Abstract
The temperature sensitive liposomal formulations are a promising tool to improve the therapeutic index of the drugs with minimal toxicity. The aim of this study was to investigate the potential of concomitant delivery of cisplatin (Cis) and doxorubicin (Dox) containing thermosensitive liposomes (TSLs) with mild hyperthermia against cancer in vitro and in vivo. The polyethylene glycol coated DPPC/DSPC, thermosensitive and DSPC, non-thermosensitive liposomes incorporating Cis and Dox were prepared and characterized. A conventional Differential Scanning Calorimetry (DSC) technique and Fourier Transform Infrared Spectroscopy (FT-IR) were applied to study drug-phospholipid interaction and compatibility. The chemotherapeutic efficacy of these formulations was evaluated in benzo[a]pyrene (BaP) induced fibrosarcoma under hyperthermic condition. The size diameter of prepared thermosensitive liposomes was measured to be 120 ± 10 nm. The DSC data exhibited the changes in the curves of DSPC + Dox and DSPC + Cis while comparing the pure DSPC and drugs. However, the FITR showed same spectrum of phospholipids and drugs individually and in the mixture as well. The data showed higher efficacy of Cis-Dox-TSL as 84% inhibition in tumor growth was recorded in this group of animals in hyperthermic condition. The Kaplan-Meir curve revealed, 100% and 80% survival of the animals in the groups treated with Cis-Dox-TSL under hyperthermia and Cis-Dox-NTSL without hyperthermia, respectively. However, Cis-TSL as well as Dox-TSL exhibited 50% survival, while only 20% survival was recorded in the groups of animals treated with Dox-NTSL and Cis-NTSL. The flow cytometry analysis revealed that Cis-Dox-NTSL augments the induction of apoptosis in the tumor cells which was recorded as 18%. As expected, Cis-Dox-TSL showed great potential as 39% of cells were measured as apoptotic cells, significantly very high in comparison to Cis-Dox-NTSL, Dox-TSL and Cis-TSL as well. The apoptotic analysis of the cells by flow cytometry clearly indicated the effect of hyperthermia during the treatment while Cis-Dox-TSL formulation was administered. Finally, the immunohistochemical analysis of the tumor tissues by confocal microscopy exhibited several fold increases in the expression of pAkt in the animals treated with vehicles in Sham-NTSL as well as Sham-TSL. However, Cis-Dox-TSL showed great reduction in the expression of Akt, as it declined by 11-fold. The results of the present study directed the role of concomitant delivery doxorubicin and cisplatin containing thermosensitive liposomes under hyperthermic conditions for the development of a novel therapeutic strategy for the treatment of cancer.
Keywords: Combination therapy, cisplatin, doxorubicin, thermosensitive liposomes, fibrosarcoma, breast cancer, prostate cancer
Introduction
The chemotherapy using a single drug often fails to achieve complete regression of cancer due to the rapid development of drug resistance in tumor cells [1-3]. Therefore, most clinical regimens comprise multiple non-cross-resistant anticancer agents [4-7]. Among all available chemotherapeutic agents, the most commonly used are arguably anthracycline and platinum-based drugs [8-15]. The reports suggested that the use of concomitant administration of two classes of drugs, improve their potential, while treating different types of cancer i.e. ovarian cancer, advanced breast cancer, endometrial carcinoma etc. [16-19]. Several in vitro as well as in vivo studies also explained the amplification in the activity of drugs due to the synergism, using the co-administration of doxorubicin and cisplatin, while comparing the effects of these drugs administered alone [20-24]. Irrespective of improved therapeutic efficacy with the co-administration of cisplatin and doxorubicin, the reports from the phase III clinical trials in endometrial carcinoma, restrict the use of this combination due to their adverse side effects [17,18].
The use of lipid-based nanocarriers for the delivery of chemotherapeutic agents has shown great impact in the development of drug formulations against different types of cancer. However, there are some principal issues that should be addressed, while optimizing liposomal anti-cancer drug formulations. The high entrapment efficiency with pertinent stability, prolonged circulation with retention of the payload until its delivery to the tumor site, are the key requirements for these liposomes [25-28]. Numerous studies suggest that the coating of liposomes with polyethylene glycol (PEG) makes them long circulating sterically stabilized, and protects from the accessibility of the enzymes. Furthermore, the preparation of nanosized PEGylated liposomes, around 200 nm size, increases their extravasation in solid tumors due to the discontinuous vasculature associated with angiogenesis [29-32]. The current developments in the field of nanomedicine are the intravascular triggered drug delivery systems (IV-DDS), that can be triggered through the internal (pH, enzymes etc.) or external stimuli (e.g., temperature, light, ultrasound, electromagnetic fields, X-rays) [33,34].
As evident from several reports, the heat triggered stimuli at hyperthermic conditions showed great potential in cancer chemotherapy. The temperature sensitive liposomal formulations of chemotherapeutic agents in hyperthermia increase the release of their payloads to the tumor cells due to enlarged extravasation and the accumulation of formulations in the heated site of the tumor [35-38]. Interestingly, the hyperthermia on the tumor site also has been reported to inhibit the cancer cells following the induction of stress, due to high acidic condition with hypoxia, and the scarcity of the nutrients as well, in tumor microenvironment [39]. Several reports suggested the presence of dipalmitoyl phosphocholine (DPPC) in the phase transition of 41-42°C with addition of other phospholipids, mainly distearoyl phosphatidylcholine (DSPC), in the preparation of thermosensitive liposomes improve heat mediated release. However, the molar ratio of different lipids and PEG plays the significant role in the development of effective thermosensitive liposomes [40-42]. Previously we, prepared and characterized doxorubicin and cisplatin encapsulated thermosensitive liposomes individually, comprising DPPC, DSPC, DPPE-PEG in the molar ratio of 95:5:0.05 W/W. The DSPC and DPPE-PEG (99.95:0.05 W/W) was used in the preparation of non-thermosensitive liposomes. We demonstrated that the cumulative release of the drug by Dox-THL was measured to be 95% after 1 hour of incubation at 42°C, while it was only 6% at 37°C. The Dox-NTSL showed less than 70% release at 37°C, whereas less than only 7.78% at 42°C. Similarly, Cis-TSL exhibited 93% release of the drugs, though it was recorded only 5.83% by the same at 37°C [43].
Keeping these facts into consideration, the liposomes mediated delivery of chemotherapeutic agents in various combinations at hyperthermal condition may amplify the potential entrapped of drugs, rather than administered alone. The present study focused on the concomitant delivery of doxorubicin and cisplatin through liposome-based thermosensitive nanoparticles as a perspective in the treatment of cancer in animal models. The efficacy of the doxorubicin and cisplatin combinations in these liposomes were investigated in vitro as well as in vivo.
Materials and methods
Materials
Distearoyl phosphatidylcholine (DSPC), 1,2-dipalmitoyl-sn-glycero-3-phosphatidyl choline (DPPC), and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(poly ethylene glycol)-5000 (DPPE-PEG5000)], were procured from Sigma-Aldrich (St. Louis, MO, USA). Doxorubicin hydrochloride and Cisplatin were procured from Tocris Bioscience (Bristol, UK). The Annexin V-FITC/PI, Cell Cytotoxicity Assay kits, α-Akt rabbit monoclonal, α-pAkt mouse monoclonal antibodies, Goat α-rabbit, α-mouse IgH H&L (Alexa Fluor 488 and 647), Benzo[a]Pyrene (BaP) were, purchased from Abcam (Cambridge, USA). MDA-MB-231 (ECACC 92020424), LNcaP (ECACC 89110211), PC-3 (ECACC 90112714), were commercially purchased from ECACC (European Collection of Cell Cultures), Salisbury, UK. SKBR3 (ATCC HTB30) was procured from ATCC (American Type Culture Collection), VA, USA.
Methods
Thermosensitive and non-thermosensitive liposome preparation and drug encapsulation
The thermosensitive liposomes composed of DPPC:DSPC:DPPE-PEG 5000 (95:5:0.05 W/W) and non-thermosensitive liposomes composed of DSPC containing doxorubicin and cisplatin were prepared by thin-film hydration method. The estimated amounts of phospholipids were dissolved in chloroform in a round bottom flask (RBF) followed by the evaporation using a rotary evaporator in N2 environment. Subsequently, the dried thin films of lipids were hydrated by doxorubicin and cisplatin individually using rotary at room temperature for 2-3 hours keeping the N2 environment. In the preparation of sham liposomes, the lipid film was hydrated with normal saline. The suspension of multilamellar liposome vesicles was then treated by freeze-thaw for ten cycles. The preparation was subsequently extruded ten times each through 800, 400, and 100 nm decreasing pore sized polycarbonate membranes with 10 cycles for each size of polycarbonate membrane using an extruder device Lipex Biomembranes Inc, heated at 50°C. The drug containing liposomal formulations were centrifuged at 30,000 rpm followed by the discard of the supernatant as unentrapped drugs. The supernatant was diluted in distilled water and the amount of drug present in the supernatant was determined by reverse phase automatic HPLC system equipped with UV/Vis detector 2489 (Waters, USA). Entrapment efficiency was calculated as the difference between the initial amount of drug added and that present in the supernatant as a free drug, according to the following formula.
Characterization of the prepared liposomes
The size diameter of the prepared thermosensitive
The size diameter of thermosensitive liposomes was measured using a laser particle size analyser (SLAD-400 from Shimadzu, Japan).
Differential scanning calorimetry
A conventional differential scanning calorimetry (DSC) technique was applied to study the effect of cisplatin and doxorubicin in DSPC phospholipids as well as to study the thermotropic properties of prepared liposomes using a Shimadzu DSC-60 calorimeter. DPPC, DSPC, DPPE-PEG 5000 separately was dissolved in chloroform. The solvents were then evaporated by a rotary evaporator under vacuum at temperatures above the transition temperature of the phospholipids. For measurements, the dry residue of phospholipid or dry thermosensitive liposomes composed of DPPC:DSPC:DPPE-PEG 5000 was dispersed in appropriate amounts of double distilled water by vortexing. An aliquot (approximately 5 mg) was sealed into stainless steel capsules obtained from Shimadzu. DSC curves were obtained on a Shimadzu DSC-60 calorimeter. Prior to scanning, the samples were held above their phase transition temperature for 1-2 minutes to ensure equilibration. All samples were scanned at least twice until identical curves were obtained using a scanning rate of 5°C/minute for all samples.
Fourier transform infrared spectroscopy (FT-IR)
Fourier transform infrared spectroscopy (FT-IR) (BRUKER, OPTIK GmbH, Germany) was used to study the compatibility of cisplatin and doxorubicin with phospholipids by comparing the spectra showing the functional groups for all ingredients before and after the preparation of the liposomes. The FT-IR spectra were collected from 4000 to 400 cm-1.
Cell cytotoxicity assay
To see the combined effect of Dox and Cis, we took the IC10 of all the formulations of Dox and Cis determined by cell cytotoxicity assay as described earlier in our previous published study [44]. All the formulations were evaluated in four types of cancer cells lines (SKBR3 & MDA-MB-231, breast cancer and PC-3% LNcaP, prostate cancer) at 37°C as well as 42°C as described in Supplementary Table 1. Briefly, the cells were seeded following 80% confluency into 96-wells cell culture plates (10,000 cells/well) for 24 hours. The cells were then treated with various formulations as stated in Supplementary Table 1, and incubated at 37°C and 42°C accordingly in 5% CO2 atmosphere for 48 hours. Then, the reagent (20 μl) from the cell cytotoxicity assay kit was added in each well and incubated at 37°C followed by the measurements at 590 nm in a microplate reader. The viability of the cells was measured using the following formula.
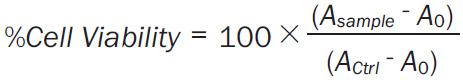 |
Asample is the absorbance of FSE treated cells.
Actrl is the absorbance of untreated cells.
A 0 is the absorbance of the background of non-cell control (only media).
In vivo studies
Considering the 5-10% accidental mortality due to the long duration of the experimental plan, a total of 180 female Swiss mice (age of 8-10 weeks) were obtained from the animal house facility of College of Pharmacy, Qassim University, Saudi Arabia. The experiments involving the animals were performed after the approval of the animal ethical committee of the College, following with the National Research Council (USA) Guide for the Care and Use of Laboratory Animals. All the animals were exposed to Benzo-a-Pyrene (BaP) with 1 mg/kg b.w subcutaneously in the corn oil (200 µl/mouse). All the surviving animals were euthanized by CO2 inhalation at the end of the study following approved procedure. However, the mice were also euthanized in a CO2 chamber within 2-4 hours, whether they were moribund, or observed by a lack of sustained purposeful response to gentle stimuli. None of the mice died during the experiment before euthanasia.
Histopathological analysis of the tumor tissues for the confirmation of the development of cancer
Three mice were sacrificed when the size of the tumor reached 200 mm3 after the administration of BaP for approximately 100 days, followed by the excision of tumor tissues for the histopathological study. Briefly, the formalin-fixed tumor tissues were processed, sectioned, and subjected to hematoxylin and eosin (H & E) staining following the standard method. The H & E staining of the tumor tissues was studied under the light microscope at 100× magnification, bar = 100 µm.
Treatment
When the tumor size reached ≈200 mm3, the animals were treated intravenously (i.v) with various formulations thrice a week for three weeks, as illustrated in Figure 1. The doses of Dox and Cis were chosen as 5 mg/kg b.w, as described in our previous studies [3,44]. The mice randomly divided into eight groups, with twenty animals in each group. The mice (5 animals from each group) were sacrificed 4 weeks after the first dose of treatment. The observational study of survival was continued for 16 weeks after the first dose of treatment for the rest of 15 mice in each group.
Figure 1.
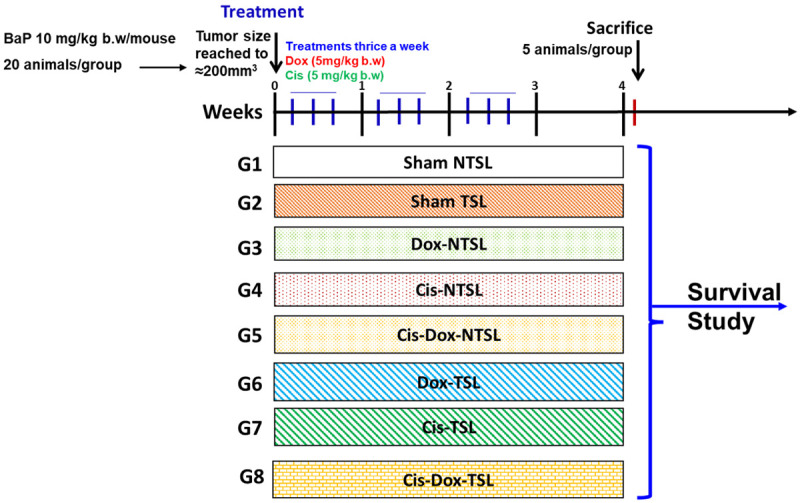
Schematic illustration of In Vivo treatment plan various formulations.
Water bath hyperthermia
The water bath hyperthermia was conducted as described by Willerding et al. 2016 and followed all the safety protocol for animals stated in the ‘Ethical Approval Section’ [45]. The animals that belonged to the hyperthermia groups were placed in a covered water bath with a constant temperature of 42°C. The tumor bearing part of the mice was submerged into the water bath and heated to the target temperature for 60 minutes after the injection. The tumor was kept covered with the plastic foil during the hyperthermia to avoid any damage to the skin.
Assessment of tumor volume
The tumor growth of each mouse was monitored every week during the experiment. The tumor volume was calculated in cubic millimeters by measuring in two directions with the help of a digital calliper according to the formula V = D × d2 × π/6, where ‘D’ and ‘d’ are large and small dimensions, respectively.
Tissue processing
After the sacrifice of the mice, tumor tissues were excised into 3-4 pieces for various examinations. One piece of the tissue from each mouse was taken in tissue cassettes in the preparation of tissue slides for immunofluorescence analysis, in 10% buffered neutral formalin, whereas the rest of the tissues were stored at -80°C.
Annexin V-FITC/PI apoptotic assay by flow cytometry
The changes in the proportion of cells in the viable, necrotic, early, and late apoptotic stages were evaluated using the Annexin V-FITC/PI apoptosis staining kit (Miltenyi Biotec, Germany), by the flow cytometry. Briefly, the single-cell suspension of tumor tissues was prepared using the gentle MACS tissue dissociator (Miltenyi Biotec, Germany), followed by filtering the cells with a 70 µm mesh cell strainer. The filtered cells were centrifuged at 300 g for 10 minutes and suspended in the binding buffer. The cells were incubated with Annexin V-FITC for 30 minutes at room temperature followed by the addition of PI before the acquisition of samples. The samples were measured on MACSQuant Analyzer 10 (Miltenyi Biotec, Germany) and analyzed using FlowJo software v10.7.
Statistical analysis
The mean values and standard errors for all samples were calculated for different treated groups. The significant difference between the groups was measured by the One-way and Two-way ANOVA, Tukey’s multiple comparison tests using Prism 9. P-value <0.05 was considered statistically significant.
Results
Schematic diagram (Figure 2) representing the process of preparation of thermosensitive liposomes and the in vitro/in vivo pharmacological evaluation for the concomitant delivery of doxorubicin and cisplatin through liposome-based thermosensitive nanoparticles. The development of the fibrosarcoma in the untreated mice was ascertained by the histopathological analysis as described in Supplementary Figure 2.
Figure 2.
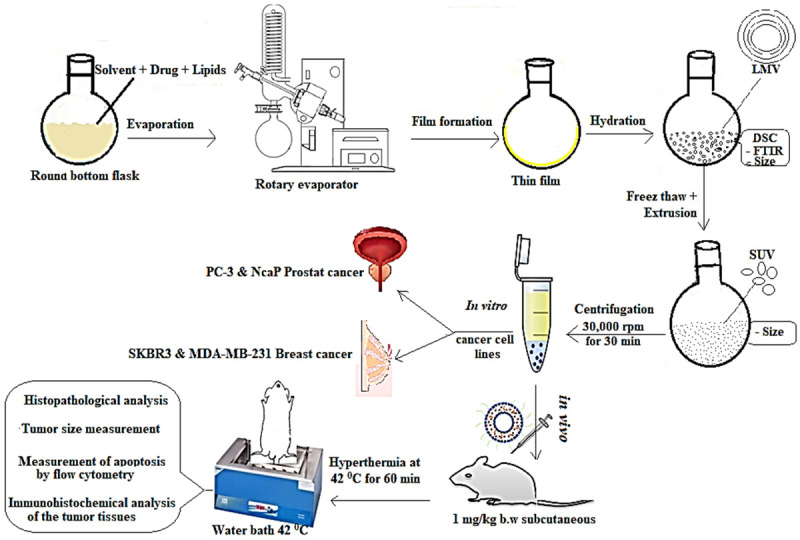
Schematic diagram representing the process of preparation of thermosensitive liposomes and the in vitro/in vivo pharmacological evaluation for the concomitant delivery of doxorubicin and cisplatin through liposome-based thermosensitive nanoparticles.
Characterization of thermosensitive liposome
The particle size distribution data revealed the size of multilamellar vesicles of TSLs within the range of 0.05 to 50 µm in diameter. However, the continuous reduction in size of TSLs was measured with the sequential extrusion using varying size of polycarbonate membranes, which was finally analysed as 120 ± 10 nm (Figure 3). In our previous study, we reported the entrapment efficiency of Dox and Cis in the TSLs as 41 ± 1.8% and 28.7 ± 1.3%, correspondingly [43]. We also demonstrated in vitro release kinetics of DOX-TSL and Cis-TSL, that were measured as 93-95% at 42°C, while it was recorded 4.7-6.5% at 37°C. The trapping efficiency for doxorubicin and cisplatin was 41 ± 1.8% and 28.7 ± 1.3%, respectively [43].
Figure 3.
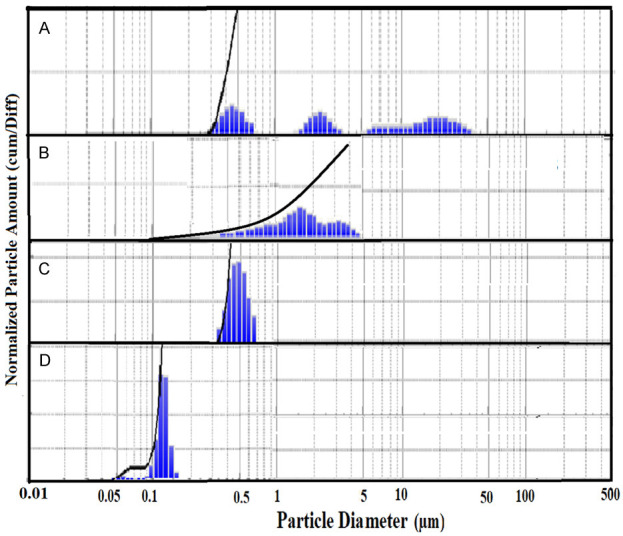
Particle size distribution (A) before extrusion, after extrusion from (B) 800 nm (C) 400 nm, and (D) 100 nm polycarbonate membrane.
The DSC curves from our previous study exhibited endothermic peaks for DPPC-DSPC:DPPE-PEG 5000 in the molar ratio of 95:5:0.05 W/W at 42.1°C [43]. As depicted in Figure 4, three endothermic peaks were measured for pure doxorubicin at 216.8°C, 243.2°C and 265.8°C (Figure 4G) while pure cisplatin showed two endothermic peaks at 286.5°C and 322.4°C followed by the peak at 337.3°C (Figure 4F). The curves for pure DSPC showed pretransition and main transition peaks at 52.3°C and 54.8°C respectively (Figure 4C). However, these were affected due to the presence of hydrophilic Dox (0.3%) as the main transition peak was shifted from 54.8°C to 50.1°C (Figure 4A). At higher concentration of Dox (0.6%), the pre-transition peak was disappeared and the width of the main peak was increased, while the height of the peak was decreased due to the incorporation of hydrophilic doxorubicin close to the hydrophilic part of phospholipid (head polar group) (Figure 4B). The incorporation of lipophilic cisplatin showed a physical interaction with the alkyl chain of phospholipids. The presence of cisplatin 0.3% into DSPC produces a shift of the main peak from 54.8°C to 46.2°C and complete disappearance of the pre-transition peak (Figure 4D). At a higher concentration of cisplatin (0.6%), the main peak was widely broadened (Figure 4E). As showed in Figure 5, the FTIR spectrum for DPPC and DSPC with and without doxorubicin and cisplatin showed no change in the FTIR spectrums while comparing pure phospholipids and pure drugs with mixture of drug-phospholipids.
Figure 4.
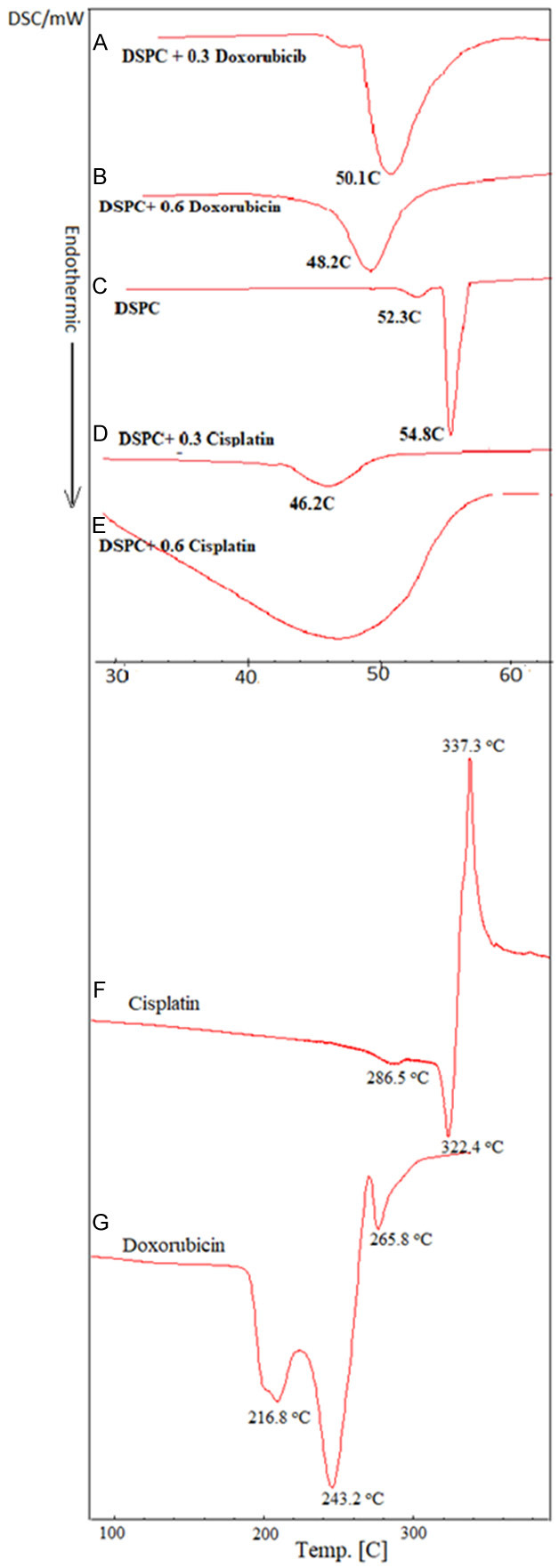
The DSC curves (A) DSPC + 0.3% Doxorubicin, (B) DSPC + 0.6% Doxorubicin, (C) DSPC, (D) DSPC + 0.3% Cisplatin, (E) DSPC + 0.6% Cisplatin, (F) Cisplatin, (G) Doxorubicin.
Figure 5.
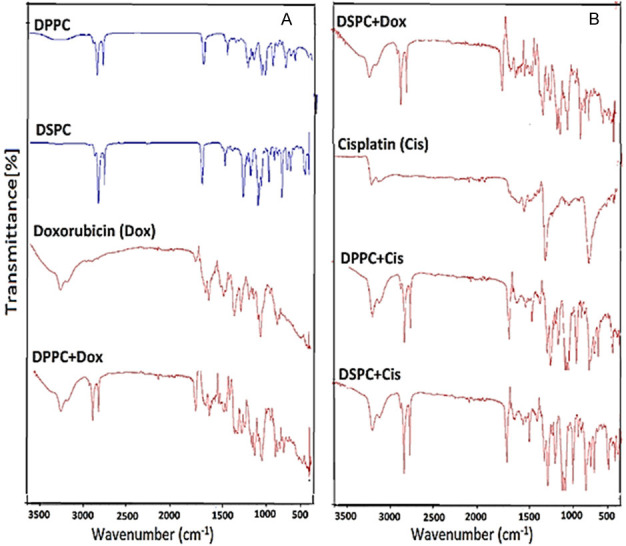
The FTIR spectrum (A) DPPC, DSPC, Doxorubicin and DPPC + Doxorubicin, (B) DSPC + Doxorubicin, Cisplatin, DPPC + Cisplatin and DSPC + Cisplatin.
Effect of various formulations of Cis and Dox on tumor growth in BaP induced fibrosarcoma
The tumor size measuring data revealed the superior efficacy of Cis and Dox co-administration containing thermosensitive liposomes in hyperthermic conditions as more than 84% growth inhibition was calculated in G8 (Figure 6A, 6B). The animals from the G5 (Cis-Dox-NTSL) groups showed more than 72% tumor growth inhibition clearly indicates the potential of the combination. Interestingly, Dox-TSL (G6) and Cis-TSL (G7) administered alone showed nearly 65% reduction in tumor growth. However, the tumor growth inhibition was recorded less than 42% in the animals treated with Dox-NTSL (G3) and Cis-NTSL (G4) (Figure 6A, 6B). As depicted in Figure 6A, the tumor growth was reduced more than 10% in week 4, one week after the last dose in G8. Interestingly all the animals from thermosensitive liposomal treated groups prevented the tumor growth as 196.6 mm3, 190.0 mm3, and 80 mm3 were estimated in G6, G7, and G8, respectively.
Figure 6.
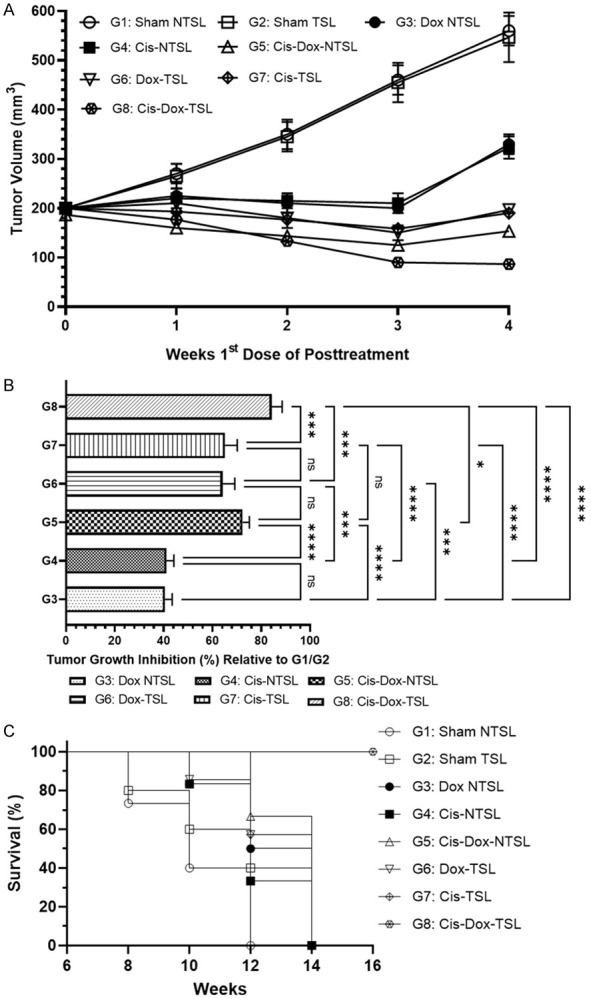
Effect of various formulations of Cis and Dox on tumor growth in BaP induced fibrosarcoma. (A) Tumor size during the treatment, (B) Tumor inhibition relative to G1/G2, (C) Frequency of survival of the animals. The values are expressed as mean ± SD of 5 mice from each group. NSNo significance within the groups, ****Significant difference between the treated groups P<0.0001, ***Significant difference between the treated groups P<0.001.
The Kaplan-Meir curve (Figure 6C) showed the 100% survival of the animals in G8 groups while it was found 50%, in G6 & G7, but 20% in G3 & G4. The survival data revealed that 80% survival in the G5, clearly indicates the efficacy of the co-administration, which is escalated by the treatment with thermosensitive liposomes during hyperthermia. The results also showed the 100% survival of the animals in G5 and G6 after 12 weeks (Figure 6C).
Effect of various formulations of Cis and Dox formulations in the induction of apoptosis by flow cytometry in the cells from the tumor tissues of the treated animals
The flow cytometry analysis revealed that G1, G2, and G4 induced no significant apoptosis in the tumor cells as clearly indicates that sham liposomes, as well as CIS-NTSL, had no effect on the induction of apoptosis. Though Dox-NTSL showed a significant number of apoptotic cells but very low as observed to be 2.3% (Figure 7A, 7B). The co-administration of Cis-Dox-NTSL augments the induction of apoptosis in the tumor cells as found 18% in G5. The co-administration of Cis-Doxo in TSL showed great potential as 39% of cells were measured as apoptotic cells in G8, significantly very high in comparison to G5, G6 and G7 as well. The flow cytometry data clearly indicated the effect of hyperthermia during the treatment while administered in combination in thermosensitive liposomes (Figure 7A, 7B).
Figure 7.
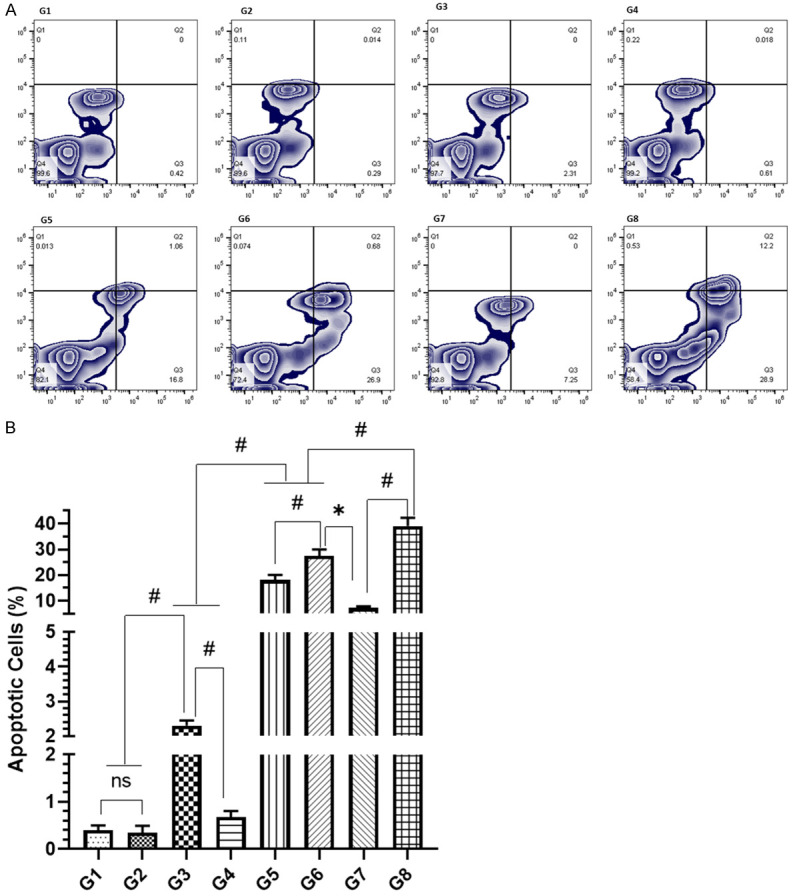
Effect of various formulations on induction of apoptosis by flow cytometry analysis (A) representative images of flowjo analysis of the cells (B) Detailed analysis of apoptotic cells. The values are expressed as mean ± SD of 5 mice from each group. NS No significance within the groups, *Significant difference between the treated groups but in reverse order, #Significant difference between the treated groups.
Effect of various formulations of cisplatin and doxorubicin on the expression Akt and pAkt by immunofluorescence analysis under a confocal microscope
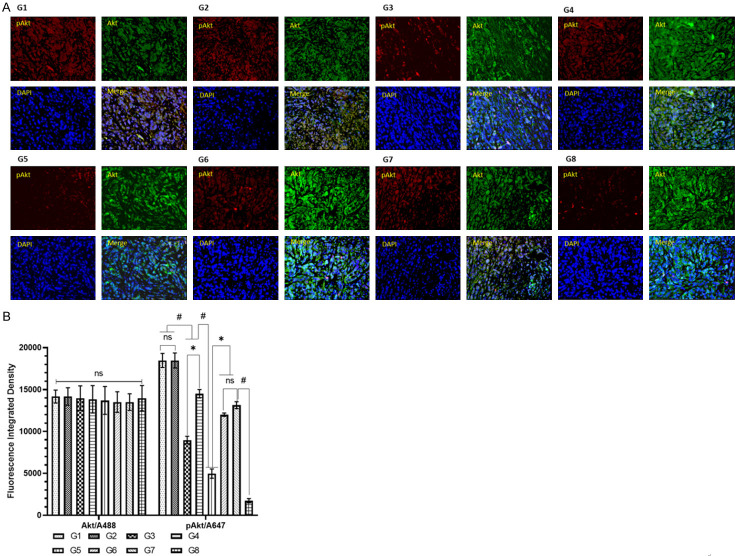
The immunohistochemical analysis of the tumor tissues by confocal microscopy revealed several fold increases in the expression of pAkt in G1 and G2 which was reduced 11-fold by G8 (Figure 8A, 8B). The data also showed the potential of G5 as it downregulated the pAkt 3.7-fold in comparison to G1 and G2 (Figure 8A, 8B).
Figure 8.
Effect of various formulations of Doxorubicin and Cisplatin on Akt and pAkt expression by immunohistochemical analysis of the tissues under a confocal microscope (A) representative immunofluorescence image of tumor with Akt (A488), pAkt (A647), nucleus/DNA (DAPI) (B) Detailed analysis of fluorescence integrated density as an expression of Akt and pAkt. The values are expressed as mean ± SD of 5 mice from each group. NS No significance within the groups, *Significant difference between the treated groups but in reverse order, #Significant difference between the treated groups.
Discussion
To date various strategies have been explored to utilize the tumor microenvironment for activating the drug release from the liposomes as thermal, magnetic, ultrasonic, chemical, electric etc. Some of the approaches exploit the presence of endogenous physiological signal as acidic pH, glutathione (GSH) redox potential, certain enzymes, hypoxia, adenosine triphosphate (ATP). However, the studies revealed the advantage of exogenous triggering over endogenous as required accuracy in the control of stimuli could be possible in the latter, while inter-patient changes have been observed by the former [38,46-52]. The therapeutic potential of thermosensitive liposomes at mild hyperthermia (above the physiological temperature but below 42°C) has shown the great impact in the development of novel formulation in the treatment of cancer. Therefore, the hyperthermia not only inhibit the tumor growth directly, but also augment the effect of chemotherapeutic agents. Moreover, this strategy amplifies the potential of drug, while entrapped in heat sensitive liposomes by maximizing the delivery of payloads at the tumor site [40,43,53]. In the current study, we evaluated the concomitant delivery of thermosensitive liposomal formulations of doxorubicin and cisplatin in hyperthermia against fibrosarcoma. As evident from several studies, the use of 16-carbon saturated fatty acid chains DPPC with phase transition temperature (Tc) more than 41°C is ideal strategy in the preparation of TSLs. But the ratio of phospholipids and the methods of preparation of liposomes play the significant role in the development of effective formulation according to the requirements. The addition of DSPC with DPPC has been shown great effect on cellular uptake and the release of the drugs [36,41,54]. Besides, the coating of the liposomes with PEG can decrease their uptake to reticular endothelial system (RES), and prolong the circulation [55,56]. The preparation of liposomes by thin layer hydration method using DPPE-DSPC-PEG500 as 95:5:0.05 W/W was measured as most suitable phospholipid ratio with a transition onset at 41°C and the DSC peak at 42.1°C as thermosensitive liposomes [43]. Due to the physical interaction observed in the DSC curves between phospholipids and drugs (Cis and Dox), it was important to investigate for any possible chemical interaction between drugs (cisplatin and doxorubicin) and phospholipids, to achieve that, FTIR spectrum was employed. However, no shift or appearance in the new peak was detected which direct the absence of any chemical interaction between phospholipids and drugs (Figures 4, 5). Li et al. 2020 showed the cytotoxic potential of oxaliplatin entrapped DPPC-DSPE-PEG2000 (10:1) TSLs in RKO colonic cancer and 4T1 breast cancer cells [57]. The Ml1 loaded DPPC-MSPC-DSPE-PEG2000 containing TSLs have been demonstrated to inhibit the cell viability upon hyperthermia treatment in CT6 colon carcinoma cells [58]. Earlier we reported the efficacy of Cis-TSL and Dox-TSL at 42°C, while the cell viability at 37°C against these cell lines [43]. The concomitant delivery of TSLs entrapping Cis and Dox in this ratio of lipids exhibited high sensitivity in tested breast cancer (SKBR3 & MDA-MB-231) and prostate cancer (PC-3 & LNcaP) cell lines at 42°C (Supplementary Figure 1).
The treatment of melphalan encapsulated TSLs in combination with hyperthermia and radiation in B16F10 transplanted murine melanoma exhibited 33% decline tumor size with 70% survival [59]. The intraperitoneal administration of gemcitabine loaded TSLs with mild hyperthermia revealed significant decrease in tumor volume in MiaPaCa-2 cells implanted xenografts [60] Recently, oxaliplatin encapsulated TSLs treatment at hyperthermia were shown to reduce 60% in the growth of 4T1 inoculated tumor xenograft, while survival was recorded to be 42.8% [57]. Earlier we reported 64% tumor growth inhibition following the administration of Dox-TSL in combination with hyperthermia. This study extended our analysis as the Cis-TSL inhibited 72% tumor size while comparing sham-TSLs. Remarkably, the concomitant delivery of Cis and Dox TSL showed 84% inhibition in tumor growth as the tumor volume was measured to be 80 mm3 (Figure 6A, 6B). The efficiency of these also could be noticed in the Kaplan Meier curve as 100% survival of the animals was recorded, while treated with Cis-Dox-TSL (Figure 6C).
The phosphorylated Akt (pAKT) also called protein kinase B (PKB) is the major downstream effector of PI3K pathway, plays the significant role in the initiation, promotion and progression of chemically induced cancer [61,62]. Several studies suggested the downregulation of pAKT leading to the apoptosis in cancer cells treated with chemotheraptic agents including Dox. The immunofluorescence analysis of tumor tissues showed several fold decreases in the expression of Akt in the animals treated with Cis-Dox-TSLs in combination with HT (Figure 8). The apoptotic cell analysis by flow cytometry also supported the efficacy of Cis-Dox-TSL with HT as it triggered 39% cancerous cells into programmed cell death (Figure 7). The results clearly revealed the potential of concomitant delivery of doxorubicin and cisplatin containing thermosensitive liposomes with hyperthermia in the sensitization of cancer cells.
Conclusion
The in vitro and in vivo results of the present study directed the role of drug combinations of thermosensitive liposomes containing doxorubicin and cisplatin and hyperthermic conditions for the development of a novel therapeutic strategy for the treatment of cancer.
Acknowledgements
This work is a part of a project supported by grants from the National Plan for Science, Technology, and Innovation (12-BIO2333-09), Qassim University.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resistance. 2019;2:141–160. doi: 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maswadeh H, Aljarbou A, Alorainy M, Rahmani A, Khan M. Coadministration of doxorubicin and etoposide loaded in camel milk phospholipids liposomes showed increased antitumor activity in a murine model. Int J Nanomedicine. 2015;10:2847–2855. doi: 10.2147/IJN.S80820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szumilak M, Wiktorowska-Owczarek A, Stanczak A. Hybrid drugs-a strategy for overcoming anticancer drug resistance? Molecules. 2021;26:2601. doi: 10.3390/molecules26092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, Yang W, Tian C, Miao Z, Wang T, Yang S. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6:201. doi: 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395:1078–1088. doi: 10.1016/S0140-6736(20)30164-1. [DOI] [PubMed] [Google Scholar]
- 7.Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 2018;834:188–196. doi: 10.1016/j.ejphar.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Xu C, Gao X, Yao Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022;12:2115–2132. doi: 10.7150/thno.69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muggia FM, Bonetti A, Hoeschele JD, Rozencweig M, Howell SB. Platinum antitumor complexes: 50 years since Barnett Rosenberg’s discovery. J. Clin. Oncol. 2015;33:4219–4226. doi: 10.1200/JCO.2015.60.7481. [DOI] [PubMed] [Google Scholar]
- 10.Sazonova EV, Kopeina GS, Imyanitov EN, Zhivotovsky B. Platinum drugs and taxanes: can we overcome resistance? Cell Death Discov. 2021;7:155. doi: 10.1038/s41420-021-00554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao Z, Shen K, Zhu L, Xu M, Yu F, Xue D, Hegen L, Cheng X. Comparisons of cardiotoxicity and efficacy of anthracycline-based therapies in breast cancer: a network meta-analysis of randomized clinical trials. Oncol Res Treat. 2019;42:405–413. doi: 10.1159/000500204. [DOI] [PubMed] [Google Scholar]
- 12.de Gregorio A, Janni W, Friedl TWP, Nitz U, Rack B, Schneeweiss A, Kates R, Fehm T, Kreipe H, Christgen M, Kümmel S, Trapp E, Wuerstlein R, Hartkopf A, Clemens M, Reimer T, Häberle L, Fasching PA, Gluz O, Harbeck N. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. 2022;126:1715–1724. doi: 10.1038/s41416-021-01690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobczuk P, Czerwińska M, Kleibert M, Cudnoch-Jędrzejewska A. Anthracycline-induced cardiotoxicity and renin-angiotensin-aldosterone system-from molecular mechanisms to therapeutic applications. Heart Fail Rev. 2022;27:295–319. doi: 10.1007/s10741-020-09977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwardson D, Narendrula R, Chewchuk S, Mispel-Beyer K, Mapletoft J, Parissenti A. Role of drug metabolism in the cytotoxicity and clinical efficacy of anthracyclines. Curr Drug Metab. 2015;16:412–426. doi: 10.2174/1389200216888150915112039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigioni M, Benzo A, Irrissuto C, Lopez G, Curatella B, Maggi CA, Manzini S, Crea A, Caroli S, Cubadda F, Binaschi M. Antitumour effect of combination treatment with Sabarubicin (MEN 10755) and cis-platin (DDP) in human lung tumour xenograft. Cancer Chemother Pharmacol. 2008;62:621–629. doi: 10.1007/s00280-007-0645-y. [DOI] [PubMed] [Google Scholar]
- 16.Martoni A, Panetta A, Angelelli B, Melotti B, Pannuti F. A phase II study of carboplatin and cyclophosphamide in advanced ovarian carcinoma. J Chemotherapy. 1993;5:47–51. doi: 10.1080/1120009x.1993.11739209. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen D, Dombernowsky P, Larsen SK, Hansen OP, Skovsgaard T. Epirubicin or epirubicin and cisplatin as first-line therapy in advanced breast cancer. A phase III study. Cancer Chemother Pharmacol. 2000;46:459–466. doi: 10.1007/s002800000178. [DOI] [PubMed] [Google Scholar]
- 18.Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, Shu L. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J. Clin. Oncol. 2004;22:3902–3908. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 19.Gilman KK, Han S, Won YW, Putnam CW. Complex interactions of lovastatin with 10 chemotherapeutic drugs: a rigorous evaluation of synergism and antagonism. BMC Cancer. 2021;21:356. doi: 10.1186/s12885-021-07963-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, Richard K, Robert AB, Annekathryn GR, Tucker B. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a gynecologic oncology group study. J. Clin. Oncol. 2004;22:2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 21.Katzenstein HM, Malogolowkin MH, Krailo MD, Piao J, Towbin AJ, McCarville MB, Tiao GM, Dunn SP, Langham MR, McGahren ED, Finegold MJ, Ranganathan S, Weldon CB, Thompson PA, Trobaugh-Lotrario AD, O’Neill AF, Furman WL, Chung N, Randazzo J, Rodriguez-Galindo C, Meyers RL. Doxorubicin in combination with cisplatin, 5-flourouracil, and vincristine is feasible and effective in unresectable hepatoblastoma: a children’s oncology group study. Cancer. 2022;128:1057–1065. doi: 10.1002/cncr.34014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsvetkova D, Ivanova S. Application of approved cisplatin derivatives in combination therapy against different cancer diseases. Molecules. 2022;27:2466. doi: 10.3390/molecules27082466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu HQ, Jin HJ, Wang C, Zhang ZH, Ruan HY, Sun LY, Yang C, Li YJ, Qin WX, Wang CC. Synergistic cisplatin/doxorubicin combination chemotherapy for multidrug-resistant cancer via polymeric nanogels targeting delivery. ACS Appl Mater Interfaces. 2017;9:9426–9436. doi: 10.1021/acsami.6b16844. [DOI] [PubMed] [Google Scholar]
- 24.Guo XL, Kang XX, Wang YQ, Zhang XJ, Li CJ, Liu Y, Du L. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019;84:367–377. doi: 10.1016/j.actbio.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Moosavian SA, Bianconi V, Pirro M, Sahebkar A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin Cancer Biol. 2021;69:337–348. doi: 10.1016/j.semcancer.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Xiao D, Zhou R. Advances in the application of liposomal nanosystems in anticancer therapy. Curr Stem Cell Res Ther. 2021;16:14–22. doi: 10.2174/1574888X15666200423093906. [DOI] [PubMed] [Google Scholar]
- 27.Barenholz Y. Doxil® - the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Chaurasiya A, Gorajiya A, Panchal K, Katke S, Singh AK. A review on multivesicular liposomes for pharmaceutical applications: preparation, characterization, and translational challenges. Drug Deliv Transl Res. 2022;12:1569–1587. doi: 10.1007/s13346-021-01060-y. [DOI] [PubMed] [Google Scholar]
- 29.Khan A, Alsahli MA, Aljasir MA, Maswadeh H, Mobark MA, Azam F, Allemailem KS, Alrumaihi F, Alhumaydhi FA, Almatroudi AA, AlSuhaymi N, Khan MA. Experimental and theoretical insights on chemopreventive effect of the liposomal thymoquinone against Benzo[a]pyrene-induced lung cancer in Swiss albino mice. J Inflamm Res. 2022;15:2263–80. doi: 10.2147/JIR.S358632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alrumaihi F, Khan MA, Babiker AY, Alsaweed M, Azam F, Allemailem KS, Almatroudi AA, Ahamad SR, AlSuhaymi N, Alsugoor MH, Algefary AN, Khan A. The effect of liposomal diallyl disulfide and oxaliplatin on proliferation of colorectal cancer cells: in vitro and in silico analysis. Pharmaceutics. 2022;14:236. doi: 10.3390/pharmaceutics14020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alrumaihi F, Khan MA, Babiker AY, Alsaweed M, Azam F, Allemailem KS, Almatroudi AA, Ahamad SR, AlSuhaymi N, Alsugoor MH, Algefary AN, Khan A. Lipid-based nanoparticle formulation of diallyl trisulfide chemosensitizes the growth inhibitory activity of doxorubicin in colorectal cancer model: a novel in vitro, in vivo and in silico analysis. Molecules. 2022;27:2192. doi: 10.3390/molecules27072192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan A, Alsahli MA, Aljasir MA, Maswadeh H, Mobark MA, Azam F, Allemailem KS, Alrumaihi F, Alhumaydhi FA, Alwashmi AS, Almatroudi AA, Alsugoor MH, Khan MA. Safety, stability, and therapeutic efficacy of long-circulating TQ-incorporated liposomes: implication in the treatment of lung cancer. Pharmaceutics. 2022;14:153. doi: 10.3390/pharmaceutics14010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ten Hagen TLM, Dreher MR, Zalba S, Seynhaeve ALB, Amin M, Li L, Dieter H. Drug transport kinetics of intravascular triggered drug delivery systems. Commun Biol. 2021;4:920. doi: 10.1038/s42003-021-02428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franco MS, Gomes ER, Roque MC, Oliveira MC. Triggered drug release from liposomes: exploiting the outer and inner tumor environment. Front Oncol. 2021;11:623760. doi: 10.3389/fonc.2021.623760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motamarry A, Asemani D, Haemmerich D. In: Thermosensitive liposomes. Catala A, editor. Vol. 7. Liposomes, Rijeka: IntechOpen; 2017. p. 68159. [Google Scholar]
- 36.Ta T, Porter TM. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J Control Release. 2013;169:112–25. doi: 10.1016/j.jconrel.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Matos MBC, Beztsinna N, Heyder C, Fens MHAM, Mastrobattista E, Schiffelers RM, Leneweit G, Kok RJ. Thermosensitive liposomes for triggered release of cytotoxic proteins. Eur J Pharm Biopharm. 2018;132:211–21. doi: 10.1016/j.ejpb.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. WIREs Nanomedicine and Nanobiotechnology. 2017;9:1450. doi: 10.1002/wnan.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crezee J, Franken NAP, Oei AL. Hyperthermia-based anti-cancer treatments. Cancers (Basel) 2021;13:1240. doi: 10.3390/cancers13061240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bi H, Xue J, Jiang H, Gao S, Yang D, Fang Y, Kai S. Current developments in drug delivery with thermosensitive liposomes. Asian J Pharm Sci. 2019;14:365–79. doi: 10.1016/j.ajps.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Cheng D, Li J, Wang Y, Guo JX, Chen ZP, Cai BC, Yang T. Influence of lipid composition on the phase transition temperature of liposomes composed of both DPPC and HSPC. Drug Dev Ind Pharm. 2013;39:197–204. doi: 10.3109/03639045.2012.668912. [DOI] [PubMed] [Google Scholar]
- 42.Maswadeh H, Demetzos C, Daliani I, Kyrikou I, Mavromoustakos T, Tsortos A, Nounesiset G. A molecular basis explanation of the dynamic and thermal effects of vinblastine sulfate upon dipalmitoylphosphatidylcholine bilayer membranes. Biochim Biophys Acta. 2002;1567:49–55. doi: 10.1016/s0005-2736(02)00564-3. [DOI] [PubMed] [Google Scholar]
- 43.Maswadeh H, Khan A, Alorainy MS, Al-Wabel NA, Demetzos C. In vitro and in vivo activity of thermosensitive liposomes loaded with doxorubicin and cisplatin. Drug Dev Ind Pharm. 2022;48:158–68. doi: 10.1080/03639045.2022.2102648. [DOI] [PubMed] [Google Scholar]
- 44.Willerding L, Limmer S, Hossann M, Zengerle A, Wachholz K, ten Hagen TLM, Oning GA, Sroka R, Lindner LH, Peller M. Method of hyperthermia and tumor size influence effectiveness of doxorubicin release from thermosensitive liposomes in experimental tumors. J Control Release. 2016;222:47–55. doi: 10.1016/j.jconrel.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Karino T, Koga S, Maeta M. Experimental studies of the effects of local hyperthermia on blood flow, oxygen pressure and pH in tumors. Jpn J Surg. 1988;18:276–283. doi: 10.1007/BF02471444. [DOI] [PubMed] [Google Scholar]
- 46.Liu M, Du H, Zhang W, Zhai G. Internal stimuli-responsive nanocarriers for drug delivery: design strategies and applications. Mater Sci Eng C Mater Biol Appl. 2017;71:1267–1280. doi: 10.1016/j.msec.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 47.Hatakeyama H. Recent advances in endogenous and exogenous stimuli-responsive nanocarriers for drug delivery and therapeutics. Chem Pharm Bull. 2017;65:612–7. doi: 10.1248/cpb.c17-00068. [DOI] [PubMed] [Google Scholar]
- 48.Alrbyawi H, Poudel I, Annaji M, Arnold RD, Tiwari AK, Babu RJ. Recent advancements of stimuli-responsive targeted liposomal formulations for cancer drug delivery. Pharm Nanotechnol. 2022;10:3–23. doi: 10.2174/2211738510666220214102626. [DOI] [PubMed] [Google Scholar]
- 49.Yuba E. Development of functional liposomes by modification of stimuli-responsive materials and their biomedical applications. J Mater Chem B. 2020;8:1093–107. doi: 10.1039/c9tb02470k. [DOI] [PubMed] [Google Scholar]
- 50.Jain A, Jain SK. Stimuli-responsive smart liposomes in cancer targeting. Curr Drug Targets. 2018;19:259–70. doi: 10.2174/1389450117666160208144143. [DOI] [PubMed] [Google Scholar]
- 51.Raza A, Rasheed T, Nabeel F, Hayat U, Bilal M, Iqbal H. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules. 2019;24:1117. doi: 10.3390/molecules24061117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding C, Tong L, Feng J, Fu J. Recent advances in stimuli-responsive release function drug delivery systems for tumor treatment. Molecules. 2016;21:1715. doi: 10.3390/molecules21121715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Yin G, Pu X, Huang Z, Liao X, Chen X. A novel tumor-targeted thermosensitive liposomal cerasome used for thermally controlled drug release. Int J Pharm. 2019;570:118660. doi: 10.1016/j.ijpharm.2019.118660. [DOI] [PubMed] [Google Scholar]
- 54.Anyarambhatla GR, Needham D. Enhancement of the phase transition permeability of DPPC liposomes by incorporation of MPPC: a new temperature-sensitive liposome for use with mild hyperthermia. J Liposome Res. 1999;9:491–506. [Google Scholar]
- 55.Mozar FS, Chowdhury EH. Impact of PEGylated nanoparticles on tumor targeted drug delivery. Curr Pharm Des. 2018;24:3283–96. doi: 10.2174/1381612824666180730161721. [DOI] [PubMed] [Google Scholar]
- 56.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Xu P, He D, Xu B, Tu J, Shen Y. Long-circulating thermosensitive liposomes for the targeted drug delivery of oxaliplatin. Int J Nanomedicine. 2020;15:6721–34. doi: 10.2147/IJN.S250773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chelvi TP, Ralhan R. Hyperthermia potentiates antitumor effect of thermosensitive-liposome-encapsulated melphalan and radiation in murine melanoma. Tumor Biol. 1997;18:250–60. doi: 10.1159/000218038. [DOI] [PubMed] [Google Scholar]
- 59.Duncan R. Drug-polymer conjugates: potential for improved chemotherapy. Anticancer Drugs. 1992;3:175–210. doi: 10.1097/00001813-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Affram K, Udofot O, Cat A, Agyare E. In vitro and in vivo antitumor activity of gemcitabine loaded thermosensitive liposomal nanoparticles and mild hyperthermia in pancreatic cancer. Int J Adv Res. 2015;3:859–74. [PMC free article] [PubMed] [Google Scholar]
- 61.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–31. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 62.Arcaro A, Guerreiro A. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Curr Genomics. 2007;8:271–306. doi: 10.2174/138920207782446160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.