Abstract
Congenital isolated ‘H-type’ tracheoesophageal fistula (TOF) is a rare disorder which is difficult to diagnose. Clinical presentation is characterised by a triad consisting of paroxysmal coughing and cyanosis during feeds; recurrent chest infections and failure to thrive; and abdominal distention secondary to gaseous loading of the bowel. It is often difficult to diagnose ‘H-type’ TOF because the continuity of the oesophagus is not interrupted. The diagnosis is often missed or delayed, leading to complications such as chronic lung disease and failure to thrive.
Keywords: tracheoesophageal fistula, H-type isolated fistula, polymerase chain reaction, microscopy and culture, Pneumocystis jirovecii pneumonia, cytomegalovirus, HIV, retroviral disease
Background
A tracheoesophageal fistula (TOF) is an abnormal connection between the trachea and oesophagus. A TOF arises as a result of failed fusion of the tracheoesophageal ridge during the third week of embryological development.[1] The incidence is 1 in 2 500 - 3 000 live births with a male: female ratio of 1.26:1.[2]
A TOF without associated oesophageal atresia, commonly called ‘H-type’ or isolated fistula, is a rare congenital anomaly.[3] The fistula runs from the posterior wall of the trachea downward to the anterior wall of the oesophagus[4] and accounts for only 4 - 5% of all congenital tracheoesophageal malformations.[5]
The clinical presentation is characterised by a triad consisting of: (i) paroxysmal coughing and cyanosis during feeds; (ii) recurrent chest infections; and (iii) abdominal distention secondary to gaseous loading of the bowel.[5] It is often difficult to diagnose ‘H-type’ TOF because the continuity of the oesophagus is not interrupted; therefore, the diagnosis is often missed or delayed.
If left untreated, this condition can lead to multiple complications such as recurrent pneumonia, failure to thrive and chronic lung disease. It is advisable to perform prone contrast oesophagraphy as well as tracheoesophageal bronchoscopy to confirm the diagnosis and identify the precise location of the fistula. Treatment of ‘H-type’ TOF is an open surgical procedure, either through a cervical approach or through a thoracotomy or thoracoscopy.[1] The patient presented here developed recurrent respiratory infections, multiple admissions and failure to thrive.
Case report
A 6-month-old female infant presented to a local hospital with a 9-day history of non-productive cough and shortness of breath without fever. She was admitted at the local hospital for a week and treated with antibiotics (azithromycin, ampicillin, co-trimoxazole) but did not respond to antibiotics clinically. She was transferred to our tertiary hospital owing to unresolving pneumonia. On further inquiry, it was found that she had had a persistent, non-productive cough since she was 5 weeks old.
According to her mother, she failed to gain weight despite normal feeding habits. There was no history of loose stools, oedema or cyanosis. She had been admitted thrice since birth, with main complaints of cough and difficulty in breathing. Her initial admission was at 5 weeks of life, when she was admitted for 2 weeks with the diagnosis of bronchiolitis. Her second admission was at 3 months, when she was diagnosed with pneumonia and managed with antibiotics. Her third admission was at 5 months, when she was first diagnosed with pneumonia and later started on treatment for pulmonary tuberculosis based on clinical presentation and a chest radiograph.
The infant was born at term by normal vaginal delivery with a birthweight of 2 830 g, Apgar score of 9/10 at one minute and 10/10 at 5 minutes. She was retroviral disease-exposed with a negative HIV-PCR at birth. She missed her immunisations at 10 and 14 weeks owing to a stock shortage at the local clinic. The patient had been exclusively formula-fed since birth and was on InfaCare formula at admission. Solid feeds had not been introduced and her milestones were appropriate for age. There was no family history of tuberculosis or asthma. On examination, she looked chronically ill with mild respiratory distress, and she was on nasal-prong oxygen therapy (2 L/ min). Her vital signs were as follows: respiratory rate, 42 breaths per minute (tachypnoeic); heart rate, 139 beats per minute (tachycardia); temperature, 36.7 °C (normal); and blood pressure, 88/44 mmHg. The infant’s anthropometric measurements were as follows: weight, 4 kg (below the third percentile); weight-for-length score, below the third percentile; and she was failing to thrive. There were no signs of clubbing, jaundice, oedema, pallor, cyanosis or hypopigmented skin lesions.
On examination of the respiratory system there were subcostal and intercostal recessions with bilateral crepitation and no deformities. The abdomen demonstrated hepatomegaly without splenomegaly. Examination of the cardiovascular system demonstrated normal pulses and no murmurs. There were no neurological abnormalities.
The initial assessment was that of a 5-month-old female infant, retroviral disease-exposed with a negative HIV-PCR at birth, recurrent chest infection, failure to thrive and pulmonary tuberculosis. The infant was admitted to the paediatric medical ward to exclude primary immune deficiency, cystic fibrosis, Pneumocystis jirovecii pneumonia and cytomegalovirus (CMV) infection. She was started on piperacillin/tazobactam, amikacin, nebulisation with fenoterol, oral prednisone and tuberculosis treatment.
The following investigations were performed: sputum microscopy, culture and sensitivity (MC&S) (no growth); GeneXpert test for tuberculosis (negative); respiratory syncytial virus and adenovirus (negative); P. jirovecii (negative); CMV DNA viral load (<1 000 copies); SARS-COVID-19 (negative). A full blood count, C-reactive protein and blood culture were normal. A stool sample for faecal elastase as well as a primary immune deficiency screen were normal. Chest X-rays showed bilateral infiltrates and a chest scan report showed bilateral multiple consolidation with no bronchiectasis. A small patent ductus arteriosus (negligible) was observed on echocardiogram.
On observation in the ward, the infant coughed excessively during feeds, a barium swallow contrast study was performed (Fig. 1) and a fistula was identified between the trachea and oesophagus. The patient was referred to paediatric surgery. Figs 2 and 3 are intraoperative images. Postoperatively, she recovered well and was discharged home after 2 weeks. At the time of publication of this report, the infant was clinically well, gaining weight and no further cough had been observed.
Fig. 1.
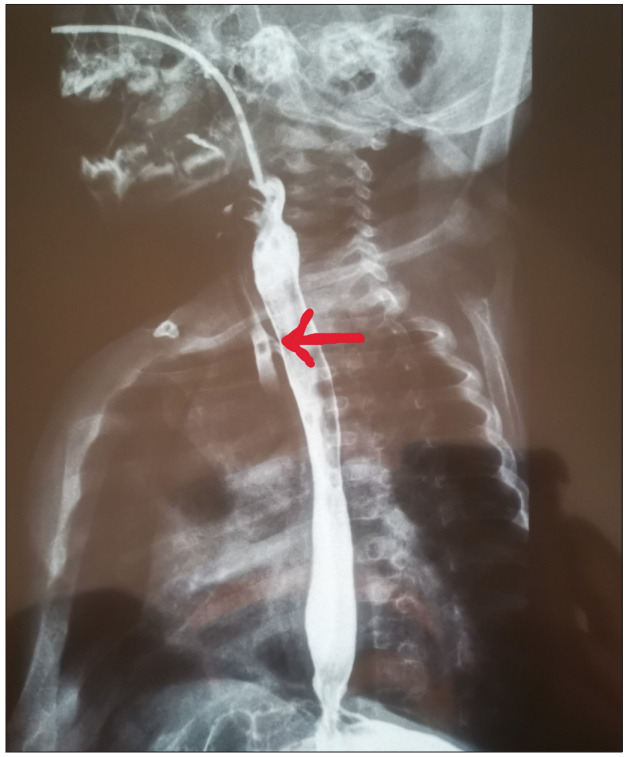
Barium swallow contrast study of the H-type tracheoesophageal fistula (red arrow).
Fig. 2.
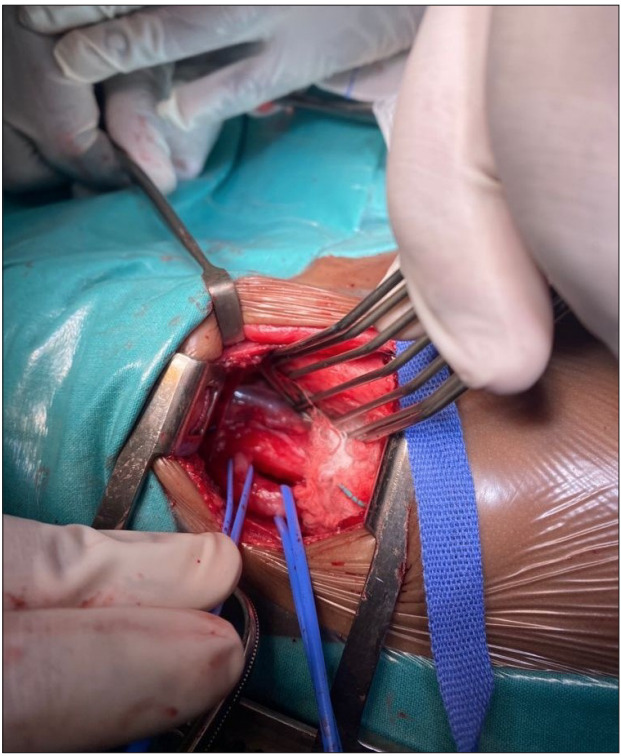
Intraoperative image of the TOF (left vascular loop) and the oesophagus (right vascular loop). The trachea is visible above the TOF. (TOF = tracheoesophageal fistula.)
Fig. 3.
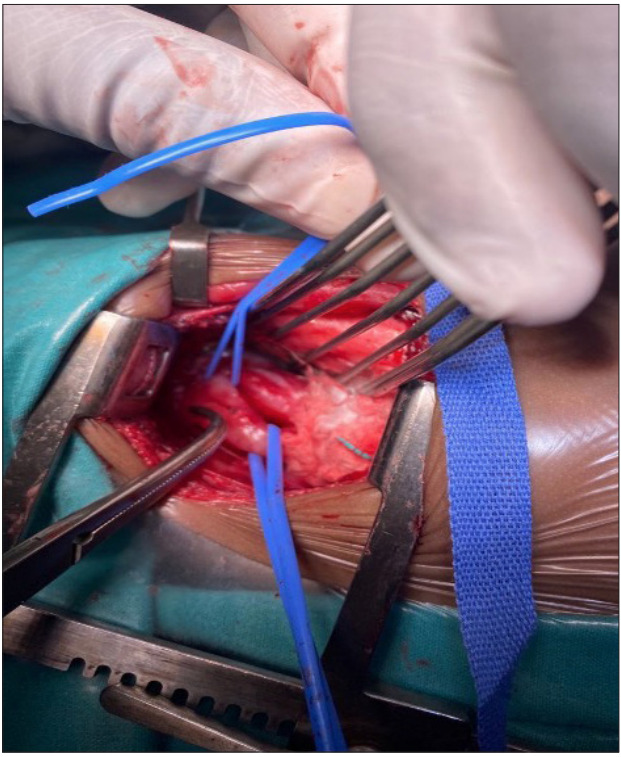
Intraoperative image demonstrating the TOF. (TOF = tracheoesophageal fistula.)
Discussion
Congenital isolated ‘H-type’ TOF is a rare disorder which is difficult to diagnose. It accounts for 4% of all congenital oesophageal malformations with an incidence of 1 in 2 500 - 3 000 livebirths.[2] It was first described by Lamb in 1873.[1] ‘H-type’ TOF is not associated with oesophageal atresia and less associated with other congenital anomalies. A high index of suspicion is necessary for diagnosis in patients that present with exacerbation of a cough during feeds and additional signs such as cyanosis, abdominal distension, choking spells, recurrent respiratory infections and failure to thrive.
Pressure changes between the trachea and oesophagus can cause entry of air into the oesophagus, or entry of oesophageal content into the trachea, giving rise to the clinical presentation. Late or missed diagnosis is due to variability in the anatomy of fistulas.[8] There is no single investigation with sufficient sensitivity and specificity to aid the accurate diagnosis of ‘H-type’ TOF,[8] although chest radiographs are helpful to check for signs of aspiration. A prone contrast oesophagram can aid diagnosis. Tracheoesophageal bronchoscopy is diagnostic and can also assist with precise location of the fistula. Special position and contrast administration techniques have been described as aiding the diagnosis. To improve sensitivity, this test should be performed with a nasogastric tube in situ and the child should be in the prone position.[6] The nasogastric tube should be withdrawn slowly while injecting contrast. Contrast-enhanced studies carry a risk of aspiration pneumonia and should be performed with adequate emergency resuscitation tools at hand. Bronchoscopy is also helpful in determining the location of the fistula, the presence of a double fistula and the position of the aortic arch.
Magnetic resonance imaging can also be used in making the diagnosis before birth. Associated congenital abnormalities are more common with other variants, whereas ‘H-type’ TOF includes least number of associated congenital abnormalities (30% of cases).[1] The associated abnormalities reported are vertebral defect, anal atresia, cardiac defect, TOF, renal anomalies and limb abnormalities (VACTERL syndrome). Common cardiac abnormalities include ventricular septal defect, tetralogy of Fallot and chromosomal abnormalities.[9] The surgical approach for ‘H-type’ TOF has been cervical, thorascopic and thoracotomy techniques. Identifying the exact level of the fistula is crucial as this will dictate the operative approach. At or above the level of the T2 vertebra, a trans-cervical approach can be utilised, while open thoracotomy or a thoracoscopic approach is necessary below the level of the T2 vertebra. [8]
The real advantage in utilising thoracoscopy over an open approach remains open for debate.[8] Endoscopic closure of ‘H-type’ TOF has also been reported with different techniques such as glue adhesion, electrocautery, the use of sclerosant agents and laser ablation.[8]
Endoscopic electrocautery provides a less invasive approach to management of ’ ‘H-type’ TOF. However, this procedure may need to be performed several times, which necessitates discussion with the family and a contingency plan in the event of failure.[5]
The most common postoperative complication is recurrent laryngeal nerve injury, with patients having difficulty in breathing, gastroesophageal reflux disease, vocal cord injury, dysphagia, wheezing and respiratory tract infections.[2]
Conclusion
Diagnosis of ‘H-type’ TOF can be challenging and is often missed in the absence of a high index of clinical suspicion. Complications such as failure to thrive and chronic lung disease are likely to occur if the diagnosis is delayed. Early diagnosis and appropriate management reduce morbidity and improve the prognosis. Tracheobronchoscopy and prone oesophagram should be performed to confirm the diagnosis. Surgical repair is the main treatment modality and a multidisciplinary approach is required for the proper management of ‘H-type’ TOF.
Acknowledgments
None.
References
- 1.Jaiswal A, Garg AK, Mohanty MK. H-type tracheo-oesophageal fistula – case reports with review of the literature. Egypt J Ear Nose Throat Allied Sci. 2014;15(2):143–148. doi: 10.1016/j.ejenta.2013.12.007. [DOI] [Google Scholar]
- 2.Pankhudi P, Ankita G. Tracheoesophageal fistula – H-type: Rare entity. Eur J Biomed Pharma Sci. 2021;8(8):508–510. [Google Scholar]
- 3.Tiwari C, Nagdeve N, Saoji R, Nama N, Khan MA. Congenital H-type tracheoesophageal fistula: An institutional review of a 10-year period. J Mother Child. 2020;24(4):2–8. doi: 10.34763/jmotherandchild,20202404.d.20-00004.24(4)2020.2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao S, Dias E. An uncommon presentation of double H-type tracheoesophageal fistula. Int J Preg Child Birth. 2020;6(2):38–39. doi: 10.15406/ipcb.2020.06.00193. [DOI] [Google Scholar]
- 5.Barbian M, Raol N, Landry A, Meisel J, Santore T, Keene T. Repair of congenital H-type tracheoesophageal fistula by electrocautery. J Paed Surg Case Rep. 2021;72(2021):101943. doi: 10.1016/j.epse.2021.101943. [DOI] [Google Scholar]
- 6.Cuesta G, Rodrigue V, Millan C, Munzon P, Munon G. H-type tracheoesophageal fistula in the neonatal period: Difficulties in diagnosis and different treatment approaches. A case series. Arch Argent Paediatric. 2020;118(1):47–60. doi: 10.5546/aap.2020.eng.56. [DOI] [PubMed] [Google Scholar]
- 7.Tanny SP, Kung SK, Omariti , Teague WJ. Double H-type tracheoesophageal fistula. J Paed Surg Case Rep. 2020;62(2020):1011662. doi: 10.1016/j.epsc,2020,101662. [DOI] [Google Scholar]
- 8.Sampat K, Losty PD. Diagnostic and management strategies for congenital H-type tracheoesophageal fistula: A systemic review. Paed Surg Int. 2021;37:539–547. doi: 10.1007/s00383-020-04853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaginuma K, Watanabe M, Tanaka H, Hosoya H. H-type trachea-oesophageal fistula in an infant. BMJ Case Rep. 2020;13:e239327. doi: 10.1136/bcr2020-239327. [DOI] [PMC free article] [PubMed] [Google Scholar]


