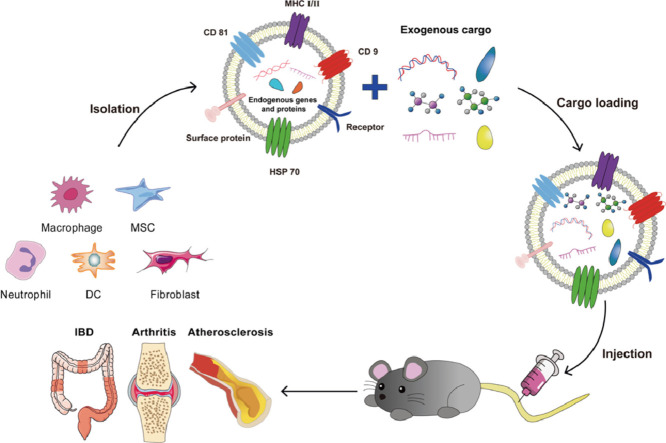
Abstract
In the inflammatory microenvironment, there are numerous exosomes secreted by immune cells (Macrophages, neutrophils, dendritic cells), mesenchymal stem cells (MSCs) and platelets as intercellular communicators, which participate in the regulation of inflammation by modulating gene expression and releasing anti-inflammatory factors. Due to their good biocompatibility, accurate targeting, low toxicity and immunogenicity, these exosomes are able to selectively deliver therapeutic drugs to the site of inflammation through interactions between their surface-antibody or modified ligand with cell surface receptors. Therefore, the role of exosome-based biomimetic delivery strategies in inflammatory diseases has attracted increasing attention. Here we review current knowledge and techniques for exosome identification, isolation, modification and drug loading. More importantly, we highlight progress in using exosomes to treat chronic inflammatory diseases such as rheumatoid arthritis (RA), osteoarthritis (OA), atherosclerosis (AS), and inflammatory bowel disease (IBD). Finally, we also discuss their potential and challenges as anti-inflammatory drug carriers.
Keywords: Exosome, Personalized delivery platform, Chronic inflammation, Therapeutic potential
Graphical abstract
Exosomes are nanoscale lipoprotein bilayer vesicles. In this paper, methods for identification, isolation, drug delivery and targeted modification are introduced. Subsequently, we reviewed the important therapeutic effects of the latest exosome-based delivery systems in chronic inflammatory diseases such as rheumatoid arthritis, osteoarthritis, atherosclerosis and inflammatory bowel disease. The significance of this study is to provide a scientific basis for the exosome delivery system to treat chronic inflammation in vivo.
1. Introduction
Inflammation is involved in many chronic and degenerative diseases, such as diabetes, arthritis, cancer and cardiovascular disease [1,2], and abnormal inflammatory responses can lead to severe pathological conditions [3]. Inflammation is often divided into acute and chronic inflammation. Acute inflammation is an immediate, adaptive and controlled response, which is usually beneficial to the body. However, if the symptoms are not effectively alleviated between six weeks, acute inflammation will develop into chronic inflammation [4]. Chronic inflammation continues to damage tissues due to the persistence of pathogenic factors. These symptoms may persist for months or even years, seriously damaging health and reducing the quality of life [5].
A large number of studies have shown that neutrophils, macrophages, fibroblasts, lymphocytes and other cells run through the occurrence and development of inflammation [6], [7], [8]. These cells regulate the inflammatory process by secreting extracellular vesicles (EVs) as communication and cargo carriers. EVs are divided into three categories: exosomes, which have diameters of 30–100 nm; microvesicles, 50–1000 nm; and apoptotic bodies, several hundred to several thousand nanometers [9], [10], [11]. Exosomes are membrane vesicles secreted by cells into the extracellular space containing a variety of biological components, such as nucleic acids, proteins and others [12]. Exosomes secreted by cells involved in inflammation have high inflammatory affinity and targeting, therefore they can deliver cargo to inflammatory cells through the interaction of surface-antibody and receptors of cell surface, which can achieve better anti-inflammatory effect [13]. In addition, exosomes derived from mesenchymal stem cells (MSCs), astrocytes and dendritic cells (DCs) from inflammatory sites with immunomodulatory functions are widely used as transport vehicles to deliver cargo to inflammatory sites for better anti-inflammatory effects [13], [14], [15].
Exosomes show good biocompatibility as well as low immunogenicity and toxicity [16], and they easily penetrate biological barriers due to their lipid bilayer [17]. In addition, exosomes can be endowed with different biological functions and targeting capabilities by surface engineering strategies. This flexibility makes them great potential as drug delivery platforms for the treatment of chronic inflammatory diseases [18]. Studies have demonstrated that exosomes can accurately deliver proteins, nucleic acids, small molecules and nanoparticles to inflammatory microenvironment, which makes them an excellent nano cargo delivery platform. After triggering the signal response, the vesicles further fused with the cell membrane, and the recipient cells phagocytose the vesicle through endocytosis, the processing of the endosomal pathway led to the release of cargo [17].
Here we review recent developments in knowledge and methods of exosome identification, isolation, drug loading and targeted modification. More importantly, we summarize the latest advances in the application of exosomes as intelligent biomimetic carriers against chronic inflammatory disorders, and we discuss the prospects and challenges of using exosomes as personalized delivery systems.
2. Methods to isolate, identify and store exosomes
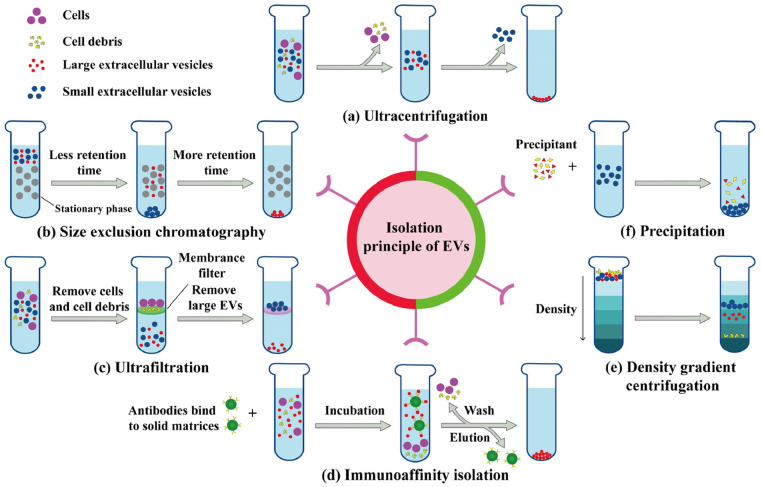
Exosomes can be isolated based on size, buoyancy density or solubility using ultracentrifugation, precipitation, ultrafiltration, immunoaffinity isolation, size-exclusion chromatography (SEC), or density gradient centrifugation (Fig. 1) [19]. Ultracentrifugation purifies exosomes from other components based on density differences, which translate into differences in sedimentation rate. This technique is currently considered the gold standard for isolating exosomes due to its simple operation, low cost, and high purity [20]. Ultrafiltration is a simple, fast isolation method that exploits size differences between exosomes and other components. This technique is usually the first step in separating exosomes, but the filter is easily blocked, and excessive force can distort or even destroy exosomes. Precipitation is a rapid and effective method using precipitators such as polyethylene glycol (PEG), protamine, sodium acetate, and organic solvents [21,22]. However, this method is time-consuming and may fail to completely remove protamine contaminants. The above isolation methods can obtain a certain quality of exosomes.
Fig. 1.
Common methods for isolating exosomes from natural cells.
It is necessary to combine multiple separation methods to isolate the exosomes of interest due to the existence of different subtypes. For example, to obtain M2 exosomes, RAW264.7 cells in serum-free medium were stimulated with 100 ng/ml interleukin (IL)−4 for a period of time to obtain M2 macrophages. Acquisition of exosomes from the M2 macrophage conditioned medium requires a combination of ultracentrifugation and ultrafiltration [23]. Briefly, cells and cell debris were removed from the collected culture medium by gradient low-speed centrifugation. To further concentrate the exosome solution, ultrafiltration of the supernatant through a molecular weight cutoff of 100 kDa was required. Finally, the supernatant was ultracentrifuged at 120,000 g for 70 min and washed with PBS. The procedure of ultracentrifugation was repeated to obtain the purified M2 exosomes.
Due to their small size, low density, and heterogeneity, different isolation methods can get different subsets of exosomes [24]. Therefore, multiple identification methods are required to evaluate the types of exosomes and determine different subtypes. Several techniques have been developed to determine the physical and biological properties of exosomes and evaluate their size distribution, concentration and integrity, including transmission electron microscopy (TEM), western blotting, confocal microscopy, and nanoparticle tracking analysis (NTA) [25]. TEM is commonly used to observe exosome surface characteristics such as size and morphology, distinguish single exosome and other particles of similar size, and evaluate their quality and concentration [26]. Western blotting is a semi-quantitative or quantitative method for monitoring target proteins [27]. The advantages of this technique are small sample size and high efficiency. Characteristic proteins such as transmembrane proteins CD9, CD63 and CD81 can be identified by this technique [28]. Confocal technology can obtain 3D images of exosomes, with the advantages of clear imaging, qualitative and quantitative analysis. In addition to being able to identify exosomes with a high signal to noise ratio, confocal microscopy also distinguishes between specific fluorescent signals and artificial signals from exosomes [29]. NTA is a straightforward technique for semi-quantitative particle characterization, which allows the analysis of exosome size distribution and concentration [30]. The method involves light scattering measurements and tracking of the Brownian motion of irradiated single particles using an optical microscope equipped with a high-definition camera [31].
For storage after isolation, the current commonly used storage method is cryopreservation (4 ℃ for short-term storage and −80 ℃ for long-term storage) [32]. Notably, due to direct freezing causes irreversible damage, cryoprotectants such as DMSO, trehalose and sucrose are often added to overcome these defects, while repeated freeze-thaw cycles should be avoided [12]. Unfortunately, long-term storage at −80 ℃ is not only costly and difficult to transport, but also changes the morphology of exosomes and reduces their biological activity [33]. Compared to cryopreservation, samples obtained by lyophilization which requires the addition of freeze-drying protectant such as mannitol can maintain their morphology and function at room temperature, raising the promise of clinical transformation of exosome-related products [34,35].
3. Methods to load cargo into exosomes
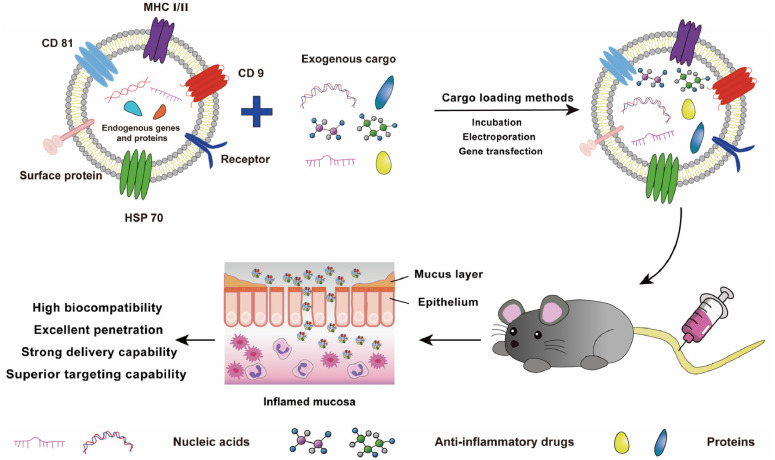
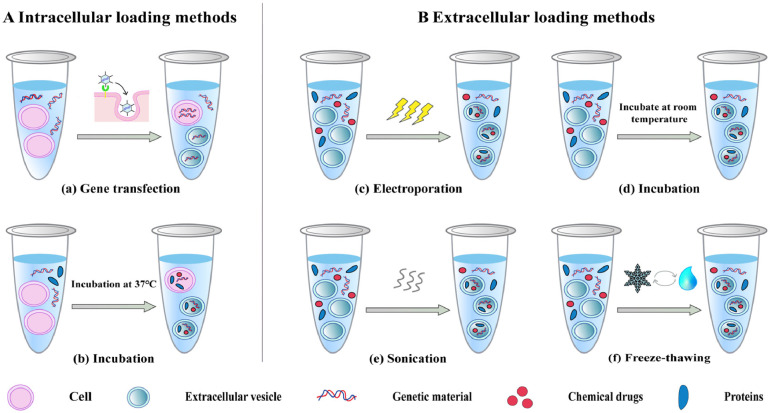
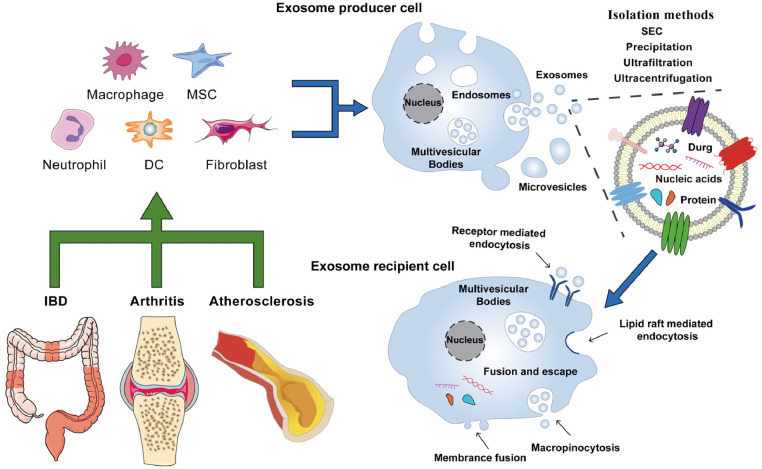
Exosomes are commonly used as carriers for a wide range of materials, such as drugs, proteins, and small nucleic acids, due to their tremendous potential for in vivo delivery and their ability to inherit signaling molecules from their parental cells that can bind specifically to receptors on the surface of target cells (Fig. 2) [36]. Cargo can be loaded into exosomes using intracellular transfection or extracellular loading (Fig. 3) [37]. Intracellular transfection involves adding genes to parental cells, which produce the desired protein cargo or simply make copies of the transfected genetic material, and then isolating exosomes containing the desired protein or genetic material. In extracellular loading, exosomes are first isolated from cells and then loaded with the required cargo by incubation, electroporation, or ultrasonication.
Fig. 2.
The nucleic acids, proteins, or anti-proteins were loaded into exosomes covered with various proteins (MHC, CD9, CD81, and HSP70) and transported to the anti-inflammatory drugs microenvironment.
Fig. 3.
Common methods for loading cargo into exosomes.
Gene transfection can introduce exogenous genes into parental cells and ensure that they or their protein products are loaded into exosomes without being secreted out of the cell, which is considered as an elegant drug delivery method [38]. This technique has the advantages of high stability and low immunogenicity [39]. The serious disadvantage, however, is its low efficiency compared to electroporation and ultrasonic loading technology.
Electroporation is another simple, fast cargo loading method commonly used to help hydrophilic drugs and nucleic acids penetrate the lipid bilayer membrane of exosomes. In this technique, a transient shock is delivered to the vesicle membrane, opening up reversible pores [40]. The loading capacity and efficiency of exosomes depend on their diameter and the size of the cargo molecules [41]. The electroporation shock lasts only a few milliseconds, so vesicle membrane components are not damaged.
Incubation is a simple, low-cost loading method in which either the parental cells are incubated with a drug solution, and then drug-loaded exosomes are isolated; or, more commonly, the drug solution is incubated directly with isolated exosomes [42,43]. Compared to hydrophilic drugs, the lipid bilayer structure of the vesicle membrane makes it easier for hydrophobic drugs such as curcumin and paclitaxel to be loaded into exosomes by incubation [44,45].
Sonication is used to help small nucleic acids and drugs penetrate into exosomes where transient pores are formed on the surface of the membrane without affecting vesicular structure [46]. Study showed that the average loading efficiency of paclitaxel in M1 macrophage-derived exosomes increased from 4.85% ± 1.65% to 19.55% ± 2.48% after sonication [47], and the integrity of exosome membranes can be restored after a brief incubation at 37 ℃.
Interestingly, freeze-thawing also can form transient holes in exosome membranes, which requires at least three repetitions of the following operations to encapsulate cargo: incubating drugs with exosomes at room temperature for a period, followed by freezing at −80 ℃ and thawing at room temperature [48].
Furthermore, extrusion and saponin treatment and other techniques can also be used to load various materials into exosomes [49], [50], [51], [52]. In fact, each loading method has its advantages and disadvantages, and suitable loading methods must be selected according to the type of cargo (nucleic acid, protein, therapeutics) and nature (hydrophilic or hydrophobic).
4. Targeted modification of exosomes
Compared to traditional nanomedicine delivery systems, the natural properties of exosomes give them some advantages in target cell uptake. Nevertheless, in order to improve the ability of exosomes to target disease sites, further modifications are needed. It is well known that genetic engineering strategies are the most mature targeted modification strategy, aiming to fuse ligands with characteristic functions to a large number of transmembrane proteins (CD9, CD63, Lamp2b) on the exosome surface. Genetic modification of parental cells can be achieved using plasmids or viruses that encode fusion ligands for transmembrane proteins. This strategy allows the expression of peptides and proteins of interest on the surface of exosomes and endows them with diverse functions [53]. The fusion of targeted peptide HSTP1 with membrane protein Lamp2b effectively promoted the uptake of exosomes by HSC-T6 cells [54]. However, conventional genetic engineering strategy has the disadvantages of low efficiency and low membrane protein expression.
Direct engineering of exosomes provides a more controllable and efficient strategy. Strategies of physical and chemical modification can bind small peptides, proteins, targeted molecules (such as folate or PEG) or chemical compounds (such as maleimide or azide derivatives) to exosome surfaces [49,55], not only preserving the integrity of exosomes, but also giving them additional functions such as fluorescence, imaging contrast or activation of the immune system [56]. During the physical surface modification process, the lipid structure of the exosome surface is temporarily destroyed by external forces (such as ultrasound or extrusion), and the vesicles will slowly return to their natural state after removal of these forces [57]. Based on the self-assembly properties, physical modification methods have been widely used in exosome imaging or fluorescence labeling [58,59].
Chemical modification is characterized by mild reactions, short time consumption, high efficiency, ease of synthesis and product separation, and does not affect size. This modification method includes covalent binding of protein groups (carboxyl, amino, and hydroxyl) through click chemistry with biologically compatible chemical molecules, or non-covalent binding through ligand-receptor or hydrophobic interactions [53,56,57,60].
Selective uptake of exosomes by target cells is mainly achieved through the following three ways: receptor-ligand interaction, membrane fusion and endocytosis [61], of which endocytosis is the most common. To further visualize exosome uptake in vitro and biological distribution in vivo, the most commonly method is to visualize lipophilic fluorescent probe or fluorescent protein labeled exosomes based on laser confocal microscopy [62]. After successful labeling of exosomes, the selective uptake of exosomes by recipient cells can be analyzed by flow cytometry [23]. With the advancement of technology, the emergence of single molecule localization microscopy (SMLM) breaks the limits of the moderate spatial resolution. SMLM enables the most efficient and clear visualization and dynamic tracking of exosomes and their miRNAs in living cells, as well as the observation of exosomes' movement within the intercellular filamentous structures [63,64]. In addition, fluorescently labeled or fluorophore-containing chemicals can be used to track the intracellular behavior of exosomes [65,66]. In addition to the methods described above, bioluminescence imaging, nuclear imaging, computed tomography (CT) and magnetic resonance imaging (MRI) can be used to track the distribution of exosomes in vivo [67], [68], [69].
5. Current progress of exosome‐based delivery platform to treat chronic inflammatory diseases
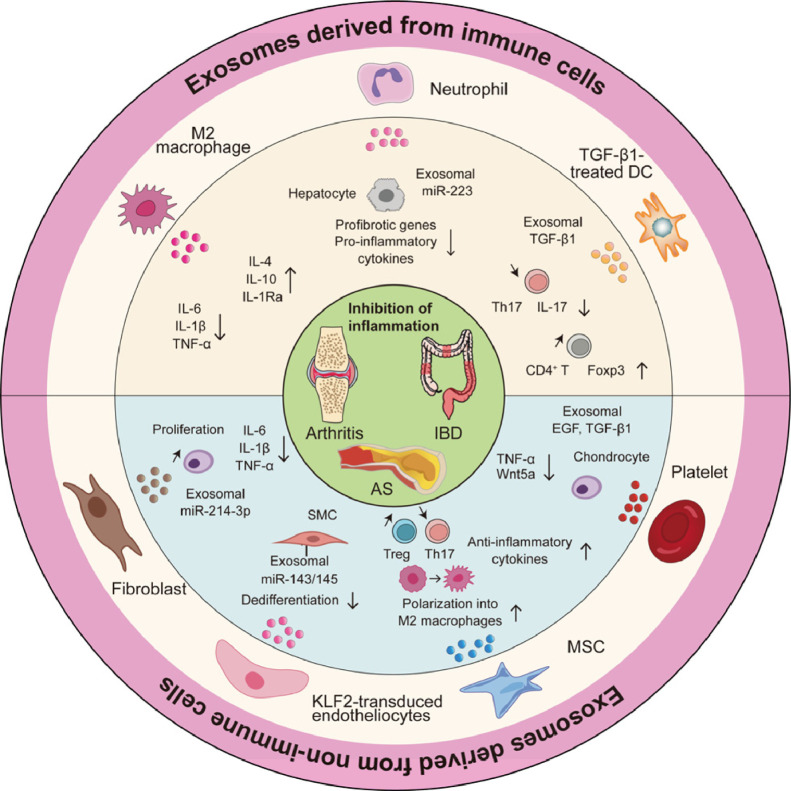
Numerous studies have shown that exosomes secreted by varieties of immune cells (DCs, macrophages, neutrophils) and non-immune cells (MSCs, platelets) participate in the occurrence and development of chronic inflammation [70,71], and play an important role in regulating the inflammatory microenvironment by regulating gene expression and releasing anti-inflammatory factors (Fig. 4) [72,73]. More importantly, in recent years, exosomes have attracted attention as optimal drug carriers for personalized nanomedicine due to their high biocompatibility, low immunogenicity, and ability to inherit many characteristics of parental cells [74]. To date, they have been used as delivery platforms for various materials in vivo, including nucleic acids, nanodrugs and proteins (Fig. 5), and have been widely studied for their effect in the treatment of chronic inflammatory diseases, including rheumatoid arthritis (RA), osteoarthritis (OA), atherosclerosis (AS), inflammatory bowel disease (IBD) (Table 1).
Fig. 4.
Inflammatory regulation of exosomes from immune and non-immune cells in chronic inflammatory diseases.
Fig. 5.
Exosomes secreted by macrophages, neutrophils, MSCs, DCs and fibroblasts deliver nucleic acids, proteins and therapeutic drugs to recipient cells with great potential for the treatment of chronic inflammatory diseases.
Table 1.
Potential therapeutic role of exosome-based or EV-based delivery strategies in inflammatory diseases.
| Diseases | Source | Cargo | Objective cells | Final effect | Ref. |
|---|---|---|---|---|---|
| RA | Macrophages | Dexamethasone sodium phosphate | RAW264.7 macrophages | Upregulated anti-inflammatory cytokines and enhanced endocytosis | [80] |
| RA | M2 macrophages | Plasmid DNA encoding IL-10 and betamethasone sodium phosphate | M1 macrophages | Promoted M1-to-M2 macrophage polarization and upregulated IL-10 | [23] |
| RA | MSCs | miR-192–5p | Not determined | Delayed inflammatory response | [84] |
| RA | MSCs | miR-150–5p | Synoviocyte | Inhibited synoviocyte hyperplasia and angiogenesis | [85] |
| RA | MSCs | Curcumin | Synovial fibroblasts | Reduced the levels of anti-apoptotic proteins and inflammatory mediators | [86] |
| RA | DCs | IL-4 | T cells | Modulated the activity of APC and T cells | [87] |
| OA | Human urine-derived stem cells | miR-140–5p | Chondrocytes | Enhanced the proliferation and migration of chondrocyte | [91] |
| OA | BMSCs | Curcumin | Chondrocytes | Inhibited the migration of IL-1β-stimulated osteoarthritic chondrocytes | [92] |
| OA | DCs | miR-140 | Chondrocytes | Inhibited cartilage-degrading proteases | [93] |
| OA | DCs | Kartogenin | SF-MSCs | Promoted the chondrogenesis of SF-MSCs | [94] |
| AS | MSCs | Small interfering LOC100129516 | THP-1 cells | Promoted cholesterol efflux and suppressed intracellular lipid accumulation | [98] |
| AS | M2 macrophages | Hexyl 5-aminolevulinate hydrochloride | Not determined | Enhanced anti-inflammatory effects and alleviated AS | [76] |
| AS | THP-1 cells | HSP 27 | Not determined | Activated NF-κB via TLR-4 and reduced inflammatory plaques | [99] |
| AS | HEK293T cells | IL-10 | Macrophages | Alleviated the AS in apolipoprotein E-deficient mice | [100] |
| UC | BMSCs | miR-146a | Not determined | Inhibited TRAF 6 and IRAK1 expression | [103] |
| UC | Bone marrow-derived DCs | IL-10 | Not determined | Reduced analyzed clinical, macroscopic and histopathologic parameters | [107] |
| UC | M2 macrophages | miR-590–3p | Colonic epithelial cells | Promoted colonic epithelial cell proliferation and regeneration | [108] |
| UC | M2 macrophages | LncRNA MEG3 | Colon epithelial cells | Promoted CREB1 transcription by competitively binding to miR-20b-5p | [109] |
| Immune response caused by titanium alloy | BMSCs | miR-181b | Macrophages | Promoted M2 polarization and enhanced osteointegration | [117] |
| Diabetic wound healing | Milk | miR-31–5p | Endothelial cells | Promoted angiogenesis and wound healing | [118] |
5.1. Therapeutic potential of exosome‐based delivery platform in RA
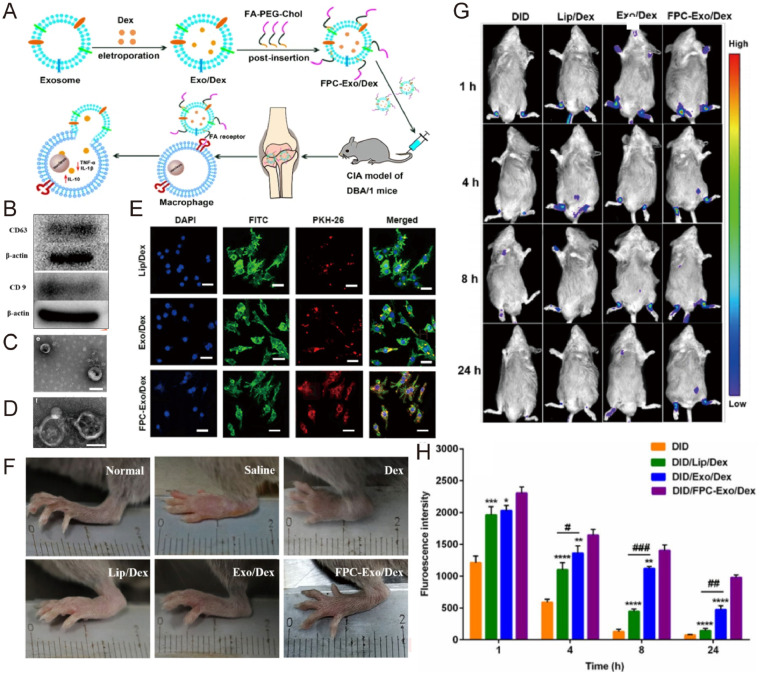
The excellent inflammatory targeting and cargo delivery capability of exosomes make themselves a promising therapeutic agent. At present, the targeted delivery of macrophage-derived exosomes to the inflammatory microenvironment has been extensively studied. Depending on the microenvironment, macrophages can be polarized into classically activated M1 types and selectively activated M2 types [75]. Exosomes secreted by M2 macrophages can produce and release anti-inflammatory cytokines (IL-4, IL-10, IL-1Ra) and transforming growth factor TGF-β, which have significant therapeutic effects in fighting inflammation and repairing tissue damage [76], [77], [78]. In addition, M2 exosomes exhibit a strong inflammatory tendency, which is an effective bionic carrier for RA targeting. Compared with the nanoparticles coated with erythrocyte membrane, this exosome-coated nanoparticle showed extremely strong in vivo targeting in collagen-induced arthritis (CIA) mice, addressing the immunogenicity and safety of the loaded drug tacrolimus and significantly inhibiting RA progression [79]. Exploiting the abundance of folate receptors on the surface of activated macrophages in the RA microenvironment, our group encapsulated dexamethasone sodium phosphate nanoparticles into macrophage-derived exosomes and modified them with folic acid-PEG-cholesterol (FPC) to enhance the active targeting ability of exosomes. The resulting delivery system significantly stabilized the drug and prolonged its circulation, allowing it to protect the articular cartilage, downregulate pro-inflammatory cytokines and upregulate anti-inflammatory cytokines (Fig. 6) [80]. M2 macrophage-derived exosomes carry varieties of anti-inflammatory cytokines, which play an important role in fighting inflammation and repairing tissue damage. To limit the consumption of chemotherapeutic drugs and improve the delivery potential of exosomes, we have developed a biomimetic drug delivery system by encapsulating betamethasone sodium phosphate and a plasmid encoding IL-10 into exosomes derived from M2 macrophages. The resulting nano-system promoted M1-to-M2 macrophage polarization by upregulating IL-10, thereby targeting the exosomes to sites of inflammation, where they exerted anti-inflammatory properties and mitigated joint damage in RA [23].
Fig. 6.
Engineered exosomes loaded with anti-inflammatory drugs were used to alleviate joint inflammation in CIA mice. (A) Exosomes encapsulated with dexamethasone sodium phosphate can be targeted into macrophages in the inflammatory microenvironment of CIA mice after FPC modification and release drugs, which alleviates the arthritis response. (B) The expression of CD9 and CD63 in exosomes was detected by Western blotting. (C) TEM of exosomes loaded with dexamethasone sodium phosphate. (D) TEM of FPC-modified exosomes loaded with dexamethasone sodium phosphate. (E) Confocal microscope image of LPS-activated RAW264.7 cells ingesting Dex preparations with different PKH 67 labels. Scale bar = 200 µm. (F) Photographs of representative hind limbs treated with different Dex preparations in CIA mice. (G) Real-time fluorescence imaging of CIA mice after intravenous injection of various preparations (n = 3). (H) Semi-quantitative analysis of the joint fluorescence intensity. Reproduced from [80]. Copyright 2020 The Authors.
In recent years, stem cell-based anti-inflammatory therapies have gained widespread popularity due to their ability to modulate macrophages, T cells and B cells to promote the secretion of regenerative cytokines, reduce inflammatory responses and repair tissues [81,82]. MSCs and their secreted exosomes have anti-fibrotic and anti-inflammatory immunomodulatory effects [83]. At present, the delivery strategy of MSC-derived exosomes has been widely studied. For instance, MSCs were infected with lentivirus to obtain exosomes overexpressing miR-192–5p, which upregulated C3 botulinum toxin substrate 2 in a mouse model of RA, delaying disease progression [84]. Similarly, exosomes with high expression of miR-150–5p reduce joint destruction by inhibiting synovial cell proliferation and angiogenesis [85]. Exosomes derived from MSCs can also encapsulate small molecule drugs for the treatment of RA. Studies have shown that exosomes loaded with curcumin not only improve the stability of free drugs, but also effectively inhibit the proliferation and inflammatory response of synovial fibroblasts by reducing the levels of anti-apoptotic proteins IAP1 and IAP2 and inflammatory factors such as IL-6 and TNF-α [86].
Exosomes derived from DCs play an important role in the immune regulation of inflammatory microenvironment through different types of immune-related proteins. Therefore, delivery strategies based on DC-derived exosomes are of great significance in the treatment of inflammatory diseases. Exosomes overexpressing IL-4 isolated from DCs transfected with virus were found to regulate antigen presenting cell and T-cell activity via MHC class II and partly Fas ligand/Fas-dependent mechanism [87]. Eventually, the inflammatory degree of CIA in mice was reduced, and the inflammation of delayed hypersensitivity was inhibited. To further strengthen the targeting of DC-derived exosomes and prolong their blood circulation time, an engineering modification is necessary. For instance, a ROS-responsive exosome was developed by hydrophobic inserting thioketal linker-embedded poly (ethylene glycol) on the vesicle surface [88]. Neutrophil-derived exosomes have strong inflammatory targeting properties and become a potential drug delivery vehicle. Given that the chemotaxis of neutrophils to inflammatory regions, Zhang et al. prepared neutrophil-derived exosomes functionalized by click chemistry with ultrasmall Prussian blue nanoparticles [89]. The exosomes can selectively accumulate in activated fibroblast-like synoviocytes and alleviate inflammatory stress by neutralizing pro-inflammatory factors and scavenging reactive oxygen species. In summary, the above cell-derived exosomes have unique intrinsic characteristics, including intrinsic inflammation-tropism, secretion of anti-inflammatory cytokines, protection of T and B lymphocytes from inflammation, and immunostimulatory or inhibitory regulatory effects. Relying on their own advantages, these exosomes can better undertake the task of drug delivery to the inflammation site and play a synergistic anti-inflammatory role, showing great potential in the treatment of RA.
5.2. Therapeutic potential of exosome‐based delivery platform in OA
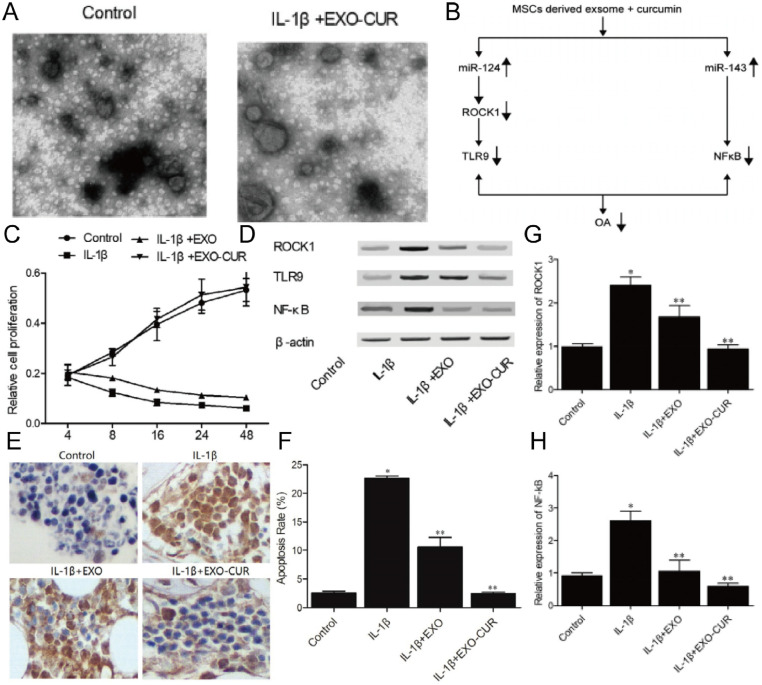
IL-1β is overexpressed in the joints, cartilage, and synovial membrane of OA patients, leading to cartilage degeneration and joint injury [90]. Therefore, reducing the effect of IL-1β on OA chondrocytes may be a treatment against the disease. The delivery strategy based on exosomes is an important solution to efficiently deliver small nucleic acids into the dense, non-vascular extracellular matrix of cartilage. Studies have shown that human urine-derived stem cells (HUSCs) can enhance the proliferation and migration of IL-1β treated chondrocytes [91]. In contrast, the same treatment of miR-140–5p transfected exosomes not only retained the above advantages, but also solves the problem of reducing extracellular matrix by targeting vascular endothelial growth factor. MSCs and MSC-derived exosomes have numerous functions, including immune regulation, homing, differentiation, anti-inflammation and tissue repair. Therefore, delivery strategies based on MSC-derived exosomes exhibit great potential in the treatment of chronic inflammatory diseases. In one research, curcumin was loaded into exosomes to enhance its bioavailability and then administered to IL-1β-stimulated human OA chondrocytes, significantly upregulated NF-kB and ROCK1 levels by restoring miR-143 and miR-124 expression in OA cells, ultimately alleviating the progression of OA (Fig. 7) [92].
Fig. 7.
Molecular mechanism of potential therapeutic effects of curcumin treated exosomes in OA treatment. (A) Morphology of exosomes derived from MSCs. (B) Schematic representation of the effects of curcumin treated MSC-derived exosomes on multiple signaling pathways. (C) The viability of primary chondrocytes after preparation treatment. (D) The expressions of ROCK1, TLR9 and NF-kB were analyzed by western blotting. (E) TUNEL detection of apoptosis in primary chondrocytes treated under different conditions. (F) Curcumin treatment of MSC-derived exosomes significantly reduced apoptosis. (G-H) Exosomes derived from MSCs treated with curcumin significantly inhibited the expression of ROCK1 and NF-kB proteins in primary chondrocytes treated with IL-1β. Reproduced from [92]. Copyright 2020 The Author(s).
Compared with MSCs, the effect of exosomes secreted by DCs on arthritis is easily overlooked. Similarly, Liang et al. successfully transmitted miR-140 into chondrocytes by constructing engineered DC-derived exosomes [93]. The microRNA was encapsulated into exosomes by electroporation technology, and the fusion of chondrocyte affinity peptide with lysosomal associated membrane glycoprotein 2b protein on the surface of exosomes endowed them with superior targeting capabilities. After intra-articular injection, the exosome penetrates the dense mesochondrium to deliver cargo to the deep cartilage region, inhibiting cartilage degradation proteases and alleviating the development of OA. Recently, an engineered targeting delivery strategy was constructed by fusing MSC-binding peptide E7 with the exosomal membrane protein Lamp 2b to generate exosomes capable of targeting synovial fluid-derived MSCs (SF-MSCs) [94]. Kartogenin was then encapsulated into exosomes by electroporation. These engineered exosomes achieve targeted delivery of small molecules and make it uniformly dispersed in cytosol, strongly promoting chondrogenesis of SF-MSCs. In the IL-1β-induced OA model, platelet-derived exosomes carry platelet-derived growth factor-Ab, TGF-β1, and endothelial growth factor, which can inhibit the release of TNF-α and alleviate cartilage degradation and inflammation progression [95]. The anti-inflammatory protein annexin A1 has a significant anti-inflammatory effect and its expression in synovial exosomes is significantly higher than in plasma exosomes [96]. Binding of annexin A1 to the receptor FPR2/ALX resulted in increased production of TGF-β1 in chondrocytes, alleviating inflammatory damage to articular cartilage. In conclusion, relying on engineering modifications, the potential of exosome-based cargo delivery strategies in the treatment of OA is constantly being explored. In addition to immunomodulatory effects, the above-mentioned exosomes can also reduce OA inflammation and repair tissues by secreting growth factors and anti-inflammatory factors. Exosome-based delivery strategies not only penetrate dense matrix layers and highly negatively charged proteoglycans to deliver cargo to inflammation sites, but also further enhance the therapeutic effect of drugs. In conclusion, the selection of the corresponding cell-derived exosome based on the pathological characteristics of a specific disease helps to better exert the coordinated therapeutic role of vector and cargo.
5.3. Therapeutic potential of exosome‐based delivery platform in AS
Exosomes display excellent cell penetration ability and play a pivotal role in cell-to-cell communication, promoting or alleviating the atherosclerotic process [97]. Therefore, they have been widely used as drug delivery carriers to target lesion sites, where they inhibit the inflammatory response in AS. In the treatment of AS, MSC therapy has shown great potential. More importantly, the therapeutic research on cargo delivery strategies based on MSC-derived exosomes is becoming more and more extensive. The exogenous small interfering LOC100129516 was successfully delivered to THP-1 cells via MSC-derived exosomes, resulting in significantly lower levels of total cholesterol, free cholesterol, and cholesteryl ester in THP-1 macrophage-derived foam cells [98]. Finally, the progression of the disease was inhibited by modulating PPARγ/LXRα/ABCA1 signaling.
M2 macrophage-derived exosomes have been selected to deliver small molecule drugs for the treatment of AS because of their anti-inflammatory and inflammation-targeting properties. Recently, it was reported that the construction of M2 macrophage-derived exosomes loaded with hexyl 5-aminolevulinate hydrochloride by electroporation [76]. The exosomes targeted sites of inflammation, where they released surface chemokine receptors and anti-inflammatory cytokines and promoted the production of carbon monoxide and bilirubin. These effects significantly enhanced the anti-inflammatory effects of the drug and alleviated AS.
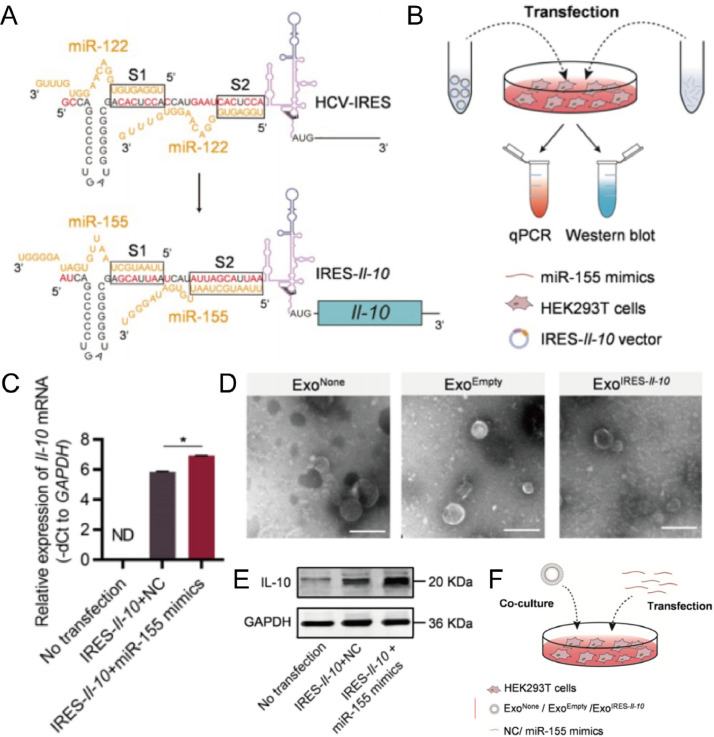
Exosomes loaded with proteins have also shown potential for treating AS. Exosomes loaded with HSP 27, an ATP-independent anti-apoptotic chaperone involved in the regulation of several signaling pathways, activated NF-κB through the TLR-4 pathway, mitigating the inflammatory response and formation of atherosclerotic plaques [99]. IL-10 is a cytokine that plays an important role in limiting inflammation and preventing tissue damage. Therefore, Bu et al. constructed a targeted delivery strategy of IL-10 mediated by exosome to significantly alleviate AS (Fig. 8) [100]. They transfected IL-10 mRNA into HEK293T cells with virus and then isolated exosomes containing IL-10 mRNA, producing large amounts of IL-10. After injection into the tail vein of mice, the exosomes were able to efficiently enter some cells, including macrophages, in apolipoprotein E-deficient plaques. Kruppel-like factor 2 (KLF2) is a shear-responsive transcription factor that plays a key role in combating AS. Thus, increased expression of miR-143/145 in the exosome secreted by KLF2-transduced endotheliocytes modulates the smooth muscle cell (SMC) phenotype in order to combat the development of atherosclerotic lesions [101]. Benefiting from the powerful in vivo cargo delivery capacity, exosomes can better help miRNA, protein and small molecule drugs to enter target cells and produce therapeutic effects. Therefore, AS treatment based on exosome delivery methods is a very promising strategy. At present, exosome delivery strategies based on nucleic acids, proteins and drugs have made some progress in the treatment of AS. According to the different types of cargo, appropriate exosomes need to be selected to better achieve synergistic therapeutic effects. For example, proteins or drugs with anti-inflammatory effects can be paired with exosomes with inflammatory regulation and tissue repair functions. This personalized delivery strategy of selective collocation is recommended.
Fig. 8.
IL-10-encapsulated exosomes targeted into macrophages significantly alleviated AS. (A) Schematic of the internal ribosome entry site IL-10mRNA activated by mir-155. (B) Schematic of miR-155-activated internal ribosome entry site IL-10mRNA verified by western blotting and qPCR. (C) IL-10 mRNA levels in transfected cells were analyzed by qPCR. (D) TEM images of exosomes derived from untransfected cells, transfected cells with empty vector and transfected cells with IL-10 mRNA. Scale bar = 200 nm. (E) Western blotting to analyze the IL-10 protein expression levels in the transfected HEK293T cells. (F) Exosomes co-cultured with HEK293T cells transfected with miR-155 mimics or negative control. Reproduced from [100]. Copyright 2021 The Authors.
5.4. Therapeutic potential of exosome‐based delivery platform in IBD
Due to their regenerative and immunosuppressive features, MSCs have recently been identified as a promising approach for ulcerative colitis (UC) treatment. Research has shown that MSC-derived exosomes can deliver cytokines and miRNAs and encapsulate external cargo to the immune microenvironment to regulate proliferation, maturation, and polarization of immune effector cells [102]. In one study, bone marrow mesenchymal stem cells (BMSCs) were transfected with lentiviruses and subsequently isolated EVs that overexpressed miR-146a significantly inhibited the expression of TNF receptor-associated factor 6 and IL-1 receptor-associated kinase 1, while mitigating colitis symptoms [103].
DCs are professional antigen-presenting cells, and the exosomes secreted by DCs can regulate immune regulation through different types of antigenic peptides [67]. Recently, DCs have been modified by various anti-inflammatory factors (including IL-10, IL4, TGF-β1) and produced various immunomodulatory exosome subtypes [87,104,105]. TGF-β1-enriched immature DC-derived exosomes alleviated inflammatory damage in IBD by suppressing Th17 responses and enhancing regulatory T cells [106]. Since IL-10 plays an important role in the development of normal mucosal immunity, IL-10-exosomes were obtained by incubating IL-10 with DC-derived exosomes [107]. The exosome delivery therapy significantly upregulated the expression of regulatory T cells and promoted the expression of IL-10 in colon tissues, and eventually inhibited the acute colitis induced by trinitrobenzene sulfonic acid.
The role of exosomes secreted by M2 macrophages in UC should also not be underestimated. M2 macrophages were found to promote the proliferation of colonic epithelial cells through the exosome pathway [108]. After transfection with plasmids, exosomes rich in miR-590–3p isolated from M2 macrophages inhibited LATS1 expression by activating YAP/β-catenin regulated transcription, which promoted epithelial regeneration and repair of damaged colon. In another research, the LncRNA maternally expressed 3 (MEG3) overexpression lentiviral vector was used to transfect M2 macrophages, and the high expression of MEG3 EVs was obtained after isolation [109]. After drug administration through the tail vein, the MEG3 enters the colon epithelial cells, and competitively binds with mir-20b-5p to promote the transcription of, mir-20b-5p/cAMP responsive element binding protein 1, further enhancing cell vitality and alleviating the inflammatory response of UC. Exosome-based nucleic acid and protein delivery strategies for the treatment of UC have been widely studied and show great potential. However, exosome delivery methods for small molecule drugs have yet to be developed.
Exosomes derived from stem cells and macrophages have strong immune regulatory and immunosuppressive properties, and have the ability of tissue regeneration and repair in the treatment of Crohn's disease (CD) [110,111]. MSCs and MSC-EVs maintained the length of the colon and the structure of the intestinal mucosa, regulated fibrosis, and promoted the healing of colitis by upregulating telomerase and HIF-1α while downregulating inflammatory cytokines [112]. However, studies on exosome-based delivery strategies for the treatment of CD are rarely reported. Although their effectiveness has been demonstrated in clinical trials for the treatment of CD [113], there are few studies on their therapeutic mechanisms. Therefore, the potential of exosome-based in vivo drug delivery in CD therapy has yet to be exploited, a novel approach to CD therapy. The various exosomes selected in the aforementioned studies can regulate the intestinal immune system, inhibit inflammatory responses, and promote wound healing. With a combination of miRNA, LncRNA and proteins, cargo-loaded exosomes can further reduce IBD inflammatory damage and promote tissue repair. However, personalized delivery strategies based on anti-inflammatory agents with suitable exosomes for the treatment of IBD are yet to be developed.
5.5. Therapeutic potential of exosome‐based delivery platform in other chronic inflammatory diseases
Due to the excellent drug delivery ability of exosomes in vivo, recent research has explored the therapeutic potential of exosomes against other chronic inflammatory diseases, such as periodontitis, pneumonia and chronic hepatitis [114], [115], [116]. Transfection of miR-181b into exosomes via lipofectamine reagent enhanced M2 macrophage polarization and inhibited the inflammatory response by activating the PRKCD/AKT signaling pathway, which subsequently promoted osteointegration in vivo [117]. Furthermore, exosomes are also considered attractive candidates for regulating wound healing. miR-31–5p was successfully encapsulated into milk-derived exosomes by electroporation, and significantly promoted the proliferation, migration and angiogenesis of endothelial cells in a mouse diabetic wound healing model [118]. In conclusion, appropriate cell-derived exosomes should be selected as delivery vehicles for different disease types, which will be more conducive to exerting the function of both carrier and cargo.
Exosomes used for cargo delivery are endogenous vesicles characterized by low immunogenicity and low toxicity [119]. Most of these exosomes are derived from human cells and are minimally reactive to the immune system. Studies have shown that the injection of exosomes isolated from allogeneic cells into patients does not trigger an immune response and body resistance [120,121]. Moreover, the excellent targeting of exosomes enhances the accumulation of cargo at disease sites and reduces damage to normal cells and the body [122]. In numerous studies of cargo-loaded exosomes in chronic inflammatory diseases, comprehensive analyses of whole blood indices and assessments of liver and kidney function have shown that exosomes seem to be well-adapted [80,123]. In summary, multiple administration of the cargo-loaded exosomes will not cause damage to major organs and tissues, and side effects might be ignored.
6. Conclusion and future perspective
Initially, exosomes were used as biomarkers for the diagnosis of inflammatory diseases. With the continuous exploration of their biological characteristics and therapeutic potential, exosomes are now used as cargo delivery vehicles. In addition, further optimization through engineering design can better achieve the purpose of treating chronic inflammatory diseases. Natural exosomes exhibit strong cargo delivery capacity and therapeutic potential due to their high stability, high biocompatibility, low immunogenicity and specific targeting. The important value of exosomes as a nano-delivery platform in chronic inflammatory diseases has been effectively demonstrated. Numerous anti-inflammatory drugs and nucleic acids have been successfully encapsulated into exosomes and then delivered to the inflammatory microenvironment for active anti-inflammatory effects.
The "without cells" delivery strategy based on exosomes has significant advantages over traditional nanomaterial delivery methods. At present, this delivery strategy is being continuously developed, and various cargo such as nucleic acids, proteins and anti-inflammatory drugs have been encapsulated into exosomes to test varieties of in vivo and in vitro anti-inflammatory models. Although research on exosomes as drug carriers is still in the early stages, their excellent targeted drug delivery ability has been effectively demonstrated. At present, most exosomes used in the treatment of chronic inflammatory diseases are derived from macrophages or pluripotent stem cells, making important research achievements. More cell-derived exosomes such as immune cells need to be developed to enrich and develop this therapeutic strategy. Recently, milk-derived exosomes and plant-derived exosome-like nanoparticles (PENs) have attracted wide attention as drug delivery carriers with great potential [124], [125], [126]. They have little cytotoxicity to healthy tissue and are widely and economically available. For example, PENs can be isolated from a variety of plants such as grapes, lemon and ginger [127]. Milk-derived exosomes and PENs represent a potentially scalable and cost-effective approach to animal cell-derived exosome delivery strategies for the treatment of chronic inflammatory diseases, although current research on this complementary strategy is still in an early stage. During this period, it is most important to figure out the uptake mechanism of milk-derived exosomes and PELNs by mammalian cells.
Most studies on cargo-loaded exosomes for the treatment of chronic inflammatory diseases are still in the preclinical stage, and no relevant products have been reported on the market, due to the difficulty in isolating various exosome subtypes, low drug loading efficiency, complicated preparation steps, and low yield to scale up production. It's reported by clinical studies that a single intra-articular injection of allogeneic MSC-derived exosome in patients with knee OA had been entered phase 1 evaluation [131]. Moreover, the safety and efficacy of human placental BMSC-derived exosomes in treating anal fistula in patients with CD was evaluated by phase 2 study [132]. In the future, it is possible to achieve large-scale production of exosomes with good stability by further improving the isolation and purification strategy, increasing the drug loading efficiency, enhancing the targeting ability, simplifying the operation steps and increasing the yield. Exosomes modified via engineering can improve targeting capability, but there are still some challenges before realizing the rationality and economic requirements of clinical commercialization and commercial promotion. For instance, the lack of corresponding quality standards and specifications for the safety, stability and reproducibility of exosome preparations. A standardized procedure is required to standardize large-scale exosome isolation, purification, modification, loading and storage processes, and a long-term drug safety assessment of the final preparation.
The inherent biological composition of different subsets of exosomes is heterogeneous, troubling the design, dosage and standardization of exosome-based products. Further identification and purification of specific subtypes of exosomes are required to achieve more effective therapeutic effects, which is exactly the technology that urgently needs to be developed. The cellular uptake mechanisms of exosomes vary with different cell types and delivery routes. Different drug delivery routes will affect the biological distribution of exosomes due to physical transport barriers, uptake of non-target tissues, and rapid clearance in vivo [128]. Further studies on the limiting factors of different drug delivery routes are required to find suitable solutions. The most likely strategy for scaling up production is to isolate exosomes from large quantities of readily available biological materials, such as biological fluids and blood samples. Second, cells such as MSCs, erythrocytes, DCs, and macrophages can preferentially meet therapeutic needs. In the early stage of development, it can be anticipated that through the combination of filtration, centrifugation, SEC and other separation methods, there will be a breakthrough in the production scale of exosomes related products and become the core separation principle. Additionally, the storage conditions of exosomes also need to be further explored. Although the storage environment of 4 ℃ or even −80 ℃ seems to have no effect on the physical and chemical properties of exosomes [129], whether and to what extent these conditions will affect the biological activity of exosomes remains to be investigated. Regulators and stakeholders should mutually contribute to the establishment of quality standards and safety specifications for clinical trial evaluation and market approval of exosome-related products within a reasonable monitoring framework.
Interestingly, encapsulation of exosomes into hydrogels can achieve the goal of slow release in the inflammatory microenvironment. Sustained release of primary chondrocyte-derived exosomes at damaged cartilage sites was achieved by loading them with an injectable heat-sensitive hydrogel [130]. However, this slow-release strategy is mostly applied to natural exosomes. In the future, it can be foreseen that inflammatory damage can be better alleviated and repaired by developing multiple sustained release vectors for loading exosomes. Furthermore, the integration of exosomes into nanomedicine, materials science, and bioengineering, combined with deeper understanding of the physiological effects of their various components, should further increase their therapeutic potential as a delivery carrier, especially against inflammatory diseases. Through in-depth exploration of the above issues and accelerating the development of clinical trials, exosome mediated drug delivery strategy is expected to make a major breakthrough in the treatment of inflammatory diseases.
Conflicts of interest
The authors have declared no conflict of interest.
Acknowledgement
This work was supported by the National Natural Science Foundation of China [grant numbers 82170459, 2021]; Sichuan Science and Technology Program [grant numbers 2022YFH0007, 2022]; Sichuan Science and Technology Program [grant numbers 23NSFSC1345, 2022]; the Key Project of Application and Basic Research of Southwest Medical University [grant numbers 2021ZKZD016, 2021]; and the Special Support Project for Young Talents of Southwest Medical University [grant numbers 2020-2022].
Contributor Information
Chunhong Li, Email: lispringhong@126.com.
Xiangyu Zhou, Email: Xiangyuzhou971@vip.126.com.
References
- 1.Gupta S.C., Kunnumakkara A.B., Aggarwal S., Aggarwal B.B. Inflammation, a double-edge sword for cancer and other age-related diseases. Front Immunol. 2018;9:2160. doi: 10.3389/fimmu.2018.02160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi J.F., Lu Z.Y., Massart C., Levon K. Dynamic immune/inflammation precision medicine: the good and the bad inflammation in infection and cancer. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.595722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varela M.L., Mogildea M., Moreno I., Lopes A. Acute inflammation and metabolism. Inflammation. 2018;41(4):1115–1127. doi: 10.1007/s10753-018-0739-1. [DOI] [PubMed] [Google Scholar]
- 4.Germolec D.R., Shipkowski K.A., Frawley R.P., Evans E. Markers of inflammation. Methods Mol Biol. 2018;1803:57–79. doi: 10.1007/978-1-4939-8549-4_5. [DOI] [PubMed] [Google Scholar]
- 5.Chiurchiù V., Leuti A., Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front Immunol. 2018;9:38. doi: 10.3389/fimmu.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oishi Y., Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30(11):511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 7.Castanheira F.V.S., Kubes P. Neutrophils and nets in modulating acute and chronic inflammation. Blood. 2019;133(20):2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 8.Croft A.P., Campos J., Jansen K., Turner J.D., Marshall J., Attar M., et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570(7760):246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L.Y., Yang X., Wang S.B., Chen H., Pan H.Y., Hu Z.M. Membrane derived vesicles as biomimetic carriers for targeted drug delivery system. Curr Top Med Chem. 2020;20(27):2472–2492. doi: 10.2174/1568026620666200922113054. [DOI] [PubMed] [Google Scholar]
- 10.Zinger A., Brozovich A., Pasto A., Sushnitha M., Martinez J.O., Evangelopoulos M., et al. Bioinspired extracellular vesicles: lessons learned from nature for biomedicine and bioengineering. Nanomaterials (Basel) 2020;10(11):2172. doi: 10.3390/nano10112172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong O.G., Kooijmans S.A.A., Murphy D.E., Jiang L., Evers M.J.W., Sluijter J.P.G., et al. Drug delivery with extracellular vesicles: from imagination to innovation. Acc Chem Res. 2019;52(7):1761–1770. doi: 10.1021/acs.accounts.9b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elashiry M., Elsayed R., Cutler C.W. Exogenous and endogenous dendritic cell-derived exosomes: lessons learned for immunotherapy and disease pathogenesis. Cells. 2021;11(1):115. doi: 10.3390/cells11010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long X., Yao X., Jiang Q., Yang Y., He X., Tian W., et al. Astrocyte-derived exosomes enriched with mir-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation. 2020;17(1):89. doi: 10.1186/s12974-020-01761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xian P., Hei Y., Wang R., Wang T., Yang J., Li J., et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956–5975. doi: 10.7150/thno.33872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang H.S., Kim H., Han G., Lee J.W., Kim K., Kwon I.C., et al. Extracellular vesicles as potential therapeutics for inflammatory diseases. Int J Mol Sci. 2021;22(11):5487. doi: 10.3390/ijms22115487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sil S., Dagur R.S., Liao K., Peeples E.S., Hu G., Periyasamy P., et al. Strategies for the use of extracellular vesicles for the delivery of therapeutics. J Neuroimmune Pharmacol. 2020;15(3):422–442. doi: 10.1007/s11481-019-09873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang T.T., Wang B., Lv L.L., Liu B.C. Extracellular vesicle-based nanotherapeutics: emerging frontiers in anti-inflammatory therapy. Theranostics. 2020;10(18):8111–8129. doi: 10.7150/thno.47865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L., Xu N., Zhang Z.L., Zhang T.C. Cell derived extracellular vesicles: from isolation to functionalization and biomedical applications. Biomater Sci. 2019;7(9):3552–3565. doi: 10.1039/c9bm00580c. [DOI] [PubMed] [Google Scholar]
- 20.Lane R.E., Korbie D., Trau M., Hill M.M. Purification protocols for extracellular vesicles. Methods Mol Biol. 2017;1660:111–130. doi: 10.1007/978-1-4939-7253-1_10. [DOI] [PubMed] [Google Scholar]
- 21.Konoshenko M.Y., Lekchnov E.A., Vlassov A.V., Laktionov P.P. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018 doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rider M.A., Hurwitz S.N., Meckes D.G. Jr. Extrapeg: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep. 2016;6:23978. doi: 10.1038/srep23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Feng Y., Zheng X., Jia M., Mei Z., Wang Y., et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30. doi: 10.1016/j.jconrel.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Doyle L.M., Wang M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arteaga-Blanco L.A., Mojoli A., Monteiro R.Q., Sandim V., Menna-Barreto R.F.S., Pereira-Dutra F.S., et al. Characterization and internalization of small extracellular vesicles released by human primary macrophages derived from circulating monocytes. PLoS ONE. 2020;15(8) doi: 10.1371/journal.pone.0237795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascucci L., Scattini G. Imaging extracelluar vesicles by transmission electron microscopy: coping with technical hurdles and morphological interpretation. Biochim Biophys Acta Gen Subj. 2021;1865(4) doi: 10.1016/j.bbagen.2020.129648. [DOI] [PubMed] [Google Scholar]
- 27.Moritz C.P. 40 years western blotting: a scientific birthday toast. J Proteomics. 2020;212 doi: 10.1016/j.jprot.2019.103575. [DOI] [PubMed] [Google Scholar]
- 28.Kowal E.J.K., Ter-Ovanesyan D., Regev A., Church G.M. Extracellular vesicle isolation and analysis by western blotting. Methods Mol Biol. 2017;1660:143–152. doi: 10.1007/978-1-4939-7253-1_12. [DOI] [PubMed] [Google Scholar]
- 29.Canan B., Melike Sever B., Nevin B., Adam P.S.B., Sefik Evren E., Turgay D. Overview of extracellular vesicle characterization techniques and introduction to combined reflectance and fluorescence confocal microscopy to distinguish extracellular vesicle subpopulations. Neurophotonics. 2022;9(2):021903. doi: 10.1117/1.NPh.9.2.021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vestad B., Llorente A., Neurauter A., Phuyal S., Kierulf B., Kierulf P., et al. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: a variation study. J Extracell Vesicles. 2017;6(1) doi: 10.1080/20013078.2017.1344087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giebel B., Helmbrecht C. Methods to analyze EVs. Methods Mol Biol. 2017;1545:1–20. doi: 10.1007/978-1-4939-6728-5_1. [DOI] [PubMed] [Google Scholar]
- 32.Wu J.Y., Li Y.J., Hu X.B., Huang S., Xiang D.X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: a comparative evaluation of storage conditions. Drug Deliv. 2021;28(1):162–170. doi: 10.1080/10717544.2020.1869866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan F., Li Y.M., Wang Z. Preserving extracellular vesicles for biomedical applications: consideration of storage stability before and after isolation. Drug Deliv. 2021;28(1):1501–1509. doi: 10.1080/10717544.2021.1951896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charoenviriyakul C., Takahashi Y., Nishikawa M., Takakura Y. Preservation of exosomes at room temperature using lyophilization. Int J Pharm. 2018;553(1–2):1–7. doi: 10.1016/j.ijpharm.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 35.Bari E., Perteghella S., Catenacci L., Sorlini M., Croce S., Mantelli M., et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine (Lond) 2019;14(6):753–765. doi: 10.2217/nnm-2018-0240. [DOI] [PubMed] [Google Scholar]
- 36.Gao J., Dong X., Wang Z. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods. 2020;177:114–125. doi: 10.1016/j.ymeth.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiklander O.P.B., Brennan M., Lötvall J., Breakefield X.O., El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):eaav8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gámbaro F., Li Calzi M., Fagúndez P., Costa B., Greif G., Mallick E., et al. Stable trna halves can be sorted into extracellular vesicles and delivered to recipient cells in a concentration-dependent manner. RNA Biol. 2020;17(8):1168–1182. doi: 10.1080/15476286.2019.1708548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orefice N.S. Development of new strategies using extracellular vesicles loaded with exogenous nucleic acid. Pharmaceutics. 2020;12(8):705. doi: 10.3390/pharmaceutics12080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Lamichhane T.N., Raiker R.S., Jay S.M. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm. 2015;12(10):3650–3657. doi: 10.1021/acs.molpharmaceut.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehryab F., Rabbani S., Shahhosseini S., Shekari F., Fatahi Y., Baharvand H., et al. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62. doi: 10.1016/j.actbio.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 43.Xi X.M., Xia S.J., Lu R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021;76(2):61–67. doi: 10.1691/ph.2021.0128. [DOI] [PubMed] [Google Scholar]
- 44.Donoso-Quezada J., Ayala-Mar S., González-Valdez J. State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: a review. Crit Rev Biotechnol. 2020;40(6):804–820. doi: 10.1080/07388551.2020.1785385. [DOI] [PubMed] [Google Scholar]
- 45.He R., Jiang Y., Shi Y., Liang J., Zhao L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ros-mediated mitochondrial apoptosis. Mater Sci Eng C Mater Biol Appl. 2020;117 doi: 10.1016/j.msec.2020.111314. [DOI] [PubMed] [Google Scholar]
- 46.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P., Wang H., Huang Q., Peng C., Yao L., Chen H., et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714–1727. doi: 10.7150/thno.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luan X., Sansanaphongpricha K., Myers I., Chen H., Yuan H., Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armstrong J.P., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11(1):69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuhrmann G., Serio A., Mazo M., Nair R., Stevens M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 51.Lamichhane T.N., Jeyaram A., Patel D.B., Parajuli B., Livingston N.K., Arumugasaamy N., et al. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng. 2016;9(3):315–324. doi: 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebrahimian M., Hashemi M., Etemad L., Salmasi Z. Thymoquinone-loaded mesenchymal stem cell-derived exosome as an efficient nano-system against breast cancer cells. Iran J Basic Med Sci. 2022;25(6):723–731. doi: 10.22038/IJBMS.2022.64092.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salunkhe S., Dheeraj Basak M, Chitkara D., Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release. 2020;326:599–614. doi: 10.1016/j.jconrel.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 54.Lin Y., Yan M., Bai Z., Xie Y., Ren L., Wei J., et al. Huc-MSC-derived exosomes modified with the targeting peptide of ahscs for liver fibrosis therapy. J Nanobiotechnology. 2022;20(1):432. doi: 10.1186/s12951-022-01636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo R., Liu M., Tan T., Yang Q., Wang Y., Men L., et al. Emerging significance and therapeutic potential of extracellular vesicles. Int J Biol Sci. 2021;17(10):2476–2486. doi: 10.7150/ijbs.59296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richter M., Vader P., Fuhrmann G. Approaches to surface engineering of extracellular vesicles. Adv Drug Deliv Rev. 2021;173:416–426. doi: 10.1016/j.addr.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Rayamajhi S., Aryal S. Surface functionalization strategies of extracellular vesicles. J Mater Chem B. 2020;8(21):4552–4569. doi: 10.1039/d0tb00744g. [DOI] [PubMed] [Google Scholar]
- 58.Abello J., Nguyen T.D.T., Marasini R., Aryal S., Weiss M.L. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics. 2019;9(8):2325–2345. doi: 10.7150/thno.30030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aryal S., Key J., Stigliano C., Ananta J.S., Zhong M., Decuzzi P. Engineered magnetic hybrid nanoparticles with enhanced relaxivity for tumor imaging. Biomaterials. 2013;34(31):7725–7732. doi: 10.1016/j.biomaterials.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Lu M., Xing H., Xun Z., Yang T., Zhao X., Cai C., et al. Functionalized extracellular vesicles as advanced therapeutic nanodelivery systems. Eur J Pharm Sci. 2018;121:34–46. doi: 10.1016/j.ejps.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 61.He C., Zheng S., Luo Y., Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aimaletdinov A.M., Gomzikova M.O. Tracking of extracellular vesicles' biodistribution: new methods and approaches. Int J Mol Sci. 2022;23(19):11312. doi: 10.3390/ijms231911312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C., Zong S., Wang Z., Lu J., Zhu D., Zhang Y., et al. Visualization and intracellular dynamic tracking of exosomes and exosomal miRNAs using single molecule localization microscopy. Nanoscale. 2018;10(11):5154–5162. doi: 10.1039/c7nr08800k. [DOI] [PubMed] [Google Scholar]
- 64.Zong S., Liu Y., Yang K., Yang Z., Wang Z., Cui Y. Eliminating nonspecific binding sites for highly reliable immunoassay via super-resolution multicolor fluorescence colocalization. Nanoscale. 2021;13(13):6624–6634. doi: 10.1039/d0nr08103e. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C., Song J., Lou L., Qi X., Zhao L., Fan B., et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioeng Transl Med. 2021;6(3):e10203. doi: 10.1002/btm2.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalimuthu S., Gangadaran P., Rajendran R.L., Zhu L., Oh J.M., Lee H.W., et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi H., Kim M.Y., Kim D.H., Yun H., Oh B.K., Kim S.B., et al. Quantitative biodistribution and pharmacokinetics study of GMP-grade exosomes labeled with 89Zr radioisotope in mice and rats. Pharmaceutics. 2022;14(6):1118. doi: 10.3390/pharmaceutics14061118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hikita T., Oneyama C. Quantification and imaging of exosomes via luciferase-fused exosome marker proteins: exoluc system. Methods Mol Biol. 2022;2524:281–290. doi: 10.1007/978-1-0716-2453-1_21. [DOI] [PubMed] [Google Scholar]
- 69.Cohen O., Betzer O., Elmaliach-Pnini N., Motiei M., Sadan T., Cohen-Berkman M., et al. Golden' exosomes as delivery vehicles to target tumors and overcome intratumoral barriers: in vivo tracking in a model for head and neck cancer. Biomater Sci. 2021;9(6):2103–2114. doi: 10.1039/d0bm01735c. [DOI] [PubMed] [Google Scholar]
- 70.Noonin C., Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11(9):4436–4451. doi: 10.7150/thno.54004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H., Wang L., Li C., Yu Y., Yi Y., Wang J., et al. Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 2019;10:1464. doi: 10.3389/fimmu.2019.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang T.H., Wu C.S., Chiou S.H., Chang C.H., Liao H.J. Adipose-derived stem cell exosomes as a novel anti-inflammatory agent and the current therapeutic targets for rheumatoid arthritis. Biomedicines. 2022;10(7):1725. doi: 10.3390/biomedicines10071725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang L., Wang T., Zhang X., Zhang H., Yan N., Zhang G., et al. Exosomes derived from human placental mesenchymal stem cells ameliorate myocardial infarction via anti-inflammation and restoring gut dysbiosis. BMC Cardiovasc Disord. 2022;22(1):61. doi: 10.1186/s12872-022-02508-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S., Dong X., Gao J., Wang Z. Targeting inflammatory vasculature by extracellular vesicles. AAPS J. 2018;20(2):37. doi: 10.1208/s12248-018-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tardito S., Martinelli G., Soldano S., Paolino S., Pacini G., Patane M., et al. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev. 2019;18(11) doi: 10.1016/j.autrev.2019.102397. [DOI] [PubMed] [Google Scholar]
- 76.Wu G., Zhang J., Zhao Q., Zhuang W., Ding J., Zhang C., et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem Int Ed Engl. 2020;59(10):4068–4074. doi: 10.1002/anie.201913700. [DOI] [PubMed] [Google Scholar]
- 77.Wang L.X., Zhang S.X., Wu H.J., Rong X.L., Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106(2):345–358. doi: 10.1002/JLB.3RU1018-378RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F., et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 79.Li R., He Y., Zhu Y., Jiang L., Zhang S., Qin J., et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19(1):124–134. doi: 10.1021/acs.nanolett.8b03439. [DOI] [PubMed] [Google Scholar]
- 80.Yan F., Zhong Z., Wang Y., Feng Y., Mei Z., Li H., et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnology. 2020;18(1):115. doi: 10.1186/s12951-020-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Behr B., Ko S.H., Wong V.W., Gurtner G.C., Longaker M.T. Stem cells. Plast Reconstr Surg. 2010;126(4):1163–1171. doi: 10.1097/PRS.0b013e3181ea42bb. [DOI] [PubMed] [Google Scholar]
- 82.An Y., Lin S., Tan X., Zhu S., Nie F., Zhen Y., et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993. doi: 10.1111/cpr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J.H., Liu F.X., Wang J.H., Cheng M., Wang S.F., Xu D.H. Mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles: potential roles in rheumatic diseases. World J Stem Cells. 2020;12(7):688–705. doi: 10.4252/wjsc.v12.i7.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng J., Zhu L., Iok In I., Chen Y., Jia N., Zhu W. Bone marrow-derived mesenchymal stem cells-secreted exosomal microrna-192-5p delays inflammatory response in rheumatoid arthritis. Int Immunopharmacol. 2020;78 doi: 10.1016/j.intimp.2019.105985. [DOI] [PubMed] [Google Scholar]
- 85.Chen Z., Wang H., Xia Y., Yan F., Lu Y. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 2018;201(8):2472–2482. doi: 10.4049/jimmunol.1800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He X., Zhang C., Amirsaadat S., Jalil A.T., Kadhim M.M., Abasi M., et al. Curcumin-loaded mesenchymal stem cell-derived exosomes efficiently attenuate proliferation and inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes. Appl Biochem Biotechnol. 2023;195:51–67. doi: 10.1007/s12010-022-04090-5. [DOI] [PubMed] [Google Scholar]
- 87.Kim S.H., Bianco N.R., Shufesky W.J., Morelli A.E., Robbins P.D. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol. 2007;179(4):2242–2249. doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- 88.Lee E.S., Sul J.H., Shin J.M., Shin S., Lee J.A., Kim H.K., et al. Reactive oxygen species-responsive dendritic cell-derived exosomes for rheumatoid arthritis. Acta Biomater. 2021;128:462–473. doi: 10.1016/j.actbio.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 89.Zhang L., Qin Z., Sun H., Chen X., Dong J., Shen S., et al. Nanoenzyme engineered neutrophil-derived exosomes attenuate joint injury in advanced rheumatoid arthritis via regulating inflammatory environment. Bioact Mater. 2022;18:1–14. doi: 10.1016/j.bioactmat.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chow Y.Y., Chin K.Y. The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm. 2020;2020 doi: 10.1155/2020/8293921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y., Zeng Y., Si H.B., Tang L., Xie H.Q., Shen B. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am J Sports Med. 2022;50(4):1088–1105. doi: 10.1177/03635465221073991. [DOI] [PubMed] [Google Scholar]
- 92.Qiu B., Xu X., Yi P., Hao Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J Cell Mol Med. 2020;24(18):10855–10865. doi: 10.1111/jcmm.15714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., et al. Chondrocyte-targeted microrna delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 94.Xu X., Liang Y., Li X., Ouyang K., Wang M., Cao T., et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- 95.Liu X., Wang L., Ma C., Wang G., Zhang Y., Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J Orthop Surg Res. 2019;14(1):470. doi: 10.1186/s13018-019-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Headland S.E., Jones H.R., Norling L.V., Kim A., Souza P.R., Corsiero E., et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7(315) doi: 10.1126/scitranslmed.aac5608. 315ra190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y.T., Yuan H.X., Ou Z.J., Ou J.S. Microparticles (exosomes) and atherosclerosis. Curr Atheroscler Rep. 2020;22(6):23. doi: 10.1007/s11883-020-00841-z. [DOI] [PubMed] [Google Scholar]
- 98.Sun L., He X., Zhang T., Han Y., Tao G. Knockdown of mesenchymal stem cell‑derived exosomal LOC100129516 suppresses the symptoms of atherosclerosis via upregulation of the PPARγ/LXRα/ABCA1 signaling pathway. Int J Mol Med. 2021;48(6):208. doi: 10.3892/ijmm.2021.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi C., Ulke-Lemée A., Deng J., Batulan Z., O'Brien E.R. Characterization of heat shock protein 27 in extracellular vesicles: a potential anti-inflammatory therapy. FASEB J. 2019;33(2):1617–1630. doi: 10.1096/fj.201800987R. [DOI] [PubMed] [Google Scholar]
- 100.Bu T., Li Z., Hou Y., Sun W., Zhang R., Zhao L., et al. Exosome-mediated delivery of inflammation-responsive IL-10 mRNA for controlled atherosclerosis treatment. Theranostics. 2021;11(20):9988–10000. doi: 10.7150/thno.64229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hergenreider E., Heydt S., Tréguer K., Boettger T., Horrevoets A.J., Zeiher A.M., et al. Atheroprotective communication between endothelial cells and smooth muscle cells through mirnas. Nat Cell Biol. 2012;14(3):249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 102.Seo Y., Kim H.S., Hong I.S. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019;2019 doi: 10.1155/2019/5126156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu H., Fan H., Shou Z., Xu M., Chen Q., Ai C., et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204–212. doi: 10.1016/j.intimp.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 104.Xu Y., Tang X., Yang M., Zhang S., Li S., Chen Y., et al. Interleukin 10 gene-modified bone marrow-derived dendritic cells attenuate liver fibrosis in mice by inducing regulatory T cells and inhibiting the TGF-β/Smad signaling pathway. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/4652596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shahir M., Mahmoud Hashemi S., Asadirad A., Varahram M., Kazempour-Dizaji M., Folkerts G., et al. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J Cell Physiol. 2020;235(10):7043–7055. doi: 10.1002/jcp.29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cai Z., Zhang W., Yang F., Yu L., Yu Z., Pan J., et al. Immunosuppressive exosomes from TGF-β1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2012;22(3):607–610. doi: 10.1038/cr.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang X., Meng S., Jiang H., Chen T., Wu W. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol. 2010;45(10):1168–1177. doi: 10.3109/00365521.2010.490596. [DOI] [PubMed] [Google Scholar]
- 108.Deng F., Yan J., Lu J., Luo M., Xia P., Liu S., et al. M2 macrophage-derived exosomal miR-590-3p attenuates DSS-induced mucosal damage and promotes epithelial repair via the LATS1/YAP/β-catenin signalling axis. J Crohns Colitis. 2021;15(4):665–677. doi: 10.1093/ecco-jcc/jjaa214. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y.X., Lin C., Cui L.J., Deng T.Z., Li Q.M., Chen F.Y., et al. Mechanism of M2 macrophage-derived extracellular vesicles carrying lncRNA MEG3 in inflammatory responses in ulcerative colitis. Bioengineered. 2021;12(2):12722–12739. doi: 10.1080/21655979.2021.2010368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo G., Tan Z., Liu Y., Shi F., She J. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res Ther. 2022;13(1):138. doi: 10.1186/s13287-022-02811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu H., Liang Z., Wang F., Zheng X., Zeng Z., He X., et al. Intestinal CD14+ macrophages protect CD4+ T cells from activation-induced cell death via exosomal membrane TNF in Crohn's disease. J Crohns Colitis. 2020;14(11):1619–1631. doi: 10.1093/ecco-jcc/jjaa083. [DOI] [PubMed] [Google Scholar]
- 112.Gómez-Ferrer M., Amaro-Prellezo E., Dorronsoro A., Sánchez-Sánchez R., Vicente Á., Cosín-Roger J., et al. HIF-overexpression and pro-inflammatory priming in human mesenchymal stromal cells improves the healing properties of extracellular vesicles in experimental crohn's disease. Int J Mol Sci. 2021;22(20):11269. doi: 10.3390/ijms222011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Altemus J., Dadgar N., Li Y., Lightner A.L. Adipose tissue-derived mesenchymal stem cells' acellular product extracellular vesicles as a potential therapy for crohn's disease. J Cell Physiol. 2022;237(7):3001–3011. doi: 10.1002/jcp.30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao J., Li Y., Jia R., Wang J., Shi M., Wang Y. Mesenchymal stem cells-derived exosomes as dexamethasone delivery vehicles for autoimmune hepatitis therapy. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.650376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng Y., Dong C., Yang J., Jin Y., Zheng W., Zhou Q., et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J Cell Physiol. 2019;234(11):20662–20674. doi: 10.1002/jcp.28671. [DOI] [PubMed] [Google Scholar]
- 116.Ridzuan N., Zakaria N., Widera D., Sheard J., Morimoto M., Kiyokawa H., et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (copd) Stem Cell Res Ther. 2021;12(1):54. doi: 10.1186/s13287-020-02088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu W., Yu M., Chen F., Wang L., Ye C., Chen Q., et al. A novel delivery nanobiotechnology: engineered miR-181b exosomes improved osteointegration by regulating macrophage polarization. J Nanobiotechnology. 2021;19(1):269. doi: 10.1186/s12951-021-01015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yan C., Chen J., Wang C., Yuan M., Kang Y., Wu Z., et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022;29(1):214–228. doi: 10.1080/10717544.2021.2023699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kimiz-Gebologlu I., Oncel S.S. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–543. doi: 10.1016/j.jconrel.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 120.Lima Correa B., El Harane N., Gomez I., Rachid Hocine H., Vilar J., Desgres M., et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc Res. 2021;117(1):292–307. doi: 10.1093/cvr/cvaa028. [DOI] [PubMed] [Google Scholar]
- 121.Wang L.T., Liu K.J., Sytwu H.K., Yen M.L., BL Yen. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl Med. 2021;10(9):1288–1303. doi: 10.1002/sctm.21-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arabpour M., Saghazadeh A., Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97 doi: 10.1016/j.intimp.2021.107823. [DOI] [PubMed] [Google Scholar]
- 123.Tang Y., Wu Z., Guo R., Huang J., Rong X., Zhu B., et al. Ultrasound-augmented anti-inflammatory exosomes for targeted therapy in rheumatoid arthritis. J Mater Chem B. 2022;10(38):7862–7874. doi: 10.1039/d2tb01219g. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Z., Yu Y., Zhu G., Zeng L., Xu S., Cheng H., et al. The emerging role of plant-derived exosomes-like nanoparticles in immune regulation and periodontitis treatment. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.896745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dad H.A., Gu T.W., Zhu A.Q., Huang L.Q., Peng L.H. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi: 10.1016/j.ymthe.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Munagala R., Aqil F., Jeyabalan J., Gupta R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J., Li S., Zhang S., Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17(1):53–69. doi: 10.1016/j.ajps.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Burnouf T., Agrahari V., Agrahari V. Extracellular vesicles as nanomedicine: hopes and hurdles in clinical translation. Int J Nanomedicine. 2019;14:8847–8859. doi: 10.2147/IJN.S225453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng Y., Zeng Q., Han Q., Xia W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell. 2019;10(4):295–299. doi: 10.1007/s13238-018-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sang X., Zhao X., Yan L., Jin X., Wang X., Wang J., et al. Thermosensitive hydrogel loaded with primary chondrocyte-derived exosomes promotes cartilage repair by regulating macrophage polarization in osteoarthritis. Tissue Eng Regen Med. 2022;19(3):629–642. doi: 10.1007/s13770-022-00437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Clinicaltrials.gov [internet]. [cited 2022 Nov 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT05060107
- 132.Clinicaltrials.gov [internet]. [cited 2022 Nov 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT05499156