Summary
Background
Due to the nature of their disease, patients with multiple myeloma (MM) often have bone disease-related pain that limits physical activity and diminishes health-related quality of life (HRQOL). Digital health technology with wearables and electronic patient reported outcome (ePRO) tools can provide insights into MM HRQoL.
Methods
In this prospective observational cohort study conducted at Memorial Sloan Kettering Cancer in NY, NY, USA, patients with newly diagnosed MM (n = 40) in two cohorts (Cohort A – patients <65 years; Cohort B – patients ≥65 years) were passively remote-monitored for physical activity at baseline and continuously for up to 6 cycles of induction therapy from Feb 20, 2017 to Sep 10, 2019. The primary endpoint of the study was to determine feasibility of continuous data capture, defined as 13 or more patients of each 20-patient cohort compliant with capturing data for ≥16 h of a 24-hr period in ≥60% of days of ≥4 induction cycles. Secondary aims explored activity trends with treatment and association to ePRO outcomes. Patients completed ePRO surveys (EORTC - QLQC30 and MY20) at baseline and after each cycle. Associations between physical activity measurements, QLQC30 and MY20 scores, and time from the start of treatment were estimated using a linear mixed model with a random intercept.
Findings
Forty patients were enrolled onto study, and activity bioprofiles were compiled among 24/40 (60%) wearable user participants (wearing the device for at least one cycle). In an intention to treat feasibility analysis, 21/40 (53%) patients [12/20 (60%) Cohort A; 9/20 (45%) Cohort B] had continuous data capture. Among data captured, overall activity trended upward cycle over cycle for the entire study cohort (+179 steps/24 h per cycle; p = 0.0014, 95% CI: 68–289). Older patients (age ≥65 years) had higher increases in activity (+260 steps/24 h per cycle; p < 0.0001, 95% CI: −154 to 366) compared to younger patients (+116 steps/24 h per cycle; p = 0.21, 95% CI: −60 to 293). Activity trends associated with improvement of ePRO domains, including physical functioning scores (p < 0.0001), global health scores (p = 0.02), and declining disease burden symptom scores (p = 0.042).
Interpretation
Our study demonstrates that feasibility of passive wearable monitoring is challenging in a newly diagnosed MM patient population due to patient use. However, overall continuous data capture monitoring remains high among willing user participants. As therapy is initiated, we show improving activity trends, mainly in older patients, and that activity bioprofiles correlate with traditional HRQOL measurements.
Funding
Grants –National Institutes of HealthP30 CA 008748, Awards – Kroll Award 2019.
Keywords: mHealth, Wearable technology, Multiple myeloma, Health related quality of life
Research in context.
Evidence before this study
Among cancer patients, multiple myeloma (MM) patients have some of the poorest health related outcomes reported. Disease symptomatology includes painful bone-based lesions, limiting physical movement and activity. Despite MM patients living longer, reduced health-related quality of life (HRQoL) remains a critical issue. Digital wearables utilizing actigraphy measurements have a unique ability to capture objective data measurements on the patient experience. Three distinct pubmed searches, utilizing the following search criteria: “multiple myeloma and actigraphy”, “multiple myeloma and remote monitoring”, and “multiple myeloma and digital health,” reveal a lack of studies reporting on the feasibility or application of continuous activity monitoring in MM cancer patients for extended time periods or how activity trends correlate with subjective HRQoL patient outcomes. Limited studies exist in utilizing actigraphy in hematologic malignant patients receiving hematopoietic stem cell transplants and recovery.
Added value of this study
In this prospective study, newly diagnosed MM patients were remotely monitored with actigraphy devices at baseline and continuously during initial 6-month chemotherapy treatments. Feasibility determined by ≥ 60% continuous capture rate of at least 4 cycles of therapy demonstrate low overall feasibility in the general myeloma population, but high capture rates among willing participants. By monitoring activity trends, we demonstrate older patients have higher activity gains cycle over cycle compared to younger patients. Improving activity trends associate with subjective patient reported outcome scores - physical functioning, global health, and declining disease burden symptoms.
Implications of all the available evidence
Digital technology solutions may provide unique insight into the cancer patient experience by passively capturing objective data measurements through more detailed comprehensive timepoints. Further work is needed to determine how such tools can evolve to actively enhance and improve cancer patient HRQoL.
Introduction
A study evaluating over 1600 cancer survivors ranked multiple myeloma (MM) among the lowest in health-related quality of life (HRQoL) outcomes.1 The true emotional and physical burden of a MM diagnosis is poorly characterized and likely underappreciated. Given the disease symptomatology of painful bone lesions and chronicity of continuous treatment, MM patients represent an ideal cancer population to study digital health technology applications that may complement subjective patient-reported outcome data (PRO) with objective functional measurements captured by actigraphy.2
The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and its module for MM patients (QLQ-MY20) are traditional well-established PRO instruments designed to assess functioning scales, symptom scales, global health status, and financial toxicity.3,4 While PRO tools provide a glimpse into a MM patient's journey of living with the disease, application has been limited to assessing HRQoL differences among treatment regimens, lacking functional integration into every day clinical practice.5,6 Digital health technology applications may bridge the gap between academic lengthy PRO questionnaires and real-time monitoring of HRQoL. Current strategies on integrating digital health technology applications in the care of MM patients include digital health coaching and actigraphy during autologous stem cell transplantation,7,8 artificial intelligence-based symptom monitoring,9 and medication adherence when transitioning subcutaneous treatments to oral-based treatments.10 To our knowledge, actigraphy has not yet been studied to evaluate physical activity trends in newly diagnosed symptomatic MM patients receiving induction chemotherapy as it correlates with HRQoL.
In other cancer survivors, mainly solid oncology tumors, actigraphy measurements have been used to objectively demonstrate a relationship between activity and physical pain and fatigue symptoms.11, 12, 13 In a study involving adult hematopoietic stem cell transplant recipients, average daily steps were associated with increases in pain, fatigue, and gastrointestinal symptoms.11 Similar studies have demonstrated altered activity patterns in cancer patients recovering postoperatively from surgery,14, 15, 16 sickle cell patients experiencing painful vaso-occlusive crises,17 and terminal cancer patients receiving pain medications.18 Additionally, past research indicates that increased physical activity may be associated with long term benefits in cancer survivors, including improved quality of life and decreased cancer recurrence.19,20 As a result, several investigations have been conducted to actively monitor and motivate cancer patients to increase their physical activity levels through patient engagement.21, 22, 23
In this prospective study of newly diagnosed MM patients, we expand on the passive application of actigraphy in MM patients by investigating the feasibility of remote monitoring activity (steps/24 h). In order to minimize effect of the wearable device on motivating the patient, we employed a passive capture strategy, where patients were unencumbered by reminders and motivational texts, and the device was running in the background. Secondary aims of the study were to gain insight on how activity and HRQoL trends with each successive therapy cycle delivered and how they relate to one another.
Methods
Study design and participants
Patients were consented and enrolled onto a prospective single-center clinical study (NCT 03006315) conducted and IRB-approved at Memorial Sloan Kettering Cancer Center (MSK) in NY, NY, USA. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and MSK regulations. Patients with newly diagnosed MM were eligible if they had not received any systemic therapy at the time of study entry and if they owned a smart phone or tablet (iOS or Android) compatible with Garmin Vivofit® device. Induction treatment was at the discretion of individual physicians and patients.
Procedures
Patients were given Garmin Vivofit® devices and asked to download a Garmin Vivofit® application and Medidata ePRO application on their phone or tablet. Patients were assigned to one of two cohorts (Cohort A – patients <65 years; Cohort B – patients ≥65 years). Patients were monitored remotely for physical activity during baseline period (1–7 days prior to chemotherapy initiation) and continuously for up to 6 cycles of chemotherapy.
Outcomes
Wearable user participants were defined as wearing the device for at least one cycle and capturing at least one data cycle. Compliance among wearable users was defined as continuous capture ≥60% of the days in at least four cycles of therapy (obtaining ≥16 h of a 24-hr period). The primary endpoint of the study was to determine successful feasibility of continuous data capture ≥60% of the days in at least four cycles of therapy (obtaining ≥16 h of a 24-hr period) in 13 or more patients of each 20-patient cohort. Patients completed mobile ePRO questionnaires (EORTC QLQ-C30 and QLQ-MY20) using the Medidata application at baseline and after each induction cycle. Compliance on ePRO data was defined as a completing at least one baseline and post-treatment questionnaire. Activity and ePRO questionnaire data were automatically synced to a Medidata Rave database through Medidata Sensorlink technology. Cohort B patients, age ≥65 years, had geriatric frailty scores assigned based on the International Myeloma Working Group frailty score into categories of fit, intermediate fitness, and frail.24 Responses at the end of the defined treatment period were scored by the IMWG response criteria.25
Statistical analyses
Categorical patient characteristics were summarized by frequency and percentage. Continuous characteristics were summarized by average and standard deviation. Associations between measurements of activity, EORTC QLQ-C30 and QLQ-MY20 scores, and time from the start of the treatment were estimated using a linear-mixed effect model with a random intercept. Similarly linear associations between EORTC QLQC-C30 and QLQ-MY20 scores and activity were estimated by a linear mixed-effect model with a random intercept. A Wald-test was used to compute p-values for the significance of association. Study findings are reported in accordance with Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.26
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. NK, ET, GH, TA, AD have directly accessed and verified data. All authors critically reviewed and provided edits to the final manuscript.
Results
Between Feb 20, 2017 and Sep 19, 2019, 40 patients with newly diagnosed MM were consented and enrolled onto the study where continuous activity (steps/24 h) and ePRO scores were tracked at baseline and cycle over cycle for up to 6 months of induction therapy. Patients were enrolled onto cohort A (age <65 years, n = 20) and cohort B (age ≥65 years, n = 20) (Fig. 1). Table 1 summarizes baseline characteristics. The mean age of cohort A was 54 years (41–64), and the mean age of cohort B was 71 years (65–82). Treatment regimens varied with the most common regimen in cohort A being carfilzomib (Kyprolis), lenalidomide (Revlimid), dexamethasone (KRd), and the most common regimen in cohort B being lenalidomide, bortezomib (Velcade), dexamethasone (RVd). Activity bioprofiles were compiled among 24/40 (60%) wearable user participants (wearing the device for at least one cycle) with 14 patients completing all 7 full datasets (baseline + 6 cycles) and 10 patients completing partial datasets (<7 datasets). Overall data capture for passive monitoring was met in 21/40 (53%) patients with 12/20 (60%) in Cohort A and 9/20 (45%) in Cohort B, thus not meeting study successful feasibility thresholds. Among the 24 wearable device users, 21/24 (88%) met threshold for continuous capture definition (at least 16 h of a 24-hr period of ≥60% during the entire primary endpoint observation period of 4 induction cycles). Three device users had low compliance rates capturing <4 partial data sets. Sixteen patients had too limited data either due to low wearable device use (8), lost devices (4), no data transfer (3), and one patient dropping out due to device band rash (1).
Fig. 1.
Consort flow diagram. ∗Wearable user participants with data wore the device and captured at least one data cycle, n = 24 patients. ∗∗Wearable user participants compliant with continuous data capture monitoring have data for ≥16 h of a 24-hr period in ≥60% of days of ≥4 induction cycles, n = 21 patients. Activity trend analysis was conducted in wearable user participants with data. Wearable user participants compliant with continuous data capture counted towards primary feasibility.
Table 1.
Patient characteristics.
| Cohort A – Age <65 Years (n = 20) | Cohort B – Age ≥65 Years (n = 20) | |
|---|---|---|
| Age, mean (range) | 54 (41–64) | 71 (65–82) |
| Sex | ||
| Female | 10 (50%) | 9 (45%) |
| Male | 10 (50%) | 11 (55%) |
| Treatment regimens | ||
| KRd | 9 (45%) | 5 (25%) |
| RVd | 4 (20%) | 8 (40%) |
| Dara-KRd | 4 (20%) | 4 (20%) |
| CyBord | 3 (15%) | 2 (10%) |
| Rd | – | 1 (5%) |
| IMW frailty score | ||
| Fit (score 0) | 8 (40%) | |
| Intermediate (score 1) | 3 (15%) | |
| Frail (score ≥2) | 9 (45%) |
KRd – carfilzomib, lenalidomide, dexamethasone; RVd – lenalidomide, bortezomib, dexamethasone; Dara-KRd – daratumamab, carfilzomib, lenalidomide, dexamethasone; CyBord – cyclophosphamide, velcade, dexamethasone; Rd – lenalidomide, dexamethasone.
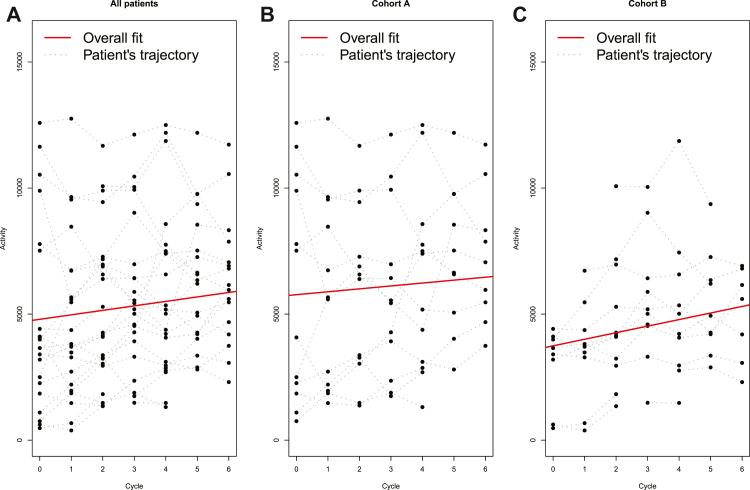
Overall, patient activity increased over time with an increase of 179 steps/24 h per cycle (p = 0.0014, 95% CI: 68–289) for the entire cohort of study participants. Mean activity at baseline was 4818 steps/24 h (SD: 3816) compared to post therapy initiation was 6278 steps/24 h (SD: 2539) (Fig. 2A). For cohort A, mean activity at baseline versus post therapy initiation was 6041 steps/24 h (SD: 4421) vs. 7266 steps/24 h (SD: 2652), respectively, with an increase of 116 steps/24 h per cycle (p = 0.21, 95% CI: −60 to 293) (Fig. 2B). For cohort B, mean activity at baseline was 2984 steps/24 h (SD: 1552) compared to 5007 steps/24 h (SD: 1838) after therapy initiation with an increase of 260 steps/24 h per cycle (p < 0.0001, 95% CI: 154–366) (Fig. 2C). Patient response to induction treatment over the course of 6 cycles of therapy were characterized into responders (≥VGPR) and sub-responders (<VGPR) groups. There was improvement in activity across both responder and sub-responder groups with an increase of 169 steps/24 h per cycle (p = 0.016, 95% CI: 31–305) and 212 steps/24 h per cycle (p = 0.0093, 95% CI: 53–371), respectively.
Fig. 2.
Activity trends over induction treatment. Panel A. Overall patient activity over 6 cycles of induction treatment regimen. Mean activity at baseline 4818 steps/24 h (SD:3816) compared to post therapy initiation 6278 steps/24 h (SD: 2539) Panel B. Cohort A (<65 Years) patient activity over 6 cycles. Mean activity at baseline 6041 steps/24 h (SD: 4421) compared to post therapy initiation 7266 steps/24 h (SD: 2652). Panel C. Cohort B (≥65 Years) patient activity over 6 cycles. Mean activity at baseline 2984 steps/24 h (SD: 1552) compared to post therapy initiation 5007 steps/24 h (SD: 1838).
Compliance rates for ePRO assessments was 35/40 (87.5%). Among the ePRO domains, mean baseline scores from cohort A to cohort B were different only in the EORTC QLQ-C30 global health status domain at 79 (SD: 20) vs. 56 (SD: 22) (p = 0.0076) (Supplemental Table S1), respectively. Table 2 summarizes trends in ePROs with treatment. Improvement was noted in the global health status domain, +1.7 score per cycle (p = 0.016, 95% CI: 0.3–3.1), and the EORTC QLQ-C30 physical functioning domain, +2.1 score per cycle (p < 0.0001, 95% CI: 1.2–3.0). Additionally, patients reported improvement in disease burden symptoms based on the QLQ-MY20 questionnaire, −1.6 score per cycle (p = 0.0013, 95% CI: −2.6 to −0.6). There were no observed changes in time over self-body image. Patients reported a worsening of future perspective over the therapy time-period, −2.8 score per cycle (p < 0.0001, 95% CI: −3.9 to −1.8). Table 2 summarizes electronic PRO trends by cohort. Improvement in physical function, global health status, and disease burden symptoms were seen across all of cohort B but limited to disease burden symptoms and physical function in cohort A. Future perspective declined in both age group cohorts.
Table 2.
ePRO Scores over 6 cycles of induction treatment regimen.
| ePRO EORTC | Domains (0–100) | Overall cohorts (A + B) |
Cohort A (age <65 years) |
Cohort B (age ≥65 years) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean baseline scores (SD) | Trend (95% CI) (score/cycle) | Net average change (95% CI) (score after 6 cycles) | p-value | Mean baseline scores (SD) | Trend (95% CI) (score/cycle) | Net average change (95% CI) (score after 6 cycles) | p-value | Mean baseline scores (SD) | Trend (95% CI) (score/cycle) | Net average change (95% CI) (score after 6 cycles) | p-value | ||
| QLQ30 | Physical function | 78 (23) | +2.1 (1.2–3.0) | +12.6 (7.2–18) | p < 0.0001 | 85 (20) | +1.9 (0.7–3.9) | +11.4 (4.2–18.6) | p = 0.0032 | 72 (24) | +2.4 (1.1–3.7) | +14.4 (6.4–22.4) | p = 0.0032 |
| QLQ30 | Global health status/QoL | 66 (24) | +1.7 (0.3–3.1) | +10.2 (1.9–18.6) | p = 0.016 | 79 (20) | +0.5 (−1.2 to 2.2) | +3.0 (−7.2 to 13.2) | p = 0.59 | 56 (22) | +3.0 (0.8–5.1) | +18 (4.8–30.6) | p = 0.0071 |
| MY20 | Disease burden symptoms | 27 (20) | −1.6 (−2.6 to −0.6) | −9.6 (−3.8 to −15.4) | p = 0.0013 | 23 (11) | −1.4 (−2.6 to −0.2) | −8.4 (−15.6 to −1.2) | p = 0.02 | 30 (24) | −1.7 (−3.2 to −0.2) | −10.2 (−19.2 to −1.2) | p = 0.03 |
| MY20 | Future perspective | 48 (23) | −2.8 (−3.8 to −1.8) | −16.8 (−23.0 to −10.6) | p < 0.0001 | 48 (25) | −3.4 (−4.8 to −2.1) | −20.4 (−28.5 to −12.3) | p < 0.0001 | 49 (23) | −2.1 (−3.7 to −0.6) | −12.8 (−22.1 to −3.6) | p = 0.0066 |
| MY20 | Self-Body image | 20 (24) | −0.6 (−2.3 to 1.1) | −3.6 (−13.8 to 6.7) | p = 0.5 | 15 (22) | −1.3 (−3.5 to 0.9) | −7.8 (−21 to 5.4) | p = 0.22 | 23 (26) | 0.1 (−2.5 to 2.8) | +0.6 (−15 to 16.8) | p = 0.91 |
Association between ePRO scores and patient activity was evaluated. Overall improvement in patient activity was associated with improvement in EORTC QLQ-C30 physical functioning scores (p < 0.0001), increasing global health status scores (p = 0.02), and decreasing disease burden scores (p = 0.042). No association was seen between activity and self-body image or future perspective (Table 3). In sub-cohort analysis, positive associations between increased patient activity and improved physical function ePRO scores were observed across both cohorts A (p = 0.0015) and B (p < 0.0001) (Table 3).
Table 3.
Association of ePRO scores with patient activity.
| Overall cohorts (A + B) |
Cohort A |
Cohort B |
||||||
|---|---|---|---|---|---|---|---|---|
| ePRO domain | Effect on activity (95% CI) | ePRO domain | Effect on activity (95% CI) | ePRO domain | Effect on activity (95% CI) | |||
| ↑ Patient activity | ↑ QLQ30 physical function | 52.6 (28.8–52.6) | ↑ Patient activity | ↑ QLQ30 physical function | 75.8 (29.0–122.6) | ↑ Patient activity | ↑ QLQ30 physical function | 37.4 (19.2–55.5) |
| ↑ Patient activity | ↑ QLQ30 global health | 20 (3.2–36.8) | ↑ Patient activity | ↑ QLQ30 global health | 42.3 (6.7–77.8) | ↑ Patient activity | ↑ QLQ30 global health | 10.5 (−2.8 to 23.8) |
| ↑ Patient activity | ↓ MY20 disease burden | −26.7 (−52.6 to −0.9) | ↑ Patient activity | ↓ MY20 disease burden | −47.2 (−97.3 to 3) | ↑ Patient activity | ↓ MY20 disease burden | 14 (−37.6 to 8.4) |
| Patient activity | MY20 future perspective | −20 (−43.4 to 3.4) | Patient activity | MY20 future perspective | −19.0 (−57.7 to 19.7) | Patient activity | MY20 future perspective | −21.5 (−44.1 to 1.1) |
| Patient activity | MY20 self-body image | −5.4 (−9 to 19) | Patient activity | MY20 self-body image | −6.5 (−1 to 17) | Patient activity | MY20 self-body image | −12.5 (−29 to 5) |
↑ Patient activity signifies increased step counts. ↑ QLQ30 physical function signifies improvement in physical function PRO scores. ↑ QLQ30 global health signifies improvement in global health PRO scores. ↓ MY20 disease burden signifies improvement in disease burden PRO scores.
Cohort B patients were assigned frailty scores at baseline (Table 1), but only 12/20 (60%) had completed activity and ePRO profiles during the study period, 6-Fit patients, 4-Intermediate, and 2-Frail. Given the lack of data among frail participants, intermediate and frail categories were combined into an intermediate/frail category in the analysis. Baseline activity and ePRO scores did not vary between fit and intermediate/frail categories (Supplemental Table S2). Upon treatment initiation, frailty score index did not impact activity trends, physical functioning, or self-body image trends cycle over cycle, but seemed to impact improvement in global health status and reduction in disease burden symptoms with a greater difference in change of ePRO scores between fit and intermediate/frail groups (Supplemental Table S3).
Discussion
In this prospective observational study, patients with newly diagnosed MM were passively biomonitored in two aged cohorts (Cohort A <65 years and Cohort B ≥ 65 years) using wearable accelerometer devices at baseline and during 6 cycles of induction chemotherapy. Patients meeting study feasibility criteria of patients capturing data at least ≥16 h of a 24-hr period in ≥60% of days of ≥4 induction cycles were 12/20 (60%) in Cohort A and 9/20 (45%) in Cohort B with an overall rate of 21/40 (53%). The study did not meet pre-specified criteria for feasibility success, largely driven by lower user rates with only 24/40 (60%) patients wearing and capturing data for at least one cycle. Of note, reminders and positive reinforcement were not provided in the study and could be a future strategy to mitigate low user rates. We were motivated to study physical activity passively in the context of induction therapy being delivered to the newly diagnosed MM patient and to minimize effectiveness that the wearable device or care team may have on actively influencing patient activity. Prior studies utilizing actigraphy monitors in hematologic malignances have been done in the setting of hematopoietic stem cell transplant, where the effect of conditioning regimens may have a broad effect on activity trends rather than the disease itself. For these reasons, our observational cohort study was conducted at diagnosis, during the height of disease symptomatology, while comparing baseline trends to post-treatment trends. Additionally, given the lack of comparator investigations in the multiple myeloma field, the study's pre-set feasibility criteria for success may have been too ambitious for the patient population. Reasons for low user rates and compliance varied from lost devices, lack of motivation or overwhelming emotional burden of a new cancer diagnosis as indicated by our HRQoL responses. Three patients had failed data transfer where patients self-reported use of devices, but uploaded data was not transferred due to sensorlink failure. Future considerations should focus on optimizing data transfer more directly between patient and provider through secure channels, allowing for feedback communication, or setting alerts if failed transfers occur. Furthermore, as the demand and clinical application of wearable technology grows, attention should be given to the validity and accuracy of the device. The decision to use the Garmin Vivofit in the current study was made based on available devices on the market at the time of study initiation and compatibility with the database vendor. It should be also noted that while Garmin devices were provided on the study, eligible patients needed to pre-own compatible smart phones or tablets, thus potentially creating a socio-economic and age bias in the study population that enrolled and adhered. Importantly, among wearable device users who used the device at least for one cycle, compliance of continuous capture rate was 21/24 (88%), thus, exhibiting a high data capture rate of more than 60% of the induction cycle observation period and showing promise for future biomonitoring strategies among willing and motivated patients.
As part of secondary aims, physical activity trends and ePRO scores were analysed to gain insight on how changes occurred during treatment. Although the study is limited in patient numbers, the quality of the continuous data streams obtained from compliant patients is high, and the associations derived from these activity trends are noteworthy to the myeloma patient experience. While overall activity improved from baseline to therapy initiation (4513 steps/24 h vs. 6278 steps/24 h) and continued to trend upward cycle over cycle in the entire study cohort (+179 steps/24 h per cycle; p = 0.0014, 95% CI: 68–289), older patients (age ≥65 years) showed a greater increase in activity (+260 steps/24 h per cycle; p < 0.0001, 95% CI: 154–366) compared to the younger cohort population (+116 steps/24 h per cycle; p = 0.21, 95% CI: −60 to 293), as demonstrated by their trend increases. This difference is likely due at least in part to poor baseline activity profiles among the older patient cohort and how impactful disease symptomatology affects elderly frail patients with less physiologic reserve. Subgroup analysis with baseline IMW frailty scores supports this possibility, given that intermediate/frail patients in cohort B had more limitations with trend improvements in ePRO scores of disease burden symptoms and global health status compared to their fit counterparts in cohort B. The absence of similar effects in activity and physical function may be due to insufficient power to detect such differences or because frailty scores capture additional factors beyond physical limitations. Interestingly, activity improvements were independent of therapy response, suggesting that especially for the frail and fragile, HRQoL and increased physical activity are integral to determining the success of therapy delivered.
Electronic PROs compiled in the study demonstrated improvement in most domains with treatment initiation, including reduction of disease burden symptoms, gains in physical function, and improved global health status scores. Unexpectedly, we found a distressing trend among patients with MM demonstrating a decline in future perspective outlook after therapy initiation. The source and reversibility for this trend is unknown but may be related to anxiety about impending autologous stem cell transplantation and/or the prospect of a chronic malignancy diagnosis with its associated uncertainties. Upcoming studies should address this future perspective trend after transplantation and during maintenance periods to evaluate long-term patient adjustments. Activity trends associated with improvement of ePRO domains, including physical functioning scores, global health scores, and declining disease burden symptom scores. Across both cohorts, the strongest association of passive capture of activity trends correlated with physical functioning scores, representing a direct objective measure of patient mobility and warrants further validation. Our findings demonstrate that “passive” wearable actigraphy may have a role in monitoring compliant motivated patients for physical functioning and symptom burden, especially at-risk elderly patients. To capture more user participants and increase compliance of continuous monitoring, further work is needed to explore whether digital technology can be utilized in an “active” capacity, where wearables directly increase or influence patient HRQoL through motivation and positive biofeedback algorithms in real time. Other potential investigational applications for wearables beyond HRQoL in the myeloma field include investigating specific symptom related outcomes associated with toxicity profiles, such as vital sign monitoring for cytokine release syndrome after T-cell directed therapies, insomnia patterns after steroid-enriched treatments, or fall events after vestibular or neuropathic agents. Despite useability and compliance issues, the role of digital wearable technology remains promising in the multiple myeloma population and should be further explored in aforementioned settings.
Contributors
NK, OL, AL, TA, JL, and AD designed research. NK, ET, DM, JL, GH, AZ, MS, AD, TA, and AL performed project administration, data collection and analyzed data. NK, ET, GH, TA, AD have directly accessed and verified data. NK, ET, DM, MS, SM, HH, ES, MH, US, CT, BD, GS, MS, OL, DC, HL, SG, AD, PS, FK, SU, OL, and AL performed patient care. AD, NK, and TA performed statistical analysis. NK, AL, and AD drafted original draft. All authors critically reviewed and provided edits to the final manuscript.
Data sharing statement
Data may be made available from corresponding author upon reasonable request and permission from Memorial Sloan Kettering Cancer Center.
Declaration of interests
N.K. – Research Funding: Amgen, Janssen; Honoraria: CCO, Onc Live, Intellisphere; S.M. – Honoraria – MJH Life Sciences, Physician Education Resource, Research Funding – Fate Therapeutics, Juno Therapeutics (Bristol-Myers Squibb Company), Allogene Therapeutics, Janssen Oncology, Takeda, Advisory Board - Abbvie; H.H. - Consultancy – Novartis, Research Funding – Celgene/BMS, Takeda; E.S. - Patents/Royalties for CAR T cells to treat multiple myeloma, BMS; Consulting: BMS, Fate Therapeutics, Precision Biosciences; M.H. Research Funding – Daiichi Sankyo, GSK, Amgen, Abbvie, Beigene, Cosette, Consultancy – Intellisphere, GSK, BMS; U.S. - Honoraria – Physicians Education Resource; Research Funding - Celgene/Bristol Myers Squibb, Janssen, Parker Institute for Cancer Immunotherapy, American Society of Hematology Clinical Research Training Institute; C.T. – Research Funding- Janssen, Honoraria - Physician Educations Resource; B.D. – Sylvester K12 ACS IRG Pilot Grant, Consulting – Janssen, Honoraria – MJH, Advisory Board – Sanofi; G.S – Research Funding – Amgen, Janssen; M.S. – Research funding – Amgen, Angiocrine Bioscience, Omeros Corporation, Honoraria – Medscape LLC, i3Health; Advisory Board – Kite, Consulting - Omeros Corporation, Angiocrine Bioscience, O.L - MorphoSys; H.L. – Research Funding – Alexion, Takeda, Janssen, Consulting/Advisory board – Takeda, Pfizer, Janssen, Celgene, Caelum, Juno, Prothena, Legend Biotech USA, Abbvie, Karyopharm; S.G. Research Funding – Actinuum, Amgen, Takeda, Miltenyi, Johnson & Johnson, Celgene/BMS, Advisory Board – Amgen, Actinuum, Celgene, Takeda, Kite, Johnson & Johnson, Jazz Pharmaceutical, Novartis; Spectrum Pharmaceutical; S.Z.U. – Research Funding – Amgen, Array Biopharma, BMS, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda, Consulting – Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, Teneobio; O.L. – Research Funding - LLS, Rising Tide Foundation, NIH Sylvester Comprehensive Cancer Center's NCI Core Grant (P30CA240139), Riney Family Foundation, MMRF, IMF, Perelman Family Foundation, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, Karyopharm; Honoraria for advisory boards for Adaptive, Amgen, Binding Site, BMS, Celgene, Cellectis, Glenmark, Janssen, Juno, Pfizer; and served on Independent Data Monitoring Committees (IDMC) for international randomized trials by Takeda, Merck, Janssen, Theradex; A.L. – Consulting/Honoraria - BMS, Janssen, Research Funding – BMS, Pfizer, Janssen; E.T., D.M, J.L. G.H., A.Z., M.S., D.C, A.D., T.M.A, P.S, F.K. declare no competing interests.
Acknowledgments
Grants –NIH P30 CA 008748, Awards – Kroll Family Fellow in Survivorship 2018–2019.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101854.
Appendix A. Supplementary data
References
- 1.Kent E.E., Ambs A., Mitchell S.A., Clauser S.B., Smith A.W., Hays R.D. Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer. 2015;121(5):758–765. doi: 10.1002/cncr.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagannath S., Mikhael J., Nadeem O., Raje N. Digital health for patients with multiple myeloma: an unmet need. JCO Clin Cancer Inform. 2021;5:1096–1105. doi: 10.1200/CCI.20.00145. [DOI] [PubMed] [Google Scholar]
- 3.Cocks K., Cohen D., Wisløff F., et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43(11):1670–1678. doi: 10.1016/j.ejca.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Kvam A.K., Fayers P., Wisloff F. What changes in health-related quality of life matter to multiple myeloma patients? A prospective study. Eur J Haematol. 2010;84(4):345–353. doi: 10.1111/j.1600-0609.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 5.Weisel K., Ludwig H., Rieth A., Lebioda A., Goldschmidt H. Health-related quality of life of carfilzomib- and daratumumab-based therapies in patients with relapsed/refractory multiple myeloma, based on German benefit assessment data. Qual Life Res. 2020;29(1):69–79. doi: 10.1007/s11136-019-02307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart A.K., Dimopoulos M.A., Masszi T., et al. Health-related quality-of-life results from the open-label, randomized, phase III ASPIRE trial evaluating carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in patients with relapsed multiple myeloma. J Clin Oncol. 2016;34(32):3921–3930. doi: 10.1200/JCO.2016.66.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee R., Chiung-Yu H., Dunn L., et al. Digital life coaching during stem cell transplantation: development and usability study. JMIR Form Res. 2022;6(3):e33701. doi: 10.2196/33701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hacker E.D., Richards R.L., Abu Zaid M., Chung S.Y., Perkins S., Farag S.S. STEPS to enhance physical activity after hematopoietic cell transplantation for multiple myeloma. Cancer Nurs. 2022;45(3):211–223. doi: 10.1097/NCC.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 9.Biran N., Kouyate R., Yucel E., et al. Adaptation and evaluation of symptom-monitoring digital health intervention for patients with relapsed and refractory multiple myeloma: pilot mixed-methods implementation study. JMIR Form Res. 2020;4(11):e18982. doi: 10.2196/18982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manda S., Yimer H., Noga S., et al. Feasibility of long-term proteasome inhibition in multiple myeloma by in-class transition from bortezomib to ixazomib. Clin Lymphoma Myeloma Leuk. 2020;20(11):e910–e925. doi: 10.1016/j.clml.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett A.V., Reeve B.B., Basch E.M., et al. Evaluation of pedometry as a patient-centered outcome in patients undergoing hematopoietic cell transplant (HCT): a comparison of pedometry and patient reports of symptoms, health, and quality of life. Qual Life Res. 2016;25(3):535–546. doi: 10.1007/s11136-015-1179-0. Epub 2015 Nov 17. PMID: 26577763. [DOI] [PubMed] [Google Scholar]
- 12.Mayo N.E., Moriello C., Scott S.C., et al. Pedometer-facilitated walking intervention shows promising effectiveness for reducing cancer fatigue: a pilot randomized trial. Clin Rehabil. 2014;28(12):1198–1209. doi: 10.1177/0269215514536209. Epub 2014 Jun 10. PMID: 24917586. [DOI] [PubMed] [Google Scholar]
- 13.Piringer G., Vormittag L., Öhler L., et al. REGO-ACT: assessment of physical activity during treatment with regorafenib for metastatic colorectal cancer. Wien Klin Wochenschr. 2020;132(15-16):423–430. doi: 10.1007/s00508-020-01703-z. Epub 2020 Jul 8. PMID: 32643016. [DOI] [PubMed] [Google Scholar]
- 14.Glassman G.E., Makhoul A.T., Zhang M., Johnson S.P., Perdikis G., Drolet B.C. Actigraphy to evaluate changes in physical activity after autologous breast reconstruction. Ann Plast Surg. 2021;86(6S Suppl 5):S610–S614. doi: 10.1097/SAP.0000000000002698. PMID: 34100822. [DOI] [PubMed] [Google Scholar]
- 15.Rossi L.A., Melstrom L.G., Fong Y., Sun V. Predicting post-discharge cancer surgery complications via telemonitoring of patient-reported outcomes and patient-generated health data. J Surg Oncol. 2021;123(5):1345–1352. doi: 10.1002/jso.26413. Epub 2021 Feb 23. PMID: 33621378; PMCID: PMC8764868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun V., Dumitra S., Ruel N., et al. Wireless monitoring program of patient-centered outcomes and recovery before and after major abdominal cancer surgery. JAMA Surg. 2017;152(9):852–859. doi: 10.1001/jamasurg.2017.1519. PMID: 28593266; PMCID: PMC5607084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittman D.D., Hines P.C., Beidler D., et al. Evaluation of longitudinal pain study in sickle cell disease (ELIPSIS) by patient-reported outcomes, actigraphy, and biomarkers. Blood. 2021;137(15):2010–2020. doi: 10.1182/blood.2020006020. PMID: 33067606; PMCID: PMC8057263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higami Y., Higuchi A., Tanaka H., et al. Nonwearable actigraphy to assess changes in motor activity before and after rescue analgesia in terminally ill patients with cancer: a pilot study. Int J Nurs Pract. 2022;28(4):e13019. doi: 10.1111/ijn.13019. Epub 2021 Oct 14. PMID: 34651388. [DOI] [PubMed] [Google Scholar]
- 19.Courneya K.S., Segal R.J., McKenzie D.C., et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46:1744–1751. doi: 10.1249/MSS.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 20.Hayes S.C., Steele M.L., Spence R.R., et al. Exercise following breast cancer: exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat. 2018;167:505. doi: 10.1007/s10549-017-4541-9. [DOI] [PubMed] [Google Scholar]
- 21.Singh B., Zopf E.M., Howden E.J. Effect and feasibility of wearable physical activity trackers and pedometers for increasing physical activity and improving health outcomes in cancer survivors: a systematic review and meta-analysis. J Sport Health Sci. 2022;11(2):184–193. doi: 10.1016/j.jshs.2021.07.008. Epub 2021 Jul 24. PMID: 34314878; PMCID: PMC9068515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnagnarella P., Marvaso G., Jereczek-Fossa B.A., et al. Life style and interaction with microbiota in prostate cancer patients undergoing radiotherapy: study protocol for a randomized controlled trial. BMC Cancer. 2022;22(1):794. doi: 10.1186/s12885-022-09521-4. PMID: 35854230; PMCID: PMC9295396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Møller T., Andersen C., Lillelund C., et al. Physical deterioration and adaptive recovery in physically inactive breast cancer patients during adjuvant chemotherapy: a randomised controlled trial. Sci Rep. 2020;10(1):9710. doi: 10.1038/s41598-020-66513-9. PMID: 32546796; PMCID: PMC7297957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palumbo A., Bringhen S., Mateos M.V., et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an international myeloma working group report. Blood. 2015;125(13):2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S., Paiva B., Anderson K.C., et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 26.Vandenbroucke J.P., von Elm E., Altman D.G., et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.