Abstract
Mouse capillary endothelial cells (1G11 cell line) embedded in type I collagen gels undergo in vitro angiogenesis. Cells rapidly reorganize and form capillary-like structures when stimulated with serum. Transforming growth factor β1 (TGF-β1) alone can substitute for serum and induce cell survival and tubular network formation. This TGF-β1-mediated angiogenic activity depends on phosphatidylinositol 3-kinase (PI3K) and p42/p44 mitogen-activated protein kinase (MAPK) signaling. We showed that specific inhibitors of either pathway (wortmannin, LY-294002, and PD-98059) all suppressed TGF-β1-induced angiogenesis mainly by compromising cell survival. We established that TGF-β1 stimulated the expression of TGF-α mRNA and protein, the tyrosine phosphorylation of a 170-kDa membrane protein representing the epidermal growth factor (EGF) receptor, and the delayed activation of PI3K/Akt and p42/p44 MAPK. Moreover, we showed that all these TGF-β1-mediated signaling events, including tubular network formation, were suppressed by incubating TGF-β1-stimulated endothelial cells with a soluble form of an EGF receptor (ErbB-1) or tyrphostin AG1478, a specific blocker of EGF receptor tyrosine kinase. Finally, addition of TGF-α alone poorly stimulated angiogenesis; however, by reducing cell death, it strongly potentiated the action of TGF-β1. We therefore propose that TGF-β1 promotes angiogenesis at least in part via the autocrine secretion of TGF-α, a cell survival growth factor, activating PI3K/Akt and p42/p44 MAPK.
Angiogenesis or the formation of new blood vessels from preexisting vasculature occurs in normal situations such as embryonic development, wound healing, and during the female reproductive cycle. However, activated blood vessel growth is also found in many diseases, such as tumor progression, diabetic retinopathy, and arthritis (24, 33). In the last few years, several studies have led to the discovery of inducers and inhibitors of the angiogenic process (6, 9, 12). Among the inducers are factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor 1 (FGF-1) and -2, which induce angiogenesis in vivo and in vitro. They can also induce the proliferation and migration of endothelial cells in two-dimensional cultures. In contrast, other factors such as transforming growth factor β (TGF-β) and tumor necrosis factor alpha induce angiogenesis in vivo and in vitro but inhibit endothelial cell proliferation in vitro (6, 9, 12).
TGF-β1 is a 25-kDa peptide belonging to a family of multifunctional cytokines that control the development and homeostasis of most tissues by regulating diverse cellular functions, such as proliferation and differentiation (49, 72). The receptors for this family are basically two transmembrane serine/threonine kinases, termed receptor type I and type II. The binding of the ligand causes the heterodimerization of receptors I and II followed by the activation by phosphorylation of receptor I. This receptor then phosphorylates and activates the Smad family of proteins, which transduce the signal to the nucleus (19, 36, 49, 72). The role of TGF-β in angiogenesis was first shown by new capillary formation after injection of the factor into mice (23, 65) and by application of the factor to the chicken chorioallantoic membrane (80). Moreover, TGF-β1 and TGF-β2 are expressed during the development of angiogenically active tissues (35, 60). This proangiogenic activity of TGF-β has been confirmed by experiments using knockout mice. The knock out of TGF-β1 (20), the type II receptor (59), and type I receptor activin receptor-like kinase 1 (ALK1) (57, 74) is lethal at 10.5 days of gestation due to defective vasculogenesis (the initial formation of the primitive vasculature in the embryo), along with defective endothelial cell differentiation and inadequate capillary tube formation. Moreover, Smad5 knockout mice also die due to defects in vasculogenesis and angiogenesis (14, 81). Finally, mutations in the human ALK1 gene and in the endoglin gene, which encodes a TGF-β1-binding protein that presents TGF-β1 to the type I and II receptors, all cause hereditary hemorrhagic telangiectasia, a disease characterized by vascular malformations (39, 50). Endoglin knockout mice also show a defective angiogenesis and die at embryonic day 11.5 (44). In vitro, TGF-β inhibits endothelial cell proliferation in two-dimensional cultures (3, 26, 34, 56) but induces tube formation when endothelial cells are cultured inside three-dimensional collagen gels (45, 53, 73). The differences between these studies have been attributed to changes in type I and II receptor expression (66). Finally, TGF-β1 promotes the in vitro differentiation of embryonic stem cells into endothelium cells as well as the formation of cord-like structures (32). However, the basic mechanisms underlying the proangiogenic action of TGF-β are largely unknown. With a mouse vascular endothelial cell model (1G11 cell line) which rapidly forms capillary-like structures in collagen and responds nicely to TGF-β1, we have investigated the basic signaling action of this angiogenic cytokine. We show that, surprisingly, TGF-β1 can stimulate the phosphatidylinositol 3-kinase (PI3K)/Akt and the p42/p44 mitogen-activated protein kinase (MAPK) pathways; however, this action is mediated by an autocrine mechanism, implicating at least the production of TGF-α. Moreover, TGF-β1-mediated stimulation of PI3K and p42/p44 MAPK signaling cascades is essential for cell survival and formation of capillary-like structures.
MATERIALS AND METHODS
Materials.
Human TGF-β1 was obtained from R&D Systems, FGF-2 and platelet-derived growth factor BB (PDGF-BB) were obtained from Pepro Tech, epidermal growth factor (EGF) and insulin were obtained from Sigma, and TGF-α was obtained from Boehringer. LY-294002 was obtained from Alexis; wortmannin and rapamycin were obtained from BioMol; PD-98059 was obtained from New England Biolabs; cycloheximide, actinomycin D, brefeldin A, and tyrphostin AG1478 were also obtained from Sigma. Genistein and SB202190 were obtained from Calbiochem. Cell culture media, fetal calf serum (FCS), glutamine, and antibiotics were obtained from Gibco-BRL. The most commonly used chemicals were purchased from Sigma.
Cell culture.
Murine lung capillary endothelial cells (1G11 cell line) were obtained from Alberto Mantovani and Annunciata Vecchi (Instituto Ricerche Farmacologiche Mario Negri, Milan, Italy) (21). They were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 20% inactivated FCS; 50 U of penicillin, 50 μg of streptomycin sulfate, 150 μg of endothelial cell growth supplement (Becton Dickinson), and 100 μg of heparin (Sigma)/ml; 1% nonessential amino acids; and 2 mM sodium pyruvate. Before the incubation with growth factors, cells were depleted for 24 h in a 1:1 mixture of DMEM and Ham's F-12 medium.
Murine heart capillary endothelial cells (H5V cell line) transformed by polyoma middle T antigen (27) were obtained from P. Huber (Grenoble, France) and cultured in DMEM containing 20% FCS.
Human umbilical vein endothelial cells (HUVEC) were obtained as previously described (5) and were cultivated in SFM (Gibco-BRL) supplemented with 20% FCS and 100 μg of heparin, 20 ng of FGF-2, and 10 ng of EGF (Sigma)/ml.
HEK293 cells were cultured in DMEM containing 7.5% inactivated FCS, 50 U of penicillin/ml, and 50 μg of streptomycin sulfate/ml. Cells were transiently transfected by the calcium phosphate method. HEK293 cells were seeded at a density of 700,000 cells per well in six-well plates and transfected with 0.25 μg of the green fluorescent protein (GFP) plasmid only or cotransfected with 0.25 μg of GFP plasmid together with 5 μg of expression vector CDM7-IgB-1, which codes for the extracellular portion of EGF receptor (ErbB-1) fused to an Fc portion of human immunoglobulin G1 (15). Forty-eight hours after transfection, the culture medium from transfected HEK293 cells was used to treat 1G11 cells.
Tubulogenesis and cell death counting assay.
To induce capillary tube formation, 1G11 cells grown to confluence were trypsinized and resuspended in 2× DMEM. Cells were added to a type I collagen solution (Becton Dickinson; 4 mg/ml) to achieve a cell concentration of 3 × 106 cells/ml and a final collagen concentration of 2 mg/ml. Sixty microliters of this preparation was placed in 24-well plates and incubated for 45 min at 37°C in a humidified incubator to allow polymerization, and complete 1G11 cell culture medium, DMEM alone, or DMEM containing 10 ng of TGF-β1/ml was added where indicated in the legends for Fig. 1, 2, 11, and 12.
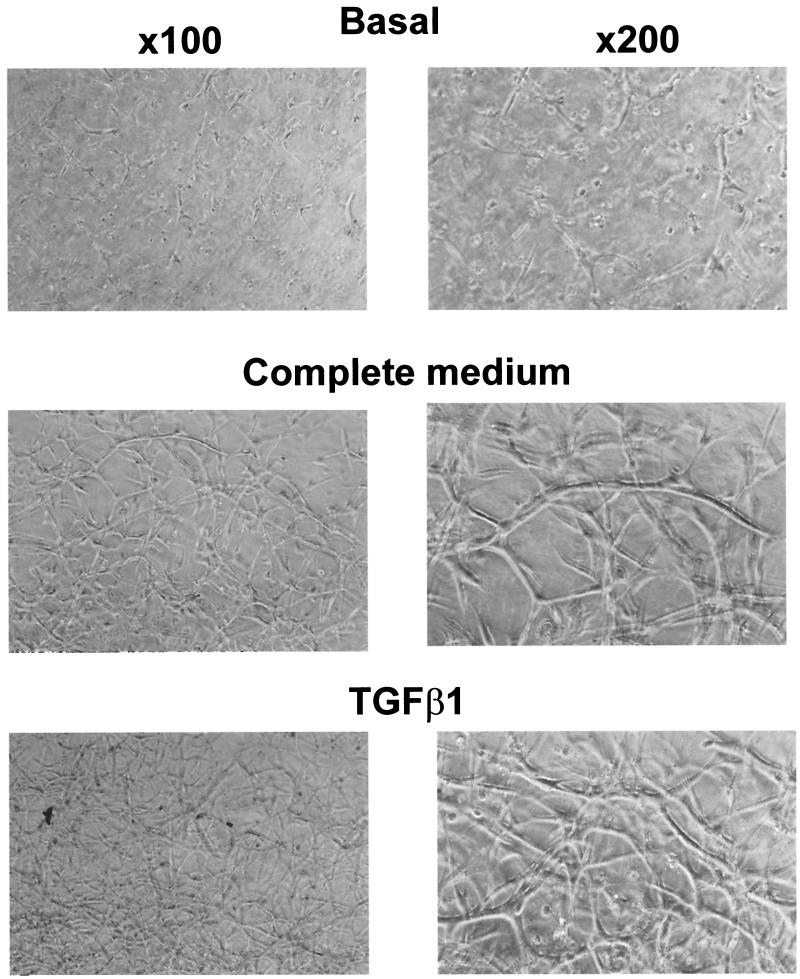
FIG. 1.
TGF-β1 and complete medium stimulate 1G11 capillary endothelial cells to form a tubular network in type I collagen gels. 1G11 cells were mixed with type I collagen and placed on culture plates to polymerize. After gel formation, DMEM alone (basal), complete medium (20% FCS and 150 μg of endothelial cell growth supplement/ml), or TGF-β1 (10 ng/ml) was added for 48 h and gels were examined by phase-contrast microscopy.
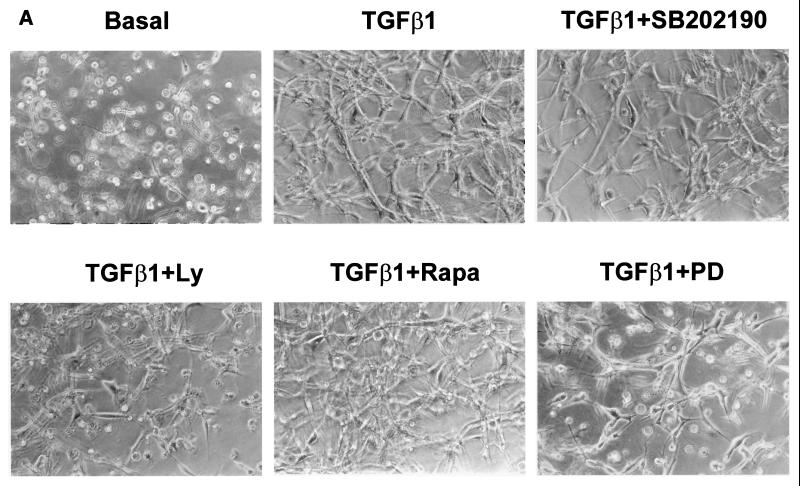
FIG. 2.
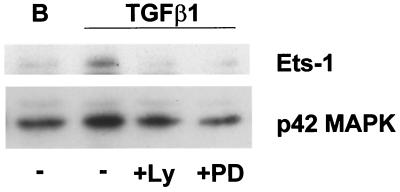
LY-294002 and PD-98059 prevent TGF-β1-induced tube formation and Ets-1 induction. (A) 1G11 endothelial cells immersed in collagen gels were cultured for 24 h in the presence of DMEM alone (basal) or 10 ng of TGF-β1/ml either alone or with 10 μM SB202190, 15 μM LY-294002 (Ly), 10 nM rapamycin (rapa), or 30 μM PD-98059 (PD). Cells were examined by phase-contrast microscopy. Magnification, ×128. (B) 1G11 cells grown in collagen gels for 24 h in the presence of 10 ng of TGF-β1/ml either alone (TGF-β1 lane −) or with 15 μM LY-294002 (lane +Ly) or 30 μM PD-98059 (lane +PD) or in the absence of TGF-β (B lane −) were lysed, and Ets-1 was detected by immunoblotting with a specific antibody. Identical amounts of protein were loaded on the gel. The decrease in p42 MAPK content in lanes +Ly and +PD reflects partial cell death. A representative Western blot of three different experiments is shown.
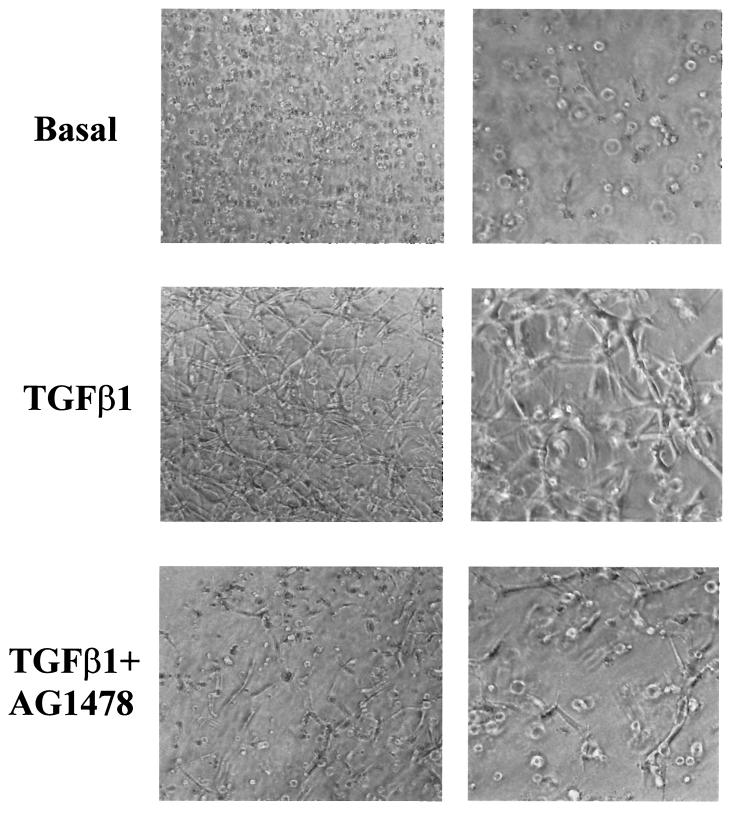
FIG. 11.
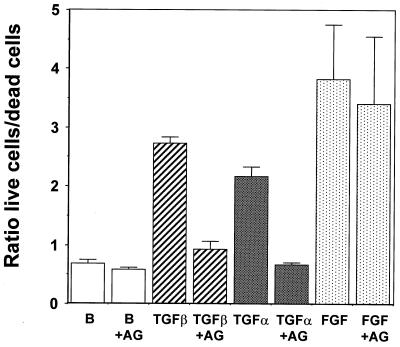
Inhibition of TGF-α signaling blocks tube formation and cell survival induced by TGF-β1 in collagen gels. (Top) 1G11 endothelial cells grown in collagen gels were cultured for 24 h in the presence of DMEM alone (basal) or 10 ng of TGF-β1/ml either alone or supplemented with 1 μM tyrphostin AG1478. After this time, cells were examined by phase-contrast microscopy. Magnifications: left, ×90; right, ×180. (Bottom) 1G11 endothelial cells grown in collagen gels were cultured for 24 h in the presence of DMEM either alone (lane B) or with 1 μM tyrphostin AG1478 (B+AG), in 10 ng of TGF-β1/ml either alone (TGFβ) or with 1 μM tyrphostin AG1478 (TGFβ+AG), in 50 ng of TGF-α/ml either alone (TGFα) or with tyrphostin AG1478 (TGFα+AG), or in 100 ng of FGF-2/ml either alone (FGF) or with 1 μM tyrphostin AG1478 (FGF+AG). Dead and living cells were counted as described in Materials and Methods. Results are averages of three different experiments.
FIG. 12.
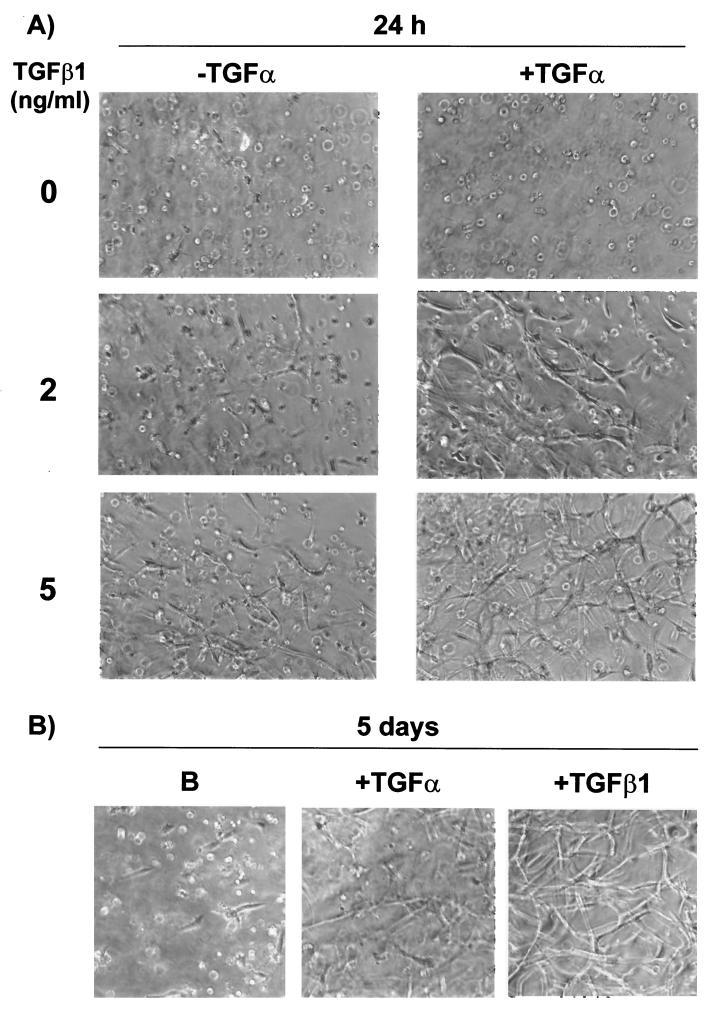
TGF-α stimulates tube formation in collagen gels in the long term and potentiates TGF-β1 action in the short term. 1G11 cells grown in collagen gels were cultured for 24 h in the presence of DMEM alone or different concentrations of TGF-β1 (2 and 5 ng/ml) in the presence or the absence of TGF-α (50 ng/ml). In parallel, 1G11 cells were grown in collagen gels for 5 days in the presence or the absence of TGF-β1 (10 ng/ml) or TGF-α (50 ng/ml). Cells were examined by phase-contrast microscopy.
To extract proteins from the collagen gels, cultures were lysed in radioimmunoprecipitation assay buffer (phosphate-buffered saline with 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 40 mM β-glycerophosphate, 200 μM sodium orthovanadate, 100 μM phenylmethylsulfonyl fluoride, 1 μM pepstatin A, 1 μg of leupeptin/ml and 4 μg of aprotinin/ml), homogenized with a minipotter homogenizer, and extracted for 15 min at 4°C. Insoluble material was removed by centrifugation at 12,000 × g for 10 min at 4°C.
To measure the numbers of live and dead cells after 24 h in collagen gels, Hoechst stain (50-μg/ml bis-benzimide; Sigma) or propidium iodide (1 μg/ml; Sigma) was added and cells were incubated for 30 min at 37°C. The live (nuclei Hoechst stained) and dead cells (nuclei with propidium iodide stain and condensed chromatin) were counted using an immunofluorescence microscope.
Western blot analysis.
Cells were washed twice with cold phosphate-buffered saline and lysed in Triton X-100 lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 50 mM NaF, 5 mM EDTA, 40 mM β-glycerophosphate, 200 μM sodium orthovanadate, 100 μM phenylmethylsulfonyl fluoride, 1 μM pepstatin A, 1 μg of leupeptin/ml, 4 μg of aprotinin/ml, 1% Triton X-100) for 15 min at 4°C. Insoluble material was removed by centrifugation at 12,000 × g for 5 min at 4°C. For conditioned medium from 1G11 cells, proteins from 10 ml were precipitated with acetone for 1 h at −20°C and centrifuged at 5,000 × g for 5 min. The final pellet was resuspended in 50 μl of Laemmli sample buffer (40), followed by protein separation on an SDS–15% polyacrylamide gel. Western blotting was performed as described previously (76). The blots were incubated with a polyclonal anti-Ets-1 antibody (Santa Cruz Biotechnology), polyclonal antiphospho-(Ser473)-Akt (New England Biolabs), a polyclonal anti-Akt antibody (New England Biolabs), a anti-poly(ADP-ribose) polymerase (anti-PARP) monoclonal antibody (BioMol), a monoclonal antiphospho-Erk1/2 antibody (Sigma), anti-p42/p44 MAPK antiserum EIB (51), a polyclonal anti-TGF-α antibody (R&D), and a polyclonal antiphospho-Smad2 antibody (Upstate Biotechnology) in blocking solution overnight at 4°C.
Where indicated (see Fig. 4B), the p70 S6K activity was determined by a mobility shift assay as described previously (76).
FIG. 4.
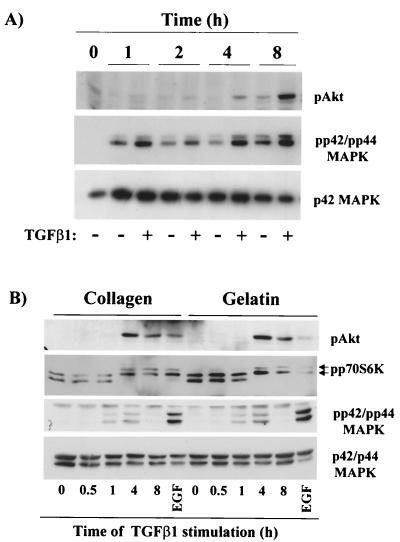
TGF-β1 stimulates PI3K, p70 S6K, and p42/p44 MAPK activities in endothelial cells grown in collagen gels and in two-dimensional cultures. (A) 1G11 cells were grown in collagen gels for the periods of time indicated. Cells were lysed, and phospho-Akt, phospho-p42/p44 MAPK (pp42/pp44 MAPK), and p42 MAPK were detected by immunoblotting with specific antibodies. A representative Western blot is shown. (B) 1G11 cells were grown on collagen- or gelatin-coated plates until confluence. After depletion of growth factors, cells were stimulated with 10 ng of TGF-β1/ml for the periods of time indicated or with 20 ng of EGF for 30 min. Cells were lysed, and Western blotting was performed using anti-phospho-Akt, anti-phospho-p42/p44 MAPK, or p42/p44 MAPK. The same extracts were loaded on an SDS–9% polyacrylamide gel (shift-up) and blotted with an anti-p70 S6K antibody. Hyperphosphorylated and active forms of p70 S6K (arrows) migrated more slowly than hypophosphorylated forms. The Western blots are representative of three independent experiments.
WGL and EGF receptor immunoprecipitation.
Quiescent cells stimulated with or without the different agonists were lysed with Triton X-100 lysis buffer for 15 min at 4°C. Insoluble material was removed by centrifugation at 12,000 × g for 5 min at 4°C. Protein lysates (500 μg) were incubated for 1 h at 4°C with wheat germ lectin (WGL) preadsorbed to Sepharose beads (Pharmacia) or for 3 h (1 mg of lysate) with a specific polyclonal anti-EGF receptor (Santa Cruz Biotechnology) preadsorbed to protein A-Sepharose beads (Pharmacia). Precipitates were then washed four times with Triton X-100 buffer. The final pellet was resuspended in 50 μl of Laemmli sample buffer (40), followed by protein separation on an SDS–7.5% polyacrylamide gel and Western blotting with an antiphosphotyrosine monoclonal antibody or an anti-EGF receptor antibody (Santa Cruz Biotechnology).
Northern blotting.
Poly(A)+ RNA was isolated from confluent 1G11 cells treated for different times with 10 ng of TGF-β1/ml or from H5V cells treated for 3 h with TGF-β1 using a Micro-FastTack 2.0 kit (Invitrogen). A Northern blot assay was performed as described previously (77). The mouse cDNA probe for TGF-α was a 190-bp PCR-amplified band obtained from 1G11 RNA using specific oligonucleotide primers for mouse TGF-α (5′-ATGGTCCCCGCGACCGGACAGCTC-3′; reverse oligonucleotide: 5′-ACATGCTGGCTTCTCTTCCTGCAC-3′). The identity of the amplified TGF-α band was confirmed by sequence analysis (Eurogentec).
Reverse transcription-PCR (RT-PCR).
Poly(A)+ RNA was isolated from confluent HUVEC treated for 2 h with 10 ng of TGF-β1/ml using a Micro-FastTack 2.0 kit (Invitrogen) or not treated. After reverse transcription using an oligo(dT) primer (Amersham), cDNAs were amplified by PCR (Amersham) using specific oligonucleotide primers for human TGF-α (5′-TTCTGGAGCTTCTCAAGGGAT-3′; reverse oligonucleotide: 5′-CCTGGTAAATCAATGGCTAGA-3′) (35 cycles) and mouse actin (5′-ATGGATGACGATATCGCTG-3′; reverse oligonucleotide: 5′-ATGAGGTAGTCTGTCAGGT-3′) (33 cycles). The mouse cDNA probe for TGF-α was a 330-bp PCR-amplified band, and that for actin was a 500-bp band.
RESULTS
Inhibition of PI3K or p42/p44 MAPK blocks TGF-β1-induced endothelial tube formation and cell survival.
Like other capillary endothelial cell models, as described previously (45, 55, 67), 1G11 lung mouse capillary endothelial cells completely reorganize when cultured with complete medium in three-dimensional type I collagen gels. The final result was a network of tubular structures with multiple cell-cell contacts (Fig. 1), which caused the retraction of the collagen gel (data not shown). A lack of this organization could be seen when cells are cultured in the presence of growth factor-free DMEM (Fig. 1). The same effect obtained in the presence of complete medium was observed when only TGF-β1 was added. This TGF-β1-induced tube formation was also observed in primary endothelial cell cultures from mouse lung cultured inside collagen gels (D. Grall and J. C. Chambard, unpublished observations) and has already been described for other microvascular endothelial cells (45, 53). The tubular-growth remodeling induced by TGF-β1 or complete medium was dependent on mRNA and protein synthesis, since preincubation in the presence of their respective inhibitors, actinomycin D and cycloheximide, completely blocked tube formation (data not shown). In contrast, when added to two-dimensional 1G11 cell cultures, TGF-β1 behaved as a growth inhibitor, as judged by the severe block in thymidine incorporation stimulated by EGF or FGF-2 (data not shown). This effect has also been described for other endothelial cell types (3, 26, 34, 56).
To determine the signal transduction pathways important for TGF-β1-induced tube formation, we preincubated 1G11 cells grown in collagen gels with specific inhibitors before addition of TGF-β1. Rapamycin, a specific inhibitor of p70 S6K activation that blocks 1G11 vascular endothelial cell proliferation (76), did not have any effect on tube formation in collagen gels (Fig. 2A). In contrast, preincubation in the presence of PI3K inhibitors LY-294002 (15 μM) or wortmannin (300 nM) blocked tube formation induced by TGF-β1 (Fig. 2A and data not shown). In the presence of PI3K inhibitors cells were not organized and appeared as isolated and refractile, similar to control cells cultured in DMEM only (Fig. 1 and 2A, basal). A similar result was obtained when p42/p44 MAPK activation was blocked by preincubating cells in the presence of 30 μM PD-98059, an inhibitor of p42/p44 MAPK kinase 1 (MEK1) (Fig. 2A). In contrast, inhibition of p38 MAPK by SB202190 did not have any effect. This blocking effect of PI3K and p42/p44 MAPK inhibitors on TGF-β1-induced tube formation was confirmed by the lack of induction of a typical angiogenic marker, transcription factor Ets-1 (73, 75) (Fig. 2B).
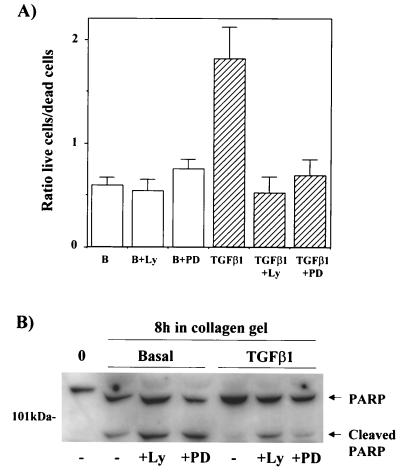
As observed in Fig. 1 and 2A, 1G11 cells cultured in collagen gels for 24 or 48 h in the absence of exogenous growth factors presented a rounded aspect, with few cells presenting extensions. To a large extent, cells presented a refractile aspect and condensed chromatin, typical of dying cells. The number of cells presenting this morphology dropped when growth factors or TGF-β1 was added to the culture medium but reappeared when cells were pretreated with LY-294002, wortmannin, or PD-98059 before addition of TGF-β1 (Fig. 2A). This result suggested that one of the effects of TGF-β1 on three-dimensional cultures of microvascular endothelial cells was to provide survival signals. To confirm this, we counted the live and dead cells (Hoechst stain- or propidium iodide-positive nuclei, respectively) in the presence and absence of TGF-β1. As shown in Fig. 3A, when cells were incubated for 24 h in DMEM only, 64% of the cells were dead (live cell/dead cell ratio of 0.6). Dead cells were round and refractile. In contrast, the addition of TGF-β1 to the cultures doubled the number of live cells (live cell/dead cell ratio of 1.85). This cell survival effect of TGF-β1 was abolished by preincubation in the presence of LY-294002 (live cell/dead cell ratio, 0.54) or in the presence of PD-98059 (live cell/dead cell ratio, 0.7). Preincubation with LY-294002 or PD-98059 alone had no effect on the basal live cell/dead cell ratio. These results were confirmed by measuring apoptosis in collagen gels. Apoptosis was measured by evaluating cleavage of PARP, a well-established substrate of caspase 3 and a marker of caspase cascade activation during apoptosis. As observed in Fig. 3B, culture of 1G11 cells in collagen gels in the presence of growth factor-free DMEM caused PARP cleavage. Treatment with TGF-β1 completely precluded the apoptotic effect of collagen immersion, and this effect was reversed by LY-294002 or PD-98059 pretreatment.
FIG. 3.
TGF-β1 stimulates endothelial cell survival in collagen gels. (A) 1G11 cells immersed in collagen gels were cultured for 24 h in the presence of DMEM alone (B), 15 μM LY-294002 alone (B+Ly), 30 μM PD-98059 alone (B+PD), 10 ng of TGF-β1/ml (TGFβ1), and TGF-β1 in the presence of 15 μM LY-294002 (TGFβ1+Ly) or 30 μM PD-98059 (TGFβ1+PD). After this time, live and dead cells were counted as described in Materials and Methods. Results are expressed as the ratios of live cells/dead cells and are averages of eight different experiments. (B) Proliferative 1G11 cells (0) or cells immersed in collagen gels for 8 h in the absence or presence of TGF-β1 (10 ng/ml), LY-294002 (15 μM), or PD-98059 (30 μM) were lysed, and PARP was detected by immunoblotting with a specific antibody. A representative Western blot is shown. The observed difference in the mobility of PARP between proliferative cells and cells immersed in collagen gels is due to the presence of collagen in the SDS-polyacrylamide gel electrophoresis.
TGF-β1 stimulates PI3K and p42/p44 MAPK in collagen gels and in two-dimensional cultures by an autocrine mechanism.
The sensitivity of tube formation to PI3K and p42/p44 MAPK inhibitors prompted us to investigate the action of TGF-β1 on these two signaling cascades, in particular during tubular network formation in collagen gels. When cells were grown in collagen gels and in the absence of growth factors, we observed a persistent activation of p42/p44 MAPK (measured with an antibody against the phosphorylated active form of the kinases) (Fig. 4A). This stimulation was probably due to integrin activation (8, 37, 69). Interestingly, under the same conditions of culture no activation of PI3K, as indicated by the phosphorylation of its downstream effector Akt, could be detected at short times; only after 8 h of culture on collagen gels was a weak increase in phospho-Akt detected (Fig. 4A). In contrast, the addition of TGF-β1 to cells grown in collagen gels led to a marked activation of Akt (Fig. 4A). Akt activation was observable at 2 h, peaked at 8 h, and persisted at least until 24 h (data not shown). For p42/p44 MAPK, increased activation could also be seen after 1 h of stimulation with TGF-β1, with a maximal difference obtained at 4 h. These results indicate that TGF-β1 stimulated PI3K and enhanced p42/p44 MAPK in collagen gels. With the objective to better characterize the observed PI3K and p42/p44 MAPK stimulation by TGF-β1, we evaluated whether the effect of TGF-β1 on p42/p44 MAPK and PI3K activity could also be observed on two- dimensional 1G11 cell cultures. Therefore, cells were cultured on plates precoated with type I collagen or gelatin and were stimulated for different periods of time with TGF-β1. The activities of Akt, p70 S6K, which also depends on the activity of PI3K, and p42/p44 MAPK were analyzed. As shown in Fig. 4B, TGF-β1 stimulated all these signaling pathways as it did in cells cultured in a three-dimensional collagen gel, but in a more transient manner. As was observed in collagen gels, the effect of TGF-β1 was only observed after stimulation periods of over 1 h. Akt and p70 S6K activation started at 2 h (data not shown), peaked at 4 h, and started to decrease at 8 h. A weak and transient stimulation of p42/p44 MAPK, starting at 1 h, reaching maximum effect at 4 h, and returning to basal levels at 8 h, was also observed.
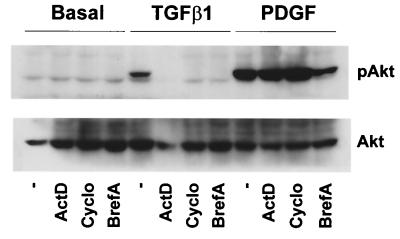
Given the latency observed for TGF-β1-stimulated PI3K and p42/p44 MAPK activities, we hypothesized that TGF-β1 could be having an indirect effect on these pathways. In view of the signaling action of TGF-β1, it was possible that an autocrine factor released by 1G11 cells after TGF-β1 stimulation was activating PI3K and p42/p44 MAPK. To validate this hypothesis, we preincubated cells in the presence of different inhibitors of mRNA synthesis (actinomycin D), protein synthesis (cycloheximide), or vesicular traffic (brefeldin A, a compound that disturbs the trans-Golgi apparatus) before addition of TGF-β1. All these inhibitors completely blocked the effect of TGF-β1 on Akt and p42/p44 MAPK stimulation (Fig. 5 and data not shown). This result suggested that the effect of TGF-β1 was caused by the synthesis and secretion of an autocrine factor. This factor would then activate PI3K and p42/p44 MAPK signaling pathways.
FIG. 5.
Effect of TGF-β1 on Akt and p42/p44 MAPK activation depends on mRNA and protein synthesis and vesicular secretion. Quiescent 1G11 cells were preincubated for 15 min in the presence of 5 μg of actinomycin D/ml, 10 μg of cycloheximide/ml, or 1 μg of brefeldin A/ml or in the absence of inhibitors (−), followed by a 4-h stimulation with 10 ng of TGF-β1/ml, a 30-min stimulation with 10 ng of PDGF-BB/ml, or no stimulation (basal). Cells were lysed, and phospho-Akt and Akt were immunodetected as previously (76) described. A Western blot representative of three different experiments is shown.
TGF-β1 stimulates the synthesis of TGF-α and EGF receptor phosphorylation.
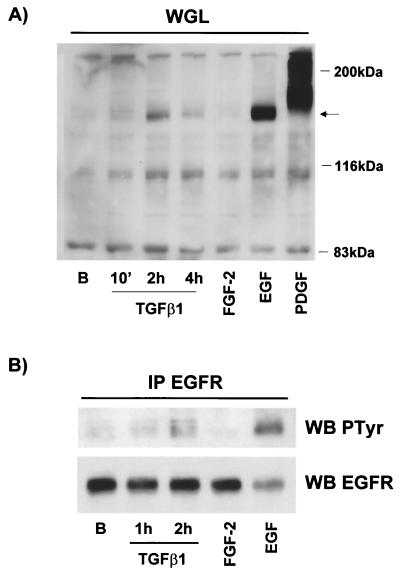
Since this autocrine factor activates the PI3K and p42/p44 MAPK pathways through cell surface receptors, we wanted to evaluate the possible activation of the cell surface tyrosine kinase receptor following TGF-β1 stimulation. Therefore, we stimulated cells for different periods of time with TGF-β1 and isolated membrane proteins by using Sepharose-coupled WGL, which binds glycoproteins. After extensive washing, purified glycoproteins were separated on a polyacrylamide gel and phosphotyrosine-containing proteins were immunodetected with a specific antibody. Incubation with TGF-β1 for 2 or 4 h induced the tyrosine phosphorylation of a number of proteins on total lysates, some of which were also detected after stimulation with FGF-2, EGF, and PDGF-BB (data not shown). Purification of glycoproteins with lectin elicited the enrichment of cell surface proteins such as growth factor receptors. The PDGF receptor and EGF receptor, which were nearly undetectable in total lysates, could clearly be seen after treatment with WGL (Fig. 6A). More interestingly, extracts from cells stimulated for 2 or 4 h with TGF-β1 increased tyrosine phosphorylation on a 160- to 170-kDa protein. This molecular mass was identical to that of the EGF receptor. This result suggested that TGF-β1 induces the phosphorylation of a member of the EGF receptor family. To confirm this hypothesis, we immunoprecipitated the EGF receptor with a specific antibody and evaluated its activity status by phosphotyrosine immunoblotting. As observed in Fig. 6B, incubation for 2 h with TGF-β1 increased the tyrosine phosphorylation of the EGF receptor, confirming the implication of a member of the EGF family of growth factors in the TGF-β1-mediated effect.
FIG. 6.
Stimulation with TGF-β1 causes EGF receptor activation. (A) Quiescent 1G11 cells were stimulated or not (lane B) for the indicated times with 10 ng of TGF-β1/ml and for 10 min with 25 ng of FGF-2/ml, 10 ng of EGF/ml, or 10 ng of PDGF-BB/ml. Cells were lysed, and proteins were incubated with Sepharose-WGL for 1 h. After being washed, the final pellet was resuspended in Laemmli sample buffer and loaded on a SDS–7.5% polyacrylamide gel. Phosphotyrosine-containing proteins were immunodetected by using a specific antibody. Arrow, phosphotyrosine-containing protein that appeared after TGF-β1 treatment. A representative Western blot is shown. (B) Cells were treated as for panel A and lysed, and the EGF receptor (EGFR) was immunoprecipitated (IP) by incubation with a specific anti-EGF receptor antibody preadsorbed to protein A-Sepharose beads. After being washed the pellet was treated as for panel A. WB, Western blot.
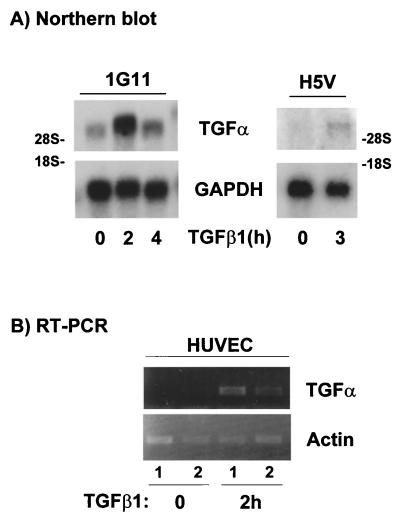
The EGF family is composed of at least six members that can activate ErbB-1 or the EGF receptor (1). Moreover, only TGF-α and EGF have been implicated in the angiogenesis process (58, 68). To identify the factor implicated in the TGF-β1-mediated effect, we performed RT-PCR experiments with mRNAs obtained from 1G11 cells treated for 2 h with TGF-β1 or not treated. A fragment corresponding to TGF-α could be amplified in these cells; in contrast there was no transcript corresponding to EGF (data not shown). We confirmed these results with Northern blot analysis using poly(A)+ RNA from 1G11 cells and the PCR fragment amplified from 1G11 cells as a specific TGF-α probe. As shown in Fig. 7A, we detected a 4.5-kb band corresponding to the TGF-α mRNA. Treatment with TGF-β1 caused a transient increase in the TGF-α transcript, which peaked at 2 h and returned to nearly basal levels after 4 h. This result indicates that TGF-β1 stimulates the synthesis of TGF-α in 1G11 capillary endothelial cells. Next, we investigated whether this effect of TGF-β1 on TGF-α synthesis is also observed in other vascular endothelial cells. We performed a Northern blot assay using poly(A)+ RNA from H5V cells (a mouse heart endothelial cell line transformed by polyoma middle T antigen [27]) treated with TGF-β1 or not treated. As observed in Fig. 7A, treatment with TGF-β1 also caused an increase in TGF-α mRNA in this endothelial cell model. Finally we performed RT-PCR experiments with mRNAs obtained from primary cultures of HUVEC treated for 2 h with TGF-β1 or not treated. As shown in Fig. 7B, stimulation with TGF-β1 for 2 h also increased TGF-α cDNA in HUVEC.
FIG. 7.
TGF-β1 induces TGF-α mRNA expression in endothelial cells. (A) 1G11 and H5V cells stimulated with 10 ng of TGF-β1/ml for the indicated times or not stimulated were lysed, and poly(A)+ mRNA was isolated. Gels were loaded with 2 μg of mRNA, and Northern blotting was performed using a 190-bp PCR-amplified fragment as a TGF-α-specific probe. Rat GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a control. A representative result is shown. (B) HUVEC stimulated with 10 ng of TGF-β1/ml for 2 h or not stimulated were lysed, and poly(A)+ mRNA was isolated. After cDNA was obtained, PCR was performed using specific primers for human TGF-α or actin (as a control). Samples were loaded on a 2% agarose gel. 1 and 2, two independent preparations of HUVEC poly(A)+ for unstimulated and stimulated cells.
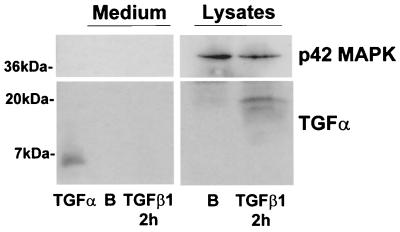
Next, we performed Western blot experiments to detect the TGF-α protein. As is observed in Fig. 8, the antibody used clearly detected commercial human TGF-α as a 6-kDa band in Western blot assays. However, we have never detected the mature and secreted form of TGF-α on conditioned medium from 1G11 cells treated for 2 (Fig. 8) or 4 h (data not shown) with TGF-β1 or not treated. In contrast, in lysates from 1G11 cells we detected a 19-kDa band that probably corresponded to an unprocessed form of the TGF-α protein. This form increased after treatment with TGF-β1 for 2 h (Fig. 8). This result indicates that TGF-β1 stimulates the synthesis of the TGF-α protein in 1G11 capillary endothelial cells; this form is then translocated to the membrane where it probably acts in an autocrine or paracrine (cell-to-cell) manner, stimulating the ErbB-1 receptor.
FIG. 8.
TGF-β1 induces TGF-α protein expression in 1G11 cells. Quiescent 1G11 cells were stimulated for 2 h with 10 ng of TGF-β1/ml or were not stimulated (lane B). After this time, medium was collected and proteins were precipitated and loaded on a 15% gel. As a control, 300 ng of human TGF-α was also loaded. In parallel, cells were lysed and 100 μg was loaded on the gel. TGF-α was immunodetected by using a specific antibody. A Western blot representative of three different experiments is shown.
EGF receptor mediates TGF-β1 stimulation of PI3K and p42/p44 MAPK and cell survival.
We next wanted to determine whether EGF receptor stimulation was responsible for PI3K and p42/p44 MAPK stimulation after treatment with TGF-β1. First, we incubated 1G11 cells in the presence of recombinant TGF-α to examine if this growth factor stimulated the same signaling pathways as TGF-β1. As shown in Fig. 9A, incubation for 1 h in the presence of 10 ng of TGF-α/ml stimulated both Akt and p42/p44 MAPK phosphorylation. Second, prior to treatment with TGF-β1, we preincubated cells with a selective inhibitor of EGF receptor tyrphostin AG1478 (43). This compound specifically inhibited TGF-α and EGF signaling in 1G11 cells, while having no effect on PDGF-BB-, insulin-, or FCS-induced PI3K or p42/p44 MAPK activities (Fig. 9A) or on the normal TGF-β1 signaling, indicated by Smad2 phosphorylation (Fig. 9B). Even more importantly, preincubation with tyrphostin AG1478 precluded the activation of p42/p44 MAPK by TGF-β1 and decreased Akt phosphorylation by 50% (Fig. 9). Moreover, the effect of AG1478 was also observed in three-dimensional collagen gels, where it blocked TGF-β1-stimulated p42/p44 MAPK and Akt activities (data not shown). Third, to sequester the possible TGF-α or other EGF-like members secreted after TGF-β1 stimulation, we used a soluble and extracellularly secreted form of the extracellular portion of the EGF receptor fused in frame to an Fc portion of human immunoglobulin G1, denoted CDM7-IgB-1 (15). This construction was expressed in HEK293 cells, and the conditioned medium of these cells was added to 1G11 cells before TGF-β1 treatment. Thus, when 1G11 cells were pretreated for 1 h in the presence of the medium from control transfected HEK293 cells, an incubation for 4 h with TGF-β1 stimulated Akt and p42/p44 MAPK (Fig. 10). 1G11 cells pretreated with CDM7-IgB-1 supernatants demonstrated a higher basal activation of Akt and p42/p44 MAPK. However TGF-β1 was no longer capable of stimulating these two signaling pathways (Fig. 10). These results reinforce the notion that TGF-β1-stimulated Akt and p42/p44 MAPK signaling in 1G11 cells is mediated mainly through the EGF receptor.
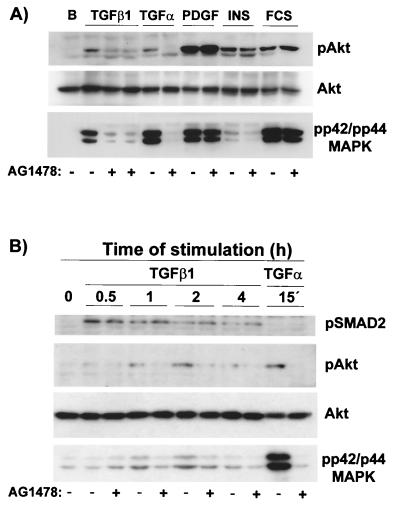
FIG. 9.
Tyrphostin AG1478, a specific inhibitor of the EGF receptor, blocks TGF-β1 stimulation of p42/p44 MAPK and Akt in the absence of changes in TGF-β signaling. (A) Quiescent 1G11 cells were preincubated for 15 min in the presence (+) or the absence (−) of tyrphostin AG1478 (1 μM). After this time, cells were stimulated for 4 h with 10 ng of TGF-β1/ml (in duplicate in the presence of tyrphostin AG1478) or for 1 h with 50 ng of TGF-α/ml, 10 ng of PDGF-BB/ml, 1 μM insulin, or 10% FCS or were left unstimulated (lane B). Cells were lysed, and phospho-Akt, Akt, and phospho-p42/p44 MAPK were immunodetected as described in Materials and Methods. A Western blot representative of four different experiments is shown. (B) Quiescent 1G11 cells were preincubated for 15 min in the presence (+) or the absence (−) of tyrphostin AG1478 (1 μM). After this time, cells were stimulated for the times indicated with 10 ng of TGF-β1/ml or for 15 min with 50 ng of TGF-α/ml. After lysis, phospho-Smad2, phospho-Akt, Akt, and phospho-p42/p44 MAPK were immunodetected as described in Materials and Methods. A representative Western blot is shown.
FIG. 10.
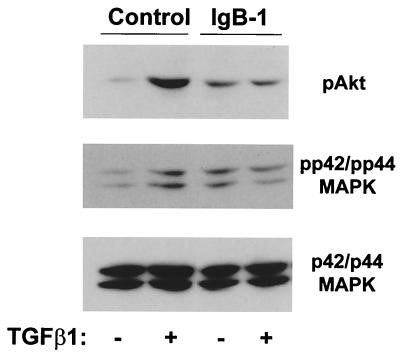
A soluble EGF receptor (IgB-1) blocks TGF-β1 stimulation of Akt and p42/p44 MAPK. Quiescent 1G11 cells were preincubated for 1 h with medium from control or soluble extracellular EGF receptor IgB-1-transfected HEK293 cells. After this period, cells were stimulated with TGF-β1 for 4 h. Cells were then lysed, and phospho-Akt, phospho p42/p44 MAPK, and p42/p44 MAPK were immunodetected as described in Materials and Methods. A representative Western blot from three different experiments is shown.
We then wanted to evaluate whether inhibition of the EGF receptor could affect tubular morphogenesis and cell survival in collagen gels. As shown in Fig. 11, top, preincubation of cells in the presence of tyrphostin AG1478 prior to the addition of TGF-β1 inhibited capillary-like formation in collagen gels. Moreover, preincubation with tyrphostin AG1478 inhibited the protective effect of TGF-β1 on cell survival (Fig. 11, bottom). Finally, it was possible that the in vitro angiogenic effect of TGF-β1 was wholly mediated by TGF-α. To evaluate this possibility, 1G11 cells were incubated in the presence or absence of TGF-α alone. In this condition TGF-α increased cell survival (Fig. 11, bottom). After a 24-h treatment TGF-α did not stimulate tubular network formation; however, long-term treatment (5 days) elicited tube formation although at a lesser level than that elicited by TGF-β1 (Fig. 12). Moreover, when TGF-β1 was added in the presence of TGF-α, we noticed a very marked synergistic effect on cell reorganization at concentrations of TGF-β1 that alone produced a limited effect (compare 2 and 5 ng of TGF-β1/ml in the presence or absence of TGF-α). These results indicate that TGF-α is necessary but not sufficient for TGF-β1-induced cell reorganization in collagen gels. Taken together, all these results suggest that TGF-α secretion induced by TGF-β1 is essential for maintaining cell survival in three-dimensional cell cultures and plays a role in the TGF-β1-induced capillary-like formation in endothelial cells.
DISCUSSION
In this study we demonstrate that, besides its effects on cell reorganization, TGF-β1 promotes endothelial cell survival during in vitro angiogenesis in collagen gels. This protective effect is mediated by the activation of the PI3K and p42/p44 MAPK signaling pathways and is obtained through an autocrine mechanism, which implicates the synthesis and secretion of TGF-α and the activation of the EGF receptor.
We show that TGF-β1 has a clear effect on 1G11 endothelial cell reorganization into capillary-like structures when cells are cultured in collagen gels. However, this effect appears to depend on an indirect antiapoptotic action of TGF-β1. If this prosurvival effect is inhibited by PI3K or p42/p44 MAPK inhibitors, cells do not organize but rather die. The proangiogenic effect of TGF-β1 reflects its role in the later stages of angiogenesis, where endothelial cells pass from a migratory and proliferative state to a more quiescent, mature, and differentiated state (6, 64). This is characterized by downregulation of endothelial cell proliferation, increased basement membrane deposition, and final morphological organization of cells into capillary tubes. These processes have been shown to be induced by TGF-β (45, 53, 61, 80), a key role confirmed by TGF-β1 and TGF-β receptor type II knockout mice. Indeed in both cases these animals die early in embryogenesis due to defects in vasculogenesis. Endothelial cell proliferation in wild-type animals was similar to that in knockout animals; however, a problem in the terminal differentiation and maintenance of the tube integrity was observed (20, 59). Differentiating cells entering growth-arrested G0 need to maintain a good balance of cell survival signals. Thus, it is not surprising that TGF-β1, which alone induces in vitro angiogenesis, can promote endothelial cell survival. Two pathways, PI3K/Akt and p42/p44 MAPK, capable of inducing survival signals have been well documented (10, 25, 41, 47). In endothelial cells these signaling cascades provide a strong antiapoptotic action. VEGF has a clear cell survival effect through the activation of PI3K/Akt and stimulation of Bcl2 (28–30, 52). Moreover, endothelial cell-specific vascular endothelial-cadherin forms a macrocomplex with the type II VEGF receptor and PI3K, and this interaction is essential for VEGF antiapoptotic signaling (13). Phorbol myristate acetate induces tube formation and survival of HUVEC on collagen gels through activation of p42/p44 MAPK and PI3K (38). In this context, it is interesting that TGF-β1 stimulates PI3K and p42/p44 MAPK (this study) and induces cell survival. A similar effect of TGF-β1 was observed in macrophages, which TGF-β1 protects from apoptosis induced by serum deprivation (16).
In the majority of cells, stimulation by TGF-β1 does not activate PI3K or p42/p44 MAPK. In 1G11 endothelial cells we do not detect any rapid activation of p42/p44 MAPK. The same results can be seen in HUVEC (data not shown). In contrast, we observe a clear stimulation of PI3K and p42/p44 MAPK by TGF-β1 in 1G11 cells, but only 2 h after TGF-β1 addition. We show that this activation is mediated by EGF receptor stimulation, probably through an autocrine loop involving TGF-α synthesis and secretion. This type of autocrine system has also been implicated in TGF-β1 induction of connective tissue cell proliferation, mediated by an autocrine PDGF loop (7, 71). This mechanism of PDGF production also exists in mouse embryo AKR-2B cells (42) but is not present in endothelial cells (6). Moreover, TGF-β1 has been shown to induce FGF-2 production in bovine corneal endothelial cells (63). Interestingly, TGF-β1 induces VEGF in different cellular types but not in HUVEC (62). Moreover, it causes the downregulation of VEGF receptor flk-1 (46). All these results reflect the role of TGF-β1 in the later stages of angiogenesis, where endothelial cells pass from a proliferative state (stimulated by VEGF) to a more quiescent and differentiated state (less sensitive to VEGF). In our study, TGF-α seems to be the major factor responsible for TGF-β1 stimulation of p42/p44 MAPK and PI3K, leading to endothelial cell survival. However, we cannot discard the synthesis of other autocrine factors that could also contribute to part of the response.
We have shown that TGF-β1 stimulates TGF-α mRNA and protein synthesis. We have only detected synthesis of a high-molecular-mass form of the TGF-α protein in 1G11 cell lysates. TGF-α is derived from a larger 20- to 22-kDa transmembrane precursor that, after shedding, generates the soluble and mature 6-kDa form (18). Higher-molecular-mass forms of biologically active TGF-α are not unusual and have been previously reported (4). Moreover, transmembrane TGF-α can activate the EGF receptor but only at neighboring cells (11, 79). We have never detected TGF-α processing and release to the medium in our experimental conditions. Moreover, Akt and p42/p44 MAPK stimulation by TGF-β1 increases with cellular confluence (data not shown). All these results indicate that probably TGF-α arrives at the cellular membrane as a high-molecular-weight precursor and there exerts its effects in an autocrine or paracrine (from a cell to its neighboring cell) manner. Further experiments are required to validate or disprove this hypothesis. TGF-α is an important angiogenic factor, more effective than EGF, and is highly expressed in neovascularized tumors (68). Thus, it is possible that the effect of TGF-α production by TGF-β1 is not restricted to the prosurvival effect and also may contribute to tube formation. In fact, different studies support a role for p42/p44 MAPK and PI3K in the angiogenic process. Sustained integrin-induced p42/p44 MAPK activity is required for FGF-2-induced angiogenesis in the chick chorioallantoic membrane (22). Virally activated Ras cooperates with integrins to induce tubulogenesis in sinusoidal endothelial cells, an effect that is blocked by PD-98059 (48). Moreover, B-Raf and MEK-1 knockout mice die during embryonic development due to defects in vascular endothelial cell differentiation and survival (B-Raf knockout) (78) or in placental angiogenesis (MEK-1 knockout) (31). The block of PI3K by wortmannin results in partial inhibition of tumor-induced angiogenesis (2). However, TGF-α-stimulated signals, such as p42/p44 MAPK and PI3K, seem to be necessary but not sufficient for tube formation in collagen gels. TGF-α alone is only partially able to reorganize endothelial cells and form tubes, and only in the long term. This demonstrates that TGF-β1 unchains other signals that are important for the reorganization of cells on collagen gels. This could be the secretion of compounds of the extracellular matrix (such as fibronectin, collagens, etc.), the stimulation of integrins (8, 17), or the release of metalloproteinases (54, 70). Future studies will help us to identify these signals more precisely and understand the mechanisms of TGF-β1-induced angiogenesis.
ACKNOWLEDGMENTS
We thank A. Vecchi for 1G11 cells, J. Madri (Yale University) for protocols for protein analysis in collagen gels; Y. Yarden (Weizmann Institute, Israel) for CDM7-IgB-1 construction; S. Pagnotta, J. P. Laugier, and G. Nicaise (Centre de Microscopie Appliquée, Université de Nice-Sophia Antipolis) for microscopic studies; and P. Huber for H5V cells. We particularly thank D. E. Richard and E. Berra for editorial help and L. Sevilla, R. Buscà, and F. Ventura and all laboratory members for discussions and technical support.
This work was supported by research grants from CNRS, University of Nice-Sophia Antipolis, INSERM, Association pour la Recherche contre le Cancer, Ligue Nationale contre le Cancer, and the European Community (EC contract B104-CT97-2071). F.V. is the recipient of a Marie Curie Research Training Grant (EC contract FMBI-CT97-2706).
REFERENCES
- 1.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 2.Arbiser J L, Moses M A, Fernandez C A, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers H R, Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird A, Durkin T. Inhibition of endothelial cell proliferation by type beta-transforming growth factor: interactions with acidic and basic fibroblast growth factors. Biochem Biophys Res Commun. 1986;138:476–482. doi: 10.1016/0006-291x(86)90305-0. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Banerjee P P, Zirkin B R, Brown T R. Regional expression of transforming growth factor-alpha in rat ventral prostate during postnatal development, after androgen ablation, and after androgen replacement. Endocrinology. 1998;139:3005–3013. doi: 10.1210/endo.139.6.6060. [DOI] [PubMed] [Google Scholar]
- 5.Barbieri B, Balconi G, Dejana E, Donati M B. Evidence that vascular endothelial cells can induce the retraction of fibrin clots. Proc Soc Exp Biol Med. 1981;168:204–207. doi: 10.3181/00379727-168-41260. [DOI] [PubMed] [Google Scholar]
- 6.Battegay E J. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med. 1995;73:333–346. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- 7.Battegay E J, Raines E W, Seifert R A, Bowen-Pope D F, Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- 8.Bazzoni G, Dejana E, Lampugnani M G. Endothelial adhesion molecules in the development of the vascular tree: the garden of forking paths. Curr Opin Cell Biol. 1999;11:573–581. doi: 10.1016/s0955-0674(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 9.Beck L, Jr, D'Amore P A. Vascular development: cellular and molecular regulation. FASEB J. 1997;11:365–373. [PubMed] [Google Scholar]
- 10.Bonni A, Brunet A, West A E, Datta S R, Takasu M A, Greenberg M E. Cell survival promoted by the ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 11.Brachmann R, Lindquist P B, Nagashima M, Kohr W, Lipari T, Napier M, Derynck R. Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell. 1989;56:691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 12.Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22:251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Lampugnani M G, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter M C, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert J M, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 14.Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk M M, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, Leitner O, Ratzkin B J, Bacus S S, Yarden Y. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–7629. [PubMed] [Google Scholar]
- 16.Chin B Y, Petrache I, Choi A M, Choi M E. Transforming growth factor beta1 rescues serum deprivation-induced apoptosis via the mitogen-activated protein kinase (MAPK) pathway in macrophages. J Biol Chem. 1999;274:11362–11368. doi: 10.1074/jbc.274.16.11362. [DOI] [PubMed] [Google Scholar]
- 17.Collo G, Pepper M S. Endothelial cell integrin alpha5beta1 expression is modulated by cytokines and during migration in vitro. J Cell Sci. 1999;112:569–578. doi: 10.1242/jcs.112.4.569. [DOI] [PubMed] [Google Scholar]
- 18.Derynck R. The physiology of transforming growth factor-alpha. Adv Cancer Res. 1992;58:27–52. doi: 10.1016/s0065-230x(08)60289-4. [DOI] [PubMed] [Google Scholar]
- 19.Derynck R, Zhang Y, Feng X H. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 20.Dickson M C, Martin J S, Cousins F M, Kulkarni A B, Karlsson S, Akhurst R J. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 21.Dong Q G, Bernasconi S, Lostaglio S, De C R, Martin P I, Breviario F, Garlanda C, Ramponi S, Mantovani A, Vecchi A. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol. 1997;17:1599–1604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- 22.Eliceiri B P, Klemke R, Stromblad S, Cheresh D A. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folkman J, Klagsburn M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 24.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 25.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 26.Frater-Schroder M, Muller G, Birchmeier W, Bohlen P. Transforming growth factor-beta inhibits endothelial cell proliferation. Biochem Biophys Res Commun. 1986;137:295–302. doi: 10.1016/0006-291x(86)91209-x. [DOI] [PubMed] [Google Scholar]
- 27.Garlanda C, Parravicini C, Sironi M, De Rossi M, Wainstok de Calmanovici R, Corozzi F, Bussolino F, Colotta F, Mantovani A, Vecchi A. Progressive growth in immunodeficient mice and host cell recruitment by mouse epithelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc Natl Acad Sci USA. 1994;91:7291–7295. doi: 10.1073/pnas.91.15.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerber H P, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 29.Gerber H P, Hillan K J, Ryan A M, Kowalski J, Keller G A, Rangell L, Wright B D, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 30.Gerber H P, McMurtrey A, Kowalski J, Yan M, Keyt B A, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 31.Giroux S, Tremblay M, Bernard D, Cardin-Girard J F, Aubry S, Larouche L, Rousseau S, Huot J, Landry J, Jeannotte L, Charron J. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr Biol. 1999;9:369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- 32.Gualandris A, Annes J P, Arese M, Noguera I, Jurukowski V, Rifkin D B. The latent transforming growth factor-β-binding protein-1 promotes in vitro differentiation of embryonic stem cells into endothelium. Mol Biol Cell. 2000;11:4295–4308. doi: 10.1091/mbc.11.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 34.Heimark R L, Twardzik D R, Schwartz S M. Inhibition of endothelial regeneration by type-beta transforming growth factor from platelets. Science. 1986;233:1078–1080. doi: 10.1126/science.3461562. [DOI] [PubMed] [Google Scholar]
- 35.Heine U, Munoz E F, Flanders K C, Ellingsworth L R, Lam H Y, Thompson N L, Roberts A B, Sporn M B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987;105:2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heldin C H, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 37.Howe A, Aplin A E, Alahari S K, Juliano R L. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 38.Ilan N, Mahooti S, Madri J A. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J Cell Sci. 1998;111:3621–3631. doi: 10.1242/jcs.111.24.3621. [DOI] [PubMed] [Google Scholar]
- 39.Johnson D W, Berg J N, Baldwin M A, Gallione C J, Marondel I, Yoon S J, Stenzel T T, Speer M, Pericak-Vance M A, Diamond A, Guttmacher A E, Jackson C E, Attisano L, Kucherlapati R, Porteous M E M, Marchuk D A. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 40.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 41.Le Gall M, Chambard J C, Breittmayer J P, Grall D, Pouyssegur J, Van Obberghen-Schilling E. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum. Mol Biol Cell. 2000;11:1103–1112. doi: 10.1091/mbc.11.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leof E B, Proper J A, Goustin A S, Shipley G D, DiCorleto P E, Moses H L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci USA. 1986;83:2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 44.Li D Y, Sorensen L K, Brooke B S, Urness L D, Davis E C, Taylor D G, Boak B B, Wendel D P. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 45.Madri J A, Pratt B M, Tucker A M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988;106:1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandriota S J, Menoud P A, Pepper M S. Transforming growth factor beta 1 down-regulates vascular endothelial growth factor receptor 2/flk-1 expression in vascular endothelial cells. J Biol Chem. 1996;271:11500–11505. doi: 10.1074/jbc.271.19.11500. [DOI] [PubMed] [Google Scholar]
- 47.Marte B M, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 48.Maru Y, Yamaguchi S, Takahashi T, Ueno H, Shibuya M. Virally activated Ras cooperates with integrin to induce tubulogenesis in sinusoidal endothelial cell lines. J Cell Physiol. 1998;176:223–234. doi: 10.1002/(SICI)1097-4652(199808)176:2<223::AID-JCP1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 49.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 50.McAllister K A, Grogg K M, Johnson D W, Gallione C J, Baldwin M A, Jackson C E, Helmbold E A, Markel D S, McKinnon W C, Murrell J, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 51.McKenzie F R, Pouyssegur J. cAMP-mediated growth inhibition in fibroblasts is not mediated via mitogen-activated protein (MAP) kinase (ERK) inhibition. cAMP-dependent protein kinase induces a temporal shift in growth factor-stimulated MAP kinases. J Biol Chem. 1996;271:13476–13483. doi: 10.1074/jbc.271.23.13476. [DOI] [PubMed] [Google Scholar]
- 52.Meeson A P, Argilla M, Ko K, Witte L, Lang R A. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development. 1999;126:1407–1415. doi: 10.1242/dev.126.7.1407. [DOI] [PubMed] [Google Scholar]
- 53.Merwin J R, Anderson J M, Kocher O, Van Itallie C M, Madri J A. Transforming growth factor beta 1 modulates extracellular matrix organization and cell-cell junctional complex formation during in vitro angiogenesis. J Cell Physiol. 1990;142:117–128. doi: 10.1002/jcp.1041420115. [DOI] [PubMed] [Google Scholar]
- 54.Miralles F, Battelino T, Czernichow P, Scharfmann R. TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol. 1998;143:827–836. doi: 10.1083/jcb.143.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983;97:1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller G, Behrens J, Nussbaumer U, Bohlen P, Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci USA. 1987;84:5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh S P, Seki T, Goss K A, Imamura T, Yi Y, Donahoe P K, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okamura K, Morimoto A, Hamanaka R, Ono M, Kohno K, Uchida Y, Kuwano M. A model system for tumor angiogenesis: involvement of transforming growth factor-alpha in tube formation of human microvascular endothelial cells induced by esophageal cancer cells. Biochem Biophys Res Commun. 1992;186:1471–1479. doi: 10.1016/s0006-291x(05)81572-4. [DOI] [PubMed] [Google Scholar]
- 59.Oshima M, Oshima H, Taketo M M. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 60.Pelton R W, Nomura S, Moses H L, Hogan B L. Expression of transforming growth factor beta 2 RNA during murine embryogenesis. Development. 1989;106:759–767. doi: 10.1242/dev.106.4.759. [DOI] [PubMed] [Google Scholar]
- 61.Pepper M S, Belin D, Montesano R, Orci L, Vassalli J D. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990;111:743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–6274. [PubMed] [Google Scholar]
- 63.Plouet J, Gospodarowicz D. Transforming growth factor beta-1 positively modulates the bioactivity of fibroblast growth factor on corneal endothelial cells. J Cell Physiol. 1989;141:392–399. doi: 10.1002/jcp.1041410221. [DOI] [PubMed] [Google Scholar]
- 64.Risau W. Differentiation of endothelium. FASEB J. 1995;9:926–933. [PubMed] [Google Scholar]
- 65.Roberts A B, Sporn M B, Assoian R K, Smith J M, Roche N S, Wakefield L M, Heine U I, Liotta L A, Falanga V, Kehrl J H, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sankar S, Mahooti B N, Bensen L, McCarthy T L, Centrella M, Madri J A. Modulation of transforming growth factor beta receptor levels on microvascular endothelial cells during in vitro angiogenesis. J Clin Investig. 1996;97:1436–1446. doi: 10.1172/JCI118565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schor A M, Schor S L, Allen T D. Effects of culture conditions on the proliferation, morphology and migration of bovine aortic endothelial cells. J Cell Sci. 1983;62:267–285. doi: 10.1242/jcs.62.1.267. [DOI] [PubMed] [Google Scholar]
- 68.Schreiber A B, Winkler M E, Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986;232:1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz M A. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sehgal I, Thompson T C. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and -2) activities by transforming growth factor-beta1 in human prostate cancer cell lines. Mol Biol Cell. 1999;10:407–416. doi: 10.1091/mbc.10.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soma Y, Grotendorst G R. TGF-beta stimulates primary human skin fibroblast DNA synthesis via an autocrine production of PDGF-related peptides. J Cell Physiol. 1989;140:246–253. doi: 10.1002/jcp.1041400209. [DOI] [PubMed] [Google Scholar]
- 72.ten Dijke I, Miyazono I, Heldin I. Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- 73.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois R N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. . (Erratum, 94:271.) [DOI] [PubMed] [Google Scholar]
- 74.Urness L D, Sorensen L K, Li D Y. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26:328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 75.Vandenbunder B, Pardanaud L, Jaffredo T, Mirabel M A, Stehelin D. Complementary patterns of expression of c-ets 1, c-myb and c-myc in the blood-forming system of the chick embryo. Development. 1989;107:265–274. doi: 10.1242/dev.107.2.265. [DOI] [PubMed] [Google Scholar]
- 76.Viñals F, Chambard J C, Pouyssegur J. p70 S6 kinase-mediated protein synthesis is a critical step for vascular endothelial cell proliferation. J Biol Chem. 1999;274:26776–26782. doi: 10.1074/jbc.274.38.26776. [DOI] [PubMed] [Google Scholar]
- 77.Viñals F, Ferré J, Fandos C, Santalucia T, Testar X, Palacin M, Zorzano A. Cyclin adenosine 3′,5′-monophosphate regulates GLUT4 and GLUT1 glucose transporter expression and stimulates transcriptional activity of the GLUT1 promoter in muscle cells. Endocrinology. 1997;138:2521–2529. doi: 10.1210/endo.138.6.5217. [DOI] [PubMed] [Google Scholar]
- 78.Wojnowski L, Zimmer A M, Beck T W, Hahn H, Bernal R, Rapp U R, Zimmer A. Endothelial apoptosis in Braf-deficient mice. Nat Genet. 1997;16:293–297. doi: 10.1038/ng0797-293. [DOI] [PubMed] [Google Scholar]
- 79.Wong S T, Winchell L F, McCune B K, Earp H S, Teixido J, Massague J, Herman B, Lee D C. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989;56:495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]
- 80.Yang E Y, Moses H L. Transforming growth factor beta 1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol. 1990;111:731–741. doi: 10.1083/jcb.111.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X, Castilla L H, Xu X, Li C, Gotay J, Weinstein M, Liu P P, Deng C X. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–1580. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]