Abstract
Objective
Current treatments for osteoarthritis do not resolve the underlying cause. Dextrose prolotherapy is an alternative method that has been proposed for treatment of osteoarthritis, due to its ability to aid tissue regeneration, improve clinical manifestations, and repair damaged tissue structures, which are pathological conditions in osteoarthritis. The aim of this systematic review was to evaluate the efficacy of dextrose prolotherapy compared with other interventions in the management of osteoarthritis.
Methods
Electronic databases PubMed, Google Scholar, Cochrane, and BioMed Central were searched from inception to October 2021. Search terms included [(prolotherapy) OR (prolotherapies) OR (dextrose prolotherapy)] AND [(osteoarthritis) OR (osteoarthritides) OR (knee osteoarthritis) OR (hip osteoarthritis) OR (hand osteoarthritis) OR (shoulder osteoarthritis)]. Randomized controlled trials that compared the use of dextrose prolotherapy with other interventions (injection, placebo, therapy, or conservative treatment) in the treatment of osteoarthritis were included. Potential articles were screened for eligibility, and data were extracted by all authors. Risk of bias was assessed using the Cochrane Risk of Bias tool. Study population, methods, and results data were extracted and tabulated by 3 authors.
Results
12 studies reported that DPT was as effective or even more effective in improving functional outcomes compared with other interventions whilst others found that HA, PRP, EP, and ACS were more effective. 14 studies assessed the effectiveness of DPT and ten of them reported that DPT was more effective in reducing pain compared with other interventions.
Conclusion
Dextrose prolotherapy in osteoarthritis confers potential benefits for pain and functional outcomes, but this systematic review found that the studies to date are at high risk of bias.
LAY ABSTRACT
Osteoarthritis is a long-term chronic illness defined by the degeneration of cartilage in joints, causing bones to rub together and causing stiffness, discomfort, and decreased movement. Current treatment options for osteoarthritis do not address the fundamental cause. Dextrose prolotherapy is a potential alternative approach for OA, due to its capacity to help tissue regeneration, improve clinical symptoms, and repair damaged tissue structures, which are pathogenic in osteoarthritis. Despite several comparison studies, the superiority of dextrose prolotherapy in osteoarthritis remains equivocal due to contradictory outcomes. Based on this review, dextrose prolotherapy should be considered as a possible treatment for osteoarthritis.
Key words: dextrose prolotherapy, osteoarthritis, evidence-based medicine, systematic review
Osteoarthritis (OA) is a long-term chronic condition characterized by deterioration of the cartilage in joints, which causes bones to rub together and creates stiffness, pain, and impaired movement. OA can affect any joint, but is most common in the knees, hands, feet and spine, and relatively common in the shoulder and hip joints (1). Based on the American college of rheumatology (ACR) Guideline for osteoarthritis (OA), only exercise, lifestyle modification, orthosis, knee brace, oral non-steroidal anti-inflammatory drugs (NSAID), topical NSAID, and intra-articular steroid are strongly recommended for treatment of OA (2). However, those treatment modalities do not resolve the underlying cause of OA.
Regenerative therapy is an alternative method proposed for OA, due to its capability to aid tissue regeneration, enhance clinical manifestations, and repair damaged tissue structures, which are pathological conditions in OA (3). Prolotherapy is a non-surgical regenerative injection technique, in which small amounts of an irritant solution are applied to painful sites and degenerated tendon attachments (entheses), joints, ligaments, and adjacent joint spaces during multiple treatment sessions to promote the growth of normal cells and tissues (4, 5). The most commonly used prolotherapeutic agent is dextrose, in concentrations ranging from 12.5% to 25% (6). The mechanism of action of prolotherapy is not fully understood. However, current theory suggests that the injected proliferant mimics the natural healing process of the body by initiating a local inflammatory cascade that triggers the release of growth factors and collagen deposits. This is achieved when induced cytokines mediate chemomodulation, which leads to the proliferation and strengthening of new connective tissue, joint stability, and reduction in pain and dysfunction (4, 5, 7).
Despite numerous comparison studies evaluating the effectiveness of dextrose prolotherapy (DPT) in OA, the superiority is inconclusive due to inconsistent results. Several previous systematic reviews and meta-analyses have examined the use of DPT in knee OA, but no recent studies have reported the effects of DPT in OA in general. The aim of this systematic review was to evaluate the efficacy of DPT compared with other interventions in the management of OA in all joints.
METHODS
A systematic review of relevant studies was conducted following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. This study was registered with PROSPERO (CRD42021286037).
Eligibility criteria
Inclusion criteria included: (i) all randomized trials that compared the use of DPT with other interventions (injection, placebo, therapy, or conservative treatment) in treatment of OA; (ii) participants at least 18 years of age; (iii) OA diagnosis as defined by the various study authors; (iv) follow-up duration of all time-points; (v) English language articles. Exclusion criteria were: articles other than randomized controlled trials (RCT), including reviews, case series, case reports, conference abstracts, non-human studies, and studies performed other than OA.
Search strategy
Potential studies were identified via a thorough systematic search of PubMed, Google Scholar, Cochrane databases, and BioMed Central (BMC). The search period spanned from inception to 12 October 2021. The search terms included [(prolotherapy) OR (prolotherapies) OR (proliferation therapy) OR (proliferation therapies) OR (therapies, proliferation) OR (therapy, proliferation)] AND [(osteoarthritis) OR (osteoarthritides) OR (osteoarthrosis) OR (osteoarthroses) OR (arthritis, degenerative) OR arthritides, degenerative) OR (degenerative arthritides) OR (degenerative arthritis) OR (arthrosis) OR (arthroses) OR (osteoarthroses deformans)].
Types of outcome measures
Eligible studies should include an assessment of self-reported pain or functional outcome. The primary outcome of interest is pain assessed using visual analogue scale (VAS) or numeric rating scale (NRS). The secondary outcome of interest is functional outcome and is evaluated by each functional outcome tool.
Selection process
One investigator (SRA) ran the search strategy and removed the duplicates. Two authors (SRA and INW) evaluated all titles and abstracts to determine if the articles met the inclusion criteria. The full text of potentially eligible articles was then retrieved and independently screened by the same 2 investigators. Any disagreement was resolved through mutual discussion. The third author (YW) would have the casting vote if a consensus could not be achieved. The reference lists of the full-text articles were further screened for relevant articles for inclusion.
Data collection
AT extracted the data independently, which was separately verified by AA. Disagreements on data extraction were resolved through consensus discussion between AT and AA. If a consensus could not be achieved, then a third author (YW) would have the casting vote. Relevant information from each included article was extracted and recorded in an electronic spreadsheet. These information were: first author and year of publication, sample size, mean age of participants, symptom duration, OA diagnosis methods, total number of injections, volume of injectate per dose, type of injectate, control, injection technique, interval of injection, and outcome measures.
Study risk of bias assessment
Two investigators (SRA and INW) independently assessed the methodological quality and risk of bias based on the Cochrane Handbook for Systematic Reviews of Interventions recommendations for each included study. The domains included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. The risk of bias for each domain was classified as low, high, or unclear. A trial was considered to have low risk of bias only when all key domains were rated as low. If all key domains were classified as low or unclear risk of bias, the trial was considered to have an unclear bias risk; if 1 or more key domains were classified as high risk of bias, then it was considered a trial with high bias risk (8). For risk of bias across included trials: if most information (> 50%) is from trials at low risk of bias it was classified as low risk of bias. It was considered a moderate risk of bias if most information is from trials at low or unclear risk of bias. A high risk of bias was considered if the proportion of information from trials at high risk of bias is sufficient to affect the interpretation of results (8).
RESULTS
Study selection
A total of 163 citations were identified from all searches, and 62 duplicates were excluded. The titles and abstracts of the remaining studies were screened, leaving 47 studies for retrieval, but only 25 were assessed for eligibility. Of these, 11 studies were excluded for the following reasons: no other intervention (n = 3), narrative review (n = 1), non-OA (n = 1), combined prolotherapy (n = 1), no functional outcome assessment (n = 1), publication in Arabic language (n = 1), non-RCT (n = 1), poster (n = 2). Fourteen studies were eligible for systematic review, 11 evaluated knee OA, 2 hand OA, and 1 study hip OA (Fig. 1).
Fig. 1.
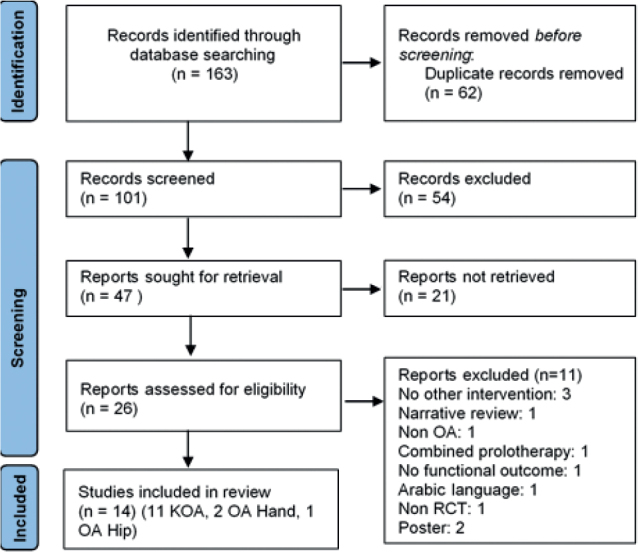
Flow chart. OA: osteoarthritis; KOA: knee osteoarthritis; RCT: randomized controlled trial.
Risk of bias
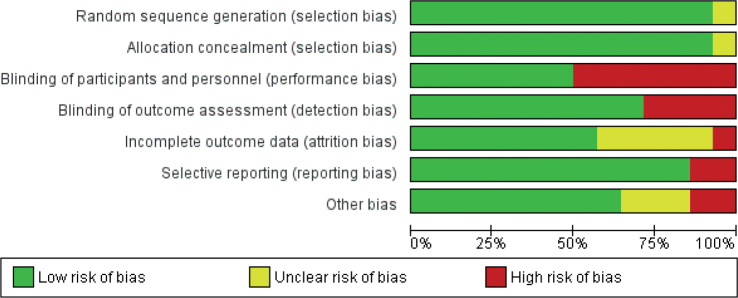
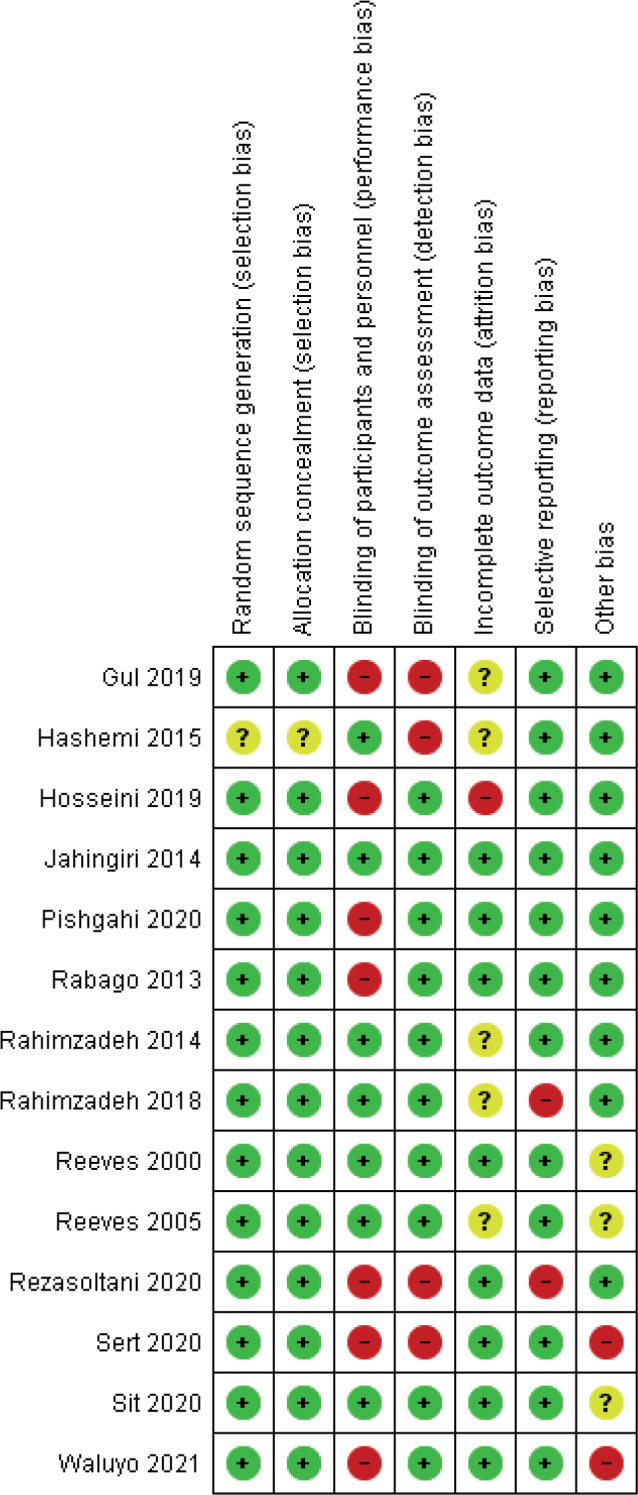
The results of the risk of bias assessment are shown in Fig. 2. One study (9) had uncertain risk in terms of selection bias due to an unclear explanation of randomization and allocation process. Seven studies (10–16) were classified as high risk in terms of performance bias because there was no blinding for participants and these studies applied different techniques or treatments to the participants. Four studies (9, 11, 15, 16) were classified as high risk in terms of detection bias because there was no blinding in the outcome assessor and the items of outcome likely to be influenced (ROM, deformity, and self-reported questionnaire). One study (14) was classified as high risk and 5 studies (3, 9, 15, 17, 18) were classified as an unclear risk in terms of attrition bias. The high-risk study has additional participants in the result without further explanation. Meanwhile, the unclear risk studies did not provide any information regarding incomplete outcome data. Two studies (3, 16) were classified as high risk in terms of reporting bias due to reported outcomes in protocol and research articles that were different. Two studies (11, 13) were classified as high risk and 3 (18–20) were classified as unclear risk in terms of other bias due to imbalance in baseline score and no data available, respectively.
Fig. 2.
Risk of bias summary.
Fig. 3.
Risk of bias graph.
Risk of bias was assessed across trials. One study was classified as low-risk (21), 4 studies were classified as unclear risk (17–20), and 9 studies were classified as high-risk (3, 9–16). Therefore, the risk of bias for all studies is classified as high risk.
Characteristics of eligible studies
All 14 included studies were RCTs, conducted in 6 different countries, with a total of 936 participants. Of the 14 studies, 11 evaluated knee OA, 2 hand OA, and 1 hip OA. Characteristics of the eligible studies are summarized in Table I.
Table I.
Characteristics of eligible studies
| Number | Author, year | Study design | Participant | Sample size | Mean age, years ± SD | Time-points | Intervention | Outcome | Result (Mean ± SD) | Significance | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPT | Other | Pain | Functional outcome | |||||||||
| 1 | Rabago, et al. (13) 2013 | RCT | Adults aged 40–76 years with knee OA. Diagnosis based on ACR criteria | DPT: 30 participants Saline: 29 participants Exercise: 31 participants |
Total: 56.7 ± 7.2 DPT: 56.8 ± 7.9 Saline: 56.8 ± 6.7 Exercise: 56.4 ± 7.0 |
Baseline, 5th week, 9th week, 12th week, 24th week, 52nd week. | Injections were performed 3 times, interval 1 month (additional session 2 times injection) 6 mL 25% dextrose injected intra-articularly. 0.5 mL 15% dextrose injected extra-articularly (at each ligament insertion) |
SALINE: Injections performed 3 times, interval 1 month (additional session 2 times injection) 6 mL 0.9% sodium chloride injected intra-articularly 0.5 mL 15% 0.9% sodium chloride injected extra-articularly (at each ligament insertion) EXERCISE: 3 sessions per week, 1 session daily, 10 repetitions per exercise gradually increase therapy as tolerated over 20 weeks (5 sessions per week, 3 times daily, 15 repetitions per exercise) and continue as desired |
Pain: pain WOMAC Functional outcome: total WOMAC |
Score changes DPT group baseline: 66.8 ± 14.9, week 5: –8.17 ± 19.12; week 9: –14.00 ± 19.28, week 12: –11.78 ± 18.81, week 24: –15.50 ± 18.84, week 52: –14.18 ± 18.46 Saline group baseline: 66.7 ± 16.1, week 5: –3.28 ± 18.85, week 9: –5.29 ± 18.15, week 12: –5.79 ± 17.98, week 24: –6.40 ± 18.15, week 52: –7.38 ± 18.35 Exercise group baseline: 63.2 ± 13.1, week 5: –4.53 ± 18.57,week 9: –3.44 ± 18.45, week 12: –4.89 ± 18.3, week 24: –8.07 ± 18.71, week 52: –9.24 ± 18.51 |
Score changes DPT group baseline: 63.1 ± 15.0; week 5: –7.94 ± 17.58; week 9: –13.91 ± 17.69; week 12: –13.31 ± 17.25; week 24: –15.85 ± 17.25; week 52: –15.32 ± 16.9) Saline group baseline: 62.7 ± 14.3; week 5: –5.22 ± 17.29; week 9: –6.75 ± 16.67; week 12: –8.19 ± 16.51; week 24: –8.12 ± 16.65; week 52: –7.59 ± 16.8 Exercise group baseline: 60.5 ± 11.3; week 5: –4.42 ± 16.99; week 9: –2.51 ± 16.94; week 12: –4.26 ± 16.8; week 24: –8.48 ± 17.04; week 52: –8.24 ± 16.98 |
Dextrose outperformed saline (p < 0.05) and exercise (p < 0.05) for pain scale and functional outcome in week 9, week 24, and week 52 Dextrose outperformed exercise (p < 0.05) for pain scale and functional outcome in week 12 There were no other side-effects or adverse events. |
| 2 | Hosseini, et al. (14) 2019 | RCT | Age 50–75 years with grade II or more knee OA. Diagnosis based on ACR criteria | DPT: 52 participants HA: 52 participants |
DPT: 61.2 ± 11.5 HA: 63.7 ± 12.2 |
Baseline, 12th week. | Injections performed 3 times, interval 1 week 10 mL 12.5% hypertonic dextrose injected extra-articularly through 4 points of injection. |
Injections performed 3 times, interval 1 week 2.5 mL hyaluronic acid injected intra-articularly via the inferomedial of patella |
Pain: VAS Functional outcome: WOMAC score |
Time-point score DPT group Baseline: 7.8 ± 1.4; 12th week: 2.5 ± 1.1 HA group Baseline: 8.2 ± 1.7; 12th week 2.1 ± 0.6 |
Time-point score DPT group Baseline: 52.7 ± 9.8; 12th week 83.7 ± 12.7; HA group Baseline: 55.9 ± 10.4; 12th week 88.5 ± 15.6 |
HA outperformed DPT for pain scale (p = 0.02) and total WOMAC (p < 0.001) at week 12 Our results have shown no serious adverse events |
| 3 | Jahangiri, et al. (21) 2014 |
RCT | Age 42–83 years with hand OA. Diagnosis based on clinical evaluation and radiological examination. | DPT: 30 participants LC: 30 participants |
Total: 63.6 ± 9.7 DPT: 63.9 ± 9.4 LC: 63.3 ± 10.1 |
Baseline, 1st month, 2nd months, and 6th months | Injections performed 3 times, interval 1 month. 0.5 mL 20% DPT mixed with 0.5 mL 2% lidocaine injected intra-articularly and extra-articularly |
Injections performed 3 times, interval 1 month. First 2 months placebo injectate 1 mL 0.9% saline administered. At third month, 40 mg methylprednisolone acetate (0.5 mL) mixed with 0.5 mL 2% lidocaine injected intra-articularly and extra-articularly |
Pain: VAS Functional outcome: total HAQ-DI |
Score changes DPT vs HA 0–1st month:– 0.7 ± 3.87; 0–2nd month: 1.0 ± 3.676; 0–6th month: 1.1 ± 3.483 |
Score changes DPT vs HA 0–1st month: 0.5 ± 2.90; 0–2nd month: 1.0 ± 3.289; 0–6th month: 1.0 ± 3.096 |
In the 2nd month, the pain score was significantly more with LC (p = 0.02). Hand function improved significantly in the group DPT compared with LC (p = 0.01). After 6 months, pain on movement more significant in the group DPT (p = 0.02). Hand function was significantly better with DPT (0.01). Adverse event info not available |
| 4 | Gül, et al. (15) 2020 | RCT | Age 18–80 years with secondary hip OA (DDH refractory) who had Crowe Type I–IV lesions | DPT: 20 participants (23 hips) Exercise: 21 participants (23 hips) |
DPT: 45.74 ± 16.86 Exercise: 47.56 ± 13.8 |
Baseline, 21st day, 3rd month, 6th month, 12th month. | Injections were repeat-ed with 21-day intervals. Injection sessions were terminated when the visual analogue scale (VAS) scores decreased to 75% of pre-injection values (maximum 6 times injection) Supine injection points: 8 mL 15% dextrose solution injected extra-articularly insertions. 8 mL 25% dextrose applied intra-articularly Lateral injection points: 12 mL 15% dextrose injected extra-articularly Home exercise: 3 times a day after 3 days of injections |
All participants received standard 12-week rehabilitation protocol and supervised progressive resistance training consisting of 30 training sessions Home exercise: 3 times a day after the 12-week rehabilitation programme |
Pain: VAS Functional outcome: HHS |
Score changes DPT group 0–21 days: – 3.1 ± 1.2; 0–3 months: – 4.0 ± 1.8; 0–6 months: – 4.6 ± 2.6; 0–12 months:– 4.5 ± 2.4. Exercise group 0–21 days: – 1.9 ± 0.9 0–3 months: – 2.6 ± 1.9 0–6 months: 2.8 ± 2.5; 0–12 months: 2.9 ± 2.5 |
Score changes DPT group 0–21 days: 16.8 ± 7.3; 0–3 months: 19.5 ± 8.9; 0–6 months: 24.2 ± 14.0; 0–12 months: 24.3 ± 13.4. Exercise group 0–21 days: 6.7 ± 6.2; 0–3 months: 19.5 ± 8.9; 0–6 months: 14.8 ± 12.4; 0–12 months: 16.5 ± 11.3 |
Dextrose injection significantly outperformed the control injection in pain improvement from 0–21 days (p = 0.001), 0–3 months (p = 0.008), 6 months (p = 0.016) and 0–12 months (p = 0.017), in dysfunction improvement from 0–21 days (p < 0.001), 0–3 months (p = 0.006), 6 months (p = 0.007) and 0–12 months (p = 0.018). Adverse event Only 3 participants in the PrT group had severe pain in the injection sites and they took acetaminophen 4 times/day for 5–7 days after injections |
| 5 | Rahimzadeh, et al. (3) 2018 |
RCT | Knee OA | DPT: 21 participants PRP: 21 participants |
DPT: 64.3 ± 5.31; PRP: 65.5 ± 6.64 |
Baseline, 1st month, 2nd months, 6th months | Injections performed 2 times, interval 1 month 7 mL dextrose 25% injected IA by USG guiding |
Injections performed 2 times, interval 1 month 7 mL PRP solution injected IA by USG guiding |
Pain: pain WOMAC Functional outcome: total WOMAC |
Time-point score DPT group Baseline: 14.6 ± 1.4; 1st month: 9.5 ± 2.3; 2nd month: 7.1 ± 1.7; 6th month: 8 ± 1.6. PRP group Baseline: 14.8 ± 1.5; 1st month: 9.2 ± 2.3; 2nd month: 5.4 ± 1.8; 6th month: 6.2 ± 2.1 |
Time-point score DPT group Baseline: 67.1 ± 7.9; 1st month: 43.8 ± 8.2; 2nd month: 34.8 ± 6.9; 6th month: 38.7 ± 6.6. PRP group Baseline: 67.9 ± 7.3; 1st month: 42.9 ± 10.85; 2nd month: 27.1 ± 9.1; 6th month: 31.4 ± 10.2 |
Better result in PRP at 2 month and 6 month for functional outcome and pain scale Functional outcome (2,6): (p = 0.004; p = 0.009) Pain (2,6): (p = 0.002; p = 0.003) Adverse effect No significant side-effects were observed |
| 6 | Reeves & Hassanein (18) 2000 |
RCT | Hand OA | DPT: 13 participants NS: 14 participants |
DPT: 64.5 ± 9.2 NS: 63.9 ± 9.4 |
Baseline, 6th month | Injections were performed 3 times, interval 2 months. 0.25–0.5 mL 10% dextrose and 0.075% xylocaine in bacteriostatic Water was injected intra-articularly |
Injections were performed 3 times, interval 2 months. 0.25–0.5 mL 0.075% xylocaine in bacteriostatic Water injected intra-articularly |
Pain: VAS (movement, rest, grip). Functional outcome: flexion motion (range) |
Score changes DPT group rest pain –0.88 ± 1.47; movement pain –1.89 ± 1.40; grip pain –1.8 ± 1.51. NS group rest pain –0.58 ± 1.45; movement pain –0.62 ± 1.38; grip pain –0.92 ± 1.53. |
Score changes DPT group Flexion: +8.01 ± 12.83; NS group Flexion: –8.65 ± 10.88 |
DPT outperformed NS in pain movement (p = 0.027) and functional outcome (p = 0.003) Side-effect information Discomfort after injection lasting a few minutes to several days. |
| 7 | Reeves & Hassanein (18) 2000 |
RCT | Knee OA with or without ACL laxity | 25 samples were analysed | 63 years | Baseline, 6th month | Injections performed 3 times, interval 2 months 9 cc 10% dextrose and 0.075% lidocaine in bacteriostatic water injected intra-articularly |
Injections performed 3 times, interval 2 months 9 cc 075% lidocaine in bacteriostatic water injected intra-articularly |
Pain: VAS (pain at rest, walking, stair use) Functional outcome: buckling and flexion range |
Score changes DPT group Pain at rest: 0.54 (0.24) Pain with walking: 1.04 (0.25) Pain with stair use: 1.37 (0.31) NS group Pain at rest: 1.04 (0.25) Pain with walking: 0.98 (0.32) Pain with stair use: 1.23 (0.32) |
Score changes DPT group Buckling 5.24 (2.23) Flexion range: 13.24 (2.15) NS group Buckling 0.79 (2.27) Flexion range: 7.69 (2.19) |
Pain at rest, pain with walking, pain with stair use, swelling, buckling episodes, and flexion range demonstrated a statistically superior effect of active solution (p = 0.015) ADVERSE EVENT Discomfort after injection did not appear. One person (control) had a flare post-injection that appeared substantial, requiring interarticular steroid and then referral to an orthopaedic surgeon. No allergic reactions or infections were noted |
| 8 | Rahimzadeh, et al. (17) 2014 |
RCT | Primary knee OA | Dextrose: 26 participants Erythropoietin: 20 participants, Pulsed radiofrequency: 24 participants |
Total: 59.90 ± 8.08 Dextrose: 60.57 ± 7.47 Erythropoietin: 61.15 ± 7.47, Pulsed radiofrequency: 56.95 ± 8.31 |
Baseline, 2nd week, 4th week, and 12th week | Single-dose injection Intra-articular injection of 5 cc 0.5% ropivacaine together with 5 cc dextrose 25% |
Single-dose injection ERYTHROPOIETIN GROUP intra-articular injection of 5 cc ropivacaine 0.5% together with 4,000 international units erythropoietin. PULSED RADIOFREQUENCY GROUP: participants underwent pulsed radiofrequency (20 ms, 2 Hz, 45 V, 15 min, 42°C, 2 cycles) intra-articular |
Pain: VAS Functional outcome: ROM |
Time-point score DPT group: Baseline: 7.11 ± 1.03 2nd week: 4.50 ± 1.36 4th week: 4.65 ± 1.38 12th week: 5.53 ± 1.60 Erythropoietin group: Baseline: 6.65 ± 0.98 2nd week: 3.15 ± 1.08 4th week: 3.15 ± 0.87 12th week: 3.50 ± 1.23 Pulsed radiofrequency group: Baseline: 7.08 ± 1.41 2nd week: 3.25 ± 2.00 4th week: 3.87 ± 1.70 12th week: 5.50 ± 1.93 |
Time-point score DPT group: Baseline: 101 ± 1.36 2nd week: 106 ± 1.43 4th week: 110 ± 1.26 12th week: 113 ± 2.16 Erythropoietin group: Baseline: 98.08 ± 1.60 2nd week: 124 ± 1.50 4th week: 124 ± 1.4 12th week: 123 ± 1.53 Pulsed radiofrequency group: Baseline: 95 ± 1.97 2nd week: 105 ± 2.06 4th week: 110 ± 2.11 12th week: 113 ± 2.16 |
Erythropoietin more efficient than other 2 interventions. DPT outperformed PRF in reducing pain at 2nd week Side-effect: No particular side-effect related to the above-mentioned interventions was observed |
| 9 | Hashemi, et al. (9) 2015 |
RCT | Age 40–75 years with knee OA diagnosed with clinical and radiographic evaluation | DPT: 40 participants OPT: 40 participants |
DPT: 57.3 ± 15.1; OPT: 59.1 ± 12.3 |
Baseline and 3rd month. | Injections repeated 3 times with 7–10 days interval Intra-articular Hypertonic dextrose prolotherapy (12.5% dextrose) |
Injections repeated 3 times with 7–10 days interval Intra-articular 15 g/mL ozone-oxygen mixture (5–7 cm3) |
Pain: VAS Functional outcome: WOMAC |
Time-point score DPT group: Baseline: 8.1 ± 1.1 3rd month: 3 ± 1.2 OPT group: Baseline: 7.6 ± 1.3 3rd month: 2.8 ± 1.1 |
Time-point score DPT group: Baseline: 58.5 ± 13.3 3rd month: 83.7 ± 15.3 OPT group: Baseline: 56.3 ± 11.5 3rd month: 81.6 ± 13.7 |
Have same effectiveness (p > 0.05) Side-effect: No available information |
| 10 | Waluyo et al. (13) 2021 |
RCT | Knee OA diagnosed based on ACR criteria | DPT: 26 participants HA: 21 participants |
Total: 62.4 ± 8.7 DPT group: 62.6 ± 6.9 HA group: 62.0 ± 10.8 |
Baseline and 12th week | Injections repeated 3 times with 4 weeks interval 5 mL 25% dextrose injected Intra-articularly and 30–40 mL 15% dextrose injected extra-articularly |
Injections repeated 5 times with 1 week interval 2 mL hyaluronic acid intra-articular injection (~10 mg) |
Pain: NRS Functional outcome: total WOMAC |
Score changes DPT group baseline: 4.85 ± 1.71 12th week: –3.38 ± 2.21 HA group baseline: 3.48 ± 1.53 12th week: –1.62 ± 1.63 |
Score changes DPT group baseline: 36.08 ± 10.06 12th week: –16.92 ± 13.86 HA group Baseline: 24.81 ± 17.25 12th week: –8.95 ± 9.79 |
More improvement in DPT group for pain scale. Side-effect: No serious adverse event occurred. All participants experienced expected mild-to moderate post-injection pain within 2–3 days. Only 1 participant, from the prolotherapy group, took paracetamol due to severe pain post-injection. |
| 11 | Sert, et al. (11) 2020 |
RCT | Aged 40–70 years with knee pain refractory to conservative therapy and diagnosed as Grade 2 or 3 KOA according to KL classification | DPT: 21 participants Saline: 22 participants Exercise: 19 participants |
DPT: 55.7 ± 6.6 Saline: 54.4 ± 7.3 Exercise: 52.0 ± 6.1 |
Baseline, 6th week and 18th week | Injections performed 3 times with 3 weeks interval and performed a home-based exercise programme 5 mL 25% dextrose applied intra-articularly 10 mL 15% dextrose solution was applied extra-articularly. |
SALINE: Injections performed 3 times with 3 weeks interval and performed a home-based exercise programme 2.5 mL 0.9% sodium chloride +2.5ml 1% lidocaine applied intra-articularly 5 mL 0.9% sodium chloride +5ml 1% lidocaine extra-articularly EXERCISE: Exercise programme was performed for at least 3 days a week and included hamstring and quadriceps stretching, isometric quadriceps strengthening exercises, and terminal knee extension exercises, each comprising 3 sets with 10 repetitions. |
Pain: VAS Functional outcome: total WOMAC |
Time-point score DPT group baseline: 7.2 ± 1.0; 6-week: 4.1 ± 1.8; 18-week: 1.1 ± 1.9 Saline group baseline: 7.4 ± 2.0; 6-week: 4.9 ± 2.2; 18-week: 4.6 ± 1.8 Exercise group baseline: 7.0 ± 0.9; 6-week: 4.9 ± 2.0; 18-week: 4.5 ± 2.0 |
Time-point change DPT group baseline: 68.7 ± 11.4; 6-week: 44.4 ± 11.5; 18-week: 32.7 ± 11.6 Saline group baseline: 69.2 ± 17.6; 6-week: 50.5 ± 16.7; 18-week: 46.7 ± 13.5 Exercise group baseline: 68.9 ± 11.9; 6-week: 61.0 ± 10.8; 18-week: 59.8 ± 10.7 |
The WOMAC and VAS-pain scores significantly decreased at 18 weeks in the DPT compared with the saline (p = 0.002 and p < 0.001, respectively) and exercise (p < 0.001 and p < 0.001, respectively) |
| 12 | Sit, et al. (20) 2020 |
RCT | Age 45–75 years, diagnosis of KOA based on ACR criteria | DPT: 38 participants Saline: 38 participants |
Total: 63.2 ± 5.5 DPT: 62.8 ± 5.8 Saline: 63.7 ± 5.2 |
Baseline, 16th week, 26th week, and 52nd week. | Injections performed 4 times, interval 4 weeks 5 mL 25% dextrose injected intra-articularly by USG guiding |
Injections were performed 4 times, interval 4 weeks 5 mL normal saline injected intra-articularly by USG guiding |
Pain: VAS Functional outcome: total WOMAC |
Pain intensity (VAS)b 16 weeks: –3.70 (–13.83 to 6.43) 26 weeks: –6.73 (–16.86 to 3.40) 52 weeks: –10.98 (–21.36 to –0.61) Overall trend: –7.02 (–14.50 to 0.46) |
WOMAC composite b 16 weeks: –4.33 (–12.27 to 3.62) 26 weeks: –7.34 (–15.28 to 0.61) 52 weeks: –9.65 (–17.77 to –1.53) Overall trend: –7.03 (–13.14 to –0.92) |
In the study’s primary linear mixed model analysis, all outcomes demonstrated a positive trend favouring the DPT group over the saline group. The composite WOMAC score at 52 weeks showed a difference-in-difference estimate of –9,65 (95% CI, –17.77 to –1.53, p = 0.020), VAS pain intensity score of –10.98 (95% CI, –21.36 to –0.61, p = 0.038) |
| 13 | Pishgahi, et al. (12) 2020 |
RCT | Knee osteoarthritis participants age 40–75 years with radiological signs of grade II, III, and IV | DPT: 30 participants PRP: 30 participants ACS: 32 participants |
DPT: 57.9 ± 1.62; PRP: 58.93 ± 1.71; ACS: 61.28 ± 1.67 |
Baseline, 1st month, and 6th month | Injections administered 3 times, interval 1 week The combination of 50% dextrose (2 mL), bacteriostatic water (2 mL), and 2% lidocaine (1 mL) injected intra-articularly by USG guidance |
PRP Injections were administered 2 times, interval 1 week 4× concentration of platelets and the lowest leukocyte of PRP was injected intra-articularly by USG guidance. ACS Injections administered 2 times, interval 1 week 2 mL ACS injected intra-articularly by USG guidance. |
Pain: VAS Functional outcome: total WOMAC |
Time-point score DPT group Basal: 67.00 ± 2.50 1 month: 63.33 ± 2.47 6 month: 63.30 ± 2.92 PRP group Basal: 61.10 ± 1.21 1 month: 56.33 ± 1.021 6 month: 55.00 ± 2.27 ACS group Basal: 61.25 ± 3.44 1 month: 46.88 ± 4.45 6 month: 35.00 ± 3.51 |
Time-point score DPT group Basal: 65.93 ± 1.67 1 month: 71.67 ± 2.95 6 month: 72.33 ± 2.57 PRP group Basal: 60.33 ± 3.70 1 month: 46.67 ± 4.30 6 month: 45.67 ± 3.82 ACS group Basal: 56.28 ± 3.13 1 month: 49.53 ± 3.67 6 month: 34.88 ± 3.35 |
ACS outperformed DPT in pain and functional outcomes PRP outperformed DPT in functional outcomes, but not significantly different in pain. |
| 14 | Rezasoltani, et al. (16) 2020 |
RCT | Knee osteoarthritis ≥50 years old with KL grade 3 or 4 | DPT: 30 participants PT: 30 participants BN: 30 participants HA: 30 participants |
DPT: 64.8 ± 5.8 PT: 70 ± 6.3 BN: 67.7 ± 7.3 HA: 66.1 ± 9.1 |
Baseline, 1st week, 4th week, 3rd month | Injections performed 3 times, 1 month interval 8 mL 20% dextrose + 2 mL 2% lidocaine injected intra-articularly by USG guidance combined with exercise programme |
PT group Participants received 20 min of superficial heat using a hot pack. Then, TENS 80−100 Hz for 100−200 ms with maximum tolerable intensity. In addition, participants received pulsed ultrasound 1 MHz, 0.8−1.0 W/cm2, 50% duty cycle, 5 min per session combined with exercise programme BN group Single-dose injection 250 units of Dysport, equivalent to 100 units of botulinum neurotoxin type A diluted with 5 mL normal saline injected intra-articularly by USG guidance combined with exercise programme HA group The injections performed 3 times, 1 week interval. 2 mL HA (Hyalgan; Fidia Farmaceutici, Abano Terme, Italy) injected intra-articularly by USG guidance combined with exercise programme |
Pain: VAS Functional outcome: KOOS |
Data was reported in linear model | Score changes DPT group Pain Baseline: 21.5 ± 5.9 3 months: 11.6 ± 6.8 Function, daily Baseline: 39.6 ± 14.1 3 months: 22.2 ± 16.1 Function, sports Baseline: 12.4 ± 2.0 3 months: 5.3 ± 4.3 Quality of life Baseline: 12.2 ± 1.5 3 months: 5.5 ± 3.0 PT group Pain Baseline: 21.3 ± 5.0 3 months: 9.2 ± 5.3 Function, daily Baseline: 34.7 ± 12.9 3 months: 8 ± 16.3 Function, sports Baseline: 13.0 ± 1.8 3 months: 4.3 ± 3.8 Quality of life Baseline: 10.2 ± 2.1 3 months: 3.8 ± 3.7 BN group Pain Baseline: 19.0 ± 6.5 3 months: 11.6 ± 6.7 Function, daily Baseline: 36.8 ± 10.0 3 months: 8 ± 16.3 Function, sports Baseline: 13.0 ± 1.8 3 months: 4.3 ± 3.8 Quality of life Baseline: 10.2 ± 2.1 3 months: 3.8 ± 3.7 HA group Pain Baseline: 20.2 ± 6.6 3 months: 2.1 ± 9.9 Function, daily Baseline: 33.7 ± 13.6 3 months: 2.8 ± 19.6 Function, sports Baseline: 10.8 ± 1.9 3 months: 1.2 ± 5.7 Quality of life Baseline: 9.5 ± 1.1 3 months: 1.7 ± 4.5 |
DPT and BN have similar effectiveness in reducing pain and improving functional outcomes. DPT outperformed PT in reducing pain, but was not significantly different in improving functional outcomes. DPT outperformed HA in both pain and functional outcomes. |
RCT: randomized controlled trial; SD: standard deviation; DPT: dextrose prolotherapy; OA: osteoarthritis; KOA: knee osteoarthritis; KL: kellgren-lawrence; ACR: American college of rheumatology; DDH: developmental dysplasia of the hip; ACL: anterior cruciate ligament; BN: botulinum neurotoxin; PT: physical therapy; HA: hyaluronic acid; ACS: autologous conditioned serum; PRP: platelet-rich plasma; VAS: visual analogue scale; WOMAC: Western and Ontario McMaster Osteoarthritis Index; USG: ultrasonography; TENS: transcutaneous electrical nerve stimulation; HHS: harris hip score.
In the control group, there were 5 studies comparing prolotherapy with saline (10, 11, 18–20), 3 studies compared prolotherapy with exercise intervention (10, 11, 15), 3 studies compared prolotherapy with intra-articular injections of hyaluronic acid (HA) (13, 14, 16), 2 studies compared prolotherapy with platelet-rich plasma (PRP) (3, 12), 1 study compared prolotherapy with ozone prolotherapy (OPT) (9), erythropoietin (EP) (17), pulsed radiofrequency (PRF) (17) and local corticosteroid (LC) (21). These studies used Western and Ontario McMaster Osteoarthritis Index (WOMAC), Harris Hip Score (HHS), Knee Injury and Osteoarthritis Outcome Score (KOOS), Health Assessment Questionnaire Disability Index (HAQ-DI), and ROM scores to assess functional outcomes based on the type of osteoarthritis and pain evaluated using visual analogue scale (VAS) and numerical rating scale (NRS).
Dextrose prolotherapy on functional outcome in generalized osteoarthritis
Fourteen studies assessed the effectiveness of DPT on functional outcomes in general OA patients with a total of 936 participants (3, 9, 18–21, 10–17). Nine studies (10, 11, 15–21) reported that DPT was more effective in improving functional outcomes compared with other interventions (saline, exercise, LC, HA, PRF), 3 other studies reported that DPT had the same effectiveness as OPT (9), HA (13), BN (16), and PT (16). Meanwhile, 4 studies reported that HA (14), PRP (3), EP (17), and ACS (12) were more effective than DPT in improving functional outcomes. These studies found that DPT shows promising results to improve functional outcomes in generalized OA.
Dextrose prolotherapy on pain in generalized osteoarthritis
Fourteen studies assessed the effectiveness of DPT with a total of 936 participants (3, 9–17, 18–21). Ten studies (10, 11, 13, 15–21) reported that DPT was more effective in reducing pain compared with other interventions (saline, exercise, LC, PRF, HA, PT), 3 other studies reported that DPT had the same effectiveness as OPT (9), PRP (12), and BN (16). Meanwhile, another study showed that HA (14), PRP (3), EP (17), and ACS (12) was more effective compared with DPT. These studies showed that DPT has potential to reduce pain in patients with generalized OA.
Dextrose prolotherapy compared with saline
Five studies compared DPT with saline in a total of 280 participants (10, 11, 18–20). Three studies used WOMAC scores for functional outcomes (10, 11, 20), 1 study used flexion ROM (19), and the rest used flexion ROM and buckling frequencies (18). One study used pain WOMAC scores to assess pain (10), while others used VAS (11, 18–20). All studies reported that pain intensity and WOMAC scores were improved significantly in DPT compared with saline.
Dextrose prolotherapy compared with exercise
Three studies, with a total of193 participants, compared DPT with exercise (10, 11, 15). Two studies used the WOMAC scale to assess functional outcomes (10, 11) and 1 study used the HHS scale (15). One study used WOMAC scores for pain (10) and 2 other studies used VAS for pain (11, 15). All studies reported that pain intensity and functional outcomes were more improved in the DPT group than in the exercise group.
Dextrose prolotherapy compared with hyaluronic acid
Three studies compared the effectiveness of DPT with intra-articular HA in a total of 271 subjects (13, 14, 16). Two studies used the WOMAC scale to assess functional outcomes (13, 14), and the other study used the KOOS scale (16). One study used NRS scores to evaluate pain (13), and the other 2 used VAS (14, 16). Two studies reported that DPT outperformed HA in reducing pain (13, 16), only 1 study found HA to be more effective than DPT (14). Regarding functional outcomes, the result was different; 1 study reported that HA outperformed DPT (14), 1 study found that HA and DPT have similar effectiveness (13), and another study reported that DPT was superior to HA (16).
Dextrose prolotherapy compared with platelet-rich plasma
Two studies compared DPT with PRP, in a total of 134 participants (3, 12). Both used the WOMAC scale to assess functional outcome. One study used pain WOMAC scores to assess pain intensity 3, and the other used VAS (12). Both studies reported that PRP outperformed DPT in improving functional outcomes. One study stated that PRP outperformed DPT in reducing pain (3). One study reported that PRP and DPT have similar effectiveness in reducing pain (12).
Dextrose prolotherapy compared with other interventions
Five studies compared DPT with other interventions (9, 12, 16, 17, 21). Three studies compared DPT with OPT, ACS, and LC (9, 12, 21), 1 study compared DPT with PT and BN (16), and 1 study compared DPT with EP and PRF (17). The total number of participants in these studies was 422. Two studies used the WOMAC scale to assess functional outcomes (9, 12), 1 study used the KOOS scale (16), 1 study used HAQ-DI (21), and 1 study used the ROM scale (17). All studies used VAS to evaluate pain. The study that compared DPT with OPT (9) and BN (16) reported that both groups had similar effectiveness in reducing pain and improving functional outcomes. Meanwhile, compared with PT, DPT was more effective in reducing pain, but both groups have similar effectiveness in functional outcomes (16). DPT outperformed PRF (17) and LC (21) in both respects. Only 1 study reported that EP outperformed DPT in both respects (17).
DISCUSSION
Prolotherapy is an alternative injection-based modality used to treat chronic musculoskeletal pain, through the use of several substances, most often dextrose (22). Despite some studies into the mechanism of action of prolotherapy, this process remains unclear. The main mechanism hypothesized by researchers is the regenerative effect. Previous studies have reported that human cells produce various growth factors after exposure to hypertonic dextrose. Normal human cells exposed to hypertonic dextrose begin to produce growth factors, such as platelet-derived growth factor, transforming growth factor-beta, epidermal growth factor, basic fibroblast growth factor, and insulin-like growth factor (23). These growth factors activate fibroblasts to form mature collagen precursors (7). In addition, a low-level chondrogenic effect of dextrose has been demonstrated by Topol et al. (24) and Waluyo et al. (13), through observation using arthroscopy and biomarker changes. In addition, dextrose is also thought to provide nutrients necessary for restoring damage cells, to exert a potential direct effect on peripheral nerves (25), and to strengthen the ligament and tendons through the production of fibrous tissue (26). This systematic review provides an update of current knowledge regarding the use of DPT in OA. Overall, it appears that DPT is effective in reducing pain and improving function in patients with OA; however, the results are at high risk of bias.
While most studies reported a positive effect of DPT in OA, this review found some inconsistent results when comparing DPT with HA. Rezasoltani et al. (16) and Waluyo et al. (13) reported that DPT outperformed HA in reducing pain, while Hosseini et al. (14) found HA to be more effective than DPT. Regarding functional outcomes, all studies reported different results. This could be due to differences in the concentration of DPT, time intervals of injection, and sites of injection. Hosseini et al. used 12.5% dextrose, peri-articularly only, injection was performed 3 times, with 1-week intervals (14). Rezasoltani et al. (16) used 16% dextrose, intra-articularly only, injection 3 times, with 4-week intervals. Waluyo et al. (13) used 25% dextrose intra-articularly and 15% peri-articularly, injection 3 times, with 4-week intervals. Therefore, the optimum effectiveness of DPT would be obtained if the concentration of dextrose was more than 15%, with at least 4-week intervals between injections. Because all the current studies about prolotherapy has concentrated on knee OA. This systematic review also found that all injections with the biological agent as the active substance (EP, PRP, and ACS) were superior to DPT. However, in clinical settings, when physicians consider cost-effectiveness in treating OA, DPT might be cheaper than biological agent-based modalities.
Based on our findings, the concentration and time-interval of DPT would differ depending on the type and severity of OA. The suggested concentration for hand OA is dextrose 10% with a 1-2 month interval. In hip and knee OA patients, Dextrose 15% was recommended for extra-articular injection and D25% for intra-articular injection. With a 21-day interval for hip OA and a 2-4-week interval for knee OA.
The limitations of this study are related to the limited number of RCTs regarding the effects of DPT on OA other than knee OA. In addition, several data were unavailable from some included studies. Despite these limitations, this systematic review discusses OA in a more comprehensive manner, not limited to knee OA, compared with previous publications.
CONCLUSION
Although DPT confers potential benefits for pain and functional outcome in OA, variation in study protocols and intervention choices, and a high risk of bias made it difficult to consolidate its therapeutic benefit. Thus, we can recommend only that DPT could be considered for use in osteoarthritis management. Further high-quality RCTs are warranted to establish the benefits of this intervention. To improve study quality, future studies should include blinding of participants, outcome assessors, and better documentation of missing data and drop-outs.
REFERENCES
- 1.World Health Organization (WHO) . Essential medicines and health products – priority diseases and reasons for inclusion – osteoarthritis. Geneva: WHO; 2013; 12: 6–8. [Google Scholar]
- 2.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019. American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol 2020; 72: 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahimzadeh P, Imani F, Faiz SHR, Entezary SR, Zamanabadi MN, Alebouyeh MR. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging 2018; 13: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linetsky FS, Manchikanti L. Regenerative injection therapy for axial pain. Tech Reg Anesth Pain Manag 2005; 9: 40–49. [Google Scholar]
- 5.Goswami A. Prolotherapy. J Pain Palliat Care Pharmacother 2012; 26: 376–378. [DOI] [PubMed] [Google Scholar]
- 6.Distel LM, Best TM. Prolotherapy: a clinical review of its role in treating chronic musculoskeletal pain. PM&R 2011; 3: S78–S81. [DOI] [PubMed] [Google Scholar]
- 7.DeChellis DM, Cortazzo MH. Regenerative medicine in the field of pain medicine: prolotherapy, platelet-rich plasma therapy, and stem cell therapy – theory and evidence. Tech Reg Anesth Pain Manag 2011; 15: 74–80. [Google Scholar]
- 8.Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashemi M, Jalili P, Mennati S, Koosha A, Rohanifar R, Madadi F, et al. The effects of prolotherapy with hypertonic dextrose versus prolozone (intraarticular ozone) in patients with knee osteoarthritis. Anesthesiol Pain Med 2015; 5: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabago D, Patterson JJ, Mundt M, Kijowski R, Grettie J, Segal NA, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med 2013; 11: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sert AT, Sen EI, Esmaeilzadeh S, Ozcan E. The effects of dextrose prolotherapy in symptomatic knee osteoarthritis: a randomized controlled study. J Altern Complement Med 2020; 26: 409–417. [DOI] [PubMed] [Google Scholar]
- 12.Pishgahi A, Abolhasan R, Shakouri SK, Zangbar MSS, Dareshiri S, Kiyakalayeh SR, et al. Effect of dextrose prolotherapy, platelet rich plasma and autologous conditioned serum on knee osteoarthritis: a randomized clinical trial. Iran J Allergy, Asthma Immunol 2020; 19: 243–252. [DOI] [PubMed] [Google Scholar]
- 13.Waluyo Y, Budu, Bukhari A, Adnan E, Haryadi RD, Idris I, et al. Changes in levels of cartilage oligomeric proteinase and urinary C-terminal telopeptide of type II collagen in subjects with knee osteoarthritis after dextrose prolotherapy: a randomized controlled trial. J Rehabil Med 2021; 53: jrm00196.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini B, Taheri M, Ardekani RP, Moradi S, Mofrad MK. Periarticular hypertonic dextrose vs intraarticular hyaluronic acid injections: a comparison of two minimally invasive techniques in the treatment of symptomatic knee osteoarthritis. Open Access Rheumatol Res Rev 2019; 11: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gül D, Orsçelik A, Akpancar S. Treatment of osteoarthritis secondary to developmental dysplasia of the hip with prolotherapy injection versus a supervised progressive exercise control. Med Sci Monit 2020; 26: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezasoltani Z, Azizi S, Najafi S, Sanati E, Dadarkhah A, Abdorrazaghi F. Physical therapy, intra-articular dextrose prolotherapy, botulinum neurotoxin, and hyaluronic acid for knee osteoarthritis: randomized clinical trial. Int J Rehabil Res 2020; 43: 219–227. [DOI] [PubMed] [Google Scholar]
- 17.Rahimzadeh P, Imani F, Faiz SHR, Entezary SR, Nasiri AA, Ziaeefard M. Investigation the efficacy of intra-articular prolotherapy with erythropoietin and dextrose and intra-articular pulsed radiofrequency on pain level reduction and range of motion improvement in primary osteoarthritis of knee. J Res Med Sci 2014; 19: 696–702. [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med 2000; 6: 68–80. [PubMed] [Google Scholar]
- 19.Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med 2000; 6: 311–320. [DOI] [PubMed] [Google Scholar]
- 20.Sit RWS, Wu RWK, Rabago D, Reeves KD, Chan DCC, Yip BHK, et al. Efficacy of intra-articular hypertonic dextrose (Prolotherapy) for knee osteoarthritis: a randomized controlled trial. Ann Fam Med 2020; 18: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahangiri A, Moghaddam FR, Najafi S. Hypertonic dextrose versus corticosteroid local injection for the treatment of osteoarthritis in the first carpometacarpal joint: a double-blind randomized clinical trial. J Orthop Sci 2014; 19: 737–743. [DOI] [PubMed] [Google Scholar]
- 22.Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care 2010; 37: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean Reeves K, Fullerton BD, Topol G. Evidence-based regenerative injection therapy (prolotherapy) in sports medicine. Sport Med Resour Man 2008: p. 611–619. [Google Scholar]
- 24.Topol GA, Podesta LA, Reeves KD, Giraldo MM, Johnson LL, Grasso R, et al. Chondrogenic effect of intra-articular hypertonic-dextrose (prolotherapy) in severe knee osteoarthritis. PM R 2016; 8: 1072–1082. [DOI] [PubMed] [Google Scholar]
- 25.Distel LM, Best TM. Prolotherapy: a clinical review of its role in treating chronic musculoskeletal pain. PMRJ 2018; 3: S78–S81. [DOI] [PubMed] [Google Scholar]
- 26.Rhatomy S, Margaretha E, Rahmadian R. Dextrose prolotherapy for muscle, tendon and ligament injury or pathology: a systematic review. Annu Res Rev Biol 2020: p. 43–62. [Google Scholar]