Abstract
Aims
Galectin-3, a β-galactoside-binding lectin, is abnormally increased in cardiovascular disease. Plasma Galectin-3 receives a Class II recommendation for heart failure management and has been extensively studied for multiple cellular functions. The direct effects of Galectin-3 on platelet activation remain unclear. This study explores the direct effects of Galectin-3 on platelet activation and thrombosis.
Methods and results
A strong positive correlation between plasma Galectin-3 concentration and platelet aggregation or whole blood thrombus formation was observed in patients with coronary artery disease (CAD). Multiple platelet function studies demonstrated that Galectin-3 directly potentiated platelet activation and in vivo thrombosis. Mechanistic studies using the Dectin-1 inhibitor, laminarin, and Dectin-1−/− mice revealed that Galectin-3 bound to and activated Dectin-1, a receptor not previously reported in platelets, to phosphorylate spleen tyrosine kinase and thus increased Ca2+ influx, protein kinase C activation, and reactive oxygen species production to regulate platelet hyperreactivity. TD139, a Galectin-3 inhibitor in a Phase II clinical trial, concentration dependently suppressed Galectin-3-potentiated platelet activation and inhibited occlusive thrombosis without exacerbating haemorrhage in ApoE−/− mice, which spontaneously developed increased plasma Galectin-3 levels. TD139 also suppressed microvascular thrombosis to protect the heart from myocardial ischaemia–reperfusion injury in ApoE−/− mice.
Conclusion
Galectin-3 is a novel positive regulator of platelet hyperreactivity and thrombus formation in CAD. As TD139 has potent antithrombotic effects without bleeding risk, Galectin-3 inhibitors may have therapeutic advantages as potential antiplatelet drugs for patients with high plasma Galectin-3 levels.
Keywords: Platelet hyperreactivity, Cardiovascular diseases, Galectin-3, Dectin-1, Antithrombotic, TD139
Structured Graphical Abstract
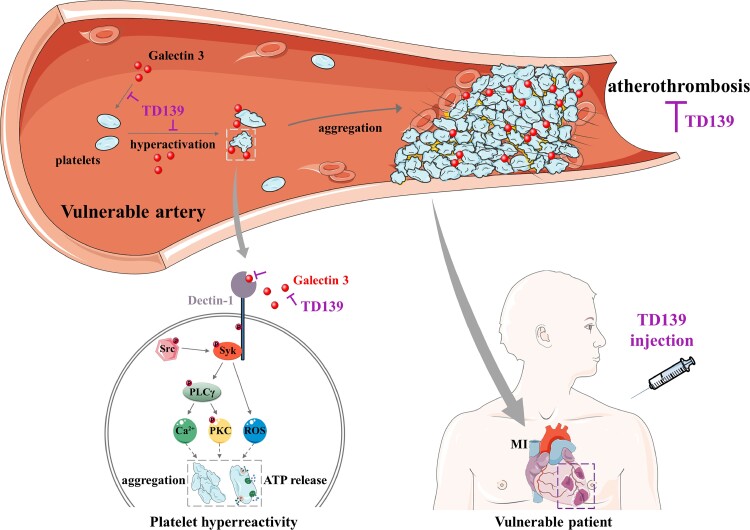
Structured Graphical Abstract.
Working model for plasma Galectin-3 directly potentiating platelet activation, and the platelet hyperreactivity suppressing effect of Galectin-3 inhibitor TD139 in coronary artery disease. Accumulated Galectin-3 in plasma directly activates Dectin-1 receptor to potentiate platelet activation (platelet aggregation and ATP release), which enhances in vivo thrombosis and myocardial ischaemia–reperfusion injury. Galectin-3 inhibitor TD139 specifically inhibits Galectin-3-induced platelet hyperreactivity to ameliorate atherothrombosis and myocardial infarction in coronary artery disease.
See the editorial comment for this article ‘Galectin-3 inhibitors as novel antithrombotic drugs with almost no bleeding risk: wishful thinking or a realistic vision?’, by Kai Jakobs and Ursula Rauch, https://doi.org/10.1093/eurheartj/ehac128.
Translational perspective.
Plasma Galectin-3 level positively correlates with platelet aggregation in patients with coronary artery disease (CAD), resulting in enhanced thrombosis potential.
Our study sheds novel insights into the mechanisms of platelet hyperreactivity and thrombotic risk in patients with high plasma Galectin-3 levels and shows that Galectin-3 activates C-type lectin-like receptor Dectin-1 (CLEC 7A)/spleen tyrosine kinase (Syk) signalling to potentiate platelet activation.
Our work introduces a new potential therapeutic strategy for patients with CAD and suggests that Galectin-3 inhibitors may be considered for patients with high plasma Galectin-3 levels to prevent platelet hyperreactivity and thrombotic events.
Abbreviations
- CAD
coronary artery disease
- MI
myocardial infarction
- PRP
platelet-rich plasma
- PPP
platelet-poor plasma
- WPs
washed platelets
- Syk
spleen tyrosine kinase
- I/R
ischaemia–reperfusion
- STEMI
ST-elevation myocardial infarction
- rGalectin
recombinant Galectin
- PCI
percutaneous coronary intervention
- PBMC
peripheral blood mononuclear cell
- PLCγ
phospholipase Cγ
- PKC
protein kinase C
- ROS
reactive oxygen species
- AAR
area at risk
Introduction
Arterial thrombotic events, such as myocardial infarction (MI) and stroke, are the leading causes of morbidity and mortality worldwide. Platelet activation and the consequent intravascular arterial thrombogenesis are the common pathological processes of acute coronary syndromes and cerebral ischaemia,1–4 while platelet hyperreactivity is an important risk factor leading to the increased incidence and mortality of arterial thrombotic events.5 Unfortunately, although the use of antiplatelet drugs has become a cornerstone for the prevention and treatment of coronary artery disease (CAD), the morbidity and mortality rates remain disappointingly high.6,7 Therefore, understanding the mechanisms of increased platelet reactivity and developing novel antiplatelet agents with a better balance between antithrombotic efficacy and bleeding are urgently needed.8
Galectin-3 (also known as Mac-2) is a 30 kDa lectin, mainly produced by immune cells and cardiomyocytes and secreted into circulation, and can be abnormally overexpressed in inflammation, atherosclerosis, cardiac remodelling, and myocardial fibrosis.9,10 Plasma Galectin-3 has been reported to be strongly associated with heart failure1–3 and receives a Class II recommendation for heart failure management according to the 2013 American College of Cardiology Foundation/American Heart Association guidelines.14 In recent years, a growing number of studies have shown that plasma Galectin-3 can predict CAD occurrence5–9 and has prognostic value for ischaemic stroke.20,21 Current evidence suggests that Galectin-3 activates resting fibroblasts into matrix-producing fibroblasts and exacerbates endothelial cell injury, which contributes to cardiac remodelling and dysfunction.2–4 In addition, Galectin-3 promotes the migration of monocytes and the infiltration of macrophages into the arterial wall, exacerbating the proinflammatory state in atherosclerosis, to enhance the development of atherosclerosis in ApoE−/− mice.5–7 However, the pleiotropic effects of Galectin-3, especially its direct effect on platelets, are not yet fully understood.
Unlike venous thrombosis which mainly relies on the coagulation cascade, following atherosclerotic plaque injury and rupture, platelets are activated by the exposed subendothelial extracellular matrix, resulting in platelet-rich thrombus generation and vessel wall erosion. These are the primary processes leading to coronary arterial thrombus formation.28 DeRoo et al.29 revealed that Galectin-3−/− mice showed a reduction in venous thrombus formation, and intravenous injection of recombinant Galectin-3 increased venous thrombus formation in wild-type mice. However, arterial thrombus formation after recombinant Galectin-3 injection was not investigated in this study. Whether Galectin-3 can directly manipulate platelet activation to potentiate arterial thrombosis, thereby providing a link between platelet hyperreactivity and the increased incidence of atherothrombotic events in CAD, remains unclear.
In this study, we reported that Galectin-3 holds a pathological relevance for platelet hyperreactivity and in vivo thrombosis in CAD patients and atherosclerotic ApoE−/− mice. We elucidated the underlying mechanism that Galectin-3 potentiates platelet activation by activating Dectin-1/spleen tyrosine kinase (Syk) signalling and demonstrated that a Galectin-3-specific inhibitor, TD139, currently in a Phase II clinical trial of idiopathic pulmonary fibrosis,0–2 can serve as a new therapeutic agent for platelet hyperreactivity, reducing arterial thrombosis and myocardial ischaemia–reperfusion (I/R) injury without increasing bleeding risk.
Methods
Detailed Materials and Methods, including study subjects and patients, are described in the Supplementary material online.
Results
Galectin-3 directly potentiates platelet function
Our study population comprised 1438 patients with a clinical diagnosis of CAD. According to the exclusion criteria, 1256 patients were excluded, leaving 182 CAD patients without antiplatelet treatment at least 14 days before blood collection. We measured plasma Galectin-3 levels in 182 CAD patients and 37 gender- and age-matched healthy subjects. Plasma concentrations of Galectin-3 were significantly increased in CAD patients (see Supplementary material online, Figure S1) and plasma Galectin-3 levels were strongly correlated with platelet aggregation in platelet-rich plasma (PRP) induced by 0.8μg/mL collagen (Spearman r = 0.65, P < 0.01, n = 182) or with 0.8 U/mL thrombin (Spearman r = 0.59, P < 0.01, n = 182), respectively (Figure 1A). The baseline characteristics of the analysed patients are summarized in Supplementary material online, Table S1.
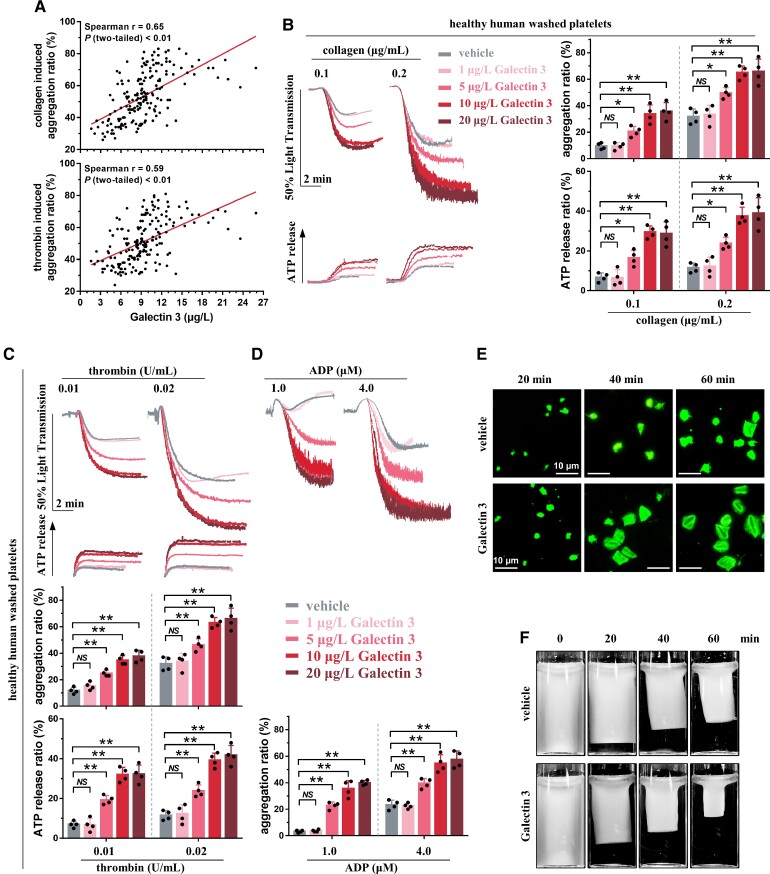
Figure 1.
Galectin-3 directly potentiates platelet function. (A) Platelet aggregation induced by 0.8 μg/mL collagen (upper panel) or 0.8 U/mL thrombin (lower panel) in platelet-rich plasma correlated well with plasma Galectin-3 levels in patients with coronary artery disease. Platelet aggregation was measured in platelet-rich plasma; plasma Galectin-3 concentration was measured using the enzyme-linked immuno sorbent assay kit. Each solid circle represents a single individual (Spearman correlation, n = 182, GraphPad Prism 7). (B–D) Recombinant Galectin-3, in a concentration-dependent manner, potentiated platelet aggregation induced by collagen (B), thrombin (C), or adenosine diphosphate (D), as well as adenosine tri-phosphate release induced by collagen (B) or thrombin (C). Representative results and summary data of four experiments are presented. (E) Representative fluorescence images (phalloidin) showing that Galectin-3 potentiated platelet spreading on immobilized fibrinogen. After preincubation with recombinant Galectin-3 (20 μg/L) for 5 min, platelets from healthy subjects were allowed to spread for the indicated times. Representative results and summary data (see Supplementary material online, Figure S3A) of three experiments are presented. Scale bar = 10 μm. (F) Galectin-3 potentiated clot retraction induced by thrombin. Washed platelets from healthy subjects were preincubated with recombinant Galectin-3 (20 μg/L) for 5 min and then stimulated with thrombin. Representative results and summary data (see Supplementary material online, Figure S3B) of three experiments are presented. Statistical analyses were performed using repeated measures one-way ANOVA followed by Dunnett's multiple comparisons test in (B), (C), and (D). Two-way ANOVA followed by Sidak's multiple comparisons test was performed in (E) and (F). NS, no significance; *P < 0.05; **P < 0.01.
Considering the strong correlation between plasma Galectin-3 levels and platelet aggregation in CAD patients, we next evaluated the effects of recombinant Galectin(rGalectin)-3 on platelet function using platelet samples from healthy subjects. rGalectin-3 treatment alone was not sufficient to induce platelet aggregation in washed platelets (WPs) from healthy subjects, even at 100 μg/L for 30 min. However, in the pathological range of 5–20 μg/L, similar to CAD patients,18 rGalectin-3 concentration dependently potentiated platelet aggregation induced by different concentrations of collagen, thrombin, or adenosine diphosphate (ADP) and increased adenosine tri-phosphate (ATP) release induced by different concentrations of collagen or thrombin (Figure 1B–D). rGalectin-3 also potentiated platelet aggregation and ATP release induced by different agonists using PRP from healthy subjects (see Supplementary material online, Figure S2; 3.1 ± 0.5 μg/L of plasma Galectin-3).
As contractile cells, platelets adhere firmly to the subendothelial components after plaque rupture and consolidate as blood clots via retractile processes, which play a pivotal role in atherothrombosis.33,34 Thus, using WP from healthy subjects, we tested whether rGalectin-3 assists in the process of human platelet spreading and clot retraction. In line with the potentiating effects of rGalectin-3 on platelet aggregation, platelet spreading was accelerated in the presence of 20 μg/L rGalectin-3 using WPs from healthy subjects (Figure 1E). A similar potentiating effect of rGalectin-3 was observed when clot contraction was performed (Figure 1F).
Galectin-3 directly potentiates ex vivo thrombosis potential
The observed positive association between Galectin-3 levels and platelet function indicates a potential effect of Galectin-3 on thrombus formation. Thus, we evaluated ex vivo thrombus formation in whole blood from 32 CAD patients and 10 healthy subjects under arterial shear stress, using a microfluidic whole-blood perfusion assay. Throughout the perfusion period, the size of thrombi developed in whole blood from CAD patients increased markedly, which was much larger than that in healthy subjects. Notably, the concentration of Galectin-3 in CAD patients again showed a positive correlation with a thrombus size at 180 s (Figure 2A). To further evaluate the effect of Galectin-3 on ex vivo thrombus formation, whole blood from healthy subjects was supplemented with rGalectin-3 and perfused over the collagen surface. Consistent with the above results from CAD patients, rGalectin-3 treatment potentiated thrombus formation in a concentration-dependent manner (Figure 2B); and, at all observed time points, whole blood supplemented with 20 μg/L rGalectin-3 presented the highest thrombus formation area.
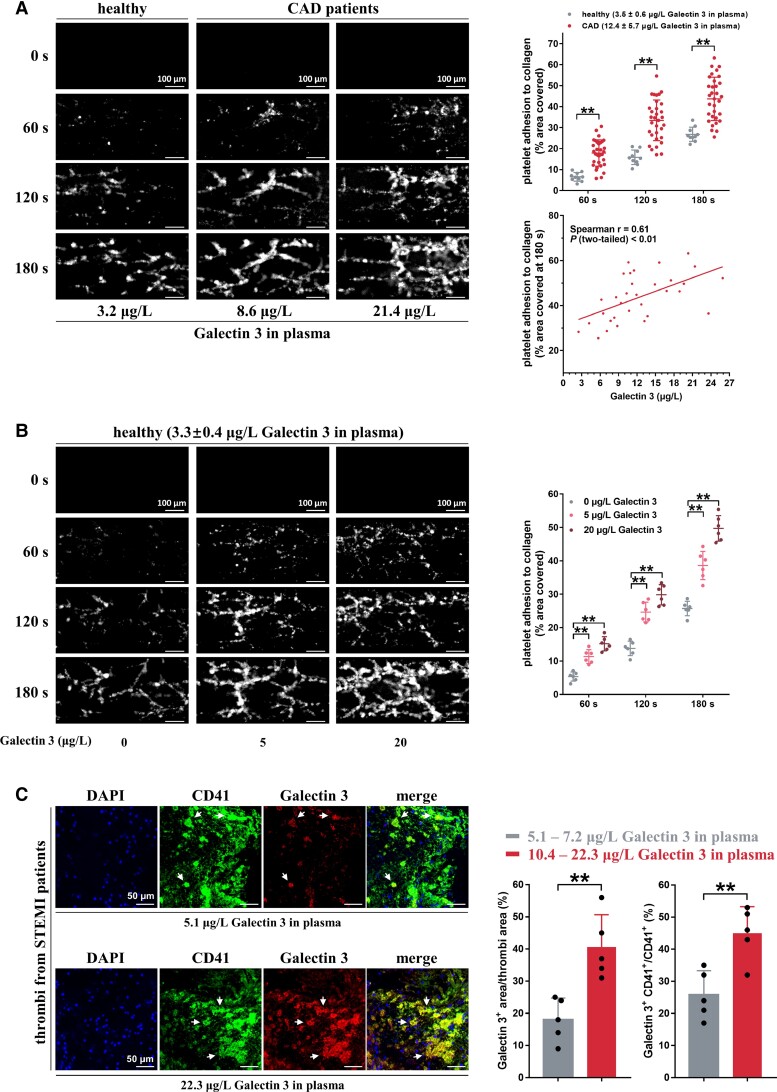
Figure 2.
Galectin-3 directly potentiates in vitro thrombosis potential. (A) Whole blood from coronary artery disease patients showed accelerated thrombus formation over an immobilized collagen surface at a shear rate of 1000 s−1 compared with that of healthy subjects (n = 10). Thrombus formation area at 180 s correlated well with plasma Galectin-3 levels in coronary artery disease patients (n = 32). The whole blood was tagged by fluorescein isothiocyanate (FITC)-labeled anti-CD41 antibody, and then perfused through fibrillar collagen-coated bioflux plates for 180 s. Representative images of thrombus formation at the indicated time points are presented. Each solid circle represents the thrombus formation area from a single individual (Spearman correlation, GraphPad Prism 7). Scale bar = 100μm. (B) Recombinant Galectin-3, in a concentration-dependent manner, accelerated thrombus formation over an immobilized collagen surface at a shear rate of 1000 s−1. Whole blood samples from healthy subjects were preincubated with recombinant Galectin-3 and FITC-labeled anti-CD41 antibody and then perfused through fibrillar collagen-coated bioflux plates. Representative images of thrombus formation are presented. Each solid circle represents the thrombus formation area from a single individual (n = 6). Scale bar = 100μm. (C) Increased Galectin-3 deposition in the thrombi from ST-elevation myocardial infarction patients with high plasma Galectin-3 levels (10.4–22.3 μg/L; n = 5) compared with those from ST-elevation myocardial infarction patients with low plasma Galectin-3 levels (5.1–7.2 μg/L; n = 5). Dual-immunofluorescence staining was carried out using anti-CD41 antibody (a platelet marker, green fluorescence) and anti-Galectin-3 antibody (red fluorescence). Blue fluorescence indicates nuclei stained with 4′,6-diamidino-2-phenylindole. White arrows (yellow fluorescence) mark co-localization of Galectin-3 and CD41. Representative results of thrombi and summary data are presented. Scale bar = 50 μm. Statistical analyses were performed using two-way ANOVA followed by Sidak's multiple comparisons test in (A) and (B) and unpaired two-tailed Student's t-test in (C). **P < 0.01.
Within the thrombi aspirated from the coronary arteries of patients with ST-elevation myocardial infarction (STEMI) during primary percutaneous coronary intervention (PCI), the presence of Galectin-3 on platelets was observed using dual-immunofluorescence (Figure 2C). Increased Galectin-3 deposition (Galectin-3+ area/thrombi area) and increased binding of Galectin-3 to platelets (Galectin-3+ CD41+/CD41+) were also observed in the thrombi from patients with high plasma Galectin-3 levels (10.4–22.3 μg/L in plasma, n = 5), compared with those from STEMI patients with low plasma Galectin-3 levels (5.1–7.2 μg/L in plasma, n = 5).
Collectively, these results provide evidence that Galectin-3 regulates platelet function and thrombus formation and highlights a possible role of Galectin-3 in platelet hyperreactivity of CAD.
Galectin-3 binds to platelet Dectin-1 to enhance platelet activation
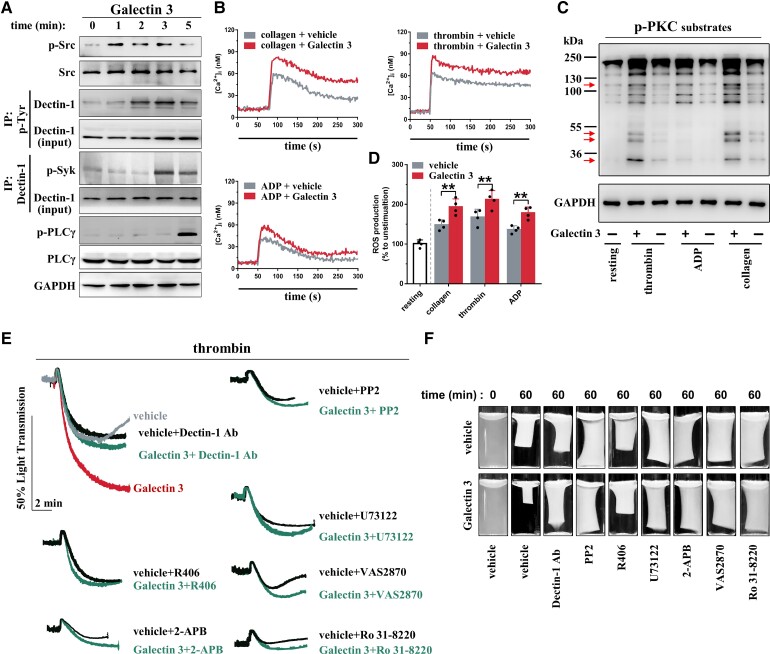
Our results thus far indicate that Galectin-3 directly enhances platelet activation and potentiates ex vivo thrombus formation. Next, we sought to elucidate the mechanisms underlying Galectin-3-mediated platelet activation. Accumulating evidence has demonstrated that Galectin-3 interacts directly with Dectin-1 [C-type lectin domain family 7 member A (CLEC7A)], which stimulates nuclear translocation of β-catenin in dendritic cells or upregulates tumour necrosis factor-α expression in macrophages.5–8 It was unclear whether platelets express Dectin-1, and we found that human platelets exhibit robust expression of Dectin-1 at both RNA and protein levels, which is comparable with that of peripheral blood mononuclear cells (PBMCs), as detected using reverse transcription polymerase chain reaction (Figure 3A and Supplementary material online, Table S2) and western blotting (Figure 3B and Supplementary material online, Figure S4). As shown in Figure 3C, Dectin-1 expression on platelets was further observed using dual-immunofluorescence staining in thrombi aspirated from the coronary arteries of STEMI patients during primary PCI. β-Glucan is a specific Dectin-1 agonist,39 and we found that it potentiated platelet aggregation and ATP release induced by different agonists in healthy WPs (Figure 3D). In contrast, laminarin, a specific Dectin-1 inhibitor, abolished the potentiating effects of β-glucan on platelet aggregation and ATP release (Figure 3D). As expected, rGalectin-3-potentiated platelet aggregation was abolished by laminarin treatment (Figure 3E).
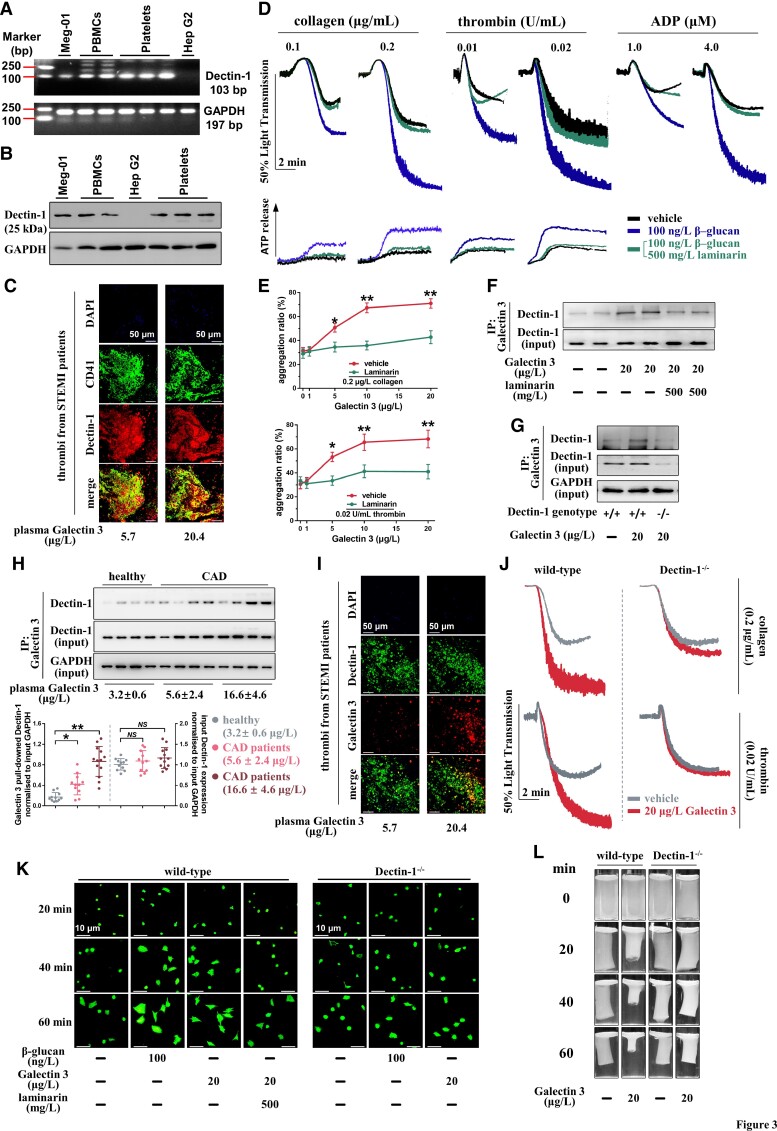
Figure 3.
Galectin-3 potentiates platelet activation by binding to platelet Dectin-1. (A) RT-PCR detection of Dectin-1 in healthy human platelets and the human megakaryoblast cell line Meg-01. (B) Western blot detection of Dectin-1 in healthy human platelets and Meg-01 cells. For (A) and (B), the human liver cell line Hep G2 was used as a negative control. Three platelet samples and two peripheral blood mononuclear cell samples were purified from different healthy blood samples. (C) Dual-immunofluorescence staining showed localization of Dectin-1 (Dectin-1+) on platelets (CD41+) within thrombi aspirated from ST-elevation myocardial infarction patients with high plasma Galectin-3 levels (10.4–22.3 μg/L; n = 5) and low plasma Galectin-3 levels (5.1–7.2 μg/L; n = 5). Scale bar = 50μm. (D) β-glucan potentiated platelet aggregation induced by collagen, thrombin, or adenosine diphosphate, as well as adenosine tri-phosphate release induced by collagen or thrombin using healthy washed platelets. Laminarin abolished the potentiating effect of β-glucan. Representative results and summary data (see Supplementary material online, Figure S5) of four experiments are presented. (E) Laminarin reversed the potentiating effect of Galectin-3 on platelet aggregation induced by collagen or thrombin using healthy washed platelets. Summary data of four experiments are presented. For (D) and (E), washed platelets from healthy subjects were pre-treated with 500 mg/L laminarin for 5 min and then treated with β-glucan (100 ng/L; 5 min) or recombinant Galectin-3 (1–20 μg/L; 5 min) before stimulation by collagen, thrombin, or adenosine diphosphate. (F) Co-immunoprecipitation shows that Galectin-3 bound to Dectin-1, which was blocked by laminarin. Representative results of at least four experiments using platelets from different healthy subjects are presented. (G) Galectin-3 bound to Dectin-1 in wild-type platelets, whereas the binding was reversed by Dectin-1 deficiency. Representative results of at least four experiments are presented. For (F) and (G), washed platelets were pre-treated with 500 mg/L laminarin for 5 min and then treated with recombinant Galectin-3 (20 μg/L) for another 5 min. (H) Binding of Galectin-3 to Dectin-1 increased in platelets from coronary artery disease patients, whereas the input Dectin-1 expression remained unchanged. The coronary artery disease patients with high plasma Galectin-3 levels (16.6 ± 4.6 μg/L) presented the highest binding of Galectin-3 to platelet Dectin-1. Representative results and summary data are presented. Each circle represents the result of a different individual from healthy subjects (n = 11), coronary artery disease patients with low plasma Galectin-3 levels (n = 11), or coronary artery disease patients with high plasma Galectin-3 levels (n = 13). (I) Co-localization of Galectin-3 and Dectin-1 was increased in thrombi from ST-elevation myocardial infarction patients with high plasma Galectin-3 levels (10.4–22.3 μg/L; n = 5) compared with those from ST-elevation myocardial infarction patients with low plasma Galectin-3 levels (5.1–7.2 μg/L; n = 5), whereas total Dectin-1 expression were similar. Scale bar = 50μm. (J) Recombinant Galectin-3 potentiated platelet aggregation induced by collagen or thrombin using platelets from wild-type mice but not from Dectin-1−/− mice. Representative results and summary data (see Supplementary material online, Figure S7) of at least four experiments are presented. (K-L) Recombinant Galectin-3 potentiated platelet spreading (K) and clot retraction (L) using platelets from wild-type mice, which was blocked by laminarin. However, Galectin-3 did not potentiate platelet spreading using platelets from Dectin-1−/− mice. Representative results and summary data (see Supplementary material online, Figure S8) of at least three experiments are presented. Statistical analyses were performed using one-way ANOVA followed by Dunnett's multiple comparison test in (H) and unpaired two-tailed Student's t-test in (L). Repeated measures one-way ANOVA followed by Sidak's multiple comparison test in (D). Two-way ANOVA followed by Sidak's multiple comparisons test was performed in (E), (J), (K), and (L). NS, no significance; *P < 0.05; **P < 0.01.
Co-immunoprecipitation assay showed that human platelet Dectin-1 was specifically pulled down with rGalectin-3 after preincubation with 20 μg/L rGalectin-3 for 5 min in WP from healthy subjects, and this binding was substantially weakened by laminarin (Figure 3F). Dectin-1 was also pulled down with rGalectin-3 in wild-type mouse platelets but not in Dectin-1−/− mouse platelets (Figure 3G). Co-immunoprecipitation assay revealed the increased binding of Galectin-3 to Dectin-1 in platelets from CAD patients compared with that in healthy subjects (Figure 3H), wherein CAD patients with high plasma Galectin-3 levels (16.6 ± 4.6 μg/L Galectin-3) presented the strongest binding. Moreover, stronger co-localization of Galectin-3 to Dectin-1 was observed in thrombi aspirated from the coronary arteries of STEMI patients with high plasma Galectin-3 levels than in those from STEMI patients with low plasma Galectin-3 levels, and the Dectin-1 expression levels (gauged by Dectin-1+ area/thrombi area) were statistically identical between the two groups (Figure 3I). When platelets were incubated with colon cancer MC38 cells, binding of tumour cell surface Galectin-3 to platelet glycoprotein VI (GPVI), another potential Galectin-3 receptor,40 was observed. However, no obvious binding of plasma Galectin-3 to GPVI was observed in platelets from CAD patients with high plasma Galectin-3 levels or healthy subjects with low plasma Galectin-3 levels. This may be due to the different conformations of plasma Galectin-3 and cell surface Galectin-3.41 Previous studies have demonstrated that conformational changes in Galectin-3 alter its interaction with receptors and the ability to participate in multivalent interactions.42,43 Moreover, the human GPVI Fc Chemira protein (hGPVI-Fc) and anti-GPVI antibody JAQ1 had no effect on rGalectin-3 potentiated human and mouse platelet aggregation in humans and mice, respectively (see Supplementary material online, Figure S6).
Next, we explored whether Dectin-1 deficiency affected platelet activation induced by Galectin-3. rGalectin-3 potentiated collagen- or thrombin-induced aggregation in wild-type platelets but not in Dectin-1−/− platelets (Figure 3J). rGalectin-3 also markedly potentiated platelet spreading in wild-type platelets (Figure 3K). This potentiating effect was Dectin-1-dependent because this effect was considerably diminished by laminarin or using Dectin-1−/− platelets. Similarly, rGalectin-3 potentiated clot retraction in wild-type platelets but not in Dectin-1−/− platelets (Figure 3L). It should be noted that Dectin-1−/− mice displayed normal blood cell parameters, including platelet count, mean platelet volume (see Supplementary material online, Table S3), and overall normal platelet morphology and bleeding potential (see Supplementary material online, Figure S9).
Galectin-3 directly activates the Dectin-1/Syk pathway to potentiate platelet activation
Upon binding to agonist ligands, Dectin-1 recruits activated Syk to mediate inflammation and anti-infection responses in white blood cells.4–6 Syk signalling has been well documented in platelet activation and thrombosis.47 Thus, we investigated whether Dectin-1/Syk signalling is involved in Galectin-3-potentiated platelet activation. Co-immunoprecipitation demonstrated that p-Syk was recruited to Dectin-1 at 3 min after rGalectin-3 stimulation (Figure 4A). Dectin-1 was phosphorylated with a peak phosphorylation apparently earlier than p-Syk recruitment, which agrees with a previous study in white blood cells,48 indicating that Syk signalling is activated downstream of Dectin-1 in Galectin-3-treated platelets. Src phosphorylation occurred earlier than Dectin-1 phosphorylation, which is consistent with the previous reports that Dectin-1 is tyrosine-phosphorylated in the hem-ITAM motif by p-Src upon agonist stimulation.45,49 Phospholipase γ (PLCγ), downstream of Syk, was phosphorylated after p-Syk recruitment.
Figure 4.
Dectin-1/Syk mediates the potentiating effects of Galectin-3 on platelet activation. (A) Galectin-3 enhanced phosphorylation of Src, Dectin-1, and PLCγ, as well as increased binding of p-Syk to Dectin-1 in human platelets. Washed platelets from healthy subjects were pre-treated with recombinant Galectin-3 (20 μg/L) for different times, as indicated. Representative results and summary data (see Supplementary material online, Figure S10A) of four experiments are presented. (B) Galectin-3 increased Ca2+ influx induced by different agonists. Washed platelets from healthy subjects loaded with Fura-2 were incubated with recombinant Galectin-3 (20 μg/L) for 5 min and measured for 300 s with collagen (0.2 μg/mL), thrombin (0.02 U/mL), or adenosine diphosphate (4.0 μM). Representative results and summary data (see Supplementary material online, Figure S10B) of four experiments are presented. (C) Galectin-3 increased p-PKC substrates induced by different agonists. Washed platelets from healthy subjects were pre-treated with recombinant Galectin-3 (20 μg/L) for 5 min and then stimulated with collagen (0.2 μg/mL), thrombin (0.02 U/mL), or adenosine diphosphate (4.0 μM) for 5 min. Red arrows mark the specific bands of p-PKC substrates. Representative results and summary data (see Supplementary material online, Figure S10C) of four experiments are presented. (D) Galectin-3 increased ROS production induced by different agonists. Washed platelets from healthy subjects loaded with DCFDA (6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate) were incubated with recombinant Galectin-3 (20 μg/L) for 5 min and then stimulated with collagen (0.2 μg/mL), thrombin (0.02 U/mL), or adenosine diphosphate (4.0 μM) for 5 min. The DCF fluorescence intensity was measured, and the levels of ROS were standardized to the percentage observed in the resting platelets. Summary data of four experiments are presented. (E-F) Galectin-3-potentiated platelet aggregation and clot reraction were abolished by inhibitors targeting Dectin-1/Syk signalling downstream of Galectin-3. Washed platelets from healthy subjects were pre-treated with 10 μg/mL Dectin-1 Ab (Dectin-1 blocking antibody), 10 μM PP2 (Src inhibitor), 1 μM R406 (Syk inhibitor), 10 μM U73122 (PLCγ inhibitor), 50 μM 2-APB (Ca2+ inhibitor), 10 μM Ro 31-8220 (PKC inhibitor) or 10 μM VAS2870 (ROS inhibitor) for 10 min and then treated with 20 μg/L recombinant Galectin-3 or vehicle for 5 min. Platelet aggregation was initiated by 0.02 U/mL thrombin, and clot retraction was initiated by 1.0 U/mL thrombin. Representative results and summary data (see Supplementary material online, Figure S12) of four experiments are presented. Statistical analyses were performed using one-way ANOVA followed by Dunnett's multiple comparison test in (A) and Sidak's multiple comparison test in (F), and two-way ANOVA followed by Sidak's multiple comparisons test in (B), (C), and (D). Repeated measures one-way ANOVA followed by Sidak's multiple comparison test was performed in (E). NS, no significance; *P < 0.05; **P < 0.01.
Dectin-1/Syk signalling activates PLCγ which subsequently increases Ca2+ influx and protein kinase C (PKC) phosphorylation, as well as enhances reactive oxygen species (ROS) production in immune cells.44,50,51 Ca2+ influx, PKC phosphorylation, and ROS production act synergistically with the downstream signalling of platelet agonists to potentiate platelet activation.2–4 Consistently, rGalectin-3 increased Ca2+ influx (Figure 4B), PKC phosphorylation (Figure 4C), and ROS production (Figure 4D) stimulated with different agonists.
Similar results were also observed in mouse platelets. rGalectin-3 stimulated the phosphorylation of Src, Dectin-1, and PLCγ, as well as increased the recruitment of p-Syk to Dectin-1 in wild-type platelets but not in Dectin-1−/− platelets (see Supplementary material online, Figure S11). rGalectin-3 also increased Ca2+ influx, PKC phosphorylation, and ROS production in wild-type platelets but not in Dectin-1−/− platelets, under stimulation with different agonists.
Inhibition of Dectin-1, Src, Syk, PLCγ, Ca2+ influx, PKC phosphorylation, and ROS production with the Dectin-1 blocking antibody, PP2, R406, U73122, 2-APB, Ro 31-8220, and VAS2870, respectively, almost abolished the potentiating effects of rGalectin-3 on thrombin-induced platelet aggregation (Figure 4E) and clot retraction (Figure 4F). These data directly indicate that Galectin-3 binds to Dectin-1 and activates Dectin-1/Src/Syk/(PLCγ/Ca2+ + PKC and ROS) signalling to potentiate platelet activation.
Galectin-3 potentiates in vivo thrombosis potential by binding to platelet Dectin-1
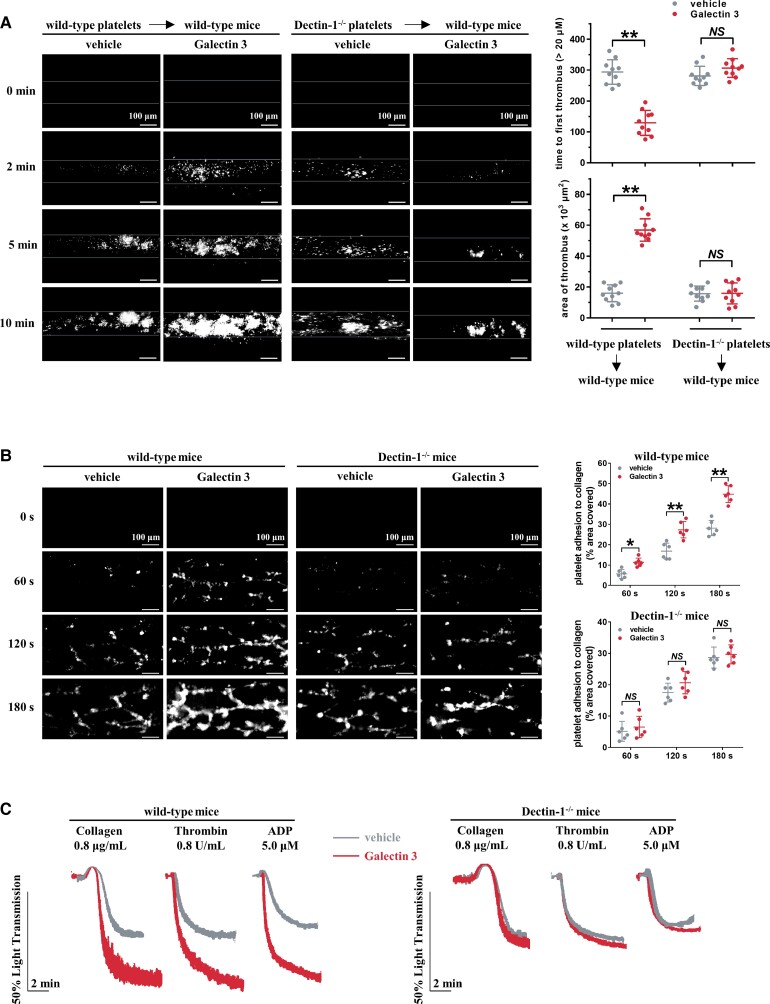
We then tested whether Galectin-3-potentiated in vivo thrombus formation was dependent on platelet Dectin-1. Approximately, 109 platelets from wild-type or Dectin-1−/− mice were injected into thrombocytopenic wild-type mice to eliminate non-platelet haemostatic or thrombotic factor variations. rGalectin-3 (10 μg/kg) was intravenously injected into mice to increase their plasma Galectin-3 level (see Supplementary material online, Figure S13) and then FeCl3-induced thrombus formation was examined in the mesenteric arterioles at different time points (Figure 5A). Notably, rGalectin-3 potentiated thrombus formation in wild-type mice transfused with wild-type platelets but had no effect in wild-type mice transfused with Dectin-1−/− platelets.
Figure 5.
Galectin-3-potentiated in vivo thrombosis potential is dependent on platelet Dectin-1. (A) After intravenous injection of 10 μg/kg recombinant Galectin-3 or vehicle (saline) into mice, FeCl3-induced arterial thrombus formation was initiated, and the thrombus area was recorded. Representative image at different time points are shown. Statistical analysis of thrombosis assessed using time to first thrombus (>20 μm) and area of thrombus at 10 min (n = 10) are shown. Scale bar = 100 μm. (B) Whole blood from Galectin-3-treated wild-type mice showed accelerated thrombus formation over an immobilized collagen surface at a shear rate of 1000 s−1, whereas whole blood from Galectin-3-treated Dectin-1−/− mice did not. After intravenous injection of 10 μg/kg recombinant Galectin-3 or vehicle into mice, whole blood was fluorescently tagged with FITC-labelled anti-CD41 antibody and then perfused through fibrillar collagen-coated bioflux plates. Representative images at the indicated time points are presented. Scale bar = 100 μm. (C) Platelet-rich plasma from Galectin-3-treated wild-type mice presented increased platelet aggregation; whereas platelet-rich plasma from Galectin-3-treated Dectin-1−/− mice did not. Representative results and summary data (see Supplementary material online, Figure S14) are presented from four experiments. Statistical analyses were performed using two-way ANOVA followed by Sidak's multiple comparisons test. NS, no significance; *P < 0.05; **P < 0.01.
The role of Dectin-1 in Galectin-3-potentiated ex vivo thrombus formation under arterial flow conditions was assessed using a microfluidic whole-blood perfusion assay. Whole blood from wild-type but not Dectin-1−/− mice formed significantly larger thrombus, when pre-treated with rGalectin-3 than without rGalectin-3 pre-treatment (Figure 5B). In addition, increased ex vivo platelet aggregation was observed in PRP from rGalectin-3-treated wild-type mice but not in those from rGalectin-3-treated Dectin-1−/− mice (Figure 5C).
Galectin-3 inhibitor TD139 concentration dependently suppresses Galectin-3-induced platelet hyperreactivity
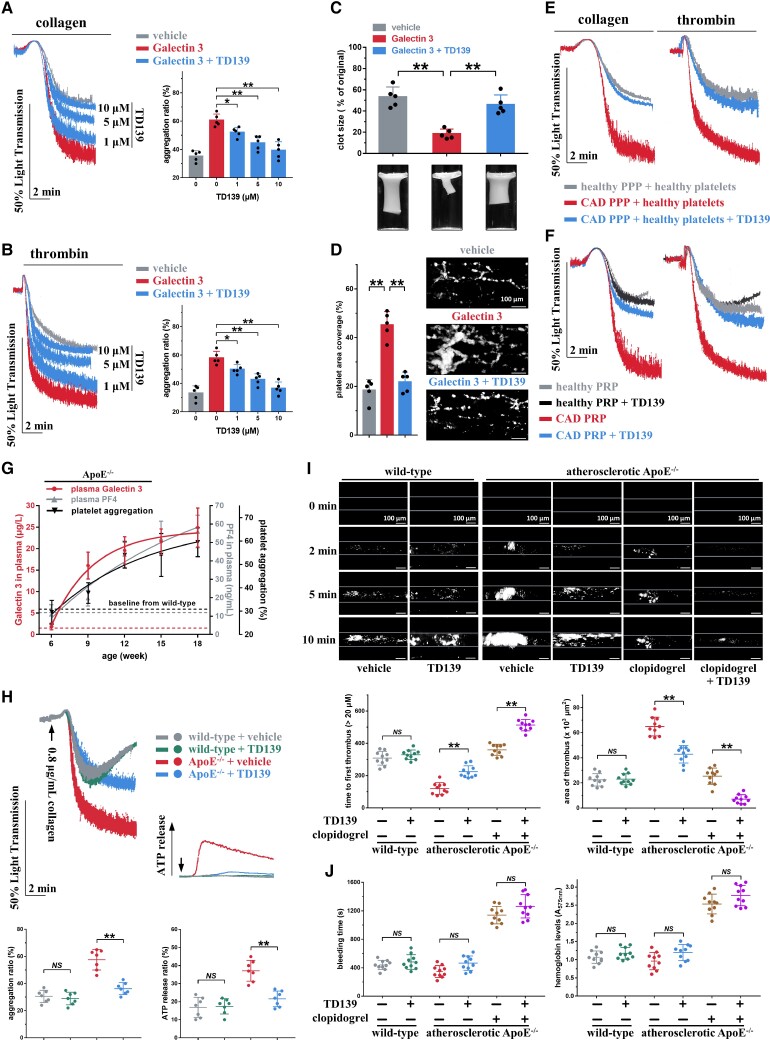
Considering our results that Galectin-3 directly potentiated platelet activation in vitro and thrombus formation in vivo, we sought to assess the potential applications of Galectin-3 inhibitors. TD139 is a novel small-molecule inhibitor of Galectin-3 currently in a Phase II clinical trial of idiopathic pulmonary fibrosis (NCT03832946),0–2 which reverses primary alveolar epithelial cell injury induced by 10μM Galectin-3 in vitro.55,56 WP from healthy subjects was preincubated with TD139 and rGalectin-3 for 5 min and then stimulated with collagen or thrombin. In the range of 1–10 μM, TD139 inhibited the potentiating effects of rGalectin-3 on platelet aggregation in a concentration-dependent manner (Figure 6A and B). Similarly, the promoted clot retraction (Figure 6C) and ex vivo thrombus formation over the immobilized collagen surface (Figure 6D) elicited by rGalectin-3 were also prevented by TD139. We next resuspended healthy WP in platelet-poor plasma (PPP) from healthy subjects with low plasma Galectin-3 levels (3.1 ± 0.5 μg/L) or in PPP from CAD patients with high plasma Galectin-3 levels (16.4 ± 3.1 μg/L), in the presence or absence of TD139. Plasma with high Galectin-3 levels significantly enhanced platelet aggregation, and the enhancing effect was markedly reversed by TD139 (Figure 6E). In addition, TD139 significantly suppressed platelet aggregation in PRP from CAD patients but not in PRP from healthy subjects (Figure 6F).
Figure 6.
TD139 inhibits the increased platelet aggregation induced by Galectin-3 and suppresses in vivo thrombosis potential in atherosclerotic ApoE−/− mice. (A and B) TD139, in a concentration-dependent manner, inhibited platelet aggregation induced by Galectin-3. Washed platelets from healthy subjects were pre-treated with TD139 (0–10 μM) and recombinant Galectin-3 (20 μg/L) for 5 min and then stimulated with collagen (0.2 μg/mL) or thrombin (0.02 U/mL). Representative results and summary data are presented (n = 5). (C) TD139 (10 μM, 5 min) suppressed the increased clot retraction induced by Galectin-3 using healthy washed platelets. Representative results and summary data are presented (n = 5). (D) TD139 (10 μM, 5 min) decreased thrombus formation induced by recombinant Galectin-3 (20 μg/L, 30 min) in whole blood from healthy subjects over an immobilized collagen surface at a shear rate of 1000 s−1. Representative results and summary data are presented (n = 5). Scale bar = 100 μm. (E) Healthy washed platelets presented enhanced platelet aggregation after preincubation with platelet-poor plasma from coronary artery disease patients with high plasma Galectin-3 levels, while the enhanced platelet aggregation was suppressed by TD139. Platelet-poor plasma from coronary artery disease patients (16.4 ± 3.1 μg/L plasma Galectin-3; n = 8) or healthy subjects (3.1 ± 0.5 μg/L plasma Galectin-3; n = 10) were prepared. Platelets from healthy subjects were resuspended within platelet-poor plasma for 10 min and then incubated with 10 μM TD139 for 5 min. Platelet aggregation was initiated by collagen (0.8 μg/mL) or thrombin (0.8 U/mL). Representative results and summary data (see Supplementary material online, Figure S15) are presented. (F) TD139 (10 μM, 30 min) inhibited platelet aggregation induced by collagen (0.8 μg/mL) or thrombin (0.8 U/mL) in platelet-rich plasma from coronary artery disease patients (17.6 ± 3.5 μg/L plasma Galectin-3; n = 8) without antiplatelet therapy but not in platelet-rich plasma from healthy subjects (3.3 ± 0.4 μg/L Galectin-3; n = 10). Representative results and summary data (see Supplementary material online, Figure S16) are presented. (G) Age-dependent increase in plasma Galectin-3 concentration, plasma PF4 concentration, and collagen-induced platelet aggregation in ApoE−/− mice at 6 weeks of age fed high-fat diet for 12 weeks was observed. Platelet-rich plasma was stimulated with 0.8 μg/mL collagen.Summary data are presented (n = 4, each time point). (H) TD139 inhibited ex vivo platelet aggregation and adenosine tri-phosphate release in platelet-rich plasma of atherosclerotic ApoE−/− mice but not in wild-type mice. Representative results and summary data are presented (n = 7). (I) TD139 suppressed FeCl3-induced thrombus formation in atherosclerotic ApoE−/− mice but not in wild-type mice. Thrombus formation in atherosclerotic ApoE−/− mice was inhibited more significantly by TD139 plus clopidogrel than by TD139 or clopidogrel alone. Representative results and summary data are presented (n = 10). Scale bar = 100μm. (J) TD139 had no effect on bleeding potential, as monitored by bleeding time and haemoglobin loss in atherosclerotic ApoE−/− mice and wild-type mice. TD139 did not increase the bleeding time and haemoglobin loss when combined with clopidogrel. The bleeding time and blood loss were measured (n = 10). For (H), (I), and (J), after 12 weeks of high-fat diet treatment, ApoE−/− mice randomly received vehicle or TD139 (15 mg/kg, i.p., twice at 12-h intervals) or clopidogrel (10 mg/kg, p.o., single dose), or TD139 (15 mg/kg, i.p., twice at 12-h intervals) plus clopidogrel (10 mg/kg, p.o., single dose). The subsequent experiments of 0.8 μg/mL collagen-induced platelet aggregation (H), FeCl3-induced thrombosis (I), and bleeding (J) were carried out at 30 min after the second TD139 administration or at 4 h after clopidogrel administration. Statistical analyses were performed using one-way ANOVA followed by Sidak's multiple comparison test in (B)–(J), and repeated measures one-way ANOVA followed by Sidak's multiple comparison test in (A). NS, no significance; *P < 0.05; **P < 0.01.
Age-dependent increase of plasma Galectin-3 levels in atherosclerotic ApoE−/− mice
ApoE−/− male mice at 6 weeks of age were fed a high-fat diet for 12 weeks, and plasma Galectin-3 level was monitored every 3 weeks. Wild-type mice fed chow diet for 12 weeks served as healthy negative controls. Intriguingly, plasma Galectin-3 concentration gradually increased after 6 weeks of age in ApoE−/− mice, whereas plasma Galectin-3 concentration in wild-type mice did not change significantly until 18 weeks of age (Figure 6G). In line with the change in plasma Galectin-3 levels, the plasma concentration of PF4, a specific platelet activation biomarker,57 and collagen-induced platelet aggregation also increased in ApoE−/− mice, and they were both correlated with the plasma Galectin-3 concentration (see Supplementary material online, Figure S17). After 12 weeks of high-fat diet treatment, the plasma Galectin-3 concentration in atherosclerotic ApoE−/− mice was similar to the pathological concentration in patients, almost 13 times higher than that in wild-type mice (see Supplementary material online, Figure S18; 22.1 ± 4.1 μg/L vs. 1.5 ± 0.8 μg/L Galectin-3 in ApoE−/− mice vs. wild-type mice, P < 0.01, n = 7 per group).
TD139 ameliorates in vivo thrombosis potential in atherosclerotic ApoE−/− mice without causing excessive bleeding
We next evaluated the antithrombotic activity of TD139 in atherosclerotic ApoE−/− mice. Atherosclerotic ApoE−/− mice (ApoE−/− mice fed a high-fat diet for 12 weeks) received two injections of TD139 (15 mg/kg, i.p.) at 12 h intervals, and then ex vivo platelet aggregation and in vivo FeCl3-induced thrombus formation were assessed at 30 min after the second TD139 injection. TD139 effectively suppressed platelet aggregation and ATP release in atherosclerotic ApoE−/− mice (Figure 6H) but not in wild-type mice. As shown in Figure 6I, occlusion occurred in all untreated atherosclerotic ApoE−/− mice within 10 min after FeCl3 injury (occlusion time was 389 ± 41 s, n = 10), whereas no occlusion occurred in untreated wild-type mice. TD139 had no effect on thrombus formation in wild-type mice; however, TD139 inhibited thrombus formation and prevented occlusion in all atherosclerotic ApoE−/− mice within 10 min after FeCl3 injury. Further analysis revealed that TD139 markedly prolonged the time to first thrombus (224 ± 37 vs. 120 ± 35 s in TD139 vs. vehicle, P < 0.01, n = 10 per group) and considerably reduced the area of thrombus at 10 min after FeCl3 injury (42.9 ± 7.0 vs. 64.9 ± 7.5 × 103 μm2, P < 0.01, n = 10 per group) in atherosclerotic ApoE−/− mice. Clopidogrel (10 mg/kg, p.o., single dose), a current standard-of-care antiplatelet drug, also prolonged the time to first thrombus mice (360 ± 34 s) and decreased the area of thrombus (25.4 ± 6.4 × 103μm2) in atherosclerotic ApoE−/− mice. We further found that the combination of TD139 and clopidogrel exhibited superior antithrombotic effect (512 ± 35 s of the time to first thrombus and 7.1 ± 3.5 × 103 μm2 of the area of thrombus) over clopidogrel or TD139 alone, suggesting a likely synergism between TD139 and clopidogrel in antithrombotic efficacy.
At the same dose used for the in vivo antithrombotic study (Figure 6I), TD139 had almost no effect on bleeding regardless of whether the mice were atherosclerotic ApoE−/− or wild-type (Figure 6J). In contrast, clopidogrel substantially increased bleeding time and haemoglobin loss. Furthermore, the bleeding time and haemoglobin loss in mice treated with TD139 combined with clopidogrel were similar to those in mice treated with clopidogrel alone, again highlighting the benefit of TD139 in minimizing bleeding risk.
Taken together, these data indicate that TD139 may be potent in inhibiting thrombosis without causing excessive bleeding in atherosclerotic ApoE−/− mice.
TD139 alleviates myocardial ischaemia–reperfusion injury in atherosclerotic ApoE−/− mice
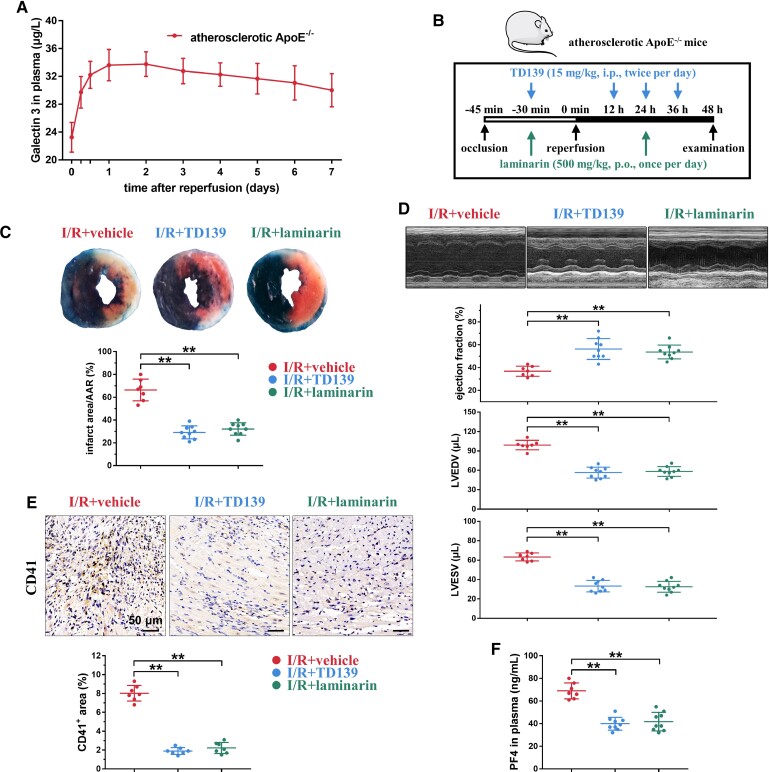
Reperfusion of ischaemic heart tissues may cause myocardial I/R injury, where platelets are activated and infiltrated into tissues, leading to the infarct expansion and a continued decline in heart function.58,59 We next investigated the effects of TD139 in a myocardial I/R injury model using atherosclerotic ApoE−/− mice after 12 weeks of high-fat diet feeding. A severe myocardial ischaemia was induced by 45 min temporary ligation of the left anterior descending coronary artery, followed by reperfusion. Plasma Galectin-3 levels increased urgently within 24 h after surgery and were maintained at a high level over 7 days (Figure 7A), which is consistent with a previous report that plasma Galectin-3 levels increased in patients when myocardial ischaemia occurs.60 TD139 (15 mg/kg, i.p., twice per day) was administered within 2 days at 12 h intervals, wherein the first administration was performed at 30 min before reperfusion (Figure 7B). TD139 treatment markedly decreased the infarct area/area at risk (AAR) ratio in atherosclerotic ApoE−/− mice compared with that in the vehicle control (Figure 7C and Supplementary material online, Figure S19). Consistently, TD139 also improved heart function, including ejection fraction and left ventricular volume indices (left ventricular end-diastolic volume, and left ventricular end-systolic volume), in atherosclerotic ApoE−/− mice (Figure 7D). Immunohistochemistry demonstrated that myocardial I/R-induced microthrombi in the cardiac tissue of atherosclerotic ApoE−/− mice were reduced by TD139 (Figure 7E). The plasma PF4 concentration, an indicator of platelet activation, was consistently reduced (Figure 7F).
Figure 7.
TD139 improves myocardial ischaemia–reperfusion injury by suppressing thrombosis and inflammation in atherosclerotic ApoE−/− mice. (A) Time-dependent increase in plasma Galectin-3 levels over 7 days after myocardial ischaemia–reperfusion (I/R) surgery in atherosclerotic ApoE−/− mice. Summary data are presented (n = 4, each time point). (B) Schematic protocol of TD139 treatment in myocardial I/R study using atherosclerotic ApoE−/− mice and wild-type mice. (C) TD139 and laminarin markedly decreased the infarct area/AAR ratio. The blue area, which was stained by Evans blue, indicates the non-infarct area, while the area not stained by Evans blue represents the AAR. The red area shows viable myocardium, which can be stained by TTC, and the white area indicates the infarct area. Representative results (Evans blue/TTC stain) of left ventricular tissue sections and summary data are presented (n = 7–9 per group). (D) TD139 and laminarin improved heart function, including ejection fraction (E-F) and left ventricular volume indices [left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV)]. Representative M-mode echocardiograms and summary data are presented (n = 7–9 per group). (E) Both TD139 and laminarin decreased myocardial I/R injury-induced microthrombi in the cardiac tissue. The representative results and summary data were presented (n = 7). (F) Both TD139 and laminarin decreased plasma PF4 concentrations after myocardial I/R injury. Summary data are presented (n = 7–9 per group). Statistical analyses were performed using one-way ANOVA followed by Dunnett's multiple comparison test in (C)–(F). **P < 0.01.
Discussion
Platelet hyperreactivity contributes to an increased prevalence of cardiovascular disease. Elevated plasma Galectin-3 level has been shown to participate in cardiovascular disease by directly enhancing endothelial cell injury, cardiac remodelling and dysfunction, and monocyte migration.2–7 However, the direct effect of plasma Galectin-3 on platelet function has not yet reported. In this study, we demonstrated that (i) elevated plasma Galectin-3 directly enhances platelet activation and thrombus formation, contributing to platelet hyperreactivity in CAD; (ii) Galectin-3 binds to platelet Dectin-1 to activate Dectin-1/Syk signalling; (iii) the Galectin-3-specific inhibitor TD139 concentration dependently suppresses Galectin-3-induced platelet hyperreactivity and thrombus formation in atherosclerotic ApoE−/− mice and synergistically enhances the antithrombotic effects of clopidogrel while minimizing the adverse effects of haemorrhage; (iv) TD139 suppresses microvascular thrombosis and improves cardiac function in a myocardial I/R injury model of atherosclerotic ApoE−/− mice. Collectively, our results indicate that elevated plasma Galectin-3 levels directly contribute to platelet hyperactivity and in vivo thrombosis and aggravate I/R-induced cardiac injury. Based on these findings, Galectin-3 inhibitors may serve as a novel antiplatelet approach for diminishing thrombosis and cardiovascular complications in CAD patients with elevated plasma Galectin-3 levels (Structured Graphical Abstract, Figure 8).
Figure 8.
Working model for plasma Galectin-3 directly potentiating platelet activation, and the platelet hyperreactivity suppressing effect of Galectin-3 inhibitor TD139 in coronary artery disease. Accumulated Galectin-3 in plasma directly activates Dectin-1 receptor to potentiate platelet activation (platelet aggregation and adenosine tri-phosphate release), which enhances in vivo thrombosis and myocardial ischaemia–reperfusion injury. Galectin-3 inhibitor TD139 specifically inhibits Galectin-3-induced platelet hyperreactivity to ameliorate atherothrombosis and myocardial infarction in coronary artery disease.
Recently, emerging evidence has demonstrated that plasma Galectin-3 is closely correlated with the severity of CAD and may act as an independent predictor of future cardiovascular events. Plasma Galectin-3 level is significantly higher in STEMI patients than in healthy control subjects, and STEMI patients with higher Galectin-3 levels have a steeper decline in left ventricular ejection fraction.61 Patients with unstable angina have higher plasma Galectin-3 levels than those with stable angina; CAD patients with three-vessel disease have higher levels of Galectin-3 than patients with one- or two-vessel disease,16 and cardiovascular deaths occur more commonly in CAD patients with high Galectin-3 levels.17 Plasma Galectin-3 levels are also correlated with carotid intima-media thickness of atherosclerosis and are independently associated with increased cardiovascular events in patients with peripheral artery disease.19 A prospective study revealed that high levels of plasma Galectin-3 can independently predict the reoccurrence of CAD after MI during 3 years of follow-up.15 Another retrospective study by Ghorbani et al.18 also showed that an increase in plasma Galectin-3 level among community-dwelling individuals predicted cardiovascular events in the Framingham Heart Study Offspring cohort. In addition, increased plasma Galectin-3 levels were reported to be associated with a decrease in left ventricular ejection fraction or an increase in infarct size from acute coronary syndrome patients,2–4 and animal studies revealed that intrapericardial injection of Galectin-3 enhanced cardiac interstitial and perivascular fibrosis and cardiac hypertrophy in rats.65 Our study further expands these findings and establishes a connection between Galectin-3 and platelet hyperreactivity, showing that Galectin-3 directly potentiates platelet activation to enhance in vivo thrombosis and MI injury, providing mechanistic insights supporting the use of Galectin-3 as an independent pathological biomarker of CAD.
In this study, we found that plasma Galectin-3 levels gradually increased with the development of atherosclerosis in ApoE−/− mice, corroborating previous reports of Galectin-3 level up-regulation in macrophages from murine atherosclerotic plaques.25,26,66 In agreement with previous studies that showed that Galectin-3 is overexpressed in murine cardiomyocytes after MI,60,67 we found that plasma Galectin-3 level increased sharply after myocardial I/R injury surgery in ApoE−/− mice. Using an in vivo FeCl3-induced thrombosis model, we found that Galectin-3 enhanced thrombus formation. A previous study found that Galectin-3 deficiency decreased venous thrombus formation in mice;29 however, the effects of Galectin-3 deficiency on arterial thrombus formation need to be further investigated. In addition, plasma Galectin-3 levels were positively correlated with platelet aggregation rates and plasma PF4 levels in ApoE−/− mice. Furthermore, there was a positive correlation between Galectin-3 levels and platelet hyperreactivity in 182 patients with CAD. These results underscore the potential clinical significance of Galectin-3, which may contribute to the increased prevalence of arterial thrombotic events in CAD.
CLEC2, a C-type lectin-like receptor, is present on platelets and is capable of directly activating platelets upon ligand binding.68 Although as another C-type lectin-like pattern recognition receptor, Dectin-1, has been well investigated in immune cells where it stimulates phagocytosis and production of inflammatory cytokines,69,70 its expression and function in platelets have never been reported. We found that platelets expressed robust Dectin-1, instead of directly activating platelets, the Dectin-1 agonist potentiated platelet function. Previously, Leclaire et al.36 reported that Galectin-3 recognizes and activates Dectin-1 via interaction between the carbohydrate recognition domain of Galectin-3 and N-glycan structures of Dectin-1. The binding of Galectin-3 onto Dectin-1 increased in immune cells and triggered proinflammatory response to pathogenic fungi and immunological tolerance.35,37 Interestingly, Fan et al.71 recently revealed that the Dectin-1-mediated immune response contributes to myocardial I/R injury; however, the endogenous agonist ligand of Dectin-1 is not clear. We found that plasma Galectin-3 bound to Dectin-1 in platelets from CAD patients. Galectin-3 stimulated Dectin-1 phosphorylation and activation, and hence potentiated platelet activation and microthrombus formation involved in MI development.
Syk signalling plays an important role in platelet activation and thrombus formation.72,73 We found that after Galectin-3 stimulation, Dectin-1 was phosphorylated earlier than p-Syk binding, which is consistent with previous reports that the immunoreceptor tyrosine-based activation motif (ITAM) motif of Dectin-1 is phosphorylated before recruiting and activating Syk.44,49 In addition, Src was phosphorylated earlier than Dectin-1, supporting that the ITAM motif of Dectin-1 is typically tyrosine-phosphorylated by p-Src.74 Previous studies have shown that Dectin-1/Syk signalling potentiates Ca2+ influx, PKC activation, and ROS production involved in cytokine production,50 antifungal immune defense,51 and macrophage chemotaxis,75 respectively. We consistently found that Galectin-3 increased Ca2+ influx, PKC phosphorylation, and ROS production in platelets, and the inhibition of Ca2+ influx, PKC phosphorylation, or ROS production abolished the potentiating effects of Galectin-3 on platelet aggregation, indicating that Ca2+ influx, PKC phosphorylation, or ROS production may be independent or synergistical to activate platelets after Galectin-3 stimulation. During acute MI, Syk activation serves as a prodromal sensor of pathophysiological arterial shear stress to potentiate thrombus formation and aggravate myocardial I/R surgery.76 Accordingly, we found that Galectin-3-activated platelet Dectin-1/Syk signalling pathways aggravated ex vivo shear stress-induced thrombus formation and in vivo arterial thrombus formation, suggesting that the Galectin-3 inhibitor may be considered to ameliorate I/R-induced cardiac injury by inhibiting platelet activation and suppressing thrombus formation.
Galectin-3 has been demonstrated to perform multiple functions, including phagocytosis77 and cell growth and proliferation,78 adhesion,79 and apoptosis80 in the pathogenesis of fibrosis in different tissues. As a potent Galectin-3-specific inhibitor, TD139 is currently being evaluated in a Phase II clinical trial (NCT03832946) for idiopathic pulmonary fibrosis.0–2 We demonstrated that TD139 inhibited Galectin-3-potentiated platelet activation in a concentration-dependent manner and suppressed thrombus formation. In agreement with the results of no bleeding side effects of TD139 reported in clinical trials, we demonstrated that TD139, which targets Galectin-3/Dectin-1/Syk signalling, does not cause apparent bleeding risk. Syk signalling seems to be dispensable for physiological haemostasis, and several Syk signalling inhibitors have been shown to protect against arterial thrombosis without increasing bleeding.1–3 However, the molecular mechanism underlying this effect needs to be further investigated.
Platelet hyperreactivity is a crucial factor that aggravates MI, which increases abnormal thrombosis formation and contributes to ongoing pathological processes, including microvascular obstruction and MI expansion. Our results further demonstrated that TD139 suppressed microvascular thrombosis in reperfused cardiac tissue and restored cardiac function in the myocardial I/R model of atherosclerotic ApoE−/− mice. It is important to note that Galectin-3 can also potentiate monocyte/macrophage chemotaxis, activate matrix-producing fibroblasts, and exacerbate endothelial cell injury, which can contribute to cardiac remodelling and dysfunction,2–4 suggesting that the Galectin-3 inhibitor TD139 may suppress Galectin-3 signalling in different cells to protect against myocardial I/R injury. These findings may explain the potent protective effect of TD139, and more basic research and additional tests are necessary to ensure an accurate depiction of the Galectin-3 inhibitor in MI. Dectin-1 inhibitor laminarin also presented multiple protective effects against myocardial I/R injury.4–6 However, the difference between the protective effect of Galectin-3 inhibition and Dectin-1 inhibition against myocardial I/R injury needs to be further investigated.
In conclusion, Galectin-3, which is increased in the plasma of CAD patients, potentiates platelet activation and thrombus formation and therefore exacerbates cardiac function deterioration and MI expansion (Figure 8). Our study also proposes a novel signalling pathway, Dectin-1/Src/Syk/(PLCγ/Ca2+ + PKC and ROS), for platelet activation, which may explain the platelet hyperreactivity in CAD patients with elevated plasma Galectin-3 levels. Our findings suggest a new potential target for antiplatelet treatment and introduce the Galectin-3 inhibitor TD139, as a potent antiplatelet agent, to prevent and treat platelet hyperactivity and thrombotic complications without causing apparent bleeding.
Supplementary Material
Acknowledgements
We thank all patients who participated in our study. We thank all the members of our team for their critical input and suggestions. We acknowledge Dr Christopher ‘Kit’ Bonin and Dr Geneva Hargis from UConn Health School of Medicine for their help in scientific writing and editing of this manuscript.
Contributor Information
Yufei Chen, Academy of Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; Department of Cardiology, Huashan Hospital, Fudan University, Shanghai, China.
Wanrong Fu, Cardiovascular Institute of Zhengzhou University, Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Yunbo Zheng, Cardiovascular Institute of Zhengzhou University, Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Jing Yang, Cardiovascular Institute of Zhengzhou University, Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Yangyang Liu, Cardiovascular Institute of Zhengzhou University, Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Zhiyong Qi, Department of Biochemistry and Molecular Biology, NHC Key Laboratory of Glycoconjugates Research, School of Basic Medical Sciences, Fudan University, Shanghai 200032, China.
Meiling Wu, Department of Biochemistry and Molecular Biology, NHC Key Laboratory of Glycoconjugates Research, School of Basic Medical Sciences, Fudan University, Shanghai 200032, China.
Zhichao Fan, Department of Immunology, School of Medicine, UConn Health, Farmington, CT, USA.
Kanhua Yin, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Yunfeng Chen, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA, USA.
Wen Gao, Department of Cardiology, Huashan Hospital, Fudan University, Shanghai, China.
Zhongren Ding, Cardiovascular Institute of Zhengzhou University, Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Jianzeng Dong, Cardiovascular Institute of Zhengzhou University, Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Qi Li, Academy of Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Si Zhang, Department of Biochemistry and Molecular Biology, NHC Key Laboratory of Glycoconjugates Research, School of Basic Medical Sciences, Fudan University, Shanghai 200032, China.
Liang Hu, Academy of Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; Cardiovascular Institute of Zhengzhou University, Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Natural Science Foundation of China to L.H. (81970305, 81803522), S.Z. (81770137, 81573423), and W.G. (81800305).
References
- 1. Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482–2494. [DOI] [PubMed] [Google Scholar]
- 2. Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013;62:2261–2273. [DOI] [PubMed] [Google Scholar]
- 3. Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J 2017;38:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. Effects of belapectin, an inhibitor of Galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology 2020;158:1334–1345.e5. [DOI] [PubMed] [Google Scholar]
- 5. Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med 1990;322:1549–1554. [DOI] [PubMed] [Google Scholar]
- 6. Szummer K, Jernberg T, Wallentin L. From early pharmacology to recent pharmacology interventions in acute coronary syndromes: JACC state-of-the-art review. J Am Coll Cardiol 2019;74:1618–1636. [DOI] [PubMed] [Google Scholar]
- 7. van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol 2019;16:166–179. [DOI] [PubMed] [Google Scholar]
- 8. Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost 2009;102:248–257. [DOI] [PubMed] [Google Scholar]
- 9. Henderson NC, Sethi T. The regulation of inflammation by Galectin-3. Immunol Rev 2009;230:160–171. [DOI] [PubMed] [Google Scholar]
- 10. de Couto G, Ouzounian M, Liu PP. Early detection of myocardial dysfunction and heart failure. Nat Rev Cardiol 2010;7:334–344. [DOI] [PubMed] [Google Scholar]
- 11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm Met al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 12. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 2017;135:e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 13. Yin QS, Shi B, Dong L, Bi L. Comparative study of Galectin-3 and B-type natriuretic peptide as biomarkers for the diagnosis of heart failure. J Geriatr Cardiol 2014;11:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 15. Lisowska A, Knapp M, Tycinska A, Motybel E, Kaminski K, Swiecki P, et al. Predictive value of Galectin-3 for the occurrence of coronary artery disease and prognosis after myocardial infarction and its association with carotid IMT values in these patients: a mid-term prospective cohort study. Atherosclerosis 2016;246:309–317. [DOI] [PubMed] [Google Scholar]
- 16. Falcone C, Lucibello S, Mazzucchelli I, Bozzini S, D’Angelo A, Schirinzi S, et al. Galectin-3 plasma levels and coronary artery disease: a new possible biomarker of acute coronary syndrome. Int J Immunopathol Pharmacol 2011;24:905–913. [DOI] [PubMed] [Google Scholar]
- 17. Maiolino G, Rossitto G, Pedon L, Cesari M, Frigo AC, Azzolini M, et al. Galectin-3 predicts long-term cardiovascular death in high-risk patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2015;35:725–732. [DOI] [PubMed] [Google Scholar]
- 18. Ghorbani A, Bhambhani V, Christenson RH, Meijers WC, de Boer RA, Levy D, et al. Longitudinal change in Galectin-3 and incident cardiovascular outcomes. J Am Coll Cardiol 2018;72:3246–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, Benito-Martin A, Burillo E, Zalba G, et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc 2014;3:e000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Venkatraman A, Hardas S, Patel N, Singh Bajaj N, Arora G, Arora P. Galectin-3: an emerging biomarker in stroke and cerebrovascular diseases. Eur J Neurol 2018;25:238–246. [DOI] [PubMed] [Google Scholar]
- 21. Wang A, Zhong C, Zhu Z, Xu T, Peng Y, Xu T, et al. Serum Galectin-3 and poor outcomes among patients with acute ischemic stroke. Stroke 2018;49:211–214. [DOI] [PubMed] [Google Scholar]
- 22. de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 2009;11:811–817. [DOI] [PubMed] [Google Scholar]
- 23. Liu X, Gu Y, Liu Y, Zhang M, Wang Y, Hu L. Ticagrelor attenuates myocardial ischaemia-reperfusion injury possibly through downregulating Galectin-3 expression in the infarct area of rats. Br J Clin Pharmacol 2018;84:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Lin J, Hu T, Ren Z, Li L, Hameed I, et al. Galectin-3 exacerbates ox-LDL-mediated endothelial injury by inducing inflammation via integrin β1-RhoA-JNK signaling activation. J Cell Physiol 2019;234:10990–11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nachtigal M, Ghaffar A, Mayer EP. Galectin-3 gene inactivation reduces atherosclerotic lesions and adventitial inflammation in ApoE-deficient mice. Am J Pathol 2008;172:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, et al. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol 2008;28:433–440. [DOI] [PubMed] [Google Scholar]
- 27. Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, et al. Human Galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol 2000;165:2156–2164. [DOI] [PubMed] [Google Scholar]
- 28. Gurbel PA, Jeong YH, Navarese EP, Tantry US. Platelet-mediated thrombosis: from bench to bedside. Circ Res 2016;118:1380–1391. [DOI] [PubMed] [Google Scholar]
- 29. DeRoo EP, Wrobleski SK, Shea EM, Al-Khalil RK, Hawley AE, Henke PK, et al. The role of Galectin-3 and Galectin-3-binding protein in venous thrombosis. Blood 2015;125:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018;379:797–798. [DOI] [PubMed] [Google Scholar]
- 31. Chan YC, Lin HY, Tu Z, Kuo YH, Hsu SD, Lin CH. Dissecting the structure-activity relationship of Galectin-ligand interactions. Int J Mol Sci 2018;19:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirani N, MacKinnon AC, Nicol L, Ford P, Schambye H, Pedersen A, et al. Target-inhibition of Galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur Respir J 2021;57:2002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stalker TJ, Welsh JD, Tomaiuolo M, Wu J, Colace TV, Diamond SL, et al. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood 2014;124:1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tutwiler V, Wang H, Litvinov RI, Weisel JW, Shenoy VB. Interplay of platelet contractility and elasticity of fibrin/erythrocytes in blood clot retraction. Biophys J 2017;112:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, Fink GR. Fungal recognition is mediated by the association of Dectin-1 and Galectin-3 in macrophages. Proc Natl Acad Sci U S A 2011;108:14270–14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leclaire C, Lecointe K, Gunning PA, Tribolo S, Kavanaugh DW, Wittmann A, et al. Molecular basis for intestinal mucin recognition by Galectin-3 and C-type lectins. FASEB J 2018;32:3301–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013;342:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers 2018;4:18026. [DOI] [PubMed] [Google Scholar]
- 39. Brown GD, Gordon S. Immune recognition. A new receptor for β-glucans. Nature 2001;413:36–37. [DOI] [PubMed] [Google Scholar]
- 40. Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived Galectin-3. Blood 2020;135:1146–1160. [DOI] [PubMed] [Google Scholar]
- 41. Cardoso AC, Andrade LN, Bustos SO, Chammas R. Galectin-3 determines tumor cell adaptive strategies in stressed tumor microenvironments. Front Oncol 2016;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mazurek N, Conklin J, Byrd JC, Raz A, Bresalier RS. Phosphorylation of the β-Galactoside-binding protein Galectin-3 modulates binding to its ligands. J Biol Chem 2000;275:36311–36315. [DOI] [PubMed] [Google Scholar]
- 43. Modenutti CP, Capurro JIB, Di Lella S, Martí MA. The structural biology of Galectin-ligand recognition: current advances in modeling tools, protein engineering, and inhibitor design. Front Chem 2019;7:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005;106:2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 2006;6:33–43. [DOI] [PubMed] [Google Scholar]
- 46. Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the Dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 2008;112:4971–4980. [DOI] [PubMed] [Google Scholar]
- 47. Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest 2019;129:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005;22:507–517. [DOI] [PubMed] [Google Scholar]
- 49. Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 2005;24:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu S, Huo J, Lee KG, Kurosaki T, Lam KP. Phospholipase Cγ2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem 2009;284:7038–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-delta to elicit Card9 adaptor-mediated innate immunity. Immunity 2012;36:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gu SX, Stevens JW, Lentz SR. Regulation of thrombosis and vascular function by protein methionine oxidation. Blood 2015;125:3851–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest 2005;115:3355–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harper MT, Poole AW. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J Thromb Haemost 2010;8:454–462. [DOI] [PubMed] [Google Scholar]
- 55. Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, et al. Regulation of transforming growth factor-β1–driven lung fibrosis by Galectin-3. Am J Respir Crit Care Med 2012;185:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cumpstey I, Sundin A, Leffler H, Nilsson UJ. C2-symmetrical thiodigalactoside bis-benzamido derivatives as high-affinity inhibitors of Galectin-3: efficient lectin inhibition through double arginine-arene interactions. Angew Chem Int Ed Engl 2005;44:5110–5112. [DOI] [PubMed] [Google Scholar]
- 57. Gremmel T, Ay C, Riedl J, Kopp CW, Eichelberger B, Koppensteiner R, et al. Platelet-specific markers are associated with monocyte-platelet aggregate formation and thrombin generation potential in advanced atherosclerosis. Thromb Haemost 2016;115:615–621. [DOI] [PubMed] [Google Scholar]
- 58. Pang A, Cheng N, Cui Y, Bai Y, Hong Z, Delaney MK, et al. High-loading Gα13-binding EXE peptide nanoparticles prevent thrombosis and protect mice from cardiac ischemia/reperfusion injury. Sci Transl Med 2020;12:eaaz7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Köhler D, Granja T, Volz J, Koeppen M, Langer HF, Hansmann G, et al. Red blood cell-derived semaphorin 7A promotes thrombo-inflammation in myocardial ischemia-reperfusion injury through platelet GPIb. Nat Commun 2020;11:1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sharma UC, Mosleh W, Chaudhari MR, Katkar R, Weil B, Evelo C, et al. Myocardial and serum Galectin-3 expression dynamics marks post-myocardial infarction cardiac remodelling. Heart Lung Circ 2017;26:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsai TH, Sung PH, Chang LT, Sun CK, Yeh KH, Chung SY, et al. Value and level of Galectin-3 in acute myocardial infarction patients undergoing primary percutaneous coronary intervention. J Atheroscler Thromb 2012;19:1073–1082. [DOI] [PubMed] [Google Scholar]
- 62. George M, Shanmugam E, Srivatsan V, Vasanth K, Ramraj B, Rajaram M, et al. Value of pentraxin-3 and Galectin-3 in acute coronary syndrome: a short-term prospective cohort study. Ther Adv Cardiovasc Dis 2015;9:275–284. [DOI] [PubMed] [Google Scholar]
- 63. van der Velde AR, Lexis CP, Meijers WC, van der Horst IC, Lipsic E, Dokter MM, et al. Galectin-3 and sST2 in prediction of left ventricular ejection fraction after myocardial infarction. Clin Chim Acta 2016;452:50–57. [DOI] [PubMed] [Google Scholar]
- 64. Meijers WC, van der Velde AR, Pascual-Figal DA, de Boer RA. Galectin-3 and post-myocardial infarction cardiac remodeling. Eur J Pharmacol 2015;763:115–121. [DOI] [PubMed] [Google Scholar]
- 65. Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004;110:3121–3128. [DOI] [PubMed] [Google Scholar]
- 66. Lee YJ, Koh YS, Park HE, Lee HJ, Hwang BH, Kang MK, et al. Spatial and temporal expression, and statin responsiveness of Galectin-1 and Galectin-3 in murine atherosclerosis. Korean Circ J 2013;43:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sanchez-Mas J, Lax A, Asensio-Lopez MC, Fernandez-Del Palacio MJ, Caballero L, Garrido IP, et al. Galectin-3 expression in cardiac remodeling after myocardial infarction. Int J Cardiol 2014;172:e98–e101. [DOI] [PubMed] [Google Scholar]
- 68. Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 2006;107:542–549. [DOI] [PubMed] [Google Scholar]
- 69. Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 2014;14:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, et al. Yeast zymosan, a stimulus for TLR2 and Dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest 2006;116:916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fan Q, Tao R, Zhang H, Xie H, Lu L, Wang T, et al. Dectin-1 contributes to myocardial ischemia/reperfusion injury by regulating macrophage polarization and neutrophil infiltration. Circulation 2019;139:663–678. [DOI] [PubMed] [Google Scholar]
- 72. Senis YA, Mazharian A, Mori J. Src family kinases: at the forefront of platelet activation. Blood 2014;124:2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Montague SJ, Andrews RK, Gardiner EE. Mechanisms of receptor shedding in platelets. Blood 2018;132:2535–2545. [DOI] [PubMed] [Google Scholar]
- 74. Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev 2010;234:335–352. [DOI] [PubMed] [Google Scholar]
- 75. Zelechowska P, Rozalska S, Wiktorska M, Brzezinska-Blaszczyk E, Agier J. Curdlan stimulates tissue mast cells to synthesize pro-inflammatory mediators, generate ROS, and migrate via Dectin-1 receptor. Cell Immunol 2020;351:104079. [DOI] [PubMed] [Google Scholar]
- 76. Speich HE, Grgurevich S, Kueter TJ, Earhart AD, Slack SM, Jennings LK. Platelets undergo phosphorylation of Syk at Y525/526 and Y352 in response to pathophysiological shear stress. Am J Physiol Cell Physiol 2008;295:C1045–C1054. [DOI] [PubMed] [Google Scholar]
- 77. Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, et al. Critical role of Galectin-3 in phagocytosis by macrophages. J Clin Invest 2003;112:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhao W, Ajani JA, Sushovan G, Ochi N, Hwang R, Hafley M, et al. Galectin-3 mediates tumor cell-stroma interactions by activating pancreatic stellate cells to produce cytokines via integrin signaling. Gastroenterology 2018;154:1524–1537.e6. [DOI] [PubMed] [Google Scholar]
- 79. Hsieh WC, Mackinnon AC, Lu WY, Jung J, Boulter L, Henderson NC, et al. Galectin-3 regulates hepatic progenitor cell expansion during liver injury. Gut 2015;64:312–321. [DOI] [PubMed] [Google Scholar]
- 80. Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, et al. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for Galectin-3 in liver. J Hepatol 2011;54:975–983. [DOI] [PubMed] [Google Scholar]
- 81. Law DA, Nannizzi-Alaimo L, Ministri K, Hughes PE, Forsyth J, Turner M, et al. Genetic and pharmacological analyses of Syk function in αIIbβ3 signaling in platelets. Blood 1999;93:2645–2652. [PubMed] [Google Scholar]
- 82. Andre P, Morooka T, Sim D, Abe K, Lowell C, Nanda N, et al. Critical role for Syk in responses to vascular injury. Blood 2011;118:5000–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Harbi MH, Smith CW, Nicolson PLR, Watson SP, Thomas MR. Novel antiplatelet strategies targeting GPVI, CLEC-2 and tyrosine kinases. Platelets 2021;32:29–41. [DOI] [PubMed] [Google Scholar]
- 84. Thiagarajan PS, Yakubenko VP, Elsori DH, Yadav SP, Willard B, Tan CD, et al. Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc Res 2013;99:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bode K, Bujupi F, Link C, Hein T, Zimmermann S, Peiris D, et al. Dectin-1 binding to annexins on apoptotic cells induces peripheral immune tolerance via NADPH oxidase-2. Cell Rep 2019;29:4435–4446.e9. [DOI] [PubMed] [Google Scholar]
- 86. Weck MM, Appel S, Werth D, Sinzger C, Bringmann A, Grunebach F, et al. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood 2008;111:4264–4272. [DOI] [PubMed] [Google Scholar]
- 87. McDonagh TA, Metra M, Adamo Met al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.