gastrointestinal endoscopy (ESGE) position statement. Endoscopy 2021; 53: 1071–1087. [DOI] [PubMed] [Google Scholar]
- 9. Lim BS, Leung JW. Training methods for endoscopic retrograde cholangiopancreatography. Tech Gastrointest Endosc 2011; 13: 132–139. [Google Scholar]
- 10. Rodrigues-Pinto E, Macedo G, Baron TH. ERCP competence assessment: miles to go before standardization. Endosc Int Open 2017; 5: E718–E721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wani S, Hall M, Wang AY, et al. Variation in learning curves and competence for ERCP among advanced endoscopy trainees by using cumulative sum analysis. Gastrointest Endosc 2016; 83: 711.e11–719.e11. [DOI] [PubMed] [Google Scholar]
- 12. Waschke KA, Anderson J, Macintosh D, et al. Training the gastrointestinal endoscopy trainer. Best Pract Res Clin Gastroenterol 2016; 30: 409–419. [DOI] [PubMed] [Google Scholar]
- 13. Waschke KA, Anderson J, Valori RM, et al. ASGE principles of endoscopic training. Gastrointestinal Endoscopy 2019; 90: 27–34. [DOI] [PubMed] [Google Scholar]
- 14. Sedlack RE. The state of simulation in endoscopy education: continuing to advance toward our goals. Gastroenterology 2013; 144: 9–12. [DOI] [PubMed] [Google Scholar]
- 15. ASGE Technology Committee, Goodman AJ, Melson J, et al. Endoscopic simulators. Gastrointest Endosc 2019; 90: 1–12. [DOI] [PubMed] [Google Scholar]
- 16. Noar MD. An established porcine model for animate training in diagnostic and therapeutic ERCP. Endoscopy 1995; 27: 77–80. [DOI] [PubMed] [Google Scholar]
- 17. Maiss J, Wiesnet J, Proeschel A, et al. Objective benefit of a 1-day training course in endoscopic hemostasis using the ‘compactEASIE’ endoscopy simulator. Endoscopy 2005; 37: 552–558. [DOI] [PubMed] [Google Scholar]
- 18. Levy N. The use of animal as models: ethical considerations. Int J Stroke 2012; 7: 440–442. [DOI] [PubMed] [Google Scholar]
- 19. Frimberger E, von Delius S, Rösch T, et al. A novel and practicable ERCP training system with simulated fluoroscopy. Endoscopy 2008; 40: 517–520. [DOI] [PubMed] [Google Scholar]
- 20. Leung JW, Lee JG, Rojany M, et al. Development of a novel ERCP mechanical simulator. Gastrointest Endosc 2007; 65: 1056–1062. [DOI] [PubMed] [Google Scholar]
- 21. Boškoski I, Costamagna G. The Boškoski-Costamagna ERCP trainer: from dream to reality. Endoscopy 2016; 48: 593. [DOI] [PubMed] [Google Scholar]
- 22. van der Wiel SE, Koch AD, Bruno MJ. Face and construct validity of a novel mechanical ERCP simulator. Endosc Int Open 2018; 6: E758–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gallo C, Boškoski I, Matteo MV, et al. Training in endoscopic retrograde cholangio-pancreatography: a critical assessment of the broad scenario of training programs and models. Expert Rev Gastroenterol Hepatol 2021; 15: 675–688 [DOI] [PubMed] [Google Scholar]
- 24. Bar-Meir S. Simbionix simulator. Gastrointest Endosc Clin N Am 2006; 16: 471–478, vii. [DOI] [PubMed] [Google Scholar]
- 25. Triantafyllou K, Lazaridis LD, Dimitriadis GD. Virtual reality simulators for gastrointestinal endoscopy training. World J Gastrointest Endosc 2014; 6: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yudkowsky R, Park YS, Downing SM. Assessment in Health Professions Education, 2nd edn. New York, NY: Routledge & CRC Press. [Google Scholar]
- 27. Khan R, Scaffidi MA, Grover SC, et al. Simulation in endoscopy: practical educational strategies to improve learning. World J Gastrointest Endosc 2019; 11: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mascagni P, Riva P, Guerriero L, et al. A curriculum to democratize and standardize flexible endoscopy fundamental knowledge and skills: a critical review of the first 5 years of a surgical endoscopy university diploma. Surg Endosc 2021; 35: 2473–2479. [DOI] [PubMed] [Google Scholar]
- 29. Chutkan RK, Ahmad AS, Cohen J, et al. ERCP core curriculum. Gastrointest Endosc 2006; 63: 361–376. [DOI] [PubMed] [Google Scholar]
- 30. Shahidi N, Ou G, Telford J, et al. When trainees reach competency in performing ERCP: a systematic review. Gastrointest Endosc 2015; 81: 1337–1342. [DOI] [PubMed] [Google Scholar]
- 31. Wani S, Han S, Simon V, et al. Setting minimum standards for training in EUS and ERCP: results from a prospective multicenter study evaluating learning curves and competence among advanced endoscopy trainees. Gastrointest Endosc 2019; 89: 1160.e9–1168.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. ASGE Training Committee, Jorgensen J, Kubiliun N, et al. Endoscopic retrograde cholangiopancreatography (ERCP): core curriculum. Gastrointest Endosc 2016; 83: 279–289. [DOI] [PubMed] [Google Scholar]
- 33. Kowalski T, Kanchana T, Pungpapong S. Perceptions of gastroenterology fellows regarding ERCP competency and training. Gastrointest Endosc 2003; 58: 345–349. [DOI] [PubMed] [Google Scholar]
- 34. Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach 2010; 32: 638–645. [DOI] [PubMed] [Google Scholar]
- 35. Ekkelenkamp VE, Koch AD, Rauws EAJ, et al. Competence development in ERCP: the learning curve of novice trainees. Endoscopy 2014; 46: 949–955. [DOI] [PubMed] [Google Scholar]
- 36. Watkins JL, Etzkorn KP, Wiley TE, et al. Assessment of technical competence during ERCP training. Gastrointest Endosc 1996; 44: 411–415. [DOI] [PubMed] [Google Scholar]
- 37. Vitale GC, Zavaleta CM, Vitale DS, et al. Training surgeons in endoscopic retrograde cholangiopancreatography. Surg Endosc 2006; 20: 149–152. [DOI] [PubMed] [Google Scholar]
- 38. Wani S, Keswani R, Hall M, et al. A Prospective multicenter study evaluating learning curves and competence in endoscopic ultrasound and endoscopic retrograde cholangiopancreatography among advanced endoscopy trainees: the rapid assessment of trainee endoscopy skills study. Clin Gastroenterol Hepatol 2017; 15: 1758.e11–1767.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Voiosu T, Bălănescu P, Voiosu A, et al. Measuring trainee competence in performing endoscopic retrograde cholangiopancreatography: a systematic review of the literature. United European Gastroenterol J 2019; 7: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wani S, Keswani RN, Petersen B, et al. Training in EUS and ERCP: standardizing methods to assess competence. Gastrointest Endosc 2018; 87: 1371–1382. [DOI] [PubMed] [Google Scholar]
- 41. Siau K, Dunckley P, Valori R, et al. Changes in scoring of direct observation of procedural skills (DOPS) forms and the impact on competence assessment. Endoscopy 2018; 50: 770–778. [DOI] [PubMed] [Google Scholar]
- 42. Voiosu T, Boskoski I, Voiosu AM, et al. Impact of trainee involvement on the outcome of ERCP procedures: results of a prospective multicenter observational trial. Endoscopy 2020; 52: 115–122. [DOI] [PubMed] [Google Scholar]
- 43. Chen J-J, Wang X-M, Liu X-Q, et al. Risk factors for post-ERCP pancreatitis: a systematic review of clinical trials with a large sample size in the past 10 years. Eur J Med Res 2014; 19: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ding X, Zhang F, Wang Y. Risk factors for post-ERCP pancreatitis: a systematic review and meta-analysis. Surgeon 2015; 13: 218–229. [DOI] [PubMed] [Google Scholar]
- 45. El Nakeeb A, El Hanafy E, Salah T, et al. Post-endoscopic retrograde cholangiopancreatography pancreatitis: risk factors and predictors of severity. World J Gastrointest Endosc 2016; 8: 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dumonceau J-M, Kapral C, Aabakken L, et al. ERCP-related adverse events: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy 2020; 52: 127–149. [DOI] [PubMed] [Google Scholar]
- 47. Cotton PB. Analysis of 59 ERCP lawsuits; mainly about indications. Gastrointest Endosc 2006; 63: 378–382. [DOI] [PubMed] [Google Scholar]
- 48. Sanders D, Bomman S, Krishnamoorthi R, et al. Endoscopic retrograde cholangiopancreatography: current practice and future research. World J Gastrointest Endosc 2021; 13: 260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim SB, Kim KH, Kim TN. Comparison of outcomes and complications of endoscopic common bile duct stone removal between asymptomatic and symptomatic patients. Dig Dis Sci 2016; 61: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 50. Xu XD, Qian JQ, Dai JJ, et al. Endoscopic treatment for choledocholithiasis in asymptomatic patients. J Gastroenterol Hepatol 2020; 35: 165–169. [DOI] [PubMed] [Google Scholar]
- 51. Kadokura M, Takenaka Y, Yoda H, et al. Asymptomatic common bile duct stones are associated with increased risk of post-endoscopic retrograde cholangiopancreatography pancreatitis. JMA J 2021; 4: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saito H, Sakaguchi M, Kadono Y, et al. Disease-based risk stratification of postendoscopic retrograde cholangiopancreatography pancreatitis for common bile duct stones. Dig Dis Sci 2022; 67: 305–314. [DOI] [PubMed] [Google Scholar]
- 53. Manes G, Paspatis G, Aabakken L, et al. Endoscopic management of common bile duct stones: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy 2019; 51: 472–491. [DOI] [PubMed] [Google Scholar]
- 54. Buxbaum JL, Abbas Fehmi SM, Sultan S, et al. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc 2019; 89: 1075.e15–1105.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cotton PB, Pauls Q, Keith J, et al. The EPISOD study: long-term outcomes. Gastrointest Endosc 2018; 87: 205–210. [DOI] [PubMed] [Google Scholar]
- 56. Dera JD. Risk stratification: a two-step process for identifying your sickest patients. FPM 2019; 26: 21–26. [PubMed] [Google Scholar]
- 57. Friedland S, Soetikno RM, Vandervoort J, et al. Bedside scoring system to predict the risk of developing pancreatitis following ERCP. Endoscopy 2002; 34: 483–488. [DOI] [PubMed] [Google Scholar]
- 58. Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54: 425–434. [DOI] [PubMed] [Google Scholar]
- 59. Haraldsson E, Kylänpää L, Grönroos J, et al. Macroscopic appearance of the major duodenal papilla influences bile duct cannulation: a prospective multicenter study by the scandinavian association for digestive endoscopy study group for ERCP. Gastrointest Endosc 2019; 90: 957–963. [DOI] [PubMed] [Google Scholar]
- 60. Zheng R, Chen M, Wang X, et al. Development and validation of a risk prediction model and scoring system for post-endoscopic retrograde cholangiopancreatography pancreatitis. Ann Transl Med 2020; 8: 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chiba M, Kato M, Kinoshita Y, et al. The milestone for preventing post-ERCP pancreatitis using novel simplified predictive scoring system: a propensity score analysis. Surg Endosc 2021; 35: 6696–6707. [DOI] [PubMed] [Google Scholar]
- 62. Fujita K, Yazumi S, Uza N, et al. New practical scoring system to predict post-endoscopic retrograde cholangiopancreatography pancreatitis: Development and validation. JGH Open 2021; 5: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park CH, Park SW, Yang MJ, et al. Pre- and post-procedure risk prediction models for post-endoscopic retrograde cholangiopancreatography pancreatitis. Surg Endosc 2022; 36: 2052–2061. [DOI] [PubMed] [Google Scholar]
- 64. Dumonceau J-M, Andriulli A, Deviere J, et al. European society of gastrointestinal endoscopy (ESGE) guideline: prophylaxis of post-ERCP pancreatitis. Endoscopy 2010; 42: 503–515. [DOI] [PubMed] [Google Scholar]
- 65. Archibugi L, Ciarfaglia G, Cárdenas-Jaén K, et al. Stark study: Machine Learning Approach To Predict Post-Ercp Pancreatitis In An International Multicenter Prospective Cohort Study. In: Endoscopy. © Georg Thieme Verlag KG, p. OP215. [Google Scholar]
- 66. Maranki J, Yeaton P. Prevention of post-ERCP pancreatitis. Curr Gastroenterol Rep 2013; 15: 352. [DOI] [PubMed] [Google Scholar]
- 67. Pekgöz M. Post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review for prevention and treatment. World J Gastroenterol 2019; 25: 4019–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Artifon ELA, Sakai P, Cunha JEM, et al. Guidewire cannulation reduces risk of post-ERCP pancreatitis and facilitates bile duct cannulation. Am J Gastroenterol 2007; 102: 2147–2153. [DOI] [PubMed] [Google Scholar]
- 69. Lella F, Bagnolo F, Colombo E, et al. A simple way of avoiding post-ERCP pancreatitis. Gastrointest Endosc 2004; 59: 830–834. [DOI] [PubMed] [Google Scholar]
- 70. de Moura ET, de Moura EG, Bernardo W, et al. Guide wire-assisted cannulation versus conventional contrast to prevent pancreatitis. A systematic review and meta-analysis based on randomized control trials. Rev Gastroenterol Peru 2016; 36: 308–319. [PubMed] [Google Scholar]
- 71. Elta GH, Barnett JL, Wille RT, et al. Pure cut electrocautery current for sphincterotomy causes less post-procedure pancreatitis than blended current. Gastrointest Endosc 1998; 47: 149–153. [DOI] [PubMed] [Google Scholar]
- 72. Macintosh DG, Love J, Abraham NS. Endoscopic sphincterotomy by using pure-cut electrosurgical current and the risk of post-ERCP pancreatitis: a prospective randomized trial. Gastrointest Endosc 2004; 60: 551–556. [DOI] [PubMed] [Google Scholar]
- 73. Hedjoudje A, Cheurfa C, Farha J, et al. Safety of different electrocautery modes for endoscopic sphincterotomy: a Bayesian network meta-analysis. Ther Adv Gastrointest Endosc 2021; 14: 26317745211062984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lee LS. ERCP and EUS: A Case-Based Approach. Springer, 2015. [Google Scholar]
- 75. Sofuni A, Maguchi H, Mukai T, et al. Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin Gastroenterol Hepatol 2011; 9: 851–858. [DOI] [PubMed] [Google Scholar]
- 76. Afghani E, Akshintala VS, Khashab MA, et al. 5-Fr vs. 3-Fr pancreatic stents for the prevention of post-ERCP pancreatitis in high-risk patients: a systematic review and network meta-analysis. Endoscopy 2014; 46: 573–580. [DOI] [PubMed] [Google Scholar]
- 77. Bhatt H. Post-endoscopic retrograde cholangiopancreatography pancreatitis: an updated review of current preventive strategies. Clin Exp Gastroenterol 2021; 14: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dubravcsik Z, Hritz I, Keczer B, et al. Network meta-analysis of prophylactic pancreatic stents and non-steroidal anti-inflammatory drugs in the prevention of moderate-to-severe post-ERCP pancreatitis. Pancreatology 2021; 21: 704–713. [DOI] [PubMed] [Google Scholar]
- 79. Anderloni A, Fugazza A, Maroni L, et al. New biliary and pancreatic biodegradable stent placement: a single-center, prospective, pilot study (with video). Gastrointest Endosc 2020; 92: 405–411. [DOI] [PubMed] [Google Scholar]
- 80. Pérez-Cuadrado Robles E, Lakhtakia S, Othman H, et al. A new biodegradable stent in bilio-pancreatic diseases: a prospective multi-center feasibility study. Rev Esp Enferm Dig 2022; 114: 529–533. [DOI] [PubMed] [Google Scholar]
- 81. Daniel P, Rana SS. Biodegradable pancreatic and biliary stents—still searching for disappearing wonder? J Dig Endosc 2020; 11: 173–176. [Google Scholar]
- 82. Liao W-C, Lee C-T, Chang C-Y, et al. Randomized trial of 1-minute versus 5-minute endoscopic balloon dilation for extraction of bile duct stones. Gastrointest Endosc 2010; 72: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 83. Chou C-K, Lee K-C, Luo J-C, et al. Endoscopic papillary balloon dilatation less than three minutes for biliary stone removal increases the risk of post-ERCP pancreatitis. PLoS One 2020; 15: e0233388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Meng W, Leung JW, Zhang K, et al. Optimal dilation time for combined small endoscopic sphincterotomy and balloon dilation for common bile duct stones: a multicentre, single-blinded, randomised controlled trial. Lancet Gastroenterol Hepatol 2019; 4: 425–434. [DOI] [PubMed] [Google Scholar]
- 85. Liu L, Li C, Huang Y, et al. Nonsteroidal anti-inflammatory drugs for endoscopic retrograde cholangiopancreatography postoperative pancreatitis prevention: a systematic review and meta-analysis. J Gastrointest Surg 2019; 23: 1991–2001. [DOI] [PubMed] [Google Scholar]
- 86. Patai Á, Solymosi N, Mohácsi L, et al. Indomethacin and diclofenac in the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis of prospective controlled trials. Gastrointest Endosc 2017; 85: 1144.e1–1156.e1. [DOI] [PubMed] [Google Scholar]
- 87. Buxbaum J, Yan A, Yeh K, et al. Aggressive hydration with lactated Ringer’s solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol 2014; 12: 303.e1–307.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shaygan-Nejad A, Masjedizadeh AR, Ghavidel A, et al. Aggressive hydration with Lactated Ringer’s solution as the prophylactic intervention for postendoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled double-blind clinical trial. J Res Med Sci 2015; 20: 838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sotoudehmanesh R, Eloubeidi MA, Asgari AA, et al. A randomized trial of rectal indomethacin and sublingual nitrates to prevent post-ERCP pancreatitis. Am J Gastroenterol 2014; 109: 903–909. [DOI] [PubMed] [Google Scholar]
- 90. Chen B, Fan T, Wang C-H. A meta-analysis for the effect of prophylactic GTN on the incidence of post-ERCP pancreatitis and on the successful rate of cannulation of bile ducts. BMC Gastroenterol 2010; 10: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Qin X, Lei WS, Xing ZX, et al. Prophylactic effect of somatostatin in preventing Post-ERCP pancreatitis: an updated meta-analysis. Saudi J Gastroenterol 2015; 21: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bai Y, Gao J, Zou D-W, et al. Prophylactic octreotide administration does not prevent post-endoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis of randomized controlled trials. Pancreas 2008; 37: 241–246. [DOI] [PubMed] [Google Scholar]
- 93. Budzyńska A, Marek T, Nowak A, et al. A prospective, randomized, placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy 2001; 33: 766–772. [DOI] [PubMed] [Google Scholar]
- 94. Rabenstein T, Fischer B, Wiessner V, et al. Low-molecular-weight heparin does not prevent acute post-ERCP pancreatitis. Gastrointest Endosc 2004; 59: 606–613. [DOI] [PubMed] [Google Scholar]
- 95. Andriulli A, Leandro G, Federici T, et al. Prophylactic administration of somatostatin or gabexate does not prevent pancreatitis after ERCP: an updated meta-analysis. Gastrointest Endosc 2007; 65: 624–632. [DOI] [PubMed] [Google Scholar]
- 96. Iqbal U, Siddique O, Khara HS, et al. Post-endoscopic retrograde cholangiopancreatography pancreatitis prevention using topical epinephrine: systematic review and meta-analysis. Endosc Int Open 2020; 8: E1061–E1067. [DOI] [PMC free article] [PubMed] [Google Scholar]


