Abstract
Biomarkers for predicting the treatment efficacy of immune checkpoint inhibitor (ICI)-based therapy in patients with unresectable hepatocellular carcinoma (uHCC) are crucial. Previous studies demonstrated that C-reactive protein and alpha-fetoprotein (AFP) in immunotherapy (CRAFITY) score at baseline predicted treatment outcomes and that patients with uHCC with AFP response, defined as > 15% decline in AFP level within the initial 3 months of ICI-based therapy, had favorable outcomes when receiving ICI-based therapy. However, whether the combination of CRAFITY score and AFP response could be used to predict treatment efficacy of programmed death-1 (PD-1) blockade-based therapy in uHCC patients remains unclear. We retrospectively enrolled 110 consecutive uHCC patients from May 2017 to March 2022. The median ICI treatment duration was 2.85 (1.67-6.63) months, and 87 patients received combination therapies. The objective response and disease control rates were 21.8% and 46.4%, respectively. The duration of progression-free survival (PFS) and overall survival (OS) was 2.87 (2.16-3.58) months and 8.20 (4.23-12.17) months, respectively. We categorized patients into three groups based on CRAFITY score (2 vs 0/1) and AFP response: patients with a CRAFITY score of 0/1 and AFP response (Group 1), those with a CRAFITY score of 2 and no AFP response (group 3), and those who did not belong to Group 1 and 3 (i.e., Group 2). The combination of CRAFITY score and AFP response could predict disease control and could predict PFS compared with CRAFITY score or AFP response alone. The combination of CRAFITY score and AFP response was an independent predictor of OS (Group 2 vs Group 1, HR: 4.513, 95% CI 1.990-10.234; Group 3 vs Group 1, HR: 3.551, 95% CI 1.544-8.168). Our findings indicated that the combination of CRAFITY score and AFP response could predict disease control, PFS, and OS in uHCC patients receiving PD-1 blockade-based immunotherapy.
Keywords: AFP response, CRAFITY score, hepatocellular carcinoma, programmed death-1 blockade, survival
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the leading cause of cancer-related death worldwide. Its incidence is higher in hepatitis B virus (HBV)- or hepatitis C virus (HCV)-endemic areas, including Southeast Asia [1]. Many patients have unresectable HCC (uHCC) at the time of HCC diagnosis [2], and immune checkpoint inhibitors (ICIs) have become part of the standard therapy against uHCC [3,4].
In a randomized multicenter phase 3 trial for patients with advanced HCC (CheckMate 459), the median overall survival (OS) was 16.4 months in the nivolumab group and 14.7 months in the sorafenib group (hazard ratio [HR] 0.85, P = .075) [5]. Although first-line nivolumab therapy did not significantly improve OS compared with sorafenib, nivolumab had an acceptable objective response rate (ORR, rate of complete response [CR] and partial response [PR]) (15.4%) and a favorable safety profile [5]. In a branch cohort of the CheckMate 040 study, patients with previous sorafenib-treated advanced HCC who were administered a combination of nivolumab (1 mg/kg) and ipilimumab (3 mg/kg) exhibited a longer median OS of 22.8 months [6]. Pembrolizumab also demonstrated antitumor activity; the median OS was 13.9 months in the pembrolizumab group and 10.6 months (HR 0.781, P = .0238) in the control group. However, immunotherapy did not reach statistical significance per prespecified criteria [7]. Combining a tyrosine kinase inhibitor (TKI) and an immunotherapeutic agent, such as lenvatinib plus pembrolizumab [8,9] and lenvatinib plus nivolumab [10], has yielded promising results.
Objective, reproducible, and applicable biomarkers are required to predict the treatment efficacy of ICI-based therapy in patients with uHCC. Patients with uHCC who exhibited a decline (> 10%-20%) in serum alpha-fetoprotein (AFP) level within the initial 4, 12, or 18 weeks of ICI therapy have favorable outcomes [11-14]. We previously demonstrated that AFP response-defined as a decline of > 15% in AFP level within the initial 3 months of ICI therapy-could predict disease control, progression-free survival (PFS), and OS in patients with uHCC receiving ICI monotherapy or combination therapy [15]. However, serial serum AFP levels need to be measured until 12 or 18 weeks after ICI initiation. Scheiner et al. indicated that the C-reactive protein (CRP) and AFP in immunotherapy (CRAFITY) score at baseline could predict treatment outcomes in patients receiving ICI therapy [16]. However, whether the combination of CRAFITY score and AFP response can be used to predict the treatment efficacy of programmed death (PD)-1 blockade-based immunotherapy in patients with uHCC remains unclear.
In this study, we investigated whether the combination of CRAFITY score and AFP response (CRAFITY/AFPr) can predict treatment response, including disease control, PFS, and OS, in patients with uHCC receiving PD-1 blockade-based immunotherapy.
Patients and methods
Patients
In this retrospective study, we enrolled 192 consecutive patients with uHCC who received at least one dose of nivolumab or pembrolizumab at China Medical University Hospital or Asia University Hospital in central Taiwan between May 2017 and March 2022. Patients with terminal HCC (Barcelona Clinic Liver Cancer [BCLC] stage D, n = 17), history of malignant cancer other than HCC, missing data showing a decline in AFP level (n = 28), no baseline CRP level (n = 58), liver transplantation, or HIV infection were excluded. Patients might have met more than one exclusion criterion. Of the 110 patients included in the final analysis, 93 had evaluable radiological imaging; 13 died and 4 were lost to follow-up before the first radiological assessment (Supplementary Figure 1).
Baseline hematologic and biochemical data, comorbidities, virological features, and tumoral characteristics were recorded. AFP kinetics were determined using the maximal difference between AFP levels at baseline and 4, 8, or 12 weeks after ICI initiation. AFP response was defined as a decline of > 15% in AFP level within the initial 3 months of ICI therapy [15]. Information regarding combination therapies with ICIs, including TKIs and locoregional therapies, such as radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and liver radiotherapy, were recorded. This study was performed in accordance with the 1975 Declaration of Helsinki and approved by the Research Ethics Committee of China Medical University Hospital, Taichung, Taiwan (CMUH108-REC3-140). Each patient’s identification number was encrypted to protect their privacy; thus, the need for informed consent was waived.
ICI and TKI doses, locoregional therapies, tumor assessment, and safety
The doses of sorafenib and lenvatinib were 400-800 mg and 8-12 mg per day, respectively, and that of regorafenib was 80 mg per day or 120-160 mg per day for the first 21 days of each 28-day cycle. The doses of ICIs were administered (2-3 mg/kg every 2 weeks for nivolumab and every 3 weeks for pembrolizumab) per the protocols of previous studies [15]. Concurrent TKI therapy was defined as the combination of an ICI and a TKI for > 7 days. Three patients received real-time ultrasound-guided RFA (Covidien, Dublin, Ireland) for viable tumors (1.3-2.0 cm in size) 10-160 days after initiating nivolumab therapy. Patients with HCC with Child-Pugh class A or B and patent main portal vein or main portal vein thrombosis with cavernous transformation were eligible for TACE. Combined radiotherapy was defined as overlapping ICI therapy with liver radiotherapy. The detailed procedures of TACE [17] and liver radiotherapy [18] have been described previously.
Tumor response was evaluated through dynamic computed tomography or magnetic resonance imaging every 8-12 weeks according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [19]. Patients with objective response were defined as those with CR or PR, and patients with disease control were defined as those with CR, PR, or stable disease (SD). Safety was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03.
Laboratory tests
Complete blood count analyses (Sysmex HST series, Kanagawa, Japan) and biochemistry tests (Beckman Coulter, Brea, CA, USA) were performed in the central laboratory of the hospitals. HBV infection was defined as the presence of serum hepatitis B surface antigen for > 6 months, and HCV infection was defined as the presence of serum anti-HCV antibody for > 6 months and detectable HCV RNA. Liver cirrhosis was defined according to unequivocal clinical, ultrasonographic, or histological analysis.
Statistical analysis
Continuous variables are presented as median (interquartile range), PFS and OS are presented as median (95% CI), and categorical variables are presented as frequency (percentage). Between-group comparisons of continuous variables were performed using the Mann-Whitney U test. Logistic regression analysis was performed to identify factors associated with disease control, and Cox regression analysis was performed to identify variables associated with PFS or OS. Variables with P < .20 in the univariate analysis were subjected to multivariate logistic or Cox regression analysis to determine their association with disease control, PFS, or OS, in accordance with the conventional approach proposed previously [20]. The predictive performance of CRAFITY score or AFP response alone or their combination for OS was examined using an area under the receiver operating characteristic curve (AUROC) analysis using the DeLong test. Kaplan-Meier analysis with the log-rank test was used to compare PFS and OS between patient subgroups. The formula of total tumor volume (TTV) was (4/3) × 3.14 × (radius of the tumor in cm)3 [21]. All statistical analyses were conducted using SPSS v25.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as two-sided P < .05.
Results
Baseline characteristics
Of the 175 patients eligible for investigation, 65 were excluded. The excluded patients had a lower platelet count; a lower proportion of alcohol consumption, macrovascular invasion (MVI); and a higher proportion of diabetes mellitus (DM); longer median OS (23.23 [16.00-30.47] months vs 8.20 [4.23-12.17] months, P = .010). The enrolled and excluded patients received a similar duration of ICI therapy and proportion of concurrent therapy (Supplementary Table 1).
The median age of the enrolled patients was 64.5 (54.6-72.4) years, and 94 of 110 (85.5%) patients were men. In total, 35 (31.8%), 62 (56.4%), 27 (24.5%), and 29 (26.4%) patients drank alcohol, had HBV infection, had HCV infection, and had DM, respectively. The median neutrophil-to-lymphocyte ratio (NLR), alanine aminotransferase (ALT) level, and AFP level were 5.34 (3.01-8.28), 35 (23-57) U/L, and 126.48 (12.25-5056.00) ng/mL, respectively. The median Child-Pugh score was 6 (5-7). Among the enrolled patients, 6 (5.5%), 16 (14.5%), and 88 (80.0%) had BCLC stages A, B, and C, respectively. The maximum tumor size was 4.7 (2.7-9.7) cm, and TTV was 740.4 (143.1-5339.6) cm3. Extrahepatic metastasis (EHM) and MVI were observed in 61 (55.5%)and 65 (59.1%) patients, respectively. Approximately one-third of the patients (n = 35, 31.8%) received ICIs as the first-line systemic therapy. Most patients received combination therapies (n = 87, 79.1%), and 76 (69.1%) patients received an ICI-TKI combination, with sorafenib being the most frequent TKI administered in the combination (n = 43, 39.1%). A total of 14 (12.7%) and 20 (18.2%) patients received ICIs combined with TACE and liver radiotherapy, respectively, for HCC (Table 1).
Table 1.
Patient demographics, baseline characteristics, and therapeutic response
| Character | All (n = 110) | CRAFITY score 0 or 1 (n = 57) | CRAFITY score 2 (n = 53) | P value |
|---|---|---|---|---|
| Age (years) | 64.5 (54.6-72.4) | 65.0 (55.8-72.2) | 63.8 (51.0-74.1) | .475 |
| Sex (male), n (%) | 94 (85.5) | 49 (86.0) | 45 (84.9) | .875 |
| Body mass index (kg/m2) | 23.43 (20.96-26.72) | 23.54 (21.12-27.05) | 23.31 (20.71-26.50) | .647 |
| NLR | 5.34 (3.01-8.28) | 5.18 (2.70-7.80) | 5.34 (3.22-8.59) | .327 |
| Platelet count (× 109/L) | 174 (109-251) | 178 (105-256) | 168 (117-252) | .914 |
| AST (U/L) | 48 (32-92) | 41 (25-65) | 69 (39-118) | < .001 |
| ALT (U/L) | 35 (23-57) | 35 (19-55) | 43 (27-61) | .036 |
| Total bilirubin (mg/dL) | 0.97 (0.66-1.60) | 0.90 (0.67-1.20) | 1.28 (0.60-1.92) | .099 |
| Albumin (g/dL) | 3.6 (3.3-4.0) | 3.7 (3.3-4.1) | 3.5 (3.2-3.9) | .106 |
| INR | 1.06 (1.01-1.17) | 1.05 (0.99-1.18) | 1.07 (1.03-1.16) | .364 |
| Etiology | ||||
| Alcohol | 35 (31.8) | 16 (28.1) | 19 (35.8) | .384 |
| HBV | 62 (56.4) | 26 (45.6) | 36 (67.9) | .019 |
| HCV | 27 (24.5) | 17 (29.8) | 10 (18.9) | .184 |
| Diabetes mellitus | 29 (26.4) | 16 (28.1) | 13 (24.5) | .675 |
| Liver cirrhosis | 84 (76.4) | 40 (70.2) | 44 (83.0) | .115 |
| Child-Pugh score | 6 (5-7) | 5 (5-7) | 6 (5-8) | .007 |
| Child-Pugh class A | 72 (66.1) | 42 (73.7) | 30 (57.7) | |
| Child-Pugh class B | 37 (33.9) | 15 (26.3) | 22 (42.3) | |
| ALBI grade | .072 | |||
| 1 | 33 (30.6) | 20 (35.1) | 13 (25.5) | |
| 2 | 67 (62.0) | 36 (63.2) | 31 (60.8) | |
| 3 | 8 (7.4) | 1 (1.8) | 7 (13.7) | |
| AFP (ng/mL) | 126.48 (12.25-5056.0) | 13.42 (3.01-57.72) | 3406.0 (526.43-54000) | < .001 |
| BCLC stage | .481 | |||
| A | 6 (5.5) | 3 (5.3) | 3 (5.7) | |
| B | 16 (14.5) | 10 (17.5) | 6 (11.3) | |
| C | 88 (80.0) | 44 (77.2) | 44 (83.0) | |
| Max. tumor size (cm) | 4.7 (2.7-9.7) | 4.0 (2.3-8.2) | 6.83 (3.79-11.09) | .003 |
| Total tumor volume (cm3) | 740.4 (143.1-5339.6) | 382.5 (76.7-2378.2) | 2242.0 (426.2-6413.7) | .001 |
| MVIa | 65 (59.1) | 29 (50.9) | 36 (67.9) | .070 |
| VP3 | 24 (21.8) | 14 (24.6) | 10 (18.9) | |
| VP4 | 34 (30.9) | 12 (21.1) | 22 (41.5) | |
| Hepatic vein | 7 (6.4) | 3 (5.3) | 4 (7.5) | |
| EHMa | 61 (55.5) | 36 (63.2) | 33 (62.3) | .168 |
| Prior therapy | ||||
| Sorafenib | 63 (57.3) | 36 (63.2) | 27 (50.9) | |
| Lenvatinib | 19 (17.3) | 10 (17.5) | 9 (17.0) | |
| Surgery | 21 (19.1) | 10 (17.5) | 11 (20.8) | |
| PEI/RFA | 7 (6.4)/20 (18.2) | 5 (8.8)/14 (24.6) | 2 (3.8)/6 (11.3) | |
| TACEb/TARE | 66 (60.0)/3 (2.7) | 36 (63.2)/1 (1.8) | 30 (56.6)/2 (3.8) | |
| Radiotherapy | 3 (2.7) | 1 (1.8) | 2 (3.8) | |
| ICI duration (months) | 2.85 (1.67-6.63) | 3.40 (1.85-9.08) | 2.37 (1.55-4.65) | .053 |
| Nivolumabc | 100 (90.9) | 48 (84.2) | 52 (98.1) | |
| Nivolumab + ipilimumabc | 3 (2.7) | 0 (0) | 3 (5.7) | |
| Pembrolizumabc | 12 (10.9) | 11 (19.3) | 1 (1.9) | |
| Reduction > 25% | 59 (53.6) | 34 (59.6) | 25 (47.2) | |
| As 1st/2nd/3rd/4th-line systemic therapy | 35 (31.8)/53 (48.2) | 16 (28.1)/30 (52.6) | 19 (35.8)/23 (43.4) | |
| 16 (14.5)/6 (5.5) | 8 (14.0)/3 (5.3) | 8 (15.1)/3 (5.7) | ||
| Concurrent therapy | 87 (79.1) | 44 (77.2) | 43 (81.1) | .613 |
| Sorafenibd | 43 (39.1) | 21 (36.8) | 22 (41.5) | |
| Lenvatinibd | 32 (29.1) | 16 (28.1) | 16 (30.2) | |
| Regorafenibd | 17 (15.5) | 11 (19.3) | 6 (11.3) | |
| RFA | 3 (2.7) | 1 (1.8) | 2 (3.8) | |
| TACE | 14 (12.7) | 9 (15.8) | 5 (9.4) | |
| Liver radiotherapy | 20 (18.2) | 10 (17.5) | 10 (18.9) | |
| Therapeutic response | ||||
| Best Response | ||||
| Complete response | 7 (6.4) | 3 (5.3) | 4 (7.5) | |
| Partial response | 17 (15.5) | 12 (21.1) | 5 (9.4) | |
| Stable disease | 27 (24.5) | 15 (26.3) | 12 (22.6) | |
| Progressive disease | 59 (53.6) | 27 (47.4) | 32 (60.4) | |
| Not evaluable | ||||
| Death before evaluation | 13 (11.8) | 5 (8.8) | 8 (15.1) | |
| Lost to follow-upe | 4 (3.6) | 2 (3.5) | 2 (3.8) | |
| Objective response | 24 (21.8) | 15 (26.3) | 9 (17.0) | .238 |
| Disease control | 51 (46.4) | 30 (52.6) | 21 (39.6) | .174 |
| Progression-free survival (months)* | 2.87 (2.16-3.58) | 4.07 (1.54-6.59) | 2.47 (2.09-2.85) | .017 |
| Overall survival (months)* | 8.20 (4.23-12.17) | 15.73 (4.76-26.71) | 4.90 (2.64-7.16) | < .001 |
Data presented as median (first quartile-third quartile).
Data presented as median (95% confidence interval).
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibitor; MVI, macrovascular invasion; NLR, neutrophil-lymphocyte ratio; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; PEI, percutaneous ethanol injection; INR, international normalized ratio; RFA, radiofrequency ablation.
Thirty-eight patients with HCC had both macrovascular invasion and extrahepatic metastasis.
The median number of TACE sessions was 3 (2-5).
Five patients received sequential ICI therapy because of progressive disease: nivolumab→pembrolizumab→atezolizumab plus bevacizumab (n = 1), nivolumab→pembrolizumab→nivolumab (n = 2), nivolumab→atezolizumab plus bevacizumab→nivolumab plus ipilimumab (n = 1), and nivolumab plus ipilimumab→nivolumab plus sorafenib (n = 1).
Eighteen patients received sequential tyrosine kinase inhibitor therapy because of progressive disease: sorafenib→regorafenib (n = 8), sorafenib→lenvatinib (n = 3), sorafenib→regorafenib→lenvatinib (n = 1), lenvatinib→sorafenib (n = 2), lenvatinib→regorafenib (n = 1), sorafenib→ramucizumab (n = 1), and lenvatinib→cabozantinib (n = 2).
Four patients were lost to follow-up because of immune-related adverse events.
Therapeutic response
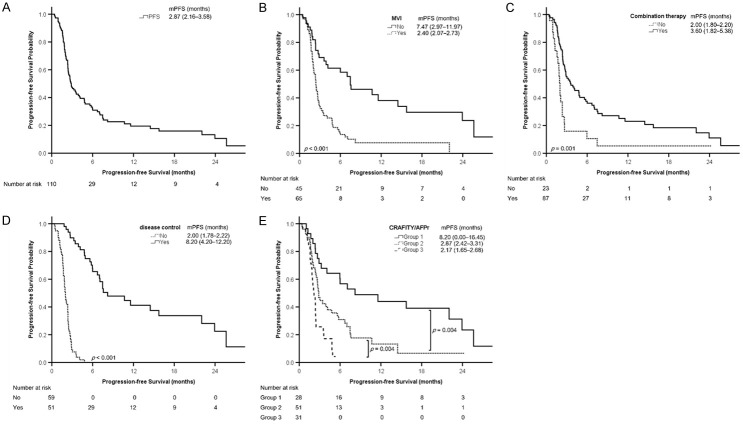
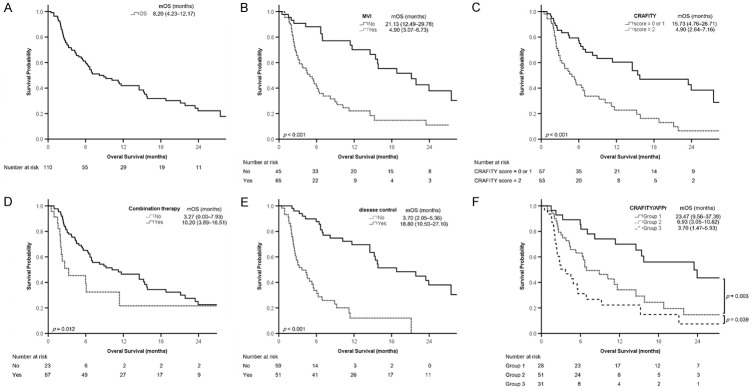
The median treatment duration of ICIs was 2.85 (1.67-6.63) months. Seventeen patients did not undergo radiological imaging; among them, 13 (11.8%) died before evaluation, and 4 (3.6%) were lost to follow-up because of treatment-related adverse events (TRAEs). In all, 7 (6.4%), 17 (15.5%), 27 (24.5%), and 59 (53.6%) patients had CR, PR, SD, and progressive disease (PD), respectively. The ORR and disease control rate (DCR, rate of CR + PR + SD) were 21.8% (24/110) and 46.4% (51/110), respectively. The duration of PFS and OS was 2.87 (2.16-3.58) and 8.20 (4.23-12.17) months, respectively (Figures 2A, 3A; Table 1).
Figure 2.
Kaplan-Meier analyses of progression-free survival. A. All patients. B. Patients with or without macrovascular invasion (MVI). C. Patients with or without combination therapy. D. Patients with or without disease control. E. Patients in different groups according to the combination of CRAFITY score and AFP response (CRAFITY/AFPr). Progression-free survival is presented as median (95% confidence interval). AFP, α-fetoprotein; CRAFITY, C-reactive protein and α-fetoprotein in immunotherapy; mPFS, median progression-free survival.
Figure 3.
Kaplan-Meier analyses of overall survival. A. All patients. B. Patients with or without macrovascular invasion (MVI). C. Patients with a CRAFITY score of 2 vs score = 0 or 1. D. Patients with or without combination therapy. E. Patients with or without disease control. F. Patients in different groups according to the combination of CRAFITY score and AFP response (CRAFITY/AFPr). Survival is presented as median (95% confidence interval). AFP, α-fetoprotein; CRAFITY, C-reactive protein and α-fetoprotein in immunotherapy; mOS, median overall survival.
Only 16 patients had a CRAFITY score of 0, so we combined patients with a CRAFITY score of 0 or 1 into one subgroup. The patients with a CRAFITY score of 0 or 1 had lower aspartate aminotransferase (AST), ALT, and AFP levels; a lower proportion of HBV infection; a lower Child-Pugh score; a smaller maximal tumor size and TTV; and a longer median PFS and OS than those with a CRAFITY score of 2 (Table 1).
Approximately two-thirds of the patients (n = 74, 67.3%) experienced at least one TRAE of any grade, with 26 experiencing grade ≥ 3 TRAEs: hepatitis (n = 12), dermatitis (n = 5), pneumonitis (n = 4), fatigue (n = 3), hand–foot syndrome (n = 3), colitis (n = 2), fever (n = 1), and gastric necrosis (n = 1). Some patients experienced more than one grade ≥ 3 TRAE. Five and two patients died from the severe TRAEs of hepatitis and pneumonitis, respectively (Supplementary Table 2).
AFP response and CRAFITY/AFPr are independent predictors of disease control
Among the 93 patients with radiological imaging data, univariate logistic regression analysis identified MVI, NLR (> 3.0 vs ≤ 3.0), and AFP response as factors associated with disease control. Multivariable logistic regression analysis indicated that AFP response (odds ratio [OR]: 7.177, 95% CI: 2.504-20.573) was an independent predictor of disease control (multivariable analysis 1, Supplementary Table 3). CRAFITY score was not a predictor of disease control in univariate (P = .282) or multivariable analysis (P = .793).
We categorized the patients into three groups based on CRAFITY score (2 vs 0/1) and AFP response: patients with a CRAFITY score of 0 or 1 and AFP response (Group 1); patients with a CRAFITY score of 2 and AFP response or patients with a CRAFITY score of 0 or 1 and no AFP response (Group 2); patients with a CRAFITY score of 2 and no AFP response (Group 3, Figure 1). The CRAFITY/AFPr was associated with disease control in univariate logistic regression analysis (Group 3 vs Group 1, OR: 0.083, 95% CI: 0.022-0.320, P < .001). Multivariable logistic regression analysis using CRAFITY/AFPr instead of either parameter alone revealed that the combination was an independent predictor of disease control (Group 3 vs Group 1, OR: 0.136, 95% CI: 0.032-0.576, P = .007; multivariable analysis 2, Supplementary Table 3).
Figure 1.
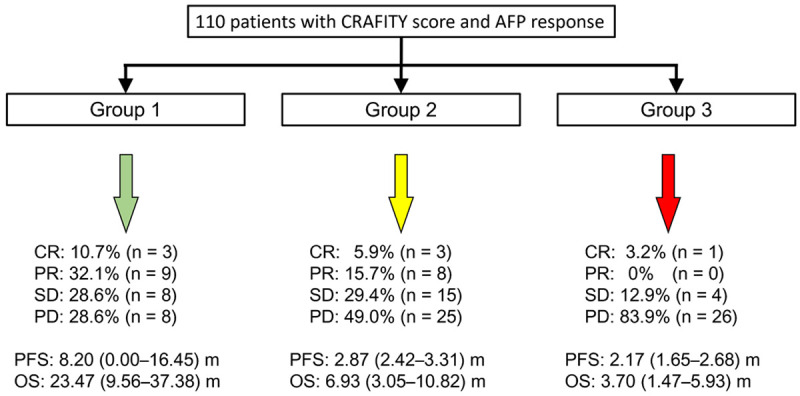
Three groups according to the combination of CRAFITY score and AFP response. Group 1: patients with a CRAFITY score of 0 or 1 and AFP response; Group 2: patients with a CRAFITY score of 2 and AFP response or patients with a CRAFITY score of 0 or 1 and no AFP response; Group 3: patients with a CRAFITY score of 2 and no AFP response. AFP, α-fetoprotein; CRAFITY, C-reactive protein and α-fetoprotein in immunotherapy.
CRAFITY/AFPr can predictor PFS
Univariate Cox regression analysis revealed that alcohol consumption, grade 1-2 TRAEs, TTV (> 1000 vs ≤ 1000 cm3), MVI, NLR (> 3.0 vs ≤ 3.0), Child-Pugh class (B vs A), AFP response, CRAFITY score (2 vs 0/1), CRAFITY/AFPr (Group 2 vs Group 1, HR: 2.342, 95% CI: 1.311-4.185, P = .004; Group 3 vs Group 1, HR: 4.905, 95% CI: 2.521-9.541, P < .001), combination therapy (including combined ICI therapy with TKIs, RFA, TACE, or liver radiotherapy vs ICI monotherapy), and disease control were significantly associated with PFS among the 110 enrolled patients. Multivariable Cox regression analysis indicated that MVI (HR: 1.823, 95% CI: 1.003-3.316), EHM (HR: 1.663, 95% CI: 1.015-2.727), combination therapy (HR: 0.458, 95% CI: 0.247-0.852), and disease control (HR: 0.087, 95% CI: 0.040-0.190) were independent predictors of PFS (multivariable analysis 1, Table 2). BCLC stage was not analyzed as a variable because most patients had BCLC stage C (n = 80, 84.2%), which confounded with MVI and EHM. In this study, we used disease control instead of objective response to prevent collinearity [15,22].
Table 2.
Factors associated with progression-free survival in 110 patients with unresectable hepatocellular carcinoma
| Character | Univariate analysis | Multivariable analysis 1 | Multivariable analysis 2 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (year) | 0.987 (0.971-1.004) | .122 | |||||
| Sex | M vs F | 0.755 (0.425-1.342) | .338 | ||||
| Alcohol | Yes vs no | 1.852 (1.180-2.907) | .007 | ||||
| HBV | Yes vs no | 0.898 (0.584-1.381) | .623 | ||||
| HCV | Yes vs no | 1.045 (0.648-1.686) | .856 | ||||
| DM | Yes vs no | 0.974 (0.611-1.552) | .912 | ||||
| Grade 1-2 TRAEs | Yes vs no | 0.586 (0.379-0.908) | .017 | ||||
| Grade ≥ 3 TRAEs | Yes vs no | 1.253 (0.773-2.029) | .360 | ||||
| TTV (cm3) | > 1000 vs ≤ 1000 | 1.719 (1.124-2.630) | .012 | ||||
| MVI | Yes vs no | 2.914 (1.793-4.736) | < .001 | 1.823 (1.003-3.316) | .049 | 1.894 (1.027-3.493) | .041 |
| EHM | Yes vs no | 1.396 (0.908-2.144) | .128 | 1.663 (1.015-2.727) | .044 | 1.877 (1.122-3.139) | .016 |
| AST (U/L) | > 40 vs ≤ 40 | 1.517 (0.952-2.419) | .080 | ||||
| ALT (U/L) | > 40 vs ≤ 40 | 1.406 (0.908-2.175) | .126 | ||||
| NLR | > 3.0 vs ≤ 3.0 | 2.247 (1.296-3.895) | .004 | ||||
| Child-Pugh class | B vs A | 1.741 (1.114-2.719) | .015 | ||||
| ALBI grade | 2/3 vs 1 | 1.289 (0.795-2.088) | .303 | ||||
| AFP decline > 15% | Yes vs no | 0.343 (0.214-0.550) | < .001 | Not assessed | |||
| CRAFITY score | 2 vs 0/1 | 1.684 (1.089-2.605) | .019 | Not assessed | |||
| Combined CRAFITY score and AFP responsea | Group 1 | Referent | Not assessed | Referent | |||
| Group 2 | 2.342 (1.311-4.185) | .004 | Not assessed | 2.091 (1.111-3.935) | .022 | ||
| Group 3 | 4.905 (2.521-9.541) | < .000 | Not assessed | 1.757 (0.838-3.684) | .136 | ||
| Combination therapyb | Yes vs no | 0.422 (0.253-0.704) | .001 | 0.458 (0.247-0.852) | .014 | 0.488 (0.267-0.893) | .020 |
| Best response | CR + PR + SD vs none | 0.053 (0.026-0.110) | < .001 | 0.087 (0.040-0.190) | < .001 | 0.074 (0.034-0.163) | < .001 |
Group 1: patients with a CRAFITY score 0 or 1 and AFP response; Group 3: patients with a CRAFITY score = 2 and no AFP response group 3; Group 2: patients who did not belong to Group 1 or 3.
Combination therapy included a tyrosine kinase inhibitor, radiofrequency ablation, transarterial chemoembolization, and liver radiotherapy.
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR + PR + SD, complete response plus partial response plus stable disease; DM, diabetes mellitus; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; M vs F, male versus female; MVI, macroscopic vascular invasion; NLR, neutrophil-lymphocyte ratio; TRAEs, treatment-related adverse events; TTV, total tumor volume.
Another multivariable Cox regression analysis using CRAFITY/AFPr instead of CRAFITY score or AFP response alone revealed that MVI, EHM, disease control, combination therapy, and CRAFITY/AFPr (Group 2 vs Group 1, HR: 2.091, 95% CI: 1.111-3.935, P = .022) were independent predictors of PFS (multivariable analysis 2, Table 2).
Kaplan-Meier analysis revealed that the probability of PFS significantly differed between the patients with and without MVI (Figure 2B), those with and without combination therapy (Figure 2C), those with and without disease control (Figure 2D), and among different groups according to CRAFITY/AFPr (Figure 2E). Patients in Group 1 had a longer PFS, followed by Groups 2 and 3 (median: 8.20, 2.87, and 2.17 months, respectively, P < .01 between groups, Figures 1 and 2E).
CRAFITY/AFPr can predict OS
Univariate Cox regression analysis indicated that TTV (> 1000 vs ≤ 1000 cm3), MVI, AST, and ALT levels (> 40 vs ≤ 40 U/L), NLR (> 3.0 vs ≤ 3.0), Child-Pugh class (B vs A), albumin-bilirubin grade (2/3 vs 1), AFP response, CRAFITY score (2 vs 0/1), CRAFITY/AFPr, combination therapy, and disease control were significantly associated with OS. Multivariable Cox regression analysis indicated that MVI (HR: 2.798, 95% CI: 1.427-5.488), EHM (HR: 2.013, 95% CI: 1.158-3.500), CRAFITY score (2 vs 0/1, HR: 2.497, 95% CI: 1.368-4.559), combination therapy (HR: 0.382, 95% CI: 0.177-0.825), and disease control (HR: 0.288, 95% CI: 0.148-0.560) were independent predictors of OS (multivariable analysis 1, Table 3).
Table 3.
Factors associated with overall survival in 110 patients with unresectable hepatocellular carcinoma
| Character | Univariate analysis | Multivariable analysis 1 | Multivariable analysis 2 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (year) | 0.994 (0.975-1.013) | .502 | |||||
| Sex | M vs F | 1.426 (0.682-2.984) | .346 | ||||
| Alcohol | Yes vs no | 1.155 (0.692-1.927) | .581 | ||||
| HBV | Yes vs no | 0.979 (0.602-1.593) | .932 | ||||
| HCV | Yes vs no | 1.089 (0.621-1.911) | .765 | ||||
| DM | Yes vs no | 0.997 (0.586-1.696) | .991 | ||||
| Grade 1-2 TRAEs | Yes vs no | 0.636 (0.390-1.038) | .070 | ||||
| Grade ≥ 3 TRAEs | Yes vs no | 1.196 (0.681-2.102) | .533 | ||||
| TTV (cm3) | > 1000 vs ≤ 1000 | 2.342 (1.441-3.805) | .001 | 1.987 (1.074-3.674) | .029 | ||
| MVI | Yes vs no | 3.413 (1.968-5.917) | < .001 | 2.798 (1.427-5.488) | .003 | 3.529 (1.714-7.269) | .001 |
| EHM | Yes vs no | 1.529 (0.936-2.498) | .090 | 2.013 (1.158-3.500) | .013 | 2.366 (1.325-4.227) | .004 |
| AST (U/L) | > 40 vs ≤ 40 | 2.397 (1.380-4.164) | .002 | ||||
| ALT (U/L) | > 40 vs ≤ 40 | 1.717 (1.059-2.784) | .028 | ||||
| NLR | > 3.0 vs ≤ 3.0 | 2.757 (1.439-5.284) | .002 | ||||
| Child-Pugh class | B vs A | 2.697 (1.647-4.419) | < .001 | ||||
| ALBI grade | 2/3 vs 1 | 2.090 (1.173-3.725) | .012 | ||||
| AFP decline > 15% | Yes vs no | 0.535 (0.327-0.874) | .013 | Not assessed | |||
| CRAFITY score | 2 vs 0/1 | 2.692 (1.637-4.429) | < .001 | 2.497 (1.368-4.559) | .003 | Not assessed | |
| Combined CRAFITY score and AFP responsea | Group 1 | Referent | Not assessed | Referent | |||
| Group 2 | 2.509 (1.296-4.860) | .006 | Not assessed | 4.513 (1.990-10.234) | < .001 | ||
| Group 3 | 4.428 (2.212-8.866) | < .001 | Not assessed | 3.551 (1.544-8.168) | .003 | ||
| Combination therapyb | Yes vs no | 0.483 (0.271-0.862) | .014 | 0.382 (0.177-0.825) | .014 | 0.306 (0.141-0.667) | .003 |
| Best response | CR + PR + SD vs none | 0.191 (0.109-0.335) | < .001 | 0.288 (0.148-0.560) | < .001 | 0.251 (0.127-0.498) | < .001 |
Group 1: patients with a CRAFITY score = 0 or 1 and AFP response; Group 3: patients with a CRAFITY score = 2 and no AFP response group 3; Group 2: patients who did not belong to Group 1 or 3.
Combination therapy included a tyrosine kinase inhibitor, radiofrequency ablation, transarterial chemoembolization, and liver radiotherapy.
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR + PR + SD, complete response plus partial response plus stable disease; DM, diabetes mellitus; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; M vs F, male versus female; MVI, macroscopic vascular invasion; NLR, neutrophil-lymphocyte ratio; TRAEs, treatment-related adverse events; TTV, total tumor volume.
Another multivariable analysis using CRAFITY/AFPr revealed that TTV (> 1000 vs ≤ 1000 cm3, HR: 1.987, 95% CI: 1.074-3.674), MVI, EHM, combination therapy, disease control, and CRAFITY/AFPr (Group 2 vs Group 1, HR: 4.513, 95% CI: 1.990-10.234, P < .001; Group 3 vs Group 1, HR: 3.551, 95% CI: 1.544-8.168, P = .003) were independent predictors of OS (multivariable analysis 2, Table 3).
A time-dependent AUROC was used to assess the predictive performance of CRAFITY score, AFP response, and their combination. The AUROCs of CRAFITY/AFPr for 1-year and 2-year OS were 0.744 (95% CI: 0.638-0.832; Supplementary Figure 2A) and 0.750 (95% CI: 0.639-0.841; Supplementary Figure 2B), respectively; the values were significantly higher than that of AFP response alone but nonsignificantly higher than that of CRAFITY score alone.
Kaplan-Meier analysis revealed that the probability of survival significantly differed between the patients with or without MVI (Figure 3B), those with a CRAFITY score of 2 versus 0/1 (Figure 3C), those with and without combination therapy (Figure 3D), those with and without disease control (Figure 3E), and among different groups according to CRAFITY/AFPr (Figure 3F). Patients in Group 1 had the longest OS, followed by Groups 2 and 3 (median: 23.47, 6.93, and 3.70 months, respectively, P < .05 between groups, Figures 1 and 3F).
In the subgroup of patients receiving concurrent ICI and TKI therapies (n = 76), Group 1 had longer OS than Group 2 (P < .001), and Group 2 also tended to have longer OS than Group 3 (P = .072, Supplementary Figure 3A). In another subgroup of patients receiving concurrent ICI and liver radiotherapy (n = 20), Group 1 also had the longest OS (Supplementary Figure 3B).
Discussion
This real-world study revealed that the combination of CRAFITY score and AFP response predicted disease control, PFS, and OS in patients with uHCC receiving PD-1 blockade-based immunotherapy. The AFP response alone (a decline of > 15% in the AFP level within the initial 3 months of ICI therapy) could predict disease control, and CRAFITY score (2 vs 0/1) alone could predict OS. Moreover, patients with a CRAFITY score of 0 or 1 and AFP response (Group 1) had the longest OS compared with other groups of patients in the subgroup analysis of concurrent ICI and TKI therapies (n = 76) or ICI and liver radiotherapy (n = 20).
Clinical parameters predicting treatment outcomes after initiating ICI therapy can help in the clinical decision-making of whether to continue or modify the ongoing therapeutic modality in patients with uHCC. Various definitions of AFP response have been proposed [11-14,23]. In previous studies, only a proportion of patients received ICI-combined therapies with ICIs [12,13]. We previously reported that AFP response could predict treatment outcomes in patients with uHCC receiving ICI with or without TKI or locoregional therapies. We extended the application of AFP response in patients receiving ICI monotherapy or combination therapies [15]. Serial serum AFP levels need to be measured until 12 or 18 weeks after ICI initiation. The CRAFITY score using baseline serum CRP and AFP levels predicts treatment outcomes in patients undergoing immunotherapy for HCC [16]. However, in our cohort, only 16 of 110 (14.5%) patients had a CRAFITY score of 0, and AFP response could serve a complementary role to CRAFITY score to categorize patients into three groups (Figure 1). AFP is a routine marker examined during HCC therapy, and the initial 3-month duration of ICI therapy is relatively short. Physicians could monitor the possible adverse effects of ICIs during this period.
Zhu et al. defined AFP response as a decline of ≥ 75% or an increase of ≤ 10% in AFP level between the baseline and 6 weeks of atezolizumab plus bevacizumab and identified AFP response as a predictor of objective response and disease control in the phase Ib GO30140 study and phase III IMbrave 150 trial [24]. On the basis of Zhu’s proposed AFP response, Teng et al. reported that the combination of CRAFITY score and AFP response at 6 weeks of therapy could predict treatment outcomes in patients with uHCC receiving atezolizumab plus bevacizumab [25]. However, the probability of survival was not different among the groups stratified by Zhu’s proposed AFP response in our cohort (Supplementary Figure 4). This difference might result from different immunotherapy regimens or patient populations.
ICIs are the frontline therapy against HCC. However, nivolumab (CheckMate 040) [26] or pembrolizumab (KEYNOTE-240) [7], used as second-line systemic therapy, had suboptimal outcomes in patients with advanced HCC. Attention has been focused on combination therapies, including two ICIs, an ICI with a TKI, or ICIs with locoregional treatments. The second-line therapy of nivolumab plus ipilimumab following first-line sorafenib therapy resulted in a longer median OS in patients with advanced HCC (CheckMate 040) [6]. Studies evaluating the ICI-TKI combination have also revealed promising results. Wu et al. concluded that the combination of lenvatinib and pembrolizumab had a high ORR (34.1%) and DCR (84.1%) in 71 patients with uHCC with or without prior target therapy or nivolumab therapy [9]. Patients receiving the combination of lenvatinib and nivolumab (n = 40) also had a higher ORR and longer PFS and OS than those receiving lenvatinib alone (n = 47) [10].
Integrated and multimodal locoregional interventions for HCC, including RFA, TACE, and liver radiotherapy, have been used for local disease control or as a bridge to curative treatment for years. These therapies induce the release of neoantigens and local inflammatory factors. Antigen-presenting cells uptake the released antigens and promote the innate and adaptive immunity of human beings [27,28]. Duffy et al. conducted a pilot study using tremelimumab in combination with RFA (n = 12) or TACE (n = 11) compared with tremelimumab monotherapy (n = 5) to explore the role of immunotherapy in combination with locoregional therapies in patients with advanced HCC [29]. A multinational registry study proved the concept, and the authors concluded that TACE could be integrated with PD-1 blockade, resulting in a delay in tumor progression and possible downstaging in selected patients [30]. The combinations of ICIs and TKIs also play a role in downstaging [31]. Kudo proposed that using upfront systemic therapy with subsequent locoregional therapy in patients with intermediate-stage HCC or the combination of atezolizumab and bevacizumab in patients with uHCC may result in curative conversion in selected patients [32].
Disease control is a significant predictor of OS in patients receiving combined TACE and sorafenib [33], ICI, or ICI-based therapies [15,22]. Scheiner et al. stated that patients with a lower CRAFITY score (0 or 1) had a higher chance of achieving an objective response or SD than those with a high CRAFITY score [16]. Our findings also indicated that patients with a CRAFITY score of 0 or 1 tended to have a higher likelihood of disease control (P = .174), and the combination of CRAFITY score and AFP response could predict disease control (Group 3 vs Group 1, OR: 0.136, 95% CI: 0.032-0.576). The differences may result from fewer enrolled patients and more advanced HCC status in the present study: 65 (59.1%) and 61 (55.5%) patients had MVI and EHM, respectively, and only 35 (31.8%) patients received ICI as first-line systemic therapy. In the original CRAFITY study, 32.4%-40.5% and 44.7%-53.9% of patients had MVI and EHM, respectively, and 34.3%-43.2% of patients received ICI as first-line systemic therapy [16].
This study has some limitations. First, this was a retrospective study that enrolled only 110 patients from two medical centers in central Taiwan. Second, this study used mRECIST [19] instead of RECIST version 1.1 [34] to assess radiological response. Approximately two-thirds of patients received prior locoregional therapy (20 [18.2%] and 66 [60%] patients receiving RFA and TACE, respectively), and mRECIST is a more suitable method with which to evaluate tumor response [35]. Third, the retrospective design precluded the evaluation of the longitudinal kinetics of CRAFITY score, which should be investigated in future studies.
In conclusion, our findings indicated that the combination of CRAFITY score and AFP response can predict disease control, PFS, and OS in patients with uHCC receiving PD-1 blockade-based immunotherapy.
Acknowledgements
This study was supported by a grant (DMR-112-190) from China Medical University Hospital, Taichung, Taiwan.
Disclosure of conflict of interest
Cheng-Yuan Peng has served as an advisory committee member for AbbVie, Bristol-Myers Squibb, Gilead, and Roche. All other coauthors have no conflicts of interest to declare.
Supporting Information
References
- 1.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, Qi X, Cheng SQ, Teng GJ. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4:721–730. doi: 10.1016/S2468-1253(19)30178-5. [DOI] [PubMed] [Google Scholar]
- 3.Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidali S, Trepo E, Sutter O, Nault JC. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J. 2022;10:765–774. doi: 10.1002/ueg2.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 6.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CJ, Lee PC, Hung YW, Lee CJ, Chi CT, Lee IC, Hou MC, Huang YH. Lenvatinib plus pembrolizumab for systemic therapy-naive and -experienced unresectable hepatocellular carcinoma. Cancer Immunol Immunother. 2022;71:2631–2643. doi: 10.1007/s00262-022-03185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu WC, Lin TY, Chen MH, Hung YP, Liu CA, Lee RC, Huang YH, Chao Y, Chen SC. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022;40:789–797. doi: 10.1007/s10637-022-01248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao YY, Liu TH, Hsu C, Lu LC, Shen YC, Lin ZZ, Cheng AL, Hsu CH. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39:2184–2189. doi: 10.1111/liv.14210. [DOI] [PubMed] [Google Scholar]
- 12.Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, Hou MC, Huang YH. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel) 2020;12:182. doi: 10.3390/cancers12010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu WF, Chuang PH, Chen CK, Wang HW, Tsai MH, Su WP, Chen HY, Yang CY, Lin CC, Huang GT, Lin JT, Lai HC, Peng CY. Predictors of response and survival in patients with unresectable hepatocellular carcinoma treated with nivolumab: real-world experience. Am J Cancer Res. 2020;10:4547–4560. [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HI, Lim J, Shim JH. Role of the alpha-fetoprotein response in immune checkpoint inhibitor-based treatment of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2022;148:2069–2077. doi: 10.1007/s00432-021-03727-y. [DOI] [PubMed] [Google Scholar]
- 15.Hsu WF, Wang HW, Chen CK, Lai HC, Chuang PH, Tsai MH, Su WP, Chen HY, Chu CS, Chou JW, Chen SH, Tsai TY, Hsiao WD, Lin CC, Huang GT, Lin JT, Peng CY. Alpha-fetoprotein response predicts treatment outcomes in patients with unresectable hepatocellular carcinoma receiving immune checkpoint inhibitors with or without tyrosine kinase inhibitors or locoregional therapies. Am J Cancer Res. 2021;11:6173–6187. [PMC free article] [PubMed] [Google Scholar]
- 16.Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, Himmelsbach V, Schulze K, von Felden J, Frundt TW, Stadler M, Heinzl H, Shmanko K, Spahn S, Radu P, Siebenhuner AR, Mertens JC, Rahbari NN, Kutting F, Waldschmidt DT, Ebert MP, Teufel A, De Dosso S, Pinato DJ, Pressiani T, Meischl T, Balcar L, Muller C, Mandorfer M, Reiberger T, Trauner M, Personeni N, Rimassa L, Bitzer M, Trojan J, Weinmann A, Wege H, Dufour JF, Peck-Radosavljevic M, Vogel A, Pinter M. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2022;76:353–363. doi: 10.1016/j.jhep.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao WD, Peng CY, Chuang PH, Lai HC, Cheng KS, Chou JW, Chen YY, Yu CJ, Feng CL, Su WP, Chen SH, Kao JT. Evaluation of dose-efficacy of sorafenib and effect of transarterial chemoembolization in hepatocellular carcinoma patients: a retrospective study. BMC Gastroenterol. 2016;16:50. doi: 10.1186/s12876-016-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol. 2019;9:1157. doi: 10.3389/fonc.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, Chiou YY, Chiang JH, Lee PC, Huo TI, Lee SD. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010;53:108–117. doi: 10.1016/j.jhep.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Kuo YH, Yen YH, Chen YY, Kee KM, Hung CH, Lu SN, Hu TH, Chen CH, Wang JH. Nivolumab versus regorafenib in patients with hepatocellular carcinoma after sorafenib failure. Front Oncol. 2021;11:683341. doi: 10.3389/fonc.2021.683341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng W, Lin CC, Ho MM, Lui KW, Wang SF, Hsu CW, Lin SM. Alpha-fetoprotein response at different time-points is associated with efficacy of nivolumab monotherapy for unresectable hepatocellular carcinoma. Am J Cancer Res. 2021;11:2319–2330. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu AX, Dayyani F, Yen CJ, Ren Z, Bai Y, Meng Z, Pan H, Dillon P, Mhatre SK, Gaillard VE, Hernandez S, Kelley RK, Sangro B. Alpha-fetoprotein as a potential surrogate biomarker for atezolizumab + bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res. 2022;28:3537–3545. doi: 10.1158/1078-0432.CCR-21-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng W, Lin CC, Su CW, Lin PT, Hsieh YC, Chen WT, Ho MM, Wang CT, Chai PM, Hsieh JC, Lin CY, Lin SM. Combination of CRAFITY score with alpha-fetoprotein response predicts a favorable outcome of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Am J Cancer Res. 2022;12:1899–1911. [PMC free article] [PubMed] [Google Scholar]
- 26.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagodinsky JC, Harari PM, Morris ZS. The promise of combining radiation therapy with immunotherapy. Int J Radiat Oncol Biol Phys. 2020;108:6–16. doi: 10.1016/j.ijrobp.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leone P, Solimando AG, Fasano R, Argentiero A, Malerba E, Buonavoglia A, Lupo LG, De Re V, Silvestris N, Racanelli V. The evolving role of immune checkpoint inhibitors in hepatocellular carcinoma treatment. Vaccines (Basel) 2021;9:532. doi: 10.3390/vaccines9050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinelli B, Kim E, D’Alessio A, Cedillo M, Sinha I, Debnath N, Kudo M, Nishida N, Saeed A, Hildebrand H, Kaseb AO, Abugabal YI, Pillai A, Huang YH, Khan U, Muzaffar M, Naqash AR, Patel R, Fischman A, Bishay V, Bettinger D, Sung M, Ang C, Schwartz M, Pinato DJ, Marron T. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study. J Immunother Cancer. 2022;10:e004205. doi: 10.1136/jitc-2021-004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran NH, Munoz S, Thompson S, Hallemeier CL, Bruix J. Hepatocellular carcinoma downstaging for liver transplantation in the era of systemic combined therapy with anti-VEGF/TKI and immunotherapy. Hepatology. 2022;76:1203–1218. doi: 10.1002/hep.32613. [DOI] [PubMed] [Google Scholar]
- 32.Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are uot suitable for TACE: upfront systemic therapy followed by curative conversion. Liver Cancer. 2021;10:539–544. doi: 10.1159/000519749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong BY, Yan ZP, Sun JH, Zhang L, Hou ZH, Zhu XL, Wen L, Ni CF. Random survival forests to predict disease control for hepatocellular carcinoma treated with transarterial chemoembolization combined with sorafenib. Front Mol Biosci. 2021;8:618050. doi: 10.3389/fmolb.2021.618050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Choi MH, Park GE, Oh SN, Park MY, Rha SE, Lee YJ, Jung SE, Choi JI. Reproducibility of mRECIST in measurement and response assessment for hepatocellular carcinoma treated by transarterial chemoembolization. Acad Radiol. 2018;25:1363–1373. doi: 10.1016/j.acra.2018.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.