Summary
Background
Data on risk factors for carbapenem-resistant Enterobacterales (CRE) with wider applicability are needed to inform preventive measures and efficient design of randomised trials.
Methods
An international matched case-control-control study was performed in 50 hospitals with high CRE incidence from March 2016 to November 2018 to investigate different aspects of infections caused by CRE (NCT02709408). Cases were patients with complicated urinary tract infection (cUTI), complicated intraabdominal (cIAI), pneumonia or bacteraemia from other sources (BSI-OS) due to CRE; control groups were patients with infection caused by carbapenem-susceptible Enterobacterales (CSE), and by non-infected patients, respectively. Matching criteria included type of infection for CSE group, ward and duration of hospital admission. Conditional logistic regression was used to identify risk factors.
Findings
Overall, 235 CRE case patients, 235 CSE controls and 705 non-infected controls were included. The CRE infections were cUTI (133, 56.7%), pneumonia (44, 18.7%), cIAI and BSI-OS (29, 12.3% each). Carbapenemase genes were found in 228 isolates: OXA-48/like, 112 (47.6%), KPC, 84 (35.7%), and metallo-β-lactamases, 44 (18.7%); 13 produced two. The risk factors for CRE infection in both type of controls were (adjusted OR for CSE controls; 95% CI; p value) previous colonisation/infection by CRE (6.94; 2.74–15.53; <0.001), urinary catheter (1.78; 1.03–3.07; 0.038) and exposure to broad spectrum antibiotics, as categorical (2.20; 1.25–3.88; 0.006) and time-dependent (1.04 per day; 1.00–1.07; 0.014); chronic renal failure (2.81; 1.40–5.64; 0.004) and admission from home (0.44; 0.23–0.85; 0.014) were significant only for CSE controls. Subgroup analyses provided similar results.
Interpretation
The main risk factors for CRE infections in hospitals with high incidence included previous colonization, urinary catheter and exposure to broad spectrum antibiotics.
Funding
The study was funded by the Innovative Medicines Initiative Joint Undertaking (https://www.imi.europa.eu/) under Grant Agreement No. 115620 (COMBACTE-CARE).
Keywords: Antimicrobial resistance, Carbapenem-resistant Enterobacterales, Risk factors, KPC, OXA, Metallo-beta-lactamases
Research in context.
Evidence before this study
We search PubMed and Scopus until June 2022 combining the terms carbapenem-resistant or carbapenemase-producing, enterobacteriaceae or enterobacterales Klebsiella, and risk factors or predisposing factors, and used systematic reviews and meta-analysis as filters. We found 4 systematic reviews of risk factors, and reviewed the primary articles included in them. The literature review of studies investigating the risk factors for carbapenem-resistant Enterobacterales (CRE) provided results for which their extrapolation is limited because they were performed mostly in one or few sites or wards, included only Klebsiella pneumoniae infections, and/or used patients with susceptible organisms as the only control group.
Added value of this study
The design of EURECA allows a better generalizability of its results as it was performed in 50 hospitals from 10 countries in Southern Europe and included two control groups (patients with carbapenem-susceptible Enterobacterales [CSE] and patients without infection), which were matched according to site of infection (for CSE), ward and previous hospital stay. This design controlled the confounding effect of the local epidemiology situation at each site and the time-dependent bias for the exposures. Also, the use of two control groups allowed to identify the variables increasing the probability of CRE infection, and to quantify their relative impact among patients with Enterobacterales infection and admitted patients, respectively.
Implications of all the available evidence
The risk factors found can be easily collected and be considered for deciding empirical treatment according to the local epidemiology and for the efficient design of future randomised trials in order to maximise the population at risk of CRE. Also, the results may help in designing preventive measures focused on patients at high risk.
Introduction
Antimicrobial resistance is recognised as one of the most important public health problems.1 Among resistant bacteria, the dramatic increase in the rate of carbapenem-resistant Enterobacterales (CRE) during the last decade is among the most worrisome phenomena because the alternatives available for their treatment are extremely limited; in fact, CRE are considered a priority for research in drug development by the World Health Organisation (WHO).2 Infections caused by these bacteria are associated with worse outcomes, longer hospitalizations and higher costs compared to their susceptible counterparts.2,3 The most frequent mechanism of resistance to carbapenems in Enterobacterales are production of class A (e.g., KPC enzymes), B (metallo-β-lactamases such as NDM or VIM, among others) and D carbapenemases (OXA-48 and OXA-48-like).4
Most previous studies investigating the risk factors for infections caused by CRE included limited number of patients, were performed in individual hospitals and some lack adequate control for key confounders,5, 6, 7, 8 therefore jeopardizing the generalizability of their results. When trying to identify risk factors for infections caused by resistant bacteria, several aspects need to be considered, including the population under consideration, the group of patients used as reference, the variables investigated and how confounding is controlled both in the design and in the analysis.
The objective of this study was to investigate the risk factors for and profile of patients with infections caused by CRE in a case-control-control multinational study in countries with a high incidence of CRE, which can be useful for the selection of high risk populations for more efficient design of randomized trials, for developing preventive strategies and for considering empirical therapy. The study targeted the most frequent infections caused by CRE, and the design was based on specified epidemiological assumptions related to CRE epidemiology and pathways for the development of infection.
Methods
Study design, participants, sites and study period
The European Prospective Cohort Study on Enterobacteriaceae Showing Resistance to Carbapenems (EURECA) is a multinational study performed in 50 European hospitals from March 2016 to November 2018 to investigate different aspects of infections caused by CRE (NCT02709408).9 The 50 participating hospitals were located in ten European countries: Albania, Croatia, Greece, Italy, Kosovo, Montenegro, Romania, Serbia, Spain and Turkey. These sites were selected based on their rate of CRE infection, experience in clinical studies and laboratory capabilities. Participating sites included consecutive cases diagnosed with the target infections (complicated urinary tract infections [cUTI], complicated intraabdominal infections [cIAI], pneumonia and bloodstream infections from other sources [BSI-OS]) caused by CRE. The study protocol is available as Supplementary Annex A.
For this analysis, a nested matched case-control-control study design was used, based on several predefined hypotheses and assumptions (Table 1). Patients included in the EURECA prospective CRE cohort were eligible as cases, and two control groups representing two different populations were chosen. The first control group included patients with infections caused by carbapenem-susceptible Enterobacteriaceae (CSE control group; one control per case); the comparison of CRE cases and CRE control represented the population of patients with infections due to Enterobacterales. The second control group included patients hospitalized without CRE infection (non-infected control group; three controls per case), and the comparison of CRE and non-infected patients represented the population of patients admitted to the hospitals. Patients in both control groups were matched to CRE cases for hospital ward and previous length of hospital stay, with an accepted difference of −3 days in the control groups (−7 days if previous stay for the case was >14 days to avoid time-dependent bias)10;CSE controls were also matched by type of infection (Table 1). CRE cases were prospectively detected in all sites by daily review of the microbiology reports. Once a CRE case was included, control patients fulfilling the above mentioned matching criteria were “prospectively” recruited at the same site, until the estimated sample size was reached.
Table 1.
Predefined hypothesis and assumptions for the design of the risk factors study.
|
STROBE recommendations for reporting results of observational studies were followed.11
Variables, data collection and quality control
The variables collected are listed and defined in Table 2; a full definition is provided in the study protocol (Annex A). The definition of timing for exposures was decided according to previous publications or the expected timeframe for their potential effect. The data were collected by local investigators in real time (i.e., while patients were admitted at hospital); all local teams at each participating site were trained remotely before the site was opened for recruitment for the study design, criteria, variables and data collection. The data were monitored remotely for missing information (i.e., any missing data prompted a query to local investigators to check if the data could be completed; unanswered queries were sent up to 3 times) and coherence by the central coordinating team at Hospital Universitario Virgen Macarena in Seville and country or region coordinators. All exposures were considered until “day 0”, which was the day when the first sample yielding CRE or CSE was obtained for the diagnosis of the infection of interest; for non-infected patients, it was the day from admission equivalent to day 0 for the correspondent CRE case.
Table 2.
Exposure to the different variables in the three patient-groups
| CRE group (n = 235) n (%) | CSE group (n = 235) n (%) | p | Non-infected group (n = 705) n (%) | p | |
|---|---|---|---|---|---|
| Demographics and epidemiological context | |||||
| Age (years), median (IQR) | 73 (62–82) | 70 (59–79) | 0.081 | 67 (53–77) | <0.001 |
| Male sex | 134 (57.0) | 126 (53.6) | 0.42 | 412 (58.4) | 0.69 |
| Caucasian ethnicity | 231 (98.3) | 229 (97.4) | 0.42 | 690 (97.9) | 0.63 |
| Present admission from: | |||||
| Home | 174 (74.0) | 203 (86.4) | 0.001 | 637 (90.4) | <0.001 |
| Nursing home | 9 (3.8) | 9 (3.8) | 1.0 | 9 (1.3) | 0.015 |
| Other long term-care facility | 19 (8.1) | 7 (3.0) | 0.01 | 10 (1.4) | <0.001 |
| Another acute care hospital | 32 (13.6) | 16 (6.8) | 0.01 | 49 (7) | 0.001 |
| Previous acute care hospitalization (last 6 months) | 150 (63.8) | 104 (44.3) | <0.001 | 224 (31.8) | <0.001 |
| Travel abroad (last 6 months) | 4 (1.7) | 3 (1.7) | 0.56 | 35 (5.0) | 0.037 |
| Household/residency mates colonized/infected by CRE | 15 (6.4) | 11 (4.7) | 0.46 | 6 (0.9) | <0.001 |
| Other patient(s) colonized/infected by CRE in the same ward during admission | 77 (32.8) | 66 (28.1) | 0.22 | 248 (35.2) | 0.35 |
| Healthcare worker or caregiver of dependant person | 1 (0.4) | 4 (1.7) | 0.21 | 7 (1.0) | 0.42 |
| Usual contact with pets, last 6 months | 33 (14.0) | 32 (13.6) | 0.88 | 110 (15.6) | 0.42 |
| Any contact with farm animals, last 6 months | 1 (0.4) | 5 (2.1) | 0.14 | 16 (2.3) | 0.092 |
| Mean days of previous stay (SD) | 9.2 (15.1) | 7.4 (13.7) | <0.001 | 7.7 (10.4) | <0.001 |
| Previous colonisation/infection by CREa | 50 (21.3) | 8 (3.4) | <0.001 | 6 (0.9) | <0.001 |
| Previous colonization/infection by other MDROb | 25 (10.6) | 18 (7.7) | 0.18 | 24 (3.4) | <0.001 |
| Type of acquisition of infectionc | |||||
| Nosocomial | 138 (58.7) | 138 (58.7) | 1.00 | – | – |
| Community-onset, healthcare-associated | 79 (33.6) | 51 (21.7) | <0.001 | – | – |
| Community-acquired | 18 (7.7) | 46 (19.6) | <0.001 | – | – |
| Chronic comorbidities and conditions | |||||
| Charlson index, median (IQR) | 3 (2–4) | 2 (1–4) | 0.008 | 2 (0–3.5) | <0.001 |
| Obesity (Body mass index >30) | 35 (15.2) | 39 (16.7) | 0.61 | 106 (15.1) | 0.98 |
| Diabetes mellitus | 70 (29.8) | 66 (28.1) | 0.66 | 170 (24.1) | 0.083 |
| Chronic pulmonary disease | 44 (18.7) | 36 (15.3) | 0.31 | 109 (15.5) | 0.22 |
| Chronic heart failure (NYHA ≥ 2) | 44 (18.7) | 28 (11.9) | 0.038 | 84 (11.9) | 0.005 |
| Dementia | 37 (15.7) | 22 (9.4) | 0.025 | 34 (4.8) | <0.001 |
| Hemiplegia | 15 (6.4) | 9 (3.8) | 0.22 | 14 (2.0) | 0.002 |
| Chronic liver disease | 15 (6.4) | 14 (6.0) | 0.83 | 64 (9.1) | 0.63 |
| Chronic renal failure (moderate or severe) | 65 (27.7) | 33 (14) | <0.001 | 88 (12.5) | <0.001 |
| Structural disease of the urinary tract | 48 (20.4) | 40 (17) | 0.21 | 0 (0) | 0.001 |
| Connective tissue disease | 8 (3.4) | 7 (3.0) | 0.79 | 26 (3.7) | 0.83 |
| Solid organ cancer | 64 (27.2) | 57 (24.3) | 0.41 | 143 (20.3) | 0.014 |
| Hematologic cancer | 12 (5.1) | 12 (5.1) | 1.00 | 35 (5.0) | 0.90 |
| Bone marrow/stem cell transplantation | 1 (0.4) | 1 (0.4) | 1.00 | 10 (1.4) | 0.17 |
| Neutropenia (<500 cels/μL) | 13 (5.8) | 8 (3.4) | 0.23 | 27 (3.8) | 0.13 |
| Solid organ transplantation | 16 (6.8) | 13 (5.5) | 0.53 | 28 (4) | 0.028 |
| HIV infection | 1 (0.4) | 2 (0.9) | 0.57 | 14 (2) | 0.14 |
| Invasive procedures or therapies | |||||
| Central venous catheter (last week) | 78 (33.2) | 60 (25.5) | 0.020 | 152 (21.6) | <0.001 |
| Urinary catheter (last week) | 153 (65.1) | 120 (51.1) | 0.001 | 216 (30.6) | <0.001 |
| Mechanical ventilation (last week) | 42 (17.9) | 45 (19.1) | 0.58 | 96 (13.6) | 0.013 |
| Major surgery last month (needing hospital admission) | 71 (30.2) | 65 (27.7) | 0.41 | 133 (18.9) | <0.001 |
| Endoscopic procedure (last week) | 16 (6.8) | 18 (7.7) | 0.72 | 30 (4.3) | 0.087 |
| Chronic dialysis | 23 (9.8) | 8 (3.4) | 0.008 | 34 (4.8) | 0.001 |
| Immunosupressive drugs (last 3 months) | 59 (25.1) | 52 (22.1) | 0.40 | 121 (17.2) | 0.002 |
| Exposure to antibacterial agents (last 3 months) | |||||
| Any antibiotic received | 186 (79.1) | 150 (63.8) | <0.001 | 370 (52.5) | <0.001 |
| Median no. of antibiotics received (IQR) | 2 (1–3) | 1 (0–2) | <0.001 | 1 (0–2) | <0.001 |
| Mean days of antibiotics (SD) | 18.3 (21.6) | 11.2 (17.1) | <0.001 | 11.0 (23.4) | <0.001 |
| Carbapenemsd | 33 (14.0) | 14 (6.0) | 0.010 | 57 (8.1) | 0.004 |
| Mean days of carbapenemsd (SD) | 1.2 (3.5) | 0.4 (2.2) | 0.011 | 0.7 (3.3) | 0.027 |
| Piperacillin-tazobactam | 45 (19.1) | 22 (9.4) | <0.001 | 64 (9.1) | <0.001 |
| Mean days of piperacillin-tazobactam (SD) | 1.5 (4.0) | 0.7 (2.6) | 0.05 | 0.7 (2.6) | <0.001 |
| Fluoroquinolonese | 87 (37.0) | 56 (23.8) | 0.003 | 146 (20.7) | <0.001 |
| Mean days of fluoroquinolonese (SD) | 3.2 (5.0) | 2.3 (5.4) | 0.007 | 1.9 (5.4) | 0.003 |
| Oxyimino β-lactamsf | 83 (35.3) | 57 (24.3) | 0.011 | 143 (20.3) | <0.001 |
| Mean days of oxyimino β-lactamsf (SD) | 3.5 (7.3) | 2.0 (4.4) | 0.015 | 1.5 (4.1) | <0.001 |
| Amoxicillin-clavulanic acid or ampicillin-sulbactam | 38 (16.2) | 36 (15.3) | 0.80 | 65 (9.2) | 0.003 |
| Mean days of amoxicillin-clavulanic acid or ampicillin-sulbactam (SD) | 1.0 (2.8) | 1.4 (4.6) | 0.89 | 0.6 (2.3) | 0.024 |
| Aminoglycosidesg | 18 (7.7) | 10 (4.2) | 0.003 | 24 (3.4) | 0.009 |
| Mean days of aminoglycosidesg (SD) | 0.5 (2.0) | 0.2 (1.2) | 0.079 | 0.1 (1.2) | 0.023 |
| Broad-spectrum anti-gram negative drugsh | 164 (69.8) | 115 (48.9) | <0.001 | 277 (39.3) | <0.001 |
| Mean days of broad-spectrum anti-gram negative drugsh (SD) | 9.4 (10.0) | 5.5 (8.2) | <0.001 | 5.0 (8.8) | <0.001 |
| Antianaerobic drugsi | 106 (45.1) | 73 (31.1) | 0.001 | 201 (28.5) | <0.001 |
| Mean days of antianaerobic drugsi (SD) | 5.4 (8.4) | 3.4 (6.8) | 0.003 | 3.5 (6.9) | <0.001 |
| Anti-gram positive drugsj | 39 (16.6) | 17 (7.2) | 0.001 | 52 (7.4) | <0.001 |
| Mean days of anti-gram positive drugsj (SD) | 1.6 (4.6) | 0.5 (2.8) | 0.008 | 0.5 (2.6) | <0.001 |
| Time of exposure to broad-spectrum drugs | <0.001 | <0.001 | |||
| No broad spectrum anti-gram negative drugs,h | 70 (29.8) | 119 (50.6) | 427 (60.6) | ||
| Broad spectrum anti-gram negative drugs,h <6 days | 24 (10.2) | 32 (13.6) | 52 (7.4) | ||
| Broad spectrum anti-gram negative drugs,h ≥6 days | 141 (60.0) | 84 (35.7) | 226 (32.1) | ||
| Number of broad-spectrum anti-gram negative drugs | <0.001 | <0.001 | |||
| None | 50 (21.3) | 96 (40.9) | 351 (49.8) | ||
| One | 121 (51.5) | 112 (47.6) | 259 (36.7) | ||
| ≥2 | 64 (27.2) | 27 (11.5) | 95 (13.5) |
Data are number of patients (percentage) except where specified.
CRE: carbapenem-resistant Enterobacterales. CSE: carbapenem-susceptible Enterobacterales. SD: standard deviation.
Evidence of any previous positive culture (screening or clinical samples) with isolation of CRE; if no evidence was available (e.g., no previous culture results with CRE), it was considered as no exposure.
As above, for ESBL- or AmpC producing Enterobacterales, carbapenem-resistant Pseudomonas aeruginosa or Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus, or vancomycin-resistant enterococci.
Nosocomial: infection occurring after 48 h of hospital admission or in <7 days after a previous hospital discharge; community-onset, healthcare-associated infection: those not nosocomially-acquired, in patients with any of the following in the last 3 months: admission to acute or long term-care facility, intravenous therapy, major surgery, specialised home care, renal replacement therapy; community-acquired: all others.
Ertapenem, meropenem, imipenem.
Ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin.
2nd, 3rd and 4th generation cephalosporins (except cephamycins), and aztreonam.
Amikacin, gentamicin, tobramycin.
Carbapenems, fluoroquinolones, piperacillin-tazobactam and oxyimino-β-lactams.
Carbapenems, β-lactam-β-lactam inhibitors, cephamycins, moxifloxacin, metronidazol, tigecycline, clindamycin.
Vancomycin, teicoplanin, linezolid, daptomycin. Definitions for other variables are in the study protocol (Annex A).
Microbiological definitions and studies
The microorganisms of interest were carbapenem-resistant (according to EUCAST breakpoints)12 and/or carbapenemase-producing (regardless of the minimum inhibitory concentration [MIC] to carbapenems) Enterobacterales (Table 1). For ease of understanding, we will refer to them as CRE. In order to detect most carbapenemase-producers, all Enterobacterales with MIC ≥1 mg/L (dilution methods) or ≤22 mm (disc-diffusion, 10 μg disks) for meropenem or imipenem were considered as putative CRE. Identification and susceptibility testing were performed by local laboratories using standard microbiological techniques after training for procedures homogeneity; putative CRE isolates were also studied locally using the CARBA NP test. All CRE isolates were preserved at −20 °C and sent to central laboratories were identification and susceptibility confirmation (Ramón y Cajal University Hospital, Madrid, Spain), and characterisation of carbapenemase genes by whole genome sequencing (Antwerp University, Belgium) were performed.
Statistical analyses
We targeted the inclusion of 248 CRE cases, distributed by type of infection according to previous studies13,14 as follows: cUTI, ≈50%; pneumonia, ≈30%; and cIAI and BSI-OS, ≈10% each, to be able to include at least 20 risk factors in a multivariate model. For each CRE case patient, one CSE and three admitted control patients were planned, in order to approximate the statistical power of full cohort data.15
Missing data were quantified (Supplementary Annex B, Table S1); the Little MCAR test was used to verify that missing data were at random, and multiple imputation was performed using the Markov chain Monte Carlo method. In order to characterize the impact of previous antibiotic use, we tested the effect of exposure to individual drugs or groups (both as dichotomous variables and as days of exposure), to drugs active only against gram positive bacteria, against anaerobic bacteria and to broad-spectrum anti-gram negative bacteria (including carbapenems, fluoroquinolones, piperacillin-tazobactam and oxyimino-β-lactams). For duration of exposure to antibiotics, in addition to being considered as continuous variables, we used classification and regression tree (CART) analyses in order to identify thresholds for duration associated with increased risk.
For the analysis of risk factors, exposure to potential risk factors among CRE and CSE patients, and among CRE and non-infected controls was performed by hierarchical conditional logistic regression for matched data. To do so, the variables were classified into hierarchical groups (Table 1). A manual backward selection method of variables was used; the variables were kept in the models if their p value was <0.1. Despite being used as a matching variable and because a rank of days was tolerated (see above), previous hospital stay was included in all models. The predictive capacity of the multivariate models was evaluated by calculating the area under the receiver operating characteristics curve (AUROC) with 95% confidence interval (CI). The analyses were performed using IBM SPSS (version 26.0) and CART software 8.0 (Salford Systems).
Ethical approval
The study was approved by the Ethics Committee of the Hospital Universitario Virgen Macarena (FIS-ATB-2015-01). The need to obtain written informed consent was waived due to the observational and epidemiological nature of the study. Approval was also gained at the participating centres according to local requirements.
Role of funding
The funders had no role in the design, conduction of the study, decision to publish or writing of the article.
Results
During the study period, 732 patients with infection caused by CRE were included; of these, the first 235 CRE case patients for whom matching controls were found were included in the cases-control-control study. These were matched to 235 CSE controls and 705 non-infected controls. The CRE case patients were recruited in Spain (104), Greece (53), Serbia (36), Turkey (16), Italy (15), Romania (9) and Montenegro (2). The median age of CRE cases was 74 years (IQR, 63–83); 135 (57.4%) were males; 174 (74%) were admitted from home, 148 (63%) were admitted to medical wards, 45 (19.1%) to surgical wards, and 42 (17.9%) to intensive care units (ICU). Their median length of previous stay in hospital was 3 days (IQR 0–14); previous stay was 0–2 days in 113 (48.1%), 3–7 days in 26 (11.1%), 8–14 days in 42 (17.9%), and >14 days in 54 (23.0%).
The type of CRE infection was cUTI in 133 (56.7%), pneumonia in 44 (18.7%), and cIAI and BSI-OR in 29 (12.3%), respectively. Klebsiella pneumoniae was the most frequent pathogen in CRE infections (206; 87.6%), followed by Enterobacter cloacae complex (11; 4.6%) and Escherichia coli (7; 2.9%); In 7 isolates (2.9%), no carbapenemase genes were found; while in the other 228, carbapenemase genes were codifying for either OXA-48 or OXA-48-like enzymes (112 isolates [47.6%]), KPC (84 [35.7%]) or metallo-β-lactamases (MBL) (44 [18.7%]) were found. Interestingly, 13 isolates produced 2 carbapenemases (10 an OXA-48 plus an MBL, and 3 a KPC plus an MBL). Overall, 191 isolates (81.2%) were carbapenem-resistant according to EUCAST breakpoints.
Risk factors for CRE infection in Enterobacterales infection population
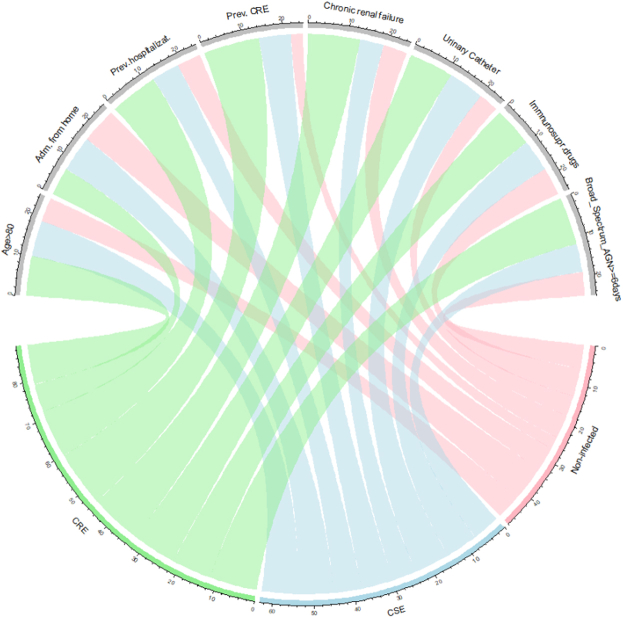
The distribution of Enterobacterales isolates was more heterogeneous among patients with CSE infection, with a predominance of E. coli (48.5%), followed by K. pneumoniae (27.5%). When exposures were compared between patients with CRE and CSE infection, the following exposures were significantly more frequent among CRE patients in univariable comparison: hospitalisation in the last three months, previous colonisation/infection by CRE, chronic heart failure, dementia, chronic renal failure, central venous and urinary catheters, dialysis, and previous use of antibiotics. Furthermore, CRE patients were less frequently admitted from home and less frequently had community-acquired infections (Table 2). The association of the populations with the key variables is shown in Fig. 1.
Fig. 1.
Chord diagram for the distribution of exposure to key variables in the CRE (green), CSE (blue) and non-infected (pink) groups. The width of the ribbons correlates with the proportion of patients exposed to each variable in the respective group.
In adjusted analysis, the final multivariate model best fitting to the data selected the following variables as independently associated with CRE infection: being admitted from home (protective), previous colonization or infection by CRE, chronic renal failure, urinary catheter, and exposure to broad-spectrum anti-gram negative antibiotics (model A; Table 3). When previous antibiotics were included as days of exposure, days of exposure to broad-spectrum anti-gram negative drugs was also associated with increased risk (model B, Table 3). CART selected 6 days of exposure to these drugs as a breakpoint for risk association, and therefore a risk category variable including no exposure, <6 days and ≥6 days of exposure was also studied; in this model, a significant association was found for the strata of >6 days of exposure to broad-spectrum anti-gram negative drugs (model C, Table 3). Finally, exposure to ≥2 broad-spectrum anti-gram negative drugs was associated with higher risk than exposure to one of these drugs. The AUROC of the three models for observed data were very similar with high predictive ability (Table 3).
Table 3.
Multivariate hierarchical conditional logistic regression analysis of risk factors for CRE infection.
| CRE vs CSE |
CRE vs non-infected |
|||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| Intrinsic features | ||||
| Age (per year) | – | – | 1.03 (1.01–1.05) | 0.001 |
| Chronic renal failure (moderate or severe) | 2.81 (1.40–5.64) | 0.004 | – | – |
| Epidemiological exposures | ||||
| Previous colonization/infection by CRE | 6.94 (2.74–17.53) | <0.001 | 13.14 (3.98–43.43) | <0.001 |
| Admission from home | 0.44 (0.23–0.85) | 0.014 | – | – |
| Previous hospitalization (last 6 months) | – | – | 1.84 (0.95–3.55) | 0.068 |
| Invasive procedures and therapies | ||||
| Urinary catheter (last week) | 1.78 (1.03–3.07) | 0.038 | 3.68 (1.86–7.28) | <0.001 |
| Immunosuppressive drugs | – | – | 3.38 (1.44–7.93) | 0.005 |
| Exposure to antibiotics | ||||
| MODEL A: Broad-spectrum anti-gram negative drugs | 2.20 (1.25–3.88)a | 0.006 | 2.89 (1.45–5.73)b | 0.002 |
| MODEL B: Days of broad-spectrum anti-gram negative drugs | 1.04 (1.00–1.07)c | 0.014 | 1.02 (0.99–1.04)d | 0.081 |
| MODEL C: Time of exposure to broad-spectrum drugs | ||||
| No broad spectrum anti-gram negative drugs | Refe | Ref | Reff | Ref |
| Broad spectrum anti-gram negative drugs, <6 days | 1.25 (0.57–2.71) | 0.56 | 3.00 (1.07–8.43) | 0.037 |
| Broad spectrum anti-gram negative drugs, ≥6 days | 2.86 (1.56–5.26) | 0.001 | 2.96 (1.44–6.06) | 0.003 |
| MODEL D: Number of broad-spectrum drugs | ||||
| None | Refg | Refh | ||
| One | 1.70 (1.00–2.90) | 0.050 | 2.03 (1.23–3.36) | 0.006 |
| ≥2 | 3.66 (1.77–7.58) | <0.001 | 2.95 (1.56–5.60) | <0.001 |
For exposures of antibiotics, different models were developed: model A included exposure to antibiotic groups as dichotomous variables; model B included duration of exposure to antibiotic groups; model C included only the risk category. Models with individual drugs did not provide significant associations and are not shown. The adjusted data for intrinsic features, epidemiological exposures, and invasive procedures and therapies were obtained for the model A, and were not significantly different for models B and C and therefore are not shown. Previous duration of hospital stay was included in all models.
Broad-spectrum anti-gram negative drugs: carbapenems, piperacillin-tazobactam, oxyimino-β-lactams, fluoroquinolones.
Reference categories for categorical exposures were “non-exposed” except where specified; for continuous variables (age, days of broad spectrum anti-gram negative drugs), the adjusted OR is per unit.
Area under the receiver operating characteristic curve (AUROC) for observed data of the models:
0.71 (95% CI: 0.67–0.75).
0.81 (95% CI: 0.78–0.84).
0.72 (95% CI: 0.68–0.76).
0.81 (95% CI: 0.78–0.84).
0.73 (95% CI: 0.69–0.77).
0.81 (95% CI: 0.78–0.84).
0.74 (0.70–0.78).
0.81 (0.78–0.84).
Risk factors for CRE in hospital-admitted patients population
All risk factors for CRE infection in the Enterobacterales infection population also applied when patients with CRE infection were compared to non-infected control patients (hospital-admitted population) in univariable analysis. Moreover, in this comparison, patients with CRE infection were older, had more frequently ambulatory contact with persons with CRE colonization or infection, more frequently had hemiplegia, solid cancer and solid organ transplantation, and exposure to mechanical ventilation, recent surgery, endoscopic procedures, dialysis and immunosuppressive drugs (Table 2).
In multivariate analysis the following variables were independently associated with CRE infection (Table 3): age, previous infection/colonisation by CRE, hospitalization in previous six months, use of urinary catheter in the last week, immunosuppressive drugs and use of broad-spectrum anti-gram negative antibiotics. As above, when antibiotics were included as days of exposure (model B), duration of broad-spectrum anti-gram negative drugs was also associated with increased risk, and when the time-categorised variable was included, both <6 and ≥6 days of broad-spectrum anti-gram negative antibiotics were associated with an increased risk for CRE. Also, exposure to ≥2 broad spectrum drugs was associated with higher risk than exposure to one drug. The AUROC of the models were similar with high predictive ability (Table 3).
Subgroups analyses
Subgroup analyses were performed in CRE patients and their matched controls for patients with infections caused by Enterobacterales producing the different carbapenemase-types, for patients with cUTI (as the most frequent type of infection), for community-onset acquisition and for patients without previous evidence of colonisation/infection by CRE. The results of these subgroups were in general consistent with those of the global analyses, with few exceptions (Table 4). Of note, previous exposure to broad-spectrum anti-gram negative drugs was associated with increased risk in all subgroups but not in the comparison of OXA-producing CRE with CSE nor in patients with community-acquisition of CRE infection against non-infected patients; and surgery was associated with increased risk of CRE among patients who had no evidence of prior colonisation/infection by CRE, and among patients with community acquisition (but only against non-infected patients).
Table 4.
Multivariate analysis of risk factors for CRE infections in subgroups of patients.
| CRE vs CSE |
CRE vs non-infected |
|||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| OXA-enzymes producers and matched controls | ||||
| Age (per year) | – | – | 1.03 (1.00–1.06) | 0.010 |
| Chronic renal failure | 5.67 (1.58–20.31) | 0.008 | – | – |
| Previous hospitalisation | – | – | 2.18 (1.23–6.41) | 0.013 |
| Admission from home | 0.24 (0.08–0.61) | 0.005 | – | – |
| Previous colonization/infection by CRE | 6.61 (1.83–23.87) | 0.004 | 16.11 (3.38–76.38) | <0.001 |
| Broad-spectrum anti-gram negative drugs | – | – | 3.26 (1.40–7.60) | 0.006 |
| KPC producers and matched controls | ||||
| Age (per year) | – | – | 1.04 (1.00–1.09) | 0.049 |
| Previous colonization/infection by CRE | 13.66 (7.62–71.12) | 0.002 | 7.59 (1.40–41.06) | 0.019 |
| Urinary catheter (last week) | 3.46 (1.16–10.30) | 0.026 | – | – |
| Broad-spectrum anti-gram negative drugs | 2.94 (1.09–7.89) | 0.032 | 6.66 (1.71–25.15) | 0.005 |
| Metallo-β-lactamase producers and matched controls | ||||
| Age (per year) | 1.05 (1.00–1.11) | 0.002 | 1.04 (0.99–1.09) | 0.064 |
| Solid tumor | 15.09 (1.40–161.74) | 0.052 | – | – |
| Chronic renal failure | – | – | 8.04 (1.32–48.75) | 0.023 |
| Broad-spectrum anti-gram negative drugs | 15.34 (2.78–84.48) | 0.0032 | 6.07 (1.51–24.42) | 0.011 |
| Complicated urinary tract infections and matched controls | ||||
| Age (per year) | 1.03 (1.00–1.06) | 0.029 | 1.03 (0.99–5.21) | 0.052 |
| Chronic renal failure (moderate or severe) | 3.24 (1.30–8.11) | 0.012 | 2.25 (0.99–5.12) | 0.052 |
| Hemiplegia | 3.24 (0.93–11.27) | 0.064 | – | – |
| Previous colonization/infection by CRE | 14.01 (3.02–65.02) | 0.001 | 16.51 (4.74–57.50) | <0.001 |
| Urinary catheter (last week) | 2.10 (1.02–4.31) | 0.042 | 7.95 (3.92–16.12) | <0.001 |
| Broad-spectrum anti-gram negative drugs | 2.60 (1.27–5.31) | 0.009 | 2.97 (1.08–5.16) | 0.001 |
| Community onset infection (community-acquired and healthcare-associated) and matched controls | ||||
| Age (per year) | – | – | 1.02 (1.00–1.04) | 0.013 |
| Previous hospitalisation | 2.58 (1.22–5.48) | 0.01 | 2.00 (1.23–3.27) | 0.005 |
| Admission from home | 0.28 (0.07–1.04) | 0.06 | 0.47 (0.26–0.87) | 0.02 |
| Chronic renal failure | 5.73 (1.83–17.90) | 0.003 | – | – |
| Dementia | 3.38 (1.02–11.20) | 0.05 | – | – |
| Previous colonization/infection by CRE | 25.50 (2.58–252.0) | 0.006 | 12.80 (4.27–38.50) | <0.001 |
| Urinary catheter (last week) | – | – | 2.10 (1.20–3.68) | 0.009 |
| Surgery (last month) | – | – | 2.14 (1.13–4.05) | 0.02 |
| Broad-spectrum anti-gram negative drugs | 3.26 (1.30–8.14) | 0.01 | – | – |
| Case patients without previous colonisation or infection by CRE and matched controls | ||||
| Age (per year) | – | – | 1.03 (1.01–1.05) | 0.004 |
| Dementia | – | – | 3.22 (1.07–9.68) | 0.037 |
| Admission from home | 0.86 (0.79–0.95) | 0.003 | – | – |
| Chronic renal failure (moderate or severe) | 3.34 (1.47–7.57) | 0.004 | 2.64 (0.94–7.36) | 0.037 |
| Urinary catheter (last week) | 1.89 (1.06–3.38) | 0.030 | 2.68 (1.25–5.41) | 0.010 |
| Surgery last month | 2.18 (1.00–4.75) | 0.048 | 2.38 (0.96–6.18) | 0.075 |
| Immunosuppressive drugs | – | – | 4.80 (1.83–12.28) | 0.001 |
| Broad-spectrum anti-gram negative drugs | 2.47 (1.36–4.47) | 0.003 | 4.27 (2.12–8.59) | <0.001 |
Reference categories for categorical exposures were “non-exposed”; for continuous variables (age, days of broad spectrum anti-gram negative drugs), the adjusted OR is per unit.
Discussion
In this multinational study, we identified risk factors and patients’ profiles for CRE infections. Some specific features of this study include: (a) the multinational participation; (b) the inclusion of infections caused by different mechanisms of carbapenem-resistance; (c) the risk factors were investigated under several specified hypothetical assumptions; (d) the risk factors were assessed in two populations: patients with Enterobacterales infection and hospital-admitted patients; and (e) a matched design was used to increase the efficiency of the study and ensure the adequate control of the confounding effect of duration of hospitalization and ward of admission, which would otherwise cause an underestimation of the effect of other variables.16 The three risk factors that were found in the two populations studied were evidence for previous colonization or infection with CRE, recent use of urinary catheter and recent use of broad spectrum anti-gram negative drugs. Also, we found a higher than expected proportion of patients with non-nosocomial acquisition of the infection and of patients admitted to non-ICU wards.
Regarding the matching variables, it was surprising to see that almost half of the CRE cases had a hospital stay of ≤2 days, contradicting the general impression that most of these infections occurred late during hospital admission, confirming some recent observations.17 Despite most of these patients having had a community-onset but healthcare-associated or a nosocomial infection (after transfer from another hospital), community circulation of CRE might be wider than previously recognised in these areas. However, we cannot reject the possibility that some healthcare-associated infections were misclassified as community-acquired. Also, it is worth noting that only 1/5 of patients were admitted to ICU, highlighting the frequency of infections caused by CRE in conventional wards.
The risk factors identified must be interpreted in the context of the two populations studied. The comparison with patients without infection provides risk estimates for exposures among all admitted patients and the risk factors found may, therefore, be partially generic for Enterobacterales infections and not fully specific for CRE. The comparison with CSE patients provides information useful for empirical therapeutic decisions but might overestimate the effect of some variables selecting for CRE over CSE, mainly exposure to antibiotics.18 Previous colonisation/infection by CRE, having a urinary catheter and having received broad-spectrum anti-gram negative drugs were associated with CRE in both comparisons, meaning they are truly specific risk factors for CRE infection. Interestingly, the adjusted ORs of previous CRE colonisation/infection and of exposure to broad-spectrum anti-gram negative antibiotics were not higher in the Enterobacterales infection population than in the hospital-admitted patient population, rejecting the hypothesis that their effect would be overestimated in the first population. In contrast, the association with chronic renal failure and being admitted from home (protective) were found in the Enterobacterales infection population only, suggesting that these variables increase risk of a CRE infection over a CSE infection; while older age, previous hospitalisation and immunosuppressive drugs were only found in the hospital-admitted patients-population, suggesting they might be risk factors for Enterobacterales infection in general. Therefore, a better characterization of CRE predictors is gained by comparing the results of both models.
Most previous studies investigating the risk factors for CRE included mostly infections caused by carbapenem-resistant K. pneumoniae but no other Enterobacterales species, and the vast majority of studies were performed in a single hospital. A minority of studies provided estimations for both CSE and non-infected control groups and some included matched controls, but overall the designs and methods for controlling the effect of confounders and the variables studied were very heterogeneous.5, 6, 7, 8 All these factors jeopardize any comparison of previous estimations with our data. Regarding the bacterial species, some 15% of the CRE cases in our study were caused by bacteria other than K. pneumoniae, but their number was insufficient to perform a specific analysis.
The strong association of CRE infection with previous CRE colonization or infection was expected but is nevertheless remarkable. In a recent study performed in high-risk patients (mostly admitted to ICU and haematological wards), all KPC-producing K. pneumoniae infections occurred in patients previously colonized.19 However, this variable was neglected in most previous studies on risk factors5, 6, 7, 8 because screening is not universally performed and therefore the information is not available for all patients. We decided to explore whether the information regarding previous colonization or infection available in the patients’ records in real life would be useful to identify patients at higher risk. Some studies have identified specific risk factors for developing a CRE infection in previously colonized, high-risk patients (again, mostly in ICU), which complement our results.20,21 Our study also provides information about risk factors in patients without evidence of previous colonisation.
Contrary to some previous studies,5, 6, 7, 8 we failed to find associations of specific drugs with increased risk of CRE infection, which might be related to an adequate control of the confounding effect of previous length of stay in our study. Since CRE are typically resistant to most β-lactams and fluoroquinolones, it is reasonable to expect that any broad spectrum drug (and not only carbapenems) would exert a selective pressure once they become endemic in a population. In fact, the antibiotic groups usually considered as the most potent resistant selectors for multidrug-resistant gram negative bacteria (carbapenems, piperacillin-tazobactam, oxyimino-β-lactams and fluoroquinolones22) were consistently associated with increased risk. We were able to characterise the impact of exposure to these drugs by analysing it as a dichotomous variable (yes/no), as a time-dependent variable and as exposure to one or more of these drugs. We found that >6-day duration of exposure was associated with increased risk, as was exposure to more than one broad-spectrum drug.
Our study had a number of limitations. It may be argued that our study design may have caused an underestimation of the impact of different risk factors due to a potential overmatching effect. However, not matching but adjusting for these variables in the analysis phase bears the risk of time-dependent bias and overestimation of the impact of many other variables,10 if control patients are selected among very low risk strata of the populations. As a consequence, the risk factors found in our study must be interpreted considering the wards of admission and length of stay for the CRE cases. Our objective was not investigating causal pathways but providing pragmatic information to help identifying patients at risk and therefore, we did not develop hypothetical direct acyclic graphs for the relation between variables. However, we performed a hierarchical analysis in groups of variables that might act in the same pathway. It should be noted that some variables might act both to facilitate colonization and infection development once colonized (e.g., some comorbidities may favour colonization because of their need of healthcare contact and also infection; also antibiotics may facilitate colonization by eliminating competing flora and infection by selecting resistant bacteria). Other variables might just be proxies for unmeasured variables (e.g., previous hospitalisation and admission from long-term care facilities may be proxies for previous colonisation). The statistical power of the study may have been limited to detect some risk factors. Nevertheless, to the best of our knowledge, this is the biggest study on risk factors for CRE including tow type of control patients, and we think the matching criteria used were efficient in identifying real risk factors. As in all case–control studies, the exposures assessment was performed retrospectively. However, the fact that cases and controls were prospectively detected allowed a better identification of exposures. Finally, the results might not be extrapolated to hospitals/areas with a different epidemiology of CRE, or even to participating sites providing low number of cases.
In conclusion, previously detected colonisation/infection by CRE, having a urinary catheter and receiving broad-spectrum anti-gram negative drugs were risk factors for CRE infection among admitted patients matched for ward of admission and length of stay. Other factors to be considered were being admitted from home (protective) and chronic renal failure for patients with Enterobacterales infections, and older age, previous hospitalisation and use of immunosuppressive drugs for hospital-admitted patients. These results might help in endemic areas, both for decisions about using empirical drugs active against CRE in patients, particularly in the case of severe infections, and for better selecting the most patients to be recruited for randomized trials testing drugs against these pathogens.
Contributors
Conceptualisation: Jesús Rodríguez-Baño, Belén Gutiérrez-Gutiérrez, Jose M. Bravo-Ferrer, Tomislav Kostyanev, Marlieke E.A. de Kraker, Jan Feifel, Rafael Cantón, Herman Goossens, Marc J. Bonten. Data curation: Salvador Pérez, Galera, María Paniagua, Jose M. Bravo-Ferrer, Tomislav Kostyanev, Joost Schotsman. Formal analysis: Belén Gutiérrez-Gutiérrez, Salvador Pérez-Galera, Jose M. Bravo-Ferrer, Marlieke de Kraker, Jan Feifel, Jesús Rodríguez-Baño. Funding acquisition: Jesús Rodríguez-Baño, Lionel K. Tan, Herman Goossens, Marc J., Bonten. Investigation: Salvador Perez-Galera, Jose M. Bravo-Ferrer, María Paniagua, Tomislav Kostyanev, Jesús Sojo-Dorado, Rafael Canton, George L. Daikos, Biljana Carevic, Gorana Dragovac, Lul Raka, Adriana Hristea, Pierluigi Viale, Murat Akova, Jose Maria Reguera, Lucia Valiente de Santis, Julian Torre-Cisneros, Angela Cano, Emmanuel Roilides, Lili Radulovic, Cenk Kirakli, Evelyn Shaw, Matthew E. Falagas, Vicente Pintado. Methodology: Jesús Rodríguez-Baño, Belén Gutiérrez-Gutiérrez, Jose M. Bravo-Ferrer, Tomislav Kostyanev, Marlieke E.A. de Kraker, Jan Feifel, Rafael Cantón, Herman Goossens, Marc J. Bonten. Project administration: Jose M. Bravo-Ferrer, Lionel Tam, Herman Goossens, Marc J. Bonten, Jesús Rodríguez-Baño. Resources: Rafael Canton, George L. Daikos, Biljana Carevic, Gorana Dragovac, Lul Raka, Adriana Hristea, Pierluigi Viale, Murat Akova, Jose Maria Reguera, Julian Torre-Cisneros, Emmanuel Roilides, Lili Radulovic, Cenk Kirakli, Evelyn Shaw, Matthew E. Falagas, Vicente Pintado, Herman Goossens, Marc J. Bonten, Jesús Rodríguez-Baño. Software: Jose M. Bravo-Ferrer, Joost Schotsman. Supervision: Jesús Rodríguez-Baño, Herman Goossens, Marc J. Bonten. Validation: Jose M. Bravo-Ferrer, Belén Gutiérrez-Gutiérrez, Jesús Rodríguez-Baño. Visualisation: Salvador Pérez-Galera, Jose M. Bravo-Ferrer, María Paniagua, Belén Gutiérrez-Gutiérrez, Jesús Rodríguez-Baño. Writing – original draft: Salvador Pérez-Galera, Jose M. Bravo-Ferrer, Belén Gutiérrez-Gutiérrez, Jesús Rodríguez-Baño. Writing – review & editing: María Paniagua, Tomislav Kostyanev, Marlieke E.A. de Kraker, Jan Feifel, Jesús Sojo-Dorado, Joost Schotsman, Rafael Canton, George L. Daikos, Biljana Carevic, Gorana Dragovac, Lionel K. Tan, Lul Raka, Adriana Hristea, Pierluigi Viale, Murat Akova, Jose Maria Reguera, Lucia Valiente de Santis, Julian Torre-Cisneros, Angela Cano, Emmanuel Roilides, Lili Radulovic, Cenk Kirakli, Evelyn Shaw, Matthew E. Falagas, Vicente Pintado, Herman Goossens, Marc J. Bonten. Salvador Pérez-Galera, Jose M. Bravo-Ferrer, María Paniagua, Marlieke E.A. de Kraker, Jan Feifel, Joost Schotsman, Belén Gutiérrez-Gutiérrez and Jesús Rodríguez-Baño had access to the data. Jose M. Bravo-Ferrer, Belén Gutiérrez-Gutiérrez and Jesús Rodríguez-Baño verified all data. All authors read and approved the final version of the manuscript.
Data sharing statement
Data collected for the study, including de-identified participant data and a data dictionary defining each field in the set, will be made available to other investigators upon request to the corresponding author, after approval of a proposal by the senior authors’ institution and the COMBACTE-CARE consortium.
Declaration of interests
George L. Daikos reports personal fees from Pfizer, personal fees from MSD, outside the submitted work. Lionel K. Tan is an employee of and holds stocks and shares in GlaxoSmithKline. Pierluigi Viale reports grants from Shionogi and Gilead; personal fees from Shionogi, MSD, Allianz, Nordic, InfectoPharm, MundiPharm and Angelini, outside the submitted work. Jose María Reguera reports non-financial support from Pfizer. Lucía Valiente de Santis reports non-financial support from Pfizer. Julián Torre-Cisneros reports personal fees from MSD, Pfizer, Menarini, and Shionogi; and non-financial support from Pfizer, Shionogi and Gilead, outside the submitted work. Ángela Cano reports personal fees from Shionogi. Emmanuel Roilides reports personal fees from Amplyx, Astellas, Gilead, MSD, Pfizer, Scynexis, GSK and Shionogi, outside the submitted work. Marc J. Bonten reports grants paid to his institution from Janssen Vaccines, Novartis, CureVac and Merck; participation in Advisory Boards with payment to his institution from Spherecydes, Pfizer, Merck and Astra-Zeneca, and participation in Data Safety Monitoring Boards with payment to his institution from Sanofi. All other authors have no conflicts to declare.
Acknowledgement
The study was funded by the Innovative Medicines Initiative Joint Undertaking (https://www.imi.europa.eu/) under Grant Agreement No. 115620 (COMBACTE-CARE), resources of which are composed of financial contributions from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in kind contribution. S.P.-G., J.M.B.-F., M.P., R.C., J.T.-C., A.C., V.P., B.G.G. and J.R.-B. receives overarching funding for research by Plan Nacional de I+D+I, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0001, RD16/0016/0008, RD16/0016/0011) and CIBERINFEC (21/13/00012, 21/13/00048, 21/13/00084) co-financed by European Development Regional Fund ‘A way to achieve Europe’, Operative Program Intelligence Growth 2014–2020.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101871.
Contributor Information
Jesús Rodriguez-Baño, Email: jesusrb@us.es.
the COMBACTE-CARE-EURECA Team:
Almudena de la Serna, Sophie Monteau, Virginia Palomo, Elena Soriano, David Gutierrez, Elisa Moreno, Zaira Palacios, Isabel Morales, Natalia Maldonado, Antonio Plata Ciezar, Juan Diego Ruiz Mesa, Beatriz Sobrino Diaz, Ignacio Marquez Gomez, Ines Perez Camacho, Azahara Frutos-Adame, Julia Guzman-Puche, Irene Gracia-Ahufinger, Elena Perez-Nadales, Julian Torre-Gimenez, Athina Pyrpasopoulou, Elias Iosifidis, Elsa Chorafa, Ivana Radovanovic, Sladjana Petrovic, Slavica Cvetkovi, Srdjan-Sanja Melentijevic, Can Bicmen, Gunes Senol, Fe Tubau, Jordi Camara, Victor Daniel Gumucio, Dimitris Bassoulis, John Deliolanis, Vassiliki Ch. Pitiriga, Nikolaos Triarides, Efstathia Argiti, Nikolaos J. Legakis, Kyriakidou Margarita, Desirée Gijón-Cordero, Patricia Ruiz-Garbajosa, Alessandro Bartoloni, Gian Maria Rossolini, Simin-Aysel Florescu, Maria Nica, Serban Benea, Daniela Talapan, Deana Medić, Sanja Maričić Prijić, Mireia Cantero Caballero, Lina M. Parra Ramírez, Volkan Korten, Hüseyin Bilgin, George N. Dalekos, Aggelos Stefos, Nikolaos Spyridis, Athanasios Michos, Francesco Giuseppe De Rosa, Rossana Cavallo, Nicola Petrosillo, Antonio Dicaro, Maria Paola Landini, Marta Luisa Ciofi degli Atti, Mileva Masanovic, Dusan Matkovic, Sotirios Tsiodras, Francesco Blasi, Marta Di pasquale, Claudio Viscoli, Andrei Vata, Olivia Dorneanu, Perlat Kapisyzi, Adriana Vince, Evdoxia Tsigou, Efstratios Maltezos, Apostolos Komnos, Charalampos Gogos, Fabio Franzetti, Massimo Antonelli, Mihaela Lupse, Dan Corneci, Dana Tomescu, Anca Georgescu, Ljiljana Bukarica, Goran Mitrović, Nataša Lukić Krstić, Arsim Kurti, Beatriz Díaz-Pollán, Julia Origüen Sabater, Patricia Muñoz, Alpay Azap, Banu Sancak, Arife Sahin, and Halis Akalin
Appendix A. Supplementary data
References
- 1.WHO . World Health Organization; Geneva: 2014. Antimicrobial resistance: global report on surveillance 2014.http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1 [Google Scholar]
- 2.Tacconelli E., Carrara E., Savoldi A., et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Bartsch S.M., McKinnell J.A., Mueller L.E., et al. Potential economic burden of Carbapenem-Resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect. 2017;23:48.e9–48.e16. doi: 10.1016/j.cmi.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M., Earley M., Chen L., et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): a prospective, multicentre, cohort study. Lancet Infect Dis. 2022;22:401–412. doi: 10.1016/S1473-3099(21)00399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrik T.C., Voor In 't Holt A.F., Vos M.C. Clinical and molecular epidemiology of extended-spectrum beta-lactamase-producing Klebsiella spp.: a systematic review and meta-analyses. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P., Li X., Luo M., et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. Microb Drug Resist. 2018;24:190–198. doi: 10.1089/mdr.2017.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W.M., Yuan Z., Zhou H.Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9:23. doi: 10.1186/s13756-020-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Li Y., Song N., Chen Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. J Glob Antimicrob Resist. 2020;21:306–313. doi: 10.1016/j.jgar.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez-Gutiérrez B., Sojo-Dorado J., Bravo-Ferrer J., et al. European prospective cohort study on Enterobacteriaceae showing REsistance to CArbapenems (EURECA): a protocol of a European multicentre observational study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyersmann J., Kneib T., Schumacher M., Gastmeier P. Nosocomial infection, length of stay, and time-dependent bias. Infect Control Hosp Epidemiol. 2009;30:273–276. doi: 10.1086/596020. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 12.Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0, valid from 2022-01-01. European Committee on Antimicrobial Susceptibility Testing. https://www.eucast.org/clinical_breakpoints/
- 13.Tumbarello M., Trecarichi E.M., De Rosa F.G., et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 14.Palacios-Baena Z.R., Oteo J., Conejo C., et al. Comprehensive clinical and epidemiological assessment of colonisation and infection due to carbapenemase-producing Enterobacteriaceae in Spain. J Infect. 2016;72:152–160. doi: 10.1016/j.jinf.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Pang D. A relative power table for nested matched case-control studies. Occup Environ Med. 1999;56:67–69. doi: 10.1136/oem.56.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris A.D., Karchmer T.B., Carmeli Y., Samore M.H. Methodological principles of case-control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin Infect Dis. 2001;32:1055–1061. doi: 10.1086/319600. [DOI] [PubMed] [Google Scholar]
- 17.van Duin D., Arias C.A., Komarow L., et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis. 2020;20:731–741. doi: 10.1016/S1473-3099(19)30755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris A.D., Samore M.H., Lipsitch M., Kaye K.S., Perencevich E., Carmeli Y. Control-group selection importance in studies of antimicrobial resistance: examples applied to Pseudomonas aeruginosa, Enterococci, and Escherichia coli. Clin Infect Dis. 2002;34:1558–1563. doi: 10.1086/340533. [DOI] [PubMed] [Google Scholar]
- 19.Cano Á., Gutiérrez-Gutiérrez B., Machuca I., et al. Association between rectal colonisation by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae and mortality: a prospective, observational study. J Glob Antimicrob Resist. 2022;29:476–482. doi: 10.1016/j.jgar.2021.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Giannella M., Trecarichi E.M., De Rosa F.G., et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect. 2014;20:1357–1362. doi: 10.1111/1469-0691.12747. [DOI] [PubMed] [Google Scholar]
- 21.Cano A., Gutiérrez-Gutiérrez B., Machuca I., et al. Risks of infection and mortality among patients colonized with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: validation of scores and proposal for management. Clin Infect Dis. 2018;66:1204–1210. doi: 10.1093/cid/cix991. [DOI] [PubMed] [Google Scholar]
- 22.Weiss E., Zahar J.R., Lesprit P., et al. Elaboration of a consensual definition of de-escalation allowing a ranking of β-lactams. Clin Microbiol Infect. 2015;21:649.e1–649.e10. doi: 10.1016/j.cmi.2015.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.