Abstract
Rationale
In the United States, donor lungs are allocated to transplant candidates on the basis of lung allocation scores (LAS). However, additional factors beyond the LAS can impact who is transplanted, including listing and donor–organ acceptance practices. These factors can result in differential selection, undermining the objectivity of lung allocation. Yet their impact on the lung transplant pathway has been underexplored.
Objectives
We sought to systematically examine sources of differential selection in lung transplantation via qualitative methods.
Methods
We conducted semistructured qualitative interviews with lung transplant surgeons and pulmonologists in the United States between June 2019 and June 2020 to understand clinician perspectives on differential selection in lung transplantation and the LAS.
Results
A total of 51 respondents (30 surgeons and 21 pulmonologists) identified many sources of differential selection arising throughout the pathway from referral to transplantation. We synthesized these sources into a conceptual model with five themes: 1) transplant center’s degree of risk tolerance and accountability; 2) successfulness and fairness of the LAS; 3) donor–organ availability and regional competition; 4) patient health versus program health; and 5) access to care versus responsible stewardship of organs.
Conclusions
Our conceptual model demonstrates how differential selection can arise throughout lung transplantation and facilitates the further study of such selection. As new organ allocation models are developed, differential selection should be considered carefully to ensure that these models are more equitable.
Keywords: lung transplantation, allocation of healthcare resources, patient selection for treatment
In May 2005, the OPTN (Organ Procurement and Transplantation Network) implemented a new policy that allocates donor lungs to candidates on the basis of lung allocation scores (LAS) (1–4). The LAS estimates the difference in days of life between transplant benefit (defined as predicted 1-year posttransplant survival minus predicted 1-year pretransplant survival) and waitlist urgency (defined as predicted 1-year pretransplant survival). Equivalently, the LAS equals predicted 1-year posttransplant survival minus two times predicted 1-year waitlist survival (1, 4). Higher values indicate greater priority for transplantation (1, 4). This score was intended to balance equity, justice, beneficence, and utility in lung allocation (2, 5).
Predictions of pre- and posttransplant survival that go into the LAS are on the basis of patient demographic and laboratory values (1, 4). Although these values are updated regularly, some can be subject to clinician interpretation (see data supplement, “Additional Details on the LAS and the Listing Decision-Making Process”.). Moreover, additional factors beyond the LAS can impact which patients receive a transplant. These factors include referral patterns (6–8) and geographic, gender, or racial or ethnic disparities in waitlist registration (8–12), pre- and posttransplant survival (8, 13–15), and donor–organ availability (8, 9, 16, 17). Collectively, such factors can give rise to differential selection for transplantation (18, 19), undermining the objectivity of lung allocation. The phrase “differential selection” is intended to capture any mechanism that can differentially impact which patients receive a transplant. Such mechanisms can include differences in referral, listing, and donor organ acceptance criteria across transplant centers. The differential selection also includes survivor bias, which captures the idea that waitlisted individuals can only receive a transplant if they survive long enough for a suitable donor organ to become available and have sufficient priority to receive this offer (17).
Although much statistical research has been conducted surrounding the LAS and differential selection (19–22), less is known about how lung transplant surgeons and pulmonologists employ the LAS in practice or how these clinicians interpret and respond to differential selection. Some qualitative studies have examined candidate (23, 24) and organ selection (25); however, these studies focused almost exclusively on patient screening rather than the complete pathway from referral through transplantation.
Given that OPTN is currently implementing a new organ allocation framework (the continuous distribution model [26–28]) in lungs, followed by other organs (e.g., kidney, liver, and heart), it is important to understand where differential selection can arise in lung transplantation and how it can impact the successfulness and fairness of lung allocation. Synthesizing this information into a conceptual model can facilitate further study of the patient- and program-level effects of differential selection in lung transplantation. Toward that end, we conducted a qualitative study of lung transplant surgeons and pulmonologists between June 2019 and June 2020 to understand clinician perspectives on differential selection in lung transplantation and the LAS. Preliminary results of this study (this abstract) were reported at the ISHLT (International Society for Heart and Lung Transplantation) 2022 conference (29).
Methods
Study Design and Target Population
We conducted a qualitative study between June 2019 and June 2020 to understand clinician perspectives on the LAS and differential selection in lung allocation. Our target population consisted of lung transplant surgeons and pulmonologists practicing in the United States. The study protocol was reviewed by the University of Pennsylvania Institutional Review Board (IRB Protocol #833089) and determined to meet exemption criteria authorized by 45 CFR 46.104, category #4,2. We also followed the Consolidated Criteria for Reporting Qualitative Research (30) checklist (see data supplement) (30).
Participants and Recruitment
Participants were recruited through the “Pulmonology and Cardiothoracic/Vascular Surgery” listserv of the ISHLT. Individuals were eligible to participate in our study if they were lung transplant surgeons or pulmonologists practicing in the United States. Individuals who did not practice in the United States, were still in training (e.g., medical student or resident), or who focused on other-organ transplant (e.g., heart) were excluded. Eligible individuals were invited to participate via email by the lead author (E.M.S.). We purposively sampled individuals to introduce variation by discipline (e.g., lung transplant surgeon and/or pulmonologist) and sought to include respondents with diverse characteristics relevant to our study question (e.g., geography and years in practice). The lead and senior author (J.E.S.) monitored for thematic saturation during data collection to determine sample size adequacy. Recruitment continued until thematic saturation across the main domains of our interview guide was reached (31–34).
Data Collection
Interviews were conducted in person or via phone by the lead author using a semistructured guide (see data supplement). The guide was piloted and refined to ensure complete capture of relevant concepts, comprehension of questions, and length (see data supplement). Questions covered the following domains: 1) how lung transplant surgeons and pulmonologists use the LAS in clinical practice; 2) factors deemed important for prioritizing lung transplant candidates but which might not be captured by the LAS; 3) how clinicians think about differential selection in lung allocation; and 4) clinician perspectives on modifying the LAS to account for differential selection. Participants consented verbally and gave permission for their interview to be audio-recorded.
Analyses
Interviews were professionally transcribed, deidentified, and uploaded to NVivo (NVivo Release 1.0, QSR International 2020). Data were analyzed by two authors (E.M.S. and J.E.S.) in consultation with the research team using a modified framework method (35). First, interview transcripts and data collection memos were reviewed to create an index codebook (36). Second, the index codebook was applied to transcripts line-by-line. As patterns emerged in the data, the index codebook was revised (in consultation with the research team), and existing codes were further developed into subcodes. Third, data from coded transcripts were charted into a framework matrix to explore overlapping codes and compare themes within and across respondents. Here, we confirmed that thematic saturation was reached overall and within subgroups defined by discipline (35). Fourth, we developed a conceptual model for sources of differential selection on the basis of the framework matrix. The model was refined through discussions among the research team and negative case analysis.
Self-reported respondent characteristics, including gender, race and ethnicity, years of experience, and UNOS (United Network for Organ Sharing) region, were ascertained at the end of each interview and summarized using counts (proportions) or medians (interquartile ranges) as appropriate (STATA 15, StataCorp LLC).
Results
Respondents
Email invitations were sent to 218 potential respondents, with 76 (35%) responding. Of these 76 individuals, 19 declined to participate, 4 did not meet study inclusion or exclusion criteria, and 2 did not respond to follow-up emails to schedule an interview. Thematic saturation was achieved in the overall sample after 20 interviews. We interviewed 51 participants (30 [59%] pulmonologists and 21 [41%] lung transplant surgeons) to ensure thematic saturation within subgroups defined by discipline and to capture a diversity of perspectives on differential selection in lung allocation. The majority of participants were male (84%), White (61%), and had practiced at more than one institution (55%). The median duration of experience was 15 years, and 47% of respondents began practicing before the LAS was implemented. At least one respondent was interviewed from each UNOS region. Finally, we interviewed respondents from low-, medium-, and high-volume centers (Table 1).
Table 1.
Demographic characteristics of interview respondents
| Factor | Value |
|---|---|
| N | 51 |
| Gender, n (%) | |
| Female | 8 (16) |
| Male | 43 (84) |
| Race/ethnicity, n (%) | |
| African American | 4 (8) |
| Asian/Indian | 6 (12) |
| Hispanic/Latino | 7 (14) |
| Other | 3 (6) |
| White | 31 (61) |
| Discipline, n (%) | |
| Pulmonologist | 30 (59) |
| Transplant surgeon | 21 (41) |
| UNOS region, n (%) | |
| 1 | 1 (2) |
| 2 | 18 (35) |
| 3 | 5 (10) |
| 4 | 1 (2) |
| 5 | 3 (6) |
| 6 | 1 (2) |
| 7 | 5 (10) |
| 8 | 2 (4) |
| 9 | 2 (4) |
| 10 | 5 (10) |
| 11 | 8 (16) |
| Transplants per yr by center (n)*, median (IQR) | 50 (26–112) |
| Respondents from centers performing, per yr, n (%)* | |
| ⩽30 transplants | 19 (37) |
| 31–59 transplants | 9 (18) |
| ⩾60 transplants | 23 (45) |
| Has practiced in more than one institution | 28 (55) |
| Experience (yr), median (IQR) | 15.0 (9.0–20.0) |
| Began practice before LAS was implemented | 24 (47) |
Definition of abbreviations: IQR = interquartile range; LAS = lung allocation score; UNOS = United Network for Organ Sharing.
As reported to the Organ Procurement and Transplantation Network in 2018, the last full year before the start of our study. See https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced/.
Sources of Differential Selection Along the Lung Transplantation Pathway
Each section below describes a step along the transplantation pathway where respondents identified that differential selection might arise. These sources of differential selection are supported by exemplar quotes (Tables 2–5) and mapped to five overarching themes (Figure 1). Note that while this study focused on differential selection that is of concern to clinicians, additional factors beyond the ones identified by our respondents influence listing and transplantation decisions; see data supplement for more context.
Table 2.
Exemplar quotes pertaining to the transition between screening and waitlist registration
| Theme | Exemplar Quote |
|---|---|
| Transplant center’s degree of risk tolerance and accountability | 1. “I think you’re interpreting somebody’s capabilities [of surviving transplant] based on how they look and how robust are they and do they look frail, and those types of things which I think are one reflector of survival that kind of goes into the gestalt when you see someone. Part of that assessment is, do they look like they’re about to keel over or not? And I think there are some patients where that probably is accurate, and there are some patients where it’s really not.” [pulmonologist] |
| 2. “The atrial pressure of your heart, that’s hard data; your PFTs [pulmonary function tests] are hard data. So [selection committees] shouldn’t be biased about how sick your heart is, or how sick your lungs are, but there is a lot of bias regarding how good of a...how compliant you’re gonna be with your medications; what kind of family support you have; what is the level of outside, your stress, on how you’re gonna manage to take care of yourself after transplant? And sometimes you can say, ‘Well, you know what? This patient might survive a transplant, but they will [be on] chronic pain medications or taking painkillers, or at some point, they smoke marijuana, or at some point...’. So, you know, all those factors come into play, particularly from the social aspects, which are the most difficult to define. And how do you define those in a way that you don’t stigmatize your patient, and you eventually don’t provide them with an opportunity.” [surgeon] | |
| 3. “I guess the fact that we do 80, 90 transplants per year allows us to reason… Well, given the number, we can be a little more aggressive and try to help these [high-risk] patients, which in a smaller center, probably that would give them a bigger hit on their overall numbers. I guess our center, we’re large enough, and also experienced enough not to make a strong decision [for or against listing a particular patient].” [surgeon] | |
| 4. “Everything has some bias involved, bias of the treatment team, bias of the center treating, how big your program is, how experienced are your surgeons, what is your workforce, and what can you take care of, because the same patient can be looked after in a different center [and] based on how they can do things there and the complexity of the patient, if they’re used to dealing with better, maybe they can pull it off as opposed to a center which is not used to doing that many transplants or it doesn’t have the facility to do that. So, I think the bias does exist in one way or the other based on available resources and experience of that center.” [surgeon] | |
| 5. “The other half of that is in when it comes to candidates. I think there is an awareness of what our current kind of mortality and waitlist statistics are as to whether we’re really capable of absorbing a really high-risk candidate. So we’re a really aggressive center, I think, compared to other centers too. But even at a certain point, if you’ve had a string of bad outcomes or if you’ve had some waitlist deaths and somebody comes up to the [listing] committee who is a marginal candidate who has the potential to have a bad outcome, depending on where the program is, I think we have to take a harder look at that case and say like, can we absorb another bad outcome in those cases? So, I think there’s a perception that it affects both candidates and recipients.” [pulmonologist] |
Table 5.
Comparing pulmonologists’ and surgeons’ perspectives on differential selection in lung transplantation
| Theme | Exemplar Quote |
|---|---|
| Optimizing the recipient | 17. “I think the main thing that we’re looking to do, and I’m not sure it’s the LAS number, or severity. I think we’re certainly always looking to maximize... I mean, maximize isn’t the right word. I think we want to be sure that the score as accurately as possible reflects the patient’s severity of illness. So whether that’s updating various testing parameters periodically, it’s certainly an awareness of the patient’s underlying condition and relatively aggressive assessment of changes to that condition in an effort to try to optimize the allocation score.” [pulmonologist] |
| Optimizing the donor | 18. “The lung allocation score only creates the potential offer. It doesn’t control the quality of the offer. If you have a situation with a patient with a high score who is likely to become a candidate for a number of offers, then a lung that is marginal may not be readily accepted. And instead, one would say, ‘Hold out for a better organ, so we have a better outcome proposition’. But what is a better organ is in the eye of the beholder. And there is some science, but no real class evidence or truly binding guidelines or regulations feasible because it remains a big black box. It’s just experienced surgeons and physicians trying to extrapolate data that helps them decide what would or wouldn’t be a functional organ. Because you want to avoid the high-risk recipient with a marginal donor lung because the combination makes for an extremely difficult postoperative course, in the vast majority of cases, and therefore, increases dramatically your chance of 30-day mortality or 1-year mortality.” [surgeon] |
Definition of abbreviation: LAS = lung allocation score.
Figure 1.
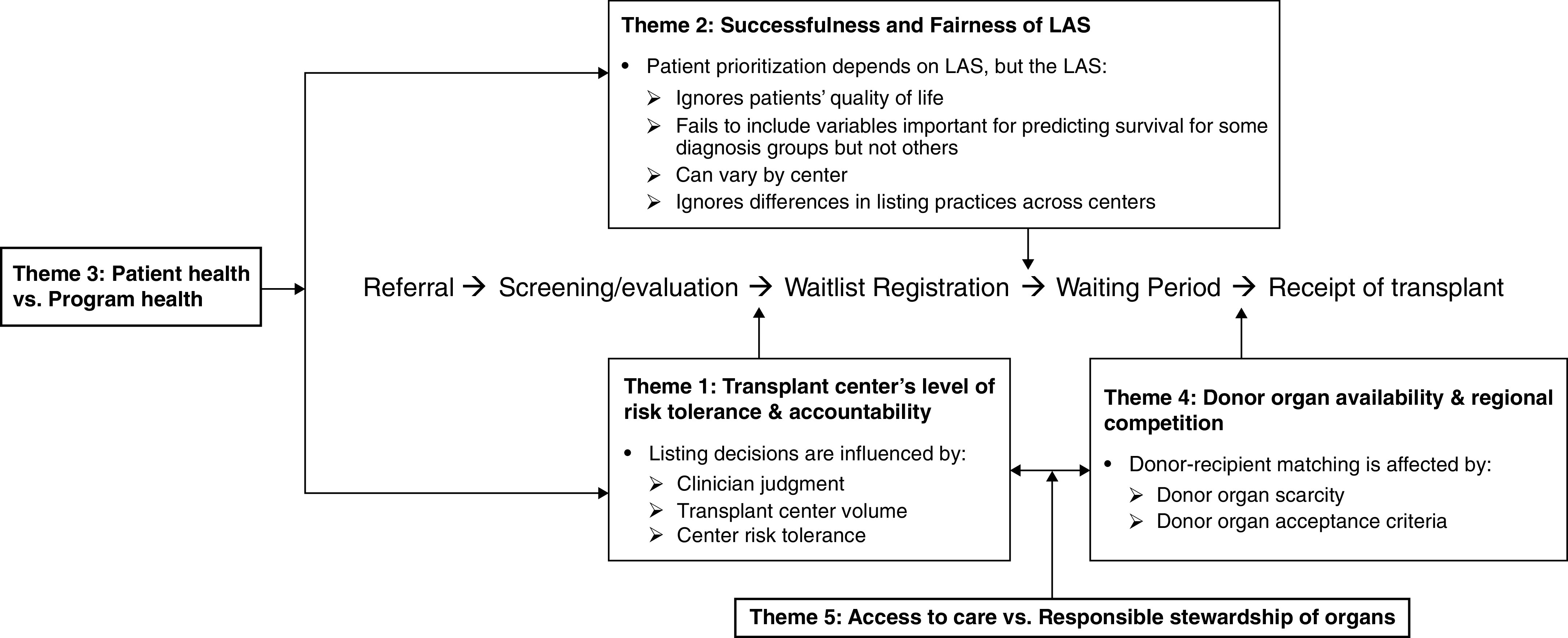
Conceptual model depicting points at which differential selection can arise in lung transplantation and synthesizing these sources of differential selection into common themes and relationships between them. LAS = lung allocation score.
Screening to Waitlist Registration
Respondents identified several points where differential selection influences lung transplantation. Although referral, which depends on how knowledgeable patients and their primary pulmonary providers are about transplantation, was recognized as a source of differential selection, respondents primarily focused on subsequent sources because they are not typically involved in the referral. Thus, respondents identified candidate screening and waitlist registration as the first major source of differential selection.
Screening involves a series of physical and mental health evaluations conducted by transplant centers to determine whether patients are sick enough to be registered on the waitlist yet healthy enough to withstand transplant and whether patients have sufficient social and financial support to undergo such a major procedure. Respondents called this timeframe the patients’ “transplant window” and described how patients listed too early or late with respect to this window may have difficulty finding a suitable donor–organ match. Although professional societies and consensus documents provide guidance on the appropriate timing and criteria for listing patients, respondents suggested that listing decisions ultimately come down to clinician judgment and subjective impression (Table 2, quote 1). Differential selection can also arise more subtly when evaluating patients’ extent of social and financial support, their degree of adherence to protocols, and how these factors might impact their transplantation outcomes (Table 2, quote 2).
Respondents suggested that listing decisions are also influenced by transplant centers’ degree of risk tolerance within the context of accountability-promoting regulatory pressures. Decisions about individual patients are made with the center’s “numbers” (e.g., average 1-year posttransplant survival calculated by the SRTR [Scientific Registry of Transplant Recipients] and acceptable performance thresholds determined by UNOS and/or CMS [Centers for Medicare and Medicaid Services]) in mind (Table 2, quote 3). Programs with larger transplant volumes may have more experienced surgical teams and increased access to medical technology (e.g., extracorporeal membrane oxygenation), which may enable them to manage high-risk patients more easily. Respondents viewed program capacity and expertise as one potential influence on decision-making (Table 2, quote 4). In addition, large transplant volume effectively dilutes the impact that poor patient outcomes might have on program-level pre- or posttransplant survival (Table 2, quote 5). Thus, large-volume centers may have greater flexibility when choosing which patients to register on their waitlist, whereas small-volume centers may be forced to be more selective to promote the continued well-being of their program.
Waitlist Registration to Waiting Period
Respondents identified waitlist registration and waiting period as the second major source of differential selection. These periods are ostensibly determined by the LAS, which aims to minimize waitlist mortality by prioritizing patients for whom transplant benefit (i.e., predicted 1-year posttransplant survival minus predicted 1-year pretransplant survival) exceeds waitlist urgency (i.e., predicted 1-year pretransplant survival); equivalently, the LAS prioritizes individuals for whom predicted 1-year posttransplant survival minus two times predicted 1-year pretransplant survival is favorable. Because the score is calculated on the basis of patient demographic and clinical characteristics, some respondents viewed the LAS as a “byproduct” that reflects the patients’ medical urgency but does not on its own influence providers’ decisions to proceed with waitlist registration or transplantation (Table 3, quote 6). Some respondents lauded the LAS’s ability to update as patients become sicker, thereby ensuring that patients receive more donor–organ offers as their waitlist urgency increases, and hence, mitigating survivor bias (because donor–organ offers are diverted away from individuals who are more likely to survive without transplant and toward individuals who are less likely to survive without transplant) (Table 3, quote 7).
Table 3.
Exemplar quotes pertaining to the transition between waitlist registration and waiting period
| Theme | Exemplar Quote |
|---|---|
| Successfulness and fairness of the LAS | 6. “The score is something that I think we’re all cognizant of in the back of our minds, but it’s not necessarily a factor to say someone should or shouldn’t be a candidate. Because the LAS is almost a byproduct, right? Someone has to be a transplant candidate, then they get the LAS score for whatever it is […] The LAS will be just whatever it is based on the specific tests of that patient.” [pulmonologist] |
| 7. “If someone’s disease advances and they end up on mechanical ventilation or something like that, their LAS goes through the roof, and they’re going to get an organ. So in many ways, the LAS mitigates survivor bias.” [pulmonologist] | |
| 8. “Yeah, it [survivor bias] is a concern. And it actually is a concern that I primarily have in regards to patients that have a relatively low score at the time of listing, especially patients with emphysema. You know, since the lung allocation score favors patients with interstitial lung disease, patients with emphysema usually get a lower score. And we certainly have a number of patients on our waitlist where I kind of wonder, ‘are they ever going to get lungs?’ And, you know, are they eventually going to be too old, or are they going to develop comorbidities that would prevent them from being suitable for transplant anymore?” [pulmonologist] | |
| 9. “Everybody is trying to get an angle for their own patients, you know, everybody feels responsible to find a strategy for their patient to get the transplant. So, they’re gonna think of things that they can do that are, in general, are almost always, I think, are honest and have integrity. I don’t think people cheat in any substantial degree, I guess, but they will use every honest angle that is available. We see that in all of our lists. But that’s what you want your doctor to do for you, right? We assume that we’ll be audited, so anything we do, we want to be able to justify and explain why or that we were completely honest with how we did it.” [surgeon] | |
| 10. “At [previous transplant center], we had this requirement that you had to be able to walk 1,000 feet in 6 min to qualify for listing. So we would give them as much oxygen as they needed, and we would push them to walk really far. And then when they changed the LAS algorithm and we saw, like, all…that, like, these patients, these people who are really, really sick, with really advanced lung disease, their scores went down significantly, and we weren’t getting offers for them anymore, then we had to change how we did it. And we had the resources there to say, ‘Okay, we’re going to do an LAS 6-minute-walk test’, which is where we did a 6-minute-walk test based on their resting oxygen requirements. But then, we would continue to do a 6-minute-walk test to assess their functional capacity. And so we would have both pieces of information, which was helpful, and then we’d use the LAS 6-minute-walk to put into a unit. Here, we don’t have those kinds of resources to be doing it twice. And so we just accept the fact that, well, we do it on something sort of in the middle and try to interpret the data as best we can.” [pulmonologist] | |
| 11. “I think the [LAS] score is reasonable, but how people populate their lists is very variable, and my concern is that people lean on the score as being a vetted, objective, consistent measure of priority, and it’s not. People will use different variables to their advantage, and listing practices are so variable that we can’t assume that an LAS of 40 means the same thing at different centers. In fact, we’ve seen patients who go to different centers have very different lung allocation scores. And when you have variability in interpretation of how to score someone, it makes the concept of broader regional sharing grossly unfair and vulnerable to gaming. This big push for broader regional sharing has to be predicated upon making listing behaviors entirely consistent across the country, or there will be gross iniquities manifest.” [surgeon] | |
| 12. “Well, the [program-level] metrics are grossly imperfect. And the reason is that there are centers that will only list one patient who is size and blood type available in a given range, a given sort of size and blood-type parameters. We don’t do that. So, we...if someone meets criteria and is listable, in a practical and medically appropriate sense, they get on the list. So, we run a large list and a pared-down list, and we do that to maintain a sense of connectivity and consideration of everyone who’s on our list. The problem is, the metric that you’re talking about is called the transplant rate, and it’s not only determined by how many transplants you do, it’s determined by the size of your list. So if I do a hundred transplants, and my transplant list is 100 patients long, I’m going to look like I’m less busy than someone who maintains a list of 10 patients and does 20 transplants per year. So it’s… it’s… It’s a thing that gets often manipulated, and it’s not an indication of how busy or aggressive a center is doing, but it’s related to more the size of that list. It’s called a gameable statistic.” [surgeon] |
Definition of abbreviation: LAS = lung allocation score.
Other respondents, however, suggested that the LAS is imperfect, mitigating some, but not all, differential selection. Specific concerns include 1) the LAS relies on predicted 1-year pre- and posttransplant survival, which may not be as relevant to patients or clinicians as longer-term survival or quality of life (e.g., focusing on 1-year posttransplant survival, as opposed to 3- or 5-year posttransplant survival, may divert donor organs away from candidates who could potentially gain more years of life from transplantation); 2) the LAS omits variables that are clinically relevant for predicting survival among some diagnoses, but not statistically significant across all diagnoses (e.g., forced expiratory volume in 1 second); and 3) discrepancies in LAS among patients dual-listed at multiple centers.
These perceived “gaps” in the LAS led many respondents to question the objectivity of lung allocation for particular patients. One respondent described being more concerned about survivor bias for patients with a low LAS than for patients with a high LAS, as the former tends to remain on the waitlist behind the latter (i.e., they never receive sufficient priority to secure a donor organ offer), eventually becoming too old or sick for transplantation (Table 3, quote 8). The challenge of advocating for patients within the LAS framework can lead to differences in patient management practices across centers.
These differences prompted concerns about the potential for gaming or exploiting the ambiguity and flexibility of the LAS to achieve desired outcomes while ostensibly honoring the framework. Two perspectives emerged: 1) patient-level: manipulating patients’ LAS to increase their chances of receiving a transplant by capitalizing on “system inefficiencies” (e.g., variables that are open to clinical interpretation and that can be changed while maintaining honesty); and 2) program-level: avoiding transplanting high-LAS patients or being more conservative with donor–organ acceptance criteria to ensure that program-level accountability metrics are maintained.
The first perspective, described as patient advocacy, involves ensuring that patients receive scores that truly reflect their waitlist urgency (Table 3, quote 9). Having the flexibility to update scores as patients’ clinical condition changes was seen as a positive attribute of the LAS. All respondents said that changes in patients’ LAS must be documented because centers are subject to audits from UNOS, CMS, and insurance companies. However, respondents mentioned that guidelines around some variables are vague (e.g., how much oxygen to use for the 6-minute-walk test) and may even have conflicting goals (e.g., the walk test assesses both patients’ disease severity and their ability to withstand transplantation). If centers have enough resources, they can conduct such tests multiple times to fulfill all goals, but if not, compromises must be made (Table 3, quote 10). Respondents also acknowledged that distinguishing between acceptable and unacceptable patient management strategies can sometimes be difficult: “it’s hard to define gaming the system versus differences in management practices, where that line is drawn”.
Respondents generally supported the idea behind exception requests, which allow physicians to petition UNOS to increase patients’ scores when the LAS does not seem to accurately capture their clinical severity or urgency. However, some expressed frustration at the inconsistency with which certain exception requests are evaluated (exception requests are reviewed by a rotating board of clinicians, and acceptance criteria vary). Others expressed concern that dual-listed patients sometimes had different LAS in different centers (this observation was attributed to a lack of communication and/or data sharing among centers rather than intentional dishonesty). Inconsistencies in LAS, while not necessarily indicative of gaming, were still viewed as having a considerable impact on donor–organ sharing, as even small differences in LAS can pull donor organs from one center to another (Table 3, quote 11).
The second perspective, conceptualized as program advocacy, involves selectivity when making listing decisions to ensure that programs do not take on patients who are too high risk for transplant teams to manage. Although such selectivity could be viewed as “cherry picking”, most respondents felt that it is sometimes necessary to preserve program integrity and ensure that program-level ratings surpass UNOS and/or CMS’s acceptability thresholds. Choosing not to list high-risk patients whom the transplant team may not be able to care for was often viewed as a favorable form of program advocacy, as it not only protects program-level metrics and integrity but also protects patients from experiencing poor transplant outcomes and ensures responsible stewardship of donor organs.
Another type of program advocacy that was viewed less favorably relates to the size of each program’s waitlist. More specifically, three surgeons suggested that well-resourced programs may operate “substitute” lists where individuals who are not currently sick enough for official listing are monitored until they become eligible. Problems with this approach arise when substitute lists are not shared with UNOS, as programs can adjust the size of their waitlist to influence their program-level transplant rate (Table 3, quote 12).
Waiting Period to Receipt of Transplant
The third major source of differential selection identified by respondents occurs between the waiting period and transplantation. Here, donor–organ availability and regional competition influence who receives a transplant (Table 4, quote 13). Specifically, transplant centers in less-competitive regions may have more flexibility in determining which patients receive which organs because they do not have to compete against other centers with patients with higher LAS. Moreover, the current allocation system encourages transplant centers to list high-risk patients but discourages centers from accepting high-risk or marginal-quality donor organs. Consequently, programs with patients primarily with lower LAS are forced to accept donor organs that were turned down by centers with patients with higher LAS: “it is unfair that you can have a very high LAS score and you could decide whatever lungs you wanna take”. Respondents acknowledged that assessments of donor organ quality could be subjective and admitted that they had no proof of higher-volume centers being overly selective with their donor organ acceptance criteria. However, they also indicated that when the best-quality donor organs continually go to higher-volume centers, “it makes you second guess the system”.
Table 4.
Exemplar quotes pertaining to the transition between waiting period and receipt of transplant
| Theme | Exemplar Quote |
|---|---|
| Donor organ availability and regional competition | 13. “I think it depends on how easy access to organs your center has and how much competition too for those organs you have. Because if you’re in a 250-mile radius, and you have 10 centers in that radius, then you’re competing for the same organs across 10 centers. If, instead, you had three centers in that radius, or two or one, then you have a lot of offers for your patients. And so, you can wait until he gets sicker or you just transplant him, or you can put them on an LAS very low, and you still will have first bid to those organs.” [surgeon] |
| Patient health vs. program health | 14. “These regulatory metrics, 30-day, 1-year mortality, have a very heavy impact on how surgeons and pulmonologists, number one, list patients, it becomes a selective bias. And number two, how they transplant them (i.e., when they get an offer, they may be very risk-averse in certain settings or with certain donors or with any additional confounders that are encountered at the time of the offer.” [surgeon] |
| 15. “We’re handcuffed because there are no donor organs, our cohort is getting sicker and sicker, and conditional survival based on your frailty or condition at the time of transplant is compromised. [With a] precious resource that’s in short supply, your cohort is likely to get sicker, and the outcomes are likely to be worse so that the longer they sit on the list, the worse the outcomes are.” [surgeon] | |
| Access to care vs. responsible stewardship of resources | 16. “I think we are intentionally biased to transplant patients that we think are gonna survive…because it’s a scarce resource. If you’re thinking about providing a treatment that’s a scarce resource that might compromise access to other patients, then I think it is reasonable to consider giving it to the patients where it may be more likely to be efficacious.” [surgeon] |
Definition of abbreviation: LAS = lung allocation score.
These concerns highlight a tension between improving patients’ health versus maintaining transplant programs’ health. Such tension was often described as being driven by program-level UNOS and/or CMS accountability metrics (Table 4, quote 14). Although centers can adjust their patient listing and donor–organ acceptance criteria to balance patient versus program health, the successfulness of these efforts is constrained by donor–organ availability (Table 4, quote 15). To many respondents, donor–organ scarcity implied an ethical duty to select patients more likely to survive transplantation to ensure the responsible stewardship of resources (Table 4, quote 16).
Conceptual Model
The sources of differential selection discussed above were synthesized into five main themes (Figure 1): 1) transplant center’s degree of risk tolerance and accountability; 2) successfulness and fairness of the LAS; 3) patient versus program health; 4) donor–organ availability and regional competition; and 5) access to care versus responsible stewardship of organs. Most themes were endorsed by both transplant surgeons and pulmonologists, lending further credibility to our conceptual model. However, respondents generally discussed differential selection from the perspective most aligned with their role in the transplant process. Specifically, pulmonologists, who provide longitudinal patient care before and after transplantation, are typically focused on “optimizing the recipient” so that the best patient is selected for each donor organ (Table 5, quote 17). Conversely, transplant surgeons, who frequently procure donor organs in addition to performing the transplant operation, typically focus on “optimizing the donor” so that the best donor organ is selected for each patient (Table 5, quote 18).
Discussion
On the basis of semistructured interviews, we developed a conceptual model (Figure 1) of transplant selection that contains five themes: 1) transplant center’s degree of risk tolerance and accountability; 2) successfulness and fairness of the LAS; 3) patient versus program health; 4) donor–organ availability and regional competition; and 5) access to care versus responsible stewardship of organs.
Although most respondents recognized that the LAS is partially successful in mitigating survivor bias at transplantation (because patients’ LAS scores, and hence, their chances of receiving donor organ offers, increase as their waitlist urgency increases), many also suggested that it ignores upstream sources of differential selection that influence which patients are registered on the waitlist and remain active candidates. Upstream sources of differential selection are typically framed as an “access to care” problem and not an issue of “differential selection”. For example, disparities in waitlist registration are often attributed to inequities in referrals without consideration of inconsistencies in screening or listing decisions. Although screening and listing guidelines exist (6, 7), recent research has advocated for earlier referral of specific patient populations (e.g., cystic fibrosis) (37–39) and greater transparency around factors that inform patient selection but which are not included in the LAS (e.g., frailty [7, 23, 40], social support [7, 23, 41, 42], and quality of life [7, 23, 42]). Our respondents suggest that the extent to which these criteria matter during listing decisions depends on centers’ transplant volume and degree of risk tolerance, as well as considerations of potential tradeoffs between patient and program health. For example, relaxing listing criteria can provide higher-risk patients with more transplant opportunities but may also necessitate greater patient management and/or donor–organ selectivity to maximize patients’ chances of surviving surgery and minimize their risk of posttransplant complications (e.g., graft rejection) (43). Conversely, whereas listing less-urgent patients lessens program-level risk, it may also hinder transplant access for higher-risk patients, who might not have enough social or financial resources to be listed at another center (44).
Such findings are consistent with and add significantly to research on decision-making in organ transplant selection committees (23, 24). Prior studies included few transplant surgeons and pulmonologists; focused solely on candidate screening rather than the complete pathway from referral through transplantation; did not examine how patients’ LAS might influence candidate selection; and only considered patient-level (not program-level) factors that might influence decision-making (23, 24). Our study also complements statistical research that addresses censoring among waitlisted patients (e.g., because of death, transplantation, etc.) (19–22, 45) and has begun to investigate the impact of delisting on patients’ long-term health (46).
Research on the receipt of transplants primarily focuses on geographic disparities in donor lung availability (9, 16, 17) and the role organ procurement organizations have in increasing donor–organ supply through broader organ sharing (47) and better procurement practices (48, 49). Yet transplant programs ultimately have the final say over which donor organs they accept, with some programs being more willing to accept marginal-quality organs (50–52) than others (25, 53). Research on pancreas donors in Germany concluded that the evaluation of donor organs was “highly inconsistent” across transplant centers and that variability in acceptance criteria can decrease the efficiency of allocation (25). For example, accepting marginal-quality donor organs can enable more patients to receive a transplant but may come at the cost of more posttransplant complications or reduced posttransplant survival (54). Such conflicts between access to care and responsible stewardship of organs have both practical and ethical implications (5, 55). Thus, understanding how clinicians decide which donor organs to accept, and making this source of differential selection more transparent, is just as important as increasing donor–organ supply.
Strengths and Limitations
Our study has several strengths. Rigorous qualitative analyses allowed us to capture a diversity of perspectives on differential selection in lung transplantation and the LAS. Including transplant surgeons and pulmonologists from transplant centers throughout the United States strengthens the robustness of our conceptual model and allows us to understand how center volume and location may shape screening, waitlisting, and transplantation decisions. Finally, considering the entire pathway from referral through transplantation enabled examination of how patient- and program-level factors influence differential selection in lung transplantation.
Our study also has limitations. First, the coronavirus pandemic necessitated switching from in-person to phone interviews midway through data collection. This change did not impact interview quality and actually increased surgeons’ participation rate. Second, the qualitative design limits generalizability to the concerns of all transplant providers. However, we purposively sampled by discipline (transplant surgeon and pulmonologist) and sought to include respondents with diverse characteristics relevant to our study question (e.g., geography and years in practice). Third, whereas our response rate was low, we were able to obtain thematic saturation both overall and within subgroups defined by discipline. Fourth, although 84% of respondents were male, this proportion is consistent with the distribution of men in the transplant workforce (56–58). Fifth, whereas some UNOS regions were underrepresented in our study, we were able to interview at least one respondent from each region and also interviewed respondents from a variety of center sizes and transplant volumes. Sixth, our purposive sampling approach and low response rate may have resulted in selection bias. Individuals who declined to participate in our study were fairly comparable to those who agreed to participate in terms of gender and region but were more likely to be transplant surgeons. Finally, our study was restricted to the U.S. lung allocation system, which relies on the LAS to prioritize patients for transplantation. Consequently, our findings around the LAS may not generalize to lung transplant allocation systems in other countries that do not use the LAS.
Conclusions
Overall, our study demonstrates how differential selection can arise throughout lung transplantation, and the proposed conceptual model can facilitate further study of such selection. Although this conceptual model was developed among lung transplant surgeons and pulmonologists, it may be applicable to other organ allocation systems that aim to determine which patients to register for transplant and how to prioritize them. With respect to lung transplantation, OPTN is currently developing a new allocation framework, the “continuous distribution model” (26–28), that will prioritize patients via composite scores consisting of the LAS alongside other patient attributes, such as candidate biology, patient access, and placement efficiency (26). This approach is a first step toward recognizing the complexity of organ allocation (59, 60) and improving equity in transplantation (8). However, our findings suggest that the other attributes included in the composite score may be susceptible to differential selection as well. Thus, the continuous distribution model should be monitored closely to ensure that the resulting allocation scheme is more equitable and does not inadvertently exacerbate differential selection in transplantation.
Acknowledgments
Acknowledgment
The authors thank the interview respondents for their willingness to participate in this study.
Footnotes
Supported by the National Heart, Lung, and Blood Institute (F31HL194338). The funder had no role in the design of the study or the collection, analysis, and interpretation of the data, or in the writing of the manuscript. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Author Contributions: All authors contributed to the study’s conception and design. E.M.S. carried out the qualitative interviews and analyses, drafted the initial manuscript, and revised it on the basis of the critical review and scientific input of E.C., S.E.K., and J.E.S.
Data availability statement: The data that support the findings of this study are available on request to the corresponding author, Ms. Erin M. Schnellinger. Data are not publicly available because of privacy and ethical restrictions.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transplant . 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 2. Gottlieb J. Lung allocation. J Thorac Dis . 2017;9:2670–2674. doi: 10.21037/jtd.2017.07.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health & Human Services. 42 CFR part 121: organ procurement and transplantation network final rule, 56649-56661;1999 [accessed 2022 Sep 11]. Available from: https://www.govinfo.gov/content/pkg/FR-1999-10-20/pdf/FR-1999-10-20.pdf.
- 4.A guide to calculating the lung allocation score. United Network for Organ Sharing https://unos.org/wp-content/uploads/unos/lung-allocation-score.pdf.
- 5.Veatch RM, Ross LF. Transplantation ethics. Washington, DC: Georgetown University Press; 2015. [Google Scholar]
- 6. Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant . 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 7. Leard LE, Holm AM, Valapour M, Galnvill AR, Attawar S, Aversa M, et al. Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant . 2021;40:1349–1379. doi: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Research Council. Realizing the promise of equity in the organ transplantation system. Washington, DC: The National Academies Press; 2022. [PubMed] [Google Scholar]
- 9. Ross-Driscoll K, Axelrod D, Lynch R, Patzer RE. Using geographic catchment areas to measure population-based access to kidney transplant in the United States. Transplantation . 2020;104:e342–e350. doi: 10.1097/TP.0000000000003369. [DOI] [PubMed] [Google Scholar]
- 10. Mooney JJ, Hedlin H, Mohabir P, Bhattacharya J, Dhillon GS. Racial and ethnic disparities in lung transplant listing and waitlist outcomes. J Heart Lung Transplant . 2018;37:394–400. doi: 10.1016/j.healun.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wille KM, Harrington KF, deAndrade JA, Vishin S, Oster RA, Kaslow RA. Disparities in lung transplantation before and after introduction of the lung allocation score. J Heart Lung Transplant . 2013;32:684–692. doi: 10.1016/j.healun.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thabut G, Munson J, Haynes K, Harhay MO, Christie JD, Halpern SD. Geographic disparities in access to lung transplantation before and after implementation of the lung allocation score. Am J Transplant . 2012;12:3085–3093. doi: 10.1111/j.1600-6143.2012.04202.x. [DOI] [PubMed] [Google Scholar]
- 13. Russo MJ, Worku B, Iribarne A, Hong KN, Yang JA, Vigneswaran W, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg . 2011;141:1270–1277. doi: 10.1016/j.jtcvs.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 14. Maxwell BG, Levitt JE, Goldstein BA, Mooney JJ, Nicolls MR, Zamora M, et al. Impact of the lung allocation score on survival beyond 1 year. Am J Transplant . 2014;14:2288–2294. doi: 10.1111/ajt.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant . 2016;35:433–439. doi: 10.1016/j.healun.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 16. Benvenuto LJ, Anderson DR, Kim HP, Hook JL, Shah L, Robbins HY, et al. Geographic disparities in donor lung supply and lung transplant waitlist outcomes: a cohort study. Am J Transplant . 2018;18:1471–1480. doi: 10.1111/ajt.14630. [DOI] [PubMed] [Google Scholar]
- 17. Drolen C, Cantu E, Goldberg HJ, Diamond JM, Courtwright A. Impact of the elimination of the donation service area on United States lung transplant practices and outcomes at high and low competition centers. Am J Transplant . 2020;20:3631–3638. doi: 10.1111/ajt.16098. [DOI] [PubMed] [Google Scholar]
- 18.Glymour MM, Greenland S. In: Modern epidemiology. 3rd. Rothman KJ, Greenland S, Lash TL, editors. Philadelphia, PA: Lippincott Williams & Wilkins; 1994. Survivor bias; pp. 197–198. [Google Scholar]
- 19. Schnellinger EM, Cantu E, Harhay MO, Schaubel DE, Kimmel SE, Stephens-Shields AJ. Mitigating selection bias in organ allocation models. BMC Med Res Methodol . 2021;21:191. doi: 10.1186/s12874-021-01379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiang F, Murray S. Restricted mean models for transplant benefit and urgency. Stat Med . 2012;31:561–576. doi: 10.1002/sim.4450. [DOI] [PubMed] [Google Scholar]
- 21. Vock DM, Tsiatis AA, Davidian M, Laber EB, Tsuang WM, Finlen Copeland CA, et al. Assessing the causal effect of organ transplantation on the distribution of residual lifetime. Biometrics . 2013;69:820–829. doi: 10.1111/biom.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vock DM, Durheim MT, Tsuang WM, Copeland CAF, Tsiatis AA, Davidian M, et al. Survival benefit of lung transplantation in the modern era of lung allocation. Ann Am Thorac Soc . 2017;14:172–181. doi: 10.1513/AnnalsATS.201606-507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blumenthal NP, Petty MG, McCorkle R. Missing domains of lung transplant patient selection. Prog Transplant . 2017;27:90–97. doi: 10.1177/1526924816679840. [DOI] [PubMed] [Google Scholar]
- 24. Volk ML, Biggins SW, Huang MA, Argo CK, Fontana RJ, Anspach RR. Decision making in liver transplant selection committees: a multicenter study. Ann Intern Med . 2011;155:503–508. doi: 10.1059/0003-4819-155-8-201110180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loss J, Drewitz KP, Schlitt HJ, Loss M. Accept or refuse? Factors influencing the decision-making of transplant surgeons who are offered a pancreas: results of a qualitative study. BMC Surg . 2013;13:47. doi: 10.1186/1471-2482-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.OPTN. Continuous distribution. US Department of Health & Human Services https://optn.transplant.hrsa.gov/governance/policy-initiatives/continuous-distribution/
- 27. Snyder JJ, Salkowski N, Wey A, Pyke J, Israni AK, Kasiske BL. Organ distribution without geographic boundaries: a possible framework for organ allocation. Am J Transplant . 2018;18:2635–2640. doi: 10.1111/ajt.15115. [DOI] [PubMed] [Google Scholar]
- 28. Kasiske BL, Pyke J, Snyder JJ. Continuous distribution as an organ allocation framework. Curr Opin Organ Transplant . 2020;25:115–121. doi: 10.1097/MOT.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 29.Schnellinger EM, Cantu E, Kimmel SE, Szymczak JE. Boston, MA: 2022. [Google Scholar]
- 30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care . 2007;19:349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 31.Miles MB, Huberman AM, Class of 1924 Book Fund . Qualitative data analysis: an expanded sourcebook. Newbury Park, CA: Sage; 1994. [Google Scholar]
- 32.Miles MB, Huberman AM, Saldana J. Qualitative data analysis: a methods sourcebook. 3rd. Newbury Park, CA: Sage; 2014. [Google Scholar]
- 33.Weiss RS. Learning from strangers: the art and method of qualitative interview studies. New York, NY: The Free Press; 1994. [Google Scholar]
- 34. Brod M, Tesler LE, Christensen TL. Qualitative research and content validity: developing best practices based on science and experience. Qual Life Res . 2009;18:1263–1278. doi: 10.1007/s11136-009-9540-9. [DOI] [PubMed] [Google Scholar]
- 35. Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol . 2013;13:117. doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deterding NM, Waters MC. Flexible coding of in-depth interviews: a twenty-first-century approach. Sociol Methods Res . 2021;50:708–739. [Google Scholar]
- 37. Ramos KJ, Smith PJ, McKone EF, Pilewski JM, Lucy A, Hempstead SE, et al. Lung transplant referral for individuals with cystic fibrosis: Cystic Fibrosis Foundation consensus guidelines. J Cyst Fibros . 2019;18:321–333. doi: 10.1016/j.jcf.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stephenson AL, Ramos KJ, Sykes J, Ma X, Stanojevic S, Quon BS, et al. Bridging the survival gap in cystic fibrosis: an investigation of lung transplant outcomes in Canada and the United States. J Heart Lung Transplant . 2021;40:201–209. doi: 10.1016/j.healun.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mooney JJ, Yang L, Hedlin H, Mohabir P, Dhillon GS. Multiple listing in lung transplant candidates: a cohort study. Am J Transplant . 2019;19:1098–1108. doi: 10.1111/ajt.15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schaenman JM, Diamond JM, Greenland JR, et al. Frailty and aging-associated syndromes in lung transplant candidates and recipients. Am J Transplant . 2021;21:2018–2024. doi: 10.1111/ajt.16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ladin K, Emerson J, Berry K, Butt Z, Gordon EJ, Daniels N, et al. Excluding patients from transplant due to social support: results from a national survey of transplant providers. Am J Transplant . 2019;19:193–203. doi: 10.1111/ajt.14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dew MA, DiMartini AF, Dobbels F, Grady KL, Jowsey-Gregoire SG, Kaan A, et al. The 2018 ISHLT/APM/AST/ICCAC/STSW recommendations for the psychosocial evaluation of adult cardiothoracic transplant candidates and candidates for long-term mechanical circulatory support. J Heart Lung Transplant . 2018;37:803–823. doi: 10.1016/j.healun.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 43. Hart A, Engels EA. Balancing uncertain risks in candidates for solid organ transplantation with a history of malignancy: who is safe to transplant? Am J Transplant . 2021;21:447–448. doi: 10.1111/ajt.16366. [DOI] [PubMed] [Google Scholar]
- 44. Wagener G. Multiple listings: good for a few, but no solution for the organ shortage. Transplantation . 2020;104:671–672. doi: 10.1097/TP.0000000000002966. [DOI] [PubMed] [Google Scholar]
- 45. Xiang F, Murray S, Liu X. Analysis of transplant urgency and benefit via multiple imputation. Stat Med . 2014;33:4655–4670. doi: 10.1002/sim.6250. [DOI] [PubMed] [Google Scholar]
- 46. Rudasill SE, Sanaiha Y, Kwon M, Mardock AL, Khoury H, Omari B, et al. Understanding lung transplant listing practices: survival in lung transplant candidates who improve clinically to delisting. Surgery . 2019;166:1142–1147. doi: 10.1016/j.surg.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 47. Mooney JJ, Bhattacharya J, Dhillon GS. Effect of broader geographic sharing of donor lungs on lung transplant waitlist outcomes. J Heart Lung Transplant . 2019;38:136–144. doi: 10.1016/j.healun.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halpern SE, McConnell A, Peskoe SB, Raman V, Jawitz OK, Choi AY, et al. A three-tier system for evaluation of organ procurement organizations' willingness to pursue and utilize nonideal donor lungs. Am J Transplant . 2021;21:1269–1277. doi: 10.1111/ajt.16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doby BL, Hanner K, Johnson S, Purnell TS, Shah MB, Lynch RJ. Results of a data-driven performance improvement initiative in organ donation. Am J Transplant . 2020;21:2555–2562. doi: 10.1111/ajt.16442. [DOI] [PubMed] [Google Scholar]
- 50. Van Pilsum Rasmussen SE, Seaman S, Brown D, Deasi N, Sulkowski M, Segev DL, et al. Patient's perspectives of experimental HCV-Positive to HCV-negative renal transplantation: report from a single site. AJOB Empir Bioeth . 2020;11:40–52. doi: 10.1080/23294515.2019.1670277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levitsky J, Formica RN, Bloom RD, Charlton M, Curry M, Friedewald J, et al. The American Society of Transplantation Consensus Conference on the use of hepatitis C viremic donors in solid organ transplantation. Am J Transplant . 2017;17:2790–2802. doi: 10.1111/ajt.14381. [DOI] [PubMed] [Google Scholar]
- 52. Whitford H, Kure CE, Henriksen A, Hobson J, Snell GI, Levvey BJ, et al. A donor PaO2/FiO2 < 300 mm Hg does not determine graft function or survival after lung transplantation. J Heart Lung Transplant . 2020;39:53–61. doi: 10.1016/j.healun.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 53. Van Pilsum Rasmussen SE, Zhou S, Thomas AG, Segev DL, Nicholas LH. Transplant community perceptions of the benefits and drawbacks of alternative quality metrics for regulation. Clin Transplant . 2019;33:e13500. doi: 10.1111/ctr.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schwarz S, Rahimi N, Kifjak D, et al. Comparison of donor scores in bilateral lung transplantation-a large single-center analysis. Am J Transplant . 2020;21:2132–2144. doi: 10.1111/ajt.16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet . 2009;373:423–431. doi: 10.1016/S0140-6736(09)60137-9. [DOI] [PubMed] [Google Scholar]
- 56. Cooke DT, Olive J, Godoy L, Preventza O, Mathisen DJ, Prager RL. The importance of a diverse specialty: introducing the STS workforce on diversity and inclusion. Ann Thorac Surg . 2019;108:1000–1005. doi: 10.1016/j.athoracsur.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 57. Erhunmwunsee L, Backhus LM, Godoy L, Edwards MA, Cooke DT. Report from the workforce on diversity and inclusion-the Society of Thoracic Surgeons members' bias experiences. Ann Thorac Surg . 2019;108:1287–1291. doi: 10.1016/j.athoracsur.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 58. Valbuena VSM, Obayemi JE, Purnell TS, Scantlebury VP, Olthoff KM, Martins PN, et al. Gender and racial disparities in the transplant surgery workforce. Curr Opin Organ Transplant . 2021;26:560–566. doi: 10.1097/MOT.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O’Connor K, Glazier A. OPO performance improvement and increasing organ transplantation: metrics are necessary but not sufficient. Am J Transplant . 2021;21:2325–2326. doi: 10.1111/ajt.16545. [DOI] [PubMed] [Google Scholar]
- 60. Hauerwaas A, Weisenfeld U. The impact of systemic innovations for transforming transplant systems. Lessons learned from the German lung transplantation system. A qualitative study. Health Syst (Basingstoke) . 2019;9:76–93. doi: 10.1080/20476965.2019.1604086. [DOI] [PMC free article] [PubMed] [Google Scholar]


