To the Editor:
Even though pulmonary arterial hypertension (PAH) is more prevalent in women, women with PAH exhibit better right ventricular (RV) function, more favorable RV treatment responses, and lower mortality than men (1, 2). These sexual dimorphisms led to a considerable, and often conflicting, body of research into the mechanisms driving sex-based differences in PAH (2). Although estrogens are major disease modifiers in PAH, significant knowledge gaps remain. For example, biological effects of estrogen and estrogen receptor (ER) signaling in pulmonary artery endothelial cells (PAECs), a cell type functionally profoundly altered in PAH, are poorly understood.
Previous studies focused on estrogen synthesis, metabolism, and downstream effects on pulmonary artery smooth muscle cell (PASMC) proliferation in PAH. One study suggested that ER-α may increase PASMC proliferation (3). However, ER-α’s effects on PAECs may differ from those in PASMCs, and ER-α may exert effects on PAECs in PAH that are vasculoprotective. For example, in systemic vessels, ER-α facilitates endothelial cell recovery after injury, blocks monocyte adhesion to endothelial cells, and inhibits vasoconstriction (4). Furthermore, loss-of-function mutations in ESR1 (encoding ER-α) associate with endothelial dysfunction, coronary artery disease, myocardial infarction, and stroke (5, 6).
Because estrogen signaling is pleiotropic and cell dependent, dissecting and identifying specific estrogenic pathways that mediate protective effects in the cardiopulmonary axis may allow a more precise and personalized medicine approach for patients of either sex with PAH. We hypothesized that loss of ER-α exacerbates experimental pulmonary hypertension (PH). We investigated 1) whether ER-α is decreased in PAECs from patients with PAH (PAH-PAECs), 2) whether ER-α loss of function affects development of experimental PH, and 3) whether activation of estrogen/ER-α signaling rescues vasculoprotective signaling in established PH as well as PAH-PAECs.
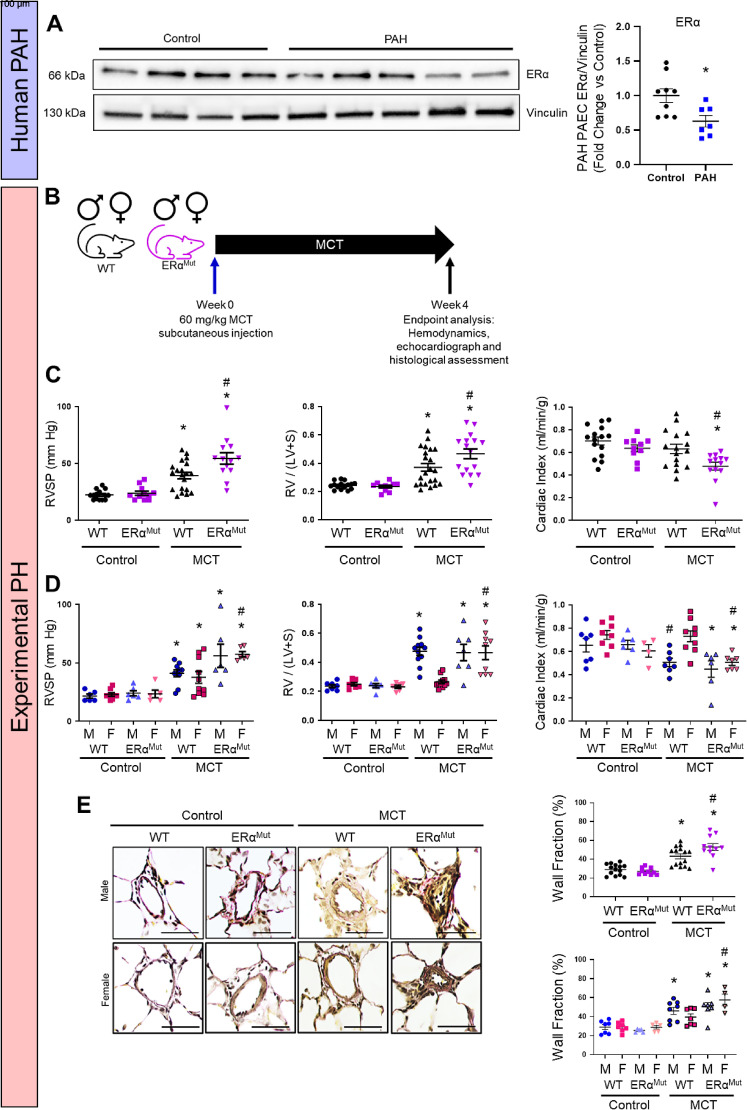
In PAH-PAECs, ER-α expression was decreased versus control PAECs (Figure 1A). To further examine the influence of ER-α on the development of experimental PH, ER-α loss-of-function mutant Sprague-Dawley rats were generated via CRISPR/Cas9 as previously described (7). Monocrotaline (MCT)-PH was induced in age-matched male and intact female ER-α mutant or wild-type (WT) littermates (Figure 1B). We measured RV systolic pressure (RVSP), RV hypertrophy (RV/left ventricular + septum [LV+S]), cardiac index (CI), and pulmonary vessel wall thickness as previously described (7, 8). Although loss of ER-α was not associated with functional changes in control animals, ER-α loss resulted in more severe MCT-induced changes in RVSP, RV/(LV+S), CI, and pulmonary vascular remodeling (Figures 1C and 1E) than in WT MCT-PH rats. As expected, when groups were separated by sex, WT female rats, as compared with WT male MCT rats, were protected against PH development (Figures 1D and 1E). However, female protection against MCT-PH was lost in the absence of ER-α (Figures 1D and 1E). Specifically, compared with WT female MCT rats, female ER-α mutant MCT rats exhibited 45–76% increases in RVSP, RV/(LV+S), and pulmonary wall thickness, as well as a 32% decrease in CI (Figures 1D and 1E). Similarly, ER-α loss tended to decrease stroke volume index and significantly increased total pulmonary resistance index (TPRI) compared with WT MCT-PH (see Figure E1A in the data supplement). When separated by sex, ER-αMut MCT females exhibited lower stroke volume index and significantly higher TPRI than WT MCT females (Figure E1B). These data indicate that loss of ER-α is associated with more severe MCT-PH, with a phenotype that exhibits a female sex bias.
Figure 1.
Estrogen receptor (ER)-α is decreased in pulmonary arterial hypertension (PAH) pulmonary artery endothelial cells (PAECs). Loss of ER-α exacerbates cardiopulmonary dysfunction in monocrotaline pulmonary hypertension (MCT-PH). (A) Western blot analysis of PAH PAECs (from Pulmonary Hypertension Breakthrough Initiative). A representative Western blot is depicted; densitometric analysis from all patient-derived cell lines is shown to the right (*P < 0.05 vs. control by Student’s t test). Each data point represents one cell line. (B) Experimental design. (C) Effects of MCT-PH (60 mg/kg) in male and female (sexes combined in groups) wild-type (WT) or ER-α loss-of-function mutants on right ventricular (RV) systolic pressure (RVSP), RV hypertrophy (RV weight divided by weight of left ventricle plus septum; RV/[LV+S]), and cardiac index (CI; echocardiographic RV cardiac output/body weight). (D) RVSP, RV hypertrophy RV/(LV+S), and CI in WT or ER-α loss-of-function mutants with groups stratified by sex. (E) Vessel wall fraction in WT and ER-α mutants measured by Verhoeff-van Gieson elastin stain in combined groups and in groups separated by sex. Fifteen to 20 vessels per rat were measured. Scale bars, 100 μm. *P < 0.05 versus same sex and genotype control; #P < 0.05 versus WT MCT by one-way ANOVA with Tukey post hoc correction. Error bars represent mean ± SEM. Each data point represents one animal. F = female; M = male.
We previously demonstrated that selectively activating ER-α signaling in established MCT-PH rescues MCT-induced alterations in RVSP, RV hypertrophy, CI, TPRI, and RV cardiomyocyte function (7); however, that study focused on ER-α signaling in the right ventricle. We now sought to identify the targets of ER-α specifically in the pulmonary vasculature. Because intact female Sprague-Dawley rats are protected against MCT-PH (Figure 1), we used male rats (7). Two weeks after MCT, a subset of rats was administered 17β-estradiol (E2) or the ER-α-selective agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (Figure E2A). At 4 weeks, lungs were evaluated for expression of pulmonary vascular homeostatic regulators bone morphogenetic protein receptor 2 (BMPR2) and apelin (7, 9, 10). We found that ER-α activation replicated effects of E2 and was sufficient to rescue MCT-induced decreases in lung BMPR2 and apelin (Figure E2B). Similarly to MCT-PH, BMPR2 and apelin mRNA was decreased in PAH-PAECs, and treatment with ER-α–selective agonist BTP-α stimulated BMPR2 and apelin protein (Figures E2C and E2D). These data indicate that E2 and ER-α enhance vasculoprotective pathways even in established PH/PAH.
Our data demonstrate a novel protective effect of ER-α in experimental PH and in PAH-PAECs. Global loss of ER-α results in more severe MCT-PH, with a female-predominant phenotype. We previously employed ER-α mutant rats to demonstrate ER-α dependence of exogenous E2 in male or ovariectomized female Sugen/hypoxia rats in the right ventricle (7). However, it remained unknown whether loss of functioning of ER-α modifies effects of endogenous sex hormones in the cardiopulmonary axis. The finding that loss of ER-α signaling sex dependently results in worse PH therefore is novel and may help explain the female predominance in PAH. In addition, ER-α abundance is decreased in human PAH-PAECs. E2 or selective ER-α activation attenuates experimental PH and rescues vasculoprotective signaling in MCT-PH lungs and PAH-PAECs. These data suggest that impaired ER-α signaling may contribute to PAH development and may provide a rationale to further study the role of this receptor in PAH. Although we identified BMPR2 and apelin as targets of ER-α in PAH-PAECs, we speculate that ER-α also regulates other biological processes, such as metabolism, inflammation, and angiogenesis. This is currently under investigation. Our work challenges the current paradigm that E2 and ER-α promote pulmonary vascular remodeling and exacerbate experimental PH (3, 11). However, previous studies employed PASMCs and hypoxic mice, thus limiting their generalizability. In fact, the finding that lung microvascular endothelial cells from Bmpr2 mutant mice exhibit aberrant ER-α trafficking (12) supports our hypothesis that ER-α signaling is impaired in PAH and indicates that proper ER-α signaling is protective in PAH. Importantly, in our study, loss of ER-α was detrimental in intact MCT female rats, demonstrating the importance of endogenous estrogen signaling in protecting against MCT-induced vascular injury.
Understanding the intricacies of estrogenic signaling in the pulmonary vasculature in response to injury and PAH pathogenesis is an urgent and unmet area of need, especially as therapeutic strategies targeting hormonal signaling in PAH progress through clinical trials. Despite their clinical importance, we currently have only an incomplete view of how estrogens impact vascular responses to injury. Deepening our understanding of how hormones affect vascular responses throughout the cardiopulmonary circuit may lead to more effective and targeted treatment approaches. Given the pleiotropic effects of estrogens, selectively targeting specific aspects of estrogenic signaling may be more efficacious than broadly targeting E2. We posit that selectively activating ER-α signaling rather than nonspecifically targeting E2 may be a novel and more precise strategy to treat PAH.
Footnotes
Supported by American Heart Association Career Development Award 19CDA34660173 (A.L.F.), American Lung Association Catalyst Award CA-629145 (A.L.F.), an Actelion Pharmaceuticals Entelligence Young Investigator Award (A.L.F.), a Pulmonary Hypertension Accelerated Bayer Level 3 Award (A.L.F.), National Institutes of Health grant 1R01HL164791-01 (A.L.F.), the Borstein Family Foundation (T.L.), U.S. Department of Veterans Affairs Merit Review Award 2 I01 BX002042-05 (T.L.), National Heart, Lung, and Blood Institute grant 1R56HL134736-01A1 (T.L.), and National Institutes of Health grant HL144727-01A1R01 (T.L.). Academic use of BTP-α was provided by Drs. Henry Bryant and Jeffrey Dodge at Eli Lilly & Co.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol . 2014;307:L7–L26. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 2.Frump AL, Shimoda LA. In: Sex-based differences in lung physiology. Silveyra P, Tigno XT, editors. Cham: Springer Nature; 2021. Sex differences in pulmonary arterial hypertension; pp. 197–249. [Google Scholar]
- 3. Wright AF, Ewart MA, Mair K, Nilsen M, Dempsie Y, Loughlin L, et al. Oestrogen receptor alpha in pulmonary hypertension. Cardiovasc Res . 2015;106:206–216. doi: 10.1093/cvr/cvv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolego C, Rossoni G, Fadini GP, Vegeto E, Pinna C, Albiero M, et al. Selective estrogen receptor-alpha agonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilization in the absence of uterotrophic action. FASEB J . 2010;24:2262–2272. doi: 10.1096/fj.09-139220. [DOI] [PubMed] [Google Scholar]
- 5. Schuit SC, Oei HH, Witteman JC, Geurts van Kessel CH, van Meurs JB, Nijhuis RL, et al. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA . 2004;291:2969–2977. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 6. Shearman AM, Cooper JA, Kotwinski PJ, Miller GJ, Humphries SE, Ardlie KG, et al. Estrogen receptor alpha gene variation is associated with risk of myocardial infarction in more than seven thousand men from five cohorts. Circ Res . 2006;98:590–592. doi: 10.1161/01.RES.0000210578.62102.a6. [DOI] [PubMed] [Google Scholar]
- 7. Frump AL, Albrecht M, Yakubov B, Breuils-Bonnet S, Nadeau V, Tremblay E, et al. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Invest . 2021;131:e129433. doi: 10.1172/JCI129433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, et al. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol . 2015;308:L873–L890. doi: 10.1152/ajplung.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alastalo TP, Li M, Perez VdeJ, Pham D, Sawada H, Wang JK, et al. Disruption of PPARγ/β-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest . 2011;121:3735–3746. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med . 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med . 2014;190:456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fessel JP, Chen X, Frump A, Gladson S, Blackwell T, Kang C, et al. Interaction between bone morphogenetic protein receptor type 2 and estrogenic compounds in pulmonary arterial hypertension. Pulm Circ . 2013;3:564–577. doi: 10.1086/674312. [DOI] [PMC free article] [PubMed] [Google Scholar]