Abstract
Background
Studies conducted during the COVID-19 pandemic have shown that crowding in nursing homes is associated with high incidence of SARS-CoV-2 infections, but this effect has not been shown for other respiratory pathogens. We aimed to measure the association between crowding in nursing homes and outbreak-associated respiratory infection incidence and related mortality before the COVID-19 pandemic.
Methods
We conducted a retrospective cohort study of nursing homes in Ontario, Canada. We identified, characterised, and selected nursing homes through the Ontario Ministry of Long-Term Care datasets. Nursing homes that were not funded by the Ontario Ministry of Long-Term Care and homes that closed before January, 2020 were excluded. Outcomes consisting of respiratory infection outbreaks were obtained from the Integrated Public Health Information System of Ontario. The crowding index equalled the mean number of residents per bedroom and bathroom. The primary outcomes were the incidence of outbreak-associated infections and mortality per 100 nursing home residents per year. We examined the incidence of infections and deaths as a function of the crowding index by use of negative binomial regression with adjustment for three home characteristics (ie, ownership, number of beds, and region) and nine mean resident characteristics (ie, age, female sex, dementia, diabetes, chronic heart failure, renal failure, cancer, chronic obstructive pulmonary disease, and activities of daily living score).
Findings
Between Sept 1, 2014, and Aug 31, 2019, 5107 respiratory infection outbreaks in 588 nursing homes were recorded, of which 4921 (96·4%), involving 64 829 cases of respiratory infection and 1969 deaths, were included in this analysis. Nursing homes with a high crowding index had higher incidences of respiratory infection (26·4% vs 13·8%; adjusted rate ratio per one resident per room increase in crowding 1·89 [95% CI 1·64–2·17]) and mortality (0·8% vs 0·4%; 2·34 [1·88–2·92]) than did homes with a low crowding index.
Interpretation
Respiratory infection and mortality rates were higher in nursing homes with high crowding index than in homes with low crowding index, and the association was consistent across various respiratory pathogens. Decreasing crowding is an important safety target beyond the COVID-19 pandemic to help to promote resident wellbeing and decrease the transmission of prevalent respiratory pathogens.
Funding
None.
Introduction
The COVID-19 pandemic has disproportionately affected residents of nursing homes in various countries, such as Canada and the UK.1, 2 Even before the COVID-19 pandemic, nursing homes were frequently and severely affected by outbreaks of respiratory infections, particularly influenza.3, 4 The congregate nature of nursing homes means that residents are often exposed to infection and are more likely than the general population to have severe negative outcomes of infection, due to their advanced age, comorbidities, and high degree of frailty.5 Several design features of nursing homes could aggravate or mitigate the potential for transmission in a home; one such important feature is the degree of crowding. Crowding has previously been defined in terms of occupants per square foot,6 proportion of occupants in single-bed rooms, ratio of occupancy to design capacity,7 and mean number of occupants per room.8 For nursing homes, one simple measure of the degree of crowding is the mean number of residents per occupied bedroom and bathroom in a home.9 Increased crowding is positively associated with SARS-CoV-2 infection and mortality rates in nursing homes.9, 10 Substantial evidence suggests that crowding is associated with transmission of various infections, particularly tuberculosis, in private dwellings and in institutional settings.6, 8 Evidence on whether crowding is also a risk factor for non-SARS-CoV-2 acute respiratory infections could influence the extension of crowding restrictions beyond the pandemic period and the design standards for the construction of new nursing homes.11 With this study, we aimed to examine the association between crowding and incidence and mortality of outbreak-associated respiratory infections in nursing homes in Ontario before the COVID-19 pandemic. We hypothesised that highly crowded nursing homes would have a greater incidence of outbreak-associated respiratory infections and deaths than would nursing homes with less crowding.
Research in context.
Evidence before this study
Viral respiratory infections are an important cause of morbidity and mortality among older people, particularly residents of nursing homes. Crowded living conditions are associated with SARS-CoV-2 incidence, specifically among residents of nursing homes. We searched PubMed and Google Scholar for original research articles published in any language from inception to Aug 15, 2022, examining the association between crowding and respiratory infection incidence or mortality among nursing home residents, using the following terms: [(“crowding” OR “overcrowding”) AND (“respiratory infection” OR “COVID” OR “SARS-CoV-2” OR “influenza” OR “respiratory syncytial virus” OR “metapneumovirus” OR “parainfluenza” OR “rhinovirus” OR “coronavirus”) AND (“long-term care” OR “care home” OR “nursing home”)].
Added value of this study
To our knowledge, this is the first study measuring the association between crowded living conditions in nursing homes and increased incidence of respiratory infection and mortality for non-SARS-CoV-2 viral respiratory pathogens, including influenza A, influenza B, non-SARS-CoV-2 coronavirus, respiratory syncytial virus, human metapneumovirus, human parainfluenza, and rhinovirus or enterovirus.
Implications of all the available evidence
Taken together, evidence suggests that residents of crowded nursing homes with a high proportion of shared bedrooms and bathrooms are at a higher risk of a wide range of viral respiratory infections and associated mortality, including but not limited to SARS-CoV-2, than are nursing homes with a low proportion of shared bedrooms and bathrooms. Crowding in nursing homes is an important public health and patient safety target and should be monitored and reduced.
Methods
Study design and participants
We conducted a retrospective cohort study of nursing homes in Ontario. All nursing homes administered by the Ontario Ministry of Long-Term Care were included, except those homes that closed before January, 2020. The data included in this study were home-level respiratory infection outbreak data, nursing home characteristics, and aggregate resident characteristics, routinely collected and used in accordance with Ontario's Personal Health Information Protection Act. Data were anonymised before being shared with the project team. Individual consent was not required for the secondary use of non-identifiable information (Canadian Tri-Council Policy Statement version 2: 5.5B). This study received ethics approval from Public Health Ontario's Research Ethics Board.
Procedures
Data on the distribution of bed types in nursing homes were obtained from the Ontario Ministry of Long-Term Care, Inspections Branch, extracted on Nov 17, 2020. The exposure of interest was the nursing home crowding index, which we defined as the mean number of residents per bedroom and bathroom in each home (ie, residents / [0·5 × bedrooms + 0·5 × bathrooms]).9 Only bathrooms intended exclusively for resident use and located in sleeping quarters were included in the calculation. A home composed exclusively of single-bed rooms with private bathrooms would have a crowding index of 1, whereas a home composed exclusively of four-bed rooms with one bathroom per room would have a crowding index of 4. If half of residents resided in single-bed rooms with private bathrooms, and the other half resided in four-bed rooms each with its own shared bathroom, then the crowding index would be 2·5. Because we did not have direct measurements of crowding in nursing homes in Ontario, we obtained information from the Ontario Ministry of Long-Term Care on the distribution of new beds and type A beds (ie, meeting the 1999 design standard)12 versus type B, C, or D beds (not meeting the 1999 design standard), and bed class according to the 1999 design standards: private beds (ie, in one-bed rooms with a private bathroom; crowding weight=1); semi-private beds (ie, in one-bed rooms with a shared bathroom; crowding weight=1·5); and basic beds (ie, in two-bed rooms with one bathroom; crowding weight=2). For older homes not meeting the 1999 design standard, private rooms are one-bed rooms that can have shared bathrooms (crowding weight=1·5) and semi-private rooms are two-bed rooms (crowding weight=2). The number of residents per basic-bed room in older homes varied substantially (ie, one room could contain up to five beds). We requested survey data from 2022 from the Ontario Ministry of Long-Term Care to establish the mean number of residents per basic-bed room in older homes. The obtained figures of 2·04 residents per room for municipal homes, 2·50 residents per room for non-profit homes, and 3·20 residents per room in for-profit homes were used as weights applied to the proportion of basic beds in old homes.
Data for respiratory infection outbreaks were retrieved from the integrated Public Health Information System of Ontario held by Public Health Ontario and were extracted by MW on June 18, 2021. Under the Ontario Health Protection and Promotion Act, respiratory infection outbreaks in nursing homes must be reported provincially. Respiratory infection outbreaks are declared when two cases of acute respiratory infection (ie, new or worsening cough or shortness of breath) with an epidemiological link (eg, on the same unit or floor) have onset within 48 h in a nursing home, including one laboratory-confirmed case, or three cases regardless of laboratory confirmation. Up to four early specimens per outbreak are tested by real-time RT-PCR for a panel of respiratory pathogens at the Public Health Ontario laboratory; most outbreaks have at least two laboratory-confirmed infections.13
Data for the distribution of nursing home characteristics were obtained from the Ministry of Long-Term Care, Inspections Branch, extracted on Nov 17, 2020. We included data for ownership (ie, private for-profit entity, private non-profit, or owned by a municipality), number of beds, and health region (ie, east, central-east, Toronto, central-west, south-west, and north) of nursing homes.
Aggregate characteristics of residents in nursing homes were obtained from the Resident Assessment Instrument Minimum Data, extracted for January, 2020, on Nov 17, 2020.14 The included variables were mean resident age, proportion of women, prevalence of each of six comorbidities (ie, dementia, diabetes, chronic heart failure, renal failure, cancer, and chronic obstructive pulmonary disease), and mean activities of daily living score (in which 0 indicates independence and 6 indicates total dependence).
Outcomes
Our primary outcomes were the incidence rate of outbreak-associated respiratory infections per 100 nursing home residents per year, recorded as part of a respiratory infection outbreak in a nursing home, and the incidence rate of outbreak-associated deaths per 100 nursing home residents per year, including only deaths as a result of the infection (as decided by the outbreak investigator or the most responsible physician). Secondary outcomes included outbreak frequency per year and outbreak size as a proportion of the number of residents in the home. We also examined incidence of infections and deaths for pathogens, classified into ten categories: coronavirus (strains OC43, 229E, NL63, and HKU1); influenza A; influenza B; human metapneumovirus; human parainfluenza virus; respiratory syncytial virus; rhinovirus or enterovirus; other (adenovirus); more than one agent; and unidentified. For descriptive purposes, we also examined case fatality (ie, the proportion of infections resulting in death).
Statistical analysis
We measured descriptive statistics (ie, median, IQR, and proportions) for homes with a high crowding index (ie, ≥2) and low crowding index (ie, <2) for each nursing home characteristic.
We used negative binomial regression with an offset consisting of the logarithm of the number of beds in the home to model the incidence rate of outbreak-associated respiratory infections and deaths occurring within a home and outbreak size, and we used negative binomial regression without an offset to model outbreak frequency. Negative binomial models were used for all outcomes because preliminary analyses suggested overdispersion relative to the Poisson distribution. All unadjusted models included the continuous crowding index as the only covariate. All adjusted models included the continuous crowding index, in addition to home characteristics (ie, ownership, number of beds, and region) and aggregate resident characteristics (ie, age, female sex, dementia, diabetes, chronic heart failure, renal failure, cancer, chronic obstructive pulmonary disease, and activities of daily living score). Number of beds in nursing homes was included as a 3-df-restricted cubic spline with knots at the 33rd and 66th percentiles. The continuous crowding index effect reflected estimated associations per one resident per room increase in the crowding index.
Statistical analysis was conducted using R version 4.1.0; negative binomial models were fit with the gam and glmer.nb functions in the mgcv and lme4 packages, and random-effects meta-analysis was done with the metafor package. Post-hoc model validation steps included a comparison of the negative binomial modelling approach with quasipoisson; a comparison of the R mgcv and R MASS packages for fitting negative binomial regression; a comparison of binary (ie, <100 vs ≥100), quintile, linear, and spline-based adjustments for number of beds in nursing homes; and a comparison of binary, linear, and spline-based modelling of the crowding effect.
We ran separate models for incidence of infections and deaths for each of ten pathogen groups. For each outcome, we recombined pathogen-specific models using random-effects meta-analysis and reported the combined meta-analytic estimate and the degree of heterogeneity between the pathogen specific estimates, using the Higgins (I 2) statistic.15 Additionally, we conducted a sensitivity analysis restricted to the eight single identified pathogens (ie, excluding outbreaks due to unidentified pathogens and outbreaks due to more than one confirmed pathogen, which could have been subject to outcome misclassification).
We used marginal standardisation to simulate the annual incidence of respiratory infections and deaths for the same population of nursing homes over the same time period, but wherein the crowding index was reduced among the more crowded homes.16 We used the fitted models for each outcome to generate predictions for the estimated incidence of infections and deaths in scenarios where the population was identical but the crowding index of nursing homes was reduced. We produced estimates for two scenarios, where all rooms with more than two beds were replaced with two-bed rooms, and where rooms with two or more beds were replaced with one-bed rooms (more than 95% of nursing home residents in Ontario are housed in one-bed, two-bed, or four-bed rooms); 95% CIs were based on the 2·5th and 97·5th percentiles of 10 000 parametric bootstrap samples.17
Role of the funding source
There was no funding source for this study.
Results
Between Sept 1, 2014, and Aug 31, 2019, 5107 respiratory infection outbreaks across 588 nursing homes in Ontario were recorded. 186 outbreaks were excluded because they corresponded to homes that were not included in the study, since they either closed or were not administered by the province, and 4921 (96·4%) were included in the study. In these outbreaks, 64 829 cases of acute respiratory infection across 376 586 resident-years of follow-up (17·2 cases per 100 resident-years) and 1969 deaths occurred (table 1 ), for a case fatality of 3·04% (1969 of 64 829).
Table 1.
Characteristics and outcomes among nursing homes with a low and high crowding index in Ontario, Canada
| All homes (n=588) | Homes with low (<2·0) crowding index (n=337) | Homes with high (≥2·0) crowding index (n=251) | ||
|---|---|---|---|---|
| Facility characteristics | ||||
| Crowding index | 1·9 (1·4–2·5) | 1·5 (1·4–1·6) | 2·5 (2·4–2·6) | |
| Ownership | ||||
| Municipal | 96 (16·3%) | 95 (28·2%) | 1 (0·4%) | |
| Private, for-profit | 343 (58·3%) | 123 (36·5%) | 220 (87·6%) | |
| Private, non-profit | 149 (25·3%) | 119 (35·3%) | 30 (12·0%) | |
| Number of beds | 130 (75–160) | 149 (101–175) | 105 (60–126) | |
| Occupancy rate, % | 98·2 (97·5–100) | 98·5 (97·9–99·6) | 97·8 (96·7–100·0) | |
| Resident characteristics | ||||
| Mean age, years | 83·5 (82·2–85·1) | 84·5 (83·4–85·7) | 82·1 (80·6–84·0) | |
| Women, % | 68·6% (64·2–73·3) | 70·2% (66·7–74·0) | 66·4% (60·7–72·6) | |
| Clinical diagnosis of dementia, % | 60·8% (54·0–67·9) | 62·7% (55·6–69·0) | 58·4% (50·0–65·2) | |
| Clinical diagnosis of diabetes, % | 28·0% (23·5–31·9) | 26·3% (22·8–29·8) | 30·2% (25·6–34·8) | |
| Clinical diagnosis of chronic heart failure, % | 3·6% (1·9–4·8) | 3·4% (1·7–4·6) | 3·9% (2·1–5·1) | |
| Clinical diagnosis of renal failure, % | 3·3% (1·4–4·3) | 3·1% (1·3–4·2) | 3·5% (1·7–4·6) | |
| Clinical diagnosis of cancer, % | 2·9% (1·2–4·0) | 2·9% (1·1–3·9) | 3·0% (1·3–4·1) | |
| Clinical diagnosis of chronic obstructive pulmonary disease, % | 4·5% (2·3–5·8) | 3·9% (2·1–5·2) | 5·3% (3·1–6·8) | |
| Mean activities of daily living score | 3·9 (3·6–4·1) | 3·9 (3·6–4·2) | 3·8 (3·6–4·1) | |
| Outcomes | ||||
| Infections, n per 100 resident-years | 19·2 (8·8–25·9) | 13·8 (7·2–18·6) | 26·4 (13·4–34·9) | |
| Deaths, n per 100 resident-years | 0·6 (0·1–0·8) | 0·4 (0·1–0·6) | 0·8 (0·3–1·1) | |
| Outbreak frequency, n per home-year | 1·7 (0·8–2·4) | 1·7 (0·8–2·2) | 1·7 (0·8–2·4) | |
| Mean outbreak size, % of home residents | 13·1% (7·3–17·0) | 9·8% (5·9–11·6) | 17·6% (11·2–21·2) | |
Data are n (%) or median (IQR). For resident characteristics and outcomes, we first measured the facility means (for continuous variables) or percentages (for binary outcomes) for all 588 facilities, and then reported the median and IQR of these facility-level variables. *Score of 0 (independence) to 6 (total dependence).
Influenza A was the most common pathogen identified among outbreak-associated cases and had the highest incidence rate, followed by rhinovirus or enterovirus and influenza B (table 2 ). Among single identified pathogens, influenza A caused the most outbreak-associated deaths, followed by influenza B and respiratory syncytial virus.
Table 2.
Viral agents identified for outbreak-associated viral respiratory infections in Ontario, Canada, 2014–19
| Resident infections, n (n per 100 resident-years) | Resident deaths, n (n per 100 resident-years) | Case fatality, % | Outbreak frequency, n (n per home per year) | Mean outbreak size, n (mean % of home residents) | ||
|---|---|---|---|---|---|---|
| Overall | 64 829 (17·2) | 1969 (0·52) | 3·04% | 4921 (1·7) | 13·2 (11·5%) | |
| Single pathogens | ||||||
| Coronavirus* | 2385 (0·6) | 25 (0·01) | 1·05% | 197 (0·1) | 12·1 (10·8%) | |
| Human metapneumovirus | 1969 (0·5) | 59 (0·02) | 3·00% | 162 (0·1) | 12·2 (12·1%) | |
| Human parainfluenza virus† | 2829 (0·8) | 80 (0·02) | 2·83% | 232 (0·1) | 12·2 (10·8%) | |
| Influenza A | 22 159 (5·9) | 937 (0·25) | 4·23% | 1414 (0·5) | 15·7 (12·8%) | |
| Influenza B | 5289 (1·4) | 233 (0·06) | 4·41% | 391 (0·1) | 13·5 (11·8%) | |
| Respiratory syncytial virus | 3954 (1·0) | 116 (0·03) | 2·93% | 309 (0·1) | 12·8 (11·7%) | |
| Rhinovirus or enterovirus | 7316 (1·9) | 108 (0·03) | 1·48% | 591 (0·2) | 12·4 (10·9%) | |
| Other | 772 (0·2) | 36 (0·01) | 4·66% | 57 (0·0) | 13·5 (13·6%) | |
| Multiple pathogens | 4501 (1·2) | 162 (0·04) | 3·60% | 280 (0·1) | 16·1 (13·6%) | |
| Unidentified | 13 655 (3·6) | 213 (0·06) | 1·56% | 1288 (0·4) | 10·6 (9·8%) | |
Coronavirus strains OC43, 229E, NL63, and HKU1 were tested.
Human parainfluenza strains 1–4 were tested.
251 (42·7%) of the 588 nursing homes included in this study were classified as crowded (ie, crowding index ≥2; table 1). Homes with a high crowding index were more often private for-profit homes and tended to have fewer beds than homes with a low crowding index. Compared with homes with a low crowding index, homes with a high crowding index tended to have younger residents, fewer women, and fewer residents with clinically diagnosed dementia, but more residents with diabetes. Race and ethnicity were not recorded in the data sources used for this study.
In previous work,9 we originally used weights of 4 for all basic beds in old homes; our new calibrated approach was strongly correlated with the original approach (Pearson's test r=0·96) but yielded lower overall estimates of crowding (median calibrated crowding index=1·7 [IQR 1·4–2·5]; median original crowding index=1·9 [1·4–2·9]) and of the overall number of beds in shared rooms with three or more beds (8402 beds vs 15 812 beds) than our original approach.
Compared with nursing homes with a low crowding index, homes with a high crowding index had higher numbers of outbreak-associated infections and deaths per 100 residents per year and a higher mean outbreak size; the frequency of outbreaks was similar between the two groups (table 1). Adjusted models (figure 1 , table 3 ) suggested that nursing homes with higher crowding index had a higher incidence of outbreak-associated infections and deaths, outbreak frequency, and outbreak size. Results of the post-hoc model validation analyses are shown in the appendix (p 2).
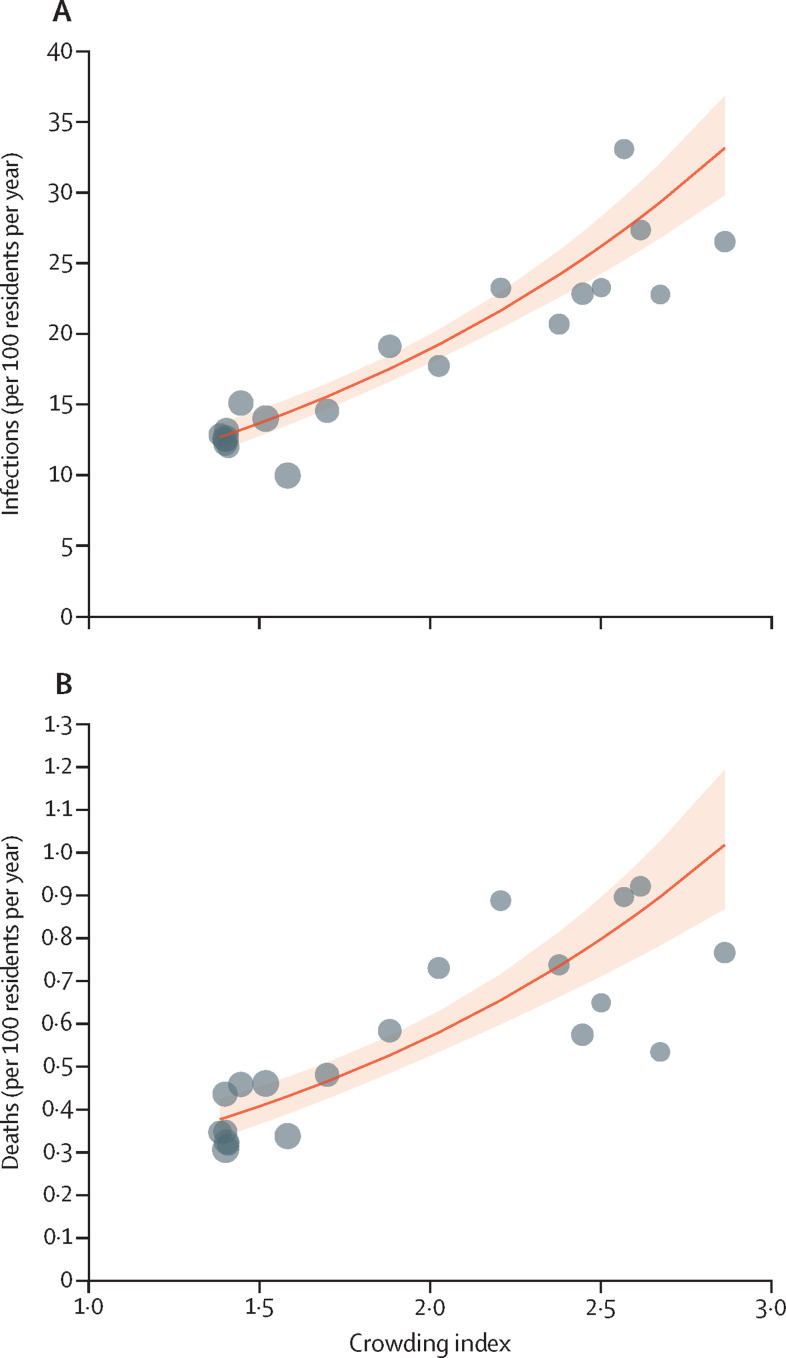
Figure 1.
Associations between nursing home crowding index and incidence of outbreak-associated respiratory infections and deaths
Associations between crowding index and infections (A) and deaths (B). To visualise trends in data for nursing homes, homes were combined into 20 equal-sized groups of 29–30 homes according to crowding index (black points). The red line shows the negative binomial model-based association with the crowding index, the shaded area showing the 95% CI. Each one-point increase in the crowding index was associated with a 1·92-times increase in incidence of infection (95% CI 1·74–2·11) and a 1·96-times increase in respiratory infection-associated mortality (1·68–2·28).
Table 3.
Associations with crowding in nursing homes in Ontario, Canada, 2014–19
| Infection incidence | Death incidence | Outbreak frequency | Outbreak size | |
|---|---|---|---|---|
| Unadjusted crowding index (per one resident per room increase) | 1·92 (1·74–2·11) | 1·96 (1·68–2·28) | 1·02 (0·92–1·13) | 1·79 (1·65–1·94) |
| Adjusted crowding index (per one resident per room increase) | 1·89 (1·64–2·17) | 2·34 (1·88–2·92) | 1·42 (1·25–1·61) | 1·28 (1·19–1·37) |
Data are risk ratio (95% CI). Models for infection incidence, death incidence, and outbreak frequency were based on negative binomial regression, and outbreak size was based on logistic regression. Unadjusted analyses were univariate, and adjusted analyses included covariates for ownership type (ie, municipal, private for-profit, private non-profit), size of home, mean age of residents, proportion of women, proportion of residents with each of six comorbidities (ie, dementia, diabetes, chronic heart failure, renal failure, cancer, and chronic obstructive pulmonary disease), mean activities of daily living score, and health region of the nursing home (six regions). Coefficient estimates for all variables included in the above models are available in the appendix (p 2).
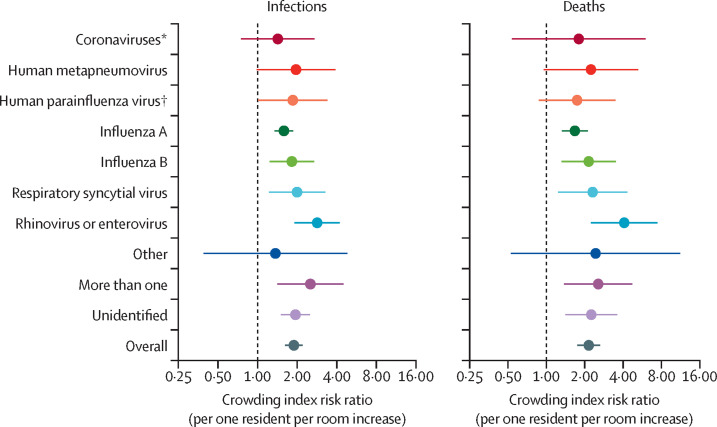
We also fitted the incidence of infections and deaths separately for each respiratory pathogen subgroup, adjusting for number of beds in the nursing home, ownership type, and mean age (adjustment for all covariates caused convergence issues for some models). For incidence of infections, the estimated associations between crowding and specific known infectious agents (figure 2 ) ranged from 1·42 (95% CI 0·75–2·71) for coronaviruses to 2·84 (1·91–4·21) for rhinovirus or enterovirus; the association for influenza A virus was 1·56 (1·35–1·86). Random-effects meta-analysis across pathogen subgroups for the incidence of infection showed a low degree of heterogeneity (adjusted risk ratio [aRR] 1·89 [95% CI 1·62–2·20]; I 2=27%), meaning that crowding effects were consistent across pathogen subgroups. For the incidence of deaths, crowding effects were also consistent across pathogen subgroups (aRR 2·15 [95% CI 1·75–2·65]; I 2=24%). When we examined only outbreaks due to the eight single and identified pathogens, we obtained results similar to those in our primary analyses (aRR 1·86 [95% CI 1·53–2·25] for infection and 2·12 [1·63–2·76] for death).
Figure 2.
Nursing home crowding index risk ratio for respiratory pathogen-specific outbreak-associated infections and deaths
Crowding index risk ratio for pathogen-specific outbreak-associated infections (A) and deaths (B). Points represent the risk ratios (per one resident per room increase in crowding) and line ranges show the corresponding 95% CIs. For each pathogen, the adjustment models included number of beds in the nursing home, ownership type, and mean resident age. The overall estimate is based on random-effects meta-analysis. *Coronavirus strains OC43, 229E, NL63, and HKU1 were tested. † Human parainfluenza strains 1–4 were tested.
We ran simulations to examine the potential effects of an intervention to change the number of people residing in shared rooms. A cap of two beds per room would have reduced infections over the 5-year period by an estimated 11 395 (95% CI 7404–14 258), from 64 829 to 53 434 infections, and outbreak-associated deaths by an estimated 433 (95% CI 275–584), from 1969 to 1536 deaths. A nursing home system with single-bed rooms (with private bathrooms) only would have reduced infections over the 5-year period by an estimated 29 003 (95% CI 23 663–33 190), from 64 829 to 35 826 infections, and outbreak-associated deaths by an estimated 1052 (95% CI 847–1220), from 1969 to 917 deaths.
Discussion
Over a 5-year period, 64 829 cases and 1969 deaths related to respiratory infection outbreaks in 588 nursing homes in Ontario were recorded. Crowded nursing homes had a greater incidence of outbreak-related acute respiratory infections and associated mortality than did less crowded nursing homes. Crowding was positively associated with increased incidence for all ten of the respiratory virus subgroups examined, including (but not limited to) influenza A, influenza B, respiratory syncytial virus, and rhinovirus or enterovirus. Simulations estimated that deaths associated with respiratory infection outbreaks would be reduced by more than 50% if nursing home occupancy was reduced to one person per room.
Systematic data on the presence of shared rooms (so-called ward rooms) in nursing homes are scarce. Although national statistics agencies in several countries collect measures of crowding for residents of private dwellings (eg, the Canadian National Occupancy Standard), the same measures are usually not collected or reported for residents of nursing homes. In the absence of systematic data from the USA, a survey from 40 randomly selected nursing homes across five US states (CA, FL, MN, NJ, and NY) indicated that 71% (1408 of 1988) of residents in nursing homes lived in shared rooms, and 28% (549 of 1988) of residents shared a bathroom between four or more residents; notably, all homes with private rooms were non-profit facilities.18 Similarly, no systematic, country-wide data for crowding in nursing homes are available in Canada. In this study of Canadian nursing homes, crowded homes were substantially more likely to be private for-profit facilities, as has been reported previously.19
Regulations, reimbursement schemes, and design standards for nursing homes have allowed crowding to persist, particularly in older homes. In the USA, Medicaid provides no additional funds for private rooms, inadvertently disincentivising construction of single-bed rooms. In the state of NY, USA, design standards indicate that only a minimum of 10% of rooms need to be single-bed.20 In Ontario, Canada's largest province, the design standards of 1999 and 2015 interdict construction of rooms for three or more residents, but set no limits on the proportion of rooms with two beds, and four-bed rooms were allowed to persist through a legacy clause.12, 21, 22 On June 10, 2020, between the first and second COVID-19 waves in Ontario, a temporary cap of two people per room was put into place for new admissions; the occupancy cap remains in place as of Feb 7, 2023.11 Consultations for new design standards for nursing homes in development in Canada indicate a strong preference for single-occupancy rooms.23
Evidence suggests that crowding is associated with increased SARS-CoV-2 incidence across a range of residential settings, including nursing homes, prisons, and households.7, 9, 24, 25 Our results support these findings, suggesting that crowding is not only a risk factor for SARS-CoV-2 virus but is also associated with increased incidence of other acute respiratory infections. We hypothesise that crowded sleeping quarters have increased transmission across a range of mechanisms, including aerosols, droplets, and direct and indirect contact. Regardless of the specific mechanisms of transmission, a reduction in crowding can be expected to reduce transmission rates, because nursing home residents spend a mean of 15 h per day in bed (11 h at night and 30% of the remaining hours of the day).26 Furthermore, crowding impedes the ability to quarantine and self-isolate, which can only be partially mitigated by infection control measures.27
A systematic review on the burden of respiratory infection in nursing homes from before the COVID-19 pandemic indicated that, for nursing home settings, “little useful guidance for decision-making to decrease respiratory infection burden” was available.28 This absence of guidance was apparent during the COVID-19 pandemic, which had devastating effects on residents of nursing homes in many countries. Our study identified an important and modifiable risk factor for non-COVID-19 respiratory infections and deaths in nursing homes, with little heterogeneity across specific respiratory infections, similar to associations identified between crowding and SARS-CoV-2.9
Although it increases costs, reducing crowding in nursing homes brings additional benefits, such as respecting the preference of most older adults of being housed in single-bed rooms29 and potentially reducing night-time disturbances (although sharing a room might not be a primary driver of poor sleep among residents of nursing homes).30 The primary cost associated with decreasing crowding is financial, with the estimated construction costs of single-bed rooms being 44% higher per bed compared with two-bed rooms and 82% higher per bed compared with four-bed rooms.29 Furthermore, nursing staff in hospitals indicate that one perceived benefit of shared rooms is decreased walking distances during nursing shifts.31
A limitation of our study is that our analysis was based on standardised outbreak surveillance procedures that might have missed some outbreaks and cases, leading to errors in the total outbreak size, and that causal pathogens might have misattributed because only the first four outbreak cases were tested in accordance with provincial guidance. In comparison, our analyses based on deaths might be less subject to misclassification errors and showed stronger associations with less heterogeneity. Data for resident and nursing home characteristics, including crowding, were extracted in November, 2020, and respiratory infection outbreaks in nursing homes included in this analysis occurred between 2014 and 2019, suggesting that the estimated resident and home characteristics, including the occupancy rate, might have been inaccurate if the resident population changed substantially between the study and data extraction. But, at a minimum, the occupancy rate of nursing homes in Ontario has been extremely stable through time; the median occupancy rate of 98·2% that we observed is consistent with that found in a 2012 Auditor General report, and the Canadian Government strongly incentivises homes to keep occupancy rates greater than 97%.32 Furthermore, the finding that 96·4% of total outbreaks identified were linked to an included nursing home suggests that few nursing homes closed during this time, since we excluded homes that closed before January, 2020. An additional limitation of this work is that nursing homes with a higher crowding index are likely to be different in terms of other building characteristics (ie, be older, have smaller rooms, be more crowded in common areas of the facility, and have lower ventilation rates), and might have differed in terms of other unmeasured characteristics, such as staffing, so that it might be difficult to attribute all differences to the crowding index. Finally, the estimated effects of reductions in crowding should be interpreted with caution, as this study was not preregistered or randomised and did not directly evaluate an intervention that reduced crowding.
The results of this analysis can inform decisions on the design and construction of nursing homes and on the use of multi-bed rooms in the future. Decreasing crowding in nursing homes is an important initiative to improve resident quality of life and patient safety beyond the COVID-19 pandemic.
Data sharing
Patient and nursing home-level data contain personal health information and cannot be shared publicly due to provincial data protection and confidentiality requirements.
Declaration of interests
AMG reports consulting fees from Sienna Senior Living; honoraria from AstraZeneca, Merck, Biogen, and Moderna; and advisory board participation for Pfizer, GlaxoSmithKline, Moderna, Medicago, Janssen, AstraZeneca, Novavax, and Sanofi. All other authors declare no competing interests.
Contributors
PL and KAB conceived and designed the study and drafted the manuscript. All authors were involved in the acquisition, analysis, or interpretation of the data and critical revision of the manuscript for important intellectual content. Statistical analyses were conducted by KAB. All authors contributed to, revised, and approved the final version of the manuscript. KAB and MW accessed and verified all the underlying data in the study. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Fisman DN, Bogoch I, Lapointe-Shaw L, McCready J, Tuite AR. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultze A, Nightingale E, Evans D, et al. Mortality among care home residents in England during the first and second waves of the COVID-19 pandemic: an observational study of 4·3 million adults over the age of 65. Lancet Reg Health Eur. 2022;14 doi: 10.1016/j.lanepe.2021.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36:870–876. doi: 10.1086/368197. [DOI] [PubMed] [Google Scholar]
- 4.Paphitis K, Achonu C, Callery S, et al. Beyond flu: trends in respiratory infection outbreaks in Ontario healthcare settings from 2007 to 2017, and implications for non-influenza outbreak management. Can Commun Dis Rep. 2021;47:269–275. doi: 10.14745/ccdr.v47i56a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X-M, Jiao J, Cao J, et al. Frailty as a predictor of mortality among patients with COVID-19: a systematic review and meta-analysis. BMC Geriatr. 2021;21:186. doi: 10.1186/s12877-021-02138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacIntyre CR, Kendig N, Kummer L, Birago S, Graham NMH. Impact of tuberculosis control measures and crowding on the incidence of tuberculous infection in Maryland prisons. Clin Infect Dis. 1997;24:1060–1067. doi: 10.1086/513632. [DOI] [PubMed] [Google Scholar]
- 7.Leibowitz AI, Siedner MJ, Tsai AC, Mohareb AM. Association between prison crowding and COVID-19 incidence rates in Massachusetts prisons, April 2020–January 2021. JAMA Intern Med. 2021;181:1315–1321. doi: 10.1001/jamainternmed.2021.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO WHO housing and health guidelines. 2018. https://apps.who.int/iris/handle/10665/276001 [PubMed]
- 9.Brown KA, Jones A, Daneman N, et al. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. JAMA Intern Med. 2021;181:229–236. doi: 10.1001/jamainternmed.2020.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres ML, Palma Díaz D, Oliver-Parra A, et al. Inequities in the incidence and mortality due to COVID-19 in nursing homes in Barcelona by characteristics of the nursing homes. PLoS One. 2022;17 doi: 10.1371/journal.pone.0269639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams D. Office of the Chief Medical Officer of Health; Toronto, ON: 2020. COVID-19 directive #3 for long-term care homes under the Long-Term Care Homes Act, 2007. [Google Scholar]
- 12.Ontario Ministry of Health and Long-Term Care Long-term care facility design manual. May, 1999. https://collections.ola.org/mon/ont/H/1999/LTCdesign_manual.pdf
- 13.MacFadden DR, McGeer A, Athey T, et al. Use of genome sequencing to define institutional influenza outbreaks, Toronto, Ontario, Canada, 2014–15. Emerg Infect Dis. 2018;24:492–497. doi: 10.3201/eid2403.171499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care. 2004;42(suppl):III50–III59. doi: 10.1097/01.mlr.0000120104.01232.5e. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43:962–970. doi: 10.1093/ije/dyu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol. 2010;63:2–6. doi: 10.1016/j.jclinepi.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Cutler LJ, Kane RA, Degenholtz HB, Miller MJ, Grant L. Assessing and comparing physical environments for nursing home residents: using new tools for greater research specificity. Gerontologist. 2006;46:42–51. doi: 10.1093/geront/46.1.42. [DOI] [PubMed] [Google Scholar]
- 19.Stall NM, Jones A, Brown KA, Rochon PA, Costa AP. For-profit long-term care homes and the risk of COVID-19 outbreaks and resident deaths. CMAJ. 2020;192:e946–e955. doi: 10.1503/cmaj.201197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.New York Codes, Rules and Regulations SubPart 713–4—standards for nursing home construction after December 31, 2010. Dec 29, 2010. https://regs.health.ny.gov/content/subpart-713-4-standards-nursing-home-construction-after-december-31-2010
- 21.Ontario Ministry of Health and Long-Term Care Long-term care home design manual 2015. February, 2015. https://health.gov.on.ca/en/public/programs/ltc/docs/home_design_manual.pdf
- 22.Office of the Auditor General of Ontario COVID-19 preparedness and management: special report on pandemic readiness and response in long-term care. April, 2021. https://www.auditor.on.ca/en/content/specialreports/specialreports/COVID-19_ch5readinessresponseLTC_en202104.pdf
- 23.CSA Group What we heard: helping inform the development of the National Standard of Canada for operation and infection prevention and control of long-term care homes (CSA Z8004) January, 2022. https://www.csagroup.org/wp-content/uploads/CSA-WWH-FinalReport-EN_Accessible.pdf
- 24.Dasgupta S, Bowen VB, Leidner A, et al. Association between social vulnerability and a county's risk for becoming a COVID-19 hotspot—United States, June 1–July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1535–1541. doi: 10.15585/mmwr.mm6942a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Ingen T, Brown KA, Buchan SA, et al. Neighbourhood-level socio-demographic characteristics and risk of COVID-19 incidence and mortality in Ontario, Canada: a population-based study. PLoS One. 2022;17 doi: 10.1371/journal.pone.0276507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yavari F, Vale B, Khajehzadeh I. In: Revisiting the role of architectural science in design and practice. Zuo J, Daniel L, Soebarto V, editors. The Architectural Science Association and The University of Adelaide; Adelaide: 2016. A time-use study of rooms and possible impact on the design of housing for an aging population; pp. 1–10. [Google Scholar]
- 27.Kain DC, McCreight LJ, Johnstone J. Dealing with coronavirus disease 2019 (COVID-19) outbreaks in long-term care homes: a protocol for room moving and cohorting. Infect Control Hosp Epidemiol. 2021;42:1402–1403. doi: 10.1017/ice.2020.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Childs A, Zullo AR, Joyce NR, et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr. 2019;19:210. doi: 10.1186/s12877-019-1236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calkins M, Cassella C. Exploring the cost and value of private versus shared bedrooms in nursing homes. Gerontologist. 2007;47:169–183. doi: 10.1093/geront/47.2.169. [DOI] [PubMed] [Google Scholar]
- 30.Kim DE, Yoon JY. Factors that influence sleep among residents in long-term care facilities. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17061889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhury H, Mahmood A, Valente M. Nurses' perception of single-occupancy versus multioccupancy rooms in acute care environments: an exploratory comparative assessment. Appl Nurs Res. 2006;19:118–125. doi: 10.1016/j.apnr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Office of the Auditor General of Ontario 2012 annual report. Nov 15, 2012. https://www.auditor.on.ca/en/content/annualreports/arreports/en12/2012ar_en.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient and nursing home-level data contain personal health information and cannot be shared publicly due to provincial data protection and confidentiality requirements.