Abstract
Over a decade of sequencing-based genomics research has unveiled a diverse somatic mutation landscape across patients with pancreatic ductal adenocarcinoma (PDAC), and the identification of druggable mutations has aligned with the development of novel targeted therapeutics. However, despite these advances, direct translation of years of PDAC genomics research into the clinical care of patients remains a critical and unmet need. Technologies that enabled the initial mapping of the PDAC mutation landscape, namely whole-genome and transcriptome sequencing, remain overly expensive in terms of both time and financial resources. Consequentially, dependence on these technologies to identify the relatively small subset of patients with actionable PDAC alterations has greatly impeded enrollment for clinical trials testing novel targeted therapies. Liquid biopsy tumor profiling using circulating tumor DNA (ctDNA) generates new opportunities by overcoming these challenges while further addressing issues particularly relevant to PDAC, namely, difficulty of obtaining tumor tissue via fine-needle biopsy and the need for faster turnaround time due to rapid disease progression. Meanwhile, ctDNA-based approaches for tracking disease kinetics with respect to surgical and therapeutic interventions offer a means to elevate the current clinical management of PDAC toward higher granularity and accuracy. This review provides a clinically focused summary of ctDNA advances, limitations, and opportunities in PDAC and postulates ctDNA sequencing technology as a catalyst for evolving the clinical decision-making paradigm of this disease.
Keywords: circulating tumor DNA, pancreatic cancer, liquid biopsy, KRAS mutation, genetic testing, molecular testing
Introduction
Pancreatic cancer incidence has steadily increased over recent years to become the third-leading cause of cancer-related death in the United States.1 Paluri et al. recently reported that over 60% of patients diagnosed with pancreatic ductal adenocarcinoma (PDAC), the most common form of pancreatic cancer, were diagnosed with incurable metastatic disease and that this number has increased to nearly 70% during the COVID-19 pandemic.2 As many patients diagnosed with metastatic PDAC are too unwell to be treated with systemic therapy,3 the average patient survival is approximately 2–3 months.4
The past decade of sequencing-based genetic research in PDAC has shown a diverse mutational landscape across patient tumors, with approximately 20% of tumors bearing mutations considered therapeutically actionable and capable of improving individual patient survival when used to guide therapy.5,6 Despite these advances in the academic field, accessibility continues to impede the delivery of genomics-guided medicine to the majority of patients with PDAC, as sequencing-based technologies capable of detecting actionable mutations are often restricted to a subset of patients receiving care through large-center clinical trials in major academic centers. Moreover, the application of bulk tissue sequencing to the clinical management of PDAC faces additional and unique challenges when compared to other cancer types, including difficulty in tissue biopsy procedure and rapid decline in patient health precluding biopsy and often utility of genomic results. As a result, the majority of patients diagnosed with PDAC do not receive any genomics testing, and clinical decision-making in PDAC remains largely centered around cytotoxic chemotherapy regimens guided by patient performance status.
Recent advances in circulating tumor DNA (ctDNA) sequencing technology present an opportunity to evolve the current clinical paradigm of PDAC care to leverage the growing knowledge base of actionable mutations through a genomics-based assay that is less invasive while having faster turnaround times and lower cost, though necessary improvements in the sensitivity and specificity of contemporary ctDNA panels remain ongoing. This review aims to delineate recent advances and ongoing limitations of ctDNA sequencing in PDAC, while positioning ctDNA-based technology as a promising catalyst for the much-needed evolution of routine clinical decision-making in PDAC.
The clinical actionability landscape of PDAC
For over a decade, DNA sequencing-based profiling of cancer using bulk tumor tissue biopsies has unveiled complex and diverse mutational landscapes across many cancer types including PDAC.7–10 Actionable mutations, in which mutation presence confers sensitivity to specific targeted agents, are harbored in approximately 20% of PDAC tumors11,12 and include alterations such as NRG1 fusion (targeted agents include afatinib13 and zenocutuzumab14), NTRK fusion (entrectinib,15 larotrectinib16), ALK fusion, DNA damage repair mutation (such as germline PALB2/BRCA mutation; PARP inhibition17), and KRAS G12C mutation (sotorasib,18 adagrasib19). Consequently, a surge of clinical trials aimed at demonstrating the efficacy of molecular-targeted drugs have emerged and include examples such as CRESTONE (NRG1 fusion inhibitor; NCT04383210), CodeBreak100 (G12C inhibitor; NCT03600883), and NAVIGATE (NTRK1 fusion inhibitor; NCT02576431). Ability to enroll eligible patients into such molecular-targeted trials, however, is faced with several ongoing challenges that greatly slow the adoption of novel therapeutics into PDAC clinical care.
Challenges faced by molecular-targeted clinical trials in PDAC
The frequency of a specific targetable mutation is low among the total population of patients diagnosed with PDAC and can vary greatly across different cohorts of patients, with NRG1 fusion rates, for example, varying from 0 of 195 patients,9 3 of 47 patients13 (6%), and 3 of 17 patients20 (18%). Despite their low frequency, however, the number of patients with PDAC capable of benefiting from targeted treatments should not be understated. In 2022, there is estimated to be 62,210 new cases of pancreatic cancer in the United States alone, and this number has risen over past years.1,21 The question remaining, then, lies in how the subgroup of patients with actionable mutations may be better identified. Exploratory, bulk tissue-based next-generation sequencing (NGS) technologies, such as whole-genome sequencing (WGS), were fundamental in establishing the current knowledge base of PDAC mutation landscapes. Continuing to apply such methods to the unselected patient population in hopes of identifying patients eligible for subgroup-targeted PDAC clinical trials, however, is suboptimal given the required time and financial costs of bulk tissue sequencing.22–24 While ctDNA sequencing offers improvement in terms of cost-effectiveness, sequencing would remain unproductive for the roughly 80% of patients with PDAC who do not harbor targetable alterations. Meanwhile, it is well established that the majority of targetable alterations are highly enriched among the approximately 10–15% of PDAC tumors that lack oncogenic KRAS mutation,25 and studies of NRG1 fusions in PDAC, for instance, have converged on the observation that such fusions are exclusively found in the KRAS wild-type subgroup of patients.13,20 For patients newly diagnosed with PDAC, clinical testing of KRAS mutation status followed by deployment of further sequencing-based testing for those with KRAS wild-type tumors represents a tiered and reflexive approach that would build upon what we have learned in PDAC genomics to better guide the appropriate subpopulation of patients toward genetic testing and greatly aid clinical trial enrollment.
Clinical trial enrollment for patients with PDAC is further hindered by inherent aggressiveness of the disease, which manifests as rapid clinical deterioration. Identification of patients with PDAC who are eligible for subgroup-targeted clinical trials is therefore time-sensitive and with turnaround times ranging between 19 and 52 (median 35) days from time of biopsy9; bulk tissue WGS is not well suited for this application. Apart from time required to perform tissue sequencing, complexities surrounding tissue biopsy further delay sequencing-based guidance in care. For a cohort of 116 patients with PDAC, Deshwar et al. reported a median diagnostic time (time from first medical visit to diagnosis) of 22 days, and individuals with longer diagnostic times were significantly less likely to be suitable candidates for resection.26 Meanwhile, delays in tissue biopsy are included among the list of negative effects by which the COVID-19 pandemic has impacted clinical care for patients with PDAC.27 While WGS possesses the breadth to cover nearly all detectable mutations in a sample, ctDNA panels are subdivided into two main categories based on how their more limited catalog of ascertainable mutations are chosen. Tumor-naïve panels rely on a predetermined list of genes commonly implicated across many cancer types, such as the 54- and 500-gene Guardant360 and GuardantOMNI (respectively) panels,28,29 while tumor-informed panels require upfront tissue biopsy and sequencing to identify the tumor mutation landscape of the individual patient, which is then used to tailor the list of ascertainable mutations. As a more rapid technology that requires only blood-based sampling, tumor-naïve ctDNA sequencing improves upon the time limitations of tissue-based WGS and offers more promise in the deliverance of sequencing-informed care for patients with PDAC. In colorectal cancer, comparison between tumor-naïve ctDNA-based GOZILA (Guardant360; n = 1687 patients) and tissue-based (non-ctDNA; mutation panel) GI-SCREEN (n = 5621) studies showed that ctDNA analysis increased the relative proportion of patients enrolled in clinical trials by 132% while shortening the median time between sample collection and trial enrollment (1.1 months for ctDNA versus 20.2 months for tissue30). Meanwhile, other tumor-naïve ctDNA studies have reported average turnaround times (from sample collection to results reporting) of 9 (Guardant360; various advanced cancer types31), 9.5 (Guardant360; various advanced rare cancers32), and 15 (Guardant360; advanced lung adenocarcinoma33) days. While the clinical benefit of reduced genomics turnaround times is certainly intuitive, clinical trials focused on ctDNA-based treatment decision-making in PDAC are currently needed to measure the degree to which clinical outcome is improved for patients receiving ctDNA versus tissue sequencing.
Rapid clinical deterioration introduces an additional challenge when identifying patients with targetable mutations, as repeat tissue biopsy procedures, the majority being fine-needle aspirate biopsy of metastatic lesions of the liver, are often too invasive for patients that have progressed on first-line therapy. Inability to perform progression biopsies represents a distinct missed opportunity in PDAC, as the evolution of tumors over time during treatment (in which acquired mutations not present in initial biopsy sampling emerge) is well known.16,34,35 The utility of minimally invasive blood sample-based ctDNA sequencing is therefore very promising for repeat tumor sampling for patients with PDAC.
ctDNA-based mutation profiling in PDAC
Cell-free, tumor-derived DNA fragments circulating in the blood stream (ctDNA) provide a window into the mutational landscape of a tumor. Early studies showed that circulating DNA levels were significantly more pronounced in plasma samples from patients with pancreatic cancer compared to healthy donors and were compatible with the notion that DNA from tumor cells could be shed into the peripheral blood stream.36,37 With the emergence of DNA sequencing technology, it was later confirmed that plasma-derived, cell-free DNA (cfDNA) detected in patients with PDAC could be attributed to tumor cells based on the presence of mutant KRAS.38,39 ctDNA typically represents only a small proportion of the total cfDNA in a plasma sample. In colorectal cancer, the proportion of ctDNA has been quantified at approximately 1% of total cfDNA,40 and this level has been shown to vary greatly across patients.41 Correlation between ctDNA levels and tumor stage is perhaps intuitive, as later tumor stages involve increased circulatory system infiltration which facilitates metastasis, and increased ctDNA levels in metastatic compared to localized tumors have been shown for several cancer types including pancreas.42 Recently, a 2022 study benchmarking ctDNA assays from five different vendors showed that variant allele frequency and DNA input levels affected accuracy at varying degrees in each of the different ctDNA platforms.43 Taken together, factors such as disease stage, sample input, and sequencing platform are reasons for which the ability to detect true genomic alterations or sensitivity has been perhaps the most challenging aspect of ctDNA sequencing that continues to impede its adoption into routine clinical practice.
Clonal, oncogenic KRAS mutations are present in approximately 90% of PDAC and represent a reasonable surrogate for assessing ctDNA sensitivity. Between 2015 and 2020, plasma-based sequencing studies in PDAC have reported KRAS mutation frequencies ranging from 40 to 77%44–49 (with a mean of 59%). More recent studies conducted in 2021 reported KRAS mutation rates of 55 and 53% across ctDNA PDAC patient cohorts of size 104 (Guardant360) and 100 (in-house NGS of plasma), respectively,50,51 while a study in 2022 reported a KRAS mutation frequency of 77% (PredicineATLAS; tumor-naïve).52 A summary of studies reporting ctDNA-based KRAS mutation frequencies is provided in Table 1. Taken together, these data indicate a need for better sensitivity of ctDNA sequencing in PDAC to adequately capture the expected number of KRAS mutations. Quality control (QC) metrics of ctDNA sequencing data typically require a minimum threshold of detectable ctDNA or circulating tumor fraction (CTF) in order to successfully pass QC and be included in downstream data analysis. Samples with inordinately low CTF may lack mutations due to limited sensitivity (therefore bearing an increased number of false negatives). By iteratively setting increasingly stringent CTF thresholds at which samples are excluded from analysis, the KRAS mutation rate of a ctDNA dataset can be brought closer to the expected 90% by excluding samples for which the lack of KRAS mutation may be attributed to data quality.52 While this sacrifices the number of analyzable samples for increased sensitivity, such a strategy may be required for near-term adoption of ctDNA sequencing in clinical care of patients with PDAC until these technologies further improve.
Table 1.
Summary of ctDNA-based KRAS mutation rates across studies.
| Study | Cohort size | Disease stage | KRAS mutation rate (%) |
|---|---|---|---|
| Renouf et al.52 | 173 | Metastatic | 77 |
| Botrus et al.50 | 104 | Locally advanced or metastatic | 55 |
| Xiong et al.51 | 100 | Resectable, locally advanced or metastatic | 53 |
| Toledano-Fonseca et al.46 | 61 | Metastatic | 77 |
| Wang et al.49 | 95 | Resectable, locally advanced or metastatic | 47 |
| Park et al.48 | 17 | Locally advanced or metastatic | 59 |
| Del Re et al.44 | 27 | Locally advanced or metastatic | 70 |
| Adamo et al.45 | 26 | Resectable, locally advanced or metastatic | 27 |
| Kinugasa et al.47 | 75 | Locally advanced or metastatic | 63 |
ctDNA: circulating tumor DNA.
Existing strategies for tracking disease kinetics in PDAC are unsatisfactory
There is a high risk of disease recurrence in patients with surgically resectable PDAC. From a cohort of 692 patients diagnosed with PDAC undergoing pancreatectomy, Groot et al. found that 76.7% of patients had disease recur within a median of 11.7 months, with the majority of recurrences detected at distant organ sites.53 In the metastatic setting, FOLFIRINOX and GEMABR have been associated with disease progression at a median of 6.4 and 5.5 months, respectively.54,55 As with other cancers, optimal clinical management of PDAC is primarily governed by alignment between therapeutic timing and disease progression and requires tools capable of granular and accurate disease monitoring, which remain a critical and unmet need in PDAC. The current paradigm for clinical disease monitoring in PDAC consists of radiographic CT scan imaging and the blood-based biomarker carbohydrate 19-9 antigen (CA19-9). While capable of high granularity due to being a blood-based test, CA19-9 is unsatisfactory for PDAC disease monitoring due to having a low sensitivity and specificity of approximately 80%.56,57 Meanwhile, CT scans benefit from higher accuracy (sensitivity and specificity of 90 and 87%, respectively58) but are resource intensive.
ctDNA to monitor disease recurrence in PDAC
Minimal residual disease (MRD), the quantification of residual tumor DNA in a sample following surgical or therapeutic intervention, represents an emerging application in ctDNA sequencing technology focused on monitoring of disease progression. Given the inherent challenge of detecting minute residual ctDNA levels, studies of MRD in PDAC to date have largely focused on detection of oncogenic KRAS mutations as they occur as frequent and highly clonal events. In resectable PDAC, KRAS-based MRD detection by ctDNA sequencing has been shown to provide prognostic information as overall or progression-free survival (OS or PFS) in patients with MRD positivity either before or after surgery is significantly lower compared to patients that are MRD-negative. In the preoperative setting, Yamaguchi et al. demonstrated reduced OS [hazard ratio (HR) = 2.35, p = 0.008] in 24/97 (25%) patients that were ctDNA-positive,59 while Groot et al. showed decreases in both the PFS (19 months versus 8 months, p < 0.001) and OS (not reached versus 14 months, p = 0.009) of 29/59 (49%) patients with ctDNA positivity.60 Additionally, Lee et al. also showed reduced PFS (HR = 4.1, p = 0.002) and OS (HR = 4.1, p = 0.015) in 23/37 (62%) of patients with preoperative ctDNA positivity.61 Postoperatively, Groot et al. demonstrated reduced PFS (15 months versus 5 months, p < 0.001) in 11/41 (27%) ctDNA-positive patients,60 while Lee et al. also showed reduced PFS (HR = 5.4, p < 0.0001) and OS (HR = 4.0, p = 0.003) in 13/35 (37%) of ctDNA-positive patients.61 In the metastatic disease setting, Kruger et al. showed the utility of ctDNA-based MRD monitoring of KRAS mutation kinetics in predicting early response to chemotherapy, with significantly higher accuracy of KRAS-mutant ctDNA detection (83% sensitivity, 100% specificity) compared to several CA19-9 and CEA biomarker thresholds (p < 0.05).62 While preliminary, these studies show significant promise for how ctDNA technologies may transform the clinical paradigm in PDAC, as accurate and molecular-informed prognostication currently remain unavailable. A major limitation of these studies, however, is that they do not apply to the approximately 10% of patients with PDAC who harbor KRAS-wild-type tumors. Jiang et al. demonstrated the prognostic significance of MRD in resectable PDAC using a ctDNA assay extending beyond oncogenic KRAS mutations, detecting clonal mutations in other genes commonly mutated in PDAC such as TP53, CDKN2A, and SMAD4.63 Using an expanded MRD panel that included other genes in addition to KRAS, the authors demonstrated a reduction in disease-free survival (HR = 5.2, p = 0.019) in 9/27 (33%) postoperative ctDNA-positive patients. The design of studies such as these, capturing a wider breadth of mutations beyond KRAS, will be important as ctDNA-based MRD monitoring moves toward clinical practice.
Besides providing prognostic information, one of the most impactful goals of ctDNA-based MRD monitoring in PDAC is to guide treatment decision-making, such as initiation, dosage, therapy switching, and precision onco-surgery.11 In the resectable and borderline resectable disease setting, surgical resection via the Whipple procedure is highly invasive and is carried out with the primary goal of eliminating a tumor prior to circulatory system infiltration and subsequent spread to distant organ sites. For patients who have completed neoadjuvant chemotherapy and are scheduled for surgical resection, ctDNA-positive status prior to surgery would indicate a high likelihood of micrometastatic disease, and such patients could be spared invasive surgical resection when such a procedure is likely futile. Meanwhile, post-surgery, changes to therapy could be considered for patients on adjuvant chemotherapy that experience persistent increases in MRD levels while on therapy, thereby avoiding prolonged treatment-related toxicities when such treatments have ceased working. Ongoing trials of chemotherapy timing in PDAC that generate retrospective ctDNA datasets will provide important insight into the utility of ctDNA during chemotherapy and include the PAC3 trial (NCT04340141) of perioperative versus adjuvant FOLFIRINOX in patients with resectable PDAC.
RNA sequencing-based molecular subtypes of metastatic PDAC have been correlated with differential response to first-line chemotherapy, such that patients with “basal-like” tumors receiving FOLFIRINOX may be better off receiving GEMABR instead.9 As RNA-based profiling is unavailable for patients with PDAC outside of a select few large-center clinical trials, sustained increases in ctDNA-based MRD levels in patients with metastatic PDAC receiving first-line FOLFIRINOX may serve as a more feasible sentinel for immediate first-line therapy switching to GEMABR.
The role of ctDNA-based sequencing in prognostication and therapeutic decision-making in PDAC remains highly preliminary and is limited by the lack of randomized, prospective clinical trial data. In stage II colorectal cancer, the DYNAMIC trial (ACTRN12615000381583) randomized patients to have postoperative treatment guided by ctDNA sequencing results versus standard clinicopathological features. Results of the trial demonstrated the ability for ctDNA-based monitoring to spare ctDNA-negative patients from unnecessary adjuvant chemotherapy while not compromising their recurrence-.64 In pancreatic cancer, DYNAMIC-pancreas (ACTRN12618000335291) remains ongoing and is poised to determine the utility of ctDNA sequencing for informing adjuvant chemotherapy in patients with early stage pancreatic cancer. Additional ongoing ctDNA-based pancreatic cancer trials are summarized in Table 2 and include observational studies such as NCT05052671, NCT04616131, and PROJECTION (NCT04246203), each of which are noninterventional studies aimed at exploring the relationship between ctDNA levels with respect to pre/postsurgery timepoints and prognosis.
Table 2.
Summary of ctDNA-based clinical trials in PDAC.
| Study name | Trial ID | Estimated enrollment | Primary outcome | Secondary outcome(s) |
|---|---|---|---|---|
| DYNAMIC-pancreas | ACTRN-12618000335291 | 438 | Patient 2-year recurrence-free survival | Correlation of ctDNA results with recurrence-free and OS |
| OU-SCC-ctDNA | NCT05052671 | 50 | Frequency of ctDNA positivity and correlation with PFS | Time to ctDNA negativity, time to variant decay, correlation with OS |
| 21-012 | NCT04616131 | 60 | Number of patients with detectable ctDNA pretreatment | ctDNA levels versus tumor stage, CA19-9 levels and response groups |
| PROJECTION | NCT04246203 | 200 | Preoperative ctDNA versus disease-free survival | NA |
| U22-02-4671 | NCT05379907 | 30 | Impact of ctDNA profiling on treatment decisions | Frequency of ctDNA testing, OS and PFS, patient satisfaction |
| KRASCIPANC | NCT04560270 | 69 | ctDNA versus chemotherapy response | OS and PFS |
| ICAPAC | NCT03435536 | 40 | ctDNA kinetics with respect to surgical timing | ctDNA versus OS and CA19-9 levels |
| LIPAC | NCT05400681 | 200 | ctDNA frequency in peritoneal lavage and peripheral blood | Prognostic value of ctDNA |
| H-2111-047-1271 | NCT05604573 | 150 | Concordance between tissue- and ctDNA-based mutation detection | Correlation between genetics data and patient prognosis |
CA19-9: carbohydrate 19-9 antigen; ctDNA: circulating tumor DNA; PDAC: pancreatic ductal adenocarcinoma; PFS: progression-free survival; OS: overall survival.
ctDNA in early detection of PDAC
Germline mutations affecting key DNA damage repair genes, such as BRCA1/2, as well as chronic pancreatitis, have been established as features that predispose individuals to developing PDAC.65–67 Individuals bearing these features are therefore considered as a high-risk group and would benefit from screening procedures capable of accurately capturing early onset of PDAC. While an early study of mutation-based ctDNA screening for early PDAC development did not show improvement compared to using CA19-9 alone,68 a more recent study demonstrated higher accuracy when ctDNA and protein biomarkers were used in combination (64% sensitivity, 99.5% specificity69). Apart from mutation-based profiling, DNA methylation-based ctDNA assays may also offer promising clinical utility in the early detection of PDAC in high-risk individuals. DNA methylation is a form of epigenetic control in which segments of DNA are structurally regulated, thus affecting transcriptional machinery access, without changes to nucleotide composition. Similar to somatic mutations affecting key genes such as KRAS and TP53, recurrent disease-associated methylation patterns have been identified in PDAC, and the utility of using ctDNA-based promoter methylation biomarkers for early PDAC detection has been demonstrated for ADAMTS1 (87.2% sensitivity, 95.8% specificity) and BNC1 (64.1 and 93.7%).70 Similar to ongoing trials in ctDNA-based detection of early tumorigenesis in colorectal cancer, such as the ECLIPSE trial (NCT04136002), prospective clinical trials investigating the utility of ctDNA-based screening are currently needed in PDAC.
Concluding remarks
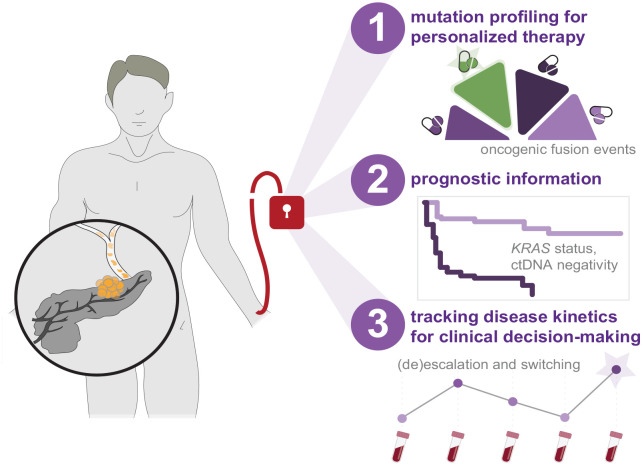
Taken together, opportunities for incorporating ctDNA sequencing-based analyses into routine clinical management of patients with PDAC include mutational profiling for personalized treatment selection, molecular-based prognostication, and tracking of disease kinetics (Figure 1). Each of these address critical needs imposed by the nature of the disease such as rapid deterioration in patient health and lack of biomarkers that are both granular and accurate. In the context of mutational profiling for personalized medicine, ctDNA sequencing offers a more rapid and less invasive alternative to tissue-based sequencing techniques. Meanwhile, pending advances in therapeutic decision-making in PDAC can be represented as a nexus of therapy selection, timing, dosage, and prognostication. While prospective clinical trials are in early stages, emerging demonstrations of the utility of ctDNA sequencing to successfully guide clinical management in colorectal cancer demonstrate highly promising avenues for the clinical utility of ctDNA in PDAC.
Figure 1.
Strategies concerning the incorporation of ctDNA sequencing-based analyses into clinical management of PDAC. Emerging studies provide indication for ctDNA sequencing to improve the clinical paradigm for patients diagnosed with PDAC through mutational profiling for personalized therapy selection, molecular-based prognostication, and tracking of disease kinetics to guide therapeutic decision-making.
ctDNA: circulating tumor DNA; PDAC: pancreatic ductal adenocarcinoma.
Acknowledgments
Not applicable.
Contributor Information
James T. Topham, Pancreas Centre BC, Vancouver, BC, Canada
Daniel J. Renouf, Pancreas Centre BC, Vancouver, BC, Canada; Division of Medical Oncology, BC Cancer, Vancouver, BC, Canada; Department of Medicine, University of British Columbia, Vancouver, BC, Canada.
David F. Schaeffer, Division of Anatomic Pathology, Vancouver General Hospital, 910 West 10th Avenue, Vancouver, BC V5Z 1M9, Canada; Pancreas Centre BC, Vancouver, BC, Canada; Department of Pathology and Laboratory Medicine, UBC, Vancouver, BC, Canada.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: James T. Topham: Conceptualization; Investigation; Visualization; Writing – original draft; Writing – review & editing.
Daniel J. Renouf: Conceptualization; Funding acquisition; Investigation; Project administration; Supervision; Visualization; Writing – original draft; Writing – review & editing.
David F. Schaeffer: Conceptualization; Funding acquisition; Investigation; Project administration; Supervision; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported through philanthropic donations received through the BC Cancer Foundation, as well as funding provided by the Terry Fox Research Institute (project 1078), Pancreatic Cancer Canada, Genome British Columbia (project B20POG), and VGH/UBC Hospital Foundation. DJR is a recipient of the MSFHR Health Professional-Investigator Award, and DFS is a recipient of the VCHRI Investigator Award.
DJR reports honoraria outside of the submitted work from Servier, Celgene, Taiho, and Ipsen and research funding and honoraria from Bayer. DFS reports honoraria outside of the submitted work from Alimentiv Inc, Pfizer, Amgen, Astellas, Merck and Diaceutics, and Satisfai Health Inc and research funding and honoraria from Bayer.
Availability of data and materials: Not applicable.
References
- 1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022; 72: 7–33. [DOI] [PubMed] [Google Scholar]
- 2. Paluri R, Laursen A, Gaeta J, et al. Impact of the COVID-19 pandemic on management of patients with metastatic pancreatic ductal adenocarcinoma in the United States. Oncologist 2022; 27: e518–e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de la Fouchardière C, Adham M, Marion-Audibert AM, et al. Management of patients with pancreatic ductal adenocarcinoma in the real-life setting: lessons from the french national hospital database. Cancers (Basel) 2021; 13: 3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pruitt SL, Tavakkoli A, Zhu H, et al. Survival of cancer survivors with a new pancreatic cancer diagnosis. Cancer Med 2023; 12: 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayashi H, Higashi T, Miyata T, et al. Recent advances in precision medicine for pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg 2021; 5: 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol 2020; 21: 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015; 518: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 2017; 32: 185–203.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aung KL, Fischer SE, Denroche RE, et al. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin Cancer Res 2018; 24: 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsang ES, Topham JT, Karasinska JM, et al. Delving into early-onset pancreatic ductal adenocarcinoma: how does age fit in? Clin Cancer Res 2021; 27: 246–254. [DOI] [PubMed] [Google Scholar]
- 11. Pietrasz D, Sereni E, Lancelotti F, et al. Circulating tumour DNA: a challenging innovation to develop “precision onco-surgery” in pancreatic adenocarcinoma. Br J Cancer 2022; 126: 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sivapalan L, Kocher HM, Ross-Adams H, et al. Molecular profiling of ctDNA in pancreatic cancer: opportunities and challenges for clinical application. Pancreatology 2021; 21: 363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones MR, Williamson LM, Topham JT, et al. NRG1 gene fusions are recurrent, clinically actionable gene rearrangements in KRAS wild-type pancreatic ductal adenocarcinoma. Clin Cancer Res 2019; 25: 4674–4681. [DOI] [PubMed] [Google Scholar]
- 14. Schram AM, Odintsov I, Espinosa-Cotton M, et al. Zenocutuzumab, a HER2xHER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discov 2022; 12: 1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pishvaian MJ, Garrido-Laguna I, Liu SV, et al. Entrectinib in TRK and ROS1 fusion-positive metastatic pancreatic cancer. JCO Precis Oncol 2018; 2: 1–7. [DOI] [PubMed] [Google Scholar]
- 16. O’Reilly EM, Hechtman JF. Tumour response to TRK inhibition in a patient with pancreatic adenocarcinoma harbouring an NTRK gene fusion. Ann Oncol 2019; 30: viii36–viii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019; 381: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019; 575: 217–223. [DOI] [PubMed] [Google Scholar]
- 19. Ning W, Yang Z, Kocher GJ, et al. A breakthrough brought about by targeting KRASG12C: nonconformity is punished. Cancers 2022; 14: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heining C, Horak P, Uhrig S, et al. NRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov 2018; 8: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 21. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin 2021; 71: 7–33. [DOI] [PubMed] [Google Scholar]
- 22. Plöthner M, Frank M, von der Schulenburg J-MG. Cost analysis of whole genome sequencing in German clinical practice. Eur J Health Econ 2017; 18: 623–633. [DOI] [PubMed] [Google Scholar]
- 23. Weymann D, Pollard S, Chan B, et al. Clinical and cost outcomes following genomics-informed treatment for advanced cancers. Cancer Med 2021; 10: 5131–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weymann D, Laskin J, Roscoe R, et al. The cost and cost trajectory of whole-genome analysis guiding treatment of patients with advanced cancers. Mol Genet Genomic Med 2017; 5: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luchini C, Paolino G, Mattiolo P, et al. KRAS wild-type pancreatic ductal adenocarcinoma: molecular pathology and therapeutic opportunities. J Exp Clin Cancer Res 2020; 39: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deshwar AB, Sugar E, Torto D, et al. Diagnostic intervals and pancreatic ductal adenocarcinoma (PDAC) resectability: a single-center retrospective analysis. Ann Pancreat Cancer 2018; 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Casolino R, Biankin AV. and PanCaCovid-19 Study Group. Impact of COVID-19 on pancreatic cancer research and the path forward. Gastroenterology 2021; 161: 1758–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Helman E, Artieri C, Vowles JV, et al. Abstract 5603: analytical validation of a comprehensive 500-gene ctDNA panel designed for immuno-oncology and DNA damage research. Cancer Res 2018; 78: 5603. [Google Scholar]
- 29. Lanman RB, Mortimer SA, Zill OA, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One 2015; 10: e0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med 2020; 26: 1859–1864. [DOI] [PubMed] [Google Scholar]
- 31. Chamberlain ED, Costantini CL, Xavier MF. Circulating tumor DNA use in a community oncology practice. Transl Cancer Res 2019; 8: 552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okuma HS, Yonemori K, Kojima Y, et al. Clinical utility of circulating tumor DNA in advanced rare cancers. Front Oncol 2021; 11: 732525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwaederlé MC, Patel SP, Husain H, et al. Utility of genomic assessment of blood-derived circulating tumor DNA (ctDNA) in patients with advanced lung adenocarcinoma. Clin Cancer Res 2017; 23: 5101–5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ioannou N, Seddon AM, Dalgleish A, et al. Acquired resistance of pancreatic cancer cells to treatment with gemcitabine and HER-inhibitors is accompanied by increased sensitivity to STAT3 inhibition. Int J Oncol 2016; 48: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Topham JT, O’Callaghan CJ, Feilotter H, et al. Circulating tumor DNA identifies diverse landscape of acquired resistance to anti-epidermal growth factor receptor therapy in metastatic colorectal cancer. J Clin Oncol 2023; 41: 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giacona MB, Ruben GC, Iczkowski KA, et al. Cell-free DNA in human blood plasma: length measurements in patients with pancreatic cancer and healthy controls. Pancreas 1998; 17: 89–97. [DOI] [PubMed] [Google Scholar]
- 37. Shapiro B, Chakrabarty M, Cohn EM, et al. Determination of circulating DNA levels in patients with benign or malignant gastrointestinal disease. Cancer 1983; 51: 2116–2120. [DOI] [PubMed] [Google Scholar]
- 38. Earl J, Garcia-Nieto S, Martinez-Avila JC, et al. Circulating tumor cells (Ctc) and kras mutant circulating free Dna (cfdna) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer 2015; 15: 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takai E, Totoki Y, Nakamura H, et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci Rep 2015; 5: 18425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A 2005; 102: 16368–16373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008; 14: 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014; 6: 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu L, Lopez G, Rassa J, et al. Direct comparison of circulating tumor DNA sequencing assays with targeted large gene panels. PLoS One 2022; 17: e0266889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Del Re M, Vivaldi C, Rofi E, et al. Early changes in plasma DNA levels of mutant KRAS as a sensitive marker of response to chemotherapy in pancreatic cancer. Sci Rep 2017; 7: 7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adamo P, Cowley CM, Neal CP, et al. Profiling tumour heterogeneity through circulating tumour DNA in patients with pancreatic cancer. Oncotarget 2017; 8: 87221–87233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toledano-Fonseca M, Cano MT, Inga E, et al. Circulating cell-free DNA-based liquid biopsy markers for the non-invasive prognosis and monitoring of metastatic pancreatic cancer. Cancers 2020; 12: E1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kinugasa H, Nouso K, Miyahara K, et al. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer 2015; 121: 2271–2280. [DOI] [PubMed] [Google Scholar]
- 48. Park G, Park JK, Son D-S, et al. Utility of targeted deep sequencing for detecting circulating tumor DNA in pancreatic cancer patients. Sci Rep 2018; 8: 11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Z-Y, Ding XQ, Zhu H, et al. KRAS mutant allele fraction in circulating cell-free DNA correlates with clinical stage in pancreatic cancer patients. Front Oncol 2019; 9: 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Botrus G, Kosirorek H, Sonbol MB, et al. Circulating tumor DNA-based testing and actionable findings in patients with advanced and metastatic pancreatic adenocarcinoma. Oncologist 2021; 26: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xiong A, Ma N, Wei G, et al. Genomic alterations in tumor tissue and ctDNA from Chinese pancreatic cancer patients. Am J Cancer Res 2021; 11: 4551–4567. [PMC free article] [PubMed] [Google Scholar]
- 52. Renouf DJ, Loree JM, Knox JJ, et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat Commun 2022; 13: 5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg 2018; 267: 936–945. [DOI] [PubMed] [Google Scholar]
- 54. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 55. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Poruk KE, Gay DZ, Brown K, et al. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med 2013; 13: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Riva F, Dronov OI, Khomenko DI, et al. Clinical applications of circulating tumor DNA and circulating tumor cells in pancreatic cancer. Mol Oncol 2016; 10: 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Toft J, Hadden WJ, Laurence JM, et al. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: a systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur J Radiol 2017; 92: 17–23. [DOI] [PubMed] [Google Scholar]
- 59. Yamaguchi T, Uemura K, Murakami Y, et al. Clinical implications of pre- and postoperative circulating tumor DNA in patients with resected pancreatic ductal adenocarcinoma. Ann Surg Oncol 2021; 28: 3135–3144. [DOI] [PubMed] [Google Scholar]
- 60. Groot VP, Mosier S, Javed AA, et al. Circulating tumor DNA as a clinical test in resected pancreatic cancer. Clin Cancer Res 2019; 25: 4973–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee B, Lipton L, Cohen J, et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann Oncol 2019; 30: 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kruger S, Heinemann V, Ross C, et al. Repeated mutKRAS ctDNA measurements represent a novel and promising tool for early response prediction and therapy monitoring in advanced pancreatic cancer. Ann Oncol 2018; 29: 2348–2355. [DOI] [PubMed] [Google Scholar]
- 63. Jiang J, Ye S, Xu Y, et al. Circulating tumor DNA as a potential marker to detect minimal residual disease and predict recurrence in pancreatic cancer. Front Oncol 2020; 10: 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tie J, Cohen JD, Lahouel K, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med 2022; 386: 2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Becker AE, Hernandez YG, Frucht H, et al. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol 2014; 20: 11182–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zimmermann MT, Mathison AJ, Stodola T, et al. Interpreting sequence variation in PDAC-predisposing genes using a multi-tier annotation approach performed at the gene, patient, and cohort level. Front Oncol 2021; 11: 606820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vujasinovic M, Dugic A, Maisonneuve P, et al. Risk of developing pancreatic cancer in patients with chronic pancreatitis. J Clin Med 2020; 9: 3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Le Calvez-Kelm F, Foll M, Wozniak MB, et al. KRAS mutations in blood circulating cell-free DNA: a pancreatic cancer case-control. Oncotarget 2016; 7: 78827–78840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cohen JD, Javed AA, Thoburn C, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A 2017; 114: 10202–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Eissa MAL, Lerner L, Abdelfatah E, et al. Promoter methylation of ADAMTS1 and BNC1 as potential biomarkers for early detection of pancreatic cancer in blood. Clin Epigenetics 2019; 11: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]