Abstract
Microorganisms colonize the human body. The lungs and respiratory tract, previously believed to be sterile, harbor diverse microbial communities and the genomes of bacteria (bacteriome), viruses (virome), and fungi (mycobiome). Recent advances in amplicon and shotgun metagenomic sequencing technologies and data-analyzing methods have greatly aided the identification and characterization of microbial populations from airways. The respiratory microbiome has been shown to play roles in human health and disease and is an area of rapidly emerging interest in pulmonary medicine. In this review, we provide updated information in the field by focusing on four lung conditions, including asthma, chronic obstructive pulmonary disease, cystic fibrosis, and idiopathic pulmonary fibrosis. We evaluate gut, oral, and upper airway microbiomes and how they contribute to lower airway flora. The discussion is followed by a systematic review of the lower airway microbiome in health and disease. We conclude with promising research avenues and implications for evolving therapeutics.
Keywords: microbiome, mucus, respiratory diseases, microbial metabolites, therapeutics
Asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and idiopathic pulmonary fibrosis (IPF) are common chronic respiratory diseases (CRDs; Figures 1A–1D) characterized by having disrupted and distinct respiratory microbiomes (1–7). There is growing evidence suggesting that viral infections exacerbate asthma (8, 9), the lung microbiome plays a significant role in COPD severity (10), and CF and IPF lungs possess unique microbiomes that impact disease transmission and progression (11, 12).
Figure 1.

Human microbiomes in health and four chronic respiratory diseases. (A) T-helper cell type 2 (Th2)-low and Th2-high inflammation characterizes two subtypes of asthma, the Th2-high subtype being more sensitive to viral infections than Th2-low. Excessive mucus and increased inflammation are the two prominent features of asthma. (B) Chronic obstructive pulmonary disease (COPD) is an inflammatory disease that is characterized by irreversible airflow obstruction and damaged alveoli. The Global Initiative for Chronic Obstructive Lung Disease spirometric staging system classifies COPD into early and advanced stages. Early COPD exhibits damaged alveolar membranes, and advanced COPD demonstrates significantly enlarged alveoli. (C) Cystic fibrosis (CF) is a genetic disease caused by four different CFTR (CF transmembrane conductance regulator) gene mutation types. Transmissible infectious microbes can be detected in patients with CF. CF bronchioles are dilated and coated with occlusive mucus. (D) IPF is of unknown etiology and pathogenesis and features damaged bronchioles and a reduction in terminal bronchioles. Microbial infection may drive the alveolar inflammation and fibrosis. (E) The anatomy of healthy human respiratory and digestive systems. Airway microbiomes, especially the lower airway microbiome, are like a desert, with fewer microbes than the gastrointestinal (GI) microbiome (lower GI; like a rainforest with diverse and abundant microbes). The gut–lung axis connects these two microbiomes. The oralome and upper airway microbiome interact with the lower airway, which form the oral–lung axis. Healthy lungs have normal bronchioles and alveoli. IPF = idiopathic pulmonary fibrosis.
Although the development and progression of these CRDs are not fully understood (6, 10, 13–19), the advent of microbial identification and characterization techniques using culture-independent, nucleic acid–based methods could provide new insights into diagnostic and therapeutic advances (12, 16, 20). Hilty and colleagues characterized the lower airway microbiome through 16S ribosomal RNA (rRNA) gene amplicon sequencing (16), discovering that Haemophilus spp. from the Proteobacteria phylum were more abundant in asthmatic bronchi than controls, a finding that encourages further investigation into a causal linkage between pathogen and disease pathogenesis (16). 16S rRNA sequencing also reveals that the presence of the Staphylococcus and Streptococcus genera in bronchoalveolar lavage (BAL) samples was associated with poorer clinical outcomes for patients with IPF (12). In addition to identifying pathogenic microbial signatures, high-throughput metagenomic sequencing helped to develop rational antibiotic therapy for lower respiratory tract infections (20). Collectively, microbiome studies are leading to novel pathogenesis and therapeutic concepts in pulmonary medicine.
Sequencing-based analysis from low-biomass samples, such as pulmonary tissue, is vulnerable to contamination. The presence of the microbial DNA in these specimens without culturable microbes raises two questions: are these true living colonies local to the lower airway microenvironment, or do they represent contaminations related to collection techniques? Microbial DNA contamination comes from both internal and external sources. Internal sources of contamination that originate from the upper airway can occur during sampling and are technically unavoidable; here, it is necessary to reach a consensus among investigators of how to best reduce contamination to an acceptable concentration by applying techniques like protected sampling (21). External sources of contamination arise through medical devices and laboratory processing, which often vary widely across different studies. Negative control samples and contaminant identification tools (e.g., Decontam) should be routinely included in evaluating and mitigating potential false-positive results (22, 23). The term “core microbiota” has been used to describe the set of dominant and stable microbes in a particular habitat that play key roles in health and disease. Given the fact that the microbes mentioned in our review are consistently present in most of the individuals within and across studies, we presumably agree that these microorganisms represent commensals or pathogens inhabiting the human body. In addition, the integration of sequencing, viability, and quantification techniques (24) provides a greater opportunity for identifying viable microorganisms in the test samples. Future microbiome studies should use these approaches more readily.
For this review, we begin by describing three well-characterized microbiome niches (gut, oral, and upper airway) and their contributions to the airway microbiota. We then give a detailed description of the microbiome in the lower airway in health and disease, with a focus on the microbial signatures and their clinical applications. Finally, we outline four promising research areas for the respiratory microbiome.
The Sources of the Lower Airway Microbiome
Gut Microbiome
The gut flora is well described. The microbial populations differ between the upper (e.g., esophagus and stomach) and lower (commonly referred to as the colon and the rectum) gastrointestinal (GI) tracts (Figure 1E). For example, Streptococcus spp. and Prevotella spp. dominate the upper GI tract (25) (Table 1). The lower GI tract contains the most diverse and abundant microbial communities in humans, with more than 1014 microbes representing a thousand species of commensal microorganisms (26). Alternatively, microbes can cause disease, such as Helicobacter pylori, which infects more than half of the world’s population and is the major cause of gastric cancer (27). The hepatitis B virus is responsible for causing hepatitis B in humans (28) (Table 1). Pathogens such as these may also trigger pathology indirectly through changes in their collective constituents, a condition known as dysbiosis. Dysbiosis is defined as a disturbance in the composition of the microbiota in a given niche and is a feature of certain colorectal diseases or syndromes. The loss of beneficial microbes, an outgrowth of pathogens, and/or reduced overall microbial diversity contribute to dysbiosis (29). For instance, the reduced abundance of certain butyrate-producing bacteria is highly prevalent in patients with irritable bowel syndrome (30). The enrichment of oral-related pathogens accounts for the increased risk for a subset of colorectal cancer (31). Microbial diversity is 50% less in control subjects than in patients with Crohn’s disease (32).
Table 1.
Microbial Species Mentioned in the Review
| Kingdom | Genus | Species | Infection Site | Health Condition | Citation |
|---|---|---|---|---|---|
| Bacteria | Streptococcus | S. oralis | OC | Healthy | 43 |
| S. mitis | OC | Healthy | 43 | ||
| S. peroris | OC | Healthy | 43 | ||
| — | UG; UA | Healthy | 25, 50 | ||
| — | LA | COPD; IPF | 12, 69 | ||
| S. pneumoniae | LA | Asthma; COPD | 66, 69 | ||
| Moraxella | M. catarrhalis | LA | Asthma | 16 | |
| M. catarrhalis | LA | COPD | 69 | ||
| — | UA | Healthy | 51 | ||
| Prevotella | — | UG; LA | Healthy | 16, 25 | |
| P. oralis | OC | Multiple diseases | 47 | ||
| Burkholderia | B. cenocepacia | LA | CF | 82, 83 | |
| B. multivorans | LA | CF | 82, 83 | ||
| Haemophilus | H. influenzae | LA | Asthma; COPD | 66, 69 | |
| Staphylococcus | S. aureus | LA | CF | 82, 83 | |
| — | LA | IPF | 12 | ||
| Corynebacterium | — | OC; UA | Healthy | 44, 51 | |
| Veillonella | — | OC; LA | Healthy | 16, 44 | |
| Coprococcus | — | LA | Healthy | 59 | |
| Dorea | — | LA | Healthy | 59 | |
| Actinomyces | — | OC | Healthy | 44 | |
| Dolosigranulum | — | UA | Healthy | 51 | |
| Neisseria | N. mucosa | LA | Asthma | 16 | |
| Mycoplasma | M. pneumoniae | LA | Asthma | 67 | |
| Chlamydia | C. pneumoniae | LA | Asthma | 67 | |
| Lachnospira | — | LA | Asthma | 63 | |
| Clostridium | C. neonatale | LA | Asthma | 63 | |
| Pseudomonas | P. aeruginosa | LA | CF | 82, 83 | |
| Helicobacter | H. pylori | UG | Gastric cancer | 27 | |
| Fusobacterium | F. nucleatum | OC | Multiple diseases | 47 | |
| Porphyromonas | P. gingivalis | OC | Multiple diseases | 47 | |
| Treponema | T. denticola | OC | Multiple diseases | 47 | |
| Campylobacter | C. gracilis | OC | Multiple diseases | 47 | |
| Viruses | Rhinovirus | — | LA | Asthma; COPD | 65, 75 |
| Pneumovirus | Respiratory syncytial virus | LA | Asthma | 65 | |
| Lymphocryptovirus | Epstein-Barr virus | UA; LA | IPF | 91 | |
| Hepacivirus | Hepatitis C virus | Liver; LA | IPF | 91 | |
| Influenza virus | Influenza A/B/C | LA; UA | MRD | 114 | |
| Betacoronavirus | SARS-CoV-2 | LA; UA | Multiple diseases | 121 | |
| Orthohepadnavirus | Hepatitis B virus | Liver | Hepatitis B | 28 | |
| Fungi | Cladosporium | C. cladosporioides | LA | Healthy | 115 |
| Eremothecium | E. sinecaudum | LA | Healthy | 115 | |
| Aspergillus | — | LA | CF; COPD | 82, 83, 116 | |
| Malassezia | — | LA | CF; asthma | 115 | |
| Candida | — | OC | Oral candidiasis | 117 | |
| C. albicans | LA | MRD | 115 | ||
| Penicillium | — | LA | MRD | 115 | |
| Pichia | P. kudriavzevii | LA | Asthma | 64 | |
| Saccharomyces | — | LA | Asthma | 64 |
Definition of abbreviations: CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; IPF = idiopathic pulmonary fibrosis; LA = lower airway; MRD = multiple respiratory disease; OC = oral cavity; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; UA = upper airway; UG = upper gastrointestinal.
Gut microbiota play essential roles in shaping the immune and metabolic status of the host. Recent evidence has suggested that the intestine directs immune responses beyond the local gut immune system, including the lung (33). Gut and lung microbes influence each other through the blood or lymphatic system (34). Immune cells and metabolites produced by gut bacteria can move through the circulation systems to stimulate immune responses in the lung, and lung microbiota can induce microbial changes in the blood and gut (34, 35). The crosstalk between gut microbiome and the lungs is known as the gut–lung axis. This communication hub influences both microbial and immune interactions in health and disease. For example, respiratory viral infections can cause disturbances in gut microbiota (36). The importance of a fiber-rich diet has been emphasized in determining the composition of the gut microbiota and as a factor affecting lung immunity (37). Moreover, components of gastroesophageal reflux, such as bile acids, pepsin, microbes, and related metabolites, are toxic to the airway epithelium (38, 39). Gastroesophageal reflux is associated with common lung diseases, including asthma, COPD, CF, and IPF (40). As such, manipulation of the gut microbiota by probiotics, fecal microbiota transplants, and proper antibiotics may represent a novel approach for treating both gut and lung diseases (34) (Figure 2B).
Figure 2.
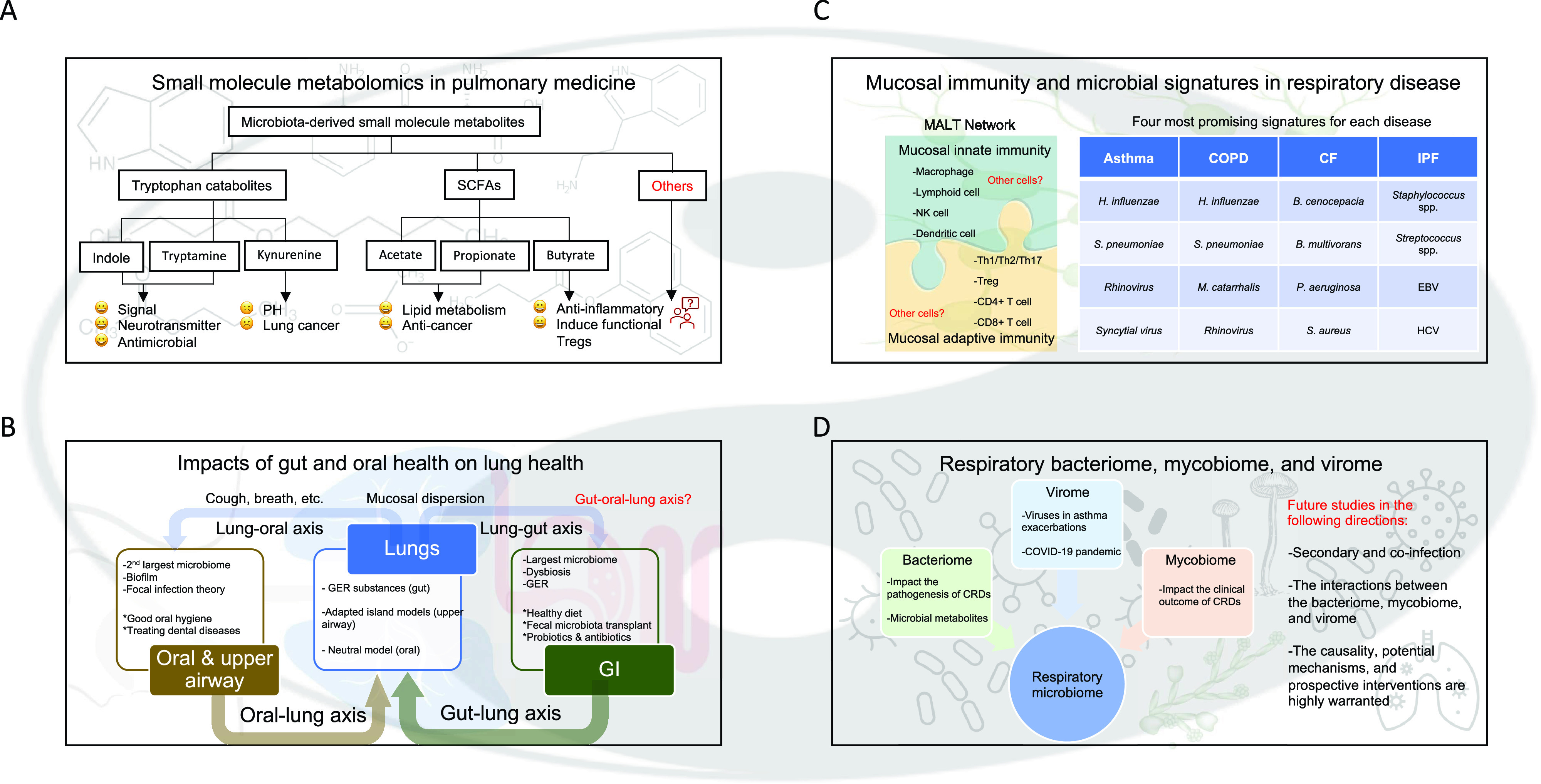
Four research directions, perspectives, and open questions in the respiratory microbiome. (A) Tai Chi diagram’s transparent background comes in yin (black) and yang (white), which indicates things are connected and affect one another. Four boxes on a second transparent background have different contents to better match the future needs of the four research areas. More specifically, (A) molecular chemical structures, (B) the anatomy of a human body, (C) mucosa-associated lymphoid tissue network, and (D) lung-associated pathogens. Open questions for each of the future directions are marked in red. (A) Tryptophan catabolites and short-chain fatty acids (SCFAs) are the two major microbiota-derived small molecule metabolites. Tryptophan catabolites, such as indole and tryptamine, are functioning as signals and antimicrobial agents; kynurenine, on the other hand, is associated with the increased risk of pulmonary hypertension and lung cancer. Acetate, propionate, and butyrate are among the three members of SCFAs, and multiple beneficial effects are associated with these metabolites. Other constituents of microbiota-derived small molecule metabolites and related mechanisms are expected. (B) The gut–lung and oral–lung axis are bidirectional at each level, connecting the lungs with the gut and the oral cavity, respectively. Gut plays a more important role in the gut–lung axis (with more weights). GI contains the largest microbiome in the human body; dysbiosis is associated with many GI-related disorders; and associated gastroesophageal reflux (GER) is a common digestive disorder. Several interventions, such as maintaining a healthy diet, fecal microbiota transplants, and probiotics and/or antibiotics can help to treat both gut and lung diseases. The oral and upper airway microbiomes are considered as a whole, which contains the second largest microbial community after the gut. The oral microbes tend to form structured multispecies communities, known as biofilms, and the focal infection theory implies that a primary localized infection in the oral cavity can cause secondary chronic diseases elsewhere in the body. Thus, practicing good oral hygiene and treating existing dental infections are critical for a healthy lung. GER substances from gut, upper airway microbes based on the adapted island models, and oral microbes based on the neutral model can better explain the sources of the lung microbiome. Lung microbes can migrate to the upper airway and gut by mucosal dispersion; cough, breath, and other mechanisms are also involved in the microbial communications between the lung and the oral and upper airway. The field of the gut–oral–lung axis appears significant but is relatively unexplored. (C) The MALT network consists of both innate and adaptive immunities, which are connected by two puzzles to indicate the interactions between them. Macrophages, lymphoid, NK, and dendritic cells form the mucosal innate immunity. Th1/Th2/Th17, Treg, CD4+, and CD8+ T cells are among the components of adaptive immunity. Both innate and adaptive immune cells form novel response networks. The four most promising microbial signatures for each respiratory disease are listed in a table on the right. (D) The respiratory bacteriome impacts the pathogenesis of chronic respiratory diseases (CRDs), and the microbial metabolites influence the host. The mycobiome has been implicated in impacting the clinical outcome of CRDs. The virome plays a role in asthma exacerbations, and the coronavirus disease (COVID-19) pandemic emphasizes the need to accelerate research on viral infections and lung disease. The respiratory bacteriome, mycobiome, and virome form the respiratory microbiome. Future studies in three different directions are listed in the right. B. cenocepacia = Burkholderia cenocepacia; B. multivorans = Burkholderia multivorans; EBV = Epstein-Barr virus; HCV = Hepatitis C virus; H. influenzae = Haemophilus influenzae; MALT = mucosa-associated lymphoid tissue; M. catarrhalis = Moraxella catarrhalis; NK = natural killer; P. aeruginosa = Pseudomonas aeruginosa; S. aureus = Staphylococcus aureus; S. pneumoniae = Streptococcus pneumoniae; Treg = T-regulatory.
Oral Microbiome
Diverse microbes residing in the oral cavity have been referred to as the oral microbiome (or oralome) (41) (Figure 1E). The oralome contains more than 700 microbial species, making it the second largest microbial community in humans after the gut (42). Streptococcus oralis, Streptococcus mitis, and Streptococcus peroris are among the three most abundant species in the healthy oral cavity (43) (Table 1). Other constitutions of a normal oralome include the genera of Actinomyces, Corynebacterium, and Veillonella (44) (Table 1). Microorganisms tend to form structured multispecies communities, the biofilms, on dental and epithelial surfaces to survive in the ever-changing oral environment (45). The structure and composition of biofilm communities are distinct in each site within the oral cavity (41). For example, a corn cob structure dominated by anaerobic and motile bacteria for supragingival biofilms (44) is distinct from a multilayered biofilm formed by microbes in different layers of subgingival plaques (46).
The oral cavity is anatomically connected to the airways. Pathogenic oral microorganisms cause periodontal diseases, such as dental caries, gingivitis, and periodontitis, and directly or indirectly lead to cardiopulmonary disease (47). The dental focal infection theory implies that a primary localized infection in the oral cavity can cause secondary chronic diseases elsewhere in the body (48). Multiple microbial species, including Porphyromonas gingivalis, Fusobacterium nucleatum, Treponema denticola, Prevotella oralis, and Campylobacter gracilis have been recognized as systemic pathogens identified from the periodontal pocket (47) (Table 1). As poor oral health and disturbances in the oral–lung axis are believed to be risk factors for developing chronic lung diseases (49), practicing good oral hygiene and treating existing dental conditions are critical for lung health (Figure 2B).
Upper Airway Microbiome
The airway, or respiratory tract, can be structurally divided into the upper and lower airways. The upper airway comprises the nasal passages and pharynx and is responsible for transporting air to and from the lower airway (i.e., larynx, trachea, bronchi, and lungs) (Figure 1E). The upper airway is in direct contact with the external environment, and although mucosal immune defenses continuously surveil it, numerous microbial species are reported to exist and have roles in human health and disease (50–54). For example, although the nasopharyngeal-associated lymphoid tissue contains a large variety of innately defensive immune cells (53), the healthy nasal cavity is nevertheless enriched with microbial species such as Corynebacterium spp., Dolosigranulum spp., and Moraxella spp. (51). (Table 1). The oropharynx microbiome is dominated by Streptococcus spp. and has higher microbial abundance than the nasopharynx (50). The upper airway microbiota may block pathogens from entering the lower airway, and the evolution of innate immune responses at mucosal barrier sites was likely influenced by commensal health-promoting bacteria (52). Upper airway microbial dysbiosis contributes to lower airway inflammation and obstruction observed in multiple pulmonary diseases, including asthma (54), CF, COPD (55), and IPF (12). With this view, one possible approach for monitoring lung diseases is to survey changes in the upper airway microbiome composition (56, 57).
Lower Airway Microbiome in Health and Disease
Lower Airway Microbiome in Health
Unlike the microbe-favoring conditions of the gut, oral cavity, and upper airways, the lower airway appears to provide an unfavorable environment (i.e., limited nutrient and high oxygen stress) for microbe survival. To develop previously used metaphors of adapted island models (55, 58), the lower airway is like a desert with respect to microbial colonization and replication (Figure 1E). Although the lower airway was previously believed to be sterile, emerging evidence shows there is a variety of microbial species in the lungs of healthy individuals (16, 59, 60). These species are primarily anaerobes, such as gram-negative Prevotella and Veillonella spp. (16) and gram-positive Coprococcus and Dorea spp. (59) (Table 1).
Together with adapted island models (55, 58), the neutral model (61) is constructed to describe the microbiome’s biogeography of the lower airway in healthy subjects. Basically, the two kinds of models propose that the healthy lung microbiome resembles those in the upper airway and the oral cavity, respectively (Figure 2B). More specifically, the adapted island model suggests that the lower airway microbial composition is largely influenced by microbial immigration (microaspiration, inhalation, direct mucosal dispersion) and elimination (cough, mucociliary clearance, innate and adaptive host immunity) from the “mainland” upper airway, and the microbial biomass decreases from upper to lower airway (55, 58). The neutral model assumes that different compositions of microorganisms are selectively equivalent, being shaped by stochastic population dynamics (61). To summarize findings of these models, the human lower airway is not sterile in health and harbors diverse microbial communities derived both from the upper airway and the oral cavity.
Asthma Microbiome
Asthma is a common long-term lung disease that can develop at any age. Asthma has been characterized into two inflammatory endotypes: T-helper cell type 2 (Th2)-high and Th2-low (9, 62). It is estimated that up to 80% of asthma exacerbations in school-aged children and 50% in adults are caused by viral infections, and the Th2-high subtype is particularly sensitive to viral infections (8, 9) (Figure 1A). The role of gut microbiota in the onset of childhood asthma is well established. For instance, two Clostridial species in the gut, namely Lachnospira and Clostridium neonatale, play contrasting roles in asthma development, and the ratio of Lachnospira/C. neonatale may serve as a biomarker for the early detection of asthma in children (63). Several fungi species detected in the gut microbiome, including Pichia kudriavzevii and Saccharomyces, were positively associated with the risk of pediatric asthma (64). The role of rhinovirus, respiratory syncytial virus (RSV), and other community-acquired viruses in causing asthma exacerbations is also well recognized (65). Viruses may disrupt the epithelial barrier, which subsequently increases bacterial infections in airways (65). In addition, Haemophilus influenzae and Streptococcus pneumoniae are linked with asthma exacerbations (66).
Other notable bacterial pathogens in asthma include species of Haemophilus, Moraxella, and Neisseria (16) (Table 1). The presence of these pathogens in asthmatic airways not only reveal the existence of distinct microbial communities in the respiratory system but also indicate that they may have some influence on chronic airway inflammation (3, 4). In a previous culture-based microbiome study, Bisgaard and colleagues (3) demonstrated that colonization of the airways with these pathogens was associated with increased blood eosinophil counts and IgE concentrations and eventually increased risk for asthma in early life. By investigating a neutrophilic treatment-resistant severe asthma cohort, Green and colleagues (4) found that these microorganisms were overrepresented and positively correlated with airway inflammatory markers (e.g., IL-8 and neutrophil). Although robust, these associations and correlations between the presence of pathogens and asthma vulnerability do not confirm causality. In addition, an increased number of mast cells are found in patients with chronic stable asthma. Most of these patients test positive for Mycoplasma pneumoniae and Chlamydia pneumoniae, suggesting a possible interaction between respiratory infection and allergen sensitization (67) (Table 1). Microbes, including bacteria, viruses, and fungi, are closely associated with asthma onset and exacerbations. Therefore, using antiinfective drugs or vaccines on these pathogens is helpful in chronic diseases like asthma (68).
COPD Microbiome
COPD is a group of inflammatory lung diseases characterized by irreversible airflow obstruction and damaged alveoli (Figure 1B). Previous culture-dependent molecular typing studies have observed bacterial outgrowth and identified key microbial signatures in patients with COPD (5, 69). Hill and colleagues (5) found that increased airway bacterial load is strongly related to several inflammation markers, including myeloperoxidase, IL-8, leukotriene B4, and leukocyte elastase, as well as albumin concentrations in patients with stable COPD. The acquisition of new bacterial pathogens such as H. influenzae, Moraxella catarrhalis, and S. pneumoniae was associated with acute exacerbations in patients with COPD (69) (Table 1 and Figure 2C). Similar to asthma, increased abundance of Haemophilus and Moraxella spp. is associated with COPD exacerbation from a longitudinal 16S rRNA sequencing in sputum samples (10). Although patients with COPD and asthma have different underlying pathophysiological processes, a partially similar microbial ecology was also observed by another two independent studies (70, 71). In particular, increased proportions of Haemophilus and Moraxella spp. were present in a subset of patients with COPD and asthma who had experienced favorable responses to antibiotics. It is unknown whether and to what extent the increased abundance of Haemophilus and Moraxella spp. in these three COPD and asthma exacerbation studies (10, 70, 71) is associated with antimicrobial interventions. To examine these associations, we will need to rely on animal models, because it is not feasible to withhold antimicrobial agents in patients undergoing acute exacerbation of their condition. Indeed, when no treatment was given in a mouse COPD model, animals’ exposure to H. influenzae was shown to induce airway inflammation and a COPD-like phenotype (72). Similar results were reported in other microbial species, such as M. catarrhalis (73) and S. pneumoniae (74). In addition, our group recently investigated a longitudinal rat microbial dataset and found that species from the Lactobacillales order showed dramatic abundance changes in lungs during the aging process (Zhao and colleagues, unpublished results), indicating that the age of the animals should be taken into consideration when performing the microbial intervention studies.
Moreover, rhinovirus infection, a common cause of asthma exacerbation, can alter the respiratory microbiome by increasing H. influenza in subjects with COPD (75). An enhanced adhesion of H. influenzae in nasal epithelial cells to further promote secondary bacterial infections has been described by Wang and colleagues (76). In summary, species like Haemophilus spp., Moraxella spp., and rhinovirus may contribute to exacerbation in a subset of patients with COPD and asthma; validations in animal models are still needed to single out potential correlations between drug administration and microbiome composition.
CF Microbiome
CF is a chronic and progressive genetic disease with variable phenotypes, which is prevalent among White individuals. The incidence of CF is 1/3,000 and 1/6,000 in Europe and across the globe, respectively (17). CF is a multiorgan disease that primarily affects the lungs and the digestive system in children and adults (77). It is caused by CFTR (CF transmembrane conductance regulator) gene mutations, which affect the synthesis and function of CFTR protein. There are four main types of mutation (class I–IV) according to the way the gene is disrupted (Figure 1C), resulting in poor mucus clearance and secondary bacterial infections in the airways (78, 79).
Airway infections in CF are polymicrobial, complex, and highly individualized (6, 80). The core microbiota of CF included five major bacteria genera, including Streptococcus, Prevotella, Veillonella, and Actinomyces (6). Bacterial infections in patients with CF, if not eradicated despite antimicrobial therapy, will lead to bronchiectasis symptoms (81). Transmissible infectious agents, such as Burkholderia cepacia complex (dominated by Burkholderia cenocepacia and Burkholderia multivorans), Pseudomonas aeruginosa, Staphylococcus aureus, and Aspergillus spp., were detected in multiple compartments from patients with CF (82, 83) (Table 1). Furthermore, CF-related intestinal dysbiosis is common. For example, Bifidobacterial and Clostridium species were underrepresented in feces from patients with CF, which may be the negative health consequence of antibiotic overuse (84). Proteobacteria and Fusobacterium were significantly increased, whereas Ruminococcaceae and Alistipes were reduced in the intestine of children with CF compared with healthy subjects (11). Viral-induced exacerbations are common in patients with both CF and non-CF bronchiectasis, although the causal relations remain to be elucidated (85). As patient-to-patient transmission of infectious agents is common in this unique patient population, safety precautions and infection control practices are critical. Accordingly, the restoration and maintenance of healthy gut microbiota may achieve special importance in CF.
IPF Microbiome
IPF is the most common type of interstitial lung disease, being distinguished from other interstitial lung diseases by its unknown etiology and pathogenesis. IPF occurs primarily in adults (>60 yr) and is characterized by irreversible lung fibrosis and epithelial cell injury and is associated with poor prognosis (86, 87). IPF remains incurable, despite advances in antifibrotic therapies (88). It is estimated that genetic factors are responsible for around 30% of IPF cases (18), whereas the remaining 70% can be attributed to other factors such as age, cigarette smoke, environmental exposure, and microbial infections.
A recent translational study in humans and mice indicated that the lung microbiome composition changes in Firmicutes phylum play a causal role in IPF progression, possibly associated with local alveolar inflammation and fibrosis (7) (Figure 1D). Lung microbial dysbiosis is believed to contribute to an inflammatory state that can drive fibrosis and functional decline. Alveolar macrophages, Th17, and CD8+ T cells promote fibrosis, whereas Th1 and tissue-resident memory CD4+ T cells provide protective immunity to IPF (89, 90). 16S rRNA sequencing studies have been undertaken to characterize the IPF microbiome. For example, members of the genera Staphylococcus and Streptococcus have been implicated in the IPF progression and associated with poorer survival outcomes according to a Cox regression followed by a principal coordinates analysis (12) (Table 1). Increased bacterial load in BAL fluid dominated by pathogenic Streptococcus, Haemophilus, Neisseria, and Veillonella spp. were seen in another IPF cohort, positively associated with a higher mortality risk (19). In addition to the clinical importance of bacteria, viruses (such as Epstein-Barr and hepatitis C) and fungi may act as cofactors driving IPF fibrosis (91) (Table 1). Investigation of the lung microbiome in IPF is just getting underway and will continue to open up new therapeutic targets for this frequently lethal pulmonary condition.
Emerging Research Areas and Translational Opportunities
Small Molecule Metabolomics in Pulmonary Medicine
Human microbiota modulate many important metabolic and immune functions, establishing the microbiota–metabolite– immune cell interactions with the host. Microbiota-derived small molecule metabolites, including tryptophan catabolites and short-chain fatty acids (SCFAs), are key mediators between the microbiota and the immune system (92). Tryptophan is an essential amino acid that cannot be synthesized in the human body. Tryptophan is acquired through the protein-rich diet and metabolized by gut microbiota into tryptophan catabolites such as indole and tryptamine, which function as signals and antimicrobial agents (93) (Figure 2A). Other major tryptophan catabolites such as kynurenine, on the other hand, were associated with an increased risk of pulmonary hypertension (94) and lung cancer (95) (Figure 2A). SCFAs, which include acetate, propionate, and butyrate, are saturated fatty acids that are metabolic byproducts from the fermentation of dietary fibers by the gut microbiota. SCFAs, particularly butyrate, have antiinflammatory effects and induce functional colonic regulatory T cells (96) (Figure 2A), thus playing essential roles in the maintenance of a healthy gut.
Gut-derived tryptophan and SCFAs can reach other organs via the vascular or lymphatic system to exert immune regulation and protective properties (93, 97). For example, serum tryptophan concentrations have been proposed as a biomarker for infection in patients with COPD (98). Dietary fiber promotes the growth of SCFA-producing bacteria and increases the concentrations of circulating SCFAs, thereby significantly reducing the inflammation in respiratory diseases (99, 100). An individual’s metabolic state is closely related to the individual’s overall health status (101). High-throughput and large-scale profiling in metabolomics have and will continue to play important roles in discovering novel biomarkers and therapeutic targets (97, 102, 103). For instance, several diagnostic metabolomic biomarkers have been shown to distinguish well between patients with asthma and healthy control subjects (102). Alterations in the gut microbiota metabolites have been linked to inflammation and pulmonary disease development (97). Metabolomic profiling and anticipating metabolism in the lungs advance novel therapeutic discoveries in inhaled medicines (103). Taken together, small molecule metabolomics is an emerging field expected to accelerate the advances in precision pulmonary medicine significantly. To make this possible, comprehensive research is needed to improve our understanding of the association between the hosts and their symbiotic microbes, as well as to recognize identified and previously unidentified metabolites and related mechanisms.
The Gut–Lung and Oral–Lung Axis: How Gut and Oral Microbiota Affect Lung Health
Although the lower airways are continuously protected by the local immunity and gut microbiota-derived metabolites, studies (3–5, 7, 11, 16, 59, 60, 75, 84, 91) have identified a diverse microbial community of bacteria and viruses that colonize the airways both in health and disease, as described above. A number of microbes, microbial metabolites, gastric contents, as well as other related substances influence host immune and inflammatory responses both locally and distally, forming a bidirectional gut–lung axis (Figure 2B). It is noteworthy that the gut microbiota play a central role in this gut–lung axis communication (Figure 2B). GI and respiratory diseases often cooccur, with many overlapping manifestations; therefore, the gut may be responsible for respiratory inflammation that contributes to CRD pathogenesis. Personalized therapeutic and dietary interventions to manipulate the gut microbiota may profoundly affect patients with pulmonary disease.
Healthy lungs are also dominated by oral-associated bacterial species such as Veillonella (44), Streptococcus (43), Actinomyces (44), and Corynebacterium (44) (Table 1), which have been implicated in their roles in maintaining pulmonary immune homeostasis (104). The term oral–lung axis refers to the communication between the oral cavity and the airways. The two systems are not only anatomically connected but also found to intimately interact with each other at the microbial level. For example, the oral cavity is an active site of infection and a reservoir for pathogens implicated in respiratory diseases (47). A small number of SCFAs derived from the oralome have been shown to modulate the host immune response in multiple lung illnesses (105). Moreover, the oral cavity and the upper airway act as a metaphorical “microbial gatekeeper” to protect respiratory health (52).
The oral, upper airway, and gut microbiota each individually establish and maintain a highly interactive microbial community with the lower airway microbiome. We previously did a meta-analysis in six independent colorectal cancer (CRC) cohorts and found that the enrichment of oral-originated pathogens was observed in the colon of a subset of CRC cases (31), indicating that a potential link between the oralome and gut microbiome is expected. It is also likely that highly dynamic microbial interactions exist in the oral cavity, airways, and gut. Although the discussion of the gut–oral–lung axis is beyond the scope of this review, future investigations are needed to elucidate these relationships better.
Mucosal Immunity and Microbial Signatures in Respiratory Disease
The lungs, nasal cavity, oral cavity, and GI tract are all derived from the ectoderm and are mucus covered. The primary function of mucus includes mucociliary clearance of foreign particles and pathogens from entering the body. The so-called mucosal immune system contains both innate and adaptive immune cellular components, which form a highly interconnected mucosa-associated lymphoid tissue network (106) (Figure 2C). In healthy states, the mucosal immune system protects the host from infection. However, microbes that are responsible for infection in CRC (107) and multiple oral (108) and airway diseases (109) can still take shelter on these surfaces. In the latter case, lung-resident innate immune cells, such as macrophages, lymphoid, natural killer, and dendritic cells, provide the first line of antimicrobial immunity (110), with adaptive immune cells including Th1, Th2, and regulatory T cells critically contributing to host defenses. Whether there are additional immune cellular components and any interactions between them are worth further investigation (Figure 2C).
Signatures of mucosal microbes have recently been determined, using culture-independent sequencing techniques, in asthma, COPD, CF, and IPF. H. influenzae and S. pneumoniae are associated with exacerbations in asthma and COPD (66, 69) (Table 1 and Figure 2C). Rhinovirus and RSV infections are important triggers of acute asthma (65) (Table 1 and Figure 2C). CF and IPF are rare respiratory diseases caused largely by genetic and environmental factors, respectively. There are at least 20 different Burkholderia gram-negative bacteria that form the B. cepacia complex, which is one of the most transmissible infectious agents in CF. The causal role of the lung microbiota in IPF progression is well documented (7, 89, 90). This includes the genera of Staphylococcus and Streptococcus (Table 1 and Figure 2C), and an increased bacterial load in BAL fluid (12, 111). Furthermore, Epstein-Barr virus and Hepatitis C virus act as cofactors driving the fibrosis in IPF (Table 1 and Figure 2C). To sum up, mucus infection, dysfunction, and impaired mucus clearance are common features of airway diseases (112), and maintaining a healthy mucus layer is crucial for a better life. Further identifying robust and prognostic microbial signatures is necessary to impact patient outcomes.
Respiratory Bacteriome, Mycobiome, and Virome
Beyond the bacteriome, the virome (including viruses and phages) and the mycobiome (fungi) form another part of the respiratory microbial ecosystem. Viral infections of the respiratory tract are very common in humans. Paramyxoviruses (mostly human RSV), picornaviruses (primarily rhinoviruses A and C), and orthomyxoviruses (influenza viruses A, B, and C) are among the most abundant viruses in the virome of patients with severe airway illness (113, 114) (Table 1 and Figure 2D). The lung mycobiome in healthy individuals includes many environmental species, like Cladosporium cladosporioides and Eremothecium sinecaudum, and the major pulmonary fungal pathogens in subjects with respiratory disease include Candida albicans, Aspergillus spp., and Penicillium spp. (115) (Table 1 and Figure 2D). The Malassezia genus is overpopulated in patients with asthma and CF (115). A high prevalence of Aspergillus spp. is detected in patients with severe COPD (116). Candida spp. are responsible for oral candidiasis and have been detected in patients with IPF (117) (Table 1).
The lung microbiome, extending beyond the bacteriome, is an emerging line of research. Characterizing the virome and mycobiome in patients with airway diseases were previously understudied and limited by technology. Recent culture-free molecular analysis of microbial communities through next-generation sequencing has been shown to characterize human microbiomes more extensively and systematically (118, 119). Although the established relationship between viral infections and asthma exacerbations is well investigated (65), the terrible impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) on pulmonary tissue emphasizes the obvious need to continue this line of research as new respiratory viruses, which persist, affect long term health. The mycobiome has also drawn much attention with respect to the clinical outcome of CRDs (115) (Figure 2D). Secondary or coinfection with bacteria, viruses, and fungi is common and usually progressive. Complex interactions between the bacteriome, mycobiome, and virome pose a great challenge to infection control in patients with CRDs. Our understanding of the respiratory microbiome is based largely on descriptive studies, with little causal and/or translational research available. Building on the knowledge gaps identified in a previous review (23), we set out the following five research priorities to accelerate both basic and translational microbiome research in the context of human health and disease:
-
1.
Given the ubiquity of contamination in low microbial biomass samples, is there a set of best practices from sampling to data analysis (120)?
-
2.
Are there core lung microbiota that are specific and conserved in mammals?
-
3.
Can the putative beneficial impacts of commensal microorganisms be understood by evaluating how the microbiome in health alters local immunity in pulmonary tissues?
-
4.
What factors influence microbial heterogeneity in lung health and disease? Can microbial heterogeneity guide forms of personalized medicine for pulmonary conditions?
Conclusions
The lungs and respiratory tract harbor diverse microbial communities of bacteria, viruses, and fungi, each individually known as the human respiratory microbiome, virome, and mycobiome. This microbial ecosystem is largely derived from the gut–lung and oral–lung axis. It plays critical roles in human health and disease. Four chronic respiratory diseases, including asthma, COPD, CF, and IPF, are characterized by having a disrupted respiratory microbiome. Distinctive mucosal microbial signatures have been identified from each of these conditions. Identifying microbiome-derived small molecule metabolites and how they modulate the host immune and inflammatory responses can revolutionize pulmonary medicine. Integrating the respiratory microbiome into pathophysiologic concepts of chronic disease will enhance our understanding of lung biology and advance therapeutic design.
Acknowledgments
Acknowledgment
The authors thank reviewers for their generous contributions, which greatly improved the review. They also thank Victor Tse, M.D., Ph.D. in the Stanford School of Medicine for assisting in proofreading before resubmission.
Footnotes
Supported by the Foundation for the National Institutes of Health grants HL095686 and HL158714; AMVETS grant BX005628; and Division Chief Startup Funds.
Author Contributions: L.Z. and M.R.N. conceived and designed the study. L.Z. wrote the draft and drew the figures. M.R.N. finalized the manuscript. L.Z., M.R.N., J.-L.L., M.K.A., and E.S. critically reviewed and revised the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2022-0208TR on December 7, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Pugin J, Auckenthaler R, Mili N, Janssens J-P, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis . 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 2. Meyer KC, Raghu G. Bronchoalveolar lavage for the evaluation of interstitial lung disease: is it clinically useful? Eur Respir J . 2011;38:761–769. doi: 10.1183/09031936.00069509. [DOI] [PubMed] [Google Scholar]
- 3. Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med . 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 4. Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One . 2014;9:e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med . 2000;109:288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 6. Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep . 2015;5:10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med . 2019;199:1127–1138. doi: 10.1164/rccm.201809-1650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gern JE, Busse WW. Relationship of viral infections to wheezing illnesses and asthma. Nat Rev Immunol . 2002;2:132–138. doi: 10.1038/nri725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuruvilla ME, Lee FE-H, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol . 2019;56:219–233. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Z, Bafadhel M, Haldar K, Spivak A, Mayhew D, Miller BE, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J . 2016;47:1082–1092. doi: 10.1183/13993003.01406-2015. [DOI] [PubMed] [Google Scholar]
- 11. Coffey MJ, Nielsen S, Wemheuer B, Kaakoush NO, Garg M, Needham B, et al. Gut microbiota in children with cystic fibrosis: a taxonomic and functional dysbiosis. Sci Rep . 2019;9:18593. doi: 10.1038/s41598-019-55028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, et al. COMET Investigators Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med . 2014;2:548–556. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature . 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 14. Himes BE, Hunninghake GM, Baurley JW, Rafaels NM, Sleiman P, Strachan DP, et al. Genome-wide association analysis identifies PDE4D as an asthma-susceptibility gene. Am J Hum Genet . 2009;84:581–593. doi: 10.1016/j.ajhg.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sleiman PMA, Flory J, Imielinski M, Bradfield JP, Annaiah K, Willis-Owen SAG, et al. Variants of DENND1B associated with asthma in children. N Engl J Med . 2010;362:36–44. doi: 10.1056/NEJMoa0901867. [DOI] [PubMed] [Google Scholar]
- 16. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One . 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scotet V, L’Hostis C, Férec C. The changing epidemiology of cystic fibrosis: incidence, survival and impact of the CFTR gene discovery. Genes (Basel) . 2020;11:589. doi: 10.3390/genes11060589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaur A, Mathai SK, Schwartz DA. Genetics in idiopathic pulmonary fibrosis pathogenesis, prognosis, and treatment. Front Med (Lausanne) . 2017;4:154. doi: 10.3389/fmed.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molyneaux PL, Cox MJ, Willis-Owen SAG, Mallia P, Russell KE, Russell A-M, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2014;190:906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diao Z, Han D, Zhang R, Li J. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J Adv Res . 2021;38:201–212. doi: 10.1016/j.jare.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grønseth R, Drengenes C, Wiker HG, Tangedal S, Xue Y, Husebø GR, et al. Protected sampling is preferable in bronchoscopic studies of the airway microbiome. ERJ Open Res . 2017;3:00019–2017. doi: 10.1183/23120541.00019-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drengenes C, Wiker HG, Kalananthan T, Nordeide E, Eagan TML, Nielsen R. Laboratory contamination in airway microbiome studies. BMC Microbiol . 2019;19:187. doi: 10.1186/s12866-019-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carney SM, Clemente JC, Cox MJ, Dickson RP, Huang YJ, Kitsios GD, et al. Methods in lung microbiome research. Am J Respir Cell Mol Biol . 2020;62:283–299. doi: 10.1165/rcmb.2019-0273TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Emerson JB, Adams RI, Román CMB, Brooks B, Coil DA, Dahlhausen K, et al. Schrödinger’s microbes: tools for distinguishing the living from the dead in microbial ecosystems. Microbiome . 2017;5:86. doi: 10.1186/s40168-017-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gall A, Fero J, McCoy C, Claywell BC, Sanchez CA, Blount PL, et al. Bacterial composition of the human upper gastrointestinal tract microbiome is dynamic and associated with genomic instability in a Barrett’s esophagus cohort. PLoS One . 2015;10:e0129055. doi: 10.1371/journal.pone.0129055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahmed I, Roy BC, Khan SA, Septer S, Umar S. Microbiome, metabolome and inflammatory bowel disease. Microorganisms . 2016;4:20. doi: 10.3390/microorganisms4020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology . 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 28. Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol . 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 29. DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis . 2016;22:1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chassard C, Dapoigny M, Scott KP, Crouzet L, Del’homme C, Marquet P, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther . 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 31. Zhao L, Cho WC, Nicolls MR. Colorectal cancer-associated microbiome patterns and signatures. Front Genet . 2021;12:787176. doi: 10.3389/fgene.2021.787176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut . 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc . 2015;12:S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 34. He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J. Gut–lung axis: the microbial contributions and clinical implications. Crit Rev Microbiol . 2017;43:81–95. doi: 10.1080/1040841X.2016.1176988. [DOI] [PubMed] [Google Scholar]
- 35. Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol . 2018;20:e12966. doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 36. Yildiz S, Mazel-Sanchez B, Kandasamy M, Manicassamy B, Schmolke M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome . 2018;6:9. doi: 10.1186/s40168-017-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anand S, Mande SS. Diet, microbiota and gut-lung connection. Front Microbiol . 2018;9:2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perng D-W, Chang K-T, Su K-C, Wu Y-C, Wu M-T, Hsu W-H, et al. Exposure of airway epithelium to bile acids associated with gastroesophageal reflux symptoms: a relation to transforming growth factor-beta1 production and fibroblast proliferation. Chest . 2007;132:1548–1556. doi: 10.1378/chest.07-1373. [DOI] [PubMed] [Google Scholar]
- 39. Savarino E, Carbone R, Marabotto E, Furnari M, Sconfienza L, Ghio M, et al. Gastro-oesophageal reflux and gastric aspiration in idiopathic pulmonary fibrosis patients. Eur Respir J . 2013;42:1322–1331. doi: 10.1183/09031936.00101212. [DOI] [PubMed] [Google Scholar]
- 40. Hu X, Lee JS, Pianosi PT, Ryu JH. Aspiration-related pulmonary syndromes. Chest . 2015;147:815–823. doi: 10.1378/chest.14-1049. [DOI] [PubMed] [Google Scholar]
- 41. Radaic A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Comput Struct Biotechnol J . 2021;19:1335–1360. doi: 10.1016/j.csbj.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol . 2019;23:122–128. doi: 10.4103/jomfp.JOMFP_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaura E, Nicu EA, Krom BP, Keijser BJF. Acquiring and maintaining a normal oral microbiome: current perspective. Front Cell Infect Microbiol . 2014;4:85. doi: 10.3389/fcimb.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kriebel K, Hieke C, Müller-Hilke B, Nakata M, Kreikemeyer B. Oral biofilms from symbiotic to pathogenic interactions and associated disease: connection of periodontitis and rheumatic arthritis by peptidylarginine deiminase. Front Microbiol . 2018;9:53. doi: 10.3389/fmicb.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aruni AW, Dou Y, Mishra A, Fletcher HM. The biofilm community: rebels with a cause. Curr Oral Health Rep . 2015;2:48–56. doi: 10.1007/s40496-014-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zijnge V, Ammann T, Thurnheer T, Gmür R. Subgingival biofilm structure. Front Oral Biol . 2012;15:1–16. doi: 10.1159/000329667. [DOI] [PubMed] [Google Scholar]
- 47. Kumar PS. From focal sepsis to periodontal medicine: a century of exploring the role of the oral microbiome in systemic disease. J Physiol . 2017;595:465–476. doi: 10.1113/JP272427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dussault G, Sheiham A. Medical theories and professional development: the theory of focal sepsis and dentistry in early twentieth century Britain. Soc Sci Med . 1982;16:1405–1412. doi: 10.1016/0277-9536(82)90135-6. [DOI] [PubMed] [Google Scholar]
- 49. Gaeckle NT, Pragman AA, Pendleton KM, Baldomero AK, Criner GJ. The oral-lung axis: the impact of oral health on lung health. Respir Care . 2020;65:1211–1220. doi: 10.4187/respcare.07332. [DOI] [PubMed] [Google Scholar]
- 50. Stearns JC, Davidson CJ, McKeon S, Whelan FJ, Fontes ME, Schryvers AB, et al. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J . 2015;9:1268. doi: 10.1038/ismej.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bosch AATM, Levin E, van Houten MA, Hasrat R, Kalkman G, Biesbroek G, et al. Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine . 2016;9:336–345. doi: 10.1016/j.ebiom.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Man WH, de Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol . 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol . 2019;17:87. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee J-J, Kim S-H, Lee M-J, Kim B-K, Song W-J, Park H-W, et al. Different upper airway microbiome and their functional genes associated with asthma in young adults and elderly individuals. Allergy . 2019;74:709–719. doi: 10.1111/all.13608. [DOI] [PubMed] [Google Scholar]
- 55. Whiteson KL, Bailey B, Bergkessel M, Conrad D, Delhaes L, Felts B, et al. The upper respiratory tract as a microbial source for pulmonary infections in cystic fibrosis: parallels from island biogeography. Am J Respir Crit Care Med . 2014;189:1309–1315. doi: 10.1164/rccm.201312-2129PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou Y, Jackson D, Bacharier LB, Mauger D, Boushey H, Castro M, et al. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat Commun . 2019;10:5714. doi: 10.1038/s41467-019-13698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang C, Zhang T, Lu W, Duan X, Luo X, Liu S, et al. Altered airway microbiota composition in patients with pulmonary hypertension. Hypertension . 2020;76:1589–1599. doi: 10.1161/HYPERTENSIONAHA.120.15025. [DOI] [PubMed] [Google Scholar]
- 58. Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc . 2015;12:821–830. doi: 10.1513/AnnalsATS.201501-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tong X, Su F, Xu X, Xu H, Yang T, Xu Q, et al. Alterations to the lung microbiome in idiopathic pulmonary fibrosis patients. Front Cell Infect Microbiol . 2019;9:149. doi: 10.3389/fcimb.2019.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Santacroce L, Charitos IA, Ballini A, Inchingolo F, Luperto P, De Nitto E, et al. The human respiratory system and its microbiome at a glimpse. Biology (Basel) . 2020;9:318. doi: 10.3390/biology9100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, et al. Lung HIV Microbiome Project Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med . 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med . 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 63. Stiemsma LT, Arrieta M-C, Dimitriu PA, Cheng J, Thorson L, Lefebvre DL, et al. Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci (Lond) . 2016;130:2199–2207. doi: 10.1042/CS20160349. [DOI] [PubMed] [Google Scholar]
- 64. Arrieta M-C, Arévalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol . 2018;142:424–434.e10. doi: 10.1016/j.jaci.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jartti T, Bønnelykke K, Elenius V, Feleszko W. Role of viruses in asthma. Semin Immunopathol . 2020;42:61–74. doi: 10.1007/s00281-020-00781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mthembu N, Ikwegbue P, Brombacher F, Hadebe S. Respiratory viral and bacterial factors that influence early childhood asthma. Front Allergy . 2021;22:692841. doi: 10.3389/falgy.2021.692841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol . 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 68. Edwards MR, Bartlett NW, Hussell T, Openshaw P, Johnston SL. The microbiology of asthma. Nat Rev Microbiol . 2012;10:459–471. doi: 10.1038/nrmicro2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sethi S, Evans N, Grant BJB, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med . 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 70. Ghebre MA, Pang PH, Diver S, Desai D, Bafadhel M, Haldar K, et al. Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J Allergy Clin Immunol . 2018;141:2027–2036.e12. doi: 10.1016/j.jaci.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Diver S, Richardson M, Haldar K, Ghebre MA, Ramsheh MY, Bafadhel M, et al. Sputum microbiomic clustering in asthma and chronic obstructive pulmonary disease reveals a Haemophilus-predominant subgroup. Allergy . 2020;75:808–817. doi: 10.1111/all.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ganesan S, Comstock AT, Kinker B, Mancuso P, Beck JM, Sajjan US. Combined exposure to cigarette smoke and nontypeable Haemophilus influenzae drives development of a COPD phenotype in mice. Respir Res . 2014;15:11. doi: 10.1186/1465-9921-15-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alnahas S, Hagner S, Raifer H, Kilic A, Gasteiger G, Mutters R, et al. IL-17 and TNF-α are key mediators of Moraxella catarrhalis triggered exacerbation of allergic airway inflammation. Front Immunol . 2017;8:1562. doi: 10.3389/fimmu.2017.01562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pichavant M, Sharan R, Le Rouzic O, Olivier C, Hennegrave F, Rémy G, et al. IL-22 defect during Streptococcus pneumoniae infection triggers exacerbation of chronic obstructive pulmonary disease. EBioMedicine . 2015;2:1686–1696. doi: 10.1016/j.ebiom.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Molyneaux PL, Mallia P, Cox MJ, Footitt J, Willis-Owen SAG, Homola D, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2013;188:1224–1231. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang JH, Kwon HJ, Jang YJ. Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope . 2009;119:1406–1411. doi: 10.1002/lary.20498. [DOI] [PubMed] [Google Scholar]
- 77. Modolell I, Guarner L, Malagelada JR. Digestive system involvement in cystic fibrosis. Pancreatology . 2002;2:12–16. doi: 10.1159/000049442. [DOI] [PubMed] [Google Scholar]
- 78. Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet . 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 79. Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med . 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Acosta N, Whelan FJ, Somayaji R, Poonja A, Surette MG, Rabin HR, et al. The evolving cystic fibrosis microbiome: a comparative cohort study spanning 16 years. Ann Am Thorac Soc . 2017;14:1288–1297. doi: 10.1513/AnnalsATS.201609-668OC. [DOI] [PubMed] [Google Scholar]
- 81. Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can’t they clear the infection? Respir Res . 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saiman L, Siegel J. Infection control in cystic fibrosis. Clin Microbiol Rev . 2004;17:57–71. doi: 10.1128/CMR.17.1.57-71.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Saiman L, Siegel JD, LiPuma JJ, Brown RF, Bryson EA, Chambers MJ, et al. Cystic Fibrous Foundation Society for Healthcare Epidemiology of America. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect Control Hosp Epidemiol . 2014;35:S1–S67. doi: 10.1086/676882. [DOI] [PubMed] [Google Scholar]
- 84. Duytschaever G, Huys G, Bekaert M, Boulanger L, De Boeck K, Vandamme P. Dysbiosis of bifidobacteria and Clostridium cluster XIVa in the cystic fibrosis fecal microbiota. J Cyst Fibros . 2013;12:206–215. doi: 10.1016/j.jcf.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 85. Schäfer J, Griese M, Chandrasekaran R, Chotirmall SH, Hartl D. Pathogenesis, imaging and clinical characteristics of CF and non-CF bronchiectasis. BMC Pulm Med . 2018;18:79. doi: 10.1186/s12890-018-0630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers . 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 87. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 88. Johannson KA, Chaudhuri N, Adegunsoye A, Wolters PJ. Treatment of fibrotic interstitial lung disease: current approaches and future directions. Lancet . 2021;398:1450–1460. doi: 10.1016/S0140-6736(21)01826-2. [DOI] [PubMed] [Google Scholar]
- 89. Hu G, Christman JW. Editorial: alveolar macrophages in lung inflammation and resolution. Front Immunol . 2019;10:2275. doi: 10.3389/fimmu.2019.02275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shenderov K, Collins SL, Powell JD, Horton MR. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J Clin Invest . 2021;131:e143226. doi: 10.1172/JCI143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fabbrizzi A, Nannini G, Lavorini F, Tomassetti S, Amedei A. Microbiota and IPF: hidden and detected relationships. Sarcoidosis Vasc Diffuse Lung Dis . 2021;38:e2021028. doi: 10.36141/svdld.v38i3.11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev . 2016;30:1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun . 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nagy B, Nagaraj C, Meinitzer A, Papp R, Foris V, Ghanim B, et al. An intrinsic tryptophan metabolite acts as pulmonary vasodilator in pulmonary hypertension [abstract] FASEB J . 2017;31:1073.8. [Google Scholar]
- 95. Chuang S-C, Fanidi A, Ueland PM, Relton C, Midttun O, Vollset SE, et al. Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol Biomarkers Prev . 2014;23:461–468. doi: 10.1158/1055-9965.EPI-13-0770. [DOI] [PubMed] [Google Scholar]
- 96. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature . 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 97. Zhang D, Li S, Wang N, Tan H-Y, Zhang Z, Feng Y. The cross-talk between gut microbiota and lungs in common lung diseases. Front Microbiol . 2020;11:301. doi: 10.3389/fmicb.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gulcev M, Reilly C, Griffin TJ, Broeckling CD, Sandri BJ, Witthuhn BA, et al. Tryptophan catabolism in acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2016;11:2435–2446. doi: 10.2147/COPD.S107844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med . 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 100. Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG. Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients . 2017;9:57. doi: 10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kouskoumvekaki I, Panagiotou G. Navigating the human metabolome for biomarker identification and design of pharmaceutical molecules. J Biomed Biotechnol . 2011;2011:525497. doi: 10.1155/2011/525497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kelly RS, Dahlin A, McGeachie MJ, Qiu W, Sordillo J, Wan ES, et al. Asthma metabolomics and the potential for integrative omics in research and the clinic. Chest . 2017;151:262–277. doi: 10.1016/j.chest.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Enlo-Scott Z, Bäckström E, Mudway I, Forbes B. Drug metabolism in the lungs: opportunities for optimising inhaled medicines. Expert Opin Drug Metab Toxicol . 2021;17:611–625. doi: 10.1080/17425255.2021.1908262. [DOI] [PubMed] [Google Scholar]
- 104. Sommariva M, Le Noci V, Bianchi F, Camelliti S, Balsari A, Tagliabue E, et al. The lung microbiota: role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cell Mol Life Sci . 2020;77:2739–2749. doi: 10.1007/s00018-020-03452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mammen MJ, Scannapieco FA, Sethi S. Oral-lung microbiome interactions in lung diseases. Periodontol 2000 . 2020;83:234–241. doi: 10.1111/prd.12301. [DOI] [PubMed] [Google Scholar]
- 106. McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol . 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhao L, Grimes SM, Greer SU, Kubit M, Lee H, Nadauld LD, et al. Characterization of the consensus mucosal microbiome of colorectal cancer. NAR Cancer . 2021;3:zcab049. doi: 10.1093/narcan/zcab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Groeger S, Meyle J. Oral mucosal epithelial cells. Front Immunol . 2019;10:208. doi: 10.3389/fimmu.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kyd JM, Foxwell AR, Cripps AW. Mucosal immunity in the lung and upper airway. Vaccine . 2001;19:2527–2533. doi: 10.1016/s0264-410x(00)00484-9. [DOI] [PubMed] [Google Scholar]
- 110. Ardain A, Marakalala MJ, Leslie A. Tissue-resident innate immunity in the lung. Immunology . 2020;159:245–256. doi: 10.1111/imm.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Molyneaux PL, Maher TM. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir Rev . 2013;22:376–381. doi: 10.1183/09059180.00000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature . 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lysholm F, Wetterbom A, Lindau C, Darban H, Bjerkner A, Fahlander K, et al. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS One . 2012;7:e30875. doi: 10.1371/journal.pone.0030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang Y, Zhu N, Li Y, Lu R, Wang H, Liu G, et al. Metagenomic analysis of viral genetic diversity in respiratory samples from children with severe acute respiratory infection in China. Clin Microbiol Infect . 2016;22:458.e1–458.e9. doi: 10.1016/j.cmi.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nguyen LDN, Viscogliosi E, Delhaes L. The lung mycobiome: an emerging field of the human respiratory microbiome. Front Microbiol . 2015;6:89. doi: 10.3389/fmicb.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Huerta A, Soler N, Esperatti M, Guerrero M, Menendez R, Gimeno A, et al. Importance of Aspergillus spp. isolation in acute exacerbations of severe COPD: prevalence, factors and follow-up. The FUNGI-COPD study. Respir Res . 2014;15:17. doi: 10.1186/1465-9921-15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cuerpo S, Moisés J, Hernández-González F, Benegas M, Ramirez J, Sánchez M, et al. Acute exacerbations of idiopathic pulmonary fibrosis: does clinical stratification or steroid treatment matter? Chron Respir Dis . 2019;16:1479973119869334. doi: 10.1177/1479973119869334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Armstrong GL, MacCannell DR, Taylor J, Carleton HA, Neuhaus EB, Bradbury RS, et al. Pathogen genomics in public health. N Engl J Med . 2019;381:2569–2580. doi: 10.1056/NEJMsr1813907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bharti R, Grimm DG. Current challenges and best-practice protocols for microbiome analysis. Brief Bioinform . 2021;22:178–193. doi: 10.1093/bib/bbz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Eisenhofer R, Minich JJ, Marotz C, Cooper A, Knight R, Weyrich LS. Contamination in low microbial biomass microbiome studies: issues and recommendations. Trends Microbiol . 2019;27:105–117. doi: 10.1016/j.tim.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 121. Crespo-Lessmann A, Plaza V, Consensus Group Multidisciplinary consensus on sputum induction biosafety during the COVID-19 pandemic. Allergy . 2021;76:2407–2419. doi: 10.1111/all.14697. [DOI] [PubMed] [Google Scholar]


